Abstract
Background.
Chronic low-level exposure to organophosphorus pesticides is associated with adverse health effects, including a decline in neurological functioning and long-term impairment. These negative effects may be more detrimental in children and adolescents due to their critical stage in development. Little work has investigated the effects of chronic exposure to pesticides, specifically chlorpyrifos (CPF) during the adolescent period.
Objectives.
To examine effects of CPF exposure over a year-long period within a group of male adolescents in Egypt (N = 242, mean age = 17.36), including both pesticide applicators and non-applicators.
Methods.
Associations between average CPF exposure (measured via urinary metabolite levels of 3,5,6-trichloro-2-pyridinol [TCPy]) and neurobehavioral functioning were examined in a 1-year longitudinal study. Given previous literature, higher levels of TCPy were expected to be associated with worse neurobehavioral functioning.
Results.
Using mixed effects linear regression, average TCPy exposure predicted deficits in more complex neurobehavioral tasks (Benton visual retention, digit span reverse, match to sample, serial digit learning, and alternating tapping) with estimates of effects ranging from −.049 to .031. Age (effects ranging from .033 to .090) and field station (effects ranging from −1.266 to −.278) were significantly predictive of neurobehavioral functioning over time. An interaction effect was found for field station and TCPy across several neurobehavioral domains.
Discussion.
Results show that occupational exposure to pesticides may have particularly deleterious effects on complex neurobehavioral domains. Additionally, differences across field stations and the age at which individuals are exposed may be important factors to investigate in future research.
Keywords: organophosphorus; neurological functioning; 3,5,6-trichloro-2-pyridinol (TCPy); mixed effects linear regression
Graphical Abstract
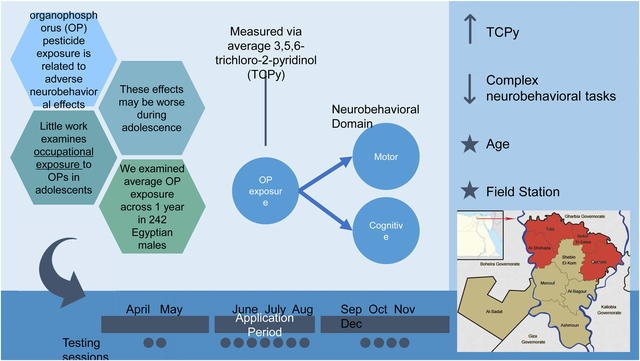
Exposure to organophosphorus (OP) pesticides can cause a host of negative acute and chronic effects. Acute effects can include detriments to neurological functioning (Keifer & Firestone, 2007) and chronic exposure can yield long-term neurobehavioral impairment, found in both human and animal studies (Naughton & Terry, 2018; Rohlman et al., 2016). Moreover, negative effects may be magnified in children and adolescents due to their critical stage in neurodevelopment (e.g., children and adolescents may absorb and metabolize harmful chemicals differently than adults). Neurocognitive and motor impairments have been found to be associated with both prenatal OP exposure (Guo et al., 2019) and early and young childhood OP exposure (Binter et al., 2020; Suarez-Lopez et al., 2017). A recent review examining prenatal through childhood exposure found, despite different methodologies, that exposure was associated with impaired neurodevelopment (Sapbamrer & Hongsibsong, 2019). Although researchers have examined adolescent-aged samples and demonstrated that these individuals may have higher biomarkers associated with exposure (e.g., Suarez-Lopez et al., 2020) relatively less work has examined effects of OP exposure on neurobehavioral outcomes during the adolescent period. Furthermore, of particular concern is understanding the effects of occupational exposure during the adolescent period. In many countries adolescents apply pesticides to agricultural fields, a more potent and chronic level of exposure compared to what one may be exposed to in everyday life (e.g., through diet or living near fields which use pesticides).
An additional concern is exposure to chlorpyrifos (CPF), an OP insecticide that is being phased out of use due to its known detrimental effects (e.g., Curl et al., 2020) and yet continues to be widely used in some areas of the world (Grube et al., 2011; Foxenberg et al., 2011). Exposure to CPF is often assessed via measuring urinary metabolite levels of 3,5,6-trichloro-2-pyridinol (TCPy), a specific metabolite of CPF. Lower dose exposures over a longer duration (e.g., occupational exposures) had been found to yield worse long-term neuropsychological performance compared to acute toxicity or high exposure (Meyer-Baron et al., 2015; Ross et al., 2013). In occupational settings, workers, including adolescents, are hired seasonally and may apply pesticides for weeks or months at a time (London et al., 2012). However, in spite of this greater risk, there have been limited studies examining occupational pesticide exposure among adolescents who may be at greater risk due to their developing brain (Rohlman et al., 2011).
Previous research with a small sample of adolescent applicators in Egypt (N = 43) found that all adolescents working with pesticides had detectable levels of urinary biomarkers, although with quite variability in exposure levels across individuals (Callahan et al., 2017). Studies examining repeated exposures to pesticides across an application season has found that adult workers show an increase in urinary metabolite levels that decrease once application ends, whereas neurobehavioral functioning is slower to recover (Arcury et al., 2010; Baldi et al., 2011). Similarly, a study with individuals ranging from adolescent to adults with both environmental and occupational exposure found neurobehavioral impairments to be worse during the application season (Ramírez-Santana et al., 2020). This work suggests that neurobehavioral functioning may be worse during the application season and that even once exposure has ended, there may be long-term negative consequences.
Early work in an observational study demonstrated that children and adolescents who applied pesticides had neurobehavioral deficits when compared to children in the same area who did not apply pesticides (Abdel-Rasoul et al., 2008). Similarly, higher urinary levels of TCPy were found to be associated with increased attention and short-term memory impairments in a small group of applicators when compared with a non-applicator group (Rohlman et al., 2014) and with more symptoms of attention deficit/hyperactivity disorder in a small sample of adolescent applicators (N = 59) from Egypt (Rohlman et al., 2019). Other work has shown that urinary metabolite levels increase and remain elevated during the application season in a cohort of adolescent applicators (Rohlman et al., 2016). Importantly, neurobehavioral functioning was more impaired in the high exposure group than the low exposure group. Although additional findings support that occupational exposure negatively affects neurobehavioral functioning in adolescents (Ismail et al., 2017a, 2017b) questions remain regarding the different facets of neurobehavioral performance that may be impaired.
