Abstract
Background
The study aimed to evaluate the distribution of circulating respiratory viral pathogens other than severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) during the first year of the coronavirus disease-2019 (COVID-19) pandemic with especially focusing on the effects of the national-based mitigation strategies.
Methods
This single-center study was conducted between March 11, 2020-March 11, 2021. All children who were tested by polymerase chain reaction on nasopharyngeal swabs for SARS-CoV-2 and other common respiratory viral pathogens were included in the study.
Results
A total of 995 children with suspected COVID-19 admitted to the study center. Of these, 513 patients who were tested by polymerase chain reaction for both SARS-CoV-2 and common respiratory viral pathogens were included in the final analysis. Two hundred ninety-five patients were (57.5%) male. The median age was 3 years of age (27 days-17 years). A total of 321 viral pathogens identified in 310 (n: 310/513, 60.4%) patients, and 11 of them (n: 11/310, 3.5%) had co-detection with more than 1 virus. The most common detected virus was rhinovirus (n: 156/513, 30.4%), and SARS-CoV-2 (n: 122/513, 23.8%) followed by respiratory syncytial virus (n: 18/513, 3.5%). The influenza virus was detected in 2 patients (0.4%). A total of 193 patients were negative for both SARS-CoV-2 and other pathogens.
Conclusions
There is a decline in the frequency of all viral pathogens like SARS-CoV-2 in correlation with the national-based mitigation strategies against COVID-19 during the pandemic.
Key Words: Co-detection, Coronavirus disease-2019, Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), Isolation strategies
Since the beginning of the coronavirus disease-2019 (COVID-19),1 it has become one of the worst infectious disease pandemics of recent times.2 Up to March 2021, more than 115 million confirmed cases of COVID-19 with 2,560,287 deaths were reported worldwide.3
Before the COVID-19 pandemic, respiratory pathogens such as influenza, parainfluenza, respiratory syncytial virus (RSV), human metapneumovirus (hMPV), and human coronavirus are reported to be responsible for the respiratory tract infections in children.4, 5, 6 For now, it has also been reported of co-infections of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and other respiratory pathogens.7, 8, 9, 10, 11 There are some studies that revealed a general reduction in the prevalence of other seasonal respiratory viruses that are attributed to the preventive measures for COVID-19.12, 13, 14 Moreover, the impact of the national based mitigation strategies including lockdowns, suspension of the schools, mandatory facial mask usage also which would change the viral pathogens circulating during the pandemic is not obvious.
This descriptive study aimed to evaluate the distribution of respiratory viral pathogens other than the SARS-CoV-2 during the first year of the COVID-19 pandemic with especially focusing on the effects of the national based mitigation strategies on the circulating respiratory viral pathogens.
METHODS
This single-center cross-sectional study was conducted in the University of Health Sciences Dr Behçet Uz Children's Hospital with a 360-bed tertiary care hospital in İzmir, Turkey, from March 11, 2020, to March 11, 2021. This hospital is a pediatric referral center in the Aegean Region of Turkey with annual approximately 600,000 outpatients and 24,000 hospitalizations.
All children (≤18 years old) who were tested by polymerase chain reaction (PCR) on nasopharyngeal swabs for SARS-CoV-2 and other common human respiratory tract pathogens were included in the study.
