Abstract
Treatments for toxoplasmosis such as pyrimethamine have shown numerous side effects. It has been reported that the likelihood of relapse associated with pyrimethamine-based therapy in patients with HIV and toxoplasmic encephalitis (TE) can have significant implications, even for patients who often develop new lesions in areas of the brain previously free of infection. This led us to research for new agents against Toxoplasma gondii. Recent findings have shown the potent biological activity of 4-thiazolidinones. We proposed to design and synthesize a new series of 2-hydrazono-4-thiazolidinones derivatives to evaluate the in vitro growth inhibition effect on T. gondii. The growth rates of T. gondii tachyzoites in Human Foreskin Fibroblast (HFF) cell culture were identified by two in vitro methodologies. The first one was by fluorescence in which green fluorescent RH parasites and cherry-red fluorescent ME49 parasites were used. The second one was a colorimetric methodology using β-Gal parasites of the RH strain constitutively expressing the enzyme beta-galactosidase. The 4-thiazolidinone derivatives 1B, 2B and 3B showed growth inhibition at the same level of Pyrimethamine. These compounds showed IC50 values of 1B (0.468–0.952 μM), 2B (0.204–0.349 μM) and 3B (0.661–1.015 μM) against T. gondii. As a measure of cytotoxicity the compounds showed a TD50 values of: 1B (60 μM), 2B (206 μM) and 3B (125 μM). The in vitro assays and molecular modeling results suggest that these compounds could act as possible inhibitors of the Calcium-Dependent Protein Kinase 1 of T. gondii. Further, our results support the fact that of combining appropriate detection technologies, combinatorial chemistry and computational biology is a good strategy for efficient drug discovery. These compounds merit in vivo analysis for anti-parasitic drug detection.
Keywords: 4-Thiazolidinones, Toxoplasma gondii, Cytotoxicity, Invasion, Growth
Graphical abstract
Highlights
-
•
The structures of 16 4-Thiazolidinones synthesized were characterized by spectral data.
-
•
The 2-pyridine that contains 5-substituted 4-thiazolidinone showed low cytotoxicity and high selectivity index.
-
•
The 2-hidroxyphenyl and 2-pyridinyl hydrazinyl derivatives showed the best anti-T. gondii activities.
1. Introduction
Toxoplasmosis is a disease produced by the Toxoplasma gondii protozoan parasite, which is a cosmopolitan food and waterborne infection with an estimated 1 to 2 billion (approximately 30%) of the world's population infected (Bahia-Oliveira et al., 2017). The most efficacious treatment for this infection is based on Pyrimethamine with Sulfadiazine. Pyrimethamine belongs to the group of amino-pyrimidines, and Sulfadiazine is related to sulfonamides. They inhibit two different reactions on the same metabolic pathway, and thus exhibit synergistic activity associated with nucleic acid synthesis. For this reason, this treatment is supplemented with folinic acid kwon as Leucovorin (Montoya and Liesenfeld, 2004). The idea of this treatment is to reduce the growth of the parasite considering that the parasite divides faster than the host cell and that T. gondii cannot synthesize nucleic acids from exogenous folinic acid, while humans can do it. However, this treatment produces severe secondary effects like hypersensitivity, hematological toxicity, teratogenicity, and allergic reactions. The development of adverse effects leads to interruption of the therapy and change for less efficacious antiparasitic drugs (de la Torre et al., 2011). Although the mix from these compounds can actively control the tachyzoite growth, they have little effect against the bradyzoite stage that forms the cyst tissue and therefore they does not cure the infection. The adverse event (AE) related profiles associated with Pyrimethamine-based treatments differ by Toxoplasma manifestation. In ocular toxoplasmosis and TE, the most frequent AEs were dermatologic, while in congenital toxoplasmosis, the most frequent AEs were associated with bone marrow suppression. Steven–Johnson syndrome was uncommon and reported in only three patients (0.1%). Hematologic AEs occurred in all manifestations and highlight the importance of monitoring the blood of patients administered Pyrimethamine-based regimens (Ben-Harari et al., 2011; Shammaa et al., 2021). Thus, there is a need to find new anti-Toxoplasma agents with better inhibitory activity and low or no cellular toxicity. The 4-thiazolidinone scaffold has been shown as a promising drug-like compound against T. gondii due to its demonstrated biological effects and the experimental information reported by several authors showing high therapeutic index (TI) values in vitro (Fig. 1) (Tenorio et al., 2005; de Aquino et al., 2008; Carvalho et al., 2010; Liesen et al., 2010; D'Ascenzio et al., 2014; Carradori et al., 2017).
Fig. 1.
4-Thiazolidinones derivatives with anti-T. gondii activity.
Modifications within the nucleus of 4-thiazolidinone greatly affect the physical-chemical properties of the molecule (e.g. solubility), for this reason, structural diversity is very important (Shelat and Guy, 2007). Modifications on positions 2 and 5 of the 4-thiazolidinone ring have been considered as a reference point to enhance the biological activity of compounds that contain this core. Additionally, the moiety hydrazone conserved in this position of the heterocyclic ring has demonstrated to enhance the effect of the 4-thiazolidinones against T. gondii, since the molecules that have this group showed high effectiveness on the host cell invasion and replication of the parasite with low cytotoxicity, to concentrations in the micro molar scale (Hamama et al., 2009; Rocha-Roa et al., 2018). On the other hand, the 2,5-disubstituted 1,3-thiazole nucleus could be an important pharmacological requirement, so the position N-3 in the 4-thiazolidinones would be important as an electron donor region (D'Ascenzio et al., 2014; Carradori et al., 2017). Because of it, the objectives of this study were to design and synthesize a new series of 2-hydrazono-4-thiazolidinones derivatives to evaluate if the molecules have in vitro growth effect of T. gondii as an alternative of new molecular entities with possible anti-Toxoplasma activity.
2. Methods
2.1. Chemistry
The solvents and chemicals were procured from commercial chemical suppliers (Merck.KGaA, 64271 Darmstadt, EDM Millipore Corporation (Germany), Alfa Aesar, 30 Bond Street, Ward Hill) and used without further purification. Melting points were recorded using an Electrothermal IA-9100 digital fuser. Thermo Scientific Nicolet 380 spectrophotometer was used for FTIR spectra. 1H (400 MHz) and 13C NMR (100 MHz) spectra were recorded on Bruker Avance 400 instrument in DMSO‑d6. HRMS spectra were recorded using the Shimadzu GCMS-QP2010 Ultra instrument. All reactions were monitored by TLC performed on silica gel 60 F254 plates. The detail of synthesis experiments are given in the Supplementary data.
