Abstract
Background:
Selenium is an essential trace element that shows beneficial or adverse health effects depending on the dose. Laboratory studies suggest that high selenium may contribute to the development of non-alcoholic fatty liver disease (NAFLD). However, human evidence is limited. We evaluated the associations of serum selenium level with serum alanine aminotransferase (ALT) activity and suspected NAFLD prevalence in U.S. adults.
Methods:
We conducted the cross-sectional analysis in 3,827 adults aged 20 years and older without viral hepatitis, hemochromatosis, or alcoholic liver disease who participated in the National Health and Nutrition Examination Survey (NHANES) 2011–2012, 2013–2014, and 2015–2016. Serum selenium was measured using inductively coupled plasma dynamic reaction cell mass spectrometry. Suspected NAFLD cases were defined in the presence of serum ALT > 30 international units (IU)/L in men and > 19 I.U./L in women in the absence of other identifiable causes of liver disease.
Results:
The median (interquartile range) of serum selenium level was 127.9 (117.9, 139.4) μg/L. Non-linear associations of serum selenium with NAFLD prevalence and serum ALT activity were observed in the generalized additive models with penalized splines. After adjustment for sociodemographic variables, lifestyle factors, body mass index, and NHANES survey cycles, positive associations were found at > ~130 μg/L serum selenium with both NAFLD and ALT, whereas the associations were flattened at < ~130 μg/L.
Conclusions:
Our findings provide evidence of non-linear associations of serum selenium with ALT activity and NAFLD prevalence. In particular, positive associations were found above serum selenium level of 130 μg/L, whereas no association was observed below this value. This finding requires confirmation in future prospective cohort studies.
Keywords: selenium, NAFLD, alanine aminotransferase, epidemiology, NHANES
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) has become a significant health concern, and its prevalence has markedly increased globally over recent decades (Mitra et al., 2020). NAFLD is currently the most common liver disease affecting approximately 2 billion people (prevalence of 25%) in the world (Younossi et al., 2018). It is characterized by excessive accumulation of lipids in the liver in the absence of alcohol abuse and may further progress to more advanced non-alcoholic steatohepatitis, fibrosis, cirrhosis, and eventually hepatocellular carcinoma (Vernon et al., 2011). NAFLD has been found more prevalent in obesity, insulin resistance, type 2 diabetes, while its cause is still largely unknown (Mitra et al., 2020). Serum alanine aminotransferase (ALT) is a widely used biomarker for liver injury, and its elevation not attributed to excessive alcohol consumption or viral hepatitis has been used as a biomarker for NAFLD (Hadizadeh et al., 2017). Epidemiologic studies have suggested that increased caloric intake and a sedentary lifestyle are important risk factors of NAFLD (Mitra et al., 2020). The potential contributions of environmental factors to the epidemic of NAFLD have received less attention.
Selenium is an essential element that plays an important role in redox homeostasis, thyroid hormone metabolism, and defense against oxidative stress and inflammation (Vinceti et al., 2018b). Acquiring from the diet, selenium is rich in organ meats and seafood, followed by grain, cereals, and dairy products (Rayman, 2008). The average dietary intake of selenium for U.S. adults ranges from 93 to 151 μg/day, which is well above the recommended dietary allowance (RDA) of 55 μg/day by the Institute of Medicine (U.S.) (Institute of Medicine, 2000) and 26 μg/day in males and 34 μg/day in females by the World Health Organization (World Health Organization, 2004). The general population can also be exposed to selenium in cigarette smoking, ambient air, soil, and drinking water (Vinceti et al., 2018b). Epidemiologic studies have shown both beneficial and adverse health effects of selenium depending on its dose (Vinceti et al., 2018b). These findings suggest that both abnormally low and high intake of this trace element should be avoided. However, the reference levels of selenium intake still lack consensus due to limited epidemiologic evidence about the dose-response relationship between selenium and specific health outcomes (Vinceti et al., 2018b).
Growing evidence from recent observational studies and randomized clinical trials highlights possible adverse cardio-metabolic effects of high selenium exposure, particularly hypertension, dyslipidemia, and type 2 diabetes (Berthold et al., 2012; Christensen et al., 2015; Stranges et al., 2010; Vinceti et al., 2018a). Biologically, selenium can contribute to the development of NAFLD. In animal models, selenium treatment impairs insulin responsiveness and disrupts lipid profiles, despite its insulin-like and antioxidant properties (Fürnsinn et al., 1996; Zhao et al., 2016). However, only a few epidemiologic studies have explored the associations between selenium and NAFLD and yield inconsistent findings (Longnecker et al., 1991; Loomba et al., 2020; Wu et al., 2020; Yang et al., 2016). Furthermore, the relationship between selenium and NAFLD remains unknown in the U.S. general population with a high average selenium intake. The objective of this study was to examine the associations of serum selenium level with serum ALT activity and the prevalence of NAFLD using the National Health and Nutrition Examination Survey (NHANES) collected in 2011–2016.
2. Materials and Methods
2.1. Study Population
The study sample consists of 3 continuous cycles (2011–2012, 2013–2014, and 2015–2016) of the NHANES, which was designed for providing a representative sample of the civilian, non-institutionalized U.S. population using a stratified, multistage probability cluster design (CDC/NCHS, 2018). All data and materials have been made publicly available at the National Center for Health Statistics website (https://www.cdc.gov/nchs/nhanes/index.htm). The protocols for NHANES were approved by the National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board, and informed consent was obtained from all participants.
