Abstract
Background:
Cannabis (marijuana) is the most widely used illicit drug in the USA, and consumption among adolescents is rising. Some animal studies show that adolescent exposure to delta 9-tetrahydrocannabinol or synthetic cannabinoid receptor 1 agonists causes alterations in affect and cognition that can persist into adulthood. It is less clear, however, whether similar alterations result from exposure to cannabis via smoke inhalation, which remains the most frequent route of administration in humans.
Aims:
To begin to address these questions, a rat model was used to determine how cannabis smoke exposure during adolescence affects behavioral and cognitive outcomes in adulthood.
Methods:
Adolescent male Long-Evans rats were assigned to clean air, placebo smoke, or cannabis smoke groups. Clean air or smoke exposure sessions were conducted daily during adolescence (from P29–P49 days of age) for a total of 21 days, and behavioral testing began on P70.
Results:
Compared to clean air and placebo smoke conditions, cannabis smoke significantly attenuated the normal developmental increase in body weight, but had no effects on several measures of either affect/motivation (open field activity, elevated plus maze, instrumental responding under a progressive ratio schedule of reinforcement) or cognition (set shifting, reversal learning, intertemporal choice). Surprisingly, however, in comparison to clean air controls rats exposed to either cannabis or placebo smoke in adolescence exhibited enhanced performance on a delayed response working memory task.
Conclusions:
These findings are consistent with a growing body of evidence for limited long-term adverse cognitive and affective consequences of adolescent exposure to relatively low levels of cannabinoids.
Keywords: Cannabis, marijuana, smoke, executive function, anxiety, working memory
Introduction
Cannabis (marijuana) is the most widely used illicit drug in the USA, with 19.8% of the population reporting past month use (National Institute on Drug Abuse (NIDA), 2015). In a national survey conducted by NIDA, consumption has risen over the past few years in adolescents between 12–17 years of age (NIDA, 2018). Cannabis use is linked to a number of serious health conditions, including greater risks for cardiovascular, lung, and gum diseases, schizophrenia, and obesity (Gordon et al., 2013; Jouanjus et al., 2017; Ross et al., 2016; Rubino and Parolaro, 2008). Additionally, cannabis use is well known to alter affect and cognition in fully mature adults. For example, among the top 10 self-reported effects of cannabis use, increased happiness and relaxation are the most prevalent (Green et al., 2003); however, several studies report that anxiety disorders are more prevalent in adults that use cannabis heavily (Crippa et al., 2009; Green et al., 2003). In mature adults, it is also well documented that several cognitive domains such as memory and decision making are impaired in the context of either acute or chronic cannabis use (Broyd et al., 2016).
Among adolescents, cannabis use is also associated with deficits in several emotional and cognitive measures including motivation (Lane et al., 2005b), depression and/or anxiety (Fergusson et al., 2002; Hayatbakhsh et al., 2007; Patton et al., 2002; Rey et al., 2002), and executive functioning (Hanson et al., 2010; Harvey et al., 2007; Medina et al., 2007; Schwartz et al., 1989; Thoma et al., 2011). Much evidence exists on the effects of acute and chronic cannabis use on emotion and cognition in adolescents and adults; however, less is known about the long-term effects of chronic cannabis use during adolescence on adult emotion and cognition. Although periods of abstinence from cannabis appear to ameliorate cognitive impairments (Hanson et al., 2010; Schwartz et al., 1989), a few studies report that cognitive impairments linked to cannabis use can persist for weeks (Medina et al., 2007) or even into early adulthood (Gruber et al., 2012a; 2012b; Solowij and Pesa, 2010; Solowij et al., 2002).
Animal studies, which provide a high degree of experimental control, report mixed results concerning the effects of adolescent cannabinoid exposure on adult behavioral outcomes. In rats, chronic administration of Δ9-tetrahydrocannabinol (THC) or synthetic cannabinoid receptor 1 (CB1R) agonists during adolescence increases anxiety-like behavior in adulthood in some (O’Shea et al., 2004, 2006), but not all (Biscaia et al., 2003), studies. Other studies show that adolescent exposure to THC can impair learning and memory in adulthood (Quinn et al., 2008; Rubino et al., 2009a, 2009b, although see Cha et al., 2006). Importantly, these prior studies employed systemic injections of THC or CB1R agonists, whereas the majority of cannabis use in humans is via inhalation, particularly smoking. Despite the prevalence of cannabis smoking in humans, there have been relatively few assessments of the effects of cannabis smoke on behavior in preclinical models. Acute cannabis smoke exposure in rodents causes hypothermia, analgesia, and changes in locomotor activity, as well as alterations in cognitive performance (Blaes et al., 2019; Bruijnzeel et al., 2016; Niyuhire et al., 2007; Varvel et al., 2005). Similar results have been obtained with models of THC or cannabis extract vapor inhalation (Freels et al., 2020; Manwell et al., 2014; Nguyen et al., 2016; Taffe et al., 2020). Chronic cannabis smoke exposure in rodents can induce dependence (as indexed by the presence of antagonist-precipitated withdrawal symptoms) as well as changes in reward thresholds (Bruijnzeel et al., 2016; Ravula et al., 2019; Wilson et al., 2006). To our knowledge, however, only a few studies have assessed the consequences of adolescent exposure to inhaled cannabinoids on adult behavioral outcomes. In one study in rats (Nguyen et al., 2020), the authors found that 2 weeks of exposure to THC vapor during adolescence enhanced food consumption (but not body weight) in adult males and enhanced fentanyl (but not oxycodone) self-administration in adult females. In another study in rats (Bruijnzeel et al., 2019), cannabis smoke exposure from P29–P49 had no effects on adult behavioral performance, including several measures of anxiety- and depression-like behavior. There were also no effects on cognition in this latter study, but as only a single measure of cognitive function (a novel object recognition task) was used, it leaves open the possibility that other measures of cognition might detect cannabis smoke-induced alterations. The present study replicated the approach used in the Bruijnzeel et al., 2019 study but used a broader range of cognitive assessments focused particularly on prefrontal cortical-dependent executive functions.
Materials and methods
Subjects
Male Long-Evans rats (n=36) were obtained from Charles River at 23 days of age (P23) and housed in the Association for Assessment and Accreditation of Laboratory Animal Care International-accredited vivarium facility in the McKnight Brain Institute building at the University of Florida. All animal procedures were conducted in accordance with the rules and regulations of the University of Florida Institutional Animal Care and Use Committee and National Institutes of Health guidelines. The facility was maintained at a consistent temperature of 25° with a 12-hour light/dark cycle (lights on at 07:00) and free access to food and water except as otherwise noted. Rats were housed three rats/cage until completion of smoke exposure (P49), after which they were housed two rats/cage until P70, at which point they were housed individually in order to facilitate food restriction protocols. Rats were randomly assigned to either a clean air (no smoke) control group (n=12), a placebo smoke group (n=12) exposed to smoke from burning placebo cannabis cigarettes from which cannabinoids were removed, and a cannabis smoke group (n=12) exposed to smoke from burning cannabis cigarettes.
Cannabis smoke delivery
Cannabis and placebo cigarettes were obtained from the NIDA Drug Supply Program. The cannabis cigarettes were the same size as standard tobacco cigarettes and weighed approximately 700 mg each. The cigarettes contained approximately 5.6% THC, 0% cannabidiol (CBD), and 0.4% cannabinol (CBN) as per the analytical data accompanying the cigarettes. The placebo cigarettes were identical in size but contained cannabis plant material from which cannabinoids were extracted, and contained 0.002% THC, 0.001% CBD, and 0.004% CBN.
Freely moving rats were exposed to smoke from burning cannabis or placebo cigarettes as described in our previous work (Blaes et al., 2019; Bruijnzeel et al., 2016; Ravula et al., 2018). Briefly, smoke exposure was conducted in a microprocessor-controlled cigarette smoking-machine (model TE-10, Teague Enterprises, Davis, California, USA) that burned cigarettes using a standardized smoking procedure (35 cm3 puff volume, 1 puff per minute, 2 s per puff; Teague et al., 1994). Mainstream and sidestream smoke was transported to a mixing chamber, where the smoke was diluted with clean air to a concentration of roughly 250 mg of total suspended particles (TSP) per m3 before being introduced into the exposure chamber. Just prior to the exposure sessions, rats were moved from their home cages into clean, standard polycarbonate rodent cages (38×28×20 cm; L×W×H) with corncob bedding, wire tops, and a water bottle (rats from the same home cage were moved into the same exposure cage). This whole-body exposure regimen allowed for free movement (i.e. not restrained) and social contact (three rats/cage) during the exposure sessions. During the sessions, the air in the chambers was monitored for carbon monoxide (CO) and TSP levels. CO levels were assessed using a continuous CO analyzer that measures CO levels between 0–2000 parts per million (Monoxor III, Bacharach, New Kensington, Pennsylvania, USA). In order to measure TSP levels, smoke was pumped out of the chamber through a pre-weighed filter for 5 min (Pallflex Emfab Filter, Pall Corporation, Port Washington, New York, USA). The TSP level was calculated by dividing the weight increase of the filter by the volume of airflow through the filter. Rats in the smoke exposure groups were exposed to smoke for 1 h per day. During this period, five cigarettes were burned (10 min per cigarette with a 2 min break between cigarettes). For cannabis cigarettes, the mean±standard error of the mean (SEM) TSP was 210.82±33.25 and CO was 249.20±26.94. For placebo cigarettes, the mean±SEM TSP was 72.60±6.12 and CO was 236.20±31.58. Rats in the clean air condition were placed in the smoke exposure chamber in the same manner described above with the machine switched on to allow continuous airflow, but no cigarettes were burned, and no smoke was delivered. For comparison, during clean air control sessions, the mean±SEM TSP was 6.57±2.53 and CO was 1.17±0.48. Exposure sessions were conducted daily (between 10:00–15:00) from P29–P49 for a total of 21 days.
Sequence of behavioral testing procedures
Behavioral testing began after P70 and started at the following ages for each test: elevated plus maze, P76; large open field, P84; probabilistic reversal learning, P98; delayed response task, P123; set-shifting task, P178; intertemporal choice task, P202; progressive ratio, P219.
Body weight measurements
Body weights were measured daily prior to each smoke exposure session. After completion of smoke exposure, rats were weighed on Mondays, Wednesdays, and Fridays until the commencement of food restriction for behavioral testing.
Large open field test
The large open field test is used to assess anxiety-like behavior (Liebsch et al., 1998). The test was conducted in a dimly lit room (75 lux) as described previously (Bruijnzeel et al., 2016, 2019; Qi et al., 2016). The open field apparatus consisted of a large arena measuring 120×120×60 cm (L×W×H). The arena was made of matte black high-density polyethylene panels that were fastened together and placed on a plastic floor plate (Faulkner Plastics, Miami, Florida, USA). The rats’ behavior was recorded with a wide-view camera mounted above the center of the arena and analyzed with EthoVision XT 11.5 software (Noldus Information Technology, Leesburg, Virginia, USA). The open field was divided into three zones: an outer zone (20 cm wide), a middle zone (20 cm wide), and a center zone (40×40 cm; L×W). The following behaviors were analyzed: total distance traveled, latency and frequency to enter the middle and center zones, and duration in each zone. Rats were placed into the outer zone of the open field and allowed to explore for 5 min. The open field was cleaned with an odorless antiseptic solution (Nolvasan, 1:100 dilution in water) between rats.