2.2. Cell line, parasites and culture conditions
2.2.1. HFF cells
Human foreskin fibroblasts (HFF; Fibroblast, Skin; foreskin, normal, human. ATCC No: SCRC-1041) obtained from the American Type Culture Collection (ATCC), Rockville, Maryland. HFF were maintained in DMEM media supplemented with 10% FBS (Fetal Bovine Serum-Gibco, USA), 1X of GlutaMAX 100X, 1 mM of sodium pyruvate 100 mM and 50 μL of penicillin (10,000 Units/mL) and streptomycin (10,000 μg/mL) per mL at 37 °C with 5% CO2.
2.2.2. Parasites
Toxoplasma gondii strains modified genetically that express enzyme substrate or fluorescent labels were used for the in vitro experiments: T. gondii RHβ1 strain (β-galactosidase tagged, kindly donated by Dr. John Boothroyd from Stanford University), T. gondii RH GFP strain (green fluorescent protein-tagged, kindly donated by Dr. David Sibley, University of Washington) and the T. gondii ME49 cherry strain (cherry fluorescent-tagged, kindly donated by Dr. Laura Knoll, University of Wisconsin) were maintained in DMEM media supplemented with 10% heat-inactivated bovine serum (GE Healthcare Life Sciences).
2.3. Infection of human cell lines and measurement of the effect of compounds on T. gondii growth
T. gondii RH-GFP and RHβ1 were passaged in confluent human foreskin fibroblast (HFF) monolayers. RH-GFP (Green Fluorescent Protein) does not require external factors and is stable throughout a wide spectrum of conditions (Striepen et al., 1998). RHβ1 is the RH strain carrying the Escherichia coli lacZ (β-galactosidase) gene under the control of the SAG1 promoter (Seeber et al., 1996). To identify the growth rates of T. gondii tachyzoites in HFF cell culture, three methodologies were used. To establish a direct effect over the parasite and its growth T. gondii GFP parasites of the strain RH (virulent) were used with the ability to emit fluorescence upon receiving excitation at 395–475 nm, so a green fluorescence can be generated and observed in a fluorescence microscope. The other two are quantitative methodologies, the first one using T. gondii β-Gal parasites of the RH (virulent) strain that is genetically modified that constitutively express beta-galactosidase, then the substrate X-Gal is added, producing a blue coloration that can be quantified by spectrophotometric reading at 615 nm wavelength. The second one is by using ME49 cherry fluorescence quantified by the IncuCyte Performing analysis, without removing cells from the incubator or disturbing cultures that automatically acquire and analyze fluorescence and phase-contrast images around the time.
2.4. HFF cell viability by Alamar blue assay
HFF cells were cultured as monolayers and maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified atmosphere with 5% CO2 at 37 °C in T-25 flasks and were subcultured twice a week. Cell lines were plated with approximately 20,000 cells per well and let grown until confluence. Once confluent, the cells were treated with a compound concentration of 10 μM for 24 h. This was followed by an Alamar Blue assay with 0.5 mM resazurin at 37 °C with 5% CO2 for 4 h. All concentrations were performed in triplicated. A fluorescent reading was then taken with a BioTek Synergy HT plate reader at 530/25 excitation and 590/25 emission. Host cell viability was determined by comparing the treatments with no compound treatment as 100% viability. A color change from blue to pink indicated the metabolically active cells. A color change in the growth control well to pink indicated proper cell growth and no color change in the sterile control well-indicated absence of contaminants.
2.5. T. gondii RH GFP tachyzoites 24h viability assay
RH-strain parasites were grown in HFF cells. Parasites to be used in the assay were prepared as follows. Cultures that had completely lysed an HFF monolayer were recollected into a centrifuge tube and were washed with 10 mL of PBS 1X centrifuging to 500 g × 5 min at room temperature to remove host cell debris and parasite aggregates. The supernatants were passed to another centrifuge tube and the pellets were collected after centrifuging to 1800 g × 10 min at room temperature. The resulting parasites were resuspended, counted, and diluted in the medium. T. gondii tachyzoites (5 × 105) were treated for 24h directly with molecules and the Pyrimethamine at 10 μM as a control. The parasites that were glowing green were considered viable. The percentage of viable cells was calculated using the following formula: (Fluorescence of the sample with treatment URF * 100)/fluorescence of the control without treatment, where URF = unit relatives of fluorescence calculated with the formula: (sample fluorescence/fluorescence in blank well) *100. Parasites in 96-well plates were directly studied by fluorescence microscopy by using a standard fluorescence EVOS Light Cube GFP. Excitation 470/22 Emission: 526/60 INVITROGEN LIFE TECHNOLOGIES with a 10× objective on EVOS FL Color Imaging System. Images were recorded with and adjusted for contrast with ImageJ. The fluorescence of the wells with parasites treated as compared with those of the control without treatment. For all the methodologies the compounds were dissolved in DMSO (Fischer Scientific). The concentration for DMSO did not exceed 1% in all assays to prevent cytotoxicity.
2.6. T. gondii RH GFP tachyzoites in 48h growth assay
HFF cells were plated in 96 well plates at approximately 20,000 cells per well and allowed to grow until confluent. Parasite cultures that had completely lysed an HFF monolayer were recollected into a centrifuge tube and were washed with 10 mL of PBS 1X centrifuging to 500 g × 5 min at room temperature to remove host cell debris and parasite aggregates. The supernatants were passed to another centrifuge tube and the pellets were collected after centrifuging to 1,800 g × 10 minutes at room temperature. The resulting parasites were resuspended, counted, and diluted in media to a concentration of 2 × 106 per mL, and 5 μL of this suspension was added to the appropriate wells of the microtiter plates, which already contained the compounds to be tested, to give an inoculum of approximately 10,000 parasites per well then we added the molecules. For the assays with the RH GFP strain, the fluorescence was quantified by fluorescence microscopy using a standard fluorescence EVOS Light Cube GFP. Excitation 470/22 Emission: 526/60 INVITROGEN LIFE TECHNOLOGIES with a 10× objective on EVOS FL Color Imaging System. Images were recorded with and adjusted for contrast with ImageJ. The plates were then incubated at 37 °C with 5% CO2 for 48h. After this time each well was gently washed and media replaced. The fluorescence of the well with parasites treated was compared with the control without treatment. GFP fluorescence was quantified by fluorescence microscopy using a standard fluoresce EVOS Light Cube GFP. Excitation 470/22 Emission 526/60 INVITROGEN LIFE TECHNOLOGIES with a 10× objective on EVOS FL Color Imaging System. Images were recorded with and adjusted for contrast with ImageJ. The plates were then incubated at 37 °C with 5% CO2 for 48h.
