Abstract
Plant breeding has developed corn genotypes with grain higher in levels of carotenoids. Dietary consumption of specific carotenoids by humans has been associated with improved eye health, notably with some protection against age-related macular degeneration. Increasing dietary sources of macular carotenoids in the standard American diet might be accomplished by using high carotenoid Orange Corn in poultry diets to increase macular carotenoid concentrations in egg yolks. Three hundred sixty laying hens (Novogen White) were fed three different diets over 31 days. Each diet had six replicates of 20 hens housed in enrichable colony cages. The only difference was the type of corn included - white, yellow, and orange, in order to assess the impact of each type of corn on egg production, yolk pigmentation, and carotenoid deposition. This study assessed yolk color and carotenoid densities using a portable colorimeter and the DSM YolkFan, and by high performance liquid chromatography (HPLC) on eggs from the feeding study and on 43 cartons of 12 eggs commercially available and produced in various production settings: conventional cage, cage-free, cage-free organic, free-range/pasture, and free-range/pasture organic. Yolks from hens fed with the Orange Corn diet produced eggs with higher (P < 0.01) DSM yolk color (6 to 10) and total xanthophylls (23.5 to 35.3 μg/g of egg yolk) compared to the yellow diet (5 to 6 DSM and 12.3 to 17.7 μg/g xanthophylls) and white diet (1 to 2 DSM and 2.5 to 3.0 μg/g xanthophylls). Egg yolks reached a maximum xanthophyll accumulation with the Orange Corn diet (35.3 μg/g of egg yolk) after twelve days of treatment and maintained steady levels at subsequent time points. In general, xanthophyll levels in yolks from the Orange Corn diet were superior (30–61% higher) to any of the commercial egg brands, suggesting that feeding high carotenoid Orange Corn increases xanthophyll density in eggs.
Key words: orange corn, macular carotenoids, yolk color, eggs, laying hens
INTRODUCTION
Lutein and zeaxanthin are xanthophylls, a class of oxygen-containing carotenoid pigments found widely in dark green leafy vegetables, colorful fruit, corn, eggs, and avocados. Lutein and zeaxanthin are isomers with an identical chemical formula, the only difference between them is the location of a double bond in one of the end rings. Consumption of higher levels of these phytonutrients is associated with a reduced risk of various health conditions, such as age-related maculopathy (Mares, 2013; Bohn, 2017; Lawler et al., 2019), oxidative stress (Bohn, 2019), cardiovascular diseases (Kulczyński et al., 2017), decline in cognitive function (Saint et al., 2018; Mewborn et al., 2018; Xavier et al., 2018; Stringham et al., 2019), Alzheimer's disease, (Obulesu et al., 2011; Honarvar et al., 2016; Das et al., 2019), and certain types of cancer (Takata et al., 2013; Kesse-Guyot et al., 2014; Bohn, 2017; Gong et al., 2018). An increase in the dietary intake of lutein and zeaxanthin is associated with improvements in visual function and a reduced risk of age-related macular degeneration (AMD) (Ma et al., 2016). The consumption of lutein and zeaxanthin is associated with increases in the macular pigment optical density and cognitive function in adolescents and older adults (Chung et al., 2004; Wenzel et al., 2006; Scott et al., 2017; Saint et al., 2018; Stringham et al., 2019; Papanikolaou and Fulgoni, 2019). Unfortunately, the National Health and Nutrition Examination Survey, 2017–2018 (NHANES) estimated that the average American adult consumes on average 1.7 mg/day of lutein and zeaxanthin combined (U.S. Department of Agriculture, 2020), which is likely not enough to attain these stated health benefits. Eggs are an essential part of the American diet with approximately 22% of the US population consuming whole eggs on a given day (Conrad et al., 2017). According to the USDA, the per capita consumption of eggs in the United States was 293.4 per person in 2019 (U.S. Department of Agriculture, 2021). Eggs have the potential to contribute significant levels of lutein and zeaxanthin, and several essential vitamins and micronutrients in the western diet (Wallace, 2018). The yellow-orange yolk color comes from lutein and zeaxanthin (Schweiggert and Carle, 2017; Titcomb et al., 2019), natural pigments present in laying hens diets. Although lutein and zeaxanthin levels in egg yolk are lower than dark green leafy vegetables, the lipid-rich matrix of the egg yolks has been shown to increase lutein and zeaxanthin bioaccessibility (Chung et al., 2004). Therefore, efforts that focus on the natural increase of lutein and zeaxanthin levels in egg yolks can benefit the health of a large proportion of the general population, given lutein and zeaxanthin's role in visual and cognitive health.
Yolk color is a crucial egg quality attribute for the egg industry and consumer preference. In most countries, consumers prefer pigmented egg yolks with golden-orange tones (Grashorn, 2016). Hens are not able to synthesize carotenoids (Schweiggert and Carle, 2015); therefore, the color of the yolk is derived from the carotenoid pigments present in their feed. To achieve a deeper colored yolk, some producers use specific feed additives with both yellow and red carotenoids from natural and artificial sources. The feed additives used might include artificially produced carotenoids, such as ethyl ester of β-apo-8′-carotenoic acid, β-apo-8′-carotenal, canthaxanthin, and citranaxanthin (Vincent et al., 2017). Although supplemental feed additives rich in carotenoids can increase the carotenoid levels in egg yolks, these additives represent a substantial cost to egg producers (Hernández-Velasco et al., 2014), and they do not provide the health benefits associated with macular carotenoids.
Corn grain exhibits natural variation in carotenoids that are associated with human health benefits: the provitamin A carotenoids β-carotene, β-cryptoxanthin, and α-carotene as well as the macular carotenoids lutein and zeaxanthin (Harjes et al., 2008; Burt et al., 2011; Pixley et al., 2012; Owens et al., 2014; Diepenbrock et al., 2021). In response to vitamin A deficiencies in human populations in developing countries the HarvestPlus program promoted the process of natural biofortification of corn to increase levels of provitamin A (Saltzman et al., 2013). Biofortification is a feasible and cost-effective process of increasing the density of vitamins and minerals in a crop through plant breeding (Bouis and Saltzman, 2017). The non-GMO corn used in this study was bred to have a dark orange color and contain significantly higher total carotenoid levels (45 to 55 μg/g) than conventional yellow corn (∼15 to 20 μg/g). While this Orange Corn was originally bred to deliver increased nutrition (increased provitamin A carotenoids) to humans in the developing world, efforts are also ongoing to develop corn germplasm with higher xanthophyll levels that could benefit the eye health of human consumers in more economically developed nations. This biofortified Orange Corn when used for dietary supplementation of xanthophylls in poultry feed diets may increase yolk color. Therefore, the main objective of the present study was to assess the inclusion of high carotenoid Orange Corn in laying hen diets and compare the lutein and zeaxanthin concentration and yolk color scores to commercially available eggs produced in various production settings.
