Abstract
Maternal systemic inflammation increases risk for neurodevelopmental disorders like autism, ADHD, and schizophrenia in offspring. Notably, these disorders are male-biased. Studies have implicated immune system dysfunction in the etiology of these disorders, and rodent models of maternal immune activation provide useful tools to examine mechanisms of sex-dependent effects on brain development, immunity, and behavior. Here, we employed an allergen-induced model of maternal inflammation in rats to characterize levels of mast cells and microglia in the perinatal period in male and female offspring, as well as social, emotional, and cognitive behaviors throughout the lifespan. Adult female rats were sensitized to ovalbumin (OVA), bred, and challenged intranasally on gestational day 15 of pregnancy with OVA or saline. Allergic inflammation upregulated microglia in the fetal brain, increased mast cell number in the hippocampus on the day of birth, and conferred region-, time- and sex- specific changes in microglia measures. Additionally, offspring of OVA-exposed mothers subsequently exhibited abnormal social behavior, hyperlocomotion, and reduced cognitive flexibility. These data demonstrate the long-term effects of maternal allergic challenge on offspring development and provide a basis for understanding neurodevelopmental disorders linked to maternal systemic inflammation in humans.
Keywords: microglia, mast cells, social play, behavior, immunology, maternal immune activation
1. INTRODUCTION
Maternal systemic inflammation increases offspring risk for neurodevelopmental and psychiatric disorders like autism spectrum disorder (ASD), schizophrenia, attention-deficit hyperactivity disorder (ADHD), and bipolar disorder (A. S. Brown, 2012; Kloiber et al., 2020; Meyer et al., 2011; Patterson, 2009, 2011). Human tissue from autistic and schizophrenic individuals as well as animal models of neurodevelopmental disorders show increased innate immune cell number, activation and downstream inflammatory signaling in the brain (Borrell et al., 2002; Giovanoli et al., 2013; Graciarena et al., 2010; Juckel et al., 2011; Monji et al., 2013; Radewicz et al., 2000; Ratnayake et al., 2013; Suzuki et al., 2013; Vargas et al., 2005; Werling et al., 2016). Maternal allergy, atopic disease and autoimmune conditions are also associated with neurodevelopmental disorders in offspring (Chen et al., 2016; Croen et al., 2005; Instanes et al., 2017; Lyall et al., 2014), and increased levels of maternal C-reactive protein are associated with psychosis in offspring (Canetta et al., 2014). Additionally, children with ASD have dysregulated levels of circulating cytokines (Ashwood et al., 2011), providing further evidence that immune function is implicated in neurodevelopmental disorders.
Many studies have implicated microglia, brain-resident macrophages, as critical players in the regulation of normal neurodevelopment (Frost & Schafer, 2016; Schwarz & Bilbo, 2012). Microglia migrate from the yolk sack and colonize the fetal brain on about E9.5, and they exhibit a more inflammatory phenotype and produce elevated levels of immune molecules during early development (Nelson et al., 2019; Schwarz et al., 2012). Microglia are involved in regulation of cell death and survival, cell proliferation, and synaptic patterning, and they conduct these activities in the developing brain (Deverman & Patterson, 2009; Frost & Schafer, 2016; Nelson et al., 2019; Schwarz et al., 2012). Previous work from our lab and others has shown that microglial depletion in the neonatal period confers persistent behavioral changes throughout life, indicating microglia program behavioral development (Nelson & Lenz, 2017; VanRyzin et al., 2016; Zhan et al., 2014). Thus, early life perturbations to microglial activity have long term consequences for development. Many models of maternal immune activation (MIA) have been used to investigate the effects on microglia or neuroimmune signaling on programming neurobehavioral outcomes in juvenile (Bilbo et al., 2018, 2018; Garay et al., 2013; Juckel et al., 2011; Manitz et al., 2013) and adult offspring (Bolton et al., 2012, 2014; Garay et al., 2013; Makinson et al., 2017; Manitz et al., 2013; Missault et al., 2014; Van den Eynde et al., 2014). Across MIA studies, findings are inconsistent with regard to whether microglia are affected by the inflammatory event, and results of MIA experiments on offspring microglia vary across rodent species, paradigms, and brain regions examined (Smolders et al., 2018).This may be because maternal immune activation shifts the microglial phenotype earlier in development. A recent study found that microglia proceed through three developmental stages, and that prenatal poly(I:C) shifts the transcriptional profile of offspring pre-microglia but not adult microglia (Matcovitch-Natan et al., 2016). All in all, few other studies have actually characterized how MIA affects microglia in the perinatal period, a time during which altered microglial function could have long-term consequences on the neuroimmune milieu.
The MIA field has largely neglected to study another innate immune cell present in the developing brain, the mast cell. Mast cells are hematopoietic immune cells that regulate blood brain barrier permeability, peripheral allergy, anaphylaxis, and atopy (Dong et al., 2014; Silver & Curley, 2013; Wernersson & Pejler, 2014). Mast cells rapidly respond to inflammatory stimuli and communicate with nearby cells via a process called degranulation, whereby they release histamine, serotonin, cytokines, proteases, and growth factors into the extracellular space. Following degranulation, mast cells can also undergo de novo synthesis of inflammatory mediators (Silver & Curley, 2013; Wernersson & Pejler, 2014). In the brain, mast cells and microglia reciprocally regulate each other’s activity (Dong et al., 2014; Zhang et al., 2016). We have previously shown that microglia and mast cells work together to facilitate sex-specific patterning of the preoptic area (Lenz et al., 2018), a brain region necessary for the display of male-typical sex behavior. Importantly, central mast cell activity has been shown to regulate anxiety and depressive-like behaviors in rodents (Nautiyal et al., 2008) as well as courting and reproductive behaviors in birds (Zhuang et al., 1993). Mast cell numbers peak in the diencephalon and surrounding pia within the first two weeks of life (Khalil et al., 2007; Lambracht-Hall et al., 1990; Lenz et al., 2018), and the neonatal hippocampal area hosts a large population of mast cells (Joshi et al., 2019). Thus, mast cell perturbations in the perinatal period may be particularly relevant for programming behavior and neurodevelopmental disorders. However, despite these data and the many signs that suggest mast cells are involved in human autism spectrum disorders (Theoharides et al., 2016), the mast cell has not been well-studied in the context of neurodevelopment and animal models of MIA.
Many neurodevelopmental disorders associated with neuroimmune dysregulation and maternal immune activation also exhibit sex differences (Estes & McAllister, 2015; Hanamsagar & Bilbo, 2016; McCarthy et al., 2017). For example, males are more likely to be diagnosed with ASD and ADHD (Baio et al., 2018; Catalá-López et al., 2012; Kim et al., 2011; Willcutt, 2012), and they are more likely to have persistent and severe negative symptoms of schizophrenia (Novick et al., 2012; Thorup et al., 2007, 2014). Notably, both microglia and mast cells are sensitive to sex steroid hormones (Lenz et al., 2013, 2018; Sierra et al., 2008), and there are sex differences in microglial colonization and brain mast cell numbers during early life (Joshi et al., 2019; Lenz et al., 2018; Schwarz et al., 2012). This suggests that investigating neuroimmune sex differences in the context of maternal inflammation may be critical for a better understanding of neurodevelopmental disorders.
Animal models of MIA enable us to study mechanisms underlying the increased risk for neurodevelopmental disorders in offspring. Many studies have focused on viral or bacterial-like models of maternal infection (Meyer, 2014; Meyer et al., 2009), and some work has been done on pollution-induced inflammation (Bilbo et al., 2018; Bolton et al., 2012, 2014). Here, we focused on a model of allergic reaction-induced inflammation, as epidemiological studies have shown correlations between risk for ASD and maternal atopic or autoimmune disease (Chen et al., 2016; Theoharides et al., 2016). Models of chronic allergic asthma have reported alterations to social, repetitive, and anxiety-like behaviors (Schwartzer et al., 2015, 2017), as well as alterations in microglial gene expression in females, particularly in gene sets associated with autism (males not examined, Vogel Ciernia et al., 2018). We have previously shown that a single-hit maternal allergic event upregulates neonatal mast cells and microglia, alters sexual differentiation of dendritic spine patterning in the neonatal preoptic area (POA), and changes adult sexual behavior (Lenz et al., 2019). Here, we expand upon our previous work and show that prenatal exposure to allergic inflammation alters the numbers of mast cells and microglia in multiple brain regions during development and produces long-term disruptions in offspring behavior, including social behavior impairments, hyperactivity, and cognitive inflexibility. These data demonstrate the long-term effects of maternal allergic challenge on offspring development and provide a basis for understanding neurodevelopmental disorders linked to maternal allergic reaction in humans.
2. METHODS
2.1. Animals:
All experimental procedures were approved by the Institutional Animal Care and Use Committee at The Ohio State University. Experiments were conducted with Sprague Dawley rats purchased from Harlan/Envigo Laboratories or bred in-house from Harlan/Envigo animals. Prior to breeding, animals were housed on a reverse 12 h light/dark cycle in standard group cages with food and water ad libitum. For breeding, adult females were paired with males and separated when vaginal lavage was sperm-positive, which was designated gestational day (GD) 0. Once sperm-positive, females were housed in groups of two until GD15. After the allergic challenge on GD15 females were housed individually and left undisturbed, with the exception of appropriate cage-maintenance practices. As GD22 approached, cages were checked daily to determine the day of birth (designated PN0). To control for litter effects and maternal care differences no more than two animals/sex/litter were assigned to each experimental endpoint.
2.2. Gestational allergic challenge and experimental timeline:
Prior to pregnancy, adult virgin females assigned to the allergic challenge group were sensitized with a subcutaneous injection of ovalbumin-Alum adjuvant, composed of 1 mg ovalbumin (OVA grade V, Sigma) prepared at 4mg/ml in pyrogen-free 0.9% saline and precipitated at a 1:1 ratio with Al(OH)3 (Thermo Scientific) according to manufacturer’s instructions (see Fig. 1 for experimental timeline). After two weeks, a second injection of ovalbumin-Alum adjuvant was given. Control females were injected with saline at the same two timepoints to control for experimental handling and stress effects. One week after the second injection, females were bred as described in section 2.1. On GD15, pregnant rats were challenged intranasally with 1% ovalbumin in saline or saline vehicle (50μl per nare), which was placed on each nare under light restraint and inhaled. We have previously shown that this procedure induces a significant increase in immunoglobulin E (IgE) levels in maternal serum 30 min following challenge (Lenz et al., 2019).
Figure 1.
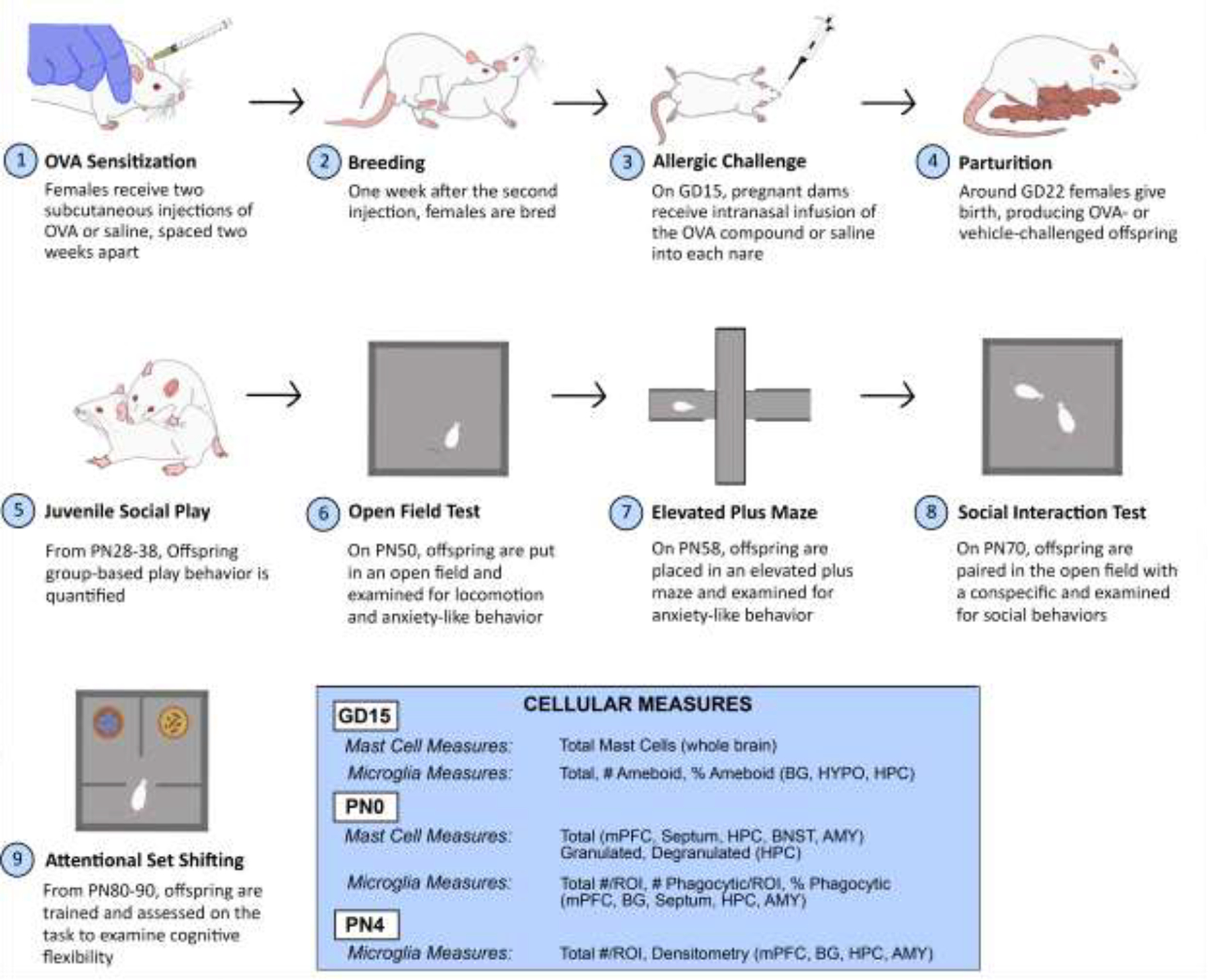
Experimental timeline. Female rats were sensitized to chicken egg ovalbumin, bred, and challenged on GD15. Offspring brains were collected, and brain mast cells and microglia were examined at three time points: two hours post-challenge, PN0, and PN4. Offspring social, emotional, and cognitive behaviors were also quantified. BG = basal ganglia, BNST = Bed Nucleus of the Stria Terminalis HYPO = hypothalamus, HPC = hippocampus, AMY = amygdala, mPFC = medial prefrontal cortex.
Two hours following the allergic challenge, dams were sacrificed via perfusion and fetal brains were harvested for mast cell and microglia analyses. The remaining dams were left undisturbed for the remainder of pregnancy and allowed to deliver naturally. On the day of birth (PN0), some offspring were sacrificed and processed for mast cell and microglia analyses. On PN4, some offspring were sacrificed and processed for microglia analyses. The remaining offspring were weaned at PN22 into same sex groups of three containing both OVA and vehicle offspring to avoid treatment and litter effects on social learning during development. These offspring were used for behavioral testing. All N’s are indicated by individual datapoints on graphs as well as in figure captions. No more than two animals/sex/litter were assigned to each experimental endpoint to control for litter effects and maternal care differences.
2.3. Tissue processing, histology, and cell counting:
2.3.1. General methods.
For all histology experiments, animals were overdosed with FatalPlus (Vortech Pharma) and transcardially perfused with saline followed by 4% paraformaldehyde. Brains were removed and postfixed for at least 12 hours. Subsequently, brains were sectioned coronally into three series at 45 μm thickness on a cryostat (Leica) and mounted onto SuperFrost charged slides (Fisherbrand) for staining procedures. Various regions implicated in cognition and social behavior were examined with experimenter blind to condition. For a summary of analyses performed and regions investigated at each experimental timepoint, refer to Figure 1.
2.3.2. Mast Cell Histology and Stereology.
Mast cells were visualized via acidic Toluidine Blue staining (Fig. 2A). Tissue was stained with 0.5% Toludine Blue O (Sigma) in 60% ethanol (pH=2.0) for 10 minutes, followed by sequential washes in ascending alcohols, defatting with xylenes (2 × 5 min) and coverslipping with Permount mounting medium. Our lab has previously employed this procedure in the developing rat brain with success (Lenz et al., 2018, 2019).
Figure 2.
