Abstract
Cigarette smoking and biomass smoke are the two main environmental risk factors of chronic obstructive pulmonary disease (COPD) worldwide. However, it remains unclear why these exposures result in two different disease phenotypes. In this study, we assessed the lung deposition from biomass and cigarette smoke exposures and examined whether differences due to inherently different particle size distributions and inhalation conditions may contribute to the differences between biomass- and tobacco-related COPD phenotypes. Using high-fidelity three-dimensional computational fluid-particle dynamics in a representative upper airway geometry, coupled to one-dimensional models of the lower airways, we computed total deposited doses and examined regional deposition patterns based on exposure data from a randomized control trial in Peru and from the literature for biomass and mainstream cigarette smoke, respectively. Our results showed that intrathoracic deposition was higher in cigarette smoking, with 36.8% of inhaled biomass smoke particles and 57.7% of cigarette smoke particles depositing in the intrathoracic airways. We observed higher fractions of cigarette smoke particles in the last few airway generations, which could explain why cigarette smoking is associated with more emphysema than biomass smoke exposure. Mean daily deposited dose was two orders of magnitude higher in cigarette smoking. Lobar distributions of the deposited dose also differed, with the left lower and right upper lobes receiving the highest doses of biomass and cigarette smoke particles, respectively. Our findings suggest that the differences between biomass- and tobacco-related COPD could, at least in part, be due to differences in total and regional lung deposition of biomass and cigarette smoke.
Keywords: Indoor air pollution, Tobacco smoke, Regional deposition, Computational fluid-particle dynamics (CFPD), Chronic obstructive pulmonary disease (COPD)
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of mortality and morbidity worldwide. In 2015, COPD affected approximately 174.5 million people and was responsible for an estimated 63.9 million disability-adjusted life years lost and 3.2 million deaths worldwide (GBD 2015 Chronic Respiratory Disease Collaborators 2017). Cigarette smoking and biomass smoke are considered the two most important risk factors for COPD (Lopez-Campos et al., 2016; Postma et al., 2015; Bruce et al., 2000; Fullerton et al., 2008; Salvi and Barnes 2010; Martin et al., 2013). However, the development of COPD from biomass smoke leads to a different disease phenotype from that of tobacco smoke (Lopez-Campos et al., 2016; Olloquequi et al., 2018; Camp et al., 2014; Rivera et al., 2008).
Biomass fuels are typically burned in low-efficiency stoves with poor ventilation, and incomplete combustion generates high concentrations of air pollutants including various noxious gases and particulate matter (PM). Biomass smoke contains many compounds similar to those present in tobacco smoke (Silva et al., 2015) and could therefore be expected to cause COPD with similar characteristics to tobacco-related COPD. However, the clinical presentation of biomass-associated COPD appears different (Lopez-Campos et al., 2016), with milder airflow obstruction and higher diffusion capacities of the lung for carbon monoxide (DLCO), but more anthracosis and pulmonary fibrosis compared to tobacco-related COPD (Olloquequi et al., 2018). Biomass smoke exposure has also been associated with less emphysema and epithelial damage, but more air trapping than tobacco smoke exposure (Camp et al., 2014; Rivera et al., 2008).
Limited data are available to explain differences between biomass- and tobacco-related COPD (Torres-Duque et al., 2016). A better understanding of the causes behind these differences is required to provide insight into the differences in pathophysiology, risk, and disease development between these two COPD phenotypes, and to help guide the development of phenotype-specific targeted treatment. We hypothesize that the differences between biomass- and tobacco-related COPD could be due, at least in part, to differences in the regional deposition of particulate matter (PM) in the lungs between biomass smoke exposure and cigarette smoking, as a result of different particle size distributions (PSD), PM concentrations and inhalation conditions. Cigarette smoke consists almost entirely of PM2.5 whereas biomass smoke contains a larger proportion of particles between 2.5 and 10 μm in size (Torres-Duque et al., 2016; Nicolaou et al., 2021). PM concentrations in mainstream cigarette smoke are also higher than those in biomass smoke, and smokers inhale through the mouth at higher flow rates, compared to individuals exposed to biomass smoke while nasally breathing. These differences can be expected to result in different total and regional deposited doses of inhaled particles in the lungs. To examine our hypothesis, we performed high-fidelity computational fluid-particle dynamics (CFPD) simulations of the airflow and particle deposition in the human upper airways, for both biomass and mainstream cigarette smoke exposure.
To our knowledge, this is the first 3D-CFPD study to examine deposition in the lungs from household exposure to biomass smoke. Recently, a couple of studies have investigated the lung-deposited doses of biomass smoke particles (BSP) using 1D models (Tigala et al., 2018; Nicolaou et al., 2021). Tigala et al. (2018) calculated the deposited fraction in the head airways, tracheobronchial airways, and alveolar region using the average particle size for different biomass fuels and liquified petroleum gas (LPG). Total deposition increased from 12.75% for LPG smoke (0.29 μm), to 15.63% for dung cakes (0.47 μm). Nicolaou et al., 2021 measured the PSD of biomass smoke across 20 kitchens in rural Puno, Peru, and found that the mean daily mass concentration consisted of a primary accumulation mode at 0.21 μm and a secondary coarse mode at 3.17 μm. Mean total deposition across ten representative airway models of adult Andean women, was 33.0 ± 0.75% of the inhaled mass, with 14.9 ± 0.76% depositing in the intrathoracic airways. The estimated mean daily lung-deposited mass (LDM) and surface area (LDSA) were 750.8 ± 1092.2 μg/day and 173.0 ± 251.9 cm2/day, respectively, with 339.0 ± 494.0 μg/day and 141.6 ± 206.2 cm2/day depositing in the intrathoracic airways.
While the literature on cigarette smoke particle (CSP) deposition is more extensive (Robinson and Yu 2001; Zhang et al., 2012; Kleinstreuer and Feng 2013; Asgharian et al., 2014; Pichelstorfer et al., 2016; Fuoco et al., 2017), no comparative assessments between CSP and BSP have yet been conducted. Predictions of the total cumulative doses from cigarette smoking and biomass smoke inhalation provide a means to quantify the relative severity of these two exposures. Furthermore, the regional deposition patterns obtained from high-fidelity simulations reveal areas in the lungs with enhanced deposition and increased likelihood of inflammation and disease initiation, which can contribute to expanding our understanding of the pathophysiology of tobacco- vs biomass-related COPD.
2. Materials and methods
The main components required for the in silico CFPD simulations are: construction of the airway geometry; specification of inhalation conditions and particle characteristics, including PM concentration and PSD; and the flow solver and particle-tracking algorithm. For generalizability, we adopted an airway geometry representative of the average adult upper airways (Shi et al., 2007; Stapleton et al., 2000; Ball et al., 2008; Nicolaou and Zaki 2016; Nicolaou 2018; Ostrovski et al., 2019; Das et al., 2018; Heller-Algazi et al., 2020; Weibel 1963; Horsfield et al., 1971). Inhalation conditions and particle characteristics were set according to previously published values (Zhang et al., 2002a, b; Fenn and Rahn 1965; Bernstein 2004; Ingebrethsen et al., 2011; Ng et al., 2014; Zhang et al., 2012; Pichelstorfer et al., 2016; Taylor et al., 1988; Reid et al., 2005; Johnson et al., 2014; Chen et al., 1990) and, in the case of biomass smoke, from kitchen area concentrations and personal exposures in 180 participants using biomass-burning cookstoves from a randomized control trial in Puno, Peru (Nicolaou et al., 2021; Checkley et al., 2020).
To solve the flow equations, we performed large-eddy simulation (LES), which offers the best trade-off between accuracy and computational cost for turbulent flows (Koullapis et al., 2018a,b). Particles were tracked through the flow field in a Lagrangian framework, by solving the equations of motion for each individual point-particle with the relevant forces acting on it (Koullapis et al., 2018a; Nicolaou 2018). Because high-fidelity simulations of the complete airway tree remain beyond current computational capabilities, we limited to modelling the extrathoracic airways (ETA) and the first seven generations of the tracheobronchial tree (G0-G6). To determine whole lung deposition, we coupled our 3D-CFPD model to a 1D-model of the lower airways.
2.1. Airway geometry
The 3D airway geometry comprises of the nasal and oral cavities, pharynx, larynx, trachea (G0) and G1-G6 (Fig. 1). The representative nasal airway geometry model was obtained from MRI scans of a healthy, non-smoking, adult male (Shi et al., 2007). The oral airway geometry is an idealized model representative of the average adult ETAs (Stapleton et al., 2000; Ball et al., 2008; Nicolaou and Zaki 2016; Nicolaou 2018). Nasal and oral ETA geometries were combined and the trachea extruded and merged with a tracheobronchial tree model for an average human (male) adult, featuring seven generations of dichotomously branching asymmetric airways (Ostrovski et al., 2019; Das et al., 2018; Heller-Algazi et al., 2020; Weibel 1963; Horsfield et al., 1971).
