Abstract
Objectives:
To develop a reliable and valid measure of social connectedness among nursing home residents with Alzheimer’s Disease and related dementias (ADRD) using items available in the Minimum Dataset 3.0 (MDS).
Methods/Design:
We conducted a retrospective scale development study using the 2016 MDS with two populations of nursing home residents with ADRD: 1) new admissions (not post-acute care) (n=146,694); 2) residents with comprehensive annual assessments (n=294,704). Twenty-nine items were included for consideration. Psychometric evaluation included content validity, item analysis, internal consistency reliability, criterion-related validity, and exploratory factor analysis. Analyses were stratified by self- or staff-assessed pain.
Results:
The resulting 5 item social connectedness index (SCI) has good content (Fleiss Kappa = 0.67), criterion-related and construct validity and adequate internal consistency reliability (Kuder Richardson-20: 0.63– 0.74) in persons with ADRD. As anticipated, younger residents, men, and those with severe cognitive impairment, anxiety, and depression were more likely to be categorized in the low social connectedness group.
Conclusion:
The SCI is a promising measure for estimating the amount of social connectedness present for nursing home residents with ADRD. Further work needs to be done to evaluate the usefulness of the SCI for evaluating health and well-being among this population over time.
Keywords: nursing homes, social connectedness, Alzheimer’s disease, dementia
Introduction
Social connectedness is an important determinant of health and well-being.1 Social connectedness is the relationship that persons have with others in their social network, community, and immediate environment2 and includes structural, functional and qualitative aspects of personal relationships.3 Lack of social connectedness leads to isolation4,5; and loneliness6 in turn results in poor psychological well-being, health, and mobility.7 There is strong evidence that feeling socially connected to others is associated with a decreased risk for all-cause mortality and comorbidity.3
Nursing home residents are especially at risk for a lack of social connectedness7–9 and little is known about what social connectedness means for nursing home residents with Alzheimer’s Disease and related dementia (ADRD). For persons with ADRD, determining which factors signal social connectedness is often difficult to determine because the usual way of measuring social connectedness is to ask individuals about their relationship and the meaning of those relationships.1,8 This can be problematic in persons with dementia due to memory and perception impairments.10 However, there is evidence that the lack of social connectedness in persons with AD is a source of mental and psychosocial stress, which may lead to social isolation-induced anxiety and negative behaviors.10 These behaviors are observable and could be used to measure the amount of social connectedness experienced by persons with ADRD.
Therefore, we chose to examine the Minimum Data Set (MDS 3.0) for the presence of data on the observable behaviors that could indicate the presence or absence of a social connection within the nursing home environment. This paper attempts to grapple with this issue by leveraging the MDS 3.0 to develop and describe a novel scale measuring social connectedness for use in this population.
Materials and Methods
Data for this analysis come from the Minimum Dataset 3.0 (MDS), a comprehensive resident assessment instrument conducted on all nursing home residents in the United States. Research data are not shared because the data were acquired under a Data Use Agreement which prohibits sharing of data. The MDS 3.0 is performed on all nursing home residents in the United States and her territories at admission, annually thereafter, and after a significant change in status, such as a new diagnosis requiring reassessment of care planning. A somewhat streamlined assessment is also conducted on a quarterly basis. The MDS 3.0 includes by design several scales to assess resident status, including various activities of daily living scales,11,12 the Patient Health Questionnaire (PHQ-9),13 the Brief Interview of Mental Status,14 the Cognitive Performance Scale,15 and the Confusion Assessment Method, Short Form (CAM-S).16 Also, several additional scales have been developed using the items included in the MDS 3.0, such as the Agitated and Restless Behavior Scale (ARBS).17
MDS 3.0 Items Considered for Inclusion in the Social Connectedness Index
We based our approach for understanding social connectedness in nursing home residents on the ecological framework described by Cotterell, Buffel & Phillipson.4 Although all levels of this framework are important for understanding the impact of social connectedness on nursing home residents, we determined that the items routinely collected in the MDS 3.0 resident assessment would be informative only at the relational (interpersonal) and individual levels; thus we focused our search to those domains. Table 1 displays the various dimensions within these domains and the potential items considered for the development of a scale to measure social connectedness among nursing home residents. We selected items based on careful review of the MDS 3.0 instrument, the MDS manual, and in consultation with expert nurse consultants.
Table 1.
