Abstract
We evaluated the supplementation of a protected complex of biofactors and antioxidants [P(BF+AOx)] on growth performance, antioxidant activity, expression of immune-related genes, and immunometabolic phenotype of broilers submitted to early life stressors. The treatments were a nutritionally complete basal diet supplemented or not with P(BF+AOx) (Jefo Nutrition Inc., Saint-Hyacinthe, QC, Canada) from 1 to 14 d of age. 720 one-day old male Ross 308 chickens were placed into pens of 30 birds (12 replicates/treatment). Birds were double-vaccinated against infectious bronchitis (IB; MILDVAC-Ma5T) at the hatchery and submitted, on d 3, to an acute reduction on environmental temperature (from 32° C to 20°C) for 48 h. Feed intake (FI), body weight gain (BWG), and feed conversion ratio (FCR) were calculated weekly. On d 7 and 15, samples were collected for expression of immune-related genes and kinome array analysis, and serum to evaluate the antioxidant status. Data were analyzed by ANOVA using SAS (SAS 9.4). From d 1 to 21 and d 1 to 28, the dietary supplementation of P(BF+AOx) significantly increased BWG (P < 0.05) by 3.6 and 3.8%, respectively, and improved FCR (P < 0.05) by 1.2 and 1.8%, respectively. From d 1 to 35, dietary supplementation enhanced BWG (P = 0.03) by 4%. Serum glutathione reductase activity on d 15 was higher in birds fed diets supplemented with P(BF+AOx) compared to the control diet-fed birds (P = 0.04). Dietary supplementation reduced the expression of IL-1β (P = 0.03) in the lungs on d 7. On d 15, dietary supplementation increased the expression of IL-6 (P = 0.02) and IL-10 (P = 0.03) in the liver. It was observed that, via decreased phosphorylation, catalase was activated in the jejunum and liver, and the phosphorylation of immunoregulatory or proinflammatory proteins was decreased. Other important cellular signaling pathways were also changed in the liver and jejunum due to the supplementation. The supplementation of P(BF+AOx) improves growth performance by promoting a general anti-inflammatory and antioxidant response in chickens undergoing early life stress.
Key words: antioxidants, broiler chickens, early life stress, immune system, infectious bronchitis
INTRODUCTION
Acute change in environmental temperature may have negative impacts on the performance of broiler flocks. It has been observed that a severe cold stress (12°C below the homeostatic temperature) throughout the life cycle of broilers negatively impacted production parameters (Su et al., 2020). Besides the negative effects on the performance of the birds, cold stress can also impair immune system development (Zhang et al., 2011; Zhao et al., 2013). For instance, Zhao et al. (2013) showed by histopathological analysis that the intestinal tissue was severely impacted, as shown by shorter villus, hemorrhagically and congested mucosa, and leukocytes infiltration, when chickens were submitted to either acute or chronic cold stress and, in another study, it led to higher oxidative stress in the intestine (Zhang et al. 2011). Additionally, cold stress may increase the susceptibility to respiratory diseases such as avian infectious bronchitis (IB) which affects the upper-respiratory tract of chickens. The disease, which is found worldwide, is often controlled by the use of attenuated live or killed vaccines (Jackwood and Wit, 2020).
The use of vitamins and other molecules with antioxidants properties has been investigated in poultry to various challenge models (Sahin et al., 2003; Ghazi Harsini et al., 2012; Hu et al., 2019). An imbalance between the pro and antioxidant systems of the body is likely to drive damage to cellular components (Estévez, 2015), including immune cells, that may impair the function of the immune response. Reactive oxygen species (ROS) production (naturally generated due to the immune response or exacerbated in stress situations) influence the activation of transcription factors, such as NF-kB, kinases and phosphatases that regulate the immune response and immune metabolism (Banerjee et al., 2020). The dietary supplementation of antioxidants, such as vitamin E, and others, may improve growth performance and have the function of protecting the cells from ROS (Ghazi Harsini et al., 2012) that are naturally generated due to the immune response, or exacerbated in stress situations. Additionally, biofactors as metabolites from a yeast cell hydrolysate showed antioxidant effects in fish hepatocytes by enhancing the activity of superoxide dismutase, catalase, and glutathione peroxidase (Rahimnejad et al., 2020). Moreover, the term postbiotic has been employed to define molecules produced during the metabolic processes of microorganisms, and has been applied to reduce the negative effects of pathogens, such as Clostridium perfringens, in chickens (Johnson et al., 2019).
Therefore, the supplementation of biofactors, which fall into the definition of postbiotic, combined with antioxidants may induce immunometabolic phenotypic alterations in different tissues to help the animal to cope with early life stressors and improve growth performance. We hypothesized that the supplementation of a protected complex of biofactors and antioxidants [P(BF+AOx)], that would be released in lower portions of the small intestine, to broilers could modulate the immunometabolic phenotype and alleviate the negative effects of early life stress. The objective of this study was to evaluate the dietary supplementation of P(BF+AOx) on the growth performance, antioxidant activity, expression of immune-related genes, and immunometabolic phenotype of the jejunum and liver of broiler chickens submitted to early life stress.