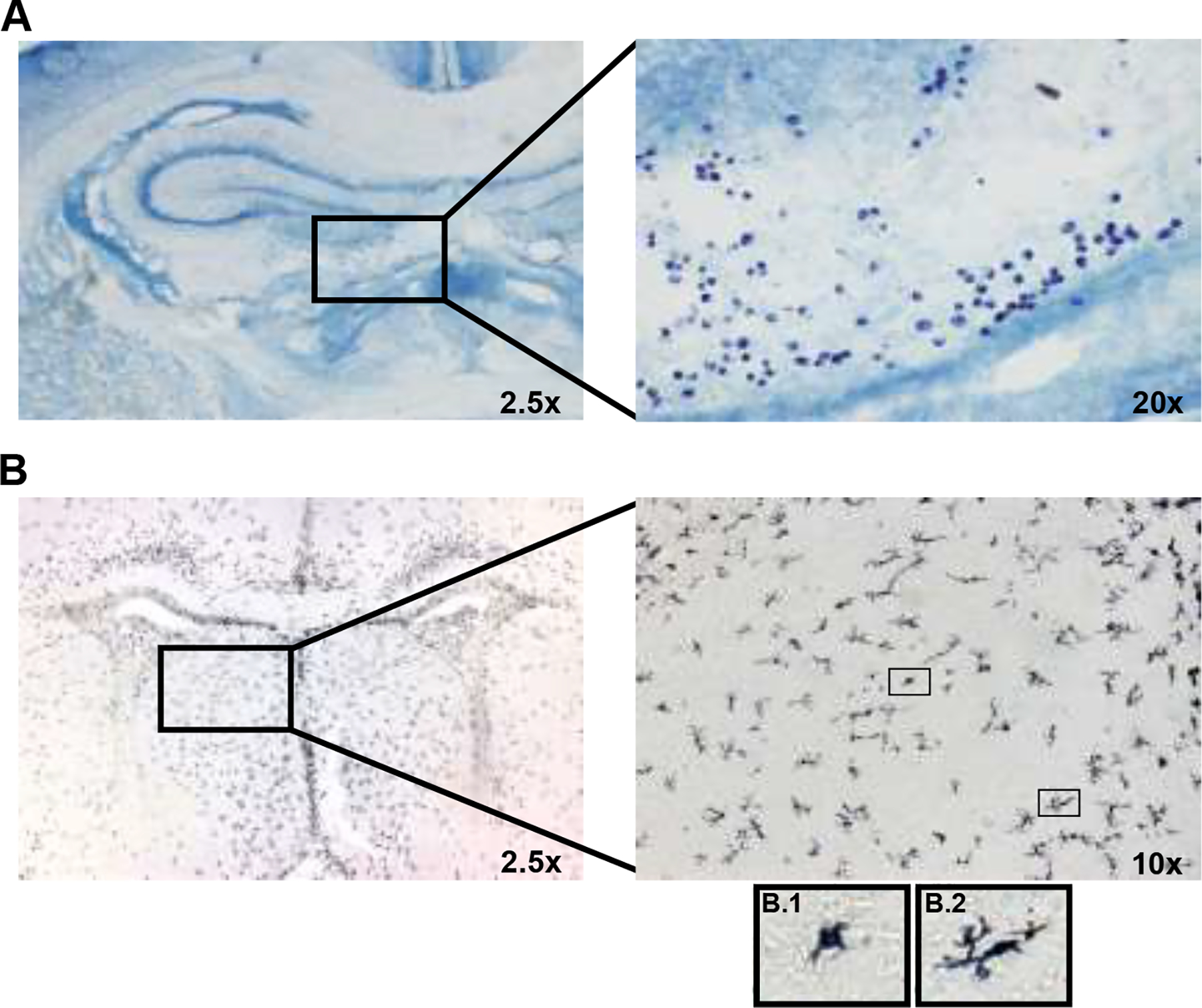
Representative images of mast cell and microglia staining in a female vehicle offspring on PN0. (A) Toluidine Blue-stained Mast cells in the perivascular area near the hippocampus. (B) DAB-stained Microglia in the lateral septum. Representative ameboid (B.1) and phagocytic (B.2) microglia are pictured.
In the fetal brain, mast cells were visualized using an Olympus CX41 bright-field microscope. All mast cells were quantified and the average number of mast cells per section was calculated manually. During the neonatal period, a large number of mast cells reside in the perivascular area bordering the hippocampus, thalamus, and amygdala (Joshi et al., 2019). Thus, our analyses at PN0 focused primarily on the space bordering the hippocampal area. Moreover, for hippocampal-related analyses at PN0, mast cells were counted using a computer-based stereology system (Stereoinvestigator, MBF Biosciences) coupled to a Zeiss Axioimager M2 microscope and a CX9000 Digital Camera. To obtain unbiased estimates of total mast cells, the optical fractionator workflow was employed. Cells were counted at 40x magnification with a 100 × 100 μm counting frame. All counted cells were classified as either granulated or degranulated, according to previously published criteria (Joshi et al., 2019). A mast cell was classified as granulated if it had a darkly stained cell body with an intact membrane border. A mast cell was considered degranulated if it had a lighter appearance, granules present, and/or a non-spherical membrane border. The total number of mast cells in four other regions was also quantified (Septum, mPFC, AMY, BNST) due to their involvement in social behavior. However, as the population of mast cells outside of the hippocampal area was fairly small, mast cell counts in these regions were conducted manually using the same microscope employed for GD15 mast cell analysis.
2.3.3. Microglia immunohistochemistry and analyses.
For immunohistochemistry staining, brain sections were rinsed with PBS, incubated in 0.3% H202 in 50% methanol to block endogenous peroxidases, permeabilized and blocked with 5% bovine serum albumin in PBS + 0.4% Triton X (PBS-T), and incubated with primary antiserum against the calcium adapter protein, Iba1 (Wako; rabbit pc 1:1000) in 2.5% BSA + 0.4% PBS-T for 24 hours at 4°C. Sections were extensively washed, and processed with biotinylated anti-rabbit secondary antibody (Vector 1:200), avidin-biotin complex (Vector 1:500), and reacted with diaminobenzidine with Nickel (Ni-DAB; Sigma) in 0.125M sodium acetate to visualize chromogen for IHC. Stained sections were cleared with ascending alcohols, defatted with xylenes, and coverslipped with Permount mounting medium (Fig. 2B).
The same microscope used for PN0 mast cell stereology was used to acquire images of microglia in various brain regions at a magnification of 4–20x. Microglia were counted and classified based on morphology in approximately four to six sections per region using one of three series. For GD15 tissue, total, number of ameboid, and percent ameboid microglia counts were obtained and means were calculated in the basal ganglia, hypothalamus, and hippocampus. We chose to examine ameboid microglia as we had previously found allergic-inflammation induced alterations to ameboid microglia in the preoptic area (Lenz et al., 2019). Microglia were classified as ameboid if they displayed an enlarged cell body with few to no processes. For PN0 tissue, mean total microglia per ROI, mean phagocytic microglia per ROI, and mean percent phagocytic microglia means were calculated in the mPFC, basal ganglia, septum, hippocampus, and amygdala. Microglia were classified as phagocytic if a phagocytic cup was visible. For PN4 tissue, average total microglia per ROI was obtained in the mPFC, basal ganglia, hippocampus, and amygdala. Densitometry measures were also taken in these regions at PN4, as described below.
To assess microglial staining density, each image used for PN4 morphology analysis was converted to grayscale and thresholded against a cell-deficient region of background staining. Following thresholding, the percentage of total black pixels per region of interest (ROI) was computed for each image and averaged across four to six sections per brain region to obtain a mean density of staining for each animal in each brain region analyzed.
2.4. Behavioral testing:
2.4.1. General methods.
For all behavioral tests, the order in which animals were run was equally balanced across treatment group. If multiple days of testing were required, the order in which animals were run was also randomized each day. For all behavioral tests performed, animals were videotaped during testing and videos coded by an experimenter who was blind to condition.
2.4.2. Juvenile Social Play.
Juvenile social play was tested in a group setting to assess for rough and tumble play behavior that is characteristic of juvenile rodents under natural circumstances (Auger & Olesen, 2009). Testing occurred daily for five days beginning at around 1000–1300 hours during the dark phase between PN28–38. Offspring were organized into mixed sex and treatment groups of six to seven animals, containing at least three males. To differentiate between animals, each subject was given a unique symbol on their dorsal side with a Sharpie marker at least 40 minutes prior to testing. For testing, the entire group was placed in a bedded 48 × 38 × 30 cm (l × w × h) Plexiglas arena under red light. Rats were left undisturbed in the arena for 13 minutes to allow three minutes for habituation and ten minutes for behavioral scoring. After testing, rats were returned to their sex-specific home cages. Frequencies of five play behaviors (pouncing, chasing, wrestling, boxing, and pinning) were quantified for each animal.
2.4.3. Open field test.
On PN50, animals were tested for 30 min in a novel 48 × 38 × 30 cm (l × w × h) Plexiglas box to test exploratory and anxiety-like behavior, as well as repetitive behavior and overall locomotion. Behaviors quantified include the time spent in the center of the arena as well as the total number of grids crossed.
2.4.4. Elevated plus maze test.
One week following the open field (around PN58), animals were placed in an elevated plus maze with four arms (width: 10cm, length: 50cm), two of which were enclosed by walls (height: 50cm), for 10 minutes to assess exploratory and anxiety-like behavior. Behaviors quantified include the amount of time spent in the open arms and the number of entries into the open arms.
2.4.5. Social interaction test.
We conducted a reciprocal social interaction test to measure social exploration behaviors. Around PN70, experimental animals were paired with an unfamiliar, treatment-naïve conspecific in a 48 × 38 × 30 cm (l × w × h) Plexiglas box under red light during the dark phase between 0900 and 1500 hr. Rats were left undisturbed for a single 10-minute session. The following behaviors were scored: number of avoidance behaviors (when the test animal was approached by the stimulus animal and the test animal moved away), number of investigative behaviors (when test animal sniffed, initiated contact, or actively investigated the stimulus animal), amount of time investigating stimulus animal, and amount of time passively interacting with the stimulus animal (test animal was in direct physical contact the stimulus animal but was directing no active investigation towards the stimulus animal).
2.4.6. Attentional set shifting.
The attentional set-shifting task (AST) is a rodent analog of the Wisconsin Card Sorting task for attention, cognitive flexibility and working memory (Birrell & Brown, 2000; V. J. Brown & Tait, 2016). The AST was performed and data analyzed as previously described (Leuner et al., 2014; Liston et al., 2006). The task requires training animals to dig in small 10 × 10 cm terracotta pots (depth × diameter) for a food reward. The pots could vary along two stimulus dimensions (texture of the pot and digging media), and the rat must learn to dig in the correct pot to earn the food reward (half of a cheerio). The testing apparatus consisted of a three chamber Plexiglass box, with an initial holding area (16 × 40 × 30 cm) separated from a two-chambered testing area (34 × 20 × 30 cm each) by a removable opaque divider. Each testing chamber contained a terracotta digging pot.
2.4.6.1. Habituation:
Beginning on PN80–90, rats were food restricted for a week to 85% of their free-feeding weight beginning on PN80–90 and maintained at 85% for at least one week prior to the beginning of training. During this week, rats were habituated to the testing arena for 30 minutes/day. On the final day of acclimation, testing pots were filled with regular bedding and a food reward (cheerio) was placed on the top of the bedding for animals to discover and consume.
2.4.6.2. Training phase 1:
On the first training day, each animal was given 4 initial exploration trials (120 s each) to explore and dig in the pots. Following the exploration trials animals underwent a four-stage training protocol, with rewards placed consecutively deeper in the bedding as follows: a) reward on top of bedding; b) buried under <1 cm bedding; c) buried under 2 cm bedding and d) buried under 4cm bedding. At each stage, animals were given 120 s to retrieve the reward successfully and had to perform six consecutive successful trials before proceeding to the next training stage. If animals could not progress through all the training phases on day one, training was repeated on the following day. If they still could not progress through training on day two, they were excluded from the experiment.
2.4.6.3. Training phase 2:
On the second training day only one of two pots was baited, but the un-baited pot contained crushed cheerio powder so animals could not use olfactory cues for discrimination. Rats were trained to discriminate between two perceptual dimensions: the texture on the outside of the pot (e.g., duct tape and sandpaper), or the type of media filling the pot (e.g., shredded paper and shredded nitrile gloves). Animals were given four exploratory trials before the testing trials. The location of the correct pot was counterbalanced across testing trials. If animals chose to dig in the incorrect pot, the divider between pot chambers was inserted so animals could not proceed to the correct pot. If animals did not dig, they were directed back to the holding chamber. Each of these behaviors was counted as an error. Animals were required to perform six consecutive trials in a row to reach training criterion for each of two discriminations. If animals failed to proceed through day two of training, they were re-run the following day. If they failed to reach criterion on this additional day of training, they were removed from the experiment.
2.4.6.4. Testing:
On testing day, animals were tested on a series of five discriminations of increasing difficulty. For the simple discrimination (SD), the rat needed to distinguish between two digging media in un-textured containers, with one medium predicting reward. For the compound discrimination (CD), a new stimulus dimension (pot texture) was introduced, but the reward-predicting medium presented in SD remained the correct stimulus. For the intradimensional shift (IDS), the exemplars of digging medium and pot texture were changed, but the digging medium remained the relevant dimensional feature. For the reversal (REV), the digging medium remained the relevant dimensional feature but the formerly incorrect digging medium from the IDS became the reward-predicting medium. For the extradimensional shift (EDS), new exemplars of pot texture and digging media were presented, and the pot texture became the relevant dimension that predicted reward. Prior to each discrimination test, animals were given four 120s exploration trials to learn the correct pot. During testing trials, animals were given 120s to dig; if they dug in the correct pot, it was counted as a correct trial, and if they dug in the incorrect pot or did not dig, it was counted as an incorrect trial and animals were removed to the holding chamber while pots were rebaited. After six consecutive correct trials, testing proceeded to the next discrimination phase. After five consecutive no-dig trials, testing was halted and resumed the following day. In accordance with previous literature (Birrell & Brown, 2000; Leuner et al., 2014; Liston et al., 2006), data are presented as the number of trials to criterion, with six being perfect performance.
2.5. Statistical analyses:
All analyses were conducted using two-way ANOVAs (sex and maternal treatment as main factors), followed by Tukey’s post-hoc tests where appropriate. GraphPad Prism software was used for data analyses. All graphs were generated in Prism and aesthetically modified in Adobe Illustrator. Data are presented as mean ± SEM with individual data points depicted over histograms. Statistical significance was set at α = 0.05 and corrected for multiple comparisons in post-hoc tests.
2. RESULTS
3.1. Mast Cell Measures
3.1.1. GD15.
Gestational allergic inflammation did not affect the number of mast cells in the fetal brain two hours post immune challenge (Fig. 3A; F(1, 26) = 0.47, n.s).
Figure 3.
