Abstract
The aim of the present study was to evaluate the association between hyperuricemia and osteoporosis in a Korean population. Data from participants of the Korean Genome and Epidemiology Study who were ≥ 40 years old were collected from 2004 to 2016. Among 173,209 participants, 11,781 with hyperuricemia (> 7.0 mg/dL in men and > 6.0 mg/dL in women) and 156,580 controls were selected based on serum measurements. Odds ratios (ORs) of osteoporosis between individuals with hyperuricemia and controls were analyzed using a logistic regression model. In the adjusted model, age, sex, income group, body mass index, smoking, alcohol consumption, hypertension, diabetes mellitus, hyperlipidemia history and nutritional intake were adjusted. The adjusted OR (aOR) of osteoporosis was 0.79 [95% confidence interval (CI) = 0.71–0.87, P < 0.001]. In subgroup analyses according to age and sex, statistical significance was observed in men > 60 years old and in women > 50 years old. In another subgroup analysis according to past medical history, significant differences were found according to hypertension (aOR = 0.83, 95% CI = 0.73–0.94, and 0.75, 95% CI = 0.64–0.87), diabetes mellitus (aOR = 0.77, 95% CI = 0.69–0.86), and hyperlipidemia (aOR = 0.74, 95% CI = 0.61–0.89, and 0.81, 95% CI = 0.72–0.91). This study demonstrated that hyperuricemia was associated with a decreased risk of osteoporosis.
Subject terms: Endocrinology, Medical research, Risk factors
Introduction
Hyperuricemia refers to elevated uric acid in the blood. Hyperuricemia that is caused by the overproduction of urate or, more commonly, by renal urate underexcretion is necessary but not sufficient to cause gout1. The prevalence of hyperuricemia has increased slightly, from 19.1% between 1988 and 1994 to 21.5% between 2007 and 2008 in the National Health and Nutrition Examination Survey (NHANES)2. Approximately 47.1 million adults in the US met the sex-specific criteria for hyperuricemia from 2015 to 20163. With increasing levels of hyperuricemia, there were graded increases in the prevalence of comorbidities4.
Osteoporosis is a metabolic bone disorder characterized by low bone mineral density (BMD) and increased skeletal fragility. Osteoporosis is common in the elderly population, and the prevalence of osteoporosis in individuals aged ≥ 50 years is estimated to be 16.0% in men and 29.9% in women in the United States5. Secondary causes of osteoporosis include chronic treatment with glucocorticoids, gastrointestinal disorders, diabetes mellitus, rheumatoid arthritis, liver disease, gluten enteropathy, multiple myeloma and other hematologic disorders6.
There has been an increase in the evidence supporting the favorable and protective effect of higher uric acid on bone metabolism in the past decade7–18. Recent studies revealed a positive relationship between uric acid and lumbar BMD among most adolescents and old adults19,20. Meta-analysis showed that subjects with hyperuricemia had significantly higher BMD values in the spine [standardized mean differences (SMD) 0.29, 95% confidence intervals (CI) 0.22–0.35], total hip (SMD 0.29, 95% CI 0.24–0.34) and femoral neck (SMD 0.25, 95% CI 0.74–0.92)21. However, Zhang et al. found that serum uric acid was independent of BMD after adjusting for confounding variables and applying multivariate analysis in a sample of 6579 American adult individuals22. Furthermore, Mendelian randomization analysis did not support a causal association between uric acid level and total femur (β = − 0.29), femoral neck (β = − 0.27), and spine (β = 0.08) BMD in people from the United States23; lumbar spine (β = 0.385), hip (β = 0.191), or femoral neck (β = 0.194) BMD in Chinese people24; or lumbar spine (β = − 0.700) BMD in Korean people25.
Although previous studies support a protective role for uric acid in bone metabolism disorders, the majority of the studies were conducted in a single institution10,26, restricted to a single sex14, limited to old participants8,9 or the sample sizes were small7,11. No study stratified the groups by sex and age, and no study performed subgroup analyses according to past medical histories. The aim of this study was to evaluate the association between hyperuricemia and osteoporosis in a nationwide population-based cross-sectional study with a significantly larger population than before. Blood tests were also performed on all asymptomatic people, and hyperuricemia was also found among those who would not have visited the hospital. In this study, we estimated the odds ratios (ORs) of osteoporosis in hyperuricemia patients compared to controls.
