Abstract
Context:
Phthalate exposure is associated with altered reproductive function, but little is known about associations of phthalate exposure with risk of hot flashes.
Objective:
To investigate associations of urinary phthalate metabolite levels with four hot flash outcomes in midlife women.
Design and Setting:
A cross-sectional study of the first year of a prospective cohort of midlife women, the Midlife Women’s Health Study (2006–2015), a convenience sample from an urban setting.
Participants:
728 multi-racial/ethnic pre- and perimenopausal women aged 45–54 years.
Outcome Measures:
Women completed questionnaires about hot flash experience and provided 1–4 urine samples over four consecutive weeks that were pooled for analysis. Phthalate metabolites were assessed individually and as molar sums representative of common compounds and exposure sources (plastics, ΣPlastic). Covariate-adjusted logistic regression models were used to assess associations of continuous natural log-transformed phthalate metabolite concentrations with hot flash outcomes. Analyses were conducted to explore whether associations differed by menopause status, body mass index (BMI), race/ethnicity, and depressive symptoms.
Results:
Overall, 45% of women reported a history of hot flashes. Compared to women who never experienced hot flashes, every two-fold increase in ΣPlastic was associated with 18% (OR: 1.18; 95%CI: 0.98, 1.43) and 38% (OR: 1.38; 95%CI: 1.11, 1.70) higher odds of experiencing hot flashes in the past 30 days and experiencing daily/weekly hot flashes, respectively. Some associations of phthalates with certain hot flash outcomes differed by menopause status, BMI, race/ethnicity, and depressive symptoms.
Conclusions:
This study suggests that phthalates are associated with hot flash experience and may impact hot flash risk in women who are susceptible to experiencing hot flashes.
Keywords: Hot flashes, menopause, phthalates, women
Graphical Abstract
1. Introduction
Hot flashes are one of the most common symptoms of menopause, but little is known about the risk factors associated with increased risk of hot flashes. Hot flashes are characterized by sudden and transient periods of intense body heat accompanied by flushing, sweating, chills, and anxiety (1). Experiencing hot flashes can impact daily life for symptomatic women for years and results in estimated medical costs of $340 million in the U.S. each year and an additional $27 million in lost work (2,3). Although the majority of peri- and postmenopausal women experience hot flashes, the dynamics of hot flashes during the menopause transition (such as age of onset, duration, intensity, and risk factors) are not well understood (2). Environmental factors (e.g. smoking), physiological factors (e.g. later stage of menopause), and decreasing estrogen levels are known to be associated with increased risk of hot flashes (4). Our own small cross-sectional analysis of a representative sample of 195 white midlife women from the Midlife Women’s Health Study (MWHS) indicated that exposure to phthalates may be associated with an increased odds of hot flashes in midlife women (5).
Phthalates are a class of synthetic chemicals composed of esters of ortho-phthalic acid with hydrocarbon side chains of varying lengths. Phthalates are used in a wide variety of consumer products, including food contact materials, medical equipment, car interiors, shower curtains, synthetic leather, and children’s toys, as well as fragranced cleaning and personal care products (6). Humans are ubiquitously and unavoidably exposed to phthalates, with 99% of urine samples from the general U.S. population containing phthalate metabolites (7). Due to greater use of personal care products by women compared to men, women typically have higher concentrations of phthalates than men (8,9).
Importantly, studies show that some phthalate metabolites exert toxicity on biological systems including the reproductive system (10–15). In both experimental and observational studies, phthalates have been shown to alter estradiol levels (13,16–18). Animal studies have also shown that mixtures of phthalates can impact hormone levels and steroidogenesis in the ovary (18–20). This is concerning given that midlife women are widely exposed to phthalates through diet and personal care products (11,14,18,21). The molecular mechanisms of action of phthalates to cause hormone disruption are hypothesized to occur through activation of peroxisome proliferator-activated receptors (13,18).
In general, factors that decrease estrogen levels in women are strongly associated with increased incidence of hot flashes (reviewed in 4). Extensive literature suggests associations between estrogen levels and hot flashes in conditions that cause acute drops in estrogen levels, such as oophorectomy (22). As phthalates are associated with decreased estrogen levels in human and animals, we hypothesized that phthalate exposure may contribute to hot flash experience in women. To our knowledge, no studies have evaluated the impact of phthalate exposure on hot flash risk in women in detail. However, this study expands upon our previous pilot study (5) to include the entire cohort of the MWHS, which contains multiple racial/ethnic groups, and includes additional analyses based on participant characteristics. Therefore, the primary objective of this study was to assess associations of urinary phthalate metabolite levels with hot flash occurrence, frequency, and severity in midlife women enrolled in the first year of the MWHS. Because risk of hot flashes may differ in women based on menopausal status, midlife body mass index (BMI), race/ethnicity, and depression status (4,23), the secondary objective of this study was to evaluate differences in associations of urinary phthalate metabolite levels with hot flash risk by these characteristics.
2. Materials and Methods
2.1. Ethical approval
All participants gave written informed consent according to procedures approved by the University of Illinois and Johns Hopkins University Institutional Review Boards (file number: 06741).
2.2. Study population
This study was a cross-sectional analysis of data collected in the first year of the MWHS, a prospective cohort study with the overall goal of evaluating risk factors of hot flashes in midlife women. A detailed study protocol of the MWHS has been published previously (24). Briefly, participants were recruited from the city of Baltimore, MD (USA) and surrounding counties from 2006 to 2015. Women were eligible to participate in the study if they were 45–54 years old and pre- or perimenopausal with or without natural hot flashes. Women were excluded if they had a history of hysterectomy or oophorectomy, were currently pregnant, were taking hormone therapy or herbal/other agents for treatment of menopause symptoms, were taking oral contraceptives, were undergoing cancer treatment, or were postmenopausal. Menopausal status was defined using the Stages of Reproductive Aging Workshop + 10 (STRAW+10) criteria (25). Briefly, menopausal status was defined as follows: pre-menopausal women were those who experienced their last menstrual period within the past 3 months and reported 11 or more periods within the past year. Perimenopausal women were those who experienced their last menstrual period within the past year, but not within the past 3 months, or their last menstrual period within the past 3 months and experienced 10 or fewer periods within the past year. Postmenopausal women were those who had not experienced a menstrual period within the past year. A total of 780 women enrolled in the study during year 1.
2.3. Collection of demographic and lifestyle characteristics
At the baseline clinic visit, women completed a detailed questionnaire and had anthropometrics measured by trained staff. Each woman’s weight and height (without shoes) were measured by trained clinic staff, and values were rounded to the nearest 0.5 pound and 0.5 inch, respectively. The baseline questionnaire collected detailed information on demographics, reproductive history, menstrual cycle characteristics, menopausal symptoms, and medical history, as well as physical activity, smoking status, and alcohol use. Women self-reported their age in years and listed the types of prescription medications used. Each woman’s racial/ethnic background was determined using the question “What is your main ethnic/racial background? (Answer, mark only one: (1) Caucasian/White, (2) African American/Black, (3) Hispanic/Latino, (4) Asian, (5) Other)”. Women reported their highest completed grade or year of schooling using the following options: (1) elementary, (2) high school, (3) technical school, (4) college training, or (5) postgraduate. Smoking status was ascertained using the questions “Have you ever smoked cigarettes?” and “Do you still smoke cigarettes?” whereas the question “In the last 12 months have you had at least 12 drinks of any kind of alcoholic beverage?” was used to determine women’s most recent alcohol consumption status. Leisure physical activity was assessed with the question: “In comparison with others my own age, I think my physical activity leisure time is” [choices: much more, more, as much, less, and much less]. Women’s depression status was assessed using the Centers for Epidemiologic Studies Depression Scale (CESD) (26), which is a validated depression score that was calculated using 20 questions that asked about how the women were feeling during the past week.
2.4. Collection and assessment of hot flash outcomes
At baseline, a detailed history of hot flashes was collected using a series of validated questions that have been used in the MWHS for over 10 years (23,24,27–30). The current study evaluated four hot flash outcomes that were obtained from the following four questions: 1) whether the woman had ever experienced hot flashes, 2) whether she experienced hot flashes in the past 30 days, 3) the usual severity of her hot flashes, and 4) the usual frequency of her hot flashes. Women were first asked “Have you ever had hot flashes?” where hot flashes were defined as “a sudden feeling of heat in the face, neck, or upper part of the chest” with accompanied “reddening or flushing of the skin followed by sweating and chills.” Women who responded “no” to ever experiencing hot flashes were prompted to skip the more detailed hot flash questions and were categorized as “never experiencing hot flashes”. Those who responded “yes” to ever experiencing hot flashes answered whether they experienced hot flashes within the past 30 days (answer: no, yes). Additionally, women who had ever experienced hot flashes were asked (in general) to describe their hot flashes as: mild (sensation of heat without sweating), moderate (sensation of heat with sweating), or severe (sensation of heat with sweating that disrupts usual activity). We categorized severity of hot flashes as either mild or moderate/severe. Similarly, women who had ever experienced hot flashes were asked (in general) to describe their hot flashes as occurring: every hour, every 2–5 h, every 6–11 h, every 12–23 h, 5–6 days per week, 1–2 days per week, 2–3 days per month, 1 day per month, less than 1 day per month, or never. We categorized frequency of hot flashes as either monthly or daily/weekly.