2.7. T. gondii expressing β-galactosidase for colorimetric assessment
We used microtiter assay for drug evaluation with a strain of T. gondii that expresses bacterial β-galactosidase. Parasites were kindly donated by John Boothroyd. This assay provides a high-throughput and nonradioactive alternative for the identification of anti-T. gondii compounds. An inoculum of approximately 10,000 parasites per well was used in wells that already contained the compounds, compared with the control without treatment. The plates were then incubated at 37 °C with 5% CO2 for 96 h. After this time 2 μl of X-Gal 30 mg per mL (Sigma-USA), was added to give a final concentration of 100 μM of X-gal. The plates were incubated at 37 °C with 5% CO2 for an additional 24 h and were then read at 420 and 615 nm on a Bio-Tek microtiter plate reader. Note that:
-
●
Previously a suitable number of parasites was standardized to perform the experiments since this number allowed to find differences in the readings and coloration produced by the X-Gal substrate. Thus, for each experiment 10,000 parasites per well were used in plates of 96 well to perform the HFF cell growth experiments after adding 4-thiazolidinone molecules.
-
●
For the colorimetric technique like X-Gal we have to take into account that the molecules can produce color when they were diluted in DMSO and mixed with the DMEM media. Then the absorbance values produced were rested with the control color of each molecule (media culture and molecules).
2.8. Effect on T. gondii ME49 strain 5 days growth assay
T. gondii growth inhibition in vitro screens: HFF cells were plated in 24 well plates and allowed to grow until confluent monolayer. Once confluency was reached, 40.000 ME49-cherry-cherry tachyzoites were added to each well and the plates were incubated at 37 °C for 24h to allow for infection. Media was then replaced, and compounds dissolved in DMSO (Fischer Scientific) were added at a concentration of 10 and 5 μM according to the HFF cytotoxicity results. The concentration of DMSO did not exceed 1% in all assays to prevent cytotoxicity. All concentrations were performed in duplicate and Pyrimethamine 10 μM was used as a positive control. A fluorescent reading was then taken with an IncuCyte S3 (Sartorius-Germany) plate reader at 530/25 excitation and 590/25 emission for 5 days.
2.9. Host cell and extracellular parasite pre-treatment assay
HFF cells were grown in 24 well plates to confluency. 10 μM of the respective compound was added to the wells (performed in triplicate). After 24 h of compound exposure, cells were then washed twice with media. Cells were then infected with 4 × 104 ME49 cherry tachyzoites/well and parasite growth was quantified by fluorescence for 5 days post-infection in the IncuCyte S3. To evaluate the effect of compound exposure to free tachyzoites, 10 μM of the respective compound was added to ME49-cherry tachyzoites isolated from culture and resuspended in media at 4 × 106 tachyzoites/mL. Exposure to the compound lasted 4 h at 37 °C. After treatment, tachyzoites were centrifuged and washed twice. Confluent HFF cells in 24 well plates were then infected with 4 × 104 treated tachyzoites per well. Tachyzoite growth was quantified by fluorescence at 5 days post-infection in the IncuCyte S3.
2.10. IC50 calculation and statistical analysis
The IC50 calculation and statistical analysis were performed on Graph pad prism 6.0. The Kruskall Wallis test and the Dunn test were applied to make multiple comparisons between the treatments and the negative control. Also, the Mann Whitney test to compare given concentrations versus the control. The Cytotoxicity assay increases concentrations to obtain the Median toxicity dose (TD50), as the concentration increases, the viability of HFF cells decreases. The cytotoxicity test allows us to approximate the mean TD50 toxicity, a measure of cytotoxicity, and helps to find the therapeutic index calculated by TD50/IC50. We use the data obtained with the strain ME49 to have a concentration gradient of the effect of molecules so that calculate the IC50 after the transformation and normalization of data (Supplementary data).
2.11. Molecular docking
Molecular docking calculations were carried out to obtain the possible binding mode of the best compounds with two molecular targets from T. gondii, the Calcium-Dependent Protein Kinase 1 (TgCDPK1) with PDB code 4JBV and the ROP18 kinase (TgROP18) with PDB code 4JRN. Previously, we suggest that these proteins may act as a target for compounds derived from the 4-thiazolidinone nucleus (Molina et al., 2018; Rocha-Roa et al., 2018). The three-dimensional structure of the compounds was drawn and optimized using Avogadro software (Hanwell et al., 2012). Both receptors (proteins) and ligands (compounds 1B, 2B, and 3B) were prepared using Autodock Tools v1.5.6 software (Morris et al., 2009), for this, polar hydrogens and charges were added; additionally, for the ligands, the torsions were also added. Molecular docking calculations were performed in triplicates and carried out using the Autodock Vina (Trott et al., 2010) and Smina (Koes et al., 2013) software. All the search boxes used had a dimension of 25 Å × 25 Å x 25 Å and were centered on the co-crystallized ligands. A value of 15 for the exhaustiveness parameter was used. To set the boxes, we did a re-docking of the co-crystallized ligands in each receptor.
3. Results and discussion
3.1. Chemistry
Previously, different research groups have reported 4-thiazolidinone molecules with increased anti-T. gondii activity (Liesen et al., 2010; D'Ascenzio et al., 2014; Molina et al., 2018; Abdizadeh et al., 2020). Thus, we designed compounds with the 4-thiazolidinone ring and different substituents in the 2-hydrazonyl fragment and the 5 positions of the main nucleus, to increase their activity anti-parasitic.