Fig. 1.
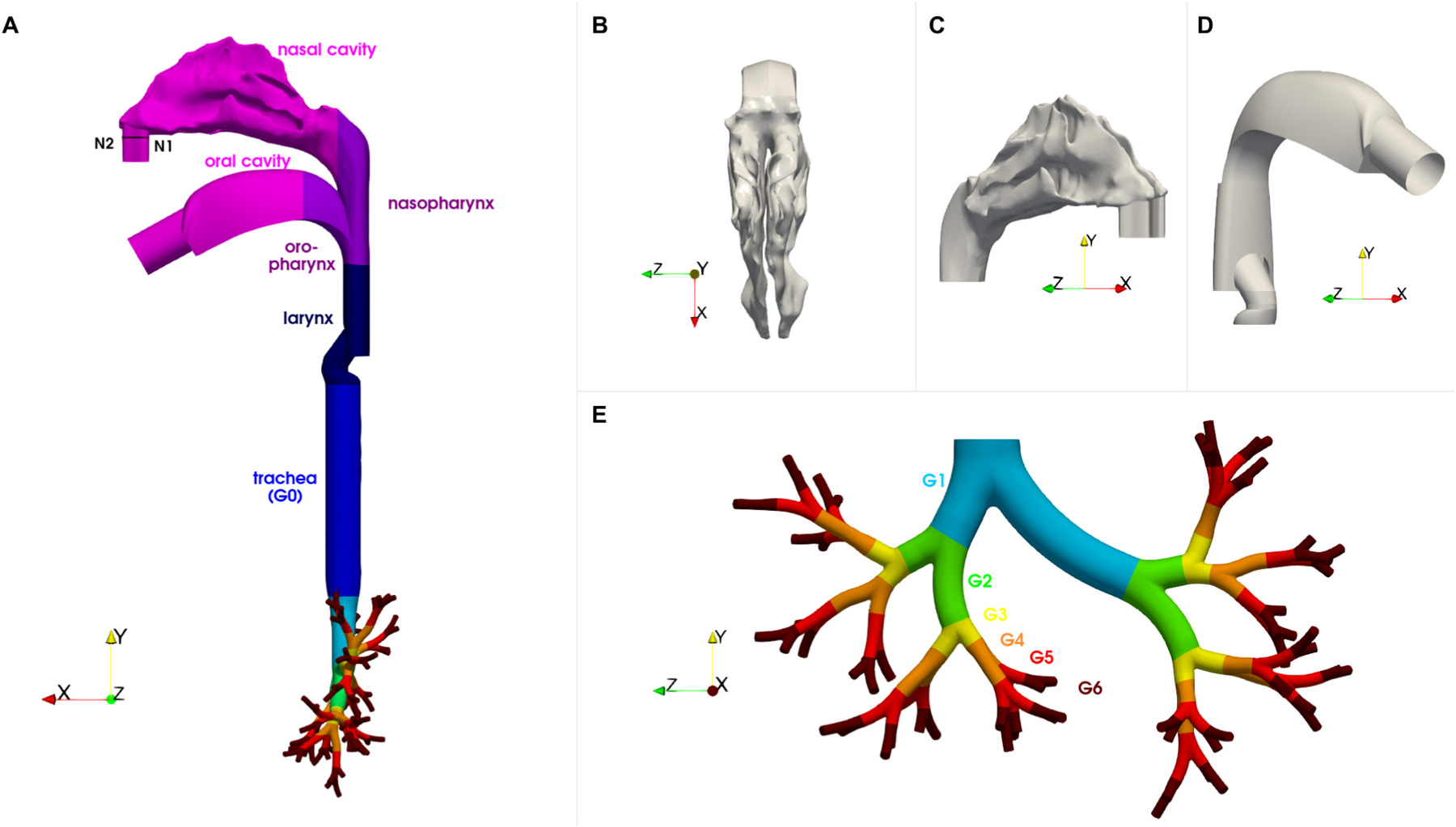
Views of the 3D airway geometry which consists of the nasal and oral airways and the tracheobronchial tree spanning seven generations: (A) side view of the full geometry; (B) top view of the nasal cavity; (C) side view of the nasal cavity; (D) side view of the oral geometry; (E) front view of the tracheobronchial tree. The tracheobronchial airways are coloured by their generation number (G0 to G6). Plane N1–N2 represents the particle injection plane for nasal inhalation.
2.2. Inhalation conditions and particle characteristics
For biomass smoke exposure (case A), we considered a typical inhalation cycle for normal breathing at rest (Zhang et al., 2002a, b; Fenn and Rahn 1965). We used the gravimetric measurements of kitchen and personal PM2.5 exposures collected at baseline from a randomized, controlled field intervention trial of LPG stoves and fuel distribution in which 180 female participants living in rural communities in Puno, Peru, and using biomass fuels daily for cooking were enrolled and followed for 2 years (Checkley et al., 2020). We collected direct-reading measurements of particle number concentrations in a subset of 20 kitchens (Nicolaou et al., 2021), and derived a continuous PSD model of the gravimetric-corrected mean PM concentration during cooking (Nicolaou et al., 2021). Daily deposited doses were computed based on the average length of time spent in the kitchen while the cookstove is on. We estimated this time based on the mean daily personal PM2.5 concentration obtained from gravimetric sampling, and the assumption that the majority of personal PM exposure in our participants occurred during cooking. This appears to be a reasonable assumption since ambient air pollution in our study setting is not likely to contribute heavily to the exposures experienced by biomass stove users, and other indoor PM concentrations are also expected to be low compared to those in the kitchen (Nicolaou et al., 2021). The average time spent in the kitchen (in minutes) while the cookstove is on, tkit, can therefore be estimated from:
| (1) |
where is the mean PM2.5 concentration in the kitchen during cooking (Table 1) and is the mean daily personal PM2.5 exposure (Nicolaou et al., 2021), which gives us an average of 25 min in the kitchen during cooking.
Table 1.
Inhalation conditions and particle parameters.
| Case A: Biomass smoke | Case B: Mainstream cigarette smoke | |
|---|---|---|
| Inhalation route | Nasal | oral |
| Inhalation time, Tinh (s) | 2.0 [22] | 2.5 [25,28] |
| Mean flow rate, Qinh, mean (L/min) | 15.0 [22] | 30.0 [27,29] |
| Peak flow rate, Qinh,max (L/min) | 20.0 [22] | 47.1 [27] |
| Matching steady flow rate, Qinh (L/min) | 17.5 | 38.6 |
| Turbulent Womersley number at trachea, Wo*trachea | 0.8 | 0.5 |
| Reynolds number at trachea, Retrachea | 1590 | 3116 |
| Mean daily exposure (number of inhalations) | 349.7 | 194.7 |
| Particle size distribution | bimodal log-normal [13] | unimodal log-normal [25] |
| PM0.1 concentration (μg/m3) | 226 | 55 |
| PM2.5 concentration (μg/m3) | 6630 | 8.92 × 106 |
| PM10 concentration (μg/m3) | 7137 | 8.92 × 106 |
| Total mass concentration (μg/m3) | 7257 | 8.92 × 106 |
| Volume fraction, Φv | 5.58 × 10−9 | 8.11 × 10−6 |
| Mass loading, Φm | 5.92 × 10−6 | 7.28 × 10−3 |
| Particle density (kg/m3) | 1300 [30] | 1100 [31,32] |
For cigarette smoking (case B), we considered the typical pattern of smoke inhalation observed in most smokers, which consists of two stages. In the first “puffing” stage, smoke is drawn into the mouth with the soft palate closed. The second stage begins with a pause of variable duration followed by inhalation of the smoke into the lungs (Bernstein 2004; Ingebrethsen et al., 2011). Due to the high number concentrations of fresh smoke particles in a puff, rapid coagulation occurs inside the mouth during the puffing stage and continues during the mouth-holding period (Ingebrethsen et al., 2011), resulting in significantly larger particles and lower number concentrations in inhaled smoke compared to fresh smoke. For our inhaled cigarette smoke, we adopted the PSD at the end of a 2 s puff and 1 s mouth-hold period for a medium ventilation cigarette smoked at a 60 ml puff volume (Ingebrethsen et al., 2011). The daily deposited dose was estimated based on a mean of 17.7 cigarettes per day (Ng et al., 2014) and 11 puffs per cigarette (Bernstein 2004).
2.3. Coupled 3D-1D flow and particle transport model
Flow solver:
To compute the air flow in the 3D airway geometry, we performed LES using the dynamic Smagorinsky subgrid scale model (Koullapis et al., 2018a). In LES, the fluid flow is described by the filtered Navier-Stokes equations for incompressible flows as follows,
| (2) |
| (3) |
where ui is the velocity component in the ith direction, p is the pressure, ρ and υ are the density and kinematic viscosity of air respectively, and υSGS is the subgrid-scale (SGS) turbulent eddy viscosity. The overbar represents the resolved quantities.