The participants were 5,085 adults aged 20 years or older who had both serum selenium levels and ALT activity measured. For this analysis, we excluded participants who had positive serum hepatitis B surface antigen or positive serum hepatitis C antibody, or with elevated transferrin saturation (> 60% for men and > 50% for women), or with high alcohol consumption (≥20 g/day for men and ≥10 g/day for women) (n=819) given that a high serum ALT in these participants may be due to other identifiable causes of liver disease (Cave et al., 2010). We further excluded participants with missing information on key covariates (n=439), leaving a total of 3,827 participants for the current study (Figure S1).
2.2. Serum selenium levels
Serum selenium was measured at the Environmental Health Sciences Laboratory of the CDC National Center for Environmental Health using the inductively coupled plasma dynamic reaction cell mass spectrometry following extensive quality control procedures (CDC/NCHS, 2018). The limit of detection (LOD) for serum selenium was 4.5 μg/dL. Of all participants in our analysis, 7.3% had serum selenium levels below the LOD. Serum selenium levels below the LOD were assigned with the LOD divided by the square root of 2.
2.3. NAFLD assessment
Suspected NAFLD cases were ascertained based on serum ALT that has been commonly used as a screening test and monitoring biomarker for NAFLD (Hadizadeh et al., 2017). Serum ALT activity was measured using the kinetic rate method on the Beckman UniCel DxC800 Synchron (Beckman Coulter, Brea, CA). NAFLD status was assumed in the presence of serum ALT activity > 30 international units (IU)/L in men and > 19 I.U./L in women in the absence of other identifiable causes of liver disease (Prati et al., 2002).
2.4. Covariates
Information on age, sex, race/ethnicity (Hispanic including Mexican American and other Hispanic, non-Hispanic White, non-Hispanic Black, non-Hispanic Asians, and others), education, poverty-income ratio (PIR), and smoking status were collected using self-administered questionnaires. Education was categorized into less than high school, high school graduate or equivalent, some college or associate degree, and college graduate or above. PIR was dichotomized at a cutoff of 1 where a value below 1 indicates that the family is living below the poverty threshold. Smoking status was categorized into self-reported current smokers, former smokers, or never smokers. Height was measured to nearest 0.1 cm, and body weight was measured to the nearest 0.1 kg at a mobile examination center by trained health technicians. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Dietary selenium intake was measured via 24-hour dietary recall interviews.
2.5. Statistical analysis
All analyses were performed accounting for the complex survey design using the appropriate subsample weights, strata, and primary sampling units per NHANES recommendation (CDC/NCHS, 2018). We used survey-weighted linear regression to evaluate the association between serum selenium level and ALT activity. Logarithmic transformation with natural base was applied to ALT given the highly skewed distribution. We used survey weight logistic regression to assess the association between serum selenium level and prevalence of NAFLD. We evaluated the associations using the quintiles of serum selenium levels, considering the possible non-linear associations. Tests for linear trend were calculated by including serum selenium quintiles as a continuous variable in the models. To further explore the shape of the relationship between serum selenium and NAFLD, we used the generalized additive model (GAM) with penalized splines, in which logarithmic transformation with base 2 was applied to selenium level given the right-skewed distribution. Potential confounders were adjusted progressively in the regression models. Initial regression models included adjustment for age, sex, race/ethnicity, and NHANES survey cycles, while the full models subsequently adjusted for education, PIR, BMI, smoking status, and alcohol consumption. Finally, we examined the possible effect modifications by age (20–39 years vs. 40 years and older) and sex on the association between serum selenium and NAFLD by stratification analysis. We chose the age cut-off of 40 years, given existing evidence of a significant increase in NAFLD risk in age 40 years and older (Gan et al., 2011).
Several sensitivity analyses were performed to evaluate the robustness of our primary findings. First, we fit the generalized additive model (GAM) with penalized splines in a subpopulation with dietary selenium intake > 55 μg/day, which is the highest RDA allowance in the U.S. (7). Second, we additionally adjusted for homeostatic model assessments for insulin resistance (HOMA-IR) in a subpopulation of 1,799 participants who had their fasting plasma glucose and insulin levels measured. HOMA-IR was calculated according to the following equation: [insulin (μU/mL) × glucose (mmol/L)]/22.5 (Matthews et al., 1985). We did not include HOMA-IR in our primary analysis in case of overadjustment bias because of its role as a risk factor of NAFLD and the fact that it could be affected by selenium levels (Fürnsinn et al., 1996; Utzschneider and Kahn, 2006). Third, we additionally adjusted for urinary arsenic level because of its potential role as a risk factor of NAFLD and shared dietary sources with selenium in a subpopulation of 3,750 participants after excluding missing information on arsenic level (Frediani et al., 2018; Wang et al., 2020a, 2020b). Finally, we additionally adjusted for pregnancy status, given an additional recommendation of selenium supplementation for pregnant women (Institute of Medicine, 2000). All analyses were conducted by SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina). Sampling weight-applied smoothing plots were created using the GAM function with penalized spline in the ‘MGCV’ package in R (version 4.0.2, www.R-proiect.org).
3. Results
Survey-weighted characteristics of the study population are summarized in Table 1. The mean (standard error, SE) age of the study participants was 47.5 (0.5) years, and 53.1% were females. Of 3,827 participants, 1,319 were identified suspected NAFLD cases (sample-weighted prevalence = 35.7%). Participants with NAFLD were more likely to be female, Hispanic, and had higher BMI. Medians (interquartile range, IQR) of serum selenium levels were 127.9 (117.9, 139.4) μg/L, 127.2 (117.7, 138.9) μg/L, and 129.4 (118.3, 140.5) μg/L in total population, participants without NAFLD, and participants with NAFLD, respectively.
Table 1.