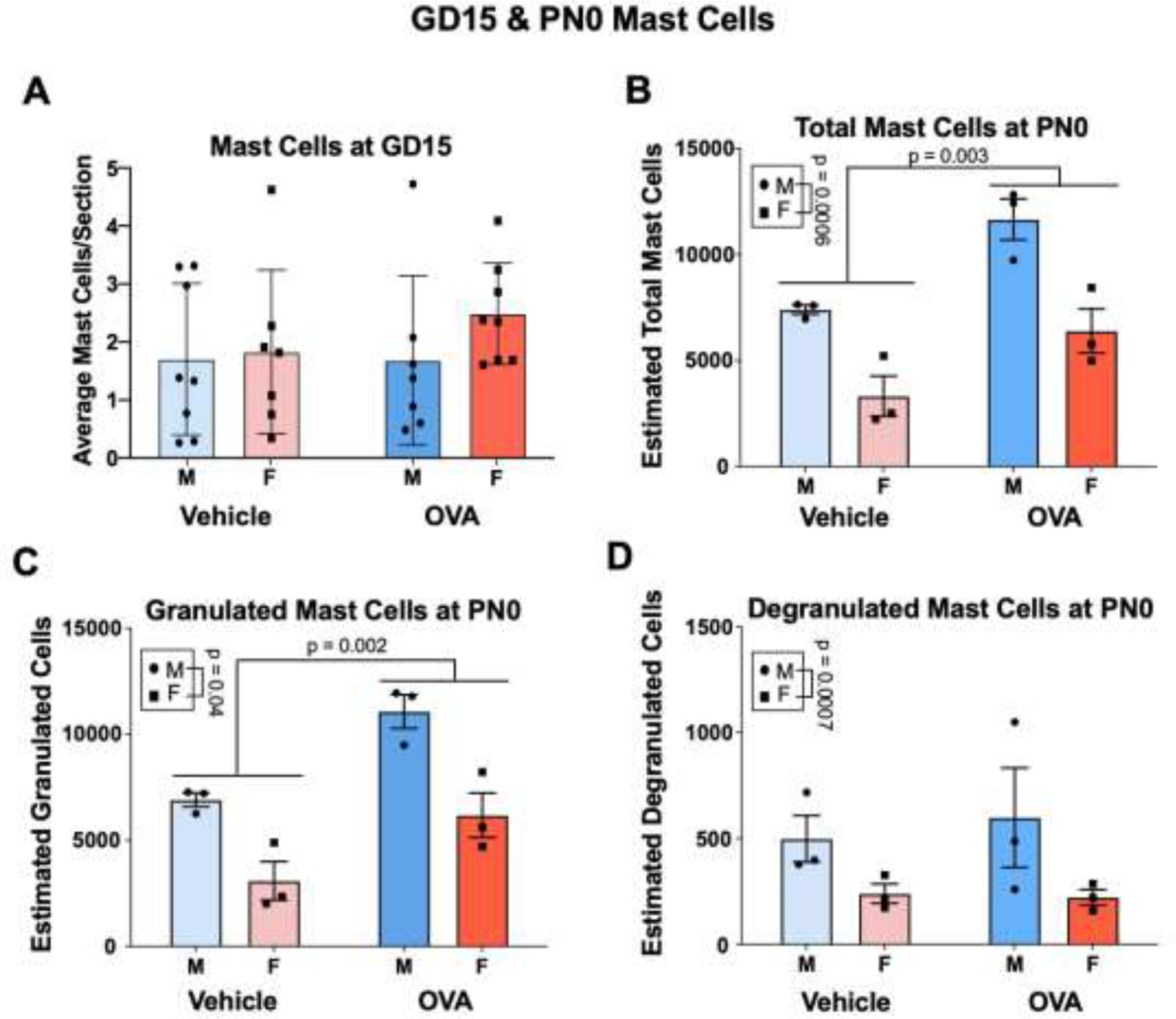
Maternal allergic inflammation increases the number of mast cells in the perinatal hippocampus. (A) OVA and vehicle offspring have similar numbers of mast cells throughout the brain two hours post-challenge on GD15. N’s = 7/group. (B – D) Males had more total, granulated, and degranulated mast cells in and near the hippocampus compared to females. (B and C) Allergic challenge increased total and granulated mast cell numbers. N = 3/group/sex. HPC = hippocampus, MC = mast cells.
3.1.2. PN0.
In the septum, mPFC, amygdala, and BNST males and females had similar numbers of mast cells (Fig. S1; sex: F’s ≤ 0.13, all n.s.), and allergic inflammation did not affect the number of mast cells present (treatment: F’s ≤ 0.81, all n.s.). There were also no interactions (interaction: F’s ≤ 2.2, all n.s.).
Consistent with previous findings (Joshi et al., 2019), males had significantly more total (sex: F(1, 8) = 29.32, p = 0.0006, η2 = 0.53), granulated (sex: F(1, 8) = 28.41, p = 0.0007, η2 = 0.50) and degranulated (sex: F(1, 8) = 5.71, p = 0.04, η2 = 0.41) mast cells in the area surrounding the hippocampus compared to females (Fig. 3B–D). Gestational allergic inflammation increased estimates of total hippocampal mast cells (treatment: F(1, 8) = 18.05, p = 0.003, η2 = 0.32), and this was likely driven by an increase in granulated mast cells (treatment: F (1, 8) = 19.68, p = 0.002, η2 = 0.35), as there were no main effects of treatment on degranulated mast cells (F (1, 8) = 0.10, n.s.). The effect of allergic inflammation did not vary according to sex for any of these measures (interactions: Fs ≤ 0.47, n.s.).
3.2. Microglia Measures
3.2.1. GD15.
Males had more total (sex: F(1, 28) = 4.29, p = 0.05, η2 = 0.12) and ameboid (sex: F(1, 28) = 4.90, p = 0.04, η2 = 0.13) microglia in the basal ganglia. However, there were no effects of sex on total (sex: F’s ≤ 2.71, all n.s.) or ameboid microglia (sex: F’s ≤ 1.39, all n.s.) in the hypothalamus or hippocampus. There were also no effects of sex on ameboid tone in any region at GD15 (sex: F’s ≤ 2.44, all n.s.).
Maternal allergic inflammation did not affect total microglia number in the basal ganglia (treatment: F(1, 28) = 1.17, n.s.) or hippocampus (treatment: F(1, 26) = 2.19, n.s.), but it increased microglia number in the hypothalamus (treatment: F(1, 28) = 14.80, p = 0.0006, η2 = 0.32). No interactions were found for total number of microglia in any region (interactions: F’s ≤ 1.44, all n.s.). Allergic inflammation tended to increase the number of ameboid microglia in the basal ganglia (treatment: F(1, 28) = 3.76, p = 0.06, η2 = 0.10) but not the hypothalamus (treatment: F(1, 28) = 2.46, n.s.) or hippocampus (treatment: F(1, 26) = 1.69, n.s.). Interestingly, while there were no interactions regarding ameboid number in the basal ganglia or hypothalamus (interactions: F’s ≤ 1.92, all n.s.), there was an interaction in the hippocampus (interaction: F(1, 26) = 10.03, p = 0.004, η2 = 0.26), whereby allergic inflammation increased the number of ameboid microglia in OVA males (p = 0.01) but not OVA females. Maternal allergic inflammation did not alter the percent of ameboid microglia in any of the three regions (treatment: F’s ≤ 2.76, all n.s.), though a significant interaction in the hippocampus (interaction: F(1, 26) = 12.37, p = 0.002, η2 = 0.31) revealed that allergic inflammation decreased the percent of ameboid microglia in OVA females (p = 0.01) but not males.
3.2.2. PN0.
Males and females had similar numbers of microglia in all regions examined at PN0 (sex: F’s ≤ 2.07, all n.s.). There was a trend for decreased phagocytic microglia in males relative to females in the hippocampus (sex: F(1, 40) = 3.30, p = 0.08, η2 = 0.06), though no other regions displayed trends for sex differences in phagocytic microglia (sex: F’s ≤ 1.75, all n.s.) or percent phagocytic microglia (sex: F’s ≤ 0.72, all n.s.).
Maternal allergic inflammation decreased microglia colonization in the mPFC (treatment: F(1, 34) = 8.73, p = 0.006, η2 = 0.20), septum (treatment: F(1, 34) = 5.68, p = 0.02, η2 = 0.14), amygdala (treatment: F(1, 51) = 31.17, p< 0.00001, η2 = 0.37), and hippocampus (treatment: F(1, 40) = 7.85, p = 0.008, η2 = 0.15), though the number of microglia in the basal ganglia was not affected (treatment: F(1, 35) = 0.004, n.s.). No interactions for total microglia were found in any region (interactions: F’s ≤ 2.05, all n.s.)
Allergic inflammation also decreased the number of phagocytic microglia in the mPFC (treatment: F(1, 34) = 9.05, p = 0.005, η2 = 0.21), septum (treatment: F(1, 34) = 9.59, p = 0.004, η2 = 0.22), amygdala (treatment: F(1, 51) = 18.22, p < 0.0001, η2 = 0.25), and hippocampus (treatment: F(1, 40) = 8.32, p = 0.006, η2 = 0.16). Conversely, allergic inflammation increased the number of phagocytic microglia in the basal ganglia (treatment: F(1, 35) = 5.84, p = 0.02, η2 = 0.13). No interactions were found in any region for phagocytic microglia (interactions: F’s ≤ 1.70, all n.s.), though one approached significance in the septum (interaction: F(1, 34) = 2.80, p = 0.10, η2 = 0.06), where maternal allergic inflammation significantly downregulated phagocytic microglia in OVA males relative to vehicle males (p = 0.02).
In terms of the overall percentage of phagocytic microglia, maternal allergic inflammation increased phagocytic tone in the basal ganglia (treatment: F(1, 35) = 9.33, p = 0.004, η2 = 0.27), and there was a trend for a decrease in the septum (treatment: F(1, 34) = 3.18, p = 0.08, η2 = 0.08). There were no main effects of allergic inflammation on phagocytic tone in the mPFC (treatment: F(1, 34) = 0.06, n.s.), amygdala (treatment: F(1, 51) = 1.50, n.s.), or hippocampus (treatment: F(1, 40) = 0.96, n.s.); however, there was an interaction in the hippocampus (interaction: F(1, 40) = 5.74, p = 0.02), whereby OVA females tended to display reduced phagocytic tone (p = 0.09), but OVA males were unaffected. No other interactions were found (interactions: F’s ≤ 0.71, all n.s.).
3.2.3. PN4.
Males and females had similar counts of microglia in all regions at PN4 (sex: F’s ≤ 1.98, all n.s.). In terms of densitometry, females tended to have increased microglial staining in the mPFC relative to males (sex: F(1, 20) = 3.62, p = 0.07, η2 = 0.13). No other regions displayed sex differences in microglial staining (sex: F’s ≤ 1.42, all n.s.).
Maternal allergic inflammation decreased the number of microglia in the basal ganglia (treatment: F(1, 16) = 24, p = 0.002, η2 = 0.56). No differences in microglial number were detected in the mPFC (treatment: F(1, 20) = 3.92 × 10−5, n.s.) or hippocampus (treatment: F(1, 8) = 0.60, n.s.), though OVA offspring tended to have decreased microglia in the amygdala (treatment: F(1, 16) = 3.08, p = 0.10, η2 = 0.12). Notably, a significant interaction in the amygdala (interaction: F(1, 16) = 6.70, p = 0.02, η2 = 0.25) indicated that this trend was driven by a significant decrease in amygdala microglia of OVA males (p = 0.03). No other interactions were found for number of microglia (interactions: F’s ≤ 0.53, all n.s.).
Allergic inflammation increased microglial staining in the mPFC (treatment: F(1, 20) = 4.51, p = 0.05, η2 = 0.17), and tended to reduce microglial staining in the basal ganglia (treatment: F(1, 16) = 3.49, p = 0.08, η2 = 0.17). No effects of maternal treatment on microglial staining were found in the amygdala (treatment: F(1, 16) = 0.07, n.s.) or hippocampus (treatment: F(1, 8) = 2.43, n.s.). However, an interaction effect was found in the amygdala (interaction: F(1, 16) = 17.96, p = 0.0006, η2 = 0.51), whereby OVA males had decreased microglial staining (p = 0.02) and OVA females tended to have increased staining (p = 0.06) relative to their vehicle counterparts. No other interactions met or approached significance for densitometry measures (interactions: F’s ≤ 0.44, all n.s.)
3.3. Juvenile Social Play and Adult Social Interaction
As juveniles, males and females initiated social chase (Fig. 7B; sex: F (1, 26) = 1.94, n.s.), rough and tumble behaviors (Fig. 7C; sex: F(1, 26) = 0.43, n.s.), and thus all social play behaviors (Fig. 7A; sex: F(1, 26) = 0.95, n.s.) at about equal frequencies per session. OVA offspring displayed fewer chase behaviors (treatment: F(1, 26) = 10.12, p = 0.004, η2 = 0.26), as well as decreased play behaviors overall (treatment: F(1, 26) = 5.75, p = 0.02, η2 = 0.17), and this effect did not vary according to sex (interactions: Fs ≤ 0.92, n.s.). Additionally, while there was no main effect of treatment on the number of rough and tumble behaviors (treatment: F(1, 26) = 1.46, n.s.), there was a significant interaction (F(1, 26) = 6.01, p = 0.021, η2 = 0.17), whereby OVA females tended to initiate fewer behaviors compared to vehicle females (p = 0.08). Thus, allergic inflammation decreased rough and tumble play behaviors in juvenile females but not males.
Figure 7.
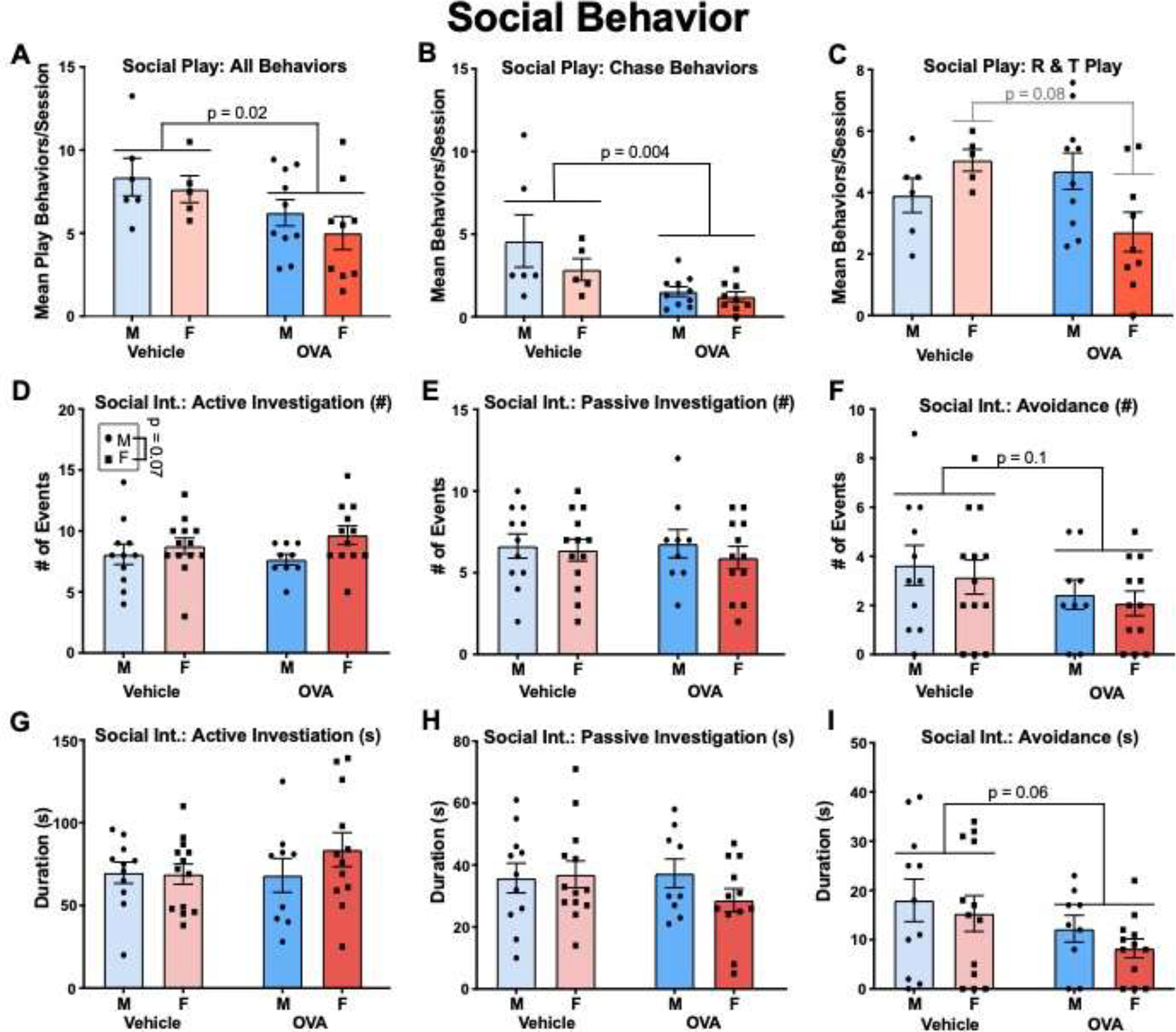
Gestational allergic inflammation alters juvenile and adult social behavior. (A) OVA offspring demonstrated fewer total play behaviors compared to vehicle. (B) Social chase was reduced in OVA offspring. (C) An interaction was found whereby allergic challenge tended to reduce rough and tumble play in females but not males. N’s = 6 MVs, 5 FVs, 10 MOVAs, 9 FOVAs. (D, E, F) display mean counts of each behavior. (G, H, I) display total amount of time (s) spent engaged in each behavior. Allergic challenge did not alter passive or active social investigation, but OVA offspring were less socially avoidant compared to vehicle offspring (F, I). N’s = 11 MVs, 13 FVs, 9 MOVAs, 12 FOVAs.
