Abstract
Bacillus subtilis is a soil bacterium that is competent for natural transformation. Genetically distinct B. subtilis swarms form a boundary upon encounter, resulting in killing of one of the strains. This process is mediated by a fast-evolving kin discrimination (KD) system consisting of cellular attack and defence mechanisms. Here, we show that these swarm antagonisms promote transformation-mediated horizontal gene transfer between strains of low relatedness. Gene transfer between interacting non-kin strains is largely unidirectional, from killed cells of the donor strain to surviving cells of the recipient strain. It is associated with activation of a stress response mediated by sigma factor SigW in the donor cells, and induction of competence in the recipient strain. More closely related strains, which in theory would experience more efficient recombination due to increased sequence homology, do not upregulate transformation upon encounter. This result indicates that social interactions can override mechanistic barriers to horizontal gene transfer. We hypothesize that KD-mediated competence in response to the encounter of distinct neighbouring strains could maximize the probability of efficient incorporation of novel alleles and genes that have proved to function in a genomically and ecologically similar context.
Subject terms: Bacterial genetics, Microbial ecology
Genetically distinct swarms of the bacterium Bacillus subtilis attack each other, forming a boundary upon encounter. Here, the authors show that these swarm antagonisms promote transformation-mediated horizontal gene transfer between strains of low relatedness.
Introduction
The spore-forming bacterium Bacillus subtilis is found in soil- and gut environments and is arguably the best-studied gram-positive model species1. B. subtilis swarms over surfaces and has diversified into a vast diversity of strains able to recognise non-kin swarms, resulting in the formation of clear swarm boundaries2. Kin discrimination (KD) in B. subtilis is mediated by a rich arsenal of intercellular attack and defence molecules with extensive variation in transcription levels upon encounter of non-kin3. KD genes are present in unique combinations in different strains and likely frequently acquired through horizontal gene transfer3. The combinatorial nature of the B. subtilis KD system means that genomically divergent strains generally also differ to a greater degree in their carriage of antimicrobial genes. As a result, swarm boundaries between unrelated strains (i.e. non-kin) are very distinct, whereas genomically highly similar strains (i.e. kin) exhibit swarm merging2.
KD-mediated barriers to swarm-merging result in the territorial sorting of strains according to genetic relatedness during the colonisation of plant roots2. However, it is not well-understood whether interference competition is the prime selective force underlying the radiation into many KD types, or whether this mechanism could have other functions. Another explanation for bacterial KD is that it could facilitate horizontal gene transfer between unrelated strains4. Recognition and lysis of neighbouring genotypes via the release of effectors by the T6SS secretion system coupled to natural transformation have been demonstrated in the gram-negative species Vibrio cholerae5 and Acinetobacter baylyi6,7. In the gram-positive species Streptococcus pneumoniae, bacteriocin release can result in lysis of neighbouring susceptible genotypes and likewise increase transformation-mediated horizontal gene transfer8,9.
B. subtilis is naturally competent10,11 but transformation has mostly been studied in the context of single clones growing in liquid medium in this species (but see refs. 12–14), precluding the action of social interactions such as swarming found in structured environments. We hypothesised that the antagonisms observed between genetically distinct B. subtilis strains could lead to transformation-mediated recombination.
Here, we show that swarm boundary interactions between non-kin B. subtilis strains result in horizontal gene transfer mediated by the cell-envelope stress-response (sigW) in the donor strain, whereas interactions between more closely related kin strains that do not form boundaries do not result in increased recombination. Our results demonstrate that competence regulation mediated by social interactions can be more important than sequence homology for successful recombination.
Results
Transformation rates are elevated at swarm boundaries between non-kin strains
To test whether transformation-mediated gene transfer occurs in swarm boundaries between different B. subtilis strains, we first tested for swarm boundary formation between all pairwise combinations of six B. subtilis strains isolated from two 1-cm3 soil samples15 and calculated Average Nucleotide Identity (ANI16), which is defined as the mean nucleotide identity of orthologous gene pairs shared between two microbial genomes17 (Table 1). Strain merging was dependent on genetic relatedness: highly related kin strains (99.93–99.99% ANI) as well as differentially marked isogenic strains (‘self’ pairings) merged during swarming (merging phenotype), whereas non-kin strains (98.73–98.84% ANI) did not merge and formed clear boundaries (Fig. 1). To test whether transformation-mediated gene transfer occurs more frequently in swarm pairs that form boundaries compared to swarm pairs that merge, differentially marked (Spectinomycin (SpR) and Chloramphenicol (CmR) pairs of B. subtilis strains (Table 2) were allowed to swarm on an agar surface, after which the swarm meeting area was sampled in order to quantify transformation frequency (the proportion of cells carrying both antibiotic markers as a consequence of transformation-mediated horizontal gene transfer) (Fig. 1a). Transformation frequencies were significantly higher for non-kin strain pairs compared to kin strain pairs or the isogenic controls (two tailed t-test; p < 0.05) (Fig. 1b and Supplementary Fig. 1a, b). Experiments using strains with inverse marker combinations yielded statistically identical results (Supplementary Figs. 1–2 and Supplementary Notes 1). Mixing of differentially marked strains (1:1) in shaken co-cultures did not result in increased transformation of non-kin DNA (Supplementary Fig. 1c, d), indicating that surface-based cell contact is required for KD-mediated transformation.
Table 1.
Average Nucleotide Identity (ANI) of strain pairs.
| PS-13 | ||||||
| PS-13 | 1.0000 | PS-18 | – | – | – | – |
| PS-18 | 0.9999 | 1.0000 | PS-216 | – | – | – |
| PS-216 | 0.9997 | 0.9994 | 1.0000 | PS-68 | – | – |
| PS-68 | 0.9996 | 0.9993 | 0.9995 | 1.0000 | PS-196 | – |
| PS-196 | 0.9882 | 0.9881 | 0.9883 | 0.9884 | 1.0000 | PS-216 |
| PS-218 | 0.9873 | 0.9875 | 0.9875 | 0.9875 | 0.9877 | 1-0000 |
Pairwise Average Nucleotide Identity (ANI) is shown for B. subtilis strain pairs.
Fig. 1. Transformation frequency as a function of genomic relatedness between strains.
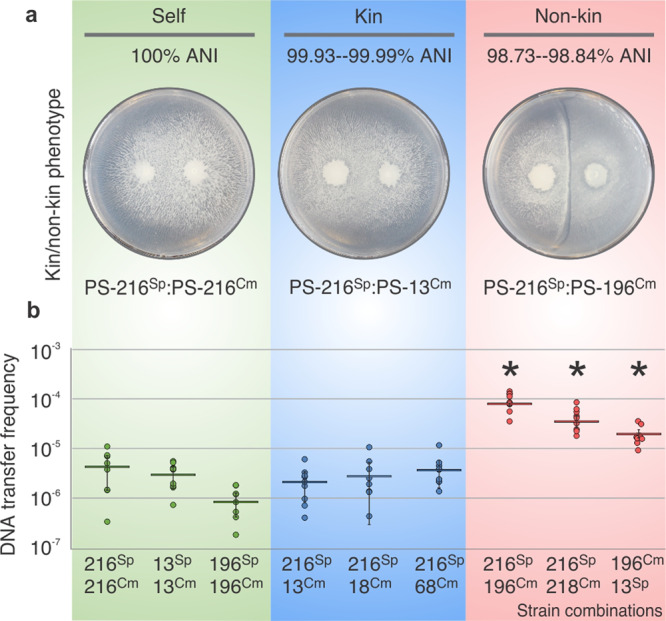
a Representative interactions of control self-interaction (green), kin interaction (blue) and non-kin interaction (red) (ANI indicated). b Transformation frequency as quantified by the frequency of double mutants isolated from the swarm boundary (n = 3 biologically independent experiments, each n performed in three technical replicates (9 data points), error bars represent SD). Asterisks represent statistically significant values compared to corresponding self and kin pairings (two tailed Student’s t-test for unpaired data assuming equal variances), (Supplementary Notes and Supplementary Fig. 2). Data are presented as mean values ± SD and error bars represent SD of the mean values (n = 3). For strain abbreviations see Table 2.
Table 2.
