Abstract
Background:
Depressive symptoms in Alzheimer’s disease (AD) predict worse cognitive and functional outcomes. Both AD and major depression inflammatory processes are characterized by shunted tryptophan metabolism away from serotonin (5-HT) and toward the neuroinflammatory kynurenine (Kyn) pathway. The present study assessed associations between Kyn and behavioral, neuroanatomical, neuropathological, and physiological outcomes common to both AD and negative affect across the AD continuum.
Methods:
In 58 cognitively normal, 396 mild cognitive impairment, and 112 AD participants from the Alzheimer’s Disease Neuroimaging Initiative-1 (ADNI1) cohort, serum markers of 5-HT, tryptophan, and Kyn were measured and their relationships investigated with immunologic markers, affect and functional outcomes, CSF markers of beta-amyloid (Aβ) and tau, and regional gray matter.
Results:
A higher Kyn/Tryptophan ratio was linked to many inflammatory markers, as well as lower functional independence and memory scores. A higher Kyn/5-HT ratio showed similar associations, but also strong relationships with negative affect and neuropsychiatric disturbance, executive dysfunction, and global cognitive decline. Further, gray matter atrophy was seen in hippocampus, anterior cingulate, and prefrontal cortices, as well as greater amyloid and total tau deposition. Finally, using moderated-mediation, several pro-inflammatory factors partially mediated Kyn/5-HT and negative affect scores in participants with subclinical Aβ (i.e., Aβ−), whereas such associations were fully mediated by Complement 3 in Aβ+ participants.
Conclusion:
These findings suggest that inflammatory signaling cascades may occur during AD, which is associated with increased Kyn metabolism that influences the pathogenesis of negative affect. Aβ and the complement system may be critical contributing factors in this process.
Keywords: Kynurenine, Serotonin, Tryptophan, Inflammation, Depression, Alzheimer’s disease
1. Introduction
Depressive affect, characterized by anhedonia, negativity, and cognitive decline, impairs overall health and is comorbid with several inflammatory and metabolic diseases. In particular, increased neuroinflammation exacerbates mood and cognitive deficits in old age (Alexopoulos and Morimoto, 2011; Loftis et al., 2010; Moussavi et al., 2007). Recent evidence supportsinflammation as being a potential contributor in the pathophysiology of psychiatric disorders like major depression. Clinical depression is typified by higher peripheral levels of cardinal pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (Dowlati et al., 2010). Administration of cytokine inducers, such as lipopolysaccharide (LPS), causes physical symptoms of sickness, depression, and fatigue in both animal models and human participants (Dantzer et al., 2008b; Reichenberg et al., 2001). Conversely, administration of cytokine antagonists to patients with chronic inflammatory conditions, or to depressed patients with elevated biomarkers of inflammation, attenuates symptoms of depression.
The link between inflammation and depressive symptoms is especially important in the context of age-related neurological disorders such as Alzheimer’s disease (AD). Approximately 30–40% of patients with AD manifest mild to major depressive symptoms (Wragg and Jeste, 1989), and depression may hasten global cognitive performance (Spalletta et al., 2012). Depressive symptoms have been associated with a subsequent diagnosis of cognitive impairment (Barnes et al., 2012; Robinson et al., 2020). These behavioral symptoms and cognitive deficits may arise due to neuroinflammation and atrophy in medial temporal and prefrontal regions (Lee et al., 2008). Indeed, neuroinflammation contributes to depressive symptoms on the Geriatric Depression Scale (GDS) in old age (van den Biggelaar et al., 2007). Further, this process may be exacerbated by amyloid plaques and neurofibrillary tau tangles that typify AD (Heneka et al., 2015; Kreisl et al., 2013), although results have been mixed (Streit et al., 2009). Global cognitive decline among individuals with mild cognitive impairment (MCI) or AD has been associated with inflammation in anterior and medial aspects of the temporal lobe, as well as tau pathology, as measured in vivo using positron emission tomography (Malpetti et al., 2020). Participants with lifelong major depression but not AD or other cognitive impairment also show modestly more amyloid deposition in the precuneus and frontal regions (Wu et al., 2014). Perhaps not coincidentally, these brain areas are the first to show accumulation of amyloid plaques in AD, which may induce or exacerbate neuroinflammation.
Several different processes stemming from neuroinflammation may lead to increased risk for both major depression and AD, including depletion of monoamines like dopamine and serotonin (Brites and Fernandes, 2015; Porter et al., 2003). Depletion of serotonin can occur as a result of many mechanisms, including deficient synthesis from its amino acid precursor tryptophan, or increased reuptake of serotonin at the synaptic level. Under normal physiological conditions, tryptophan is utilized for protein synthesis, neurotransmitter formation (Schwarcz et al., 2012), and energy production via NAD/NADPH synthesis (Beadle et al., 1947). Tryptophan can be hydroxylated to form 5-hydroxytryptophan, which subsequently undergoes decarboxylation to synthesize 5-hydroxytryptamine (5-HT) or serotonin. Elevated levels of Kyn have been observed with higher BMI (Favennec et al., 2015), which has also been tied to neuroinflammation. Alternatively, neuroinflammation and chronic stress (Campbell et al., 2014) can increase tryptophan 2,3-dioxygenase (TDO) expression in the liver and extrahepatic indoleamine 2,3-dioxygenase (IDO) expression. Activation of these enzymes shunts tryptophan through the kynurenine (Kyn) pathway and therefore potentially compromises the synthesis of serotonin (Dantzer and Capuron, 2017). In addition, activation of the Kyn pathway can generate neurotoxic Kyn metabolites that accumulate in the brain during both depression and AD (Braidy et al., 2009; Capuron et al., 2011; Leonard, 2007; Miller et al., 2006; Wright et al., 2005).
Increased IDO expression has been reported in the hippocampus and neocortex of AD patients (Gulaj et al., 2010) and is correlated with beta-amyloid (Aβ) plaque load (Guillemin et al., 2003), one of the hallmarks of the disease. In addition, AD subjects demonstrate an activation of the Kyn pathway at the periphery, potentially making tryptophan less available for the synthesis of 5-HT (Widner et al., 2000). Taken together, these findings point to the possible role of tryptophan metabolism in the pathophysiology of AD and AD comorbid depression. Activation of IDO by inflammation can be measured by the ratio of kynurenine to tryptophan (Kyn/Tryptophan). This ratio increases in inflammation, HIV, AD, and cancer (Huengsberg et al., 1998; Suzuki et al., 2010; Widner et al., 2000). In the brain, increased Kyn/5-HT ratios have also been used, to measure the relative decrease in the synthesis of 5-HT that is due to the increased metabolism of tryptophan into Kyn (Miura et al., 2009).
Thus, the present study was carried out to determine associations between common behavioral and biological correlates of AD and depression in conjunction with the Kyn pathway. Primarily, we examined how the inflammation-induced activation of the Kyn pathway and serotonin metabolism contribute to negative affect, both domain-specific and global cognitive impairment across the AD spectrum, and AD neuropathological features such as cerebrospinal fluid (CSF) biomarkers and brain atrophy. As a secondary outcome, because Kyn synthesis, Aβ load, and inflammation are interconnected (Guillemin et al., 2003; Huengsberg et al., 1998; Suzuki et al., 2010; Widner et al., 2000), we also used mediation and moderated-mediation (Hayes, 2018) to see if associations between these factors accounted for negative affect scores.
2. Materials and Methods
2.1. Setting
The present study used Alzheimer’s Disease Neuroimaging Initiative (ADNI) data. ADNI is a multicenter longitudinal study examining clinical, imaging, genetic, and biochemical markers funded by public and private partnerships in part by the National Institute on Aging, pharmaceutical companies, and foundations through the Foundation for the National Institutes of Health.
2.2. Participants
ADNI1 data was obtained from 566 participants including 58 cognitively normal (CN), 396 MCI and 112 AD who had metabolite markers of tryptophan metabolism. Data of interest included: 1) demographics; 2) serum, plasma, and CSF biomarkers, including immunologic markers like pro- and anti-inflammatory cytokines, Aβ, and the neurodegeneration marker tau (Tosun et al., 2010); 3) Magnetic Resonance Imaging (MRI) volumetric scans; 4) neuropsychiatric assessments including self-reported affect and activities of daily living; and 5) neuropsychological performance. Participants were clinically diagnosed at every visit based on standardized criteria described in the protocol manual (http://adni.loni.usc.edu/). Participants taking SSRIs, cholinesterase inhibitors, or NMDA antagonists were excluded to prevent confounding effects, as these medication may influence serotonin metabolite values. For this report, importantly, ADNI1 excluded prospective participants who had GDS scores reflecting major depression (GDS≥6) and a 1–2 year history of major depression.