Items Considered for Social Connectedness Index After Initial Nursing Expert Review
| Concept | MDS 3.0 Item(s) and Wording |
|---|---|
| Isolation | O0100m2: Isolation or quarantine for active infectious disease in past 14 days |
| Use of Restorative Nursing | Any restorative nursing performed in the last 7 days O0500a: Passive range of motion O0500b: Active range of motion O0500c: Splint or brace assistance Training and Skill Practice in: O0500d: Bed mobility O0500e: Transfer O0500f: Walking O0500g: Dressing and/or grooming O0500h: Eating and/or swallowing O0500i: Amputation/prostheses care O0500j: Communication |
| Presence of Behavioral Symptoms | E0200a: Physical behavioral symptoms directed towards others (e.g., hitting, kicking, pushing, scratching, grabbing, abusing others sexually) E0200b: Verbal behavioral symptoms directed toward others (e.g., threatening others, screaming at others, cursing at others) E0200c: Other behavioral symptoms directed at others (e.g., physical symptoms such as hitting or scratching self, pacing, rummaging, public sexual acts, disrobing in public, throwing or smearing food or bodily wastes, or verbal/vocal symptoms like screaming, disruptive sounds) |
| Behavioral Symptoms Interfere with Social Participation | E0500c: Behavioral symptoms significantly interfere with the resident’s participation in activities or social interactions E0300: Were any behavioral symptoms in questions E0200 coded 1, 2, or 3? |
| Behavioral Symptoms Impact Others | E0600a: Behavioral symptoms put others at significant risk for physical injury E0600b: Behavioral symptoms significantly intrude on the privacy or activity of others E0600c: Behavioral symptoms significantly disrupt care or living environment E0300: Were any behavioral symptoms in questions E0200 coded 1, 2, or 3? |
| Intrusive Wandering | E1000b: Does the {resident’s} wandering significantly intrude on the privacy or activities of others? E0900: Has the resident wandered? |
| Rejection of Care | E0800: Did the resident reject evaluation of care (e.g., bloodwork, taking medications, ADL assistance) that is necessary to achieve the resident’s goals for health and well-being? |
| Prefers Group Activities | F0500e: How important is it to you to do things with groups of people? F0800p: {Staff} Resident prefers doing things with groups of people |
| Others Involved in Care Decisions | F0400f: How important is it to you to have your family or a close friend involved in discussions about your care? F0800i: {Staff} Resident prefers family or significant other involvement in care discussions |
| Prefers Spending Time Away | F0800r: {Staff} Resident prefers spending time away from the nursing home |
| Prefers Religious Participation | F0500h: How important is it to you to participate in religious activities or practices? F0800t: {Staff} Resident prefers participating in religious activities or practices |
| Presence of Depressive Symptoms | D0300: {sum of PHQ-9 symptom frequency scores} D0600: {sum of PHQ-9-OV symptom frequency scores} |
| Staff Needs Interpretation Services | A1100: Does the resident need or want an interpreter to communicate with a doctor or health care staff? |
| Vision | B1000: Ability to see in adequate light (with glasses or other visual appliances) |
| Hearing | B0200: Ability to hear (with hearing aid or hearing appliances if normally used) |
| Speech Clarity | B0600: Select best description of speech pattern |
| Makes Self Understood | B0700: Ability to express ideas and wants, consider both verbal and non-verbal expression |
| Understands Others | B0800: Understanding verbal content, however able (with hearing aid or device if used) |
| Daily Decision-Making Capacity | C1000: {Staff} Made decisions regarding tasks of daily file |
| Serious Mental Illness | A1510a: Level II Preadmission Screening and Resident Review Conditions: Serious mental illness I5900: Manic depression (bipolar disease) I5950: Psychotic disorder (other than schizophrenia) I6000: Schizophrenia (e.g., schizoaffective and schizophreniform disorders) |
| Intellectual Disability | A1510b: Level II Preadmission Screening and Resident Review Conditions: Intellectual disability A1550a: Down syndrome A1550b: Autism A1550d: Other organic condition related to ID/DD A1550e: ID/DD with no organic condition I8000a-I8000j: Intellectual disability (ICD-10: F70*-F70; ICD-9: 317*-319*) I8000a-I8000j: Pervasive developmental disorder (ICD-10: F84*; ICD-9: 299*) I8000a-I8000j: Down syndrome (ICD-10: Q90; ICD-9: 758.0*) |
| Mobility On Unit | G0110e1: {Self-performance} Locomotion on unit – how resident moves between locations in his/her room and adjacent corridor on same floor. If in wheelchair, self-sufficiency once in chair. G0110e2: {Support provided} |
| Mobility Off Unit | G0110f1: {Self-performance} Locomotion off unit – how resident moves to and returns from off-unit locations {e.g., areas set aside for dining, activities or treatments). If facility has only one floor, how resident moves to and from distant areas on the floor. If in wheelchair, self-sufficiency once in chair. G0110f2: {Support provided} |
| Urinary Continence | H0300: Urinary continence – select the one category that best describes the resident |
| Bowel Continence | H0400: Bowel continence – select the one category that best describes the resident |
| Hallucinations | E0100a: Hallucinations (perceptual experiences in the absence of real external sensory stimuli) |
Within the relational domain, Cotterell, Buffel & Phillipson4 describe four dimensions: 1) contact with family, 2) contact with friends (hereafter interpreted as contact with other residents), 3) contact with staff, and 4) conflict with others. In terms of contact with family, we identified several items: marital status and the importance of family or significant other involvement in care decisions. Because marital status itself is entwined in multiple dimensions (e.g., companionship, societal approval, likelihood of children), and can vary greatly in persons with ADRD, we elected not to develop a measure of the dimension of contact with family further.