MATERIAL AND METHODS
Birds, Housing, and Treatments
The animals have been cared according to the recommended code of practice of Chicken Farmers of Canada and under the supervision of a poultry veterinarian. Seven hundred twenty (720) 1-day-old male Ross x Ross 308 chickens were used in this study. At the hatchery, the birds were vaccinated against Marek's disease (HVT). The experiment was conducted at the experimental station of Jefo Nutrition Inc., in Saint-Hyacinthe, QC, Canada. The feed was formulated based on corn and soybean meal. The feeding program was divided into two phases: starter (0–14) and grower (14–35 d; Table 1). Feed additives were mixed separately in the feed. The experiment consisted of a completely randomized design with two treatments: feed supplemented or not with 150 mg/kg of Protected Biofactors and Antioxidants P(BF+AOx) added on top, from 1 to 14 d of age. All the birds were fed the non-supplemented diet from 14 to 35 d of age. The P(BF+AOx) is a complex of vitamins and fermentation extract (vitamin A, vitamin D3, vitamin E, menadione, thiamine, riboflavin, niacin, pantothenic acid, vitamin B6, biotin, folic acid, vitamin B12, L-tryptophan, and fermentation extract of dried Bacillus subtilis, Aspergillus niger and A. oryzae) microencapsulated in a matrix of triglycerides from hydrogenated vegetable oil (Jefo Nutrition Inc., Saint-Hyacinthe, QCS, Canada). Each treatment consisted of 12 replicate pens with 30 birds each. The birds were placed onto floor pens with new litter. Each pen was provided with supplemental heat, and ad libitum access to water and feed in mash form.
Table 1.
Starter (1–21 d) and grower (21–35 d) diets formulation, and formulated energy and nutrient composition.
| Ingredient, % | Starter | Starter | Grower | Grower |
|---|---|---|---|---|
| Corn | 30.6 | 30.6 | 34.0 | 34.0 |
| Soybean meal, 48% CP | 26.0 | 26.0 | 18.3 | 18.3 |
| Wheat | 31.0 | 31.0 | 34.3 | 34.3 |
| DDGS | 5.0 | 5.0 | 5.0 | 5.0 |
| Animal fat | 2.8 | 2.8 | 4.4 | 4.4 |
| Monocalcium phosphate | 0.98 | 0.98 | 1.01 | 1.01 |
| Calcium carbonate | 2.13 | 2.13 | 1.73 | 1.73 |
| NaCl | 0.31 | 0.31 | 0.28 | 0.28 |
| L-lysine HCl | 0.315 | 0.315 | 0.310 | 0.310 |
| DL-Methionine, 99% | 0.305 | 0.305 | 0.245 | 0.245 |
| L-threonine | 0.090 | 0.090 | 0.045 | 0.045 |
| Choline, 60% | 0.076 | 0.076 | 0.076 | 0.076 |
| L-Valine | 0.259 | 0.259 | 0.076 | 0.076 |
| L-Tryptophane | 0.029 | 0.029 | 0.024 | 0.024 |
| Vitamin-Mineral Premix1 | 0.15 | 0.15 | 0.15 | 0.15 |
| Sodium bicarbonate | - | - | 0.04 | 0.04 |
| P(BF+AOx)2 | - | 0.015 | - | - |
| Formulated energy and nutrient composition | ||||
| ME Kcal/Kg | 2,950 | 2,950 | 3,097 | 3,097 |
| Crude Protein, % | 20.5 | 20.5 | 17.5 | 17.5 |
| Fat, % | 5.34 | 5.34 | 6.98 | 6.98 |
| Lysine, % | 1.200 | 1.200 | 1.003 | 1.003 |
| Thr, % | 0.797 | 0.797 | 0.639 | 0.639 |
| Met+Cys, % | 0.938 | 0.938 | 0.810 | 0.810 |
| Non phytate phosphorus, % | 0.440 | 0.440 | 0.440 | 0.440 |
| Total Ca, % | 1.11 | 1.11 | 0.95 | 0.95 |
| Na, % | 0.15 | 0.15 | 0.15 | 0.15 |
Supplied per kg of diet: vitamin A, 10,005 IU; vitamin D3, 3,000 IU; vitamin E, 30 IU; vitamin K, 2.55 mg; vitamin B12, 15 mg; biotin, 201 mg; thiamine, 3 mg; riboflavin, 6 mg; pantothenic acid, 14.1 mg; pyridoxine, 3.6 mg; niacin, 49.95 mg; folic acid, 1 mg; Zn, 100; Fe, 49.5 mg; Cu, 15 mg; I, 0.09 mg; Se, 0.45 mg, Mn, 100 mg.
Minimum Supplied per kg of diet: vitamin A, 900 IU; vitamin D3, 450 IU; vitamin E, 12 IU; vitamin K, 0.135 mg; vitamin B12, 0.00525 mg; biotin, 0.03 mg; thiamine, 0.9 mg; riboflavin, 1.35 mg; pantothenic acid, 3 mg; pyridoxine, 0.75 mg; niacin, 12 mg; folic acid, 0.3 mg.
Challenge
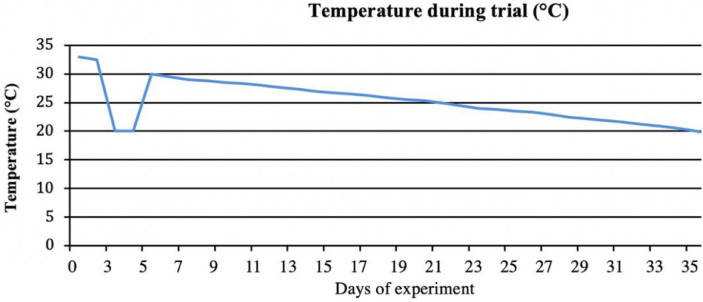
On d 0, all the birds received a 2X dose of live bronchitis vaccine (MILDVAC-Ma5) by spray at the hatchery. In addition, on day 3 all the chickens were submitted to an acute reduction of environmental temperature from 30–32°C to 20°C (10–12°C below the thermoneutral temperature for this age) during 48 h, returning to a normal temperature afterwards, following the breeder-recommended temperature profile. The temperature was automatically controlled (Vari-Vent; Model CVS-22HA), and monitored daily with thermometers evenly distributed inside the facility. The temperature profile registered throughout the study is shown in Figure 1.