The governing equations (Eqs. (2) and (3)) were discretized and solved in OpenFOAM using a finite-volume scheme for unsteady incompressible flow based on a merged PISO-SIMPLE (pressure implicit split operator-semi-implicit method for pressure-linked equations) algorithm. The scheme was second-order accurate in both space and time. We used a geometric-algebraic multi-grid algorithm with Gauss-Seidel relaxation to solve the pressure Poisson equation, and a diagonal incomplete LU-preconditioned bi-conjugate gradient solver for the velocity equations. The residual tolerances were set to 1 × 10−7 and 1 × 10−8 for pressure and velocity respectively, and the residual control for the outer correction loop was set to 1 × 10−4. Convergence was typically obtained within 12–13 outer iterations.
To assess the effect of unsteadiness on the flow, we determined the turbulent counterpart of the Womersley number given by,
| (4) |
where D is the airway diameter, ω is the angular frequency of the oscillatory flow, and υt is the eddy viscosity (Pedley 1977).
In the trachea, is at most 0.8 and 0.5 for Case A and B, respectively. In the smaller airways, these values are smaller. The effects of unsteadiness are therefore not expected to be large and the flow may be assumed quasi-steady. Steady airflow inhalation was prescribed for both biomass and cigarette smoke exposure, with equal flow rates to both nostrils in the former case. To obtain similar deposition efficiencies as in cyclic inspiratory flow, we adopted matching inhalation flow rates ( for Case A; for Case B), which lie between the mean and peak flows of the corresponding inhalation waveforms (Zhang et al., 2002a). Previous studies in the extrathoracic and upper airways have shown no significant differences in deposition for nano- and micron-sized particles between cyclic inspiratory flow and constant flow at a matching flow rate (Zhang et al., 2002b; Zhang and Kleinstreuer. 2004; Shi et al., 2006).
In Case A, we prescribed an inflow rate of to each of the nasal inlets, and a zero mass flow rate through the oral cavity. In Case B, we applied to the oral cavity inlet and zero mass flow rate through both nasal inlets. In both cases, we adopted uniform velocity profiles, and zero gradient conditions for pressure at all inlets. Constant pressure was assumed at the outlets. To eliminate the development effect of plug flow, we extended the nostril and oral cavity inlets with constant-area tubes (Fig. 1). Although the inlet condition is an approximation, previous experimental and numerical studies have shown that a given nostril or oral inlet airflow does not alter the downstream flow patterns significantly (Subramaniam et al., 1998; Ball et al., 2008; Nicolaou and Zaki 2013). Regarding the outflow condition, outlet pressures in a G6 airway model computed based on prescribed flow rates and the impedances of the downstream small airways have been found to be very similar to one another (Ma and Lutchen 2006). Constant pressure is therefore a reasonable approximation.
Particle-tracking scheme:
A Lagrangian particle-tracking approach was adopted to compute the particle trajectories and determine deposition. For biomass smoke, the mean volume fraction of PM in the air during cooking is 5.6 × 10−9, indicating that only the fluid flow has an effect on the particles (one-way coupling). For cigarette smoke, the volume fraction inhaled into the lungs is 8.1 × 10−6, which, according to the standard paradigm, falls in the two-way coupling regime where the particles also have an effect on the flow (Elghobashi 1994). However, studies have shown particle feedback on the flow to be negligible at volume fractions, , and mass loadings, (Costa et al., 2020; Capecelatro et al., 2018; Wang and Richter 2019), so we can therefore consider both cases to fall into the one-way-coupling assumption where the effects of the particles on the flow and particle-particle interactions are negligible.
Particles were tracked through the flow field by solving the equations of motion that describe the change in position, xp, and velocity, up, along their trajectory:
| (5) |
| (6) |
where mp is the mass of the particle and represents all the forces acting on the particle. For the size range of particles considered, aerodynamic drag (FD), gravity (FG), and Brownian diffusion (FB) were taken as the dominant forces on the particles (Koullapis et al., 2018b). Other forces such as the Saffman lift, added mass, pressure gradient, and Basset forces were considered negligible (Finlay et al., 1996). Particles were assumed to be spherical, rigid and non-rotating.
The drag force acting on the particles was determined by
| (7) |
where Rep is the particle Reynolds number,
| (8) |
dp is the particle diameter, ρf and ρp are the fluid and particle densities respectively, μf is the fluid viscosity, uf is the fluid velocity at the particle location, CD is the drag coefficient and CC is the Cunningham correction factor. For the drag coefficient, we adopted the correlation proposed by Schiller and Naumann (1933):
| (9) |
The Cunningham correction factor, applied to the drag to account for slip at the particle surface due to non-continuum effects, was computed as
| (10) |
where λ = 0.070 μm is the mean free path of air. For 1 μm particles at standard atmospheric conditions, drag is 15% lower than that predicted without taking into account the slip correction. As particle size decreases beyond 1 μm, slip rapidly increases.
The combined gravity and buoyancy force is given by
| (11) |
where the gravitational vector, g, acts in the downward vertical direction.
For submicron particles, Brownian motion caused by random collisions with the gas molecules, becomes important (Ounis et al., 1991). The Brownian force was modelled as a Gaussian white-noise random process (Li and Ahmadi 1992), with the amplitudes of the Brownian force components at time t evaluated from
| (12) |
where Gi is a zero mean variant from a Gaussian probability density function, T = 310 K is the absolute temperature, is the Brownian diffusivity, kB = 1.3806488 × 1023 J/K is the Boltzmann constant, and Δt is the time step used in the integration of the particle equations of motion.
In case A, biomass smoke particles (BSP) were uniformly distributed on planes offset from the extruded inlet patches to minimize flow development effects (see Fig. 1), and released into the flow at every time step over a time period equal to approximately half a flow-through in the trachea (0.025s). The initial velocity was set equal to the fluid velocity at the particle location. In case B, aged cigarette smoke particles (CSP) post puff and mouth-hold, were uniformly distributed throughout the oral cavity with zero initial velocity at the start of inhalation. Particles were tracked until they exited the airway geometry through G6 or deposited on the wall. An implicit Euler scheme was used to integrate the particle equations of motion (Eqs. (5) and (6)), and the fluid velocity at the particle location was obtained via inverse-distance weighted interpolation. Deposition was assumed once a particle came into contact with the airway wall. Reflection and re-suspension were not included as the existence of a saliva or mucus layer on the inner walls of the airways ensures that particles colliding with the surface deposit (Koullapis et al., 2018b).
One-dimensional model of the lower airways:
To model whole lung deposition, we coupled the 3D geometry with a 1D model of the airways beyond G6. Specifically, we attached airways from the five-lobe typical-path model of Yeh and Schum (1980) to the end of each G6 airway of the 3D geometry and adopted the multiple-path deposition model by Anjilvel and Asgharian (1995). In this model, the airways are considered straight cylindrical tubes with constant cross-sectional area, A, connected in series in a bifurcating tree (Supplementary Material: Figure S1). The number of airways and the average geometrical parameters (length, L, diameter, d, branching angle, φ, and gravity angle, θ) of the airways in each generation differ for each lobe. Note that Yeh and Schum (1980) labeled the airway generations starting at G1 instead of G0, so our G7 corresponds to G8 in the five-lobe model.
The aerosol concentration at the entrance of the G7 airways in a given lobe was set to the concentration exiting that lobe of the 3D geometry. The time the inspiratory front of air crosses the distal end of generation k, or crossing time tk, was calculated factoring in the time to clear the upper airways, based on our 3D geometry. The concentration of particles at a given location was assumed to be zero before tk. Traversing down the tree, the proximal, , and distal concentrations, , in the airways after the front of air crosses that location were computed based on theoretically-derived efficiencies for deposition by impaction, εi (Cai and Yu 1988), sedimentation, εs (Wang 1975), and diffusion, εd (Ingham 1975; Yeh and Schum 1980) within each airway tube or bifurcation (Anjilvel and Asgharian, 1995):
| (13) |
The total mass entering an airway in generation k over the inhalation period, , was then determined by
| (14) |
where is the flowrate at the proximal end of the airway and is the particle concentration at the distal end of the parent airway (Supplementary Material: Figure S1). Similarly, the total mass exiting the airway, , was computed by
| (15) |
where and are the flowrate and particle concentration, respectively, at the distal end of the airway. The mass remaining in the airway at the end of inhalation, , was approximated by
| (16) |
Finally, the mass depositing in the airway, , was computed from a mass balance:
| (17) |
where is the initial mass in the airway at the beginning of inhalation, which was assumed to be zero.