Elevated plus maze test
The elevated plus maze test is used to assess anxiety-like behavior and was conducted as described previously (Bruijnzeel et al., 2019; Qi et al., 2016; Rylkova et al., 2009). The test apparatus consisted of four black polypropylene arms (Coulbourn Instruments, Holliston, MA). The two “open” arms had 0.5 cm walls and the two “closed” arms had 30 cm walls. The open arms were oriented opposite from each other. The arms were 10 cm wide, 50 cm long, and were placed on 55 cm tall acrylic legs. Testing occurred in a quiet, dimly-lit (75 lux) room. At the beginning of each test, the rats were placed in the center of the apparatus facing an open arm. Rats were allowed to explore the apparatus for 5 min, and their behavior was recorded with a camera mounted above the maze. The elevated plus maze was divided into five zones (two open arms, two closed arms, and center). Behavior was scored manually using EthoVision XT 11.5 software by an experienced observer who was blind to the treatment conditions. The following behaviors were analyzed: time spent in the open and closed arms and the number of entries into open and closed arms. An arm entry was counted when the rat had all four paws in that arm. The apparatus was cleaned with a Nolvasan solution between rats.
Operant behavioral testing
Apparatus and shaping procedures.
Testing on all operant tasks (progressive ratio, delayed response, set-shifting, probabilistic reversal learning, intertemporal choice) was conducted in eight identical standard rat behavioral test chambers (Coulbourn Instruments, Holliston, Massachusetts, USA) with metal front and back walls, transparent Plexiglas side walls, and a floor composed of steel rods (0.4 cm in diameter) spaced 1.1 cm apart. Each test chamber was housed in a sound-attenuating cubicle and was equipped with a recessed food pellet delivery trough located 2 cm above the floor in the center of the front wall. The trough was fitted with a photobeam to detect head entries and a 1.12 W lamp for illumination. Food rewards consisted of 45 mg grain-based food pellets (5TUL; Test Diet, Richmond, Indiana, USA). Two retractable levers were positioned to the left and right of the food trough (11 cm above the floor), and a 1.12 W cue lamp was located 3.8 cm above each lever. An additional 1.12 W house light was mounted near the top of the rear wall of the sound-attenuating cubicle. A computer interfaced with the behavioral test chambers and equipped with Graphic State 3 software (Coulbourn Instruments) was used to control experiments and collect data. Prior to shaping, rats were food restricted to 85% of their ad libitum weight. This target weight was increased by 5 g/week during the period of restriction to allow for growth. On the day prior to the first day of shaping, rats were given five of the 45 mg food pellets in their home cages to reduce neophobia to the food rewards used in operant testing. Following a single session of magazine training, rats were shaped to lever press to initiate delivery of a food pellet into the food trough and were then trained to nosepoke to initiate lever extension. Each nosepoke initiated the extension of either the left or right lever (randomized across pairs of trials), a press on which yielded a single food pellet. After two consecutive days of reaching criterion performance (50 presses on each lever), rats began testing in the operant tasks.
Progressive ratio task.
This task evaluated rats’ motivation to press a lever to obtain a food reward, and was based on designs used previously by our laboratory and others (Barr and Phillips, 1999; Cetin et al., 2004; Hernandez et al., 2017; Kheramin et al., 2005; Mendez et al., 2009). Instrumental responding for food reward was assessed using a progressive ratio schedule of reinforcement, on which the number of lever presses required to earn a reward increased with each successive reward earned. The number of responses required for reward delivery increased in a geometric progression, in the sequence 1, 4, 10, 20, 35, 56, 84, 120, 165, 220, 286 ( where N is the number of responses required to earn a reward r and where r is the ordinal number of the reward; i.e. for the second reward, r=2; for the third reward, r=3). Only a single lever was extended into the test chamber, and rats were tested in the progressive ratio task for seven consecutive sessions (one session per day). These sessions varied in length, ending only after 30 min elapsed with no reward delivery.
Delayed response working memory task.
The design of this task was based on a previous study (Sloan et al., 2006) and has been used before by our laboratory to evaluate prefrontal cortical function (e.g. Bañuelos et al., 2014; Beas et al., 2013; Blaes et al., 2019; Hernandez et al., 2017; McQuail et al., 2016). Each 40 min session began with illumination of the house light, which remained illuminated throughout the entire session except during timeout periods. Rats received a single test session each day. Each trial in the task began with the extension of a single “sample” lever into the chamber. The sample lever (left or right) was randomly selected within each pair of trials to ensure equal representation of both levers across the test session. A press on the sample lever caused it to retract and initiated the delay interval. During the delay interval, rats were required to nosepoke into the food trough to minimize their use of mediating strategies (e.g. positioning themselves in front of the sample lever during the delay). The first nosepoke executed after the delay interval expired initiated the “choice” phase, in which both levers were extended. During the choice phase, a response on the same lever pressed during the sample phase was “correct” and resulted in the retraction of both levers and delivery of a food pellet into the food trough. A nosepoke into the food trough to retrieve the food initiated a 5 s intertrial interval, after which the next trial began. A response on the opposite lever from that chosen during the sample phase was “incorrect” and resulted in the retraction of both levers and initiation of a 5 s “timeout” period during which the house light was extinguished. Immediately following this timeout, the house light was re-illuminated, signaling the start of the next trial.
During initial sessions in this task, there were no delays between the sample and choice phases, and a correction procedure was used such that the sample lever was repeated on the same side following an incorrect response to reduce the development of side biases. Once rats reached a criterion of 80% correct choices across a test session for two consecutive sessions, this correction procedure was discontinued, and a set of seven delays was introduced. The presentation of delay durations was randomized within each block of seven trials, such that each delay was presented once within a block. Upon establishing >80% correct responses across two consecutive sessions in a “delay set”, rats were progressed to the next set, which contained increasingly longer delays (delay set one: 0, 1, 2, 3, 4, 5, 6 s; delay set two: 0, 2, 4, 8, 12, 16 s; delay set three: 0, 2, 4, 8, 12, 18, and 24 s).
Set shifting task.
This task was originally developed by Floresco et al. (2008) and was used previously in our laboratory to assess cognitive flexibility (Beas et al., 2013, 2017; Hernandez et al., 2017). Rats were first assessed on a protocol designed to determine their individual “side bias” or inherent preference for the left or right lever. This protocol was composed of 45 trials, each of which consisted of two phases. In the first phase of each trial, the house light was illuminated, and both levers extended into the test chamber. A response on either lever resulted in the retraction of both levers and the delivery of a single food pellet. In the second phase of each trial, both levers were extended into the chamber, but only a response on the lever opposite to the choice made in the first phase was rewarded. If the rat made an “incorrect” response (i.e. chose the same lever as in the first phase), the levers were retracted and the house light was extinguished. The second phase of the trial was then repeated until the rat made a correct response, and then a new trial was initiated. An individual rat’s “side bias” reflected the lever position on which it made the greatest number of responses across the entire test session.
The day after side bias determination, rats began discrimination training on the initial (visual cue) discrimination learning rule. Each 20 s trial began with illumination of one of the cue lights positioned over the left or right lever for 3 s (the position was randomly selected within each pair of trials). Both levers were then inserted into the chamber for 4 s, during which the cue light remained illuminated (the house light was also illuminated during this phase). If the rat made a correct response (pressed the lever beneath the cue light), both levers were retracted, the cue light was extinguished, and a single food pellet was delivered. If the rat made an incorrect response (pressed the lever opposite from the cue light), the levers were retracted, and the house light extinguished, but no food was delivered. As in our previous work (Beas et al., 2013, 2017), rats were trained on the visual cue discrimination for a minimum of 30 trials and until reaching criterion performance of 10 consecutive correct choices. The visual discrimination task included a maximum of 120 trials/session. If a rat failed to reach criterion performance in a single session, the task was repeated on subsequent days. Upon reaching criterion performance, the session was ended. To reinforce the formation of an attentional “set,” rats received one additional session of 120 trials of visual discrimination training on the day after reaching criterion performance.
The day after completing visual cue discrimination training, rats received a “set shift” in which the contingencies for making a correct (reinforced) choice were changed. The presentation of the trials during the set shift was identical to that in the visual cue discrimination phase of the task; however, to receive a food reward, rats were now required to ignore the cue light and instead respond to a particular lever position (either left or right, whichever was not their “biased” side as determined during the side bias assessment) to receive a food reward. Rats were trained on the set shift until achieving criterion performance of 10 consecutive correct trials (Beas et al., 2013, 2017). As in the initial discrimination phase, each session included a maximum of 120 trials.
Probabilistic reversal learning task.
Procedures were adapted from Dalton et al. (2014). Rats were initially trained in 90-trial sessions designed to familiarize them with the task procedures. Each trial began with the levers retracted and the house light off. Trials occurred every 40 s, with the house light being illuminated prior to insertion of a single lever (left or right, randomized within each pair of trials). Failure to respond within 10 s resulted in lever retraction, termination of the house light, and the trial being scored as an omission. If a response occurred within 10 s of lever insertion, a single pellet was delivered with a 50% probability. This ensured equivalent familiarity with the levers and the probabilistic schedule of reinforcement. This initial training lasted approximately 3–4 sessions, and rats were progressed to the probabilistic reversal learning task after reaching a criterion of approximately 80 successful trials in a session.
Following the initial training, rats were tested in the probabilistic reversal learning task. Each session began with illumination of the house light, after which both levers extended into the chamber. One lever was associated with an 80% probability of reward delivery (a single food pellet), and the other lever was associated with a 20% probability of reward delivery. After eight consecutive choices on the 80% lever (the correct choice, resulting in a food reward 80% of the time), the lever contingencies were reversed, such that the previously correct lever became incorrect and vice versa. Once levers were extended during a trial, rats had 10 s to press a lever, after which the levers retracted, a 5 s intertrial interval ensued, and the trial was scored as an omission. Omissions did not count as incorrect choices (and thus did not reset the correct choice counter). Sessions lasted up to 56 min with a maximum of 255 trials, with each trial lasting up to 15 s.
Intertemporal choice task.
The intertemporal choice (delay discounting) task was based on a design by Evenden and Ryan (1996) and has been used previously by our laboratories to evaluate the effects of age and drugs of abuse on impulsive choice (Hernandez et al., 2017; Mendez et al., 2012; Mitchell et al., 2014). Each 100 min session consisted of five blocks of 12 trials each. Each 100 s trial began with a 10 s illumination of the food trough and house lights. A nosepoke into the food trough during this time extinguished the food trough light and triggered extension of either a single lever (forced-choice trials) or of both levers simultaneously (free-choice trials). Each block consisted of two forced choice trials (one for each lever) followed by 10 free-choice trials. The forced-choice trials were designed to remind rats of the delay contingencies in effect for that block. A press on one lever (either left or right, counterbalanced across exposure groups) resulted in one food pellet (the small reward) delivered immediately. A press on the other lever resulted in four food pellets (the large reward) delivered after a variable delay. The identities of the levers remained consistent throughout testing. Failure to press either lever within 10 s of their extension resulted in the levers being retracted and lights extinguished, and the trial was scored as an omission. Once either lever was pressed, both levers were retracted for the remainder of the trial (i.e. the inter-trial interval). The duration of the delay preceding large reward delivery increased between each block of trials (0, 10, 20, 40, 60 s), but remained constant within each block.