Materials and methods
Study population and data collection
The Institutional Review Board and Ethics Committee of Hallym University Sacred Heart Hospital (IRB No. 2019-02-020) approved the use of these data. The requirement for written informed consent was waived by the Institutional Review Board and Ethics Committee of Hallym University Sacred Heart Hospital. All methods were performed in accordance with the relevant guidelines and regulations. This prospective cross-sectional study relied on data from the Korean Genome and Epidemiology Study (KoGES) from 2004 through 2016. A detailed description of these data was provided in a previous study27. Among the KoGES Consortium, we used KoGES health examinee (HEXA) data consisting of participants living in urban areas who were ≥ 40 years old. The data consisted of the baseline data from 2004 to 2013 and follow-up data from 2012 to 2016.
Participant selection
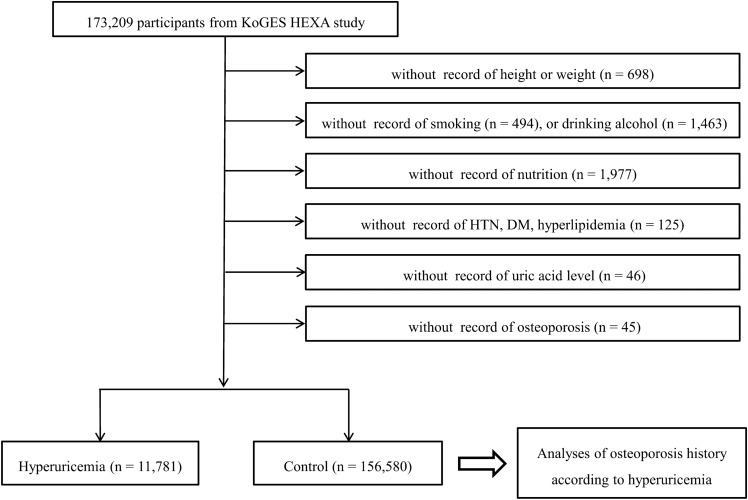
Among 173,209 participants, we excluded participants who lacked records of height or weight (n = 698), smoking history (n = 494), alcohol consumption habits (n = 1,463), nutrition (n = 1,977), hypertension, diabetes mellitus, and hyperlipidemia history (n = 125), uric acid measurements (n = 46), and osteoporosis history (n = 45). Finally, 11,781 hyperuricemia and 156,580 control (nonhyperuricemia) participants were selected (Fig. 1). Then, we analyzed the histories of osteoporosis among the hyperuricemia and control participants.
Figure 1.
A schematic illustration of the participant selection process used in the present study. Of a total of 173,209 participants, 11,781 hyperuricemia patients and 156,580 control participants were included.
Survey
The participants were asked about their previous histories of hypertension, diabetes mellitus, hyperlipidemia, and osteoporosis by trained interviewers. We defined hyperuricemia as uric acid > 7.0 mg/dL in men2 and > 6.0 mg/dL in women28 following previous studies. Body mass index (BMI) was calculated as kg/m2 using the health checkup data. Smoking history was categorized as nonsmokers (< 100 cigarettes over their entire life), past smokers (quit more than one year ago), and current smokers. Participants were categorized according to their alcohol consumption habits as nondrinkers, past drinkers, and current drinkers. Their nutritional intake [total calories (kcal/day), protein (g/day), fat (g/day), carbohydrates (g/day), calcium (mg/d), and phosphorus (mg/d)] was surveyed by a food-frequency questionnaire that was validated by a previous study29. The income group was categorized as nonrespondent, low income (< ~ $2000 per month), middle income (~ $2000–$3999 per month), and high income (~ ≥ $4000 per month) by their household income.
Statistical analyses
Chi-square tests were used to compare the rates of sex, income group, smoking, alcohol consumption, and hypertension, diabetes mellitus, and hyperlipidemia history30. Independent t-tests were used to compare age, BMI, and nutritional intake7.
To analyze the OR of hyperuricemia for osteoporosis, a logistic regression model was used13. Crude and adjusted models [age, sex, income group, BMI, smoking, alcohol consumption, hypertension, diabetes mellitus, and hyperlipidemia history and nutritional intake (total calorie, protein, fat, carbohydrate, calcium, and phosphorous intake)] were used31. In the subgroup analyses according to age and the sex, we divided age into 10-year increments.
Two-tailed analyses were conducted, and P values less than 0.05 were considered to indicate significance30. The results were statistically analyzed using SPSS v. 24.0 (IBM, Armonk, NY, USA).
Results
The general characteristics of participants were different between hyperuricemia and control participants (Table 1).
Table 1.