Figure 1.
Temperature (°C) profile throughout the study.
Growth Performance Parameters
Body weight (BW) and feed intake (FI) averaged by pen were recorded weekly. Based on these data, body weight gain (BWG) and feed conversion ratio (FCR) of birds were calculated. FCR was corrected for the weight of dead birds. Growth performance was evaluated weekly and for the cumulative periods. Survivability was calculated by the ratio between the number of birds at the end and number of birds at the beginning of the experiment. The European Production Efficiency Factor (EPEF) was calculated by the formula: body weight (Kg) x % survivability x 100/FCR x trial duration in days.
Sample Collection
At 7 and 15 d, one bird per pen was randomly selected and euthanized by cervical dislocation. Blood (serum), liver, lung, jejunum, and ileum were collected from the same birds in order to evaluate the antioxidant status and expression of immune-related genes. Jejunum and liver from 15 d old birds were also submitted to kinome array analysis.
Oxidative Stress Markers
Serum samples from 7 and 15 d were analyzed for biomarkers of oxidative stress such as the concentration of thiobarbituric acid reactive substances (TBARS), glutathione peroxidase (GPx), and glutathione reductase (GR) activities using colorimetric assays kits (Cayman Chemicals, Ann Arbor, MI). Among these parameters, GR was only analyzed at 15 d due to the low volume of serum obtained at 7 d. The serum samples were kept at −80°C until the analyses were performed. All bioassays were performed according to the protocol outlined by the manufacturer.
Gene Expression
Lung, liver, jejunum and ileum were evaluated for expression of immune-related genes, according to Kogut and Arsenault (2015). Briefly, the mRNA was isolated using from 25 mg of tissue using the RNeasy Plus mini kit (Qiagen). The total isolated mRNA was eluted with 50 µL of RNase-free water and stored at −80°C for qRT-PCR analysis. RNA was quantified and the quality evaluated using a spectrophotometer (NanoDrop Products, Wilmington, DE, USA).
The prime and probe sets used in the qRT-PCR are shown in Table 2. The PCR was performed using the TaqMan fast universal PCR master mix and one-step RT-PCR master mix reagents (Applied Biosystems). Normalization was carried out using 28S rRNA as a housekeeping gene, and the corrected cytokine mean change in mRNA levels were calculated as follow: mean 40-Ct*slope of the standard curve of the target cytokine/slope of the standard curve of the 28S gene*differential factor of the 28S gene (Kogut and Arsenault, 2015). Lung tissue was analyzed for expression of IL-10, IL-1β, and IFN- γ genes. Liver, jejunum, and ileum were tested for expression of IL-6 and IL-10 genes.
Table 2.
Real-time quantitative PCR probe and primers used.
| Target gene | Probe/Prime sequence | Accession number | |
|---|---|---|---|
| 28S | Probe | AGGACCGCTACGGACCTCCACCA | X59733 |
| Forward | GGCGAAGCCAGAGGAAACT | ||
| Reverse | GACGACCGATTGCACGTC | ||
| IL-6 | Probe | AGGAGAAATGCCTGACGAAGCTCTCCA | AJ250838 |
| Forward | GCTCGCCGGCTTCGA | ||
| Reverse | GGTAGGTCTGAAAGGCGAACAG | ||
| IL-10 | Probe | CGACGATTCGGCGCTGTCACC | AJ621614 |
| Forward | CATGCTGCTGGGCCTGAA | ||
| Reverse | CGTCTCCTTGATCTGCTTGATG | ||
| IL-1β | Probe | CCACACTGCAGCTGGAGGAAGCC | AJ245728 |
| Forward | GCTCTACATGTCGTGTGTGATGAG | ||
| Reverse | TGTCGATGTCCCGCATGA | ||
| INF-γ | Probe | TGGCCAAGCTCCCGATGAACGA | Y07922 |
| Forward | GTGAAGAAGGTGAAAGATATCATGGA | ||
| Reverse | GCTTTGCGCTGGATTCTCA |
Kinome Array Analysis
Tissue samples were collected and immediately flash-frozen into liquid nitrogen to preserve kinase enzymatic activity and stored at −80°C prior to further processing. Samples were shipped overnight on dry ice to University of Delaware for kinome analysis. The kinome peptide array was carried out as described by Arsenault et al. (2017). To summarize, 40 mg of tissue samples were lysed in 100 uL of lysis buffer containing protease and phosphatase inhibitors. The supernatant of the lysed tissue was mixed with 10 uL of activation mix containing ATP and applied to the JPT peptide microarray (Berlin, Germany). Microarrays were incubated in a humidity chamber at 40°C and 5% CO2 for two hours and washed in phosphate-buffered saline - 1% Triton, 2M NaCl-1% Triton twice and a final wash in ddH20 with agitation, each for a minimum of 60 s. The arrays were submerged in phosphospecific fluorescent ProQ Diamond Phosphoprotein Stain (Life Technologies, Carlsbad, CA) in a large dish and placed on a shaker table at 50 rpm for one hour. Arrays were then placed in a new dish and submerged in destaining solution (20% acetonitrile (EMD Millipore Chemicals, Billerica, MA) and 50 mM sodium acetate (Sigma)) for 10 min with agitation at 50 rpm. This process was repeated 2 times. A final wash was then done with ddH2O. The arrays were spun dried. Arrays were then scanned using a Tecan PowerScanner microarray scanner (Tecan Systems, San Jose, CA) at 532 to 560 nm with a 580-nm filter to detect dye fluorescence to collect the array image.