Diagnosis of COVID-19 was documented by quantitative real-time reverse transcriptase-PCR positivity with detection of was positive for SARS-CoV-2 with detection of double targets, N-gene and ORF ab1 region at cycling threshold value under 35 cycles [SARS-CoV-2 (2019-nCoV) qPCR Detection Kit, Bio-Speedy, Turkey].15 The detection of the respiratory viruses was performed by the multiplex real-time PCR assay (Bosphore Respiratory Pathogens Panel Kit V4, Anatolia Geneworks, Turkey) that is capable of identifying viral pathogens including influenza viruses (influenza A, pandemic H1N1 influenza A, seasonal H1N1 influenza A, and influenza B), parainfluenza viruses (PIVs; PIV-1, PIV-2, PIV-3, and PIV-4), human coronaviruses (CoV OC43, CoV NL63, CoV HKU1, and CoV 229E), RSV A/B, rhinovirus, hMPV, enterovirus, bocavirus, adenovirus, and parechovirus. For detection of respiratory viruses’ specific master mix reagents which include targeted genomic regions of microorganisms were used and cycling threshold values under 35 cycles was considered as positive.16
Demographic and microbiological data of the patients were obtained from hospital medical records. Statistical analysis was performed using SPSS statistical software (version 22; SPSS, Chicago, IL). Measurement data with normal distribution are expressed as mean ± standard deviation (mean ± SD).
Timeline of national mitigation strategies
In Turkey, the first case of COVID-19 was announced on March 11, 2020.17 As a result of pandemic, some preventive measures were implemented by Ministry of Health. First, face-to-face educations in primary, secondary, and high schools were suspended since March 16, 2020.18 On April 3, 2020, children were restricted from leaving their houses which achieved to reduce their contact with each other and with society. In addition to the lockdown, mandatory face mask usage in public areas is a general rule among the country, since April 3, 2020.19 On October 12, 2020, face-to-face education was started in all primary schools, and 8th and 12th classes of secondary schools. However, on November 17, 2020, face-to-face educations in all schools were suspended again because of the increased numbers of COVID-19 cases.20 Considering the number of COVID-19 cases, curfews measures were implemented between April 11, 2020 and June 01, 2020, on weekends in Turkey. Although this restriction is left on June 1, 2020, it was started to be applied again on November 17, 2020. Children were allowed to leave their house on particular days of the week and particular hours of the day for less than 4 hours during the curfews.
This study was approved by the Local Ethical Committee of University of Health Science Dr. Behcet Uz Children's Training and Research Hospital.
RESULTS
During the study period, a total of 995 children with suspected COVID-19 admitted to the study center. Of these, 513 patients who were tested by PCR for both SARS-CoV-2 and other common respiratory viral pathogens were included in the final analysis. Two hundred ninety-five patients were (57.5%) male and 218 patients (42.5%) were female. The median age was 3 years of age (ranging from 27 days of life to 17 years of age). One hundred sixty-nine patients (32.9%) of the patients were younger than 1 year of age, 132 patients (68.0%) were under 6 years of age, and 98 patients (18.1%) were older than 12 years of age.
Of 513 patients, 122 (23.8%) were positive for SARS-CoV-2. The mean age of these patients with COVID-19 was 7.2 ± 5.8 years (ranging from 27 days to 17 years) and the mean age of patients with negative SARS-CoV-2 was 4.5 ± 4.8 years (ranging from 28 days to 17.5 years). The difference of the patients’ ages was significantly higher in children with COVID-19 (P < .05).
A total of 321 viral pathogens including respiratory viral pathogen and/or SARS-CoV-2 were identified in 310 (n: 310/513, 60.4%) patients and of them 11 (n: 11/310, 3.5%) had co-detection with more than 1 virus. A total of 193 (37.6%) patients were negative for both SARS-CoV-2 and the other viral pathogens.
During the study period, the most common detected viral pathogen was rhinovirus (n: 156/513, 30.4%), and SARS-CoV-2 (n: 122/513, 23.8%) followed by RSV (n: 18/513, 3.5%) and enteroviruses (n: 9/513, 1.7%). Remarkably, the influenza virus was detected in only 2 samples (0.4%) with influenza infection at the first month of the pandemic. A total of 193 patients were negative for both SARS-CoV-2 and other pathogens. The distribution of the detected viral pathogens was shown in Table 1 .
Table 1.