Survey-weighted characteristics of the study population by non-alcoholic fatty liver disease (NAFLD) status (n=3,827).
| Characteristics | Total (n=3827) | No NAFLD (n=2508) | NAFLD (n=1319) |
|---|---|---|---|
| Age (years), mean (SE) | 47.5 (0.5) | 47.9 (0.6) | 46.8 (0.6) |
| Sex, % (SE) | |||
| Male | 46.9 (1.1) | 50.7 (1.6) | 40.2 (1.8) |
| Female | 53.1 (1.1) | 49.3 (1.6) | 59.8 (1.8) |
| Race/ethnicity, % (SE) | |||
| Non-Hispanic White | 64.9 (2.3) | 66.0 (2.2) | 63.1 (3.0) |
| Non-Hispanic Black | 11.1 (1.2) | 12.6 (1.5) | 8.4 (1.0) |
| Non-Hispanic Asian | 5.9 (0.7) | 5.8 (0.8) | 6.0 (0.7) |
| Hispanic | 15.5 (1.7) | 12.9 (1.4) | 20.0 (2.5) |
| Other | 2.6 (0.4) | 2.7 (0.4) | 2.5 (0.5) |
| Education, % (SE) | |||
| < High school | 15.4 (1.2) | 15.2 (1.3) | 15.9 (1.5) |
| High school | 21.3 (1.0) | 21.5 (1.3) | 21.0 (1.6) |
| Some college | 33.0 (1.1) | 33.4 (1.3) | 32.3 (1.5) |
| College or above | 30.2 (1.6) | 29.9 (1.3) | 30.8 (2.4) |
| Poverty-income ratio, % (SE) | |||
| < 1.0 | 16.2 (1.1) | 16.9 (1.1) | 14.9 (1.5) |
| ≥ 1.0 | 83.8 (1.1) | 83.1 (1.1) | 85.1 (1.5) |
| Smoking status, % (SE) | |||
| Never smoker | 59.1 (1.1) | 58.3 (1.3) | 60.5 (1.7) |
| Former smoker | 24.1 (0.9) | 24.0 (1.1) | 24.4 (1.8) |
| Current smoker | 16.8 (0.9) | 17.7 (1.1) | 15.1 (1.3) |
| Alcohol consumption (g/day), mean (SE) | 1.4 (0.1) | 1.5 (0.1) | 1.3 (0.2) |
| BMI (kg/m2), % (SE) | 29.4 (0.2) | 28.4 (0.2) | 31.3 (0.3) |
| HOMA-IR, Median (IQR) | 2.6 (1.5, 434) | 2.3 (1.4, 3.5) | 3.5 (1.9, 5.8) |
| Serum selenium (μg/L), Median (IQR) | 127.9 (117.9, 139.4) | 127.2 (117.7, 138.9) | 129.4 (118.3, 140.5) |
BMI, body mass index; HOMA-IR, homeostatic model assessments for insulin resistance; IQR, interquartile range.
The association between serum selenium levels and ALT activity is presented in Table 2. After multivariable adjustments for age, sex, race/ethnicity, NHANES survey cycles, education, poverty-income-ratio, BMI, smoking, and alcohol consumption, participants in the highest quintile of serum selenium (142.5–297.9 μg/L), on average, had a 9.25% (95% CI: 2.23%, 16.76%) and a 12.24% (95% CI: 5.83%, 19.04%) higher serum ALT activity, compared to the participants in the first (58.1–114.9 μg/L) and second (115.0–123.6 μg/L) lowest quintiles, respectively (P-trend=0.0003). In the flexible dose-response analysis based on the GAM with penalized splines in both initial and full models, a non-linear association between selenium and ALT was observed (Figure 1, Figure S2). A positive association was found at > ~130 μg/L serum selenium (log2-transformed serum selenium > ~7.0), whereas the association was flattened at < ~130 μg/L serum selenium.
Table 2.
Adjusted percentage changes (95% CI) in serum alanine aminotransferase (ALT) activitya with quintile changes in serum selenium levels in the survey-weighted multivariable-adjusted linear regressions (n=3,827).
| Quintiles of serum selenium levels, μg/L | ||||||
|---|---|---|---|---|---|---|
| Models | Quintile 1 (58.1–114.9) | Quintile 2 (115.0–123.6) | Quintile 3 (123.7–131.7) | Quintile 4 (131.8–142.4) | Quintile 5 (142.5–297.9) | P-trend |
| Initial modelb | Ref | −3.18 (−9.29, 3.34) | 3.01 (−2.48, 8.81) | 3.68 (−1.98, 9.66) | 9.53 (2.93, 16.56) | 0.0005 |
| Full modelc | Ref | −2.67 (−8.65, 3.71) | 3.71 (−1.71, 9.43) | 4.16 (−0.89, 9.47) | 9.25 (2.23, 16.76) | 0.0003 |
ALT activity was log-transformed.
Initial model: adjustments for age, sex, race/ethnicity, and NHANES survey cycles.
Full model: initial model with additional adjustments for education, poverty-income-ratio, body mass index, smoking status, and alcohol consumption.
Figure 1.
