Abstract
Age-related deterioration in turnover of collagen proteins accelerates extracellular matrix fibrosis and hinders adaptation to external stimuli. This project sought to understand factors that increase skeletal muscle fibrosis with age by studying what we term the dynamic protein pool. We hypothesized that the dynamic protein pool size of muscle collagen decreases with age, thus indicating a decrease in proteostatic maintenance (ie, ability to maintain proteostasis), and that failure to account for these changes impacts the interpretation of tracer-measured synthesis rates. We used deuterium oxide (D2O) labeling for up to 60 days in adult (6 months) and old (23 months) mice. The dynamic protein pool in adult skeletal muscle was 65% in tibialis anterior (TA), but only 28% in gastrocnemius (Gastroc). In aged muscle, the dynamic protein pool was further decreased to only 35% and 14% for TA and Gastroc, respectively. We showed that this loss in dynamic pool size was associated with increases in markers of fibrosis and decreased proteostatic maintenance. We demonstrate that aged muscle has higher rates of collagen protein synthesis and lower rates of collagen protein breakdown, which causes collagen accumulation. We further demonstrated that the normal assumption of complete protein renewal and the standard practice of taking a single sample with isotope labeling have profound impacts on interpretation of the genesis of fibrosis. Strategies to maintain muscle function with aging should focus on the dynamic protein pool with attention to methodological strategies to assess those changes.
Keywords: stable isotope, incomplete product renewal, fibrosis, extracellular matrix, collagen, muscle, aging
Graphical Abstract
Graphical Abstract.
Introduction
The loss of muscle mass only partially explains the loss of muscle function during aging.1 Therefore, there is an increased focus on factors outside myofiber size that lead to the loss of muscle function with age. One such factor is skeletal muscle extracellular matrix (ECM). Once thought of being a rather inert tissue component, it is now accepted that ECM is dynamic, responsive to environmental queues, and responsible for coordinating tissue responses.2 ECM has important roles in maintaining muscle structure,3 mechanotransduction,4 and other forms of signaling.5,6 There remains a need to explore changes in skeletal muscle ECM with age to understand age-related decline in muscle function.
Fibrosis refers to the overgrowth and hardening of tissue that results from excess deposition of ECM components, including collagen.7 Because enzymatic repair of protein is low,8 protein turnover is required to reduce the accumulation of protein modifications, such as advanced glycation end products (AGEs), that lead to cross bridging and fibrotic accumulation.9 Although a stable protein structure is advantageous for some aspects of collagen within ECM (eg, the deep zones of cartilage), a progressive decline in collagen protein turnover would hinder adaptation to outside stimuli. Recent studies indicate that increased ECM stiffness with age causes physical and secretory alterations in resident fibroblasts that enhance ECM deposition, further stiffen muscle ECM, and promote the fibrogenic conversion of satellite cells.6 Therefore, a loss of collagen turnover with aging is expected to be an important contributor to fibrosis with aging.
Most studies that have examined age-related changes in skeletal muscle collagen have done so by examining transcriptional changes.10–17 However, changes in mRNA expression often do not predict protein expression changes,18,19 which is especially true in collagen because of the extensive posttranscriptional regulation during synthesis.2 Direct measurements of skeletal muscle collagen protein synthesis have largely relied on short-term amino acid labeling,20–26 and only a couple of these have examined age-related changes.23,24 Although studies using short-term labeling have highlighted that collagen adapts to stimuli such as exercise and disuse, the labeling approach biases results to proteins undergoing rapid turnover and misses important changes in long-lived proteins.27 Therefore, the effect of aging on skeletal muscle collagen turnover is still largely unknown.
The use of the stable isotope deuterium oxide (D2O) has facilitated the capture of slowly turning over proteins because it lends itself to long-term (days to weeks) labeling.28 The assumptions, limitations, and technical application of the method have been detailed to ensure valid results.28 When calculating synthesis rates from a single time point, standard tracer methodology assumes that the product undergoes complete renewal (ie, turnover).29 However, this assumption of complete renewal may not be correct for some proteins, like collagen, that can become resistant to turnover during aging.30 Not accounting for a potential change in the size of the protein pool that is undergoing turnover, such as collagen that becomes resistant to turnover, could impact the interpretation of the resultant data.28,29 Since mass spectrometry analysis by itself cannot determine which proteins are resistant to turnover, alternative strategies are needed.
In addition to potentially impacting calculation of protein synthesis rates, incomplete renewal is an important physiological outcome. Maintaining the function of proteins over time requires the coordination of multiple dynamic processes collectively termed proteostatic maintenance.31 The decline of proteostatic maintenance is characterized by increased protein aggregation, resistance to turnover, and increased fibrosis, which are prominent features of aging collagen. With aging, there is a decline in the ability to maintain proteostasis.31,32 Since a decline in turnover and increased aggregation are hallmarks of the loss of proteostatic maintenance, we propose here that a decline in the size of the pool of proteins that are actively turning over would indicate a decline in proteostatic maintenance. Reciprocally, a treatment that prevents a decline or increases the pool size of actively turning over proteins would indicate improved proteostatic maintenance. In this manuscript, we refer to the pool of proteins that actively turn over as the dynamic protein pool, to distinguish it from the protein pool that is resistant to turnover. We also propose that when studying protein turnover as a mechanism to maintain proteostatic,33 the size of the dynamic protein pool is just as important as the rate of protein turnover.
In this manuscript, we seek to understand contributing factors to increased skeletal muscle fibrosis with age by examining skeletal muscle collagen synthesis. We hypothesize that the dynamic pool size of collagen proteins decreases with age, thus indicating a decrease in proteostatic maintenance, and that failure to account for these changes impacts the interpretation of tracer-measured synthesis rates. To address our hypothesis, we present a tracer-based time-course approach that overcomes limitations of single-duration labeling approaches for assessment of collagen turnover by focusing on the dynamic protein pool. We also demonstrate that not accounting for changes in the dynamic protein pool can greatly impact the quantitative assessment of collagen protein turnover, and therefore potentially impact strategies to minimize the decline in proteostatic maintenance and skeletal muscle fibrosis.
Materials and Methods
Ethical Approval
All animal procedures were conducted in accordance with institutional guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at the Oklahoma Medical Research Foundation (OMRF). The study was conducted in adherence to the American Physiological Society's Guiding Principles for the Care and Use of Vertebrate Animals in Research.