Strain abbreviations.
| Strain name | Strain genotype | Strain abbreviation |
|---|---|---|
| PS-216 |
Wt p43-cfp (Sp) p43-yfp (Cm) |
216 216Sp 216Cm |
| PS-18 |
Wt p43-cfp (Sp) p43-yfp (Cm) |
18 18Sp 18Cm |
| PS-68 |
Wt p43-cfp (Sp) p43-yfp (Cm) |
68 68Sp 68Cm |
| PS-13 |
Wt p43-cfp (Sp) p43-yfp (Cm) |
13 13Sp 13Cm |
| PS-196 |
Wt p43-cfp (Sp) p43-yfp (Cm) |
196 196Sp 196Cm |
| PS-218 |
Wt p43-cfp (Sp) p43-yfp (Cm) |
218 218Sp 218Cm |
Transformation is largely unidirectional, with one swarm principally acting as a recipient and the other as a donor
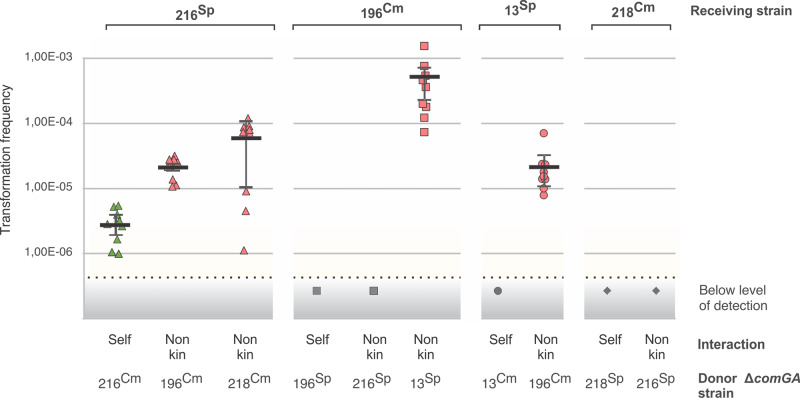
The previous experiment (Fig. 1) was based on the detection of double recombinants, which in theory could arise from successful DNA uptake and recombination by both strains or by only one strain. To test the directionality of DNA transfer, we constructed comGA mutants with impaired DNA uptake efficiency18,19. Four wild-type strains were paired both with a comGA self- control and with a non-kin comGA mutant and double transformants at the swarm boundary between strains were enumerated. The transformation frequencies were below the level of detection for some wild-type strains (zero transformants were obtained), especially in self and kin setting, but transformation frequency generally increased above the detection limit in non-kin pairings (Fig. 2). When PS-216 was paired with a competence defective PS-196 ΔcomGA, its transformation frequency increased significantly (~7-fold) relative to the isogenic control (two tailed t-test, p = 0.0002). PS-216 also showed an increased transformation frequency when paired with PS-218 ΔcomGA, however not significantly (two tailed t-test, p = 0.1161). Similarly, PS-196 and PS-13 displayed increased transformation frequencies when paired with the PS-13 and PS-196 ΔcomGA strains, respectively, even though the transformation frequencies of self- controls were below the detection limit (Fig. 2). In the reversed examples above, where the PS-196 and the PS-218 strains were paired with PS-216 ΔcomGA, no recombination could be detected in the recipient strains PS-196 and PS-218, respectively. No transformants could be detected when pairing two differentially marked ∆comGA mutants (data not shown), confirming that horizontal gene transfer during swarming is mediated by the activation of competence. This experiment demonstrates that gene flow is primarily unidirectional.
Fig. 2. Transformation frequencies of wt B. subtilis DNA receiving strains paired with donor ΔcomGA mutants.
Transformation frequency was measured as the proportion of double transformants at swarm boundaries. Self-interactions are shown in green, non-kin interactions in red and transformation frequencies below level of detection in grey colour. Data are presented as mean values ± SD and error bars represent SD of the mean values (n = 3 biologically independent experiments, each n performed in three technical replicates (9 data points). Strains are depicted by different shapes: PS-216 triangle, PS-196 square, PS-13 circle and PS-218 a diamond. For strain abbreviations see Table 2 and for strain combinations see Supplementary Table 3.
Swarm boundaries are a site of asymmetric cell lysis
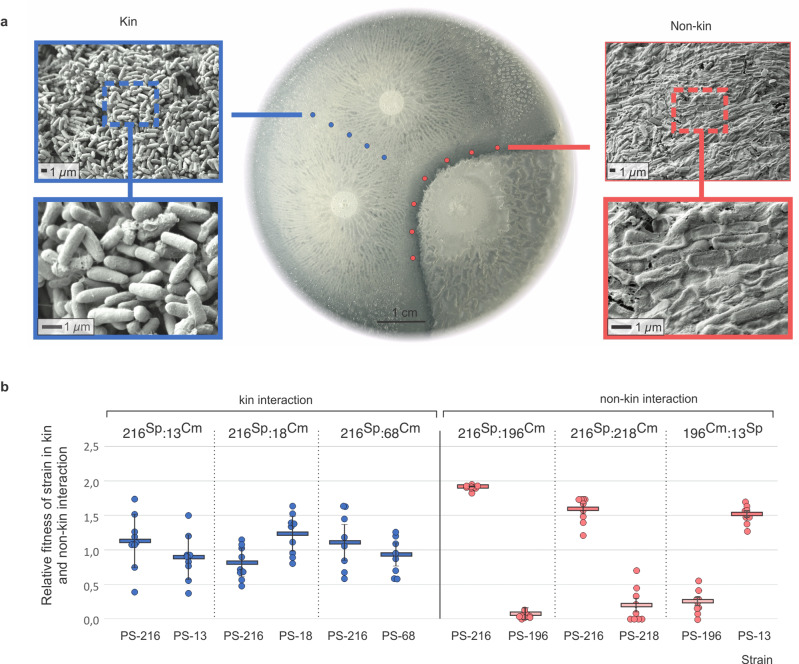
Scanning electron microscopy revealed that areas where kin strains merged contained healthy intact cells, whereas swarm boundaries between non-kin strains showed deflated and empty cells, pointing to cell lysis (Fig. 3a). To test whether cell lysis occurred predominantly in the strain serving as the DNA donor for transformation (Fig. 2), we first compared the fitness of strains (i.e. cell survival–CFUstrainA/CFUstrainA+B) during non-kin vs self-interaction and the fitness of strains in kin vs self- interaction. Fitness of strains in kin setting was not significantly different from the fitness of self-paired strains (isogenic marked mutants) (PS-216:PS-13, p216 = 0.58, p13 = 0.55; PS-216:PS-18, p216 = 0.92, p18 = 0.94; PS-216:PS-68, p216 = 0.95, p68 = 0.53). However, in non-kin strain pairs, fitness of interacting strains was significantly different from that during self-interaction (PS-216:PS-196, p216 = 0.01, p196 = 0.01; PS-216:PS-218, p216 = 0.003, p218 = 0.03; PS-13:PS-196, p13 = 0.004, p196 = 0.005).
Fig. 3. Kin discrimination mediates fitness via cell lysis.
a Swarm assay on B agar. The left and right panels are scanning electron microscopy micrographs taken at the meeting point of kin strains PS-216Sp and PS-13Cm (blue, left) and non-kin strains PS-216Sp and PS-196Cm (red, right). b Relative fitness of strains in kin pairings (blue) and non-kin pairings (red) (n = 3 biologically independent experiments, each n performed in three technical replicates (9 data points)). See Table 2 for strain abbreviations. All experiments (in a and b) were performed in three biologically independent experiments. Data are presented as mean values ±SD and error bars represent SD of the mean values (n = 3).
Furthermore, we compared the relative fitness of three non-kin strain pairs at the swarm interface to the relative fitness of each strain staged with a kin strain (Fig. 3b). For example, relative fitness of PS-216 in non-kin setting was determined by calculating fitness of strain PS-216Sp when paired with a non-kin PS-196Cm strain and divided by the fitness of the PS-216Sp strain when paired with a differentially marked version of itself (PS-216Cm), see Eq. (1). Similarly, relative fitness of strain PS-216Sp in a kin setting was calculated as fitness of the PS-216Sp strain when paired with PS-13Cm and divided by the fitness of the PS-216Sp when paired with differentially marked self-strain PS-216Cm. We observed an increase in the relative fitness of one strain and a decrease of relative fitness in the other strain in all three non-kin interactions (Fig. 3b). This is consistent with the scanning electron microscope observations (Fig. 3a) and suggests lysis of one strain by the other strain in non-kin encounters: the PS-216 strain kills the PS-196 and PS-218 strain, and PS-13 kills the PS-196 strain.
