Abstract
The uncontrolled growth and spread of abnormal cells because of activating protooncogenes and/or inactivating tumor suppressor genes are the hallmarks of cancer. The PI3K/Akt signaling is one of the most frequently activated pathways in cancer cells responsible for the regulation of cell survival and proliferation in stress and hypoxic conditions during oncogenesis. Non-coding RNAs are a large family of RNAs that are not involved in protein-coding, and microRNAs (miRNAs) are a sub-set of non-coding RNAs with a single strand of 18–25 nucleotides. miRNAs are extensively involved in the post-transcriptional regulation of gene expression and play an extensive role in the regulatory mechanisms including cell differentiation, proliferation, apoptosis, and tumorigenesis. The impact of cancer on mRNA stability and translation efficiency is extensive and therefore, cancerous tissues exhibit drastic alterations in the expression of miRNAs. miRNAs can be modulated by utilizing techniques such as miRNA mimics, miRNA antagonists, or CRISPR/Cas9. In addition to their capacity as potential targets in cancer therapy, they can be used as reliable biomarkers to diagnose the disease at the earliest stage. Recent evidence indicates that microRNA-mediated gene regulation intersects with the Akt pathway, forming an Akt-microRNA regulatory network. miRNAs and Akt in this network operate together to exert their cellular tasks. In the current review, we discuss the Akt-associated miRNAs in several cancers, their molecular regulation, and how this newly emerging knowledge may contribute greatly to revolutionize cancer therapy.
Keywords: Akt, Biomarkers, Cancer, RNA-therapeutics, MicroRNAs
1. Introduction
Cancer is one of the major causes of death globally with an estimated diagnosis rate of 16 million annually [1,2]. Cancer is characterized by uncontrolled growth and spread of abnormal cells [3] because of the hyperactivation of protooncogenes and/or inactivation of tumor suppressor genes [4,5]. Among various cancers, lung cancer is the primary cause of morbidity and mortality [6,7] with around 1.8 million new cases, and 1.6 million deaths reported annually [7]. Hepatocellular carcinoma (HCC) is another deadly disease with very high mortality [8], which is more prevalent in Asian countries [7]. According to GLOBOCAN 2012, prostate cancer is the most diagnosed cancer among males in 87 countries analyzed [9,10]. Prostate cancer is expected to top the total number of new cancer cases in men in 2020 [11]. Breast cancer, on the other hand, is the most frequently diagnosed cancer in women [12], which along with cervical cancer are two leading cancer types in females from the Latin Americas, Caribbean, Africa, and most of Asia [9]. Among the gynecological malignancies, ovarian cancer is the most common cancer associated with morbidity and mortality in women [7,13].
Cancer was initially proposed with a simple and precise description of a collection of progressive diseases of “acquired genetic lability” [14]. Later in 1889, Stephen Paget articulated that “seeds” (cancer cells) preferentially grow in the conducive “soil” (microenvironment) of select organs, where the composition and transformation of cells depend on the genetic and environmental factors [15,16]. Numerous internal and external factors have been implicated in the pathogenesis of cancer with the latter including tobacco, infectious organisms, and an unhealthy diet while the former include hormones, immune conditions, and inherited genetic mutations [4]. These elements may act together or in sequence to trigger cancer. Medical and socio-cultural factors such as psychosocial and behavioral interventions, active screening behaviors, and healthier lifestyles may also play an integral role in the length and quality of that survival [17].
Cancer involves significant changes in chromatin structures [18] and molecular regulatory networks [2]. Often, more than 10 years pass between exposure to external factors and obvious cancer [4]. Most therapeutic modalities for cancer are associated with resistance [19] and a spectrum of late complications ranging from minor and treatable to serious and, occasionally, lethal [17]. Several different growth factors, cytokines, their receptors, and intracellular signaling molecules have been targeted in various clinical trials [20] for cancer since the middle of the 20th century. Although decades of research have developed several modalities of cancer treatments, reliable cancer therapies remain elusive. Non-coding RNAs, microRNAs (miRNAs) in particular, have been at the forefront of cancer research lately and provides us with a new tool in the treatment of various cancers [21]. In the current chapter, we review the Akt pathway-associated miRNAs in cancer and their potential utility in cancer therapeutics as compared to the other existing modalities of cancer treatments.
2. Cancer prevention and diagnosis
Improved screening methods for the early detection of cancers [22] and the significant advances in surgical and radiation oncology have resulted in an increase in the overall 5-year survival rates of several cancers in the United States to 66%, compared to just over 50% in 1990 [23]. Cervical cancer was recently the leading cause of cancer death in women in the developing world. However, its mortality burden has been dramatically reduced with the introduction of the Papanicolaou (Pap) smear as a screen for the identification of high-grade pre-cancerous lesions which can be excised or ablated [14]. Therefore, early detection and treatment of cancer are essential for better health management [1]. Biomarkers may be helpful in this regard and have been consistently being used in several types of cancer. For instance, elevated serum thyroglobulin level is a prognostic marker for thyroid cancer as it strongly correlates with disease persistence or recurrence; hence physicians directing the patients for imaging until the lesions are localized [24]. Probing consistently up or down-regulated genes, proteins, or clusters of genes has been the mainstream in recognizing potential biomarkers for early cancer detection. In a study, messenger RNA (mRNA) expressions of three cancer-related genes PIK3C3, PIM3, and PTEN, all of which the upstream regulators of the Akt pathway, were correlated during cancer progression and the correlation coefficients showed the potential to be used for cancer diagnosis [5]. Studies from the past have shown that human cancers contain activated oncogenes and the expression of these genes is not only required for the initiation of cancer but also for the maintenance of the disease thereby signifying its role as therapeutic targets [25]. These oncogenes are targeted by a type of miRNAs referred to as onco-miRNAs (OncomiRs) which regulate various hallmarks of cancer with their impacts on stability and translation efficiency of the mRNA (e.g. miR15, miR17, and let-7, etc.) [2]. A Web-based portal oncomiRDB stores and displays all the curated data entries where users can easily browse and search all the entries for the oncomiRs in terms of cancer types, targeted genes or cellular processes. OncomiRDB is a valuable resource for the computational analysis as well as the experimental study of miRNA functions and regulatory networks in cancer [2].
3. Modalities of cancer therapies
The fundamental rationale of a cancer treatment plan is to have a cure for cancer and, when a complete cure is not achievable, the treatment plan should be to contain cancer to a subclinical state and maintaining homeostasis so the patient can enjoy a good quality of life [1]. Various forms of treatments are available for cancer depending on their type and stage. These include chemotherapy, surgery, radiation therapy, hormonal therapy, targeted therapy including immunotherapy [4]. Targeted therapies also include signal transduction inhibitors, angiogenesis inhibitors, gene expression modulators, and apoptosis inducers. The precision of the targeted therapy approach is attractive and has been successful for given cancers in a subset of patients [23]. The treatment plan may use a combination of the treatment methods to have maximum effectiveness for the treatment [1].
Chemotherapy is generally considered an effective way of cancer treatment that employs anti-cancer drugs to kill or destroy the cancerous cells by interfering with their growth. When a blend of these drugs is used, it is referred to as combination chemotherapy [1].
Radiation therapy makes use of high doses of radiation generally ionizing radiation to kill cancer cells by damaging their DNA directly [26] or by creating free radicals within the cells thereby destroying the tumor tissues [27]. It is approximately used in 50% of all cancer patients during their course of illness [28]. In combination with surgery, it helps in removing or reducing the size of the tumors [1]. Surgical resection involves the removal of cancer tissues from the body resulting in complete or partial cure ideally in tumors that are localized and of small size [1,29]. Ablative therapies is a technique that uses precise targeting to deliver high doses of radiation capable of ablating tumors directly and [30] have the advantages of negligible invasion with enhanced targeting and reduced pain [31]. Other types of surgical interventions include radiofrequency ablation (RFA) and microwave ablation. Woefully, RFA inflicts a high risk to the adjacent tissues and its impact on patient survival needs further clinical research [31].
Angiogenesis is the formation of new blood vessels from pre-existing vessels, which has been considered an essential process for the proliferation and viability of tumor cells [32]. The tumor environment is, therefore, characterized by abnormal angiogenesis and permeable blood vessels [33–35]. Certain pharmacological agents can disrupt the vascular supply and starve the tumor of nutrients and oxygen, primarily through blockade of vascular endothelial growth factor (VEGF) or its receptor signaling [36] including tyrosine kinase inhibitors (TKI) such as sorafenib, sunitinib, and axitinib which has been approved as monotherapy for cancer [37,38]. Akt1 is another kinase, whose activation is linked with VEGF [39], that reportedly modulate physiological and tumor angiogenesis [40].
Cancers are sensitive to hormonal therapy because it modulates the extent of hormones in the body to suppress cancer growth as cancer cells essentially rely on these hormones for their growth and spread [1]. Hence, hormonal therapies are replacing conventional cancer therapies for better efficacy [41].
Immunotherapy involves the concept of boosting the immune system to target and kill cancer cells [42]. This is also known as biologic therapy, which stimulates the disease-fighting mechanism within the patient’s body to fight cancer such as monoclonal antibodies that block specific protein function by binding to cancer cells, which train the immune system to recognize and attack the cancer cells. This method of treatment is safe and does not have any major side effects [1]. This has been a goal of cancer treatment for over a century. However, inadequate success has been attained with this therapy, as cancer cells tend to develop mechanisms that evade immune recognition [43].