2.4. Assessment of urinary phthalate metabolites
Urinary phthalate metabolite concentrations are the preferred biomarkers of phthalate exposure (31). Humans are exposed to phthalate diesters (i.e. parent compounds), which are rapidly metabolized to monoester metabolites in the body (32). Therefore, epidemiological studies measuring human urinary phthalate metabolites often measure one or more metabolites for each parent phthalate and report as sums of metabolite concentrations based on parent compound, exposure source, or biological activity (33–37).
Participants provided spot urine specimens at the initial baseline clinic visit and at visits during the next three consecutive weeks, which were used for urinary phthalate metabolite assessment. Each woman provided samples at 1–4 visits in the 4 week timeframe, which were pooled due to the short half-lives of phthalates in the body and the high daily and weekly intra-variability of measured concentrations (38). Pooled samples were analyzed for the following 9 phthalate metabolites: mono-2-ethylhexyl phthalate (MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5 -carboxypentyl) phthalate (MECPP), mono-(2-ethyl-5-oxohexyl)phthalate (MEOHP), mono-(3-carboxypropyl) phthalate (MCPP), monobenzyl phthalate (MBzP), monoethyl phthalate (MEP), monobutyl phthalate (MBP), and monoisobutyl phthalate (MiBP). Analyses were performed using isotope dilution high-performance liquid chromatography negative-ion electrospray ionization-tandem mass spectrometry (HPLC–MS/MS) at the Metabolomics Lab of the Roy J. Carver Biotechnology Center, University of Illinois at Urbana-Champaign, using methods adapted from the Center for Disease Control and Prevention (39) and are described in the supporting information (40).
2.5. Statistical analysis
Out of 780 women enrolled during year 1 of the MWHS, 10 were missing information about hot flashes, an additional 10 were missing information about urinary phthalate metabolite concentrations and/or specific gravity, and an additional 32 women were missing information about covariates (which are described below). Therefore, the current study included a total of 728 women with information about baseline covariates, urinary phthalate metabolite concentrations, and hot flashes. Covariates for associations of midlife urinary phthalate metabolite concentrations with risk of hot flashes were chosen a priori and using previous literature that informed a directed acyclic graph (4,23). To reduce potential for multicollinearity issues, we assessed correlations among all selected covariates (with none being strongly correlated). Therefore, final statistical models evaluating overall associations of urinary phthalate metabolite concentrations with four hot flash outcomes (objective 1) were adjusted for age, race, education, current drinking status, smoking status, medication use, menopause status, BMI, and CESD score. Age and CESD score were included as continuous variables, whereas the other variables were categorized with reference group set as shown in Table 1. Our second objective was to assess differences in associations of urinary phthalate metabolite concentrations with 4 hot flash outcomes by menopausal status, BMI, race/ethnicity, and CESD score (objective 2)—factors that may influence the experience of hot flashes in midlife women (4). In addition to including the previously listed covariates in all stratified models, we a priori stratified our analyses as follows: pre- versus perimenopausal women, under-/normal weight (BMI<25kg/m2) versus overweight/obese (BMI≥25kg/m2) women, non-Hispanic white versus Black/other women, and women with fewer (CESD<16) versus more (CESD≥16) depressive symptoms (26).
Table 1.
Demographic and lifestyle characteristics of 45-54 year-old women from the Midlife Women’s Health Study (n=728).
| Demographic or Lifestyle Characteristic | n (%)2 |
|---|---|
| Age (years)1 | |
| 45 to 49 | 477 (65.5) |
| 50 to 54 | 251 (34.5) |
| Race1 | |
| Non-Hispanic White (ref) | 484 (66.5) |
| Black | 215 (29.5) |
| Other | 29 (4.0) |
| Employment status | |
| Unemployed | 146 (20.1) |
| Employed | 582 (79.9) |
| Education1 | |
| Some college or less | 255 (35.0) |
| College graduate or higher (ref) | 473 (65.0) |
| Annual family income ($) | |
| <20,000 | 45 (6.2) |
| 20,000 to 39,999 | 113 (15.5) |
| 40,000 to 99,999 | 241 (33.1) |
| ≥100,000 | 308 (42.3) |
| Marital status | |
| Single | 133 (18.3) |
| Married/Living with Partner | 476 (65.4) |
| Widowed/divorced/separated | 118 (16.2) |
| Menopausal status1 | |
| Premenopausal (ref) | 468 (64.3) |
| Perimenopausal | 260 (35.7) |
| Alcohol consumption status (>1 drink/month on average)1 | |
| No | 252 (34.6) |
| Yes (ref) | 476 (65.4) |
| Smoking status1 | |
| Current | 69 (9.5) |
| Former | 256 (35.2) |
| Never (ref) | 403 (55.4) |
| Leisure physical activity compared to others | |
| Much more/more | 257 (35.3) |
| As much | 230 (31.6) |
| Less/much less | 235 (32.3) |
| Body mass index (kg/m2)1 | |
| <25 | 290 (39.8) |
| ≥25 (ref) | 438 (60.2) |
| Current medication use1 | |
| No | 311 (42.7) |
| Yes (ref) | 417 (57.3) |
| CES depression score1 | |
| Fewer depressive symptoms (<16) | 581 (79.8) |
| More depressive symptoms (≥16) | 147 (20.2) |
Variables included in logistic regression models.
Percentages may not add up to 100% due to missing values.
Nine urinary phthalate metabolites were assessed from pooled urine samples (Table 2). Urinary phthalate metabolite concentrations below the level of detection (LOD) were converted to the LOD/√(2). To account for urine dilution, we used the following formula to adjust all urinary phthalate metabolite concentrations: Pc = P[(1.018 − 1)/(SGi − 1)], where Pc is the specific gravity adjusted phthalate metabolite concentration, P is the measured phthalate metabolite concentration (ng/mL), 1.018 is the median specific gravity of MWHS population included in this analysis, and SGi is the specific gravity of each woman’s pooled urine sample (41). Specific gravity-adjusted urinary phthalate metabolite concentrations were used to approximate women’s midlife exposure to phthalate parent compounds. Exposure to DEHP was approximated as the molar converted sum of 4 urinary metabolites using the following equation: ΣDEHP = (MEHP/278) + (MEHHP/294) + (MEOHP/292) + (MECPP/308). Exposure to DOP, BzBP, DEP, DBP, and DiBP was approximated directly using the non-molar converted concentrations of their major urinary metabolites MCPP, MBzP, MEP, MBP, and MiBP, respectively. Additional phthalate sums were created based on primary sources of phthalate exposure, and sum of plasticizer (ΣPlastic) and personal care product (ΣPCP) phthalate metabolites were estimated as follows: ΣPlastic = (MEHHP/294) + (MEHP/278) + (MEOHP/292) + (MECPP/308) + (MCPP/252) + (MBzP/256) and ΣPCP = (MEP/194) + (MBP/222) + (MiBP/222). Previous experimental and some epidemiological studies suggest that certain phthalate metabolites have anti-androgenic activity in the body (18,42,43). Therefore, the sum of anti-androgenic phthalate metabolites (ΣAA) was calculated as (MEHP/278) + (MEHHP/294) + (MEOHP/292) + (MECPP/308) + (MBzP/256) + (MBP/222) + (MiBP/222). Lastly, all 9 urinary phthalate metabolites were molar converted and summed to approximate total midlife phthalate exposure (ΣPhthalates).
Table 2.