Statistical Analysis
The growth performance, gene expression, and antioxidant activity were submitted to ANOVA using the software SAS (SAS 9.4). All the data were tested for normality and homogeneity. Nonparametric data were submitted to the Kruskal-Wallis test (P < 0.05). Trends are indicated when P ≤ 0.10. Regarding the kinome array, the data collection and analysis processes were carried out as described in Johnson et al. (2019). Data was then analyzed by the PIIKA2 peptide array analysis software (Trost et al., 2013). Briefly, the resulting data points were normalized to eliminate variance due to technical variation, for example, random variation in staining intensity between arrays or between array blocks within an array. Variance stabilization normalization was performed. As the arrays were printed in triplicate with triplicate peptide blocks there are 9 technical values for each peptide per array. Using the normalized data set, comparisons between treatment and control groups were performed, calculating fold change and a significance P-value. The P-value is calculated by conducting a paired t-test between treatment and control values for a given peptide. Signficant peptides were analyzed using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database's (Szklarczyk et al., 2019) output of Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa et al., 2017) pathways and false discovery ratio (FDR). Human uniport accession and phosphorylation site complementary to chicken are reported in this study.
RESULTS
Growth Performance
From 0 to 7 and 0 to 14 d, no significant effects were observed for any of the growth performance parameters evaluated (Table 3). There was a trend, however, toward improved FCR at 7 d (P = 0.10) and increased BW gain at 14 d (P = 0.06) in birds fed supplemented diets. From 0 to 21 and 0 to 28 d, the dietary supplementation of biofactors and antioxidants increased BW gain (P < 0.05) by 3.6 and 3.8%, respectively, and improved FCR (P < 0.05) by 1.2 and 1.8%, respectively. Considering the overall experimental period (0–35 d), supplementation enhanced body weight gain (P = 0.03) by 4% and tended to improve FCR (P = 0.07) by 2%.
Table 3.
Cumulative growth performance of broiler chickens fed control or diets supplemented with a complex of biofactors and antioxidants undergoing early life stress.
| BWG, g | FI, g | FCR | BWG, g | FI, g | FCR | |
|---|---|---|---|---|---|---|
| Treatment | 0 to 7 d | 0 to 14 d | ||||
| Control | 92 | 118 | 1.266 | 345 | 456 | 1.322 |
| P(BF+AOx)1 | 95 | 117 | 1.229 | 359 | 469 | 1.306 |
| SEM | 1.39 | 0.84 | 0.01 | 4.14 | 3.79 | 0.01 |
| P value | 0.13 | 0.55 | 0.10 | 0.06 | 0.68 | 0.25 |
| Treatment | 0 to 21 d | 0 to 28 d | ||||
| Control | 770b | 1,069 | 1.389a | 1,367b | 2,042 | 1.545a |
| P(BF+AOx)1 | 799a | 1,096 | 1.372b | 1,422a | 2,107 | 1.516b |
| SEM | 8.02 | 8.04 | 0.01 | 13.03 | 16.50 | 0.01 |
| P value | 0.03 | 0.74 | 0.02 | 0.01 | 0.81 | 0.01 |
| Treatment | 0 to 35 d | Survivability, % | EPEF | |||
| Control | 2,051b | 3,281 | 1.686 | 98 | 358.4 | |
| P(BF+AOx)1 | 2,136a | 3,390 | 1.647 | 97 | 373.0 | |
| SEM | 22.74 | 27.65 | 0.01 | 0.01 | 6.39 | |
| P value | 0.03 | 0.35 | 0.07 | 0.25 | 0.34 | |
Protected complex of biofactors and antioxidants; BWG: Body weight gain; FI: Feed intake; FCR: Feed conversion ratio; SEM: Standard error of mean; EPEF: European production efficiency factor.
P < 0.05; n = 12 replicate pens/treatment and 30 birds/pen.
Oxidative Stress
The serum concentration of TBARS, GPx, and GR are shown in Table 4. There was no difference between treatments on 7 or 15 d for TBARS and GPx activity. The GR activity at 15 d was higher in birds fed diets supplemented with biofactors and antioxidants compared to the control diet-fed birds (P = 0.04). At 7 d, however, we were unable to perform the GR analysis due to the low volume of serum obtained from birds of this age.
Table 4.
TBARS, glutathione peroxidase, and glutathione reductase in the serum of broiler chickens fed control or diets supplemented with a complex of biofactors and antioxidants undergoing early life stress.
| Treatment | MDA (uM) |
Gpx nmol/min/mL |
GR nmol/min/mL |
||
|---|---|---|---|---|---|
| 7 d | 15 d | 7 d | 15 d | 15 d | |
| Control | 21.7 | 25.0 | 18.5 | 18.5 | 34.9b |
| P(BF+AOx)1 | 23.3 | 23.4 | 17.5 | 19.4 | 45.3a |
| SEM | 1.80 | 1.14 | 1.13 | 1.11 | 2.72 |
| P value | 0.56 | 0.38 | 0.55 | 0.69 | 0.04 |
Protected complex of biofactors and antioxidants; MDA: Malondialdehyde; Gpx: glutathione peroxidase; GR: glutathione reductase SEM: Standard error of mean.
P < 0.05; n = 12 replicate pens/treatment and 30 birds/pen.