Nucleic-acid therapies are comprised of two distinct classes related to the central dogma of molecular biology, gene therapy, and RNA therapy. While the former deals with DNA, the latter alters the RNA to help effect a cure [44]. The evolving field of cancer gene therapy presents several exciting potential treatments. Several RNA-based therapeutics are being investigated at the basic research and late-stage clinical trials with a few of them already approved for treatment [45]. miRNAs and small interference RNAs (siRNAs) compose a vital class of these RNA-based therapies [46]. miRNAs, discovered in 1993 by Lee and colleagues [47], have been recognized considerably in the past two decades for their roles in development and diseases especially cancers [48] as depicted in Fig. 1 [49–51]. They have the potential to regulate several genes and signaling pathways because of their unique patterns of expression and ability to target numerous transcripts [52]. Interestingly, certain miRNAs have reached the clinical trial stage of research for their potential use in various cancers (Table 1) [53]. Preclinical gene therapy tests have also been performed on gliomas, pancreatic cancer, and liver cancer, as well as many other cancers [43]. Information about the transcription and translation processes in addition to the miRNA biosynthesis is critical for understanding the RNA therapeutics and hence these are delineated ahead.
Fig. 1.
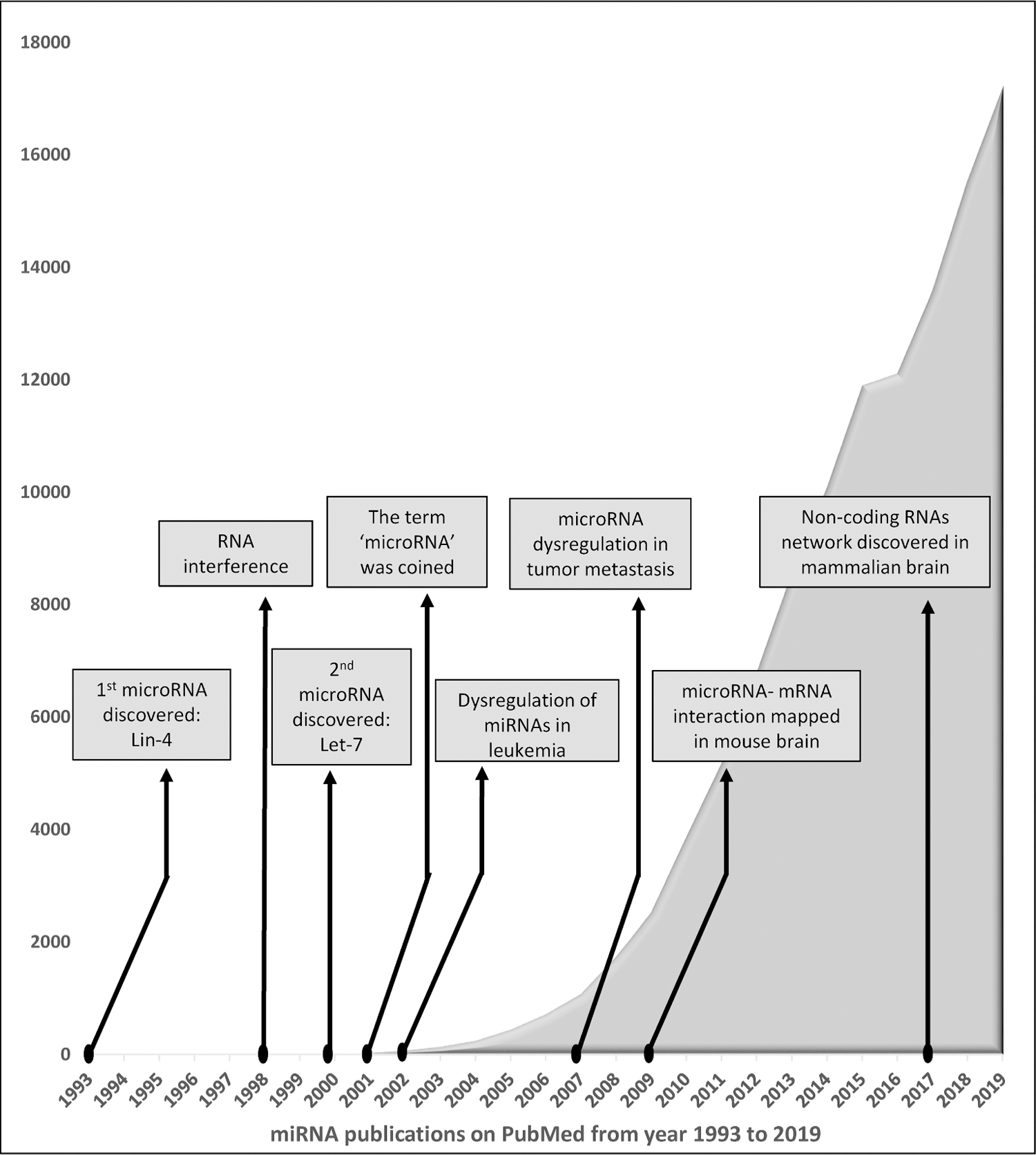
History of miRNAs and milestones in miRNA research.
Table 1.
miRNAs in the clinical trials.
| miRNA | Drug Name | Disease |
|---|---|---|
| miR-34 | MRX34 | Liver cancer Lymphoma Melanoma |
| miR-92 | MRG 110 | Wound healing Heart failure |
| miR-16 | MesomiR-1 | Mesothelioma Lung cancer |
| miR-122 | Miravirsen | Hepatitis C virus |
| miR-29 | MRG-201 | Keloid, fibrous scar tissue formation |
| miR-21 | RG-012 | Alport syndrome |
| miR-155 | Cobomarsen (MRG-106) | T-cell lymphoma/ mycosis fungoides |
4. miRNAs in the regulation of protein synthesis
Cell growth and proliferation entail ordered progression through various cell-cycle stages. During cell-cycle progression, the genome undergoes considerable changes, which affect its structure and function [54]. DNA is a part of the central dogma of molecular biology which was coined in the early days of modern biology [55]. Transcription and translation are two main pillars of gene expression that are involved in the conversion of DNA to RNA and RNA to protein, respectively [55,56]. Additionally, a series of processes lie between transcription and translation, which in many stages involve RNA regulators. To produce a functional protein, the nascent mRNA receives a cap and a poly-A tail. It is spliced, checked for premature stop codons, and finally exported. Even then, siRNAs may control the destruction of the final transcript or modify its expression level via miRNAs before the mRNA reaches the ribosome [55].
5. miRNAs in cancer
Non-coding RNAs are a large family of RNAs that are not involved in coding for any known proteins [57,58]. About 17 categories of non-coding RNA molecules have been identified so far that include transfer RNAs, ribosomal RNAs, siRNAs, miRNAs, circular RNAs, snoRNAs, and several others [59,60]. miRNAs are single-stranded RNAs of 18–25 nucleotides [61] that are extensively involved in post-transcriptional regulation of gene expression in diverse organisms from plant to animal [61–64].
5.1. miRNA biosynthesis
A small portion of miRNAs is encoded by their genes whereas ~80% of annotated miRNAs are obtained from various large coding and non-coding transcripts. These initial transcripts, referred to as pre-miRNAs, are processed to ~70 bp pre-miRNAs by the Microprocessor consisting of DROSHA and DGCR8 in the nucleus [65] (Fig. 2). Pre-miRNAs are exported out of the nucleus by Exportin 5 to get further processed into mature miRNAs [64]. Once in the cytoplasm, the RNase III Dicer cuts the pre-miRNA into a form of ~20 bp miRNA/miRNA coupled pair. One strand represents the 5′ miRNA, whereas the other strand represents the 3′ miRNA. The last step of the process requires the participation of the RNA-induced silencing complex (RISC), which can unwind both strands (Fig. 2). In most cases, while one of the strands gets degraded, the other gets incorporated into the RISC and guide it to its mRNA target [60,66]. miRNAs can be isolated from cells, tissues, and body fluids such as serum, plasma, tears, or urine [61].
Fig. 2.

Biosynthesis of miRNA and inhibition of mRNA.
5.2. miRNA functions
miRNAs play a significant role in the regulatory mechanisms operating in various organisms, including developmental timing and host-pathogen interactions as well as cell differentiation, proliferation, apoptosis, and tumorigenesis [67,68]. Additionally, they act as post-transcriptional regulators of their mRNA targets [60,68]. Whereas it’s binding at 3′ untranslated regions (UTRs) of mRNAs suppresses protein translation [69] and degrades mRNA [70], 5′ UTR region binding causes an inverse function that increases the translation of specific targets of the same strand [66,70]. miRNA achieves repression by reducing the stability of mRNAs and attenuating the translation machinery [60,67,68]. They also play an important role in the ceRNA (competing endogenous RNA) network via combining with target mRNA thus inhibiting the action of mRNA expression [71].