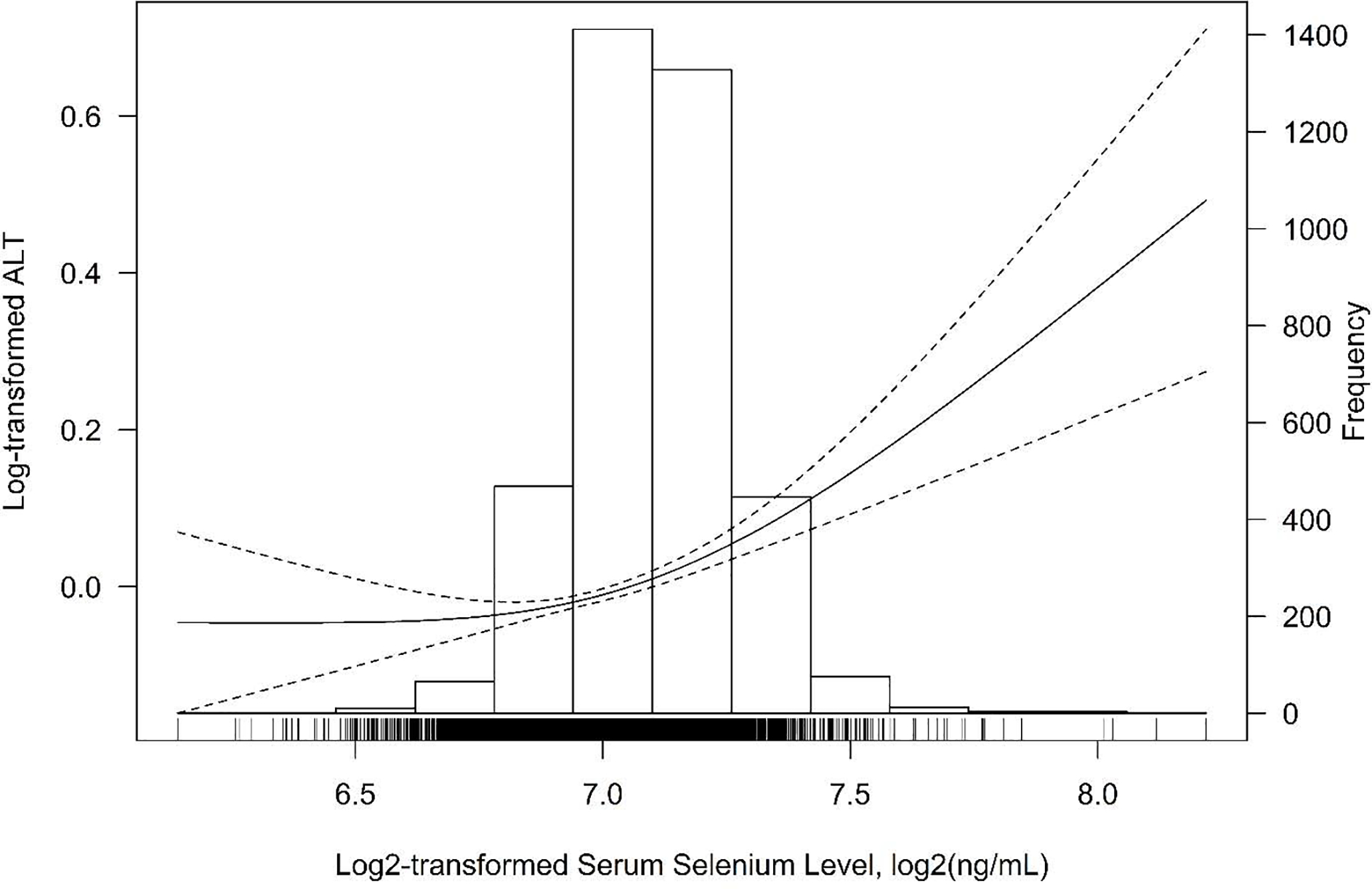
Smoothing curve of the relationship between log2-transformed serum selenium level and log-transformed serum alanine aminotransferase (ALT) activity (95% CI) based on the generalized additive model (GAM) with penalized splines. The GAM was adjusted for age, sex, race/ethnicity, NHANES survey cycles, education, poverty-income-ratio, body mass index, smoking status, and alcohol consumption (n=3,827).
Table 3 summarizes the associations between serum selenium level and NAFLD prevalence. After full adjustment for covariates, the ORs for NAFLD comparing the highest selenium quintile with the first and second lowest quintiles were 1.36 (95% CI: 1.00, 1.88) and 1.64 (95% CI: 1.223 2.19), respectively (P-trend=0.02). A similar non-linear association between selenium and NAFLD was observed in the GAM with penalized splines (Figure 2, Figure S3). A positive association was found at > ~130 μg/L serum selenium (log2-transformed serum selenium > ~7.0); in contrast, no association was observed at < ~130 μg/L serum selenium. No significant difference in the association between serum selenium and NAFLD was found in the stratification analysis by age (Figure S4) and sex (Figure S5).
Table 3.
Adjusted odds ratios (ORs) (95% CI) for prevalence of non-alcoholic fatty liver disease (NAFLD) with quintile changes in serum selenium levels in the survey-weighted multivariable-adjusted logistic regressions (n=3,827).
| Quintiles of serum selenium levels, μg/L | ||||||
|---|---|---|---|---|---|---|
| Models | Quintile 1 (58.1–114.9) | Quintile 2 (115.0–123.6) | Quintile 3 (123.7–131.7) | Quintile 4 (131.8–142.4) | Quintile 5 (142.5–297.9) | P-trend |
| Initial modela | Ref | 0.82 (0.59, 1.13) | 0.97 (0.70, 1.33) | 1.03 (0.80, 1.33) | 1.31 (0.96, 1.78) | 0.02 |
| Full modelb | Ref | 0.83 (0.59, 1.17) | 1.00 (0.73, 1.36) | 1.05 (0.82, 1.35) | 1.36 (1.00, 1.88) | 0.02 |
Initial model: adjustments for age, sex, race/ethnicity, and NHANES survey cycles.
Full model: initial model with additional adjustments for education, poverty-income-ratio, body mass index, smoking status, and alcohol consumption.
Figure 2.