MATERIALS AND METHODS
Animals and Housing
Experimental procedures were approved by the Purdue Animal Care and Use Committee (Protocol #1709001622). A total of 360 Novogen White laying hens at 32 wk of age were randomly allocated to three experimental diet groups at the Purdue Animal Sciences Research and Education Center (ASREC) in West Lafayette, IN. Each diet had six replicates of 20 hens housed in enrichable colony cages. Hens were provided 464.5 cm2 space per hen with 6.1 cm feeder space and six pin-metered drinkers per colony cage. Three different diets were provided, with the only difference being the type of corn included - white, yellow, or Orange Corn. Diets were formulated to the Novogen White Management Guide (Novogen, 2018). Tables 1 and 2 provides diet formulation and analyzed nutrients details.
Table 1.
Main ingredients and nutrient composition of the experimental diets fed to the Novogen White laying hens.
| Parameter | Treatment1 |
||
|---|---|---|---|
| White | Yellow | Orange | |
| Ingredients, g/kg | |||
| Corn | 565 | 565 | 565 |
| Soybean meal, 48% CP | 230 | 230 | 230 |
| Wheat midds | 43.8 | 43.8 | 43.8 |
| Soy oil | 43.5 | 43.5 | 43.5 |
| L-lysine HCl | 0.4 | 0.4 | 0.4 |
| DL-methionine | 1.85 | 1.85 | 1.85 |
| L-threonine | 0.55 | 0.55 | 0.55 |
| Salt | 3.75 | 3.75 | 3.75 |
| Limestone | 95.7 | 95.7 | 95.7 |
| Dicalcium phosphate | 12.9 | 12.9 | 12.9 |
| Vitamin-mineral premix2 | 2.5 | 2.5 | 2.5 |
| Calculated values | |||
| Protein, g/kg | 160.7 | 160.7 | 160.7 |
| Metabolizable energy, Kcal/kg | 2959.2 | 2959.2 | 2959.2 |
| Calcium, g/kg | 40.1 | 40.1 | 40.1 |
| Phosphorus, g/kg | 6.1 | 6.1 | 6.1 |
| Lysine, g/kg | 8.6 | 8.6 | 8.6 |
| Methionine, g/kg | 4.3 | 4.3 | 4.3 |
| Methionine + cysteine, g/kg | 7.0 | 7.0 | 7.0 |
| Threonine, g/kg | 6.6 | 6.6 | 6.6 |
Diets were formulated with the assumption corn nutrients were the same.
Vitamin and mineral premix provided the following (per kg of diet): vitamin A, 12,320 IU; vitamin D3, 4,620 IU; vitamin E, 15.4 IU; vitamin K, 3.08 mg; riboflavin, 6.16 mg; niacin, 46.2 mg; vitamin B12, 23.1 mg; pantothenic acid, 15.4 mg; folic acid, 0.31 mg; choline, 401 mg; iron, 36 mg; zinc, 51 mg; manganese, 90 mg; copper, 5 mg; iodine, 0.7 mg; and selenium, 0.25 mg.
Table 2.
Analyzed diet values for nutrients and carotenoid composition expressed on dry matter basis fed to the Novogen White laying hens.
| Parameter | Treatment |
||
|---|---|---|---|
| White | Yellow | Orange | |
| Analyzed values | |||
| Crude protein, % | 21.8 | 21.5 | 24.1 |
| Crude fat, % | 6.40 | 6.31 | 7.03 |
| Crude fiber, % | 2.00 | 1.80 | 2.30 |
| Metabolizable energy, Kcal/kg | 3505.3 | 3527.4 | 3505.3 |
| Phosphorus, % | 0.73 | 0.76 | 0.70 |
| Calcium, % | 1.21 | 1.31 | 1.17 |
| Threonine, % | 0.75 | 0.72 | 0.84 |
| Methionine, % | 0.69 | 0.89 | 0.66 |
| Lysine, % | 1.19 | 1.14 | 1.30 |
| Analyzed carotenoid composition1 | |||
| Lutein, µg/g | 0.5 ± 0.0 | 3.0 ± 0.1 | 4.1 ± 0.0 |
| Zeaxanthin, µg/g | 0.3 ± 0.0 | 2.2 ± 0.0 | 15.6 ± 0.3 |
| β-cryptoxanthin, µg/g | 0.1 ± 0.0 | 0.3 ± 0.0 | 2.29 ± 0.0 |
| Total xanthophylls, µg/g2 | 0.9 ± 0.1 | 5.7 ± 0.1 | 22.9 ± 0.0 |
| Total Carotenoids, µg/g3 | 0.9 ± 0.1 | 5.7 ± 0.1 | 24.9 ± 0.3 |
Carotenoid data are mean ± SEM; n = 2.
Total xanthophylls = lutein + zeaxanthin + α-cryptoxanthin + β-cryptoxanthin.
Total carotenoid content was calculated by the summary of each individual carotenoid identified and quantified by analysis.
Performance of Laying Hens and Eggs Quality Parameters
Hen body weights were measured at the start and the end of the 31-day experimental period (Table 3). Egg production was collected daily and expressed as a hen day percentage. Each week, case weights were recorded for each diet replicate. Daily, 24 eggs per treatment (4 eggs per replicate) were randomly collected and stored at 4°C overnight prior to assessment. Individual eggs had Haugh Unit (Haugh, 1937) measured using a TSS QCD system (Technical Services and Supplies, Dunnington, York, UK), subjective yolk color assessed using the DSM YolkFan, and egg component weights as described by Karcher et al. (2019). Egg yolks were combined into four 6-egg pools per treatment for colorimeter and carotenoid assessment.
Table 3.