5.3. Role of miRNAs in cancer
As cancer involves significant changes in chromatin structures and molecular regulatory networks, its impact on mRNA stability and translation efficiency is extensive [2]. Therefore, cancerous tissues exhibit drastic alterations in the expression of miRNAs [67,72]. micro-RNAs, as expert gene regulators, impact varied cellular pathways and they may function as oncogenes or tumor suppressors [72]. Several studies have shown the correlation of certain miRNAs with the progression and prognosis of tumors [73]. Breast cancer tissues, for instance, showed elevated miR-106b and miR-93 levels which promoted cell migration, invasion, and proliferation in vitro and tumor growth in vivo [74,75]. The researchers also identified PTEN (phosphatase and tensin homolog) located on chromosome 10, which is widely acknowledged as one of the most frequently deleted or mutated genes in the diverse human tumor, as the common target of these miRNAs that negatively regulates the activity of the PI3K/Akt pathway to restrain tumor progress [74]. Overexpression of human miR-21 was also associated with numerous cancers [76] while miR-15 and miR-16 expressions were mostly correlated with B-cell neoplasm [77]. Further, miRNAs such as miR-143 and miR-145 are considered as potential anti-tumor candidates as they are known to directly regulate other oncomiRs [67].
5.4. Cellular quantities of miRNA
The widespread perception is that the quantity of mature miRNAs in the cell is critical for cell regulation [78]. Precisely, excess of a specific type of miRNA potentially titrates out valid mRNA targets. As a secondary effect, an overflow of specific miRNA will most likely result in its binding to non-genuine targets, the so-called ‘off-target’ effect [67].
5.5. Modulation of miRNAs for cancer therapy
miRNA can be modulated by utilizing transient transfections of miRNA mimics or miRNA antagonists in vitro or topical delivery of the miRNA mimics/inhibitors intranasally that require a much lower dose than systemic has been described and this can be potentially be used in study and treatment [61,79]. Stable miRNA targeting can be achieved with the help of CRISPR/Cas9 technology [80] without using a lentiviral facility [61]. Additionally, in vivo miRNA modulation can be performed by using cholesterol-conjugated anti-miRNAs that can be administered via an intravenous infusion using a weight-based regimen [61]. These anti-miRNAs have the efficiency to be uptaken by all tissues except the brain [61,81]. Another method to deliver miRNA in vivo involves liposomal mediated delivery or usage of polymer-based nanoparticle delivery vehicles [61,82]. Other possibilities to utilize miRNAs for therapeutic interventions include isosequential-exogenous miRNAs for substituting endogenously expressed miRNA, and oligonucleotides or small molecule antagonists to lower the gene regulatory effect of natural miRNAs [83].
6. The PI3K-Akt pathway in miRNA regulation
Akt is a serine-threonine kinase involved in the regulation of cell growth, survival, proliferation, protection from apoptosis, control of DNA damage responses, metabolism, migration, and metastasis, all of which are hallmarks of cancer [84–87]. Akt exists in three isoforms as Akt1–3 with similar homology but different, or even opposite, roles in tumorigenesis. While Akt1 is overexpressed in breast cancer, Akt2 is highly expressed in CRC and Akt3 in melanoma [84]. Interestingly, human T24 bladder cancer cells reveal the presence of Akt1 at the membrane, cytoplasm, and nucleus, and that genetic deletion or pharmacological inhibition of Akt1 results in the inhibition of bladder cancer cell functions, Akt2 in cytoplasmic and Akt3 in the nucleus and may be involved in different pathways in the regulation of T24 cell proliferation at specific stages of bladder cancer [88].
6.1. The PI3K/Akt pathway
PI3Ks are a distinctive family of intracellular lipid kinases responsible for phosphorylation of the 3′-hydroxyl group of the inositol ring of phosphatidylinositides (PtdIns) [89]. These are divided into three classes [90,91] and the most studied is class I PI3K which stimulates the conversion of phosphatidylinositol-(4,5)-bisphosphate [PtdIns(4,5)P2; PIP2] to phosphatidylinositol-(3,4,5)-trisphosphate [PtdIns(3,4,5)P3; PIP3] [89]. PIP3 acts as a secondary messenger that facilitates the recruitment and activation of kinases such as PDK1 that possess the pleckstrin homology (PH) domain. The PIP3 signaling is regulated by PTEN which acts to oppose PI3K activity. Akt also possesses a PH domain and is phosphorylated at amino acid residues T308 and S473 by PDK1 and mTORC2, respectively, for activation. In turn, Akt can phosphorylate many other target proteins. [89]. PI3K/Akt is one of the most frequently activated pathways in cancer cell signaling and a key regulator of cell survival in stress conditions such as oncogenesis [69,92].
PTEN is the second most frequently mutated tumor suppressor gene and a pivotal effector of the PI3K/Akt/GSK3β signaling pathway [93,94]. It is known to inactivate the PI3K/Akt pathway and therefore has been associated with multiple cancers and alterations in cell cycle regulation and apoptosis [5,69]. Whereas its down-regulation promotes several cancers and tumor aggressiveness, its up-regulation suppresses tumor cell growth and promotes tumor cell apoptosis [74,93]. PI3K integrates Akt and Erk signaling in prostate cancer [95], akin to the integration of Akt and ERK signaling by the P21 activated kinase-1 (PAK-1) [86], another oncogene [96].
6.2. The link between Akt and miRNA in cancer
Carcinogenesis is a highly intricate and heterogenic process coordinated by many deregulated signal transduction pathways including the PI3K/Akt pathway [97]. Akt, also referred to as protein kinase B was discovered in 1977 by Staal and team [98]. Since then, Akt has evolved into a significant gene as described in Fig. 3 [98–102]. The review focuses on the Akt pathway since its activation relies on PTEN which is reportedly one of the most frequently disrupted tumor suppressors [103]. Increasing evidence reveals that miRNAs play a critical role in the activation and functioning of all major signaling pathways [7]. Dysregulated expression of miRNAs often distorts the normal functioning of the abovementioned signaling machinery associated with human cancer pathogenesis [7,104]. Evidence also indicates that miRNA-mediated gene regulation intersects with the Akt pathway, forming an Akt-miRNA regulatory network [72]. miRNAs and Akt in this network operate together to exert their cellular tasks. The miRNAs associated with Akt can be broadly categorized into upstream and downstream miRNAs utilizing their ability in regulating Akt activity and serving as Akt effectors, respectively [72]. Interestingly, a miR-PTEN network has been established in recent times. Evidence illustrates that PTEN-regulating miRNAs, including miR-141-3p, miR-29a, miR-21, miR-19a, miR-92a, and miR-486 contribute to anti-tumor treatment resistance [105].
Fig. 3.

Milestones in Akt research.
7. Akt-regulated miRNAs and miRNAs regulating the Akt pathway
The PI3K/Akt signaling pathway is one of the leading pathways that have been linked to dysregulated miRNA expression levels [72]. Many studies revealed that miRNAs processing and stability are dependants on post-transcriptional mechanisms that induce the rapid fluctuation in its levels. Interestingly, Akt is one of the few signaling pathways that can explicitly alter the translation of a gene while averting the necessity for transcription at the time of transient signaling events [106]. Conversely, miRNAs also have the potential to regulate Akt via its positive or negative factors. Whereas miRNAs induced PTEN targeting may lead to activation of Akt, PP2A modulation can cause a turnaround effect since phosphatases inactivate Akt by removing the phosphate group [72]. The most predominant PI3K/Akt related miRNA dysregulation include miR-143, miR-145, miR-133a [72], and miR-233 [107]. Other miRNAs associated with genes in PI3K/Akt-signaling pathway include miR-590-5p, 106b, and miR-93 with PTEN. miR-497 with IGF1R, miR-451 with LKB1, Akt, PI3K, and BCL2, and miR-126 with Akt [69]. Here are the details about Akt associated microRNAs involved in the modulation of various cancers (Fig. 4).
Fig. 4.

Diagrammatic representation of microRNAs involved in suppression and progression of various cancers through Akt regulated pathways.
7.1. Prostate cancer
Research from our laboratory on the transgenic adenocarcinoma of the mouse prostate (TRAMP) has demonstrated for the first time that Akt1 suppression during the early and the advanced stages of prostate cancer stimulates stage-specific expression changes in the repertoire of miRNAs involved in the differential regulation of oncogenic transformation, tumor growth, and metastasis [10]. Akt1 deficiency in the early prostatic inter-epithelial neoplasia stages suppressed tumor and resulted in a significant increase of miR-155-5p, -199a-5p, -29b-3p, and -30a-3p in addition to a decrease in the expression of mir-485-5p, -493-3p, and -467e-5p, all of which are known regulators of cell survival [10]. However, Akt1 inhibition in the advanced stages promoted metastasis and led to increased expression of miR-669h-3p and -3104-3p along with a reduction in expression of miR-375-3p, -le7a-5p, -10a-5p, and -143-3p, all of which are the signature microRNAs in the alteration of TGFβ and EMT (epithelial to mesenchymal transformation) pathways [10,108]. Several other researchers have also reported modulation of Akt associated miRNAs in prostate cancer [7,109]. Tumor suppressor miR-7 was linked to suppression of prostate cancer stem cells (CSCs) growth and associated carcinogenesis by inhibition of KLF4. Its restoration abolished the cellular and molecular alterations associated with prostate cancer development via targeting KLF4/PI3K/Akt/p21 signaling pathways [110]. Other microRNAs reported to be beneficial in prostate cancer therapeutics via Akt pathway inhibition are miR-34b and -133a-3p which act as a suppressor of the tumor and bone metastasis, respectively [111,112]. On the contrary, the expression of miR-146b and -32-5p are inversely linked to beneficial effects in prostate cancer. Whereas miR-146b inhibits autophagy through activation of PTEN/Akt/mTOR signaling pathway, miR-32-5p contributes to castration resistance, radio-resistance, and chemoresistance [105,113]. Also, miR-410-3p was found to be associated with the promotion of prostate cancer progression via the PTEN/AKT/mTOR signaling pathway [114].