Concentrations of individual urinary phthalate metabolites and molar sums from 45-54-year-old women from the Midlife Women’s Health Study and the National Health and Nutrition Examination Survey.
| Name | Abbreviation | MWHS (2006–2015) n=728 |
NHANES (2005–2016) n=7571 |
|---|---|---|---|
| Phthalate metabolite | Median (25th, 75th percentiles) in ng/mL | ||
| Monoethyl phthalate | MEP | 97.3 (48.2, 192.0)2 | 58.9 (20.0, 179.8) |
| Mono-n-butyl phthalate | MBP | 19.8 (13.0, 32.8) | 11.6 (5.4, 25.3) |
| Mono-isobutyl phthalate | MiBP | 16.4 (10.0, 26.1) | 5.8 (2.6, 13.2) |
| Mono(3-carboxypropyl) phthalate | MCPP | 2.5 (1.3, 5.4) | 1.5 (0.6, 3.4) |
| Monobenzyl phthalate | MBzP | 9.4 (5.4, 16.1) | 4.2 (1.8, 10.4) |
| Mono(2-ethylhexyl) phthalate | MEHP | 4.5 (2.7, 9.3) | 1.2 (0.6, 3.1) |
| Mono(2-ethyl-5-hydroxyhexyl) phthalate | MEHHP | 33.5 (20.5, 58.7) | 9.1 (3.5, 22.6) |
| Mono(2-ethyl-5-oxohexyl) phthalate | MEOHP | 12.0 (7.3, 22.4) | 5.6 (2.1, 13.3) |
| Mono(2-ethyl-5-carboxypentyl) phthalate | MECPP | 25.9 (15.9, 48.0) | 13.4 (5.6, 31.7) |
| Phthalate molar-converted sum | Median (25th, 75th percentiles) in nmol/mL | ||
| Di(2-ethylhexyl) phthalate | DEHP | 0.3 (0.2, 0.5) | 0.1 (0.04, 0.2) |
| Sum of all phthalate metabolites | ΣPhthalates | 1.2 (0.7, 2.1) | 0.7 (0.3, 1.8) |
| Sum of all personal care product phthalate metabolites | ΣPCP | 0.7 (0.4, 1.3) | 0.4 (0.2, 1.2) |
| Sum of all plastic phthalate metabolites | ΣPlastic | 0.3 (0.2, 0.6) | 0.1 (0.1, 0.3) |
| Sum of anti-androgenic phthalate metabolites | ΣAA | 0.5 (0.3, 0.8) | 0.2 (0.1, 0.5) |
Weighted phthalate metabolite concentrations for 45-54-year-old US women from combined NHANES survey years 2005–06, 2007–08, 2009–10, 2011–12, 2013–14, and 2015–16.
Two samples (0.3%) were <LOD for MEP.
We used logistic regression models to evaluate overall and stratified associations of midlife urinary phthalate metabolite concentrations with the following 4 hot flash outcomes: 1) ever experiencing hot flashes, 2) experiencing hot flashes in the past 30 days, 3) experiencing daily/weekly or monthly hot flashes, and 4) experiencing moderate/severe or mild hot flashes (Table 3). All statistical analyses were conducted in SAS 9.4 (version 14.3, SAS Institute) using PROC LOGISTIC. Specifically, binary logistic regression models assessed overall and stratified associations of continuous midlife urinary phthalate levels with the odds of ever experiencing and experiencing hot flashes in the past 30 days compared to never experiencing hot flashes. Women who did experience hot flashes at some point, but not in the past 30 days (n=87), were excluded from binary logistic regression models comparing women who experienced hot flashes in the past 30 days to those who never experienced hot flashes. Multinomial logistic regression models assessed overall and stratified associations of continuous midlife urinary phthalate levels with the odds of experiencing daily/weekly or monthly hot flashes and of experiencing moderate/severe or mild hot flashes compared to never experiencing hot flashes. Because all phthalate individual metabolites and molar sums were right-skewed, phthalates were natural log-transformed in all logistic regression models. All odds ratios (ORs) and 95% confidence intervals (CIs) were back transformed using the equation [eln(OR)*ln(2.00)] and data in Table 4, Figures 1–4, and Supplementary Tables 1–4 are presented as the OR of experiencing these hot flash outcomes with every two-fold increase in phthalate metabolite or molar sum concentration with a priori alpha level of p < 0.05 (40). For logistic regression models evaluating stratified associations of phthalates with hot flashes, we provided formal test for effect modification in Supplementary Tables 1–4 (40), but reported on all relevant associations regardless of the interaction p-value.
Table 3.
Prevalence of hot flashes self-reported by women from the Midlife Women’s Health Study (n=728).
| Hot Flashes | n (%) |
|---|---|
| History of hot flashes | |
| No | 399 (54.8) |
| Yes | 329 (45.2) |
| Hot flashes during past 30 days | |
| Never had hot flashes | 399 (54.8) |
| Had hot flashes and experienced in past 30 days | 236 (32.4) |
| Had hot flashes but did not experience in past 30 days | 87 (12.0) |
| Missing | 6 (0.8) |
| Frequency of hot flashes | |
| Never had hot flashes | 399 (54.8) |
| Monthly hot flashes | 139 (19.1) |
| Daily/weekly hot flashes | 159 (21.8) |
| Missing | 31 (4.3) |
| Severity of hot flashes | |
| Never had hot flashes | 399 (54.8) |
| Mild hot flashes | 108 (14.8) |
| Moderate/severe hot flashes | 213 (29.3) |
| Missing | 8 (1.1) |
Table 4.
Overall associations of urinary phthalate concentrations with hot flashes.
| Outcomes | ||||||
|---|---|---|---|---|---|---|
| Ever experiencing hot flashes1 | Experiencing hot flashes in the past 30 days1 | Experiencing daily/weekly or monthly hot flashes2 | Experiencing moderate/severe or mild hot flashes2 | |||
| n | 728 | 635 | 697 | 720 | ||
| Reference | Never experiencing hot flashes | Never experiencing hot flashes | Never experiencing hot flashes | Never experiencing hot flashes | ||
| Exposure3 | Daily/weekly | Monthly | Moderate/severe | Mild | ||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| MEP | 1.09 (0.98, 1.22) | 1.05 (0.93, 1.18) | 1.1 (0.96, 1.27) | 1.1 (0.96, 1.25) | 1.06 (0.94, 1.2) | 1.12 (0.97, 1.3) |
| MBP | 1.01 (0.85, 1.21) | 1.1 (0.89, 1.35) | 1.17 (0.93, 1.47) | 0.92 (0.74, 1.16) | 1 (0.81, 1.23) | 0.98 (0.76, 1.25) |
| MiBP | 0.98 (0.82, 1.16) | 1.03 (0.85, 1.26) | 1.03 (0.82, 1.3) | 0.98 (0.79, 1.21) | 1 (0.82, 1.22) | 0.91 (0.72, 1.16) |
| mCPP | 0.92 (0.83, 1.03) | 0.94 (0.82, 1.06) | 1.02 (0.88, 1.18) | 0.87 (0.76, 1) | 0.92 (0.81, 1.05) | 0.92 (0.79, 1.07) |
| MBzP | 0.95 (0.82, 1.1) | 0.93 (0.79, 1.11) | 1.08 (0.89, 1.3) | 0.94 (0.79, 1.12) | 0.95 (0.81, 1.13) | 0.94 (0.77, 1.15) |
| MEHP | 1.05 (0.92, 1.19) | 1.07 (0.92, 1.24) | 1.18 (0.99, 1.4) | 0.99 (0.85, 1.17) | 1.05 (0.9, 1.22) | 1.05 (0.88, 1.25) |
| MEHHP | 1.14 (0.97, 1.33) | 1.19 (1, 1.43) | 1.34 (1.09, 1.65) | 1.09 (0.9, 1.32) | 1.17 (0.98, 1.39) | 1.1 (0.88, 1.36) |
| MEOHP | 1.07 (0.93, 1.23) | 1.11 (0.95, 1.3) | 1.23 (1.03, 1.48) | 1.04 (0.87, 1.23) | 1.07 (0.91, 1.25) | 1.08 (0.9, 1.31) |
| MECPP | 1.12 (0.97, 1.3) | 1.17 (0.99, 1.38) | 1.28 (1.06, 1.54) | 1.09 (0.92, 1.3) | 1.13 (0.96, 1.33) | 1.12 (0.93, 1.37) |
| ΣDEHP | 1.13 (0.96, 1.31) | 1.18 (0.99, 1.42) | 1.32 (1.08, 1.61) | 1.08 (0.89, 1.3) | 1.14 (0.96, 1.37) | 1.1 (0.89, 1.36) |
| ΣPhthalates | 1.13 (0.96, 1.32) | 1.12 (0.93, 1.35) | 1.26 (1.03, 1.54) | 1.09 (0.89, 1.33) | 1.07 (0.89, 1.29) | 1.19 (0.97, 1.47) |
| ΣPCP | 1.08 (0.94, 1.23) | 1.04 (0.89, 1.22) | 1.12 (0.94, 1.33) | 1.07 (0.91, 1.27) | 1.03 (0.88, 1.2) | 1.12 (0.93, 1.34) |
| ΣPlastic | 1.12 (0.95, 1.32) | 1.18 (0.98, 1.43) | 1.38 (1.11, 1.71) | 1.05 (0.85, 1.29) | 1.14 (0.95, 1.37) | 1.1 (0.88, 1.38) |
| ΣAA | 1.08 (0.9, 1.31) | 1.16 (0.93, 1.44) | 1.36 (1.07, 1.73) | 1 (0.79, 1.27) | 1.09 (0.88, 1.35) | 1.07 (0.83, 1.39) |
Binary logistic regression models evaluated associations of every 2-fold increase in urinary phthalate concentrations with the odds of ever experiencing and experiencing hot flashes in the last 30 days compared to never having hot flashes.