Data analyses
Data analyses were conducted in GraphPad Prism 8. Prior to statistical analyses, the robust regression and outlier removal (ROUT; Motulsky and Brown, 2006) method was used to identify and remove outliers as in our previous work (Hernandez et al., 2019). Data across all measures were analyzed using a one-factor analysis of variance (ANOVA) (with exposure group as a between-subjects variable) or a two-factor repeated-measures ANOVA (with group as a between-subjects variable and within-subjects variables as appropriate for the measure). Specifically, body weights were analyzed using a two-factor repeated-measures ANOVA, with exposure group (clean air, placebo smoke, cannabis smoke) as a between-subjects variable and day as a within-subjects variable. Open field data were analyzed using a two-factor repeated-measures ANOVA, with exposure group as a between-subjects variable and zone as a within-subjects variable. Elevated plus maze data were analyzed using a two-factor repeated-measures ANOVA, with exposure group as a between-subjects variable and arm as a within-subjects variable. Additional elevated plus maze measures were compared between groups using a one-factor ANOVA. Progressive ratio data were compared between groups using a one-factor ANOVA. Delayed response task data were analyzed using a two-factor repeated-measures ANOVA, with exposure group as a between-subjects variable and delay as a within-subjects variable. Set shifting task data were compared between groups using either a one-factor ANOVA (trials-to-criterion) or a two-factor repeated-measures ANOVA, with exposure group as a between-subjects variable and error type as a within-subjects variable. Probabilistic reversal learning data were analyzed using a two-factor repeated-measures ANOVA, with exposure group as a between-subjects variable and day as a within-subjects variable. Intertemporal choice task data were analyzed using either a two-factor repeated-measures ANOVA, with exposure group as a between-subjects variable and delay as a within-subjects variable, or compared between groups (for indifference point data) using a one-factor ANOVA. The alpha value was set at 0.05, and effect sizes for factors associated with group are denoted by the partial eta squared (ηp2) value wherever significant differences were observed. For all ANOVAs, the Greenhouse-Geisser correction was used in cases in which homogeneity of variance was violated.
Results
Effects of adolescent smoke exposure on adult body weight
To determine if adolescent exposure to cannabis smoke affected growth (body weight change as a function of age), a two-factor repeated-measures ANOVA (group×day) was used, with group (three levels: air, placebo, and cannabis) as the between-subjects factor and day (22 levels, days 29–50) as the within-subjects factor. This analysis confirmed an expected main effect of day such that all rats gained weight as they aged (F(21,693)=3699.019, p<0.001; Figure 1(a)). Although there was no main effect of group (F(2,33)=2.013, p=0.150), there was a group×day interaction (F(42,693)=3.092, p<0.001, ηp2=0.16). A follow-up comparison specifically between the cannabis smoke and placebo smoke groups using a two-factor repeated-measures ANOVA with group as the between-subjects factor and day as the within-subjects factor revealed a significant main effect of day (F(21,462)=2038.00, p<0.001) and a group×day interaction (F(21,462)=1.793, p=0.017, ηp2 =0.08), but no main effect of group (F(1,22)=1.001, p=0.329). A similar comparison between the placebo smoke and clean air control groups revealed the expected main effect of day (F(21,462)=2572.00, p<0.001) but no other effects (main effect of group: F(1,22)=0.994, p=0.330; group×day: F(21,462)=1.305, p=0.165).
Figure 1.
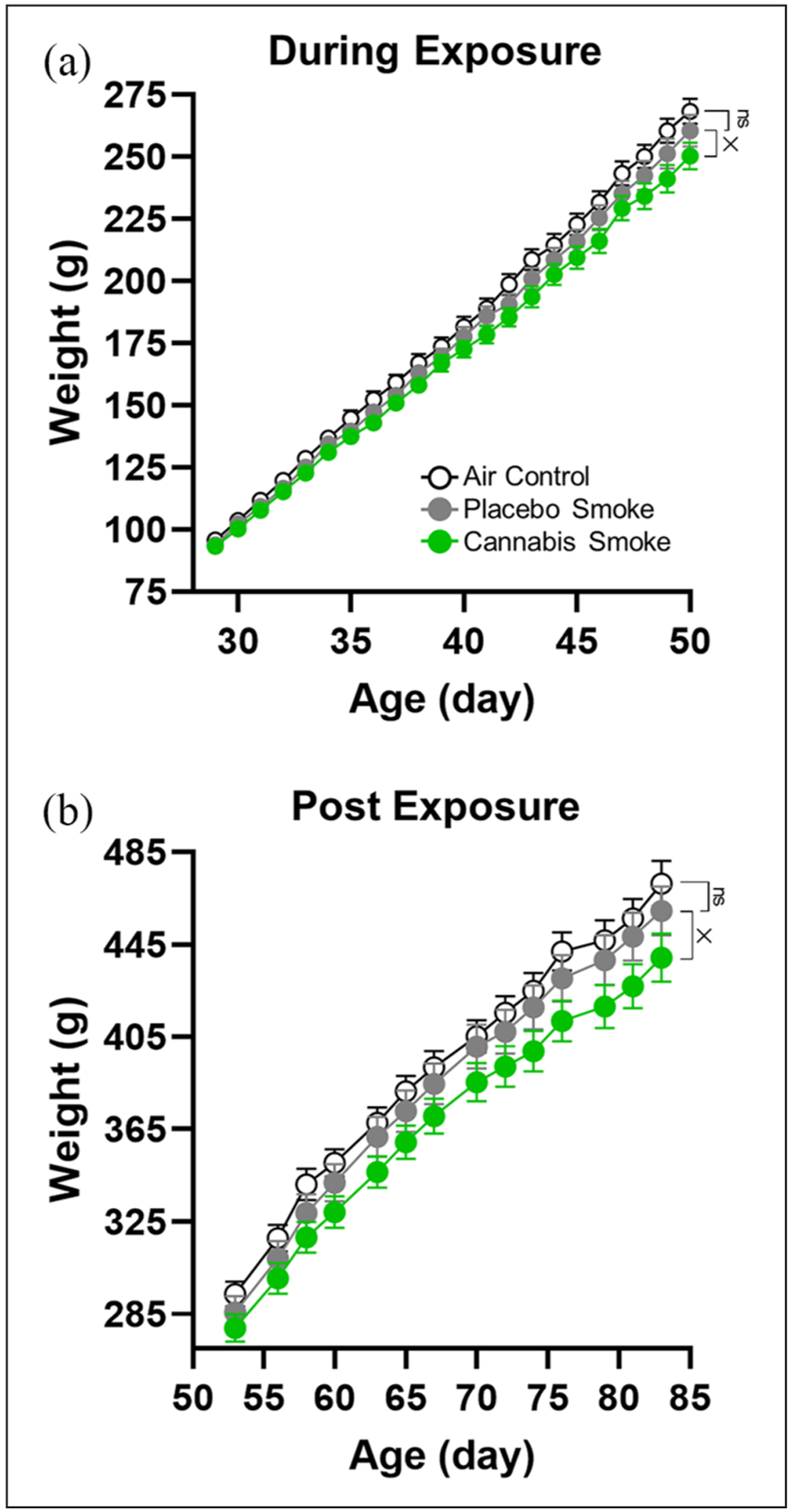
Body weights during and after exposure. (a) Body weights during the adolescent exposure period increased with age, but this increase was attenuated in smoke exposed rats (significant group × day interaction). Follow-up comparisons between placebo and clean air controls revealed no differences, whereas there was a significant group × day interaction in the comparison between placebo smoke and cannabis smoke groups. (b) Rats continued to gain weight into adulthood. As with the exposure period, the increase in body weight was attenuated in cannabis smoke-exposed rats (significant group×day interaction in the comparison between placebo smoke and cannabis smoke groups, but not in the comparison between clean air and placebo smoke groups). In all figure panels, open circles and black lines represent the clean air control group, filled grey circles and lines represent the placebo smoke group, and filled green circles and lines represent the cannabis smoke group (n=12 rats/group for all measures). Error bars represent standard error of the mean (SEM). The “×” symbol denotes a significant group delay interaction: ×p<0.05. No significant difference is indicated by “ns.”
A similar analysis of rats’ body weight during adulthood (group×day: 14 levels, days 53–83) confirmed the expected main effect of day (i.e. all rats continued to gain weight; F(13,429)=1716.320, p<0.001; Figure 1(b)). There was also a group×day interaction (F(26,429)=1.729, p=0.015, ηp2 =0.10) but no main effect of group (F(2,33)=2.254, p=0.121). A follow-up comparison between the cannabis smoke and placebo smoke groups using a two-factor repeated-measures ANOVA with group as the between-subjects factor and day as the within-subjects factor revealed a significant main effect of day (F(13,286)=1168.00, p<0.001) and a group×day interaction (F(13,286)=2.164, p=0.011, ηp2=0.09), but no main effect of group (F(1,22)=1.604, p=0.219). A similar comparison between the placebo smoke and clean air control groups revealed the expected main effect of day (F(13,286)=1136.00, p<0.001) but no other effects (main effect of group: F(1,22)=0.569, p=0.459; group×day: F(13,286)=0.463, p=0.944). Considered together, these data show that adolescent exposure to cannabis smoke slows normal developmental patterns of weight gain, both during the period of exposure and for at least several weeks afterward.
Effects of adolescent smoke exposure on open field testing
The amount of time spent near the walls of an open field arena can be a measure of anxiety-like behavior (Lezak et al., 2017). For example, relative to the outer area of an open field, the middle or center areas may be perceived as more dangerous (e.g. due to an increased risk of predation), and thus, greater time spent in the center relative to the outer or middle zones of the arena may be indicative of a decreased sense of vulnerability to open spaces (i.e. less anxiety-like behavior). A two-factor repeated-measures ANOVA (group×zone) with group (three levels: air, placebo, and cannabis) as the between-subjects factor and zone (three levels: outer, middle, and center) as the within-subjects factor was used to analyze the duration of time each group spent per zone. As expected, there was a main effect of zone such that all rats spent the most time in the outer zone and the least time in the center zone (F(2,66)=2653.794, p<0.001; Figure 2(a)); however, there was neither a main effect of group (F(2,33)=1.921, p=0.162) nor a group×zone interaction (F(4,66)=0.970, p=0.430) on these measures.
Figure 2.