Gene Expression
The results regarding the expression of immune-related genes are shown in Table 5 as corrected cytokine means. At 7 d, the dietary supplementation of biofactors and antioxidants downregulated (P = 0.03) the expression of IL-1β in the lungs and tended to upregulate the expression of IL-6 (P = 0.06) and IL-10 (P = 0.06) in the liver. At 15 d, dietary supplementation upregulated the expression of IL-6 (P = 0.02) and IL-10 (P = 0.03) in the liver; moreover, there was a trend toward downregulation of IL-10 in the jejunum (P = 0.10) and upregulation of IL-6 in the ileum (P = 0.06) of broilers supplemented with antioxidants and biofactors.
| Corrected Cytokine Mean2 - 15 d of age | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lungs |
Jejunum |
Ileum |
Liver |
||||||
| Treatment | IL-10 | IL-1β | IFN- γ | IL-6 | IL-10 | IL-6 | IL-10 | IL-6 | IL-10 |
| Control | 9.77 | 10.77 | 13.28 | 10.53 | 9.54 | 8.27 | 9.62 | 9.87 b | 7.82 b |
| P(BF+AOx)1 | 9.50 | 10.37 | 13.46 | 9.09 | 8.17 | 9.30 | 9.00 | 12.03 a | 9.39 a |
| SEM | 0.25 | 0.61 | 0.36 | 0.93 | 0.55 | 0.38 | 0.37 | 0.60 | 0.79 |
| P value | 0.51 | 0.68 | 0.74 | 0.32 | 0.10 | 0.06 | 0.33 | 0.02 | 0.03 |
P < 0.05; n = 6 replicate pens/treatment and 30 birds/pen.
Protected complex of biofactors and antioxidants.
Calculated by the ratio between the mean 40-Ct*slope of the standard curve of the target cytokine/slope of the standard curve of the 28S gene*differential factor of the 28S gene; SEM: Standard error of mean.
Table 5.
mRNA expression of cytokines in the lung, jejunum, ileum and liver of broiler chickens fed control or diets supplemented with a complex of biofactors and antioxidants undergoing early life stress.a,b
| Corrected Cytokine Mean2 - 7 d of age | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lungs |
Jejunum |
Ileum |
Liver |
||||||
| Treatment | IL-10 | IL-1β | IFN- γ | IL-6 | IL-10 | IL-6 | IL-10 | IL-6 | IL-10 |
| Control | 9.82 | 10.50a | 8.63 | 10.19 | 8.95 | 9.59 | 7.43 | 8.24 | 8.15 |
| P(BF+AOx)1 | 9.17 | 9.04b | 8.07 | 9.04 | 8.30 | 9.12 | 6.43 | 11.09 | 5.88 |
| SEM | 0.45 | 0.48 | 0.47 | 0.59 | 0.48 | 0.40 | 0.45 | 0.85 | 0.66 |
| P value | 0.35 | 0.03 | 0.45 | 0.20 | 0.38 | 0.45 | 0.14 | 0.06 | 0.06 |
P < 0.05; n = 6 replicate pens/treatment and 30 birds/pen.
Kinome Array Analysis
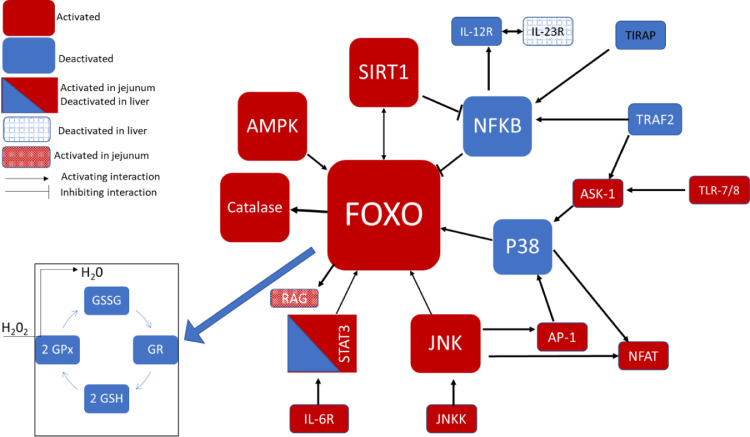
The results of the kinome peptide array performed on jejunum and liver tissues showed that the treatment had significant effect on oxidative stress resistance (Figure 2). Both jejunum and liver tissues at 15 d showed the Forkhead box protein O (FOXO) signaling pathway in the top 25 KEGG output with a FDR significance of 5.38E-21 and 6.79E-14, respectively (Supplementary Tables 1 and 2). Although the phosphorylation status of FOXO was not determined, the results show the phosphorylation status of proteins upstream and downstream of FOXO for both jejunum and liver samples at 15 d (Supplementary Tables 1 and 2). Catalase was activated downstream of FOXO via decreased phosphorylation on sites Y231 and Y386 (Supplementary Tables 1 and 2) which induce protein degradation (Cao et al., 2003a; b). Also, we observed the decrease in phosphorylation of immunoregulatory or proinflammatory proteins in the FOXO pathway. The proteins upstream and downstream of FOXO like NF-kB, P38, NFAT STAT3 and AP-1 (Table 6 and 7) are also found in the T cell receptor signaling pathway which shows up in the top 25 list of KEGG pathways for both liver (FDR 1.55E-13) and jejunum (FDR 4.37E-16) at 15 d (Supplementary Tables 1 and 2). These proteins have been linked to the increased expression of the anti-inflammatory marker IL-10 (Hermann-Kleiter and Baier, 2010; Sanin et al., 2015). Other important cellular signaling observed included adipocytokine and chemokine signaling observed in the top 25 KEGG pathways for liver while cellular senescence and relaxin signaling were also observed for the jejunum (Supplementary Tables 1 and 2).
Figure 2.
Summary of FOXO signaling during oxidative stress resistance. Shown here are the kinome peptide array and oxidative stress (smaller diagram at the lower left) results that were activated or deactivated by the supplementation of a complex of biofactors and antioxidants. Pathway diagram adapted from data in the KEGG pathway database (Kanehisa, et al., 2017).
Table 6.