2.3. Standard protocol approvals, registrations, and patient consents
Written informed consent was obtained from all ADNI participants at their respective sites. Site-specific Institutional Review Boards approved the ADNI protocol.
2.4. Serum, Plasma, and CSF Biomarkers
Kyn, tryptophan, and 5-HT metabolites were assayed in serum using a Biocrates AbsoluteIDQ p180 kit with liquid chromatography/mass spectrometry from the Alzheimer’s Disease Metabolomics Consortium, as described in white papers (http://adni.loni.usc.edu). In a subset of 58 CN, 396 MCI, and 112 AD, inflammatory markers were assayed from plasma sent to Rules-Based Medicine (RBM, Austin, TX, USA) for analysis using a Luminex xMAP multiplex array (Austin, TX, USA). This array examined 49 biomarkers of immunologic activation, as described in Supplementary Table 1. CSF Aβ1–42, total tau and phosphorylated (P)Tau-181 were analyzed using xMAP Luminex (Innogenetics/Fujirebio AlzBio3 Ghent, Belgium) immunoassay kits. Each analyte has a validation report after independent evaluation by Myriad RBM with a multianalyte panel (Human Discovery MAP version 1.0; Myriad RBM). Quality control information and the detection limits for assays can be found here: http://adni.loni.ucla.edu/wp-ontent/uploads/2010/11/BC_Plasma_Proteomics_Data_Primer.pdf. For moderation-mediation analyses, Aβ1–42 CSF levels were categorized as being Aβ negative (Aβ−, >192 pg/mL) or positive (Aβ+, ≤192 pg/mL) to reflect clinically meaningful amyloid load for AD (Shaw et al., 2009).
2.5. Apolipoprotein E (APOE) Haplotype
The ADNI Biomarker Core at the University of Pennsylvania conducted APOE ε4 haplotyping. We characterized participants as having zero vs. one or two APOE4 alleles.
2.6. Outcome Measures
2.6.1. MRI Acquisition and Pre-Processing
T1-weighted MR volumetry scans [1.25×1.25×1.25mm] were acquired from 1.5T units within 10–14 days of the screening visit, following a back-to-back 3D magnetization prepared rapid gradient echo (MP-RAGE) scanning protocol described elsewhere (Jack et al., 2008). Images were pre-processed using FreeSurfer 4.3 (Fischl et al., 2004). As described previously (Willette et al., 2015), this software corrects for motion, deskulls, bias corrects, segments, and parcellates gray and white matter into labeled areas. Mean gray matter volume was derived from subcortical and archiocortical regions of interest (ROIs), chosen a priori for their relevance to major depression and associations with kynurenine metabolites (Savitz et al., 2015a; Savitz et al., 2015b; Young et al., 2016). ROIs included: hippocampus, amygdala, caudate, and putamen. Several frontal and cingulate regions were also chosen based on prior studies (Meier et al., 2016), including Superior Frontal Gyrus, Caudal Anterior Cingulate Gyrus, Medial Orbital Frontal Gyrus, Lateral Orbital Frontal Gyrus, Caudal Middle Frontal Gyrus, and Rostral Middle Frontal Gyrus. In these neocortical areas, we examined cortical thickness (CT) instead of volume. CT is typically a more sensitive index of gray matter pathology in participants with AD risk (Burggren et al., 2008) or who have AD (Querbes et al., 2009).
2.6.2. Neuropsychological Assessments
All subjects underwent clinical and neuropsychological assessment at the time of scan acquisition. Global tests included Clinical Dementia Rating sum of boxes (CDR-sob), Mini-Mental State Examination (MMSE), AD Assessment Schedule – Cognition 11 (ADAS-Cog). Memory assessments included the Rey Auditory Verbal Learning Test (RAVLT) and a composite memory factor (Crane et al., 2012). A composite executive function score (Gibbons et al., 2012) was also used.
2.6.3. Neuropsychiatric Assessment
The primary outcome measure of depressive symptoms was the Geriatric Depression Scale, or GDS (Yesavage, 1988). One sub-score of the GDS asked whether or not a participant felt like they have more memory problems than others. This sub-score was not included in the GDS total due to the high frequency of memory complaints in ADNI. The 12-item Neuropsychiatric Inventory Questionnaire (NPI-Q) and a sub-score examining Apathy/Anxiety were assessed (Cummings, 1997). The Functional Assessment Questionnaire (FAQ) measured the ability to carry out 10 activities of daily living according to dependence on a caregiver (Gunel et al., 2010). Higher scores indicated more severe neuropsychiatric symptoms or greater functional impairment.
2.6.4. Body Mass Index (BMI)
BMI (kilograms/meters2) was calculated from weight meaurements at baseline and height measurements at the screening visit.
2.7. Statistical Analysis
All analyses were conducted using SPSS 23 (IBM Corp., Armonk, NY). ANOVA and follow-up LSD tests examined differences in metabolite, cognitive, and other outcomes among CN, MCI, and AD subjects (see Table 1). Logistic regression analyses were performed to see if Kyn/5-HT or Kyn/Tryptophan predicted cognitive status (i.e. MCI or AD diagnosis). Subsequently, linear mixed effects models tested the main effects of a Kyn/5-HT or Kyn/Tryptophan ratio on outcomes of interest. Covariates included age at baseline and sex, as well as education for cognitive and affective measures. For subcortical brain volumes, total intracranial volume was also used as a covariate to correct for whole brain size. Other outcomes included: peripheral inflammatory markers (see Supplementary Table 1), neuropsychiatric stability, neuropsychological performance, CSF AD biomarkers including Aβ1–42, total tau and PTau-181, and subcortical and cortical ROIs.
Table 1.
Demographics and Sample Characteristics
| CN (n=58) |
MCI (n=396) |
AD (n=112) |
|
|---|---|---|---|
| Age (years) | 75.1 ± 5.77 | 74.7 ± 7.40 | 74.80 ± 8.08 |
| Gender (% Male) | 51.7 | 64.6 | 58 |
| Education (years) | 15.7 ± 2.78 | 15.6 ± 3.03 | 15.09 ± 3.20 |
| BMI | 26.89 ± 3.84 | 26.01 ± 3.84 | 25.54 ± 3.84 |
| APOE4 (% carriers) | 8.6a | 53.3b | 67.8c |
| Trytophan (μM) | 71.6 ± 13.4a | 71.8 ± 16.00b | 72.55 ± 14.66b |
| Kyn (μM) | 3.33 ± 0.94a | 3.15 ± 1.01a | 3.31 ± 1.11a |
| 5-HT (μM) | 0.675 ± 0.37a | 0.535 ± 0.340b | 0.445 ± 0.407b |
| Kyn/5-HT | 9.19 ± 12.60a | 13.1 ± 18.8b | 23.4 ± 29.4c |
| Kyn/Tryptophan | 0.048 ± 0.015a | 0.045 ± 0.016a | 0.047 ± 0.019a |
| CDR-sob | 0.026 ± 0.11a | 1.60 ± 0.877b | 4.32 ± 1.56c |
| MMSE | 28.9 ± 1.15a | 27.0 ± 1.78b | 23.6 ± 1.91c |
| ADAS-cog11 | 6.25 ± 2.79a | 11.5 ± 4.42b | 18.3 ± 6.42c |
| ADNI Memory Factor | 0.87 ± 0.46a | −0.086 ± 0.586b | −0.814 ± 0.547c |
| ADNI EF Factor | 0.71 ± 0.58a | −0.043 ± 0.783b | −0.934 ± 0.816c |
| GDS Total | 0.86 ± 1.23a | 1.58 ± 1.37b | 1.72 ± 1.36b |
| FAQ Total | 0.05 ± 0.22a | 3.82 ± 4.46b | 12.54 ± 6.71c |
| NPI-Q Total | 0.28 ± 0.72a | 1.85 ± 2.67b | 3.39 ± 3.31c |
| Aβ1–42 (pg/mL) | 251 ± 21.1a | 168 ± 55.6b | 144 ± 40.8c |
| Total Tau (pg/mL) | 63.6 ± 21.8a | 97.4 ± 58.1b | 119 ± 56.8c |
| P-Tau (pg/mL) | 21.1 ± 8.43a | 35.5 ± 18.8b | 41.8 ± 19.7c |
Numbers represent frequency or unadjusted mean ± SD. Superscript letters indicate if a given value for a clinical group is significantly different from other clinical groups. For example, CDR-sob differs between each clinical group, while 5-HT only differs between the CN versus MCI and AD groups. Aβ = beta-amyloid; AD = Alzheimer’s disease; ADAS-Cog = Alzheimer’s Disease Assessment Scale-cognition 11; APOE4 = apolipoprotein E4; BMI = Body Mass Index; CDR-sob=Clinical Dementia Rating – sum of boxes; CN = Cognitively normal; EF = executive function; GDS = Geriatric Depression Scale; FAQ=Functional Assessment Questionnaire; Kyn = Kynurenine; MCI = Mild Cognitive Impairment; MMSE = Mini-Mental State Exam; NPI-Q=Neuropsychiatric Inventory Questionnaire.