Novogen White laying hens performance and egg quality parameters from 32 to 36 wk of age. Hens were fed a white, yellow or Orange Corn diet.
| Parameter | Week | Treatment |
|||
|---|---|---|---|---|---|
| White | Yellow | Orange | P-value | ||
| Egg weight, g1 | 1 | 61.9 ± 0.7 | 61.4 ± 0.9 | 61.4 ± 0.8 | 0.77 |
| 2 | 61.6 ± 0.8 | 62.0 ± 0.9 | 62.2 ± 0.8 | 0.78 | |
| 3 | 61.4 ± 0.7 | 61.7 ± 0.7 | 62.3 ± 0.9 | 0.77 | |
| 4 | 62.9 ± 0.7 | 63.2 ± 0.6 | 62.5 ± 0.7 | 0.78 | |
| 5 | 60.9 ± 1.2 | 60.6 ± 1.1 | 62.3 ± 1.0 | 0.46 | |
| Yolk weight, g1 | 1 | 16.2 ± 0.2 | 16.0 ± 0.3 | 15.9 ± 0.2 | 0.67 |
| 2 | 15.9 ± 0.3 | 16.1 ± 0.2 | 15.8 ± 0.3 | 0.76 | |
| 3 | 15.8 ± 0.3 | 15.9 ± 0.4 | 16.2 ± 0.3 | 0.71 | |
| 4 | 17.0 ± 0.3 | 16.4 ± 0.2 | 16.5 ± 0.3 | 0.18 | |
| 5 | 16.1 ± 0.5 | 16.6 ± 0.3 | 16.6 ± 0.3 | 0.58 | |
| Shell weight, g1 | 1 | 5.9 ± 0.1 | 5.7 ± 0.1 | 5.8 ± 0.1 | 0.58 |
| 2 | 5.9 ± 0.1 | 5.9 ± 0.1 | 6.0 ± 0.1 | 0.59 | |
| 3 | 6.0 ± 0.1⁎ | 5.5 ± 0.1 | 5.9 ± 0.1 | 0.00 | |
| 4 | 6.1 ± 0.1 | 5.8 ± 0.1 | 6.0 ± 0.1 | 0.17 | |
| 5 | 5.7 ± 0.1 | 5.6 ± 0.1 | 6.0 ± 0.1 | 0.06 | |
| Haugh unit1 | 1 | 87.5 ± 1.3 | 89.9 ± 1.4 | 88.8 ± 1.1 | 0.45 |
| 2 | 85.7 ± 1.4 | 90.4 ± 1.3 | 88.8 ± 0.8 | 0.03 | |
| 3 | 89.4 ± 2.1 | 89.7 ± 1.5 | 95.7 ± 1.2⁎ | 0.02 | |
| 4 | 88.6 ± 1.1 | 93.7 ± 1.4⁎ | 87.9 ± 1.3 | 0.00 | |
| 5 | 89.7 ± 1.6 | 88.7 ± 1.4 | 88.7 ± 1.9 | 0.89 | |
| Egg production rate, %2 | 1 | 91.4 ± 2.1 | 91.9 ± 1.9 | 94.0 ± 2.1 | 0.45 |
| 2 | 97.1 ± 2.1 | 96.8 ± 1.9 | 97.7 ± 2.1 | 0.74 | |
| 3 | 99.9 ± 2.1 | 97.4 ± 1.9 | 98.7 ± 2.1 | 0.63 | |
| 4 | 100.1 ± 2.1 | 95.9 ± 1.9 | 99.7 ± 2.1 | 0.55 | |
| 5 | 98.3 ± 2.1 | 95.8 ± 1.9 | 99.1 ± 2.1 | 0.71 | |
| 32 wk. body weight3 | 1.5 ± 0.0 | 1.5 ± 0.0 | 1.5 ± 0.0 | 0.42 | |
| 36 wk. body weight4 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.51 | |
Data are means ± SEM based on 24 replicates per treatment.
Egg production rate are mean ± SEM; based on 6 replicates per treatment, with twenty hens per replicate.
32 wk. body weight are mean ± SEM; n = 60.
36 wk. body weight are mean ± SEM; n = 30.
Significant difference within a row according to Tukey's HSD test (P < 0.05).
Commercially Available Eggs
In March and May 2018, a total of 43 cartons consisting of 12 large eggs were purchased at four different grocery stores in West Lafayette and Lafayette, IN. The samples were produced by different brands and production practices: conventional cage (9 cartons), cage-free (10 cartons), cage-free organic (9 cartons), free-range/pasture (11 cartons), and free-range/pasture organic (4 cartons). Individual eggs from each sample were broken and had yolk color assessed using the DSM YolkFan. Following the measurement, albumen was removed, and egg yolks were combined to create two 6-egg pools per carton for colorimeter and carotenoid assessment.
Colorimeter Assessment of Eggs
All egg pools were homogenized in a stomacher (Seward, West Sussex, UK) for 2 min. The bag was placed flat on a countertop, and the color analyzed in three different locations of the plastic bag using a portable Konica Minolta CR-400 Chroma Meter (Konica Minolta Sensing Americas, Inc., Ramsey, NJ). The colorimeter was calibrated with the plastic bag prior to the measurements. Color space, often referred to as L*a*b*, was used for assessment of yolk color. The L* values represent lightness, with a value of 0 to 100 (L* = 0 indicates darkness, L* = 100 lightest). The a* values represent redness on a scale from -60 (green) to +60 (red). The b* values represent yellowness on a scale from -60 (blue) to +60 (yellow). These measurements correspond to the CIELAB color space defined by the International Commission on Illumination (CIE). It expresses color as three values: L* for perceptual lightness, and a* and b* for the four unique colors of human vision: red, green, blue, and yellow. Colorimeter settings used the standard illuminant D65 and an observer angle of 2° during the measurements. After color analysis, we transferred egg pools into conical tubes and stored them at -80°C. Golden-orange tones in egg yolks were of interest for this study, so only the green/red or a* axis color space will be presented here. A higher a* axis color space indicates more red coloration present in the egg yolk. The b* and L* axis color spaces were minimally significant for each of the diet treatments, further justifying not presenting the b* and L* axis color space results.
Egg Pool Carotenoid Analysis
All sample preparations and extractions were performed under yellow-filtered light to minimize the potential for photo-isomerization reactions. Ground corn and diets were used for carotenoid extractions using the procedure previously described (Ortiz and Ferruzzi, 2019) with minor modification. Briefly, samples were ground to < 0.5 mm particle size with a Foss CT 1093 Cyclotec sample mill (Foss Analytical Co, Suzhou, China). The ground samples (0.6 g) were spiked with 80 μL internal standards (150 μM or 30 μM β-apo-8′-carotenal in ethyl acetate), in 15 mL conical tubes. Samples were hydrated with 1 mL of distilled water on ice for 10 min, and carotenoids were extracted twice with 5 mL of cold acetone and then once with 2 mL of methyl tert-butyl ether. Extracts were dried under a stream of nitrogen, resolubilized in 1:1 methanol:ethyl acetate, filtered through a 0.45 µm PTFE syringe filter (Macherey-Nagel, Düren, Germany) and then 10 μL was injected into the HPLC system.