Phosphorylation status of proteins in the FOXO signaling pathways of jejunum at 15 d of broiler chickens fed control or diets supplemented with a complex of biofactors and antioxidants undergoing early life stress.
| Human Ortholog Uniprot number | Protein name | Phosphorylation status | Function |
|---|---|---|---|
| Q96EB6 | SIRT1 | + | + |
| P36897 | TGF-B receptor | + | + |
| Q9NR97 | TLR7/8 | + | unknown |
| P04040 | Catalase (Y231) | - | + |
| O95644 | NFAT | + | + |
| Q9Y478 | AMPK | + | + |
| P45985 | JNKK | + | + |
| P05412 | AP-1 | + | + |
| P46108 | P38 | - | + |
| Q00653 | NF-kB | - | altered |
| P40189 | IL-6R | + | altered |
| P29460 | IL-12 | - | unknown |
| P58753 | TIRAP | - | + |
| P40763 | STAT3 | + | + |
| Q12933 | TRAF2 | - | + |
| P55895 | RAG | + | altered |
The phosphorylation status of the proteins found upstream or downstream of FOXO. The phosphorylation status of each significant protein in the jejunum at 15 d was determined by entering the respective Uniprot accession into phosphosite database (Hornbeck et al., 2015), finding the annotation of the site of interest and accounting for the phosphorylation fold change (increased or decreased) of that site.
Table 7.
Phosphorylation status of proteins in the FOXO signaling pathway of liver at 15 d of broiler chickens fed control or diets supplemented with a complex of biofactors and antioxidants undergoing early life stress.
| Human Ortholog Uniprot number | Protein name | Phosphorylation status | Function |
|---|---|---|---|
| Q96EB6 | SIRT1 | - | + |
| P36897 | TGF-B receptor | + | + |
| Q7L0×0 | TLR7/8 | + | unknown |
| P04040 | Catalase (Y231, 386) | - | + |
| Q60591 | NFAT | + | + |
| Q13131 | AMPK | + | + |
| P45985 | JNKK | + | + |
| P05412 | AP-1 | + | + |
| P46108 | P38 | - | + |
| Q00653 | NF-kB | - | altered |
| P40189 | IL-6 | + | altered |
| P29460 | IL-12 | - | unknown |
| Q5VWK5 | IL-23 | + | unknown |
| P58753 | TIRAP | - | + |
| P40763 | STAT3 | - | + |
| Q9UKE5 | TRAF2 | - | + |
| Q99683 | ASK1 | + | + |
The phosphorylation status of the proteins found upstream or downstream of FOXO. The phosphorylation status of each significant protein in the jejunum at 15 d was determined by entering the respective Uniprot accession into phosphosite database (Hornbeck et al., 2015), finding the annotation of the site of interest and accounting for the phosphorylation fold change (increased or decreased) of that site.
DISCUSSION
In the present study, a positive effect of P(BF+AOx) supplementation was observed on the growth performance of the birds at 21, 28, and 35 d, on antioxidant defenses and expression of immune-related genes. It is important to take into consideration that the supplementation was provided from 1 to 14 d only, when the birds may be more susceptible to stressors and the immune system may not be completely mature (Klasing, 2007). Therefore, a longer time of supplementation may be even more beneficial to the birds, especially when facing challenges later in life. Compared to control diet fed birds, the chickens supplemented with P(BF+AOx) had a lower expression of IL-1β in the lungs at 7 d, and 26 and 18% higher mRNA expression of IL-6 in the liver at 7 and 15 d, respectively. The birds were double vaccinated against IB on day of hatch and exposed to 48 h of cold stress (from 3 to 5 d); therefore, the lower expression of IL-1β in the lungs of P(BF+AOx) supplemented birds at 7 d may represent a lessened inflammatory response. Stressors lead to the activation of transcription factors, such as NF-kB, which will ultimately promote the expression of proinflammatory cytokines (Banerjee et al., 2020), including IL-1β. Additionally, the higher expression IL-6 by P(BF+AOx) indicates that it may have a strong metabolic effect, as IL-6 has been shown to have metabolic effects, including glucose disposal, lipolysis, oxidative metabolism, and energy expenditure (Han et al., 2020), apart from its immune-mediating effects. The gene expression data obtained in the present study agrees with the kinome analysis performed in both jejunum and liver, wherein the phosphorylation of several proteins, related to the immune and antioxidant defenses, as well as their function, were changed by the supplementation.
The interplay between immunity and metabolism, known as immunometabolism, is a relatively recent research approach that can be considered from different perspectives: the effect of the immunity on the whole organism metabolism or the role of metabolic pathways on the host immunity (Arsenault and Kogut, 2013). Although this is of paramount importance when evaluating the mechanism of action of novel feed additives, there are few studies using this concept in poultry (Arsenault et al., 2017; Johnson et al., 2019). From an industry point of view, performance parameters are the main indicators when deciding whether or not to use a product in commercial poultry production; however, the decision making process may be complemented notably with support of other science related parameters. The main advantages of kinome analysis using peptide arrays involves site-specific information on the immune and metabolic alterations, similar biochemical properties to the full-length protein, and provide a means for defining phosphorylation-mediated events (Ouyang et al., 2003) and chicken-specific kinome arrays are available (Arsenault and Kogut, 2013). Phosphorylation is an important mechanism of post-translational modification regulating protein function and regulates fundamental biological processes (Manning et al., 2002). Therefore, we explored the immune and metabolic changes observed in broiler chickens undergoing early life stress that could help to explain the improvement in growth performance and glutathione reductase activity, as well as the changes in gene expression, obtained by the supplementation of P(BF+AOx).