2.7.1. Error Correction
To correct for type 1 error, omnibus MANCOVA testing was used for a given family of outcome variables (e.g., neuropsychological tests). If the omnibus was significant, all follow-up tests were judged at p<.05 because the family-wise error rate stays below Alpha of .05 (Wilkinson, 1975). When the omnibus was non-significant, a stricter Holm-Bonferroni correction (Holm, 1979) was used. This closed test procedure maintains a family-wise Alpha = 0.05 by requiring unadjusted P values of 0.05 divided by x, x being the number of null hypotheses tested. For four cognitive tests, for example, P values of .0125, .025, .0375, and .050 would be successively needed when testing outcomes in the closed set.
2.7.2. Mediation and Moderation
Finally, it was of interest to conduct mediation and moderated mediation analyses using the PROCESS macro (Hayes, 2018). The objective was to test if immunologic or AD biomarkers accounted for significant associations between Kyn/5-HT and GDS scores. Kyn/Tryptophan was not considered because it was not related to GDS scores. First, to constrain type 1 error, all immunologic mediators were entered and then backwards selection used to retain biomarkers at p<.05. In turn, Kyn/5-HT was regressed onto each selected inflammatory marker, yielding a beta coefficient (e.g., path A). The inflammatory marker was separately regressed onto depression scores (i.e., GDS), yielding a second beta coefficient (e.g., path B). The indirect effect was estimated as the product between the two beta coefficients. The direct effect (path C) was estimated by regressing Kyn/5-HT against GDS scores. The size of the mediation effect was estimated using the variance percentage attributed to the complete model explained by the mediator (Fairchild et al., 2009). For moderated mediation, we exclusively focused on Aβ because protein oligomers can influence neuroinflammation and tryptophan metabolism (Guillemin et al., 2003; Huengsberg et al., 1998; Suzuki et al., 2010; Widner et al., 2000; Wu et al., 2014). Aβ status (Aβ− vs. Aβ+) was tested as a moderator of path B and path C. Covariates in the models included age and gender.
3. Results
3.1. Data Summary
Clinical, demographic, and other data and differences among CN, MCI, or AD participants are presented in Table 1. As expected in this ADNI sub-sample, there were stepwise declines in global cognition, memory, executive function, amyloid and tau markers; those with cognitive impairment were more likely to be APOE4 positive. While ADNI1 did not recruit participants with GDS scores in the major depression range, MCI and AD nonetheless had mild depressive symptoms and more neuropsychiatric disturbances vs. CN.
For CSF metabolites of interest, tryptophan [F=4.29, p=.014] and 5-HT [F=9.27, p<.001] levels were higher in CN participants than MCI or AD. There was also a marked dose-response difference in Kyn/5-HT between CN, MCI, and AD [F=10.93, p<.001]. No differences were noted for Kyn or the Kyn/Tryptophan ratio, by contrast. For peripheral immune biomarkers, Supplementary Table 1 shows the mean, standard deviation, unit of measurement, and percentage of missingness due to values being below the detection threshold. Many of these variables were log transformed to achieve normality for use in parametric tests.
3.2. Risk of Cognitive Impairment
A higher Kyn/Tryptophan ratio was not associated with baseline clinical diagnosis. A higher Kyn/5-HT ratio, however, was significantly associated with being diagnosed as MCI or AD versus CN [F=26.0, P<0.001], but not MCI conversion to AD. Logistic regression models indicated that per point increase in the Kyn/5-HT ratio, risk doubled for having MCI or AD [Wald=10.18, OR=1.953, p<0.001].
3.3. Peripheral Immunologic Biomarkers
Linear mixed effect models tested whether Kyn/5-HT or Kyn/Tryptophan ratios were related to pro- and anti-inflammatory immune markers available in an ADNI multiplex serum panel (see Supplemental Table 1). While analyses were done for all markers, caution is warranted for interpreting markers with 25% or greater missingness. Such markers were not included in mediation or moderated mediation analyses with GDS.
For the Kyn/Tryptophan ratio, a significant multivariate omnibus [F=10.3, P< 0.001] allowed for follow-up linear mixed model tests at a family-wise error rate of p<.05 (Wilkinson, 1975). As noted in Supplementary Table 2, a higher Kyn/Tryptophan ratio was related to higher levels of most peripheral immune biomarkers. Representative associations (all p<.001) are depicted for IL-1ra [β±SE=674±153; Figure 1A], IL-12p40 [β±SE=1.97±0.29; Figure 1B], and IL-18 [β±SE=2560±304; Figure 1C]. Likewise, an omnibus for Kyn/5-HT [F=2.09, p< 0.001] and linear mixed models showed that a higher Kyn/5-HT ratio was related to higher levels of many multiplex biomarkers (Supplementary Table 3), though fewer in number than for the Kyn/Tryptophan ratio. Representative associations (all p<.001) are shown for IL-1ra [β=0.45±0.12] (Figure 1D), IL-12p40 [β=.0007±.0002] (Figure 1E), and IL-18 [β=0.69±0.24] (Figure 1F).
Figure 1.
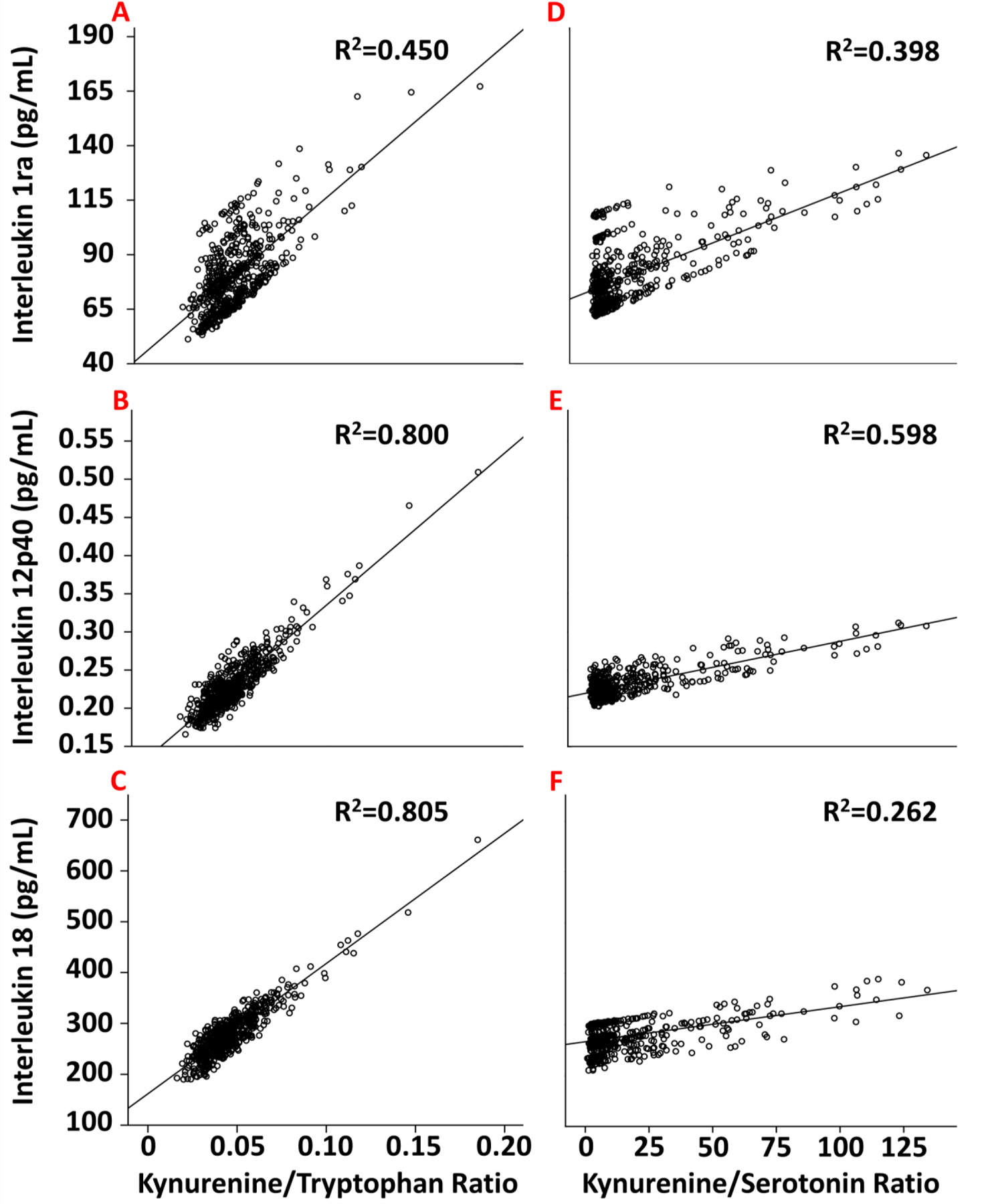
The relationship between Kyn/Tryptophan (A–C, left column) or Kyn/5-HT (D–F, right column) ratios with peripheral immune markers, including IL-1ra (A & D); IL-12p40 (B & E); and IL-18 (C & F). R2 is the proportion of variance explained by a given ratio.