Multinomial logistic regression models evaluated associations of every 2-fold increase in urinary phthalate concentrations with the odds of experiencing daily/weekly or monthly hot flashes and experiencing moderate/severe hot flashes compared to never experiencing hot flashes. All logistic regression models were adjusted for age, race/ethnicity, education, alcohol consumption, smoking status, medication use, menopausal status, body mass index, and CESD score. CI, confidence interval; OR, odds ratio.
ΣDEHP = (MEHP/278) + (MEHHP/294) + (MEOHP/292) + (MECPP/308); ΣPhthalates = (MEP/194) + (MBP/222) + (MiBP/222) + (MEHP/278) + (MEHHP/294) + (MEOHP/292) + (MECPP/308) + (MBzP/256) + (MCPP/252); ΣPCP = (MEP/194) + (MBP/222) + (MiBP/222); ΣPlastic = (MEHHP/294) + (MEHP/278) + (MEOHP/292) + (MECPP/308) + (MCPP/252) + (MBzP/256); ΣAA = (MEHP/278) + (MEHHP/294) + (MEOHP/292) + (MECPP/308) + (MBzP/256) + (MBP/222) + (MiBP/222)
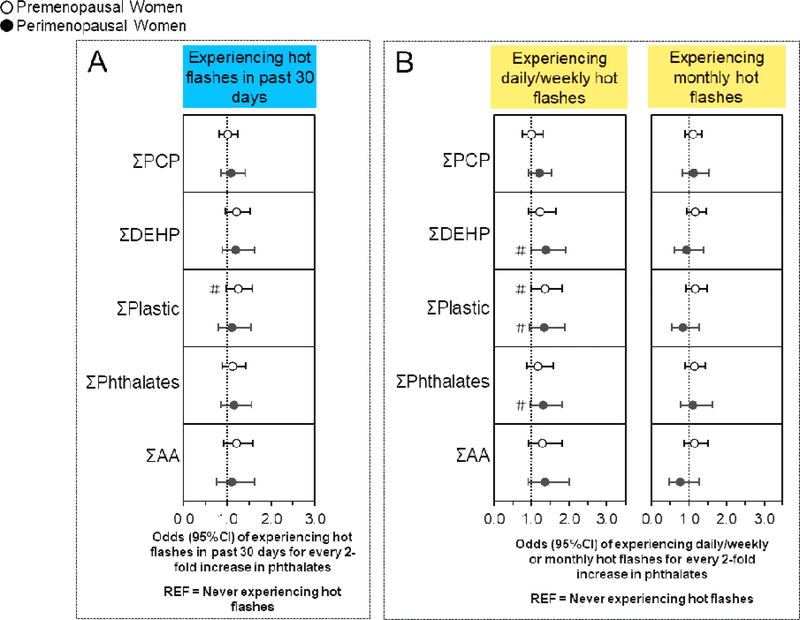
Figure 1. Associations of urinary phthalate concentrations with hot flashes stratified by menopause status.
Binary logistic regression models evaluated associations of urinary phthalate concentrations with the odds experiencing hot flashes in the last 30 days compared to never having hot flashes (n=635), while multinomial logistic regression models evaluated associations of urinary phthalate concentrations with the odds of experiencing daily/weekly or monthly hot flashes compared to never experiencing hot flashes (n=697). All models were stratified by menopause status and were adjusted for age, race/ethnicity, education, alcohol consumption, smoking status, medication use, body mass index, and CESD score. Data are presented as odds ratio (circles) and 95% confidence interval (solid lines) for every two-fold increase in urinary phthalate concentrations. Confidence intervals that do not cross the null are significant at #P<0.10 and *P<0.05.
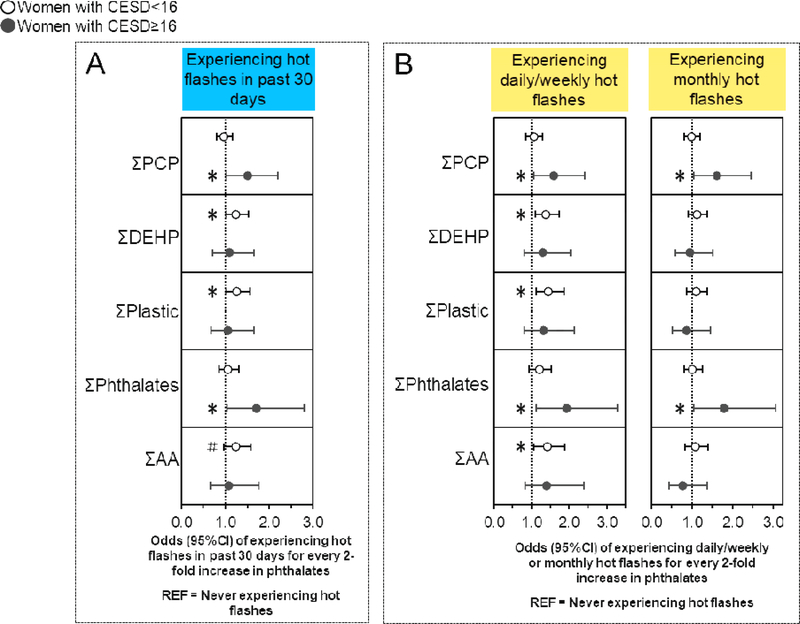
Figure 4. Associations of urinary phthalate concentrations with hot flashes stratified by CESD score.
Binary logistic regression models evaluated associations of urinary phthalate concentrations with the odds of experiencing hot flashes in the last 30 days compared to never having hot flashes (n=635), while multinomial logistic regression models evaluated associations of urinary phthalate concentrations with the odds of experiencing daily/weekly or monthly hot flashes compared to never experiencing hot flashes (n=697). All models were stratified by CESD score and were adjusted for age, race/ethnicity, education, alcohol consumption, smoking status, medication use, menopause status, and body mass index. Data are presented as odds ratio (circles) and 95% confidence interval (solid lines) for every two-fold increase in urinary phthalate concentrations. Confidence intervals that do not cross the null are significant at #P<0.10 and *P<0.05.
3. Results
3.1. Baseline MWHS population characteristics and hot flash prevalence
Baseline characteristics of 728 women in the MWHS are reported in Table 1. Overall, 64% of women were premenopausal, whereas 36% were perimenopausal. Most women were white (67%), whereas 33% were Black or of another race/ethnicity. Most women were employed (80%), college educated (65%), married or cohabiting (65%), and had an annual family income ≥$40,000 (75%). The prevalence of baseline healthy lifestyle characteristics in this study population were as follows: 35% did not regularly consume alcohol within the past year, 55% never smoked, 67% reported leisure time physical activity as more/much more than others, 43% were not taking any medications, and 80% did not meet criteria for depression based on the CESD. Over one-third of the women were under- or normal weight (40%) and over half were overweight or obese (60%).
The self-reported baseline prevalence of hot flashes in the MWHS is presented in Table 3. Out of 728 total women in the current study, all women provided information about ever experiencing hot flashes, 722 had information about experiencing hot flashes within the past 30 days, 697 had information about hot flash frequency, and 720 had information about hot flash severity. Approximately 55% of women never experienced hot flashes, whereas 45% had experienced hot flashes and 32% had experienced hot flashes in the past 30 days. Overall, 22% had daily/weekly hot flashes and 19% had monthly hot flashes, whereas 29% had moderate/severe hot flashes and 15% had mild hot flashes.
3.2. Baseline urinary phthalate metabolite concentrations
Table 2 presents median (25th, 75th percentiles) concentrations of individual urinary phthalate metabolites and phthalate molar sums during the first year of the MWHS. Concentrations of most urinary phthalate metabolites measured in the MWHS were ≥ LOD in 100% of women, except for MEP, for which 99.7% of women had concentrations ≥ LOD. Median urinary phthalate metabolite and molar sum concentrations in the MWHS are compared to those measured from a nationally representative sample of 45–54-year-old U.S. women from the 2005–2016 National Health and Nutrition Examination Survey (NHANES). Median metabolite levels are slightly higher in the MWHS than NHANES with overlapping 25–75th percentiles. Metabolite levels are similar to recently reported results from the Study of Environment, Lifestyle, and Fibroids (SELF) from non-Hispanic Black women (23–35 years old) (44). SELF also reports slightly higher DEHP metabolites than the corresponding NHANES samples (44).
3.3. Overall associations of phthalates with hot flashes
Midlife urinary phthalate metabolite concentrations were not associated with ever experiencing hot flashes or experiencing moderate/severe or mild hot flashes compared to never experiencing hot flashes (Table 4). However, phthalates were associated with experiencing hot flashes in the past 30 days. Specifically, women had 19% higher odds of the experiencing hot flashes in the past 30 days with every two-fold increase in MEHHP (OR: 1.19; 95%CI: 1.00, 1.43). Additionally, phthalates were associated with experiencing daily/weekly, but not monthly hot flashes. Specifically, women had 23–38% higher odds of experiencing daily/weekly hot flashes with every two-fold increase in the DEHP metabolites MEHHP (OR: 1.34, 95%CI: 1.09, 1.65), MEOHP (OR: 1.23, 95%CI: 1.03, 1.48), MECPP (OR: 1.28, 95%CI: 1.06, 1.54) and the summary measures ΣDEHP (OR: 1.32, 95%CI: 1.08, 1.61), ΣPlastic (OR: 1.38; 95%CI: 1.11, 1.71), ΣPhthalates (OR: 1.26; 95%CI: 1.03, 1.54), and ΣAA (OR: 1.36; 95%CI: 1.07, 1.73).