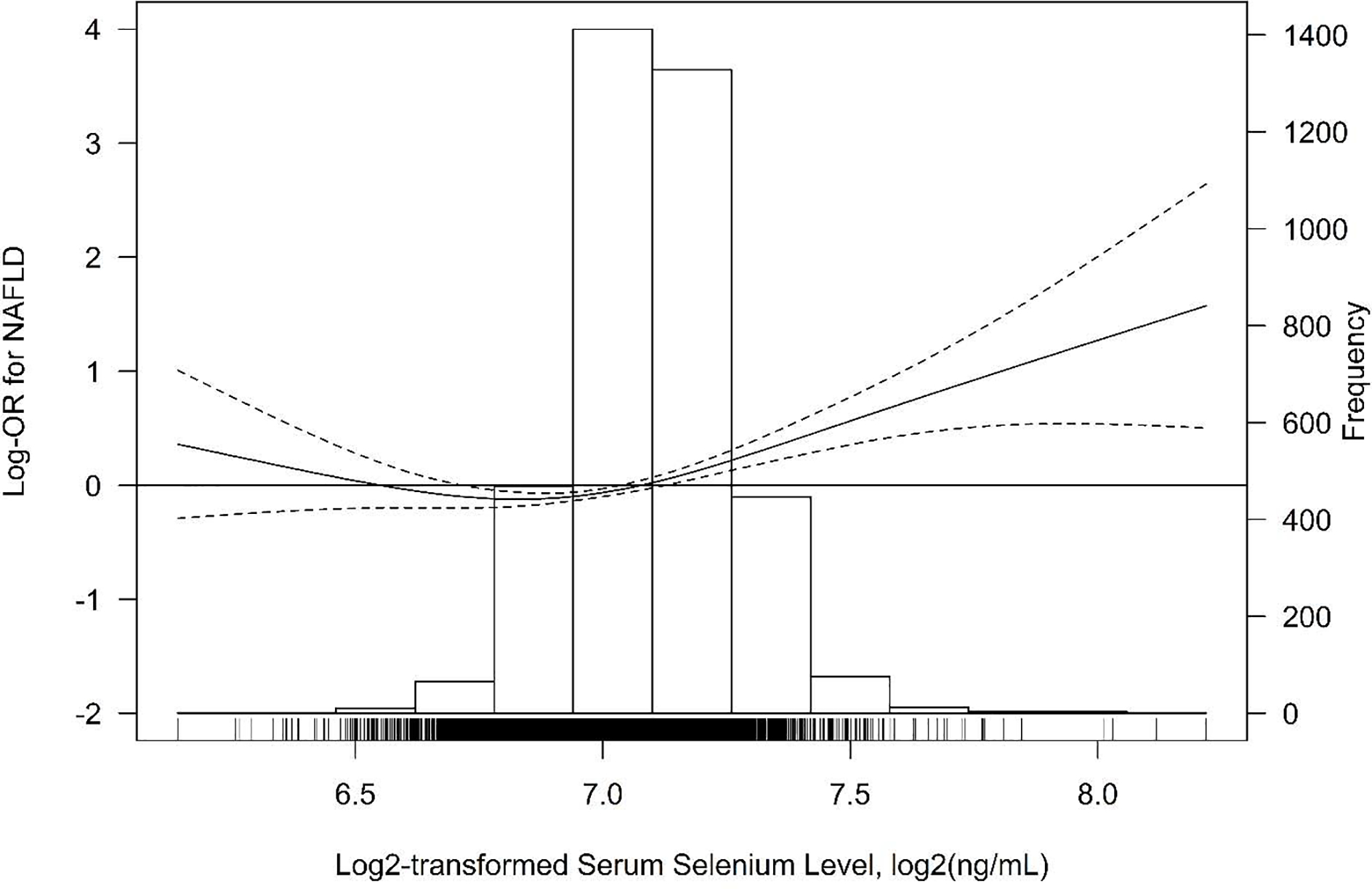
Smoothing curve of the relationship between log2-transformed serum selenium level and log-transformed odds ratio of non-alcoholic fatty liver disease (NAFLD) (95% CI) based on the generalized additive model (GAM) with penalized splines. The GAM was adjusted for age, sex, race/ethnicity, NHANES survey cycles, education, poverty-income-ratio, body mass index, smoking status, and alcohol consumption (n=3,827).
In the sensitivity analysis, associations of serum selenium with ALT and NAFLD were evaluated in a subpopulation of 3,497 (91.3%) participants with dietary selenium intake > 55 μg/day. Similar associations were observed in GAMs (Figure S6, Figure S7) as those in the primary analyses. In the sensitivity analysis of 1,799 participants with HOMA-IR available, associations of serum selenium with ALT and NAFLD after further adjustment of with HOMA-IR were also similar as observed in the primary analyses (Figure S8, Figure S9). In the sensitivity analysis of 3,750 participants with urinary arsenic level measured, additional adjustment for arsenic did not alter the associations (Figure S10, Figure S11). In the sensitivity analysis, after further adjusting for pregnancy status (35 pregnant women in our study), similar associations were observed in GAMs (Figure S12, Figure S13) as those in the primary analyses.
4. Discussion
Our study investigated the association between serum selenium level and NAFLD in U.S. adults using nationally representative data. We found non-linear associations of serum selenium with serum ALT activity and NAFLD prevalence after adjusting for sociodemographic variables, lifestyle factors, and BMI. Below ~ 130 μg/L, we did not have enough evidence suggesting associations of serum selenium with ALT and NAFLD. We observed positive associations above 130 μg/L. This study is the first to investigate the association between serum selenium and NAFLD in the U.S.