General characteristics of participants.
| Characteristics | Total participants | P value | |
|---|---|---|---|
| Hyperuricemia | Control | ||
| Age (mean, SD, y) | 54.9 (8.7) | 53.0 (8.3) | < 0.001* |
| Sex (n, %) | < 0.001* | ||
| Men | 7775 (66.0) | 50,002 (31.9) | |
| Women | 4006 (34.0) | 106,578 (68.1) | |
| BMI (mean, SD, kg/m2) | 25.5 (3.0) | 23.8 (2.9) | < 0.001* |
| Income (n, %) | < 0.001* | ||
| Missing, no response | 2086 (17.7) | 24,549 (15.7) | |
| Lowest | 3240 (27.5) | 42,806 (27.3) | |
| Middle | 4018 (34.1) | 56,589 (36.1) | |
| Highest | 2437 (20.7) | 32,636 (20.8) | |
| Smoking status (n, %) | < 0.001* | ||
| Nonsmoker | 5774 (49.0) | 116,888 (74.7) | |
| Past smoker | 3507 (29.8) | 21,218 (13.6) | |
| Current smoker | 2500 (21.2) | 18,474 (11.8) | |
| Alcohol consumption (n, %) | < 0.001* | ||
| Non drinker | 4,000 (34.0) | 81,519 (52.1) | |
| Past drinker | 734 (6.2) | 5,899 (3.8) | |
| Current drinker | 7047 (59.8) | 69,162 (44.2) | |
| Hypertension | 4771 (40.5) | 32,706 (20.9) | < 0.001* |
| Diabetes mellitus | 1240 (10.5) | 12,049 (7.7) | < 0.001* |
| Hyperlipidemia | 2037 (17.3) | 20,156 (12.9) | < 0.001* |
| Nutritional intake | |||
| Total calories (kcal/d) | 1786.6 (595.9) | 1756.7 (590.3) | < 0.001* |
| Protein (g/d) | 61.1 (27.6) | 59.8 (27.2) | < 0.001* |
| Fat (g/d) | 29.0 (19.0) | 28.1 (18.7) | < 0.001* |
| Carbohydrate (g/d) | 315.5 (96.6) | 312.0 (96.5) | < 0.001* |
| Calcium (mg/d) | 431.9 (262.3) | 453.5 (276.9) | < 0.001* |
| Phosphorus (mg/d) | 902.4 (375.6) | 900.5 (379.5) | 0.600 |
| Osteoporosis (n, %) | 502 (4.3) | 11,020 (7.0) | < 0.001* |
*Independent T-test or Chi-square test. Significance at P < 0.05.
The adjusted OR (aOR) of hyperuricemia for osteoporosis was 0.79 [95% confidence interval (CI) = 0.71–0.87, P < 0.001, Table 2]. In subgroup analyses, the finding was consistent only in older women. The aORs were 0.86 (95% CI = 0.41–1.82) in ≤ 50-year-old men, 1.18 (95% CI = 0.80–1.72) in ≤ 50-year-old women, 1.08 (95% CI = 0.68–1.71) in 51–60 years old men, 0.76 women (95% CI = 0.64–0.90, P = 0.002) in 51–60 years old, 0.60 (95% CI = 0.38–0.94, P = 0.026) in > 60-year-old men, and 0.79 (95% CI = 0.69–0.91, P = 0.001) in > 60-year-old women. Statistical significance was observed in men > 60 years old and in women > 50 years old.
Table 2.
Crude and adjusted odd ratios (95% confidence interval) of hyperuricemia for osteoporosis.
| Characteristics | Odd ratios for osteoporosis | |||
|---|---|---|---|---|
| Crude | P value | Adjusted† | P value | |
| Total participants (n = 168,361) | ||||
| Hyperuricemia | 0.59 (0.54–0.64) | < 0.001* | 0.79 (0.71–0.87) | < 0.001* |
| Control | 1.00 | 1.00 | ||
| Age ≤ 52 years old, men (n = 26,485) | ||||
| Hyperuricemia | 0.62 (0.30–1.29) | 0.203 | 0.67 (0.32–1.39) | 0.282 |
| Control | 1.00 | 1.00 | ||
| Age ≤ 52 years old, women (n = 57,795) | ||||
| Hyperuricemia | 1.14 (0.85–1.53) | 0.370 | 1.01 (0.75–1.37) | 0.935 |
| Control | 1.00 | 1.00 | ||
| Age ≥ 53 years old, men (n = 31,292) | ||||
| Hyperuricemia | 0.77 (0.56–1.06) | 0.104 | 0.81 (0.59–1.12) | 0.198 |
| Control | 1.00 | 1.00 | ||
| Age ≥ 53 years old, women (n = 52,789) | ||||
| Hyperuricemia | 0.80 (0.72–0.90) | < 0.001* | 0.77 (0.69–0.86) | < 0.001* |
| Control | 1.00 | 1.00 | ||
*Logistic regression model, Significance at P < 0.05.
