Abstract
Ximenia americana is among wild edible fruit indigenous to Ethiopia. The fruit reported as indispensable source of phytochemicals and health imparting components. However, the phytochemicals constituents, antioxidant activities and fatty acid profile of Ethiopian X. americana cultivar studies are still scarce. This research aimed at evaluation of flesh and seed of Ethiopian X. americana yellow and red colored cultivars for bioactive component, antioxidant activities, fatty acid profiles and physicochemical characteristics. The red fruit flesh had higher total phenol (3292.60 mg GAE/100 g) while yellow fruit flesh showed higher total flavonoid (173.95 mg quercetin/100g). The higher DPPH∗ radical scavenging activity (97%) was observed in red fruit flesh due to its higher phenolic content. The yellow X. americana seed (90.69 mg QE/100g) had higher total flavonoid contents than the red seed (18.64 mg QE/100g). The dominant unsaturated fatty acid found in red X. americana flesh was oleic acid (26.29%), and the main saturated fatty acid detected was palmitic acid (29.78%). The seed also had high unsaturated fatty acid which was elaidic acid, (84.32%). Ximeninic acid (12.78%) which is expected to be detected in Ximenia americana seed oil was also observed. The highest content of fat (52.23%) and protein (16.59%) was obtained in yellow fruit seed and the lowest fat content observed in yellow flesh (7.25%). Most abundant minerals were calcium (42.45–49.55 mg/100g) and manganese (26.73–33.92 mg/100g) in seeds. Whereas, fleshs displayed higher magnesium (76.3–98.27 mg/100g) and copper (1.35–1.52 mg/100g) contents. The Ethiopian X. americana fruit cultivars represent high nutritional and medicinal values despite variation related to genetic variability.
Keywords: Ximenia americana, Flesh, Seed, Phenol, Flavonoids, Antioxidants, Fatty acid
Ximenia americana; Flesh; Seed; Phenol; Flavonoids; Antioxidants; Fatty acid
1. Introduction
Wild edible fruits can be considered as interesting high-value sources of nutrients as dietary fiber and bioactive compounds with high antioxidant activity, which could provide the basis for nutraceuticals, food supplements or functional foods (Heinrich et al., 2006) . These wild edible fruits can be used as food source for the native people (Hegazy et al., 2019). Deficiencies of essential micronutrients can increase the risk of illness or death from infectious diseases by reducing immune and non-immune defenses. Such nutrient deficiencies are widespread in low and middle-income countries where wild fruits are a source of these compounds (Fernández-Ruiz et al., 2017). Scholars suggest that the consumption of wild fruit helps for healing potential of numerous diseases such as diabetes, cardiovascular problems, digestive and urinary tract disorder as a result of their phytochemical content with diverse bioactivities Erşan et al. (2020); Islary et al. (2016).
Ximenia americana is one of the wild edible fruit which belongs to Olacaceae family. The fruit can be used for innovative drug formulation due to its medicinal value. Generally, the fruit is obtained in Africa, India, New Zealand, Central America and South America (Mohamed and Feyissa, 2020).
Ethiopia is known for X. americana fruit potential. The fruit is found in different parts of the country in which the southern region takes the upper hand with high dense quantity (Hunde, 2012). Inkoy is commonly known local name for the fruit. It is green colored at the early stage of ripening and turn to yellow or red colored when ripen (Maikai et al., 2009). The fruit is found in shrub form with spine. Both the yellow and red cultivars of the X. americana fruit had been identified in the region. The harvesting time and frequency varies from place to place depending on the availability of matured edible fruit due to ecological and climatic conditions (Zewdu Yilma, Ethiopian Forest and Environment Research Institute). It was observed that the red cultivar is bitter than the yellow one.