3.4. Associations of phthalates with hot flashes stratified by menopause status
Associations of urinary phthalate metabolite concentrations with ever experiencing hot flashes, experiencing hot flashes in the past 30 days, or experiencing daily/weekly hot flashes did not significantly differ by menopause status in any of the interaction terms (Figure 1A, B and Table S1) (40).
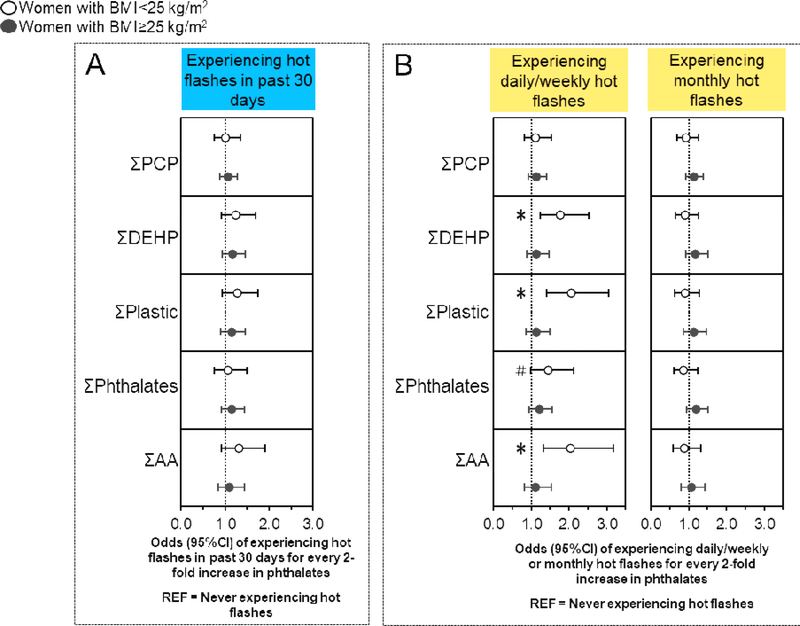
3.5. Associations of phthalates with hot flashes stratified by midlife BMI
Associations of urinary phthalate metabolite concentrations with ever experiencing hot flashes, experiencing hot flashes in the past 30 days, and experiencing moderate/severe or mild hot flashes were not different by midlife BMI. (Figure 2A, Table S2) (40). However, some associations of phthalates with experiencing daily/weekly (but not monthly) hot flashes were only observed in under-/normal weight women (Figure 2B, Table S2) (40), who had 57–107% higher odds of experiencing daily/weekly hot flashes with every two-fold increase in MBP (OR: 1.57; 95%CI: 1.04, 2.38), MBzP (OR: 1.63; 95%CI: 1.18, 2.25), ΣDEHP (OR: 1.77; 95%CI: 1.24, 2.52), ΣPlastic (OR: 2.07; 95% CI: 1.40, 3.05), and ΣAA (OR: 2.05; 95%CI: 1.31, 3.19).
Figure 2. Associations of urinary phthalate concentrations with hot flashes stratified by midlife BMI.
Binary logistic regression models evaluated associations of urinary phthalate concentrations with the odds of experiencing hot flashes in the last 30 days compared to never having hot flashes (n=635), while multinomial logistic regression models evaluated associations of urinary phthalate concentrations with the odds of experiencing daily/weekly or monthly hot flashes compared to never experiencing hot flashes (n=697). All models were stratified by midlife BMI and were adjusted for age, race/ethnicity, education, alcohol consumption, smoking status, medication use, menopause status, and CESD score. Data are presented as odds ratio (circles) and 95% confidence interval (solid lines) for every two-fold increase in urinary phthalate concentrations. Confidence intervals that do not cross the null are significant at #P<0.10 and *P<0.05. BMI, body mass index.
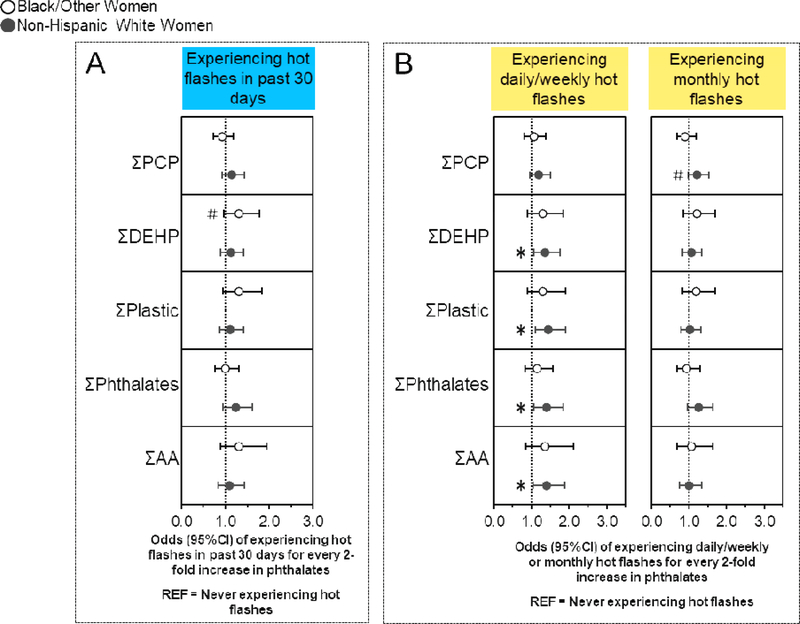
3.6. Associations of phthalates with hot flashes stratified by race/ethnicity
Some associations of urinary phthalate metabolite concentrations with ever experiencing hot flashes, experiencing hot flashes in the past 30 days, experiencing daily/weekly or monthly hot flashes, and experiencing mild (but not moderate/severe) hot flashes also differed by race/ethnicity (Figure 3A, B, Table S3) (40). Non-Hispanic white women had 19–27% higher odds of ever experiencing hot flashes with every two-fold increase in MEP (OR: 1.19; 95%CI: 1.03, 1.38) and ΣPhthalates (OR: 1.27; 95%CI: 1.01, 1.58) (Table S3) (40). Additionally, non-Hispanic white women had 37–45% higher odds of experiencing daily/weekly hot flashes with every two-fold increase in ΣDEHP (OR: 1.37; 95%CI: 1.06, 1.76), ΣPlastic (OR: 1.45; 95%CI: 1.11, 1.90), ΣPhthalates (OR: 1.40; 95%CI: 1.06, 1.85), and ΣAA (OR: 1.40; 95%CI: 1.04, 1.89), as well as higher odds of experiencing monthly hot flashes with every two-fold increase in MEP (OR: 1.23; 95%CI: 1.03, 1.47) (Figure 3B, Table S3) (40). Conversely, Black/other women had 20% (OR: 0.80; 95%CI: 0.66, 0.98) and 28% (OR = 0.72, 95% CI 0.55, 0.95) lower odds of ever experiencing or experiencing mild hot flashes, respectively, with every two-fold increase in MCPP, but had 51% higher odds of experiencing hot flashes in the past 30 days with every two-fold increase in MEHHP (OR: 1.51; 95%CI: 1.08, 2.10) (Table S3) (40).
Figure 3. Associations of urinary phthalate concentrations with hot flashes stratified by race/ethnicity.
Binary logistic regression models evaluated associations of urinary phthalate concentrations with the odds of experiencing hot flashes in the last 30 days compared to never having hot flashes (n=635), while multinomial logistic regression models evaluated associations of urinary phthalate concentrations with the odds of experiencing daily/weekly or monthly hot flashes and compared to never experiencing hot flashes (n=697). All models were stratified by race/ethnicity and were adjusted for age, education, alcohol consumption, smoking status, medication use, menopause status, body mass index, and CESD score. Data are presented as odds ratio (circles) and 95% confidence interval (solid lines) for every two-fold increase in urinary phthalate concentrations. Confidence intervals that do not cross the null are significant at #P<0.10 and *P<0.05.