The distribution of the viral pathogens at the patients during COVID-19 pandemics
| Detected viral agents | Frequency of detected viral agents (n) | Percentage of viruses among detected viral agents (%) n: 321* | Percentage of viruses detected in patients included in the study (%) n: 513* |
|---|---|---|---|
| SARS-CoV-2 | 122 | 38 | 23.8 |
| Rhinovirus | 156 | 48.6 | 30.4 |
| RSV | 18 | 5.6 | 3.5 |
| Enterovirus | 9 | 2.8 | 1.7 |
| Human metapneumovirus | 7 | 2.18 | 1.4 |
| Adenovirus | 7 | 2.18 | 1.4 |
| Parainfluenza | 5 | 1.55 | 1.0 |
| Bocavirus | 2 | 0.6 | 0.4 |
| Coronavirus OC43 | 2 | 0.6 | 0.4 |
| Influenza virus | 2 | 0.6 | 0.4 |
| Paraechovirus | 1 | 0.3 | 0.2 |
| Negative PCR results | 193 | 37.6 | |
| Total | 524* |
PCR, polymerase chain reaction; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
A total of 11 patients had co-detection with more than 1 virus.
Among 122 patients with COVID-19, 10 (8.2%) was also positive for other respiratory viruses including rhinovirus in 6 patients, followed by RSV, enterovirus, adenovirus, and hMPV for one each patient associated with COVID-19 infection. In 1 patient with negative SARS-CoV-2, was detected as positive of both parechovirus and rhinovirus
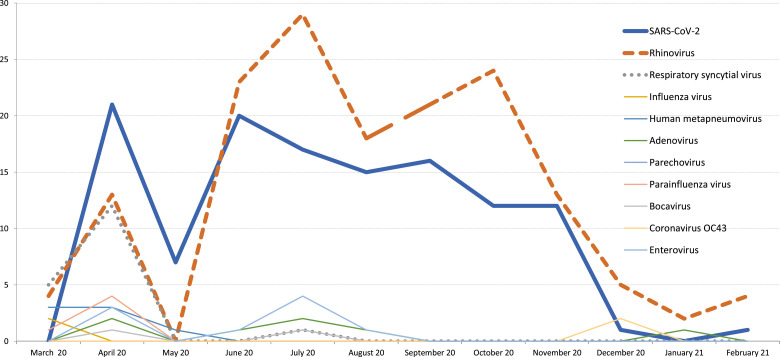
As summarized in Figure 1 , during the period March to August, 2020, a wide spectrum of viral pathogens was observed at the hospitalized children whereas since September 2020, we observed only viral respiratory infections related to either rhinovirus or SARS-CoV-2. As seen in Figure 1, with the increasing implementation of curfew and lockdown measures, a decrease in the frequency of spreading viruses was observed. Another noticeable finding is that spread of respiratory viral pathogens other than SARS-CoV-2 and rhinovirus have almost disappeared. The only influenza infections were observed at March 2020 at the initial phase of COVID-19 pandemic and no influenza virus was detected at the 2020 late fall-winter period.
Fig 1.
Distribution of spreading viruses during coronavirus disease-2019 pandemic.
DISCUSSION
In this study, co-detection with other viral respiratory pathogens in children with SARS-CoV-2 infections was found to be 8.2%. In children with suspected COVID-19 during the pandemic period, the most common detected viral respiratory pathogens were rhinovirus (30.4%), SARS-CoV-2 (23.8%) followed by RSV (3.5%) and enteroviruses (1.7%). Remarkably, influenza virus detected only in 2 children which were found to be in the initial period of pandemic. There is a decline in the frequency of all viral pathogens like SARS-CoV-2 in correlation with the national-based mitigation strategies against COVID-19 during the pandemic.