The yellow colored fruits of Ximenia americana grown in different countries was extensively analyzed for their nutritional, anti-nutritional composition, phytochemicals and medicinal values (Almeida et al., 2016; Lamien-Meda et al., 2008; Muhammad et al., 2019). These aforementioned reports showed that X. americana fruit flesh possesses considerable amount of total polyphenol, vitamin C and free radical scavenging activity. Not only the fruit flesh but also the seed presents high polyphenols and antioxidant activity which makes it potential raw material for medicinal use (Sarmento et al., 2015). Unlike other countries, the flesh and seed of the yellow cultivar of Ethiopian X. americana fruit has not been studied for its phytochemicals, bioactive components and fatty acid profiles yet. Besides, as far as our knowledge is concerned, the red cultivar of X. americana was not studied elsewhere.
Therefore, the objective of this study was to evaluate the bio-active composition, free radical scavenging activity, fatty acid profile and physicochemical characteristics of red and yellow X. americana fruit fleshs and their respective seeds.
2. Material and methods
All chemicals and solvents used in this study were analytical grades. Methods used for the analyses were officially approved.
2.1. Sample collection
Red and yellow X. americana fruit cultivars were collected from Arbaminch area, Ethiopia. It is located at 5°58′57.04″ N and 37° 32′20.4″ E, an altitude of 1269 m above sea level. Fruits were randomly collected from the shrubs at pick harvesting time and stored at 4 °C.
2.2. Sample preparation
The whole fruit flesh (includes exocarp and mesocarp) and seed of X. americana fruit cultivars were separated with the aid of stainless-steel knives. The flesh was homogenized (IKA, Germany) and dried using oven (FW 100, China) until constant weight was obtained. The seed sample was ground using mortar and pestle and dried using oven (FW 100, China) until constant weight was obtained. Finally, the samples were stored in cool and dry place until further analysis.
For phytochemical analysis, the flesh and seed of each cultivar samples were freeze dried at -50 °C and 0.05 mbar for 72 h using freeze dryer (Grenco N.V. S-Hertogenbosch, Holland). The samples were then ground using mortar and pestle and allowed to pass through a sieve (20 meshes). Then, it kept in a sealed polyethylene bag and stored in the dark place at ambient temperature until further analysis.
2.3. Phytochemical determination
2.3.1. Extraction of bio-active compounds
The fruit fleshs and seeds extract were prepared using the maceration extraction according to Vongsak et al. (2013) method. The freeze dried flesh and seed powder (2 g) each was placed in 200 ml flask. Then, 40 mL of 70% Ethanol was added in to the flask. The sample was kept in a sealed flask and covered with aluminum foil to avoid light exposure. Subsequently, it was placed in the shaker (ES-20) at 100 rpm and at a temperature of 30 °C for 24 h. Whatman No.1 filter paper was used to filter the extract and dried using rotary evaporator (HS-2005S). The sample was kept in a refrigerator at 4 °C until analysis.
2.3.2. Total phenolic compound
The total phenolic compound (TPC) was determined according to Dadi et al. (2018). A mixture of 0.5 mL of the extract and 2.5 mL of the 10 % aqueous (by volume) Folin–Ciocalteu reagent was prepared. 8 min later, 2.0 mL of the 7.5 % (m/v) sodium carbonate was added. Then, it mixed with the extract mixture and kept in the dark at room temperature for 2 h. Similar method was used for the blank and gallic acid standard prepared at different concentrations (0, 20, 40, 60, 80, 100, 120, 140, 160 and 180 μg/mL) to develop a standard curve. UV-Vis spectrophotometer (PerkinElmer, Lambda 950, UK) was used to measure the absorbance at 765 nm. The result was stated in terms of mg gallic acid equivalent per 100 g (mg GAE/100 g).