We identified two groups of items that could potentially measure contact with other residents; Of these items, only importance of group activity and importance of participation in religious activities were retained after expert nursing review; both of these items achieved 100% agreement as very important by the content experts.
We identified a large number of items potentially indicative of contact with staff: Of these, only restorative nursing care was retained after expert nursing review. Restorative nursing care was rated as very important by 86% of the expert panel. Items related to conflict with others were identified and of these, the presence of behavioral symptoms, impact of behaviors on other residents, impact of wandering on other residents, and rejection of care were retained after initial expert nursing review, as was item E0500c – “did the identified behavioral symptoms significantly interfere with the resident’s participation in activities or social interactions?”
Within the individual domain, Cotterell, Buffel & Phillipson4,7 describe twelve dimensions: advanced age, spending significant time alone, marital status, limited finances, psychological vulnerabilities, minority status, language barriers, visual barriers, hearing barriers, speaking barriers, comprehension barriers, decision-making barriers, and the presence of physical, mental, and/or intellectual disabilities. We did not further consider advanced age for several reasons; first, in the context of a nursing home, both younger and older age may result in diminished social connectedness, but more importantly for this project, we wished to examine our measures in relation to age as a covariate in an exploratory descriptive analysis.
In terms of psychological vulnerabilities, we considered the Patient Health Questionnaire-9 (PHQ-9), an assessment of depressed mood.13 This item was retained after the initial expert nursing review. In terms of language barriers, we identified item A1100, indicating whether or not the resident’s care staff requires a translator to communicate effectively. This item was retained after initial expert nursing review. In terms of visual barriers, hearing barriers, speaking barriers, comprehension barriers, and decision-making barriers, we identified items for vision and hearing impairment, speech clarity, ability to make self understood, and ability to understand others.
In terms of the presence of physical, mental, and/or intellectual disabilities, we identified many items in this grouping (i.e., activities of daily living, intellectual disability, severe mental illness and specific medical conditions); however of these potential items, only incontinence, intellectual disability, severe mental illness, and hallucinations were retained after initial expert nursing review, as were two items relating to activities of daily living indicating support provided for locomotion on unit and off unit.
Populations
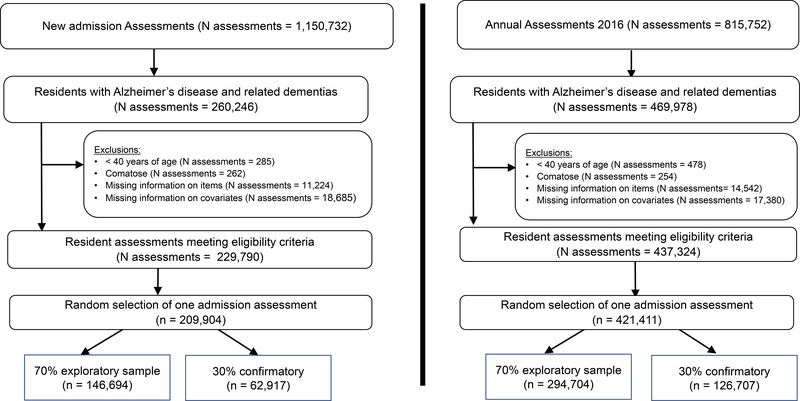
We identified two populations of nursing home residents with ADRD from 2016 using identical eligibility criteria aside from one distinction: one population is composed of comprehensive admission assessments (not post-acute care) (Figure 1, left panel), the other of comprehensive annual assessments (Figure 1, right panel). We made this distinction for several reasons. First, we believed that as nursing home staff get to know the residents, documentation and measurement may improve. Second, we believed that residents may experience greater social connectedness with extended time in the nursing home. Lastly, we believed that such an index may be helpful to assess at admission to heighten nursing home staff awareness of lack of social connectedness so that appropriate care plans may be developed to address this. Annual assessments from any anniversary were eligible for this analysis. The same resident could, theoretically, be eligible for both populations, but given that the study period is one calendar year, this is an infrequent occurrence. Exclusion criteria include resident age less than 40 years, comatose, missing data on items, and missing data on key covariates. For residents with multiple assessments, one was randomly selected. In anticipation of needing to conduct a confirmatory factor analysis, we split each population of eligible residents into an “exploratory” and a “confirmatory” data set, randomly in a 7:3 ratio. However, we only used the exploratory population as a result of the factor analysis findings described below. These exploratory populations in turn were divided into four populations on the basis of whether pain was assessed by staff (hence referred to as staff-assessed), or reported by the resident (hence referred to as self-reported) and described in the next section.