A review examining the impact of occupational exposure on neurobehavioral functioning found that across 14 studies, low-level exposure was consistently associated with poorer neurobehavioral performance, specifically, motor speed and domains of executive functioning (Ross et al., 2013). Contrastingly, two additional review studies were unable to determine the exact effects of OP exposure. The first, a systematic review of 24 studies, concluded that effects of OP exposure could not be fully understood due to the differences in neuropsychological tests and OP exposure measurement methods across studies (Takahashi & Hashizume. 2014). The second, a review of 33 studies, again indicated that although chronic exposure to OPs is associated with neuropsychological effects, it is unclear which specific domains are impacted (Muñoz-Quezada et al., 2016).
Present Study
In sum, few studies have used longitudinal approaches in adolescents, with most that have examined changes over time only including a few time points and this work has lacked such detailed exposure metrics with a range of neurobehavioral outcomes. As such, the goal of the present study is to examine effects of CPF exposure over a year-long period within a group of male adolescents in Egypt (N = 242). Changes in urinary levels of TCPy, the CPF-specific metabolite, were examined to understand how TCPy was associated with specific facets of neurobehavioral functioning over the same period. These indices were collected before, during, and after exposure for a total of 13 data collection timepoints. This allows for an assessment of change in neurobehavioral function over time as it relates to overall CPF exposure. Based on previous literature, higher levels of TCPy were expected to be associated with worse motor and cognitive functioning.
Methods
Study Population and Setting
A longitudinal prospective design was used to collect data from male adolescents in Egypt across four years (2014 – 2017, with follow-up testing in 2018 and 2019). Females do not apply pesticides and were not included in the study. The present study will focus on the pesticide application cycle that took place in 2016, which included 13 data collection time points; only participants with urinary TCPy and neurobehavioral data were included (N = 242). To be eligible in the study, participants had to be between the ages of 12 and 18 at recruitment, work in pesticide application (for the applicator group) or be non-applicators and living in the same communities as the applicators. Participants were recruited for this study from field stations in the Nile delta region in Egypt (Quesna, Alshohada, Tala, Berket El Sabe). Participants were excluded if they had any diagnosed neurological or cognitive disorders (no participants met this exclusion criteria).
Applicators (n = 177) were either 1) employed seasonally during the summer months of June, July, and August (i.e., during the summer break from school) with informal contracts and were paid regularly by the Ministry of Agriculture for the days that they worked or 2) worked as private applicators applying pesticides to their family or neighbors’ fields or other farms. Duties of the applicators included mixing pesticides, filling backpack sprayers, which were then used to apply pesticides to cotton fields, and holding flags to mark the edge of the field. Importantly, while types of pesticide and equipment used was standardized by the Ministry of Agriculture, there may be individual differences among adolescents working as private applicators. Additionally, a non-applicator group (n = 65) was recruited from the same communities as the applicators. Finally, during each session, participants completed neurobehavioral tasks and provided a urine specimen for later analysis of TCPy, a biomarker of exposure to CPF. Approximately 33% of adolescents were missing all neurobehavioral measures and were thus excluded from analyses, resulting in the final sample of N = 242. However, given that this study included multiple follow-up time points, some attrition was expected. Study participants completed informed consent to participate in the study and this study was approved by the local institutional review boards.
Measures
Markers of Exposure
Urinary TCPy levels throughout the study period were used to estimate average exposure to chlorpyrifos. As described in Rohlman et al. (2019), urine samples were collected during each of the 13 test sessions at the beginning of the work shift and stored in a cooler with wet ice until transported to the laboratory at Menoufia University at the end of the test session. Samples were then aliquoted into two 5 ml cryovials; one to be shipped to the University at Buffalo for analysis and one to be stored at −20 °C at the Menoufia University laboratory. The method for analysis of urinary TCPy has been described elsewhere (Crane et al., 2013; Farahat et al., 2010, 2011). Briefly, urinary TCPy was measured using negative-ion chemical ionization gas chromatography-mass spectrometry (GC–MS) using 13C-15N-3,5,6-TCPy as an internal standard. The within run imprecision of this assay is very low, as shown by a < 2% coefficient of variation and an intraclass correlation coefficient of 0.997. Urine samples from all participants were above the limit of detection for TCPy.(0.5 μg/ml urine). Colorimetric analysis of creatinine was done by the Jaffe reaction (Fabiny & Ertingshausen, 1971) and urine TCPy concentrations are expressed as μg TCPy/g creatinine. Due to missing data, a mean TCPy score was created for each participant and used in analyses.
Neurobehavioral Tasks
Computer-based neurobehavioral tasks were completed via the Behavioral Assessment and Research System (BARS; Rohlman et al., 2003). The BARS is a battery of tests used to detect neurotoxicity in special populations. Specifically, the BARS has been shown to be applicable to determining level of neurobehavioral functioning in adolescents with pesticide exposure (Rohlman et al., 2014). Rohlman et al. (2003) describes each of the BARS tasks and the included measures are described below. In addition, a series of standardized non-computerized tests were administered including the Benton visual retention, Similarities, Pegboard, trail making, and visual motor integration, described further below.
Of note, given the repeated measures nature of the current data structure, practice effects may be expected. However, to combat this issue alternating forms, sequences, and varying number of stimuli were used across testing sessions for tasks as appropriate. Additionally, given that exposure to pesticides has been shown to degrade neurobehavioral functioning, practice effects would only attenuate findings, potentially underestimating the true level of impairment after exposure.
Cognitive Measures.
Match-to-Sample measures visual memory. Participants are shown 15 stimuli for three seconds each and then are shown three stimuli again and must match them to the original set of stimuli. For the current study, correct count and average correct latency variables were used as a measure of accuracy and time on this task. Continuous Performance Test measures sustained attention for which adolescents are shown 75 stimuli, 30 of which are targets the adolescent should select (i.e. press a key). DPrime is a measure from the continuous performance task which shows how well a participant distinguishes targets from non-targets; Digit Span Task measures working memory and memory span through the participant having to type from memory a series of digits that were presented visually. There is both a forward and reverse recall and both total scores were used as indicators of overall memory span abilities. Symbol Digit Task measures processing speed and working memory during which adolescents are asked to match numbers to a specific symbol as quickly as they can. The average correct latency was used specifically to examine adolescents’ overall accuracy. Serial Digit Learning is a measure of learning abilities for which adolescents learn and remember a sequence of digits over several trials. The total score was included in analyses. Similarities as a verbal and abstract reasoning task during which adolescents are asked to state how two words are alike. The total score from this test was used in analyses. Benton Visual Retention is a task during which adolescents are shown a drawing for 10 seconds and then given 15 seconds to reproduce the drawing after the card is removed. Total score from this task was used in analyses. Trails A and B measure processing speed and task-switching, respectively. Total time on each condition was used in analyses.