DNA release through cell lysis is not required for efficient transformation
As transformation is dependent on the presence of extracellular DNA, we next sought to test whether transformation in swarm boundaries is facilitated because cell lysis increases DNA availability. We first confirmed that extracellular DNA is a prerequisite for DNA uptake by measuring transformation on agar plates supplemented with DNases which degrade free DNA (Supplementary Fig. 3a). Cells were harvested from swarm boundaries sections of the agar surface that were either treated with DNaseI or were left untreated in order to quantify double recombinants (Supplementary Fig. 3a). Numerous transformants were obtained from non-DNase-treated agar sections, but no transformants could be recovered from the DNase treated portion of the agar plate, confirming that extracellular DNA is a prerequisite for the observed horizontal gene transfer events (Supplementary Fig. 3b).
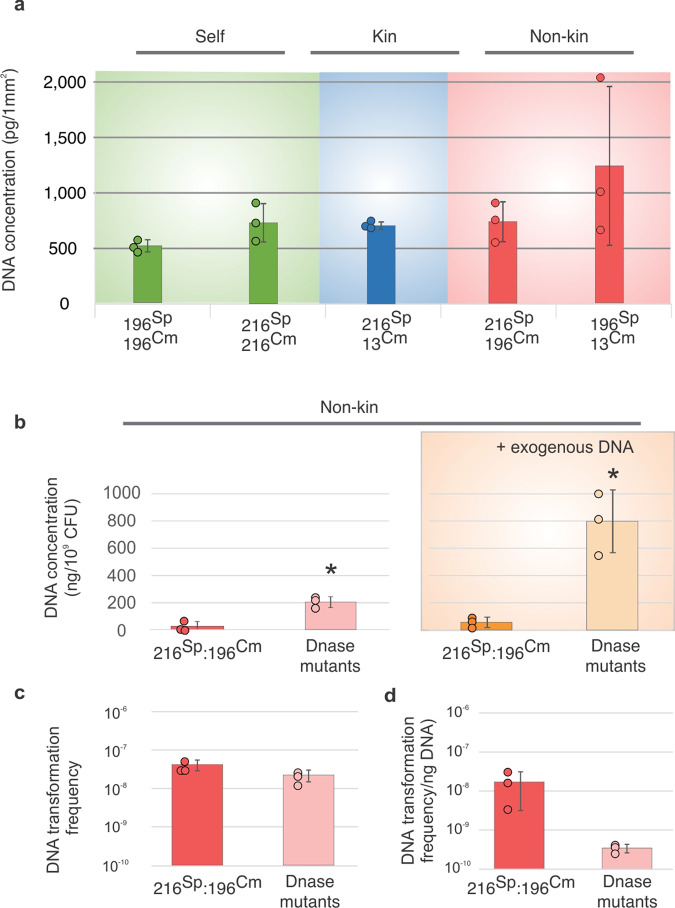
Next, we tested whether cell lysis might increase extracellular DNA concentrations at the boundary and promote DNA transfer with non-kin. Surprisingly, extracellular DNA concentrations measured at the boundary between non-kin strains were comparable to those between staged kin- or isogenic strains (Fig. 4a and Supplementary Fig. 4). To test for the possibility that extracellular DNA is degraded by DNases to the extent markers cannot be successfully taken up and recombined, we sampled the swarm meeting area and extracted DNA from the agar samples following a spectrophotometric DNA quantification (Supplementary Notes 2). We measured DNA concentrations at the boundary of two wt strains (PS-216 and PS-196) and compared it to the DNA concentration measured between two nuclease mutant strains (PS-216Sp ΔyhcR, ΔnucB and PS-196Cm ΔyhcR; see Supplementary Table 3). Extracellular DNA concentrations were significantly higher at the boundary between non-kin nuclease mutant strains compared to wt strains (two tailed t-test, p = 0.004). The activity of extracellular nucleases was furthermore demonstrated in another experiment where 30 µg of DNA extracted from PS-216 ΔepsA-O (TetR) was added in the middle of agar plates inoculated with non-kin strains. Significantly lower DNA concentrations were measured on the boundary between two wt non-kin strains, whereas the non-kin nuclease mutants showed elevated DNA concentrations at the boundary (p+DNA = 0.005) (Fig. 4b and Supplementary Fig. 4). However, elevated concentrations of extracellular DNA did not significantly affect transformation frequency between two non-kin nuclease mutant strains. In fact, transformation frequency between nuclease mutants was not significantly different from corresponding wild-type strains in the presence of added DNA (two tailed t-test, p = 0.093) (Fig. 4c and Supplementary Fig. 4b). These combined results indicate that the concentrations of extracellular DNA in swarms are saturating (i.e. not limiting transformation) and that cell lysis in swarm boundaries is not a prerequisite for efficient recombination (Fig. 4 and Supplementary Notes 2).
Fig. 4. Transformation frequency at swarm boundaries is largely unaffected by DNA concentration.
a DNA concentrations at the meeting points of self-controls (green), kin (blue) and non-kin strains (red), b DNA concentration at the swarm boundary between non-kin wt PS-216Sp and PS-196Cm (red) and between nuclease mutants (DNase mutants) PS-216Sp ΔyhcR ΔnucB and PS-196Cm ΔyhcR without exogenously added DNA (pink) (left panel) and with 30 µg DNA added at the swarm boundary (right panel, wt orange, DNase mutants yellow), asterisks represent statistically significant values compared to corresponding wt pairings (two tailed Student’s t-test for unpaired data assuming equal variances), c transformation frequency at the boundary of two non-kin wt (PS-216Sp and PS-196Cm) (red) and between two nuclease mutant strains (PS-216Sp ΔyhcR ΔnucB and PS-196Cm ΔyhcR) (pink), d transformation frequency at the boundary of two non-kin wt strains (PS-216Sp and PS-196 m) (red) and between two nuclease mutants strains (PS-216Sp ΔyhcR ΔnucB and PS-196Cm ΔyhcR) (pink) calculated per ng available DNA. Assays were replicated three times. See Table 2 for strain abbreviations. Data are presented as mean values ±SD and error bars represent SD of the mean values (n = 3). For statistical parameters see Supplementary Notes.
Increased transformation rate in swarm boundaries is a consequence of cell envelope stress-induced upregulation of competence
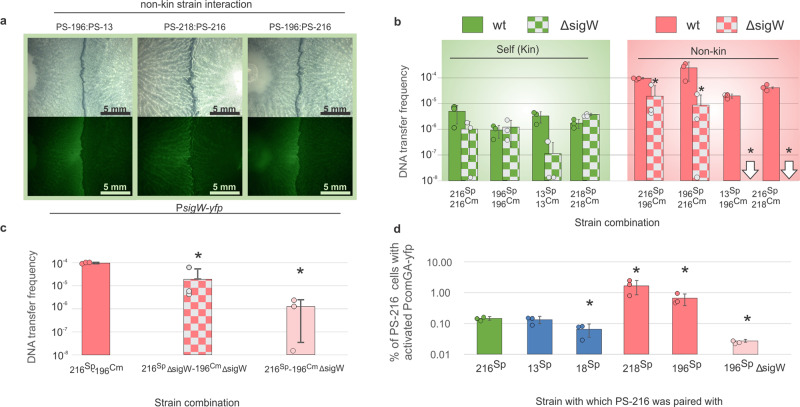
Stress and the release of antimicrobials have been shown to induce competence in a variety of bacterial species20–23. In B. subtilis, the sigW (σW) gene is expressed in response to cell envelope stress24 and was previously shown to be upregulated at the non-kin swarm boundary3. To examine the connection between stress and competence, we visualised the expression of PsigW-yfp (σW) and measured transformation rates in ∆sigW (σW) mutants, both in non-kin swarm boundaries and in merging areas between kin strains. First, we tagged strains PS-13, PS-196, PS-216 and PS-218 with PsigW-yfp and observed an increased YFP signal in non-kin swarm boundaries, whereas kin and self-interactions showed no PsigW-yfp induction (Supplementary Fig. 5). sigW was predominantly induced in the strain that was antagonised (Fig. 5a and Supplementary Fig. 5), corresponding with the results of the pairwise fitness assays (Fig. 3b).