3.4. Neuropsychiatric Assessments
We next examined Kyn ratios with affect and quality of life outcomes. For Kyn/Tryptophan, the multivariate omnibus was marginally significant [F=2.34, p=.054]. After Holm-Bonferroni correction, higher Kyn/Tryptophan levels were only associated with worse FAQ total scores [F=8.31, β=40.1±13.9, p=.004], indicating less quality of life due to disability. The beta coefficient here is large because the analyte ratio is in the decimal range.
For Kyn/5-HT, the multivariate omnibus was significant [F=3.06, p=.016]. Relationships are noted in Figure 2 for: A) GDS Total; B) NPI-Q Total; C) FAQ Total; and D) the NPI-Q Anxiety sub-score. Subjects with higher Kyn/5-HT ratios had worse scores for the GDS [F=6.28, β=.006±.002, p=.012], NPI-Q [F=15.5, β=.016±.004, p<.001], FAQ total [F=42.10, β=.062±.011, p<.001], and NPI-Q anxiety sub-score [F=16.60, β=.002±.001, p<.001].
Figure 2.
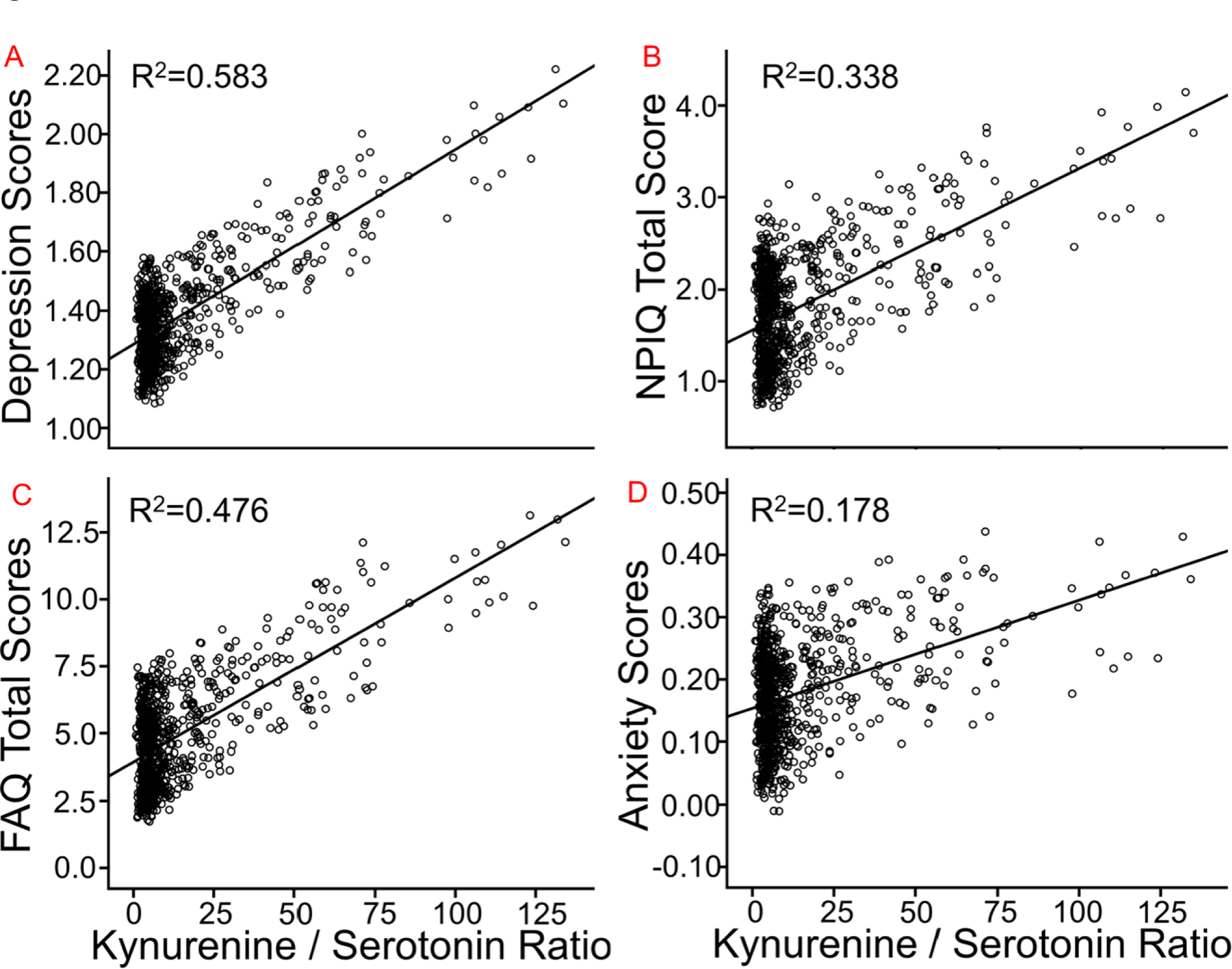
The relationship between Kyn/5-HT ratios and neuropsychiatric assessments, such as: A) GDS total score; B) NPI-Q total score; C) FAQ total score; and D) the anxiety NPI-Q sub-score. R2 is the proportion of variance explained by the Kyn/5-HT ratio. FAQ=Functional Assessment Questionnaire; GDS=Geriatric Depression Scale; NPI-Q=Neuropsychiatric Inventory Questionnaire. Note that the depression and anxiety scores respectively represent the GDS and a sub-scale of the NPI-Q. Note also that total GDS score is lower than usual because of removing a question regarding memory concerns, and that several subjects were excluded who took anti-depressive medication.
3.5. Neuropsychological Assessments: Memory and Executive Function
For the Kyn/Tryptophan ratio, the multivariate omnibus was marginally significant. Note again that large beta values are due to the decimal range of the Kyn/Tryptophan ratio. After Holm-Bonferroni correction, a higher ratio corresponded to worse immediate memory on RAVLT Trials 1–5 [F=6.16, β=−60.2±24.3, p=.013] and short delay memory [F=8.26, β=−16.00±5.57, p=.004], as well as a higher percentage of items forgotten during long delay [F=4.32, β=152±73, p=.038]. A higher ratio was also linked to lower Z-scores for the memory factor [F=5.09, β=−4.00±1.77, p=.024].
For Kyn/5-HT, among neuropsychological indices, the multivariate omnibus [F= 5.25, p=0.002], followed by linear mixed models, indicated that higher Kyn/5-HT ratios were related to worse performance on RAVLT Trials 1–5 (F=14.9, β=−.075±.019, p<0.001) and RAVLT short delay (F=11.5, β=−.016±.004, p<0.001), the memory factor (F=20.4, β=−.006±.001, p<0.001) (Figure 3A), and the executive function factor (P<0.001, β=−.006±.002, F=13.9) (Figure 3B).
Figure 3.