†Models adjusted for age, sex, income group, BMI, smoking, alcohol consumption, hypertension, diabetes mellitus, hyperlipidemia histories and nutritional intake (total calories, protein, fat, carbohydrate intake, calcium, and phosphorous intake).
In another subgroup analysis according to the past medical histories of hypertension, diabetes mellitus, and hyperlipidemia, the results showed statistical significance except for in the participant with diabetes mellitus (Table 3).
Table 3.
Crude and adjusted odd ratios (95% confidence interval) of hyperuricemia for osteoporosis according their past medical histories.
| Characteristics | Odd ratios for osteoporosis | |||
|---|---|---|---|---|
| Crude | P value | Adjusted† | P value | |
| With hypertension (n = 37,477) | ||||
| Hyperuricemia | 0.59 (0.53–0.67) | < 0.001* | 0.83 (0.73–0.94) | 0.005* |
| Control | 1.00 | 1.00 | ||
| Without hypertension (n = 130,884) | ||||
| Hyperuricemia | 0.45 (0.39–0.51) | < 0.001* | 0.75 (0.64–0.87) | < 0.001* |
| Control | 1.00 | 1.00 | ||
| With diabetes mellitus (n = 13,289) | ||||
| Hyperuricemia | 0.83 (0.66–1.04) | 0.106 | 0.88 (0.69–1.13) | 0.315 |
| Control | 1.00 | 1.00 | ||
| Without diabetes mellitus (n = 155,072) | ||||
| Hyperuricemia | 0.55 (0.50–0.61) | < 0.001* | 0.77 (0.69–0.86) | < 0.001* |
| Control | 1.00 | 1.00 | ||
| With hyperlipidemia (n = 22,193) | ||||
| Hyperuricemia | 0.48 (0.41–0.57) | < 0.001* | 0.74 (0.61–0.89) | 0.002* |
| Control | 1.00 | 1.00 | ||
| Without hyperlipidemia (n = 146,168) | ||||
| Hyperuricemia | 0.59 (0.53–0.66) | < 0.001* | 0.81 (0.72–0.91) | < 0.001* |
| Control | 1.00 | 1.00 | ||
*Logistic regression model, Significance at P < 0.05.
†Models adjusted for age, sex, income group, BMI, smoking, alcohol consumption, hypertension, diabetes mellitus, hyperlipidemia histories and nutritional intake (total calories, protein, fat, carbohydrate intake, calcium, and phosphorous intake).
Discussion
In our study, the OR for osteoporosis in the hyperuricemia group was lower than that in the control group (aOR 0.79, 95% CI = 0.71–0.87). The strengths of this study are that it is the largest study investigating risk factors in not only subjects with hyperuricemia but also controls with normal serum uric acid levels. This cross-sectional study included asymptomatic participants regardless of their past medical history, possibly revealing undiagnosed hyperuricemia patients. Additionally, the inclusion of a sufficient number of participants allowed us to define hyperuricemia with > 7.0 mg/dL in men and > 6.0 mg/dL in women, which are clinically meaningful reference values.
In our study, the prevalence of hyperuricemia was 7.5% (13.5% in men and 3.8% in women), which was slightly lower than the 11.4% (17.0% in men and 5.9% in women) identified in a sample of 5,548 individuals from the general Korean population32 and much lower than that of the general American population (21.4% from 2007 to 2008)2. Our study included participants ≥ 40 years old, and a previous report included participants ≥ 19 years old32. As the prevalence was high in individuals under 40 years old in both sexes in a previous report32, age was probably a major contributor to the differences in prevalence rates.
Since elderly people have the coexistence of common conditions of hypertension, diabetes mellitus and hyperlipidemia, these conditions may confound the association between hyperuricemia and osteoporosis. To our knowledge, this is the first study that has analyzed subgroups in association with hyperuricemia and osteoporosis. Many studies have reported that smoking30, alcohol consumption33 and nutritional intake34 are factors influencing bone metabolism. In our study, there were still significant findings after adjusting for confounders.