During the adult social interaction test, females tended to initiate active social investigation more than males (Fig. 7D; sex: F(1, 41) = 3.50, p = 0.07, η2 = 0.08). Both sexes participated in passive social investigation (Fig. 7E; sex: F(1, 41) = 0.57, n.s.) and avoidance (Fig. 7F; sex: F(1, 41) = 0.39, n.s.) at about the same frequency. The duration of time spent in active (Fig. 7G; sex: F(1, 41) = 0.76, n.s.) and passive investigation (Fig. 7H; sex: F(1, 41) = 0.72, n.s.), as well as avoidance (Fig. 7I; sex: F(1, 41) = 0.98, n.s.) also did not vary according to sex. Allergic inflammation did not affect the frequency or duration of active (treatment: Fs ≤ 0.62, n.s.) or passive (treatment: Fs ≤ 0.60, n.s.) investigation, and there were no interactions (Fs ≤ 1.24, n.s.). However, OVA offspring tended to show decreased frequency (treatment: F(1, 41) = 2.79, p = 0.1, η2 = 0.06) and duration (treatment: F(1, 41) = 3.64, p = 0.06, η2 = 0.08) of avoidance behaviors, and these trends were not affected by sex (interactions: Fs ≤ 0.04, n.s.).
3.4. Open Field and Elevated Plus Maze
Females spent more time in the center (Fig. 8A; sex: F(1, 39) = 4.056, p = 0.05, η2 = 0.08) and crossed more grids (Fig. 8B; sex: F(1, 28) = 19.32, p = 0.0001, η2 = 0.34) compared to males during the open field test in adolescence. Allergic inflammation decreased anxiety like behaviors, with OVA offspring spending more time in the center (treatment: F(1, 39) = 4.45, p = 0.04, η2 = 0.09) and crossing more grids (treatment: F(1, 28) = 6.41, p = 0.02, η2 = 0.11) compared to vehicle offspring. The effect of allergic inflammation did not vary significantly according to sex for either measure (interactions: Fs ≤ 0.91, n.s.).
Figure 8.
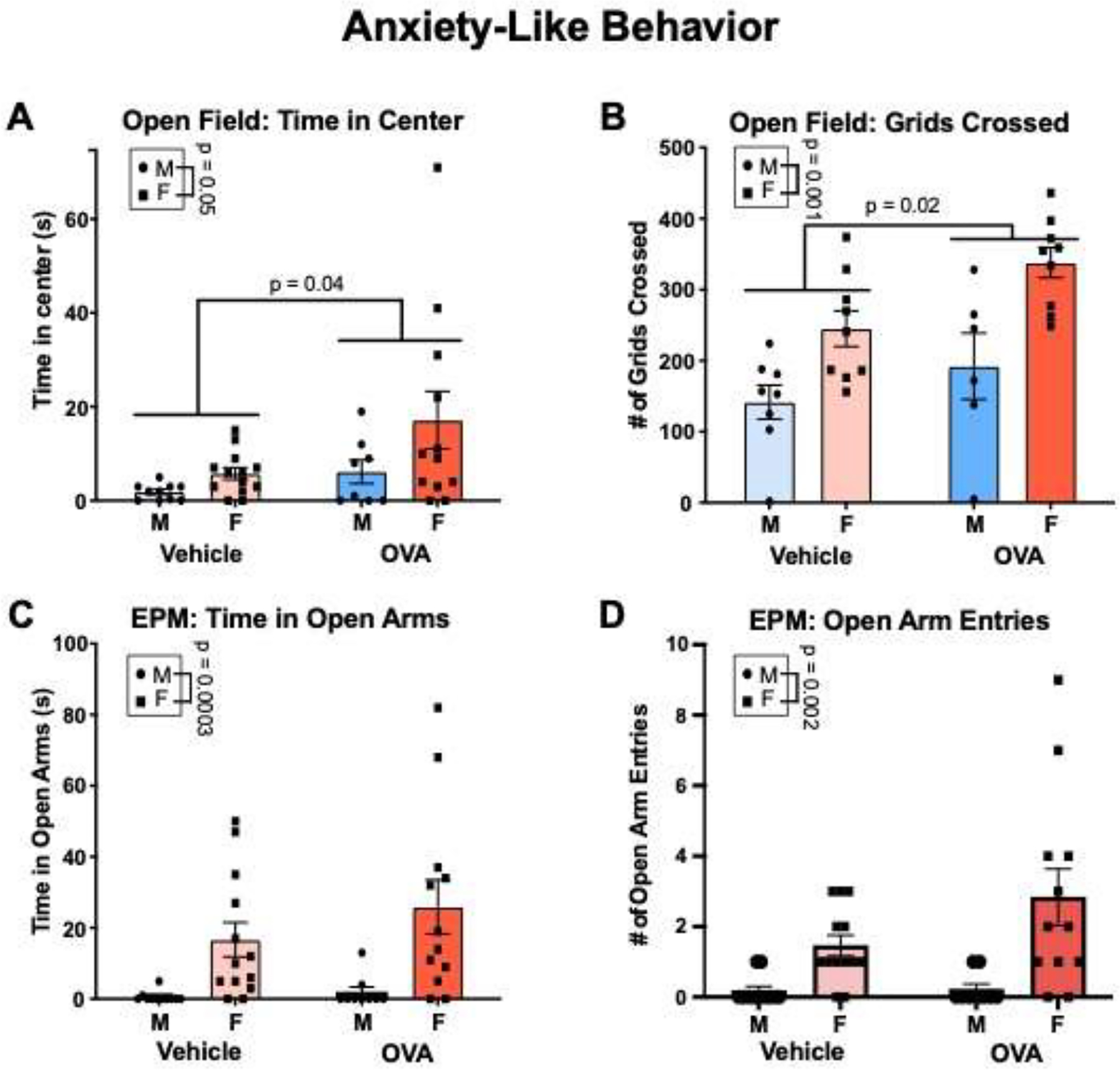
Gestational allergic inflammation increases locomotion in late adolescence/early adulthood. (A) Females spent more time in the center of the open field. OVA offspring spent more time in the center. N’s = 10 MVs, 13 FVs, 8 MOVAs, 12 FOVAs. (B) Females crossed more grids in the open field compared to males. OVA offspring crossed more grids compared to vehicle offspring. N’s = 8 MVs, 9 FVs, 6 MOVAS, 9 FOVAs. (C and D) Females spent more time in the open arms and had higher numbers of open arm entries. N’s = 11 MVs, 13 FVs, 9 MOVAs, 12 FOVAs.
In the elevated plus maze, females spent significantly more time on the open arms (Fig. 8C; sex: F(1, 41) = 15.93, p = 0.0003, η2 = 0.27), and entered the open arms with higher frequency (Fig. 8D; sex: F(1, 41) = 16.78, p = 0.002, η2 = 0.27). There was no effect of allergic inflammation on open arm time (treatment: F(1, 41) = 1.10, n.s), or open arm entries (treatment: F(1,41) = 2.21, n.s.). There were also no interactions (Fs ≤ 1.97, n.s.).
3.5. Attentional Set-Shifting Task
There were no main effects of sex on any phase of the AST (Fig. 9; sex: Fs ≤ 0.76, n.s.). OVA and vehicle offspring performed equally well on the simple discrimination (Fig. 9A; treatment: F(1, 23) = 1.60, n.s.), compound discrimination (Fig. 9B; treatment: F(1, 23) = 0.03, n.s.), and intradimensional shift (Fig. 9C; treatment: F(1, 23) = 1.09, n.s.), and this was not moderated by sex (interactions: Fs ≤ 2.25, n.s.). However, OVA offspring were impaired on the reversal (Fig. 9D; treatment: F(1, 23) = 4.38, p = 0.05, η2 = 0.14) and extradimensional shift (Fig. 9E; treatment: F(1, 23) = 4.98, p = 0.04, η2 = 0.18), taking more trials to reach criteria. Sex tended to moderate the effect of allergic inflammation on the reversal task (interaction: F(1, 23) = 2.99, p = 0.1, η2 = 0.10), whereby OVA females tended to take more trials compared to vehicle females (p = 0.09). Conversely, OVA males and females were equally impaired on the extradimensional shift relative to their vehicle counterparts (interaction: F(1, 23) = 0.15, n.s.).
Figure 9.
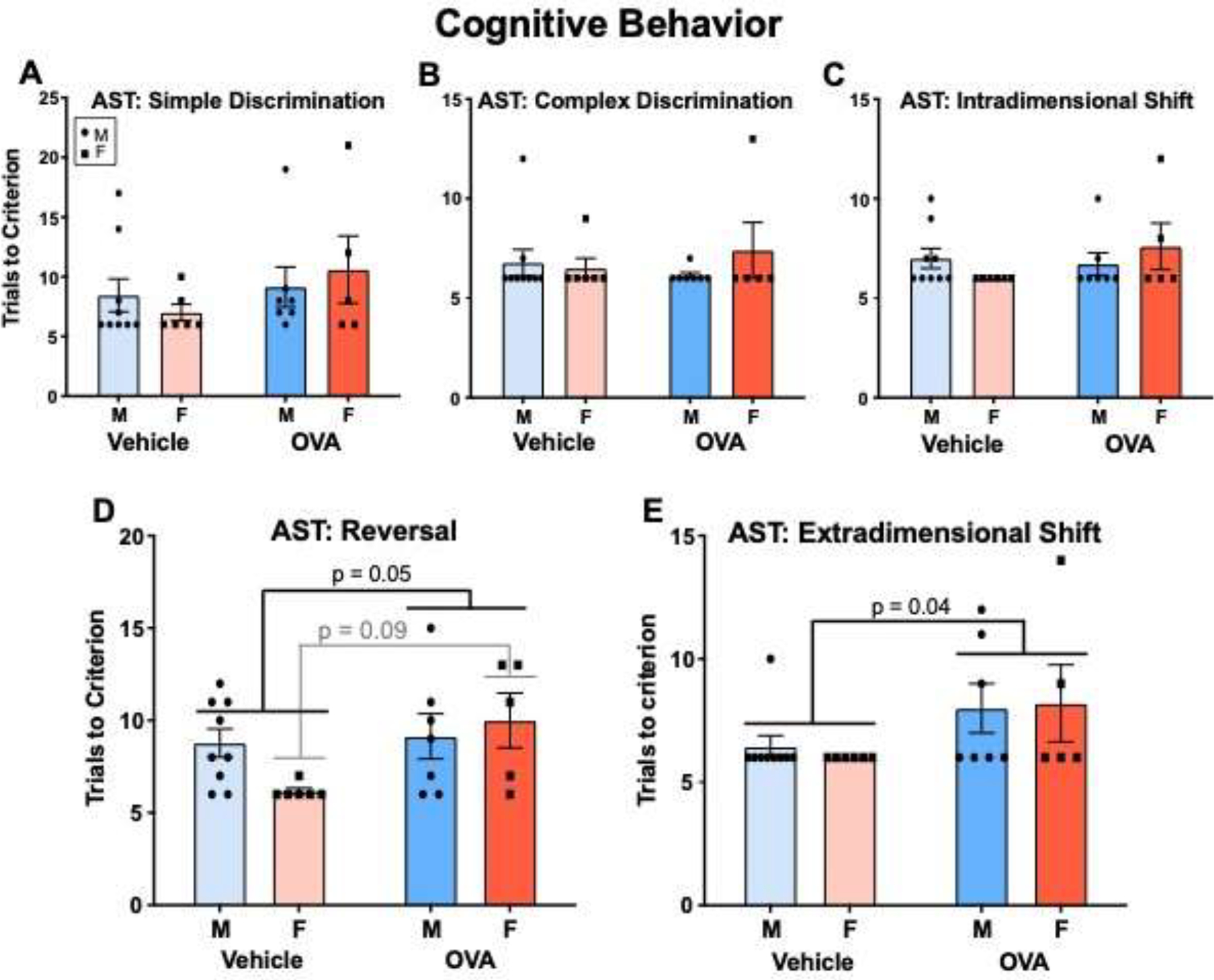
Gestational allergic inflammation reduces adult cognitive flexibility in offspring. (A - C) OVA and vehicle offspring performed similarly on the simple discrimination, compound discrimination, and intradimensional shift tasks. (D). OVA offspring displayed worse performance in the reversal task, and this may have been driven by females. (E) OVA offspring displayed worse performance on the extradimensional shift task. N’s = 9 MVs, 6 FVs, 7 MOVAs, 5 FOVAs.
DISCUSSION
The current experiments tested whether prenatal exposure to allergic inflammation led to changes in the brain’s innate immune system and behavioral outcomes throughout life. Our primary findings were 1) Gestational allergic inflammation increases mast cells in the neonatal hippocampus, 2) Gestational allergic inflammation confers time-, region- and sex- dependent changes in developmental microglia, 3) Gestational allergic inflammation produces hyperlocomotive and abnormal social behavior in offspring, and 4) Gestational allergic inflammation decreases adult cognitive flexibility. We also found sex differences that merit consideration. These findings highlight the effects that maternal allergic inflammation has on the fetal neuroimmune milieu and adds to mounting evidence suggesting that early changes in microglia and mast cells may underlie behavioral outcomes in rodent models of MIA.
4.1. Gestational allergic inflammation increases mast cells in the neonatal hippocampus
OVA offspring had more mast cells in the space surrounding the hippocampus and thalamus on PN0. Mast cells are the brain’s first responders, releasing histamine, serotonin, cytokines, and proteases in response to insult via a process called degranulation (Wernersson & Pejler, 2014). These molecules bind to receptors on microglia to induce subsequent microglial release of inflammatory mediators (Lenz et al., 2018; Zhang et al., 2016). While few in number, mast cells contribute disproportionately to hippocampal function (Nautiyal et al., 2012), and mast cell granules can travel up to 50um upon release (Silver & Curley, 2013), potentially enabling them to communicate with multiple neurons, astrocytes and microglia. As mast cells can be found in the leptomeninges near the hippocampus, they can release mediators into the CSF and thus have a wider effect on the parenchyma (Silver & Curley, 2013). Consequently, an upregulation in mast cells of OVA offspring may represent a heightened neuroimmune tone and increased drive on the inflammatory response, suggestive of a more primed immune system. This change in tone also likely alters brain development, as we have previously shown that mast cell – microglia cross talk during early postnatal development is required for male-typical synaptic patterning of the mPOA and male-typical sex behaviors (Lenz et al., 2018). We have also previously demonstrated that this maternal allergic inflammation paradigm increases mast cell number and alters mPOA development and adult sociosexual behavior (Lenz et al., 2019). It is possible that the mast cell alterations documented here may have influenced the behavioral phenotypes presenting later in life via alteration of neurodevelopmental trajectory and long-term immune function. Future studies should examine whether such alterations in early mast cell numbers are causally implicated in subsequent behavioral abnormalities.
4.2. Gestational allergic inflammation confers time-, region-, and sex- dependent changes in developmental microglia
At GD15, allergic inflammation increased microglial colonization of the hypothalamus, though there were no changes in colonization in the basal ganglia or hippocampus. However, significant interactions indicated that allergic inflammation increased the number of ameboid microglia in the hippocampus of male offspring, and decreased ameboid tone in the hippocampus of female offspring (discussed further below). Moreover, it is reasonable to expect that the hypothalamus would be most sensitive to inflammation-induced upregulations in microglial colonization. The hypothalamus receives peripheral immune input via cytokine signaling (Czura et al., 2007; Ganong, 2000; Steinberg et al., 2016), lending vulnerability to the immediate effects of an immune challenge. Additionally, the maternal allergy-induced immune response may have crossed or induced further cytokine release at the level of the placenta, with the immune response subsequently acting on the fetal hypothalamus to induce microglial recruitment to the region.
At PN0, OVA offspring displayed reduced microglial colonization in the mPFC, septum, amygdala, and hippocampus. All of these regions also showed reductions in phagocytic microglia, though only the septum displayed a trend towards a decrease in overall phagocytic tone. Conversely, OVA offspring had more phagocytic microglia and increased phagocytic tone in the basal ganglia. Interestingly, we saw different patterns at PN4, when there were no differences in microglia number in the hippocampus or mPFC, though we found heightened microglia staining in the mPFC in OVA offspring, suggesting an increase of microglial ramification. Conversely, we found reduced microglial colonization and staining in the basal ganglia, suggesting reduced ramification. A trend for a decrease in microglial colonization of the amygdala was observed, and OVA males had reduction in microglial staining whilst females had increased staining. Overall, these data suggest that gestational allergic inflammation produces variable changes in microglia number throughout a critical period of neurodevelopment.