FOXO is an immunometabolic and dual inflammatory signaling pathway (Figure 2) with focus on oxidative stress and oxidative stress resistance (Akasaki et al., 2014; Sundaresan and Puthanveetil, 2017). Under oxidative stress, FOXO has anti-inflammatory effects by promoting ROS detoxification and apoptosis (ASK-1 and AP-1) and decreased activation of proinflammatory cytokines. The phosphorylation of catalase on 2 of its phosphorylation sites Y231 and Y386 promotes proteasome activity and ubiquitination (Cao et al., 2003b), therefore the decreased phosphorylation of catalase on these sites as observed in the kinome peptide array results indicate preservation of the enzyme allowing its activity within the tissue. Research suggest that FOXO expression may increase glutathione peroxidase activity and reduced ROS (Akasaki et al., 2014). Research also shows that glutathione peroxidase and glutathione reductase are equally important in ROS detoxification (Yang et al., 2006). Thus, the oxidative stress results showing increased glutathione reductase activity further supports glutathione activity and a relation to FOXO signaling during oxidative stress. Moreover, FOXO can be phosphorylated and inhibited by AKT on 3 sites to inhibit its activity and can be activated by JNK and AMPK (Klotz et al., 2015; Wang et al., 2017). Therefore, the phosphorylation status of the upstream and downstream signaling proteins indicates activation of the FOXO pathway. Further support of active responses and resistance to oxidative stress was observed by the activation of cellular senescence which can also be induced via oxidative stress and TGFRB signaling (Hubackova et al., 2012).
Liver results showed more innate immunoregulatory pathways (Supplementary Table 2) and upon further peptide analysis, it was observed that these proteins’ phosphorylation status indicated less activation than in control (nonsupplemented) broilers. These proteins include STAT3, IL12-R, IL-23R, and TIRAP (Table 7). These results along with the increase IL-10 expression (Table 5) and phosphorylation of IL-6R and TGF-β receptor on their active sites may be an indication of reduced inflammation or anti-inflammatory responses. Although IL-23R is phosphorylated, the effects of phosphorylation on this specific site S121 remains unknown. Also, the induction of IL-23R's functional pathway requires phosphorylation and activation of both IL12-RB and IL-23R (Parham et al., 2002; Floss et al., 2016) which is not supported by this data. Similar to liver samples collected on day 15, the kinome peptide array results of the jejunum at d 15 showed decreased phosphorylation associated with reduced activation of TIRAP, IL-12R and NF-kB (Table 6). Jejunum also shows increased phosphorylation of dual/anti-inflammatory indicators such as IL-6R and TGF-beta receptor (Table 6). However, proinflammatory factors like RAG and STAT3 were increasingly phosphorylated, indicating activation. Thus, there is more of a mixed immunomodulatory response in the jejunum as compared to the liver.
In conclusion, the supplementation of P(BF+AOx) improved the growth performance of broiler chickens undergoing early life stress. Further analyses presented herein, demonstrated that this novel feed additive was beneficial in modulating the immune and antioxidant defense systems of the birds. Overall, the kinome data functionally agreed with the gene expression and antioxidant results and indicates a general anti-inflammatory and antioxidant response in birds supplemented with P(BF+AOx).
DISCLOSURES
C. Bortoluzzi, L. Lahaye, and E. Santin are employees at Jefo Nutrition Inc. The other authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2021.101176.
Appendix. Supplementary materials
REFERENCES
- Akasaki Y., Alvarez-Garcia O., Saito M., Caramés B., Iwamoto Y., Lotz M.K. FOXO transcription factors support oxidative stress resistance in human chondrocytes. Arthritis Rheumatol. 2014;66:3349–3358. doi: 10.1002/art.38868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault R.J., Lee J.T., Latham R., Carter B., Kogut M.H. Changes in immune and metabolic gut response in broilers fed β-mannanase in β-mannan-containing diets. Poult. Sci. 2017;96:4307–4316. doi: 10.3382/ps/pex246. [DOI] [PubMed] [Google Scholar]
- Arsenault R., Kogut M. Chicken-specific peptide arrays for kinome analysis: flight for the flightless. Curr. Top. Biotechnol. 2013;7:79–89. [Google Scholar]
- Banerjee S., Ghosh S., Mandal A., Ghosh N., Sil P.C. ROS-associated immune response and metabolism: a mechanistic approach with implication of various diseases. Arch. Toxicol. 2020;94:2293–2317. doi: 10.1007/s00204-020-02801-7. [DOI] [PubMed] [Google Scholar]
- Cao C., Leng Y., Kufe D. Catalase activity is regulated by c-Abl and Arg in the oxidative stress response. J. Biol. Chem. 2003;278:29667–29675. doi: 10.1074/jbc.M301292200. [DOI] [PubMed] [Google Scholar]
- Cao C., Leng Y., Liu X., Yi Y., Li P., Kufe D. Catalase is regulated by ubiquitination and proteosomal degradation. Role of the c-Abl and arg tyrosine kinases. Biochem. 2003;42:10348–10353. doi: 10.1021/bi035023f. [DOI] [PubMed] [Google Scholar]
- Estévez M. Oxidative damage to poultry: from farm to fork. Poult. Sci. 2015;94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Floss D.M., Klöcker T., Schröder J., Lamertz L., Mrotzek S., Strobl B., Hermanns H., Scheller J. Defining the functional binding sites of interleukin 12 receptor β1 and interleukin 23 receptor to Janus kinases. MBoC. 2016;27:2301–2316. doi: 10.1091/mbc.E14-12-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi Harsini S., Habibiyan M., Moeini M.M., Abdolmohammadi A.R. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol. Trace Elem. Res. 2012;148:322–330. doi: 10.1007/s12011-012-9374-0. [DOI] [PubMed] [Google Scholar]