Study Design and Animal Experimentation
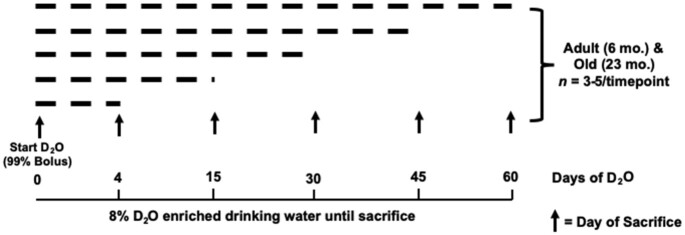
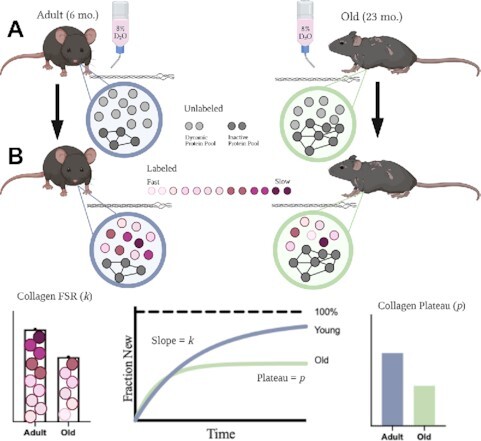
Female C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were housed in the vivarium at the OMRF under the care of the Department of Comparative Medicine. Animals were group housed (3–5 per cage) with ad libitum access to food and water in a room on a 14:10 h light:dark cycle under constant temperature and humidity control. Mice were randomly assigned to a D2O labeling time point of 4, 15, 30, 45, or 60 days of D2O, such that all adult (6 months) and old (23 months) animals were sacrificed at the same age (Figure 1). All mice received an initial i.p. bolus injection of 99% D2O equivalent to 5% of the body water pool followed by 8% D2O-enriched drinking water for the remainder of the experiment until their designated time point.
Figure 1.
Overview of Experimental Design. Arrows indicate days when mice were euthanized. Dashed lines indicate the period of D2O labeling. All labeling periods started with an i.p. injection of 99% D2O.
For tissue and blood collection, mice were anesthetized with isoflurane followed by exsanguination through cardiac puncture for euthanasia. Blood was collected and then centrifuged at 2000 g for 10 min at 4°C. Plasma was then aliquoted and frozen at −80°C for further analyses. Gastrocnemius (Gastroc) and tibialis anterior (TA) muscles from both hindlimbs were quickly harvested, trimmed of fat and connective tissues, and flash frozen in liquid nitrogen.
Protein Synthesis Rate and Plateau Fraction New
Protein synthesis was determined using our established methods.28,34 Briefly, ∼30–50 mg of skeletal muscle tissue was homogenized 1:20 in isolation buffer (100 mm KCl, 40 mm Tris–HCl, 10 mm Tris Base, 5 mm MgCl2, 1 mm EDTA, 1 mm ATP, pH = 7.6) with phosphatase and protease inhibitors (HALT, Thermo Fisher Scientific) using a bead homogenizer (Next Advance Inc., Averill Park, NY, USA). After homogenization, subcellular fractions were isolated via differential centrifugation to obtain myofibrillar and collagen protein fractions. Protein fractions were derivatized for analysis of deuterium enrichment in alanine using Gas Chromatography-Mass Spectroscopy (7890A GC-Agilent 5975C MS, Agilent, Santa Clara, CA).35To determine the precursor pool enrichment, plasma samples were prepared for analysis of deuterium enrichment on a liquid water isotope analyzer (Los Gatos Research, Los Gatos, CA, USA) using 0%–12% deuterium standards.36 The precursor enrichment of alanine was then adjusted by mass isotopomer distribution analysis.37 The deuterium enrichments of both the protein (product) and the precursor were used to calculate fraction new: Fraction new = Eproduct/Eprecursor, where the Eproduct is the enrichment (E) of protein-bound alanine and Eprecursor is the calculated maximum alanine enrichment from equilibration of the body water pool.
For adult and old mice, the fraction new data were then plotted across the time points and curves were fit to the data using one-phase associations.38,39 Two parameters of interest were then calculated from the curves using Graphpad Prism 9 and the one-phase association function. The software calculates rate parameter (k, 1/day), which reflects the protein synthetic rate, and plateau fraction new (p), which represents the proportion of the protein pool that is actively turning over (ie, the dynamic protein pool), with 1.0 equal to 100% of the protein pool.38,39 We constrained the plateau (p) to 1.0 when assuming the protein pool fully renews, and ≤1.0 when not assuming full renewal. The rate parameter (k) was therefore calculated as rise to these separate plateau values.
Pepsin Collagen Solubility and Hydroxyproline Assay
To quantify the proportion of mature cross-linked collagen from noncross-linked and immature cross-linked collagen in Gastroc muscles, we treated ∼30 mg of tissue with pepsin as previously described with minor modifications.40 Samples were rinsed in ice-cold phosphate buffered saline (PBS) using a microcentrifuge tube rotator for 30 min at 4°C. Samples were centrifuged at 16 000g for 30 min at 4°C and the resulting supernatant was discarded. The resultant pellets were digested with 1 mg/mL pepsin (AAJ6167903, Fisher Scientific) in 0.5 M acetic acid using a tube rotator overnight at 4°C. The next day, samples were centrifuged at 16 000g for 30 min at 4°C to obtain a pepsin soluble fraction (supernatant) and pepsin insoluble fraction (pellet). To the pepsin soluble fraction, we added an equal volume of 4 M NaCl, which was mixed on a tube rotator for 30 min at 4°C. The pepsin soluble fraction was centrifuged at 16 000g for 30 min at 4°C and the supernatant was discarded. The pepsin soluble and pepsin insoluble fractions were hydrolyzed in 6 M HCl overnight at 110°C. The collagen concentration in the pepsin soluble and pepsin insoluble fractions were determined using a hydroxyproline assay exactly as described.40 Ten microliters of hydrolyzed sample was mixed with 150 μL isopropanol and 75 μL of chloramine-T (EMD Millipore Sigma, St. Louis, MO) in citrate buffer and oxidized for 10 min at room temperature. The oxidized samples were then mixed with 1 mL of a 3:13 solution of Ehrlich reagent (3 g of p-dimethylaminobenzaldehyde (Sigma-Aldrich, St. Louis, MO), 10 mL 100% ethanol, and 675 μL sulfuric acid) to isopropanol and incubated for 45 min at 55°C. Quantification was then determined by measuring absorbance at 558 nm on a 96-well plate reader in duplicate. Hydroxyproline concentration (μg/mg tissue) was then determined using a standard curve of trans-4-hydroxy-l-proline (Sigma-Aldrich, St. Louis, MO).