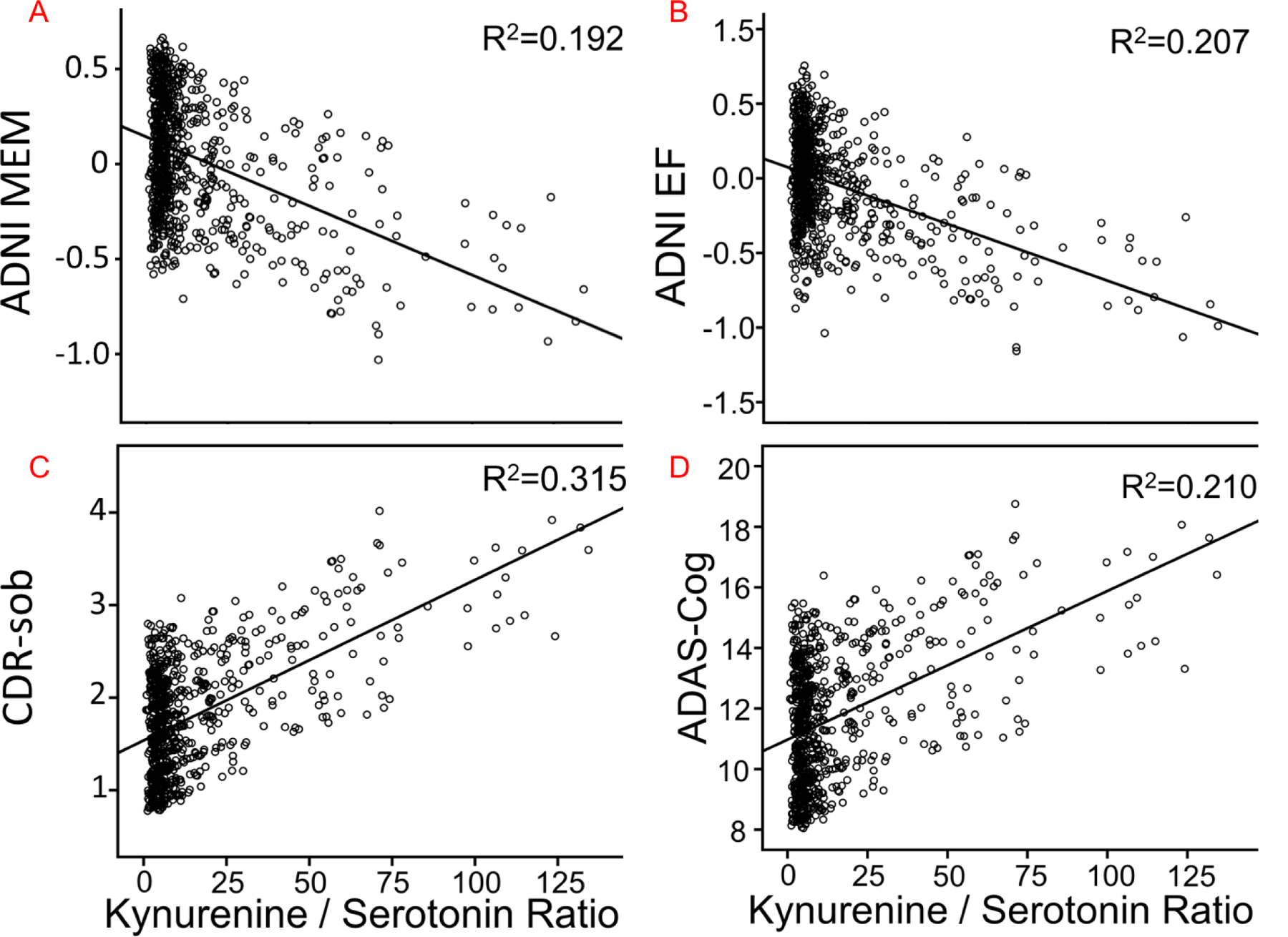
The relationship between Kyn/5-HT ratios and neuropsychological assessments, including: A) ADNI-MEM; B) ADNI-EF; C) CDR-sob; D) ADAS-Cog. R2 is the proportion of variance explained by the Kyn/5-HT ratio. ADAS-cog=Alzheimer’s Disease Assessment Scale-cognition 11; CDR-sob=Clinical Dementia Rating – sum of boxes; EF=Executive function factor; MEM=Memory factor.
3.6. Neuropsychological Assessments: Global Domains
For Kyn/Tryptophan, the multivariate omnibus was non-significant and a result with CDR-sob [F=4.391, p=.036] did not survive Holm-Bonferroni correction. For Kyn/5-HT, a significant omnibus [F=8.70, p=0.001] and follow-up linear mixed models showed that higher Kyn/5-HT ratios were related to worse cognition on CDR-sob (F= 25.0, β=.015±.003, p<0.001) (Figure 3C), MMSE (F=14.0, β=−.016±.004, p<0.001), and ADAS-cog (F=14.7, β=.041±.011, p<0.001) (Figure 3D).
3.7. CSF Amyloid and Tau
The AD biomarkers of T-Tau, PTau-181 and Aβ1–42 were next evaluated. Higher Kyn/Tryptophan ratios were not associated with these indices. By contrast, a multivariate omnibus [F=4.77, p=.003] for Kyn/5-HT and follow-up tests indicated that a higher ratio was associated with lower CSF Aβ1–42 (F=11.74, β=−.353±.103, p=0.001) and higher CSF T-Tau (F=3.87, β=.237±.120, p=.05) but not PTau-181, corresponding to increased amyloid and total tau deposition in brain parenchyma.
3.8. Regional Grey Matter Volume
Next, subcortical, hippocampus, and neocortical ROIs implicated in past studies of depression and kynurenine metabolites were investigated. As indicated in Table 2, higher Kyn/Tryptophan ratios were not significantly related to gray matter atrophy in any region, though there was a marginal negative association with hippocampus. By contrast, higher Kyn/5-HT corresponded to less hippocampal volume and thinner cingulate and frontal areas chosen a priori.
Table 2.
MRI Analyses
| Ratio | Gray Matter Index | Brain Region | F Value | β Estimate | Standard Error | P value |
|---|---|---|---|---|---|---|
| Kyn/5-HT | Sub-Cortex and Archiocortex Volumes |
Hippocampus | 8.70 | −5.30 | 1.80 | 0.003 |
| Putamen | 0.43 | −1.51 | 2.30 | 0.511 | ||
| Caudate | 0.09 | −0.54 | 1.84 | 0.769 | ||
| Amygdala | 0.41 | −0.46 | 0.71 | 0.524 | ||
| Neocortex Cortical Thickness |
Superior Frontal Gyrus | 12.1 | −1.4E-3 | 4.0E-4 | 0.001 | |
| Caudal Anterior Cingulate Gyrus | 6.34 | −1.3E-3 | 5.2E-4 | 0.012 | ||
| Medial Orbital Frontal Gyrus | 12.1 | −1.3E-3 | 3.7E-4 | 0.001 | ||
| Lateral Orbital Frontal Gyrus | 10.1 | −1.2E-3 | 3.7E-4 | 0.002 | ||
| Caudal Middle Frontal Gyrus | 8.04 | −1.1E-3 | 4.0E-4 | 0.005 | ||
| Rostral Middle Frontal Gyrus | 9.16 | −1.0E-3 | 3.3E-4 | 0.003 | ||
| Kyn/TRP | Sub-Cortex and Archiocortex Volumes |
Hippocampus | 3.40 | −4383 | 2379 | 0.066 |
| Putamen | 0.18 | −1279 | 3033 | 0.673 | ||
| Caudate | 0.05 | −545 | 2429 | 0.823 | ||
| Amygdala | 0.60 | −730 | 942 | 0.439 | ||
| Neocortex Cortical Thickness |
Superior Frontal Gyrus | 0.95 | −0.52 | 0.53 | 0.330 | |
| Caudal Anterior Cingulate Gyrus | 1.00 | 0.69 | 0.69 | 0.318 | ||
| Medial Orbital Frontal Gyrus | 1.10 | −0.52 | 0.50 | 0.295 | ||
| Lateral Orbital Frontal Gyrus | 0.11 | −0.16 | 0.49 | 0.738 | ||
| Caudal Middle Frontal Gyrus | 0.01 | 0.48 | 0.54 | 0.929 | ||
| Rostral Middle Frontal Gyrus | 0.52 | −0.31 | 0.44 | 0.472 |
Brain regions implicated in Region of Interest analyses examining the relationship between either Kyn/5-HT or Kyn/TRP ratios and less gray matter in sub-cortical volumes or thickness of the neocortex. Kyn = kynurenine. TRP = tryptophan. Please note that Beta values are large for Kyn/TRP because values are in the decimal range.
3.9. BMI
Linear mixed effects models also tested the relationship between BMI and Kyn, tryptophan, and 5-HT metabolites. After removing outliers >3.29 SDs from the mean (n=3), associations were not observed between BMI and Kyn or Kyn/5-HT ratio. However, higher BMI predicted a greater Kyn/tryptophan ratio [F=4.62, β=.0004±.0002, P=0.032].
3.10. Moderation-Mediation Analyses of Negative Affect
Given that Kyn/5-HT but not Kyn/Tryptophan was correlated with negative affect, the following analyses were restricted to Kyn/5-HT. Mediation and moderated-mediation models determined: 1) which peripheral immunologic markers significantly mediated the Kyn/5-HT relationship with negative affect scores (i.e., GDS); and 2) if clinically significant Aβ load (i.e., Aβ+ vs. Aβ−) specific to AD (Shaw et al., 2009) acted as a moderator, modifying how immunologic markers were related to GDS. Among all participants, the following markers significantly predicted GDS total scores after error correction: Complement 3 (C3), eotaxin 1 (EO-1), TNF-related apoptosis-inducing ligand receptor (TRAIL), and vascular endothelial growth factor (VEGF). These results were superseded by significant moderation through Aβ load (all p’s<.01 to .05). Specifically, as shown in Figure 4, Aβ− participants showed higher GDS scores when VEGF levels were higher and C3 levels were lower. For Aβ+ participants through full mediation, by contrast, higher C3 drove the association between higher Kyn/5-HT and worse GDS scores.