3.7. Associations of phthalates with hot flashes stratified by CESD score
Some associations of urinary phthalate metabolite concentrations with ever experiencing hot flashes, experiencing hot flashes in the past 30 days, experiencing daily/weekly or monthly hot flashes, and experiencing mild (but not moderate/severe) hot flashes differed by CESD score (Figure 4A, B, Table S4) (40). For example, women with fewer depressive symptoms (CESD<16) had 24% higher odds of experiencing hot flashes in the past 30 days with every two-fold increase in ΣDEHP (OR: 1.24; 95%CI: 1.02, 1.49) (Table S4) (40), and had 38–45% higher odds of experiencing daily/weekly hot flashes with every two-fold increase in ΣDEHP (OR: 1.38; 95%CI: 1.09, 1.73), ΣPlastic (OR: 1.45; 95%CI: 1.14, 1.86), and ΣAA (OR: 1.42; 95%CI 1.07, 1.88) (Figure 4B, Table S4) (40). Conversely, women with more depressive symptoms (CESD≥16) had 42–71% higher odds of experiencing hot flashes in the past 30 days with every two-fold increase in MEP (OR: 1.42; 95%CI: 1.02, 1.97), ΣPCP (OR: 1.50; 95%CI: 1.03, 2.19), and ΣPhthalates (OR: 1.71; 95%CI: 1.04, 2.79) (Figure 4A, Table S4) (40). Additionally, these women had 47–93% and 60–78% higher odds of experiencing daily/weekly or monthly hot flashes, respectively, for every two-fold increase in MEP (daily/weekly OR: 1.47; 95%CI: 1.03, 2.11; monthly OR: 1.60; 95%CI: 1.10, 2.33), ΣPCP (daily/weekly OR: 1.59; 95%CI: 1.06, 2.41; monthly OR: 1.60; 95%CI: 1.05, 2.46), and ΣPhthalates (daily/weekly OR: 1.93; 95%CI: 1.13, 3.28; monthly OR: 1.78; 95%CI: 1.04, 3.06), but also had 43% lower odds of experiencing monthly hot flashes with every two-fold increase in MBzP (OR: 0.57; 95%CI: 0.35, 0.94) (Figure 4B, Table S4) (40).
4. Discussion
In a cross-sectional analysis of year 1 data from the MWHS, a prospective cohort of pre- and perimenopausal women from Baltimore and its surrounding counties, urinary phthalate metabolite concentrations were associated with experiencing recent and experiencing more frequent hot flashes, but not associated with ever experiencing hot flashes or hot flash severity. Generally, we found that phthalate metabolites of parent compounds found in plastics were associated with higher risk of experiencing hot flashes in the past 30 days and experiencing daily/weekly hot flashes. Some associations of phthalates with certain hot flash outcomes were different by menopause status, midlife BMI, race/ethnicity, and depressive symptoms. Most notably, we found that associations of personal care product phthalates with most hot flash outcomes were strongest in women with more depressive symptoms. Interestingly, MCPP (and to a lesser extent MBzP) was associated with lower risk of hot flashes, especially in perimenopausal and Black/other women.
Overall, these results are consistent with our previous findings that phthalate metabolites are associated with increased frequency of hot flashes in midlife women (5). In our pilot study of a representative sample of 195 white women from the MWHS cohort, ΣPCP was associated with higher odds of ever experiencing hot flashes, experiencing hot flashes in the past 30 days, and experiencing daily/weekly hot flashes (5). In the current study, we found that associations of ΣPCP with risk of hot flashes only emerged when associations were stratified by CESD score. In the pilot study, ΣAA and ΣDEHP were not associated with hot flash frequency. However, in the current study, we found that ΣDEHP and ΣPlastic were consistently associated with experiencing hot flashes in the past 30 days and hot flash frequency, whereas ΣAA was only associated with hot flash frequency. The pilot study did not evaluate associations of individual phthalate metabolites, ΣPhthalates, or ΣPlastic with hot flash experience.
To our knowledge, no other observational studies have reported on the associations between phthalates and hot flashes in midlife women. However, phthalates are associated with disorders of the female reproductive system, including premature reproductive senescence and altered hormone levels, and these disorders may contribute to the risk of hot flashes (4,23,28,45,46). Studies in mice have shown premature reproductive senescence following adult exposure to DEHP and diisononyl phthalate (DiNP) (47,48). Observational studies have identified associations between urinary phthalate metabolite levels and earlier onset of menopause (49) and premature ovarian failure (50,51). Women who undergo earlier menopause report more frequent and severe hot flashes than premenopausal women (46,52). In addition, higher urinary DEHP metabolite levels were associated with decreased levels of the hormones inhibin B and anti-Müllerian hormone, markers of ovarian reserve in women (53).
Hot flashes and declining estradiol levels occur simultaneously during the menopause transition and other physiological transitions such as the postpartum period (4). Clinical trials have shown that estrogen therapy can alleviate hot flashes, suggesting a causal association, although the mechanism remains unknown (54–56). Numerous observational studies have also identified associations of altered estradiol and progesterone levels with risk of hot flashes (4,23,28,45,57). Using the MWHS cohort, the close relationship between hot flashes and estradiol was recently modeled using a Bayesian network (54). Animal studies have demonstrated altered estradiol and progesterone levels following phthalate exposure (11,18,19,21,58), and future studies from the MWHS will investigate the association between urinary phthalate metabolites and hormone levels in midlife women. Other potential mechanisms through which phthalates could be linked to hot flashes include direct disruption of hypothalamic or thyroid function (4,59,60). However, as the etiology of hot flashes is not well understood, it is difficult to speculate on causal links between phthalates and hot flashes.
When we evaluated stratified associations of phthalates with these hot flashes outcomes by menopause status, BMI, and race/ethnicity, the strongest associations were observed in perimenopausal women, non-Hispanic white women, and under-/normal weight women. These sub-group associations were most consistent between DEHP metabolites and experiencing daily/weekly hot flashes. Black women generally have higher phthalate exposure than white women (61,62), which may be due to the phthalate content in personal care products used by Black women (63). In the MWHS, Black women had higher urinary PCP phthalate concentrations than non-Hispanic white women (data not shown). However, we did not identify any consistent associations of ΣPCP with risk of hot flashes in Black/other women despite this being a high-risk exposure group, which may indicate that phthalates in personal care products do not contribute to hot flash experience in non-white women. Previously, we have identified high BMI as a risk factor for experiencing perimenopausal hot flashes (45). However, we found stronger associations between phthalates and risk of hot flashes in women with lower BMI. Interestingly, MBzP was associated with increased risk of hot flashes in normal weight women and trending towards decreased risk of ever experiencing hot flashes in overweight women. These differences suggest that women with higher body weight may be less susceptible to endocrine disruption by phthalates. One possible explanation is that estrogen levels are already decreased in perimenopausal women with high BMI (45).
Previous studies have identified bidirectional associations of depression with risk of hot flashes, and suggest that they may be linked through sleep disruption (64). In addition, phthalates may be associated with depression in adults. MECPP, MBP, MiBP, and MBzP were associated with depression in adults from NHANES (65). In elderly populations (ages 59–93), DEHP metabolites, MCPP, and MBP have also been associated with depression (66,67). When associations of phthalates with risk of hot flashes were stratified by CESD scores in the MWHS, we observed different associations of phthalate metabolites, with risk of hot flashes in women experiencing fewer and more depressive symptoms. DEHP metabolites and phthalates found in plastics were consistently associated with hot flashes in women experiencing fewer depressive symptoms, whereas MEP and phthalates associated with personal care products were associated with hot flashes in women with more depressive symptoms. The different observations in women in more vs. fewer depressive symptoms suggests that depression symptoms may related to hormonal changes or that the physiology of depression may play a role, possibly hormonal, in phthalate mechanism of action. Additional studies are needed to investigate the role of depression in environmental chemical action. In addition, the interaction of each depression group with a different phthalate category may be evidence that the high molecular weight phthalates found in plastics act through different mechanisms than the low molecular weight phthalates found in personal care products.
Across multiple hot flashes measures, MCPP and to a lesser extent MBzP were associated with decreased risk of experiencing hot flashes. MCPP is a downstream oxidized metabolite and may be produced from multiple phthalates including MBP, BzBP, and phthalates with long n-hydrocarbon side chains (68). One hypothesis for the negative association between MCPP and hot flashes is that the presence of highly oxidized metabolites is a marker of the overall efficacy of metabolism and detoxification. Greater capacity to metabolize and excrete phthalates (and other environmental chemicals) could reduce their effects on multiple sensitive endpoints. MBzP, containing a benzyl group on its side chain, has a unique structure for a phthalate that is more similar to steroid hormones. As a result, MBzP may act through different mechanisms than other phthalate metabolites.
This study has several strengths. Of note, four urine samples taken over consecutive weeks at similar times of day were pooled for phthalate measurement. Within-subject pooling has been shown to decrease exposure misclassification for phthalates and improves the credibility of estimated exposure based on urine concentrations (69). Other strengths of this study include the large size of the population and the detailed information collected from each participant on hot flash experience using validated questionnaires that are accepted by the National Institute of Health to assess hot flashes (70).
