Abstract
Vaccination plays an important role in the fight against SARS-CoV-2 to minimie the spread of coronavirus disease 2019 (COVID-19) and its life-threatening complications. Myocarditis has been reported as a possible and rare adverse consequence of different vaccines, and its clinical presentation can range from influenza-like symptoms to acute heart failure. We report a case of a 30-year-old man who presented progressive dyspnea and constrictive retrosternal pain after receiving SARS-CoV-2 vaccine. Cardiac magnetic resonance and laboratory data revealed typical findings of acute myopericarditis.
Résumé
La vaccination joue un rôle important dans la lutte contre le SARS-CoV-2 afin de minimiser la propagation de la maladie à coronavirus 2019 (COVID-19) et ses complications potentiellement mortelles. La myocardite a été signalée comme une conséquence potentielle, indésirable et rare, de différents vaccins, et sa présentation clinique peut aller de symptômes de type grippal à une insuffisance cardiaque aiguë. Nous rapportons le cas d'un homme de 30 ans qui a présenté une dyspnée progressive et une douleur rétrosternale constrictive après avoir reçu le vaccin contre le SARS-CoV-2. La résonance magnétique cardiaque et les données de laboratoire ont révélé des résultats typiques d'une myopéricardite aiguë.
A 30-year-old man presented at the emergency department complaining dyspnea, constrictive retrosternal pain, nausea, and profuse sweating. Of note, the patient had suffered from fever (38.8°C) and arthralgia 72 hours earlier when he received his second dose of SARS-CoV-2 vaccine (mRNA BNT162b2), which was injected 21 days after the first dose.
The patient tested negative at nasopharyngeal swab testing for SARS-CoV-2, as required before hospital admission.
Anamnesis was negative for cardiovascular or metabolic disorders and recent infectious diseases.
At physical examination he was afebrile, with moderate tachycardia (heart rate 93 beats/min) and normal blood pressure (115/58 mm Hg). At auscultation, neither lung alterations nor heart murmurs were identified; oxygen saturation was of 99% on room air.
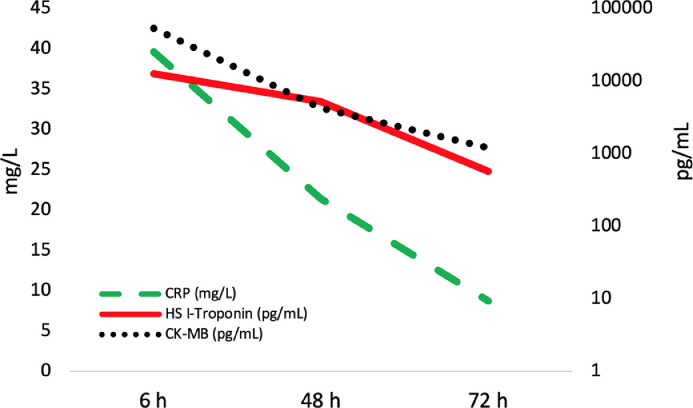
Laboratory data revealed elevated cardiac troponin I (12,564.80 pg/mL; normal < 34.2 pg/mL), creatine kinase-MB (53.8 ng/mL; normal 0-5.2), lactate dehydrogenase (228 U/L; normal 125-220), activated partial thromboplastin time (75.2 seconds; normal 20-40), and C-reactive protein (39.6 mg/L; normal 0-5). White blood cells were 10.4 103/µL (normal 4.0-10.0), with mild eosinophilia (0.9 × 103/µL, normal 0.0-0.5 × 103). Serum levels of cardiac troponin I, creatine kinase-MB, and C-reactive protein during the first 72 hours from hospital admission are shown in Figure 1 .
Figure 1.
Graph showing the patient's serum levels of cardiac troponin I, creatine kinase-MB, and C-reactive protein during the first 72 hours after hospital admission.