Figure 4.
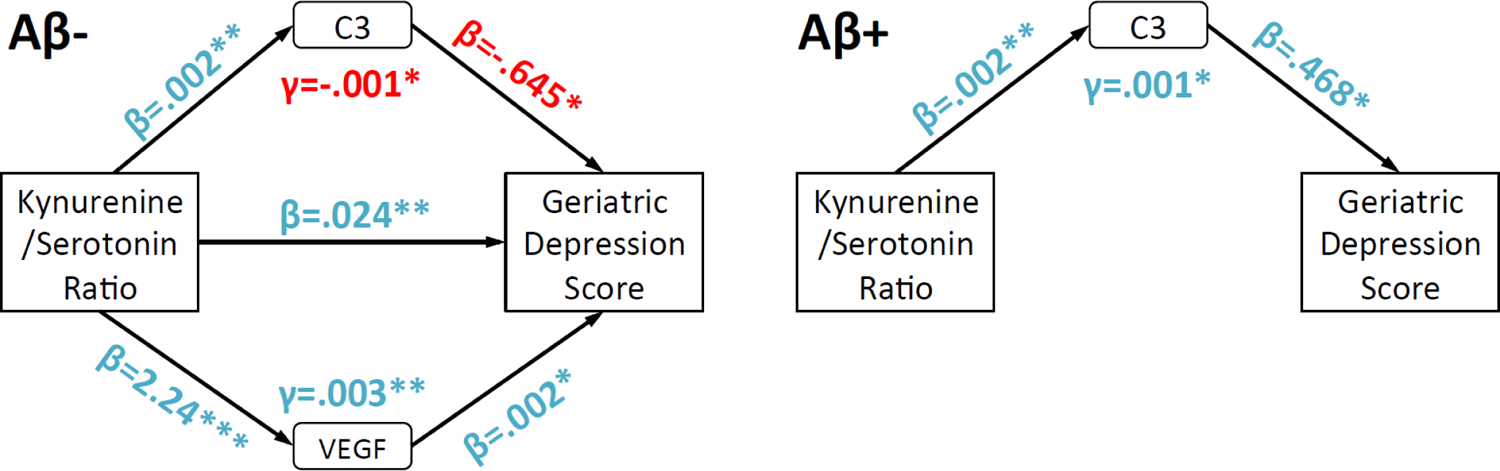
Path diagrams highlighting how immunologic factors and Aβ status modify Kyn/5-HT and Geriatric Depression Scale (GDS) total score associations. Separate path diagrams for Aβ− and Aβ+ are used to illustrate how Aβ status led to different patterns of mediation by immunologic factors. “Blue” and “red” colors respectively highlight positive or negative associations between variables. For clarity, only C3 and VEGF are displayed because they were significant mediators for Aβ− and/or Aβ+. Covariates included age and gender. *,**=p<.05, .01.
4. Discussion
Overall, the results suggest that shifted tryptophan metabolism toward the kynurenine pathway is related to not only more self-reported negative affect and behavioral disturbances, but also clinical risk and cognitive impairment, more amyloid and tau deposition, and gray matter atrophy in AD- and depression-sensitive regions. Curiously, for the classic Kyn/Tryptophan ratio, higher values did not correspond to negative affect, but as predicted were strongly associated with peripheral immunologic markers. Higher Kyn/Tryptophan was also strongly related to impaired memory. By contrast, for the first time in a large human sample, we found that a Kyn/5-HT ratio showed immunologic ties similar to Kyn/Tryptophan, but also correlated with negative affect, global cognition and both memory and executive function, AD biomarkers, and regional gray matter in key areas related to emotion regulation and memory. A rodent model study indicated a shift in the Kyn pathway, as measured by increased Kyn/5-HT levels in the brain, resulted in part due to inflammatory insult from LPS administration (Miura et al., 2009). Furthermore, Aβ status modified how Kyn/5-HT was linked to negative affect scores, with results suggesting that the complement system fully accounted for this linkage in participants who were Aβ+.
A meta-analysis conducted by Howren et al. (2009) confirmed that circulating concentrations of pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α are elevated during depression in humans. Further, the production of IL-1 may be impacted by the concentration of Kyn metabolites (Zunszain et al., 2012).We similarly found elevated IL-1ra among subjects with a higher Kyn/Tryptophan or Kyn/5-HT ratio. This increase in peripheral inflammation may upregulate production of Kyn metabolites and corresponds to our pattern of results.
4.1. Cognition, affect, and Kyn pathways
A primary finding of interest was the strong relationship between a higher Kyn/5-HT ratio with worse global and domain-specific cognition, while Kyn/Tryptophan associations were found exclusively for memory. While progressive memory loss typifies AD, loss of global function and cognition in multiple domains are required for diagnosis. Acute tryptophan deficiency is linked with decreased word recall and impaired memory consolidation (Riedel et al., 2002). The present study shows similar results and supports the hypothesis that less tryptophan is related to worse memory (Park et al., 1994), implicating the serotonergic system in these processes. Additionally, only variation in the Kyn/5-HT ratio was strongly related to depression and anxiety measures, which is in agreement with other studies (Maes et al., 2002). By contrast, Kyn/Tryptophan ratios were not correlated with affect-related outcomes. This result was unexpected because Kyn/Tryptophan typically tracks depressive affect in participants with major depression. While a Kyn/5-HT ratio has only been sparingly investigated previously, it was consistently related to pro- and anti-inflammatory cytokines, chemokines, and other immunologic signaling molecules that show increased concentrations among patients with depressive symptoms (Howren et al., 2009), including IL-6 and IL-1.
4.2. Inflammatory markers, affect, and Kyn pathways
It is also well known that endotoxin-induced sickness behavior consists of energy-conserving, withdrawal-oriented behaviors driven by neuroinflammation (Dantzer et al., 2008a). Indeed, inflammatory and neuroendocrine markers can mediate affective symptoms in rodents (Ball et al., 2007), rhesus macaques (Willette et al., 2012), and humans (Wright et al., 2005). We found that higher Kyn/5-HT was related to higher levels of peripheral C3, EO-1, TRAIL, and VEGF, which in turn were linked with higher GDS scores. Curiously, Aβ status moderated which of these inflammatory or angiogenic factors mediated negative affect. For Aβ− individuals, VEGF and C3 were related to more and less negative affect respectively. For Aβ+ adults, strikingly, C3 was instead related to more negative affect and fully accounted for the Kyn/5-HT and GDS score association. Aβ can inhibit the angiogenic functionality of VEGF and its receptors (Patel et al., 2010), which may explain why it only arose in Aβ− adults as a relevant partial mediator. Additionally, higher levels of VEGF and Kyn have been observed in individuals with depression and coronary heart disease (Nikkheslat et al., 2015). C3, meanwhile, is a biomarker of early stage activation of the complement system (Janeway et al., 2001), where the CR1 gene underlies C3b receptor synthesis and has been consistently implicated in AD (Lambert et al., 2009). C3 activation optimizes Aβ opsinization and clearance via red blood cells to the liver for degradation, as well as mediates pro-inflammatory responses via the classic and alternate complement systems (Crane et al., 2018). In our study, for Aβ− participants, more C3 reduced the relationship between higher Kyn/5-HT and GDS scores, which may be due its Aβ clearance properties. For Aβ+, C3 may instead represent chronic neuroinflammation that is correlated with neurodegeneration and cognitive decline in rodent models (Yin et al., 2019) and humans (Bonham et al., 2016).
4.3. Regional brain volume, CSF markers of AD, and Kyn pathways
Higher Kyn/5-HT, but not Kyn/Tryptophan, was also related to less gray matter in hippocampal, precuneus, and prefrontal cortex volumes or cortical thickness, which complement relationships with affect and cognitive outcomes. It is important to note that Kyn/Tryptophan and regional atrophy have been consistently found, but only in patients with major depression (Meier et al., 2016; Savitz et al., 2015a; Savitz et al., 2015b; Young et al., 2016), which unfortunately were excluded from ADNI1 enrollment. For the first time in aged humans, we also found that Kyn metabolism was correlated with lower Aβ1–42 and higher total tau. These data reflect increased amyloid and tau deposition in brain parenchyma and may corroborate how Kyn metabolism impacts amyloid plaque formation (Wu et al., 2013). It was particularly interesting that clinically significant Aβ (i.e., Aβ+) may influence Kyn metabolism and negative affect via the complement system. Future work in rodent models should determine if these correlations are meaningful and relevant to common comorbid negative affect in MCI and AD (Grigsby et al., 2002; Ryan et al., 2012).