Fig. 5. Cell envelope stress and competence induction in swarm boundaries.
a Meeting points of non-kin sigW-yfp swarms. b The effect of stress response inactivation (∆sigW) on transformation rate. Green depicts control self-combinations and red depicts non-kin combinations; full columns and chequered columns represent wt controls and ∆sigW mutants respectively. Asterisks represent statistically significant values (two tailed Student’s t-test for unpaired data assuming equal variances, p216-196 = 0.01, p196-216 = 0.05). c The effect of sigW inactivation in a non-kin donor strain on transformation frequency in recipient strain PS-216. Red column = PS-216 and PS-196 wt control donor, chequered column = PS-216 ∆sigW and PS-196 ∆sigW donor, pink column = PS-216 and PS-196 ∆sigW donor. Asterisks represent statistically significant values (two tailed Student’s t-test for unpaired data assuming equal variances, pWT-216ΔsigW:196ΔsigW = 0.01; pWT-216:196ΔsigW = 0.00002). d Activation of competence genes in strain PS-216 comGA-yfp at the meeting point with self (PS-216Sp)(green), kin strains (PS-13, PS-18) (blue) and non-kin strains (PS-196, PS-218 (red) and ∆sigW PS-196 (pink)). Bars represent fractions of PS-216 cells expressing the comGA gene at swarm meeting points. Data are presented as mean values ± SD and error bars represent SD of the mean values. Assays were replicated three times. Asterisks represent statistically significant values (two tailed Student’s t-test for unpaired data assuming equal variances, p216-18 = 0.02, p216-218 = 0.03, p216-196 = 0.03, p216-196ΔsigW = 0.0005, p196-196ΔsigW = 0.02, see Supplementary Notes). See Table 2 for strain abbreviations and for strain combinations see Supplementary Table 3.
Since stress could induce competence, we next tested whether an inability to respond to stress (∆sigW) prevents the induction of competence and lowers transformation frequency. Indeed, when non-kin PS-216Sp∆sigW and PS-196Cm∆sigW were staged, transformation rates decreased significantly compared to the wt control pairing (two tailed t-tests, p = 0.01). The decreased transformation rates of ∆sigW mutants were statistically indistinguishable to transformation frequencies between wt self- strain pairs (Fig. 5b) (Supplementary Notes 3). Similarly, when we compared two other non-kin ∆sigW strain pairs (PS-13:PS-196 and PS-216:PS-218), transformation frequencies decreased dramatically compared to wt strains as no transformants were detected in non-kin ∆sigW strain pairs (Fig. 5b). No significant changes occurred to the rate of transformation in isogenic combinations, apart from PS-13, where ∆sigW strains pairs showed reduced transformation (Fig. 5b and see Supplementary Notes 3).
How can the fact that the dominant strain acts as the recipient of DNA (Fig. 2) be reconciled with the finding that DNA uptake and recombination is associated with a stress response (sigW expression) which can be expected to be most prominent in the lysed (not the lysing) strain (Fig. 2)? We hypothesised that the sigW expression in the lysed strain induces competence in the lysing strain. To test this, we staged the wt PS-216 strain with a PS-196 ∆sigW mutant (the latter strain undergoes lysis in this pairing), (Fig. 3b) and measured transformation rates). Consistent with our hypothesis, wt PS-216 transformation frequency was significantly lower in this strain combination compared to its transformation frequency when staged with the PS-196 wt strain (p = 0.00002) (Fig. 5b). Transformation frequency of PS-216 paired with PS-196 ∆sigW mutant was statistically indistinguishable to transformation frequency between strains both carrying the ∆sigW mutation (p = 0.288) (Fig. 5c). This indicates that sigW induction in the lysed strain is the cause of increased transformation rate in the strain causing lysis.
To verify whether higher transformation frequencies during non-kin encounters are due to the upregulation of competence, we tagged the central competence gene comGA in strain PS-216 with a YFP reporter and paired this strain with: (i) a wt version of itself (PS-216), (ii) two wt kin strains (PS-13 and PS-18) and (iii) two wt non-kin strain (PS-196 and PS-218) (Fig. 5d). Significant upregulation of comGA in PS-216 was observed at the boundary with non-kin strains, compared to comGA-yfp activity of self (two tailed t-test, PS-216:PS-196, p = 0.031; PS-216:PS-218, p = 0.032). comGA expression of PS-216 in kin and self- encounters was either statistically the same (two tailed t-test, PS-216:PS-13, p = 0.598) or even lower (two tailed t-test, PS-216:PS-18, p = 0.017) (Fig. 5d). To further test the link between stress response and competence upregulation, we paired the PS-216 strain carrying the comGA-yfp with a PS-196 ∆sigW and observed down regulation of comGA (Fig. 5d). These experiments demonstrate that in non-kin swarm boundaries competence in the recipient strain is induced by cell envelope stress in the antagonised donor strain.
KD-mediated transformation has the potential to speed up adaptation
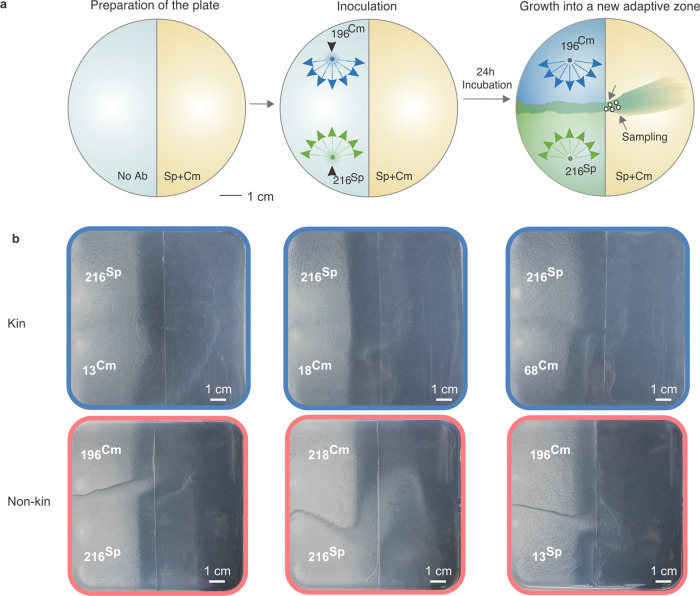
The evolutionary benefits of competence and transformation are still the subject of active debate and several, non-mutually exclusive benefits have been proposed including nutrient acquisition, repair and sex-like benefits25,26. As the uptake and recombination of extracellular DNA is an integral part of transformation, it is often assumed that recombination-based benefits are at least partly responsible for its evolutionary maintenance25. A proof-of-principle experiment was devised to test whether KD-mediated transformation could aid adaptation through the uptake of beneficial alleles originating from the encounter with a non-kin swarm. Agar plates were prepared such that only one half contained Sp and Cm antibiotics (Fig. 6a). Pairs of either kin- or non-kin strains differentially marked with Sp and Cm were inoculated on the half of the agar plate unamended with antibiotic and allowed to swarm (Fig. 6a). Staged kin strain pairs mainly stayed on the half of the plate unamended with antibiotics, whereas non-kin combinations were able to invade the antibiotic-amended portion in the plate (Fig. 6b and Supplementary Fig. 6). In all, 100% and 98% of cells sampled from two non-kin pairing-derived invading swarms were resistant to both antibiotics and carried double fluorescence markers CFP and YFP (Supplementary Fig. 7 and Supplementary Notes 4). These findings confirm that invading lineages were the result of transformation-mediated recombination events between non-kin strains. A control experiment where paired non-kin strains did not carry Sp and Cm resistance genes, did not lead to swarm invasions (Supplementary Fig. 8).
Fig. 6. Transformation at swarm boundaries can result in recombinants with novel adaptive capabilities.
a Experimental design. Samples were taken from the swarm that spread onto the part of plate containing both antibiotics (Ab): Chloramphenicol (Cm) and Spectinomycin (Sp) (marked with arrows and with circles). b Meeting points of kin (blue) and non-kin (red) strain pairs after 24 h of incubation on 12 cm B media agar plates. See Table 2 for strain abbreviations.