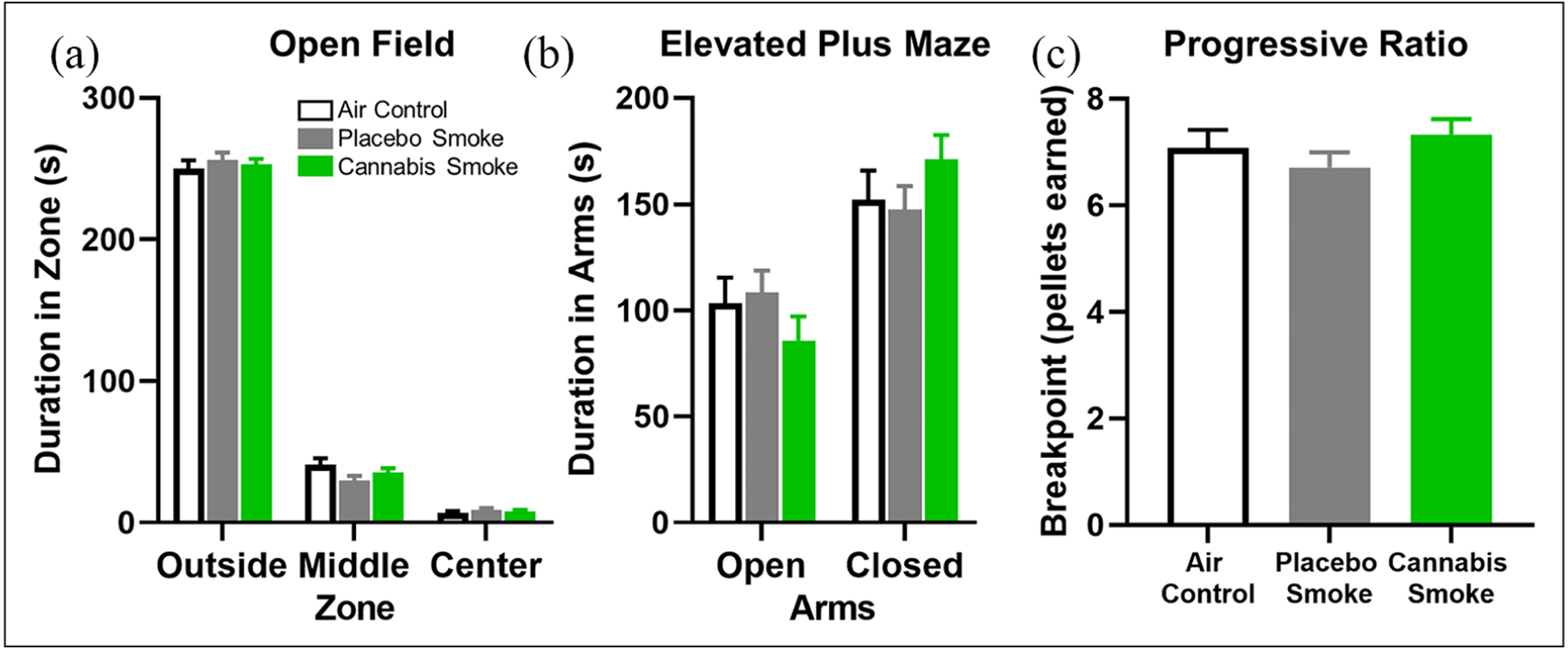
Measures of anxiety-like behavior and motivation in adult rats after repeated clean air, placebo, or cannabis smoke exposure during adolescence. (a) Open field arena. There were no group differences in the duration spent in each zone. (b) Elevated plus maze. There were no group differences in the time spent in each arm. (c) Progressive ratio. There were no group differences in breakpoint (expressed as the number of food pellets earned). In all figure panels, open bars and black lines represent the clean air control group, filled grey bars and lines represent the placebo smoke group, and filled green bars and lines represent the cannabis smoke group (n=12 rats/group for all measures). Error bars represent standard error of the mean (SEM).
Zone entry latency may be a more sensitive measure of anxiety than duration spent in a zone (i.e. longer latencies to enter could suggest greater caution upon approach to the zone). A two-factor repeated-measures ANOVA (group×zone) was used to analyze zone entry latency (outer, middle, center) and confirmed an expected main effect of zone such that all rats were slower to enter the center zone (F(2,64)=19.72, p<0.001; Table 1). There was, however, no main effect of group (F(2,33)=0.369, p=0.694) and no group×zone interaction (F(4,64)=0.282, p=0.889). Furthermore, while the frequency of entries differed across zones (F(2,66)=130.033, p<0.001; Table 1), with the fewest entries into the center zone, the frequency of zone entries did not differ by group (group: F(2,33)=1.038, p=0.366; group×zone: F(4,66)=1.359, p=0.258). Finally, a one-factor ANOVA confirmed there were no group differences in total distance traveled in the arena (F(2,33)=0.939, p=0.401). Taken together, these data suggest that adolescent exposure to cannabis smoke did not alter anxiety-like behavior in adulthood.
Table 1.
Effects of adolescent cannabis smoke exposure on open field arena measures.
| Open field zone entry latency | |||
|---|---|---|---|
| Group | Zone | Mean | Standard error |
| Air | Outer | 2.294 | 1.19 |
| Middle | 8.745 | 2.778 | |
| Center | 44.781 | 19.854 | |
| Placebo | Outer | 3.971 | 1.304 |
| Middle | 8.041 | 3.044 | |
| Center | 64.698 | 21.749 | |
| Cannabis | Outer | 2.169 | 1.19 |
| Middle | 6.076 | 2.778 | |
| Center | 61.367 | 19.854 | |
| Open field zone entry frequency | |||
| Air | Outer | 14.917 | 1.432 |
| Middle | 18.833 | 1.422 | |
| Center | 5.167 | 0.785 | |
| Placebo | Outer | 13.667 | 1.432 |
| Middle | 14.417 | 1.422 | |
| Center | 4.833 | 0.785 | |
| Cannabis | Outer | 13.917 | 1.432 |
| Middle | 16.167 | 1.422 | |
| Center | 4.917 | 0.785 | |
| Total distance | |||
| Mean | Standard error | ||
| Air | 3717.9 | 158.6 | |
| Placebo | 3523.1 | 162.7 | |
| Cannabis | 3811.1 | 131.8 | |
| Total time | |||
| Air | 298.4 | 0.9 | |
| Placebo | 294.8 | 1.7 | |
| Cannabis | 296.7 | 1.2 | |
There were no group effects on zone entry latencies, entry frequencies, total distance traveled, or total time spent in arena. See text for statistical details.
Effects of adolescent smoke exposure on elevated plus maze
Similar to the open field arena, the elevated plus maze is used to evaluate anxiety-like behavior (Lezak et al., 2017). A two-factor repeated-measures ANOVA (group×arm) with group (three levels: air, placebo, and cannabis) as the between-subjects factor and arm (two levels: open and closed) as the within-subjects factor was used to analyze the time spent in each arm. Results of the analysis confirmed the expected main effect of arm such that all rats spent more time in the closed arms compared to the open arms (F(1,33)=18.595, p<0.001; Figure 2(b)). There was no main effect of group, however (F(2,33)=0.046, p=0.955), and no group×arm interaction (F(2,33)=1.108, p=0.342). In addition to the time spent in each arm, the latency to enter the closed arms was analyzed with a one-factor ANOVA to determine group differences. Shorter latencies to enter closed arms, possibly reflecting the desire to return to a safe location, could be indicative of greater anxiety; however, there was no effect of group on closed arm entry latency (F(2,33)=2.109, p=0.138; Table 2). The analysis of arm entry frequencies (open and closed) using a two-factor repeated-measures ANOVA (group×arm) confirmed that all rats entered the closed arms more frequently (F(1,33)=4.718, p=0.037; Table 2) but that this effect did not differ by group (group: F(2,33)=0.205, p=0.816; group×arm interaction: F(2,33)=0.061, p=0.941). Finally, one-factor ANOVAs confirmed that there were no group differences in total distance traveled (F(2,33)=1.286, p=0.290), mean velocity (F(2,33)=1.204, p=0.313), and maximum velocity (F(2,33)=1.649, p=0.208). These data are congruent with the open field data (as well as with previous work from our labs; Bruijnzeel et al. 2019) and suggest that adolescent exposure to cannabis smoke does not affect anxiety-like behavior in adulthood.
Table 2.
Effects of adolescent cannabis smoke exposure on elevated plus maze measures.
| Closed arm entry latency | |||
|---|---|---|---|
| Mean | Standard error | ||
| Air | 45.09 | 11.85 | |
| Placebo | 78.84 | 11.67 | |
| Cannabis | 66.47 | 11.75 | |
| Arm entry frequency | |||
| Group | |||
| Air | Open | 9.25 | 1.44 |
| Closed | 12.42 | 1.21 | |
| Placebo | Open | 9.00 | 1.44 |
| Closed | 11.25 | 1.21 | |
| Cannabis | Open | 9.08 | 1.44 |
| Closed | 11.42 | 1.21 | |
| Total distance traveled | |||
| Air | 1460.69 | 85.62 | |
| Placebo | 1336.44 | 51.71 | |
| Cannabis | 1475.64 | 60.11 | |
| Total time spent on maze | |||
| Air | 296.84 | 1.86 | |
| Placebo | 295.74 | 0.95 | |
| Cannabis | 295.16 | 1.52 | |
| Mean velocity | |||
| Air | 4.95 | 0.29 | |
| Placebo | 4.55 | 0.18 | |
| Cannabis | 5.02 | 0.21 | |
| Max velocity | |||
| Air | 49.28 | 3.21 | |
| Placebo | 42.66 | 1.83 | |
| Cannabis | 48.66 | 3.27 | |
There were no group effects on closed arm entry latencies, arm entry frequencies, total distance traveled, or mean or maximum velocities. See text for statistical details.
Effects of adolescent smoke exposure on instrumental responding on a progressive ratio schedule
The primary dependent variable in the progressive ratio task was the number of lever presses completed before rats reached their breakpoint, or the point at which they were no longer willing to lever press for a food reward. A one-factor ANOVA revealed no group differences in breakpoint, expressed as either as the highest ratio achieved (F(2,33)=0.833, p=0.444) or the number of food pellets earned (F(2,33)=1.079, p=0.352; Figure 2(c)). These results suggest that adolescent exposure to cannabis smoke does not affect motivation to work for food in adulthood.
Effect of adolescent smoke exposure on working memory (delayed response task)
The delayed response task assesses working memory, or the ability to maintain and update information “in mind” for relatively brief periods of time (Sloan et al., 2006). One rat’s data from the clean air group was identified as an outlier using the ROUT method (Hernandez et al., 2019) and was removed from subsequent analyses. A two-factor repeated-measures ANOVA (group×delay), with group (three levels: air, placebo, and cannabis) as a between-subjects factor and delay (seven levels: 0, 2, 4, 8, 12, 18, 24 s) as a within-subjects factor, was used to analyze choice accuracy as a function of delay. This analysis confirmed the expected main effect of delay such that as delays increased, choice accuracy decreased (F(6,192)=146.552, p<0.001; Figure 3(a)). In addition, there was a significant main effect of group (F(2,32)=4.073, p=0.027, ηp2=0.20), as well as a group×delay interaction (F(12,192)=2.390, p=0.007, ηp2=0.13). Follow-up testing comparing the cannabis smoke and placebo smoke groups using a two-factor repeated measures ANOVA with group as the between-subjects factor and day as the within-subjects factor revealed a significant main effect of delay (F(6,132)=74.58, p<0.001) but no other effects (main effect of group: F(1,22)=1.530, p=0.229; group×delay: F(6,132)=0.667, p=0.677). A similar two-factor ANOVA comparing the placebo smoke and clean air control groups revealed the expected main effect of delay (F(6,126)=121.5, p<0.001) but no other effects (main effect of group: F(1,21)=2.812, p=0.108; group×delay: F(6,126)=2.105, p=0.057). To test whether the effects revealed in the parent ANOVA were driven by group differences between the cannabis smoke and clean air control group, a similar follow-up two-factor ANOVA was used. In this instance, there were significant effects of group (F(1,21)=8.324, p=0.009, ηp2=0.28) and delay (F(6,126)=102.00, p<0.001), in addition to a group×delay interaction (F(6,126)=4.616, p<0.001, ηp2=0.18). Finally, a one-factor ANOVA comparing the numbers of trials completed/session between groups revealed no group differences (clean air mean±SEM: 136.0±2.28; placebo smoke: 134.80±1.73; cannabis smoke: 136.30±1.52; F(2,32)=0.187, p=0.831), suggesting that the differences among groups in choice accuracy were not secondary to differences in task engagement.