2.3.3. Total flavonoid content
The total flavonoid content (TFC) was analyzed following Adom and Liu (2002) method. First the extract was mixed with 0.15 mL of 5% (m/V) sodium nitrite. Then, 2.5 mL of de-ionized water was used and mixed. The mixture was kept for 6 min. 10 percent (m/v) aluminum chloride (0.3 mL) was added and mixed. This was followed by addition of 1 mL of 1.0 M sodium hydroxide and subsequently 0.55 mL of distilled water. The solution was kept for 15 min in stirring. UV-Vis spectrophotometer (PerkinElmer, Lambda 950, UK) was used for reading of the concentration at 510 nm. The same procedure was applied for the blank and quercetin as standard at different concentrations (25, 50, 75, 100, 125, and 150 μg/mL) to develop the standard curve. The TFC was expressed as mg quercetin equivalent per 100 g of dry mass (mg QE/100g) of the sample.
2.4. DPPH∗ free radical scavenging
It was quantified using the 2,2-diphenyl-1-picrylhydrazyl (DPPH∗) according to Brand-Williams et al. (1995). A mixture of 2.4 mg of DPPH∗ solution in 100 mL of 80 % ethanol was prepared. The absorbance was ensured for the reading of less than one at 517 nm. One tenth milliliter of the sample and standard (ascorbic acid) of different concentrations was mixed with 3.9 mL of DPPH∗ solution. The mixtures were kept in the dark for 30 min. Absorbance reading was taken using UV-Vis spectrophotometer (PerkinElmer, Lambda 950, UK) at 517 nm. The solution for the blank was prepared using 80% methanol instead of the sample solution and assayed under the same conditions. The absorbance was then read. The DPPH∗ scavenging capacity was calculated against blank (Eq.1). The concentration providing 50% scavenging of DPPH∗ free radicals (IC50) was calculated graphically using a calibration curve in the linear range by plotting the extract concentration versus the corresponding scavenging effect.
| (1) |
where: is control sample absorbance.
is test sample absorbance.
2.5. Fourier transform infrared spectroscopy
The Fourier Transform Infrared Spectroscopy (FTIR) analysis was done according to Park et al. (2015). A Perkin Elmer IR SUBTECH Spectrum ASCII PEDS spectrophotometer from 4000 to 400 cm−1 range, by Fourier Transformed and KBr pellets was used to obtain the absorption spectra in infrared region for the yellow and red X. americana fruit flesh and seed.
2.6. Fatty acid profile
Fatty Acid Methyl Esters (FAMEs) were prepared by trans- esterification of the red X. americana flesh and its seed oil obtained after Soxhlet extraction according to David et al. (2005). About 100-mg sample was weighed in a 20-mL test tube. The sample was mixed in a 10-mL hexane. 100-μL 2 N potassium hydroxide in methanol (11.2 g in 100 mL) was added. The test tube was vortexed for 30 s and centrifuged. The clear supernatant was transferred in to 2-mL auto sampler vial. The results were presented in relative percentage of each fatty acid.
The quantification of fatty acids was performed using automated gas chromatograph (Agilent 6890 GC) with a flame ionization detector (FID). Automated split-splitless injector (Agilent 7683) was used for sample injection. The separation was conducted on a 60 m × 0.25 mm ID, 0.15 μm columns. The inlet and detector temperatures were 250 °C and 280 °C respectively. Helium was used as carrier gas at the pressure of 230 Kpa. The injection volume was 1 μL and the Split ratio was 1/50.
2.7. Chemical compositions
2.7.1. Moisture content
Moisture content was determined gravimetrically according to an AOAC method No. 925.09 (2000) method with some modifications immediately after harvesting on fresh weight basis. 5 ± 0.05 g flesh and seed samples from both X. americana were dried in a vacuum oven (Model 4567, Kimya Pars Co., Iran) at 65 °C until a constant weight was obtained. The percent moisture content was computed by weight difference after oven drying to that of the original sample weight.