Discussion
Here we demonstrate that kin discrimination promotes horizontal gene transfer in B. subtilis through upregulation of competence in response to cell envelope stress. B. subtilis antimicrobial genes (sunA, sdpC, sboA, yobL, skf, wapA, srfA) have been shown to be upregulated at the non-kin boundary3. It is possible that antibiotics that affect cell-envelope integrity induce sigW at the boundary which, together with competence development, increases tolerance to antimicrobials and augment the more competent strain27. It could also be speculated that sigW activated cells with damaged membranes release stress-related metabolites that upregulate competence in the dominant strain as was documented during inter-species transformation experiments where lysis of E. coli cells and subsequent release of cell metabolites affected the transformation ability of B. subtilis recipient cells28. Similarly, it was recently shown that exposure to antibiotics increases cell-to-cell natural transformation in B. subtilis by affecting the donor strain through as yet unknown mechanism29.
Induction of competence in B. subtilis requires activation of a quorum sensing (QS) system, encoded by the comQXPA operon30,31, that ensures activation of the competence genes via the induction of ComS32,33. We and others have shown that B. subtilis genotypes evolved extensive polymorphism in the ComQXPA QS system15,34–36. In liquid competence media, competence can only be induced if two strains express the same pheromone (i.e. both strains belong to the same pherotype)15,34,37. Limited cross-talk and sometimes even inhibition of competence has been observed between different pherotypes34, leading to the expectation that transformation between pherotypes is lower than within pherotypes. In contrast, the non-kin strains that engage in transformation in this study all belong to different pherotypes2,15, for example, PS-216 and other kin strains belong to “pherotype 168”, whereas PS-218 and PS-196 belong to “NAF4 pherotype”15, yet still show higher transformation efficiency when paired than do more closely related kin strain pairs belonging to the same pherotype. This discrepancy with earlier studies could potentially result from the fact that different mechanisms could be important governing HGT in structured (agar) environments versus unstructured (broth) environments.
Interestingly, we found the rate of horizontal gene transfer to increase with genomic divergence. This result seems counterintuitive at first, as the efficiency of transformation-mediated recombination of more divergent DNA fragments has been shown to decrease log-linearly with increasing sequence dissimilarity in Bacillus38–40. Social interactions leading to competence development thus can play a more significant role in the efficiency of uptake of foreign DNA than constraints imposed by the recombination machinery in this species. This highlights the importance of considering social interactions in the study of bacteria generally, and in the study of horizontal gene transfer specifically. A variety of (non-mutually exclusive) explanations for the evolutionary benefits of natural transformation have been put forward, one of which is that increased genetic variation through recombination with foreign DNA facilitates adaptation25,26 or that it can aid curing the genome of selfish deleterious elements41. The observed increase in recombination between genomically more distant strains is not predicted by the DNA for food hypothesis and poses a problem for the DNA repair hypothesis but would be consistent with the sex hypothesis. The coupling of competence to strain-specific killing could ensure that recombination is not upregulated in (near) clonal swarms (which would not introduce any genetic variation) nor upon encounter of dissimilar species (which would unlikely result in successful recombination). Instead, lysis of distinct but closely related strains occupying the same patch maximises the probability of efficient incorporation of novel alleles and genes that have proved to function in a similar genomic and ecological context42. Such non-kin interactions are common in natural populations of B. subtilis: 84% of strain combinations isolated from two microscale soil aggregates were shown to be non-kin2. Evolution experiments incorporating ecological realism are needed to shed light on the adaptive benefits of KD-mediated antagonism and transformation.
Methods
Strains and media
Strains used in this study and their mutant derivatives are presented in Supplementary Table 3. Strain combinations used in experiments are shown in Supplementary Table 3. Briefly, 6 Bacillus subtilis wild-type strains isolated from sandy bank of the Sava River in Slovenia11 were used (Table 2). For strains carrying antibiotic markers appropriate antibiotics at the following concentrations were used: 5 μg/ml of Chloramphenicol (Cm), 100 μg/ml of Spectinomycin (Sp), 20 µg/ml of Tetracycline (Tet), 25 µg/ml of Kanamycin (Kn) and 20 μg/ml of Erythromycin (Ery). Overnight cultures were prepared in liquid LB medium and swarming assays were performed on swarming agar (final agar concentration 0.7%), which was based on B-medium composed of 15 mM (NH4)2SO4, 8 mM MgSO4 × 7H2O, 27 mM KCl, 7 mM sodium citrate × 2H2O, 50 mM Tris-HCl, pH 7.5; 2 mM CaCl2 × 2H2O, 1 μM FeSO4 × 7H2O, 10 μM MnSO4 × 4H2O, 0.6 mM KH2PO4, 4.5 mM sodium glutamate, 0.86 mM lysine, 0.78 mM tryptophan and 0.2% glucose.
Strain construction
For fluorescence visualisation experiments wild-type B. subtilis strains were tagged with a yfp or a cfp gene linked to a constitutive promoter (p43), inserted at the sacA or amyE locus, and for observation of competence induction a yfp gene was linked to the comGA promoter. P43-yfp construct from Pkm3-p43-yfp plasmid2 was digested with EcoRI and BamHI and ligated into the ECE17443 plasmid yielding the pEM1071 plasmid carrying a p43-yfp fusion inside the sacA integration site. For construction of p43-cfp fusion PkM8 plasmid was used, carrying the cfp gene linked to spoIIQ region44. spoIIQ region from the original plasmid was removed by EcoRI and HindIII digestion and replaced by p43 promoter sequence, which was amplified with primers p43-F1-EcoRI and p43-R1-HindIII2 (Supplementary table 2) and was ligated into the PkM8 EcoRI and HindIII sites yielding the Pkm8-p43-cfp pEM1069 plasmid.
Strains carrying PcomGA-yfp (Cm) and knockouts in ∆nucB (kn), ∆yhcR (Ery), ∆comGA (Ery), ∆epsA-O (Tet) and ∆comQXP (Kn), sigW-yfp (Ery) and ∆sigW were obtained by transforming the wt B. subtilis strains with DNA isolated from B. subtilis 11A79 (PcomGA-yfp)44, BD2121 (ΔcomK)45, BKK25750 (ΔnucB)46, BKE09190 (ΔyhcR)46, BKK24730 (ΔcomGA, kn)46, BKE24730 (ΔcomGA)46, ZK4300 (ΔepsA-O)3, plasmid pED302 (ΔcomQXP)37, NL362 (∆sigW)3 and ZK4860 (sigW-yfp)3 (Supplementary Table 3). The DNA (plasmid or genomic) was introduced to the B. subtilis using standard transformation protocol.
Harvesting spores
For swarming assay spores were inoculated on semi solid media and spore crops were prepared by inoculating an overnight B. subtilis culture from LB medium to 2 x SG sporulation medium47. Cultures were incubated with shaking (200 rpm) at 37 °C for 3 days, after which spores were washed twice with saline solution and stored in glycerol at −20° degrees.
Frequency of DNA exchange between B. subtilis strains in liquid CM media
To determine the frequency of DNA exchange between kin or non-kin B. subtilis strains, strain pairs were inoculated into a liquid competence medium (CM). CM medium was inoculated with spores (final concentration 1%), where spores of each strain represented half of the inoculum. Inoculated media was shaken (200 rpm) at 37 °C and was sampled after 8 h. Using appropriate antibiotic selection, CFUs of each strain, as well as the number of cells that have taken up each other’s DNA, were determined. DNA exchange frequency was calculated as the number of total CFU/ml of transformants (Cm + Sp) divided by total CFU count (sum of CFUs of both strains (Cm and Sp) minus the number of transformants (Cm + Sp)). The frequency of DNA exchange in liquid media was performed in three biologically independent experiments each in three technical replicates for every strain combination.