The dimensions of the airways in the five-lobe model correspond to a lung inflated to total lung capacity (TLC). We adjusted these dimensions to equal a normal lung volume by setting an airway size midway between lung volume at rest and maximum inhalation following the scaling procedure described in Schum and Yeh (1980) and assuming that (i) there is a 15% increase in the conducting airway diameters between 60% of TLC and TLC, (ii) both lengths and diameters in the respiratory region are proportional to the cube root of the lung volume (Schum and Yeh 1980; Yeh and Schum 1980), and (iii) functional residual capacity (FRC) is 60% of TLC (Yeh and Schum 1980). Functional residual capacity was 3216 ml. The inhaled volume, Vinh, was calculated from Vinh = QinhTinh, where Tinh is the inhalation time and Qinh is the inhalation flow rate. Deposition was computed at FRC + Vinh/2. Volumes inhaled during regular nasal breathing (case A) and cigarette smoking (case B) were 588 ml and 1608 ml, respectively.
3. Results and discussion
3.1. Inhalation and exposure conditions
Inhalation conditions and particle parameters for biomass and mainstream cigarette smoke exposure are summarized in Table 1. Inhalation flow rates were higher for cigarette smoke, with mean and maximum flow rates of 30 L/min and 47.1 L/min, respectively, when compared to 15 L/min and 20 L/min during biomass smoke. The inhalation route also differs: while biomass smoke is inhaled nasally, tobacco smoke is inhaled through the mouth, which has a lower filtration efficiency compared to the nasal cavity.
Differences in the PSD and PM concentrations between biomass and cigarette smoke are also evident. Mean PSD of biomass smoke during cooking consists of a bimodal log-normal distribution with a primary accumulation mode at 0.20 μm containing 84% and of the total mass, and a secondary coarse mode at 2.81 μm. Primary and secondary modes have geometric standard deviations of 1.50 and 2.79, respectively (Supplementary Material: Figure S1A). For inhaled cigarette smoke, the adopted mass concentration follows a log-normal distribution with geometric mean diameter, dg = 0.38 μm, and geometric standard deviation, GSD = 1.36 (Supplementary Material: Figure S1B). Concentrations of inhaled CSP are approximately three orders of magnitude higher than BSP.
3.2. In silico models
A grid convergence and validation study were performed for the flow and particle deposition in the nasal cavity (Supplementary Material: Results). Based on the results from the mesh independence test, we adopted a mesh with approximately 10 million elements for our upper airway geometry. To ensure numerical stability, the time step was set to 10−5 s. Simulations were performed for a total physical time of 0.9 s. The first 0.3 s were discarded to remove the initial transient development of the flow, and particle tracking was performed for the last 0.6 s, which was sufficient to ensure all particles had either deposited on the airway walls or exited through the outlet branches in G6. Simulations were computed in parallel on 64 processors and the overall computational time was approximately 6000 CPU hours for each of the two cases, including the initial time to remove the transient and the particle-tracking time. Approximately 1.2 million particles were tracked in each simulation.
3.3. Deposition in the upper airways
The deposition patterns in the upper airways of biomass smoke particles (BSP) and cigarette smoke particles (CSP) show distinct differences between the two exposures (Fig. 2). For biomass smoke high filtration (45.7% of deposited PM in the upper airways) occurs in the nasal cavity, as the geometrical constriction of the nasal passages results in an increase in air velocities and higher deposition via impaction for larger particles. Despite this filtering effect, large particles still penetrate down to the intrathoracic airways (ITA) and beyond the outlet branches in G6 (Fig. 2A,B,D). For cigarette smoke, higher levels of deposition are observed in the pharynx and larynx, as the higher inhalation rate results in stronger jet impingement on the back wall (Supplementary Material: Figure S6). The larger concentrations of small particles and higher levels of turbulence compared to biomass exposure (Supplementary Material: Figure S7) also result in higher levels of deposition along the trachea due to dispersion (Fig. 2E–H). In general, larger particles display more concentrated deposition patterns, or hot spots, due to an increase in impaction and gravitational sedimentation. Examples can be seen along the bottom wall of the nasal cavity and the left main bronchus for biomass smoke (Fig. 2A,D). Small particles on the other hand, exhibit higher levels of dispersion. In the ITA, particles have a higher tendency to deposit on the carinal ridges of the bifurcations.
Fig. 2.

Particle deposition patterns in the upper airways: (A–D) Biomass smoke; and (E–H) cigarette smoke. (A,E) Front view of the full geometry; (B,F) rear view of the full geometry; (C,G) side view of the extrathoracic airways; (D,H) front view of the intrathoracic airways.
In Fig. 3 we show the deposition and penetration efficiencies and fractions of BSP and CSP in the upper airways, as a function of particle size. Deposition efficiency in the upper airways follows the characteristic S-curve behaviour as a function of particle size (Fig. 3A). Although penetration efficiency decreases as particle size increases, particles up to ~15 μm still penetrate the lower airways, with ~15% of 10 μm particles not depositing in the upper airways (Fig. 3B). For sizes smaller than ~4 μm, over 80% of particles penetrate beyond G6. The deposition and penetration fractions for biomass and cigarette smoke display main peaks around 0.2 μm and 0.4 μm, respectively, which correspond to the geometric mean diameters of the accumulation modes (Fig. 3C and D). A secondary peak in deposition exists around 10 μm for biomass smoke, while the penetration fractions above this particle size are negligible as these larger particles deposit in the upper airways (Fig. 3D).
Fig. 3.

Deposition and penetration of BSP and CSP by particle size in the upper airways: (A) Deposition and (B) penetration efficiencies; (C) Deposition and (D) penetrations fractions. The deposition efficiency, , represents the fraction of inhaled particles of a given size i that deposit in the upper airways. Similarly, the penetration efficiency is the fraction of inhaled particles of a given size that penetrate beyond G6. The deposition fraction, , is defined as the fraction of total inhaled mass that deposits in the upper airways at a given particle size i, and the penetration fraction is the fraction of total inhaled mass penetrating beyond G6 at a given particle size.
In Fig. 4, we show the fractions of inhaled PM10–2.5, PM2.5–0.3 and PM0.3 depositing in the different regions of the upper airway geometry. The nasal cavity filters the largest portion of inhaled BSP, with 12.6%, 1.4% and 3.8% of PM10–2.5, PM2.5–0.3 and PM0.3 respectively, depositing in this region. On the other hand, deposition of CSP in the mouth is minor, due to the low filtration efficiency of the oral cavity. A relatively high deposition fraction in the larynx is observed across all 3 p.m. sizes in both cases, which can be attributed to the laryngeal jet (Supplementary Material: Figure S6). Beyond the trachea, deposition increases with generation number. While mean flow velocities and probability of impaction remain fairly constant in the first 7 generations, the decrease in airway diameters increases deposition via diffusion and, compounded by larger gravity angles, increases sedimentation.
Fig. 4.
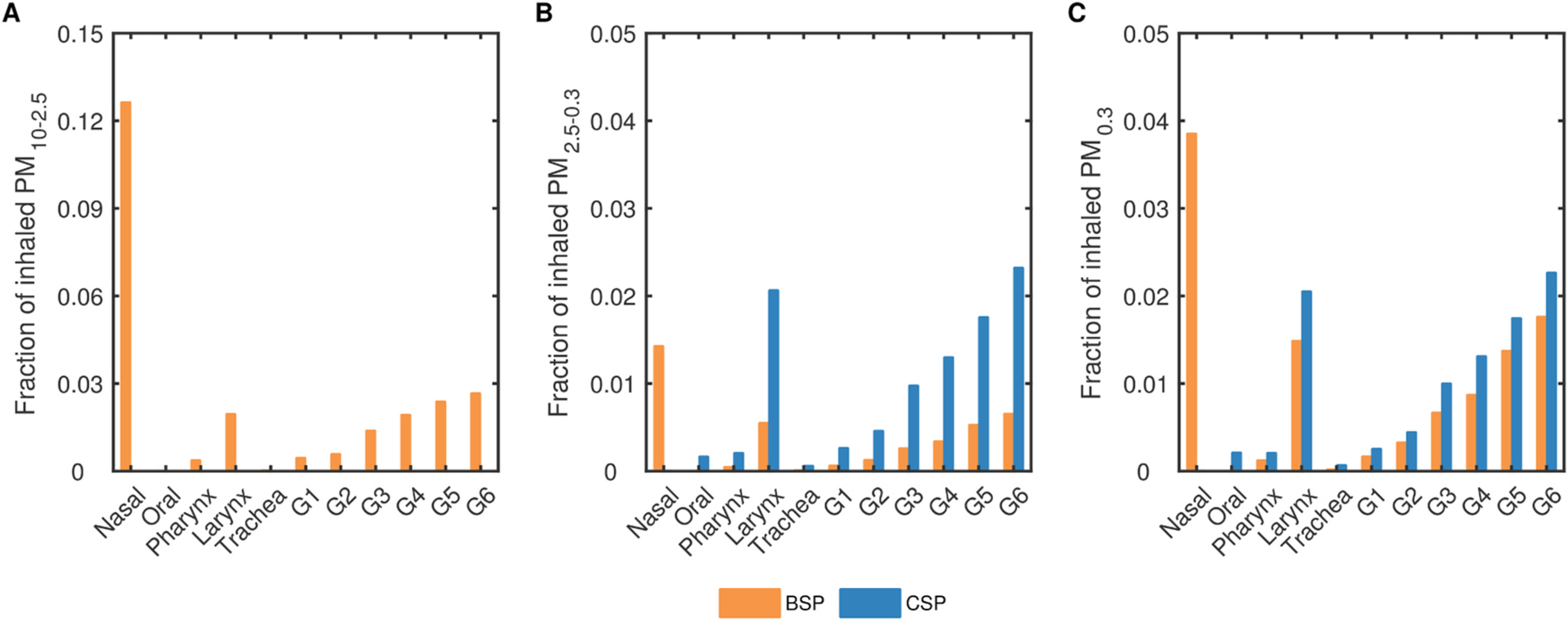
Regional deposition fractions of BSP and CSP in the upper airways: (A) Fraction of inhaled PM10 – 2.5 mass depositing in each region from the ETA to G6; (B) Fraction of inhaled PM2.5 – 0.3 mass depositing in each region from the ETA to G6; (C) Fraction of inhaled PM0.3 mass depositing in each region from the ETA to G6.