Motor Control Measures.
Finger Tapping measures motor speed and coordination during which adolescents are asked to tap with each hand as quickly as possible for 20 seconds. Mean scores for the right, left, and alternating hands were computed and used in analyses. Santa Ana Pegboard is a task during which adolescents are asked to place pegs on a board in a certain way using only one hand, as quickly as possible. Total time to complete this task was used for both left and right hand. Visual Motor Integration is a task during which adolescents are asked to copy drawings as accurately as possible and is a measure of hand-eye coordination. The total score from this task was used in analyses. Simple Reaction Time measures adolescents’ response speed. Only the correct latency was included to capture motor speed.
Statistical Analyses
First, all dependent variables were standardized to Z-scores to be in the same metric. Next, symbol digit task latency, simple reaction time latency, and Trails A and B total time were multiplied by −1 so that higher scores indicated better performance across all variables. Both processes were to aid in interpretation of results. Descriptive statistics (mean and standard deviation by field station and by TCPy quartile groups) were performed across all model variables. An average TCPy exposure was calculated by taking the mean of all available TCPy data for each participant, thus a single TCPy value was created for each participant. TCPy was recoded into quartile groups to aid in visualization of differences across high and low exposure for each neurobehavioral task. Next, mixed effects linear regressions (MLR) were run separately for each neurobehavioral task in SPSS version 26 using the “Mixed” command. TCPy as a continuous variable was used as the predictor and time (13 timepoints) was accounted for by adding it as a factor. Models were run with age and field station as covariates with interaction effects between these variables and TCPy. A model trimming approach was used in that non-significant interaction effects with a p >.100 were removed, one at a time, leaving the most parsimonious model for each neurobehavioral task. A second approach was taken to modeling this data using latent variable models. Thus, confirmatory factor analyses were modeled for all 13 time points including all neurobehavioral tasks at each time. A two-factor structure (cognitive and motor latent variables) were examined at each time point. Factor scores from each time point were saved and used in the MLR, one model for each latent variable outcome. The same predictor, covariates, interactions, and model trimming approach described above were used with the latent variables. Of note, the samples size of N = 242 gave power estimates of 85% to detect a moderate effect size (i.e., Cohen’s d = 0.5) at each time point at an alpha level of 0.05. (Cohen, 1988). Similar samples of this size have been used to examine questions such as these and have provided adequate power (e.g., Rohlman et al., 2016).
Results
Means (M) and standard deviations (SD) for quartile groups and each neurobehavioral task, the two latent variables, and model covariates are depicted in Tables 1 and 2. First, given that 33% of the sample was missing all neurobehavioral data, differences were assessed between those with and without that data. Individuals that did not complete the neurobehavioral measures were significantly older (M age = 23.50, SD = 5.24) compared to participants that did complete the neurobehavioral data (M age = 17.36, SD = 2.34, p <.001). Additionally, there was a significant difference between those missing and not missing all neurobehavioral data and field station such that more individuals than expected with complete data were from the Alshohadaa station (p < .05) compared to the other three stations. There were no significant differences between applicator and non-applicator status and those with and without neurobehavioral data.
Table 1.
Means and SDs for all model variables by TCPy quartile groups
| Lowest 25% | 25–50% | 50–75% | Highest 25% | Full Sample | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model Variables | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Average TCPy (μg /g creatinine) | 9.25 | 2.15 | 14.90 | 1.47 | 21.48 | 2.25 | 49.87 | 44.22 | 24.00 | 27.20 |
| Cognitive Latent Variable | .09 | .36 | .03 | .41 | .01 | .36 | −.13 | .42 | .00 | .40 |
| BVRT | .06 | .93 | −.01 | 1.04 | .03 | .97 | −.11 | 1.03 | −.01 | 1.00 |
| DPrime | .19 | .91 | −.19 | 1.00 | .03 | 1.06 | .01 | .97 | .00 | 1.00 |
| DST Forward | .29 | 1.13 | −.06 | .97 | −.11 | .96 | −.09 | .89 | .00 | 1.00 |
| DST Reverse | .36 | 1.10 | −.05 | 1.00 | −.11 | .92 | −.15 | .90 | .00 | 1.00 |
| MTS Correct Count | .21 | 1.06 | −.06 | .94 | −.09 | .98 | −.06 | .98 | .00 | 1.00 |
| SDL | .17 | .81 | .10 | .91 | −.03 | 1.02 | −.24 | 1.16 | .00 | 1.00 |
| SDT Latency | .17 | .98 | .10 | .99 | .04 | .84 | −.29 | 1.09 | .01 | .99 |
| Similarities | .03 | .96 | .12 | 1.05 | .00 | .94 | −.20 | 1.01 | −.01 | 1.00 |
| VMI | .16 | .88 | −.02 | 1.07 | .02 | .99 | −.15 | 1.02 | .00 | 1.00 |
| Trails A | .09 | .80 | .09 | .88 | .11 | .89 | −.30 | 1.26 | .00 | .99 |