Western Blotting
The same portion (∼30–50 mg) of Gastroc and TA muscles that was used for protein synthesis was also used for western blot analysis. During differential centrifugation, the cytosolic protein fraction was saved, and protein concentration was determined by bicinchoninic acid assay (BCA) assay and prepared in Laemmli sample buffer, as described previously.38,41 For quantification of protein abundance, samples were electrophoretically separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) using 4%–15% TGX gels (Bio-Rad, Hercules, CA) and then transferred to a Polyvinylidene difluoride (PVDF) (Bio-Rad, Hercules, CA) membrane. Transfer efficiency and equal loading among lanes were then determined by staining membranes with Ponceau S (Sigma-Aldrich, St. Louis, MO) and imaging. After blocking membranes (5% nonfat dry milk in tris-buffered saline with tween (TBS-T)) for 1 h, proteins of interest (below) were probed with primary antibody overnight at 4°C. Membranes were then serially washed in TBS-T, incubated with appropriate secondary antibody in blocking buffer for 1 h, and again serially washed in TBS-T. For all proteins, horseradish peroxidase (HRP) activity was detected by enhanced chemiluminescence substrate (SuperSignal West Dura, Thermo Fisher Scientific, Waltham, MA). Images were taken for quantification by densitometry using a ChemiDoc imager (Bio-Rad, Hercules, CA). Protein content was quantified and corrected for local background using Image Lab analysis software (Bio-Rad, Hercules, CA).
Primary antibodies and dilutions were as follows: lysyl oxidase (LOX) (Novus Biologicals, no. NBP2-24877, 1:1000) and AGEs,42 1:500. For each protein, we used a goat anti-rabbit, HRP-linked secondary antibody (Cell Signaling Technology no. 7074) at 1:5000.
Protein Carbonyls
Protein carbonylation, a marker of oxidative modification to proteins, was determined in Gastroc muscle as we have previously described, using 25 mg of frozen tissue.43 The carbonyl content of the protein samples was expressed as the ratio of Fluorescein (FITC) fluorescence (carbonyls) to Coomassie Blue absorption (protein concentration).
Picrosirius Staining, Imaging, and Analyses
Collagen content and collagen packing density were measured as a fraction of total muscle area using picrosirius red staining, as described previously,44 with modifications. Briefly, TA muscle cross sections of 10 μm were cut at the midbelly using a cryostat at −20°C (Thermo Fisher, Waltham, MA) and stored at −80°C until further analyses. On the day of staining, sections were incubated in 4% paraformaldehyde in PBS for 5 min, rinsed in running deionized (DI) H2O for 3 min, and then air dried at room temperature for 45 min. Samples were then cleared in xylenes for 10 min, dehydrated in 10 s changes of 100%, 96%, 80%, and 70% ethanol, rinsed in DI H2O, and then stained in 0.1% Sirius red solution (Sigma-Aldrich, St. Louis, MO) at room temperature for 1 h. Excess Sirius red solution was removed and samples were acidified in 0.01 N HCl for 2 min. Samples were then rehydrated in 10 s changes of 70%, 80%, 96%, and 100% ethanol, cleared in 2 changes of xylenes for 10 min at room temperature, and then cover slipped and mounted with Permount.
Whole stained cross sections were then imaged at 10× magnification using both brightfield and polarized light on a confocal microscope (Zeiss LSM-710, Oberkochen, Germany) using the tiling function in the Zeiss Zen Blue software in OMRF's Imaging Core. To evaluate collagen content, Image J software was used to determine the % area of red staining (collagen) relative to the whole muscle cross section of the brightfield images. Similarly, to evaluate collagen packing density, Image J software was used to determine the % area of red staining to green staining relative to the whole-muscle cross section of the nonpolarized and polarized light images. Red and green staining are indicative of densely and loosely packed collagen, respectively.44
Statistical Analyses
Prior to any statistical analyses, the data were tested for normal distribution and equal variances to determine the appropriate statistical test. Adult and old comparisons were made between outcome variables using two-tailed independent sample t-tests. The data in Figure 2 were analyzed by a two-way analysis of variance (age × time point). Curve fitting was done using Graphpad Prism 9 (San Diego, CA, USA) with constraints noted where appropriate. All values reported are mean ± standard error (SE) and statistical significance was based at P < 0.05.
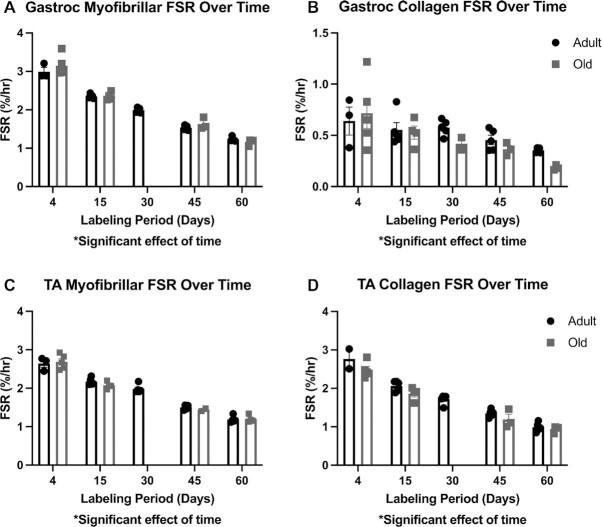
Figure 2.
Demonstration of How the Period of Labeling Impacts Fractional Synthesis Rate (FSR) in Myofibrillar and Collagen Fractions of Gastrocnemius (A, B) and TA (C, D) Muscles. Calculation of FSR (%/day) using the precursor–product calculation at each individual time point. There was a significant effect of time indicating that as the period of labeling increased, the FSR became slower. The progressive decrease in FSR is caused by the decreased contribution of faster synthesized proteins (that fully turn over early) and the increased contribution of slower synthesized proteins. Filled boxes = adult mice; open boxes = old mice. N = 3–5 mice/time point (16–23 mice/age). Values expressed as mean ± SEM. Significance was determined by a two-way (age × time) analysis of variance.