3.4. Deposition in the lower airways
In Fig. 5 we show the fractions of inhaled BSP and CSP entering and depositing in the lower airway (LA) model, beyond G6, for PM10–2.5, PM2.5–0.3 and PM0.3. The majority of the inhaled aerosol clears the upper airways, with 70.0% of PM10–2.5, 85.3% of PM2.5–0.3, and 85.4% of PM0.3 from biomass smoke, and 87.0% of both PM2.5–0.3 and PM0.3 from cigarette smoke entering the LA model. Overall, 42.9% of the inhaled PM10–2.5, 30.7% of PM2.5–0.3 and 31.5% of PM0.3 deposit in the LA during biomass exposure. In the case of cigarette smoking, a higher fraction of the inhaled mass (56.7% of PM2.5–0.3 and 57.4% of PM0.3) deposits in the LA. The differences in the deposited fractions between biomass and cigarette smoke demonstrate the importance of accounting for PSD and not just traditional PM size metrics. The results also show that, while exposure assessment studies typically focus on PM2.5, PM above 2.5 μm should not be neglected as a substantial fraction is able to penetrate into the lower airways.
Fig. 5.

Fractions of inhaled BSP and CSP entering and depositing in the lower airways (beyond G6) of the left upper (LU), left lower (LL), right upper (RU), right middle (RM), and right lower (RL) lobes: (A) Fractions of inhaled PM10 – 2.5 mass per lobe; (B) Fraction of inhaled PM2.5 – 0.3 mass per lobe; (C) Fraction of inhaled PM0.3 mass per lobe.
The lower airways in the lower left (LL) lobe receive the highest fraction of inhaled particles for both biomass and cigarette smoke. However, while deposition of BSP is also highest in the LL lobe, the highest deposition of CSP occurs in the right upper (RU) lobe. Lowest doses entering the lower airways are in the right lower (RL) and right middle (RM) lobes for BSP and CSP, respectively, and the lowest deposition occurs in the RL lobe for both. These results show a non-linear relationship between the doses entering and those depositing in each lobe, which is likely due to the difference in PSD entering the lower airways of each lobe as well as the geometric variation across lobes.
3.5. Lung deposited doses
Deposited fraction (DF), mass (DM) and surface area (DSA) are plotted by generation number in Fig. 6A, B, and C, respectively, for the whole lung. Two peaks in deposition are observed: a primary one in the pulmonary region, with maximum deposition occurring in G21, and a secondary one in the upper bronchial airways, in G6. While BSP and CSP follow similar trends in deposition, CSP displays higher fractions in the last few airway generations as a result of the higher air flowrates which allow particles to penetrate further during inhalation. The inhaled concentrations of CSP are significantly higher than those of BSP, and so deposited mass and surface area are approximately two orders of magnitude larger. Normalizing by unit tissue surface area, we observe that the upper airways (in particular G3-G6) receive the highest tissue doses, and that doses per tissue surface area rapidly decay beyond G6 (Fig. 6D–F). Since adverse effects of inhaled aerosols are related to the local tissue dose (Hofmann et al., 1989), these results demonstrate the importance of taking into account not only the regional deposited doses but also the airway surface areas for risk assessment.
Fig. 6.
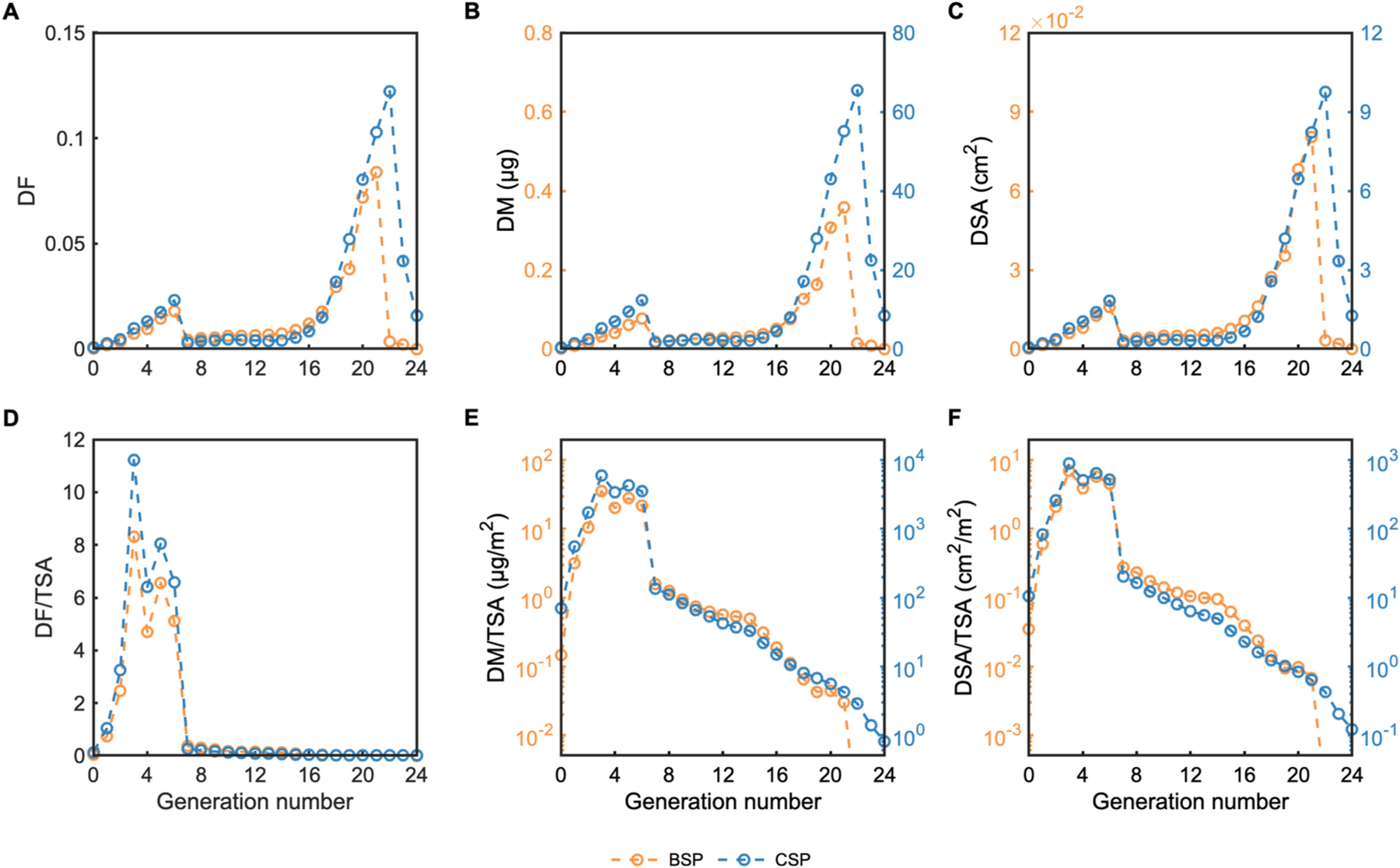
Deposition of CSP and BSP per airway generation in one inhalation: (A) Deposited fraction (DF), (B) Deposited mass (DM), (C) Deposited surface area (DSA), (D) Deposited fraction per tissue surface area (DF/TSA), (E) Deposited mass per tissue surface area (DM/TSA), (F) Deposited surface area per tissue surface area (DSA/TSA).
Finally, in Fig. 7 we show the daily total, regional and lobar deposition of CSP and BSP. Overall, 44.4% and 60.2% of the inhaled mass deposits in the airways for BSP and CSP, respectively. The larger sized particles in BSP, along with the nasal inhalation route, result in a three-fold increase in deposition fraction in the extrathoracic airways for BSP (7.6%) compared to CSP (2.5%). We also observe a substantially larger fraction of CSP depositing in the alveolar ducts (AD) and sacs (AS): 43.8% compared to 21.9% of BSP (Fig. 7A).