Selenium is an essential nutrient for human health, with a narrow range between essentiality and toxicity (Rayman, 2012). U.S. adults are considered a population with adequate selenium status (F. Combs, Jr., 2015). Despite the nutritional benefits, selenium supplementation has been a growing concern, especially given the termination of the Selenium and Vitamin E Cancer Prevention Trial that an excess incidence of type 2 diabetes and high-grade prostate cancer found in the selenium supplementation arm (Kristal et al., 2014; Lippman et al., 2009). Elevated selenium levels are associated with higher blood pressure, lipid levels, and neurological diseases (Christensen et al., 2015; Laclaustra et al., 2009; Naderi et al., 2021). In the current study, we found a positive association between serum selenium and NAFLD at a serum selenium concentration of above 130 μg/L; this concentration can be approximately translated into a selenium intake of 87 μg/day based on the previous link between serum selenium level and the dietary intake (Haldimann et al., 1996). Notably, this is much lower than the Institute of Medicine established tolerable upper intake level of 400 μg/day for adults 19 years and older, guiding the highest level of daily selenium intake that is likely to pose no risk of adverse health effects in almost all individuals (Institute of Medicine, 2000). Thus, our findings suggest the reference for a possible upper safe limit of selenium status/intake in terms of risk of NAFLD; however, more dose-response research, especially prospective cohort studies in different populations or already performed selenium clinical trials with NAFLD available as a secondary outcome, are needed to confirm our findings.
The potential hepatotoxicity of selenium overexposure has been first reported in the 1930s (Smith and Westfall, 1937), though the scope of the biological mechanism underlying the association between selenium and NAFLD is still largely unknown. Selenium exerts antioxidant effects through selenium-dependent glutathione peroxidases and other selenoproteins (Burk, 2002). However, serum selenoproteins levels are maximized between 70 and 90 μg/L (F. Combs, Jr., 2015). Above these levels, increases in serum selenium do not capture increases in selenoprotein levels but reflect predominantly non-specific incorporation of selenomethionine into albumin and other proteins in place of methionine (F. Combs, Jr., 2015). Given our participants having a median serum level of 127.9 μg/L, higher serum selenium levels in our study probably capture increases in non-specific incorporation of selenomethionine proteins rather than the incorporation into selenoproteins. Selenomethionine is an organic derivative of selenium widely used as supplements in our diets (Institute of Medicine, 2000). Consumption of diets rich in selenomethionine will raise blood selenium concentrations because this form of selenium is not influenced by homeostatic regulation (Burk, 2002). Once inside the cell, selenomethionine can be either incorporated into proteins in place of methionine or metabolized to several selenocompounds such as selenols, selenohomocysteine and selenocysteine, through the methionine cycle and the trans-sulfuration pathway. Such selenocompounds can generate superoxide radicals, thereby inducing oxidative stress (Lazard et al., 2017). Thus, it is conceivable that high serum selenium may impact the pathogenesis of NAFLD through the toxicity of selenomethionine. Studies on the speciation of selenium in blood warrant further investigations in the future.
Only a few epidemiologic studies have evaluated the associations between selenium and NAFLD. A positive log-linear dose-response relationship was observed in a large cross-sectional study of 8,550 Chinese adults, where median plasma selenium level in this study was 213.0 μg/L, which was higher than that in our study (Yang et al., 2016). Another cross-sectional study of 5,436 middle-aged and elderly Chinese adults also reported a positive association between dietary intake of selenium and prevalence of NAFLD (OR = 1.58, 95% CI: 1.39, 1.89, comparing the highest (⩾ 30.6 μg/1,000 kcal/day) to the lowest (≤ 21.3 μg/1,000 kcal/day) quartile) (Wu et al., 2020). A positive association between dietary selenium intake and ALT has also been reported in seleniferous areas of the U.S. (Longnecker et al., 1991). In contrast, no association of blood, hair, or nail selenium with ALT was found in 680 Indian adults living in a high selenium environment (Loomba et al., 2020).
With sampling weights, strata, and units considered in the analyses, we were able to assess the association between selenium and NAFLD in a representative sample of the general U.S. adults. Our findings provide impetus to confirm this association in different populations over a broader range of exposure, eventually contributing to a consensus regarding the safe range of selenium exposure and intake. However, we acknowledge that the cross-sectional nature of NHANES data precludes the ability to determine the chronicity of serum status and persistence of NAFLD. Therefore reverse causation could be an explanation for our results since participants with NAFLD may have adapted lifestyle changes, including diet and supplement use (Nseir et al., 2014). Though studies with the prospective design are needed to make a causal inference, our findings are still important and add the reference of potential hepatotoxicity of selenium overexposure. Additionally, suspected cases of NAFLD were primarily based on ALT activity in our study. We acknowledge that liver biopsies would provide a better NAFLD assessment; however, this is not feasible for large population-based studies. Although the liver biopsies were not provided in NHANES, the ALT-based NAFLD should be relatively specific because the prevalence of myopathy, the most important extrahepatic source of ALT, is considered low in the U.S. general population (Green and Flamm, 2002). Moreover, we cannot eliminate residual confounding due to the observational nature of the study, although we have controlled for many known confounders. There are possibly other environmental toxicants that shared common sources with selenium, such as diets that may also impact NAFLD (Oddy et al., 2013; Wang et al., 2017). Finally, only total selenium was measured in serum samples, and data on selenium speciation were not available. The source and toxicity of selenium species and compounds may differ markedly (Vinceti et al., 2018b). The association between selenium and health outcomes may also depend on the use of specific biomarkers (whole blood, serum/plasma, urine, nails, toenails, hair selenium content) or exposure assessment methods (dietary assessment through questionnaires or daily records). In future studies, selenium speciation and a comparison of different biomarkers will be critical to providing a better understanding of selenium exposures and associated health risks, including NAFLD.