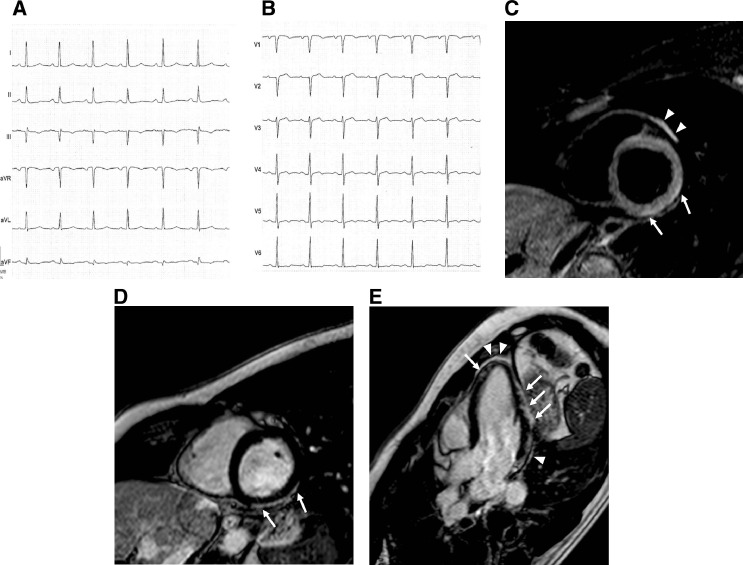
Electrocardiography (ECG) showed subtle ST-segment elevation suggestive of potential myocardial injury or pericarditis in V2-V4 and nonspecific T-wave changes in V5 and V6. Transthoracic echocardiography revealed preserved ejection fraction, mild pericardial effusion, and segmental wall motion abnormality of the apical portion of interventricular septum. No coronary artery disease was found at coronary angiography. Cardiac magnetic resonance imaging (MRI), performed 72 hours after hospital admission, revealed good systolic function and increased myocardial and pericardial signal intensity on T2-weighted short tau inversion recovery sequences (T2 ratio 2.1; normal < 2). T1-weigheted phase-sensitive inversion recovery sequences, performed 15 minutes after intravenous injection of gadolinium, showed subepicardial enhancement of the myocardium, suggesting a provisional diagnosis of myopericarditis (Fig. 2 ).
Figure 2.
(A, B) Twelve-lead electrocardiography on admission showing subtle ST-segment elevation suggestive of potential myocardial injury or pericarditis in V2-V4 and nonspecific T-wave changes in V5 and V6. (C) Cardiac magnetic resonance imaging T2-weighted short tau inversion recovery sequence acquired along the basal short-axis view shows increased subepicardial signal intensity of the inferolateral myocardial segments (arrows). Increased thickness and signal intensity of pericardium is also shown (arrowheads). T1-weighted phase-sensitive inversion recovery sequences performed along (D) basal short-axis view, (E) 3-chamber view, and (F) 4-chamber view show diffuse myocardial late gadolinium enhancement with subepicardial distribution (arrows) and sparing of the basal and mid-septal segments; thickening and enhancement of pericardium can also be seen (arrowheads).
Extensive infectious and rheumatologic workup was unremarkable. Virus serology did not show IgM antibodies nor 4-fold increase of IgG antibodies for Epstein-Barr virus, Cytomegalovirus, Adenovirus, Enterovirus (Coxsackie A, Coxsackie B1), and human Herpes 1 and 2 viruses.
A quantitative SARS-CoV-2 antibody assay was performed on an Abbott Architect platform (Abbott, Abbott Park, IL). The Abbott SARS-CoV-2 IgG II Quant test (Abbott S IgG) quantified antispike post-vaccination titers of > 40,000 AU/mL (range 21,000-40,000 U/mL) at hospital admission and 8,392 U/mL at 1-month follow-up. On the other hand, the patient tested negative for SARS-CoV-2 infection (antinucleocapsid IgG antibodies) at both time points.