Figure 3.
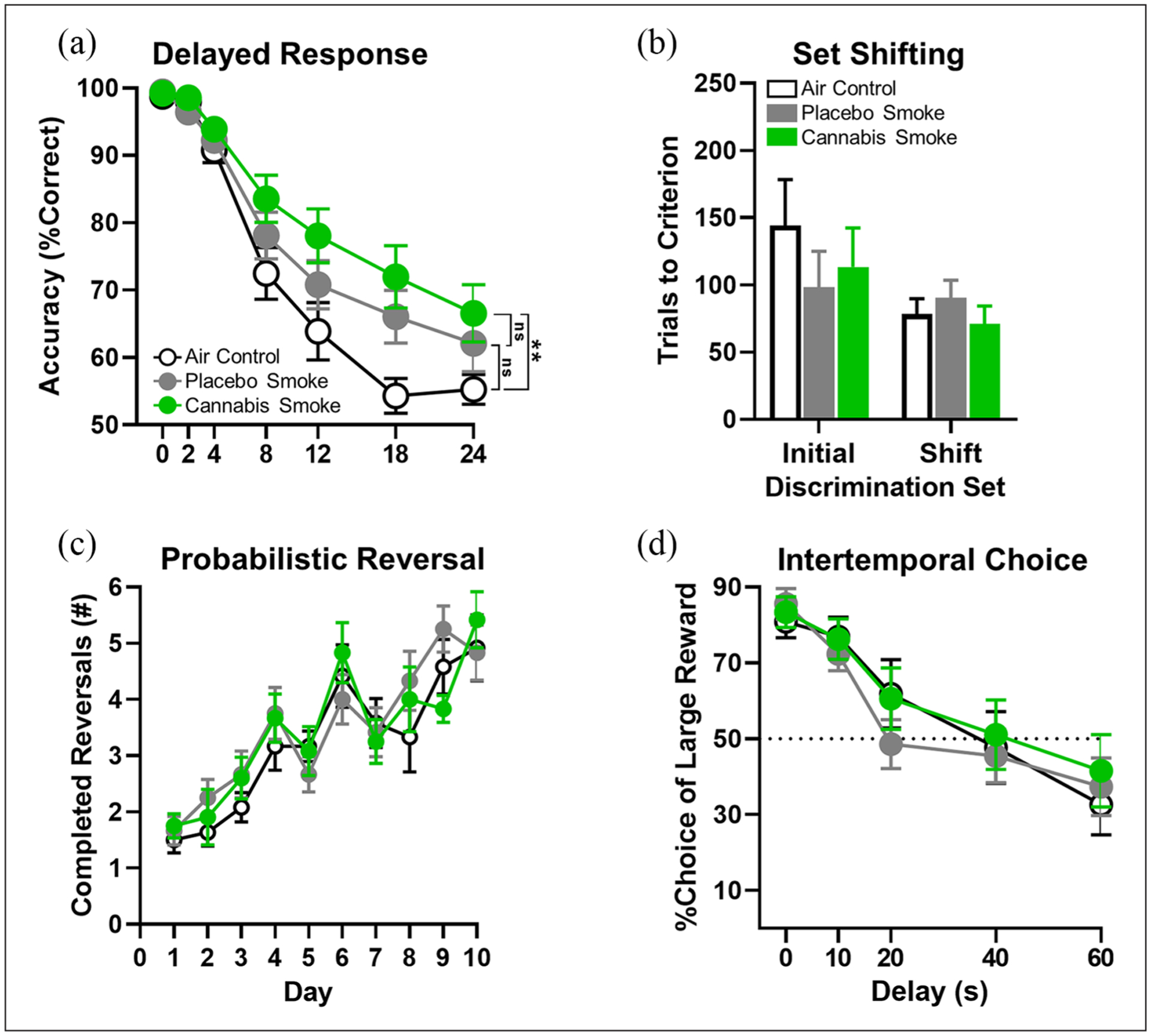
Measures of executive function in adult rats after repeated clean air, placebo, or cannabis smoke exposure during adolescence. (a) Delayed response task (working memory). The cannabis smoke group (n=12) performed significantly better than the clean air control (n=11), but not better than the placebo smoke group (n=12). (b) Set-shifting task (cognitive flexibility). There were no group differences in trials to criterion on either the initial discrimination or the set shift (air control, n=11; placebo smoke, n=11; cannabis smoke, n=10). (c)Probabilistic reversal learning (cognitive flexibility). There were no group differences in the number of reversals performed (air control, n=11; placebo smoke, n=12; cannabis smoke, n=10). (d) Intertemporal choice task (decision making). There were no group differences in preference for the large, delayed over the small, immediate reward (n=12/group). In all figure panels, open circles/bars and black lines represent the clean air control group, filled grey circles/bars and lines represent the placebo smoke group, and filled green circles/bars and lines represent the cannabis smoke group. Error bars represent standard error of the mean (SEM). No significant difference is indicated by “ns” and **p<0.01 for main effects of group.
Effects of adolescent smoke exposure on cognitive flexibility (set-shifting task)
The set-shifting task evaluates one form of cognitive flexibility, or the ability to adapt behavioral strategies in response to changes in environmental contingencies (Floresco et al., 2008). Data from four rats were excluded from analysis due to malfunctions in the operant chambers (n=1 clean air control, n=1 placebo smoke, n=2 cannabis smoke). A one-factor ANOVA showed no group differences in the number of trials required to reach criterion performance (10 consecutive correct trials) on the initial discrimination rule (F(2,29)=0.609, p=0.551; Figure 3(b)), demonstrating that visual discrimination learning was unaffected by adolescent smoke exposure. There were also no group differences in trials to criterion to adapt to the new rule (set shift; F(2,29)=0.575, p=0.569; Figure 3(d)). To further evaluate acquisition of the new rule during set shifting, the types of errors made during learning were divided into “previously reinforced” (responses that correspond to the previously-correct but now-incorrect rule) and “never reinforced” (responses that do not correspond to either the initial or the new rule). A two-factor repeated-measures ANOVA (group×error type), with group (three levels: air, placebo, and cannabis) as a between-subjects factor and error type (two levels: previously-reinforced and never-reinforced) as a within-subjects factor, confirmed the expected main effect of error type, such that all rats made more previously-reinforced than never-reinforced errors (F(1,29)=81.426, p<0.001; Table 3). There was, however, no effect of group (F(1,29)=0.575, p=0.569) and no group×error type interaction (F(2,29)=0.736, p=0.488). Considered together, these data suggest that cognitive flexibility is unaffected by smoke exposure.
Table 3.
Set-shifting errors by type.
| Set shift errors by type | |||
|---|---|---|---|
| Group | Mean | Standard error | |
| Air | Previously reinforced | 20.091 | 3.491 |
| Never reinforced | 5.273 | 1.028 | |
| Placebo | Previously reinforced | 24.182 | 3.491 |
| Never reinforced | 4.455 | 1.028 | |
| Cannabis | Previously reinforced | 18.800 | 3.662 |
| Never reinforced | 3.500 | 1.079 | |
There were no group differences in errors on the set-shifting task. See text for statistical details.
Effects of adolescent smoke exposure on cognitive flexibility (probabilistic reversal learning)
The probabilistic reversal learning task measures cognitive flexibility and learning. The number of completed reversals was the primary measure of performance. As a consequence of incomplete data collection due to computer errors, n=1 air control, and n=2 cannabis smoke rats were excluded from this analysis. A two-factor repeated-measures ANOVA (group×day) was used to analyze the number of completed reversals as a function of day. This analysis confirmed an expected main effect of day such that rats increased the number of reversals completed per session across testing days (F(9,278)=23.92, p<0.001; Figure 3(c)); there was, however, no effect of group (F(2,31)=0.141, p=0.866) and no group×day interaction (F(18,278)=0.877, p=0.608). In addition to accuracy, lever press response latencies (the time from lever extension to lever press) were assessed to evaluate smoke exposure effects on task motivation. A two-factor repeated-measures ANOVA (group×day), with group (three levels: air, placebo, and cannabis) as the between-subjects factor and day (10 levels: days 1–10) as the within-subjects factor, confirmed that rats responded with shorter latencies across days (F(9,279)=5.376, p<0.001) but that this did not differ across groups (group: F(2,31)=0.683, p=0.513; group×day: F(18,279)=1.126, p=0.326). Together with the set-shifting data, these results indicate that adolescent exposure to cannabis does not impair cognitive flexibility in adulthood.
Effects of adolescent smoke exposure on intertemporal choice
To evaluate the effects of adolescent smoke exposure on decision making and one form of impulsivity (impulsive choice), rats were tested on an intertemporal choice (delay discounting) task. A two-factor repeated-measures ANOVA (group×delay), with group (three levels: air, placebo, and cannabis) as the between-subjects factor and delay (five levels: 0, 10, 20, 40, 60 s) as a within-subjects factor, was used to analyze the percent choice of the large reward as a function of delay. This analysis revealed a main effect of delay such that rats reduced their choice of the large reward at longer delays (F(4,132)=60.183, p<0.001; Figure 3(d)); however, there was neither a main effect of group (F(2,33)=1.024, p=0.370) nor an interaction between group and delay (F(8,132)=1.079, p=0.382). To further evaluate effects of smoke exposure on intertemporal choice, a one-factor ANOVA compared choice indifference points and showed no differences across groups (clean air mean±SEM: 37.98±5.018; placebo smoke: 26.20±5.403; cannabis smoke: 43.91±7.365; F(2,33)=2.245, p=0.123), suggesting that adolescent exposure to cannabis smoke does not alter intertemporal decision making in adulthood.
Discussion
The current study evaluated the effects of adolescent exposure to cannabis smoke on measures of affect and cognition. Briefly, adolescent exposure to cannabis smoke led to slower growth (attenuated body weight gain) but had no effect on anxiety-like behavior, either in an open field arena or an elevated plus maze, nor did it affect food motivation under a progressive ratio schedule of instrumental responding for food reward. While there was no effect of adolescent smoke exposure on measures of cognitive flexibility (set-shifting and probabilistic reversal learning), surprisingly, it appeared to enhance working memory relative to the clean air control condition. Finally, smoke exposure had no effect on impulsive choice as measured with an intertemporal choice task.
Cannabis smoke and body weight
Previous studies have reported inconsistent effects of cannabis use on body weight. For example, one study reports that adolescent cannabis use is linked with increased risk of obesity in early adulthood (Huang et al., 2013), whereas other studies suggest that adolescent cannabis use does not impact weight in middle life (Jin et al., 2017), and that cannabis use may even produce weight loss in adults (Le Foll et al., 2013). The results of the current study show that adolescent exposure to cannabis smoke can reduce weight gain both during the period of smoke exposure and for at least several weeks after exposure (during young adulthood). This finding is consistent with previous work showing that adolescent exposure to THC reduces body weight in rats (Bruijnzeel et al., 2019; Klein et al., 2011), an effect that can persist up to 21 weeks after exposure (Klein et al., 2011). Notably, however, previous work from our laboratory (Bruijnzeel et al., 2019) failed to observe changes in body weight during and after adolescent exposure to cannabis smoke in either male or female rats, suggesting that the effects of cannabis smoke on body weight are not robust. Importantly, however, the current data provide a “positive control” for the efficacy of the smoke exposure regimen in affecting developmental physiology, which is particularly important considering the largely negative data on measures of affect and cognition.