In the first step (Scheme 1) for the synthesis of modified compounds, the aryl thiosemicarbazones (3) were synthesized with 80–90% yield through condensation of aldehyde and acetophenones (1) with thiosemicarbazide (2) in a mixture of dry ethyl alcohol containing a catalytic amount of glacial acetic acid (Hussein et al., 2019; Xun-Zhong et al., 2020). The procedure for the synthesis of 2-(hydrazono)-4-thiazolidin-5-ylidene acetate derivatives (5) consisted on an intramolecular cyclocondensation of thiosemicarbazones (2) in methanol solution with dimethyl acetylenedicarboxylate (DMAD) (Yavari et al., 2007; Aly et al., 2014). Initially, the stirring of an equimolar mixture of both compounds in methanol at room temperature, after 1 h of stirring afforded a precipitated. The orange solid (9B) was isolated and purified after several washes with ice water. To explore the generality and scope of the approach previously described, intramolecular cyclocondensation of DMAD with various thiosemicarbazones were tested under the optimal conditions found.
Scheme 1.
General synthesis of the derivatives 1–16B. Reagents and conditions: (a) EtOH, microwave at 40W of power; (b) MeOH, stirring at 500 rpm and room temperature.
In all cases reactions proceeded with the same behavior, affording the expected 2-(hydrazono)-4-thiazolidin-5-ylidene acetate derivates (1–16B) in excellent yields, and characterized based on their physical, analytical, and spectral data (Table 1). The structures of the synthesized compounds were confirmed by FTIR, 1H, and 13C NMR spectroscopy and HRMS. The IR spectrum of 9B exhibited a characteristic absorption at 3467 cm−1 y 3136 cm−1 due to the stretching of the OH and NH, respectively at about 1713 and 1608 cm−1 due to carbonyl groups. The presence of C N group was confirmed by the characteristic for the stretching band at 1515 cm−1. The 1H NMR spectrum of 9B showed downfield two singlets at 12.83 ppm for NH and 9.58 ppm for the OH group; one doublet at 7.49 ppm, one doublet of doublets at 7.37 ppm, and on doublet at 6.89 ppm for aromatic protons. The vinyl proton was observed at 6.67 ppm and three CH3 groups at 3.86–2.42 ppm. The 13CNMR spectrum of 9B showed 15 signals, which is in agreement with the proposed structure. The 1H and 13C NMR spectra of 1–16B were similar to those of 9B, except for the substituted phenyl moiety that exhibited characteristic resonances in the appropriate regions of the spectra. Finally, the mass spectrum of 9B exhibited an intense molecular ion peak at m/z = 349.36 consistent with the molecular formula of C15H15N3O5S.
Table 1.
Main characteristics of the 2-hydrazono-4-thiazolidinones compounds.
| Compound |  |
Reaction time | Melting points (°C) | M. weight (g/mol) | Yield (%) | Color |
|---|---|---|---|---|---|---|
| 1B | 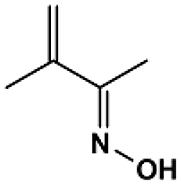 |
2h-40′ | 258–260 | 284129 | 91.4 | Beige |
| 2B |  |
1h-13′ | 254–256 | 304.32 | 91.3 | Yellow |
| 3B | 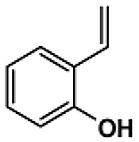 |
2h-30′ | 296–298 | 305.31 | 91.5 | Yellow |
| 4B |  |
2h-35′ | 295–297 | 405.11 | 90.7 | Orange |
| 5B |  |
10h-30′ | 278–280 | 368.75 | 78.9 | Beige |
| 6B |  |
2h-45′ | 289–291 | 305.31 | 88.8 | Orange |
| 7B |  |
2h | 288–289 | 349.36 | 95.2 | Yellow |
| 8B |  |
1h-10′ | 265–267 | 319,34 | 94.3 | Yellow |
| 9B | 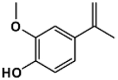 |
1h | 229–231 | 349.36 | 88.1 | Orange |
| 10B |  |
1h-35′ | 238–240 | 317.00 | 92.8 | Yellow |
| 11B | 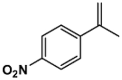 |
1h-20′ | 284–286 | 348.33 | 86.6 | Yellow |
| 12B |  |
1h-45′ | 230–232 | 319.34 | 90.5 | Yellow |
| 13B |  |
2h-30′ | 344–346 | 390.8 | 96 | Yellow |
| 14B | 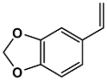 |
1h-25′ | 299–301 | 333.32 | 92.5 | Gray |
| 15B |  |
2h-15′ | 295–297 | 335.34 | 76.4 | Yellow |
| 16B |  |
1h-30′ | 290–291 | 330.32 | 87 | Orange |
3.2. Evaluation of biological activities
In vitro assay systems to screen drugs for anti-T. gondii is focused on growth assays of the parasite or infection of host cells. It is very important to be able to identify anti-T. gondii candidates using different in vitro assay systems. In this evaluation, we used different systems to filter molecules, taking into account that each technique has its effectiveness and limitations (Carey et al., 2004; Jin et al., 2012).
3.2.1. HFF cell viability by Alamar blue assay
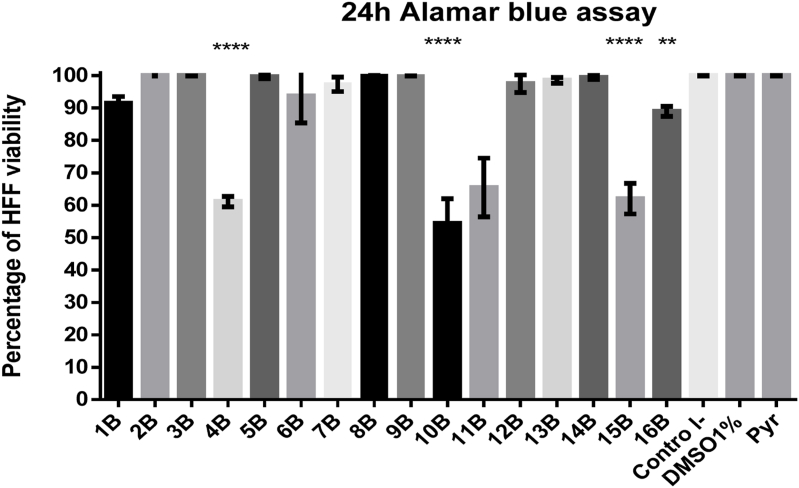
The treatment of HFF cells with molecules 4B, 10B, 11B and 15B at a single compound concentration of 10 μM for 24 h were toxic because reduced more than 50% percent of the cell viability when compared to the negative control (Fig. 2). However, treatment with these same molecules at a concentration of 5 μM were not toxic.