None of the patient's close contacts had tested positive for SARS-CoV-2 by means of nasopharyngeal swab test or presented any COVID-19–related symptoms. Family history was also negative for rheumatologic or genetic diseases.
The patient was initially treated with bisoprolol and acetylsalicylic acid, with progressive resolution of his symptoms. During his hospitalisation, he also received prednisolone. Seven days after hospital admission a nasopharyngeal swab for SARS-CoV-2 was repeated, and the patient tested negative. Cardiac-specific troponin I and creatine kinase-MB progressively decreased and he was discharged home with the recommendation to avoid intense physical activity.
Discussion
The coronavirus disease 2019 (COVID-19) has spread rapidly into a pandemic. The global death toll has surpassed 3 million (https://coronavirus.jhu.edu). Meanwhile, infections have neared 150 million worldwide, and different reports have been suggesting that the cardiovascular system is prominently affected by the infection.1
Vaccination is playing a crucial role to protect from COVID-19 and its life-threating complications. However, vaccines can induce adverse reactions that in rare cases may lead to lethal consequences, such as myocarditis. These adverse events have been described more frequently in patients receiving smallpox vaccination, and very rarely in patients receiving vaccines for single-stranded RNA viruses such as influenza virus.2 , 3
MRI has become a mainstay in the diagnostic workup of patients with an infarction-like clinical presentation, allowing for a valid and noninvasive alternative to endomyocardial biopsy for diagnosis.4 The presence of multiple areas of subepicardial late gadolinium enhancement, myocardial edema, and pericardial thickening together with patient's ECG and laboratory data fulfilled the diagnostic criteria for myopericarditis.4
The pathophysiology of our case was more likely related to an autoimmune phenomenon. Although the exact trigger for autoimmune myocarditis is unknown, literature evidence suggests a “molecular mimicry” when the viral antigen resembles proteins on the myocardium. When autoreactive sensitisation occurs, cytokines and lymphocytes migrate into the myocardial interstitial space, inducing an inflammatory response.3
Another possible hypothesis is represented by a delayed hypersensitivity reaction, such as serum sickness–like reaction. In fact, the first vaccine dose may have presumably acquired sensitisation. Moreover, the hypothesis of a delayed hypersensitivity after the second dose would be concordant either with the timing of symptoms, and with the mild peripheral eosinophilia seen in our case. A further hypothesis can be represented by eosinophilic myocarditis directly after immunisation, which has been reported as an extremely rare event, despite the possible underdiagnosis due to its delayed development.5
Several cases of myocarditis associated with different vaccine administrations have been previously reported. In our case, we speculate that adverse reaction to the COVID-19 vaccine was responsible for the development of myocarditis, owing to its temporal relationship. However, substantial evidence other than temporal aspects still need to be provided to demonstrate the causality, such as histologically proven cases of autoimmune myocarditis following vaccination.
Finally, in light of the vast number of subjects receiving different doses of SARS-CoV-2 vaccine in the next few months, clinicians should remain vigilant and suspect myocarditis in patients who present with cardiopulmonary symptoms after a recent vaccination.
Acknowledgments
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 1667 for disclosure information.
References
- 1.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. Erratum: JAMA Cardiol 2020;5:1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halsell JS, Riddle JR, Atwood JE, et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA. 2003;289:3283–3289. doi: 10.1001/jama.289.24.3283. [DOI] [PubMed] [Google Scholar]
- 3.Nagano N, Yano T, Fujita Y, et al. Hemodynamic collapse after influenza vaccination: a vaccine-induced fulminant myocarditis? Can J Cardiol. 2020;36 doi: 10.1016/j.cjca.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto H, Hashimoto T, Ohta-Ogo K, et al. A case of biopsy-proven eosinophilic myocarditis related to tetanus toxoid immunization. Cardiovasc Pathol. 2018;37:54–57. doi: 10.1016/j.carpath.2018.10.003. [DOI] [PubMed] [Google Scholar]