Furthermore, microglia phagocytose cells in the cortex, hippocampus, and amygdala (Cunningham et al., 2013; Nelson et al., 2017; VanRyzin et al., 2019), participate in synaptic sculpting and formation (Kopec et al., 2018; Miyamoto et al., 2016; Schafer et al., 2012; Vanderschuren et al., 2016; Weinhard et al., 2018), and support developmental cell genesis in the subventricular zone, hippocampus, amygdala, and cortex (Shigemoto-Mogami et al., 2014; Nelson et al., 2021), among other things. Microglial depletion in the neonatal period impairs prefrontally- and hippocampally-mediated cognition, induces hyperlocomotion, and reduces social play behavior (VanRyzin et al., 2016; Nelson & Lenz, 2017). Given that region-specific microglial manipulations in the nucleus accumbens and amygdala sculpt social play behavior (Kopec et al., 2018; VanRyzin et al., 2019), it is likely that the region-dependent perturbations to microglial numbers documented here are responsible for long term organizational changes in synaptic patterning, brain structure, neuroimmune function, and behavior.
4.3. Gestational allergic inflammation produces hyperlocomotive and abnormal social behavior in offspring.
OVA offspring showed reduced social play behavior in the juvenile period. Interestingly, allergic inflammation reduced social chase in both sexes while it reduced rough and tumble play in females only. Previous studies employing an LPS model of maternal immune activation found male-specific reductions in social behaviors (Hava et al., 2006; Kirsten, Taricano, Maiorka, et al., 2010; Kirsten et al., 2012; Taylor et al., 2012). Likewise, studies using a viral mimetic paradigm (poly(I:C)) found reduced sociability and reduced ultrasonic vocalization responses to social stimuli in adult male offspring (females not examined; Malkova et al., 2012). It is possible that social interactions become less rewarding in offspring of immune-challenged moms, as studies employing poly(I:C) and LPS models of MIA have found alterations to the dopaminergic system in offspring (Aguilar-Valles et al., 2020; Kirsten et al., 2012; Kirsten, Taricano, Flório, et al., 2010; Ling et al., 2002; Vuillermot et al., 2010), which may affect reward processing. Interestingly, microglial activity is involved in shaping the dopaminergic system (Squarzoni et al., 2014), and pro-inflammatory cytokines are toxic to rat fetal dopaminergic neurons in culture (Jarskog et al., 1997). Thus, the reduction in microglia in various brain regions discussed section 4.2 could be directly implicated in shaping abnormal social behaviors.
In late adolescence, OVA offspring spent more time in the center of the open field and crossed more grids compared to vehicle offspring. This hyperlocomotive activity aligns with some studies employing other models of maternal immune activation, suggesting maternal immune activation may increase locomotion and risk-taking behavior and potentially decrease anxiety-like behaviors in offspring (Makinson et al., 2017; Vuillermot et al., 2012). However, other studies have found MIA-induced either increases in anxiety-like behaviors (Canetta et al., 2016; Depino, 2015) or no alterations (Kirsten, Taricano, Maiorka, et al., 2010; Meyer, 2006; Penteado et al., 2014; Schwartzer et al., 2015). Contradictory results could be due to the different immune stimulants applied, the varied timing of the immune challenge, age at time of analysis, and/or the different rodent species or strains used. Alternatively, it is possible that hyperactivity may not necessarily mean reduced anxiety. For example, in a model of maternal stress and pollutant-induced prenatal inflammation, male offspring spent less time exploring unfamiliar bedding and demonstrated hyperlocomotive activity in their home cage bedding relative to vehicles (Bilbo et al., 2018), which could be indicative of anxiety. Nevertheless, since allergic inflammation did not induce alterations to anxiety-like behavior in the elevated plus maze, open-field hyperactivity could be indicative of increased risk-taking behavior or novelty seeking, which would align well with the reduced social avoidance displayed by adult OVA offspring in the social interaction test. A caveat to note, however, is that males rarely left the closed arms of the elevated plus maze at all. Moreover, we were unable to detect increases in anxiety-like behaviors in males in the elevated plus maze, and this impedes our ability to interpret the hyperlocomotive activity in the open field, at least in the OVA males.
As mentioned earlier, previous studies have found that microglial depletion in the early postnatal period produces socially impaired and hyperlocomotive offspring (Nelson & Lenz, 2017; VanRyzin et al., 2016). Another study examining the effect of a CX3CR1 knockout, which reduces neuron-microglia signaling and causes a transient developmental reduction of microglia, found decreased PFC-HPC functional connectivity, and this was correlated with reduced social investigation (Zhan et al., 2014). Thus, microglial action during early development shapes circuits involved in anxiety and social behaviors and is likely implicated here.
Interestingly, multiple studies have also implicated mast cell activity in anxiety-like behavior. For example, central, not peripheral, injections of a mast cell stabilizer decreased exploratory behavior in the open field and elevated plus maze (Nautiyal et al., 2008). Mast cell degranulation in the meninges correlates positively with motor activity (Larson et al., 2011), and histamine receptor (HR) 1 activation results in behavioral hyperactivity (Chiavegatto et al., 1998). Thus, prolonged dysregulated mast cell activity in OVA offspring could underlie this hyperlocomotive phenotype. It has been demonstrated previously that developmental mast cell activity can regulate adult sexual behaviors (Lenz et al., 2018); it is likely that other behaviors, including social behavior and anxiety-like behaviors, can be affected as well.
The allergic inflammation-induced upregulation of mast cells and downregulation of microglia in various cognitive, social and emotional brain regions during early postnatal development likely contribute to the abnormal social and locomotive behaviors documented here. Future studies should characterize the specific contributions that each of these changes in microglial and mast cell populations makes to these behaviors and whether the effects are dependent on one another.
4.4. Gestational allergic inflammation decreases adult cognitive flexibility in the AST
Adult OVA offspring required more trials to meet criterion during the reversal and extradimensional shift phases of the attentional set shifting task. Studies have found that the reversal task is mediated by the orbitofrontal cortex (McAlonan & Brown, 2003). whereas the extradimensional shift is mediated by prelimbic and infralimbic PFC (Birrell & Brown, 2000). It is possible that the hyperlocomotive phenotype displayed by OVA offspring in the open field may have contributed to their impaired ability to learn these two tasks, although it is unlikely to have played a significant role as the open field test as OVA offspring learned the simple discrimination, complex discrimination, and intradimensional shift tasks just as well as vehicle offspring, which suggests that their motor behavior itself did not interfere with their overall ability to learn. Moreover, akin to the current study, other MIA models have found impairments in prefrontally-mediated behaviors in offspring (Canetta et al., 2016; Vuillermot et al., 2012). Interestingly, rats that are deprived of juvenile social play display impaired flexibility in unpredictable environments (Vanderschuren & Trezza, 2014), as well as disruptions in other measures of cognition (Vanderschuren et al., 2016). Perhaps the reduced social play exhibited by juvenile OVA offspring relates to this later life reduction in cognitive flexibility.
Cognitive flexibility deficits are characteristic of various neurodevelopmental disorders linked to prenatal immune activation, including autism, ADHD, and schizophrenia (Canetta et al., 2016; Deverman & Patterson, 2009; Estes & McAllister, 2015; Knuesel et al., 2014; Meyer, 2019). Given that we found decreased microglial colonization in the prefrontal cortex and given the data that microglial depletion decreases cognitive flexibility (Nelson & Lenz, 2017; Vanryzin et al., 2016), more investigation into how developmental microglia depletion may sculpt adult cognition in this model of allergic inflammation is merited.
4.5. Does sex matter?
The various sex biases in neurodevelopmental disorders necessitate the study of sex differences in rodent models of MIA. Many MIA studies have found male offspring to be more vulnerable to detrimental effects (Bolton et al., 2012, 2014; Makinson et al., 2017; Santos-Galindo et al., 2011; Taylor et al., 2012; Wynne et al., 2011). Additionally, male fetuses have compromised placental function (McCarthy, 2019; Nugent et al., 2018; Nugent & Bale, 2015), and glial and inflammatory genes dysregulated in ASD are more highly expressed in the fetal male brain (Werling et al., 2016), potentially rendering them more vulnerable to alterations in immune function. Here, we found the presence and absence of multiple sex differences that merit discussion.
With regard to baseline sex differences, we replicated previous data from our lab showing that males have more mast cells in the neonatal hippocampus compared to females (Joshi et al., 2019), suggesting males may be more primed to react to allergen-related immune perturbations. It is possible this sex difference could be driven by heightened hippocampal cell proliferation at this time point (Khalaf-Nazzal & Francis, 2013), particularly in males (Bowers et al., 2010).
In terms of microglia, males had more total and ameboid microglia compared to females in the GD15 basal ganglia, a region that has previously not been analyzed in the context of sex differences. However, this effect was transient, as no sex differences were found in the basal ganglia at PN0 or 4. We have found that females have fewer dendritic spines in the basal ganglia at P5 and P30 (unpublished data). Perhaps the increased microglial number in fetal males patterns this postnatal sex difference. We also reproduced previous findings whereby females tended to have more phagocytic microglia in the neonatal hippocampus relative to males, and this occurs in the absence of sex differences in total microglial colonization (Nelson et al., 2017). Additionally, females tended to have increased microglial staining at PN4 in the mPFC, indicating higher ramification. Outside of these findings, there were no other sex differences in microglia at baseline for any timepoint or region. This outcome seemingly contrasts with data from a previous study, which found that females had more microglia in the amygdala at PN0 and that males had more microglia in the amygdala and the hippocampus at PN4 (Schwarz et al., 2012). Reasons for these discrepancies likely lie within our different tissue processing methods. Here, we sectioned our tissue into 45 μm thick sections, whereas Schwarz et al. utilized 14 μm sections. Ramified microglial processes can frequently extend past 14 μm in the z-dimension, thus thinner sections could cut off these processes, as well as truncate cell bodies to potentially interfere with accurate quantification and classification. Moreover, we have previously found no sex differences in total microglia number in the neonatal hippocampus or amygdala (Nelson et al., 2017; Nelson et al., 2021), supporting our current null findings within that region. Regardless, we can conclude that males and females show sex-dependent differences in mast cell number as well as region-dependent and some sex-dependent differences in microglial colonization.
We found a few sex-dependent effects of maternal allergic inflammation that also merit discussion. At GD15, allergic inflammation increased the number of ameboid microglia in the hippocampus of OVA males but not OVA females. However, only OVA females showed a change in overall ameboid tone, exhibiting a decrease in ameboid tone rather than an increase. These patterns occurred in the absence of any alterations to total microglial colonization in the hippocampus, suggesting that there are sex-specific microglial activation responses following a fetal immune challenge, rather than sex-specific alterations to recruitment or proliferation. We also found a trend for reduced phagocytic tone in the hippocampus of OVA females at PN0. These data together suggest that allergic inflammation reduces microglial activation state in the hippocampus of females during the perinatal period. Additionally, allergic inflammation decreased microglial colonization and the density of microglial staining in the amygdala of OVA males at PN4. In comparison, OVA females did not show any significant changes in microglial colonization in the amygdala at PN4, and a trend for increased microglial staining approached significance. It is likely these perturbations in microglia measures coincide with sex-specific changes in microglia physiological function, as poly(I:C) injection on GD14.5 alters the transcriptomic activity of microglia in offspring, representing a shift towards a more mature developmental stage, as well as an upregulation of inflammatory genes, at least in males (Matcovitch-Natan et al., 2016). Given that microglia phagocytose neural progenitor cells (Nelson et al., 2017) and support cell genesis (Nelson et al., 2021) in the neonatal hippocampus in a sex-dependent manner and given that microglia also support cell genesis in the amygdala (Nelson et al., 2021), the sex-dependent changes in microglial measures documented here likely have consequences for long-term brain development and function that could lead to sex-specific behavioral sequalae.
Some previous studies have found that males exhibit more social play compared to females (Meaney, 1981; Pellis & Pellis, 1990; Taylor et al., 2012; VanRyzin et al., 2019), though not all studies report a sex difference (Argue & McCarthy, 2015). Here, we did not find any baseline sex differences in social play. We tested play in treatment- and sex- mixed groups in a novel cage environment over the course of ten days. We argue that this method is just as - if not more - ecologically valid compared to measures of social play in dyads, as rats play in mix-sexed litters under normal circumstances. However, partner composition has been shown to influence play frequency. Both sexes respond to playful contacts more frequently when contacted by males rather than females (Pellis & Pellis, 1990), and play has been shown to be “contagious” whereby less playful animals increase their play behavior when paired with more playful partners (Argue & McCarthy, 2015). Similarly, in a different MIA study, Taylor et al. found that when animals were paired with a familiar sex, age, and treatment-matched conspecific, males displayed more social play than females. However, when animals were paired with an unfamiliar, treatment-unmatched conspecific, males and females demonstrated the same amount of play behaviors (Taylor et al., 2012). Thus, it is possible that interactions between partner sex and treatment groups masked sex differences in play. Novel environments have also been shown to decrease social play in males (Vanderschuren, 1995), though it is unknown whether females are similarly affected. Thus, it is possible that the novel environment reduced male play to female levels.
While we did not find baseline sex differences in play, we did find a significant interaction whereby allergic inflammation tended to decrease rough and tumble play behavior in females but not males. These data seemingly contrast with previous studies, which suggest males may be more susceptible to LPS-induced MIA (Hava et al., 2006; Kirsten, Taricano, Maiorka, et al., 2010; Kirsten et al., 2012; Taylor et al., 2012). Perhaps males displayed deficits in one specific sub-behavior of rough and tumble play (pouncing, wresting, boxing, or pinning), but compensated for this with increases in other behaviors. Females also appear primarily responsible for OVA-induced increases in open field activity, which has not been documented previously. Future studies should investigate whether female behavior is particularly affected in models of maternal allergic inflammation versus models of maternal infection. Furthermore, while we did not find major behavioral sex differences in the current study, it does appear that males and females may be differentially sensitive to changes in neuroimmune cell number as a result of gestational allergic inflammation, warranting further rigorous investigation into these behavioral phenotypes and any mediating influence of sex as a variable.
3. CONCLUSION:
Here, we demonstrated that a single maternal allergic challenge on GD15 significantly alters the perinatal neuroimmune milieu in multiple brain regions and confers life-long effects on offspring social, emotional, and cognitive behaviors. The activity of brain-resident immune cells during the perinatal period has been causally implicated in the organization of later life behavior (Lenz et al., 2013, 2019; Nelson & Lenz, 2017; VanRyzin et al., 2016, 2019; Zhan et al., 2014), and we have previously shown that our maternal allergic inflammation paradigm affects the masculinization process in the preoptic area and confers sex-dependent changes in adult sexual behavior (Lenz et al., 2019). Furthermore, it is possible that our histological and behavioral findings are causally related, though this remains a subject for future investigation. Our results function as a basis for understanding the consequences of maternal allergic inflammation and provide directions for future study on the mechanisms underlying neurodevelopmental disorders linked to maternal immune activation in humans.
Supplementary Material
Supplementary Figure 1. Allergic inflammation does not affect the number of mast cells in the septum (A), mPFC (B), amygdala (C) or BNST(D). N’s = 3/group/sex for septum, AMY, and BNST; N’s = 5 MV, 5 FV, 5 MOVA, and 9 FOVA for the mPFC. AMY = amygdala, BNST = bed nucleus of the stria terminalis, mPFC = medial prefrontal cortex.
Figure 4.
