Abstract
Preventing lipid oxidation, especially with the polyunsaturated fat-based products, is a major concern in sectors as agri-food and cosmetic. Even though the efficiency of synthetic antioxidants has been recognized, both consumers and manufacturers are looking for more innovative, healthy and quality products while rejecting synthetic additives due to their concern about safety, along with their environmental impact issues. In this context, plant biomass, which have shown to be rich in compounds, have raised interest for the isolation of novel naturally occurring antioxidants. Among their myriad of molecules, bioactive peptides, which are biologically active sequence of amino acid residues of proteins, seem to be of a great interest. Therefore, the number of identified amino acids sequences of bioactive peptides from plant biomass with potential antioxidant action is progressively increasing. Thus, this review provides a description of 129 works that have been made to produce bioactive peptides (hydrolysate, fraction and/or isolate peptide) from 55 plant biomass, along with the procedure to examine their antioxidant capacity (until 2019 included). The protein name, the process, and the method to concentrate or isolate antioxidant bioactive peptides, along with their identification and/or specificity were described. Considering the complex, dynamic and multifactorial physico-chemical mechanisms of the lipid oxidation, an appropriate in-vitro methodology should be better performed to efficiently probe the antioxidant potential of bioactive peptides. Therefore, the results were discussed, and perspective for antioxidant applications of bioactive peptides from plant biomass was argued.
Keywords: Plant biomass, Proteins, Peptides, Antioxidants, Lipid oxidation
Graphical abstract
Highlights
-
•
Exhaustive description of 129 works made to produce bioactive peptides from 55 plant biomass.
-
•
Controlled proteolysis can be used as a sustainable strategy to produce peptides.
-
•
Presentation of the important points to consider when estimating the antioxidant capacity of peptides.
-
•
Methodology should be improved to better probe the antioxidant potential of peptides.
Abbreviations:
- DH
degree of hydrolysis
- E/S
enzyme/substrate ratio
- Da
Dalton
- LOOH
lipid hydroperoxides
- HAT
hydrogen atom transfer
- (RP)-HPLC
(reverse phase) high-Performance liquid chromatography
- MW
molar weight
- PUFA
polyunsaturated fatty acid
- SET
single electron transfer
- BHA
butylated hydroxyanisole
- BHT
butylated hydroxytoluene
- TBHQ
tert-butylhydroquinone
- SDG
sustainable development goals
- BP
bioactive peptides
- UF
ultra-filtration
- HPP
high hydrostatic pressure
- SEC
size exclusion chromatography
- EMR
enzyme membrane reactor
- RP-UFLC
reversed phase ultra-flow liquid chromatograph
- MAR
macroporous adsorption resins
- IMAC
immobilized metal affinity chromatography
- NI
not investigated
- ESR
electron spin resonance
- DPPH
diphenyl-picrylhydrazyle
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid
- FRAP
ferric reducing antioxidant power
- HSRA
hydroxyl radical scavenging activity
- TBARS
thiobarbituric acid reactive substances
- SRSA
superoxide radical scavenging activity
- CAA
cellular antioxidant assay
- PCL-ACW
photochemiluminescence-antiradical capacity of water soluble substances
- PV
peroxides value
- Tpx
total peroxides
- GSH
glutathione
- EDTA
ethylenediaminetetraacetic acid
- ED50
median effective dose
- IC50
half maximal inhibitory concentration
- EC50
half maximal effective concentration
- TE
Trolox equivalent
- TEAC
Trolox equivalent antioxidant capacity
- SOD
superoxide dismutase
- TAC
total antioxidant capacity
- CAT
catalase
- ABAP
2,2-azobis(2-amidinopropane) dihydrochloride
- LDL
low density lipoprotein
1. Introduction
Lipids are important food components because of their many advantages (Sikorski et al., 2010). For instance, they contain essential fatty acids (e.g. ω3 fatty acids), or they can be used as a vehicle to dissolve and help the absorption of fat-soluble vitamins or other nutrients (Akoh, 2017). They can also enhance the organoleptic perception, since the texture, the color, the structure or the flavor of food, are positively influenced by the presence of lipids (Gunstone and Norris, 1983). However, lipids are chemically unstable compounds and thus, can easily deteriorate due to many undesired reactions through different mechanisms, so called “lipid oxidation” (Decker et al., 2010b).
Thus, preventing lipid oxidation, especially with the polyunsaturated fat-based products, is a major concern in sectors as agri-food and cosmetic (Decker et al., 2010a). In order to overcome this problem, several lines of research are growing, mainly focused on the retention of endogenous antioxidants or addition of exogenous antioxidants, in addition to the use of active and intelligent packaging to prevent the oxidation. In addition, many synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) or tert-butylhydroquinone (TBHQ) are used in food products to preserve their stability and quality. Although they effectiveness has been recognized, their safety has been questioned many times, which may lead to restrictions of use. Indeed, consumers are looking for innovative, healthy and quality products with extended shelf life while rejecting synthetic additives due to their concern about safety, and/or to mitigate the environmental impact issues. Thus, naturally occurring antioxidants from plant biomass (byproducts, coproducts and wastes) are more valued than synthetic ones, for safety, for potential health benefits, but also for many other reasons that align with several Sustainable Development Goals (SDGs) identified by the United Nations. Moreover, although attention continues to be focused on the waste minimization, agricultural waste is unavoidable, therefore its effective utilization is essential. Animal and plant biomass have shown to be rich in molecules and have therefore raised interest for the isolation of novel naturally occurring antioxidant. Ascorbic acid, carotenoids, flavonoids, phenolic acids, tocopherols, tocotrienols, etc., or natural extracts showing antioxidants properties, have been the subject of significant research over the past decades (Brewer, 2011). Although amino acids such as Trp has been pointed out as a potent antioxidant (Ma et al., 2010), free amino acids were not found to be effective antioxidants, which limit their incorporation for stabilizing food products (Chan et al., 1994; Østdal et al., 1999). Yet, bioactive peptides (BP), which are biologically active sequence of amino acid residues of proteins that are linked by peptide bonds, could be of a better interest (Korhonen and Pihlanto, 2006; Pihlanto and Korhonen, 2003; Waseem et al., 2018). Those BP exhibited many interesting properties that have already demonstrated antiproliferative, antimutagenic, anti-inflammatory, anticoagulant, immunomodulatory, cytomodulatory, opioid, mineral binding, antioxidative, hypoglycemic, hypocholesterolemic, antihypertensive, and antimicrobial activities, which can help in many diseases, including cancer, diabetes and inflammatory disorders (Bhandari et al., 2020; Marczak et al., 2003; Xue et al., 2009a, Xue et al., 2009b; Zhang et al., 2008). They are also considered as regulators that can prevent oxidation and microbial degradation in foods (Maestri et al., 2016; Samaranayaka and Li-Chan, 2011).
Various BP have been reported in recent years as naturally present, or produced, from food proteins of different origins (from animal, plant or marine organisms). The main food sources reported are dairy products, eggs, meat, fish and cereals (Kitts and Weiler, 2005; Nasri, 2017; Piovesana et al., 2018; Waseem et al., 2018). That being said, plant resources have shown increasing interest in recent years compared to animal resources. This is explained by their more sustainable and ecological production, and also their good image for the consumer. BP consist of protein fragments, usually 2–20 amino acids long, already encoded as amino acid sequences, but inactive when encrypted in the parent protein. The BP can be released by proteolytic processes using exogenous proteases, acid or alkali hydrolysis, enzymatic hydrolysis during gastrointestinal digestion by endogenous enzymes (autolysis), but some BP can also be released during food processing (microbial fermentation, ripening, cooking) or storage (Marciniak et al., 2018; Nasri, 2017; Piovesana et al., 2018).
Several BP have been investigated for their antioxidant activities (Sarmadi and Ismail, 2010; Wong et al., 2020). BP, along with the parent proteins, expressed their antioxidant activities through different mechanisms, including radical scavenging, metal chelation, electron or hydrogen transfer reduction, and aldehyde quenching. This review intends to synthesize the recent progress in the discovery of plant biomass-derived antioxidant BP. Information about their production, purification and isolation are summarized, as well as the identification of their amino acids’ composition. A special attention was given to the studies that have successfully determined the sequence of antioxidant peptides. Those antioxidant activities were mostly estimated in absence of lipid substrates through recognized in vitro assays in homogenous solutions (e.g. water or organic solvents), typically to evaluate free radical scavenging, electron transfer capacity or metal chelation. Although sparsely investigated, antioxidant activities in lipid dispersion systems (bulk oils or emulsions) were also presented. Considering the multifaceted mechanisms of the lipid oxidation, a more suitable in-vitro methodology should be performed to efficiently probe the antioxidant potential of BP. Therefore, the results were discussed, and perspective for antioxidant applications of BP from plant biomass was argued.
2. Lipid oxidation
Lipid oxidation is a natural phenomenon in foods, but also in biological systems, and has become a major problem for human health, along with in sectors of agri-food and cosmetics (Decker et al., 2010a). In living organisms, lipid oxidation is caused by oxidative stress via some reactions induced by reactive oxygen or nitrogen species (Pizzino et al., 2017). Yet, cells have an endogenous antioxidant defense system including antioxidant enzymes, uric acid, glutathione or metal-binding proteins, which can help to reduce oxidative stress and its damages (Birben et al., 2012). Many food processing operations can damage and destroy the endogenous antioxidants, leaving food lipids unprotected. Thus, lipids in food deteriorate during all the processing steps, including raw product selection, harvesting, storage, refining, manufacturing and distribution (German, 1999). In addition, consumers are becoming more aware of the unhealthy effects of a high amount of fat and saturated fatty acids in food products. Therefore, a growing interest exists in developing new products with enriched content in high polyunsaturated fatty acids (PUFA), including ω3 enriched products. Yet, adding PUFA to food products lead to a negative effect on the shelf life and oxidative stability of products.
Lipid oxidation itself is a very complex, dynamic and multifactorial physico-chemical mechanism that is affected by numerous internal factors. It may be influenced by the type of systems, rather homogeneous (e.g. bulk oil) or heterogeneous (e.g. emulsions), the chemical structure and composition of lipids, as well as the presence of other molecules (e.g. antioxidants, pro-oxidants, surfactants). In addition, the reactivity, the mobility, the partitioning, and the diffusion of molecules constituting each phase (e.g. lipid phase, water phase, interfaces or membranes), along with the physico-chemical properties of the media (e.g. pH, temperature, aw) are important points to conceptualize the lipid oxidation (Berton-Carabin et al., 2014; Decker et al., 2017; Waraho et al., 2011). Thus, many parameters are of crucial importance regarding lipids oxidation mechanisms and the resulting efficiency of the antioxidant solution. That is why for example, the lipid oxidation of an oil in a bulk state may differ from its oxidation in the form of droplets dispersed in an aqueous environment, as observed in an oil in water emulsion (McClements and Decker, 2000). Lipid oxidation is assumed to proceed along a nonenzymatic free radical chain reaction (called autoxidation), a photo-oxidation route, or an enzyme-mediated mechanism (e.g. with lipoxygenases). These three mechanisms differ at the initiation stage, since the oxidation of unsaturated lipids may be catalyzed by heat, light, ionizing radiation, free radicals, trace metals, metalloproteins, oxygen pressure, or enzymes. To explain it simply, unsaturated fatty acids react with molecular oxygen, mostly via a free radical mechanism, to lead to hydroperoxides that are considered to be the first oxidation products. Hydroperoxides are odorless and do not contribute any aroma. However, these compounds are highly unstable and decompose in a large number of secondary oxidation products that include hydrocarbons, aldehydes, ketones, alcohols, esters and acids (Schaich, 2020). Thus, the lipid deterioration leads to the production of oxidative fragments (responsible for the off-flavors of rancidity), but also the degradation of proteins, vitamins and pigments, or cross-link molecules into non-nutritive polymers. Consequently, lipid oxidation affects the sensory attributes, such as the odor, the flavor, the color and the texture, and as the reactions induce losses of some essential components of food, it can also reduce the nutritional value and generate toxic compounds, such as hydroperoxides, aldehydes, epoxides, trans fatty acids, Maillard type products, among others. In addition, the lipid oxidation in foods decreases the technological suitability, like the emulsifying activity of proteins or their solubility. Thus, providing efficient and natural antioxidant strategy to counteract lipid oxidation, especially with the polyunsaturated fat-based products, is challenging.
3. Production and identification of antioxidant bioactive peptides from plant biomass
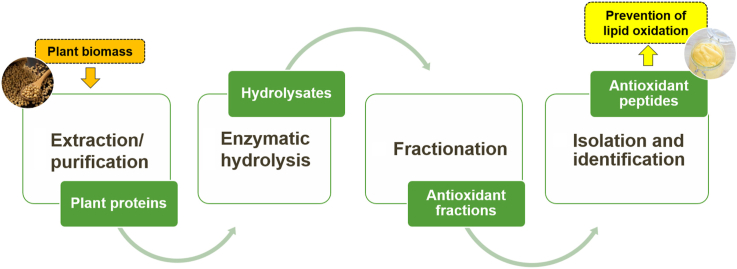
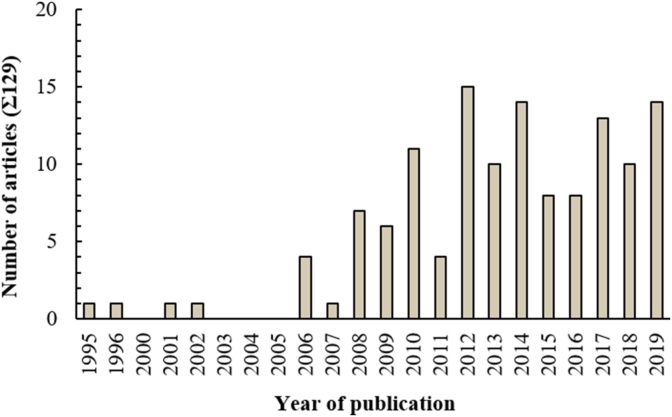
Over the last fifteen years, several studies have been made to develop a series of methods with the aim at optimizing the enzymatic proteolysis, concentration, isolation, purification and identification of novel antioxidant bioactive peptides (BP) from plant biomass (Fig. 1).
Fig. 1.
Number of articles per year of publication, dealing with the production and the identification of antioxidant bioactive peptides from plant biomass.
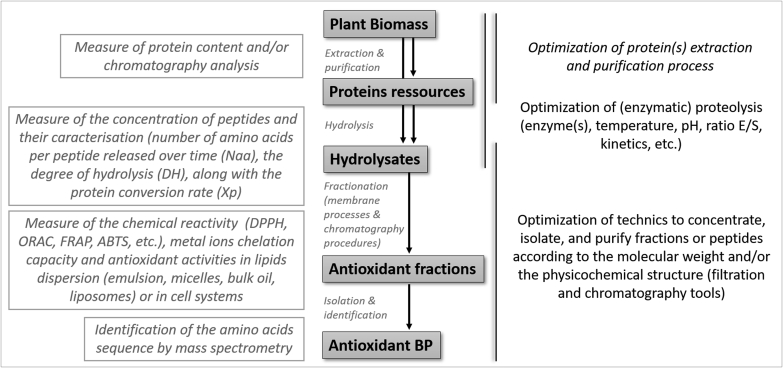
Based on the literature, the main flowchart for the investigation of antioxidant BP from plant biomass is illustrated in Fig. 2. These researches led to the identification of BP, and the number of identified amino acids sequences with potential antioxidant action is progressively increasing.
Fig. 2.
Flowchart for the production and the identification of antioxidant bioactive peptides from plant biomass.
3.1. Sources of antioxidant bioactive peptides from plant biomass
Many different plant sources with high or moderate concentration of proteins have been already investigated for their production in BP. Table 1 provides a description of 129 works that have been made to produce BP (hydrolysate, fraction and/or isolate peptide) from 55 plant biomass, which were further evaluated for their antioxidant capacity. These articles are the result of an in-depth bibliographic study. They were selected because they deal with peptides production from plant biomass, which has been tested with at least one antioxidant activity assay. The protein name, the process, and the procedure to concentrate or isolate antioxidant BP, along with their identification and/or specificity were described. Plant proteins have been classified in different ways. BP may originate from various natural proteins present in biomass, such as cereals, herbs, fruits, legumes, in either seeds, leaves or fruits. Cereals have been reported many times as a source of antioxidant BP. Indeed, they have been isolated and identified from wheat (Coda et al., 2012; Park et al., 2012; Wang et al., 2007; Zhu et al., 2006), corn (Jiang et al., 2018; Jin et al., 2016; Kong and Xiong, 2006; Li et al., 2010; Liu et al., 2015; Ortiz-Martinez et al., 2017; Wang et al., 2014; Wang et al., 2015; Wang et al., 2014; Zheng et al., 2012; Zheng et al., 2006; Zhou et al., 2015; Zhou et al., 2012; Zhu et al., 2008; Zhuang et al., 2013;Zhuang et al., 2013), rice (Adebiyi et al., 2008; 2009; Phongthai et al., 2018; Selamassakul et al., 2016; 2018; Wang et al., 2017; Wattanasiritham et al., 2016; Yan et al., 2015), barley (Bamdad and Chen, 2013; Chanput et al., 2009; Xia et al., 2012), millet (Agrawal et al., 2016, 2019; Amadou et al., 2013), oat (Coda et al., 2012; Tsopmo et al., 2010) or sorghum (Agrawal et al., 2017; Xu et al., 2019) among others. Moreover, beans generally contain high protein contents, such as soybeans that received the greatest research attention (Beermann et al., 2009; Chen et al., 1995; Chen et al., 1996; Indiano-Romacho et al., 2019; Moure et al., 2006; Park et al., 2010; Penta-Ramos and Xiong, 2002; Yang, Wang, et al., 2017; Zhang et al., 2010; Zhang et al., 2018; 2019; Zhao and Xiong, 2015), or, more rarely, with black (Betancur-Ancona et al., 2014; Carrasco-Castilla et al., 2012a, 2012b; Evangelho et al., 2017; Torruco-Uco et al., 2009; Valdez-Ortiz et al., 2012), pinto (Ngoh and Gan, 2016), azufrado (Valdez-Ortiz et al., 2012), mung (Sonklin et al., 2018), kidney (Mundi and Aluko, 2014) or Mucuna pruriens beans (Herrera Chalé et al., 2014). Seeds and nuts have also shown to contain peptides with good antioxidant activities. Among seeds, rapeseed (Alashi et al., 2014; Cumby et al., 2008; He et al., 2012, 2013; Pan et al., 2011;Xue et al., 2009a, Xue et al., 2009b; Yu et al., 2013; Zhang et al., 2008, 2009), hemp seed (Girgih et al., 2014; Girgih et al., 2013,2014; Lu et al., 2010), flaxseed (Karamać et al., 2016; Silva et al., 2017), sesame (Das et al., 2012; Lu et al., 2019; Wang et al., 2012), palm (Ng et al., 2013; Zarei et al., 2012, 2014) and amaranth seeds (Orsini Delgado et al., 2011; Sabbione et al., 2016) are the most reported. Chia (Silveira Coelho et al., 2019), pumpkin (Venuste et al., 2013), watermelon (Wen et al., 2019), quinoa (Ren et al., 2017), perilla (Yang et al., 2018), moringa (Aderinola et al., 2019), sunflower (Megías et al., 2008) and cassia (Chai et al., 2019) have been also explored. In addition, oilseed natural resources (e.g. flaxseed, rapeseed, sunflower, sesame, perilla, palm or chia) as a starting material for the production of antioxidant BP are particularly interesting in a biorefinery approach of sustainable valorization of agri-food by-products. Indeed, its solid byproduct (meal) of oil extraction process has an important protein content (around 34% on dry matter basis for rapeseed meal, as example), that is extensively produced every year (408 million tons in world in 2018 (FAO, 2020)). Peanut (Hwang et al., 2001; 2010; Yu et al., 2012; Zheng et al., 2012), walnut (Chen et al., 2012; Feng et al., 2019; Gu et al., 2015; Lv et al., 2017; Ren et al., 2018) and pine nut (Liang et al., 2017; Yang, Li, et al., 2017) are the most documented nuts containing antioxidant BP. Leaves (e.g. from alfalfa or mulberry) can also contain antioxidant BP but are not receiving much attention (Sun et al., 2019; Xie et al., 2008). Other plants, such as chickpea (Arcan & Yemenicioǧ;lu, 2007; Jamdar et al., 2017; Li et al., 2008; Torres-Fuentes et al., 2015; Zhang et al., 2011), pea (Pownall et al., 2010), potato (Cheng et al., 2010; 2014; Zhang et al., 2014; Zhang and Mu, 2017), and cowpea (Marques et al., 2015; Segura-Campos et al., 2013; Segura Campos et al., 2010) have shown to contain antioxidant peptides, among others. Researchers have also explored green tea dregs (Zhao et al., 2014), African legume crops (Ajibola et al., 2011; Arise et al., 2016) as sources of antioxidant BP, since valorizing agricultural by-products has become a main priority. Likewise, antioxidant BP have been also identified from medicinal plants, such as arrowhead (Wen et al., 2018), moringa oleifera (Aderinola et al., 2019), Chinese leek (Hong et al., 2014), along with marine fungi such as schizochytrium sp. (Cai et al., 2017; Hu et al., 2019; Yang et al., 2019).
Table 1.
Description of the bioactive peptides produced from plant biomass and tested for their antioxidant capacity.