Cannabis smoke, anxiety, and motivation
Adolescent cannabis use is associated with increased prevalence of anxiety and/or depression in teenagers (Patton et al., 2002) as well as in adults (Fergusson et al., 2002; Hayatbakhsh et al., 2007; Rey et al., 2002). In the current study, chronic cannabis smoke exposure during adolescence did not alter measures of anxiety-like behavior in adulthood in either an open field arena or elevated plus maze. These findings replicate the results of previous work showing a similar absence of effects of adolescent cannabis smoke exposure in these tasks, as well as findings that adolescent exposure to THC or synthetic cannabinoids has no effect on these tasks in adulthood (Bruijnzeel et al., 2019; Higuera-Matas et al., 2009; Rubino et al., 2008). In contrast to the absence of long-term effects of adolescent cannabis smoke, acute administration of either cannabis smoke or THC in adulthood can alter anxiety-like behavior. For example, acute exposure to cannabis smoke in adult rats increases time spent in the center of an open field, potentially indicating an anxiolytic effect (Bruijnzeel et al., 2016). In contrast, acute injection of THC in adolescent rats has the opposite effect in an open field arena and also reduces entries into the open arms of the elevated plus maze (Klein et al., 2011). These contrasting effects likely depend on the dose of THC administered. Doses of THC greater than 1–3 mg/kg in rats can be aversive (and thus may produce acute anxiogenesis or conditioned place aversion; Braida et al., 2004; Elsmore and Fletcher, 1972; Parker and Gillies, 1995), whereas doses below 1 mg/kg can produce rewarding effects (Braida et al., 2004). Blood THC levels in rats following the cannabis smoke exposure regimen used here peak at 10–12 ng/mL, which is estimated to correspond to a dose of 0.05 mg/kg (Ravula et al., 2018, 2019). Given that cannabis users are unlikely to intentionally ingest anxiogenic doses of THC, it seems probable that the doses of THC obtained through the cannabis smoke exposure regimen used here are a reasonable model of recreational levels of use.
Similar to the measures of anxiety-like behavior, there was no effect of adolescent cannabis smoke exposure on willingness to expend effort to obtain food rewards (food motivation), as assessed by breakpoint on a progressive ratio (PR) schedule of reinforced responding for food. These data are consistent with those from a recent study in which adolescent exposure to THC vapor failed to affect PR breakpoint for an oxycodone reinforcer in adulthood (despite a small increase in fentanyl self-administration selectively in adult females (Nguyen et al., 2020)). A similar absence of effect on PR breakpoints (for both food and opioids) has been observed following perinatal THC exposure (González et al., 2003), although see (Ellgren et al., 2007) for contrasting results with adolescent THC injections. In contrast, a prior study found that repeated administration of the synthetic cannabinoid WIN 55,212–2 (WIN) during late adolescence in rats caused a reduction in breakpoint in adulthood on a PR schedule for a food reinforcer, whereas the same WIN administration regimen in adult rats had no effect (Schneider and Koch, 2003). There were several significant differences between this prior work and the current results, however, including the drug used, the relatively high dose of WIN, and the short period (10 days) between cessation of WIN administration and PR testing, making it difficult to directly compare the two findings. Intriguingly, the absence of effects of cannabis smoke on food motivation is not in line with reports from studies in human subjects that show moderate increases in food intake during cannabis administration (Foltin et al., 1986; Rodondi et al., 2006). Importantly, the null effects of adolescent cannabis smoke exposure on food motivation in the current study are not likely due to non-behaviorally effective doses of THC. Aside from the fact that growth was attenuated, our prior work shows that the smoke exposure regimen used here produces blood THC levels that are behaviorally active (Bruijnzeel et al., 2016; Ravula et al., 2019) and within the range observed following cannabis self-administration in humans (1.9–43.6 ng/mL; Lee et al., 2015). Taken together, these results suggest that cannabis smoke exposure in adolescent rats does not produce lasting alterations in anxiety-like behavior and motivation in adulthood.
Cannabis smoke and executive functions
In contrast to the null effects of adolescent cannabis smoke exposure on the open field, elevated plus maze, and progressive ratio tasks, smoke exposure in adolescence appeared to enhance working memory in adulthood. It is important to note that while the cannabis smoke group showed better working memory performance relative to the clean air control group, the cannabis smoke group did not perform significantly better than the placebo smoke group, nor did the placebo smoke group perform significantly better than the clean air control group. This pattern of results suggests that while cannabis smoke exposure appears to improve working memory, the better performance in the cannabis smoke group is unlikely to be explained by the cannabinoid content of the smoke alone, as the cannabis and placebo smoke groups were not different. It would appear then that placebo smoke does not markedly improve performance but instead produces an intermediate phenotype that can be enhanced by the active components of cannabis smoke (or potentially by the greater quantity of particulate matter produced by burning the cannabis compared to placebo cigarettes). Indeed, exposure to inhaled particulate matter or CO from the smoke likely produced some degree of stress, which can have lasting effects on cognitive development (Gunnar and Quevedo, 2007). Intriguingly, however, several previous reports in rats indicate that intravenous self-administration of the synthetic CB1R agonist WIN during adolescence results in a lasting enhancement in working memory performance similar to that observed here (Kirschmann et al., 2017a, 2017b). These data show that developmental cannabinoid exposure (at voluntarily ingested doses) has the potential to facilitate at least some forms of cognitive function (see also Higuera-Matas et al. (2009) for potential enhancing effects of adolescent synthetic cannabinoid injections in the Morris water maze). Moreover, previous work from our laboratory showed that acute exposure to cannabis (but not placebo) smoke enhances performance in the same delayed response task employed in the present study (Blaes et al., 2019), indicating that the dose of THC obtained from cannabis smoke is sufficient to potentiate working memory.
Studies in humans have reported inconsistent effects of acute THC (or CB1R agonist) administration on working memory, with different studies reporting impairments (Bedi et al., 2013; D’Souza et al., 2004; Englund et al., 2013; Freeman et al., 2015; Hunault et al., 2009; Lane et al., 2005a; Lile et al., 2014; Morrison et al., 2009; Ramesh et al., 2013; Theunissen et al., 2015; Wesnes et al., 2010), improvements (Makela et al., 2006), or no effects (D’Souza et al., 2008; Kollins et al., 2015; Lile et al., 2011; Ranganathan et al., 2012; reviewed in Broyd et al., 2016). Similar to the mixed effects of acute THC on working memory, studies of chronic cannabis use in humans also report either impairments (Fried et al., 2005; Herzig et al., 2014; Wadsworth et al., 2006) or no effects (Becker et al., 2014; Grant et al., 2012). Furthermore, those who abstained after chronic use showed no difference in performance relative to non-users (Fried et al., 2005), suggesting that after prolonged cannabis use, abstinence can rescue working memory impairments.
It is thought that the prefrontal cortex (PFC) supports working memory via recurrent excitation of local excitatory pyramidal networks (Goldman-Rakic, 1995). Inhibitory interneurons within the PFC are well-positioned to regulate these local networks of excitatory neurons (McQuail et al., 2015). Neurobiological reports suggest that CB1Rs are enriched at inhibitory neurons within the PFC (Wędzony and Chocyk, 2009) and function to inhibit presynaptic release of the primary inhibitory neurotransmitter, gamma-amino butyric acid (GABA) (McLaughlin et al., 2014). Indeed, inhibition of GABA signaling ameliorates age-related impairments in working memory performance (Bañuelos et al., 2014), likely in part via blunting of tonic inhibition onto excitatory neurons of the PFC (Carpenter et al., 2016). Though the mechanisms by which adolescent cannabis smoke appears to facilitate working memory are not entirely clear, one possibility is that chronic exposure to cannabis smoke during adolescence may result in prolonged dampening of inhibition over the recurrent excitation necessary for information maintenance in working memory. Consistent with this interpretation and with the findings of the current study, chronic WIN self-administration during adolescence enhances working memory performance in adult rats, likely via alterations to PFC glutamatergic and GABAergic signaling (Kirschmann et al., 2017b). More specifically, Kirschmann et al. found selective increases in expression of both the synaptic GABA uptake transporter (GAT-1) and the N-methyl-D-aspartate receptor subunit NR2B, which would promote increased removal of GABA at inhibitory synapses and increased excitability, respectively. The results of the present study suggest that both the cannabinoid content of smoke and smoke exposure itself may act synergistically to potentiate working memory functions in adulthood. Future studies will be necessary to determine the mechanisms (e.g. decreased GABAergic signaling) underlying these enhancements.
Chronic exposure to cannabis smoke during adolescence did not impact cognitive flexibility in adulthood in either set-shifting or probabilistic reversal learning tasks. Prior studies have reported mixed effects of acute CB1R agonist administration on cognitive flexibility (Egerton et al., 2005; Hill et al., 2006; reviewed in Cohen and Weinstein, 2018). Specifically, Hill et al. (2006) reported that acute intraperitoneal administration of a high dose (20 μg/kg) of the CB1R agonist HU-250 impaired performance in a set-shifting task, whereas a low dose (5 μg/kg) of the drug improved performance. Egerton et al. (2005) reported that acute intraperitoneal administration of 1.0 mg/kg THC impaired reversal learning in rats, whereas a dose of 0.01 mg/kg had no effect. Differences between previously reported effects and the findings of the current study likely arise from pharmacokinetic differences in drug, doses, routes of administration, and duration of administration; however, the null effects of low doses of THC in the Egerton et al. study are consistent with the results presented here. Considering the working memory results in the current study and others, the null effects on cognitive flexibility suggest that the effects of cannabis use in adolescence do not broadly impact adult performance on tasks assessing executive function.
Cannabis smoke and intertemporal choice
Chronic adolescent exposure to cannabis smoke did not alter impulsive choice in adulthood as assessed by an intertemporal choice task (i.e. no changes in rats’ preference for a small, immediate reward vs a large, delayed reward). Previous studies have reported that chronic cannabis use can impair decision making assessed in the Iowa Gambling Task in humans (Becker et al., 2014; Grant et al., 2012; Whitlow et al., 2004). Furthermore, acute cannabis use was shown to increase risky decisions in adults given choices between a small but certain monetary gain vs a larger monetary gain with a risk of loss (Lane et al., 2005c). In contrast, several studies have reported no effects of inhaled or oral cannabis on intertemporal choice in humans (Churchwell et al., 2010; Gonzalez et al., 2012; McDonald et al., 2003). In rats, activation of CB1Rs via acute intraperitoneal administration of WIN did not alter performance in an intertemporal choice task (Pattij et al., 2007), whereas acute intraperitoneal administration of THC dose-dependently decreased impulsive choice in rats (Wiskerke et al., 2011). Compared to the Wiskerke et al. (2011) study, the absence of an effect of cannabis smoke on impulsive choice in the current study could be due to differences in the timing, chronicity, or route of administration. Indeed, the results of the present study more closely resemble the null findings in human subjects, in which drug administration occurred mostly by cannabis smoke inhalation. To our knowledge, no other studies have investigated effects of adolescent cannabis exposure on adult impulsive choice, and the current results would suggest there are no long-term impairing effects. The results of the current study, in combination with the literature on cannabis use and decision making, suggest that although cannabis may have selective effects on other forms of decision-making, it appears to spare intertemporal choice.