Figure 1.
Sample selection procedure for nursing home residents with Alzheimer’s disease and related dementias, 2016
Covariates
To assess comparability of the admission and annual samples, we examined the populations in terms of demographics (age, gender (men/women), race/ethnicity), cognitive impairment, physician-documented limited life expectancy, and pain assessment. Age was categorized as 40 to 64 years, 65 to 74 years, 75 to 84 years, or ≥ 85 years. Race/ethnicity was categorized as Hispanic of any race(s) (if A1000d-Hispanic or Latino was checked), or non-Hispanic of a single indicated race for Whites, Blacks, Asian, or Other (American Indians and Alaska Natives, Native Hawaiian or Pacific Islander, or any other non-Hispanic multiracial combination). Cognitive impairment was assessed using the Cognitive Function Scale, a four-point scale categorized as intact, mildly impaired, moderately impaired, or severely impaired.15 Physician-documented limited life expectancy was categorized based on item J1400-“Does the resident have a condition or chronic disease that may result in a life expectancy of less than 6 months?” Pain assessment was based on whether pain was present and pain intensity (for self-reported only).
Analytic Methods
Within each of the dimensions described above, we next considered which items could reasonably be collapsed into a coherent scale (e.g., ratings that are compatible or can be collapsed into comparable categorizations). Each item response was categorized as 0 or 1 with higher values indicating greater social connectedness. Notations in Table 1 indicate which items were carried forward for further scale development on the basis of this review. We then performed a content validity assessment. Seven experts, in the fields of nursing, medicine, social work, public health and policy related to nursing home residents, were recruited and asked to rate each of the initial 29 items on its relatedness to the concept of social connectedness in the nursing home context. Next, we performed item analysis and internal consistency reliability testing using Kuder Richardson-20 (KR-20). Then an exploratory factor analysis with varimax rotation was performed to evaluate the underlying dimensions of the new scale.
We examined the distribution of residents by age, sex, pain, cognitive function, anxiety, depression, and scores on the PHQ-9 (measure of depressive symptoms) to evaluate the criterion-related validity assumptions that younger residents, men, those with pain, severe cognitive impairment, anxiety, depression and scores ≥ 10 on the PHQ-9 would be less socially connected. The PHQ-9 cutoff of 10 has been shown to be highly specific for depression (above 0.9) in nursing home residents.18 For ease of visualizing these findings, we dichotomized the social connectedness index (SCI) so that 0–3 would equal low social connectedness and 4–5 would equal high social connectedness. We acknowledge that further research is needed to determine the appropriate categorization of the SCI into a binary variable.
Results
Content Validity
Six experts (out of seven recruited) completed the content validity review process. After initial evaluation of the items, the research team discovered that five of the items needed to be removed because they were (a) missing from the majority of assessments, (b) didn’t have both a resident- and staff-assessment correlate, or (c) needed to be combined to include both resident- and staff-assessed items, leaving 24 items to evaluate for content validity. The overall percent agreement for these 24 items was 83.6%; the Fleiss Kappa was 0.67 indicating substantial agreement.19
Description of study sample
The majority of newly admitted residents with ADRD were able to self-report pain, as were the majority of residents participating in the annual MDS assessment process. Table 2 shows that regardless of admission status (newly admitted or annual assessment), most residents were aged of ≥ 85 years, non-Hispanic White, with a life expectancy of greater than 6 months and either moderate or severe cognitive impairment. Pain was present for slightly more than half of these residents.
Table 2.