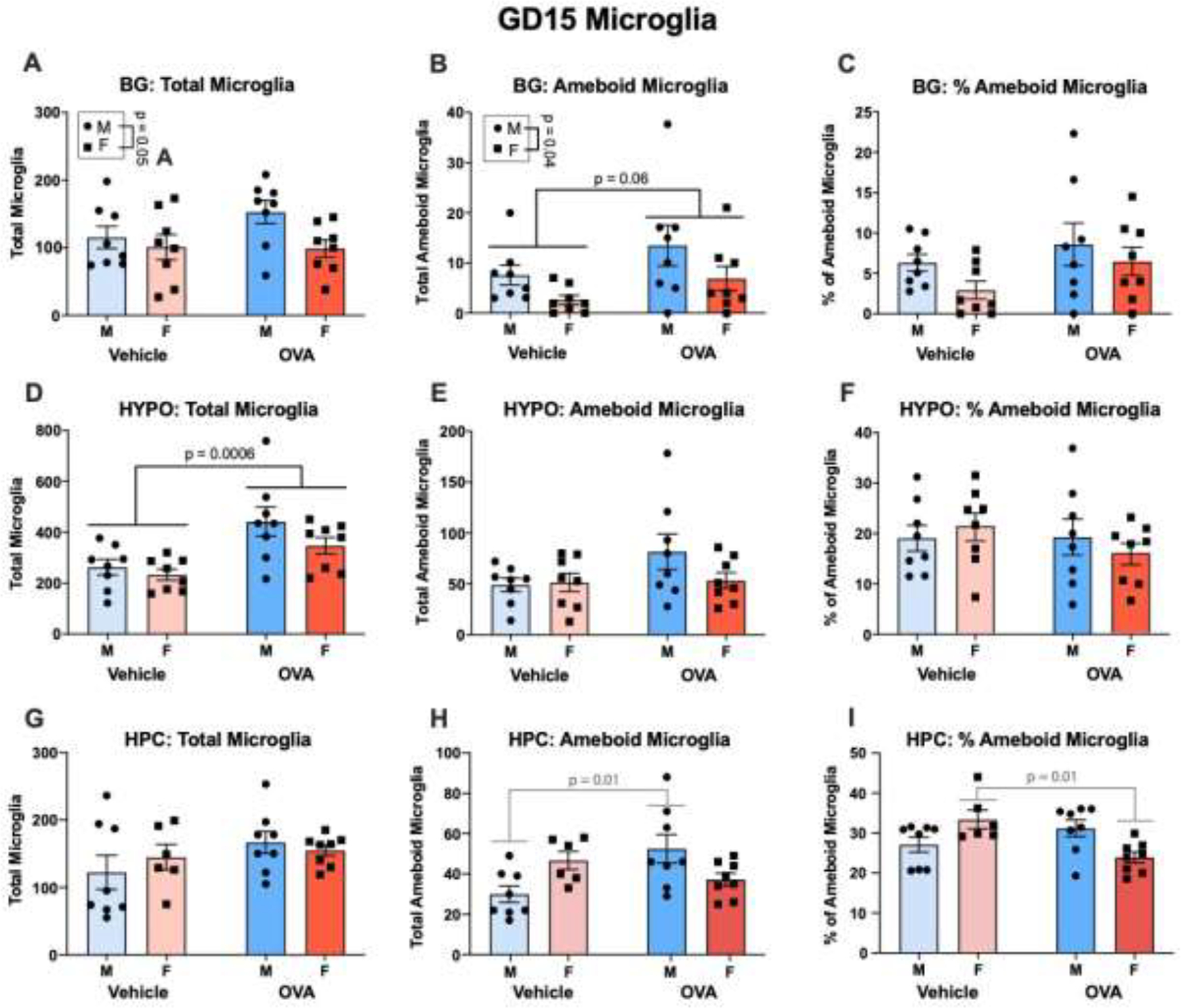
Maternal allergic inflammation acutely produces sex- and region- specific changes in fetal brain microglia. (A-C) In the basal ganglia, males had more total and phagocytic microglia than females, and maternal allergic inflammation increased ameboid microglia without changing ameboid tone. N’s = 8/group/sex. (D-F) Allergic inflammation increased microglial colonization of the hypothalamus, without changing the number of ameboid microglia or ameboid tone. N’s = 8/group/sex. (G-I) Allergic inflammation increased the number of ameboid microglia in the male hippocampus only, yet decreased ameboid tone in the female hippocampus. N’s = 8 MV, 6 FV, 8 MOVA, 8 FOVA. BG = basal ganglia, HYPO = hypothalamus, HPC = hippocampus.
Figure 5.
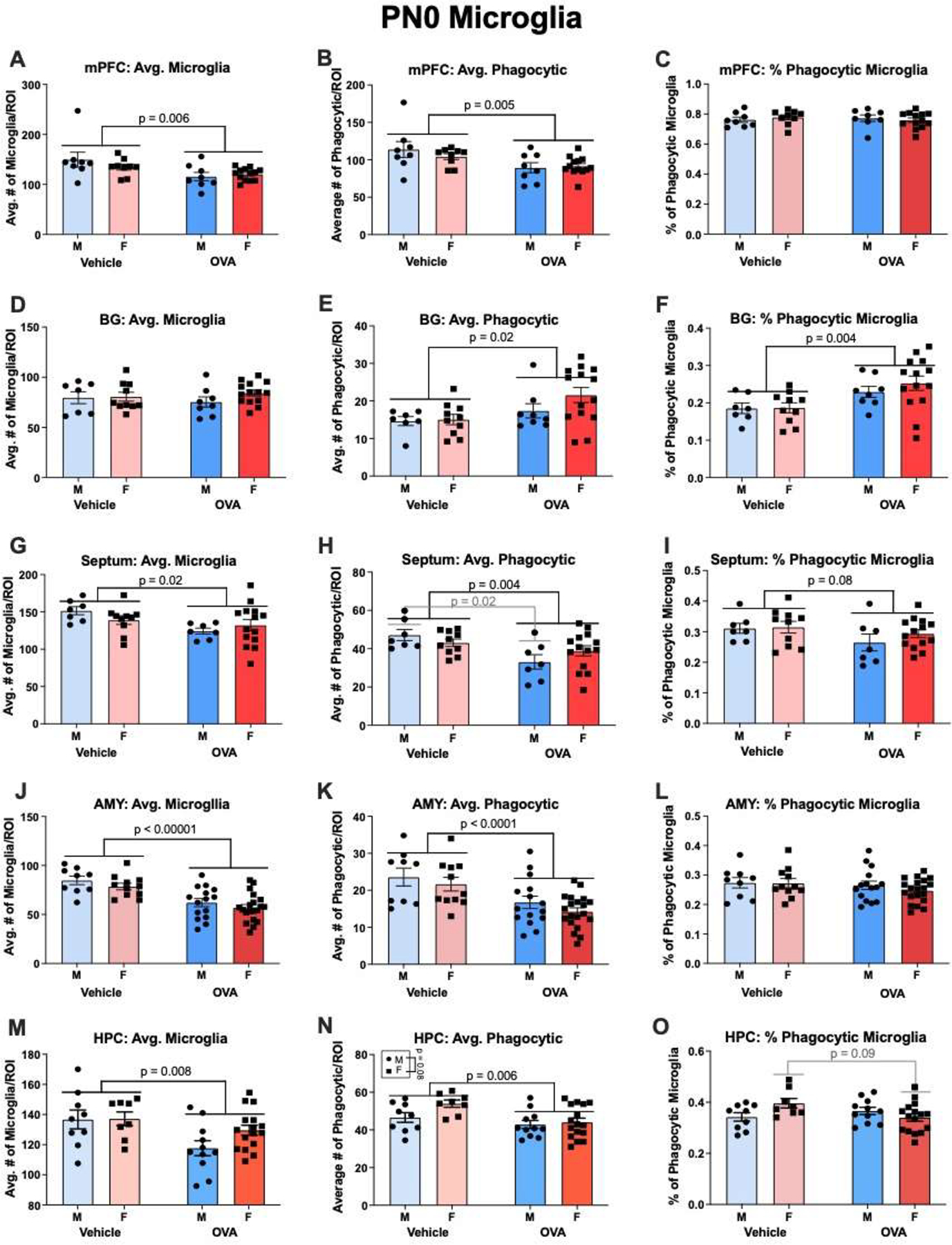
Gestational allergic inflammation alters offspring microglia in a region-dependent manner on PN0. (A-C) OVA offspring had lower counts of total and phagocytic microglia, but no change in phagocytic tone in the mPFC. N’s = 8 MV, 9 FV, 8 MOVA, 13 FOVA. (D-F) OVA offspring had increased phagocytic microglia and thus an increase in phagocytic tone in the basal ganglia. N’s = 7 MVs, 10 FVs, 8 MOVAs, 14 FOVAs. (G-I) OVA offspring had fewer total and phagocytic microglia in the septum, and this contributed to a decrease in phagocytic tone that approached significance. Notably, an interaction approached significance whereby OVA males, but not OVA females had fewer phagocytic microglia. N’s = 7 MV, 10 FV, 7 MOVA, 14 FOVA. (J-L) As with the mPFC, OVA offspring had fewer total and phagocytic microglia in the amygdala, but there was no change in phagocytic tone. N’s = 9 MV, 11 FV, 15 MOVA, 20 FOVA. (M-O) OVA offspring displayed fewer total and phagocytic microglia in the hippocampus. A significant interaction found that Allergic inflammation also tended to decrease the phagocytic tone in the hippocampus of females, but not males. N’s = 9 MV, 8 FV, 11 MOVA, 16 FOVA. mPFC = medial prefrontal cortex, BG = basal ganglia, AMY = amygdala, HPC = hippocampus.
Figure 6.
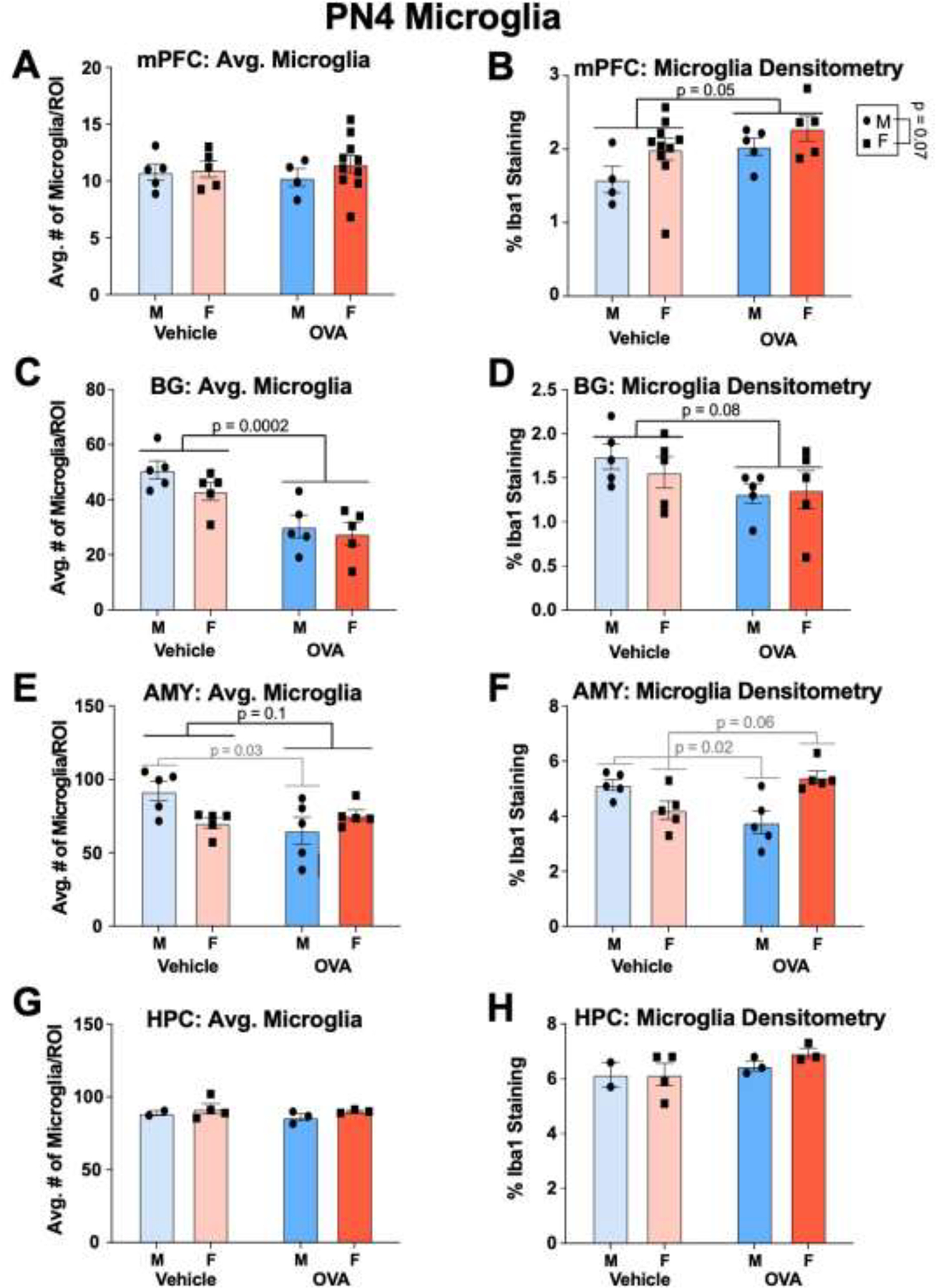
Gestational allergic inflammation alters offspring microglia in a region-dependent manner on PN4. (A-B) OVA offspring did not show changes in the number of microglia in the mPFC, but microglial staining was increased. Males tended to have increased staining relative to females. N’s = 5 MVs, 5 FVs, 4 MOVAs, 10 FOVAs for A, and N’s = 4 MVs, 10 FVs, 5 MOVAs, and 4 FOVAs for B. (C-D) OVA offspring had fewer microglia in the basal ganglia relative to vehicle offspring, and there was a trend for a decrease in microglial staining. N’s = 5/group/sex. (E-F) OVA offspring tended to have fewer microglia in the amygdala, and this was due to a significant decrease in microglia in the males. Conversely, allergic inflammation decreased microglial staining in the amygdala of male offspring and increased staining in females. N’s = 5/group/sex. (G-H) Allergic inflammation did not affect microglial number or staining in the P4 hippocampus. N’s = 2 MV, 4 FV, 3 MOVA, 3 FOVA. mPFC = medial prefrontal cortex, BG = basal ganglia, AMY = amygdala, HPC = hippocampus.
5. ACKNOWLEDGEMENTS
The authors would like to thank Angela Saulsberry for thoughtful comments on the manuscript. MRB would like to thank Tracy and Maureen Breach for their support during her graduate school application process.
Funding:
This work was supported by NIH R21MH105826 to KML.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing: The data presented here are available from the corresponding author upon reasonable request.
Conflict of Interest Statement: The authors declare no competing financial interests.