Yolk pool samples in duplicate (0.3 g) were spiked with 80 μL of an internal standard (150 μM or 30 μM β-apo-8′-carotenal in ethyl acetate) and mixed with 1 mL deionized water using a vortex, followed by 3 mL ethanol (0.1% butylated hydroxytoluene as an antioxidant) addition. The carotenoids from the egg yolks were extracted using a modification of the method of Sowa et al. (2017). Samples were mixed with 1 mL of distilled water to each sample and incubated on ice for 10 min. Then, 4 mL of a mixture of hexane/ethyl acetate (1:1) was added to each sample and vortexed the tubes for 1 min. Tubes were centrifuged (3000g, 5 min, 4°C) and the upper organic phase was collected from the tubes. Samples were mixed two more times with the hexane/ethyl acetate until the extract was colorless. Extracts were dried under a stream of nitrogen, reconstituted with 500 μL 50:50 methanol: ethyl acetate, filtered through a 0.45 µm PTFE syringe filter (Macherey-Nagel, Düren, Germany), and 10 μL was injected into the HPLC system. A YMC C30 column (3μm 2.0 mm × 150 mm) with a YMC carotenoid guard column (2.0 mm × 23 mm) was used to separate carotenoids in a Shimadzu HPLC Prominence UFLC XR series system coupled with a diode array detector at 450 nm as previously described by Ortiz and Ferruzzi (2019). Carotenoids were separated with a gradient elution profile based on a binary mobile phase system consisting of Methanol:1 M ammonium acetate (98:2 v/v) in phase A and ethyl acetate in phase B. Flow rate of 0.37 mL/min was settled with initial conditions set at 0 min 80:15 v/v, phase A: phase B; 6 min 20:80 v/v, phase A: phase B; 8 min 0:100 v/v, phase A: phase B; 12 min 0:100 v/v, phase A: phase B, 14 min 80:15 v/v, phase A: phase B. Carotenoids were identified by comparing spectral information in the literature (Britton et al., 2004) and retention times with authentic all-trans-carotenoid standards. Carotenoids quantification was carried out with external standards on seven-point calibration curves prepared spectrophotometrically with authentic all-trans-standards with a concentration range between 0.01 to 7.67 μM.
Statistical Analysis
Data were analyzed using the Fit Model Platform of JMP Pro v14.0 (SAS Institute Inc, 2018). Results are presented as mean ± standard error. The performance, egg data, egg yolk color, and carotenoid concentrations were analyzed by one-way ANOVA, posthoc comparisons between treatments were made by Tukey's test. Statistical significance was considered at P < 0.05. The impact of diet and time on yolk color (DSM yolk color index and colorimeter a* axis values) and carotenoid deposition were analyzed using a completely randomized two-way mixed ANOVA (diet, day, and diet x day) design followed by the Tukey−Kramer method for pairwise comparison. Total xanthophyll content was calculated by summing of all-trans-lutein, all-trans-zeaxanthin, and β-cryptoxanthin concentrations.
RESULTS
Performance of Laying Hens and Egg Quality Parameters
The mean values of laying hen's weekly performance and egg quality parameters are shown in Table 3. Dietary treatments had no effect on all the measured parameters.
Color Assessment on Egg Yolks
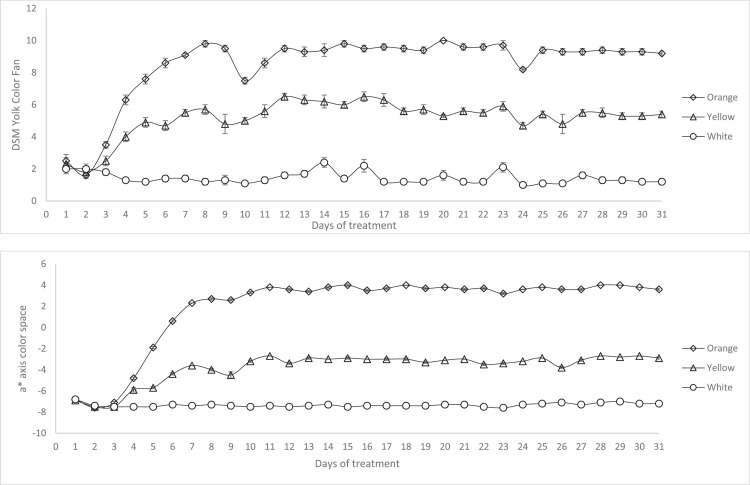
The effect of diet and time on DSM YolkFan score and colorimeter a* axis values are shown in Figure 1 and Table 4. There were differences among diet, day, and the diet x day interaction (P < 0.001). Yolks from hens fed with the Orange Corn had the highest DSM YolkFan score (9 ± 0.3), followed by egg yolks from hens fed with yellow corn (6 ± 0.2) on the DSM YolkFan color index. Egg yolks from hens’ fed a diet with white corn scored a low light-yellow DSM YolkFan color, ranging from a 1 to 2 (Figure 1). The YolkFan color index scored 7 ± 0.2 for commercial eggs. Free-range/pasture and free-range/pasture-organic eggs had the highest DSM YolkFan scores (P < 0.01) among all commercial egg classes (Table 4). Free-range/pasture and free-range/pasture-organic DSM YolkFan index was not different from the yolk scores of conventional caged hens fed the Orange Corn diet (P > 0.05).
Figure 1.
Changes in yolk color from Novogen White laying hens fed a white, yellow or Orange Corn diet starting at 32 wk of age. Each dot on the upper graph represents a mean of individual DSM YolkFan assessment on 24 yolks per treatment. Each dot on the bottom graph represents a mean of four colorimeter determinations (a-axis) on four 6-egg yolk pools, stomached prior to assessment.
Table 4.
Yolk DSM YolkFan scores and colorimeter readings (L*a*b*) from Novogen White laying hens fed white, yellow and orange diets compared to commercially available eggs.