To our knowledge, our study had the largest sample with 11,781 hyperuricemia and 156,580 control participants, followed by 607 hyperuricemia and 4,592 control participants in the study by Yan et al.13. Most previous studies analyzed the association between serum uric acid levels and BMD rather than evaluating the association between hyperuricemia and osteoporosis. In recent studies, the categories of serum uric acid were not described7,26,35, and the authors divided participants according to their median serum uric acid level11, tertiles8, quantiles15,20 or quintiles10. Few studies have included subgroups with serum uric acid levels > 7 mg/dL, indicating hyperuricemia9,10, but the subgroup was as small as 25/10809 and the participants were limited to men aged 40 to 60 years10.
The aOR of 0.79 was lower than that for females reported in a preceding study conducted with a Chinese cohort (aOR 0.874, 95% CI = 0.824–0.928)13. We believe that these differences may be due to the study design and included participants. We excluded participants who were younger than 40 years at the time of the diagnosis of hyperuricemia and divided age into 10-year increments. However, a previous Chinese study included participants of all ages and did not divide groups by age13. In our study, we used an adjusted model that adjusted not only for age but also for sex, income group, BMI, smoking, alcohol consumption, hypertension, diabetes mellitus, hyperlipidemia and nutritional intake. Four more studies presented results with ORs15–18 but used the median or quartiles as subgroups rather than the hyperuricemia cutoff. In another matched cohort study (with 36,458 with gout and 71,602 without gout), the gout cohort showed a 1.2-fold increase in the incidence of osteoporosis (adjusted hazard ratio 1.2, 95% CI = 1.06–1.35)36. However, since the study examined the medical claim of gout, the exact level of uric acid is unknown.
According to past medical history, hyperuricemia participants with diabetes mellitus did not show a statistically significant decrease in OR in the present study. Type 2 diabetes mellitus affects bone metabolism and strength in a direct way, and certain antidiabetic medications affect bone metabolism37. Hyperuricemia can aggravate the progression of diabetes and lead to bone fragility; thus, hyperuricemia can indirectly accelerate bone loss in type 2 diabetes patients38. In a study with Chinese type 2 diabetes mellitus patients, the association of uric acid with osteoporosis varied by sex, BMI and skeletal site39. In their study, multiple linear regression analysis demonstrated a significant association between serum uric acid levels and BMD in the BMI < 25 group (beta: 0.146 to 0.218). Further investigations are needed to explain this issue.
In the subgroup analysis in our study, the OR of hyperuricemia for osteoporosis was significantly lower than that of the control group in older men (> 60 years) and women (> 50 years). Statistically significant findings were found for men and women of different age groups. The prevalence of hyperuricemia and osteoporosis differs by age and sex distribution; the prevalence of hyperuricemia is higher in men than in women32, and osteoporosis is more prevalent in older women5. This association indicates that hyperuricemia may have a protective effect against osteoporosis in older women. Uric acid levels increase more significantly in postmenopausal females than in males40. It was hypothesized that the antioxidant effect of uric acid antagonizes oxidative stress-induced bone metabolism12. Although a positive association between uric acid and BMD was observed in some studies with males10,18, the association between hyperuricemia and osteoporosis was not studied in their studies.
Previously, the association between hyperuricemia and osteoporosis was not fully understood pathologically, while some plausible explanations may be possible. At the physiological level, uric acid is considered an important endogenous antioxidant that scavenges reactive oxygen species (ROS) and mitigates cellular and vascular damage caused by oxidative stress41. Due to its antioxidant properties, uric acid may inhibit osteoclastic bone resorption and contribute to higher BMD42. In an in vitro study with mice, uric acid significantly suppressed osteoclastogenesis in a dose-dependent manner by reducing ROS production in osteoclast-precursor cells17. Additionally, a previous study found a significant positive correlation between serum 25(OH)D and serum uric acid levels18. However, uric acid also promotes oxidative stress by superoxide free radicals produced via NADPH oxidase (NOX4), and the imbalance between oxidative stress and antioxidation affects bone remodeling and causes osteoporosis43.