Limitations and conclusions
The current study sought to determine the effects of chronic cannabis smoke exposure during adolescence on measures of anxiety, motivation, and cognition in adulthood. Despite the fact that cannabis smoke exposure produced a lasting reduction in body weight, it had no effects on measures of affect or executive function. The findings suggest that adolescent cannabis smoke exposure does not cause adverse affective and cognitive effects in adulthood, and are consistent with prior descriptions of the effects of chronic adolescent exposure to low doses of cannabinoid receptor agonists (Bruijnzeel et al., 2019; Kirschmann et al., 2017a, 2017b).
One limitation of the current study is that all of the rats were tested in the behavioral tasks in the same order, rendering it possible that experience with one task occluded detection of group differences in subsequent tasks. Although this possibility cannot be entirely ruled out, in a previous study that also found no effects of adolescent cannabis smoke exposure on the open field and elevated plus maze tasks (Bruijnzeel et al., 2019), the rats were tested in these tasks in the opposite order from that used here. This suggests that at least within the domains of locomotion and anxiety-like behavior, the order of testing did not influence test outcomes.
Another limitation of the current study, which leaves the door open to future work, is that rats were not tested during the period of cannabis exposure. It is highly probable that in humans, cannabis use starting in adolescence continues through at least early adulthood, and thus future rat models of cannabis use should take this into consideration. In addition, the current study was limited to male rats. Although the null effects of cannabis smoke observed here mimicked those in our previous study that employed both sexes (Bruijnzeel et al., 2019), there are significant sex differences on several of the tasks used here (intertemporal choice, working memory, progressive ratio; Blaes et al., 2019; Hernandez et al., 2020; Orsini et al., 2016), and thus it is possible that adolescent smoke exposure would affect females differently. In addition, the current study employed only a single rat strain (Long-Evans) with a single smoke exposure regimen. It will be important in future work to determine whether these findings are replicated with additional strains and species (although note that a similar absence of impairing effects on adult working memory was observed following adolescent cannabinoid self-administration in Sprague Dawley rats; Kirschmann et al., 2017b).
Finally, the relatively low, “recreational use”-like levels of THC employed in the current study may not entirely model current trends in cannabis use. Commercially available cannabis can contain THC levels of 15% or higher, in addition to a rich mix of other cannabinoids. Thus, it would be valuable in future studies to be able to use cannabis that better reflects currently available cannabinoid contents. Self-administration models will be useful in this regard (e.g. self-administration of cannabis extracts), as they can address these issues as well as those concerning potential aversive effects of higher levels of cannabinoid exposure (Freels et al., 2020; Kruse et al., 2019). Given the contrast between the present results and those of previous work employing adolescent exposure to higher doses of cannabinoid receptor agonists, as well as the growing availability of cannabis products to adolescents, the current findings highlight the need for modeling of adolescent exposure to a range of cannabinoid doses in order to better understand how such exposure affects the brain and behavior.
Acknowledgements
The authors wish to thank Vicky S Kelly, Matt M Bruner, Shannon C Wall, Sara Heshmati, Darin Jagnarine, and Kailey L Simpson for technical assistance, and the NIDA Drug Supply Program for kindly providing cannabis and placebo cigarettes.
Funding
The author(s) declared the following support for the research, authorship, and/or publication of this article: Supported by the McKnight Brain Research Foundation and NIH DA039349 (BS, AWB, and MF).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Bañuelos C, Beas BS, McQuail JA, et al. (2014) Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J Neurosci 34: 3457–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM and Phillips AG (1999) Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology 141: 99–106. [DOI] [PubMed] [Google Scholar]
- Beas BS, McQuail JA, Bañuelos C, et al. (2017) Prefrontal cortical GABAergic signaling and impaired behavioral flexibility in aged F344 rats. Neurosci 345: 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beas BS, Setlow B and Bizon JL (2013) Distinct manifestations of executive dysfunction in aged rats. Neurobiol Aging 34: 2164–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MP, Collins PF and Luciana M (2014) Neurocognition in college-aged daily marijuana users. J Clin Exp Neuropsychol 36: 379–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Cooper ZD and Haney M (2013) Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict Biol 18: 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscaia M, Marín S, Fernández B, et al. (2003) Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology 170: 301–308. [DOI] [PubMed] [Google Scholar]
- Blaes SL, Orsini CA, Holik HM, et al. (2019) Enhancing effects of acute exposure to cannabis smoke on working memory performance. Neurobiol Learn Mem 157: 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Iosuè S, Pegorini S, et al. (2004) Δ9-Tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol 506: 63–69. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, van Hell HH, Beale C, et al. (2016) Acute and chronic effects of cannabinoids on human cognition: A systematic review. Biol Psychiatry 79: 557–567. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Knight P, Panunzio S, et al. (2019) Effects in rats of adolescent exposure to cannabis smoke or THC on emotional behavior and cognitive function in adulthood. Psychopharmacology 236: 2773–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Qi X, Guzhva LV, et al. (2016) Behavioral characterization of the effects of cannabis smoke and anandamide in rats. PLoS One 11: e0153327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter HE, Kelly KB, Bizon JL, et al. (2016) Age-related changes in tonic activation of presynaptic versus extrasynaptic γ-amniobutyric acid type B receptors in rat medial prefrontal cortex. Neurobiol Aging 45: 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin T, Freudenberg F, Füchtemeier M, et al. (2004) Dopamine in the orbitofrontal cortex regulates operant responding under a progressive ratio of reinforcement in rats. Neurosci Lett 370: 114–117. [DOI] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, et al. (2006) Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav 83: 448–455. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Lopez-Larson M and Yurgelun-Todd DA (2010) Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol 1: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen K and Weinstein A (2018) The effects of cannabinoids on executive functions: Evidence from cannabis and synthetic cannabinoids—A systematic review. Brain Sci 8: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Martín-Santos R, et al. (2009) Cannabis and anxiety: A critical review of the evidence. Hum Psychopharmacol 24: 515–523. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Phillips AG and Floresco SB (2014) Preferential involvement by nucleus accumbens shell in mediating probabilistic learning and reversal shifts. J Neurosci 34: 4618–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, et al. (2004) The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology 29: 1558–1572. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Braley G, Blaise R, et al. (2008) Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Δ−9-tetrahydrocannabinol in humans. Psychopharmacology 198: 587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Brett RR and Pratt JA (2005) Acute Δ 9 -Tetrahydrocannabinol-induced deficits in reversal learning: Neural correlates of affective inflexibility. Neuropsychopharmacology 30: 1895–1905. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Spano SM and Hurd YL (2007) Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 32: 607–615. [DOI] [PubMed] [Google Scholar]
- Elsmore TF and Fletcher GV (1972) Δ9-Tetrahydrocannabinol: Aversive effects in rat at high doses. Science 175: 911–912. [DOI] [PubMed] [Google Scholar]
- Englund A, Morrison PD, Nottage J, et al. (2013) Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol 27: 19–27. [DOI] [PubMed] [Google Scholar]
- Evenden JL and Ryan CN (1996) The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 128: 161–170. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ and Swain-Campbell N (2002) Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction 97: 1123–1135. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE and Tse MTL (2008) Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res 190: 85–96. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Brady JV and Fischman MW (1986) Behavioral analysis of marijuana effects on food intake in humans. Pharmacol Biochem Behav 25: 577–582. [DOI] [PubMed] [Google Scholar]
- Freels TG, Baxter-Potter LN, Lugo JM, et al. (2020) Vaporized cannabis extracts have reinforcing properties and support conditioned drug-seeking behavior in rats. J Neurosci 40: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Dunn G, Murray RM, et al. (2015) How cannabis causes paranoia: Using the intravenous administration of Δ9-tetrahydrocannabinol (THC) to identify key cognitive mechanisms leading to paranoia. Schizophr Bull 41: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B and Gray R (2005) Neurocognitive consequences of marihuana: A comparison with pre-drug performance. Neurotoxicol Teratol 27: 231–239. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1995) Cellular basis of working memory. Neuron 14: 477–485. [DOI] [PubMed] [Google Scholar]
- González B, de Miguel R, Martín S, et al. (2003) Effects of perinatal exposure to Δ9-tetrahydrocannabinol on operant morphine-reinforced behavior. Pharmacol Biochem Behav 75: 577–584. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, et al. (2012) Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. J Clin Exp Neuropsychol 34: 962–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Conley JW and Gordon JM (2013) Medical consequences of marijuana use: a review of current literature. Curr Psychiatry Rep 15: 419. [DOI] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Schreiber L, et al. (2012) Neuropsychological deficits associated with cannabis use in young adults. Drug Alcohol Depend 121: 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B, Kavanagh D and Young R (2003) Being stoned: A review of self-reported cannabis effects. Drug Alcohol Rev 22: 453–460. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, et al. (2012a) Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett 511: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, et al. (2012b) Age of onset of marijuana use and executive function. Psychol Addict Behav 26: 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M and Quevedo K (2007) The neurobiology of stress and development. Annu Rev Psychol 58: 145–173. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, et al. (2010) Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav 35: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, et al. (2007) The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev 26: 309–319. [DOI] [PubMed] [Google Scholar]
- Hayatbakhsh MR, Najman JM, Jamrozik K, et al. (2007) Cannabis and anxiety and depression in young adults: A large prospective study. J Am Acad Child Adolesc Psychiatry 46: 408–417. [DOI] [PubMed] [Google Scholar]
- Hernandez AR, Hernandez CM, Truckenbrod LM, et al. (2019) Age and ketogenic diet have dissociable effects on synapse-related gene expression between hippocampal subregions. Front Aging Neurosci 11: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Vetere LM, Orsini CA, et al. (2017) Decline of prefrontal cortical-mediated executive functions but attenuated delay discounting in aged Fischer 344× brown Norway hybrid rats. Neurobiol Aging 60: 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Orsini CA, Wheeler A-R, et al. (2020) Testicular hormones mediate robust sex differences in intertemporal choice in rats. ELife 9: e58604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig DA, Nutt DJ and Mohr C (2014) Alcohol and relatively pure cannabis use, but not schizotypy, are associated with cognitive attenuations. Front Psychiatry 5: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuera-Matas A, Botreau F, Miguéns M, et al. (2009) Chronic periadolescent cannabinoid treatment enhances adult hippocampal PSA-NCAM expression in male Wistar rats but only has marginal effects on anxiety, learning and memory. Pharmacol Biochem Behav 93: 482–490. [DOI] [PubMed] [Google Scholar]
- Hill MN, Froese LM, Morrish AC, et al. (2006) Alterations in behavioral flexibility by cannabinoid CB1 receptor agonists and antagonists. Psychopharmacology 187: 245–259. [DOI] [PubMed] [Google Scholar]
- Huang DYC, Lanza HI and Anglin MD (2013) Association between adolescent substance use and obesity in young adulthood: A group-based dual trajectory analysis. Addict Behav 38: 2653–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunault CC, Mensinga TT, Böcker KBE, et al. (2009) Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg Δ−9-tetrahydrocannabinol (THC). Psychopharmacology 204: 85–94. [DOI] [PubMed] [Google Scholar]