| Trails B | .07 | .96 | .00 | 1.03 | .14 | .89 | −.24 | 1.07 | .00 | 1.00 |
| Motor Latent Variable | .12 | .40 | .01 | .43 | −.01 | .41 | −.12 | .41 | .00 | .43 |
| SAP Right | .08 | 1.08 | .00 | .87 | −.03 | .99 | −.06 | 1.04 | −.01 | .99 |
| SAP Left | .17 | 1.13 | −.03 | .93 | −.07 | .91 | −.06 | .98 | −.01 | .99 |
| SRT Timely Latency | .22 | 1.06 | −.03 | .97 | −.07 | .98 | −.07 | .98 | .00 | 1.00 |
| Tapping, Alternating | .35 | .96 | .04 | .87 | −.11 | .93 | −.26 | 1.13 | −.01 | 1.00 |
| Tapping, Left | .19 | .94 | −.05 | .92 | .02 | 1.18 | −.16 | .87 | −.01 | 1.00 |
| Tapping, Right | .23 | .99 | −.02 | 1.00 | −.07 | 1.10 | −.13 | .84 | −.01 | 1.00 |
| Covariates | ||||||||||
| Age | 18.52 | 2.82 | 17.09 | 2.18 | 17.04 | 2.69 | 17.36 | 2.48 | 17.50 | 2.60 |
| Education | 11.61 | 1.90 | 11.08 | 2.16 | 10.62 | 2.29 | 10.69 | 2.12 | 11.00 | 2.15 |
| Parent Education | 11.17 | 3.82 | 11.13 | 4.20 | 10.72 | 3.87 | 10.59 | 4.45 | 10.90 | 4.08 |
| Income, Subjective | 2.31 | .90 | 2.28 | .86 | 2.23 | .92 | 2.39 | .92 | 2.30 | .90 |
| Income, Items | 11.66 | 1.35 | 11.87 | 1.67 | 11.67 | 1.67 | 11.95 | 1.77 | 11.79 | 1.62 |
| Group | N = 19, 40 | N = 18, 43 | N = 18, 43 | N = 10, 51 | N = 65, 177 | |||||
| Field Station | ||||||||||
| Quesna | N = 22 | N = 20 | N = 12 | N = 14 | N = 68 | |||||
| Alshohadaa | N = 10 | N = 21 | N = 25 | N = 14 | N = 70 | |||||
| Tala | N = 14 | N = 12 | N = 10 | N = 18 | N = 54 | |||||
| Berket El Sabe | N = 13 | N = 8 | N = 14 | N = 15 | N = 50 | |||||
Note. Neurobehavioral tasks are z-scored, with higher values indicating better performance. Latent variables are standardized with higher values indicating better performance. SD = standard deviation; BVRT = Benton visual retention task; DST = digit span task; MTS = match to sample; SAP = santa ana pegboard; SDL = serial digit learning; SDT = symbol digit task; SRT = simple reaction time; VMI = visual motor integration. Education is presented in years. Subjective income is rated on a 4-point scale from inadequate to enough money to save. Income based on items is a possible of 19 items the family may own, with higher scores indicating better income standing. Group is presented as non-applicators, applicators.
Table 2.
Means and standard deviations for demographics by field station.
| Quesna | Alshohadaa | Tala | Berket El Sabe | Full Sample | |
|---|---|---|---|---|---|
| Age | |||||
| Mean | 16.79 | 17.47 | 17.44 | 18.31 | 17.36 |
| N | 68 | 66 | 45 | 16 | 195 |
| SD | 1.89 | 2.28 | 2.69 | 3.03 | 2.34 |
| Hours worked per day | |||||
| Mean | 6.57a | 9.80b | 7.26 a | 7.83 a | 7.91 |
| N | 51 | 48 | 31 | 20 | 150 |
| SD | 4.69 | 6.64 | 5.25 | 6.22 | 5.80 |
| Days worked per week | |||||
| Mean | 7.26 | 9.04 | 8.85 | 6.88 | 8.02 |
| N | 54 | 51 | 33 | 33 | 171 |
| SD | 3.06 | 5.36 | 4.41 | 2.86 | 4.18 |
| Years of Education | |||||
| Mean | 10.87 | 11.30 | 10.59 | 11.04 | 10.97 |
| N | 68 | 70 | 54 | 50 | 242 |
| SD | 1.83 | 2.07 | 2.23 | 2.50 | 2.14 |
| Parent Years of Education | |||||
| Mean | 10.16 | 10.67 | 11.28 | 11.82 | 10.90 |
| N | 68 | 70 | 54 | 50 | 242 |
| SD | 4.88 | 3.73 | 3.73 | 3.55 | 4.08 |
| Income, subjective | |||||
| Mean | 2.25 | 2.44 | 2.09 | 2.40 | 2.30 |
| N | 68 | 70 | 54 | 50 | 242 |
| SD | .80 | .91 | .81 | 1.05 | .90 |
| Income, items | |||||
| Mean | 11.25a | 12.74b | 11.50 a | 11.50 a | 11.79 |
| N | 68 | 70 | 54 | 50 | 242 |
| SD | 1.34 | 1.56 | 1.50 | 1.63 | 1.62 |
Note. Subjective income is rated on a 4-point scale from inadequate to enough money to save; income based on items is based on a list of 19 items a family may own; significant differences between field station is noted by superscript letters such that participants from Alshohadaa worked significantly more hours in a day and had a significantly higher income than the other three field stations.
Next, using the final dataset (N = 242) Pearson Chi square tests of independence were performed to analyze the association between group (applicator or non-applicator) and TCPy quartile membership. Chi square tests showed there were no significant differences between applicator and non-applicator group status and quartile membership (Χ2 (3, N = 245) = 4.360, p = .225). Additionally, using the continuous average TCPy variable for all participants, results of a t-test indicated the applicator group had significantly higher levels of TCPy (Mean = 26.26 μg TCPy/g creatinine, SD = 31.17) than the non-applicator group (Mean = 17.84 μg TCPy/g creatinine, SD = 8.45; t(243) = −2.11, p =.036). The applicator and non-applicator group did not differ based on age (Mean = 17.47 and 17.00, SD = 2.22 and 2.68, respectively; t(196) = −1.49, p =.137) or education (Mean years = 11.10 and 10.62, SD = 2.01 and 2.44 for applicators and non-applicators, respectively; t(243) = −1.69, p =.092). Finally, using analysis of variance, no significant differences were found in average TPCy values based on field station (F(3, 241) = 1.35, p = .258). However, results of chi square testing did show significantly more participants in the 50–75 quartile at Alshohadaa compared to the three other field stations (p <.05) though the overall chi square test was not significant (Χ2 (9, N = 245) = 16.33, p = .060).