Characteristics of US nursing home residents with Alzheimer’s disease or related dementia (2016)
| Admission Assessments | Annual Assessments | |||
|---|---|---|---|---|
| Pain Self-Reported | Pain Staff-Assessed | Pain Self-Reported | Pain Staff-Assessed | |
| (N=121,534) | (N=25,160) | (N=225,758) | (N=68,946) | |
| Characteristic | Percentages | |||
| Age group (years) 40 to 64 | 5.4 | 6.0 | 6.5 | 5.7 |
| 65 to 74 | 13.5 | 13.6 | 13.2 | 11.8 |
| 75 to 84 | 34.2 | 34.2 | 28.3 | 29.5 |
| ≥85 | 46.9 | 46.2 | 52.0 | 53.1 |
| Women | 62.5 | 64.5 | 70.2 | 76.9 |
| Race/ethnicity Hispanic -any race(s) | 6.2 | 6.7 | 5.8 | 6.5 |
| Non-Hispanic White | 79.9 | 76.7 | 77.6 | 76.3 |
| Non-Hispanic Black | 11.5 | 12.9 | 14.2 | 14.2 |
| Non-Hispanic Asian | 1.7 | 2.8 | 1.7 | 2.3 |
| Non-Hispanic Other | 0.7 | 0.8 | 0.6 | 0.8 |
| Cognitive Impairment Intact/Mild | 49.7 | 13.6 | 44.9 | 6.4 |
| Moderate | 46.2 | 47.7 | 49.0 | 35.2 |
| Severe | 4.1 | 38.7 | 6.0 | 58.4 |
| Limited Life Expectancy | 4.1 | 12.6 | 2.0 | 6.1 |
| Any pain documented, Past 5 days | 58.3 | 57.3 | 55.5 | 53.9 |
| Pain Intensity, Past 5 days among those with self-assessed pain only | ||||
| None or Managed | 67.3 | 82.1 | ||
| Mild | 12.8 | 8.4 | ||
| Moderate | 14.7 | 7.3 | ||
| Severe | 5.1 | 2.3 | ||
Reliability and validity
Item analysis and reliability assessments (KR-20) were conducted for the total scale scores (24 items) for each group (i.e., newly admitted self-reported pain, newly admitted staff-assessed pain, annual assessment self-reported pain, annual assessment staff-assed pain). The corrected item to total correlations ranged from 0.008 to 0.412, with the majority being below the desired level of 0.3. The KR-20 estimates were 0.58 (admission- self), 0.59 (admission – staff), 0.59 (annual – self) and 0.57 (annual- staff), suggesting low reliability, the presence of random error and values lower than the minimally acceptable value of 0.65.20
Exploratory factor analyses with varimax rotation were conducted for each of the four samples (admission self-reported, admission staff-assessed, annual self-reported, and annual staff-assessed). All analyses provided similar results; therefore, the admission self-reported results are provided here as an illustration of these findings. The Kaiser-Meyer-Olkin (KMO) measure was 0.65, the Bartlett’s Test was statistically significant (p<.0001), and the initial factor solution suggested eight possible factors, explaining 52.9% of the variance in social connectedness. However, only the first factor, with 5 items, demonstrated substantial factor loadings (all >0.4) and adequate reliability (items shaded in Table 1); the remaining factors were spurious or demonstrated numerous cross-loadings and had poor reliability estimates (all KR-20 values <0.5). Several versions of exploratory factor analysis were then conducted, including 4 and 6 factor solutions, as suggested by the scree plot; all analyses resulted in similar findings. However, the exploratory factor analysis consistently suggested that one group of items worked well together for all 4 samples; these items measured behavioral conflict with others and included: (1) presence of behavioral acting out symptoms, (2) presence of behavioral symptoms interfering with social interactions or activities, (3) negative behavior impacting others, (4) intrusive wandering behavior and (5) rejection of attempts to provide care. Items 1 and 3 are composites of 3 items in the MDS 3.0. The reliability assessments of these 5 items ranged between 0.63 and 0.74, with the admission assessments demonstrating higher reliability estimates (Table 3).
Table 3.
Standardized factor loadings, percent of variance explained and Cronbach’s alpha for the social connectedness index items for residents with Alzheimer’s Disease or related dementia
| Admission Assessments | Annual Assessments | |||
|---|---|---|---|---|
| Pain Self-Assessed | Pain Staff-Assessed | Pain Self-Assessed | Pain Staff-Assessed | |
| Behavioral Symptoms | 0.79 | 0.79 | 0.76 | 0.77 |
| Behaviors interfere with social Interactions | 0.76 | 0.76 | 0.77 | 0.76 |
| Behaviors impact others | 0.84 | 0.84 | 0.83 | 0.83 |
| Wandering intrudes on others | 0.44 | 0.46 | 0.34 | 0.40 |
| Resident rejects care | 0.59 | 0.65 | 0.49 | 0.55 |
| Percent of Variance Explained | 48.9 | 51.5 | 44.3 | 46.3 |
| Cronbach’s Alpha | 0.70 | 0.74 | 0.63 | 0.67 |
As anticipated, younger residents, men, and those with severe cognitive impairment, anxiety, depression and depressive symptoms were more likely to be categorized in the low social connectedness group (Table 4). For example, among newly admitted residents with staff-assessed pain, 29.2% of those with current depressive symptoms (PHQ ≥10) and 17.4% of residents without depressive symptoms had SCI scores between 0 and 3 (PHQ <10). Similar patterns were observed on annual assessments.
Table 4.