| Husbandry | Egg Pools1 | L* axis |
a* axis |
b* axis |
DSM Yolk Color |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean2 | Min | Max | Mean2 | Min | Max | Mean2 | Min | Max | Mean2 | Min | Max | ||
| Conventional cage | 18 | 63.2 ± 0.9 | 56.4 | 72.1 | -0.6 ± 0.4 | -4.7 | 2 | 59.0 ± 0.7 | 53.5 | 64.8 | 6 ± 0.3 | 4 | 8 |
| Cage Free | 20 | 62.4 ± 0.6 | 58.1 | 68 | -0.1 ± 0.8 | -12.2 | 4.2 | 59.4 ± 0.6 | 54.1 | 63.5 | 6 ± 0.3 | 4 | 9 |
| Cage free-organic | 18 | 65.1 ± 0.8 | 61.8 | 73.9 | -2.1 ± 0.4 | -6.3 | 0.4 | 59.0 ± 1.2 | 47.4 | 66.7 | 5 ± 0.2 | 4 | 8 |
| Free range/pasture | 22 | 64.4 ± 0.7 | 59.7 | 72.7 | 1.4 ± 0.7 | -2.7 | 6.1 | 59.4 ± 0.7 | 53.4 | 66.2 | 8 ± 0.4 | 5 | 11 |
| Free Range/Pasture-Organic | 8 | 63.2 ± 1.2 | 59.0 | 68.4 | 3.3 ± 1.7 | -1.9 | 9.9 | 59.3 ± 0.9 | 56 | 63.7 | 8 ± 0.8 | 5 | 11 |
| White3 | 12 | 62.3 ± 0.3 | 60.4 | 63.5 | -7.5 ± 0.0 | -7.7 | -7.3 | 32.0 ± 0.3 | 30.4 | 33.4 | 1 ± 0.1 | 1 | 2 |
| Yellow3 | 11 | 59.9 ± 0.1 | 59.3 | 60.5 | -3.4 ± 0.1 | -3.7 | -2.8 | 52.7 ± 0.2 | 51.7 | 53.7 | 6 ± 0.2 | 5 | 6 |
| Orange3 | 12 | 57.8 ± 0.2 | 56.6 | 58.6 | 3.2 ± 0.3 | 0.9 | 4.2 | 54.0 ± 0.3 | 51.7 | 56.1 | 9 ± 0.3 | 6 | 10 |
An egg pool consisted of 6 egg yolks, stomached prior to assessment.
Data are means ± SEM.
Egg collected after 12 days of treatments.
The CIELAB color space values of eggs from the different diet treatments, and commercial production settings are presented in Table 4. There were significant differences on L*, a*, b* axis values among the diet treatments and commercial production settings (P < 0.001). Egg yolks from hens fed with the Orange Corn diet were darker, redder, and less yellow than eggs from yellow and white corn treatment groups (Figure 2) and all eggs from commercial production settings, based on color differences using the CIELAB coordinates (Table 4).
Figure 2.
Representative yolks illustrate carotenoid deposition from Novogen White laying hens fed white, yellow and orange diets starting at 32 wk of age.
Egg Yolk Carotenoid Composition
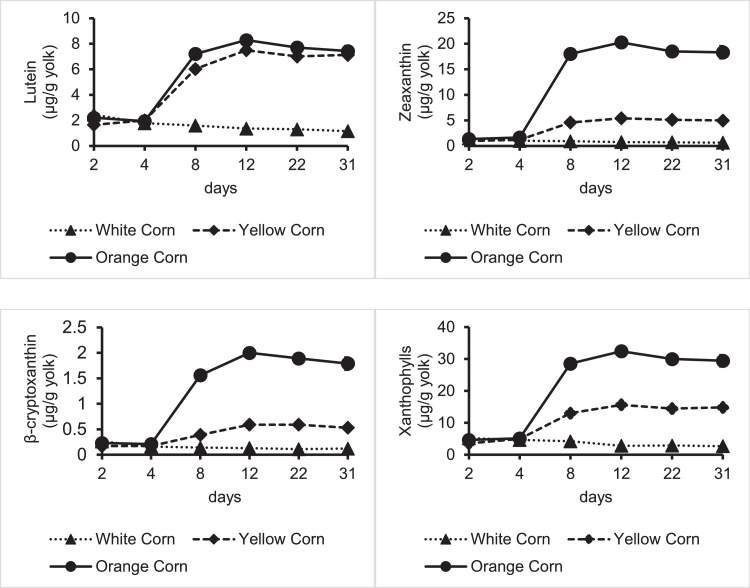
The diet and time effect on egg yolk carotenoid composition is shown in Figure 3 and Table 5. Lutein, zeaxanthin, β-cryptoxanthin, and total xanthophyll levels were different among diets after 8 days of feeding (P < 0.01). Maximum carotenoid accumulation was reached at day 12 of the three different diets and maintained steady levels at subsequent time points. Zeaxanthin was the predominant carotenoid in egg yolks from the Orange Corn treatment, showing higher zeaxanthin concentration (20.3 ± 0.7 μg/g of egg yolk) than any of the commercially available eggs evaluated (P < 0.01). The highest xanthophyll concentration in yolks from hens fed an Orange Corn diet was 35.3 μg/g of egg yolk. The mean xanthophyll level in yolks from the Orange Corn diet was 30% higher than the mean xanthophyll levels in free-range/pasture-organic yolks, which was the egg class with highest DSM YolkFan scores among all commercial egg classes.
Figure 3.
Carotenoid content in egg yolks over a 31d period of Novogen White laying hens fed white, yellow, or Orange Corn diet. Each dot on the graph represents the mean of a duplicate carotenoid determination on four 6-egg yolk pools, stomached prior to assessment.
Table 5.
Carotenoid profile from Novogen White laying hens fed white, yellow and orange diets compared to commercially available eggs (mean value ± standard error).
| Husbandry | Egg pools1 | All-trans-lutein, µg/g |
All-trans-zeaxanthin, µg/g |
β-cryptoxanthin, µg/g |
Total xanthophylls4, µg/g |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean2 | Min | Max | Mean2 | Min | Max | Mean2 | Min | Max | Mean2 | Min | Max | ||
| Conventional cage | 18 | 11.5 ± 0.7 | 4.8 | 18.9 | 8.30 ± 0.6 | 3.80 | 15.6 | 0.9 ± 0.1 | 0.3 | 1.5 | 21.5 ± 1.3 | 9.20 | 33.9 |
| Cage free | 20 | 13.6 ± 1.3 | 4.5 | 27.8 | 6.90 ± 0.3 | 2.70 | 10.0 | 0.9 ± 0.1 | 0.3 | 1.5 | 22.1 ± 1.5 | 7.80 | 38.7 |
| Cage free-organic | 18 | 11.2 ± 1.2 | 2.8 | 27.0 | 5.80 ± 0.4 | 1.50 | 8.10 | 0.7 ± 0.1 | 0.1 | 1.2 | 18.2 ± 1.6 | 4.60 | 37.6 |
| Free range/pasture | 22 | 11.6 ± 0.6 | 3.9 | 18.2 | 5.90 ± 0.3 | 2.40 | 7.90 | 0.8 ± 0.0 | 0.3 | 1.1 | 18.8 ± 0.9 | 6.80 | 27.2 |
| Free range/pasture-organic | 8 | 14.1 ± 0.6 | 10 | 17.3 | 7.00 ± 0.6 | 4.90 | 9.90 | 1.1 ± 0.1 | 0.7 | 1.5 | 22.6 ± 0.8 | 18.0 | 26.7 |
| White3 | 7 | 1.10 ± 0.1 | 0.9 | 1.30 | 0.60 ± 0.0 | 0.50 | 0.70 | 0.1 ± 0.0 | 0.1 | 0.1 | 2.70 ± 0.1 | 2.50 | 3.00 |
| Yellow3 | 8 | 7.10 ± 0.3 | 6.0 | 8.20 | 5.00 ± 0.2 | 4.30 | 5.70 | 0.5 ± 0.0 | 0.4 | 0.6 | 14.8 ± 0.6 | 12.3 | 17.7 |
| Orange3 | 8 | 7.40 ± 0.4 | 6.2 | 8.90 | 18.3 ± 1.3 | 14.7 | 22.5 | 1.8 ± 0.1 | 1.4 | 2.2 | 29.4 ± 1.9 | 23.5 | 35.3 |
An egg pool consisted of 6 egg yolks, stomached prior to assessment.