Next, MLRs were run with each neurobehavioral task, with the final model for each task presented in Supplemental Table 1 and estimates of fixed effects presented in Table 3. Age and field station were included in the models as covariates. Of note, education and age were highly correlated and thus only age was retained in the final models. Models were run separately using age and education and results did not substantially change. Across all tasks, there was no significant main effect of time in predicting neurobehavioral functioning. Main effects of age were significantly predictive of all task performance except for Dprime, serial digit learning and both trails A and B conditions. However, estimates of effects were small across tasks (ranging from .046 for tapping, alternating to .090 for simple reaction time; see Table 3). A significant main effect for field station was found for digit span forward and reverse, match to sample correct count, santa ana pegboard left, symbol digit task, similarities, finger tapping with alternating hands, visual motor integration, and both trails conditions A and B. Estimates of effect for field station were larger, with Tala showing overall worse performance across the neurobehavioral tasks (ranging from −1.266 for tapping, alternating to .286 for visual motor retention). Main effects of average TCPy values were found only for Benton visual retention, digit span reverse, match to sample correct count, serial digit learning, and finger tapping with alternating hands. These effects ranged from −.049 for serial digit learning to .038 for Benton visual retention. A significant but small age by TCPy interaction effect was found only for Benton visual retention (−.002) and serial digit learning (.002). Lastly, a field by TCPy interaction effect was found for serial digit learning, symbol digit task, similarities, finger tapping with alternating hands, and visual motor integration, again with small effects (ranging from −.021 for visual motor integration at Quesna field station to .049 for tapping, alternating, at Tala field station; presented in Figure 1).
Table 3.
Estimates (β) of fixed effects for each neurobehavioral outcome.
| Outcome | Age | Field 1 | Field 2 | Field 3 | TCPy | Age*TCPy | Field 1*TCPy | Field 2*TCPy | Field 3*TCPy |
|---|---|---|---|---|---|---|---|---|---|
| Cognitive | .033 | −.217 | −.241 | −.416 | −.009 | — | .006 | .008 | .012 |
| BVRT | .065 | .257 | .297 | .200 | .031 | −.002 | — | — | — |
| DPrime | −.023 | .264 | .298 | .267 | −.002 | — | — | — | — |
| DSTF | .065 | −.407 | −.359 | −.123 | −.002 | — | — | — | — |
| DSTR | .082 | −.963 | −.830 | −.706 | −.025 | — | .028 | .023 | .024 |
| MTS | .066 | −.620 | −.582 | −.198 | −.005 | — | — | — | — |
| SDL | .014 | −.028 | −.124 | −.317 | −.049 | .002 | .006 | .016 | .014 |
| SDT | .072 | −.479 | −.300 | −.956 | −.009 | — | .002 | −.004 | .019 |
| Similarities | .053 | −.451 | −.495 | −.849 | −.017 | — | .012 | .021 | .027 |
| VMI | .061 | .455 | .077 | .286 | .006 | — | −.021 | −.005 | −.008 |
| Trails A | .025 | −.013 | .108 | −.278 | −.003 | — | — | — | — |
| Trails B | .021 | −.181 | −.107 | −.304 | −.002 | — | — | — | — |
| Motor | .042 | −.168 | −.247 | −.391 | −.009 | — | .006 | .008 | .014 |
| SAPR | .059 | .093 | −.072 | −.265 | −.005 | — | .011 | .005 | .022 |
| SAPL | .061 | .272 | −.008 | .079 | −.001 | — | — | — | — |
| SRT | .090 | −.264 | −.240 | −.248 | .000 | — | — | — | — |
| TAPA | .046 | −.619 | −.977 | −1.266 | −.041 | — | .032 | .038 | .049 |
| TAPL | .050 | −.126 | −.212 | −.266 | −.003 | — | — | — | — |
| TAPR | .085 | −.119 | −.220 | −.092 | −.003 | — | — | — | — |
Note. Field 4 (Berket el Sabe) was used as the reference category. Field 1 = Quesna; Field 2 = Alshohadaa; Field 3 = Tala. Significant estimates are bolded, all ps <.05. Cognitive = cognitive latent variable; BVRT = Benton visual retention task; DST = digit span task forward (F) and reverse (R); MTS = match to sample; SDL = serial digit learning; SDT = symbol digit task; VMI = visual motor integration; motor = motor latent variable; SAP = santa ana pegboard right (R) and left (L); SRT = simple reaction time; TAP = tapping alternating (A), left (L), and right (R).
Figure 1.
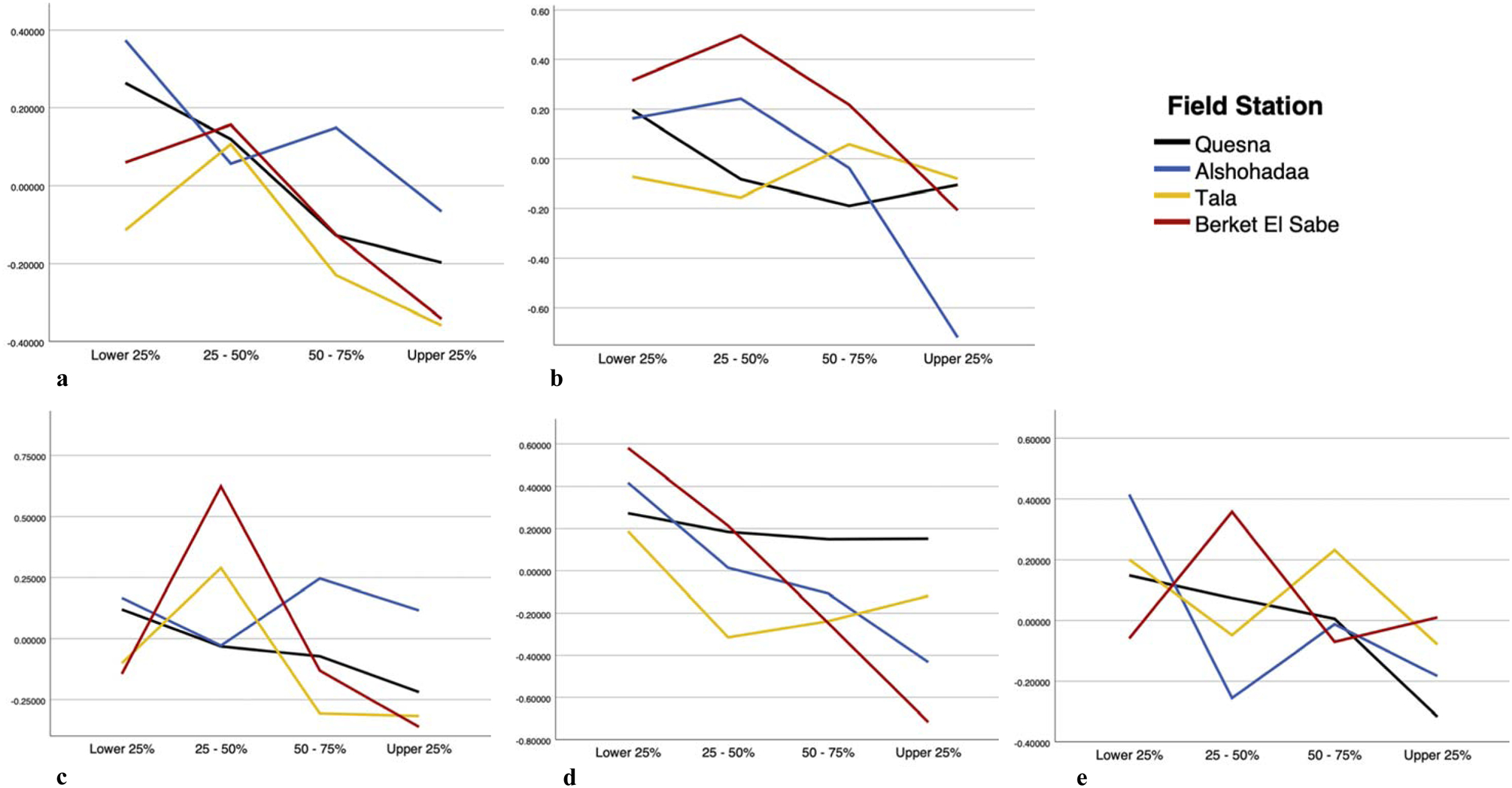
Each panel shows the impact of chlorpyrifos exposure, as assessed by quartiles of the mean level of the urinary metabolite, TCPy, on neurocognitive outcomes in the four field stations for those that had a significant field*tcpy interaction effect (p <.05). Specifically, Serial Digit Learning (panel a), Symbol Digit Task (panel b), Similarities (panel c), Tapping, Alternating (panel d), and Visual Motor Integration (panel e) are shown. Lower values indicate worse performance.
To create the latent variables, confirmatory factor analyses were run next. Across all 13 time points model fit was adequate (see Supplemental Table 2) resulting in a cognitive latent variable and motor latent variable at each time point. Factor scores for each latent variable at each time point were saved and used in analyses. Main effects of age and field station were found for both the motor latent variable and cognitive latent variable, with small effects (see Table 3). There were no other significant results.