| Plant Biomass | Protein name | Process | Samples characteristic | Peptides information (sequence, amino acids content, etc.) | Ref. |
|---|---|---|---|---|---|
| A. mantegazzianus (Pass cv Don Juan) | orsin | Enzymatic (simulated gastrointestinal digestion with Pepsin + Pancreatin and Alcalase sequentially) | Hydrolysates and 80 fractions (RP-HPLC): Fractions 7, 16, 20, 22, 25 and 30 contained peptides with MW ranging from 800 to 1700 Da (7–15 amino acids). | A total of 54 peptides were identified, 4 peptides with interesting activity: AWEEREQGSR, YLAGKPQQEH, IYIEQGNGITGM, TEVWDSNEQ. | (Orsini Delgado et al., 2011, 2016) |
| Amaranthus hypochondriacus | Amaranth seed proteins | Enzymatic (endogenous aspartic protease) | Hydrolysate with 75% of MW peptides between 100 and 5 kDa, and 25% with MW < 5 kDa. | Not identified. | Sabbione et al. (2016) |
| African Yam Bean Seed (Sphenostylis stenocarpa) | African yam bean seed proteins | Enzymatic (Alcalase) | Hydrolysates and 4 fractions (UF): MW < 1 kDa, 1 kDa < MW < 3 kDa, 3 kDa < MW < 5 kDa, and 5 kDa < MW < 10 kDa. |
Glu + Gln, Asp + Asn, Gly, Leu, Lys and Ala were the most predominant amino acids. Ala, Met, Leu, and Trp were highest in MW < 1 kDa fraction, but had less contents of Glu + Gln, and Asp + Asn. The MW < 1 kDa had the least content of His, Lys, and Pro, but higher total hydrophobic and aromatic amino acids. | Ajibola et al. (2011) |
| Alfalfa | Alfalfa leaf proteins | Enzymatic (Alcalase) | Hydrolysate and 1 fraction (UF): MW < 3 kDa analyzed with SEC. The MW of peptides were concentrated in MW < 1 kDa. | Large proportion of peptides with 2–6 amino acid residues, with the presence of His, Tyr, Met, and Cys.3 low-MW peptides have been isolated but not described. | Xie et al. (2008) |
| Arrowhead (Sagittaria sagittifolia L.) | Arrowhead proteins | Enzymatic (Pepsin, trypsin, Alcalase) and with ultrasound pretreatment proteolysis | Hydrolysates. | Not identified. | Wen et al. (2018) |
|
Bambara groundnut (Vigna subterranea) |
Bambara proteins |
Enzymatic (Alcalase, trypsin and Pepsin) |
Hydrolysates and 4 fractions (UF): MW < 1 kDa, 1 kDa < MW < 3 kDa, 3 kDa < MW < 5 kDa, and 5 kDa < MW < 10 kDa. |
Not identified. |
Arise et al. (2016) |
|
Barley |
Glutelin |
Enzymatic (Alcalase and Flavourzyme) |
Hydrolysates and 3 fractions (UF) from Alcalase: | 4 peptides: QKPFPQQPPF, PQIPEQF, LRTLPM, SVNVPL. |
Xia et al. (2012) |
| MW > 10 kDa, 1 kDa < MW < 10 kDa and MW < 1 kDa. | |||||
|
Barley |
Hordein |
Enzymatic (Alcalase) |
Hydrolysate and 4 fractions (UF): UF1 (MW > 10 kDa), UF2 (10–5 kDa), UF3 (5–1 kDa), and UF4 (MW < 1 kDa). | 10 peptides: QSYPVQPQ, QQTPLPQ, QPQPYPQ, TQQPYPQ, SPLQPQ, QQPYPQ, QPVLSQ, QVPQ, LLPQ, HVLQ in UF2–F2. 5 peptides: KPFPQQPPF, QPPFWQ, SVNVPLY, AELIIPQ, YRIVPL in UF4–F4. The intermittent Pro and Gln residues, and the pentapeptide QPYPQ was predominant. |
Bamdad & Chen (2013) |
| UF2 and UF4 were purified into 4 fractions (RP-HPLC). | |||||
|
Barley grains (Hordeum vulgare L. ssp. Vulgare) and rice bran (Oryza sativa L.) |
3 hordein fractions of barley (B, C and D). Rice bran proteins (Albumin, globulin, prolamin and glutelin fractions). |
Enzymatic (Pepsin followed by Trypsin) |
Barley hordein (from SEC): Hordein fraction, partially purified B hordein, partially purified C hordein and partially purified D hordein. | Not identified. |
Chanput et al. (2009) |
| Hordein is composed of three sub-fractions and their approximate MW were 35–46, 55–75, and more than 100 kDa for B, C, and D hordeins, respectively. | |||||
| Bean, black (Phaseolus vulgaris) | Bean proteins (isolate and phaseolin) | Enzymatic (simulated gastrointestinal digestion with Pepsin + Pancreatin) | Hydrolysate and fraction (UF) with MW < 1 kDa fractionated by (SEC): 0.7 kDa < MW < 1.0 kDa, 0.43 kDa < MW < 0.7 kDa and MW < 0.43 kDa (A1, A2, and A3 for protein isolate, and B1, B2, and B3 for phaseolin). | Presence of Arg and Leu in A2 and B2; Phe and Trp in A3 and B3; and Lys in B2, in the carboxy-terminal end of peptides. | Carrasco-Castilla et al. (2012b) |
| Bean, black (Phaseolus vulgaris) | Bean seed proteins | Enzymatic (Pepsin, Alcalase) | Hydrolysates. | Not identified | Evangelho et al. (2017) |
| Bean, black (Phaseolus vulgaris) and lima (Phaseolus lunatus) | Bean seed proteins | Enzymatic (Alcalase, Flavourzyme) | Hydrolysates. | High content of hydrophobic amino acids: Val (55.8–61.7 g/kg), Ile (42.2–50.4 g/kg), Pro (6.6–9.8 g/kg), Met (17.1–20.9 g/kg), Phe (64.4–77.4 g/kg), Leu (92.8–100.4 g/kg), Trp (7.3–12.8 g/kg). | Torruco-Uco et al. (2009) |
| Bean, black (Phaseolus vulgaris) | Bean seed proteins | Enzymatic (sequential Alcalase-Flavourzyme and pepsin–pancreatin) | Hydrolysates. | Not identified. | Betancur-Ancona et al. (2014) |
|
Beans, azufrado (Phaseolus vulgaris) |
Bean seed proteins |
Enzymatic (Alcalase, Thermolysin, Pancreatin) |
Hydrolysates. |
Large amino acid content compared to other bean and pea hydrolysates. Good balance of essential amino acids (His, Thr, Tyr, Phe, Ile, Leu, Lys). |
Valdez-Ortiz et al. (2012) |
|
Bean, black (Phaseolus vulgaris) |
Bean seed proteins (isolate, phaseolin and lectins) |
Enzymatic (Pepsin and Pancreatin sequentially) |
Hydrolysates. |
Asp + Asn, Pro and Tyr are lower in phaseolin than isolate, but Ser, Phe, Lys and Leu are higher. |
Carrasco-Castilla et al. (2012a) |
| Cys, Ser, Thr, and Ala content were high in the lectin extract, while Glu + Gln, His, Ile and Phe were lower. | |||||
| Bean, pinto (Phaseolus vulgaris) | Bean proteins | Enzymatic (Protamex) | Hydrolysates and 6 fractions (UF): UF1 (MW > 100 kDa), UF2 (100–50 kDa), UF3 (50–30 kDa), UF4 (30–10 kDa), UF5 (10–3 kDa) and UF6 (MW < 3 kDa). | 6 peptides in UF6: PPHMLP, PPMHLP, PLPPHMLP, PLPLHMLP, ACSNHSPLGWRGH, LSSLEMGSLGALFVCM. | Ngoh & Gan (2016) |
| Buckwheat | Low-fat buckwheat flour proteins | Enzymatic (simulated gastrointestinal digestion with Pepsin + Pancreatin) | Hydrolysate and 6 fractions (SEC). MW of fractions were 3611 Da (I), 960 Da (II), 529 Da (III), 456 Da (IV), 365 Da (V), and 362 Da (VI). I, was a mixture of peptides. II, III, IV and V or VI had a preponderance of heptameric, tetrameric, trimeric, or dimeric peptides. VI also contained free amino acids | 3 peptides: WPL, VPW, VFPW in fraction IV. 1 peptide: PW in fraction V. W in fraction VI. |
Ma et al. (2010) |
| Cassiae semen (cassia obtusifolia L.) | Semen cassiae proteins (seed) | Enzymatic (Alcalase) | Hydrolysate and fraction (UF): MW < 3 kDa. | 4 peptides: PMPVR, FETLPF, KMRDNL, LDESKRF. | Chai et al. (2019) |
|
Cereals (Whole wheat, Durum wheat, Rye, Spelt, Oat, Rice, Kamut, Barley, Maize) |
Cereal (Whole wheat, Durum wheat, Rye, Spelt, Oat, Rice, Kamut, Barley, Maize) proteins |
Sourdough fermentation (Selected lactic acid bacteria) |
Hydrolysates and 37 fractions (RP-HPLC). |
25 peptides from 8 to 57 amino acid residues. Whole wheat: MAPAAVAAAEAGSK, DNIPIVIR. Spelt:AIAGAGVLSGYDQLQILFFGK, GNQEKVLELVQR, PAGSAAGAAP, EALEAMFLAAGAAAAARSAGQCGR, ITFAAYRR, HPVPPKKK. Rye: VFVDEGLEVLGWRPVPFNVSVVGRNAK, RLSLPAGAPVTVAVSP, NANGELCPNNMCCSQWGYCGLGSEFCGNGCQSGACCPEK, LCPVHRAADL, PAEMVAAALDR, KVALMSAGSMH, DLADIPQQQRLMAGLALVVATVIFLK, KNGSIFNSPSATAATIIHGHNYSGLAYLDFVTSK, GTIFFSQEGDGPTSVTGSVSGLKPGLHGFHVHALGDTTNGCMSTGPHFNPTGK. Kamut:YEWEPTVPNFDVAKDVTDM, GVSNAAVVAGGH, DAQEFKR, PPGPGPGPPPPPGAAGRGGGG, HKEMQAIFDVYIMFIN, TGGGSTSSSSSSSSSLGGGASRGSVVEAAPPATQGAAAAN, APAVPVVVVDTQEAGIR, DTAAGYVAPPDPAVSTGDYGLAGAEAPHPHESAVMSGAAAAAVAPGGEAYTR. |
Coda et al. (2012) |
|
Chenopodium quinoa Willd. |
Quinoa seed proteins |
Enzymatic (protease cocktail) |
Hydrolysate and fraction (UF): 1 kDa < MW < 10 kDa. | 1 peptide: Lunasin, 43 amino-acids, with 9 Asp in C-terminal, a cell adhesion motif with RGD, and a helix with structural homology to a region of chromatin proteins. |
(Ren et al., 2017) |
| Fraction was purified (SEC, ion-exchange chromatography and RP-HPLC). | |||||
|
Chia (Salvia hispanica L.) |
Chia seed proteins |
Enzymatic (Alcalase, Flavourzyme and Alcalase-Flavourzyme sequentially) |
3 fractions (UF): | Not identified |
Silveira Coelho et al. (2019) |
| Retentate 1 (F1: MW > 10 kDa), retentate 2 (F2: 3 < MW < 10 kDa, and the permeate (F3: MW < 3 kDa). | |||||
|
Chickpea |
Chickpea proteins |
Enzymatic (simulated gastrointestinal digestion with Pepsin + Pancreatin) |
Purification by affinity chromatography (FPLC AKTA-purifier system). Purified peptide fractions (F1, F2 and F3) were fractioned (F1A–F1F; F2A–F2D; F3A–F3E) by SEC and all eluted peptide fractions were collected and concentrated in distillated water using a nanofiltration system. |
Main sequences, ALEPDHR, TETWNPNHPEL, FVPH and SAEHGSLH, corresponded to legumin, the main seed protein. Most peptides contained His. 2 peptides also included Try and Phe. |
Torres-Fuentes et al. (2015) |
|
Chickpea (Cicer arietinum L.) |
Chickpea proteins |
Enzymatic (Alcalase) |
Hydrolysate and 4 fractions (SEC): | Rich in Arg, Phe, Lys, Leu, Ala, Asp. The hydrophobicity exhibited by Fra.IV (125.61 kcal/mol amino acid residue (AAR)) was higher than that of Fra.I (114.34 kcal/mol AAR), Fra.II (103.77 kcal/mol AAR) and Fra.III (103.64 kcal/mol AAR). |
(Li et al., 2008) |
| Fra.I, Fra.II, Fra.III and Fra.IV, Fra.IV showed MW of 940–2622 Da (49.21%) and 220–940 Da (40.63%). | |||||
|
Chickpea (Cicer arietium L.) |
Chickpea proteins |
Enzymatic (Alcalase) |
Hydrolysate and 4 fractions (SEC). CPH-IV (fraction 4) was divided into 11 fractions. |
1 peptide: NRYHE in fraction 7. |
(Zhang et al., 2011) |
|
Chinese leek (Allium tuberosum Rottler) seeds |
Chinese leek seeds |
Chemical extraction of peptides (CLP) with acetic acid from Chinese leek seed powder |
2 fractions (SEC): a and b eluted according to the MW | 1 peptide: GSQ. |
Hong et al. (2014) |
| Fraction b was further divided into 12 peaks (RP-HPLC). | |||||
| Corn | Albumin, Globulin, Prolamin, Glutelin | Enzymatic (Alcalase) | Hydrolysates. | Not identified. | Ortiz-Martinez et al. (2017) |
|
Corn |
Corn gluten meal |
Fermentation (Bacillus natto) |
Hydrolysates (different fermentation times). |
The MW of the peptides in the optimal hydrolysate distributed mainly over 860–5300 Da determined by gel filtration chromatography. |
(Zheng et al., 2012) |
|
Corn |
Corn gluten meal and zein |
Enzymatic (Alcalase) |
Hydrolysates: The MW distributions were from 0.31 >MW > to 10.3 kDa. | Rich in Glu, Leu, Ala and Pro. |
(Li et al., 2010) |
| The highest relative abundance of peptides was ~0.5 kDa. | |||||
|
Corn |
Corn gluten meal |
Enzymatic (Alcalase, Trypsin, Papain, Flavourzyme) |
Hydrolysates and 2 fractions (SEC) from Alcalase and Flavourzyme: F1 and F2. | Possible peptide in F2: GHKPS. |
Zhuang et al. (2013) |
| Peptides in F2 were separated (RP-HPLC). | |||||
|
Corn |
Corn gluten meal |
Enzymatic (Alcalase) |
Hydrolysate and the fraction (UF): 30 kDa > MW > 10 kDa. |
Not identified. |
(Wang et al., 2014) |
|
Corn |
Corn gluten meal |
Enzymatic (Alcalase and Protamex) |
Hydrolysates and 4 fractions (UF): FA (>20 kDa), FB (20 kDa > MW > 10 kDa), FC (10 KDa > MW > 6 kDa), FD (<6 kDa). |
QQPQPW. |
(Wang et al., 2014) |
| FD was purified with anion exchange, SEC and RP-HPLC. | |||||
| Corn | Corn gluten meal | Enzymatic (Alcalase) | Hydrolysates after 30 min (MW = 5–1.19 kDa), 60 min (MW = 3.8–0.66 kDa) and 120 min (MW = 3.71–0.66 kDa) of hydrolysis time. After anion exchange chromatography and SEC, the fraction of MW = 0.7–1.4 kDa were collected and purified with RP-HPLC. | FPLEMMPF. | (Zheng et al., 2006) |
|
Corn |
Corn gluten meal |
Enzymatic (Protamex) |
Hydrolysates (different hydrolysis time). |
Presence (in 24 h) of Lys, His, Tyr and Met with higher concentration of hydrophobic amino acids and moderate amounts of aromatic amino acids and negatively charged amino acids. |
(Zhou et al., 2015) |
|
Corn |
Corn gluten meal |
Enzymatic (Alcalase, Protamex, Flavourzyme, and combinations) |
Hydrolysates: According to conditions, MW were ranging from ~6 kDa > MW > to < 0.160 kDa. | Not identified. |
(Liu et al., 2015) |
| Two-step hydrolysis catalyzed by Alcalase and Protamex enriched low MW peptides, 79.62% of which were less than 1.2 kDa. | |||||
|
Corn |
Corn gluten meal |
Enzymatic (Alcalase, Flavourzyme, and Alcalase + Flavourzyme) |
Hydrolysate and fraction (UF): MW < 6 kDa. |
CSQAPLA, YPKLAPNE, and YPQLLPNE. |
Jin et al. (2016) |
| The active fraction from permeate was separated by RP-HPLC. | |||||
| Corn | Corn gluten meal |
Enzymatic (Flavourzyme and alkaline protease) | 2 fractions (UF): fraction 1 hydrolysate (MW > 30 kDa) and permeate (MW < 30 kDa). The permeate was further ultrafiltered with a cut- off of 10 kDa to obtain the second retentate (fraction 2, 30 kDa > MW > 10 kDa) and permeate (fraction 3 MW < 10 kDa). 5 fractions of hydrolysates with MW < 10 kDa and 30 kDa > MW > 10 kDa (SEC): F1–F5. |
3 peptides: LPF, LLPF and FLPF in F3. |
Zhuang et al. (2013) |
|
Corn |
Corn proteins |
Enzymatic (Protease Validase FP concentrate from Aspergillus oryzae, Alkaline Protease concentrate from Bacillus licheniformis, Neutral Protease from Bacillus Subtilis) |
4 fractions for each protease (UF): | Not identified. |
(Zhou et al., 2012) |
| F1 (MW > 10 kDa), F2 (10 kDa > MW > 3 kDa), F3 (3 KDa > MW > 1 kDa), F4 (MW < 1 kDa). | |||||
|
Corn |
Zein |
Enzymatic (Alcalase + simulated gastrointestinal digestion with Pepsin + Pancreatin (trypsin and chymotrypsin)) |
Hydrolysate and 3 fractions (SEC) representing 39.9, 23.6, and 35.9% of the total mass, respectively. |
Tetra-, tri-, and dipeptides with MW of 449, 338, and 257 Da rich in Glu (27.2%), Leu (19.2%), Ala (9.5%), Pro (7.8%), Phe (7.2%), and Ser (5.6%). |
(Zhu et al., 2008) |
|
Corn |
Corn gluten proteins |
Enzymatic (Alcalase) |
Hydrolysate and 2 fractions (UF): | 4 peptides: AGI/LPM and HAI/LGA in CPH2. |
Jiang et al. (2018) |
| CPH1 (MW < 1 kDa) and CPH2 (10 kDa < MW < 30 kDa). | |||||
|
Corn |
Corn gluten meal |
Enzymatic (Alcalase) |
Hydrolysate and 2 fractions (UF): | Rich in hydrophobic amino acids, more than 45% of total amino acids. YFCLT in CPF1. |
(Wang et al., 2015) |
| CPF1 (MW < 1 kDa), CPF2 (1 kDa < MW < 3 kDa). | |||||
|
Corn |
Zein |
Enzymatic (Alcalase and Papain) |
Hydrolysates. |
Nonhydrolyzed zein had a relatively high content of Gln, Glu and nonpolar amino acids such as Leu, Ala, and Pro. Cys and Met had 2-3-fold increases in hydrolyzed samples, a slight increase in Lys and Val was also noted. |
Kong & Xiong (2006) |
|
Corn |
Zein |
Enzymatic (Alcalase) |
Hydrolysate and 5 fractions (UF): F1 (>10 kDa), F2 (10 kDa > MW > 5 kDa), F3 (5 KDa > MW > 3 kDa), F4 (3 KDa > MW > 1 kDa), F5 (MW < 1 kDa). | 3 peptides: YA, LMCH, YFYPEL. |
(Tang et al., 2010) |
| F5 was purified into 23 fractions (RP-HPLC). | |||||
|
Corn |
Zein |
Enzymatic (Alcalase, Trypsin, Papain, Flavourzyme) |
Hydrolysates and 2 fractions (UF) from Alcalase: MW > 3 kDa and MW < 3 kDa. | 2 peptides in F3: PF and LPF. |
(Tang and Zhuang, 2014) |
| MW < 3 kDa was purified into 6 fractions (F1–F6) with SEC. | |||||
| Cowpea (Vigna unguiculata L.) | Cowpea seed proteins | Enzymatic (simulated gastrointestinal digestion with Pepsin + Pancreatin) | Hydrolysates (raw and cooked) and the fraction (UF): MW < 3 kDa. | Not identified. | Marques et al. (2015) |
| Cowpea (Vigna unguiculata), black bean (Phaseolus vulgaris) | Cowpea seed proteins and bean proteins | Enzymatic (Alcalase, Flavourzyme, Alcalse + Flavourzyme, and simulated gastrointestinal digestion with Pepsin + Pancreatin | Hydrolysates. | Rich in Asp, Glu, Ser, Arg, Val, Ile, Leu, Phe, Lys. | Segura-Campos et al. (2013) |
| Cowpea (Vigna unguiculata) | Cowpea seed proteins | Enzymatic (Alcalase, Flavourzyme, and simulated gastrointestinal digestion with Pepsin + Pancreatin) | Hydrolysates and 5 fractions (UF): MW < 1 kDa, 1 kDa < MW < 3 kDa, 3 kDa < MW < 5 kDa, 5 kDa < MW < 10 kDa, and MW > 10 kDa. | Not identified. | Segura Campos et al. (2010) |
| Finger millet (Eleusine coracana) | Finger millet proteins | Enzymatic (Trypsin and Pepsin) | Hydrolysates and 3 fractions (UF) from Trypsin: F1 (MW > 10 kDa), F2 (10–3 kDa), and F3 (MW < 3 kDa). F3 was further purified by SEC into 5 fractions, and then RP-HPLC. | The major amino acid was Ser in all fractions, and the ratio of hydrophobic amino acid was higher in F3 and F2 compared to F1. 2 peptides: TSSSLNMAVNGGLTR, STTVGLGISMNSASVN |
Agrawal et al. (2019) |
|
Foxtail millet (Setaria italica) |
Foxtail millet proteins |
Fermentation (solid state fermentation with Lactobacillus paracasei Fn032) |
Hydrolysate and 13 fractions (RP-HPLC). |
3 peptides FFMp4 (SGYYMH), FFMp6 (LGTFQN) and FFMp10 (LHALLL). |
Amadou et al. (2013) |
|
Flaxseed (Linum usitatissimum L.) |
Flaxseed cake proteins |
Enzymatic (Alcalase, Papain, Flavourzyme, Trypsin and Pancreatin) |
Flavourzyme and Pancreatin hydrolysates: had peptides MW < 1 kDa. | Not identified. |
Karamać et al. (2016) |
| Predominance of MW ~390 Da in Flavourzyme, and ~450 Da with the Pancreatin. | |||||
| Trypsin and papain hydrolysates: 45 > had peptides with wide MW > 0.3 kDa (from 0.3 to 45 kDa). | |||||
| Alcalase degraded proteins to hydrolysates: MW < 6.5 kDa. | |||||
| Flaxseed (Linum usitatissimum L.) | Flaxseed cake proteins | Enzymatic (Alcalase) | Hydrolysate and 6 fractions (RP-HPLC). F2 and F5 comprised peptides with the whole range of molecular weights (0.5–4 kDa), F3 and F6 showed predominantly small size peptides (0.5–1 kDa). F1 and F4 showed an intermediate mass profile. | 5 peptides: QGRGGQGGQGQ in F2, NGSGYPGSDLDSSPPGAKVP, GREEIGNVMRSLM in F5, GVKVEGDGGLVRRDEI, GFPGRLDHWCASE in F6. | Silva et al. (2017) |
| Green tea | Tea dregs proteins | Enzymatic (Alcalase, Protamex or Neutrase) | Hydrolysates and 3 fractions (nanofiltration tubular membrane system) of protamex: MW > 8 kDa, 3.5 kDa < MW < 8 kDa and MW < 3.5 kDa. | Peptides contain His, Pro, Ala, Val, Met and Leu. | (Zhao et al., 2014) |
| Hazelnut (C. heterophylla Fisch) | Hazelnut proteins | Enzymatic (Alcalase) | Hydrolysate and fractions (SEC): A1-A3 and then B1–B2 from A3. The fraction B2 with the strongest antioxidant activity was separated through RP-HPLC: C1–C5. | 6 peptides in C2: ADGF, AGGF, AWDPE, DWDPK, ETTL, SGAF. | (Liu et al., 2018) |
| Hemp seed (Cannabis sativa L.) | Hemp seed proteins |
Enzymatic (simulated gastrointestinal digestion with Pepsin + Pancreatin) |
Hydrolysate. |
Hydrolysate has higher contents of aromatic amino acids (20.67%) and hydrophobic (29.13%) when compared to the protein (8.14 and 25.97%). Tyr (from 12.56 to 1.39%) and Arg (from 2.11 to 13.91%) significantly increased in the hydrolysate. |
(Girgih et al., 2014) |
|
Hemp seed (Cannabis sativa L.) |
Hemp seed proteins |
Enzymatic (simulated gastrointestinal digestion with Pepsin + Pancreatin) |
Hydrolysate and 8 fractions (RP-HPLC). | 23 short-chain (≤5 amino acids) peptides in F6–P1 and F6–P2: LPL, LQL, YNL, YNI, WSY, LPAGV, IPAGV, VSYT, PSIPA, LALPA, IPAGM, FEQL, FEQI, EFQL, EFLQ, EFQI, EFIQ, LEEAF, IEEAF, WVYY, PSLPA, WYT, SVYT. Presence of substantial amount of hydrophobic, branched-chain or aromatic amino acids such as Phe, Pro, Gly, Ile, Leu, Tyr, and Trp. |
(Girgih et al., 2014) |
| F4 to F8 were further purified (RP-HPLC). | |||||
| Two pooled peaks for each of the four fractions (F4–P1, F4–P2; F5–P1, F5–P2; F6–P1, F6–P2; and F7–P1, F7–P2) were collected. | |||||
| Hemp seed (Cannabis sativa L.) | Hemp seed proteins | Enzymatic (Alcalase) | Hydrolysate and fractions using MAR + 6 fractions (SEC): FA-FF. Fractions (RP-HPLC) from FA (0.35 KDa < MW < 2 kDa): A1-A6, and 2 fractions from A4 (0.4 KDa < MW < 1.2 kDa): A4a-A4b. | 2 peptides in A4a: NHAV and HVRETALV. Higher amount of hydrophobic amino acids (Tyr, Val, Met, Phe, Ile, Leu, Pro) in fractions using MAR, with enriched Asp, Glu and Arg. | (Lu et al., 2010) |
|
Hemp seed (Cannabis sativa L.) |
Hemp seed proteins |
Enzymatic (simulated gastrointestinal digestion with Pepsin + Pancreatin) |
Hydrolysate and reverse-phase HPLC separation into 8 peptide fractions (F1–F8). |
Leu and Tyr increased 3 and 4-fold in F2. Trp and Phe increased 2 and 3-folds in F5 and F3. Positively charged amino acids (His, Arg and Lys) increased in F1 by 1.7, 1.9 and 2.2-fold. Negatively charged amino acids were pronounced in almost all the fractions, F1 (42.9%), F2 (42.8%) and F4 (40.5%). |
(Girgih et al., 2013) |
|
Jiupei (fermented grains) |
Jiupei proteins |
Chemical/physical (ultrasounds) |
Hydrolysate and 2 fractions (UF): | 2 Tripeptides: VNP in E1 and YGD in D1. |
Jiang et al. (2019) |
| MW > 5 kDa and MW < 5 kDa. Fraction with MW < 5 kDa was fractionated in 5 fractions (SEC), A-E, that were subdivided in RP-HPLC (A1-A4, B1–B3, C1–C3, D1-D3, E1-E1) | |||||
|
Kidney bean |
Kidney bean proteins of the seed globulin protein |
Enzymatic (Alcalase) |
4 fractions (UF): | Hydrolysate and peptide fractions contained low levels of Met and Cys, and high contents of Glu, Gln, Asp, Asn, Lys, and Ala. |
Mundi & Aluko (2014) |
| MW < 1 kDa, 1 kDa < MW < 3 kDa, 3 kDa < MW < 5 kDa, and 5 kDa < MW < 10 kDa. | |||||
| Legumes, Horse gram Lentil, Cow pea, Chickpea, Black pea, White pea, Green gram. | Legumes, Horse gram Lentil, Cowpea, Chickpea, Black pea, White pea, Green gram powders or fractionated aqueous extracts. | Enzymatic (Pepsin, Trypsin and Chymotrypsin) | Hydrolysates. | Not identified. | Jamdar et al. (2017) |
| Manchurian walnut (Juglans mandshurica Maxim.) | Walnut proteins | Enzymatic (Neutrase and Alcalase) | Hydrolysates and 3 fractions (UF): MW > 10 kDa, 3 kDa < MW < 10 kDa, and MW < 3 kDa. | Not identified. | (Ren et al., 2018) |
|
Moringa oleifera |
Moringa seed protein globulin |
Hydrolysis of the GPI (globulin protein fraction) by Alcalase |
4 fractions (UF): MW < 1 kDa, 1 kDa < MW < 3 kDa, 3 kDa < MW < 5 kDa, 5 kDa < MW < 10 kDa. |
Not identified |
Aderinola et al. (2019) |
|
Mucuna pruriens. |
Mucuna pruriens bean proteins |
Enzymatic (Alcalase + Flavourzyme and Pepsin + Pancreatin) |
Hydrolysates and 5 peptide fractions (UF): | Abundance of hydrophobic and hydrophilic amino acids in peptide fractions (MW < 1 kDa). |
Herrera Chalé et al. (2014) |
| MW > 10 kDa, 5 kDa < MW < 10 kDa, 3 kDa < MW < 5 kDa, 1 kDa < MW < 3 kDa, and MW < 1 kDa. | |||||
|
Mulberry (Morus atropurpurea Roxb.) |
Mulberry leaf proteins |
Enzymatic (Neutrase) |
Hydrolysate and 9 fractions (ion exchange chromatography): F1−1, F1−2, F2−1, F2−2, F3, F4, F5, F6, and F7. | 3 peptides: SVL, EAVQ, RDY in R1. |
Sun et al. (2019) |
| 2 fractions (SEC) from F5: G1 and G2. | |||||
| Then, 2 fractions from G1 (RP-HPLC): R1 and R2. | |||||
|
Mungbean |
Mungbean meal protein |
Enzymatic (Bromelain) |
Hydrolysate and 4 fractions (UF): | High content of Glu, Asp, Lys, Arg, Leu, Ser, Pro and Phe in hydrolysate. Asp and Glu were the highest in peptide fractions. F4 had the highest content of Glu, Arg, Gly, Leu, Met, Tyr, Phe, Trp and Ser, and in aromatic amino acid (Tyr, Phe, Trp). |
Sonklin et al. (2018) |
| F1 (MW > 10 kDa), F2 (5 kDa < MW < 10 kDa), F3 (1 kDa < MW < 5 kDa) and F4 (MW < 1 kDa). | |||||
|
Oat |
Oat flour proteins |
Enzymatic (Alcalase and Trypsin) |
Hydrolysates and 3 fractions (UF): MW < 2 kDa, 2 kDa < MW < 10 kDa and MW > 10 kDa. |