This study also has several limitations. The MWHS cohort is composed of primarily white (67%) and Black (30%) midlife women. Few women of other races/ethnicities were enrolled in the study; therefore, the results of this study are most applicable to non-Hispanic white and Black women. Due to the cross-sectional analyses performed here, some outcomes may have occurred before phthalate exposure, obscuring temporality. The measure of “ever experiencing hot flashes” is the most imprecise and mostly likely to differ in temporal ordering with respect to phthalate exposure. Therefore, prospective studies are needed to confirm whether phthalates influence risk of experiencing hot flashes. We were unable to adjust for co-pollutants or diet, although we adjusted for a number of relevant and important confounder, including age, race, education, current drinking status, smoking status, medication use, menopause status, BMI, and CESD score. However, other environmental chemical exposures may be correlated with phthalates and hot flashes. Diet quality may be important given that we observed strong associations between plasticizing phthalates and hot flashes risk and exposure to plasticizing phthalates occurs primarily through diet. In addition, we did not evaluate non-linear associations.
5. Conclusions
In midlife women from the MWHS, some urinary phthalate metabolites were associated with higher risk of recently experiencing and experiencing frequent hot flashes, but not of ever experiencing or experiencing severe hot flashes. We observed that urinary phthalate metabolites of plasticizer parent compounds are associated with higher odds of experiencing hot flashes in the past 30 days and experiencing daily or weekly hot flashes. Additionally, we found that some associations of urinary phthalate metabolites with hot flashes were different by menopause status, midlife BMI, race/ethnicity, and CESD score. Although this is one of the first studies to assess the relationship between phthalate exposure and risk of hot flashes, these results are consistent with previous studies showing that phthalates can interfere with normal female reproductive function (37). Our results suggest specific relationships between phthalates from common exposure sources and the evaluated hot flash outcomes. Future studies should investigate the mechanisms through which phthalates may be acting to facilitate the development of interventions to alleviate hot flashes in midlife women.
Supplementary Material
Highlights.
The Midlife Women’s Health Study is a cohort of pre- and perimenopausal women
Phthalate exposure is associated with hot flash experience in midlife women
Phthalates found in plastics are associated with recent hot flash experience
Associations differed by race/ethnicity, menopause status, body mass, and depressive symptoms
Acknowledgments
The authors thank the MWHS participants for their time, information, and biological samples and the staff members at Johns Hopkins University and the University of Illinois at Urbana-Champaign who helped with this study. Thank you to Yonatan (Yoni) Segev and Tyler Beers for their help with aliquoting samples.
Funding: This work was supported by the National Institute of Environmental Health Sciences (R01 ES026956 and T32 007326).
Abbreviations:
- CI
confidence interval
- OR
odds ratio
- CESD
Center for Epidemiological Studies Depression Score
- PCP
personal care products
- BMI
body mass index
- MWHS
Midlife Women’s Health Study
- NHANES
National Health and Nutrition Examination Survey
- DEP
diethyl phthalate
- DBP
dibutyl phthalate
- DiBP
diisobutyl phthalate
- BzBP
benzylbutyl phthalate, DEHP, di(2-ethylhexyl)phthalate
- DOP
dioctyl phthalate
- MECPP
mono-(2-ethyl-5-carboxypentyl) phthalate
- MBP
monobutyl phthalate
- MEP
monoethyl phthalate
- MEOHP
mono-(2-ethyl-5-oxohexyl)phthalate
- MBzP
monobenzyl phthalate
- MiBP
monoisobutyl phthalate
- MCPP
mono-(3-carboxypropyl) phthalate
- MEHHP
mono-(2-ethyl-5-hydroxyhexyl) phthalate
- MEHP
mono-2-ethylhexyl phthalate
- LOQ
limit of quantitation
- AA
anti-androgenic
- DiNP
diisononyl phthalate
Footnotes
Disclosure: The authors have no conflicts of interest to declare.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freedman RR. Physiology of hot flashes. Am. J. Hum. Biol. 2001;13(4):453–464. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg F Hot Flashes: Epidemiology and Physiologya. Ann. N. Y. Acad. Sci. 1990;592(1):52–86. [DOI] [PubMed] [Google Scholar]
- 3.Sarrel P, Portman D, Lefebvre P, Lafeuille MH, Grittner AM, Fortier J, Gravel J, Duh MS, Aupperle PM. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause 2015;22(3):260–266. [DOI] [PubMed] [Google Scholar]
- 4.Ziv-Gal A, Flaws JA. Factors that may influence the experience of hot flushes by healthy middle-aged women. J. Women’s Heal. 2010;19(10):1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziv-Gal A, Gallicchio L, Chiang C, Ther SN, Miller SR, Zacur HA, Dills RL, Flaws JA. Phthalate metabolite levels and menopausal hot flashes in midlife women. Reprod. Toxicol. 2016;60:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007;210(5):623–634. [DOI] [PubMed] [Google Scholar]
- 7.Center for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. Atlanta, GA; 2018. Available at: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Mar2018.pdf. Accessed September 4, 2018. [Google Scholar]
- 8.Biesterbos JWH, Dudzina T, Delmaar CJE, Bakker MI, Russel FGM, Von Götz N, Scheepers PTJ, Roeleveld N. Usage patterns of personal care products: Important factors for exposure assessment. Food Chem. Toxicol. 2013;55:8–17. [DOI] [PubMed] [Google Scholar]
- 9.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 2004;112(3):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis BJ, Weaver R, Gaines LJ, Heindel JJ. Mono-(2-ethylhexyl) Phthalate Suppresses Estradiol Production Independent of FSH-cAMP Stimulation in Rat Granulosa Cells. Toxicol Appl Pharmacol 1994;128:224–228. [DOI] [PubMed] [Google Scholar]
- 11.Hannon PR, Brannick KE, Wang W, Flaws JA. Mono(2-Ethylhexyl) Phthalate Accelerates Early Folliculogenesis and Inhibits Steroidogenesis in Cultured Mouse Whole Ovaries and Antral Follicles. Biol. Reprod. 2015;92(5):120–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lake BG, Gray TJ b., Lewis DF v., Beamand JA, Hodder KD, Purchase R, Gangolli SD. Structure-activity relationships for induction of peroxisomal enzyme activities by phthalate monoesters in primary rat hepatocyte cultures. Toxicol. Ind. Health 1987;3(2):165–183. [DOI] [PubMed] [Google Scholar]
- 13.Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 2003;111(2):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol. Appl. Pharmacol. 2001;172(3):217–224. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Craig ZR, Basavarajappa MS, Hafner KS, Flaws JA. Mono-(2-Ethylhexyl) Phthalate Induces Oxidative Stress and Inhibits Growth of Mouse Ovarian Antral Follicles1. Biol. Reprod. 2012;87(6):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y-X, Zeng Q, Sun Y, You L, Wang P, Li M, Yang P, Li J, Huang Z, Wang C, Li S, Dan Y, Li Y-F, Lu W-Q. Phthalate exposure in association with serum hormone levels, sperm DNA damage and spermatozoa apoptosis: A cross-sectional study in China. Environ. Res. 2016;150:557–565. [DOI] [PubMed] [Google Scholar]
- 17.Meeker JD, Missmer SA, Altshul L, Vitonis AF, Ryan L, Cramer DW, Hauser R. Serum and follicular fluid organochlorine concentrations among women undergoing assisted reproduction technologies. Environ. Health 2009;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front. Endocrinol. (Lausanne). 2015;6(FEB):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou C, Flaws JA. Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicol. Sci. 2017;156(1):217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meling DD, Warner GR, Szumski JR, Gao L, Gonsioroski A V., Rattan S, Flaws JA. The effects of a phthalate metabolite mixture on antral follicle growth and sex steroid synthesis in mice. Toxicol. Appl. Pharmacol. 2020;388(December 2019):114875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannon PR, Brannick KE, Wang W, Gupta RK, Flaws JA. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 2015;284(1):42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sturdee DW. The menopausal hot flush-Anything new? Maturitas 2008;60(1):42–49. [DOI] [PubMed] [Google Scholar]
- 23.Gallicchio L, Miller SR, Kiefer J, Greene T, Zacur HA, Flaws JA. Risk factors for hot flashes among women undergoing the menopausal transition: Baseline results from the Midlife Women’s Health Study. Menopause 2015;22(10):1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziv-Gal A, Smith RL, Gallicchio L, Miller SR, Zacur HA, Flaws JA. The Midlife Women’s Health Study – a study protocol of a longitudinal prospective study on predictors of menopausal hot flashes. Women’s Midlife Heal. 2017;3(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, De Villiers TJ. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging Methods-Scientists from five countries and multiple disciplines evaluated data from cohort studies of midlife women and in. Menopause 2012;19(4):387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977;1(3):385–401. [Google Scholar]
- 27.Flaws JA, Cochran CJ, Gallicchio L, Miller SR, Zacur H. Cigarette smoking, androgen levels, and hot flushes in midlife women. Obstet. Gynecol. 2008;112(5):1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallicchio L, Miller SR, Visvanathan K, Lewis LM, Babus J, Zacur H, Flaws JA. Cigarette smoking, estrogen levels, and hot flashes in midlife women. Maturitas 2006;53(2):133–143. [DOI] [PubMed] [Google Scholar]
- 29.Visvanathan K, Gallicchio L, Schilling C, Babus JK, Lewis LM, Miller SR, Zacur H, Flaws JA. Cytochrome gene polymorphisms, serum estrogens, and hot flushes in midlife women. Obstet. Gynecol. 2005;106(6):1372–1381. [DOI] [PubMed] [Google Scholar]
- 30.Ziv-Gal A, Gallicchio L, Miller SR, Zacur HA, Flaws JA. Genetic polymorphisms in the aryl hydrocarbon receptor signaling pathway as potential risk factors of menopausal hot flashes. Am. J. Obstet. Gynecol. 2012;207(3):202.e9–202.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Högberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Håkansson H. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ. Health Perspect. 2008;116(3):334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staples CA, Peterson DR, Parkerton TF, Adams WJ. The environmental fate of phthalate esters: A literature review. Chemosphere 1997;35(4):667–749. [Google Scholar]
- 33.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008;116(8):1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radke EG, Braun JM, Meeker JD, Cooper GS. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ. Int. 2018;121:764–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson KK, Rosen EM, Rosario Z, Feric Z, Calafat AM, McElrath TF, Vélez Vega C, Cordero JF, Alshawabkeh A, Meeker JD. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ. Int. 2019;132(July):105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radke EG, Glenn BS, Braun JM, Cooper GS. Phthalate exposure and female reproductive and developmental outcomes: a systematic review of the human epidemiological evidence. Environ. Int. 2019;130(December 2018):104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environ. Int. 2015;85:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Center for Disease Control and Prevention. Metabolites of phthalates and phthalate alternatives: Urine; 2013. Available at: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/PHTHTE_G_met.pdf.