The present study has several limitations. First, we could not determine whether a causal relationship exists between hyperuricemia and osteoporosis due to its cross-sectional study design. Thus, further longitudinal studies adjusting for potential confounding factors are indicated to confirm our findings. Second, our data include a self-administered questionnaire and might have involved a recall bias of previous history. Third, we could not analyze the effects of physical exercise, waist circumference or the use of medication because this information was not available and could not be elicited due to the retrospective nature of our study. Furthermore, there was no questionnaire on gout in the survey. Additional longitudinal studies that include this information are needed. Fourth, according to the definition of osteoporosis, there may have been diagnosis errors because the diagnosis relied on the patient's memory. Diagnosis errors according to the definition of osteoporosis have already been pointed out in previous papers. In a paper comparing patients’ responses to diagnosis questions and T-scores, only 7.6% of men and 37.8% of women were aware of their diagnosis44. However, in studies with fragility fracture patients, the concordance rate between self-report and a clinical diagnosis of osteoporosis varied by country, ranging from 30 to 80%45. Among the countries, Korea showed highest rate of osteoporosis diagnosis. Osteoporosis had a prevalence of 6.8% in our paper, which is lower than that in previous reports (7.8 to 22.1%)46,47. Since our data showed a relatively high proportion of older men over the age of 60, we speculate that the overall number of participants with osteoporosis may have been low. Finally, the participants in this study included adults over 40 years old, so the findings might not be generalizable to younger people. Despite these limitations, the present study demonstrated that the associations between hyperuricemia and osteoporosis differ by age and sex group. We found a protective effect of hyperuricemia against osteoporosis, particularly in older women. While prior studies observed a positive association between serum uric acid and BMD, our study demonstrated the OR of hyperuricemia and osteoporosis. The examined participants were also a low-risk, asymptomatic large population of Koreans and had laboratory results, which is an additional strength of our study.
Conclusion
In conclusion, our study showed that hyperuricemia was associated with a decreased risk of osteoporosis after adjusting for confounding factors. In subgroup analyses according to age and sex, statistical significance was observed only in the group of older women.
Acknowledgements
This work was supported in part by a research Grant (NRF-2018-R1D1A1A0-2085328) from the National Research Foundation (NRF) of Korea, and Hallym University Research Fund (HURF).
Author contributions
J.W.L.: Formal Analysis, Investigation, Validation, Visualization, Writing—Original Draft Preparation. B.C.K.: Supervision, Writing—Review and Editing. H.G.C.: Conceptualization, Data curation, Formal Analysis, Funding Acquisition, Investigation, Methodology, Project Administration, Software, Supervision, Validation, Visualization, Writing—Review and Editing.
Data availability
Data in this study are from the Korean Genome and Epidemiology Study (KoGES; 4851-302), National Research Institute of Health, Centers for Disease Control and Prevention, Ministry for Health and Welfare, Republic of Korea. These data are available online with permission from the Division of Epidemiology and Health Index of the Korea Centers for Disease Control and Prevention.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neogi T. Clinical practice. Gout. N. Engl. J. Med. 2011;364:443–452. doi: 10.1056/NEJMcp1001124. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheumatol. 2011;63:3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 3.Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019;71:991–999. doi: 10.1002/art.40807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am. J. Med. 2012;125:679–687.e671. doi: 10.1016/j.amjmed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Wright NC, Saag KG, Dawson-Hughes B, Khosla S, Siris ES. The impact of the new National Bone Health Alliance (NBHA) diagnostic criteria on the prevalence of osteoporosis in the USA. Osteoporos. Int. 2017;28:1225–1232. doi: 10.1007/s00198-016-3865-3. [DOI] [PubMed] [Google Scholar]
- 6.Rosen, C. J. The Epidemiology and Pathogenesis of Osteoporosis. In Endotext (eds. Feingold, K. R., et al.) (MDText.com, Inc. Copyright © 2000–2020, MDText.com, Inc., South Dartmouth (MA), 2000).