4.4. Limitations
Several limitations of this study should be highlighted. Foremost, Kyn/Tryptophan was not related to affect or brain volumetry, as is typically shown in this literature. Rather, it was only correlated with immune markers, memory function, and functional independence. One reason may be the ADNI1 sample’s intentionally limited range of GDS scores, the range of which is sub-depressive to mild depression. This contrast may be due to Kyn/Tryptophan representing the degree of inflammation present and how much tryptophan is shunted toward the Kyn pathway, which may not necessarily reflect downstream effects on emotional processes. Markers of the Kyn pathway were also measured in serum rather than CSF, which could influence the interpretation of our results. However, recent research has suggested that plasma and CSF levels of Kyn are highly correlated among individuals with depression (Haroon et al., 2020). Another limitation is that many Kyn metabolism end-products, including quinolinic acid or 3-hydroxy-Kyn, have not been measured in ADNI. These metabolites would have provided a more complete picture of the Kyn pathway and which neurotoxic products were associated with neural, cognitive, affective, and AD biomarker outcomes. Several hundred subjects also lacked multiplex data, with many of them being cognitively intact. Thus, caution is warranted for extrapolating the mediation analyses to CN subjects, as most subjects with Kyn metabolites, GDS, and inflammatory marker data were MCI or AD.
5. Conclusion
Taken together, results suggest that Kyn/Tryptophan and especially Kyn/5-HT may be relevant biomarkers of inflammation pertinent to the pathogenesis of negative affect, cognitive decline, AD biomarkers, and clinical impairment diagnosis in AD. In particular, Aβ may impact the complement system, shifting itfrom mitigating Kyn-related associations with negative affect and instead exacerbating and fully driving them. Future work will shed light on the role of tryptophan metabolism in functional connectivity among brain networks, as well as further examining associations with peripheral inflammatory markers in relation to affect outcomes.
Supplementary Material
Higher Kyn/Trytophan and Kyn/5-HT were linked to poorer memory, executive function, and global cognition.
A higher Kyn/5-HT ratio was linked to less gray matter in AD-sensitive brain regions
Higher Kyn/5-HT was related to worse neuropsychiatric outcomes
Inflammatory and angiogenic markers mediated the relationship between Kyn/5-HT and depressive affect, which varied between Aβ− vs Aβ+ individuals.
Acknowledgements:
This study was funded in part by the College of Human Sciences at Iowa State University, a Big Data Brain Initiative grant through the Iowa State University Office of Vice President for Research, NIH grant AG047282, and the Alzheimer’s Association Research Grant to Promote Diversity grant AARGD-17–529552. No funding source had any involvement in the report. Data collection and sharing for this project were funded by the ADNI (National Institutes of Health Grant U01-AG-024904) and Department of Defense ADNI (award number W81XWH-12–2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the Alzheimer’s Association and the Alzheimer’s Drug Discovery Foundation. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private-sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuroimaging at the University of Southern California. The data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. Portions of this report were presented at the Psychoneuroimmunology Research Society in 2017 in Galveston, Texas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Auriel Willette - Reports no disclosures.
Brandon Klinedinst – Reports no disclosures.
Ana Collazo-Martinez – Reports no disclosures.
Scott Le – Reports no disclosures.
Colleen Pappas – Reports no disclosures.
Nathan Hoth – Reports no disclosures.
Qian Wang – Reports no disclosures.
Brittany Larsen – Reports no disclosures.
Amy Pollpeter – Reports no disclosures.
Tianqi Li – Reports no disclosures.
Sara A. Willette – Reports no disclosures.
Karin Allenspach – Reports no disclosures.
Jonathan Mochel – Reports no disclosures.
Robert Dantzer – Received honorarium from Danone Nutricia Research France unrelated to this work.
References
- Alexopoulos GS, Morimoto SS, 2011. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry 26, 1109–1118. 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH, 2007. Characterization of an indoleamine 2,3dioxygenase-like protein found in humans and mice. Gene 396, 203–213. 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA, 2012. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 69, 493–498. 10.1001/archgenpsychiatry.2011.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle GW, Mitchell HK, Nyc JF, 1947. Kynurenine as an Intermediate in the Formation of Nicotinic Acid from Tryptophane by Neurospora. Proc Natl Acad Sci U S A 33, 155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham LW, Desikan RS, Yokoyama JS, 2016. The relationship between complement factor C3, APOE ε4, amyloid and tau in Alzheimer’s disease. Acta Neuropathol Commun 4, 65–65. 10.1186/s40478-016-0339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidy N, Grant R, Adams S, Brew BJ, Guillemin GJ, 2009. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox Res 16, 77–86. 10.1007/s12640-009-9051-z. [DOI] [PubMed] [Google Scholar]
- Brites D, Fernandes A, 2015. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front Cell Neurosci 9, 476. 10.3389/fncel.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY, 2008. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage 41, 1177–1183. 10.1016/j.neuroimage.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BM, Charych E, Lee AW, Moller T, 2014. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci 8, 12. 10.3389/fnins.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P, Laye S, Fuchs D, 2011. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry 70, 175–182. 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Crane A, Brubaker WD, Johansson JU, Trigunaite A, Ceballos J, Bradt B, Glavis-Bloom C, Wallace TL, Tenner AJ, Rogers J, 2018. Peripheral complement interactions with amyloid β peptide in Alzheimer’s disease: 2. Relationship to amyloid β immunotherapy. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 14, 243–252. 10.1016/j.jalz.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D, Alzheimer’s Disease Neuroimaging I., 2012. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 6, 502–516. 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, 1997. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 48, S10–16. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Capuron L, 2017. Inflammation-associated depression : evidence, mechanisms and implications. Current topics in behavioral neurosciences,. Springer, pp. 1 online resource (x, 356 pages). [Google Scholar]
- Dantzer R, Capuron L, Irwin MR, Miller AH, Ollat H, Perry VH, Rousey S, Yirmiya R, 2008a. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology 33, 18–29. 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008b. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9, 46–56. 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry 67, 446–457. 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Fairchild AJ, Mackinnon DP, Taborga MP, Taylor AB, 2009. R2 effect-size measures for mediation analysis. Behav Res Methods 41, 486–498. 10.3758/BRM.41.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favennec M, Hennart B, Caiazzo R, Leloire A, Yengo L, Verbanck M, Arredouani A, Marre M, Pigeyre M, Bessede A, Guillemin GJ, Chinetti G, Staels B, Pattou F, Balkau B, Allorge D, Froguel P, Poulain-Godefroy O, 2015. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity (Silver Spring) 23, 2066–2074. 10.1002/oby.21199. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM, 2004. Automatically parcellating the human cerebral cortex. Cereb Cortex 14, 11–22. 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SM, Mungas D, Crane PK, Alzheimer’s Disease Neuroimaging, I., 2012. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav 6, 517–527. 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ, 2002. Prevalence of anxiety in adults with diabetes: a systematic review. Journal of psychosomatic research 53, 1053–1060. 10.1016/s0022-3999(02)00417-8. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Croitoru-Lamoury J, Dormont D, Armati PJ, Brew BJ, 2003. Quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia 41, 371–381. 10.1002/glia.10175. [DOI] [PubMed] [Google Scholar]
- Gulaj E, Pawlak K, Bien B, Pawlak D, 2010. Kynurenine and its metabolites in Alzheimer’s disease patients. Adv Med Sci 55, 204–211. 10.2478/v10039-010-0023-6. [DOI] [PubMed] [Google Scholar]
- Gunel MK, Tarsuslu T, Mutlu A, Livanelioglu A, 2010. Investigation of interobserver reliability of the Gillette Functional Assessment Questionnaire in children with spastic diparetic cerebral palsy. Acta Orthop Traumatol Turc 44, 63–69. 10.3944/AOTT.2010.2218. [DOI] [PubMed] [Google Scholar]
- Haroon E, Welle JR, Woolwine BJ, Goldsmith DR, Baer W, Patel T, Felger JC, Miller AH, 2020. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology 45, 998–1007. 10.1038/s41386-020-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, 2018. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. The Guilford Press, New York, NY. [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP, 2015. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14, 388–405. 10.1016/S1474-4422(15)700165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S, 1979. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 6, 65–70. [Google Scholar]