Overall, results indicated higher levels of TCPy in applicators compared to non-applicators, per study hypotheses. Importantly, however, there was still large individual variation in levels of TCPy in non-applicators with some showing elevated levels including membership in the top 25% group of TCPy. Generally, as TCPy levels increased, neurobehavioral performance worsened. In particular, the more complex neurobehavioral tasks showed the greatest level of impairment, such as Tapping, alternating (but not left and right) and digit span reverse (but not forward). Additionally, both age and field station had multiple significant main effects on neurobehavioral outcomes as well as interaction effects with TCPy. This may indicate that differences across field stations (hours worked, hygiene practices, how pesticides are applied, tools used) account for some of the variation in pesticide exposure with Tala field station showing significantly worse performance across most neurobehavioral domains. Similarly, the age at which an individual is exposed contributes to the heterogeneity of deficits. Interestingly, no main effects of time were found across neurobehavioral task performance. That is, deficits in neurobehavioral performance over the one-year time-span did not increase or decrease. Differences across motor and cognitive domains broadly were quite heterogeneous, with both having main effects of age and field, but no other significant associations.
Discussion
These results should be interpreted in the context of prior research, despite there being limited work investigating occupational exposure to pesticides in adolescent samples. The results of the present study are consistent with work that has examined exposure in human adolescents and has found resulting deficits in neurocognition (e.g., Ramírez-Santana et al., 2020; Rohlman et al., 2016; Ross et al., 2013). Preliminary work investigating effects of environmental exposure in adolescent females has found similar deficits as the present study in motor functioning, specifically using the tapping task, though different cognitive functioning outcomes with no common deficits across tasks, despite similar tasks being used (Abdel-Rasoul et al., 2019). As such, the existing literature base shows there are inconsistent results regarding 1) the specific type of deficits found and 2) the amount of exposure that is associated with these deficits (e.g., Sapbamrer & Hongsibsong, 2019; Takahashi & Hashizume. 2014) Interestingly, recent work in adults suggests the typically used markers for exposure (i.e., TCPy, AChE, and BChE), which reflect current or recent exposure levels, may not accurately predict the neurobehavioral deficits resulting from chronic exposure (Anger et al., 2020). As such, incorporating additional methods of capturing levels of exposure (e.g., observational and self-report data of associated factors such as hygiene, hours worked, use of protective equipment, etc.) and using consistent methodologies to measure both exposure and neurobehavioral functioning across studies may help to gain a more complete picture.
Moving forward, it will be important to understand if neurobehavioral deficits due to pesticide exposure are reversible, long-lasting, and potentially if more exposure will continue to degrade abilities. Results of the present study did not find significant changes in neurobehavioral performance over time indicating that functioning was not continuing to decline as exposure continued or increased. It is also possible repeated completion of the same tasks led to practice effects which may have impacted individuals’ scores. Future work should continue to investigate these effects. It may also be that a recovery period could occur if an individual is no longer occupationally exposed to pesticides, as was seen in adult samples (Arcury et al., 2010; Baldi et al., 2011). However, it will be important to examine how developmental and biological changes occurring during adolescence effect potential recovery. Adolescent pesticide applicators may have already been exposed during the prenatal period or early childhood. Exposure this early in life has been associated with biological changes in the brain which may affect neurodevelopment later in life (van den Dries et al., 2020). This additional risk, combined with occupational exposure during adolescence, may place these individuals at much heightened risk of experiencing detrimental effects. Additionally, per results of the present study it may be that more complex neurobehavioral functioning is impacted most. Of course, this has unique concerns for adolescents who are often still in the midst of developing executive functioning abilities. Although the present study had mixed findings across the domains of neurobehavioral functioning, future work should continue to investigate if certain domains are more or less affected than others (i.e. motor functioning, learning and memory, attention) and if they may recover at different rates once exposure ends.