- 7.Simon D, et al. Bone microstructure and volumetric bone mineral density in patients with hyperuricemia with and without psoriasis. Osteoporos. Int. 2020;31:931–939. doi: 10.1007/s00198-019-05160-x. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, et al. Specific higher levels of serum uric acid might have a protective effect on bone mineral density within a Chinese population over 60 years old: a cross-sectional study from northeast China. Clin. Interv. Aging. 2019;14:1065–1073. doi: 10.2147/CIA.S186500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babaei M, Shamsi R, Heidari B, Bijani A, Hosseini SR. Serum uric acid status and its association with bone mineral density in the elderly people aged 60 years and more. Int. J. Endocrinol. Metab. 2019;17:e80780. doi: 10.5812/ijem.80780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang J, Hwang JH, Ryu S, Ahn JK. Higher serum uric acid is associated with higher lumbar spine bone mineral density in male health-screening examinees: a cross-sectional study. J. Bone Miner. Metab. 2019;37:142–151. doi: 10.1007/s00774-018-0905-4. [DOI] [PubMed] [Google Scholar]
- 11.Kaushal N, Vohora D, Jalali RK, Jha S. Raised serum uric acid is associated with higher bone mineral density in a cross-sectional study of a healthy Indian population. Ther. Clin. Risk Manag. 2018;14:75–82. doi: 10.2147/TCRM.S147696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyazit F, Pek E. Effects of vitamin B(12), folate, uric acid, and serum biomarkers of inflammation on bone mineral density in postmenopausal women. Prz. Menopauzalny. 2018;17:69–76. doi: 10.5114/pm.2018.77305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan DD, et al. Association of serum uric acid levels with osteoporosis and bone turnover markers in a Chinese population. Acta Pharmacol. Sin. 2018;39:626–632. doi: 10.1038/aps.2017.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirro M, et al. Uric acid and bone mineral density in postmenopausal osteoporotic women: the link lies within the fat. Osteoporos. Int. 2017;28:973–981. doi: 10.1007/s00198-016-3792-3. [DOI] [PubMed] [Google Scholar]
- 15.Han W, et al. Association between lumbar bone mineral density and serum uric acid in postmenopausal women: a cross-sectional study of healthy Chinese population. Arch. Osteoporos. 2017;12:50. doi: 10.1007/s11657-017-0345-0. [DOI] [PubMed] [Google Scholar]
- 16.Lin X, et al. Association between serum uric acid and bone health in general population: a large and multicentre study. Oncotarget. 2015;6:35395–35403. doi: 10.18632/oncotarget.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn SH, et al. Higher serum uric acid is associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in healthy postmenopausal women. Osteoporos. Int. 2013;24:2961–2970. doi: 10.1007/s00198-013-2377-7. [DOI] [PubMed] [Google Scholar]
- 18.Nabipour I, et al. Serum uric acid is associated with bone health in older men: a cross-sectional population-based study. J. Bone Miner. Res. 2011;26:955–964. doi: 10.1002/jbmr.286. [DOI] [PubMed] [Google Scholar]
- 19.Pan K, Yao X, Liu M, Zhu Z. Association of serum uric acid status with bone mineral density in adolescents aged 12–19 years. Front. Med. (Lausanne) 2020;7:255. doi: 10.3389/fmed.2020.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao X, Chen L, Xu H, Zhu Z. The association between serum uric acid and bone mineral density in older adults. Int. J. Endocrinol. 2020;2020:3082318. doi: 10.1155/2020/3082318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veronese N, et al. Hyperuricemia protects against low bone mineral density, osteoporosis and fractures: a systematic review and meta-analysis. Eur. J. Clin. Investig. 2016;46:920–930. doi: 10.1111/eci.12677. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, et al. Relationship between serum uric acid and bone mineral density in the general population and in rats with experimental hyperuricemia. J. Bone Miner. Res. 2015;30:992–999. doi: 10.1002/jbmr.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalbeth N, et al. Mendelian randomization analysis to examine for a causal effect of urate on bone mineral density. J. Bone Miner. Res. 2015;30:985–991. doi: 10.1002/jbmr.2434. [DOI] [PubMed] [Google Scholar]
- 24.Xiong A, et al. No causal effect of serum urate on bone-related outcomes among a population of postmenopausal women and elderly men of Chinese Han ethnicity—a Mendelian randomization study. Osteoporos. Int. 2016;27:1031–1039. doi: 10.1007/s00198-015-3341-5. [DOI] [PubMed] [Google Scholar]
- 25.Lee YH, Song GG. Uric acid level, gout and bone mineral density: a Mendelian randomization study. Eur. J. Clin. Investig. 2019;49:e13156. doi: 10.1111/eci.13156. [DOI] [PubMed] [Google Scholar]