Swarm boundary assay
Swarming assays were performed as previously described2, with a few minor adaptations. B. subtilis spores were diluted 1:1 with LB media and 1–2 µl was inoculated in parallel on semisolid 0.7% agar B media. Pairs of inoculated kin or non-kin strains were labelled with different antibiotic resistance and fluorescence marker. When spores were unavailable, overnight cultures, prepared from the freezing stocks (−80°) were transferred to fresh liquid LB media (1% inoculum) and incubated for 2 h with shaking (200 rpm) at 37 °C. After 2 h incubation, the culture was again transferred to fresh liquid LB media (1% inoculum) and incubated with shaking (200 rpm) at 37 °C for additional 2 h. After the second incubation the exponential phase cell suspension was used for B media inoculation (for experimental scheme see Supplementary Fig. 9). Samples were taken in the same manner for CFU or DNA quantification). Inoculated agar plates were incubated overnight (22–24 h) at 37 °C and 80% humidity. If not stated otherwise, experiments were performed in three biological replicates and each replicate was sampled in three technical replicates.
Frequency of DNA exchange between swarms of B. subtilis strains on semisolid B media
To determine the frequency of DNA exchange between kin or non-kin B. subtilis swarms, pairs of strains were inoculated parallel to each other on semisolid B medium and incubated overnight as described above. Ten samples (per plate) were taken at the swarm meeting point with cut pipette tips and resuspended in 500 µl 0.9% NaCl solution. Using appropriate antibiotic selection, CFUs of each approaching swarms at the meeting point was determined as well as the number of cells that have taken up each other’s DNA. DNA exchange frequency at the border was calculated as the number of total CFU/ml of transformants (Cm + Sp) divided by total CFU count (sum of CFUs of both strains (Cm and Sp) minus the number of transformants (Cm + Sp)). Experiment was performed in three biological replicates and each replicate was sampled in three technical replicates.
DNA exchange in the presence/absence of DNaseI
In order to quantify DNA exchange in the presence of DNaseI, 20 µl of DNaseI (5 mg/ml) was spread on one half of the semisolid B media (concentration on the surface of media was 50 µg/ml) (Supplementary Fig. 3). B. subtilis strains were inoculated parallel to each other and incubated overnight for swarms to spread as described above. Sampling was performed at the swarm meeting point of non-kin strains in the area containing DNaseI and in the area without DNaseI present (Supplementary Fig. 3). Ten samples were picked from each area (with/without DNaseI) using cut pipette tips and resuspended in 500 µl 0.9% NaCl solution. CFUs were determined and DNA exchange was calculated as described above. Experiment was performed in three biological replicates.
comGA gene expression
The comGA-yfp gene activation was observed at meeting points of kin and non-kin swarms. Pairs of kin and non-kin strains were inoculated parallel to each other on semisolid B media and incubated overnight as described above. Samples were taken at the meeting points of two swarms with an inoculation loop, resuspended in 200 µl of 0.9% NaCl solution and mixed with a vortex mixer. In total, 20 µl of each sample was pipetted onto a glass slide coated with a solution of poly-l-lysine (0.1% (w/v). Before microscopic observation, slides were dried and antifade reagent SlowFade (Invitrogen, ZDA) was added. Zeiss Axio Observer Z1 with DIC technique (differential interference contrast) and fluorescence technique with fluorescence filter (Zeiss, Göttingen, Nemčija) was used for observing fluorescent protein yfp (Ex 430 nm/Em 474 nm). For every technical replicate 20 fields of view were analysed and photographed totalling to ~30,000 cells per studied interaction (self, kin, non-kin and ΔsigW). Pictures were analysed with Fiji (1.51d). Overall number of cells (DIC technique) and number of cells expressing comGA-yfp (yellow glowing cells under microscope with fluorescence filter for observing yfp) were determined. The ratio of cells expressing comGA-yfp was calculated. Experiment was performed in three biological replicates and each replicate was observed under microscope in two technical replicates as described above.
DNA quantification
DNA concentration was determined in individual B. subtilis swarms and at swarm meeting points of kin or non-kin strains. Pairs of strains were inoculated parallel to each other on semisolid B media and incubated overnight as described above. Twenty samples were taken at the swarm meeting points and within each swarm with cut pipette tips (Supplementary Fig. 4). Samples were first resuspended in 800 µl Gel Solubilization Buffer and lightly mixed. Samples were heated for 20 min at 50 °C for the agar to melt. Next, they were centrifuged at 10,000 × g for 5 min and filtered through a 0.2-μm pore Millipore filter (Merck, KGaA, Germany) previously wetted with 1 ml of 0.9% NaCl solution, to remove the cells. DNA was isolated using PureLink® Quick Gel Extraction Kit (Thermo Scientific, ZDA). Further DNA isolation process was performed as specified in PureLink® Quick Gel Extraction Kit manual. DNA was eluted with 330 µl 1x TE buffer and 100 µl of DNA samples were pipetted into a 96-well microtiter plate where DNA concentration was determined using QuantiFluor® dsDNA System (Promega Corporation, ZDA) according to the manual. The DNA concentration in the agar was calculated using calibration curve obtained from DNA isolation of Lambda DNA standard (QuantiFluor® dsDNA System) added to the agar plate in concentrations: 0 pg/µl, 10 pg/µl, 50 pg/µl and 100 pg/µl. Experiment was performed in three biological replicates and the DNA was measured in three technical replicates.
DNA quantification and DNA exchange in the presence of exogenous DNA
To test whether DNA at the meeting point gets digested by B. subtilis nucleases exported to the media we measured DNA concentration in plates with/without added DNA at the centre of the plate (Supplementary Fig. 5) where PS-216 (amy:p43-cfp (Sp) and PS-196 (sacA:p43-yfp (Cm) swarms met. Approximately 30 µg of DNA (PS-216 ΔepsA-O (Tet)) was added to a 1-cm-wide stripe in the middle of the B medium agar plates, where the strains usually meet and 20 samples from the meeting point on B medium plate with/without added DNA were resuspended in 700 µl of 0.9% NaCl instead of Gel Solubilization Buffer from the PureLink® Quick Gel Extraction Kit (Thermo Scientific, ZDA) to prevent damaging viable cells. Sample was centrifuged at 10,000 × g for 5 min and filtered through a 0.2-μm pore Millipore filter (Merck, KGaA, Germany) previously wetted with 1 ml of 0.9% NaCl solution. DNA concentration was determined using QuantiFluor® dsDNA System as described above. Experiment was performed in three biological replicates and the DNA was measured in three technical replicates.
DNA quantification and DNA exchange between nuclease mutant strains in the presence of exogenous DNA
To test whether more DNA is found in the agar if nuclease mutant strains are used, and how this affects DNA exchange of the nuclease mutant strains, we simultaneously measured DNA concentration and DNA exchange at the boundary of PS-216 (amy:p43-cfp (Sp), ΔnucB (Kn) ΔyhcR (Ery)) and PS-196 (sacA:p43-yfp (Cm), ΔyhcR (Ery)) with/without added DNA at the centre of the plate. DNA quantification and the following procedures are the same as decribed above for wild-type strains. Experiment was performed in three biological replicates and each replicate was sampled in three technical replicates. The DNA concentration was measured in three replicates for each technical replicate.
Double plate setup for investigating ecological advantage of transformants
The spread of transformants, cells which acquired the genes from the opposite swarm (amyE::p43-cfp or sacA::p43-yfp) on the boundary, that now carry both markers, into area containing two antibiotic markers was tested on B medium agar plate. One half contained semi solid B medium (0.7% agar) and the other half contained semi solid B medium supplemented with two antibiotics: 10 μg/ml of Chloramphenicol (Cm) and 100 μg/ml of Spectinomycin (Sp) (Fig. 5a). First, B media without antibiotics was poured and allowed to solidify, after which one half was carefully removed with a sterile scalpel and B media containing both antibiotics was poured into the plate. Strains (kin and non-kin pairs) were inoculated onto the agar containing no antibiotics and the spread of transformants was observed and sampled after 24 h of incubation at 37 °C at 80% humidity (Fig. 5a). The number of transformants colonising the area containing antibiotics and total CFU was determined by selective plating. In total, 50 colonies carrying both Sp and Cm from three independent experiments were further tested for YFP and CFP fluorescence to confirm the transfer of DNA. Experiment was performed in three biological replicates.