- Howren MB, Lamkin DM, Suls J, 2009. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71, 171–186. 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Huengsberg M, Winer JB, Gompels M, Round R, Ross J, Shahmanesh M, 1998. Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clin Chem 44, 858–862. [PubMed] [Google Scholar]
- Jack CR Jr., Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, J, L.W., Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW, 2008. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 27, 685–691. 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, Shlomchik M, 2001. Immunobiology: The Immune System in Health and Disease. Garland Science, New York. [Google Scholar]
- Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, Corona W, Morse CL, Zoghbi SS, Pike VW, McMahon FJ, Turner RS, Innis RB, Biomarkers Consortium, P.E.T.R.P.T., 2013. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 136, 2228–2238. 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fiévet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanché H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alpérovitch A, Lathrop M, Amouyel P, 2009. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nature genetics 41, 1094–1099. 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT, 2008. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation 5, 37. 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE, 2007. Inflammation, depression and dementia: are they connected? Neurochem Res 32, 1749–1756. 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Huckans M, Morasco BJ, 2010. Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies. Neurobiol Dis 37, 519–533. 10.1016/j.nbd.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Verkerk R, Bonaccorso S, Ombelet W, Bosmans E, Scharpe S, 2002. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci 71, 1837–1848. 10.1016/s0024-3205(02)01853-2. [DOI] [PubMed] [Google Scholar]
- Malpetti M, Kievit RA, Passamonti L, Jones PS, Tsvetanov KA, Rittman T, Mak E, Nicastro N, Bevan-Jones WR, Su L, Hong YT, Fryer TD, Aigbirhio FI, O’Brien JT, Rowe JB, 2020. Microglial activation and tau burden predict cognitive decline in Alzheimer’s disease. Brain 143, 1588–1602. 10.1093/brain/awaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, Bodurka J, Teague TK, Dantzer R, Savitz J, 2016. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun 53, 39–48. 10.1016/j.bbi.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Weis S, 2006. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res 1073–1074, 25–37. 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Miura H, Shirokawa T, Isobe K, Ozaki N, 2009. Shifting the balance of brain tryptophan metabolism elicited by isolation housing and systemic administration of lipopolysaccharide in mice. Stress 12, 206–214. 10.1080/10253890802252442. [DOI] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B, 2007. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 370, 851–858. 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Nikkheslat N, Zunszain PA, Horowitz MA, Barbosa IG, Parker JA, Myint AM, Schwarz MJ, Tylee AT, Carvalho LA, Pariante CM, 2015. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain Behav Immun 48, 8–18. 10.1016/j.bbi.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, Cowen PJ, 1994. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology 33, 575–588. 10.1016/00283908(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Patel NS, Mathura VS, Bachmeier C, Beaulieu-Abdelahad D, Laporte V, Weeks O, Mullan M, Paris D, 2010. Alzheimer’s beta-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. Journal of neurochemistry 112, 66–76. 10.1111/j.1471-4159.2009.06426.x. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Lunn BS, O’Brien JT, 2003. Effects of acute tryptophan depletion on cognitive function in Alzheimer’s disease and in the healthy elderly. Psychol Med 33, 41–49. 10.1017/s0033291702006906. [DOI] [PubMed] [Google Scholar]
- Querbes O, Aubry F, Pariente J, Lotterie JA, Demonet JF, Duret V, Puel M, Berry I, Fort JC, Celsis P, Alzheimer’s Disease Neuroimaging I., 2009. Early diagnosis of Alzheimer’s disease using cortical thickness: impact of cognitive reserve. Brain 132, 2036–2047. 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T, 2001. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 58, 445–452. 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Riedel WJ, Klaassen T, Schmitt JA, 2002. Tryptophan, mood, and cognitive function. Brain Behav Immun 16, 581–589. 10.1016/s0889-1591(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Robinson AC, Roncaroli F, Davidson YS, Minshull J, Heal C, Montaldi D, Payton A, Horan MA, Pendleton N, Mann DMA, 2020. Mid to late-life scores of depression in the cognitively healthy are associated with cognitive status and Alzheimer’s disease pathology at death. Int J Geriatr Psychiatry. 10.1002/gps.5470. [DOI] [PMC free article] [PubMed]
- Ryan JP, Sheu LK, Critchley HD, Gianaros PJ, 2012. A neural circuitry linking insulin resistance to depressed mood. Psychosom Med 74, 476–482. 10.1097/PSY.0b013e31824d0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Dantzer R, Meier TB, Wurfel BE, Victor TA, McIntosh SA, Ford BN, Morris HM, Bodurka J, Teague TK, Drevets WC, 2015a. Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology 62, 54–58. 10.1016/j.psyneuen.2015.07.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Dantzer R, Wurfel BE, Victor TA, Ford BN, Bodurka J, Bellgowan PS, Teague TK, Drevets WC, 2015b. Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology 52, 200–211. 10.1016/j.psyneuen.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ, 2012. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13, 465–477. 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ, 2009. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Annals of neurology 65, 403–413. 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalletta G, Caltagirone C, Girardi P, Gianni W, Casini AR, Palmer K, 2012. The role of persistent and incident major depression on rate of cognitive deterioration in newly diagnosed Alzheimer’s disease patients. Psychiatry Res. 198, 263–268. 10.1016/j.psychres.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS, Bechmann I, 2009. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol 118, 475–485. 10.1007/s00401-0090556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, Nakamura Y, Inui N, Nakamura H, Chida K, 2010. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 67, 361–365. 10.1016/j.lungcan.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Tosun D, Schuff N, Truran-Sacrey D, Shaw LM, Trojanowski JQ, Aisen P, Peterson R, Weiner MW, 2010. Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: a longitudinal MRI study. Neurobiology of aging 31, 1340–1354. 10.1016/j.neurobiolaging.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Biggelaar AH, Gussekloo J, de Craen AJ, Frolich M, Stek ML, van der Mast RC, Westendorp RG, 2007. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol 42, 693–701. 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D, 2000. Tryptophan degradation and immune activation in Alzheimer’s disease. J Neural Transm (Vienna) 107, 343–353. 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- Wilkinson L, 1975. Response variable hypotheses in the multivariate analysis of variance. Psychological Bulletin 82, 408–412. [Google Scholar]
- Willette AA, Coe CL, Colman RJ, Bendlin BB, Kastman EK, Field AS, Alexander AL, Allison DB, Weindruch RH, Johnson SC, 2012. Calorie restriction reduces psychological stress reactivity and its association with brain volume and microstructure in aged rhesus monkeys. Psychoneuroendocrinology 37, 903–916. 10.1016/j.psyneuen.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Johnson SC, Birdsill AC, Sager MA, Christian B, Baker LD, Craft S, Oh J, Statz E, Hermann BP, Jonaitis EM, Koscik RL, La Rue A, Asthana S, Bendlin BB, 2015. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement 11, 504–510 e501. 10.1016/j.jalz.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wragg RE, Jeste DV, 1989. Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry 146, 577–587. 10.1176/ajp.146.5.577. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A, 2005. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun 19, 345–350. 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Wu KY, Hsiao IT, Chen CS, Chen CH, Hsieh CJ, Wai YY, Chang CJ, Tseng HJ, Yen TC, Liu CY, Lin KJ, 2014. Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on 18F-florbetapir (AV-45/Amyvid) positron emission tomography. Eur J Nucl Med Mol Imaging 41, 714–722. 10.1007/s00259-013-2627-0. [DOI] [PubMed] [Google Scholar]
- Wu W, Nicolazzo JA, Wen L, Chung R, Stankovic R, Bao SS, Lim CK, Brew BJ, Cullen KM, Guillemin GJ, 2013. Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS One 8, e59749. 10.1371/journal.pone.0059749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, 1988. Geriatric Depression Scale. Psychopharmacol Bull 24, 709–711. [PubMed] [Google Scholar]
- Yin C, Ackermann S, Ma Z, Mohanta SK, Zhang C, Li Y, Nietzsche S, Westermann M, Peng L, Hu D, Bontha SV, Srikakulapu P, Beer M, Megens RTA, Steffens S, Hildner M, Halder LD, Eckstein H, Pelisek J, Herms J, Roeber S, Arzberger T, Borodovsky A, Habenicht L, Binder CJ, Weber C, Zipfel PF, Skerka C, Habenicht AJR, 2019. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nature medicine 25, 496–506. 10.1038/s41591-018-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD, Drevets WC, Dantzer R, Teague TK, Bodurka J, Savitz J, 2016. Kynurenine pathway metabolites are associated with hippocampal activity during autobiographical memory recall in patients with depression. Brain Behav Immun 56, 335–342. 10.1016/j.bbi.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, Thuret S, Price J, Pariante CM, 2012. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 37, 939–949. 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