5. Conclusions
In summary, the present analysis provides evidence of non-linear associations of serum selenium with serum ALT activity and NAFLD prevalence. In particular, positive associations were found above the serum selenium level > 130 μg/L, whereas no association was observed below this value. This finding requires confirmation in future prospective cohort studies. Such association, if confirmed, will be of considerable public health relevance given the epidemic of NAFLD.
Supplementary Material
Highlights.
Non-linear associations of serum selenium with ALT activity and NAFLD were found.
A positive association between selenium and ALT was observed at serum selenium level > 130 μg/L.
A positive association between selenium and NAFLD was observed at serum selenium level > 130 μg/L.
No association was observed below serum selenium level of 130 μg/L.
Acknowledgement
This study was supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01-ES026578 and R01-ES026964 to Sung Kyun Park, and National Institute of Health (NIH) R01DK123022 and R21NS112974 to Young Ah Seo. The authors would also like to thank the NHANES participants and the staff members for their contribution to data collection and for making the data publicly available.
Abbreviations:
- ALT
alanine aminotransferase
- GAM
generalized additive model
- HOMA-IR
homeostatic model assessments for insulin resistance
- I.U.
international units
- LOD
limit of detection
- NAFLD
non-alcoholic fatty liver disease
- NHANES
National Health and Nutrition Examination Survey
- RDA
recommended dietary allowance
- PIR
poverty-income ratio
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare they have no actual or potential competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berthold HK, Michalke B, Krone W, Guallar E, Gouni-Berthold I, 2012. Influence of serum selenium concentrations on hypertension: the Lipid Analytic Cologne cross-sectional study. J. Hypertens 30, 1328–1335. 10.1097/HJH.0b013e32835414df [DOI] [PubMed] [Google Scholar]
- Burk RF, 2002. Selenium, an antioxidant nutrient. Nutr. Clin. Care 5, 75–79. 10.1046/j.1523-5408.2002.00006.x [DOI] [PubMed] [Google Scholar]
- Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G, 2010. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in american adults: NHANES 2003–2004. Environ. Health Perspect. 118, 1735–1742. 10.1289/ehp.1002720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC/NCHS, 2018. NHANES - Questionnaires, Datasets, and Related Documentation.
- Christensen K, Werner M, Malecki K, 2015. Serum selenium and lipid levels: Associations observed in the National Health and Nutrition Examination Survey (NHANES) 2011–2012. Environ. Res 140, 76–84. https://doi.org/10.1016Zj.envres.2015.03.020 [DOI] [PubMed] [Google Scholar]
- F. Combs G Jr., 2015. Biomarkers of Selenium Status. Nutrients 7, 2209–2236. 10.3390/nu7042209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frediani JK, Naioti EA, Vos MB, Figueroa J, Marsit CJ, Welsh JA, 2018. Arsenic exposure and risk of nonalcoholic fatty liver disease (NAFLD) among U.S. adolescents and adults: an association modified by race/ethnicity, NHANES 2005–2014. Environ. Heal 17, 6. 10.1186/s12940-017-0350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürnsinn C, Englisch R, Ebner K, Nowotny P, Vogl C, Waldhäusl W, 1996. Insulin-like vs. non-insulin-like stimulation of glucose metabolism by vanadium, tungsten, and selenium compounds in rat muscle. Life Sci. 59, 1989–2000. 10.1016/S0024-3205(96)00550-4 [DOI] [PubMed] [Google Scholar]
- Gan L, Chitturi S, Farrell GC, 2011. Mechanisms and implications of age-related changes in the liver: Nonalcoholic fatty liver disease in the elderly. Curr. Gerontol. Geriatr. Res 2011. 10.1155/20n/831536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RM, Flamm S, 2002. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 123, 1367–1384. 10.1053/gast.2002.36061 [DOI] [PubMed] [Google Scholar]
- Hadizadeh F, Faghihimani E, Adibi P, 2017. Nonalcoholic fatty liver disease: Diagnostic biomarkers. World J. Gastrointest. Pathophysiol 8, 11. 10.4291/wjgp.v8.i2.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldimann M, Venner TY, Zimmerli B, 1996. Determination of selenium in the serum of healthy Swiss adults and correlation to dietary intake. J. Trace Elem. Med. Biol. 10, 31–45. 10.1016/S0946-672X(96)80006-X [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids, Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academies Press. 10.17226/9810 [DOI] [PubMed] [Google Scholar]
- Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, Thompson IM, Meyskens FL, Goodman GE, Minasian LM, Parnes HL, Lippman SM, Klein EA, 2014. Baseline Selenium Status and Effects of Selenium and Vitamin E Supplementation on Prostate Cancer Risk. JNCI J. Natl. Cancer Inst. 106. 10.1093/jnci/djt456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E, 2009. Serum selenium concentrations and hypertension in the US population. Circ. Cardiovasc. Qual. Outcomes 2, 369–376. 10.1161/CIRCOUTCOMES.108.831552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazard M, Dauplais M, Blanquet S, Plateau P, 2017. Recent advances in the mechanism of selenoamino acids toxicity in eukaryotic cells. Biomol. Concepts 8, 93–104. 10.1515/bmc-2017-0007 [DOI] [PubMed] [Google Scholar]
- Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Baker LH, Coltman CA, 2009. Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers. JAMA 301, 39–51. 10.1001/jama.2008.864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Taylor PR, Levander OA, Howe M, Veillon C, McAdam PA, Patterson KY, Holden JM, Stampfer MJ, Morris JS, Willett WC, 1991. Selenium in diet, blood, and toenails in relation to human health in a seleniferous area. Am. J. Clin. Nutr 53, 1288–1294. 10.1093/ajcn/53.5.1288 [DOI] [PubMed] [Google Scholar]
- Loomba R, Filippini T, Chawla R, Chaudhary R, Cilloni S, Datt C, Singh S, Dhillon KS, Vinceti M, 2020. Exposure to a high selenium environment in Punjab, India: Effects on blood chemistry. Sci. Total Environ. 716, 135347. https://doi.org/10.10167j.scitotenv.2019.135347 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–9. [DOI] [PubMed] [Google Scholar]
- Mitra S, De A, Chowdhury A, 2020. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 5. 10.21037/TGH.2019.09.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi M, Puar P, Zonouzi-Marand M, Chivers DP, Niyogi S, Kwong RWM, 2021. A comprehensive review on the neuropathophysiology of selenium. Sci. Total Environ. 767, 144329. https://doi.org/10.1016Zj.scitotenv.2020.144329 [DOI] [PubMed] [Google Scholar]
- Nseir W, Hellou E, Assy N, 2014. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J. Gastroenterol. 20, 9338–9344. 10.3748/wjg.v20.i28.9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddy WH, Herbison CE, Jacoby P, Ambrosini GL, O’Sullivan TA, Ayonrinde OT, Olynyk JK, Black LJ, Beilin LJ, Mori TA, Hands BP, Adams LA, 2013. The Western Dietary Pattern Is Prospectively Associated With Nonalcoholic Fatty Liver Disease in Adolescence. Am. J. Gastroenterol 108, 778–785. 10.1038/ajg.2013.95 [DOI] [PubMed] [Google Scholar]
- Prati D, Taioli E, Zanella A, Torre E. Della, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G, 2002. Updated Definitions of Healthy Ranges for Serum Alanine Aminotransferase Levels. Ann. Intern. Med 137, 1. 10.7326/0003-4819-137-1-200207020-00006 [DOI] [PubMed] [Google Scholar]
- Rayman MP, 2012. Selenium and human health. Lancet 379, 1256–1268. 10.1016/S0140-6736(11)61452-9 [DOI] [PubMed] [Google Scholar]
- Rayman MP, 2008. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr 100, 254–268. 10.1017/S0007114508939830 [DOI] [PubMed] [Google Scholar]
- Smith MI, Westfall BB, 1937. Further Field Studies on the Selenium Problem in Relation to Public Health. Public Heal. Reports 52, 1384. 10.2307/4582321 [DOI] [Google Scholar]
- Stranges S, Navas-Acien A, Rayman MP, Guallar E, 2010. Selenium status and cardiometabolic health: State of the evidence. Nutr. Metab. Cardiovasc. Dis 20, 754–760. 10.1016/j.numecd.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Utzschneider KM, Kahn SE, 2006. The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab 91, 4753–4761. 10.1210/jc.2006-0587 [DOI] [PubMed] [Google Scholar]
- Vernon G, Baranova A, Younossi ZM, 2011. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther 34, 274–285. 10.1111/j.1365-2036.2011.04724.x [DOI] [PubMed] [Google Scholar]
- Vinceti M, Filippini T, Rothman KJ, 2018a. Selenium exposure and the risk of type 2 diabetes: a systematic review and meta-analysis. Eur. J. Epidemiol 33, 789–810. 10.1007/s10654-018-0422-8 [DOI] [PubMed] [Google Scholar]
- Vinceti M, Filippini T, Wise LA, 2018b. Environmental Selenium and Human Health: an Update. Curr. Environ. Heal. reports 5, 464–485. 10.1007/s40572-018-0213-0 [DOI] [PubMed] [Google Scholar]
- Wang X, Ding N, Tucker KL, Weisskopf MG, Sparrow D, Hu H, Park SK, 2017. A Western Diet Pattern Is Associated with Higher Concentrations of Blood and Bone Lead among Middle-Aged and Elderly Men. J. Nutr 147, 1374–1383. 10.3945/jn.117.249060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Karvonen-Gutierrez CA, Herman WH, Mukherjee B, Harlow SD, Park SK, 2020a. Urinary metals and incident diabetes in midlife women: Study of Women’s Health Across the Nation (SWAN). BMJ Open Diabetes Res. Care 8, e001233. 10.1136/bmjdrc-2020-001233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mukherjee B, Karvonen-Gutierrez CA, Herman WH, Batterman S, Harlow SD, Park SK, 2020b. Urinary metal mixtures and longitudinal changes in glucose homeostasis: The Study of Women’s Health Across the Nation (SWAN). Environ. Int 145, 106109. https://doi.org/10.10167j.envint.2020.106109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2004. Vitamin and mineral requirements in human nutrition. World Health Organization. [Google Scholar]
- Wu J, Zeng C, Yang Z, Li X, Lei G, Xie D, Wang Y, Wei J, Yang T, 2020. Association Between Dietary Selenium Intake and the Prevalence of Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study. J. Am. Coll. Nutr 39, 103–111. 10.1080/07315724.2019.1613271 [DOI] [PubMed] [Google Scholar]
- Yang Z, Yan C, Liu G, Niu Y, Zhang W, Lu S, Li X, Zhang H, Ning G, Fan J, Qin L, Su Q, 2016. Plasma selenium levels and nonalcoholic fatty liver disease in Chinese adults: A cross-sectional analysis. Sci. Rep 6, 1–8. 10.1038/srep37288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E, 2018. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol 15, 11–20. 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Barcus M, Kim J, Lum KL, Mills C, Lei XG, 2016. High Dietary Selenium Intake Alters Lipid Metabolism and Protein Synthesis in Liver and Muscle of Pigs. J. Nutr 146, 1625–1633. 10.3945/jn.116.229955 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