2.7.2. Crude fat
Crude fat content was determined according to AOAC method No. 925.38 (2000). Soxhlet extractor (Soxtec™ 8000) was used following the procedure on the manufacturer user guide manual. 2.0 ± 0.05 g of partially oven dried flesh and seed samples from both X. americana fruit cultivars were weighed in a filter bag and sealed. The fat was extracted by refluxing the filter bags containing the samples for 60 min in a petroleum ether. After extraction, filter bags were dried in the oven at 102 ± 2 °C for 15 min and the crude fat content was computed by the weight difference to that of the initial sample weight.
2.7.3. Crude fiber
The crude fiber was determined according to AOAC method No. 962.09 (2000) standard methods using auto-fiber analyzer (Fibertec ™ 8000). 2 ± 0.05 g of oven dried flesh and seed samples were defatted using 250 ml petroleum ether. The sample digestion for crude fiber was carried out using 1N of sulfuric acid and potassium hydroxide, according to manufacturer's instructions. After digestion, bags were rinsed with boiling water followed with acetone and air dried. The crude fiber was calculated based on the weight difference after digestion to that of the initial sample weight.
2.7.4. Crude protein content
The total nitrogen content of sample was determined using Kjeldahl method AOAC No. 920.87 (2000). 0.5 ± 0.05 g of flesh and seed samples of both fruits were used for crude protein analysis. Crude protein content was calculated by multiplying the total nitrogen with a factor of 6.25.
2.7.5. Determination of ash and mineral composition
Ash and mineral compositions had been determined as described by Bouhlali et al. (2017). Briefly, about 2 g of each X. americana fruit samples were placed in a previously weighed porcelain crucible and heated at 550 °C in a furnace for 4 h until the residue was uniformly white. The resulting ashes were dissolved in 5 mL of concentrated hydrochloric acid and the mixtures were heated on a hot plate until complete dryness. Then, little drops of H2O and 5 mL of distilled water were added and made up to 25 mL in a standardized flask. The resultant solutions have been used for mineral content analysis. An atomic absorption spectrometry (Agilent AAS 240 series, USA, 2017) was used to determine Ca, Cu, Fe, Mg, and Mn contents.
2.7.6. pH and total soluble solid determination
pH was determined as described by (Holcroft and Kader, 1999) method with some modifications. About 10 g of sample was diluted with 100 mL of deionized water and analyzed using a pH meter (LE-409, Germany) with automatic temperature adjustment. Digital refractometer (RFM C-60, Germany) was used to examine the total soluble solid content.
2.8. Statistical analysis
All experiments were conducted in triplicate and values were reported as mean ± standard deviation. JMP software (Version 13.0, SAS institute Inc.) was used to analyze the data. The mean values (P < 0.05) for the data was compared using one way analysis of variance (ANOVA) and the Tukey's test.
3. Result and discussion
3.1. Phytochemical components
The total phenolic and flavonoid content of the yellow and red colored X. americana fruit cultivars were depicted in Table 1. Both fruits' seeds and flesh s of X. americana showed significant (P < 0.05) differences in total phenol and total flavonoid compound.
Table 1.
Total phenol and flavonoid content of two cultivars of X. americana fruit.
| Parameters | Red X. americana |
Yellow X. americana |
||
|---|---|---|---|---|
| Seed | Flesh | Seed | Flesh | |
| Total Phenol (mg GAE/100 g) | 704.31 ± 19.84c | 3292.60 ± 259.21a | 544.04 ± 34.56d | 2880.39 ± 198.49b |
| Total Flavonoid (mg QE/100 g) | 18.64 ± 4.27d | 58.34 ± 6.62c | 90.69 ± 7.57b | 173.95 ± 8.24a |
The same letters in the same row represents no significant difference (P < 0.05). All values are expressed as mean ± standard deviation (n = 3).