Not identified. |
Tsopmo et al. (2010) |
|
Palm (Elaeis guineensis) |
Palm kernel cake |
Enzymatic (Alcalase, Papain, Pepsin, Flavourzyme, Trypsin, Chymotrypsin and Bromelain) |
Hydrolysates and 31 fractions (RP-HPLC) from Papain. | 9 peptides: YLLLK, YGIKVGYAIP, GGIF, GIFE, WAFS, GVQEGAGHYALL, WAF, AWFS, LPWRPATNVF. High percentage (>50%) of hydrophobic residues Trp, Ala, Phe, Tyr, Leu, Ile, Val and Ala. |
Zarei et al. (2014) |
| Fractions were further fractionated by extracted ion chromatogram. | |||||
| The MW of all peptides were ranging of 0.39–1.20 kDa. | |||||
| Palm (Elaeis guineensis) | Palm kernel cake | Enzymatic (Trypsin) | Hydrolysate. | Tyr, Met, His and Lys were important, along with hydrophobic amino acids (47.46 g/99.06 g) protein. | Ng et al. (2013) |
|
Palm (Elaeis guineensis) |
Palm kernel cake |
Enzymatic (Papain, Alcalase, Pepsin, Trypsin, Flavourzyme, Bromelain, Chymotrypsin) |
Hydrolysates and 31 fractions (RP-HPLC) from the papain hydrolysate. |
Small and hydrophobic peptides with basic or neutral isoelectric point. |
Zarei et al. (2012) |
|
Pea (Pisum sativum L.) |
Pea seed proteins |
Enzymatic (Thermolysin) |
Hydrolysate and fraction (UF) MW < 3 kDa. | Hydrophobic amino acids (Val, Leu, Ile, Phe, Trp, Pro) increased from F1 to F5, while Ala, Ser, Lys, Arg, His, Asp, Asn decreased. |
Pownall et al. (2010) |
| The resulting permeate was divided into 5 fractions (RP-HPLC). | |||||
| Peanut | Roasted and defatted peanut kernels | Enzymatic (Esperase and Neutrase) | Hydrolysates. | Not identified | Hwang et al. (2001) |
|
Peanut (Arachin conarachin L.) |
Peanut proteins |
Enzymatic (Alcalase) and with ultrasound assisted proteolysis |
Hydrolysates. |
Not identified |
(Yu et al., 2012) |
|
Peanut (Arachis hypogaea L.) |
Peanut kernel proteins |
Enzymatic (Esperase, Neutrase, Pepsin, Protease, Protease N) |
Hydrolysates and 3 fractions (UF) from Esperase: FI (MW < 3 kDa), FII (3 kDa < MW < 5 kDa), FIII (5 kDa < MW). | Not identified |
Hwang et al. (2010) |
| FII was further purified by SEC and RP-HPLC. | |||||
|
Peanut (Arachis hypogaea L.) |
Defatted peanut meal |
Fermentation (from A. oryzae under solid state fermentation with defatted peanut meal as culture medium). |
Hydrolysate and 2 fractions (UF): DPMH-I (MW > 3 kDa) and DPMH-II (MW < 3 kDa). | 1 peptide: YGS in F4. |
(Zheng et al., 2012) |
| DPMH-II was purified (SEC) in 4 fractions (F1 – F4). F4, highest antioxidant activity, was purified (RP-HPLC, 5 fractions). | |||||
| Pearl millet (Pennisetum glaucum) | Pearl millet proteins | Enzymatic (Trypsin) | Hydrolysate and 25 fractions (SEC). Highest antioxidant fractions (F6) were further purified with RP-UFLC (F6A, F6B, F6C, F6D). | 1 peptide: SDRDLLGPNNQYLPK. | Agrawal et al. (2016) |
| Perilla (Perilla frutescens L. Britton) | Perilla seed proteins | Enzymatic (Alkaline protease) | Hydrolysate and 3 fractions (SEC): a, b, c. Fraction c with the lowest MW was purified (RP-HPLC) and separated in 50 fractions. | 2 peptides: YL and FY. | (Yang et al., 2018) |
| Pigeon pea (Cajanus cajan) | Pigeon pea seed proteins | Enzymatic (Alcalase, Pancreatin and Pepsin + Pancreatin sequentially) | Hydrolysates and 5 fractions (UF): MW < 1 kDa, 1 kDa < MW < 3 kDa, 3 kDa < MW < 5 kDa, 5 kDa < MW < 10 kDa, and MW > 10 kDa. | Not identified. | Olagunju et al. (2018) |
| Pine nut (Pinus koraiensis) | Pine nut meal proteins | Enzymatic (Alcalase) | Hydrolysate and 4 fractions (UF): MW < 1 kDa, 1 kDa < MW < 3 kDa, 3 kDa < MW < 10 kDa, and MW > 30 kDa 3 kDa < MW < 10 kDa peptides were purified (SEC) and 4 fractions were obtained: F1, F2, F3, F4. | 2 peptides: KWFCT, QWFCT in F4. | (Yang, Li, et al., 2017) |
| Pine nut (Pinus koraiensis) | Pine nut proteins | Not mentioned | Hydrolysate with MW of 3–10 kDa. | 1 peptide: QDHCH | Liang et al. (2017) |
| Potato | Potato proteins | Enzymatic (Alcalase) | Hydrolysate, fractionated with ammonium sulfate precipitation (P30, P50, P70, P90, PR) and 27 fractions (P50–F1 to P50–F27) from P50 (RP-HPLC). | 6 peptides from P50–F10, P50–F12, and P50–F13: TSNLLT, SSGFTMQ, KPYVFRATGAL, LMRWMR, SSGFTY, IYLGQ. |
Cheng et al. (2010) |
| Potato | Potato proteins | Enzymatic (Alcalase) | Hydrolysate and 78 fractions (SEC). | 21 peptides from 200 to 800 Da, dipeptides to heptapeptides: ALEPRP, VTLLADKQ, KPSQSLQ, DLKSV, RLCAK, LTEPGR, NSVDLQ, KNGKAM, LFVRAS, DF, PLAL, RPALM, SFDL(I)K, TEVL, GPVNLL, SFY, SFDL(I)K, PDE, AQRVP, ENYKT, RTQ. | Cheng et al. (2014) |
| Pumpkin | Pumpkin seed meal proteins | Enzymatic (Alcalase, Flavourzyme, Protamex and Neutrase) | Hydrolysates with distribution (SEC) ~15–20% (MW < 0.180 Da), 34–57% (0.180 < MW < 1 kDa), 23–38% (1 < MW < 5 kDa), 1–7% (5< MW < 10 kDa), 1–2% (MW > 10 kDa) for all, expect for the Flavourzyme that was ~12% (MW < 0.180 Da), 26% (0.180< MW < 1 kDa), 22% (1 < MW < 5 kDa), 12% (5 < MW < 10 kDa), 29% (MW > 10 kDa). | About 20% of hydrophobic amino acids. Rich in Arg (~12–1313%), Val (~2–33%), Lys (~2.52.5%), Glu (~15–1616%), Tyr + Phe (~5–66%) and Leu (~3–55%) | Venuste et al. (2013) |
|
Rapeseed (Brassica napus L.) |
Rapeseed proteins |
Fermentation (solid state fermentation with Bacillus subtilis) |
Hydrolysate and 5 fractions (UF): MW < 5 kDa (5.5 kDa, 3.1 kDa, 1.7 kDa, 0.61 kDa, 0.18 kDa size range). |
Glu (19.5%), Lys (7.6%) and Pro (7.3%) were the most dominant amino acids but Ser (1.5%), Trp (1.3%) and Cys (0.5%) were present in least amounts. |
He et al. (2012) |
|
Rapeseed (Brassica napus L.) |
Rapeseed proteins |
Enzymatic (combination of pectinase, cellulase, and β-glucanase + Alcalase) |
Hydrolysate and 3 fractions (RP25, RP55, and RP85). | 1 peptide: PAGPF. |
(Zhang et al., 2009) |
| RP55 was fractionated by anion-exchange chromatography into 3 fractions (E1, E2, and E3). | |||||
| E2 with higher protein content was sequentially purified by SEC and RP-HPLC. | |||||
| Rapeseed (Brassica napus L.) | Rapeseed proteins | Enzymatic (Thermolysin, Proteinase K, Alcalase, Pepsin + Pancreatin, and Flavourzyme) | Hydrolysates and 4 fractions (UF): MW < 1 kDa, 1 kDa < MW < 3 kDa, 3 kDa < MW < 5 kDa, and 5 kDa < MW < 10 kDa. Alcalase, Thermolysin and Proteinase K hydrolysates contained more peptides in the MW range of 143–2639 Da. | Not identified. | He et al. (2013) |
| Rapeseed (Brassica napus L.) | Rapeseed proteins | Enzymatic (Alcalase) | Hydrolysate. | High contents of hydrophobic amino acids (Ile, Leu, and Lys, ~21.45%), relatively high levels of Glu (14.74%), Pro (8.30%), Gly (7.83%), aroma amino acids (8.46%), sulfur-containing amino acids (3.43%) and rich in His (4.95%). | Pan et al. (2011) |
|
Rapeseed (Brassica napus L.) |
Rapeseed proteins |
Enzymatic (combination of Pectinase, Cellulase, and β-glucanase, followed by sequential treatments of alkaline extraction and alkaline protease (Alcalase)) |
Hydrolysate and 3 fractions RP25, RP55, and RP85 (desorption from the resin column) with different levels of bitterness and protein content. |
Highest contents of hydrophobic amino acids in RP55. |
( Zhang et al., 2008) |
|
Rapeseed (Brassica napus L.) |
Australian canola meal proteins |
Enzymatic (Alcalase, Chymotrypsin, Pepsin, Trypsin and Pancreatin) |
Hydrolysates and 4 fractions (UF): | High levels of Lys and Leu, along with Thr, Val, Ile, Tyr, and Lys. |
Alashi et al. (2014) |
| MW < 1 kDa, 1 kDa < MW < 3 kDa, 3 kDa < MW < 5 kDa, and 5 kDa < MW < 10 kDa. | |||||
|
Rapeseed (Brassica napus L.) |
Albumin |
Enzymatic (Alcalase and Flavourzyme sequentially) |
Hydrolysate and 4 fraction (SEC): | 1 peptide: PFDSYFVC in RSP-4. |
(W. Yu et al., 2013) |
| RSP-1, RSP-2, RSP-3, and RSP-4. | |||||
|
Rapeseed (Brassica napus L.) |
Rapeseed proteins |
Enzymatic (Alcalase and Flavourzyme sequentially) |
Hydrolysate and 3 fractions (SEC): | RSP fractions are rich in Arg, Glu, Phe, Leu, Tyr, and Pro. Hydrophobic amino acids accounted for <35% for all three RSP fractions |
Xue et al., 2009a, Xue et al., 2009b |
| RSP1, RSP2 and RSP3. | |||||
| Rapeseed (Brassica napus L.) | Rapeseed proteins | Enzymatic (Alcalase, Flavourzyme, and Alcalase + Flavourzyme) | Hydrolysates. | Not identified. | Cumby et al. (2008) |
| Brown rice | Brown rice proteins | Enzymatic (Bromelain and Protease FP51®) | Hydrolysates. MW peptides were low (~57, 37 and less than 5 kDa) with Protease FP51®. Bromelain led to larger peptides (~37 to less than 10 kDa), but absence of rice glutelin (~57 kDa) due to a bromelain-specific cleavage at the Arg-Ala and Ala-Glu bonds. | Not identified. | Selamassakul et al. (2016) |
| Organic Thai rice | Rice bran proteins | Enzymatic (gastrointestinal digestion with Pepsin, and Pepsin + Trypsin) | Hydrolysates and 3 fractions (UF): F1 (MW < 3 kDa, F2 (MW 3–5 kDa, and F3 (MW 5–10 kDa) | Peptides in F1 were detected at m/z 553 and m/z 2773. Peptides with m/z at 609, 944, and 1088 were three major fragments. The majority of peptides with m/z at 1088 were likely octapeptides. | Phongthai et al. (2018) |
| Thai brown rice | Protein fractions: Albumin (2.18%), globulin (3.98%), glutelin (84.23%), and prolamin (9.61%). | Enzymatic (Bromelain) | Hydrolysates. Low MW, less than 15 kDa, and mainly composed of oligopeptides (500–2000 Da, 60–70%), followed by polypeptides (>2000 Da, 20–30%). 6 fractions (F1–F6) by RP-HPLC. | 6 peptides in F4: SPFWNINAHS, MPVDVIANAYR, HIAGKSSIFRA, VVYFDQTQAQA, FDTADLPSGKGYL, AVYVYDVNNNANQ. 3 peptides in F5: VEVGGGARAP, YNILSGFDTEL, VVSNFGKTVFDGVL. |
Selamassakul et al. (2018) |
|
Rice |
Rice residue proteins |
Enzymatic (Alcalase, Trypsin, Protamex, Flavourzyme, Pepsin, Papain (alone and combination)) |
Hydrolysates and fraction (UF) from Papain:Flavourzyme:Protamex = 2:3:1 with MW < 3 KDa. Permeate was fractionated with SEC in 4 fractions (RRPA-RRPD). RRPB was separated with RP-HPLC in 5 fractions (RRPB1–RRPB5). |
4 peptides: RPNYTDA, TSQLLSDQ, TRTGDPFF and NFHPQ in RRPB3. |
Yan et al. (2015) |
|
Rice bran |
Albumin, globulin, glutelin, prolamine (native and denturated) |
Enzymatic (Papain and Trypsin) |
Hydrolysates and 24 fractions from trypsin/albumin denaturated (RP-HPLC). |
3 peptides in F14: VAGAEDAAK, AAVQGQVEK, and GGPAAAMESAASR. |
Wattanasiritham et al. (2016) |
| 3 peptides in F15: EAAANVGASAR, NAADKDAAEVR and AKDAADMAQGTAR. | |||||
| 3 peptides in F16: GQTVVPGGTGGK, DKAVAADQGGGGG-DLR and IPGPGSGGAGAGAAAGEGK. | |||||
| Peptides were rich in hydrophobic amino acids, Gly and Ala, with MW of 800–2100 Da and with 6–21 amino acid residues. | |||||
|
Rice bran |
Rice bran proteins (Phytate-free) |
Enzymatic (Protease M from Aspergillus oryzae, protease N from Bacillus subtilis, protease P from Aspergillus melleus, protease S from Bacillus and Pepsin) |
Hydrolysates. | 3 peptides: AIRQGDVF, VLEANPRSF, YFPVGGDRPESF. 100% homologies with the globulin-like Oryza sativa japonica cultivar group. |
Adebiyi et al. (2008) |
| Protease P hydrolysate was further purified and fractionated into fractions using several RP-HPLC. | |||||
|
Rice bran |
Rice bran protein fractions: Albumin, globulin, glutelin and prolamin |
Enzymatic (Protease M from Aspergillus oryzae, protease N from Bacillus subtilis, protease P from Aspergillus melleus, protease S from Bacillus and Pepsin) |
Hydrolysate and 63 fractions (RP-HPLC) for 2 h-pepsin hydrolyzed globulin. | 19 peptides: 6–30 amino acids, MW from 670 to 3611 Da. LVDTGRGPIMY, EEEQVGQGYETIRARL, FVAPAGTINY, YEADARSFHDLAEHDIRV, YEADARSFHDLAEHDIRV, LRGIKNYRVAVL, AAVGGYRVAVL, YVAQGEGVVA, YLAGMN, IIENGEKWS, IAPNYNTRATKL, YLAGMN, IIENGEKWS, WSRRGEREEEDERRRHGGEGGRPYHLGEES, YVAQGEGVVA, VAVDKHDYEPLGHSDIGVY, FFAPGRNPTSFS, EEYFPVGGDRPESF, WEIKPSSLTGKSPYFSNNHGKL. |
Adebiyi et al. (2009) |
| 11 peptide fractions were subjected to the second step of purification (RP-HPLC). | |||||
| Rice bran | Rice bran proteins | Enzymatic (Trypsin) | Hydrolysate and 3 fractions (UF): MW < 4 kDa, 4 kDa < MW<6 kDa, MW>6 kDa. MW < 4 kDa was purified with SEC (3 fractions, F1, F2, F3) and RP-HPLC. | 1 peptide: YSK in F2. | (Wang et al., 2017) |
| Schizochytrium Limacinum | Schizochytrium Limacinum residue proteins | Enzymatic (Papain, Trypsin, Flavourzyme, Protamex, Alcalase and their combination) | Hydrolysate and 3 fractions from Protamex–Alcalase (UF): SLH-I (MW < 50 kDa), SLH-II (MW < 10 kDa), and SLH-III (MW < 5 kDa). SLH- III was further separated into 5 fractions (A–E) by SEC. SLH–III–A was separated into 8 fractions (RP-HPLC). | 1 peptide in SLH–III–A4: PYK. | Hu et al. (2019) |
| Schizochytrium sp. | Schizochytrium sp. meal proteins | Enzymatic (Flavourzyme) | Hydrolysate with MW from 71 to 21814 Da with 57.2% of MW < 5 kDa. | Asp and Glu constituted approximately 26.32% of the total amino acids, and Glu was the most abundant. | (Yang et al., 2019) |
| Schizochytrium sp. | Schizochytrium sp. meal proteins | Enzymatic (Alcalase and Flavourzyme sequentially) | 2 fractions (UF): SPH-I (MW < 3 kDa) and SPH-II (MW > 3 kDa). | Hydrolysate was mainly composed of Glu (17.66%), Asp (15.89%), Leu (9.96%), and Arg (7.81%) along with small amounts of Phe (5.27%), Tyr (2.71%), and His (1.57%). | Cai et al. (2017) |
|
Sesame (Sesamum indicum L.) |
Sesame seeds protein |
Enzymatic (Papain, Alcalase and Trypsin) |
4 fractions (IMAC-Zn2+): Fractions 2 and 3 were purified by RP-HPLC: Fraction 2-p1 and 3-p2 were responsible for the highest metal chelating activities. |
6 peptides in 2-p1 and 3-p2: SM, LAN, IAN, RKR, RQR, NCS. |
(Wang et al., 2012) |
|
Sesame (Sesamum indicum L.) |
Sesame seeds protein |
Enzymatic (Alcalase + Trypsin 3:1) |
Hydrolysate and 4 fractions (UF): | 7 peptides in P4: RDRHQKIG, TDRHQKLR, MNDRVNQGE, RENIDKPSRA, SYPTECRMR, GGVPRSGEQEQQ, AGEQGFEYVTFR |
(Lu et al., 2019) |
| DSPH-I (MW > 10 kDa), DSPH-II (8 < MW < 10 kDa), DSPH-III (5 < MW < 8 kDa), DSPH-IV (3 < MW < 5 kDa) and DSPH-V (MW < 3 kDa). DSPH-V was purified into 9 fractions (P1–P9) by RP-HPLC. | |||||
| Sesame (Sesamum indicum L.) | Sesame seeds protein | Enzymatic (Protease A Amano 2G from Aspergillus oryzae) | Hydrolysate and 3 fractions (UF): MW < 5 kDa, MW < 2 kDa, MW < 1 KDa. | Not identified | Das et al. (2012) |
| Sorghum | Kafirin | Enzymatic (Alcalase, Flavourzyme, Neutrase, Everlase, Protamex, trypsin, Pepsin, bromelain, Ficin, and Papain) | Hydrolysates and 3 fractions (UF) from Neutrase: MW < 3 kDa, 3 kDa < MW < 10 kDa, and MW > 10 kDa 3 kDa < MW < 10 kDa was purified by SEC in 4 fractions (F1–F4). | 23 peptides in F2: QAMCGVV, VAQNMP, MRMMDMQS, MDMQSRCQAM, AMCGVVQ, VQSVVQ, GGGLYPCAEY, MMDMQSRCQA, SASALQM, QPQCSP, VAQVAQNMPA, TPLAMAVAQVAQ, PAAQALTPL, AVAQVAQNMP, QQMRMMDMQ, LPAAQALTP, TPCATSAAIPP, LPSYCTTP, FLYPCAEYL, SAAIPPYY, VQSVVQQLQ, CGLYQLPS, YALREQT. Glu and Ala the most abundant amino acids. MDMQ and VAQ the most frequent sequences. | Xu et al. (2019) |
| Sorghum | Green tender sorghum proteins | Enzymatic (Alcalase) | Hydrolysate and 3 fractions (UF): UF3 (MW < 3 kDa), UF2 (3 kDa < MW < 10 kDa), and UF1 (MW > 10 kDa). The major amino acids were Arg and Lys. The amount of aromatic (Phe and Tyr), hydrophobic (Ala, Ile, Leu, Tyr, Pro and Cys) and positively charged amino acids in UF3 was significantly higher. UF3 was purified by SEC (6 fractions GF1-FF6) and GF2 was divided by RP-HPLC into 5 sub-fractions (F2A-F2E). | 7 peptides: VPPSKLSP (in F2A), VAITLTMK (in F2B), GLLGKNFTSK (in F2C), LDSCKDYVME (in F2D), HQTSEFK (in F2E), VSKSVLVK (in F3A), TSVEIITSSK (in F3B). | Agrawal et al. (2017) |
| Soybean | Soybean proteins | Enzymatic (Flavourzyme) | Hydrolysate and 3 fractions (UF): SPUF-10 (MW < 10 kDa), SPUF-50 (MW < 50 kDa), SPR (MW > 50 kDa). | Not identified. | Moure et al. (2006) |
| Soybean | Soybean proteins | Enzymatic (neutral protease from Bacillus subtilis, validase from Aspergillus oryze, and alkaline protease from Bacillus licheniformis) | Hydrolysates and 4 fractions (UF): F1 (MW > 10 kDa), F2 (MW > 3 kDa), F3 (MW > 1 kDa), F4 (MW < 1 kDa). | Not identified. | (Zhang et al., 2010) |
| Soybean | Soybean proteins | Enzymatic (Alcalase) | Hydrolysate. | Not identified. | (Zhao and Xiong, 2015) |
| Soybean | Soybean proteins | Enzyme-assisted aqueous extraction (Alcalase) | Hydrolysate and 4 fractions (UF): SPH-I (MW < 3 kDa), SPH-II (3–5 kDa), SPH-III (5–10 kDa), and SPH-IV (MW > 10 kDa). | 4 peptides: VVFVDRL (SPH-IA), VIYVVDLR (SPH-IB), IYVVDLR (SPH-IC), and IYVFVR (SPH-ID). | (Zhang et al., 2019) |
| Soybean | Soybean protein (glycinin) | Enzymatic (Alcalase + simulated gastrointestinal digestion with Pepsin + Pancreatin) | Hydrolysate and 2 fractions (SEC): 1 kDa < MW < 5 kDa and MW > 10 kDa. After digestion, the larger peptide fractions were degraded into oligopeptides and amino acids. The two fractions of 5–10 kDa and 1–5 kDa were reduced, while the MW < 1 kDa fraction was significantly increased. | Peptides contained at least one amino acid residues such as Pro, Asp, Leu, Val, Arg and His, + several repeating amino acids, such as Pro-Pro, Glu-Glu and Asn-Asn. | (Zhang et al., 2018) |
|
Soybean |
Soybean proteins |
Enzymatic (3 purified (Pepsin, Papain, and Chymotrypsin) and 3 crude (Alcalase, Protamex, and Flavourzyme)). |
Hydrolysates. |
Not identified. |
Penta-Ramos & Xiong (2002) |
|
Soybean |
β-conglycinin (7s protein) |
Enzymatic (from protease M Aspergillus melleus, P Aspergillus oryzae, S Bacillus sp., and N Bacillus subtilis) |
Hydrolysates and 3 fractions (SEC) for protease S. | 6 peptides: LLPHH, VNPHDHQN, LVNPHDHQN, LLPHHADADY, VIPAGYP, LQSGDALRVPSGTTYY. |
(Chen et al., 1995) |
| Fraction 3 was separated with RP-HPLC into 5 fractions (F1–F5). | |||||
| Soybean | Soybean (2 S albumin) | Synthesized by the conventional Fmoc solid-phase synthesis method | Synthetic peptides. | Lunasin (SKWQHQQDSCRKQLQGVNLTPCEKHIMEKIQGRGDDDDDDDDD) and lunasin-derived peptides (SKWQHQQDSC, RKQLQGVN, VNLTPCEKHIME, LTPCEKHIME, KIQGRGDDDDDDD, KIQGRGDDDDDDDDD). | Indiano-Romacho et al. (2019) |
| Soybean | Soybean proteins | Enzymatic (Alcalase) | Hydrolysate and 4 fractions (UF): MW < 3 kDa, 3 kDa < MW < 10 kDa, 10 kDa < MW < 30 kDa, and MW > 30 kDa. | Not identified. | (Park et al., 2010) |
|
Soybean |
Soybean proteins (glycinin, β-conglycinin and 7 S globulin) |
Enzymatic (Protease S from Bacillus sp.) |
Hydrolysate. |
1 isolated antioxidative peptide: LLPHH. 28 synthetic peptides: HPLH, HHLP, HL, HLPH, LLPH, PLHH, HPHL, HH, LLHH, HHPLL, HLHP, LPHH, HHPL, LHPH, LH, LLPHH, HLH, LLPHHH, LHH, PHH. |
(Chen et al., 1996) |
|
Soybean |
Soybean proteins |
Enzymatic (Alcalase) |
Hydrolysate and 5 fractions (UF): MW > 30 kDa (SPH-1), 10 kDa < MW < 30 kDa (SPH-2), 3 kDa < MW < 10 kDa (SPH-3), 1 kDa < MW < 3 kDa (SPH-4) and MW < 1 kDa (SPH-5). | 2 peptides: | (Yang, Wang, et al., 2017) |
| SPH-4 was fractionated using SEC. |
SHECN and LPFAM |
||||
|
Soybean |
Soybean proteins |
Enzymatic (simulated gastrointestinal digestion with Trypsin + Pancreatin) |
Hydrolysate and fraction (UF) with MW < 10 kDa. | 9 peptides in fraction 7: WNLNAN, SLDFPALW, FESFFL, FQTLF, SYLQGF, TTYY, LFF, LY, IY. |
Beermann et al. (2009) |
| Permeate was purified in 7 fractions (SEC). |
Originate from storage-proteins β-conglycinin and glycinin, corresponding to 7 S and 11 S globulins. |
||||
|
Sunflower |
Sunflower proteins |
Enzymatic (simulated gastrointestinal digestion with Pepsin + Pancreatin) |
Hydrolysate and 7 fractions (RP-HPLC). |
Peptides contains amino acids such as His and Arg. |
Megías et al. (2008) |
|
Sweet potato |
Sweet potato proteins |
Enzymatic (Alcalase) |
Hydrolysate and 4 fraction (UF): | 5 peptides in fractions IV-5c and IV-5i: TYQTF, SGQYFL and YMVSAIWG matched the sequence of sporamin A, while YYIVS and YYDPL matched the sequence of sporamin B. |
(Zhang et al., 2014) |
| F–I (MW > 10 kDa), F-II (5 kDa < MW < 10 kDa), F-III (3 kDa < MW < 5 kDa) and F-IV (MW < 3 kDa). | |||||
| Sweet potato | Sweet potato proteins | Enzymatic (Alcalase) | Hydrolysate and 3 fractions (UF): FI (MW > 10 kDa), FII (3 kDa < MW < 10 kDa) and FIII (MW < 3 kDa). | 5 peptides: HDSASGQY, YYMVSA, HDSESGQY, YYIVS, RYYDPL. | (Zhang and Mu, 2017) |
| Walnut | Defatted Walnut meal proteins | Enzymatic (simulated gastrointestinal digestion with Pepsin + Pancreatin) | Hydrolysate and 5 fractions (UF): DWMPH-I (MW > 10 kDa), DWMPH-II (5 kDa < MW < 10 kDa), DWMPH-III (3 kDa < MW < 5 kDa), DWMPH-IV (1 kDa < MW < 3 kDa) and DWMPH-V (MW < 1 kDa). | 6 peptides: VRN, NPAN, AHSVGP, SSE, TY, SGGY. | Feng et al. (2019) |
| Walnut | Defatted Walnut meal proteins | Enzymatic (Neutral protease) | Hydrolysate and adsorbed iron-chelated peptides (IMAC-Fe3+ column). The iron-binding peptides were separated into ~10 fractions by RP-HPLC. | 2 peptides: LAGNPDDEFRPQ, VEDELVAVV in F32min. | Lv et al. (2017) |
| Walnut (Juglans regia L.) | Walnut proteins | Enzymatic (Neutrase, Alcalase and Pepsin) | Hydrolysates and 2 fractions (UF): WPH-I (MW > 3 kDa) and WPH-II (MW < 3 kDa). WPPH-II was separated in 4 fractions (F1–F4) by SEC. The active fractions F3 were fractionated (14 fractions) with a RP-HPLC. | 1 peptide (Pepsin hydrolysates) with the highest antioxidative activity: ADAF. | (Chen et al., 2012) |
|
Walnut (Juglans Sigillata Dode) |
Walnut defatted meal protein |
Enzymatic (Pancreatin) |
Hydrolysate and 5 fractions (SEC): A > B > C > D > E. The fraction D was purified (RP-HPLC) into five portions (D1-D5). |
14 peptides containing high amount of Tyr and Cys. 6 peptides (YS, YSVH, YK, YT, LPC, QM) in D2 and 8 peptides (CA, SQK, CR, CHC, GHC, YA, YG) in D3. |
Gu et al. (2015) |
|
Watermelon |
Watermelon seed proteins |
Enzymatic (Alcalase) and with ultrasound pretreatment proteolysis |
Hydrolysate and 3 fractions (UF): | WSPH-I was rich in hydrophobic amino acids (15.642%), including Gly (7.799%), Ala (1.778%), Val (1.757%), Met (0.467%), Ile (1.577%), Leu (2.777%), Tyr (1.345%), and Phe (1.886%); and high proportion of aromatic amino acids (4.517%), including Tyr (1.345%), Phe (1.886%), His (1.286%), Pro (1.125%). |
Wen et al. (2019) |
| WSPH-I (MW < 1 kDa), WSPH-II (1 kDa < MW < 5 kDa) and WSPH-III (MW > 5 kDa). | |||||
|
Wheat |
Gluten |
Enzymatic (Papain) |
Hydrolysate and 2 fractions (UF): | Contain Glu, His, Leu, Val, Ala. |
(Wang et al., 2007) |
| MW > 5 kDa and MW < 5 kDa. | |||||
| Wheat | Wheat germ proteins | Enzymatic (Alcalase) | Hydrolysate with high contents of Gly, Lys, Ala, Pro, hydrophobic amino acids. | Glu, Arg and Asp were the major amino acids. High proportion of peptides from 1500 Da to free amino acids. | (Zhu et al., 2006) |
| Wheat | Gluten | Enzymatic (commercial production from Nisshin Pharma, Tokyo, Japan) | Hydrolysate and 4 fractions (autofocusing). GP1 (acidic, Fr. 1–4), GP2 (weak acidic, Fr. 5), GP3 (neutral, Fr. 6–7), and GP4 (basic, Fr. 8–10). More than 85% of the peptides were between Fr. 5 and 7 (4 < pH < 7), ~10% in basic fractions (pH > 8.0, GP4), and a low % in the acidic fractions (pH < 4.0, GP1). | The peptides in the acidic and basic fractions are characterized by a higher content of acidic and basic amino acids, respectively. | (Park et al., 2012) |
UF: Ultra-filtration, MW: Molecular weight, RP-HPLC: Reverse phase chromatography, RP-UFLC: reversed phase ultra-flow liquid chromatograph, SEC: Size exclusion chromatography, MAR: Macroporous adsorption resins, IMAC: immobilized metal affinity chromatography.