7.2. Colorectal cancer
In colorectal CSCs, miRNAs have been reported to be involved in regulating many signaling pathways [7] as genetic variation in PIK3CA and Akt1 is associated with a strongly increased risk of colon cancer [69]. Overexpression of mir-21 is critically associated with increased colon CSCs [7] and CRC proliferation [115] through activation of the Akt signaling pathway [115]. miR-708, one of the novel reported downregulated miRNAs, has been considered as a potential negative biomarker in the diagnosis of the progression of multiple types of cancer. While overexpression of the miR-708 in HCT-116 cells led to a decrease in protein expression of phosphorylated Akt, miR-708 inhibitor caused a reversal thereby increasing its level [7]. Another study revealed that miR-125a-3p overexpression leads to inhibition of proliferation, migration, invasion, and angiogenesis of colorectal cancer cells via downregulation of fucosyltransferase (FUT)-5 and -6 and modulation of the PI3K/Akt signaling pathway [116]. Further, miR-424 was identified as a tumor suppressor in colorectal cancer growth in vitro and in vivo. Human colorectal cancer cell lines and patient biopsies revealed downregulation of miR-424 and microarray evidence shows that it directly targets the 3′ UTR of the Akt3 [84].
7.3. Lung cancer
Dysregulated expression of miRNA has been shown to play a crucial role in the regulatory mechanisms related to the initiation and progression of lung cancer [117]. They are believed to be the central regulatory molecules with a key role in the maintenance of lung CSCs and are associated with metastasis, drug resistance, and tumor self-renewal. miR-128, a well-known tumor suppressor frequently suppressed/deregulated in various human cancers including lung cancer, plays an essential role in the self-renewal of CSCs and resistance via targeting Akt/ERK signaling pathways. Conversely, uncontrolled upregulation of miR-23a has been linked to erlotinib resistance whereas its inhibition accelerated the anticancer effect of erlotinib via modulating the functioning of PTEN/PI3K/Akt pathways [118]. MiR-200c and -708 were found to suppress the expression of ZEB1 through AKT/mTOR signaling pathway thereby inhibiting proliferation, migration, and invasion [7].
Epidermal growth factor receptor (EGFR) is an important component for regulating tumor cell proliferation, invasion, angiogenesis, adhesion, metastasis, and apoptosis. Data collected from NSCLC patients and volunteers at Tsinghua Changgung Hospital revealed downregulated serum levels of miRNA-223 in NSCLC. Overexpression of miRNA-223 in vitro induced apoptosis while decreasing cell proliferation. In contrast, its downregulation inhibited apoptosis whereas increased cell proliferation. Overexpression of miRNA-223 reportedly suppresses the protein expression of EGFR, PI3K, and phosphorylated Akt in non-small cell lung cancer (NSCLC) cells. As a result, inhibitors of both EGFR and PI3K increased the anticancer effects of miRNA-223 in NSCLC cells [92,119].
Another miRNA that is modulated upon oncogenic activation of the PI3K/Akt pathway is miR-196a. It can induce anchorage-dependent and -independent proliferation and migration in NSCLC cells downstream PI3K, thus alleviating the comprehension of the pathogenesis of this neoplasia. It is believed to target FoxO1, p27, and HOXA9 thereby playing an important role in mediating the effects exerted by abnormal activation of PI3K/Akt signaling [119]. miR-181 [120] and miR-29c [121] are other non-coding RNAs that negatively regulate Akt to reduce cisplatin resistance in lung cancer. miR-153 is also known to inhibit Akt to induce anti-tumor activity [122]. On the contrary, miR-21 activates Akt to promote cancer progression [93].
7.4. Breast cancer
Studies propose that miRNAs play the chief regulatory role for the growth and proliferation of breast cancer cells and breast CSCs as they control the expression and functioning of genes associated with a series of signaling pathways crucial for normal cellular homeostasis and pathogenesis of various human diseases [123]. miR-29a is one such element upregulated in breast CSCs, MCF-7, and breast cancer tissues. Evidence suggests that basic fibroblast growth factor (bFGF)-induces upregulation of miR-29a to enhance the migration and metastasis of breast CSCs and other aggressive breast cancer cells by inhibiting the expression of SUV420H2-mediated downregulation of H4K20me3. Downregulated expression of miR-1287-5p in mammospheres (BCSCs) and human breast cancer tissue was reportedly found to be critical in poor prognosis and survival via modulation of PI3K signaling cascade. Whereas its upregulation inhibited cell proliferation and induced cell cycle arrest, apoptosis, and tumor formation in triple-negative breast cancer [7]. miR-221/222 are also reported to enhance breast cancer growth, migration, and invasion, through PTEN inhibition and activating Akt [104]. On the contrary, triple-negative breast cancer pathogenesis revealed the downregulation of miR-1287-5p both in mammospheres and human breast cancer tissue. It is believed to be critical for poor prognosis and survival via modulation of the PI3K signaling cascade [7]. Altered expression of miR-106b and -93 reportedly led to changes in the capability of migration, invasion, and proliferation in breast cancer. PTEN was found to be reduced in breast cancer tissues or MDA-MB-231 cells associated with high levels of miR-106b and miR-93. These miRNAs induced migration, invasion, and proliferation and concurrently enhanced the activity of the PI3K/Akt pathway of MCF-7 cells which could be blocked by upregulation of PTEN [71,74]. Moreover, PTEN is known to be regulated by miR-20 [71] and its expression level is known to be decreased on miR-27a overexpression in triple-negative breast cancer cells [94].
Overexpression of miR-204 and -205 are associated with suppression of cell growth and migration in breast cancer. While the latter targets ErbB3 and VEGF-A, the former triggers apoptosis and G2/M cell cycle arrest, Bioinformatic analysis revealed PTEN to be the target. Since, PTEN regulates the PI3K/AKT signaling pathway, the effect of overexpression was also assessed on this pathway and showed that they inhibit the expression of p-AKT and p-PI3K significantly in breast cancer cells. [74,124]. Inversely, miR-130b, -133a-3p, and -181a were found to promote breast cancer when upregulated [71,74].
7.5. Hepatocellular carcinoma
Abnormally high expression of miR-6875-3p and -106b-5p was reportedly detected in HCC tissues and cell lines against the normal controls. While miR-6875-3p was linked to BTG2/FAK/Akt signaling pathway, miR-106b-5p targeted PTEN via PI3K/Akt pathway [7]. Overexpression of another microRNA, miR-32-5p, is believed to activate the PI3K/Akt pathway through PTEN suppression thereby inducing multidrug resistance in HCC via angiogenesis and EMT [105]. Also, miR-192-5p was found to be responsible for the high expression of TRIP13 which drives the progression of HCC via the TRIP13/ACTN4/Akt/mTOR axis [125]. Conversely, miR-302a/d and -302b negatively regulate self-renewable capability and tumor growth in HCC, respectively. Whereas miR-302a/d targets the E2F7 gene and its downstream Akt/β-catenin/CCND1 signaling pathway, miR-302b utilizes the EGFR/Akt2/CCND1 pathway [105]. Another miRNA, miR-370 was linked with inhibition of cell proliferation through Akt suppression and FoxO3a activation [126].
7.6. Ovarian cancer
EMT is an important phenomenon associated with stemness-related tumor aggressiveness, has been demonstrated to enhance the stemness of ovarian cancer. Interestingly, miR-20a and -200c were found to be capable of regulating EMT through the modulation of the PI3K/AKT pathway [7]. They reportedly downregulate PTEN to facilitate activation of the PI3K/AKT pathway thereby promoting proliferation and invasion of human ovarian cancer cells. miR-214 is another microRNA that can enhance ovarian cancer cell survival and cisplatin resistance by interfering with the Akt pathway. Also, reports suggest that Twist deals with the differentiation of ovarian CSCs through the PTEN/Akt pathway by regulating miR-199a [127].
CircPLEKHM3 is recognized as the most significantly downregulated circular RNA in ovarian cancer tissues. Its overexpression inhibited cell growth, migration, and EMT, and its knockdown, undoubtedly, exerted an opposite role. It was revealed that circPLEKHM3 binds to miR-9 to augment the endogenous inhibitive effect of BRCA1, DNAJB6, and KLF4. Moreover, the circPLEKHM3-miR-9 axis is considered to be an important mediator of the crosstalk between Wnt/β-catenin and Akt1 signaling pathways that promote the progression of ovarian cancer [73]. Therefore, Akt inhibitor MK-2206 [128] could block the tumor-promoting effect of circPLEKHM3 depletion and escalate Taxol-induced growth inhibition of ovarian cancer cells [73].