Fig. 2.
Evaluation of cell viability by Alamar Blue assay. Cells were treated with a single compound concentration of 10 μM for 24 h. Molecules 4B, 10B, 11B, 15B reduced the percentage of viability of the HFF cells. The results in the bar graph are the average ± standard deviation of the percentage of viability in triplicate of a representative experiment. Once confluent, cells were treated with a compound concentration of 10 μM for 24 h. This was followed by an Alamar Blue assay with 0.5 mM resazurin at 37 °C with 5% CO2 for 4 h. The results in the bar graph are the average ± standard deviation of the percentage of triplicate viability of a representative experiment.
3.2.2. Viability assay of T. gondii RH GFP tachyzoite at 24h
T. gondii RH GFP tachyzoites were treated with compounds at the concentration of 5 μM and 10 μM for 24 h according to with the previous HFF cell viability test (Fig. 2) and Pyrimethamine at the concentration of 10 μM as seen in Fig. 3. The parasites that were glowing green were considered viable and the percentage of tachyzoites viability was compared with parasites without treatment. Molecules labeled as 1B, 2B, 3B, 4B, 6B, 9B and 12B reduced in more than 50% the viability of the parasites.
Fig. 3.
Viability of RH-GFP tachyzoites in host-cell free medium, parasites were treated with compounds at 10 μM and 5 μM for 24 h at 37 °C with 5% CO2. Pyrimethamine (Pyr) and DMSO at 1% were used as controls. Molecules 1B, 2B, 3B, 4B, 6B, 9B and 12B reduce the viability of parasites. Pyrimethamine didn't reduce the viability of the parasites. The results in the bar graph are the average ± standard deviation of the percentage of the percentage of viability in triplicated of a representative experiment.
3.2.3. Effect of compounds on T. gondii RH GFP tachyzoites 48h growth assay
Molecules with group 2-hydrazono-4-thiazolidinone (1B, 2B, 3B, 4B, 6B and 12B) reduced the percentage of growth at the same level of the control Pyrimethamine measuring GFP fluorescence in T. gondii RH-GFP tachyzoites (Fig. 4).
Fig. 4.
48h growth assay of RH-GFP tachyzoites. Microtiter plates with the compounds were infected with an inoculum of approximately 10,000 parasites per well. Molecules 1B, 2B, 3B, 4B, 6B and 12B reduce the percentage of growth similarly to pyrimethamine. Fluorescence of GFP RH parasites was quantified by fluorescent microscopy after 48h of treatment by using a standard fluorescence EVOS Light Cube GFP. Excitation 470/22 Emission: 526/60 with a 10× objective on EVOS FL Color Imaging System. Images were recorded with and adjusted for contrast with ImageJ.
3.2.4. Effect of compounds on T. gondii expressing beta-galactosidase
Toxoplasma strains stably expressing bacterial β-galactosidase makes possible to analyze after 96h of incubation using a colorimetric growth assays to evaluate a given drug's activity (McFadden et al., 1997). We analyzed the same molecules with both techniques T. gondii GFP growth (Fig. 3, Fig. 4) and β-galactosidase RH strain (Fig. 5A). The 4B, 6B and 12B molecules reduced more than 50% of the viability of the parasites and the percentage of growth, at the same level that the control Pyrimethamine by GFP assay, but they were not selected for further experiments with ME49 because they did not reduce the growth in more than 50% in β-galactosidase assay. The molecules 1B, 2B and 3B were selected for further profiling using T. gondii ME49 because they show interesting results; low toxicity in HFF measure by resazurin, direct effect and proliferation effect, assessed by fluorimetric and colorimetric technique (Fig. 5B).
Fig. 5.
Colorimetric assessment using T. gondii expressing β-galactosidase. (A). Growth inhibition screen with T. gondii β-galactosidase parasites (1B, 2B, 3B) from the group 2-hydrazono-4-thiazolidinone reduce more that 50% of the growth of the parasite. 4B, 6B, 9B, 12B didn't have an effect on the strain β-galactosidase. (B) We identified promising derivatives (1B, 2B, 3B) and assessed for further profiling in ME49 strain.
3.2.5. Effect on T. gondii RH cherry and ME49 strains at 5 days assay
Then, the RH cherry parasites were used to test the reproducibility of 1B, 2B, and 3B molecules (Fig. 6A). These molecules reduce the proliferation of a 24h after established infection. The molecules 1B, 2B, 3B were selected for further profiling using T. gondii ME49 (Type II lineage) which is considered less virulent and more representative of natural infections (Howe and Sibley, 1995). Fig. 6B shows the reduction of proliferation of a 24h established infection and we also use this strain to calculate the IC50 (Table 2).
Fig. 6.
(A) Effect on T. gondii RH cherry strain 5 days assay. The RH cherry parasites were used to test reproducibility of 1B, 2B, and 3B molecules. All concentrations were performed in duplicate at 10 μM this concentration also was used for Pyrimethamine as a positive control. The results on the bar chart are the percentage of growth of a representative experiment. (B) Effect on T. gondii ME49 strain 5 days growth assay. The molecules 1B, 2B and 3B were selected for further profiling using T. gondii ME49 (Type II lineage) which is considered less virulent and more representative of natural infections. (Supplementary data Figure 6 concentration gradient to find IC50).
Table 2.
Anti-Toxoplasma activity, Median toxicity dose, Therapeutic index and molecular docking results for the best 4-thiazolidinone compounds.
| Molecule | IC50 (μM) |
TD50 (μM) |
TI | Affinity TgCDPK1 (kcal/mol) |
Affinity TgROP18 (kcal/mol) |
||
|---|---|---|---|---|---|---|---|
| A. Vina | Smina | A. Vina | Smina | ||||
| 1B | 0.46 | 60 | 128 | −7.16 ± 0.03 | −7.26 ± 0.03 | −6.30 ± 0.00 | −6.40 ± 0.10 |
| 2B | 0.20 | 206 | 1000 | −7.90 ± 0.00 | −7.90 ± 0.00 | −6.56 ± 0.03 | −6.60 ± 0.00 |
| 3B | 0.66 | 125 | 188 | −7.63 ± 0.12 | −7.93 ± 0.23 | −6.96 ± 0.03 | −7.10 ± 0.00 |
3.2.6. Host cell pre-treatment and extracellular tachyzoite exposure results
To describe a possible mode of action, both HFF cells and tachyzoites were pre-treated with 10 μM of the molecules. When HFF cells were pre-treated with molecules before infection, there was no change in parasite growth; when the parasite was pre-treated with molecules before infection, there was decrease growth of the parasite. When HFF cells were treated with 2B, there was no significant change in growth. However, when tachyzoites were treated with molecule 2B before infection, the growth percentage was reduced to 40.3% (Fig. 7). From these data, it can be reasoned that 2B molecule can be taken up by the host cell to affect the growth of T. gondii while that molecules, 1B and 3B can have a direct effect on the extracellular parasite before the invasion and on the host cell.