Fig. 7.
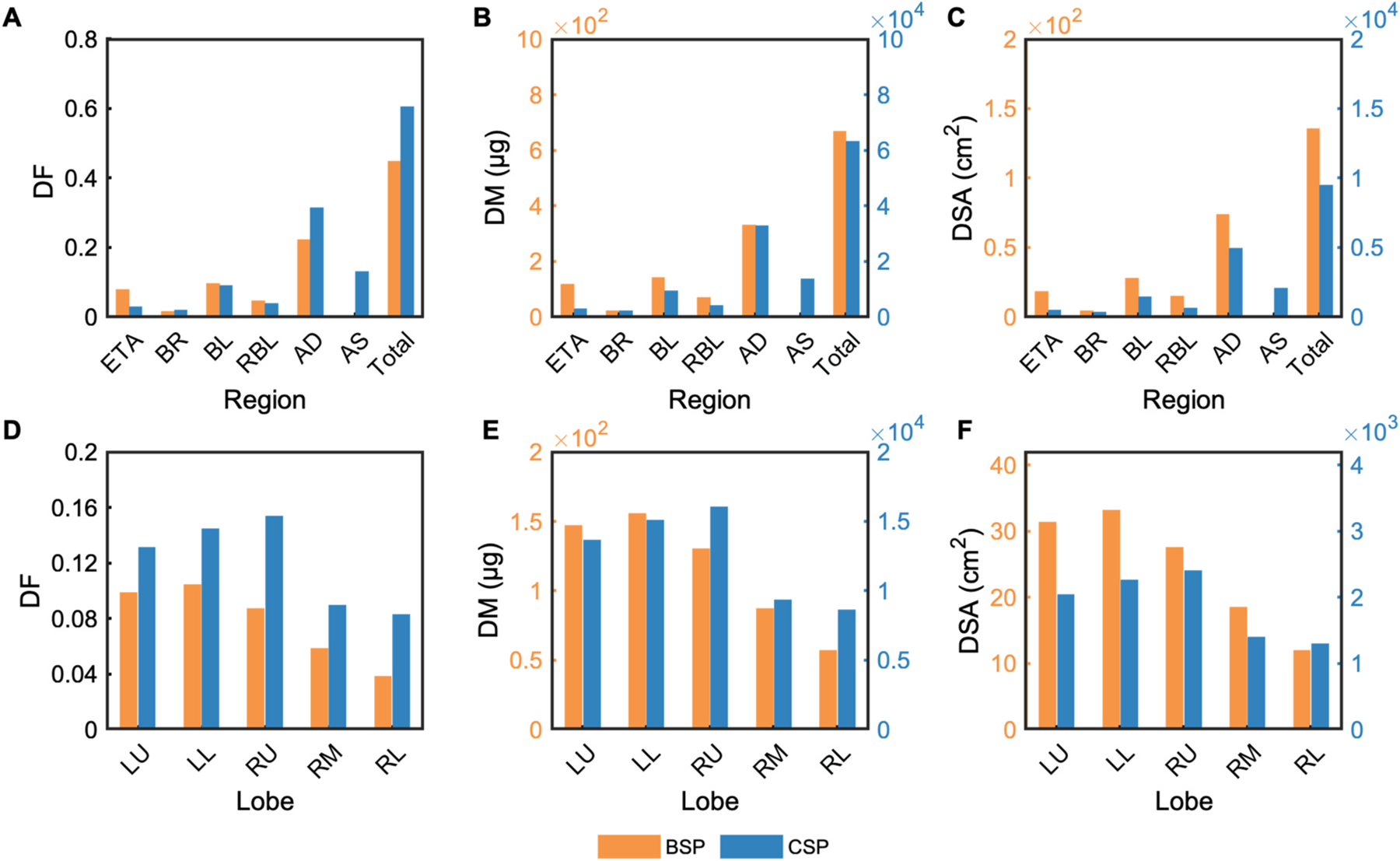
Regional and lobar deposited fractions (DF), daily deposited mass (DM) and daily deposited surface area (DSA) of CSP and BSP: (A–C) Total and regional deposition in the extrathoracic airways (ETA), bronchi (BR), bronchioles (BL), respiratory bronchioles (RBL), alveolar ducts (AD), and alveolar sacs (AS); (D–F) Deposition in the left upper (LU), left lower (LL), right upper (RU), right middle (RM), and right lower (RL) lobes.
Daily deposited doses, in terms of both mass and surface area, are approximately two orders of magnitude larger for CSP, as the higher PM concentration and inhalation rate result in higher inhaled doses despite the shorter exposure duration compared to BSP (Fig. 7B and C). The lobar distribution of the deposited dose also differs between BSP and CSP, with the LL and RU lobes receiving the highest doses during biomass exposure and cigarette smoking, respectively (Fig. 7D–F). In general, higher deposition fractions are observed across all lobes for CSP (Fig. 7D).
4. Conclusions
We present the first in silico CFPD experiment to examine particulate lung deposition from household exposure to biomass smoke, and to provide detailed data on the differences between biomass and tobacco smoke exposure. Using high-fidelity CFPD, we demonstrate that mainstream tobacco smoke exposure resulted in higher doses of PM depositing in the lungs compared to biomass smoke, which may explain differences in the phenotypes and the risk of developing COPD between these two exposures. Our research concludes that differences in concentration and size distribution of inhaled particles, as well as the inhalation conditions, are important factors resulting in different lung-deposited doses and regional deposition patterns for biomass and cigarette smoke exposure. Indeed, biomass smoke consisted of a bimodal log-normal distribution with particles in the PM50–0.05 range, while aged cigarette smoke contained a narrower distribution of particle sizes, with most particles in the PM1–0.1 range. Concentrations of inhaled CSP were also three orders of magnitude higher than BSP.
Intrathoracic deposition fraction was higher in the case of cigarette smoking, with 36.8% of inhaled BSP and 57.7% of CSP depositing in the ITAs, of which 59.6% and 76.0%, respectively, deposited in the alveolar region. Due to the larger particle sizes and higher filtration efficiency of the nasal cavity, the fraction of inhaled PM depositing in the ETAs was three times higher in biomass smoke exposure. While BSP and CSP followed similar trends in deposition fraction with generation number, with tissue doses being highest in G3 to G6, we observed higher fractions of CSP in the last few airway generations as a result of the higher air flowrates which allow particles to penetrate further during inhalation. This decreased deposition of biomass smoke in the lung parenchyma is in agreement with CT studies which have found that biomass-related COPD is associated with less emphysema than tobacco smoke exposure (Camp et al., 2014; Rivera et al., 2008).
While the daily exposure duration was shorter on average in the case of cigarette smoking, the daily inhaled dose was significantly higher due to higher PM concentrations and a higher inhalation rate compared to biomass smoke exposure. The fraction of inhaled mass depositing in the airways was also higher during smoking. As a result, daily deposited mass was found to be two orders of magnitude higher for cigarette smoking than for household exposure to biomass smoke. We also observed different lobar distributions of the deposited dose between biomass smoke exposure and cigarette smoking, with the LL and RU lobes receiving the highest doses of BSP and CSP, respectively. Since airflow distribution across the lobes is the same in both cases, albeit the higher flow rate in cigarette smoking, these results indicate that the differences in lobar deposition arise due to the difference in PSDs reaching the intrathoracic airways.
A few recent studies have shown that differences also exist in the clinical characteristics of COPD between active and passive smoking, with exposure to secondhand smoke associated with milder (Li et al., 2020) or no emphysema (Putcha et al., 2016) compared to active smoking. While we did not model secondhand smoke exposure in this study, we know that inhalation conditions differ to those in active smoking and that exhaled cigarette smoke, a major contributor to secondhand smoke, has a larger count median diameter and geometric standard deviation than mainstream smoke (Sahu et al., 2013). Concentrations of secondhand smoke across different settings have also been found to be between two to three orders of magnitude lower than the concentration of inhaled mainstream smoke (Braun et al., 2019; Fuoco et al., 2017). Based on these exposure conditions, we expect deposited doses from passive smoking to be substantially lower than those from active smoking, and closer to those from biomass smoke exposure, which could explain the milder emphysema observed in passive smokers.
We note that the effect of hygroscopic growth on particles was not considered in this study. In a recent study on soot particles, deposition efficiencies during the first 25 h of the aerosol aging process were found to be between 0.15% and 0.78% and between 0.5% and 2.4% higher in the tracheobronchial and alveolar regions, respectively, when hygroscopic growth was neglected (Ching and Kajino 2018). Including hygroscopic effects but assuming an internally-mixed state however, underestimated alveolar deposition by up to 5% for aged soot, and up to 20% for fresh soot (Stevens and Dastoor 2019). These results suggest that neglecting hygroscopicity has a relatively small effect on the regional deposition of BSP. In the case of cigarette smoke, we took into account the particle growth that occurs in the mouth during the puffing and mouth-hold stage. While CSP may continue to grow beyond the mouth, studies have reported no significant impact of hygroscopic growth on droplet size and deposition (Zhang et al., 2012; Robinson and Yu 2001).