- 40.Warner GR, Pacyga D, Strakovsky R, Smith R, James-Todd T, Williams P, Hauser R, Meling D, Li L, Flaws J. Data from: Phthalates and Hot Flashes. Illinois Databank 2020. Deposited 6 June 2020. 10.13012/B2IDB-9238850_V1. [DOI] [Google Scholar]
- 41.Meeker, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Téllez-Rojo MM. Urinary phthalate metabolites in relation to preterm birth in Mexico City. Environ. Health Perspect. 2009;117(10):1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ. Health Perspect. 2006;114(6):805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marie C, Vendittelli F, Sauvant-Rochat MP. Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environ. Int. 2015;83:116–136. [DOI] [PubMed] [Google Scholar]
- 44.Wesselink AK, Fruh V, Hauser R, Weuve J, Taylor KW, Orta OR, Claus Henn B, Bethea TN, McClean MD, Williams PL, Calafat AM, Baird DD, Wise LA. Correlates of urinary concentrations of phthalate and phthalate alternative metabolites among reproductive-aged Black women from Detroit, Michigan. J. Expo. Sci. Environ. Epidemiol. 2020. doi: 10.1038/s41370-020-00270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallicchio L, Visvanathan K, Miller SR, Babus J, Lewis LM, Zacur H, Flaws JA. Body mass, estrogen levels, and hot flashes in midlife women. Am. J. Obstet. Gynecol. 2005;193(4):1353–1360. [DOI] [PubMed] [Google Scholar]
- 46.Edwards BJ, Li J. Endocrinology of menopause. Periodontol. 2000 2013;61(1):177–194. [DOI] [PubMed] [Google Scholar]
- 47.Chiang C, Lewis LR, Borkowski G, Flaws JA. Exposure to di(2-ethylhexyl) phthalate and diisononyl phthalate during adulthood disrupts hormones and ovarian folliculogenesis throughout the prime reproductive life of the mouse. Toxicol. Appl. Pharmacol. 2020;393(March):114952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hannon PR, Niermann S, Flaws JA. Acute Exposure to Di(2-Ethylhexyl) Phthalate in Adulthood Causes Adverse Reproductive Outcomes Later in Life and Accelerates Reproductive Aging in Female Mice. Toxicol. Sci. 2016;150(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grindler NM, Allsworth JE, Macones GA, Kannan K, Roehl KA, Cooper AR. Persistent organic pollutants and early menopause in U.S. women. PLoS One 2015;10(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao M, Pan W, Shen X, Li C, Zhou J, Liu J. Urinary levels of phthalate metabolites in women associated with risk of premature ovarian failure and reproductive hormones. Chemosphere 2020;242:125206. [DOI] [PubMed] [Google Scholar]
- 51.Özel Ş, Tokmak A, Aykut O, Aktulay A, Hançerlioğulları N, Engin Ustun Y. Serum levels of phthalates and bisphenol-A in patients with primary ovarian insufficiency. Gynecol. Endocrinol. 2019;35(4):364–367. [DOI] [PubMed] [Google Scholar]
- 52.Gibson-Helm M, Teede H, Vincent A. Symptoms, health behavior and understanding of menopause therapy in women with premature menopause. Climacteric 2014;17(6):666–673. [DOI] [PubMed] [Google Scholar]
- 53.Du YY, Guo N, Wang YX, Hua X, Deng TR, Teng XM, Yao YC, Li YF. Urinary phthalate metabolites in relation to serum anti-Müllerian hormone and inhibin B levels among women from a fertility center: A retrospective analysis. Reprod. Health 2018;15(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith RL, Gallicchio LM, Flaws JA. Understanding the complex relationships underlying hot flashes. Menopause 2018;25(2):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bansal R, Aggarwal N. Menopausal Hot Flashes: A Concise Review. J. Midlife. Health 2019;10(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson HD. Commonly Used Types of Postmenopausal Estrogen for Treatment of Hot Flashes: Scientific Review. J. Am. Med. Assoc. 2004;291(13):1610–1620. [DOI] [PubMed] [Google Scholar]
- 57.Smith RL, Gallicchio L, Miller SR, Zacur HA, Flaws JA. Risk Factors for Extended Duration and Timing of Peak Severity of Hot Flashes. PLoS One 2016;11(5):e0155079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HHC. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol. Appl. Pharmacol. 2010;242(2):224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol a concentrations and serum thyroid measures in u.s. adults and adolescents from the national health and nutrition examination survey (NHANES) 2007–2008. Environ. Health Perspect. 2011;119(10):1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim MJ, Moon S, Oh B-C, Jung D, Choi K, Park YJ. Association Between Diethylhexyl Phthalate Exposure and Thyroid Function: A Meta-Analysis. Thyroid 2019;29(2):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varshavsky JR, Zota AR, Woodruff TJ. A Novel Method for Calculating Potency-Weighted Cumulative Phthalates Exposure with Implications for Identifying Racial/Ethnic Disparities among U.S. Reproductive-Aged Women in NHANES 2001–2012. Environ. Sci. Technol. 2016;50(19):10616–10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.James-Todd TM, Chiu Y-H, Zota AR. Racial/Ethnic Disparities in Environmental Endocrine Disrupting Chemicals and Women’s Reproductive Health Outcomes: Epidemiological Examples Across the Life Course. Curr. Epidemiol. Reports 2016;3(2):161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helm JS, Nishioka M, Brody JG, Rudel RA, Dodson RE. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ. Res. 2018;165(November 2017):448–458. [DOI] [PubMed] [Google Scholar]
- 64.Natari RB, Clavarino AM, McGuire TM, Dingle KD, Hollingworth SA. The bidirectional relationship between vasomotor symptoms and depression across the menopausal transition. Menopause 2018;25(1):109–120. [DOI] [PubMed] [Google Scholar]
- 65.Shiue I Urinary heavy metals, phthalates and polyaromatic hydrocarbons independent of health events are associated with adult depression: USA NHANES, 2011–2012. Environ. Sci. Pollut. Res. 2015;22(21):17095–17103. [DOI] [PubMed] [Google Scholar]
- 66.Kim KN, Choi YH, Lim YH, Hong YC. Urinary phthalate metabolites and depression in an elderly population: National Health and Nutrition Examination Survey 2005–2012. Environ. Res. 2016;145:61–67. [DOI] [PubMed] [Google Scholar]
- 67.Lee KS, Lim YH, Kim KN, Choi YH, Hong YC, Lee N. Urinary phthalate metabolites concentrations and symptoms of depression in an elderly population. Sci. Total Environ. 2018;625:1191–1197. [DOI] [PubMed] [Google Scholar]
- 68.Silva MJ, Samandar E, Reidy JA, Hauser R, Needham LL, Calafat AM. Metabolite profiles of Di-n-butyl phthalate in humans and rats. Environ. Sci. Technol. 2007;41(21):7576–7580. [DOI] [PubMed] [Google Scholar]
- 69.Shin HM, Bennett DH, Barkoski J, Ye X, Calafat AM, Tancredi D, Hertz-Picciotto I. Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ. Int. 2019;122(July 2018):222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller HG, Li RM. Measuring hot flashes: Summary of a National Institutes of Health Workshop. In: Mayo Clinic Proceedings. Vol 79.; 2004:777–781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.