Results
Because we had independent samples at multiple time points, we calculated the fractional synthesis rate (FSR) at each time point to demonstrate that the length of labeling impacts FSR. In Figure 2, we show that there is a significant effect of time, indicating that as the labeling period gets longer, the calculated FSR for myofibrillar proteins (Figure 2A) and collagen proteins (Figure 2B) becomes slower.
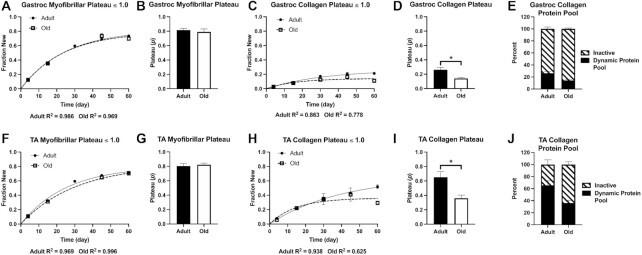
We next plotted the fraction new over time for myofibrillar (Figure 3A and F) and collagen (Figure 3C and H) proteins of Gastroc and TA muscles to calculate synthesis rates using a one-phase association. In this case, the curve was fit with the constraint that the plateau value (p) was less than or equal to 1.0. Therefore, we did not make the a priori assumption that all protein pools fully renew. The curve fit for myofibrillar proteins were excellent and ranged from R2 of 0.969–0.996. For the myofibrillar pool, the curve fit predicted p values of approximately 0.80 (Figure 3B and G), which were not different between adult and old. Unlike myofibrillar protein fraction new, the curve fit for collagen fraction new was different between adult and old (Figure 3C and H). Whereas the curve was reasonably fit for adult (R2 = 0.863 and 0.938), the fit for old animals was lower (0.778 and 0.625). The p for collagen was significantly lower in old compared with adult mice (Figure 3D and I). In Figure 3E and J, we used p to show the fraction of the collagen protein pool turning over, ie, the dynamic protein pool, to demonstrate that even in adult animals there is a significant portion of the collagen protein pool that is resistant to turnover.
Figure 3.
Time-Course Approach to Demonstrate Incomplete Renewal. Rise to plateau of fraction new of Gastroc myofibrillar (A) and collagen (C) proteins, and TA myofibrillar (F) and collagen (H) proteins. Calculated plateau values (p) for Gastroc myofibrillar (B) and collagen (D), and TA myofibrillar (G) and collagen (I) protein pools when it is assumed that p was ≤1.0. Representation of the dynamic protein pool size of the collagen in Gastroc (E) and TA (J), as determined from the p. Filled circles = adult mice; open squares = old mice. N = 3–5 mice/time point (18–20 mice/age). Values expressed as mean ± SEM. Significance was determined by an unpaired t-test.
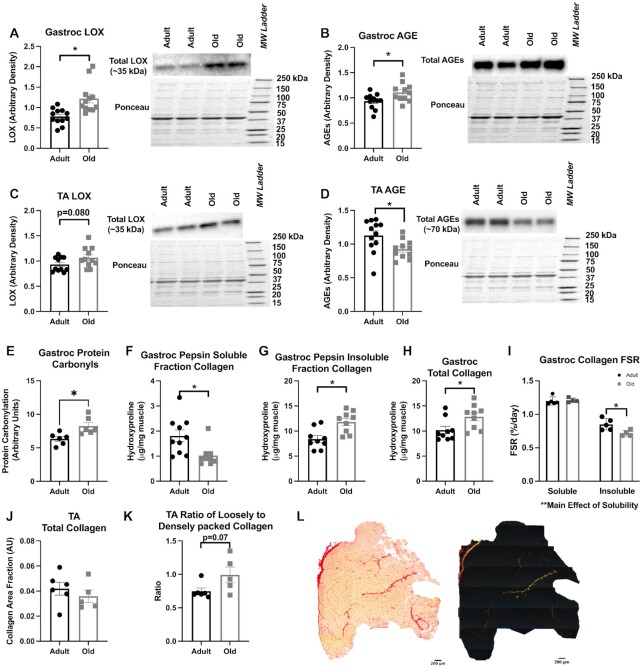
In Figure 4, we use several methods to support our tracer-measured differences in the dynamic protein pool. Consistent with the tracer-based results, the protein content of the cross-linking enzyme LOX was significantly greater in old Gastroc compared with adult (Figure 4A) and trended (p = 0.080) toward greater in the TA (Figure 4C). AGEs were greater in old Gastroc compared with adult (Figure 4B), but this was opposite in TA (Figure 4D). Protein carbonylation was significantly higher in old compared with adult (Figure 4E). In the Gastroc, the collagen solubility assay confirmed that a reduced concentration of collagen remained soluble in old versus adult skeletal muscle (Figure 4F), and the concentration of insoluble collagen was greater in old compared with adult skeletal muscle (Figure 4G). Furthermore, the overall concentration of collagen was greater in old compared with adult skeletal muscle (Figure 4H). When we looked at the FSR of soluble and insoluble fractions, there was no difference between adult and old in the soluble fraction, while there was lower FSR in the insoluble fraction of old compared with adult (Figure 4I). Because pepsin was added to the digestion, thus adding an unlabeled protein, it is possible that the FSR of the soluble (but not insoluble) fraction was underestimated, and that the true difference between soluble and insoluble fractions was greater. Finally, using immunohistochemistry on the TA, there were no differences in total collagen (Figure 4J) although there was a trend (p = 0.07) for the ratio of loosely to densely packed collagen to be greater in the old (Figure 4K and L).
Figure 4.
Secondary Measurements to Confirm Changes in Collagen Proteostasis, Including LOX Content (A, C), AGEs (B, D), and Protein Carbonylation (E). Protein solubility was performed in Gastroc followed by measurement of the collagen content of pepsin soluble fraction (F), pepsin insoluble fraction (G), total collagen content (H) by OHP assay, and collagen FSR of the soluble and insoluble muscle (I). TA collagen content (J), the ratio of collagen packing (K), and representative images of using polarized-light imaging picrosirius red staining (L). N = 5–12 muscles age group. Values expressed as mean ± SEM. Significance was determined by an unpaired t-test.