- Jin LZ, Rangan A, Mehlsen J, et al. (2017) Association between use of cannabis in adolescence and weight change into midlife. PLoS One 12: e0168897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanjus E, Raymond V, Lapeyre-Mestre M, et al. (2017) What is the current knowledge about the cardiovascular risk for users of cannabis-based products? A systematic review. Curr Atheroscler Rep 19: 26. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Herrera FM, et al. (2005) The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: Implications for models of inter-temporal choice. Behav Brain Res 156: 145–152. [DOI] [PubMed] [Google Scholar]
- Kirschmann EK, McCalley DM, Edwards CM, et al. (2017a) Consequences of adolescent exposure to the cannabinoid receptor agonist WIN55,212–2 on working memory in female rats. Front Behav Neurosci 11: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschmann EK, Pollock MW, Nagarajan V, et al. (2017b) Effects of adolescent cannabinoid self-administration in rats on addiction-related behaviors and working memory. Neuropsychopharmacology 42: 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Karanges E, Spiro A, et al. (2011) Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology 218: 443–457. [DOI] [PubMed] [Google Scholar]
- Kollins SH, Schoenfelder EN, English JS, et al. (2015) An exploratory study of the combined effects of orally administered methylphenidate and delta-9-tetrahydrocannabinol (THC) on cardiovascular function, subjective effects, and performance in healthy adults. J Subst Abuse Treat 48: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse LC, Cao JK, Viray K, et al. (2019) Voluntary oral consumption of Δ9 -tetrahydrocannabinol by adolescent rats impairs reward-predictive cue behaviors in adulthood. Neuropsychopharmacology 44: 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Lieving LM, et al. (2005a) Marijuana effects on human forgetting functions. J Exp Anal Behav 83: 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Pietras CJ, et al. (2005b) Performance of heavy marijuana-smoking adolescents on a laboratory measure of motivation. Addict Behav 30: 815–828. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, et al. (2005c) Acute marijuana effects on human risk taking. Neuropsychopharmacology 30: 800–809. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Trigo JM, Sharkey KA, et al. (2013) Cannabis and Δ9-tetrahydrocannabinol (THC) for weight loss? Med Hypotheses 80: 564–567. [DOI] [PubMed] [Google Scholar]
- Lee D, Bergamaschi MM, Milman G, et al. (2015) Plasma cannabinoid pharmacokinetics after controlled smoking and ad libitum cannabis smoking in chronic frequent users. J Anal Toxicol 39: 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak KR, Missig G and Carlezon WA (2017) Behavioral methods to study anxiety in rodents. Dialogues Clin Neurosci 19: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebsch G, Montkowski A, Holsboer F, et al. (1998) Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav Brain Res 94: 301–310. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH and Hays LR (2011) Separate and combined effects of the cannabinoid agonists nabilone and Δ9-THC in humans discriminating Δ9-THC. Drug Alcohol Depend 116: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH and Hays LR (2014) Separate and combined effects of the GABAA positive allosteric modulator diazepam and Δ9-THC in humans discriminating Δ9-THC. Drug Alcohol Depend 143: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, et al. (2003) Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 28: 1356–1365. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN and Gorzalka BB (2014) A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev 42: 116–131. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Frazier CJ and Bizon JL (2015) Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends Mole Med 21: 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Beas BS, Kelly KB, et al. (2016) NR2A-containing NMDARs in the prefrontal cortex are required for working memory and associated with age-related cognitive decline. J Neurosci 36: 12537–12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela P, Wakeley J, Gijsman H, et al. (2006) Low doses of δ−9 tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology 31: 462–470. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Ford B, Matthews BA, et al. (2014) A vapourized Δ9-tetrahydrocannabinol (Δ9-THC) delivery system part II: Comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J Pharmacol Toxicol Methods 70: 112–119. [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, et al. (2007) Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc 13: 807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Gilbert RJ, Bizon JL, et al. (2012) Effects of acute administration of nicotinic and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision making tasks in rats. Psychopharmacology 224: 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Williams MT, Bhavsar A, et al. (2009) Long-lasting sensitization of reward-directed behavior by amphetamine. Behav Brain Res 201: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Beas BS, et al. (2014) Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacology 39: 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PD, Zois V, McKeown DA, et al. (2009) The acute effects of synthetic intravenous Δ9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med 39: 1607–1616. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ and Brown RE (2006) Detecting outliers when fitting data with nonlinear regression: A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 7: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA) (2015) Nationwide Trends. Available at: https://www.drugabuse.gov/publications/drugfacts/nationwide-trends (accessed 19 December 2019).
- National Institute on Drug Abuse (NIDA) (2018) National Survey of Drug Use and Health. Available at: https://www.drugabuse.gov/national-survey-drug-use-health (accessed 19 December 2019).
- Nguyen JD, Aarde SM, Vandewater SA, et al. (2016) Inhaled delivery of Δ9-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology 109: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Creehan KM, Kerr TM, et al. (2020) Lasting effects of repeated Δ9-tetrahydrocannabinol vapour inhalation during adolescence in male and female rats. Br J Pharmacol 177: 188–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Martin BR, et al. (2007) Exposure to marijuana smoke impairs memory retrieval in mice. J Pharmacol Exp Ther 322: 1067. [DOI] [PubMed] [Google Scholar]
- O’Shea M, McGregor IS and Mallet PE (2006) Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol 20: 611–621. [DOI] [PubMed] [Google Scholar]
- O’Shea M, Singh ME, Mcgregor IS, et al. (2004) Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol 18: 502–508. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Gilbert RJ, Willis M, et al. (2016) Sex differences in a rat model of risky decision making. Behav Neurosci 130: 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA and Gillies T (1995) THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behav Neurosci 109: 71–78. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MCW, Schepers I, et al. (2007) Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology 193: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, et al. (2002) Cannabis use and mental health in young people: Cohort study. BMJ 325: 1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Guzhva L, Yang Z, et al. (2016) Overexpression of CRF in the BNST diminishes dysphoria but not anxiety-like behavior in nicotine withdrawing rats. Euro Neuropsychopharmacol 26: 1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, et al. (2008) Adolescent rats find repeated Δ9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 33: 1113–1126. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Haney M and Cooper ZD (2013) Marijuana’s dose-dependent effects in daily marijuana smokers. Exp Clin Psychopharmacol 21: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Carbuto M, Braley G, et al. (2012) Naltrexone does not attenuate the effects of intravenous Δ9-tetrahydrocannabinol in healthy humans. Int J Neuropsychopharmacol 15: 1251–1264. [DOI] [PubMed] [Google Scholar]
- Ravula A, Chandasana H, Jagnarine D, et al. (2019) Pharmacokinetic and pharmacodynamic characterization of tetrahydrocannabinol-induced cannabinoid dependence after chronic passive cannabis smoke exposure in rats. Cannabis Cannabinoid Res 4: 240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravula A, Chandasana H, Setlow B, et al. (2018) Simultaneous quantification of cannabinoids tetrahydrocannabinol, cannabidiol and CB1 receptor antagonist in rat plasma: An application to characterize pharmacokinetics after passive cannabis smoke inhalation and co-administration of rimonabant. J Pharm Biomed Anal 160: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey JM, Sawyer MG, Raphael B, et al. (2002) Mental health of teenagers who use cannabis: Results of an Australian survey. Br J Psychiatry 180: 216–221. [DOI] [PubMed] [Google Scholar]
- Rodondi N, Pletcher MJ, Liu K, et al. (2006) Marijuana use, diet, body mass index, and cardiovascular risk factors (from the CARDIA study). Am J Cardiol 98: 478–484. [DOI] [PubMed] [Google Scholar]
- Ross JM, Graziano P, Pacheco-Colón I, et al. (2016) Decision-making does not moderate the association between cannabis use and body mass index among adolescent cannabis users. J Int Neuropsychol Soc 22: 944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T and Parolaro D (2008) Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol 286(1 Suppl 1): S108–S113. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, et al. (2009a) Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus 19: 763–772. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, et al. (2009b) The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox Res 15: 291–302. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano’ D, Realini N, et al. (2008) Chronic Δ 9 -tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: Behavioral and biochemical correlates. Neuropsychopharmacology 33: 2760–2771. [DOI] [PubMed] [Google Scholar]
- Rylkova D, Shah HP, Small E, et al. (2009) Deficit in brain reward function and acute and protracted anxiety-like behavior after discontinuation of a chronic alcohol liquid diet in rats. Psychopharmacology 203: 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M and Koch M (2003) Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 28: 1760–1769. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Gruenewald PJ, Klitzner M, et al. (1989) Short-term memory impairment in cannabis-dependent adolescents. Am J Dis Child 143: 1214–1219. [DOI] [PubMed] [Google Scholar]
- Sloan HL, Good M and Dunnett SB (2006) Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res 171: 116–126. [DOI] [PubMed] [Google Scholar]
- Solowij N and Pesa N (2010) Cognitive abnormalities and cannabis use. Rev Bras Psiquiatr 32(Suppl 1): S31–S40. [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, et al. (2002) Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 287: 1123–1131. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Creehan KM, Vandewater SA, et al. (2020) Effects of Δ9-tetrahydrocannabinol (THC) vapor inhalation in Sprague-Dawley and Wistar rats. Exp Clin Psychopharmacol. Epub ahead of print 16 April 2020. DOI: 10.1037/pha0000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague SV, Pinkerton KE, Goldsmith M, et al. (1994) Sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhalation Toxicology 6: 79–93. [Google Scholar]
- Theunissen EL, Heckman P, de Sousa Fernandes Perna EB, et al. (2015) Rivastigmine but not vardenafil reverses cannabis-induced impairment of verbal memory in healthy humans. Psychopharmacology 232: 343–353. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Monnig MA, Lysne PA, et al. (2011) Adolescent substance abuse: The effects of alcohol and marijuana on neuropsychological performance. Alcohol Clin Exp Res 35: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Bridgen DT, Tao Q, et al. (2005) Δ9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther 314: 329. [DOI] [PubMed] [Google Scholar]
- Wadsworth EJK, Moss SC, Simpson SA, et al. (2006) Cannabis use, cognitive performance and mood in a sample of workers. J Psychopharmacol 20: 14–23. [DOI] [PubMed] [Google Scholar]
- Wędzony K and Chocyk A (2009) Cannabinoid CB1 receptors in rat medial prefrontal cortex are colocalized with calbindin but not parvalbumin- and calretinin-positive GABA-ergic neurons. Pharmacol Rep 61: 1000–1007. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Annas P, Edgar C, et al. (2010) Nabilone produces marked impairments to cognitive function and changes in subjective state in healthy volunteers. J Psychopharmacol 24: 1659–1669. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Brooke Livengood L, et al. (2004) Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend 76: 107–111. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Varvel SA, Harloe JP, et al. (2006) SR 141716 (Rimonabant) precipitates withdrawal in marijuana-dependent mice. Pharmacol Biochem Behav 85: 105–113. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Stoop N, Schetters D, et al. (2011) Cannabinoid CB1 receptor activation mediates the opposing effects of amphetamine on impulsive action and impulsive choice. PLoS One 6: e25856. [DOI] [PMC free article] [PubMed] [Google Scholar]