3.1.1. Total phenolic compound
The total phenolic content showed significant difference (P < 0.05) between the flesh of the red (3292.60 mg GAE/100 g) and yellow X. americana fruit (2880.39 mg GAE/100 g). This variation perhaps explained by the taste of both fruits in which the red colored fruit flesh tasted bitter than the yellow one. Almeida et al. (2016) and Sarmento (2015) reported that the total phenolic content of the yellow X. americana fruit grown in different region of Brazil were 1298.22 mg GAE/100 g and 3002.08 mgGAE/100 g, respectively. Lamien-Meda et al. (2008) reported lower total phenol content of X. americana fruit (2086.67 mg GAE/100 g) grown in Burkina Faso as compared to the present study.
The red and yellow X. americana seeds contained 704.31 mg GAE/100 g and 544.04 mg GAE/100 g of total phenolic content, respectively. Differences (P < 0.05) were observed in total phenolic content within the seeds and fleshs of the red and yellow X. americana fruits, with the fleshs contained higher mean values (Supplementary material, Table 1). The total phenolic content of the red fruit might contain anthocyanin pigment at low pH (2.84 in this case) which was responsible for its reddish color. pH is an important factor for color expression of anthocyanin, being these compounds more stable in acidic than alkaline or neutral medium (de Freitas et al., 2017).
Folin–Ciocalteu method used for determination of polyphenol may have some errors in increasing the yield due to reactivity of the reagent with other compounds like vitamin C (Everette et al., 2010). However, Almeida et al. (2016) observed this effect for the yellow X. americana fruit and concluded that there was no vitamin C interference in polyphenol yield. The author reported that no change was seen in vitamin C during maturing stages as the polyphenol decreased by half.
The difference in total phenolic content between the two fruits could be explained by their genetic factors and different ability to synthesize secondary metabolites. The substantial content of phenolic compound of the red colored X. americana fruit can be an important raw material for functional foods, food additives and pharmaceuticals.
3.1.2. Total flavonoid content
The total flavonoid content of the yellow X. americana fruit flesh (173.95 mg QE/100 g) was significantly higher (P < 0.05) than the red X. americana flesh (58.34 mg QE/100 g). The obtained result in this study were higher than reported value of 30.95 mg QE/100 g by Lamien-Meda et al. (2008) in Burkina Faso. The total flavonoid content of yellow X. americana seed (90.69 mg QE/100 g) was almost four times higher than the red X. americana seed (18.64 mg QE/100 g), with significant difference (P < 0.05). There was significant difference (P < 0.05) in total flavonoid content between the two X. americana fruits in terms of flesh and seed which might be described due to their color variation (Supplementary material, Table 1). It is rational to accept as true that the yellow X. americana fruit is rich in yellow flavonoid content. This was confirmed by Almeida et al. (2016) who reported that the yellow flavonoid was almost twenty times higher than the total anthocyanin for the yellow X. americana fruit. These differences in flavonoid content might be emanated from the dependency of either on the cultivar or on the growing condition (Al-Alawi et al., 2017).
The fruit cultivars color showed variations during maturation from green to red and yellow. These variations of fruits during maturation are dependent on the concentrations of anthocyanin pigments, yellow flavonoid content along with chlorophyll and carotenoids as well as the interactions between them (Xu et al., 2016). The presence of significant quantity of total phenols and flavonoids in the fruits have role in radical scavenging activities, lowering some chronic diseases, prevention of some cardiovascular disease and certain kinds of cancer development (Metoui et al., 2019).