- 26.Bonaccorsi G, et al. Changes in adipose tissue distribution and association between uric acid and bone health during menopause transition. Int J Mol Sci. 2019;20:6321. doi: 10.3390/ijms20246321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y, Han BG. Cohort profile: the Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2017;46:e20. doi: 10.1093/ije/dyv316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin KC, Lin HY, Chou P. Community based epidemiological study on hyperuricemia and gout in Kin-Hu, Kinmen. J. Rheumatol. 2000;27:1045–1050. [PubMed] [Google Scholar]
- 29.Ahn Y, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007;61:1435–1441. doi: 10.1038/sj.ejcn.1602657. [DOI] [PubMed] [Google Scholar]
- 30.Trevisan C, et al. The impact of smoking on bone metabolism, bone mineral density and vertebral fractures in postmenopausal women. J. Clin. Densitom. 2019;23:381–389. doi: 10.1016/j.jocd.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Wee JH, et al. Association between asthma and cardiovascular disease. Eur. J. Clin. Investig. 2020;51:e13396. doi: 10.1111/eci.13396. [DOI] [PubMed] [Google Scholar]
- 32.Kim Y, Kang J, Kim GT. Prevalence of hyperuricemia and its associated factors in the general Korean population: an analysis of a population-based nationally representative sample. Clin. Rheumatol. 2018;37:2529–2538. doi: 10.1007/s10067-018-4130-2. [DOI] [PubMed] [Google Scholar]
- 33.Luo Z, et al. Cellular and molecular mechanisms of alcohol-induced osteopenia. Cell Mol. Life Sci. 2017;74:4443–4453. doi: 10.1007/s00018-017-2585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986. doi: 10.3390/nu12071986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishii S, et al. Association between serum uric acid and lumbar spine bone mineral density in peri- and postmenopausal Japanese women. Osteoporos. Int. 2014;25:1099–1105. doi: 10.1007/s00198-013-2571-7. [DOI] [PubMed] [Google Scholar]
- 36.Kok VC, et al. Gout as a risk factor for osteoporosis: epidemiologic evidence from a population-based longitudinal study involving 108,060 individuals. Osteoporos. Int. 2018;29:973–985. doi: 10.1007/s00198-018-4375-2. [DOI] [PubMed] [Google Scholar]
- 37.Paschou SA, et al. Type 2 diabetes and osteoporosis: a guide to optimal management. J. Clin. Endocrinol. Metab. 2017;102:3621–3634. doi: 10.1210/jc.2017-00042. [DOI] [PubMed] [Google Scholar]
- 38.Zhao X, Yu X, Zhang X. Association between uric acid and bone mineral density in postmenopausal women with type 2 diabetes mellitus in China: a cross-sectional inpatient study. J. Diabetes Res. 2020;2020:3982831. doi: 10.1155/2020/3982831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu M, et al. Positive association between serum uric acid and bone mineral density in Chinese type 2 diabetes mellitus stratified by gender and BMI. J. Bone Miner. Metab. 2018;36:609–619. doi: 10.1007/s00774-017-0877-9. [DOI] [PubMed] [Google Scholar]
- 40.Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women–the Third National Health and Nutrition Examination Survey. Arthritis Res. Ther. 2008;10:R116. doi: 10.1186/ar2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vassalle C, Mazzone A, Sabatino L, Carpeggiani C. Uric acid for cardiovascular risk: Dr. Jekyll or Mr. Hide? Diseases. 2016;4:12. doi: 10.3390/diseases4010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lane NE, et al. Association of serum uric acid and incident nonspine fractures in elderly men: the Osteoporotic Fractures in Men (MrOS) study. J. Bone Miner. Res. 2014;29:1701–1707. doi: 10.1002/jbmr.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin KM, et al. The paradoxical role of uric acid in osteoporosis. Nutrients. 2019;11:2111. doi: 10.3390/nu11092111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim Y, Kim JH, Cho DS. Gender difference in osteoporosis prevalence, awareness and treatment: based on the Korea national health and nutrition examination survey 2008–2011. J. Korean Acad. Nurs. 2015;45:293–305. doi: 10.4040/jkan.2015.45.2.293. [DOI] [PubMed] [Google Scholar]
- 45.Kung AW, et al. Factors influencing diagnosis and treatment of osteoporosis after a fragility fracture among postmenopausal women in Asian countries: a retrospective study. BMC Womens Health. 2013;13:7. doi: 10.1186/1472-6874-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi MH, Yang JH, Seo JS, Kim YJ, Kang SW. Prevalence and diagnosis experience of osteoporosis in postmenopausal women over 50: Focusing on socioeconomic factors. PLoS ONE. 2021;16:e0248020. doi: 10.1371/journal.pone.0248020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi, Y. J., Shin, H. B., Park, B., Kim, D. J. & Chung, Y. S. Temporal change in the diagnosis and treatment rates of osteoporosis: results from the Korea National Health and Nutrition Examination Survey. Osteoporos. Int. PMID: 33630130 10.1007/s00198-021-05864-z (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data in this study are from the Korean Genome and Epidemiology Study (KoGES; 4851-302), National Research Institute of Health, Centers for Disease Control and Prevention, Ministry for Health and Welfare, Republic of Korea. These data are available online with permission from the Division of Epidemiology and Health Index of the Korea Centers for Disease Control and Prevention.