The studies focusing on viral co-infections with SARS-CoV-2 are mostly in adults. Some studies generally show the rates of co-infection as 3.2%-4.3%.21 , 22 In another study, of 21 patients infected with SARS-CoV-2, 2 samples were also positive for influenza A (9.5%) and 1 positive for PIV-3 (4.76%).23 Wu et al reported that a total of 19 patients (51.35%) among the 34 pediatric patients with COVID-19 were also positive for other common respiratory pathogens.9 In another study from China, which included 20 pediatric patients, found the rate of co-infection to be 40%.24 In the current study, co-detection with other viral pathogens and SARS-CoV-2 in children was found to be as 8.2% and rhinovirus was the most common co-detected virus. According to a review that evaluated several aspects of co-infection in COVID-19 comprehensively, overall the co-infection frequency with other viral pathogens was detected low, and rhinovirus/enterovirus, respiratory syncytial virus, and influenza viruses were more detected common co-virus pathogens.11
Interestingly, the current study showed influenza virus positivity in only 2 patients (0.4%) during the study period. This result is extremely low that influenza viruses are one of the most detected pathogens in patients with respiratory infections. As an example, according to the previous study which was conducted at the same center before the pandemic, 131 patients were hospitalized for influenza-related lower respiratory tract infections between January 2016 and March 2018.25 This decrease in influenza rate is thought to be related to the infection control measures performed to reduce the spreading of SARS-CoV-2. Data from the countries in Southern Hemisphere indicated also decreased influenza activity mostly due to the widespread mitigation measures.26 In our country, influenza vaccination is not mandatory and influenza vaccine shortage was present due to the high demand during the pandemic. Thus, the low influenza detection rate in our country might be as a result of the suspending of the schools throughout the COVID-19 pandemic at the winter period.
In this study, RSV was detected in 3.5% of the patients that was similar to recent studies which showed the detection rates of RSV approximately 3%–9%.10 , 27 , 28 Moreover, some studies also pointed out a decline in the incidence of RSV detection similar to our findings.13 , 14 According to a study which aimed to describe the effects of the nationwide lockdown strategy on the incidence of influenza A, influenza B, and RSV infections among children in Finland, influenza and RSV seasons ended more rapidly compared with previous seasons among the pediatric population in 2020.13 Yeoh et al revealed that incidence of RSV and influenza declined as 98% and 99.4%, respectively, after the initiation of local COVID-19 restrictions compared with data of prepandemic years.14
Studies have reported the declined rates of infectious diseases during the pandemic such as influenza, chickenpox, herpes zoster, rubella, or measles which have transmission route of droplet and/or airborne.29, 30, 31 However, according to the literature, rhinovirus-related respiratory tract infection outbreaks continue to be seen especially in children.32 In this study, it was detected that rhinovirus is the most common pathogen during the pandemic as shown in Figure 1. While there is a decline in rates of other viral pathogens during the pandemics, the spread of the rhinovirus seems to be as unaffected and so it is still the most frequently detected pathogen during the study year. Leung et al identified that the viruses in coughs and exhaled breath of children and adults with acute respiratory illness by seasonal human coronaviruses, influenza viruses, and rhinoviruses.33 According to this study, surgical face masks significantly reduced the detection of influenza virus RNA in respiratory droplets and coronavirus RNA in aerosols, but for rhinovirus, there were no significant differences between detection of virus with or without surgical face masks, both in respiratory droplets and in aerosols.33 These findings support the results of our study.
After a short time after the first announcement of the first COVID-19 case in Turkey, the schools including day care centers, primary and high schools were suspended, and later the daily care centers were also suspended. In addition, facial mask was set to be mandatory even in the social daily lifetime. These measures reduced the frequency of all viral respiratory pathogens as well as SARS-CoV-2. The correlation between the number of cases and the rigidity of limitation strategies is seen in Figure 1. Thus the viral pathogens which are expected be more contagious in the crowded placed were not observed during COVID-19 pandemic.