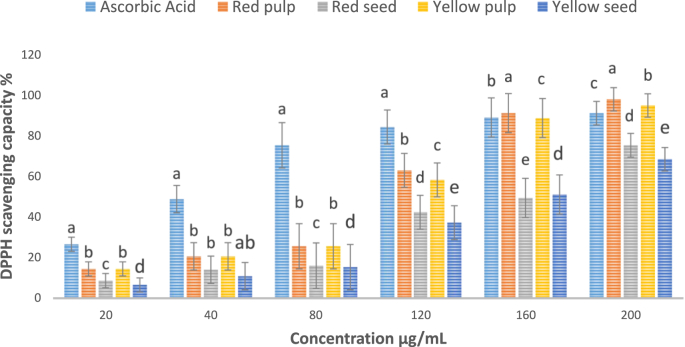
3.2. DPPH∗ free radical scavenging
A stable free radical used to determine the ability of fruit extract to scavenge free radicals is known as DPPH∗ (Silva and Sirasa, 2018). The scavenging of free radicals both in fruit parts in this study was dependent on the concentration of the flesh and seed extract (Figure 1). Both the red and yellow X. americana fruit fleshs depicted more than 90% of DPPH∗ free radical scavenging at a concentration of 200 μg/mL. The red flesh of X. americana fruit showed higher radical scavenging (97%) as compared to the yellow X. americana flesh (94%) at 200 μg/mL concentration. Researchers from different countries Lamien-Meda et al. (2008); Le et al. (2012) and Sarmento (2015) reported that the yellow X. americana fruit had high antioxidant activity which agree with the current study.
Figure 1.
DPPH∗ scavenging capacity (%) of X. americana fruit parts at different concentrations of extract.
The yellow and red X. americana seeds also showed significant amount of DPPH∗ radical scavenging at 200 μg/mL concentration. The red X. americana seed had higher scavenging activity (75%) than the yellow X. americana seed (68%). This might be due to the presence of the relative higher inherent bioactive compound like total phenol in the fruit which enables to be used in food and pharmaceuticals to prevent oxidation of cells in human body.
The quantity of antioxidant needed to decrease the original amount of free radicals by half is called IC50. The IC50 for the red X. americana flesh was 99 μg/mL which indicated the highest antioxidant activity among all studied X. americana fruit parts. It was obtained that the IC50 value for the yellow X. americana flesh was (102 μg/mL), which had no significant difference with the red X. americana flesh. The red X. americana seed also showed higher antioxidant capacity (IC50 = 147 μg/mL) as compared to yellow fruit seed (IC50 = 154 μg/mL) without significant variations. Sarmento (2015) reported that the flesh and seed of the yellow X. americana fruit grown in Mossoró-Assu, RN, Brazilian semiarid region had also significant antioxidant activities. Hence, the fruit can be applied for the value addition of food processing and pharmaceutical industry due to the high free radical scavenging activities (Schubert et al., 2007).
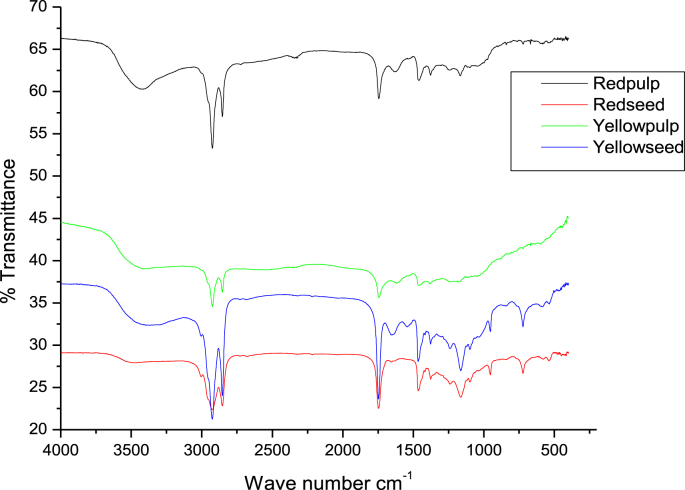
3.3. FTIR
From the FTIR spectrum, it was observed that similarities in the absorption bands with slight differences in the intensity of the peaks for the red and yellow X. americana fruit fleshs and seeds (Figure 2).
Figure 2.
IR spectrum of the flesh and seed of the red and yellow X. americana fruit cultivar.