We also note that deposition was computed during inhalation only, and not exhalation. Generally, deposition is much higher during the inspiratory than expiratory period because of the bifurcating airway morphology (Kim et al., 1989). However, while inspiratory deposition plays a dominant role for very small submicron (≤0.01 μm) and large (>5 μm) particles, Choi and Kim (2007) found that deposition takes place throughout the whole breathing cycle for particles between 0.01 and 5 μm. Daily deposited doses are therefore likely to be higher than our current estimates. Finally, the respiratory health effects of inhaled particles depend not only on the deposited dose and site of deposition but also on the retention times, which were not computed in this study.
Despite these limitations, our study provides novel, detailed data on the regional deposition of BSP and CSP in the lungs and contributes to expanding our understanding of the differences between biomass- and tobacco-related COPD. Our study also demonstrates the role that in silico modeling can play in exposure risk assessment and in helping to understand respiratory disease as a non-invasive complement to in vivo and ex vivo studies. Given that only the delivered dose can do harm and that deposition is not a linear function of personal exposure, estimates of the deposited dose provide a more biologically-relevant and accurate metric to use in exposure-response analyses compared to exposure concentrations. Additionally, the site-specific predictions of deposited dose obtained from high-fidelity simulations can help determine areas in the lungs with enhanced deposition and increased likelihood of inflammation and disease initiation. Future work will include the use of subject-specific airway geometries and personal exposure measurements to determine subject-specific deposition patterns. Comparison of the in silico results with imaging data will also allow us to establish whether regions of high deposition correlate with areas of lung injury.
Supplementary Material
Acknowledgements
The authors would like to acknowledge C. Kleinstreuer for sharing the nasal cavity geometry and Y. Ostrovski for the upper airway tree model.
Funding sources
Research reported in this publication was supported by the United States National Institutes of Health through the following Institutes and Centers: Fogarty International Center, National Institute of Environmental Health Sciences, National Cancer Institute, and the Centers for Disease Control and Prevention under award numbers U01TW010107 and U2RTW010114 (MPIs: Checkley, Gonzales, Naeher, Steenland). The authors also received generous support from Mr. William and Bonnie Clarke III and the COPD Discovery Award from Johns Hopkins University.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.111116.
References
- Anjilvel S, Asgharian B, 1995. A multiple-path model of particle deposition in the rat lung. Fund. Appl. Toxicol 28, 41–50. [DOI] [PubMed] [Google Scholar]
- Asgharian B, Price OT, Yurteri CU, Dickens C, McAughey J, 2014. Component-specific, cigarette particle deposition modeling in the human respiratory tract. Inhal. Toxicol 26 (1), 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball CG, Uddin M, Pollard A, 2008. Mean flow structures inside the human upper airway. Flow, Turbul. Combust 81, 155–188. [Google Scholar]
- Bernstein DM, 2004. A review of the influence of particle size, puff volume, and inhalation pattern on the deposition of cigarette smoke particles in the respiratory tract. Inhal. Toxicol 16 (10), 675–689. [DOI] [PubMed] [Google Scholar]
- Braun M, Fromm EL, Gerber A, Klingelhöfer D, Müller R, Groneberg DA, 2019. Particulate matter emissions of four types of one cigarette brand with and without additives: a laser spectrometric particulate matter analysis of secondhand smoke. BMJ Open 9 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce N, Perez-Padilla R, Albalak R, 2000. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull. World Health Organ 78 (9), 1078–1092. [PMC free article] [PubMed] [Google Scholar]
- Cai FS, Yu CP, 1988. Inertial and interceptional deposition of spherical particles and fibers in a bifurcating airway. J. Aerosol Sci 19, 679–688. [Google Scholar]
- Camp PG, Ramirez-Venegas A, Sansores RH, Alva LF, McDougall JE, Sin DD, Paré PD, Müller NL, Silva CI, Rojas CE, Coxson HO, 2014. COPD phenotypes in biomass smoke-versus tobacco smoke-exposed Mexican women. Eur. Respir. J 43 (3), 725–734. [DOI] [PubMed] [Google Scholar]
- Capecelatro J, Desjardins O, Fox RO, 2018. On the transition between turbulence regimes in particle-laden channel flows. J. Fluid Mech 845, 499. [Google Scholar]
- Checkley W, Williams KN, Kephart JL, Fandino-Del-Rio M, Steenland NK, Gonzales GF, Naeher LP, Harvey SA, Moulton LH, Davila-Roman VG, Goodman D, 2020. Effects of a cleaner energy intervention on cardiopulmonary outcomes in Peru: a randomized controlled trial. Am. J. Respir. Crit. Care Med (ja). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Namenyi J, Yeh HC, Mauderly JL, Cuddihy RG, 1990. Physical characterization of cigarette smoke aerosol generated from a Walton smoke machine. Aerosol. Sci. Technol 12 (2), 364–375. [Google Scholar]
- Ching J, Kajino M, 2018. Aerosol mixing state matters for particles deposition in human respiratory system. Sci. Rep 8 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JI, Kim CS, 2007. Mathematical analysis of particle deposition in human lungs: an improved single path transport model. Inhal. Toxicol 19 (11), 925–939. [DOI] [PubMed] [Google Scholar]
- Costa P, Brandt L, Picano F, 2020. Interface-resolved simulations of small inertial particles in turbulent channel flow. J. Fluid Mech 883, A54. [Google Scholar]
- Das P, Nof E, Amirav I, Kassinos SC, Sznitman J, 2018. Targeting inhaled aerosol delivery to upper airways in children: insight from computational fluid dynamics (CFD). PloS One 13 (11), e0207711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghobashi S, 1994. On predicting particle-laden turbulent flows. Appl. Sci. Res 52, 309–329. [Google Scholar]
- Fenn WO, Rahn H, 1965. Handbook of Physiology, Section 3: Respiration American Physiological Society, Washington, D.C. [Google Scholar]
- Finlay W, Stapleton K, Yokota J, 1996. On the use of computational fluid dynamics for simulating flow and particle deposition in the human respiratory tract. J. Aerosol Sci 9, 329–341. [Google Scholar]
- Fullerton DG, Bruce N, Gordon SB, 2008. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg 09 102 (9), 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuoco FC, Stabile L, Buonanno G, Scungio M, Manigrasso M, Frattolillo A, 2017. Tracheobronchial and alveolar particle surface area doses in smokers. Atmosphere 8 (1), 19. [Google Scholar]
- GBD 2015 Chronic Respiratory Disease Collaborators, 2017. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med 5 (9), 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller-Algazi M, Nof E, Das P, Bhardwaj S, Kassinos SC, Sznitman J, 2020. In silico optimization of targeted aerosol delivery in upper airways via Inhaled Volume Tracking. Clin. BioMech 80, 105138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Martonen T, Graham R, 1989. Predicted deposition of nonhygroscopic aerosols in the human lung as a function of subject age. J. Aerosol Med 2 (1), 49–68. [Google Scholar]
- Horsfield K, Dart G, Olson DE, Filley GF, Cumming G, 1971. Models of the human bronchial tree. J. Appl. Physiol 31 (2), 207–217. [DOI] [PubMed] [Google Scholar]
- Ingham DB, 1975. Diffusion of aerosols from a stream flowing through a cylindrical tube. J. Aerosol Sci 6, 125–132. [Google Scholar]
- Ingebrethsen BJ, Alderman SL, Ademe B, 2011. Coagulation of mainstream cigarette smoke in the mouth during puffing and inhalation. Aerosol. Sci. Technol 45 (12), 1422–1428. [Google Scholar]
- Johnson TJ, Olfert JS, Cabot R, Treacy C, Yurteri CU, Dickens C, McAughey J, Symonds JP, 2014. Steady-state measurement of the effective particle density of cigarette smoke. J. Aerosol Sci 75, 9–16. [Google Scholar]
- Kim CS, Iglesias AJ, Garcia L, 1989. Deposition of inhaled particles in bifurcating airway models: II. Expiratory deposition. J. Aerosol Med 2 (1), 15–27. [Google Scholar]