3.2. Production, isolation and identification of the bioactive peptides
The processes for production, isolation and identification of antioxidant BP are different but some of them are common to many studies. The first step for producing antioxidant BP is the extraction of the protein content from the plant biomass. Three plant proteins enriched materials are distinguished according to their protein concentration on a dry base: flours (50–65%), concentrates (65–90%) and isolate (>90%) (Guéguen et al., 2016). Many reports describe processes of extraction and isolation of protein from plant biomass (Rodrigues et al., 2012). For most of them, a solid/liquid extraction in aqueous media at alkaline pH was employed to reach high protein extraction yields. Then, to reach an isolate protein content, a second step of protein purification should be applied to eliminate microsolutes. Isoelectric precipitation (acidic conditions), membrane process (ultrafiltration), chromatographic techniques or micellization are commonly implemented.
The second step for producing antioxidant BP is commonly the enzymatic hydrolysis of the plant protein materials obtained. Enzymatic protein hydrolysis involves a protease which catalyzes the hydrolysis reaction of certain peptide bonds, resulting in a complex mixture (hydrolysate) of various peptides and amino acids and may also contain residual intact proteins. Multiple conditions involving both the initial protein substrate, the enzyme and the operating conditions of the hydrolytic process contribute to the composition and functional activities of BP. Proteases recognize more or less specific cleavage sites in the protein chain. The protease cleavage specificity and the amino acid sequence of the initial protein play thus an important role in the hydrolysate composition. The composition and characteristics of the peptides obtained (the average peptide length and exposition of the side chains) are also influenced by the hydrolysis reaction advancement and the enzymatic mechanism. The proteolysis advancement is usually monitor with the degree of hydrolysis (DH) which represents the ratio of peptide bonds cleaved on protein peptide bonds. Limited hydrolysis is thus commonly characterized by a low DH value (<10%), and extensive hydrolysis by a high DH value (>10%). The extent of the enzymatic proteolysis process can also be quantified by the protein conversion rate (Beaubier et al., 2019).
The frequently used hydrolytic enzymes for producing BP from plant biomass, were commercially available proteases from microbial or animal resources, such as Flavourzyme (EC 3.4.15.1; microbial), Neutrase (EC 3.4.24.28; microbial), Trypsin (EC 3.4.21.4; animal), Thermolysin (EC 3.4.24.27; microbial), and Pepsin (EC 3.4.23.15; animal). The most reported hydrolytic enzyme was the commercial preparation Alcalase (EC 3.4.21.14), a non-specific serine endoprotease that consists primarily of subtilisin A from Bacillus licheniformis. Cysteine proteases from plants, such as Bromelain (EC 3.4.22.33) from pineapple, Ficin (EC 3.4.22.3) from fig-tree latex, and Papain (EC 3.4.22.2) from papaya latex, have been also tested (Karamać et al., 2016; Kong and Xiong, 2006; Penta-Ramos and Xiong, 2002; Selamassakul et al., 2016; 2018; Wang et al., 2012; Wang et al., 2007; Wattanasiritham et al., 2016; Xu et al., 2019). Moreover, sequential proteolysis by different enzymes has been examined, especially to simulate gastrointestinal digestion (e.g. Pancreatin, Pepsin, trypsin, chymotrypsin) in order to investigate the fate of digested peptides or hydrolysates with respect to their antioxidant action (Carrasco-Castilla et al., 2012b; Chanput et al., 2009; Feng et al., 2019; Girgih et al., 2014; Girgih et al., 2013; 2014; He et al., 2013; Herrera Chalé et al., 2014; Ma et al., 2010; Marques et al., 2015; Megías et al., 2008; Segura Campos et al., 2010; Torres-Fuentes et al., 2015; Zhang et al., 2018; Zhang et al., 2008; Zhu et al., 2008). Yet, one disadvantage of extended proteolysis is the release of hydrophobic groups leading to bitter peptides. Sequential proteolysis by Alcalase followed by Flavourzyme is known to overcome this issue. A combination of hydrolytic enzymes such as Pectinase, Cellulase or β-glucanase have been also tested in addition to proteases (e.g. Alcalase) to improve the production of BP (Zhang et al., 2008, 2009). Another way to obtain antioxidant peptides by protein hydrolysis, which is not widely reported, was the fermentation by different bacteria (Amadou et al., 2013; Coda et al., 2012; He et al., 2012; Zheng et al., 2012). Other methods such as ultrasonic-assisted hydrolysis (Jiang et al., 2019), in combination with enzymes (Yu et al., 2012), or for the pretreatment of biomass proteins (Wen et al., 2018, 2019), have been tested, but with less attention. However, it has been found that the ultrasound treatment could significantly improve antioxidant activities of the hydrolysate, likely by affecting the protein structure and increasing their susceptibility to proteolysis. In this regard, technologies such as high hydrostatic pressure (HHP) has been recognized as one of the most promising emerging technologies, with growing commercial interest, to improve enzymatic hydrolysis of proteins and generation of BP (Marciniak et al., 2018; Zhang and Mu, 2017).
After enzymatic hydrolysis step, peptide fractions were, in most cases, further purified by fractionation, based on the size of the molecules, in either membrane processes or in column chromatography. Indeed, enzymatic hydrolysis produces very complex mixtures which often lead to a low activity of hydrolysate containing bioactive peptides. Ultrafiltration was usually applied for the peptide enrichment from protein hydrolysates and allows for the elimination of the residual intact protein fraction if needed. This membrane process separates the peptide fractions according to the selectivity of the microporous membrane used. The membrane allows for the transmission and retention of certain component according to their hydrodynamic volume (or Stoke radius) and the membrane pore diameters, under a pump-delivered pressure gradient (Bazinet and Firdaous, 2009). An original method was recently proposed to simulate the performances of ultrafiltration aimed at enriching a hydrolysate in a bioactive peptide, validated with an alfalfa white protein hydrolysate (Kapel et al., 2011). The separation was then often completed by reverse phase chromatography separation (RP-HPLC) and/or gel filtration (size exclusion chromatography, SEC). Enzyme membrane reactor (EMR) could be also applied for the production of antioxidant BP. This is a continuous process in which ultrafiltration membranes are added in the production process allowing the separation of the enzymes and the end product (Bazinet and Firdaous, 2009; Das et al., 2012). EMR are of interest because of the recycling of the enzyme and have been already investigated for the production of anti-hypertensive BP from alfalfa proteins for example (Romain Kapel et al., 2006), but studies were very rare for the production of antioxidant BP from plant biomass (Das et al., 2012). Also, when BP exhibited metal chelating activity, affinity chromatography, such as immobilized metal affinity chromatography (IMAC), was applied for the peptide fractionation (Lv et al., 2017; Wang et al., 2012). After the peptides fractionation, the amino acid sequence of the antioxidant BP was usually identify by mass spectrometry with the help of bioinformatics tools.
Different peptides have been identified from different fractions, going from less than 1 kDa–30 kDa, and the presence of hydrophobic, branched-chain and/or aromatic amino-acids have often been reported in antioxidant BP. Some antioxidant peptides have been synthesized after identification in previous works, as observed with the lunasin that is a 43-amino-acid antioxidant peptide presents in many different plants (Indiano-Romacho et al., 2019). It has been already found in soybean and quinoa (Indiano-Romacho et al., 2019; Ren et al., 2017) as well as in other plants, such as wheat (Jeong et al., 2007), barley (Bamdad and Chen, 2013; Jeong et al., 2002), oat (Nakurte et al., 2013), rye (Jeong et al., 2009) and amaranth seed (Maldonado-Cervantes et al., 2010).
3. Focus on the antioxidant activities of bioactive peptides obtained from plant biomass
As already stressed in the introduction part, the BP may have different activities. Here, the attention was on the antioxidant properties and the mechanisms stressing such activities. An antioxidant will be considered herein as any compound (e.g. BP) that, in lipid-based formulations, prevents unsaturated lipids from oxidative degradations. Thus, the mechanisms in living biological systems won't be discussed, and we refer the reader to previous reviews which debate this point for more details (Wong et al., 2020). The relationships between the precursor protein that releases the antioxidant peptides (correct sequence in amino acid and tridimensional structure) and the prediction of the antioxidant activity cannot be easily predicted. First, because the knowledge of underlying mechanism and the structure-activity relationships of BP are very important (Samaranayaka and Li-Chan, 2011; Zou et al., 2016), and second, because this has to be confronted with the knowledge on lipid oxidation, a phenomenon that remain complex, still on debate and in deep research (Berton-Carabin et al., 2014; Decker et al., 2017; Villeneuve et al., 2018).
The antioxidant capacities of BP obtained from plant biomass are summed up in Table 2. The reported results were almost exclusively obtained through in vitro assays (DPPH, ABTS, HRSA, SRSA, ORAC) to evaluate the radical scavenging capacity, or the FRAP assay to estimate the metal reducing/electron transfer. The singlet oxygen quenching, along with metal chelating tests for iron and copper were estimated. The lipid oxidation assessment was performed by measuring the inhibition of linoleic acid oxidation (PV and/or TBARS values) in different lipid systems (e.g. emulsions, micelles, liposomes). The precursor protein structures and their hydrolytic process (enzymes specificities, operating conditions, etc.) will affect the characteristic and the peptide chemical structures. As a result, the composition and the sequence in amino acids, together with the size and the tridimensional structure of the antioxidant BP, has been identified as the main factors affecting the antioxidant activity (Nwachukwu and Aluko, 2019; Samaranayaka and Li-Chan, 2011; Zou et al., 2016). This makes sense, since the chemical reactivity toward free radicals, the metal chelating capacity, and the distribution in (or close to) the active sites of lipid oxidation are both, linked with those characteristics and responsible of the antioxidant capacity. Yet, the different tests used for estimating the antioxidant capacity make the comparison of experimental results obtained from various studies and plant biomass very difficult. In addition, for a same test, various protocols have been used in the literature and have differed in operating conditions (i.e. the concentration of radical species, the incubation time, the properties of solvent, the pH of the reaction, etc.) and results interpretation (e.g. EC50 values, percentages, equivalent concentrations, kinetics). As a result, strong dissimilarities even for antioxidants molecules commonly used as references could be observed. The structure-activity relationship of the antioxidant peptides has been very well documented in recent reviews (Nwachukwu and Aluko, 2019; Samaranayaka and Li-Chan, 2011; Zou et al., 2016). Therefore, this review did not aim to go into detail about what has already been described. Yet, besides the exhaustive presentation of the antioxidant results of BP obtained from plant biomass (Table 2), we would like to stress three important points that must be considered while estimating the antioxidant capacity of BP.
Table 2.
Description of methods and results of the antioxidant capacity of bioactive peptides produced from plant biomass.
| Systems | Antioxidant methods | Metal chelation | Results | Ref. |
|---|---|---|---|---|
| In vitro (solution) | ORAC, ABTS | NI | Antioxidant activity increased after simulated gastrointestinal digestion, but further hydrolysis of digested hydrolysates with Alcalase did not improve the activity. Highest hydrolysate activities were ABTS (IC50 = 1.16 ± 0.09 mg/mL) and ORAC value of 0.308 ± 0.007 μg Trolox/μg sample. ORAC (IC50) for peptides were AWEEREQGSR (6.7 μg/mL) > YLAGKPQQEH (16 μg/mL) ~ IYIEQGNGITGM (17 μg/mL) ~ TEVWDSNEQ (20 μg/mL) | (Orsini Delgado et al., 2011, and Delgado et al., 2016) |
| In vitro (solution) | ORAC, ABTS | NI | The hydrolysate presented a higher (~2-fold) antioxidant capacity than isolate proteins. ORAC (IC50 = 0.058 ± 0.027 mg/mL TE) and ABTS (IC50 = 2.1 ± 0.3 mg/mL TE). | Sabbione et al. (2016) |
| In vitro (solution and linoleic acid model system) | DPPH, SRSA, HRSA, FRAP, and linoleic acid oxidation (PV) | Fe | Small peptide size (MW < 1 kDa) and high level of hydrophobicity seemed to be important for DPPH, SRSA, HSRA and FRAP. Peptides had better metal chelating properties than GSH but significantly lower FRAP, SRSA, HRSA and DPPH. | Ajibola et al. (2011) |
| In vitro (solution) | FRAP, DPPH, SRSA, HRSA | Fe | Fraction with MW < 1 kDa improved antioxidant activity. GSH exhibited better antioxidant activity in all assays. Fraction showed 65.15% chelating effect on Fe2+ at 0.50 mg/mL, much lower than EDTA. | Xie et al. (2008) |
| In vitro (solution) and in cellulo | DPPH, ABTS | NI | The peptide fractions obtained by Alcalase hydrolysis had the best antioxidant capacity. | Wen et al. (2018) |
| In vitro (solution) | DPPH, SRSA, HRSA, FRAP, | Fe | The activity increased with MW of peptides, except for DPPH results. Pepsin hydrolysate and its fractions did not show any metal chelating activity. Fractions with MW (5–10 kDa) of trypsin showed the highest metal chelation (90%). | Arise et al. (2016) |
| In vitro (solution) | DPPH, SRSA, HRSA, FRAP | Fe | Alcalase hydrolysates exhibited excellent Fe2+ chelating activity and strong DPPH and HRSA capacities. The large-sized peptides (MW > 10 kDa) possessed stronger DPPH activity and reducing power, whereas small-sized peptides (MW < 1 kDa) were more effective in Fe2+ chelating and HRSA. | Xia et al. (2012) |
| In vitro (solution) | DPPH, FRAP, SRSA | Fe | Large-sized peptides (MW > 10 kDa) exhibited strong DPPH, FRAP and SRSA capacities. Low MW fractions (1–5 kDa and <1 kDa) displayed comparable, even lower, EC50 chelation activities than EDTA (0.45 mg/mL). | Bamdad & Chen (2013) |
| In vitro (solution and linoleic model system) | FRAP, TPC (FC), linoleic acid oxidation (PV) | NI | Partially purified C hordein demonstrated the most powerful reducing activity in comparison with those of B and D hordeins. Yet, hydrolysates of hordein fractions and rice bran protein fractions using Pepsin and Trypsin, showed much greater antioxidative activity and reducing power than the original proteins. | Chanput et al. (2009) |
| In vitro (solution, β-carotene/linoleic acid model system) | Inhibition of β-carotene bleaching, FRAP, ABTS | Fe/Cu | Highest antioxidant activity in the smaller MW fractions. A1 and B1 had the highest copper chelating activity (78% and 82%, respectively), while iron chelating activity was the highest in fractions A1 and B3 (36% and 16%, respectively). A2 and B3 had the highest FRAP capacity and inhibition of β-carotene bleaching, while the highest ABTS activity was found in A3 and B3. Phaseolin is the major contributor to the antioxidant and copper chelating activities of the hydrolyzed protein. | Carrasco-Castilla et al. (2012b) |
| In vitro (solution) | DPPH, ABTS | NI | Hydrolysate from pepsin showed higher DPPH activity than protein concentrate and Alcalase hydrolysate. Opposite results were obtained with ABTS activities. | Evangelho et al. (2017) |
| In vitro (solution) | ABTS | NI | The highest ABTS activities were 11.55 mmol/L TEAC/mg protein for P. lunatus Flavourzyme hydrolysate and 10.09 mmol/L TEAC/mg protein for P. vulgaris Alcalase hydrolysate. | Torruco-Uco et al. (2009) |
| In vitro (solution) | ABTS | NI | Hydrolysate with Alcalase–Flavourzyme was the most active with a TEAC of 8.1 mM/mg sample. The TEAC value for Pepsin–Pancreatin hydrolysate was 6.4 mM/mg sample. | Betancur-Ancona et al. (2014) |
| In vitro (solution) | DPPH, ABTS | NI | The DPPH activity values of hydrolysates ranged from 24 to 44% (0.0146–0.027 mmol/L TEAC/mg protein). The best treatment was Azufrado Higuera/Alcalase. ABTS was ranging from 50.2 to 99.9% (1.903–3.788 mmol/L TEAC/mg protein). The treatment Regional ‘87/Alcalase was the more effective | Valdez-Ortiz et al. (2012) |
| In vitro (solution) and In cellulo | TPC (FC) and free radicals scavenging capacity in Caco-2-cells | Fe/Cu | Phaseolin and lectins have 18% and 32% of Fe2+ chelating activity. The lectin and phaseolin hydrolysates, especially the latter, had higher copper and iron (81%) chelating activity. The highest antioxidant activity in cells was found in the hydrolysates of whole protein isolates. | Carrasco-Castilla et al. (2012a) |
| In vitro (solution) | FRAP, ABTS | NI | Lower MW fraction (<3 kDa) showed the highest antioxidant (ABTS and FRAP assays). | Ngoh & Gan (2016) |
| In vitro (solution) | ABTS, HRSA | NI | Free radical scavenging activities was accentuated by in vitro digestion, especially after 2 h Pancreatin following the 1 h Pepsin treatment. Fractions IV, V, VI enriched with di-, tri- and tetrameric peptides containing Trp and Pro exhibited the strongest activity. Dominant existence of Trp in fraction VI suggested that it had an antioxidant activity. | Ma et al. (2010) |
| In vitro (solution) | ABTS | Fe | The MW < 3 kDa fraction after 2 h of hydrolysis by Alcalase had the strongest ABTS (EC50 = 229 μg/mL) and iron chelating (EC50 = 89 μg/mL) activities. It retained its radical scavenging activity after simulated GI digestion and thermal treatments. | Chai et al. (2019) |
| In vitro (solution and linoleic acid model system) | DPPH, inhibition of linoleic acid oxidation (PV) | NI | The radical-scavenging activity of water/salt-soluble extracts from sourdoughs was significantly higher than that of chemically acidified doughs. The highest activity was found for whole wheat, spelt, rye, and kamut sourdoughs. Almost the same results were found for the inhibition of linoleic acid autoxidation. | Coda et al. (2012) |
| In vitro (solution) | DPPH, ABTS, ORAC | NI | The lunasin exhibited a weak DPPH activity (no IC50 value), but a strong ABTS activity (IC50 = 1.45 g/L) and ORAC value (40.06 mol/L TE/g protein when the concentration was 3.20 g/L). | (Ren et al., 2017) |
| In vitro, cooked ground beef and in vivo | DPPH, ABTS, FRAP, meat lipid oxidation (TBARS) | NI | Hydrolysates can effectively inhibit lipid oxidation in food models. F3 inhibited lipid oxidation in meat and presented small peptides (MW < 3 kDa). F2 fraction (3 kDa < MW < 10 kDa) also inhibited lipid oxidation in the highest concentration. F2 and F3 had the greatest DPPH values compared to the hydrolysates and fraction F1 (MW > 10 kDa). F2 and F3 prepared using Alcalase performed better in ABTS, followed by the Alcalase-Flavourzyme sequential system and, lastly, Flavourzyme. | Silveira Coelho et al. (2019) |
| In vitro (solution) and in cellulo | FRAP, DPPH, CAA | NI | F1, F2 and F3, showed a relatively low activity in comparison to BHT. Subfractions from F1 did not show reducing power. F2C, F2D, F3D and F3E were the most active fractions, with a significant increase in reducing power, comparable to BHT. | Torres-Fuentes et al. (2015) |
| In vitro (solution and linoleic acid model system) | DPPH, SRSA, HRSA, FRAP, linoleic acid oxidation (PV) | NI | The lipid peroxidation inhibitory ratio of Fra.IV (81.13%) was closer to that of α-tocopherol (83.66%) but lower than that of BHT (99.71%). Fra.IV had the strongest antioxidant activity compared with the other three fractions. | (Li et al., 2008) |
| In vitro (solution and linoleic acid model system) | DPPH, HRSA, SRSA, linoleic acid oxidation (PV) | Fe/Cu | The lipid peroxidation inhibitory ratio of fraction 7 was 88.81% at the 8th day, which was higher than α-tocopherol (58.85%). The Cu2+ and Fe2+ chelating capabilities of 76.92% and 63.08%, respectively, were lower than EDTA (at 50 μg/mL). | (Zhang et al., 2011) |
| In vitro (solution and linoleic acid model system) and in cellulo | DPPH, ABTS and SRSA, FRAP, linoleic acid oxidation (PV). | NI | GSQ exhibited significant scavenging antioxidant activities and cellular protective effect against oxidative stress. The reducing power of GSQ was lower than that of GSH. | Hong et al. (2014) |
| In vitro (solution) | ORAC | NI | The albumin and globulin hydrolysates showed higher ORAC values than their whole protein counterparts (3-fold increase). The glutelins presented a higher antioxidant potential but did not improve after hydrolysis. | Ortiz-Martinez et al. (2017) |
| In vitro (solution) | SRSA | NI | The fermentative hydrolysate possessed better solubility and antioxidative activity. The antioxidant activity increased (from 10.28 to 259.21%) with fermentation time (from 0 to 32 h), the soluble protein content (from 5.16 to 24.95 mg/mL) and the solubility of the fermentative hydrolysate (from 29.78 to 74.16%). | (Zheng et al., 2012) |
| In vitro (solution and linoleic acid model system) | DPPH, SRSA, HRSA, FRAP, inhibition of linoleic acid oxidation (PV) | NI | Hydrolysate from corn gluten meal possessed the same in vitro antioxidative activities as the zein hydrolysate. EC50 values were about 1.27 and 1.26 mg/mL (DPPH), 12.82 and 12.49 mg/mL (SRSA), 0.81 and 0.80 mg/mL (HRSA), respectively. | (Li et al., 2010) |
| In vitro (solution and linoleic acid model system) | DPPH, FRAP, inhibition of linoleic acid oxidation (PV) | Fe | Alcalase hydrolysate had the highest DPPH (16.67 ± 0.67%), chelation (39.32 ± 0.25%), lipid peroxidation inhibition (34.06 ± 1.62%) and reducing power. F2 exhibited the highest activity, and GHKPS was responsible of the antioxidant activity. | Zhuang et al. (2013) |
| In vitro (solution) | DPPH | NI | The DPPH inhibition of peptide fraction (30 kDa > MW > 10 kDa) treated by pulsed electric field increased 32.1%, compared to the sample untreated. Thus, this technology could improve the antioxidant activity of antioxidant peptides. | (Wang et al., 2014) |
| In vitro (solution) | DPPH, ABTS, HRSA | Fe | QQPQPW exhibited EC50 values of 0.95 (DPPH), 0.0112 (ABTS) and 4.43 (HRSA) mg/mL. It also exhibited notable reducing power of 0.54 (Abs 700 nm) at 2.0 mg/mL, but showed weaker Fe2+ chelating capacity (EC50 = 6.27 mg/mL). | (Wang et al., 2014) |
| In vitro (solution) | SRSA | NI | Hydrolysate showed similar increasing trend as with protein contents until hydrolysis of 10 min and then decreased as hydrolysis time increased. FPLEMMPF exhibited a SRSA of 78.1 U/ml. | (Zheng et al., 2006) |
| In vitro (solution) | DPPH, HRSA, SRSA, FRAP | NI | The antioxidant activities of hydrolysate are highly correlated to small peptides and content of antioxidative amino acids. The IC50 values obtained were 1.42 mg/mL (DPPH), 41.83 mg/mL (HRSA), 26.60 mg/mL (SRSA), and 3.37 mg/mL (FRAP). | (Zhou et al., 2015) |
| In vitro (solution) and in vivo | SRSA. SOD, GPx and MDA measurement | NI | Peptides with low MW exhibited higher antioxidant activities compared to high MW peptides. | (Liu et al., 2015) |
| In vitro (solution) | DPPH, SRSA, HRSA, FRAP | Fe | The hydrolysate (Alcalase + Flavourzyme) exhibited the best antioxidant activities. CSQAPLA exhibited good reducing power and excellent scavenging capacities with IC50 values of 0.116 (DPPH) and 0.39 mg/mL (SRSA). | Jin et al. (2016) |
| In vitro (solution and linoleic acid model system) | DPPH, HRSA, ABTS, SRSA, inhibition of linoleic acid oxidation (PV) | Fe/Cu | Hydrolysates with MW < 10 kDa exhibited the highest antioxidant activities in all relevant assays. F3 exhibited the highest antioxidant action. IC50 of peptides LPF, LLPF, FLPF were 2.07, 2.22 and 1.51 mM (DPPH); 2.70, 2.11 and 2.83 mM (ABTS); respectively. In addition, these peptides effectively inhibited lipid peroxidation in the linoleic acid model system. | Zhuang et al. (2013) |
| In vitro (solution and cooked ground beef) | ORAC, DPPH, lipid oxidation (TBARS) | Fe | ORAC values of hydrolysates varied between 65.6 and 191.4 μmol TE/g. F3 produced by neutral protease possesses the highest activity. DPPH of fractions varied between 18.4 and 38.7 μmol TE/g. F2 and F3 produced by alkaline protease showed the strongest activity. F3 (>1 kDa) from Neutral Protease was the only fraction that inhibited lipid oxidation in ground beef at 250 and 500 μg/g. Fractions exerted modest chelating activities (0.15–0.43 mg EDTA equivalents/g). | (Zhou et al., 2012) |
| In vitro (solution) | DPPH, ABTS, FRAP | Cu | The ABTS of hydrolysate was decreased by 27% after Pepsin treatment but was fully recovered after Pancreatin digestion. DPPH was lower than ABTS activity and showed a 7-fold reduction following Pancreatin treatment. The reducing power of hydrolysates increased 2-fold after Pancreatin digestion. Cu2+ chelation was reduced by Pepsin but was reestablished after Pancreatin treatment. Activities of hydrolysates (1–8 mg/mL) was comparable to, or exceeded, that of 0.1 mg/mL of ascorbic acid or BHA. | (Zhu et al., 2008) |
| In vitro (solution) + in cellulo | DPPH, ORAC, cytotoxicity and CAA on HepG2 cells, ESR. | NI | Peptides AGLPM and HALGA showed significantly better ORAC capacities than AGIPM and HAIGA. ESR showed that the AGLPM and HALGA peptides had strong abilities to scavenge hydroxyl radicals. | Jiang et al. (2018) |
| In vitro (solution) + in cellulo | HRSA, SRSA, ABTS, ORAC, CAA, intracellular ROS clearance capacity | NI | CPF1 (MW < 1 kDa) and CPF2 (1 kDa < MW < 3 kDa) exhibited good HRSA, SRSA, ABTS and ORAC values (CPF1 was slightly higher). YFCLT exhibited excellent ABTS activity (EC50 = 37.63 μM), but was much lower than that of Trolox. | (Wang et al., 2015) |
| In vitro (solution and liposomal system) | FRAP, ABTS. Liposome oxidation (PV, TBARS) | Fe/Cu | Nonhydrolyzed zein was incapable of sequestering either copper (Cu2+) or ferrous (Fe2+) ions. Marked enhancement of the Cu2+ chelation activity of hydrolysates; but no significant improvement in the Fe2+ binding ability. Hydrolysates possessed strong Cu2+ chelation ability and marked reducing power that were accentuated with hydrolysis time. | Kong & Xiong (2006) |