There are several limitations of this study due to its retrospective design and including only a single pediatric center. Moreover, data of this study include only hospitalized pediatric patients and the findings may not be attributed to the whole population. However, we must emphasize that the present study includes one of the largest pediatric population which may give an opinion about the distribution in population. From the beginning of the COVID-19 pandemic, most of the hospitals preferred only performing PCR tests for SARS-CoV-2, instead of other viral pathogens due to more efficient use of resources and laboratories and safety precautions. Thus this study provides the first and initial sight of view about the distribution of other viral respiratory pathogens during the SARS-CoV-2 outbreak in pediatric patients in Turkey.
CONCLUSIONS
In conclusion, rates and distribution of detected concomitant viral respiratory tract pathogens with SARS-CoV-2 are similar with adults and are not as much higher than early reports during the initial period of pandemic. In addition, the circulating viruses during the COVID-19 pandemic can change due to multifactorial factors including season, geographical variance, and also the national mitigation strategies in which changed the distribution of the viruses them we expected or observed in the previous year.
Footnotes
Funding/support: No funding was secured for this study.
Conflicts of interest: The authors have no example conflict of interest and no relevant financial relationships to disclose.
Author contributions: Prof. Dr Devrim conceptualized and designed the study, carried out the initial analyses, and reviewed and revised the manuscript. Dr Kıymet collected data, carried out the initial analyses, and drafted the initial manuscript. Assoc. Prof. Bayram conceptualized and designed the study, coordinated, supervised data collection, and reviewed and revised the manuscript. Dr Böncüoğlu, Dr Şahinkaya, Dr Cem, Dr Çelebi, Dr Düzgöl, Dr Kara, Dr Arıkan, Dr Aydın, Assoc. Prof. İşgüder, Assoc. Prof. Yılmazer, Dr Ayhan, Dr Gülfidan, Dr Bayram, Dr Çelik, Dr Alp Alp designed the data collection instruments, collected data, and contributed essential intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. (World Health Organization): Coronavirus disease (COVID-2019) situation reports. 2020;2019 (March):1-19. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed July 13, 2021.
- 3.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University of Medicine. John Hopkins University of Medicine. Available at: https://coronavirus.jhu.edu/map.html. Accessed March 4, 2021.
- 4.Korsun N, Angelova S, Trifonova I. Viral pathogens associated with acute lower respiratory tract infections in children younger than 5 years of age in Bulgaria. Braz J Microbiol. 2019;50:117–125. doi: 10.1007/s42770-018-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Upadhyay BP, Banjara MR, Shrestha RK, Tashiro M, Ghimire P. Etiology of coinfections in children with influenza during 2015/16 winter season in Nepal. Int J Microbiol. 2018;2018:1–6. doi: 10.1155/2018/8945142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cimolai N. Complicating infections associated with common endemic human respiratory coronaviruses. Health Secur. 2021;19:195–208. doi: 10.1089/hs.2020.0067. [DOI] [PubMed] [Google Scholar]
- 7.Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323:2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang S, Liu P, Xiong G. Coinfection of SARS-CoV-2 and multiple respiratory pathogens in children. Clin Chem Lab Med. 2020;58:1160–1161. doi: 10.1515/cclm-2020-0434. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q, Xing Y, Shi L. Co-infection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;146 doi: 10.1542/peds.2020-0961. [DOI] [PubMed] [Google Scholar]
- 10.Zhang DD, Acree ME, Ridgway JP. Characterizing coinfection in children with COVID-19: a dual center retrospective analysis. Infect Control Hosp Epidemiol. 2020;23:1–3. doi: 10.1017/ice.2020.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cimolai N. The complexity of co-infections in the era of COVID-19. SN Compr Clin Med. 2021:1–13. doi: 10.1007/s42399-021-00913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang JW, Bialasiewicz S, Dwyer DE. Where have all the viruses gone? Disappearance of seasonal respiratory viruses during the COVID-19 pandemic. J Med Virol. 2021;93:4099–4101. doi: 10.1002/jmv.26964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuitunen I, Artama M, Mäkelä L, Backman K, Heiskanen-Kosma T, Renko M. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J. 2020;39:e423–e427. doi: 10.1097/INF.0000000000002845. [DOI] [PubMed] [Google Scholar]
- 14.Yeoh DK, Foley DA, Minney-Smith CA. The impact of COVID-19 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2021;72:2199–2202. doi: 10.1093/cid/ciaa1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SARS-CoV-2 Double Gene RT-qPCR Kit, Bio Speedy Package Insert Release and Revision Date: 21.01.2021/19.04.2021, Cataloge no: BS-SY-WCOR-307-100/BS-SY-WCOR-307-250 BS-SY-WCOR-307-500/BS-SY-WCOR-307-1000 Available at: https://www.bioeksen.com.tr/en/biospeedy%c2%ae-sars-cov2-double-gene-rtqpcr.