Fig. 7.
Parasite Growth inhibition with Pre-treatment of Host cell and extracellular tachyzoite. (A) HFF cells were pre-treated with compounds at 10 μM and incubated at 37 °C for 24 h. Cells were then washed and infected with 40000 ME49 cherry tachyzoites. (B) ME49 cherry tachyzoites in host-cell free medium were pre-treated with compounds at 10 μM for 4 h at room temperature, then 40,000 parasites were used to infect HFF. Fluorescent readings were taken 5 days post-infection for both experiments.
3.2.7. Inhibitory concentration calculation and statistical analysis
Sixteen N-functionalized derivatives-4-thiazolidinones 5-acetates were synthesized and evaluated in vitro to determine their antiparasitic effect against T. gondii in different strains such as TgRH GFP and TgME49 cherry-red. The results of HFF cell viability show that compounds 1B, 2B and 3B at a concentration of 10 μM do not alter cellular metabolism on HFF cells measure by Alamar Blue (Fig. 2). Likewise, the TgRH GFP parasite viability assay after 24 h of incubation shows that molecules 1B, 2B, 3B and 4B significantly reduce tachyzoites viability between 60 and 85% (Fig. 3).
The parasite intracellular growth assay shows that compounds 1-4B, 6B and 12B significantly reduce the multiplication of the parasite at a concentration of 10 μM and are similar to Pyrimethamine by GFP (Fig. 4) The graph of the growth percentage of T. gondii in strain ME49 highlights that molecules 1B, 2B and 3B at a concentration of 10 μM presented a similar effect as the Pyrimethamine control (10 μM), with which, the correlation and reproducibility of the activity results of the 4-thiazolidinone derivatives in TgRH and TgME49 (Fig. 6A and B).
Due to the good correlation that the previous tests presented, the intracellular growth effect test on TgME49 was carried out in 5 days of exposure, this experiment determined that compounds 1B, 2B and 3B presented similar effects than Pyrimethamine control at a concentration of 10 μM.
To know the mode of action of the molecules synthesized molecules in both HFF cells and tachyzoites, pre-treatment tests were carried out with the best compounds at a concentration of 10 μM. The mean toxic dose (TD50) was calculated for the three molecules 1B (60 μM), 2B (206 μM) and 3B (125 μM) (Fig. 8). Similarly, the IC50 was calculated for 1B (0.46 μM), 2B (0.20 μM) and 3B (0.66 μM). The lower a therapeutic index (TI), the greater the toxicity of the molecule on the host cell. On the contrary, a high TI indicates that the drug is less toxic to HFF cells. The therapeutic index as a measure of efficacy is calculated by TD50/IC50. The TI for the molecules were: 1B (128), 2B (1,000) and 3B (188). The maximum mean inhibitory concentration is a measure of the potency of a substance to inhibit a specific biological or biochemical function. The fluorescence units for the cherry-ME49 strain were plotted. The transformation and normalization of the data were made to find the IC50 (Table 2). The results indicate that the 2B molecule exhibits greater selectivity for tachyzoites relative to host cells.
Fig. 8.
HFF Cytotoxicity assay by increasing concentrations in order to obtain the TD50 Median toxicity dose, a measure of cytotoxicity and helps to find the therapeutic index.
3.3. Molecular docking
We have carried out molecular docking to describe a possible binding mode of our best compounds in the parasite T. gondii. We use proteins for which some reports suggest that they may act as a target for compounds derived from the 4-thiazolidinone nucleus (Molina et al., 2018; Rocha-Roa et al., 2018). The results for molecular dockings (Table 2) suggest that compounds 1B, 2B and 3B would have a greater affinity (more negative value) for TgCDPK1 protein than for TgROP18. TgCDPK1 protein is one of the most studied molecular targets in drug design against T. gondii, this can be reflected in the most of 40 crystallizations deposited in the Protein Data Bank. The chemical structures of the compounds that have been most studied as inhibitors of TgCDPK1 are derivatives of the pyrazolopyrimidine nucleus (Deng et al., 2019). However, there were are also some crystallizations with inhibitors that contain thiazole rings in their structures (PDB codes 5T6K, 5T6I and 5T6A), which allows us to think that possibly our 4-thiazolidinone derivatives could also interact with TgCDPK1. Since T. gondii is an obligate intracellular parasite, steps such as invasion and egress from the host cell are crucial for its survival. Inhibition of proteins such as TgCDPK1 has been shown to alter these and other processes of the parasite (Lourido et al., 2010; Sugi et al., 2013; Shrestha et al., 2019). TgCDPK1 has particular characteristics that make it a promising molecular target against T. gondii, for example, it is not expressed in animals and has changes in its binding site to ATP that make it different from human kinase proteins. Its gatekeeper residue has low bulky (glycine), compared to the bulky gatekeeper of human kinases (generally methionine, leucine, or phenylalanine) (Billker et al., 2009; Hui et al., 2015), this allows that a possible drug presents specific interactions with the parasite. Thus, it is possible to associate the in vitro effects of our compounds on the parasite with a possible inhibitory effect of the TgCDPK1 protein.