REFERENCES
- Aguilar-Valles A, Rodrigue B, & Matta-Camacho E (2020). Maternal Immune Activation and the Development of Dopaminergic Neurotransmission of the Offspring: Relevance for Schizophrenia and Other Psychoses. Frontiers in Psychiatry, 11. 10.3389/fpsyt.2020.00852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argue KJ, & McCarthy MM (2015). Utilization of same- vs. Mixed-sex dyads impacts the observation of sex differences in juvenile social play behavior. Current Neurobiology, 6(1), 17–23. 10.4172/0975-9042.000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, & Van de Water J (2011). Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, Behavior, and Immunity, 25(1), 40–45. 10.1016/j.bbi.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger AP, & Olesen KM (2009). Brain Sex Differences and the Organisation of Juvenile Social Play Behaviour. Journal of Neuroendocrinology, 21(6), 519–525. 10.1111/j.1365-2826.2009.01871.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Rosenberg CR, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee L-C, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, … Hall-Lande J (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. 67(6), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, & Wicks B (2017). Sex-specific mechanisms for responding to stress. Journal of Neuroscience Research, 95(1–2), 75–82. 10.1002/jnr.23812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Block CL, Bolton JL, Hanamsagar R, & Tran PK (2018). Beyond infection—Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Experimental Neurology, 299, 241–251. 10.1016/j.expneurol.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, & Brown VJ (2000). Medial Frontal Cortex Mediates Perceptual Attentional Set Shifting in the Rat. Journal of Neuroscience, 20(11), 4320–4324. 10.1523/JNEUROSCI.20-11-04320.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Auten RL, & Bilbo SD (2014). Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brain, Behavior, and Immunity, 37, 30–44. 10.1016/j.bbi.2013.10.029 [DOI] [PubMed] [Google Scholar]
- Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, & Bilbo SD (2012). Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. The FASEB Journal, 26(11), 4743–4754. 10.1096/fj.12-210989 [DOI] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arévalo-Martin A, Molina-Holgado E, & Guaza C (2002). Prenatal immune challenge disrupts sensorimotor gating in adult rats: Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology, 26(2), 204–215. 10.1016/S0893-133X(01)00360-8 [DOI] [PubMed] [Google Scholar]
- Bowers JM, Waddell J, & McCarthy MM (2010). A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biology of Sex Differences, 1(1), 8. 10.1186/2042-6410-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredewold R, Schiavo JK, van der Hart M, Verreij M, & Veenema AH (2015). Dynamic changes in extracellular release of GABA and glutamate in the lateral septum during social play behavior in juvenile rats: Implications for sex-specific regulation of social play behavior. Neuroscience, 307, 117–127. 10.1016/j.neuroscience.2015.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredewold R, Smith CJW, Dumais KM, & Veenema AH (2014). Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Frontiers in Behavioral Neuroscience, 8. 10.3389/fnbeh.2014.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS (2012). Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Developmental Neurobiology, 72(10), 1272–1276. 10.1002/dneu.22024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, & Tait DS (2016). Attentional Set-Shifting Across Species. In Robbins TW & Sahakian BJ (Eds.), Translational Neuropsychopharmacology (pp. 363–395). Springer International Publishing. 10.1007/7854_2015_5002 [DOI] [PubMed] [Google Scholar]
- Canetta S, Bolkan S, Padilla-Coreano N, Song LJ, Sahn R, Harrison NL, Gordon JA, Brown A, & Kellendonk C (2016). Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Molecular Psychiatry, 21(7), 956–968. 10.1038/mp.2015.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta S, Sourander A, Surcel H-M, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C, McKeague IW, & Brown AS (2014). Elevated Maternal C-Reactive Protein and Increased Risk of Schizophrenia in a National Birth Cohort. American Journal of Psychiatry, 171(9), 960–968. 10.1176/appi.ajp.2014.13121579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalá-López F, Peiró S, Ridao M, Sanfélix-Gimeno G, Gènova-Maleras R, & Catalá MA (2012). Prevalence of attention deficit hyperactivity disorder among children and adolescents in Spain: A systematic review and meta-analysis of epidemiological studies. BMC Psychiatry, 12(1), 168. 10.1186/1471-244X-12-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. wei, Zhong X. shan, Jiang L. na, Zheng X. yan, Xiong Y. quan, Ma S. juan, Qiu M, Huo S. ting, Ge J, & Chen Q (2016). Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. Behavioural Brain Research, 296, 61–69. 10.1016/j.bbr.2015.08.035 [DOI] [PubMed] [Google Scholar]
- Chiavegatto S, Nasello AG, & Bernardi MM (1998). Histamine and spontaneous motor activity: Biphasic changes, receptors involved and participation of the striatal dopamine system. Life Sciences, 62(20), 1875–1888. 10.1016/S0024-3205(98)00154-4 [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, & De Water J. Van. (2005). Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: A case-control study. Archives of Pediatrics and Adolescent Medicine, 159(2), 151–157. 10.1001/archpedi.159.2.151 [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Martinez-Cerdeno V, & Noctor SC (2013). Microglia Regulate the Number of Neural Precursor Cells in the Developing Cerebral Cortex. Journal of Neuroscience, 33(10), 4216–4233. 10.1523/JNEUROSCI.3441-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czura CJ, Rosas-Ballina M, & Tracey KJ (2007). Cholinergic Regulation of Inflammation. In Psychoneuroimmunology (pp. 85–96). Elsevier. 10.1016/B978-012088576-3/50007-1 [DOI] [Google Scholar]
- Daenen EWPM, Wolterink G, Gerrits MAFM, & Van Ree JM (2002). The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behavioural Brain Research, 136(2), 571–582. 10.1016/S0166-4328(02)00223-1 [DOI] [PubMed] [Google Scholar]
- Davies W (2014). Sex differences in Attention Deficit Hyperactivity Disorder: Candidate genetic and endocrine mechanisms. Frontiers in Neuroendocrinology, 35(3), 331–346. 10.1016/j.yfrne.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Depino AM (2015). Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience, 299, 56–65. 10.1016/j.neuroscience.2015.04.065 [DOI] [PubMed] [Google Scholar]
- Deverman BE, & Patterson PH (2009). Cytokines and CNS Development. Neuron, 64(1), 61–78. 10.1016/j.neuron.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Dong H, Zhang X, & Qian Y (2014). Mast Cells and Neuroinflammation. Medical Science Monitor Basic Research, 20, 200–206. 10.12659/MSMBR.893093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes ML, & McAllister AK (2015). Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nature Reviews Neuroscience, 16(8), 469–486. 10.1038/nrn3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, & Tye KM (2014). Amygdala Inputs to the Ventral Hippocampus Bidirectionally Modulate Social Behavior. Journal of Neuroscience, 34(2), 586–595. 10.1523/JNEUROSCI.4257-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, González MI, Wilson CA, & File SE (1999). Factor Analysis Shows That Female Rat Behaviour Is Characterized Primarily by Activity, Male Rats Are Driven by Sex and Anxiety. Pharmacology Biochemistry and Behavior, 64(4), 731–736. 10.1016/S0091-3057(99)00139-2 [DOI] [PubMed] [Google Scholar]
- File SE (2001). Factors controlling measures of anxiety and responses to novelty in the mouse. Behavioural Brain Research, 125(1–2), 151–157. 10.1016/S0166-4328(01)00292-3 [DOI] [PubMed] [Google Scholar]
- Frick LR, & Pittenger C (2016). Microglial Dysregulation in OCD, Tourette Syndrome, and PANDAS. Journal of Immunology Research, 2016, 1–8. 10.1155/2016/8606057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick LR, Rapanelli M, Abbasi E, Ohtsu H, & Pittenger C (2016). Histamine regulation of microglia: Gene-environment interaction in the regulation of central nervous system inflammation. Brain, Behavior, and Immunity, 57, 326–337. 10.1016/j.bbi.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JL, & Schafer DP (2016). Microglia: Architects of the Developing Nervous System. Trends in Cell Biology, 26(8), 587–597. 10.1016/j.tcb.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganong WF (2000). Circumventricular Organs: Definition And Role In The Regulation Of Endocrine And Autonomic Function. Clinical and Experimental Pharmacology and Physiology, 27(5–6), 422–427. 10.1046/j.1440-1681.2000.03259.x [DOI] [PubMed] [Google Scholar]
- Garay PA, Hsiao EY, Patterson PH, & McAllister AK (2013). Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain, Behavior, and Immunity, 31, 54–68. 10.1016/j.bbi.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Feldon J, Schedlowski M, & Meyer U (2013). Stress in Puberty Unmasks Latent Neuropathological Consequences of Prenatal Immune Activation in Mice. In Science (Vol. 339, Issue 6123). American Association for the Advancement of Science. 10.1126/science.1231897 [DOI] [PubMed] [Google Scholar]
- Graciarena M, Depino AM, & Pitossi FJ (2010). Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFβ1 downregulation. Brain, Behavior, and Immunity, 24(8), 1301–1309. 10.1016/j.bbi.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, & Shansky RM (2015). Sexually divergent expression of active and passive conditioned fear responses in rats. ELife, 4, e11352. 10.7554/eLife.11352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, & Kreitzer AC (2016). Cortico–Basal Ganglia Circuit Function in Psychiatric Disease. Annual Review of Physiology, 78(1), 327–350. 10.1146/annurev-physiol-021115-105355 [DOI] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA, & Selbach O (2008). Histamine in the Nervous System. Physiological Reviews, 88(3), 1183–1241. 10.1152/physrev.00043.2007 [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, & Bilbo SD (2016). Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. The Journal of Steroid Biochemistry and Molecular Biology, 160, 127–133. 10.1016/j.jsbmb.2015.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S, & Guerre-Millo M (2006). The Placenta Cytokine Network and Inflammatory Signals. Placenta, 27(8), 794–798. 10.1016/j.placenta.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Hava G, Vered L, Yael M, Mordechai H, & Mahoud H (2006). Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Developmental Psychobiology, 48(2), 162–168. 10.1002/dev.20116 [DOI] [PubMed] [Google Scholar]
- Imhof J (1993). Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behavioural Brain Research, 56(2), 177–180. 10.1016/0166-4328(93)90036-P [DOI] [PubMed] [Google Scholar]
- Instanes JT, Halmøy A, Engeland A, Haavik J, Furu K, & Klungsøyr K (2017). Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers With Inflammatory and Immune System Diseases. Biological Psychiatry, 81(5), 452–459. 10.1016/j.biopsych.2015.11.024 [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Xiao H, Wilkie MB, Lauder JM, & Gilmore JH (1997). Cytokine regulation of embryonic rat dopamine and serotonin neuronal survival in vitro. International Journal of Developmental Neuroscience, 15(6), 711–716. 10.1016/S0736-5748(97)00029-4 [DOI] [PubMed] [Google Scholar]
- Johnston AL, & File SE (1991). Sex differences in animal tests of anxiety. Physiology & Behavior, 49(2), 245–250. 10.1016/0031-9384(91)90039-Q [DOI] [PubMed] [Google Scholar]
- Joshi A, Page CE, Damante M, Dye CN, Haim A, Leuner B, & Lenz KM (2019). Sex differences in the effects of early life stress exposure on mast cells in the developing rat brain. Hormones and Behavior, 113, 76–84. 10.1016/j.yhbeh.2019.04.012 [DOI] [PubMed] [Google Scholar]
- Juckel G, Manitz MP, Brüne M, Friebe A, Heneka MT, & Wolf RJ (2011). Microglial activation in a neuroinflammational animal model of schizophrenia—A pilot study. Schizophrenia Research, 131(1–3), 96–100. 10.1016/j.schres.2011.06.018 [DOI] [PubMed] [Google Scholar]
- Katayama T, Jodo E, Suzuki Y, Hoshino K-Y, Takeuchi S, & Kayama Y (2009). Phencyclidine affects firing activity of basolateral amygdala neurons related to social behavior in rats. Neuroscience, 159(1), 335–343. 10.1016/j.neuroscience.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Khalaf-Nazzal R, & Francis F (2013). Hippocampal development – Old and new findings. Neuroscience, 248, 225–242. 10.1016/j.neuroscience.2013.05.061 [DOI] [PubMed] [Google Scholar]
- Khalil M, Ronda J, Weintraub M, Jain K, Silver R, & Silverman A-J (2007). Brain mast cell relationship to neurovasculature during development. Brain Research, 1171, 18–29. 10.1016/j.brainres.2007.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh Y-J, Fombonne E, Laska E, Lim E-C, Cheon K-A, Kim S-J, Kim Y-K, Lee H, Song D-H, & Grinker RR (2011). Prevalence of Autism Spectrum Disorders in a Total Population Sample. American Journal of Psychiatry, 168(9), 904–912. 10.1176/appi.ajp.2011.10101532 [DOI] [PubMed] [Google Scholar]
- Kirsten TB, Chaves-Kirsten GP, Chaible LM, Silva AC, Martins DO, Britto LRG, Dagli MLZ, Torrão AS, Palermo-Neto J, & Bernardi MM (2012). Hypoactivity of the central dopaminergic system and autistic-like behavior induced by a single early prenatal exposure to lipopolysaccharide. Journal of Neuroscience Research, 90(10), 1903–1912. 10.1002/jnr.23089 [DOI] [PubMed] [Google Scholar]
- Kirsten TB, Taricano M, Flório JC, Palermo-Neto J, & Bernardi MM (2010). Prenatal lipopolysaccharide reduces motor activity after an immune challenge in adult male offspring. Behavioural Brain Research, 211(1), 77–82. 10.1016/j.bbr.2010.03.009 [DOI] [PubMed] [Google Scholar]
- Kirsten TB, Taricano M, Maiorka PC, Palermo-Neto J, & Bernardi MM (2010). Prenatal Lipopolysaccharide Reduces Social Behavior in Male Offspring. Neuroimmunomodulation, 17(4), 240–251. 10.1159/000290040 [DOI] [PubMed] [Google Scholar]
- Kloiber S, Rosenblat JD, Husain MI, Ortiz A, Berk M, Quevedo J, Vieta E, Maes M, Birmaher B, Soares JC, & Carvalho AF (2020). Neurodevelopmental pathways in bipolar disorder. Neuroscience & Biobehavioral Reviews, 112, 213–226. 10.1016/j.neubiorev.2020.02.005 [DOI] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, & Prinssen EP (2014). Maternal immune activation and abnormal brain development across CNS disorders. Nature Reviews Neurology, 10(11), 643–660. 10.1038/nrneurol.2014.187 [DOI] [PubMed] [Google Scholar]
- Kokras N, & Dalla C (2014). Sex differences in animal models of psychiatric disorders: Sex differences in models of psychiatric disorders. British Journal of Pharmacology, 171(20), 4595–4619. 10.1111/bph.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec AM, Smith CJ, Ayre NR, Sweat SC, & Bilbo SD (2018). Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nature Communications, 9(1), 3769. 10.1038/s41467-018-06118-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H-Y, & Liu F-C (2019). Synaptic Wiring of Corticostriatal Circuits in Basal Ganglia: Insights into the Pathogenesis of Neuropsychiatric Disorders. Eneuro, 6(3), ENEURO.0076–19.2019. 10.1523/ENEURO.0076-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambracht-Hall M, Dimitriadou V, & Theoharides TC (1990). Migration of mast cells in the developing rat brain. Developmental Brain Research, 56(2), 151–159. 10.1016/0165-3806(90)90077-C [DOI] [PubMed] [Google Scholar]
- Larson AA, Thomas MJ, McElhose A, & Kovács KJ (2011). Spontaneous locomotor activity correlates with the degranulation of mast cells in the meninges rather than in the thalamus: Disruptive effect of cocaine. Brain Research, 1395, 30–37. 10.1016/j.brainres.2011.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R, & McCarthy MM (2013). Microglia Are Essential to Masculinization of Brain and Behavior. Journal of Neuroscience, 33(7), 2761–2772. 10.1523/JNEUROSCI.1268-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Pickett LA, Wright CL, Davis KT, Joshi A, & McCarthy MM (2018). Mast Cells in the Developing Brain Determine Adult Sexual Behavior. Journal of Neuroscience, 38(37), 8044–8059. 10.1523/JNEUROSCI.1176-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Pickett LA, Wright CL, Galan A, & McCarthy MM (2019). Prenatal Allergen Exposure Perturbs Sexual Differentiation and Programs Lifelong Changes in Adult Social and Sexual Behavior. Scientific Reports, 9(1), 4837. 10.1038/s41598-019-41258-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Fredericks PJ, Nealer C, & Albin-Brooks C (2014). Chronic Gestational Stress Leads to Depressive-Like Behavior and Compromises Medial Prefrontal Cortex Structure and Function during the Postpartum Period. PLOS ONE, 9(3), e89912. 10.1371/journal.pone.0089912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Gayle DA, Ma SY, Lipton JW, Tong CW, Hong J-S, & Carvey PM (2002). In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Movement Disorders, 17(1), 116–124. 10.1002/mds.10078 [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, & McEwen BS (2006). Stress-Induced Alterations in Prefrontal Cortical Dendritic Morphology Predict Selective Impairments in Perceptual Attentional Set-Shifting. Journal of Neuroscience, 26(30), 7870–7874. 10.1523/JNEUROSCI.1184-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Ashwood P, Van De Water J, & Hertz-Picciotto I (2014). Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. Journal of Autism and Developmental Disorders, 44(7), 1546–1555. 10.1007/s10803-013-2017-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinson R, Lloyd K, Rayasam A, McKee S, Brown A, Barila G, Grissom N, George R, Marini M, Fabry Z, Elovitz M, & Reyes TM (2017). Intrauterine inflammation induces sex-specific effects on neuroinflammation, white matter, and behavior. Brain, Behavior, and Immunity, 66, 277–288. 10.1016/j.bbi.2017.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, & Patterson PH (2012). Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain, Behavior, and Immunity, 26(4), 607–616. 10.1016/j.bbi.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitz MP, Esslinger M, Wachholz S, Plümper J, Friebe A, Juckel G, & Wolf R (2013). The role of microglia during life span in neuropsychiatric disease—An animal study. Schizophrenia Research, 143(1), 221–222. 10.1016/j.schres.2012.10.028 [DOI] [PubMed] [Google Scholar]