Low social connectedness index scores by age group, sex, pain, cognitive function, anxiety diagnosis, depression diagnosis, depressive symptoms (PHQ-9/PHQ-9OV)
| Admission Assessments | Annual Assessments | ||||
|---|---|---|---|---|---|
| Social connectedness index 0–3 = low | Pain Self-reported | Pain Staff-Assessed | Pain Self-reported | Pain Staff-Assessed | |
| Characteristic | percentage | ||||
| Age group (years) | 40 to 64 | 11.4 | 22.7 | 10.5 | 14.0 |
| 65 to 74 | 10.1 | 20.6 | 8.9 | 12.6 | |
| 75 to 84 | 9.0 | 19.2 | 7.7 | 12.2 | |
| ≥85 | 7.9 | 16.4 | 6.7 | 11.9 | |
| Gender | Women | 7.9 | 16.8 | 7.0 | 11.4 |
| Men | 10.2 | 21.1 | 8.7 | 14.6 | |
| Any Pain | No | 9.5 | 17.6 | 7.4 | 10.0 |
| Yes | 8.2 | 18.9 | 7.6 | 14.0 | |
| Cognitive Impairment | Intact/Mild | 4.7 | 9.2 | 4.7 | 7.0 |
| Moderate | 12.0 | 20.0 | 9.3 | 14.4 | |
| Severe | 20.9 | 19.5 | 14.0 | 11.4 | |
| Anxiety Diagnosis | No | 7.5 | 15.6 | 6.3 | 9.9 |
| Yes | 12.7 | 25.1 | 10.0 | 16.5 | |
| Depression Diagnosis | No | 8.6 | 17.2 | 7.0 | 10.4 |
| Yes | 9.1 | 20.2 | 7.9 | 13.9 | |
| Current Depression Symptoms | No (score <10) | 8.3 | 17.4 | 7.0 | 11.5 |
| Yes (score ≥10) | 14.9 | 29.2 | 15.6 | 21.1 | |
Discussion
The findings suggest that data measuring residents’ conflict with others was the most salient indicator (as opposed to contacts) of social connectedness in this sample. This is consistent with Buckley and McCarthy’s1 finding that many of the contacts with other nursing home residents were considered superficial and lacked connection. Thus, we propose that observable behaviors, and not number of contacts, are most important for evaluating social connectedness among nursing home residents with ADRD.
Social connectedness in nursing home residents is understudied among residents with ADRD. Residents on dementia-specific units have social networks much smaller than other residents.21 Residents with dementia are often willing conversationalists, especially with prompting to foster between-resident communication.22 For residents on dementia special care units, positive social integration was associated with improved quality of life.23 The same may not hold for residents without dementia. In a qualitative research study with residents without dementia, expectations and capacity to interact with peer residents varied, and some residents noted that personal relationships were not essential to “thrive”.24 Closer social bonds may occur between residents with similar degrees of cognitive function.23 This warrants further study as many studies to date have included small samples and may be restricted to very few nursing homes.
The availability of the SCI using existing data resources such as the MDS 3.0, opens future avenues for research. Understanding the extent of social connectedness in nursing homes and how it varies across nursing home context may provide insights to inform nursing home care practices. For example, one small study showed that the development of social relationships may be hindered by the tendency of a nursing home to place residents in groups with similar conditions and needs.25 Whether such findings hold with large-scale studies needs to be examined.
We were able to derive the SCI using existing items in the MDS 3.0. Although the SCI contains several items similar to the Agitated and Reactive Behavior Scale,17 it differs in several important ways. First, the SCI includes E0500c (do the behaviors interfere with the resident’s participation in activities or social interactions?), E0600 (do the behaviors have a negative impact on others?), and E1000b (do wandering behaviors intrude on the privacy or activities of others?); items which attempt to measure whether the observable behaviors interfere with the resident’s social connectedness with others. Second, the SCI is less concerned about the presence of individual behaviors (thus, combining E0200 a, b, and c into a composite item indicating whether any observed negative behaviors that may reflect isolation and distress are occurring). The focus of the SCI is not on the behaviors themselves, but what these observables mean for the resident’s social connection to others within the nursing home environment.
We hypothesized that MDS items indicative of rrelationships with staff would be an essential component of the index. This was not supported by our analyses. Such relationships occur as an unintentional consequence of clinical interactions, and the nature of these interactions strongly influence the degree to which residents perceive these relationships as friendly or unfriendly.26 Relationships between residents and staff are often oriented around strategies to meet needs and avoid conflict, but dialog and active listening can result in deeper caring relationships.27 Staff intentionality about developing relationships, such as acknowledging residents as special or providing “extras”, fostered more positive relationships with residents.28 Reasons why inclusion of these items was not supported by the data may be a reflection of the guidance the MDS manual provides (e.g., for restorative care) and how homes choose to deliver the care), measurement issues, or simply that the items were not consistent with the underlying construct. We were unable to explore explanations for this with the secondary data sources available.