Relative DNA exchange frequencies in non-kin vs. self
In order to determine whether DNA exchange frequency of swarming B. subtilis PS-216 strain in non-kin combination is significantly higher compared to DNA exchange frequency with an isogenic strain, we used pairs of strains in which one of the strain carried a ΔcomGA mutation and was not able to accept DNA. This enabled us to determine DNA exchange frequency of only one strain in a pair (kin or non-kin pair) and not total DNA exchange frequency as described above, where transformants of both strains were summed in the equation. Pairs of strains (PS-216 (Sp) was staged with PS-216 ΔcomGA and PS-196 (Cm) was staged with PS-196 ΔcomGA) were inoculated parallel to each other on semisolid B medium, incubated overnight and sampled as described above. DNA exchange frequency was calculated as the number of total CFU/ml of transformants (Sp + Cm) divided by CFU count of the strain not carrying the comGA mutation. Experiment was performed in three biological replicates and each replicate was sampled in three technical replicates.
Scanning electron microscopy
For observation of the bacterial surface, the selected pieces of colonies were cut from agar plates and fixed in 1% glutaraldehyde and 0.5% formaldehyde in 0.1 M cacodylate buffer, pH 7.3 at 4 °C overnight. After washing of the fixative with 0.1 M cacodylate buffer, the samples were postfixed in 1% aqueous solution of OsO4 for 1 h. Postfixed samples were dehydrated in an ascending ethanol series (30, 50, 70, 90 and 96%) and transferred into pure acetone that was gradually replaced by hexamethyldisilazane (HMDS) and allowed to air-dry overnight. Dried samples were attached to metal holders with silver paint, coated with platinum and observed with a JEOL JSM-7500F field-emission scanning electron microscope.
Fitness calculations
The boundary between non-kin strains PS-216 amyE::P43-cfp (Sp) and PS-196 sacA::P43-yfp (Cm) strains was sampled and CFU of each strain was determined in the boundary (CFU216non-kin, CFU196non-kin) and total CFU of both strains (CFUnon-kin(216+196)) was determined. Each strain was also staged with a self strain carrying a different Ab marker and CFU of each strain was determined (CFU216kin, CFU196kin), and total CFU was determined (CFUkin(216(Sp)+216(Cm), CFUkin(196(Sp)+196(Cm))). Fitness was calculated as relative change in strain frequency in non-kin versus kin setting. Relative change in the frequency of strain PS-216 in non-kin setting versus kin setting is shown in the Equation (1) below.
Fitness (relative change in strain frequency in non-kin vs kin setting) =
| 1 |
Calculations were performed for data of three biological replicates each in three technical replicates.
sigW-YFP expression at the meeting area
Envelope stress response was observed at the swarm meeting points using kin, non-kin and isogenic strain combinations using a yfp (yellow fluorescent protein) gene linked to the promotor of sigW, thus cells under envelope stress produced YFP and glowed yellow. Strains were inoculated in parallel on semisolid B media as described above. Fluorescence was observed under stereomicroscope (Leica Microsystems, Germany). Pictures were taken under stereomicroscope of each plate and experiment was performed in at least three biological replicates.
Statistics and reproducibility
Data was presented as a mean of three biological replicates ±standard deviation (error bars) or as individual data points (9 technical replicates–3 individual replicates of three biologically independent experiments). Biological replicates were used to calculate mean and sample standard deviation (n = 3). For statistical comparison, we performed two tailed Student’s t-test for unpaired data assuming equal variances. Exact p values are stated in Results. Statistically significant (p < 0.05) difference between strain combinations is designated by “*”. Mean, standard deviation and t-test calculations were performed using Microsoft Excel 12.00 (2016, Microsoft, Redmond, WA, USA).
Bioinformatics
Raw reads for each isolate were obtained after sequencing of dual-indexed Nextera XT libraries (Illumina, USA) prepared from single isolates DNA extracts. Sequencing of the pooled libraries was performed using an Illumina MiSeq benchtop sequencer and 2 × 250 bp paired-end reads MiSeq v2 kit-500 cycles reagents (Illumina, USA). Raw reads were trimmed of remnant sequencing adaptors sequences and low-quality regions (Phred score < Q20) using CLC Genomics Workbench 9 (https://www.qiagenbioinformatics.com/). Assembly was performed on adaptors and quality trimmed reads with CLC Genomics Workbench 9 assembler using default parameters.
Genome analysis
The degree of relatedness between the 6 B. subtilis isolates was evaluated using Average Nucleotide Identity48, using BLAST similarity scores of 1020 bp long genome segments between all genome pairs (ANIb) as described by Goris et al.49. The ANI computation was run using FASTani17. Genome sequences are available in the NCBI database under genome accession numbers VBRL00000000, VBRM00000000, VBRN00000000, VBRO00000000, VBRQ00000000 and VBRR00000000.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by grants from Slovenian Research Agency (ARRS): the Programme Grant P4-0116, the Slovenia-USA collaboration grant bilateral ARRS project US/18-19-091 and ARRS projects: J4-9302, J4-8228, J4-7637 and the University infrastructural centre “Microscopy of biological samples” located in Biotechnical faculty, University of Ljubljana.
Source data
Author contributions
P.S. and I.M.M conceived the project, P.S., K.B. and R.K. performed the experiments. P.S., J.K., J.N., S.S. and J.M. performed the genome sequencing and assembly analysis. P.S., B.K., R.K., J.N. and J.M. contributed with the methods and P.S. produced the figures. P.S. and I.M.M. supervised the experiments. P.S., M.V. and I.M.M. wrote the manuscript, with all authors contributing to the final version.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request and in Source Data file. Genome sequences are available in the NCBI database under genome accession numbers VBRL00000000, VBRM00000000, VBRN00000000, VBRO00000000, VBRQ00000000 and VBRR00000000. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Rosemary Redfield and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Polonca Stefanic, Katarina Belcijan.
These authors jointly supervised this work: Polonca Stefanic, Ines Mandic-Mulec.
Contributor Information
Polonca Stefanic, Email: Polonca.Stefanic@bf.uni-lj.si.
Ines Mandic-Mulec, Email: Ines.MandicMulec@bf.uni-lj.si.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-23685-w.
References
- 1.Errington J, Aart LTV. Microbe profile: Bacillus subtilis: model organism for cellular development, and industrial workhorse. Microbiology. 2020;166:425–427. doi: 10.1099/mic.0.000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefanic P, Kraigher B, Lyons NA, Kolter R, Mandic-Mulec I. Kin discrimination between sympatric Bacillus subtilis isolates. Proc. Natl Acad. Sci. USA. 2015;112:14042–14047. doi: 10.1073/pnas.1512671112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyons NA, Kraigher B, Stefanic P, Mandic-Mulec I, Kolter R. A combinatorial kin discrimination system in Bacillus subtilis. Curr. Biol. 2016;26:733–742. doi: 10.1016/j.cub.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veening JW, Blokesch M. Interbacterial predation as a strategy for DNA acquisition in naturally competent bacteria. Nat. Rev. Microbiol. 2017;15:629. doi: 10.1038/nrmicro.2017.89. [DOI] [PubMed] [Google Scholar]
- 5.Borgeaud S, Metzger LC, Scrignari T, Blokesch M. The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science. 2015;347:63–67. doi: 10.1126/science.1260064. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, R. M., Tsimring, L. & Hasty, J. Inter-species population dynamics enhance microbial horizontal gene transfer and spread of antibiotic resistance. eLife6, e25950 (2017). [DOI] [PMC free article] [PubMed]
- 7.Ringel PD, Hu D, Basler M. The role of type VI secretion system effectors in target cell lysis and subsequent horizontal gene transfer. Cell Rep. 2017;21:3927–3940. doi: 10.1016/j.celrep.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Wholey WY, Kochan TJ, Storck DN, Dawid S. Coordinated bacteriocin expression and competence in Streptococcus pneumoniae contributes to genetic adaptation through neighbor predation. PLoS Pathog. 2016;12:e1005413. doi: 10.1371/journal.ppat.1005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjos, M. et al. Expression of Streptococcus pneumoniae bacteriocins is induced by antibiotics via regulatory interplay with the competence system. PLoS Pathog.12, e1005422 (2016). [DOI] [PMC free article] [PubMed]