Downregulation of miR-195 was observed in ovarian cancer and its expression was reportedly associated with apoptosis and decreased cell proliferation through inhibition of VEGFR2 and phosphorylated Akt [129]. Expression of miR-203a-3p was also found to be decreased in ovarian cancer associated with an upregulation of ATM, a serine/threonine-protein kinase closely linked to poor prognosis in ovarian cancer patients. The microRNA reportedly targets ATM thereby inhibiting proliferation, invasion, and migration through blockage of the Akt/GSK-3β/Snail pathway [130]. miR-126-3p is another miRNA whose over-expression reportedly suppressed cell proliferation and invasion through inhibition of Akt phosphorylation [131].
7.7. Gastric cancer
Gastric cancer is a major health burden across the world with adenocarcinomas constituting about 90% of cases [132]. The expression of miRNAs is critical for the progression of gastric cancer [133]. Whereas miR-139-5p [134], miR-589 [135], miR-107 [136], and miR-19a [137] through Akt activation are associated with tumor-promoting effects, miR-567 [138] and miR-340 [139] are linked to tumor suppression via negative regulation of Akt.
7.8. Other cancers
Esophageal cancer was shown to be promoted by miR-203a [140] and miR-502 [141] through the activation of Akt signaling. In contrast, miR-30a [142] and miR-608 [143] were inversely linked to the promotion of pancreatic and bladder cancers, respectively. Akt was also found to be targeted by miR-564 in osteosarcoma thereby resulting in reduced glycolysis and cell proliferation [144]. Human glioma is the most common tumor of the brain and its prognosis is disapproving despite advances in surgical intervention and treatment. Studies report the upregulation of mir-21 in glioblastoma tumors, indicating its major role in cell growth, proliferation, and apoptosis in glioblastoma. mir-21 was found to be inhibiting the PTEN expression along with the promotion of PI3K, Akt, P53, and p-GSK3 [145].
Isoflurane, a commonly used inhalational anesthetic in clinical practice, may generate significant risks of neurotoxicity in the developing brains. Neonatal exposure to isoflurane is reported to induce widespread neuro-apoptosis and oxidative stress in developing brains associated with subsequent long-term neurocognitive impairments. Interestingly, miR-241 induced activation of the PI3K/Akt pathway exhibited potential neuroprotective effects against oxidative stress and brain injury [146].
8. Conclusions
Its high time the miRNAs should be considered over the conventional therapies, alone or in combination with other modalities, for cancer therapeutics. miRNAs are critical elements in driving the key signaling pathways responsible for cellular homeostasis. In addition to their capacity as potential targets in cancer therapy, they can be used as reliable biomarkers to diagnose the disease at the earlier stages. Taken together, Akt-regulated miRNAs, specifically, regulate several cancers, and therefore understanding its molecular regulation would contribute greatly to a revolution in cancer therapy. With substantial progress in the field of miRNAs, the near future seems promising for RNA therapeutics where techniques such as RNA mimics would cure fatal cancers.
Acknowledgements
Funds were provided by the NHLBI (United States) grant R01HL103952, NCATS (United States) grant UL1TR002378, Wilson Pharmacy Foundation (intramural), and Translational Research Initiative grant (intramural) to PRS.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Wang JJ, Lei KF, Han F, Tumor microenvironment: recent advances in various cancer treatments, Eur. Rev. Med. Pharmacol. Sci 22 (12) (2018) 3855–3864. [DOI] [PubMed] [Google Scholar]
- [2].Wang D, Gu J, Wang T, Ding Z, OncomiRDB: a database for the experimentally verified oncogenic and tumor-suppressive microRNAs, Bioinformatics 30 (15) (2014) 2237–2238. [DOI] [PubMed] [Google Scholar]
- [3].Zhang WL, Zhao YN, Shi ZZ, Cong D, Bai YS, Lutein inhibits cell growth and activates apoptosis via the PI3K/AKT/mTOR signaling pathway in A549 human non-small-cell lung cancer cells, J. Environ. Pathol. Toxicol. Oncol 37 (4) (2018) 341–350. [DOI] [PubMed] [Google Scholar]
- [4].Qadir MI, Usman M, Akash MSH, Transposable elements (human endogenous retroviruses) in cancer, Crit. Rev. Eukaryot. Gene Expr 27 (3) (2017) 219–227. [DOI] [PubMed] [Google Scholar]
- [5].Ling B, Chen L, Liu Q, Yang J, Gene expression correlation for cancer diagnosis: a pilot study, Biomed. Res. Int 2014 (2014), 253804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hirsh V, Skeletal disease contributes substantially to morbidity and mortality in patients with lung cancer, Clin. Lung Cancer 10 (4) (2009) 223–229. [DOI] [PubMed] [Google Scholar]
- [7].Khan AQ, Ahmed EI, Elareer NR, Junejo K, Steinhoff M, Uddin S, Role of miRNA-regulated cancer stem cells in the pathogenesis of human malignancies, Cells 8 (8) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP Jr., Hepatocellular carcinoma: a review, J. Hepatocell Carcinoma 3 (2016) 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Torre LA, Siegel RL, Ward EM, Jemal A, Global cancer incidence and mortality rates and trends – an update, Cancer Epidemiol. Biomarkers Prev 25 (1) (2016) 16–27. [DOI] [PubMed] [Google Scholar]
- [10].Alwhaibi A, Gao F, Artham S, Hsia BM, Mondal A, Kolhe R, Somanath PR, Modulation in the microRNA repertoire is responsible for the stage-specific effects of Akt suppression on murine neuroendocrine prostate cancer, Heliyon 4 (9) (2018), e00796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Verma A, Artham S, Alwhaibi A, Adil MS, Cummings BS, Somanath PR, PAK1 inhibitor IPA-3 mitigates metastatic prostate cancer-induced bone remodeling, Biochem. Pharmacol 177 (2020), 113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alkabban FM, Ferguson T, Breast Cancer, StatPearls, StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC., Treasure Island (FL), 2020. [Google Scholar]
- [13].Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H, Ovarian cancer in the world: epidemiology and risk factors, Int. J. Womens Health 11 (2019) 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Elshamy WM, Duhé RJ, Overview: cellular plasticity, cancer stem cells and metastasis, Cancer Lett 341 (1) (2013) 2–8. [DOI] [PubMed] [Google Scholar]
- [15].Liao Z, Tan ZW, Zhu P, Tan NS, Cancer-associated fibroblasts in tumor microenvironment – accomplices in tumor malignancy, Cell. Immunol 343 (2019), 103729. [DOI] [PubMed] [Google Scholar]
- [16].Langley RR, Fidler IJ, The seed and soil hypothesis revisited – the role of tumor-stroma interactions in metastasis to different organs, Int. J. Cancer 128 (11) (2011) 2527–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aziz NM, Cancer survivorship research: state of knowledge, challenges and opportunities, Acta Oncol 46 (4) (2007) 417–432. [DOI] [PubMed] [Google Scholar]
- [18].Natesan R, Aras S, Effron SS, Asangani IA, Epigenetic regulation of chromatin in prostate cancer, Adv. Exp. Med. Biol 1210 (2019) 379–407. [DOI] [PubMed] [Google Scholar]
- [19].Zeng Y, Advances in mechanism and treatment strategy of cancer, Cell Mol. Biol. (Noisy-le-grand) 64 (6) (2018) 1–3. [PubMed] [Google Scholar]
- [20].Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, Rodríguez-Ruiz ME, Ponz-Sarvise M, Castañón E, Melero I, Cytokines in clinical cancer immunotherapy, Br. J. Cancer 120 (1) (2019) 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang T, Alvarez A, Hu B, Cheng SY, Noncoding RNAs in cancer and cancer stem cells, Chin. J. Cancer 32 (11) (2013) 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Loud JT, Murphy J, Cancer screening and early detection in the 21(st) century, Semin. Oncol. Nurs 33 (2) (2017) 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gdovin MJ, Kadri N, Rios L, Holliday S, Jordan Z, Focal photodynamic intracellular acidification as a cancer therapeutic, Semin. Cancer Biol 43 (2017) 147–156. [DOI] [PubMed] [Google Scholar]
- [24].Mirghani H, Lang Kuhs KA, Waterboer T, Biomarkers for early identification of recurrences in HPV-driven oropharyngeal cancer, Oral Oncol 82 (2018) 108–114. [DOI] [PubMed] [Google Scholar]
- [25].Martín-Lorenzo A, Gonzalez-Herrero I, Rodríguez-Hernández G, García-Ramírez I, Vicente-Dueñas C, Sánchez-García I, Early epigenetic cancer decisions, Biol. Chem 395 (11) (2014) 1315–1320. [DOI] [PubMed] [Google Scholar]
- [26].Mehta SR, Suhag V, Semwal M, Sharma N, Radiotherapy: basic concepts and recent advances, Med. J. Armed Forces India 66 (2) (2010) 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW, Biological response of cancer cells to radiation treatment, Front. Mol. Biosci 1 (2014) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Baskar R, Lee KA, Yeo R, Yeoh KW, Cancer and radiation therapy: current advances and future directions, Int. J. Med. Sci 9 (3) (2012) 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lippman SM, Hawk ET, Cancer prevention: from 1727 to milestones of the past 100 years, Cancer Res 69 (13) (2009) 5269–5284. [DOI] [PubMed] [Google Scholar]
- [30].Folkert MR, Timmerman RD, Stereotactic ablative body radiosurgery (SABR) or Stereotactic body radiation therapy (SBRT), Adv. Drug Deliv. Rev 109 (2017) 3–14. [DOI] [PubMed] [Google Scholar]
- [31].Luo XM, Niu LZ, Chen JB, Xu KC, Advances in cryoablation for pancreatic cancer, World J. Gastroenterol 22 (2) (2016) 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rajabi M, Mousa SA, The role of angiogenesis in cancer treatment, Biomedicines 5 (2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Caporarello N, Lupo G, Olivieri M, Cristaldi M, Cambria MT, Salmeri M, Anfuso CD, Classical VEGF, Notch and Ang signalling in cancer angiogenesis, alternative approaches and future directions (Review), Mol. Med. Rep 16 (4) (2017) 4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Adil MS, Somanath PR, Endothelial permeability assays in vitro, Methods Mol. Biol (2020). [DOI] [PubMed]
- [35].Adil MS, Somanath PR, Vascular permeability assays in vivo, Methods Mol. Biol (2020). [DOI] [PubMed]
- [36].Niu G, Chen X, Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy, Curr. Drug Targets 11 (8) (2010) 1000–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ramjiawan RR, Griffioen AW, Duda DG, Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 20 (2) (2017) 185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Qin S, Li A, Yi M, Yu S, Zhang M, Wu K, Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy, J. Hematol. Oncol 12 (1) (2019) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Adil MS, Narayanan SP, Somanath PR, Cell-cell junctions: structure and regulation in physiology and pathology, Tissue Barriers (2020) 1848212. [DOI] [PMC free article] [PubMed]
- [40].Goc A, Liu J, Byzova TV, Somanath PR, Akt1 mediates prostate cancer cell microinvasion and chemotaxis to metastatic stimuli via integrin β3 affinity modulation, Br. J. Cancer 107 (4) (2012) 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lupulescu AP, Hormones, vitamins, and growth factors in cancer treatment and prevention. A critical appraisal, Cancer 78 (11) (1996) 2264–2280. [PubMed] [Google Scholar]
- [42].Ventola CL, Cancer immunotherapy, Part 1: current strategies and agents, P T 42 (6) (2017) 375–383. [PMC free article] [PubMed] [Google Scholar]
- [43].Cross D, Burmester JK, Gene therapy for cancer treatment: past, present and future, Clin. Med. Res 4 (3) (2006) 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Peters J, Haven’t heard of RNA therapy yet? You will, Massive Sci (2018).