- Han M.S., White A., Perry R.J., Camporez J.-P., Hidalgo J., Shulman G.I., Davis R.J. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. USA. 2020;117:2751–2760. doi: 10.1073/pnas.1920004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann-Kleiter N., Baier G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood. 2010;115:2989–2997. doi: 10.1182/blood-2009-10-233585. [DOI] [PubMed] [Google Scholar]
- Hornbeck P.V., Zhang B., Murray B., Kornhauser J.M., Latham V., Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512–D520. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., He Y., Arowolo M.A., Wu S., He J. Polyphenols as potential attenuators of heat stress in poultry production. Antioxidants. 2019;8:67. doi: 10.3390/antiox8030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubackova S., Krejcikova K., Bartek J., Hodny Z. IL1- and TGFβ-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine ‘Bystander senescence. Aging. 2012;4:932–951. doi: 10.18632/aging.100520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W., Wit S.de. Infectious Bronchitis. In: Swayne D.E., Boulianne M., Logue C.M., Mcdougald L.R., Nair V., Suarez D.L., editors. Diseases of Poultry. 14th Ed. Wiley-Blackwell; Hoboken, NJ: 2019. [Google Scholar]
- Johnson C.N., Kogut M.H., Genovese K., He H., Kazemi S., Arsenault R.J. Administration of a postbiotic causes immunomodulatory responses in broiler gut and reduces disease pathogenesis following challenge. Microorganisms. 2019;7:268. doi: 10.3390/microorganisms7080268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing K.A. Nutrition and the immune system. Br. Poult. Sci. 2007;48:525–537. doi: 10.1080/00071660701671336. [DOI] [PubMed] [Google Scholar]
- Klotz L.-O., Sánchez-Ramos C., Prieto-Arroyo I., Urbánek P., Steinbrenner H., Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H., Arsenault R.J. A role for the non-canonical Wnt-β-catenin and TGF-β signaling pathways in the induction of tolerance during the establishment of a Salmonella enterica serovar enteritidis persistent cecal infection in chickens. Front. Vet. Sci. 2015;2:1–11. doi: 10.3389/fvets.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Sci. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Ouyang Z., Takáts Z., Blake T.A., Gologan B., Guymon A.J., Wiseman J.M., Oliver J.C., Davisson V.J., Cooks R.G. Preparing protein microarrays by soft-landing of mass-selected ions. Sci. 2003;301:1351–1354. doi: 10.1126/science.1088776. [DOI] [PubMed] [Google Scholar]
- Parham C., Chirica M., Timans J., Vaisberg E., Travis M., Cheung J., Pflanz S., Zhang R., Singh K.P., Vega F., To W., Wagner J., O'Farrell A.-M., McClanahan T., Zurawski S., Hannum C., Gorman D., Rennick D.M., Kastelein R.A., de W. Malefyt R., Moore K.W. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- Rahimnejad S., Yuan X.-Y., Liu W.-B., Jiang G.-Z., Cao X.-F., Dai Y.-J., Wang C.-C., Desouky H.E. Evaluation of antioxidant capacity and immunomodulatory effects of yeast hydrolysates for hepatocytes of blunt snout bream (Megalobrama amblycephala) Fish & Shellfish Immunol. 2020;106:142–148. doi: 10.1016/j.fsi.2020.06.019. [DOI] [PubMed] [Google Scholar]
- Sahin K., Sahin N., Kucuk O. Effects of chromium, and ascorbic acid supplementation on growth, carcass traits, serum metabolites, and antioxidant status of broiler chickens reared at a high ambient temperature (32°C) Nutr. Res. 2003;23:225–238. [Google Scholar]
- Sanin D.E., Prendergast C.T., Mountford A.P. IL-10 Production in macrophages is regulated by a TLR-driven CREB-mediated mechanism that is linked to genes involved in cell metabolism. J. Immunol. 2015;195:1218–1232. doi: 10.4049/jimmunol.1500146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Li S., Xin H., Li J., Li X., Zhang R., Li J., Bao J. Proper cold stimulation starting at an earlier age can enhance immunity and improve adaptability to cold stress in broilers. Poult. Sci. 2020;99:129–141. doi: 10.3382/ps/pez570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan S., Puthanveetil P. Is FoxO1 the culprit, partner in crime, or a protector in systemic inflammation? Am. J. Cell Physiol. 2017;313:C239–C241. doi: 10.1152/ajpcell.00194.2016. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., Jensen L.J., von Mering C. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost B., Kindrachuk J., Määttänen P., Napper S., Kusalik A. PIIKA 2: an expanded, web-based platform for analysis of kinome microarray data. PloS one. 2013;8(11):e80837. doi: 10.1371/journal.pone.0080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hu S., Liu L. Phosphorylation and acetylation modifications of FOXO3a: Independently or synergistically? Oncol. Lett. 2017;13:2867–2872. doi: 10.3892/ol.2017.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.S., Chan H.W., Yu L.C. Glutathione peroxidase and glutathione reductase activities are partially responsible for determining the susceptibility of cells to oxidative stress. Toxicol. 2006;226:126–130. doi: 10.1016/j.tox.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Zhang Z.W., Lv Z.H., Li J.L., Li S., Xu S.W., Wang X.L. Effects of cold stress on nitric oxide in duodenum of chicks. Poult. Sci. 2011;90:1555–1561. doi: 10.3382/ps.2010-01333. [DOI] [PubMed] [Google Scholar]
- Zhao F., Zhang Z., Yao H., Wang L., Liu T., Yu X., Li S., Xu S.-W. Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res. Vet. Sci. 2013;95:146–155. doi: 10.1016/j.rvsc.2013.01.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.