Furthermore, the results of the present study add to the growing body of work indicating chronic pesticide exposure indeed has detrimental, and potentially long-lasting, neurobehavioral effects. These negative effects may be heightened when exposure occurs during the adolescent period. This is particularly important given the significant interaction effects found between TCPy exposure and field station in predicting neurocognitive deficits because some adolescents, depending upon where they live and work, may be at even greater risk. Although data was not collected in the present study to understand what may be causing differences between field stations leading to more or less exposure, this is an important potential area for future research as well as target for intervention. Moreover, these risks speak to efforts to ban the use of pesticides which have increased in some countries, although many countries have yet to adopt these policies. The data of the present study provide additional evidence that pesticide use should indeed be limited and more research working to improve safe working conditions to limit potential negative effects is indicated (e.g., Rohlman et al., 2020).
Limitations
Findings from the present study should be considered in light of several limitations. First, the included sample was non-probabilistic and the study did not include a comparison group with no exposure to pesticides. As such, it may be that the present sample suffers from a selection bias whereby workers that are healthier or take better precautions chose to participate in the study. Additionally, although the non-applicator group was not occupationally exposed to pesticides, this group still displayed elevated levels of TCPy (e.g., through diet, distance of home from fields, or home pesticide use) with several participants within the highest quartile of TCPy exposure. As such, there is need to understand the effects of environmental exposures in addition to comparing both groups to individuals with low, background TCPy levels, such as those in the United States to have a full understanding of how effects of pesticide exposure degrade neurobehavioral functioning compared to typical development. Similarly, although all participants have worked at least one year applying pesticides, some adolescents in the study may have been exposed to pesticides before the study start date and have thus taken part in multiple application seasons. Moreover, it is possible some participants were exposed during the prenatal period or as infants. It is extraordinarily difficult to ascertain for how long someone has been exposed to pesticides, at what times, and in what magnitude; nonetheless, this is important work which should be evaluated further. The present data also includes additional potential confound which may have affected study results (e.g., hygiene, eating while applying, methods of mixing and applying pesticides, exposure to other types of pesticides which were not included in the present study).
Conclusion
This was one of the first studies to examine how occupational exposure to pesticides affects a broad range of neurobehavioral domains in an adolescent population. Individuals who applied pesticides showed higher levels of pesticide exposure than those who did not, though both applicators and non-applicators displayed large amounts of individual variation. Deficits in neurobehavioral functioning were affected by age, field, and the interaction of age and TCPy and field and TCPy. Over time, however, individuals did not show increasingly worse neurobehavioral functioning. Additionally, future work would benefit from the inclusion of neuroimaging techniques to strengthen the understanding of potential neurobehavioral deficits present in this population. Additionally, it will be important to examine individual variability in the metabolization and elimination of harmful chemicals. Finally, understanding the pattern of neurobehavioral deficits due to pesticide exposure is still unclear. It may be that motor and cognitive impairments are incurred at different rates or severity and will show different recovery times, if at all, and thus be an important question for future work.
Supplementary Material
Highlights.
Little work has investigated the effects of chronic exposure to pesticides during the adolescent period
The aim of this study was to examine effects of pesticide exposure on neurobehavioral functioning over a year within a group of male adolescent pesticide applicators and non-applicators in Egypt
Higher levels of urinary TCPy (3,5,6-trichloro-2-pyridinol) were associated with poorer functioning on neurobehavioral tasks that require complex functioning
Neurobehavioral tasks showed no changes over time and there were significant effects of age and field station
Occupational exposure to pesticides may have particularly deleterious effects on complex neurobehavioral domains during the adolescent period
Acknowledgements:
This research was funded by the National Institutes of Health (5R01ES022163-05).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests: The authors declare they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdel-Rasoul GM, Abou Salem ME, Mechael AA, Hendy OM, Rohlman DS, & Ismail AA (2008). Effects of occupational pesticide exposure on children applying pesticides. Neurotoxicology, 29(5), 833–838. [DOI] [PubMed] [Google Scholar]
- Abdel-Rasoul GM, Salem EA, Elbadry AS, Hendy OM, Rohlman DS, and Abdel-Latif AA (2019). Neurobehavioral and menstrual disorders among adolescent females environmentally exposed to pesticides, Menoufia Governorate, Egypt. Egyptian journal of occupational medicine, 43(3), 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anger WK, Farahat FM, Lein PJ, Lasarev MR, Olson JR, Farahat TM, & Rohlman DS (2020). Magnitude of behavioral deficits varies with job-related chlorpyrifos exposure levels among Egyptian pesticide workers. Neurotoxicology, 77, 216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcury TA, Grzywacz JG, Talton JW, Chen H, Vallejos QM, Galván L, … & Quandt SA (2010). Repeated pesticide exposure among North Carolina migrant and seasonal farmworkers. American journal of industrial medicine, 53(8), 802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi I, Gruber A, Rondeau V, Lebailly P, Brochard P, & Fabrigoule C (2011). Neurobehavioral effects of long-term exposure to pesticides: results from the 4-year follow-up of the PHYTONER study. Occupational and environmental medicine, 68(2), 108–115. [DOI] [PubMed] [Google Scholar]
- Binter AC, Bannier E, Saint-Amour D, Simon G, Barillot C, Monfort C, … & Chevrier C (2020). Exposure of pregnant women to organophosphate insecticides and child motor inhibition at the age of 10–12 years evaluated by fMRI. Environmental Research, 188, 109859. [DOI] [PubMed] [Google Scholar]