- Marín-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, & Mallat M (2004). Microglia Promote the Death of Developing Purkinje Cells. Neuron, 41(4), 535–547. 10.1016/S0896-6273(04)00069-8 [DOI] [PubMed] [Google Scholar]
- Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada Gonzalez F, Perrin P, Keren-Shaul H, Gury M, Lara-Astaiso D, Thaiss CA, Cohen M, Bahar Halpern K, Baruch K, Deczkowska A, Lorenzo-Vivas E, … Amit I (2016). Microglia development follows a stepwise program to regulate brain homeostasis. Science, 353(6301), aad8670–aad8670. 10.1126/science.aad8670 [DOI] [PubMed] [Google Scholar]
- Mazzoni IE, & Kenigsberg RL (1996). Microglia from the developing rat medial septal area can affect cholinergic and GABAergic neuronal differentiation in vitro. Neuroscience, 76(1), 147–157. 10.1016/S0306-4522(96)00235-7 [DOI] [PubMed] [Google Scholar]
- McAlonan K, & Brown VJ (2003). Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research, 146(1), 97–103. 10.1016/j.bbr.2003.09.019 [DOI] [PubMed] [Google Scholar]
- McCarthy MM (2019). Sex differences in neuroimmunity as an inherent risk factor. Neuropsychopharmacology, 44(1), 38–44. 10.1038/s41386-018-0138-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM, & Lenz KM (2017). Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nature Reviews Neuroscience, 18(8), 471–484. 10.1038/nrn.2017.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M (1981). Neonatal androgens influence the social play of prepubescent rats. Hormones and Behavior, 15(2), 197–213. 10.1016/0018-506X(81)90028-3 [DOI] [PubMed] [Google Scholar]
- Meyer U (2006). The Time of Prenatal Immune Challenge Determines the Specificity of Inflammation-Mediated Brain and Behavioral Pathology. Journal of Neuroscience, 26(18), 4752–4762. 10.1523/JNEUROSCI.0099-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U (2014). Prenatal Poly(I:C) Exposure and Other Developmental Immune Activation Models in Rodent Systems. Biological Psychiatry, 75(4), 307–315. 10.1016/j.biopsych.2013.07.011 [DOI] [PubMed] [Google Scholar]
- Meyer U (2019). Neurodevelopmental Resilience and Susceptibility to Maternal Immune Activation. Trends in Neurosciences, 42(11), 793–806. 10.1016/j.tins.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, & Fatemi SH (2009). In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neuroscience & Biobehavioral Reviews, 33(7), 1061–1079. 10.1016/j.neubiorev.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Meyer U, Schwarz MJ, & Müller N (2011). Inflammatory processes in schizophrenia: A promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacology and Therapeutics, 132(1), 96–110. 10.1016/j.pharmthera.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Missault S, Van den Eynde K, Vanden Berghe W, Fransen E, Weeren A, Timmermans JP, Kumar-Singh S, & Dedeurwaerdere S (2014). The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain, Behavior, and Immunity, 42, 138–146. 10.1016/j.bbi.2014.06.013 [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, & Nabekura J (2016). Microglia contact induces synapse formation in developing somatosensory cortex. Nature Communications, 7(1), 12540. 10.1038/ncomms12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, Yamauchi Y, Yamada S, & Kanba S (2013). Neuroinflammation in schizophrenia especially focused on the role of microglia. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 42, 115–121. 10.1016/j.pnpbp.2011.12.002 [DOI] [PubMed] [Google Scholar]
- Nautiyal KM, Dailey CA, Jahn JL, Rodriquez E, Son NH, Sweedler JV, & Silver R (2012). Serotonin of mast cell origin contributes to hippocampal function: Hippocampal serotonin of mast cell origin. European Journal of Neuroscience, 36(3), 2347–2359. 10.1111/j.1460-9568.2012.08138.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Ribeiro CA, Pfaff DW, & Silver R (2008). Brain mast cells link the immune system to anxiety-like behavior. Proceedings of the National Academy of Sciences, 105(46), 18053–18057. 10.1073/pnas.0809479105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LH, & Lenz KM (2017). Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behavioural Brain Research, 316, 279–293. 10.1016/j.bbr.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LH, Saulsbery AI, & Lenz KM (2019). Small cells with big implications: Microglia and sex differences in brain development, plasticity and behavioral health. Progress in Neurobiology, 176(March 2018), 103–119. 10.1016/j.pneurobio.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LH, Warden S, & Lenz KM (2017). Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain, Behavior, and Immunity, 64, 11–22. 10.1016/j.bbi.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick D, Haro JM, Hong J, Brugnoli R, Lepine JP, Bertsch J, Karagianis J, Dossenbach M, & Alvarez E (2012). Regional differences in treatment response and three year course of schizophrenia across the world. Journal of Psychiatric Research, 46(7), 856–864. 10.1016/j.jpsychires.2012.03.017 [DOI] [PubMed] [Google Scholar]
- Nugent BM, & Bale TL (2015). The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Frontiers in Neuroendocrinology, 39, 28–37. 10.1016/j.yfrne.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, O’Donnell CM, Epperson CN, & Bale TL (2018). Placental H3K27me3 establishes female resilience to prenatal insults. Nature Communications, 9(1), 2555. 10.1038/s41467-018-04992-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2011). The Vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. The Journal of Comparative Neurology, 519(18), 3599–3639. 10.1002/cne.22735 [DOI] [PubMed] [Google Scholar]
- Panula P, & Nuutinen S (2013). The histaminergic network in the brain: Basic organization and role in disease. Nature Reviews Neuroscience, 14(7), 472–487. 10.1038/nrn3526 [DOI] [PubMed] [Google Scholar]
- Patterson PH (2009). Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behavioural Brain Research, 204(2), 313–321. 10.1016/j.bbr.2008.12.016 [DOI] [PubMed] [Google Scholar]
- Patterson PH (2011). Modeling autistic features in animals. Pediatric Research, 69(5 PART 2), 34–40. 10.1203/PDR.0b013e318212b80f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis SM, & Pellis VC (1990). Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Developmental Psychobiology, 23(3), 215–231. 10.1002/dev.420230303 [DOI] [PubMed] [Google Scholar]
- Penteado SHW, Teodorov E, Kirsten TB, Eluf BP, Reis-Silva TM, Acenjo MK, de Melo RC, Suffredini IB, & Bernardi MM (2014). Prenatal lipopolysaccharide disrupts maternal behavior, reduces nest odor preference in pups, and induces anxiety: Studies of F1 and F2 generations. European Journal of Pharmacology, 738, 342–351. 10.1016/j.ejphar.2014.05.058 [DOI] [PubMed] [Google Scholar]
- Radewicz K, Garey LJ, Gentleman SM, & Reynolds R (2000). Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. Journal of Neuropathology and Experimental Neurology, 59(2), 137–150. 10.1093/jnen/59.2.137 [DOI] [PubMed] [Google Scholar]
- Ratnayake U, Quinn T, Walker DW, & Dickinson H (2013). Cytokines and the neurodevelopmental basis of mental illness. Frontiers in Neuroscience, 7(7 OCT), 1–9. 10.3389/fnins.2013.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, & Garcia-Segura LM (2011). Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biology of Sex Differences, 2(1), 7. 10.1186/2042-6410-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, & Stevens B (2012). Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron, 74(4), 691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Chang C, Onore CE, & Ashwood P (2015). Allergic fetal priming leads to developmental, behavioral and neurobiological changes in mice. Translational Psychiatry, 5(4), e543–e543. 10.1038/tp.2015.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Coburn MA, Rose DR, Hughes HK, & Ashwood P (2017). Behavioral impact of maternal allergic-asthma in two genetically distinct mouse strains. Brain, Behavior, and Immunity, 63, 99–107. 10.1016/j.bbi.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, & Bilbo SD (2012). Sex, glia, and development: Interactions in health and disease. Hormones and Behavior, 62(3), 243–253. 10.1016/j.yhbeh.2012.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, & Bilbo SD (2012). Sex differences in microglial colonization of the developing rat brain. Journal of Neurochemistry, 120(6), 948–963. 10.1111/j.1471-4159.2011.07630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM (2015). Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiology of Stress, 1, 60–65. 10.1016/j.ynstr.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, & Sato K (2014). Microglia Enhance Neurogenesis and Oligodendrogenesis in the Early Postnatal Subventricular Zone. Journal of Neuroscience, 34(6), 2231–2243. 10.1523/JNEUROSCI.1619-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, & Bulloch K (2008). Steroid hormone receptor expression and function in microglia. Glia, 56(6), 659–674. 10.1002/glia.20644 [DOI] [PubMed] [Google Scholar]
- Silver R, & Curley JP (2013). Mast cells on the mind: New insights and opportunities. Trends in Neurosciences, 36(9), 513–521. 10.1016/j.tins.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Smolders S, Notter T, Smolders SMT, Rigo J-M, & Brône B (2018). Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders. Brain, Behavior, and Immunity, 73, 51–65. 10.1016/j.bbi.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, & Garel S (2014). Microglia Modulate Wiring of the Embryonic Forebrain. Cell Reports, 8(5), 1271–1279. 10.1016/j.celrep.2014.07.042 [DOI] [PubMed] [Google Scholar]
- Steinberg BE, Silverman HA, Robbiati S, Gunasekaran MK, Tsaava T, Battinelli E, Stiegler A, Bouton CE, Chavan SS, Tracey KJ, & Huerta PT (2016). Cytokine-specific Neurograms in the Sensory Vagus Nerve. Bioelectronic Medicine, 3(1), 7–17. 10.15424/bioelectronmed.2016.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, Yoshihara Y, Omata K, Matsumoto K, Tsuchiya KJ, Iwata Y, Tsujii M, Sugiyama T, & Mori N (2013). Microglial activation in young adults with autism spectrum disorder. Archives of General Psychiatry, 70(1), 49–58. 10.1001/jamapsychiatry.2013.272 [DOI] [PubMed] [Google Scholar]
- Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, & de Vries GJ (2012). Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biology of Sex Differences, 3(1), 15. 10.1186/2042-6410-3-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Stewart JM, Panagiotidou S, & Melamed I (2016). Mast cells, brain inflammation and autism. European Journal of Pharmacology, 778, 96–102. 10.1016/j.ejphar.2015.03.086 [DOI] [PubMed] [Google Scholar]
- Thorup A, Albert N, Bertelsen M, Petersen L, Jeppesen P, Quack PL, Krarup G, Jørgensen P, & Nordentoft M (2014). Gender differences in first-episode psychosis at 5-year follow-up – two different courses of disease? Results from the OPUS study at 5-year follow-up. European Psychiatry, 29(1), 44–51. 10.1016/j.eurpsy.2012.11.005 [DOI] [PubMed] [Google Scholar]
- Thorup A, Petersen L, Jeppesen P, Ohlenschlæger J, Christensen T, Krarup G, Jorgensen P, & Nordentoft M (2007). Gender Differences in Young Adults With First-Episode Schizophrenia Spectrum Disorders at Baseline in the Danish OPUS Study: The Journal of Nervous and Mental Disease, 195(5), 396–405. 10.1097/01.nmd.0000253784.59708.dd [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, & Vanderschuren LJMJ (2011). Evaluating the rewarding nature of social interactions in laboratory animals. Developmental Cognitive Neuroscience, 1(4), 444–458. 10.1016/j.dcn.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, & Yamashita T (2013). Layer V cortical neurons require microglial support for survival during postnatal development. Nature Neuroscience, 16(5), 543–551. 10.1038/nn.3358 [DOI] [PubMed] [Google Scholar]
- Van den Eynde K, Missault S, Fransen E, Raeymaekers L, Willems R, Drinkenburg W, Timmermans J-P, Kumar-Singh S, & Dedeurwaerdere S (2014). Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behavioural Brain Research, 258, 179–186. 10.1016/j.bbr.2013.10.005 [DOI] [PubMed] [Google Scholar]
- van Kerkhof LWM, Trezza V, Mulder T, Gao P, Voorn P, & Vanderschuren LJMJ (2013). Cellular activation in limbic brain systems during social play behaviour in rats. Brain Structure and Function. 10.1007/s00429-013-0558-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ (1995). Influence of environmental factors on social play behavior of juvenile rats. Physiology & Behavior, 58(1), 119–123. 10.1016/0031-9384(94)00385-I [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Achterberg EJM, & Trezza V (2016). The neurobiology of social play and its rewarding value in rats. Neuroscience & Biobehavioral Reviews, 70, 86–105. 10.1016/j.neubiorev.2016.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, & Trezza V (2014). What the Laboratory Rat has Taught us About Social Play Behavior: Role in Behavioral Development and Neural Mechanisms. In Andersen SL & Pine DS (Eds.), The Neurobiology of Childhood (pp. 189–212). Springer. 10.1007/7854_2013_268 [DOI] [PubMed] [Google Scholar]
- VanRyzin JW, Marquardt AE, Argue KJ, Vecchiarelli HA, Ashton SE, Arambula SE, Hill MN, & McCarthy MM (2019). Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron, 102(2), 435–449.e6. 10.1016/j.neuron.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin JW, Yu SJ, Perez-Pouchoulen M, & McCarthy MM (2016). Temporary Depletion of Microglia during the Early Postnatal Period Induces Lasting Sex-Dependent and Sex-Independent Effects on Behavior in Rats. Eneuro, 3(6), ENEURO.0297–16.2016. 10.1523/ENEURO.0297-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, & Pardo CA (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology, 57(1), 67–81. 10.1002/ana.20315 [DOI] [PubMed] [Google Scholar]
- Vogel Ciernia A, Careaga M, LaSalle JM, & Ashwood P (2018). Microglia from offspring of dams with allergic asthma exhibit epigenomic alterations in genes dysregulated in autism. Glia, 66(3), 505–521. 10.1002/glia.23261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillermot S, Joodmardi E, Perlmann T, Ove Ogren S, Feldon J, & Meyer U (2012). Prenatal Immune Activation Interacts with Genetic Nurr1 Deficiency in the Development of Attentional Impairments. Journal of Neuroscience, 32(2), 436–451. 10.1523/JNEUROSCI.4831-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillermot S, Weber L, Feldon J, & Meyer U (2010). A Longitudinal Examination of the Neurodevelopmental Impact of Prenatal Immune Activation in Mice Reveals Primary Defects in Dopaminergic Development Relevant to Schizophrenia. Journal of Neuroscience, 30(4), 1270–1287. 10.1523/JNEUROSCI.5408-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Béchade C, Roumier A, Bernard D, Triller A, & Bessis A (2008). Developmental Neuronal Death in Hippocampus Requires the Microglial CD11b Integrin and DAP12 Immunoreceptor. Journal of Neuroscience, 28(32), 8138–8143. 10.1523/JNEUROSCI.1006-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y, & Gross CT (2018). Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nature Communications, 9(1), 1228. 10.1038/s41467-018-03566-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling DM, Parikshak NN, & Geschwind DH (2016). Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nature Communications, 7. 10.1038/ncomms10717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernersson S, & Pejler G (2014). Mast cell secretory granules: Armed for battle. Nature Reviews Immunology, 14(7), 478–494. 10.1038/nri3690 [DOI] [PubMed] [Google Scholar]
- Willcutt EG (2012). The Prevalence of DSM-IV Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Neurotherapeutics, 9(3), 490–499. 10.1007/s13311-012-0135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolterink G, Daenen LEWPM, Dubbeldam S, Gerrits MAFM, van Rijn R, Kruse CG, Van Der Heijden JAM, & Van Ree JM (2001). Early amygdala damage in the rat as a model for neurodevelopmental psychopathological disorders. European Neuropsychopharmacology, 11(1), 51–59. 10.1016/S0924-977X(00)00138-3 [DOI] [PubMed] [Google Scholar]
- Wynne O, Horvat JC, Osei-Kumah A, Smith R, Hansbro PM, Clifton VL, & Hodgson DM (2011). Early life infection alters adult BALB/c hippocampal gene expression in a sex specific manner. Stress, 14(3), 247–261. 10.3109/10253890.2010.532576 [DOI] [PubMed] [Google Scholar]
- Zaretsky MV, Alexander JM, Byrd W, & Bawdon RE (2004). Transfer of Inflammatory Cytokines Across the Placenta Obstetrics & Gynecology, 103(3), 546–550. 10.1097/01.AOG.0000114980.40445.83 [DOI] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, & Gross CT (2014). Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature Neuroscience, 17(3), 400–406. 10.1038/nn.3641 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang Y, Dong H, Xu Y, & Zhang S (2016). Induction of Microglial Activation by Mediators Released from Mast Cells. Cellular Physiology and Biochemistry, 38(4), 1520–1531. 10.1159/000443093 [DOI] [PubMed] [Google Scholar]
- Zhao C, Eisinger B, & Gammie SC (2013). Characterization of GABAergic Neurons in the Mouse Lateral Septum: A Double Fluorescence In Situ Hybridization and Immunohistochemical Study Using Tyramide Signal Amplification. PLoS ONE, 8(8), e73750. 10.1371/journal.pone.0073750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Silverman A-J, & Silver R (1993). Reproductive Behavior, Endocrine State, and the Distribution of GnRH-like Immunoreactive Mast Cells in Dove Brain. Hormones and Behavior, 27(3), 283–295. 10.1006/hbeh.1993.1021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Allergic inflammation does not affect the number of mast cells in the septum (A), mPFC (B), amygdala (C) or BNST(D). N’s = 3/group/sex for septum, AMY, and BNST; N’s = 5 MV, 5 FV, 5 MOVA, and 9 FOVA for the mPFC. AMY = amygdala, BNST = bed nucleus of the stria terminalis, mPFC = medial prefrontal cortex.