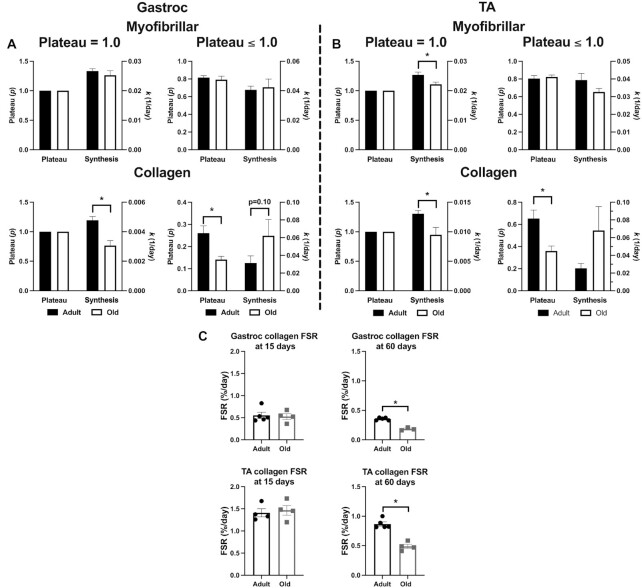
We next compared the calculated rates of synthesis (k) when it was assumed that the pool undergoes complete renewal (p = 1.0) versus when complete renewal was not assumed (p ≤ 1.0). This comparison allowed us to examine the impact of this assumption on qualitative and quantitative outcomes. We performed calculations on both the myofibrillar and collagen protein fractions for both the Gastroc (Figure 5A) and TA (Figure 5B). In the Gastroc, the assumption of complete renewal did not impact the influence of age on myofibrillar protein synthesis, but the assumption of complete turnover did impact the conclusion of whether age altered TA synthesis rates or not. Further, the synthesis rates were faster when empirically determined plateau values were used rather than assumed to be 1.0. The assumption of complete renewal also impacted the interpretation of the synthesis of collagen. If full renewal was assumed in both the Gastroc and TA, the collagen synthesis rate in old animals was slower than adult animals. However, if full renewal was not assumed, the synthesis rate of collagen was not different between adult and old, and in fact it trended (p = 0.10) toward higher in old compared with adult muscle. Finally, in Figure 5C, we demonstrate why single time point calculations of FSR may not be appropriate for studies of proteins that do not undergo complete renewal. In both Gastroc and TA, if FSR was calculated after 15 days of labeling, there was no difference in collagen synthesis between adult and old muscle. However, if taken at 60 days, the calculation of FSR shows that collagen synthesis was lower in old skeletal muscle compared with adult. This discrepancy compared with the 15-day time point is because the dynamic protein pool was smaller in the older muscle and tracer incorporation failed to increase any further with the longer labeling period. Thus, the fraction new reached a limit and dividing by a longer period of time simply made the FSR lower.
Figure 5.
Demonstration That Not Accounting for Differences in the Dynamic Protein Pool Impacts Qualitative and Quantitative Outcomes. In panels(A) (Gastroc) and (B) (TA), we show outcomes when it is assumed that the pool undergoes complete renewal (p = 1.0) or noncomplete renewal (p ≤ 1.0) of myofibrillar and collagen protein pools. Each figure shows p on the left axis and synthesis (k) on the right axis. In panel (C), we demonstrate how the commonly used approach of calculating FSR from a single time point of 15 or 60 days also changes the interpretation of the data. For panels (A) and (B), N = 3–5 mice/time point (18–20 mice/age). For panel (C), N = 3–5 mice/age group. Values expressed as mean ± SEM. Significance was determined by an unpaired t-test.
Discussion
There are several changes in tissue proteins that characterize the loss of proteostatic maintenance with age, such as an increase in insoluble proteins, protein aggregation, and resistance to turnover. Although it is generally recognized that proteins can become resistant to turnover, current tracer approaches to measure protein turnover assume that the whole protein pool renews. In the current study, we examined collagen proteins in the ECM of skeletal muscle because of their role in the age-related increase in skeletal muscle fibrosis. We demonstrate that stable isotopes can be used to quantify changes in the dynamic protein pool that occur with aging. Further, we demonstrate that not accounting for alterations in the dynamic protein pool can change the qualitative and quantitative assessment of collagen protein synthesis. When applied to skeletal muscle collagen proteins, we demonstrate that the size of the dynamic protein pool is smaller in older skeletal muscle compared with adult muscle, and that not accounting for this change can lead to misleading conclusions about the contribution of protein synthesis to age-related fibrosis. In the discussion, we highlight why maintaining the size of the dynamic protein pool may be a strategy to maintain proteostasis in skeletal muscle ECM.
Methodological Considerations
The use of D2O as a stable isotopic tracer expanded the capacity for long-term measurements of protein turnover.28 This increased capacity facilitated measurements during free-living conditions and expanded the ability to measure proteins that turn over slowly. However, the ability to label long term also comes with new methodological concerns. Many studies in the literature have simply applied the precursor–product approach used during short-term studies with labeled amino acids to long-term studies using D2O. However, under many conditions, this is inappropriate and can lead to erroneous results. Here, we discuss 2 such reasons in the context of collagen protein synthesis.
In a previous publication, we discussed why the period of labeling is important for interpreting tracer-measured synthesis rates.27 By using a modeling approach and validating with real data, we demonstrated that when measuring the synthesis rates of a mixed protein pool, shorter labeling periods bias synthesis rates to rapidly synthesized proteins, while longer labeling periods have increased contribution from slowly synthesized proteins.27 In Figure 2, we confirmed the findings from our previous study by showing that as the labeling period increases, the FSR at each time point becomes slower. There are several practical consequences of this phenomenon for long-term labeling studies. First, studies in the literature that use serial biopsies (eg, human muscle biopsies) in the same organism to capture early versus late responses, or untrained state followed by the trained state, are not comparing the same proteins at each time point. The early time points are overrepresented by rapidly synthesized proteins, which over time become fully turned over, and as a consequence the later time points are more represented by proteins that turn over slowly. Measuring the change in enrichment between repeated biopsies does not circumvent this limitation. Second, when making direct comparisons between groups or between tissues, the same labeling period must be used. Finally, when comparing outcomes between studies, the period of labeling can impact the interpretation of the results. For example, the question of whether aging slows protein synthesis in skeletal muscle is complicated by discrepant findings where short-term amino acid–labeling methods show a slowing of muscle protein synthesis with age,45,46 whereas long-term labeling shows no difference or increased protein synthesis with age.34 It is possible that both answers are true because they are measuring different subsets of proteins.