| In vitro (solution) | DPPH, ABTS, SRSA | NI | Hydrophobic amino acids contributed to the DPPH activity, whereas hydrophilic residues were responsible for the ABTS activity. Low MW peptides had stronger activity to prevent SRSA but high MW had stronger DPPH and ABTS activities. | (Tang et al., 2010) |
| In vitro (solution and linoleic model system) | DPPH, ABTS, FRAP, linoleic acid oxidation (PV) | Fe/Cu | Alcalase hydrolysate with the lowest MW fraction (<3 kDa) had the best activity. Iron chelation was high, whereas the copper chelation was very poor. PF and LPF were the active peptides with DPPH of IC50 = 3.2 mM and 2.07 mM, and ABTS of IC50 = 3.37 mM and 2.7 mM. | (Tang and Zhuang, 2014) |
| In vitro (solution) | ORAC | NI | The ORAC values were 426.7 and 783.8 μmol TE/g for hydrolysate and its fraction (MW < 3 kDa), and 250.6 and 500.8 μmol TE/g after cooking. Fractions had better ORAC values, and cooking reduced activity. | Marques et al. (2015) |
| In vitro (solution) | ABTS, DPPH, FRAP | Fe/Cu | The ABTS values of the hydrolysates ranged from 14.3 to 15.1 mM/mg of sample, while DPPH ranged from 86.3 to 98.2% (Pepsin-Pancreatin). FRAP were similar and equivalent to BHT. Alcalase hydrolysate of Vigna unguiculata had the highest chelating activities 70.1% (Fe) and 71.4% (Cu), while Pepsin-Pancreatin hydrolysates had the lowest, ≤49.2%. | Segura-Campos et al. (2013) |
| In vitro (solution) | ABTS | NI | ABTS values were not significantly different between hydrolysates (~14 TE mmol/L/mg). Decreasing MW increased ABTS values. The highest capacity was obtained with MW < 1 kDa with Flavourzyme (2830 TE mmol/L/mg). | Segura Campos et al. (2010) |
| In vitro (solution) | ABTS, DPPH, HRSA | Fe | Antioxidant activity increased after proteolysis. Trypsin hydrolysate showed higher DPPH (38.41 ± 0.02%), ABTS (40.15 ± 0.19%) and metal chelating (35.11 ± 0.05%) activity as compared to Pepsin. Smallest fraction with MW < 3 kDa (F3) possessed the highest DPPH, ABTS, HRSA and chelation activities. | Agrawal et al. (2019) |
| In vitro (solution) | DPPH, SRSA | NI | Short peptides with 2–10 amino acids exhibit significant antioxidant activity than their parent proteins or large polypeptide. Tyr, Leu, His, Gly and Pro were involved in the antioxidant activity of identified peptides. | Amadou et al. (2013) |
| In vitro (solution) | ABTS, SRSA, FRAP | Fe | Hydrolysis slightly increased antioxidant activities. Alcalase and Pancreatin were the best hydrolysates with ABTS ~0.22 mmol TE/g, SRSA ~35 μmol TE/g, FRAP ~0.23 mmol Fe2+/g, and 70% of Fe2+ chelation capacity at 1.54 mg/mL. | Karamać et al. (2016) |
| In vitro (solution) | ORAC, FRAP | NI | The most antioxidant fraction comprised only small size peptides (MW < 1.5 kDa). Potent reduction capacity of GFPGRLDHWCASE (3.20 ± 0.24 μmol TE/μmol), higher than BHA (2.43 μmol Trolox equivalents/μmol). | Silva et al. (2017) |
| In vitro (solution and linoleic acid model system) and chicken patties | HRSA, DPPH, linoleic acid oxidation (PV), lipid oxidation (PV, TBARS) | NI | HRSA and DPPH activity increased drastically after hydrolysis. The fraction with MW > 8 kDa had the strongest scavenging activity on DPPH, HRSA, and antioxidant activity on chicken products (effect similar to BHT). | (Zhao et al., 2014) |
| In vitro (solution) and in cellulo | DPPH, ABTS, cytotoxicity, CAA | NI | C2 showed the most potent antioxidant activities. ADGF demonstrated the strongest radicals scavenging activity, but still lower than ascorbic acid. | (Liu et al., 2018) |
| In vitro (solution) + in vivo (TAC, SOD, CAT, Tpx) | DPPH, HRSA, SRSA. | Fe | The hydrolysate was able to scavenge up to 52%, 32% and 2% of the DPPH, hydroxyl, and superoxide radicals, respectively. DPPH value was similar to GSH, while HRSA and SRSA results was lower. It exhibited moderate metal chelation activity. | (Girgih et al., 2014) |
| In vitro (solution) | DPPH | Fe | At 0.5 mg/mL, WVYY and PSLPA were the most active antioxidant peptides with 67% and 58% DPPH scavenging activity, and metal chelation activity of 94% and 96%, respectively. | (Girgih et al., 2014) |
| In vitro (solution) and in cellulo | HRSA, SRSA, DPPH, FRAP | NI | IC50 values (DPPH, SRSA, HRSA) of the MAR-treated fractions were significantly lower than hydrolysate. At 10 μg/mL, A4a increased cell survival for 60%. | (Lu et al., 2010) |
| In vitro (solution and linoleic acid model system) | ORAC, DPPH, HRSA, SRSA, FRAP, and lipid oxidation (PV) | Fe | Compared to hydrolysate, fractions had higher DPPH, HRSA, SRSA activities, but similar metal reducing, chelating activities, and ability to inhibit linoleic acid oxidation. F2 and F6 were the most active in SRSA (F2 and F6), HRSA (F2), FRAP (F6), and metal chelation (F2 and F6) activities. | (Girgih et al., 2013) |
| In vitro (solution) + in cellulo | DPPH, ABTS, FRAP, ORAC, induced oxidative damage in HepG2 cells | Fe | VNP had only strong ORAC activity (1.5 μmol TE). YGD exhibited high abilities in ABTS (0.95 μmol TE) and ORAC (1.2 μmol TE). In FRAP, YGD and VNP exhibited low abilities. In chelation assay, they exhibited ~0.5 μmol TE. In the cell model, VNP and YGD exert antioxidant effect. | Jiang et al. (2019) |
| In vitro (solution and linoleic acid model system) | DPPH, HRSA, FRAP, linoleic acid oxidation (PV) | Fe | The MW < 1 kDa and 5 < MW < 10 kDa fractions exhibited significantly highest ability to scavenge DPPH, inhibition of the peroxidation of linoleic acid and the reduction of Fe3+ to Fe2+. | Mundi & Aluko (2014) |
| In vitro (solution) | TPC (FC), ABTS, FRAP, TAC (reduction of MoVI), CAA | Fe | Soaking decreased ABTS, TAC, FRAP and chelation activity in all legumes. Cooking of the soaked seeds led to further decrease. Protein digestion increased ABTS and TAC of legumes. The scavenging and reducing activities were correlated with TPC. The potential can be summarized as horse gram > lentils > white pea > black pea > green gram > cowpea > chickpea. | Jamdar et al. (2017) |
| In vitro (solution), in cellulo and in vivo | HRSA, ORAC, cytotoxicity, CAA | Fe | The activities of MW < 3 kDa and 3 kDa < MW < 10 kDa fractions were generally higher than those of MW > 10 kDa. The highest ORAC value was 1452 μmol TE/g for MW < 3 kDa. For the ferrous ion chelation rate, the <3 kDa and 3–10 kDa MW fractions achieved over 90% at 1.2 mg/mL, whereas the >10 kDa MW was lower than 80%. | (Ren et al., 2018) |
| In vitro (solution) | DPPH, HRSA, FRAP | Fe | MW < 1 kDa increased DPPH by 67.77%, 3 kDa < MW < 5 kDa increased HRSA by 44.15%, 5 kDa < MW < 10 kDa increased FRAP (0.153 mmol Fe2+). Hydrolysate and the 5 kDa < MW < 10 kDa fraction showed no metal chelating ability, while 3 kDa < MW < 5 kDa showed inhibition up to 11%. | Aderinola et al. (2019) |
| In vitro (solution) + in vivo | ABTS, FRAP | NI | Pepsin–Pancreatin hydrolysate was the most active in ABTS (102.8 mM/mg TE) and in FRAP (IC50 = 67.2 g/mL). The fraction MW < 1 kDa was the most active (709.8 mM/mg TE) and a FRAP (IC50 = 54.9 g/mL). | Herrera Chalé et al. (2014) |
| In vitro (solution) and in cellulo | DPPH, ABTS, cytotoxicity, CAA | NI | F5 (1.4 kDa > MW > 0.3 kDa) showed the best antioxidant activity. RDY showed higher DPPH and CAA activities in comparison to SVL and EAVQ. SVL, EAVQ and RDY have synergistic antioxidant effects. | Sun et al. (2019) |
| In vitro (solution) | DPPH, HRSA, SRSA, FRAP | Fe | Highest antioxidant potential for the lowest MW fractions. F4 (MW < 1 kDa) exhibited the highest DPPH, along with HRSA and SRSA activities (54 and 65.1%), but moderate activity for FRAP (0.102 mmol Fe2+/g protein) compared to other fractions and the hydrolysate. Chelation activity was generally weak, except for F4 (43.94% at 5 mg/mL). | Sonklin et al. (2018) |
| In vitro (solution and linoleic acid model system) | DPPH, ORAC, linoleic acid oxidation (PV) | Fe | The Alcalase fraction, MW < 2 kDa demonstrated the highest DPPH. The linoleic acid oxidation was equally and significantly inhibited by Trypsin and Alcalase hydrolysates. Hydrolysates showed better chelating properties (Trypsin > Alcalase) than their fractions (MW < 2 kDa, and MW between 2 and 10 kDa). | Tsopmo et al. (2010) |
| In vitro (solution) | DPPH | Fe | AWFS showed the highest radical scavenging activity (71%) followed by peptide WAF, LPWRPATNVF, WAFS and YGIKVGYAIP with 55.7%, 50%, 47.3% and 44%, respectively. GGIF and GIFE showed the lowest DPPH activity. Chelation activity was LPWRPATNVF > AWFS > YGIKVGYAIP. | Zarei et al. (2014) |
| In vitro (solution) | TPC (FC), DPPH, ABTS, FRAP, PCL-ACW | NI | Strongest antioxidant capacity for the highest degree of hydrolysis (50%). DPPH value was 0.14 mg/mL (EC50), ABTS was 326 ± 5.77 μmol TEAC/g, TPC was 45.94–50.36 mg GAE/g, and the FRAP values were 10-fold lower than ascorbic acid. | Ng et al. (2013) |
| In vitro (solution) | DPPH | NI | Papain hydrolysate after 38 h hydrolysis exhibited both the highest degree of hydrolysis (91 ± 0.1%) and DPPH activity (73.5 ± 0.25%) compared to the other hydrolysates. Activity had reverse correlation with peptide size. | Zarei et al. (2012) |
| In vitro (solution and linoleic acid model system) | HRSA, DPPH, H2O2, SRSA, FRAP, linoleic acid oxidation (PV) | Fe | F4 and F5 showed the strongest scavenging and electron transfer capacities in comparison to F1, F2, F3. F5 possessed the strongest metal chelating activity. In comparison to GSH, fractions had less ability to scavenge free radicals but better capacity to chelate metals and inhibit linoleic acid oxidation. | Pownall et al. (2010) |
| In vitro (solution and linoleic acid and liposome model system) | FRAP, DPPH, linoleic acid oxidation (PV) and human LDL oxidation | Fe | The roasting enhanced the antioxidant activity and roasted products were much stronger when hydrolyzed by proteases. Hydrolysates showed reducing powers at a concentration of 1.0 mg/mL, almost equivalent to 0.02 mg BHA or α-tocopherol/ml. At a concentration of 1.0 mg/mL, hydrolysates showed chelating effect almost equal to 0.0075 mg/mL of EDTA. | Hwang et al. (2001) |
| In vitro (solution) | DPPH, HRSA, SRSA, Reducing power (Fe, Mo), anti-lipid peroxidation | Fe/Cu | Comparing the ultrasonic-assisted proteolysis with Alcalase hydrolysis, the former required less time and had higher antioxidant activities. | (Yu et al., 2012) |
| In vitro (solution, linoleic acid model system) | DPPH, FRAP, linoleic acid oxidation (PV) and human LDL oxidation | Fe | Proteolysis incubation time increase (up to 2 h), and then decrease antioxidative activity (up to 12 h). FII (3 kDa < MW < 5 kDa) had the highest antioxidant and chelation activities. Basic peptides from fraction FII exhibited higher antioxidative activity than the neutral or acidic peptides. | Hwang et al. (2010) |
| In vitro (solution and linoleic acid model system) and in cellulo | DPPH, FRAP, ORAC, linoleic acid oxidation (PV), CAA | Fe | The ORAC value of YGS was 3-fold higher than GSH, and it displayed a stronger protective effect on linoleic acid peroxidation. YGS showed negligible DPPH, FRAP, and no metal chelating ability. | (Zheng et al., 2012) |
| In vitro (solution) | ABTS, DPPH, HRSA, FRAP | Fe | Antioxidant activities of the peptide SDRDLLGPNNQYLPK was higher than hydrolysate, that was higher than the protein isolate. Except for chelation activity (51.20% vs 10.59%), the DPPH, ABTS, FRAP and HRSA activities of the peptide were lower than BHT or Trolox at the concentration of 1 mg/mL. | Agrawal et al. (2016) |
| In vitro (solution and linoleic acid model system), in cellulo and in vivo | DPPH, ABTS, HRSA, SRSA, ORAC, linoleic acid oxidation (PV), cytotoxicity, CAA | NI | FY efficiently quenched free radicals. YL and FY showed high ORAC (~3.5 μmol TE/mg). YL and FY had the ability to scavenge superoxide anion radicals, although weaker than GSH. YL and FY reduced lipid peroxidation (similar to GSH, much lower than BHA). | (Yang et al., 2018) |
| In vitro (solution and linoleic acid model system) | DPPH, HRSA, SRSA, ORAC, ABTS, FRAP, linoleic acid oxidation (PV) | NI | Strongest DPPH activity for the lowest MW with Pancreatin (MW < 1 kDa) whereas it was the reverse for other hydrolysates (MW > 10 kDa). Highest SRSA, HRSA, ABTS, ORAC activities for all hydrolysates at the lowest MW fractions, whereas the reduction capacity (FRAP) was better with high-MW fraction (>10 kDa). The low MW fractions (<5 kDa) were able to inhibit the initial lipid peroxidation. GSH was more effective than hydrolysates and fractions. | Olagunju et al. (2018) |
| In vitro (solution) and in cellulo | DPPH, ABTS, FRAP, CAA | NI | KWFCT had a higher FRAP and DPPH values than acetylated-QWFCT. Acetylated-QWFCT had a higher ABTS and cellular antioxidant activity. This indicated that Lys at N-terminal easily reacts with DPPH free radicals and Fe3+ (FRAP), and acetylated-Gln at N-terminal is sensitive to ABTS and ABAP free radicals. | (Yang, Li, et al., 2017) |
| In vitro (solution) and in cellulo | DPPH, ABTS, HRSA, cytotoxicity, CAA | NI | After pulsed electric field treatment, the DPPH and ABTS radical inhibition values of QDHCH were increased to 85.13 ± 0.17% and 95.45 ± 0.12%. The HRSA of QDHCH was increased by 10.53%. | Liang et al. (2017) |
| In vitro (solution and oil-in-water emulsion) | ABTS and lipid oxidation (TBARS) | NI | Low-polarity, or less hydrophilic peptides (rich in hydrophobic amino acids), were prevalent in strongly antiradical and antioxidative fractions. P50–F10, P50–F12, and P50–F13 exhibited the greatest ABTS activity (~350 μM TE at 200 μg/mL). | Cheng et al. (2010) |
| In vitro (solution and oil-in-water emulsion) | ABTS, emulsion physical stability | Fe/Cu | Pro and Leu, were contained in the peptides that exhibited high ABTS ability. Lys, Arg, Glu and Asp were able to chelate metal ions such as Fe2+ and Cu2+. Adsorbed peptides were mostly short oligopeptides composed of two to seven amino acids, of which SFDL(I)K matched the sequence of patatin. | Cheng et al. (2014) |
| In vitro (solution and linoleic acid model system) | DPPH, FRAP, linoleic acid oxidation (PV) | Fe | At 10 mg/mL, hydrolysates had increased DPPH activities from 21.89 to 85.27%, the reducing power increased from 0.21 to 0.48 (Abs700nm). Iron chelating ability was improved from 30.50 to 80.03% at 1 mg/mL. Hydrolysates showed better lipid peroxidation inhibition in the linoleic acid model system. Flavourzyme was the best to produce antioxidative peptides. | Venuste et al. (2013) |
| In vitro (solution and linoleic acid model system) | DPPH, FRAP, linoleic acid oxidation (PV) | Fe | The lowest MW peptides had stronger radical scavenging activity. DPPH value (IC50) of hydrolysate was 165 μg/mL (NB: phenolics still present), chelation capacity was of 7 mg/mL (IC50), the reducing power (1 nm at 0.1 mg/mL) was lower than BHA, ascorbic acid or tocopherol (1.2, 1.7, 0.6 nm respectively at 100 μg/mL). | He et al. (2012) |
| In vitro (solution) | DPPH | NI | The peptide PAGPF exhibited DPPH radical scavenging of 0.063 mg/mL (ED50). | (Zhang et al., 2009) |
| In vitro (solution) | ORAC | NI | Hydrolysates from Alcalase and Proteinase K, had high levels of free radical scavenging capacity. MW of peptide fractions were inversely related to the capacity. Peptides with sizes MW < 3 kDa had significantly reduced surface hydrophobicity, but showed much higher ORAC ability compared to the MW > 3 kDa peptides. | He et al. (2013) |
| In vitro (solution) | FRAP, DPPH, SRSA, HRSA | NI | The EC50 values of hydrolysate for DPPH, SRSA, and HRSA were 0.71, 1.05, and 4.92 mg/mL, respectively. The FRAP value was 0.51 (at 700 nm) at 2.00 mg/mL. | Pan et al. (2011) |
| In vitro (solution + liposome model system) | DPPH, HRSA, FRAP, inhibition of lipid peroxidation in a liposome model system (TBARS) | NI | The reducing power of RP55 and hydrolysate was higher than RP25. The ED50 of hydrolysate, RP25 and RP55 for DPPH were 72, 499 and 41 mg/mL, respectively. The ED50 for RP25 and RP55 for HRSA were 2.53 and 6.79 mg/mL, while the ED50 of RP55 and hydrolysate for inhibition of lipid peroxidation in liposomes system were 4.06 and 4.69 mg/mL. The inhibitory effect on lipid oxidation of RP55 was similar to that of ascorbic acid at a concentration of 5.0 mg/mL. | (Zhang et al., 2008) |
| In vitro (solution and linoleic acid model system) | ABTS, DPPH, SRSA, ORAC, linoleic acid oxidation (PV) | NI | Low MW peptides were the most effective. The DPPH and SRSA of the fraction (MW < 1 kDa) with Pepsin followed the same trend as GSH. | Alashi et al. (2014) |
| In vitro (solution) | ABTS | NI | The ABTS values (TE) of hydrolysate and each fraction (RSP-1, RSP-2, RSP-3, or RSP-4) were 0.168, 0.186, 0.140, 0.120 and 0.240 mg/mL, respectively. | (Yu et al., 2013) |
| In vitro (solution) | FRAP, SRSA, HRSA, lipid peroxidation (MDA) | NI | Hydrolysate had reducing activity (FRAP assay increases of ~1 Abs at 700 nm at 100 mg/mL), and scavenged > 9, 80 and 87% of hydroxyl radicals at 0.1, 100 and 250 mg/mL, respectively. The highest SRSA of hydrolysate and RSP1-3 were 80, 90, 35, and 80%, respectively, at concentrations of 0.5 (RSCH), 0.5 (RSP1), 0.05 (RSP2), and 2 mg/mL (RSP3). | Xue et al. (2009) |
| In vitro (solution) | FRAP, DPPH | NI | All three hydrolysates exhibited a concentration dependent DPPH activity with a maximum around 70% at 10 mg/mL. Reducing power were also concentration dependent with an absorbance increases <1 (700 nm) at 10 mg/.mL. | Cumby et al. (2008) |
| In vitro (solution) | ABTS, DPPH | NI | The scavenging activity of bromelain and protease against the DPPH radical (0.81 ± 0.002 and 0.60 ± 0.009 mM TE, respectively) was lower than the ABTS radical (2.89 ± 0.03 and 2.02 ± 0.06 mM TE, respectively). The enhanced antioxidant activity is likely the consequence of some low MW peptides or free amino acids with bromelain. | Selamassakul et al. (2016) |
| In vitro (solution) | DPPH, ABTS, FRAP | Fe | Digestion increased activities. Pepsin hydrolysate had DPPH = 80.18 ± 6.18 μmoL TE/g sample. F1 (MW < 3 kDa) showed the highest DPPH (66.25 ± 2.60 μmoL TE/g sample), FRAP (96.43 ± 3.88 μmoL FeSO4 equivalent/g sample) and ABTS (425.81 ± 2.59 TE/g sample). Yet, smallest fraction (F1) had the lowest chelating activity (22.23 ± 0.93 μmoL EDTA equivalent/g sample). | Phongthai et al. (2018) |
| In vitro (solution) | ABTS | Cu | Glutelin hydrolysates exhibited the highest ABTS (0.69 ± 0.04 μM trolox) and copper chelating (4.12 ± 0.01 mg EDTA) activities. The F4 fraction showed the highest ABTS (1.08 ± 0.03 mM trolox) and copper chelating (5.00 ± 0.02 mg EDTA) activities. The peptides with MW < 1500 Da and hydrophobic or aromatic N-terminal residues (e.g. SPFWNINAHS, MPVDVIANAYR, VVYFDQTQAQA, VEVGGGARAP) possibly contributed to the highest antioxidant activity. | Selamassakul et al. (2018) |
| In vitro (solution) | DPPH, ABTS, FRAP | NI | F4 of RRPB3 exhibited the highest antioxidant activity, with DPPH (IC50 = 0.144 mg/mL), ABTS (IC50 = 0.107 mg/mL) and FRAP (0.165 ± 0.011 mg/mL). The antioxidant activities of peptides within MW 500–1500 Da are higher than that of peptides above 1500 Da and peptides below 500 Da. RPNYTDA and TRTGDPFF showed a synergistic effect. | Yan et al. (2015) |
| In vitro (solution) | ORAC | NI | The antioxidant activities of denatured hydrolysates are significantly higher compared to native hydrolysates. The trypsin hydrolysate possessed the highest antioxidant activity (4.067 μmol of TE/mg protein). Highest antioxidant activity for peptides in F14/F15/F16, with an ORAC value of 22.9–24.9 nmol of TE. | Wattanasiritham et al. (2016) |
| In vitro (linoleic acid model system) | Linoleic acid oxidation (PV) | NI | Higher antioxidative activity of crude protein was due to the presence of phytic acid. The antioxidative activity did not increase with increasing DH, and hydrolysates with DH below 10% had higher antioxidative activity than those above 20%. | Adebiyi et al. (2008) |
| In vitro (solution) | ABTS | NI | DH inversely correlates with antioxidant capacity. Albumin hydrolysates had higher antioxidative activity than other protein fractions. YLAGMN had the highest antioxidative activity. | Adebiyi et al. (2009) |
| In vitro (solution) | DPPH, FRAP | NI | The best DPPH (IC50 = 0.98 mg/mL) and FRAP (IC50 = 0.159 mg/mL at 0.05 mg/mL) values were obtained with the lowest MW (<4 kDa). YSK exhibited strong scavenging activity on DPPH (IC50 = 0.15 mg/mL) and FRAP (0.125 at 0.05 mg/mL). | (Wang et al., 2017) |
| In vitro (solution) | FRAP, DPPH, HRSA | NI | The lowest MW (<5 kDa) fraction of the Protamex-Alcalase hydrolysate had the highest activity. PYK exhibited a DPPH value of IC50 = 0.12 mg/mL, a HRSA value of IC50 = 0.75 mg/mL, and a FRAP of ~1 abs (700 nm) at 0.5 mg/mL. Results were similar to GSH. | Hu et al. (2019) |
| In vitro (solution) | DPPH, HRSA, ABTS, FRAP | NI | Hydrolysate had certain HRSA activity (IC50 = 3.0 mg/mL), strong ABTS activity (IC50 = 35 μg/mL), scavenging DPPH ability (IC50 = 420 μg/mL), and FRAP absorption value reached 0.5 (700 nm) at 3.20 mg/mL. | (Yang et al., 2019) |
| In vitro (solution and linoleic acid model system) and in vivo | DPPH, ABTS, FRAP and linoleic acid oxidation (PV) | NI | SPH-I (MW < 3 kDa) exhibited the highest DPPH and ABTS radicals scavenging activities (IC50 of 350 μg/mL and 17.5 μg/mL, respectively), reducing power and the lipid peroxidation inhibition potential. | Cai et al. (2017) |
| In vitro (solution) | NI | Zn/Fe | The hydrolysates treated by trypsin had the highest metal chelating ability. The NCS peptide showed the highest zinc and iron chelating ability, which was higher than GSH. | (Wang et al., 2012) |
| In vitro (solution) | DPPH, ABTS | NI | The lowest MW (<3 kDa) fraction DSPH-V showed the highest efficiency. P4 had the best DPPH (IC50 = 2.793 ± 0.104 mg/mL) and ABTS (IC50 = 2.949 ± 0.069 mg/mL). SYPTECRMR, fragment of sesame 2 S albumins, showed the strongest DPPH (0.105 ± 0.018 mg/mL) and ABTS (0.004 mg/mL) radical scavenging activities. | (Lu et al., 2019) |
| In vitro (solution and linoleic acid model system) | DPPH, linoleic acid oxidation (TBARS) | NI | The lowest MW (<1 kDa) fraction showed the highest inhibition of lipid peroxidation (~60%) and the best DPPH radical scavenging activity (IC50 = 0.038 ± 0.002 mg/mL). In comparison, tocopherol and TBHQ were 0.018 ± 0.002 mg/mL and 0.016 ± 0.001 mg/mL, with inhibition of lipid peroxidation between 75 and 85%. | Das et al. (2012) |
| Ground meat model and in vitro (solution and oil-in-water emulsion) | DPPH, ORAC, FRAP, TPC (FC), lipid oxidation (PV, TBARS) | Fe | Hydrolysates displayed promising antioxidant capacity. Medium MW (3–10 kDa) hydrolysates exhibited the highest TPC, the best antioxidant activities, and effectively retarded lipid autoxidation in model emulsion and ground meat systems. | Xu et al. (2019) |
| In vitro (solution) | DPPH, ABTS, FRAP | Fe | Low MW fraction UF3 (<3 kDa) exhibited the strongest DPPH, ABTS, FRAP and Fe2+ chelating ability. Peptides VAITLTMK and VSKSVLVK exhibited the dominating radical scavenging capacity. | Agrawal et al. (2017) |
| In vitro (solution, β-carotene model system) | HRSA, ABTS, SRSA, FRAP, β-carotene bleaching | NI | Low MW fractions were the most active in β-carotene model system and as radical scavengers. | Moure et al. (2006) |
| In vitro (solution) and fat-rich food model (cooked ground beef) | ORAC, DPPH, lipid oxidation (TBARS) | Fe | NP-F1 (MW > 10 kDa) from neutral protease and AP-F3 (MW > 1 kDa) from alkaline protease exhibited good results with respect to meat peroxidation. Fractions MW > 10 kDa from Validase and neutral protease exhibited a good chelating activity. | (Zhang et al., 2010) |
| In vitro (oil-in-water emulsion) | TBARS | NI | Oxidized soybean protein hydrolysate and oxidized soybean protein were still able to retard TBARS formation in emulsions by as much as 52%. Oxidation of peptides and proteins did not significantly affect the emulsion physical stability, their distribution and the peptide adsorption at water-oil interface. | (Zhao and Xiong, 2015) |
| In vitro (solution) and In cellulo | DPPH, ABTS, ORAC, FRAP. Cell studies | NI | The peptides displayed DPPH (from 16.5 ± 0.5 to 20.3 ± 1.0 μM TE/μM), ABTS (from 3.42 ± 0.2 to 4.24 ± 0.4 mM TE/μM), ORAC (from 143 ± 2.1 to 171 ± 4.8 μM TE/μM), and FRAP (from 54.7 ± 1.2 to 79.0 ± 0.6 mM Fe2+/μM) activities. They showed inhibitory effects against intracellular reactive oxygen species generation in Caco-2 cells. | (Zhang et al., 2019) |
| In vitro (solution) and In cellulo | DPPH, ABTS, ORAC, FRAP, cytotoxicity, CAA. | Fe | Hydrolysate displayed DPPH (IC50 = 4.22 mg/mL), ABTS (IC50 = 2.93 mg/mL), reducing power, metal ion-chelating activities (IC50 = 0.67 mg/mL) and significantly inhibited the generation of intracellular reactive oxygen species in Caco-2 cells. After simulated gastrointestinal digestion, the activities were enhanced, except for the ABTS capacity. | (Zhang et al., 2018) |
| In vitro (liposomal system) | Liposome oxidation (TBARS) | NI | Nonhydrolyzed soy protein isolate possessed antioxidant activity. Preheated and hydrolyzed samples of Chymotrypsin and Flavourzyme (0.5 h) had the greatest inhibitory effect on lipid oxidation. | Penta-Ramos & Xiong (2002) |
| In vitro (linoleic acid model system) | Linoleic acid oxidation (PV) | NI | Strong antioxidant activity of histidine-containing peptides. LLPHH was the most active. | (Chen et al., 1995) |
| In vitro (solution) and in cellulo | ABTS, ORAC, CAA. | NI | Antioxidant activities has been demonstrated for fragments released from N-terminal and central regions of lunasin. | Indiano-Romacho et al. (2019) |
| In vitro (linoleic acid model system) | Linoleic acid oxidation (PV and TBARS) | NI | Antioxidant activities increased with decreasing MW of hydrolysate (highest with MW < 3 kDa). The strongest antioxidant peptide showed activity of 108.13%/mg for TBARS, and contained hydrophobic amino acids, such as Phe, Ala, and Pro. | (Park et al., 2010) |
| In vitro (linoleic acid model system) | Linoleic acid oxidation (PV + HPLC = linoleic acid, linoleic acid hydroperoxides, secondary oxidation products | NI | His and Pro play important roles in the antioxidant activity of synthetic peptides. Tocopherol, BHA, and BHT, potentiated the antioxidative activities of the peptides. | (Chen et al., 1996) |