Data are means ± SEM.
Egg collected after 12 days of treatments.
Total xanthophylls = lutein + zeaxanthin + α-cryptoxanthin + β-cryptoxanthin.
Yolk carotenoid content from eggs purchased at supermarkets is presented in Table 5. Lutein and zeaxanthin were the predominant xanthophylls present in commercial egg yolks; concentrations ranged from 2.8 to 27.8 μg/g, and from 1.5 to 15.6 μg/g, respectively. Mean total xanthophyll concentration in commercial egg yolks ranged from 18.2 to 22.6 μg/g across different production practices. The highest total xanthophyll concentration in commercial eggs was from one brand of cage-free eggs with 38.7 μg/g of egg yolk (Table 5). Mean lutein, zeaxanthin, and xanthophyll concentration among all commercial eggs were not discriminable on the same level of significance.
DISCUSSION
This study compared the impact of using biofortified high carotenoid Orange Corn initially bred for human consumption versus standard yellow and white corn in a poultry diet to determine the impact of each diet on egg production, yolk pigmentation, and carotenoid deposition. We compared yolk color pigmentation and carotenoid deposition of eggs produced from the experimental diets to commercially available eggs from different production practices. Yolk color has been previously found to reflect the content of carotenoids in the hens feed, and yolk color has a visual impact on consumer perceptions (Grashorn, 2016; Moreno et al., 2020). The diet with Orange Corn resulted in egg yolks with a higher DSM yolk color index value than diets with commercial white and yellow corn. Moreno et al. (2020) obtained similar results when hens were fed a diet including genetically engineered corn enriched in carotenoids. The naturally bred high carotenoid Orange Corn used in the hens feed in our experiment provided the same level of yolk pigmentation (DSM yolk color index) obtained by Moreno et al. (2020), even with a lower total carotenoid concentration available in the diet. The Moreno et al. (2020) diet including genetically engineered corn had higher total carotenoid levels (31.05 μg/g) compared to the diet in this study made with Orange Corn (24.9 μg/g). This suggests that Orange Corn provided a better coloring efficiency, and/or there is a maximum effect from yellow/orange carotenoids in the feed beyond which there is no additional coloration in yolks. The incorporation of Orange Corn in the hens diet resulted in yolks not statistically different in color (DSM yolk color index and a* value) than yolks of commercial free-range/pasture and free-range/pasture-organic eggs. This result indicates that when Orange Corn is included in laying hens diets, egg yolks have the same level of coloration as commercially produced pasture-raised and free-range eggs.
Consumers in various countries prefer yolks with intense golden-orange color tones because they associate deeper yolk color with better quality and a more intense taste (Grashorn, 2016). However, some studies have reported that when hens are fed diets supplemented with only yellow carotenoids, yolk color will not reach high-intensity golden-orange levels (Grashorn, 2016). This is supported by the study of Moreno et al. (2020) and this study that both fed corn with a balance of yellow and red carotenoids to laying hens and both reported resultant yolks with intense golden-orange color.
Lutein, zeaxanthin, and the lutein metabolite meso-zeaxanthin are collectively referred to as macular carotenoids. They preferentially accumulate in high concentration in the central retina (macula lutea) of the human eye (Bone et al., 1985; Landrum et al., 2013; Bernstein et al., 2016). Landrum and colleagues (2013) reported high serum levels of lutein and zeaxanthin can reduce the risk of age-related macular degeneration by protecting the retina against harmful blue light. The macular carotenoid concentration in yolks produced with the Orange Corn diet was higher than in yolks produced with the yellow corn diet and higher than any of the commercial eggs tested (P < 0.05). Levels of lutein, zeaxanthin and total xanthophylls in commercial egg yolks were similar to levels previously reported (Schlatterer and Breithaupt, 2006). Moreno et al. (2020) reported a higher macular carotenoid concentration in yolks produced with a diet supplemented with genetically engineered corn. Of note, macular carotenoid concentration in the yolks from the Orange Corn diet in this study was higher than in yolks from hens fed high β-cryptoxanthin Orange Corn (Heying et al., 2014) or commercially available marigold fortificant (Titcomb et al., 2019).
Summary
A feeding study found that a diet including Orange Corn resulted in eggs with deeper yolks color than a diet with yellow corn (DSM 9 versus 6, respectively). The carotenoid analysis revealed that Orange Corn is a good feed source for depositing more zeaxanthin in egg yolks and potentially increase yolk pigmentation compared to feeding yellow corn. Comparisons to store-bought eggs showed that Orange Corn fed eggs had color comparable to the highest type (Free Range/Pasture and Free Range/Pasture-Organic), and zeaxanthin levels higher than all the commercially bought eggs (P < 0.01). A well-balanced composition of yellow and red carotenoids in hens' diets appears necessary to achieve the consumer desired levels of yolk color. Our results indicate that Orange Corn could enhance lutein and zeaxanthin levels in eggs for consumers without much diet modification. It is still unknown what the minimal Orange Corn inclusion rate in diets would be to produce a yolk color score in conventional cage eggs similar to eggs produced in commercial settings. Therefore, further work needs to be conducted with Orange Corn since it is a promising feed source for laying hens, including diets that use different Orange Corn levels that may result in different DSM scores and levels of carotenoids.
Acknowledgments
Special thanks to Jason Fields and his staff at the Purdue University ASREC Poultry Unit for day-to-day daily assistance at the farm, and Marsha Kern for assistance in producing and handling the corn for the diets. This project was supported by a USDA SBIR grant awarded to NutraMaize (Award Number 2017-33610-26980) with a subaward to Purdue, Patterson Chair funds, and the USDA National Institute of Food and Agriculture, Hatch project 1024772. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funding organizations.