The strength of this study is the use of a large national dataset of nursing home residents in the United States. Study limitations include lack of data on other aspects of social connectedness such as loneliness or social support. We were restricted to items available in the MDS 3.0. MDS items may be prone to misclassification. However, many nursing homes have dedicated staff trained in the completion of the MDS 3.0. In previous version of the MDS, there were items that potentially measured social connection, it would be helpful if future versions of the MDS consider either reinstating these items or adding contemporary items that measure social connectedness or isolation in nursing home residents.
In addition, future research needs to be done to examine the relationship between SCI scores and interviews or observation of social connection in this population. Finally, the SCI includes only one domain from the ecological framework – conflict with others. Further work is needed, perhaps collecting original data, to capture the other domains of social connectedness implied by this framework.
Conclusion
Nursing homes are an important site of care during life’s final chapter for persons with ADRD.29–31 There is a need to understand facets of residents’ lives, such as social connectedness, that impact their quality of life, mortality, and other important health outcomes. Five items from the MDS 3.0 make up a novel social connectedness index that could be used to explore the impact of social connectedness in nursing home residents.
Key Points:
Social connectedness is an important determinant of health and well-being among nursing home residents;
Little is known about social connectedness in nursing home residents with Alzheimer’s Disease and related dementias;
The Minimum Data Set 3.0 is a vital source of data that can be leveraged to measure social connectedness among a national sample of nursing home residents with Alzheimer’s Disease and related dementias;
The Social Connectedness Index (SCI) has adequate reliability, and good content, criterion-related, and construct validity for use in this population.
Acknowledgments
Funding:
This work was funded by the National Institutes of Nursing Research (NR016977 grant to Dr. Lapane).
Sources of funding:
National Institutes of Health (NIH) National Institute on Aging (NIA) 1R01NR019677 (PI: Kate Lapane).
Conflicts of interest: Dr. Lapane reports grants from the National Institutes of Health during the conduct of the study. For the remaining authors none were declared.
Footnotes
Institutional Review Board (IRB): The University of Massachusetts Medical School IRB approved this study.
Data Availability Statement:
The data that support the findings of this study are available from the Centers for Medicare and Medicaid Services. Restrictions apply to the availability of these data, which were used under a data use agreement for this study. Data are available from www.resdac.org with the permission of the Centers for Medicare and Medicaid Services.
References
- 1.Buckley C, McCarthy G. An exploration of social connectedness as perceived by older adults in a long-term care setting in Ireland. Geriatr Nurs. 2009;30(1528–3984 (Electronic)):390–396. [DOI] [PubMed] [Google Scholar]
- 2.Poey J, Burr J, Roberts J. Social Connectedness, Perceived Isolation, and Dementia: Does the Social Environment Moderate the Relationship Between Genetic Risk and Cognitive Well-Being? The Gerontologist. 2017;6(1758–5341 (Electronic)):1031–1040. [DOI] [PubMed] [Google Scholar]
- 3.Holt-Lunstad J, Robles T, Sbarra D. Advancing social connection as a public health priority in the United States. Am Psychol. 2017;72(1935–990X (Electronic)):517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotterell N, Buffel T, Phillipson C. Preventing social isolation in older people. Maturitas. 2018;113(1873–4111 (Electronic)):80–84. [DOI] [PubMed] [Google Scholar]
- 5.Zavaleta D, Samuel K, Mills C. Social isolation: A conceptual and measurement proposal. OPHI Working Paper No. 67. https://www.ophi.org.uk/wp-content/uploads/ophi-wp-67.pdf. Published 2015. Accessed April 1, 2020.
- 6.O’Rourke H, Collins L, Sidani S. Interventions to address social connectedness and loneliness for older adults: a scoping review. BMC Geriatrics. 2018;18(1471–2318 (Electronic)):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansson A, Muurinen S, Savikko N, et al. Loneliness in nursing homes and assisted living facilities: Prevalence, associated factors and prognosis. Jour Nursing Home Res. 2017;3:43–49. [Google Scholar]
- 8.Paque K, Bastiaens H, Van Bogaert P, Dilles T. Living in a nursing home: a phenomenological study exploring residents’ loneliness and other feelings. Scand J Caring Sci. 2018;32(1471–6712 (Electronic)):1477–1484. [DOI] [PubMed] [Google Scholar]
- 9.Trybusińska D, Saracen A. Loneliness in the Context of Quality of Life of Nursing Home Residents. Open Med. 2019;14(2391–5463 (Print)):354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsiao Y, Chang C, Gean P. Impact of social relationships on Alzheimer’s memory impairment: mechanistic studies. J of Biomed Sci. 2018;25(1423–0127 (Electronic)):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long-term care facility resident assessment instrument 3.0 user’s manual: version 1.16. Centers for Medicare and Medicaid Services (CMS). Web site. https://downloads.cms.gov/files/1-MDS-30-RAI-Manual-v1-16-October-1-2018.pdf. Published 2018. Accessed 1.14.