| In vitro (solution) and in cellulo | DPPH, ABTS, ORAC, CAA | NI | SHECN had significantly higher antioxidant activity than LPFAM. The CAA value of SHECN was 776.22 μmol QE/100 g. SHECN also showed significant DPPH inhibition (70.18 ± 4.06%), ABTS inhibition (88.16 ± 0.76%), ORAC value of 0.090 ± 0.002 μmol TE/mg. | (Yang, Wang, et al., 2017) |
| In vitro (solution) | ABTS | NI | Highest MW fractions did not show significant activity. F7 (enriched in Tyr) was the highest with 78% scavenging property (~113 mg TEAC/g). TTYY showed 13.6% up to 59.6% radical scavenging property within a range of 0.18 mM–18 mM. | Beermann et al. (2009) |
| In vitro (solution) | β-carotene bleaching | Cu | Peptides with higher chelating activity contained His and Arg. More hydrophilic fractions were the most antioxidative. | Megías et al. (2008) |
| In vitro (solution) | HRSA, DNA damage assay | Fe | Low-MW fraction F-IV (<3 kDa) exhibited the strongest HRSA and Fe2+ chelating ability. Synthesized peptide YYIVS showed the highest HRSA. | (Zhang et al., 2014) |
| In vitro (solution) | HRSA, ORAC | Fe | Hydrolysate at 300 MPa for 60 min showed the strongest antioxidant activity. Low MW peptide fractions (<3 kDa) were mainly the contributors to antioxidant activity. ORAC values were HDSASGQY (123.06 μg TE/mL) ≥ YYMVSA (117.30 μg TE/mL) ≥ HDSESGQY ~ YYIVS ~ RYYDPL (113.97, 111.90 and 109.24 μg TE/mL, respectively). | (Zhang and Mu, 2017) |
| In vitro (solution) and in cellulo | ABTS, ORAC, CAA | NI | Highest activity for the lowest MW fractions (<3 kDa). ORAC values were in the order of TY > SGGY > SSE > VRN > NPAN > AHSVGP. TY and SGGY exhibited excellent ABTS (~6300 μmol TE/g) and ORAC (~800 μmol TE/g) values, equivalent to GSH. SGGY was effective to protect SH-SY5Y cells against oxidative damage induced by H2O2. | Feng et al. (2019) |
| In vitro (solution) | NI | Fe | LAGNPDDEFRPQ and VEDELVAVV showed strong chelation capacity. | Lv et al. (2017) |
| In vitro (solution and linoleic acid model system) | DPPH, HRSA, FRAP, linoleic acid oxidation (PV) | Fe | Pepsin hydrolysate (3 h) exhibited the highest antioxidant activities (IC50 of HRSA = 5.04 ± 0.19 mg/mL). Its metal chelating activity was higher than GSH, but lower (~20-fold) than EDTA. Highest antioxidant activity with the lowest MW fraction (<3 kDa), and the DPPH activity of ADAF (IC50 = 0.31 ± 0.03 mg/mL) was 6.65-fold higher than hydrolysate. | (Chen et al., 2012) |
| In vitro (solution) and in cellulo | ABTS, DPPH, ORAC | Fe | Higher ORAC values (mmoL TE/mg) with fraction D2 (4248 ± 62) compared to hydrolysate (1389.26 ± 57.13). Hydrolysate chelation of Fe2+ ions was 74 ± 2.03% at 2.00 mg/mL, much higher than GSH. | Gu et al. (2015) |
| In vitro (solution) and in cellulo | DPPH | Fe | Fraction MW < 1 kDa (WSPHs-I) showed the highest antioxidant activities. Peptides showed good antioxidant activity stability against the heat, pH and simulated gastro-intestinal digestion treatment. | Wen et al. (2019) |
| In vitro (solution and linoleic acid model system) | DPPH and inhibition of linoleic acid oxidation (TBARS) | NI | The hydrolysate and its UF fractions showed strong antioxidative activities in both assays. UF fractions were superior to the hydrolysate. Fraction MW < 5 kDa showed the best activity (similar to α-tocopherol). | (Wang et al., 2007) |
| In vitro (solution and linoleic acid model system) | DPPH, SRSA, HRSA, FRAP, inhibition of linoleic acid oxidation (PV) | Fe | The hydrolysate showed a relatively higher free radical-scavenging activities, a notable reducing power, and an antioxidant activity close to that of α-tocopherol in the linoleic acid model system. The hydrolysate had a strong iron binding capacity. | (Zhu et al., 2006) |
| In vitro (solution, β-carotene-linoleic acid model system) and cooked meat patty | DPPH, HRSA, ORAC, inhibition of β-carotene-linoleic acid oxidation (UV), lipid oxidation (TBARS) | Fe | Hydrolysate can suppress lipid oxidation in cooked pork meat patties during room temperature and chilled storage, and did not adversely affect sensory properties. All autofocusing fractions (especially the acids) showed higher chelating ability than hydrolysate. Acidic and basic fractions showed higher antioxidant activity than the hydrolysate. | (Park et al., 2012) |
NI: Not investigated, ESR: electron spin resonance, DPPH: diphényl-picrylhydrazyle, ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid, FRAP: ferric reducing antioxidant power, HSRA: hydroxyl radical scavenging activity, CAA: cellular antioxidant assay, PCL-ACW: Photochemiluminescence-antiradical capacity of water soluble substances, PV: peroxides value, TBARS: thiobarbituric acid reactive substances, SRSA: superoxide radical scavenging activity, SOD: superoxide dismutase, TAC: total antioxidant capacity, CAT: catalase, Tpx: total peroxides, GSH: glutathione, ABAP: 2,2-azobis(2-amidinopropane) dihydrochloride, BHA: butylated hydroxyanisole, DH; degree of hydrolysis, BHT: butylated hydroxytoluene, EDTA: ethylenediaminetetraacetic acid, ED50: median effective dose, IC50: half maximal inhibitory concentration, EC50: half maximal effective concentration, TE: Trolox equivalent, TEAC: Trolox equivalent antioxidant capacity.
3.1. Chemical reactivity of bioactive peptides
One of the main mechanisms of action of an antioxidant lies to its capacity to limit the lipid oxidation propagation by reducing free radicals. This ability to prevent free radicals induced oxidation was evaluated through the capacity of BP to transfer a hydrogen (H) atom or an electron (e−) to reactive species by using different methods such as ABTS, DPPH, HRSA, SRSA, ORAC, FRAP.
This chemical reactivity of peptides is directly associated with their amino acid composition. Basic and hydrophobic amino acids, along with amino acids containing aromatic rings, have exposed positive effects on the antioxidant capacity of the BP, likely due to their better capacity to scavenge radicals in comparison with hydrophilic ones. Therefore, peptides containing His, Lys, Arg, Tyr, Trp, Phe, Pro, Met, may have good results in the aforementioned in-vitro assays (Chen et al., 1995; Cheng et al., 2014; Liu et al., 2018; Pan et al., 2011; Park et al., 2010; Torres-Fuentes et al., 2015; Xie et al., 2008; Yu et al., 2013; Zhang and Mu, 2017; Zhang et al., 2009). His residues are recognized with strong radical scavenging activity due to the presence of an imidazole ring, which explain the antioxidant activity of BP from chickpea proteins that commonly contains such residues (Torres-Fuentes et al., 2015). The presence of aromatic amino acids (Tyr and Phe), or indole and pyrrolidine ring in Trp and Pro, could also serve as hydrogen donors to electron deficient radicals. For example, Phe has an allylic hydrogen that is very active and easily abstracted by free radical. Moreover, sulfur-containing amino acids such as Met and Cys also exhibit scavenging ability. Cys, with a thiol, is likely the most potent because it may transfer hydrogen atom from the SH group or the loss of an electron from its sulfur atom. The negatively charged acidic amino acids, such as Glu or Asp, have also demonstrated free radical quenching activity, as it was shown with rapeseed BP produced by solid state fermentation (He et al., 2012).
In addition, studies have shown that the chemical reactivity of BP depends of the amino acids sequence, that is to say their position and their order in the peptide backbone. For instance, hydrophobic amino acids such as Val, Tyr, Met or Phe at the C-and N-termini may promote the antioxidant activity of BP (Jiang et al., 2019; Liu et al., 2018; Selamassakul et al., 2018; Sun et al., 2019). Therefore, the YGS peptide displayed interesting ORAC value after a hydrogen atom transfer mechanism of the Tyr at the N-terminal position to the water-soluble radicals (Zheng et al., 2012). Moreover, it has been shown in the sequence of peptide Leu-Leu-Pro-His-His that the deletion of the C-terminal His decreased the antioxidant activity, whereas the deletion of the N-terminal Leu had no effect (Chen et al., 1996). In addition, the interaction and influence of the neighboring amino acid residues (e.g. indole ring of Trp) in the peptide sequence, may contribute to the antioxidant capacity of the BP (Tian et al., 2015). For instance, the appropriate combination of Gln, Pro and Tyr in the peptide sequence provided a proper cooperation of structural requirements for the antioxidant action (Bamdad et al., 2015). Similarly, Gly-Pro sequence in the active peptides played an important role in addition to the unique amino acid composition (Zhang et al., 2009).
The majority of hydrolysate produced from plant biomass, identified in Table 1, was often separated and concentrated according to the molecular weight (MW) of BP. The fractions with the best antiradical scavenging and antioxidant activities (Table 2) were frequently the fractions with the lowest MW. In addition, the BP with the strongest activities were dominantly composed of 3–8 amino acids with MW below 1000 Da (1 kDa), which support the direct relationship between MW and the antioxidant activity (Zou et al., 2016). In this context, appropriately low MW can exert a significant effect on the antioxidant activities of peptides. That being said, the low-MW peptides cannot be systematically connected with a better activity since the inherent composition of the amino acid sequences and thus, the protease specificities, remain fundamental (Adebiyi et al., 2008; Karamać et al., 2016; Tang et al., 2010). In addition, the conformation may also influence the chemical properties of BP. Indeed, diverse attractive forces between amino acids can stabilize structures and contribute to the chemical reactivity of BP. For instance, the results of the circular dichroism analysis demonstrated that, compared to LPFAM, which had much lower antioxidant activity, SHECN had a high β-sheet content and reduced α-helix content (R. Yang, Wang, et al., 2017). Another example is the much lower antioxidant activities of the linear chemically-synthesized peptides, in comparison to the natural analogue peptides with specific spatial conformations (Sun et al., 2019). One may say that this effect should be minor for the most active BP that were composed of few amino acids, but the secondary structure may be an important factor for the antioxidant peptides with higher MW. That could explain why studies have found that large-sized peptides (>10 kDa) revealed strongest scavenging and/or reducing power than the smallest ones, likely due to a specific conformation that exposed hydrophobic and/or active antioxidant amino acids at the surface of peptides (Arise et al., 2016; Bamdad and Chen, 2013; Olagunju et al., 2018; Tang et al., 2010; Xia et al., 2012). Lastly, the amphiphilic nature of BP also seems to contribute to the radical-scavenging activities by facilitating interaction and reduction of radical species, especially in multiphasic systems. This characteristic would be discussed in 3.3.
3.2. Metal binding capacity of bioactive peptides
The capacity of BP to prevent the initiation or the acceleration of oxidation by chelating transition metals is of a great importance. Indeed, the catalysis of lipid oxidation is frequently attributed to cyclic boost of LOOH decomposition by Fe2+. Yet, the chemical mechanisms for metal catalysis is more complex, especially in multiphasic or compartmentalized reaction systems (Schaich, 1992). It might arise thought the direct oxidation of unsaturated lipids by the higher valence state metals via electron transfer, or by lower valence state metals via formation of metal oxygen transition complexes or autoxidation (Ingold, 1961). Metals may also get involved in the oxidation or reduction of preformed lipid hydroperoxides or decompose the hydrogen peroxide, leading to reactive oxygen radicals that will contribute to the overall increasing in lipid oxidation because the rates of hydrogen abstraction by those substances are much faster than the rates of ab initio L• formation (Kremer, 1963; Waters, 1971). In addition, metals may also change the oxidation product distributions, the degree of chain branching and secondary reaction, and the nature of termination reactions (e.g. rearrangement of LOOH to epoxides) (Schaich, 2020). Copper has received less attention than iron, but it is known to be as or even more effective in accelerating the decomposition of peroxides. For instance, among the studies reported (129 articles), only ~43% (56 articles) have investigated the chelation capacity of BP, with ~95% (53 articles) dealing with iron and only ~21% (12 articles) with copper.
Metal chelators decrease (or suppress) the lipid oxidation by preventing the multifaceted chemical mechanisms catalyzed by transition metals. Chelators such as BP may form insoluble metal complexes, or provide steric hindrance between metals and oxidizable food components or their oxidation products. Binding metal may also physically separate the metal from the lipid, away of the droplet surface, as observed in an oil-in-water emulsion with the presence of chelating agents in the aqueous phase (Berton-Carabin et al., 2014; Decker et al., 2017). A positive correlation between His, Lys and Arg contents of BP with their metal chelating activity has been already reported (Torres-Fuentes et al., 2012; Zarei et al., 2014), along with the total aromatic and hydrophobic amino acids (Pownall et al., 2010). The metal chelating effect of BP containing His residues was largely related to the –NH group of the imidazole ring. Moreover, this capacity was enhanced when such amino acids were located at the C-terminal extremity (Canabady-Rochelle et al., 2015; Zarei et al., 2014). Similarly, it has been evidenced that Tyr at N-terminal and Asp at C-terminal may contribute to this ability, while Val at N-terminal and Pro at C-terminal showed no ability (Jiang et al., 2019). Peptides containing Glu and Asp could also contribute to the metal ion chelating activity, as observed with African Yam Bean Seed protein hydrolysates (Ajibola et al., 2011) or with mungbean meal protein hydrolysates (Sonklin et al., 2018). In addition, exposed Gln residues in BP could be converted to glutamic acid (deamidation), thus exposing charged groups responsible of strong chelating capacities (Bamdad and Chen, 2013). The interaction of amino acids and the relative location in the sequence can also influence the chelation capacity of BP. For instance, located at the N-terminal of the peptides, Ala can drastically enhance the chelation capacity of His (Canabady-Rochelle et al., 2015). Moreover, the single indole ring of the Trp residue was not involved in the iron chelation mechanism (Canabady-Rochelle et al., 2015). Yet, the combined effect of the indole ring of Trp, together with benzene and phenol rings existing in Phe and Tyr could contribute to the metal chelating activity of BP (Zarei et al., 2014). Moreover, adjacent position of Asp and Glu (e.g. Asp-Asp-Glu and Glu-Asp-Glu) in the sequence might be the crucial part for the peptides binding with iron (Lv et al., 2017). Last but not the least, the conditions in the sequential proteolysis treatments, could significantly affect the chelation property of hydrolysates, as observed with Pepsin followed by Pancreatin on the Black Jamapa bean seed proteins (Carrasco-Castilla et al., 2012a). These variations were due to hydrolysis by Pancreatin of some of the chelating peptides that were released by Pepsin. This result is useful for explaining how peptides released by hydrolysis of different protein fractions contribute to the chelating activities of whole protein hydrolysates. This would be also true for the chemical reactivity, as observed with rice proteins hydrolysates, where an increase of DPPH activity after pepsin hydrolysis was observed, whereas further digestion by trypsin led to a decrease (Phongthai et al., 2018).
It is worth mentioning that the BP-ion complex can also alter both, the electron density at the metallic center and so its redox potential, along with the ion distribution in the system. These two remarks may be fundamental to figure out contradictory effects of metal binding BP, with respect to lipid oxidation in multiphasic environments, since the balance between reactivity and distribution has to be understood. For instance, EDTA complexation may remove free or weakly complexed iron from critical oxidation area, but in opposite lower the Fe3+/Fe2+ redox potential from 0.77 V to 0.12 V, making it a better and faster reducing agent to lipid hydroperoxides (Schaich, 1992). Nevertheless, most of the antioxidant prediction of BP from plant biomass (chelation or chemical reactivity assessments) was performed in the absence of lipids. Indeed, only very few studies have addressed the antioxidant capacity of BP with lipid substrates. Among these experiments, most of them used the linoleic acid dispersion in micelles, and only few works (~6% of the total) has tested the antioxidant power of BP in complex lipid dispersion systems (Cheng et al., 2010; 2014; Silveira Coelho et al., 2019; Xu et al., 2019; Zhang et al., 2010; Zhao and Xiong, 2015; Zhao et al., 2014; Zhou et al., 2012). However, lipid oxidation does not have the same mechanism whether it occurs in homogenous aqueous or organic solvents, as predicted with in-vitro assays (DPPH, FRAP, ABTS, ORAC, etc.), or emulsions and bulk lipids, as the most common forms of lipids dispersion in foods (milk, sauces, soups, beverages, etc.). Indeed, emulsions have an aqueous phase that contains both prooxidants and antioxidants, along with oil-water interface that impact interactions between oil and water components. Thus, the ability of antioxidants (e.g. BP) to inhibit lipid oxidation in food emulsions depends on many factors such as antioxidant concentration, reactivity, partitioning between oil, water and interfacial phases, interactions with other food components, and many other environmental conditions such as pH, ionic strength and temperature. In this context, evaluating or even trying to predict a BP antioxidant efficiency should not be limited to the evaluation of one single, or combination, of these in-vitro assays. To combat lipid oxidation, antioxidant may have different mode of action and mechanisms. The large majority of in vitro methods focus on chemical reactivity and do not consider the effect of the physical state of the formulated system; especially when different phases coexist. However, the system itself has crucial influence on physico-chemistry of lipid oxidation and resulting efficiency of antioxidants.
3.3. Distribution of bioactive peptides in active sites of the lipid oxidation
Anticipating the antioxidant efficiency in real food systems from the unique measure of chemical reactivity is extremely risky. For instance, the DPPH assay has not been correlated with the antioxidant activity of rice hulls extracts in model food matrices (Park et al., 2019). Similarly, the efficiency of diverse antioxidants was found to be significant with such conventional in vitro assays but not in more complex matrices such as oil-in-water emulsions (Martinović et al., 2019). Besides the difference in chemical reactivities between synthetic DPPH and lipid radicals (e.g. physical steric hindrance), the complex physical organization of the system where different phases coexist (e.g. oil and water in emulsions) may become more important than the chemical reactivity. It alters the local concentration of components, and therefore the dynamic equilibrium of the system. In their “polar paradox” theory, Porter et al. suggested that the polar antioxidants, that is to say a molecule having strong water affinity, are more effective in low surface-to-volume ratio displays of lipids (e.g. bulk oils), whereas molecules that are rather nonpolar with strongest lipid phase affinity, are more effective antioxidants in high surface-to-volume lipid environments (e.g. emulsions) (Porter, 1993). This paradox was supported by the works of Frankel et al. that demonstrated that the hydrophilic antioxidants such as trolox and ascorbic acid, were more efficient in bulk oil than in an oil-in-water emulsion, while the reverse trend was observed for their lipophilic derivatives (α-TOH and ascorbyl palmitate) (Frankel et al., 1994). This opposing behavior is typically related with the capacity of antioxidant molecules to distribute in the multiphasic system (molecular partitioning and interphase diffusion), and their proximity with oxidant and prooxidant species. It is worth mentioning that the chemical reactivity between molecules (e.g. BP and oxidant species) lies on their capacity to interact, that is to say, be in the vicinity to each other. This condition is of particular relevance when compartmentalized regions or microstructures exist. In addition, surface-active molecules such as lipid hydroperoxides (LOOH) or phospholipids may stimulate the formation of microstructures (e.g. reversed micelles in bulk oils) and thus, change the molecular partitioning and physical interaction between components, which in turn affect the oxidative chemical pathway (Huang et al., 1996). In addition, the capacity of micelles to be involved in lipid oxidation pathways, by assisting the transport of lipid oxidation products (e.g. LOOH), antioxidants, and other surface-active compounds, from one lipid droplet to another, is getting more and more attention (Li et al., 2020). In this perspective, one may think that the antioxidant efficiency of BP may also connect with their capacity to interact with surfactant micelles, which in turn may be affected by their concentration, since structure and composition may influence the co-micelles formation with other surfactant molecules. In 2014, Cheng et al. evidenced that a cooperativity between BP from potato (rich in Leu, Met, Phe, and Tyr) and surfactants (Tween 20) was responsible of the improved antioxidant activity in oil-in-water emulsions. Indeed, peptides partitioned in the interface, filled in the space between individual Tween 20 molecules, thus provided steric hindrances and electrostatic effects to inhibit oxidation by means of physical obstacles in addition to chemical mechanisms (Cheng et al., 2014). This result supported early study (Chen et al., 1995), which mentioned that peptide with hydrophobic amino acids, valine or leucine, at the N-terminal could retard oxidation by interaction with the free fatty acids (e.g. linoleic acid). In this context, the amphiphilic nature of BP, increase the antioxidant capacity in oil-in-water emulsion by promoting their accumulation at the droplets interface where oxidation is prevalent (Berton-Carabin et al., 2014; Decker et al., 2017).
4. Conclusion and perspectives
The antioxidative potential of BP from plant biomass has been evidenced by many researches, especially during last decade. Considering the myriad of possible sources from plant biomass and the diversity of technology and means to obtain BP from protein materials, it is reasonable to expect more applications. Even though much of the published studies on BP have not taken a systematic approach to optimize the multiple parameters affecting the production and/or the purification of these peptides, this part is rather well mastered with processes that may be scale-up to industrial productions. Yet, to date, very few commercial products with BP have been developed, which should be attributed to a variety of other reasons such as the production cost, allergenicity, or bitter off-flavors. BP could also cause problems in food products by altering the texture (e.g. increased viscosity or gelation) or the color (light scattering or undesirable Maillard reactions) (Elias et al., 2008) and decreasing their techno-functional properties (e.g. foaming, emulsifying properties). In addition, problems in making a reproducible product and/or confirming the real antioxidant efficacy of peptides in the end-product should be better considered. For instance, BP can degrade during food processing (e.g. thermal processing) or lose their antioxidant activity because of interaction with other components of the food matrix like lipids or carbohydrates. In this context, and as discussed above, it is important to study antioxidant properties of peptides in lipid-based formulations. Indeed, most of the “antioxidant” conclusion has been made after measuring the ability of BP to scavenge synthetic radicals in absence of lipids. Moreover, those assays do not take into consideration the complex organization of the food matrix. This remark should carefully be taken into consideration when looking for possible applications of antioxidant peptides. Moreover, most of these 129 works claimed, with data based on these approximate in-vitro antioxidant studies, that the BP from plant biomass could be used in functional foods, although no deep studies have been conducted to really appreciate their health benefits. Therefore, it is necessary to develop model functional foods containing BP to study their activities (e.g. to preserve the lipid oxidation), in addition to their interaction with other food components, and then, their contribution to health. In addition, the effects of incorporating these BP, along with the processing conditions on their bioactivity after formulation in the food matrixes, must be investigated. Finally, in vivo studies should also be conducted to help for the evaluation of the safety, bioavailability and thus, the bioactivity of the BP as food ingredient. In addition, the form (purified peptide vs active fraction vs hydrolysate) in which the antioxidant peptides will be incorporated into food matrices has to be better discussed. Besides the differences in production costs, this will open new scientific interrogations such as the antioxidant peptides’ stability, the synergistic effects, the involvement and interaction with the other trace components, etc. Last but not the least, BP have been proved as possibly beneficial compounds against several life-style related diseases. Thus, the identification of the fate of the plant biomass-derived BP after human digestion would be of a great interest, and the possibility to develop hydrolysates with different peptides that could contribute to both, the stabilization of food products and beneficial effect on human health, would be challenging but very helpful task.