Disclosures
The authors declare that there are no conflicts of interest.
REFERENCES
- Bernstein P.S., Li B., Vachali P.P., Gorusupudi A., Shyam R., Henriksen B.S., Nolan J.M. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye. Res. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn T. Carotenoids, chronic disease prevention and dietary recommendations. Int. J. Vitam. Nutr. Res. 2017;87:121–130. doi: 10.1024/0300-9831/a000525. [DOI] [PubMed] [Google Scholar]
- Bohn T. 2019. Carotenoids and markers of oxidative stress in human observational studies and intervention trials: implications for chronic diseases. Antioxidants 8:179. [DOI] [PMC free article] [PubMed]
- Bone R.A., Landrum J.T., Tarsis S.L. Preliminary identification of the human macular pigment. Vision Res. 1985;25:1531–1535. doi: 10.1016/0042-6989(85)90123-3. [DOI] [PubMed] [Google Scholar]
- Bouis H.E., Saltzman. A. Improving nutrition through biofortification. A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017;12:49–58. doi: 10.1016/j.gfs.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton G., Liaanen-Jensen S., Pfander H. 2004. Carotenoids. Handbook. Basle, Switzerland. [Google Scholar]
- Burt A.J., Grainger C.M., Smid M.P., Shelp B.J., Lee E.A. Allele mining of exotic maize germplasm to enhance macular carotenoids. Crop. Sci. 2011;51 991–14. [Google Scholar]
- Chung H.-Y., Rasmussen H.M., Johnson E.J. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J. Nutr. 2004;134:1887–1893. doi: 10.1093/jn/134.8.1887. [DOI] [PubMed] [Google Scholar]
- Conrad Z., Johnson L.K., Roemmich J.N., Juan W., Jahns L. Time trends and patterns of reported egg consumption in the US by sociodemographic characteristics. Nutrients. 2017;9:333. doi: 10.3390/nu9040333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B.C., Dasgupta S., Ray S.K. Potential therapeutic roles of retinoids for the prevention of neuroinflammation and neurodegeneration in Alzheimer's disease. Neural. Regen. Res. 2019;14:1880–1892. doi: 10.4103/1673-5374.259604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X., Smith J., Swanson H., Rubin L. Carotenoid lutein selectively inhibits breast cancer cell growth and potentiates the effect of chemotherapeutic agents through ROS-mediated mechanisms. Molecules. 2018;23:905–918. doi: 10.3390/molecules23040905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashorn M. Feed additives for influencing chicken meat and egg yolk color. In: Carle R., Schweigert R.M., editors. Handbook on Natural Pigments in Food and Beverages (pp. 283–302) Woodhead Publising; 2016. [Google Scholar]
- Haugh R.R. The Haugh unit for measuring egg quality. US Egg Poult. Mag. 1937;43:552–573. [Google Scholar]
- Harjes C.E., Rocheford T.R., Bai L., Brutnell T.P., Kandianis C.B., Sowinski S.G., Stapleton A.E., Vallabhaneni R., Williams M., Wurtzel E.T., Yan J., Buckler E.S. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science. 2008;319:330–333. doi: 10.1126/science.1150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Velasco X., Chapman H.D., Owens C.M., Kuttappan V.A., Fuente-Martínez B., Menconi A., Latorre J.D., Kallapura G., Bielke L.R., Rathinam T., Hargis B.M., Tellez G. Absorption and deposition of xanthophylls in broilers challenged with three dosages of Eimeria acervulina oocysts. Brit. Poult. Sci. 2014;55:167–173. doi: 10.1080/00071668.2013.879095. [DOI] [PubMed] [Google Scholar]
- Heying E.K., Tanumihardjo J.P., Vasic V., Cook M., Palacios-Rojas N., Tanumihardjo S.A. Biofortified orange maize enhances β-cryptoxanthin concentrations in egg yolks of laying hens better than tangerine peel fortificant. J. Agr. Food Chem. 2014;62:11892–11900. doi: 10.1021/jf5037195. [DOI] [PubMed] [Google Scholar]
- Honarvar N.M., Saedisomeolia A., Abdolahi M., Shayeganrad A., Sangsari G.T., Rad B.H., Muench G. Molecular anti-inflammatory mechanisms of retinoids and carotenoids in Alzheimer's disease: a review of current evidence. J. Mol. Neurosci. 2016;61:289–304. doi: 10.1007/s12031-016-0857-x. [DOI] [PubMed] [Google Scholar]
- Karcher D.M., Jones D.R., Robison C.I., Eberle K.N., Gast R.K., Anderson K.E. Production and well-being resulting from delayed movement of pullets to the hen facility. J. Appl. Poult. Res. 2019;28:278–289. [Google Scholar]
- Kesse-Guyot E., Andreeva V.A., Ducros V., Jeandel C., Julia C., Hercberg S., Galan P. Carotenoid-rich dietary patterns during midlife and subsequent cognitive function. Brit. J. Nutr. 2014;111:915–923. doi: 10.1017/S0007114513003188. [DOI] [PubMed] [Google Scholar]
- Kulczyński B., Gramza-Michałowska A., Kobus-Cisowska J., Kmiecik D. The role of carotenoids in the prevention and treatment of cardiovascular disease - current state of knowledge. J. Funct. Foods. 2017;38:45–65. [Google Scholar]
- Landrum J.T., Bone R.A., Neuringer M., Cao Y. Macular pigment: from discovery to function. In: Landrum J.T., Nolan J.M., editors. Carotenoids and Retinal Disease. 2013. [Google Scholar]
- Lawler T., Liu Y., Christensen K., Vajaranant T.S., Mares J. Dietary antioxidants, macular pigment, and glaucomatous neurodegeneration: a review of the evidence. Nutrients. 2019;11:1002–1015. doi: 10.3390/nu11051002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Liu R., Du J.H., Liu T., Wu S.S., Liu X.H. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients. 2016;8:426. doi: 10.3390/nu8070426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares, J. Relationships of lutein and zeaxanthin to age-related macular degeneration. In Carotenoids and Retinal Disease (pp. 63–74). CRC Press.
- Mewborn C., Lindbergh C., Robinson T., Gogniat M., Terry D., Jean K., Hammond B., Renzi-Hammond L., Miller L. Lutein and zeaxanthin are positively associated with visual-spatial functioning in older adults: an fMRI study. Nutrients. 2018;10 doi: 10.3390/nu10040458. 458–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J.A., Díaz-Gómez J., Fuentes-Font L., Angulo E., Gosálvez L.F., Sandmann G., Portero-Otin M., Capell T., Zhu C., Christou P., Nogareda C. Poultry diets containing (keto) carotenoid-enriched maize improve egg yolk color and maintain quality. Anim. Feed Sci. Tech. 2020;260 [Google Scholar]
- Novogen. 2018. Management guide of Novogen chickens. Accessed Dec. 2020. https://novogen-layers.com/wp-content/uploads/2020/11/202010-PS-Management-guide-Novogen-White-GB-.pdf.