- [45].Coutinho MF, Matos L, Santos JI, Alves S, Therapeutics RNA, How far have we gone? Adv. Exp. Med. Biol 1157 (2019) 133–177. [DOI] [PubMed] [Google Scholar]
- [46].Zhong X, Zhang D, Xiong M, Zhang L, Noncoding RNA for cancer gene therapy, Recent Results Cancer Res 209 (2016) 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bhaskaran M, Mohan M, MicroRNAs: history, biogenesis, and their evolving role in animal development and disease, Vet. Pathol 51 (4) (2014) 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rupaimoole R, Slack FJ, MicroRNA therapeutics: towards a new era for the management of cancer and other diseases, Nat. Rev. Drug Discov 16 (3) (2017) 203–222. [DOI] [PubMed] [Google Scholar]
- [49].Navarro A, Monzo M, MicroRNAs in human embryonic and cancer stem cells, Yonsei Med. J 51 (5) (2010) 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li H, Gupta S, Yang BB, MicroRNA regulated stress responses in cancer, in: Wondrak GT (Ed.), Stress Response Pathways in Cancer: From Molecular Targets to Novel Therapeutics, Springer, Netherlands, Dordrecht, 2015, pp. 107–126. [Google Scholar]
- [51].Thomas KT, Gross C, Bassell GJ, microRNAs sculpt neuronal communication in a tight balance that is lost in neurological disease, Front. Mol. Neurosci 11 (2018) 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bajan S, Hutvagner G, RNA-based therapeutics: from antisense oligonucleotides to miRNAs, Cells 9 (1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hanna J, Hossain GS, Kocerha J, The potential for microRNA therapeutics and clinical research, Front. Genet 10 (2019) 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Teloni F, Michelena J, Lezaja A, Kilic S, Ambrosi C, Menon S, Dobrovolna J, Imhof R, Janscak P, Baubec T, Altmeyer M, Efficient pre-mRNA cleavage prevents replication-stress-associated genome instability, Mol. Cell 73 (4) (2019) 670–683.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schneider-Poetsch T, Yoshida M, Along the central dogma-controlling gene expression with small molecules, Annu. Rev. Biochem 87 (2018) 391–420. [DOI] [PubMed] [Google Scholar]
- [56].Slobodin B, Han R, Calderone V, Vrielink J, Loayza-Puch F, Elkon R, Agami R, Transcription impacts the efficiency of mRNA translation via co-transcriptional N6-adenosine methylation, Cell 169 (2) (2017) 326–337.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hubé F, Francastel C, Coding and non-coding RNAs, the Frontier has never been so blurred, Front. Genet 9 (2018) 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mattick JS, Makunin IV, Non-coding RNA, Hum. Mol. Genet 15 Spec No. 1 (2006) R17–R29. [DOI] [PubMed] [Google Scholar]
- [59].Ratti M, Lampis A, Ghidini M, Salati M, Mirchev MB, Valeri N, Hahne JC, MicroRNAs (miRNAs) and long non-coding RNAs (lncRNAs) as new tools for cancer therapy: first steps from bench to bedside, Target Oncol 15 (3) (2020) 261–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Catalanotto C, Cogoni C, Zardo G, MicroRNA in control of gene expression: an overview of nuclear functions, Int. J. Mol. Sci 17 (10) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lu TX, Rothenberg ME, MicroRNA, J. Allergy Clin. Immunol 141 (4) (2018) 1202–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lu Z, Wang M, Wu S, Ye M, Lin Z, Shun T, Duan C, MicroRNA-137-regulated AKT serine/threonine kinase 2 inhibits tumor growth and sensitizes cisplatin in patients with non-small cell lung cancer, Oncol. Lett 16 (2) (2018) 1876–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A, Identification of mammalian microRNA host genes and transcription units, Genome Res 14 (10a) (2004) 1902–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jiang L, Shao C, Wu QJ, Chen G, Zhou J, Yang B, Li H, Gou LT, Zhang Y, Wang Y, Yeo GW, Zhou Y, Fu XD, NEAT1 scaffolds RNA-binding proteins and the Microprocessor to globally enhance pri-miRNA processing, Nat. Struct. Mol. Biol 24 (10) (2017) 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bofill-De Ros X, Kasprzak WK, Bhandari Y, Fan L, Cavanaugh Q, Jiang M, Dai L, Yang A, Shao TJ, Shapiro BA, Wang YX, Gu S, Structural differences between pri-miRNA paralogs promote alternative drosha cleavage and expand target repertoires, Cell Rep 26 (2) (2019) 447–459.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Morales S, Monzo M, Navarro A, Epigenetic regulation mechanisms of microRNA expression, Biomol. Concepts 8 (5–6) (2017) 203–212. [DOI] [PubMed] [Google Scholar]
- [67].Rasnic R, Linial N, Linial M, Enhancing identification of cancer types via lowly-expressed microRNAs, Nucleic Acids Res 45 (9) (2017) 5048–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cai Y, Yu X, Hu S, Yu J, A brief review on the mechanisms of miRNA regulation, Genomics Proteomics Bioinformatics 7 (4) (2009) 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Slattery ML, Mullany LE, Sakoda LC, Wolff RK, Stevens JR, Samowitz WS, Herrick JS, The PI3K/AKT signaling pathway: associations of miRNAs with dysregulated gene expression in colorectal cancer, Mol. Carcinog 57 (2) (2018) 243–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].O’Brien J, Hayder H, Zayed Y, Peng C, Overview of MicroRNA biogenesis, mechanisms of actions, and circulation, Front. Endocrinol. (Lausanne) 9 (2018) 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gao X, Qin T, Mao J, Zhang J, Fan S, Lu Y, Sun Z, Zhang Q, Song B, Li L, PTENP1/miR-20a/PTEN axis contributes to breast cancer progression by regulating PTEN via PI3K/AKT pathway, J. Exp. Clin. Cancer Res 38 (1) (2019) 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Xu M, Mo YY, The Akt-associated microRNAs, Cell. Mol. Life Sci 69 (21) (2012) 3601–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cui M, Wang H, Yao X, Zhang D, Xie Y, Cui R, Zhang X, Circulating microRNAs in cancer: potential and challenge, Front. Genet 10 (2019) 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li N, Miao Y, Shan Y, Liu B, Li Y, Zhao L, Jia L, MiR-106b and miR-93 regulate cell progression by suppression of PTEN via PI3K/Akt pathway in breast cancer, Cell Death Dis 8 (5) (2017), e2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fang L, Du WW, Yang W, Rutnam ZJ, Peng C, Li H, O’Malley YQ, Askeland RW, Sugg S, Liu M, Mehta T, Deng Z, Yang BB, MiR-93 enhances angiogenesis and metastasis by targeting LATS2, Cell Cycle 11 (23) (2012) 4352–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Feng YH, Tsao CJ, Emerging role of microRNA-21 in cancer, Biomed. Rep 5 (4) (2016) 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lovat F, Fassan M, Gasparini P, Rizzotto L, Cascione L, Pizzi M, Vicentini C, Balatti V, Palmieri D, Costinean S, Croce CM, miR-15b/16–2 deletion promotes B-cell malignancies, Proc. Natl. Acad. Sci. U.S.A 112 (37) (2015) 11636–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Michlewski G, Cáceres JF, Post-transcriptional control of miRNA biogenesis, RNA 25 (1) (2019) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Titze-de-Almeida R, David C, Titze-de-Almeida SS, The race of 10 synthetic RNAi-based drugs to the pharmaceutical market, Pharm. Res 34 (7) (2017) 1339–1363. [DOI] [PubMed] [Google Scholar]
- [80].Chang H, Yi B, Ma R, Zhang X, Zhao H, Xi Y, CRISPR/cas9, a novel genomic tool to knock down microRNA in vitro and in vivo, Sci. Rep 6 (2016) 22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kim DG, Kim KH, Seo YJ, Yang H, Marcusson EG, Son E, Lee K, Sa JK, Lee HW, Nam DH, Anti-miR delivery strategies to bypass the blood-brain barrier in glioblastoma therapy, Oncotarget 7 (20) (2016) 29400–29411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Muthiah M, Park IK, Cho CS, Nanoparticle-mediated delivery of therapeutic genes: focus on miRNA therapeutics, Expert Opin. Drug Deliv 10 (9) (2013) 1259–1273. [DOI] [PubMed] [Google Scholar]