Funding
The authors acknowledge the financial support of SOFIPROTEOL under the BIOPEPTIDE project.
CRediT authorship contribution statement
Erwann Durand: Conceptualization, Writing – original draft. Sophie Beaubier: Conceptualization, Writing – original draft. Isidora Ilic: Writing – original draft. Romain Kapel: Supervision, Writing – review & editing. Pierre Villeneuve: Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adebiyi A.P., Adebiyi A.O., Ogawa T., Muramoto K. Purification and characterisation of antioxidative peptides from unfractionated rice bran protein hydrolysates. Int. J. Food Sci. Technol. 2008;43(1):35–43. doi: 10.1111/j.1365-2621.2006.01379.x. [DOI] [Google Scholar]
- Adebiyi A.P., Adebiyi A.O., Yamashita J., Ogawa T., Muramoto K. Purification and characterization of antioxidative peptides derived from rice bran protein hydrolysates. Eur. Food Res. Technol. 2009;228(4):553–563. doi: 10.1007/s00217-008-0962-3. [DOI] [Google Scholar]
- Aderinola T.A., Fagbemi T.N., Enujiugha V.N., Alashi A.M., Aluko R.E. In vitro antihypertensive and antioxidative properties of alcalase-derived Moringa oleifera seed globulin hydrolysate and its membrane fractions. J. Food Process. Preserv. 2019;43(2) doi: 10.1111/jfpp.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal H., Joshi R., Gupta M. Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016;204:365–372. doi: 10.1016/j.foodchem.2016.02.127. [DOI] [PubMed] [Google Scholar]
- Agrawal H., Joshi R., Gupta M. Isolation and characterisation of enzymatic hydrolysed peptides with antioxidant activities from green tender sorghum. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;84:608–616. doi: 10.1016/j.lwt.2017.06.036. [DOI] [Google Scholar]
- Agrawal H., Joshi R., Gupta M. Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Res. Int. 2019;120:697–707. doi: 10.1016/j.foodres.2018.11.028. [DOI] [PubMed] [Google Scholar]
- Ajibola C.F., Fashakin J.B., Fagbemi T.N., Aluko R.E. Effect of peptide size on antioxidant properties of african Yam bean seed (sphenostylis stenocarpa) protein hydrolysate fractions. Int. J. Mol. Sci. 2011;12(10):6685–6702. doi: 10.3390/ijms12106685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoh C. 2017. Food Lipids: Chemistry, Nutrition, and Biotechnology, Fourth Edition. [DOI] [Google Scholar]
- Alashi A.M., Blanchard C.L., Mailer R.J., Agboola S.O., Mawson A.J., He R., Girgih A., Aluko R.E. Antioxidant properties of Australian canola meal protein hydrolysates. Food Chem. 2014;146:500–506. doi: 10.1016/j.foodchem.2013.09.081. [DOI] [PubMed] [Google Scholar]
- Amadou I., Le G.W., Amza T., Sun J., Shi Y.H. Purification and characterization of foxtail millet-derived peptides with antioxidant and antimicrobial activities. Food Res. Int. 2013;51(1):422–428. doi: 10.1016/j.foodres.2012.12.045. [DOI] [Google Scholar]
- Arcan I., Yemenicioǧlu A. Antioxidant activity of protein extracts from heat-treated or thermally processed chickpeas and white beans. Food Chem. 2007;103(2):301–312. doi: 10.1016/j.foodchem.2006.07.050. [DOI] [Google Scholar]
- Arise A.K., Alashi A.M., Nwachukwu I.D., Ijabadeniyi O.A., Aluko R.E., Amonsou E.O. Antioxidant activities of Bambara groundnut (: vigna subterranea) protein hydrolysates and their membrane ultrafiltration fractions. Food and Function. 2016;7(5):2431–2437. doi: 10.1039/c6fo00057f. [DOI] [PubMed] [Google Scholar]
- Bamdad F., Chen L. Antioxidant capacities of fractionated barley hordein hydrolysates in relation to peptide structures. Mol. Nutr. Food Res. 2013;57(3):493–503. doi: 10.1002/mnfr.201200252. [DOI] [PubMed] [Google Scholar]
- Bamdad F., Ahmed S., Chen L. Specifically designed peptide structures effectively suppressed oxidative reactions in chemical and cellular systems. Journal of Functional Foods. 2015;18:35–46. doi: 10.1016/j.jff.2015.06.055. [DOI] [Google Scholar]
- Bazinet L., Firdaous L. Membrane processes and devices for separation of bioactive peptides. Recent Pat. Biotechnol. 2009;3(1):61–72. doi: 10.2174/187220809787172623. [DOI] [PubMed] [Google Scholar]
- Beaubier S., Framboisier X., Ioannou I., Galet O., Kapel R. Simultaneous quantification of the degree of hydrolysis, protein conversion rate and mean molar weight of peptides released in the course of enzymatic proteolysis. J. Chromatogr. B: Analytical Technologies in the Biomedical and Life Sciences. 2019;1105:1–9. doi: 10.1016/j.jchromb.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Beermann C., Euler M., Herzberg J., Stahl B. Anti-oxidative capacity of enzymatically released peptides from soybean protein isolate. Eur. Food Res. Technol. 2009;229(4):637–644. doi: 10.1007/s00217-009-1093-1. [DOI] [Google Scholar]
- Berton-Carabin C.C., Ropers M.-H., Genot C. Lipid oxidation in oil-in-water emulsions: involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2014;13(5):945–977. doi: 10.1111/1541-4337.12097. [DOI] [Google Scholar]
- Betancur-Ancona D., Sosa-Espinoza T., Ruiz-Ruiz J., Segura-Campos M., Chel-Guerrero L. Enzymatic hydrolysis of hard-to-cook bean ( Phaseolus vulgaris L.) protein concentrates and its effects on biological and functional properties. Int. J. Food Sci. Technol. 2014;49(1):2–8. doi: 10.1111/ijfs.12267. [DOI] [Google Scholar]
- Bhandari D., Rafiq S., Gat Y., Gat P., Waghmare R., Kumar V. A review on bioactive peptides: physiological functions, bioavailability and safety. Int. J. Pept. Res. Therapeut. 2020;26(1):139–150. doi: 10.1007/s10989-019-09823-5. [DOI] [Google Scholar]
- Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organization Journal. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer M.S. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011;10(4):221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- Cai X., Yan A., Fu N., Wang S. In vitro antioxidant activities of enzymatic hydrolysate from schizochytrium sp. and its hepatoprotective effects on acute alcohol-induced liver injury in vivo. Mar. Drugs. 2017;15(4) doi: 10.3390/md15040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canabady-Rochelle L.S., Harscoat-Schiavo C., Kessler V., Aymes A., Fournier F., Girardet J.M. Determination of reducing power and metal chelating ability of antioxidant peptides: revisited methods. Food Chem. 2015;183:129–135. doi: 10.1016/j.foodchem.2015.02.147. [DOI] [PubMed] [Google Scholar]
- Carrasco-Castilla J., Hernández-Álvarez A.J., Jiménez-Martínez C., Jacinto-Hernández C., Alaiz M., Girón-Calle J., Vioque J., Dávila-Ortiz G. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem. 2012;131(4):1157–1164. doi: 10.1016/j.foodchem.2011.09.084. [DOI] [PubMed] [Google Scholar]
- Carrasco-Castilla J., Hernández-Álvarez A.J., Jiménez-Martínez C., Jacinto-Hernández C., Alaiz M., Girón-Calle J., Vioque J., Dávila-Ortiz G. Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chem. 2012;135(3):1789–1795. doi: 10.1016/j.foodchem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Chai T.T., Soo Z.Y., Hsu K.C., Li J.C., Abd M.F., Wong F.C. Antioxidant activity of semen cassiae protein hydrolysate: thermal and gastrointestinal stability, peptide identification, and in silico analysis. Modern Food Science and Technology. 2019;35(9):38–48. doi: 10.13982/j.mfst.1673-9078.2019.9.004. [DOI] [Google Scholar]
- Chan W.K., Decker E.A., Lee J.B., Butterfield D.A. EPR spin-trapping studies of the hydroxyl radical scavenging activity of carnosine and related dipeptides. J. Agric. Food Chem. 1994;42(7):1407–1410. doi: 10.1021/jf00043a003. [DOI] [Google Scholar]
- Chanput W., Theerakulkait C., Nakai S. Antioxidative properties of partially purified barley hordein, rice bran protein fractions and their hydrolysates. J. Cereal. Sci. 2009;49(3):422–428. doi: 10.1016/j.jcs.2009.02.001. [DOI] [Google Scholar]
- Chen H.-M., Muramoto K., Yamauchi F. Structural analysis of antioxidative peptides from soybean beta-conglycinin. J. Agric. Food Chem. 1995;43(3):574–578. doi: 10.1021/jf00051a004. [DOI] [Google Scholar]
- Chen H.M., Muramoto K., Yamauchi F., Nokihara K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J. Agric. Food Chem. 1996;44(9):2619–2623. doi: 10.1021/jf950833m. [DOI] [Google Scholar]
- Chen N., Yang H., Sun Y., Niu J., Liu S. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides. 2012;38(2):344–349. doi: 10.1016/j.peptides.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Xiong Y.L., Chen J. Fractionation, separation, and identification of antioxidative peptides in potato protein hydrolysate that enhance oxidative stability of soybean oil emulsions. J. Food Sci. 2010;75(9):C760–C765. doi: 10.1111/j.1750-3841.2010.01864.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chen J., Xiong Y.L. Interfacial adsorption of peptides in oil-in-water emulsions costabilized by tween 20 and antioxidative potato peptides. J. Agric. Food Chem. 2014;62(47):11575–11581. doi: 10.1021/jf5038135. [DOI] [PubMed] [Google Scholar]
- Coda R., Rizzello C.G., Pinto D., Gobbetti M. Selected lactic acid bacteria synthesize antioxidant peptides during sourdough fermentation of cereal flours. Appl. Environ. Microbiol. 2012;78(4):1087–1096. doi: 10.1128/AEM.06837-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumby N., Zhong Y., Naczk M., Shahidi F. Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chem. 2008;109(1):144–148. doi: 10.1016/j.foodchem.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Das R., Ghosh S., Bhattacharjee C. Enzyme membrane reactor in isolation of antioxidative peptides from oil industry waste: a comparison with non-peptidic antioxidants. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2012;47(2):238–245. doi: 10.1016/j.lwt.2012.01.011. [DOI] [Google Scholar]
- Decker E.A., Elias R.J., McClements D.J. 2010. Oxidation in Foods and Beverages and Antioxidant Applications | Management in Different Industry Sectors (Woodhead P) [Google Scholar]
- Decker E.A., Elias R.J., McClements D.J. 2010. Oxidation in Foods and Beverages and Antioxidant Applications | Understanding Mechanisms of Oxidation and Antioxidant Activity (Woodhead P. [Google Scholar]
- Decker E.A., McClements D.J., Bourlieu-Lacanal C., Durand E., Figueroa-Espinoza M.C., Lecomte J., Villeneuve P. Hurdles in predicting antioxidant efficacy in oil-in-water emulsions. Trends Food Sci. Technol. 2017;67 doi: 10.1016/j.tifs.2017.07.001. [DOI] [Google Scholar]
- Delgado Orsini, Nardo A., Pavlovic M., Rogniaux H., Añón M.C., Tironi V.A. Identification and characterization of antioxidant peptides obtained by gastrointestinal digestion of amaranth proteins. Food Chem. 2016;197:1160–1167. doi: 10.1016/j.foodchem.2015.11.092. Part B. [DOI] [PubMed] [Google Scholar]
- Elias R.J., Kellerby S.S., Decker E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008;48(5):430–441. doi: 10.1080/10408390701425615. [DOI] [PubMed] [Google Scholar]
- Evangelho J. A. do, Vanier N.L., Pinto V.Z., Berrios J.J.D., Dias A.R.G., Zavareze E. da R. Black bean (Phaseolus vulgaris L.) protein hydrolysates: physicochemical and functional properties. Food Chem. 2017;214:460–467. doi: 10.1016/j.foodchem.2016.07.046. [DOI] [PubMed] [Google Scholar]
- Fao FAO (2020). FAOSTAT-production, last update 4 march 2020. FAO statistics division 2009. 2020. http://www.fao.org/faostat/fr/#data/QC
- Feng L., Peng F., Wang X., Li M., Lei H., Xu H. Identification and characterization of antioxidative peptides derived from simulated in vitro gastrointestinal digestion of walnut meal proteins. Food Res. Int. 2019;116:518–526. doi: 10.1016/j.foodres.2018.08.068. [DOI] [PubMed] [Google Scholar]
- Frankel E.N., Huang S., Kanner J., German J.B. Interfacial phenomena in the evaluation of antioxidants: bulk oils vs emulsions. J. Agric. Food Chem. 1994;42:1054–1059. doi: 10.1021/jf00041a001. [DOI] [Google Scholar]
- German J.B. vol. 459. 1999. Food processing and lipid oxidation; pp. 23–50. (Advances in Experimental Medicine and Biology). Kluwer Academic/Plenum Publishers. [DOI] [PubMed] [Google Scholar]
- Girgih A.T., Udenigwe C.C., Aluko R.E. Reverse-phase HPLC separation of hemp seed (cannabis sativa L.) protein hydrolysate produced peptide fractions with enhanced antioxidant capacity. Plant Foods Hum. Nutr. 2013;68(1):39–46. doi: 10.1007/s11130-013-0340-6. [DOI] [PubMed] [Google Scholar]
- Girgih A., Alashi A., He R., Malomo S., Raj P., Netticadan T., Aluko R. A novel hemp seed meal protein hydrolysate reduces oxidative stress factors in spontaneously hypertensive rats. Nutrients. 2014;6(12):5652–5666. doi: 10.3390/nu6125652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgih A.T., He R., Malomo S., Offengenden M., Wu J., Aluko R.E. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. Journal of Functional Foods. 2014;6(1):384–394. doi: 10.1016/j.jff.2013.11.005. [DOI] [Google Scholar]
- Gu M., Chen H.-P., Zhao M.-M., Wang X., Yang B., Ren J.-Y., Su G.-W. Identification of antioxidant peptides released from defatted walnut (Juglans Sigillata Dode) meal proteins with pancreatin. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2015;60(1):213–220. doi: 10.1016/j.lwt.2014.07.052. [DOI] [Google Scholar]
- Guéguen J., Walrand S., Bourgeois O. Les protéines végétales : contexte et potentiels en alimentation humaine. Cah. Nutr. Diet. 2016;51(4):177–185. doi: 10.1016/j.cnd.2016.02.001. [DOI] [Google Scholar]
- Gunstone F.D., Norris F.A. 1983. Lipids in Foods - 1st Edition, Chemistry, Biochemistry and Technology. Elsevier Ltd. All Rights Reserved. [Google Scholar]
- He R., Ju X., Yuan J., Wang L., Girgih A.T., Aluko R.E. Antioxidant activities of rapeseed peptides produced by solid state fermentation. Food Res. Int. 2012;49(1):432–438. doi: 10.1016/j.foodres.2012.08.023. [DOI] [Google Scholar]
- He R., Alashi A., Malomo S.A., Girgih A.T., Chao D., Ju X., Aluko R.E. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013;141(1):153–159. doi: 10.1016/j.foodchem.2013.02.087. [DOI] [PubMed] [Google Scholar]
- Herrera Chalé F.G., Ruiz Ruiz J.C., Acevedo Fernández J.J., Betancur Ancona D.A., Segura Campos M.R. ACE inhibitory, hypotensive and antioxidant peptide fractions from Mucuna pruriens proteins. Process Biochem. 2014;49(10):1691–1698. doi: 10.1016/j.procbio.2014.06.021. [DOI] [Google Scholar]
- Hong J., Chen T.T., Hu P., Yang J., Wang S.Y. Purification and characterization of an antioxidant peptide (GSQ) from Chinese leek (Allium tuberosum Rottler) seeds. Journal of Functional Foods. 2014;10:144–153. doi: 10.1016/j.jff.2014.05.014. [DOI] [Google Scholar]
- Hu X., Yang X., Wu Q., Li L., Wu Y., Chen S., Li R., Ren J. Purification and identification of antioxidant peptides from Schizochytrium limacinum hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Molecules. 2019;24(16):3004–3015. doi: 10.3390/molecules24163004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.W., Hopia A., Schwarz K., Frankel E.N., German J.B. Antioxidant activity of α-tocopherol and trolox in different lipid substrates: bulk oils vs oil-in-water emulsions. J. Agric. Food Chem. 1996;44(2):444–452. doi: 10.1021/jf9505685. [DOI] [Google Scholar]
- Hwang J.Y., Shue Y.S., Chang H.M. Antioxidative activity of roasted and defatted peanut kernels. Food Res. Int. 2001;34(7):639–647. doi: 10.1016/S0963-9969(01)00083-7. [DOI] [Google Scholar]
- Hwang J.Y., Shyu Y.S., Wang Y.T., Hsu C.K. Antioxidative properties of protein hydrolysate from defatted peanut kernels treated with esperase. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2010;43(2):285–290. doi: 10.1016/j.lwt.2009.08.020. [DOI] [Google Scholar]
- Indiano-Romacho P., Fernández-Tomé S., Amigo L., Hernández-Ledesma B. Multifunctionality of lunasin and peptides released during its simulated gastrointestinal digestion. Food Res. Int. 2019;125:108513. doi: 10.1016/j.foodres.2019.108513. [DOI] [PubMed] [Google Scholar]
- Ingold K.U. Inhibition of the autoxidation of organic substances in the liquid phase. Chem. Rev. 1961;61(6):563–589. doi: 10.1021/cr60214a002. [DOI] [Google Scholar]
- Jamdar S.N., Deshpande R., Marathe S.A. Effect of processing conditions and in vitro protein digestion on bioactive potentials of commonly consumed legumes. Food Bioscience. 2017;20:1–11. doi: 10.1016/j.fbio.2017.07.007. [DOI] [Google Scholar]
- Jeong H.J., Lam Y., De Lumen B.O. Barley lunasin suppresses ras-induced colony formation and inhibits core histone acetylation in mammalian cells. J. Agric. Food Chem. 2002;50(21):5903–5908. doi: 10.1021/jf0256945. [DOI] [PubMed] [Google Scholar]
- Jeong H.J., Jeong J.B., Kim D.S., Park J.H., Lee J.B., Kweon D.H., Chung G.Y., Seo E.W., de Lumen B.O. The cancer preventive peptide lunasin from wheat inhibits core histone acetylation. Canc. Lett. 2007;255(1):42–48. doi: 10.1016/j.canlet.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Jeong H.J., Lee J.R., Jeong J.B., Park J.H., Cheong Y.K., De Lumen B.O. The cancer preventive seed peptide lunasin from rye is bioavailable and bioactive. Nutr. Canc. 2009;61(5):680–686. doi: 10.1080/01635580902850082. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Zhang M., Lin S., Cheng S. Contribution of specific amino acid and secondary structure to the antioxidant property of corn gluten proteins. Food Res. Int. 2018;105:836–844. doi: 10.1016/j.foodres.2017.12.022. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Zhao D., Sun J., Luo X., Li H., Sun X., Zheng F. Analysis of antioxidant effect of two tripeptides isolated from fermented grains (Jiupei) and the antioxidative interaction with 4-methylguaiacol, 4-ethylguaiacol, and vanillin. Food Sci. Nutr. 2019;7(7):2391–2403. doi: 10.1002/fsn3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D.X., Liu X.L., Zheng X.Q., Wang X.J., He J.F. Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chem. 2016;204:427–436. doi: 10.1016/j.foodchem.2016.02.119. [DOI] [PubMed] [Google Scholar]
- Kapel Romain, Chabeau A., Lesage J., Riviere G., Ravallec-Ple R., Lecouturier D., Wartelle M., Guillochon D., Dhulster P. Production, in continuous enzymatic membrane reactor, of an anti-hypertensive hydrolysate from an industrial alfalfa white protein concentrate exhibiting ACE inhibitory and opioid activities. Food Chem. 2006;98(1):120–126. doi: 10.1016/j.foodchem.2005.05.062. [DOI] [Google Scholar]
- Kapel R., Klingenberg F., Framboisier X., Dhulster P., Marc I. An original use of size exclusion-HPLC for predicting the performances of batch ultrafiltration implemented to enrich a complex protein hydrolysate in a targeted bioactive peptide. J. Membr. Sci. 2011;383(1–2):26–34. doi: 10.1016/j.memsci.2011.08.025. [DOI] [Google Scholar]
- Karamać M., Kosińska-Cagnazzo A., Kulczyk A. Use of different proteases to obtain flaxseed protein hydrolysates with antioxidant activity. Int. J. Mol. Sci. 2016;17(7):1027. doi: 10.3390/ijms17071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts D., Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharmaceut. Des. 2005;9(16):1309–1323. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- Kong B., Xiong Y.L. Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J. Agric. Food Chem. 2006;54(16):6059–6068. doi: 10.1021/jf060632q. [DOI] [PubMed] [Google Scholar]
- Korhonen H., Pihlanto A. Bioactive peptides: production and functionality. Int. Dairy J. 2006;16(9):945–960. doi: 10.1016/j.idairyj.2005.10.012. [DOI] [Google Scholar]
- Kremer M.L. Oxidation reduction step in catalytic decomposition of hydrogen peroxide by ferric ions. Trans. Faraday Soc. 1963;59:2535–2542. doi: 10.1039/tf9635902535. 0. [DOI] [Google Scholar]
- Li Y., Jiang B., Zhang T., Mu W., Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106(2):444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Li H.-M., Hu X., Guo P., Fu P., Xu L., Zhang X.-Z. Antioxidant properties and possible mode of action of corn protein peptides and zein peptides. J. Food Biochem. 2010;34(1):44–60. doi: 10.1111/j.1745-4514.2009.00292.x. [DOI] [Google Scholar]
- Li P., McClements D.J., Decker E.A. Application of flow cytometry as novel technology in studying lipid oxidation and mass transport phenomena in oil-in-water emulsions. Food Chem. 2020;315:126225. doi: 10.1016/j.foodchem.2020.126225. [DOI] [PubMed] [Google Scholar]
- Liang R., Zhang Z., Lin S. Effects of pulsed electric field on intracellular antioxidant activity and antioxidant enzyme regulating capacities of pine nut (Pinus koraiensis) peptide QDHCH in HepG2 cells. Food Chem. 2017;237:793–802. doi: 10.1016/j.foodchem.2017.05.144. [DOI] [PubMed] [Google Scholar]
- Liu X., Zheng X., Song Z., Liu X., kumar Kopparapu N., Wang X., Zheng Y. Preparation of enzymatic pretreated corn gluten meal hydrolysate and in vivo evaluation of its antioxidant activity. Journal of Functional Foods. 2015;18:1147–1157. doi: 10.1016/j.jff.2014.10.013. [DOI] [Google Scholar]
- Liu C., Ren D., Li J., Fang L., Wang J., Liu J., Min W. Cytoprotective effect and purification of novel antioxidant peptides from hazelnut (C. heterophylla Fisch) protein hydrolysates. Journal of Functional Foods. 2018;42:203–215. doi: 10.1016/j.jff.2017.12.003. [DOI] [Google Scholar]
- Lu R.R., Qian P., Sun Z., Zhou X.H., Chen T.P., He J.F., Zhang H., Wu J. Hempseed protein derived antioxidative peptides: purification, identification and protection from hydrogen peroxide-induced apoptosis in PC12 cells. Food Chem. 2010;123(4):1210–1218. doi: 10.1016/j.foodchem.2010.05.089. [DOI] [Google Scholar]
- Lu X., Zhang L., Sun Q., Song G., Huang J. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2019;116:707–716. doi: 10.1016/j.foodres.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Lv Y., Wei K., Meng X., Huang Y., Zhang T., Li Z. Separation and identification of iron-chelating peptides from defatted walnut flake by nanoLC-ESI–MS/MS and de novo sequencing. Process Biochem. 2017;59(May):223–228. doi: 10.1016/j.procbio.2017.05.010. [DOI] [Google Scholar]
- Ma Y., Xiong Y.L., Zhai J., Zhu H., Dziubla T. Fractionation and evaluation of radical scavenging peptides from in vitro digests of buckwheat protein. Food Chem. 2010;118(3):582–588. doi: 10.1016/j.foodchem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestri E., Marmiroli M., Marmiroli N. Bioactive peptides in plant-derived foodstuffs. Journal of Proteomics. 2016;147:140–155. doi: 10.1016/j.jprot.2016.03.048. [DOI] [PubMed] [Google Scholar]
- Maldonado-Cervantes E., Jeong H.J., León-Galván F., Barrera-Pacheco A., De León-Rodríguez A., González De Mejia E., De Lumen B.O., Barba De La Rosa A.P. Amaranth lunasin-like peptide internalizes into the cell nucleus and inhibits chemical carcinogen-induced transformation of NIH-3T3 cells. Peptides. 2010;31(9):1635–1642. doi: 10.1016/j.peptides.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Marciniak A., Suwal S., Naderi N., Pouliot Y., Doyen A. Enhancing enzymatic hydrolysis of food proteins and production of bioactive peptides using high hydrostatic pressure technology. Trends Food Sci. Technol. 2018;80:187–198. doi: 10.1016/j.tifs.2018.08.013. [DOI] [Google Scholar]
- Marczak E.D., Usui H., Fujita H., Yang Y., Yokoo M., Lipkowski A.W., Yoshikawa M. New antihypertensive peptides isolated from rapeseed. Peptides. 2003;24(6):791–798. doi: 10.1016/s0196-9781(03)00174-8. http://www.ncbi.nlm.nih.gov/pubmed/12948830 [DOI] [PubMed] [Google Scholar]
- Marques M.R., Soares Freitas R.A.M., Corrêa Carlos A.C., Siguemoto É.S., Fontanari G.G., Arêas J.A.G. Peptides from cowpea present antioxidant activity, inhibit cholesterol synthesis and its solubilisation into micelles. Food Chem. 2015;168:288–293. doi: 10.1016/j.foodchem.2014.07.049. [DOI] [PubMed] [Google Scholar]
- Martinović N., Poklar Ulrih N., Abramovič H. Sinapic acid and its derivatives increase oxidative stability in different model lipid systems. Eur. J. Lipid Sci. Technol. 2019;121(4):1800326. doi: 10.1002/ejlt.201800326. [DOI] [Google Scholar]
- McClements D., Decker E. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000;65(8):1270–1282. doi: 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- Megías C., Pedroche J., Yust M.M., Girón-Calle J., Alaiz M., Millán F., Vioque J. Production of copper-chelating peptides after hydrolysis of sunflower proteins with pepsin and pancreatin. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2008;41(10):1973–1977. doi: 10.1016/j.lwt.2007.11.010. [DOI] [Google Scholar]