- 16.Available at:http://www.anatoliageneworks.com/en/kitler.asp?id=208&baslik=Bosphore%20Respiratory%20Pathogens%20Panel%20Kit%20v4&bas=Bosphore%20Respiratory%20Pathogens%20Panel%20Kit%20v4.
- 17.AA AGENCY. 2020; Turkey confirms first case of coronavirus. Available at: https://www.aa.com.tr/en/latest-on-coronavirus-outbreak/turkey-confirms-first-case-of-coronavirus/1761522. Accessed March 9, 2021.
- 18.Available at: http://www.meb.gov.tr/bakan-selcuk-koronaviruse-karsi-egitim-alaninda-alinan-tedbirleri-acikladi/haber/20497/tr. Accessed March 19, 2021.
- 19.AA AGENGY. Türkiye'nin koronavirüsle mücadelesinde son 24 saatte yaşananlar. Available at: https://www.aa.com.tr/tr/koronavirus/turkiyenin-koronavirusle-mucadelesinde-son-24-saatte-yasananlar/1791816. Accessed March 19, 2021.
- 20.Available at: https://tr.wikipedia.org/wiki/Türkiye%27de_COVID-19_pandemisi#Eğitim. Accessed March 19, 2021.
- 21.Lin D, Liu L, Zhang M. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci China Life Sci. 2020;63:606–609. doi: 10.1007/s11427-020-1668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020;92:1549–1555. doi: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;4720:2019–2021. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Böncüoğlu E, Kıymet E, Çağlar İ. Influenza-related hospitalizations due to acute lower respiratory tract infections in a tertiary care children's hospital in Turkey. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104355. [DOI] [PubMed] [Google Scholar]
- 26.Olsen SJ, Azziz-Baumgartner E, Budd AP. Decreased influenza activity during the COVID-19 pandemic—United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1305–1309. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson S, Hirsch JS, Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wee LE, Ko KKK, Ho WQ, Kwek GTC, Tan TT, Wijaya L. Community-acquired viral respiratory infections amongst hospitalized inpatients during a COVID-19 outbreak in Singapore: co-infection and clinical outcomes. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto H, Ishikane M, Ueda P. Seasonal influenza activity during the SARS-CoV-2 outbreak in Japan. JAMA. 2020;323:1969–1971. doi: 10.1001/jama.2020.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu D, Lu J, Liu Y, Zhang Z, Luo L. Positive effects of COVID-19 control measures on influenza prevention. Int J Infect Dis. 2020;95:345–346. doi: 10.1016/j.ijid.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu D, Liu Q, Wu T, Wang D, Lu J. The impact of COVID-19 control measures on the morbidity of varicella, herpes zoster, rubella and measles in Guangzhou, China. Immun Inflamm Dis. 2020;8:844–846. doi: 10.1002/iid3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu D, Lu J, Sun Z. Rhinovirus remains prevalent in school teenagers during fight against COVID-19 pandemic. Immun Inflamm Dis. 2021;9:76–79. doi: 10.1002/iid3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung NHL, Chu DKW, Shiu EYC. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]