- [83].Baumann V, Winkler J, miRNA-based therapies: strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents, Future Med. Chem 6 (17) (2014) 1967–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fang Y, Liang X, Xu J, Cai X, miR-424 targets AKT3 and PSAT1 and has a tumor-suppressive role in human colorectal cancer, Cancer Manage. Res 10 (2018) 6537–6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Alwhaibi A, Verma A, Adil MS, Somanath PR, The unconventional role of Akt1 in the advanced cancers and in diabetes-promoted carcinogenesis, Pharmacol. Res 145 (2019), 104270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Somanath PR, Vijai J, Kichina JV, Byzova T, Kandel ES, The role of PAK-1 in activation of MAP kinase cascade and oncogenic transformation by Akt, Oncogene 28 (25) (2009) 2365–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gao F, Alwhaibi A, Sabbineni H, Verma A, Eldahshan W, Somanath PR, Suppression of Akt1-β-catenin pathway in advanced prostate cancer promotes TGFβ1-mediated epithelial to mesenchymal transition and metastasis, Cancer Lett 402 (2017) 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sabbineni H, Alwhaibi A, Goc A, Gao F, Pruitt A, Somanath PR, Genetic deletion and pharmacological inhibition of Akt1 isoform attenuates bladder cancer cell proliferation, motility and invasion, Eur. J. Pharmacol 764 (2015) 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yu JS, Cui W, Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination, Development 143 (17) (2016) 3050–3060. [DOI] [PubMed] [Google Scholar]
- [90].Duncan L, Shay C, Teng Y, PI3K isoform-selective inhibitors in cancer, Adv. Exp. Med. Biol 1255 (2020) 165–173. [DOI] [PubMed] [Google Scholar]
- [91].Bilanges B, Posor Y, Vanhaesebroeck B, PI3K isoforms in cell signalling and vesicle trafficking, Nat. Rev. Mol. Cell Biol 20 (9) (2019) 515–534. [DOI] [PubMed] [Google Scholar]
- [92].Alwhaibi A, Kolhe R, Gao F, Cobran EK, Somanath PR, Genome atlas analysis based profiling of Akt pathway genes in the early and advanced human prostate cancer, Oncoscience 6 (5–6) (2019) 317–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dai L, Chen F, Zheng Y, Zhang D, Qian B, Ji H, Long F, Cretoiu D, miR-21 regulates growth and EMT in lung cancer cells via PTEN/Akt/GSK3β signaling, Front. Biosci. (Landmark Ed) 24 (2019) 1426–1439. [DOI] [PubMed] [Google Scholar]
- [94].Wu J, Sun Z, Sun H, Li Y, MicroRNA-27a promotes tumorigenesis via targeting AKT in triple negative breast cancer, Mol. Med. Rep 17 (1) (2018) 562–570. [DOI] [PubMed] [Google Scholar]
- [95].Goc A, Al-Husein B, Kochuparambil ST, Liu J, Heston WW, Somanath PR, PI3 kinase integrates Akt and MAP kinase signaling pathways in the regulation of prostate cancer, Int. J. Oncol 38 (1) (2011) 267–277. [PubMed] [Google Scholar]
- [96].Kichina JV, Goc A, Al-Husein B, Somanath PR, Kandel ES, PAK1 as a therapeutic target, Expert Opin. Ther. Targets 14 (7) (2010) 703–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Giancotti FG, Deregulation of cell signaling in cancer, FEBS Lett 588 (16) (2014) 2558–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Brazil DP, Hemmings BA, Ten years of protein kinase B signalling: a hard Akt to follow, Trends Biochem. Sci 26 (11) (2001) 657–664. [DOI] [PubMed] [Google Scholar]
- [99].Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV, The Akt/PKB pathway: molecular target for cancer drug discovery, Oncogene 24 (50) (2005) 7482–7492. [DOI] [PubMed] [Google Scholar]
- [100].Arafeh R, Samuels Y, PIK3CA in cancer: the past 30 years, Semin. Cancer Biol 59 (2019) 36–49. [DOI] [PubMed] [Google Scholar]
- [101].Goc A, Al-Husein B, Katsanevas K, Steinbach A, Lou U, Sabbineni H, DeRemer DL, Somanath PR, Targeting Src-mediated Tyr216 phosphorylation and activation of GSK-3 in prostate cancer cells inhibit prostate cancer progression in vitro and in vivo, Oncotarget 5 (3) (2014) 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, Baird RD, Delgado L, Taylor A, Lupinacci L, Riisnaes R, Pope LL, Heaton SP, Thomas G, Garrett MD, Sullivan DM, de Bono JS, Tolcher AW, First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors, J. Clin. Oncol 29 (35) (2011) 4688–4695. [DOI] [PubMed] [Google Scholar]
- [103].Dillon LM, Miller TW, Therapeutic targeting of cancers with loss of PTEN function, Curr. Drug Targets 15 (1) (2014) 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Li B, Lu Y, Wang H, Han X, Mao J, Li J, Yu L, Wang B, Fan S, Yu X, Song B, miR-221/222 enhance the tumorigenicity of human breast cancer stem cells via modulation of PTEN/Akt pathway, Biomed. Pharmacother 79 (2016) 93–101. [DOI] [PubMed] [Google Scholar]
- [105].Fu X, Liu M, Qu S, Ma J, Zhang Y, Shi T, Wen H, Yang Y, Wang S, Wang J, Nan K, Yao Y, Tian T, Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway, J. Exp. Clin. Cancer Res 37 (1) (2018) 52. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [106].Sayed D, Abdellatif M, AKT-ing via microRNA, Cell Cycle 9 (16) (2010) 3213–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Han J, Zhao F, Zhang J, Zhu H, Ma H, Li X, Peng L, Sun J, Chen Z, miR-223 reverses the resistance of EGFR-TKIs through IGF1R/PI3K/Akt signaling pathway, Int. J. Oncol 48 (5) (2016) 1855–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Alwhaibi A, Verma A, Artham S, Adil MS, Somanath PR, Nodal pathway activation due to Akt1 suppression is a molecular switch for prostate cancer cell epithelial-to-mesenchymal transition and metastasis, Biochem. Pharmacol 168 (2019) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Balacescu O, Dumitrescu RG, Marian C, MicroRNAs role in prostate cancer, Methods Mol. Biol 2018 (1856) 103–117. [DOI] [PubMed] [Google Scholar]
- [110].Chang YL, Zhou PJ, Wei L, Li W, Ji Z, Fang YX, Gao WQ, MicroRNA-7 inhibits the stemness of prostate cancer stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21 pathway, Oncotarget 6 (27) (2015) 24017–24031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Majid S, Dar AA, Saini S, Shahryari V, Arora S, Zaman MS, Chang I, Yamamura S, Tanaka Y, Chiyomaru T, Deng G, Dahiya R, miRNA-34b inhibits prostate cancer through demethylation, active chromatin modifications, and AKT pathways, Clin. Cancer Res 19 (1) (2013) 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tang Y, Pan J, Huang S, Peng X, Zou X, Luo Y, Ren D, Zhang X, Li R, He P, Wa Q, Downregulation of miR-133a-3p promotes prostate cancer bone metastasis via activating PI3K/AKT signaling, J. Exp. Clin. Cancer Res 37 (1) (2018) 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gao S, Zhao Z, Wu R, Wu L, Tian X, Zhang Z, MiR-146b inhibits autophagy in prostate cancer by targeting the PTEN/Akt/mTOR signaling pathway, Aging (Albany NY) 10 (8) (2018) 2113–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhang Y, Zhang D, Lv J, Wang S, Zhang Q, miR-410-3p promotes prostate cancer progression via regulating PTEN/AKT/mTOR signaling pathway, Biochem. Biophys. Res. Commun 503 (4) (2018) 2459–2465. [DOI] [PubMed] [Google Scholar]
- [115].Liu H, Wang J, Tao Y, Li X, Qin J, Bai Z, Chi B, Yan W, Chen X, Curcumol inhibits colorectal cancer proliferation by targeting miR-21 and modulated PTEN/PI3K/Akt pathways, Life Sci 221 (2019) 354–361. [DOI] [PubMed] [Google Scholar]