The second methodological consideration, which is especially important in the context of collagen synthesis, is that not all protein pools undergo complete renewal. Zhou et al. previously discussed the limitations of the assumption that protein pools undergo complete renewal.29 The authors of this paper nicely demonstrated that the incomplete equilibration of enrichment between the precursor and product pools of liver collagen was not because of a lack of equilibration between the body water pool and amino acids, but rather the incomplete renewal of proteins.29 Around the time of this publication, we began to use a time-course approach to understand the incomplete renewal of some protein pools.38,39,47 Although the paper of Zhou et al. clearly demonstrated the theoretical implications of not accounting for incomplete renewal of certain protein pools,29 we believe that a change in the dynamic protein pools is an important proteostatic outcome.38,39,47 Therefore, we performed the present study to test the implications of the theoretical considerations posed by Zhou et al. to collagen synthesis in aging skeletal muscle.
Skeletal Muscle Collagen Turnover and Incomplete Renewal
Studies from our lab22,34 and others48 have repeatedly demonstrated that muscle collagen synthesis is slower than muscle myofibrillar protein synthesis. Therefore, we used a time-course approach that extended to 60 days to capture a plateau (p) in the protein fraction new. As expected, we found that there is a smaller dynamic protein pool in collagen of aged muscle compared with adult muscle. What was unexpected to us was how small the dynamic protein pool was in both adult and aged skeletal muscle and how much it varied between the Gastroc and TA. The dynamic protein pool in adult skeletal muscle was 65% in TA, but only 26% in Gastroc. In aged muscle, the dynamic protein pool was only 36% and 14% for TA and Gastroc, respectively. Therefore, in aged muscle, and some adult muscle, most of the collagen pool is not actively turning over. We did not see the same change with aging in the myofibrillar fraction, although plateau values did not reach 100% within the myofibrillar protein pool either. Therefore, assuming complete renewal is not correct for all protein pools in the body.
We took 2 approaches to demonstrate how failing to account for the change in dynamic protein pool size impacts measured synthesis rates: (1) the calculation of the rate of synthesis (k) in a time-course study, and (2) the calculation of FSR using a single time point. When using graphing software such as Graphpad Prism, the one-phase-association curve fitting can be constrained for certain parameters. If we assume a protein pool undergoes a complete renewal, the plateau is constrained to 1.0 (ie, the line eventually goes to 100% new). If we do not assume that the protein pool undergoes complete renewal, the plateau needs to be constrained to ≤1.0 (ie, the line plateaus at some value less than or equal to 100% new). In Figure 5A and B, we demonstrate how these constraints impact the calculated slope of the line (k), and thus the synthesis rate. For the myofibrillar protein pool, the differences in the qualitative outcomes between adult and aged muscle were restricted to TA myofibrillar protein synthesis. In addition, the actual rates (1/day) of synthesis were different between the 2 approaches because the line advances to the plateau value of 0.8 faster than 1.0. For the collagen protein pool, not accounting for the difference in the dynamic protein pool during aging had a substantial qualitative and quantitative impact on conclusions. If it is assumed that the protein pool undergoes complete renewal, aged muscle has a slower collagen synthesis rate than adult muscle. Yet, if the decrease in the dynamic protein pool is accounted for, the rate of collagen protein synthesis is not different and in fact trends higher in aged compared with adult muscle. Since we measured hydroxyproline content (adult: 10.19 μg/mg muscle; old: 12.84 μg/mg muscle) we also estimated absolute collagen synthesis by using the dynamic protein pool size and the synthesis rate. From that calculation, the absolute rate of collagen synthesis is approximately 0.074 μg/mg muscle/day in adult muscle and 0.110 μg/mg muscle/day in aged muscle. Importantly, not accounting for incomplete protein turnover would have led to the opposite conclusion regarding the effect of age on muscle collagen synthesis.
In Figure 5C, we used the standard approach to measure collagen protein FSR based on a single time point. Of note, when using a single time point measurement it is not possible to get information about the dynamic protein pool, so no adjustments can be made. We compared the FSR of adult and old Gastroc and TA at day 15 (early time point) and day 60 (late time point). At day 15, there were no differences in collagen synthesis rates (%/day) between adult and old muscle. However, at day 60, the FSR of aged muscle was lower than adult muscle. Again, the period of labeling profoundly impacted the measured outcome and interpretation. More importantly, neither the 15-day nor the 60-day measurement of FSR qualitatively agreed with the outcome using time-course data when the difference in the dynamic protein pool was accounted for. Therefore, when a protein pool does not undergo complete renewal, or the change in the dynamic protein pool is not accounted for, FSR calculated from a single time point is likely to provide an erroneous conclusion.
Finally, it is worth noting potential differences in collagen protein breakdown that contribute to age-related collagen accumulation. Again, using the absolute quantity of collagen in adult and old Gastroc (adult: 10.19 μg/mg muscle; old: 12.84 μg/mg muscle), and the fraction of the pool that is resistant to turnover (adult: 0.74; old: 0.86), we can calculate that the absolute collagen protein pool resistant to turnover is 7.54 μg/mg muscle for adult and 11.04 μg/mg muscle for old. Since both the fraction of the collagen pool and the absolute concentration of collagen that was resistant to turnover were greater in old compared with adult, and synthesis rates were not different, the collagen protein breakdown must have been slower in old muscle.
How Does This Inform Proteostatic Maintenance with Aging?
With a loss of proteostatic maintenance, there is an increase in protein aggregation, insolubility, and resistance to turnover31,32 eventually leading to proteostatic collapse.49 Protein synthesis and breakdown (ie, turnover) are primary proteostatic processes.33,49 Protein turnover can only be captured with methods such as stable isotopes that measure rates of change. Protein content is a poor indicator of proteostatic processes since it only indicates whether the net balance of synthesis and breakdown increased, decreased, or stayed the same. In this study, we advance the notion that in addition to protein turnover, the change in the dynamic protein pool size is also an important proteostatic outcome. Although we have previously reported dynamic protein pool sizes,38,39,47 we have not explored whether this measurement was indicative of proteostatic maintenance.
In Figure 4, we presented several indicators of proteostatic maintenance of skeletal muscle collagen. It is thought that proteins accumulate damage over time and that this accumulation contributes to age-related decline in cellular function.50,51 In addition, it is thought that long-lived proteins, such as collagen, are especially susceptible to the accumulation of damage.52 As a marker of collagen protein damage, we measured AGEs, which were higher in the aged Gastroc muscle compared with adult muscle, but unexpectedly lower in TA. The greater synthesis rates and maintenance of the TA collagen pool may help explain this unexpected difference compared with Gastroc. In addition, protein carbonylation, a marker of oxidative protein damage, was also greater in old muscle compared with adult. Finally, LOX, an enzyme that facilitates cross-linking in collagen proteins, was greater in old muscles compared with adult muscle. Overall, these data are indicative of increased protein damage, an indicator of proteostatic decline.