- 12.Morris J, Fries B, Morris S. Scaling ADLs Within the MDS. J Gerontol A Biol Sci Med Sci 1999;54A:M546–M553. [DOI] [PubMed] [Google Scholar]
- 13.Kroenke K, Spitzer R, Williams J. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(0884–8734 (Print)):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saliba D, Buchanan J, Edelen M, et al. MDS 3.0: brief interview for mental status. J Am Med Dir Assoc. 2012;13(1538–9375 (Electronic)):611–617. [DOI] [PubMed] [Google Scholar]
- 15.Thomas K, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. 55. 2017;9:e68–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inouye S The Short Confusion Assessment Method (Short CAM): Training Manual and Coding Guide. Hospital Elder Life Program Web site. https://www.hospitalelderlifeprogram.org/uploads/disclaimers/Short_CAM_Training_Manual_8-29-14.pdf.. Published 2003. Accessed Downloaded April 15, 2020.
- 17.McCreedy E, Ogarek J, Thomas K, Mor V. The Minimum Data Set Agitated and Reactive Behavior Scale: Measuring Behaviors in Nursing Home Residents With Dementia. J Am Med Dir Assoc. 2019;20(1538–9375 (Electronic)):1548–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bélanger E, Thomas K, Jones R, Epstein-Lubow G, Mor V. Measurement validity of the Patient-Health Questionnaire-9 in US nursing home residents. Int J Geriatr Psychiatry. 2019;34(1099–1166 (Electronic)):700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.J. L, Koch G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(0006–341X (Print)):159–174. [PubMed] [Google Scholar]
- 20.DeVellis R Scale development: Theory and Applications. Fourth ed ed: Thousand Oaks: Sage; 2017. [Google Scholar]
- 21.Casey A, Low L, Jeon Y, Brodaty H. Residents Perceptions of Friendship and Positive Social Networks Within a Nursing Home. Gerontologist 2016;56(1758–5341 (Electronic)):855–867. [DOI] [PubMed] [Google Scholar]
- 22.Mok Z, Müller N. Staging casual conversations for people with dementia. Dementia. 2014;13(1741–2684 (Electronic)):834–853. [DOI] [PubMed] [Google Scholar]
- 23.Abbott K, Pachucki M. Associations between social network characteristics, cognitive function, and quality of life among residents in a dementia special care unit: A pilot study. Dementia. 2017;16:1004–1019. [DOI] [PubMed] [Google Scholar]
- 24.Bergland Å, Kirkevold M. The significance of peer relationships to thriving in nursing homes. J Clin Nurs. 2008;17:1295–1302. [DOI] [PubMed] [Google Scholar]
- 25.Bonifas R, Simons K, Biel B, Kramer C. Aging and place in long-terms care settings: influence on social relationships. J Aging Health 2014;26:1320–1339. [DOI] [PubMed] [Google Scholar]
- 26.Roberts T, Bowers B. How nursing home residents develop relationships with peers and staff: A grounded theory study. Int J Nurs Stud. 2015(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palacios-Ceña D, Losa-Iglesias M, Gómez-Calero C, Cachón-Pérez J, Brea-Rivero M, Fernández-de-las-Peñas. A qualitative study of the relationships between residents and nursing home nurses.. J Clin Nurs 2014;23:550–559. [DOI] [PubMed] [Google Scholar]
- 28.Roberts T Nursing home resident relationship types: What supports close relationships with peers & staff?. J Clin Nurs. 2018;27:4361–4372. [DOI] [PubMed] [Google Scholar]
- 29.Arrighi H, Neumann P, Lieberburg I, Townsend R. Lethality of Alzheimer disease and its impact on nursing home placement.. Alzheimer Dis Assoc Disord. 2010;24:90–95. [DOI] [PubMed] [Google Scholar]
- 30.Taylor C, Greenlund S, McGuire L, Lu H, Croft J. Deaths from Alzheimer’s Disease — United States, 1999–2014.. MMWR 2017;66:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zissimopoulos J, Crimmins E, St Clair P. The Value of Delaying Alzheimer’s Disease Onset. Forum Health Econ Policy. Forum Health Econ Policy. 2014;18:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the Centers for Medicare and Medicaid Services. Restrictions apply to the availability of these data, which were used under a data use agreement for this study. Data are available from www.resdac.org with the permission of the Centers for Medicare and Medicaid Services.