- [116].Liang L, Gao C, Li Y, Sun M, Xu J, Li H, Jia L, Zhao Y, miR-125a-3p/FUT5-FUT6 axis mediates colorectal cancer cell proliferation, migration, invasion and pathological angiogenesis via PI3K-Akt pathway, Cell Death Dis 8 (8) (2017), e2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wu KL, Tsai YM, Lien CT, Kuo PL, Hung AJ, The roles of microRNA in lung cancer, Int. J. Mol. Sci 20 (7) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Han Z, Zhou X, Li S, Qin Y, Chen Y, Liu H, Inhibition of miR-23a increases the sensitivity of lung cancer stem cells to erlotinib through PTEN/PI3K/Akt pathway, Oncol. Rep 38 (5) (2017) 3064–3070. [DOI] [PubMed] [Google Scholar]
- [119].Guerriero I, D’Angelo D, Pallante P, Santos M, Scrima M, Malanga D, De Marco C, Ravo M, Weisz A, Laudanna C, Ceccarelli M, Falco G, Rizzuto A, Viglietto G, Analysis of miRNA profiles identified miR-196a as a crucial mediator of aberrant PI3K/AKT signaling in lung cancer cells, Oncotarget 8 (12) (2017) 19172–19191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Liu J, Xing Y, Rong L, miR-181 regulates cisplatin-resistant non-small cell lung cancer via downregulation of autophagy through the PTEN/PI3K/AKT pathway, Oncol. Rep 39 (4) (2018) 1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sun DM, Tang BF, Li ZX, Guo HB, Cheng JL, Song PP, Zhao X, MiR-29c reduces the cisplatin resistance of non-small cell lung cancer cells by negatively regulating the PI3K/Akt pathway, Sci. Rep 8 (1) (2018) 8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yuan Y, Du W, Wang Y, Xu C, Wang J, Zhang Y, Wang H, Ju J, Zhao L, Wang Z, Lu Y, Cai B, Pan Z, Suppression of AKT expression by miR-153 produced anti-tumor activity in lung cancer, Int. J. Cancer 136 (6) (2015) 1333–1340. [DOI] [PubMed] [Google Scholar]
- [123].Loh HY, Norman BP, Lai KS, Rahman N, Alitheen NBM, Osman MA, The regulatory role of microRNAs in breast cancer, Int. J. Mol. Sci 20 (19) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Fan X, Fang X, Liu G, Xiong Q, Li Z, Zhou W, MicroRNA-204 inhibits the proliferation and metastasis of breast cancer cells by targeting PI3K/AKT pathway, J. Buon 24 (3) (2019) 1054–1059. [PubMed] [Google Scholar]
- [125].Zhu MX, Wei CY, Zhang PF, Gao DM, Chen J, Zhao Y, Dong SS, Liu BB, Elevated TRIP13 drives the AKT/mTOR pathway to induce the progression of hepatocellular carcinoma via interacting with ACTN4, J. Exp. Clin. Cancer Res 38 (1) (2019) 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sun G, Hou YB, Jia HY, Bi XH, Yu L, Chen DJ, MiR-370 promotes cell death of liver cancer cells by Akt/FoxO3a signalling pathway, Eur. Rev. Med. Pharmacol. Sci 20 (10) (2016) 2011–2019. [PubMed] [Google Scholar]
- [127].Luo X, Dong Z, Chen Y, Yang L, Lai D, Enrichment of ovarian cancer stem-like cells is associated with epithelial to mesenchymal transition through an miRNA-activated AKT pathway, Cell Prolif 46 (4) (2013) 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Artham S, Verma A, Alwhaibi A, Adil MS, Manicassamy S, Munn DH, Somanath PR, Delayed Akt suppression in the lipopolysaccharide-induced acute lung injury promotes resolution that is associated with enhanced effector regulatory T cells, Am. J. Physiol. Lung Cell. Mol. Physiol 318 (4) (2020) L750–L761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Chen J, miRNA-195 suppresses cell proliferation of ovarian cancer cell by regulating VEGFR2 and AKT signaling pathways, Mol. Med. Rep 18 (2) (2018) 1666–1673. [DOI] [PubMed] [Google Scholar]
- [130].Liu HY, Zhang YY, Zhu BL, Feng FZ, Zhang HT, Yan H, Zhou B, MiR-203a-3p regulates the biological behaviors of ovarian cancer cells through mediating the Akt/GSK-3β/Snail signaling pathway by targeting ATM, J. Ovarian Res 12 (1) (2019) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Xiang G, Cheng Y, MiR-126-3p inhibits ovarian cancer proliferation and invasion via targeting PLXNB2, Reprod. Biol 18 (3) (2018) 218–224. [DOI] [PubMed] [Google Scholar]
- [132].Correa P, Gastric cancer: overview, Gastroenterol. Clin. North Am 42 (2) (2013) 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Zhang Z, Dong Y, Hua J, Xue H, Hu J, Jiang T, Shi L, Du J, A five-miRNA signature predicts survival in gastric cancer using bioinformatics analysis, Gene 699 (2019) 125–134. [DOI] [PubMed] [Google Scholar]
- [134].Zhang Y, Bai J, Si W, Yuan S, Li Y, Chen X, SLC39A7, regulated by miR-139-5p, induces cell proliferation, migration and inhibits apoptosis in gastric cancer via Akt/mTOR signaling pathway, Biosci. Rep 40 (2) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zhang F, Li K, Pan M, Li W, Wu J, Li M, Zhao L, Wang H, miR-589 promotes gastric cancer aggressiveness by a LIFR-PI3K/AKT-c-Jun regulatory feedback loop, J. Exp. Clin. Cancer Res 37 (1) (2018) 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wang L, Li K, Wang C, Shi X, Yang H, miR-107 regulates growth and metastasis of gastric cancer cells via activation of the PI3K-AKT signaling pathway by down-regulating FAT4, Cancer Med 8 (11) (2019) 5264–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Lu WD, Zuo Y, Xu Z, Zhang M, MiR-19a promotes epithelial-mesenchymal transition through PI3K/AKT pathway in gastric cancer, World J. Gastroenterol 21 (15) (2015) 4564–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Zhang F, Li K, Yao X, Wang H, Li W, Wu J, Li M, Zhou R, Xu L, Zhao L, A miR-567-PIK3AP1-PI3K/AKT-c-Myc feedback loop regulates tumour growth and chemoresistance in gastric cancer, EBioMedicine 44 (2019) 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Yu J, Wang R, Chen J, Wu J, Dang Z, Zhang Q, Li B, miR-340 inhibits proliferation and induces apoptosis in gastric cancer cell line SGC-7901. Possibly via the AKT pathway, Med. Sci. Monit 23 (2017) 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wang L, Zhang Z, Yu X, Li Q, Wang Q, Chang A, Huang X, Han X, Song Y, Hu J, Pang L, Hou J, Li F, SOX9/miR-203a axis drives PI3K/AKT signaling to promote esophageal cancer progression, Cancer Lett 468 (2020) 14–26. [DOI] [PubMed] [Google Scholar]
- [141].Xu J, Pan X, Hu Z, MiR-502 mediates esophageal cancer cell TE1 proliferation by promoting AKT phosphorylation, Biochem. Biophys. Res. Commun 501 (1) (2018) 119–123. [DOI] [PubMed] [Google Scholar]
- [142].Wang T, Chen G, Ma X, Yang Y, Chen Y, Peng Y, Bai Z, Zhang Z, Pei H, Guo W, MiR-30a regulates cancer cell response to chemotherapy through SNAI1/IRS1/AKT pathway, Cell Death Dis 10 (3) (2019) 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Liang Z, Wang X, Xu X, Xie B, Ji A, Meng S, Li S, Zhu Y, Wu J, Hu Z, Lin Y, Zheng X, Xie L, Liu B, MicroRNA-608 inhibits proliferation of bladder cancer via AKT/FOXO3a signaling pathway, Mol. Cancer 16 (1) (2017) 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ru N, Zhang F, Liang J, Du Y, Wu W, Wang F, Liu X, MiR-564 is down-regulated in osteosarcoma and inhibits the proliferation of osteosarcoma cells via targeting Akt, Gene 645 (2018) 163–169. [DOI] [PubMed] [Google Scholar]
- [145].Chai C, Song LJ, Han SY, Li XQ, Li M, MicroRNA-21 promotes glioma cell proliferation and inhibits senescence and apoptosis by targeting SPRY1 via the PTEN/PI3K/AKT signaling pathway, CNS Neurosci. Ther 24 (5) (2018) 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [146].Wu Q, Shang Y, Shen T, Liu F, Xu Y, Wang H, Neuroprotection of miR-214 against isoflurane-induced neurotoxicity involves the PTEN/PI3K/Akt pathway in human neuroblastoma cell line SH-SY5Y, Arch. Biochem. Biophys 678 (2019), 108181. [DOI] [PubMed] [Google Scholar]