Based on the above hypothesis, we analyzed the possible molecular interactions between compounds with the best in vitro results and TgCDPK1 (Fig. 9). All three compounds were docked right in the elongated area of the ATP-binding pocket available thanks to the presence of the small gatekeeper (Gly128), occupying it mainly with the fragments attached to the hydrazone moiety. Specifically, compound 2B (a compound with the best in vitro results) would be using its pyridine fragment to form mainly hydrophobic interactions with amino acids such as Leu96, Leu103, Leu114, Leu126, and Phe202, which would be favoring the anchoring of the compound, turning the pyridine ring into an important fragment in 2B. Given that this region is mainly hydrophobic, it is feasible to think that modifications of the same type on the pyridine ring could contribute to increasing its affinity for this site. Upon entering the hydrophobic region of the ATP-binding pocket, 2B would be expected to act as a competitive inhibitor.
Fig. 9.
Predicted binding modes of the best in vitro compounds in TgCDPK1. (A) General view of the binding mode of compounds 1B (cyan), 2B (orange) and 3B (green) in the TgCDPK1 protein. 2D interactions between 1B (B), 2B (C) and 3B (D) compounds with the TgCDPK1 protein. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In general, this research shows that the presence of fragments such as 2-pyridinyl, 2-hydroxyphenyl, and monoxime groups on the 2-hydrazonyl position substantially enhanced anti-T. gondii activity compared to the compounds developed by D'Ascenzio et al. (2014) and Carradori et al. (2017). Furthermore, the presence of a fragment of α,β-unsaturated ester on the 5-position of the 4-thiazolidinone ring was shown to improve the anti-Toxoplasma activity since a compound labeled 27A by D'Ascenzio et al. (2014), whose only structural difference was the absence of this ester group that showed an IC50 value of 2.9 μM compared to 0.2 μM obtained for our compound 2B, which suggests that fragments of this type on position 5 of the 4-thiazolidinone ring would be an interesting moiety to conserve or modify in future studies. Similarly, Carvalho et l., (2010) reported a similar series of 4-thiazolidinone derivatives with a potential effect on T. gondii, however, the effects there reached concentration scales of mM, showing that our structural modifications effectively improve the anti-Toxoplasma effect of 4-thiazolidinone core derivatives.
4. Conclusion
In this study, different functional groups were used to modify the structure of 4-thiazolidinone analogs and were then identified by 1H NMR, 13C NMR, IR, and HR-MS analyses. The anti-Toxoplasma activity of the synthesized compounds was measured by TgGFP and TgME49 assays in vitro. The 1B, 2B and 3B target compounds had not only activity against T. gondii but also lower toxicity towards the host cells. For these molecules, the fluorescence methods showed correlation with the β-galactosidase assay. The 2B molecule had an IC50 value for parasite growth of 0.204 μM, and had no effect on host cell viability at 10 μM and this compound could be considered in vitro leads for anti-Toxoplasma therapy. Also, based on our in vitro and silico results we suggest that our best compounds could act as inhibitors of the TgCDPK1 protein. The use of different methods to analyze the inhibition of T. gondii growth on human cells by small molecules increases the power of selectivity tests.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
This project was funded by the Colombian Agency for Science, Technology and Innovation, COLCIENCIAS, grant number 57104, and contract 777–2017. The authors acknowledge to Dr. Laura Knoll for the internship, reagents and lab space. C.R.R. has been supported by MinCiencias, University of Antioquia and Ruta N, Colombia, the Max Planck Society, Germany.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2021.05.004.
Contributor Information
Diego A. Molina, Email: damolinal@uqvirtual.edu.co.
Edwar Cortes, Email: ecortes@uniquindio.edu.co.
Author contributions statement
D.A.M. and L.K., J.E.G. and E.C. conceived and designed the study. D.A.M., G.A.R and E.C. performed chemical experiment part. D.A.M., A.O.Z. and G.M.G.L performed biological experiment part. D.A.M. and C.R.R. performed the molecular docking part. D.A.M., C.R.R. and E.C. wrote the manuscript. All authors have read and approved the final version of the manuscript. University of Costa, Research Group in Natural and Exact Sciences, GICNEX.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdizadeh R., Hadizadeh F., Abdizadeh T. In silico studies of novel scaffold of thiazolidin-4-one derivatives as anti-Toxoplasma gondii agents by 2D/3D-QSAR, molecular docking, and molecular dynamics simulations. Struct. Chem. 2020;31:1149–1182. [Google Scholar]
- Aly A.A., Ishak E.A., Brown A.B. Reaction of arylidenehydrazono-4-aryl-2,3-dihydrothiazole-5-carbonitriles with diethyl acetylenedicarboxylate. Synthesis of (Z)-ethyl 2-[((Z)-2-(E)-arylidenehydrazono)-4-oxo-thiazolidine-5-ylidene]acetates. NMR investigation. J. Sulfur Chem. 2014;35:382–392. [Google Scholar]
- Bahia-Oliveira L., Gomez-Marin J., Shapiro K. Toxoplasma gondii. In: Rose J.B., Jiménez-Cisneros B., editors. Global Water Pathogens Project. 2017. http://www.waterpathogens.orghttp://www.waterpathogens.org/book/toxoplasma-gondii.Michigan.State.University,E.Lansing,MI,UNESCOgens.org/book/toxoplasma-gondii.M (R. Fayer and W. Jakubowski, (eds) Part 3 Protists) [Google Scholar]
- Ben-Harari R.R., Goodwin E., Casoy J. Adverse event profile of Pyrimethamine-based therapy in Toxoplasmosis: a systematic review. Drugs R. 2011;17:523–544. doi: 10.1007/s40268-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billker O., Lourido S., Sibley L.D. Calcium-Dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe. 2009;5:612–622. doi: 10.1016/j.chom.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey K.L., Westwood N.J., Mitchison T.J., Ward G.E. A small-molecule approach to studying invasive mechanisms of Toxoplasma gondii. Proc. Natl. Acad. Sci. Unit. States Am. 2004;101:7433–7438. doi: 10.1073/pnas.0307769101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carradori S. Synthesis and biological evaluation of anti-Toxoplasma gondii activity of a novel scaffold of thiazolidinone derivatives. J. Enzym. Inhib. Med. Chem. 2017;32:746–758. doi: 10.1080/14756366.2017.1316494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C.S., de Melo E.J.T., Tenório R.P., Góes A.J.S. Anti-parasitic action and elimination of intracellular Toxoplasma gondii in the presence of novel thiosemicarbazone and its 4-thiazolidinone derivatives. Braz. J. Med. Biol. Res. 2010;43:139–149. doi: 10.1590/s0100-879x2009005000038. [DOI] [PubMed] [Google Scholar]