- Kleinstreuer C, Feng Y, 2013. Lung deposition analyses of inhaled toxic aerosols in conventional and less harmful cigarette smoke: a review. Int. J. Environ. Res. Publ. Health 10 (9), 4454–4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koullapis P, Nicolaou L, Kassinos SC, 2018a. In silico assessment of mouth-throat effects on regional deposition in the upper tracheobronchial airways. J. Aerosol Sci 117, 164–188. [Google Scholar]
- Koullapis P, Kassinos SC, Muela J, Perez-Segarra C, Rigola J, Lehmkuhl O, Cui Y, Sommerfeld M, Elcner J, Jicha M, Saveljic I, Filipovic N, Lizal F, Nicolaou L, 2018b. Regional aerosol deposition in the human airways: the SimInhale benchmark case and a critical assessment of in silico methods. Eur. J. Pharmaceut. Sci 113, 77–94. [DOI] [PubMed] [Google Scholar]
- Li A, Ahmadi G, 1992. Dispersion and deposition of spherical particles from point sources in a turbulent channel flow. Aerosol. Sci. Technol 16, 209–226. [Google Scholar]
- Li X, Wu Z, Xue M, Du W, 2020. Smoking status affects clinical characteristics and disease course of acute exacerbation of chronic obstructive pulmonary disease: a prospectively observational study. Chron. Respir. Dis 17, 1479973120916184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Campos JL, Tan W, Soriano JB, 2016. Global burden of COPD. Respirology 21 (1), 14–23. [DOI] [PubMed] [Google Scholar]
- Ma B, Lutchen KR, 2006. An anatomically based hybrid computational model of the human lung and its application to low frequency oscillatory mechanics. Ann.Biomed. Eng 34, 1691–1704. [DOI] [PubMed] [Google Scholar]
- Martin II WJ, Glass RI, Araj H, Balbus J, Collins FS, Curtis S, Diette GB, Elwood WN, Falk H, Hibberd PL, Keown SE, Mehta S, Patrick E, Rosenbaum J, Sapkota A, Tolunay HE, Bruce NG, 2013. Household air pollution in low- and middle-income countries: health risks and research priorities. PLoS Med 10 (6) e1001455–e1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E, 2014. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. J. Am. Med. Assoc 311 (2), 183–192. [DOI] [PubMed] [Google Scholar]
- Nicolaou L, Fandino-Del Rio M, Koehler K, Checkley W, 2021. Size distribution and lung-deposited doses of particulate matter from household exposure to biomass smoke. Indoor Air 31 (1), 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou L, Zaki TA, 2013. Direct numerical simulations of flow in realistic mouth-throat geometries. J. Aerosol Sci 57, 71–87. [Google Scholar]
- Nicolaou L, Zaki TA, 2016. Characterization of aerosol Stokes number in 90° bends and idealized extrathoracic airways. J. Aerosol Sci 102, 105–127. [Google Scholar]
- Nicolaou L, 2018. Inertial and gravitational effects on aerosol deposition in the conducting airways. J. Aerosol Sci 120, 32–51. [Google Scholar]
- Olloquequi J, Jaime S, Parra V, Cornejo-Córdova E, Valdivia G, Agustí À, Silva R, 2018. Comparative analysis of COPD associated with tobacco smoking, biomass smoke exposure or both. Respir. Res 19 (1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrovski Y, Dorfman S, Mezhericher M, Kassinos S, Sznitman J, 2019. Targeted drug delivery to upper airways using a pulsed aerosol bolus and inhaled volume tracking method. Flow, Turbul. Combust 102 (1), 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounis H, Ahmadi G, McLaughlin JB, 1991. Brownian diffusion of submicrometer particles in the viscous sublayer. J. Colloid Interface Sci 143, 266–277. [Google Scholar]
- Pedley TJ, 1977. Pulmonary fluid dynamics. Annu. Rev. Fluid Mech 9, 229–274. [Google Scholar]
- Pichelstorfer L, Hofmann W, Winkler-Heil R, Yurteri CU, McAughey J, 2016. Simulation of aerosol dynamics and deposition of combustible and electronic cigarette aerosols in the human respiratory tract. J. Aerosol Sci 99, 125–132. [Google Scholar]
- Postma DS, Bush A, van den Berge M, 2015. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet 385 (9971), 899–909. [DOI] [PubMed] [Google Scholar]
- Putcha N, Barr RG, Han MK, Woodruff PG, Bleecker ER, Kanner RE, Martinez FJ, Smith BM, Tashkin DP, Bowler RP, Eisner MD, 2016. Understanding the impact of second-hand smoke exposure on clinical outcomes in participants with COPD in the SPIROMICS cohort. Thorax 71 (5), 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JS, Koppmann R, Eck TF, Eleuterio DP, 2005. A review of biomass burning emissions part II: intensive physical properties of biomass burning particles. Atmos. Chem. Phys 5, 799–825. [Google Scholar]
- Rivera RM, Cosio MG, Ghezzo H, Salazar M, Perez-Padilla R, 2008. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int. J. Tubercul. Lung Dis 12 (8), 972–977. [PubMed] [Google Scholar]
- Robinson RJ, Yu CP, 2001. Deposition of cigarette smoke particles in the human respiratory tract. Aerosol. Sci. Technol 34 (2), 202–215. [Google Scholar]
- Sahu SK, Tiwari M, Bhangare RC, Pandit GG, 2013. Particle size distribution of mainstream and exhaled cigarette smoke and predictive deposition in human respiratory tract. Aerosol and Air Quality Research 13 (1), 324–332. [Google Scholar]
- Salvi S, Barnes PJ, 2010. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest 138 (1), 3–6. [DOI] [PubMed] [Google Scholar]
- Schiller L, Naumann A, 1933. A drag coefficient correlation. VDI Zeitschrift 77, 318–320. [Google Scholar]
- Schum M, Yeh HC, 1980. Theoretical evaluation of aerosol deposition in anatomical models of mammalian lung airways. Bull. Math. Biol 42, 1–15. [DOI] [PubMed] [Google Scholar]
- Shi H, Kleinstreuer C, Zhang Z, 2006. Laminar airflow and nanoparticle or vapor deposition in a human nasal cavity model. J Biomech Eng 03 128 (5), 697–706. [DOI] [PubMed] [Google Scholar]
- Shi H, Kleinstreuer C, Zhang Z, 2007. Modeling of inertial particle transport and deposition in human nasal cavities with wall roughness. J. Aerosol Sci 38 (4), 398–419. [Google Scholar]
- Silva R, Oyarzun M, Olloquequi J, 2015. Pathogenic mechanisms in chronic obstructive pulmonary disease due to biomass smoke exposure. Arch.Bronconeumol 51 (6), 285–292. [DOI] [PubMed] [Google Scholar]
- Stapleton KW, Guentsch E, Hoskinon MK, Finlay WH, 2000. On the suitability of k ε turbulence modeling for aerosol deposition in the mouth and throat: a comparison with experiment. J. Aerosol Sci 31 (6), 739–749. [Google Scholar]
- Stevens R, Dastoor A, 2019. A review of the representation of aerosol mixing state in atmospheric models. Atmosphere 10 (4), 168. [Google Scholar]
- Subramaniam R, Richardson R, Morgan K, Kimbell J, Guilmette R, 1998. Computational fluid dynamics simulations of inspiratory airflow in the human nose and nasopharynx. Inhal. Toxicol 10, 91–120. [Google Scholar]
- Taylor DR, Reid WD, Pare PD, Fleetham JA, 1988. Cigarette smoke inhalation patterns and bronchial reactivity. Thorax 43 (1), 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigala S, Sharma AR, Sachdeva K, 2018. Health risk assessment due to biomass smoke exposure in Indian indoor environment: an empirical approach using lung deposition model. Sci. Total Environ 640, 935–942. [DOI] [PubMed] [Google Scholar]
- Torres-Duque CA, Garcia-Rodriguez MC, Gonzalez-Garcia M, 2016. Is chronic obstructive pulmonary disease caused by wood smoke a different phenotype or a different entity? Arch. Bronconeumol 52 (8), 425–431. [DOI] [PubMed] [Google Scholar]
- Wang CS, 1975. Gravitational deposition of particles from laminar flows in inclined channels. J. Aerosol Sci 6, 191–204. [Google Scholar]
- Wang G, Richter D, 2019. Modulation of the turbulence regeneration cycle by inertial particles in planar Couette flow. J. Fluid Mech 861, 901–929. [Google Scholar]
- Weibel ER, 1963. Morphometry of the Human Lung Springer, Berlin. [Google Scholar]
- Yeh HC, Schum GM, 1980. Models of human lung airways and their application to inhaled particle deposition. Bull. Math. Biol 42, 461–480. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Kleinstreuer C, Kim CS, 2002a. Aerosol deposition efficiencies and upstream release positions for different inhalation modes in an upper bronchial airway model. Aerosol. Sci. Technol 36 (7), 828–844. [Google Scholar]
- Zhang Z, Kleinstreuer C, Kim CS, 2002b. Cyclic micron-size particle inhalation and deposition in a triple bifurcation lung airway model. J. Aerosol Sci 33 (2), 257–281. [Google Scholar]
- Zhang Z, Kleinstreuer C, 2004. Airflow structures and nano-particle deposition in a human upper airway model. J. Comput. Phys 198, 178–210. [Google Scholar]
- Zhang Z, Kleinstreuer C, Feng Y, 2012. Vapor deposition during cigarette smoke inhalation in a subject-specific human airway model. J. Aerosol Sci 53, 40–60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