- 10.Dubnau D. Genetic competence in Bacillus subtilis. Microbiol. Rev. 1991;55:395–424. doi: 10.1128/MR.55.3.395-424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson GA, Bott KF. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J. Bacteriol. 1968;95:1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham JB, Istock CA. Genetic exchange in Bacillus subtilis in soil. Mol. Gen. Genet. 1978;166:287–290. doi: 10.1007/BF00267620. [DOI] [PubMed] [Google Scholar]
- 13.Khanna M, Stotzky G. Transformation of Bacillus subtilis by DNA bound on montmorillonite and effect of DNase on the transforming ability of bound DNA. Appl. Environ. Microbiol. 1992;58:1930–1939. doi: 10.1128/aem.58.6.1930-1939.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallori E, et al. Transformation of Bacillus subtilis by DNA bound on clay in non-sterile soil. FEMS Microbiol. Ecol. 1994;15:119–126. doi: 10.1111/j.1574-6941.1994.tb00236.x. [DOI] [Google Scholar]
- 15.Stefanic P, Mandic-Mulec I. Social interactions and distribution of Bacillus subtilis pherotypes at microscale. J. Bacteriol. 2009;191:1756–1764. doi: 10.1128/JB.01290-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konstantinidis KT, Tiedje JM. Genomic insights that advance the species definition for prokaryotes. Proc. Natl Acad. Sci. USA. 2005;102:2567–2572. doi: 10.1073/pnas.0409727102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain C, Rodriguez RL, Phillippy AM, Konstantinidis KT, Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018;9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haijema BJ, Hahn J, Haynes J, Dubnau D. A ComGA-dependent checkpoint limits growth during the escape from competence. Mol. Microbiol. 2001;40:52–64. doi: 10.1046/j.1365-2958.2001.02363.x. [DOI] [PubMed] [Google Scholar]
- 19.Chung YS, Dubnau D. All seven comG open reading frames are required for DNA binding during transformation of competent Bacillus subtilis. J. Bacteriol. 1998;180:41–45. doi: 10.1128/JB.180.1.41-45.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claverys JP, Prudhomme M, Martin B. Induction of competence regulons as a general response to stress in Gram-positive bacteria. Annu. Rev. Microbiol. 2006;60:451–475. doi: 10.1146/annurev.micro.60.080805.142139. [DOI] [PubMed] [Google Scholar]
- 21.Prudhomme M, Attaiech L, Sanchez G, Martin B, Claverys JP. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science. 2006;313:89–92. doi: 10.1126/science.1127912. [DOI] [PubMed] [Google Scholar]
- 22.Dorer, M. S., Fero, J. & Salama, N. R. DNA damage triggers genetic exchange in Helicobacter pylori. PLoS Pathog.6, e1001026 (2010). [DOI] [PMC free article] [PubMed]
- 23.Charpentier X, Kay E, Schneider D, Shuman HA. Antibiotics and UV radiation induce competence for natural transformation in Legionella pneumophila. J. Bacteriol. 2011;193:1114–1121. doi: 10.1128/JB.01146-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butcher BG, Helmann JD. Identification of Bacillus subtilis sigma-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by Bacilli. Mol. Microbiol. 2006;60:765–782. doi: 10.1111/j.1365-2958.2006.05131.x. [DOI] [PubMed] [Google Scholar]
- 25.Vos M. Why do bacteria engage in homologous recombination? Trends Microbiol. 2009;17:226–232. doi: 10.1016/j.tim.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Ambur, O. H., Engelstadter, J., Johnsen, P. J., Miller, E. L. & Rozen, D. E. Steady at the wheel: conservative sex and the benefits of bacterial transformation. Philos. Trans. R. Soc. Lond. B Biol. Sci371, 20150528 (2016). [DOI] [PMC free article] [PubMed]
- 27.Hahn J, Tanner AW, Carabetta VJ, Cristea IM, Dubnau D. ComGA-RelA interaction and persistence in the Bacillus subtilis K-state. Mol. Microbiol. 2015;97:454–471. doi: 10.1111/mmi.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, et al. Across genus plasmid transformation between Bacillus subtilis and Escherichia coli and the effect of Escherichia coli on the transforming ability of free plasmid DNA. Curr. Microbiol. 2007;54:450–456. doi: 10.1007/s00284-006-0617-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, X. et al. Stress-induced, highly efficient, donor cell-dependent cell-to-cell natural transformation in Bacillus subtilis. J. Bacteriol. 200, e00267–18 (2018). [DOI] [PMC free article] [PubMed]
- 30.Dubnau D, Hahn J, Roggiani M, Piazza F, Weinrauch Y. Two-component regulators and genetic competence in Bacillus subtilis. Res. Microbiol. 1994;145:403–411. doi: 10.1016/0923-2508(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 31.Hahn J, Dubnau D. Growth stage signal transduction and the requirements for srfA induction in development of competence. J. Bacteriol. 1991;173:7275–7282. doi: 10.1128/JB.173.22.7275-7282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Sinderen D, et al. comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol. Microbiol. 1995;15:455–462. doi: 10.1111/j.1365-2958.1995.tb02259.x. [DOI] [PubMed] [Google Scholar]
- 33.Turgay K, Hamoen LW, Venema G, Dubnau D. Biochemical characterization of a molecular switch involving the heat shock protein ClpC, which controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes Dev. 1997;11:119–128. doi: 10.1101/gad.11.1.119. [DOI] [PubMed] [Google Scholar]
- 34.Ansaldi M, Marolt D, Stebe T, Mandic Mulec I, Dubnau D. Specific activation of the Bacillus quorum-sensing systems by isoprenylated pheromone variants. Mol. Microbiol. 2002;44:1561–1573. doi: 10.1046/j.1365-2958.2002.02977.x. [DOI] [PubMed] [Google Scholar]
- 35.Oslizlo A, et al. Exploring ComQXPA quorum-sensing diversity and biocontrol potential of Bacillus spp. isolates from tomato rhizoplane. Micro. Biotechnol. 2015;8:527–540. doi: 10.1111/1751-7915.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stefanic P, et al. The quorum sensing diversity within and between ecotypes of Bacillus subtilis. Environ. Microbiol. 2012;14:1378–1389. doi: 10.1111/j.1462-2920.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 37.Tortosa P, et al. Specificity and genetic polymorphism of the Bacillus competence quorum sensing system. J. Bacteriol. 2001;183:451–460. doi: 10.1128/JB.183.2.451-460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts MS, Cohan FM. The effect of DNA sequence divergence on sexual isolation in Bacillus. Genetics. 1993;134:401–408. doi: 10.1093/genetics/134.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zawadzki P, Roberts MS, Cohan FM. The log-linear relationship between sexual isolation and sequence divergence in Bacillus transformation is robust. Genetics. 1995;140:917–932. doi: 10.1093/genetics/140.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majewski J. Sexual isolation in bacteria. FEMS Microbiol. Lett. 2001;199:161–169. doi: 10.1111/j.1574-6968.2001.tb10668.x. [DOI] [PubMed] [Google Scholar]
- 41.Croucher NJ, et al. Horizontal DNA transfer mechanisms of bacteria as weapons of intragenomic conflict. PLoS Biol. 2016;14:e1002394. doi: 10.1371/journal.pbio.1002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vos M, Buckling A, Kuijper B. Sexual selection in bacteria? Trends Microbiol. 2019;27:972–981. doi: 10.1016/j.tim.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Middleton R, Hofmeister A. New shuttle vectors for ectopic insertion of genes into Bacillus subtilis. Plasmid. 2004;51:238–245. doi: 10.1016/j.plasmid.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Doan T, Marquis KA, Rudner DZ. Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum. Mol. Microbiol. 2005;55:1767–1781. doi: 10.1111/j.1365-2958.2005.04501.x. [DOI] [PubMed] [Google Scholar]
- 45.Berka RM, et al. Microarray analysis of the Bacillus subtilis K-state: genome-wide expression changes dependent on ComK. Mol. Microbiol. 2002;43:1331–1345. doi: 10.1046/j.1365-2958.2002.02833.x. [DOI] [PubMed] [Google Scholar]
- 46.Koo BM, et al. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst. 2017;4:291–305. doi: 10.1016/j.cels.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldrick S, Setlow P. Expression of a Bacillus megaterium sporulation-specific gene during sporulation of Bacillus subtilis. J. Bacteriol. 1983;155:1459–1462. doi: 10.1128/JB.155.3.1459-1462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 49.Goris J, et al. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request and in Source Data file. Genome sequences are available in the NCBI database under genome accession numbers VBRL00000000, VBRM00000000, VBRN00000000, VBRO00000000, VBRQ00000000 and VBRR00000000. Source data are provided with this paper.