- Callahan CL, Hamad LA, Olson JR, Ismail AA, Abdel-Rasoul G, Hendy O, … & Bonner MR (2017). Longitudinal assessment of occupational determinants of chlorpyrifos exposure in adolescent pesticide workers in Egypt. International journal of hygiene and environmental health, 220(8), 1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (Revised ed.). Hillsdale, NJ: Lawrence Earlbaum Associates. [Google Scholar]
- Crane AL, Rasoul GA, Ismail AA, Hendy O, Bonner MR, Lasarev MR, … & Rohlman DS (2013). Longitudinal assessment of chlorpyrifos exposure and effect biomarkers in adolescent Egyptian agricultural workers. Journal of exposure science & environmental epidemiology, 23(4), 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl CL, Spivak M, Phinney R, & Montrose L (2020). Synthetic pesticides and health in vulnerable populations: agricultural workers. Current environmental health reports, 7(1), 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiny DL, & Ertingshausen G (1971). Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clinical chemistry, 17(8), 696–700. [PubMed] [Google Scholar]
- Farahat FM, Fenske RA, Olson JR, Galvin K, Bonner MR, Rohlman DS, Lein PJ, and Anger WK (2010). A Preliminary Study of Chlorpyrifos Exposures in Egyptian Cotton Field Workers. NeuroToxicology 31: 297–304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske RA, … & Olson JR (2011). Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environmental health perspectives, 119(6), 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxenberg RJ, Ellison CA, Knaak JB, Ma C, & Olson JR (2011). Cytochrome P450-specific human PBPK/PD models for the organophosphorus pesticides: chlorpyrifos and parathion. Toxicology, 285(1–2), 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube A, Donaldson D, Kiely T, & Wu L (2011). Pesticides industry sales and usage. US EPA, Washington, DC. [Google Scholar]
- Guo J, Zhang J, Wu C, Lv S, Lu D, Qi X, … & Chang X (2019). Associations of prenatal and childhood chlorpyrifos exposure with Neurodevelopment of 3-year-old children. Environmental Pollution, 251, 538–546. [DOI] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal, 6(1), 1–55. [Google Scholar]
- Ismail AA, Bonner MR, Hendy O, Abdel Rasoul G, Wang K, Olson JR, & Rohlman DS (2017a). Comparison of neurological health outcomes between two adolescent cohorts exposed to pesticides in Egypt. PLoS One, 12(2), e0172696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AA, Wang K, Olson JR, Bonner MR, Hendy O, Abdel Rasoul G, & Rohlman DS (2017b). The impact of repeated organophosphorus pesticide exposure on biomarkers and neurobehavioral outcomes among adolescent pesticide applicators. Journal of Toxicology and Environmental Health, Part A, 80(10–12), 542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer MC, & Firestone J (2007). Neurotoxicity of pesticides. Journal of Agromedicine, 12(1), 17–25. [DOI] [PubMed] [Google Scholar]
- London L, Beseler C, Bouchard MF, Bellinger DC, Colosio C, Grandjean P, … & Meijster T (2012). Neurobehavioral and neurodevelopmental effects of pesticide exposures. Neurotoxicology, 33(4), 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Baron M, Knapp G, Schäper M, & van Thriel C (2015). Meta-analysis on occupational exposure to pesticides–Neurobehavioral impact and dose–response relationships. Environmental research, 136, 234–245. [DOI] [PubMed] [Google Scholar]
- Muñoz-Quezada MT, Lucero BA, Iglesias VP, Muñoz MP, Cornejo CA, Achu E, … & Villalobos M (2016). Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: a review. International journal of occupational and environmental health, 22(1), 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton SX, & Terry AV Jr (2018). Neurotoxicity in acute and repeated organophosphate exposure. Toxicology, 408, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Santana M, Zúñiga-Venegas L, Corral S, Roeleveld N, Groenewoud H, Van der Velden K, … & Pancetti F (2020). Reduced neurobehavioral functioning in agricultural workers and rural inhabitants exposed to pesticides in northern Chile and its association with blood biomarkers inhibition. Environmental Health, 19(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, Gimenes LS, Eckerman DA, Kang SK, Farahat FM, & Anger WK (2003). Development of the Behavioral Assessment and Research System (BARS) to detect and characterize neurotoxicity in humans. Neurotoxicology, 24(4–5), 523–531. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Anger WK, & Lein PJ (2011). Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology, 32(2), 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, Ismail AA, Abdel-Rasoul G, Lasarev M, Hendy O, & Olson JR (2014). Characterizing exposures and neurobehavioral performance in Egyptian adolescent pesticide applicators. Metabolic brain disease, 29(3), 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, Ismail AA, Rasoul GA, Bonner MR, Hendy O, Mara K, … & Olson JR (2016). A 10-month prospective study of organophosphorus pesticide exposure and neurobehavioral performance among adolescents in Egypt. Cortex, 74, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, Ismail A, Bonner MR, Rasoul GA, Hendy O, Dickey LO, … & Olson JR (2019). Occupational pesticide exposure and symptoms of attention deficit hyperactivity disorder in adolescent pesticide applicators in Egypt. NeuroToxicology, 74, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, Davis JW, Ismail A, Abdel Rasoul GM, Hendy O, Olson JR, & Bonner MR (2020). Risk perception and behavior in Egyptian adolescent pesticide applicators: an intervention study. BMC public health, 20, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SM, McManus IC, Harrison V, & Mason O (2013). Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Critical reviews in toxicology, 43(1), 21–44. [DOI] [PubMed] [Google Scholar]
- Sapbamrer R, & Hongsibsong S (2019). Effects of prenatal and postnatal exposure to organophosphate pesticides on child neurodevelopment in different age groups: a systematic review. Environmental Science and Pollution Research, 26(18), 18267–18290. [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Checkoway H, Jacobs DR Jr, Al-Delaimy WK, & Gahagan S (2017). Potential short-term neurobehavioral alterations in children associated with a peak pesticide spray season: The Mother’s Day flower harvest in Ecuador. Neurotoxicology, 60, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Nazeeh N, Kayser G, Suárez-Torres J, Checkoway H, López-Paredes D, … & de la Cruz F (2020). Residential proximity to greenhouse crops and pesticide exposure (via acetylcholinesterase activity) assessed from childhood through adolescence. Environmental Research, 188, 109728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, & Hashizume M (2014). A systematic review of the influence of occupational organophosphate pesticides exposure on neurological impairment. BMJ open, 4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Dries MA, Guxens M, Spaan S, Ferguson KK, Philips E, Santos S, … & Pronk A (2020). Phthalate and bisphenol exposure during pregnancy and offspring nonverbal IQ. Environmental health perspectives, 128(7), 077009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