- D'Ascenzio M. Design, synthesis and biological characterization of thiazolidin-4-one derivatives as promising inhibitors of Toxoplasma gondii. Eur. J. Med. Chem. 2014;86:17–39. doi: 10.1016/j.ejmech.2014.08.046. [DOI] [PubMed] [Google Scholar]
- de Aquino T.M. Synthesis, anti-Toxoplasma gondii and antimicrobial activities of benzaldehyde 4-phenyl-3-thiosemicarbazones and 2-[(phenylmethylene)hydrazono]-4-oxo-3-phenyl-5-thiazolidineacetic acids. Bioorg. Med. Chem. 2008;16:446–456. doi: 10.1016/j.bmc.2007.09.025. [DOI] [PubMed] [Google Scholar]
- de la Torre A., Stanford M., Curi A., Jaffe G.J., Gomez-Marín J.E. Therapy for ocular toxoplasmosis. Ocul. Immunol. Inflamm. 2011;19:314–320. doi: 10.3109/09273948.2011.608915. [DOI] [PubMed] [Google Scholar]
- Deng Y., Wu T., Zhai S.Q., Li C.H. Recent progress on anti-Toxoplasma drugs discovery: design, synthesis and screening. Eur. J. Med. Chem. 2019;183:111711. doi: 10.1016/j.ejmech.2019.111711. [DOI] [PubMed] [Google Scholar]
- Hamama W.S., Ismail M.A., Shaaban S., Zoorob H.H. Progress in the chemistry of 4‐ thiazolidinones. J. Heterocycl. Chem. 2009;45:939–956. [Google Scholar]
- Hanwell M.D., Curtis D.E., Lonie D.C., Vandermeersch T., Zurek E., Hutchison G.R. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 2012;4:17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe D.K., Sibley L.D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- Hui R., Bakkouri M.E., Sibley L.D. Designing selective inhibitors for calcium-dependent protein kinases in apicomplexans. Trends Pharmacol. Sci. 2015;36:452–460. doi: 10.1016/j.tips.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M.A. Synthesis, structural elucidation and cytotoxicity of new thiosemicarbazone derivatives. Arabian Journal of Chemistry. 2019;12:3183–3192. [Google Scholar]
- Jin C., Jung S.Y., Kim S.Y., Song H.O., Park H. Simple and efficient model systems of screening anti-Toxoplasma drugs in vitro. Expet Opin. Drug Discov. 2012;7:195–205. doi: 10.1517/17460441.2012.660479. [DOI] [PubMed] [Google Scholar]
- Koes D.K., Baumgartner M.P., Camacho C.J. Lessons learned in empirical scoring with smina from the CSAR 2011 benchmarking exercise. J. Chem. Inf. Model. 2013;53:1893–1904. doi: 10.1021/ci300604z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesen A.P. Synthesis and evaluation of anti-Toxoplasma gondii and antimicrobial activities of thiosemicarbazides, 4-thiazolidinones and 1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010;45:3685–3691. doi: 10.1016/j.ejmech.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Lourido S., Shuman J., Zhang C., Shokat K.M., Hui R., Sibley L.D. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–362. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D.C., Seeber F., Boothroyd J.C. Use of Toxoplasma gondii expressing beta-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrob. Agents Chemother. 1997;41:1849–1853. doi: 10.1128/aac.41.9.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina D. Protein targets of thiazolidinone derivatives in Toxoplasma gondii and insights into their binding to ROP18. BMC Genom. 2018;19:856. doi: 10.1186/s12864-018-5223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya J.G., Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- Morris G.M. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Roa C., Molina D., Cardona N. A perspective on thiazolidinone scaffold development as a new therapeutic strategy for toxoplasmosis. Front. Cell Infect Microbiol. 2018;8:360. doi: 10.3389/fcimb.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeber F., Boothroyd J.C. Escherichia coli beta-galactosidase as an in vitro and in vivo reporter enzyme and stable transfection marker in the intracellular protozoan parasite Toxoplasma gondii. Gene. 1996;169:39–45. doi: 10.1016/0378-1119(95)00786-5. [DOI] [PubMed] [Google Scholar]
- Shammaa A.M., Powell T.G., Benmerzouga I. Adverse outcomes associated with the treatment of Toxoplasma infections. Sci. Rep. 2021;11:1035. doi: 10.1038/s41598-020-80569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelat A.A., Guy R.K. Scaffold composition and biological relevance of screening libraries. Nat. Chem. Biol. 2007;3:442–446. doi: 10.1038/nchembio0807-442. [DOI] [PubMed] [Google Scholar]
- Shrestha A., Ojo K.K., Koston F., Ruttkiwski B., Vidadala R.S.A., Dorr C.S. Bumped kinase inhibitor 1369 is effective against Crytoisospora suis in vivo and in vitro. Int. J. Paraasitol. Drugs Drug Resist. 2019;10:9–19. doi: 10.1016/j.ijpddr.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepen S., He C.Y., Matrajt M., Roos D.S. Expression, selection, and organellar targeting of the green fluorescent protein in Toxoplasma gondii. Mol. Biochem. Parasitol. 1998;92:325–338. doi: 10.1016/s0166-6851(98)00011-5. [DOI] [PubMed] [Google Scholar]
- Sugi T., Kobayashi K., Takemae H., Gong H., Ishiwa A., Murakoshim F. Identification of mutations in TgMAPK1 of Toxoplasma gondii conferring resistance to 1NM-PP1. Int. J. Paraasitol. Drugs Drug Resist. 2013;3:93–101. doi: 10.1016/j.ijpddr.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenóri R.P. Synthesis of thiosemicarbazone and 4-thiazolidinone derivatives and their in vitro anti-Toxoplasma gondii activity. Bioorg. Med. Chem. Lett. 2005;15:2575–2578. doi: 10.1016/j.bmcl.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun-Zhong Y. Synthesis, crystal structures, and antimicrobial and antitumor studies of two zinc(II) complexes with pyridine thiazole derivatives. Bioinorgan. Chem. Appl. 2020;17:1–9. doi: 10.1155/2020/8852470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavari I., Ali-Asgari S., Porshamsian K., Bagheri M. Efficient synthesis of functionalized bis-(4-oxo-1,3-thiazolan-5-ylidene)acetates. J. Sulfur Chem. 2007;28:477–482. doi: 10.1007/s11030-007-9061-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.