- Obulesu M., Dowlathabad M.R., Bramhachari P.V. Carotenoids and Alzheimer's disease: an insight into the therapeutic role of retinoids in animal models. Neurochem. Int. 2011;59:535–541. doi: 10.1016/j.neuint.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Ortiz D, Ferruzzi. M.G. Identification and quantification of carotenoids and tocochromanols in sorghum grain by high-performance liquid chromatography. Methods Mol. Biol. 2019;1931:141–151. doi: 10.1007/978-1-4939-9039-9_10. [DOI] [PubMed] [Google Scholar]
- Owens, B. F., A. E. Lipka, M. Magallanes-Lundback, T. Tiede, C. H. Diepenbrock, C. B. Kandianis, E. Kim, J. Cepela, M. Mateos-Hernandez, C. R. Buell, E. S. Buckler, D. Dellapenna, M. A. Gore, and T. Rocheford. 2014. A foundation for provitamin A biofortification of maize: genome-wide association and genomic prediction models of carotenoid levels. Genetics 198:1699–1716. [DOI] [PMC free article] [PubMed]
- Papanikolaou, Y., and V. L. Fulgoni 3rd 2019. Egg Consumption in U.S. Children is Associated with Greater Daily Nutrient Intakes, including Protein, Lutein + Zeaxanthin, Choline, α-Linolenic Acid, and Docosahexanoic Acid. Nutrients 11:1137–11 [DOI] [PMC free article] [PubMed]
- Pixley, K., N. P. Rojas, R. Babu, R. Mutale, R. Surles, and E. Simpungwe. 2012. Biofortification of maize with provitamin a carotenoids. Pages 271–292 in Carotenoids and Human Health. S. Tanumihardjo, ed. Humana Press, New York, NY.
- Saint S., Renzi-Hammond L., Khan N., Hillman C., Frick J., Hammond B. The macular carotenoids are associated with cognitive function in preadolescent children. Nutrients. 2018;10 doi: 10.3390/nu10020193. 193–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman A.E.Birol, Bouis H.E., Boy E., De Moura F.F., Islam Y., Pfeiffer W.H. Biofortification: progress toward a more nourishing future. Glob. Food Sec. 2013;2:9–17. [Google Scholar]
- Schlatterer J., Breithaupt. D.E. Xanthophylls in commercial egg yolks: quantification and identification by HPLC and LC-(APCI)MS using a C30 phase. J. Agr. Food Chem. 2006;54:2267–2273. doi: 10.1021/jf053204d. [DOI] [PubMed] [Google Scholar]
- Schweiggert R.M., Carle. R. Carotenoid deposition in plant and animal foods and its impact on bioavailability. Crit. Rev. Food Sci. Nutr. 2017;57:1807–1830. doi: 10.1080/10408398.2015.1012756. [DOI] [PubMed] [Google Scholar]
- Scott, T. M., Rasmussen, H. M., Chen, O., and Johnson, E. J. (2017). Avocado Consumption Increases Macular Pigment Density in Older Adults: A Randomized, Controlled Trial. Nutrients, 9(9), 919. [DOI] [PMC free article] [PubMed]
- Sowa M., Yu J., Palacios-Rojas N., Goltz S.R., Howe J.A., Davis C.R., Rocheford T., Tanumihardjo S.A. Retention of carotenoids in biofortified maize flour and β-cryptoxanthin-enhanced eggs after household cooking. ACS Omega. 2017;2:7320–7328. doi: 10.1021/acsomega.7b01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringham J.M., Johnson E.J., Hammond B.R. Lutein across the lifespan: from childhood cognitive performance to the aging eye and brain. Curr. Dev. Nutr. 2019;3:nzz066. doi: 10.1093/cdn/nzz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata Y., Xiang Y.-B., Yang G., Li H., Gao J., Cai H., Gao Y.-T., Zheng W., Shu X.-O. Intakes of fruits, vegetables, and related vitamins and lung cancer risk: results from the Shanghai Men's Health Study (2002-2009) Nutr. Cancer. 2013;65:51–61. doi: 10.1080/01635581.2013.741757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titcomb T.J., Kaeppler M.S., Cook M.E., Simon P.W., Tanumihardjo S.A. Carrot leaves improve color and xanthophyll content of egg yolk in laying hens but are not as effective as commercially available marigold fortificant. Poult. Sci. 2019;63:9740. doi: 10.3382/ps/pez257. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. 2021. World Agricultural Supply and Demand Estimates. WASDE Report. Accessed May 2021. https://www.usda.gov/oce/commodity/wasde/wasde0421.pdf
- U.S. Department of Agriculture, Agricultural Research Service. 2020. Total nutrient intakes: percent reporting and mean amounts of selected vitamins and minerals from food and beverages and dietary supplements, by gender and age, what we eat in America, NHANES 2017-2018. Accessed May 2021. http://www.ars.usda.gov/nea/bhnrc/fsrg
- Vincent U., Serano F., von Holst C. Development and validation of a multi-analyte method for the regulatory control of carotenoids used as feed additives in fish and poultry feed. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017;34:1285–1297. doi: 10.1080/19440049.2017.1315651. [DOI] [PubMed] [Google Scholar]
- Wallace T.C. A comprehensive review of eggs, choline, and lutein on cognition across the lifespan. J. Am. Coll. Nutr. 2018;37:1–18. doi: 10.1080/07315724.2017.1423248. [DOI] [PubMed] [Google Scholar]
- Wenzel A.J., Gerweck C., Barbato D., Nicolosi R.J., Nicolosi R.J., Handelman G.J., Curran-Celentano J. A 12-Wk Egg Intervention Increases Serum Zeaxanthin and Macular Pigment Optical Density in Women. J. Nutrition. 2006;136:2568–2573. doi: 10.1093/jn/136.10.2568. [DOI] [PubMed] [Google Scholar]
- Xavier A., Díaz-Salido E., Arenilla-Vélez I., Aguayo-Maldonado J., Garrido-Fernández J., Fontecha J., Sánchez-García A., Pérez-Gálvez A. Carotenoid content in human colostrum is associated to preterm/full-term birth condition. Nutrients. 2018;10 doi: 10.3390/nu10111654. 1654–12. [DOI] [PMC free article] [PubMed] [Google Scholar]