Since proteostatic decline is also characterized by increases in protein aggregation and a loss of protein solubility, we performed a protein solubility assay40,44 and quantified collagen content in each fraction. As expected, the quantity of insoluble collagen protein was greater in aged muscle compared with adult muscle, as was overall collagen content. To further confirm that what we measured as an insoluble fraction is indicative of the proteins' resistant to turnover, we measured tracer incorporation into the soluble and insoluble fractions. As expected, the FSR of the insoluble fraction was lower than the soluble fraction; and within the insoluble fraction, old was lower than adult. Although we expected almost no tracer incorporation into the insoluble fraction, the solubility assay was performed on muscle tissue that contained both collagen and noncollagen tissues. Therefore, we cannot be certain of which proteins were labeled. Further, a protein that was synthesized in the soluble pool could end up in the insoluble pool by the conclusion of the prolonged labeling period.
Lastly, we measured collagen packing on the TA using circularly polarized light of picrosirius red–stained sections as previously described by the Barton lab.44 In contrast to the Gastroc, the total collagen content of the TA was not increased. However, there was a trend (p = 0.07) toward an overall higher ratio of loosely packed to densely packed collagen. Although how differences in collagen structure impact muscle function are not yet understood, our findings do agree with the observation that limb muscles of mdx mice, which contain significant muscle fibrosis, had an increased fraction of loosely packed collagen.44 In sum, our secondary markers of muscle damage, solubility, and fibrosis lend strong support for the use of tracer-measured changes in the dynamic protein pool size as an important indicator of proteostasis.
Practical Recommendations and Conclusions
Although it is generally accepted that skeletal muscle collagen content increases with age,2 the dynamics of how this increase occurs have been difficult to ascertain. Using a stable isotope approach that accounts for the dynamic protein pool size, we demonstrate that aged muscle does not change or trends toward higher rates of collagen protein synthesis, while collagen protein breakdown slows. The result of these changes was an increase in muscle collagen content in the old. However, more importantly, in the aged muscle there was a greater amount of collagen that was resistant to turnover, which indicated a loss of proteostatic maintenance. Moving forward, strategies to maintain muscle function with aging should focus on maintaining the dynamic protein pool size. Further, assessing the efficacy of interventional strategies will require careful attention to methodological strategies to avoid erroneous results. Finally, we suggest the use of our approach to help assess proteostatic decline in other protein and tissue types.
Supplementary Material
Acknolwedgments
M.M.L.'s current institution is the Department of Kinesiology and Outdoor Recreation at Southern Utah University in Cedar City, UT, USA. All experimentation and analyses were performed at the OMRF. C.B.A. and M.M.L. were responsible for acquisition, analysis, interpretation of data, and contributed to the writing of the manuscript. K.A.K., E.B.P.L., F.F.P., and E.J.D. were responsible for acquisition, analysis, and interpretation of data. T.M.G. and B.F.M. were responsible for conception and design of the experiments, acquisition of the data, and interpretation of the results. B.F.M. wrote the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Contributor Information
Claire B Abbott, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104, USA.
Marcus M Lawrence, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104, USA.
Kamil A Kobak, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104, USA.
Erika Barboza Prado Lopes, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104, USA.
Frederick F Peelor, III, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104, USA.
Elizabeth J Donald, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104, USA.
Holly Van Remmen, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104, USA.
Timothy M Griffin, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104, USA.
Benjamin F Miller, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104, USA.
Funding
C.B.A. was supported by a Fleming Scholarship from OMRF. M.M.L. was supported by an American Physiological Society (APS) Postdoctoral Fellowship and NIA Training Grant AG052363. The protein carbonylation measurements were done by the Integrative Redox Biology Core in the Oklahoma Nathan Shock Center (P30AG050911). Support for T.M.G. and E.B.P.L. was provided by NIH grants P30GM114731 and R01AG049058. Support for B.F.M., F.F.P., and E.J.D. was provided by R56AG067754.
Conflict of Interest
None declared.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67(1):28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84(2):649–698. [DOI] [PubMed] [Google Scholar]
- 3. Purslow PP. The structure and role of intramuscular connective tissue in muscle function. Front Physiol. 2020;11:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10(1):75–82. [DOI] [PubMed] [Google Scholar]
- 5. Fry CS, Kirby TJ, Kosmac Ket al. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell. 2017;20(1):56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stearns-Reider KM, D'Amore A, Beezhold Ket al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell. 2017;16(3):518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mary J, Vougier S, Picot CRet al. Enzymatic reactions involved in the repair of oxidized proteins. Exp Gerontol. 2004;39(8):1117–1123. [DOI] [PubMed] [Google Scholar]
- 9. Ott C, Jacobs K, Haucke Eet al. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alnaqeeb MA, Al Zaid NS, Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat. 1984;139(Pt 4):677–689. [PMC free article] [PubMed] [Google Scholar]
- 11. Goldspink G, Fernandes K, Williams PEet al. Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord. 1994;4(3):183–191. [DOI] [PubMed] [Google Scholar]
- 12. Heinemeier KM, Olesen JL, Haddad Fet al. Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J Appl Physiol. 2009;106(1):178–186. [DOI] [PubMed] [Google Scholar]
- 13. Kovanen V, Suominen H. Age- and training-related changes in the collagen metabolism of rat skeletal muscle. Eur J Appl Physiol Occup Physiol. 1989;58(7):765–771. [DOI] [PubMed] [Google Scholar]
- 14. Kovanen V, Suominen H, Peltonen L. Effects of aging and life-long physical training on collagen in slow and fast skeletal muscle in rats. A morphometric and immuno-histochemical study. Cell Tissue Res. 1987;248(2):247–255. [DOI] [PubMed] [Google Scholar]
- 15. Mohan S, Radha E. Age-related changes in rat muscle collagen. Gerontology. 1980;26(2):61–67. [DOI] [PubMed] [Google Scholar]