- Moure A., Domínguez H., Parajó J.C. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2006;41(2):447–456. doi: 10.1016/j.procbio.2005.07.014. [DOI] [Google Scholar]
- Mundi S., Aluko R.E. Inhibitory properties of kidney bean protein hydrolysate and its membrane fractions against renin, angiotensin converting enzyme, and free radicals. J. Nutr. Food Sci. 2014;2(1):1008. [Google Scholar]
- Nakurte I., Kirhnere I., Namniece J., Saleniece K., Krigere L., Mekss P., Vicupe Z., Bleidere M., Legzdina L., Muceniece R. Detection of the lunasin peptide in oats (Avena sativa L) J. Cereal. Sci. 2013;57(3):319–324. doi: 10.1016/j.jcs.2012.12.008. [DOI] [Google Scholar]
- Nasri M. Protein hydrolysates and biopeptides: production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017;81:109–159. doi: 10.1016/bs.afnr.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Ng K.L., Ayob M.K., Said M., Osman M.A., Ismail A. Optimization of enzymatic hydrolysis of palm kernel cake protein (PKCP) for producing hydrolysates with antiradical capacity. Ind. Crop. Prod. 2013;43(1):725–731. doi: 10.1016/j.indcrop.2012.08.017. [DOI] [Google Scholar]
- Ngoh Y.Y., Gan C.Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto) Food Chem. 2016;190:331–337. doi: 10.1016/j.foodchem.2015.05.120. [DOI] [PubMed] [Google Scholar]
- Nwachukwu I.D., Aluko R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019;43(1) doi: 10.1111/jfbc.12761. [DOI] [PubMed] [Google Scholar]
- Olagunju A.I., Omoba O.S., Enujiugha V.N., Alashi A.M., Aluko R.E. Pigeon pea enzymatic protein hydrolysates and ultrafiltration peptide fractions as potential sources of antioxidant peptides: an in vitro study. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2018;97:269–278. doi: 10.1016/j.lwt.2018.07.003. [DOI] [Google Scholar]
- Orsini Delgado M.C., Tironi V.A., Añón M.C. Antioxidant activity of amaranth protein or their hydrolysates under simulated gastrointestinal digestion. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2011;44(8):1752–1760. doi: 10.1016/j.lwt.2011.04.002. [DOI] [Google Scholar]
- Ortiz-Martinez M., Otero-Pappatheodorou J.T., Serna-Saldívar S.O., García-Lara S. Antioxidant activity and characterization of protein fractions and hydrolysates from normal and quality protein maize kernels. J. Cereal. Sci. 2017;76:85–91. doi: 10.1016/j.jcs.2017.05.021. [DOI] [Google Scholar]
- Østdal H., Andersen H.J., Davies M.J. Formation of long-lived radicals on proteins by radical transfer from heme enzymes - a common process? Arch. Biochem. Biophys. 1999;362(1):105–112. doi: 10.1006/abbi.1998.0988. [DOI] [PubMed] [Google Scholar]
- Pan M., Jiang T.S., Pan J.L. Antioxidant activities of rapeseed protein hydrolysates. Food Bioprocess Technol. 2011;4(7):1144–1152. doi: 10.1007/s11947-009-0206-y. [DOI] [Google Scholar]
- Park S.Y., Lee J.-S., Baek H.-H., Lee H.G. Purification and characterization of antioxidant peptides from soy protein hydrolysate. J. Food Biochem. 2010;34(Suppl. 1):120–132. doi: 10.1111/j.1745-4514.2009.00313.x. [DOI] [Google Scholar]
- Park E.Y., Imazu H., Matsumura Y., Nakamura Y., Sato K. Effects of peptide fractions with different isoelectric points from wheat gluten hydrolysates on lipid oxidation in pork meat patties. J. Agric. Food Chem. 2012;60(30):7483–7488. doi: 10.1021/jf301532e. [DOI] [PubMed] [Google Scholar]
- Park J.H., Gim S.Y., Jeon J.Y., Kim M.J., Choi H.K., Lee J.H. Chemical profiles and antioxidant properties of roasted rice hull extracts in bulk oil and oil-in-water emulsion. Food Chem. 2019;272:242–250. doi: 10.1016/j.foodchem.2018.08.054. [DOI] [PubMed] [Google Scholar]
- Penta-Ramos E.A., Xiong Y.L. Antioxidant activity of soy protein hydrolysates in a liposomal system. J. Food Sci. 2002;67(8):2952–2956. doi: 10.1111/j.1365-2621.2002.tb08844.x. [DOI] [Google Scholar]
- Phongthai S., D'Amico S., Schoenlechner R., Homthawornchoo W., Rawdkuen S. Fractionation and antioxidant properties of rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion. Food Chem. 2018;240:156–164. doi: 10.1016/j.foodchem.2017.07.080. [DOI] [PubMed] [Google Scholar]
- Pihlanto A., Korhonen H. Bioactive peptides and proteins. Adv. Food Nutr. Res. 2003;47:175–276. doi: 10.1016/S1043-4526(03)47004-6. [DOI] [PubMed] [Google Scholar]
- Piovesana S., Capriotti A.L., Cavaliere C., La Barbera G., Montone C.M., Zenezini Chiozzi R., Laganà A. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal. Bioanal. Chem. 2018;410(15):3425–3444. doi: 10.1007/s00216-018-0852-x. [DOI] [PubMed] [Google Scholar]
- Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Hindawi Limited; 2017. Oxidative stress: harms and benefits for human health. (Oxidative Medicine and Cellular Longevity). 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter W.L. vol. 9. SAGE PublicationsSage UK; London, England: 1993. Paradoxical behavior of antioxidants in food and biological systems; pp. 93–122. (Toxicology and Industrial Health). Issues 1–2. [DOI] [PubMed] [Google Scholar]
- Pownall T.L., Udenigwe C.C., Aluko R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) Enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010;58(8):4712–4718. doi: 10.1021/jf904456r. [DOI] [PubMed] [Google Scholar]
- Ren G., Zhu Y., Shi Z., Li J. Detection of lunasin in quinoa ( Chenopodium quinoa Willd.) and the in vitro evaluation of its antioxidant and anti-inflammatory activities. J. Sci. Food Agric. 2017;97(12):4110–4116. doi: 10.1002/jsfa.8278. [DOI] [PubMed] [Google Scholar]
- Ren D., Zhao F., Liu C., Wang J., Guo Y., Liu J., Min W. Antioxidant hydrolyzed peptides from Manchurian walnut ( Juglans mandshurica Maxim.) attenuate scopolamine-induced memory impairment in mice. J. Sci. Food Agric. 2018;98(13):5142–5152. doi: 10.1002/jsfa.9060. [DOI] [PubMed] [Google Scholar]
- Rodrigues I.M., Coelho J.F.J., Carvalho M.G.V.S. Journal of Food Engineering. vol. 109. 2012. Isolation and valorisation of vegetable proteins from oilseed plants: methods, limitations and potential; pp. 337–346. Elsevier Ltd. [DOI] [Google Scholar]
- Sabbione A.C., Ibañez S.M., Martínez E.N., Añón M.C., Scilingo A.A. Antithrombotic and antioxidant activity of amaranth hydrolysate obtained by activation of an endogenous protease. Plant Foods Hum. Nutr. 2016;71(2):174–182. doi: 10.1007/s11130-016-0540-y. [DOI] [PubMed] [Google Scholar]
- Samaranayaka A.G.P., Li-Chan E.C.Y. Food-derived peptidic antioxidants: a review of their production, assessment, and potential applications. Journal of Functional Foods. 2011;3(4):229–254. doi: 10.1016/j.jff.2011.05.006. [DOI] [Google Scholar]
- Sarmadi B.H., Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31(10):1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Schaich K.M. Metals and lipid oxidation. Contemporary issues. Lipids. 1992;27(3):209–218. doi: 10.1007/BF02536181. [DOI] [PubMed] [Google Scholar]
- Schaich K.M. Lipid oxidation: new perspectives on an old reaction. In: Shahidi F., editor. Bailey's Industrial Oil and Fat Products, Seventh Edition. John Wiley & Sons, Ltd. 2020. [DOI] [Google Scholar]
- Segura Campos M.R., Chel Guerrero L.A., Betancur Ancona D.A. Angiotensin-I converting enzyme inhibitory and antioxidant activities of peptide fractions extracted by ultrafiltration of cowpea Vigna unguiculata hydrolysates. J. Sci. Food Agric. 2010;90(14):2512–2518. doi: 10.1002/jsfa.4114. [DOI] [PubMed] [Google Scholar]
- Segura-Campos M., Ruiz-Ruiz J., Chel-Guerrero L., Betancur-Ancona D. Antioxidant activity of Vigna unguiculata L. walp and hard-to-cook Phaseolus vulgaris L. protein hydrolysates. CyTA - J. Food. 2013;11(3):208–215. doi: 10.1080/19476337.2012.722687. [DOI] [Google Scholar]
- Selamassakul O., Laohakunjit N., Kerdchoechuen O., Ratanakhanokchai K. A novel multi-biofunctional protein from brown rice hydrolysed by endo/endo-exoproteases. Food and Function. 2016;7(6):2635–2644. doi: 10.1039/c5fo01344e. [DOI] [PubMed] [Google Scholar]
- Selamassakul O., Laohakunjit N., Kerdchoechuen O., Yang L., Maier C.S. Isolation and characterisation of antioxidative peptides from bromelain-hydrolysed brown rice protein by proteomic technique. Process Biochem. 2018;70(March):179–187. doi: 10.1016/j.procbio.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski Z., Kolakowska A., Kolakowska A. 2010. Chemical and Functional Properties of Food Lipids. [DOI] [Google Scholar]
- Silva F.G. D.e., Hernández-Ledesma B., Amigo L., Netto F.M., Miralles B. Identification of peptides released from flaxseed (Linum usitatissimum) protein by Alcalase® hydrolysis: antioxidant activity. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;76:140–146. doi: 10.1016/j.lwt.2016.10.049. [DOI] [Google Scholar]
- Silveira Coelho M., de Araujo Aquino S., Machado Latorres J., de las Mercedes Salas-Mellado M. In vitro and in vivo antioxidant capacity of chia protein hydrolysates and peptides. Food Hydrocolloids. 2019;91:19–25. doi: 10.1016/j.foodhyd.2019.01.018. [DOI] [Google Scholar]
- Sonklin C., Laohakunjit N., Kerdchoechuen O. Assessment of antioxidant properties of membrane ultrafiltration peptides from mungbean meal protein hydrolysates. PeerJ. 2018;(7):e5337. doi: 10.7717/peerj.5337. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Tang X., Ren Y., Wang E., Shi L., Wu X., Wu H. Novel antioxidant peptides purified from mulberry (morus atropurpurea roxb.) leaf protein hydrolysates with hemolysis inhibition ability and cellular antioxidant activity. J. Agric. Food Chem. 2019;67(27):7650–7659. doi: 10.1021/acs.jafc.9b01115. [DOI] [PubMed] [Google Scholar]
- Tang N., Zhuang H. Evaluation of antioxidant activities of zein protein fractions. J. Food Sci. 2014;79(11):2174–2184. doi: 10.1111/1750-3841.12686. [DOI] [PubMed] [Google Scholar]
- Tang X., He Z., Dai Y., Xiong Y.L., Xie M., Chen J. Peptide fractionation and free radical scavenging activity of zein hydrolysate. J. Agric. Food Chem. 2010;58(1):587–593. doi: 10.1021/jf9028656. [DOI] [PubMed] [Google Scholar]
- Tian M., Fang B., Jiang L., Guo H., Cui J.Y., Ren F. Structure-activity relationship of a series of antioxidant tripeptides derived from β-Lactoglobulin using QSAR modeling. Dairy Sci. Technol. 2015;95(4):451–463. doi: 10.1007/s13594-015-0226-5. [DOI] [Google Scholar]
- Torres-Fuentes C., Alaiz M., Vioque J. Iron-chelating activity of chickpea protein hydrolysate peptides. Food Chem. 2012;134:1585–1588. doi: 10.1016/j.foodchem.2012.03.112. [DOI] [PubMed] [Google Scholar]
- Torres-Fuentes C., Contreras M.D.M., Recio I., Alaiz M., Vioque J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015;180:194–202. doi: 10.1016/j.foodchem.2015.02.046. [DOI] [PubMed] [Google Scholar]
- Torruco-Uco J., Chel-Guerrero L., Martínez-Ayala A., Dávila-Ortíz G., Betancur-Ancona D. Angiotensin-I converting enzyme inhibitory and antioxidant activities of protein hydrolysates from Phaseolus lunatus and Phaseolus vulgaris seeds. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2009;42(10):1597–1604. doi: 10.1016/j.lwt.2009.06.006. [DOI] [Google Scholar]
- Tsopmo A., Cooper A., Jodayree S. Enzymatic hydrolysis of oat flour protein isolates to enhance antioxidative properties. Adv. J. Food Sci. Technol. 2010;2(4):206–212. [Google Scholar]
- Valdez-Ortiz A., Fuentes-Gutiérrez C.I., Germán-Báez L.J., Gutiérrez-Dorado R., Medina-Godoy S. Protein hydrolysates obtained from Azufrado (sulphur yellow) beans (Phaseolus vulgaris): nutritional, ACE-inhibitory and antioxidative characterization. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2012;46(1):91–96. doi: 10.1016/j.lwt.2011.10.021. [DOI] [Google Scholar]
- Venuste M., Zhang X., Shoemaker C.F., Karangwa E., Abbas S., Kamdem P.E. Influence of enzymatic hydrolysis and enzyme type on the nutritional and antioxidant properties of pumpkin meal hydrolysates. Food and Function. 2013;4(5):811–820. doi: 10.1039/c3fo30347k. [DOI] [PubMed] [Google Scholar]
- Villeneuve P., Durand E., Decker E.A. The need for a new step in the study of lipid oxidation in heterophasic systems [In-brief] J. Agric. Food Chem. 2018;66(32):8433–8434. doi: 10.1021/acs.jafc.8b03603. [DOI] [PubMed] [Google Scholar]
- Wang J.S., Zhao M.M., zhong Zhao Q., Jiang Y. ming. Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems. Food Chem. 2007;101(4):1658–1663. doi: 10.1016/j.foodchem.2006.04.024. [DOI] [Google Scholar]
- Wang C., Li B., Ao J. Separation and identification of zinc-chelating peptides from sesame protein hydrolysate using IMAC-Zn2+ and LC-MS/MS. Food Chem. 2012;134(2):1231–1238. doi: 10.1016/j.foodchem.2012.02.204. [DOI] [PubMed] [Google Scholar]
- Wang K., Wang Y., Lin S., Liu X., Yang S., Jones G.S. Analysis of DPPH inhibition and structure change of corn peptides treated by pulsed electric field technology. J. Food Sci. Technol. 2014;52(7):4342–4350. doi: 10.1007/s13197-014-1450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.J., Zheng X.Q., Kopparapu N.K., Cong W.S., Deng Y.P., Sun X.J., Liu X.L. Purification and evaluation of a novel antioxidant peptide from corn protein hydrolysate. Process Biochem. 2014;49(9):1562–1569. doi: 10.1016/j.procbio.2014.05.014. [DOI] [Google Scholar]
- Wang L., Ding L., Wang Y., Zhang Y., Liu J. Isolation and characterisation of in vitro and cellular free radical scavenging peptides from corn peptide fractions. Molecules. 2015;20(2):3221–3237. doi: 10.3390/molecules20023221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chen H., Fu X., Li S., Wei J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: biochemical characterization and molecular docking study. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;75:93–99. doi: 10.1016/j.lwt.2016.08.047. [DOI] [Google Scholar]
- Waraho T., McClements D.J., Decker E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011;22(1):3–13. doi: 10.1016/j.tifs.2010.11.003. [DOI] [Google Scholar]
- Waseem M., Kumar S., Kumar A. Secondary Metabolite and Functional Food Components: Role in Health and Disease. 2018. Bioactive peptides; pp. 259–287. Nova Science Publisher Inc. [DOI] [Google Scholar]
- Waters W.A. The kinetics and mechanism of metal-catalyzed autoxidation. JAOCS (J. Am. Oil Chem. Soc.) 1971;48(9):427–433. doi: 10.1007/BF02544654. [DOI] [Google Scholar]
- Wattanasiritham L., Theerakulkait C., Wickramasekara S., Maier C.S., Stevens J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016;192:156–162. doi: 10.1016/j.foodchem.2015.06.057. [DOI] [PubMed] [Google Scholar]
- Wen C., Zhang J., Zhou J., Duan Y., Zhang H., Ma H. Effects of slit divergent ultrasound and enzymatic treatment on the structure and antioxidant activity of arrowhead protein. Ultrason. Sonochem. 2018;49:294–302. doi: 10.1016/j.ultsonch.2018.08.018. [DOI] [PubMed] [Google Scholar]
- Wen C., Zhang J., Zhang H., Duan Y., Ma H. Effects of divergent ultrasound pretreatment on the structure of watermelon seed protein and the antioxidant activity of its hydrolysates. Food Chem. 2019;299:125165. doi: 10.1016/j.foodchem.2019.125165. [DOI] [PubMed] [Google Scholar]
- Wong F.C., Xiao J., Wang S., Ee K.Y., Chai T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020;99(January):44–57. doi: 10.1016/j.tifs.2020.02.012. [DOI] [Google Scholar]
- Xia Y., Bamdad F., Gänzle M., Chen L. Fractionation and characterization of antioxidant peptides derived from barley glutelin by enzymatic hydrolysis. Food Chem. 2012;134(3):1509–1518. doi: 10.1016/j.foodchem.2012.03.063. [DOI] [PubMed] [Google Scholar]
- Xie Z., Huang J., Xu X., Jin Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008;111(2):370–376. doi: 10.1016/j.foodchem.2008.03.078. [DOI] [PubMed] [Google Scholar]
- Xu S., Shen Y., Chen G., Bean S., Li Y. Antioxidant characteristics and identification of peptides from sorghum kafirin hydrolysates. J. Food Sci. 2019;84(8):2065–2076. doi: 10.1111/1750-3841.14704. [DOI] [PubMed] [Google Scholar]
- Xue Z., Yu W., Liu Z., Wu M., Kou X., Wang J. Preparation and antioxidative properties of a rapeseed (Brassica napus) protein hydrolysate and three peptide fractions. J. Agric. Food Chem. 2009;57(12):5287–5293. doi: 10.1021/jf900860v. [DOI] [PubMed] [Google Scholar]
- Xue Z., Yu W., Wu M., Wang J. In vivo antitumor and antioxidative effects of a rapeseed meal protein hydrolysate on an S180 tumor-bearing murine model. Biosci. Biotechnol. Biochem. 2009;73(11):2412–2415. doi: 10.1271/bbb.90374. [DOI] [PubMed] [Google Scholar]
- Yan Q.J., Huang L.H., Sun Q., Jiang Z.Q., Wu X. Isolation, identification and synthesis of four novel antioxidant peptides from rice residue protein hydrolyzed by multiple proteases. Food Chem. 2015;179:290–295. doi: 10.1016/j.foodchem.2015.01.137. [DOI] [PubMed] [Google Scholar]
- Yang R., Li X., Lin S., Zhang Z., Chen F. Identification of novel peptides from 3 to 10 kDa pine nut (Pinus koraiensis) meal protein, with an exploration of the relationship between their antioxidant activities and secondary structure. Food Chem. 2017;219:311–320. doi: 10.1016/j.foodchem.2016.09.163. [DOI] [PubMed] [Google Scholar]
- Yang R., Wang J., Lin S., Ye H., Chen F. In vitro antioxidant activities of the novel pentapeptides Ser-His-Glu-Cys-Asn and Leu-Pro-Phe-Ala-Met and the relationship between activity and peptide secondary structure. J. Sci. Food Agric. 2017;97(6):1945–1952. doi: 10.1002/jsfa.8000. [DOI] [PubMed] [Google Scholar]
- Yang J., Hu L., Cai T., Chen Q., Ma Q., Yang J., Meng C., Hong J. Purification and identification of two novel antioxidant peptides from perilla (Perilla frutescens L. Britton) seed protein hydrolysates. PloS One. 2018;13(7) doi: 10.1371/journal.pone.0200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Cai X., Liu Z., Wang S. Antioxidant assessment of schizochytrium meal protein enzymatic hydrolysate and its potential application. J. Aquat. Food Prod. Technol. 2019;28(4):413–426. doi: 10.1080/10498850.2019.1595799. [DOI] [Google Scholar]
- Yu L., Sun J., Liu S., Bi J., Zhang C., Yang Q. Ultrasonic-assisted enzymolysis to improve the antioxidant activities of peanut (arachin conarachin L.) antioxidant hydrolysate. Int. J. Mol. Sci. 2012;13(7):9051–9068. doi: 10.3390/ijms13079051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Gao J., Xue Z., Kou X., Wang Y., Zhai L. Radical-scavenging activity, ACE-inhibiting capability and identification of rapeseed albumin hydrolysate. Food Science and Human Wellness. 2013;2(2):93–98. doi: 10.1016/j.fshw.2013.05.002. [DOI] [Google Scholar]
- Zarei M., Ebrahimpour A., Abdul-Hamid A., Anwar F., Saari N. Production of defatted palm kernel cake protein hydrolysate as a valuable source of natural antioxidants. Int. J. Mol. Sci. 2012;13(7):8097–8111. doi: 10.3390/ijms13078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M., Ebrahimpour A., Abdul-Hamid A., Anwar F., Bakar F.A., Philip R., Saari N. Identification and characterization of papain-generated antioxidant peptides from palm kernel cake proteins. Food Res. Int. 2014;62:726–734. doi: 10.1016/j.foodres.2014.04.041. [DOI] [Google Scholar]
- Zhang M., Mu T.H. Identification and characterization of antioxidant peptides from sweet potato protein hydrolysates by Alcalase under high hydrostatic pressure. Innovat. Food Sci. Emerg. Technol. 2017;43:92–101. doi: 10.1016/j.ifset.2017.08.001. [DOI] [Google Scholar]
- Zhang S.B., Wang Z., Xu S.Y. Antioxidant and antithrombotic activities of rapeseed peptides. JAOCS (J. Am. Oil Chem. Soc.) 2008;85(6):521–527. doi: 10.1007/s11746-008-1217-y. [DOI] [Google Scholar]
- Zhang S.B., Wang Z., Xu S.Y., Gao X.F. Purification and characterization of a radical scavenging peptide from rapeseed protein hydrolysates. JAOCS (J. Am. Oil Chem. Soc.) 2009;86(10):959–966. doi: 10.1007/s11746-009-1404-5. [DOI] [Google Scholar]
- Zhang L., Li J., Zhou K. Chelating and radical scavenging activities of soy protein hydrolysates prepared from microbial proteases and their effect on meat lipid peroxidation. Bioresour. Technol. 2010;101(7):2084–2089. doi: 10.1016/j.biortech.2009.11.078. [DOI] [PubMed] [Google Scholar]
- Zhang T., Li Y., Miao M., Jiang B. Purification and characterisation of a new antioxidant peptide from chickpea (Cicer arietium L.) protein hydrolysates. Food Chem. 2011;128(1):28–33. doi: 10.1016/j.foodchem.2011.02.072. [DOI] [PubMed] [Google Scholar]
- Zhang M., Mu T.H., Sun M.J. Purification and identification of antioxidant peptides from sweet potato protein hydrolysates by Alcalase. Journal of Functional Foods. 2014;7(1):191–200. doi: 10.1016/j.jff.2014.02.012. [DOI] [Google Scholar]
- Zhang Q., Tong X., Qi B., Wang Z., Li Y., Sui X., Jiang L. Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. Journal of Functional Foods. 2018;42:298–305. doi: 10.1016/j.jff.2018.01.017. [DOI] [Google Scholar]
- Zhang Q., Tong X., Li Y., Wang H., Wang Z., Qi B., Sui X., Jiang L. Purification and characterization of antioxidant peptides from alcalase-hydrolyzed soybean (Glycine max L.) hydrolysate and their cytoprotective effects in human intestinal caco-2 cells. J. Agric. Food Chem. 2019 doi: 10.1021/acs.jafc.9b01235. [DOI] [PubMed] [Google Scholar]
- Zhao J., Xiong Y.L. Interfacial peptide partitioning and undiminished antioxidative and emulsifying activity of oxidatively stressed soy protein hydrolysate in an O/W emulsion. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2015;61(2):322–329. doi: 10.1016/j.lwt.2014.12.022. [DOI] [Google Scholar]
- Zhao L., Wang S., Huang Y. Antioxidant function of tea dregs protein hydrolysates in liposome-meat system and its possible action mechanism. Int. J. Food Sci. Technol. 2014;49(10):2299–2306. doi: 10.1111/ijfs.12546. [DOI] [Google Scholar]
- Zheng X.Q., Li L.T., Liu X.L., Wang X.J., Lin J., Li D. Production of hydrolysate with antioxidative activity by enzymatic hydrolysis of extruded corn gluten. Appl. Microbiol. Biotechnol. 2006;73(4):763–770. doi: 10.1007/s00253-006-0537-9. [DOI] [PubMed] [Google Scholar]
- Zheng L., Su G., Ren J., Gu L., You L., Zhao M. Isolation and characterization of an oxygen radical absorbance activity peptide from defatted peanut meal hydrolysate and its antioxidant properties. J. Agric. Food Chem. 2012;60(21):5431–5437. doi: 10.1021/jf3017173. [DOI] [PubMed] [Google Scholar]
- Zheng X., Liu X., Liu Z. Production of fermentative hydrolysate with antioxidative activity of extruded corn gluten meal by bacillus natto. Appl. Mech. Mater. 2012;138–139:1142–1148. doi: 10.4028/www.scientific.net/AMM.138-139.1142. [DOI] [Google Scholar]
- Zhou K., Sun S., Canning C. Production and functional characterisation of antioxidative hydrolysates from corn protein via enzymatic hydrolysis and ultrafiltration. Food Chem. 2012;135(3):1192–1197. doi: 10.1016/j.foodchem.2012.05.063. [DOI] [PubMed] [Google Scholar]
- Zhou C., Hu J., Ma H., Yagoub A.E.A., Yu X., Owusu J., Ma H., Qin X. Antioxidant peptides from corn gluten meal: orthogonal design evaluation. Food Chem. 2015;187:270–278. doi: 10.1016/j.foodchem.2015.04.092. [DOI] [PubMed] [Google Scholar]
- Zhu K., Zhou H., Qian H. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006;41(6):1296–1302. doi: 10.1016/j.procbio.2005.12.029. [DOI] [Google Scholar]
- Zhu L., Jie C., Tang X., Xiong Y.L. Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. J. Agric. Food Chem. 2008;56(8):2714–2721. doi: 10.1021/jf703697e. [DOI] [PubMed] [Google Scholar]
- Zhuang H., Tang N., Dong S.-T., Sun B., Liu J.-B. Optimisation of antioxidant peptide preparation from corn gluten meal. J. Sci. Food Agric. 2013;93(13):3264–3270. doi: 10.1002/jsfa.6170. [DOI] [PubMed] [Google Scholar]
- Zhuang H., Tang N., Yuan Y. Purification and identification of antioxidant peptides from corn gluten meal. Journal of Functional Foods. 2013;5(4):1810–1821. doi: 10.1016/j.jff.2013.08.013. [DOI] [Google Scholar]
- Zou T.B., He T.P., Li H.B., Tang H.W., Xia E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules. 2016;21(1):1–14. doi: 10.3390/molecules21010072. [DOI] [PMC free article] [PubMed] [Google Scholar]