- 16. Pattison JS, Folk LC, Madsen RWet al. Transcriptional profiling identifies extensive downregulation of extracellular matrix gene expression in sarcopenic rat soleus muscle. Physiol Genomics. 2003;15(1):34–43. [DOI] [PubMed] [Google Scholar]
- 17. Zimmerman SD, McCormick RJ, Vadlamudi RKet al. Age and training alter collagen characteristics in fast- and slow-twitch rat limb muscle. J Appl Physiol. 1993;75(4):1670–1674. [DOI] [PubMed] [Google Scholar]
- 18. Miller BF, Konopka AR, Hamilton KL. The rigorous study of exercise adaptations: why mRNA might not be enough. J Appl Physiol. 2016;121(2):594–596. [DOI] [PubMed] [Google Scholar]
- 19. Schwanhausser B, Busse D, Li Net al. Global quantification of mammalian gene expression control. Nature. 2011;473(7347):337–342. [DOI] [PubMed] [Google Scholar]
- 20. Miller BF, Hansen M, Olesen JLet al. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am J Physiol Endocrinol Metab. 2006;290(1):E163–E168. [DOI] [PubMed] [Google Scholar]
- 21. Miller BF, Hansen M, Olesen JLet al. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol. 2007;102(2):541–546. [DOI] [PubMed] [Google Scholar]
- 22. Miller BF, Olesen JL, Hansen Met al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567(3):1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Babraj JA, Cuthbertson DJ, Smith Ket al. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab. 2005;289(5):E864–E869. [DOI] [PubMed] [Google Scholar]
- 24. Mays PK, McAnulty RJ, Campa JSet al. Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. Biochem J. 1991;276(2):307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barlow AD, Xie J, Moore CEet al. Rapamycin toxicity in MIN6 cells and rat and human islets is mediated by the inhibition of mTOR complex 2 (mTORC2). Diabetologia. 2012;55(5):1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trommelen J, Holwerda AM, Senden JMet al. Casein ingestion does not increase muscle connective tissue protein synthesis rates. Med Sci Sports Exerc. 2020;52(9):1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller BF, Wolff CA, Peelor FF 3rdet al. Modeling the contribution of individual proteins to mixed skeletal muscle protein synthetic rates over increasing periods of label incorporation. J Appl Physiol. 2015;118(6):655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller BF, Reid JJ, Price JCet al. CORP: the use of deuterated water for the measurement of protein synthesis. J Appl Physiol. 2020;128(5):1163–1176. [DOI] [PubMed] [Google Scholar]
- 29. Zhou H, Wang SP, Herath Ket al. Tracer-based estimates of protein flux in cases of incomplete product renewal: evidence and implications of heterogeneity in collagen turnover. Am J Physiol Endocrinol Metab. 2015;309(2):E115–E121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heinemeier KM, Schjerling P, Heinemeier Jet al. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb (14)C. FASEB J. 2013;27(5):2074–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balch WE, Morimoto RI, Dillin Aet al. Adapting proteostasis for disease intervention. Science. 2008;319(5865):916–919. [DOI] [PubMed] [Google Scholar]
- 32. Hipp MS, Kasturi P, Hartl FU. The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol. 2019;20(7):421–435. [DOI] [PubMed] [Google Scholar]
- 33. Miller BF, Drake JC, Naylor Bet al. The measurement of protein synthesis for assessing proteostasis in studies of slowed aging. Ageing Res Rev. 2014;18:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller BF, Baehr LM, Musci RVet al. Muscle-specific changes in protein synthesis with aging and reloading after disuse atrophy. J Cachexia Sarcopenia Muscle. 2019;10(6):1195–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lawrence MM, Van Pelt DW, Confides ALet al. Muscle from aged rats is resistant to mechanotherapy during atrophy and reloading. Geroscience. 2021;43(1):65–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Groennebaek T, Jespersen NR, Jakobsgaard JEet al. Skeletal muscle mitochondrial protein synthesis and respiration increase with low-load blood flow restricted as well as high-load resistance training. Front Physiol. 2018;9:1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am J Physiol. 1999;276(6):E1146–E1170. [DOI] [PubMed] [Google Scholar]
- 38. Wolff CA, Lawrence MM, Porter Het al. Sex differences in changes of protein synthesis with rapamycin treatment are minimized when metformin is added to rapamycin. Geroscience. 2021;43(2):809–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reid JJ, Linden MA, Peelor FFet al. Brain protein synthesis rates in the UM-HET3 mouse following treatment with rapamycin or rapamycin with metformin. J Gerontol A Biol Sci Med Sci. 2020;75(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith LR, Hammers DW, Sweeney HLet al. Increased collagen cross-linking is a signature of dystrophin-deficient muscle. Muscle Nerve. 2016;54(1):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lawrence MM, Pelt DWV, Confides ALet al. Massage as a mechanotherapy promotes skeletal muscle protein and ribosomal turnover but does not mitigate muscle atrophy during disuse in adult rats. Acta Physiol. 2020;229(3):e13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fleenor BS, Ouyang A, Olver TDet al. Saxagliptin prevents increased coronary vascular stiffness in aortic-banded mini swine. Hypertension. 2018;72(2):466–475. [DOI] [PubMed] [Google Scholar]
- 43. Qaisar R, Bhaskaran S, Premkumar Pet al. Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J Cachexia Sarcopenia Muscle. 2018;9(5):1003–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith LR, Barton ER. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am J Physiol Cell Physiol. 2014;306(10):C889–C898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Balagopal P, Rooyackers OE, Adey DBet al. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997;273(4):E790–E800. [DOI] [PubMed] [Google Scholar]
- 46. Rooyackers OE, Adey DB, Ades PAet al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA. 1996;93(26):15364–15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolff CA, Reid JJ, Musci RVet al. Differential effects of rapamycin and metformin in combination with rapamycin on mechanisms of proteostasis in cultured skeletal myotubes. J Gerontol A Biol Sci Med Sci. 2020;75(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilkinson DJ, Franchi MV, Brook MSet al. A validation of the application of D(2)O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am J Physiology Endocrinol Metab. 2014;306(5):E571–E579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84(1):435–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stadtman ER. Protein oxidation and aging. Free Radic Res. 2006;40(12):1250–1258. [DOI] [PubMed] [Google Scholar]
- 51. Hamilton KL, Miller BF. Mitochondrial proteostasis as a shared characteristic of slowed aging: the importance of considering cell proliferation. J Physiol. 2017;595(20):6401–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Toyama BH, Savas JN, Park SKet al. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154(5):971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.