The narrow and weak peak at 2834 cm−1 and 2828 cm−1 wave number for the red and yellow X. americana fleshs , respectively represents the presence of symmetric stretching vibrations of aldehyde (–CH2) bond and methoxy groups which confirmed the presence of phenolic compounds (Stuart, 2000). The peak at 2541 cm−1 and 2545 cm−1 for the red and yellow X. americana fleshs, respectively revealed the presence of S–H stretching bond (thiols) functional group (Stuart, 2005). The strong and narrow peak at 1076 cm−1 for both X. americana fleshs was attributed to the presence of –C–O stretching (primary alcohol) functional group (Stuart, 2000). Presence of aldehyde functional group confirmed the presence of xylose and arabinose which contribute to the sweetness of the X. americana fruit flesh. –C–O stretching (primary alcohol) may indicate presence of hydroxyl group in phenolic compound and sugar component.
The weak peak at 3462 cm−1 for the red X. americana seed revealed the presence of N–H stretches (amine) functional group (Stuart, 2000). According to the red and yellow X. americana seeds spectrum, the vibrational frequencies found around 3278 cm−1 for both seeds and 3047 cm−1 for the red seed represents the C–H stretching vibration of cis double bond (=CH) alkene functional groups (Stuart, 2000). The peak at 2838 cm−1 and 2837cm−1 for the red and yellow X. americana seeds respectively refers the presence of symmetric stretching vibrations of aldehyde (–CH2) bond (Stuart, 2000). The peak at 2535 cm−1 for both fruit seeds revealed the presence of S–H stretching bond (thiols) functional group (Stuart, 2000). The presence of –C≡N (nitrile) functional group attributed by a peak at 2254 cm−1 on both seeds spectrum (Stuart, 2000). A peak found at 1075 cm−1 and 1076 cm−1 for the red and yellow X. americana seeds, respectively indicated the presence of –C–O stretching functional group (Stuart, 2000). Aldehyde and C–O functional groups confirmed the presence of phenolic and flavone hydroxyl group.
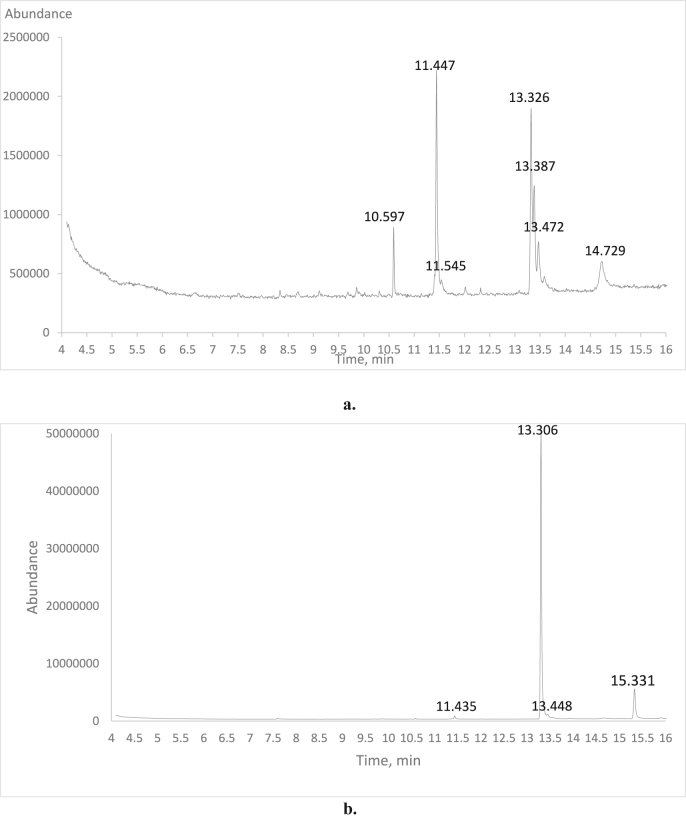
3.4. Fatty acid profile
Figure 3 shows GC-MS chromatogram for the fatty acid profile of the red X. americana cultivar for its flesh and seed. The dominant saturated fatty acid found in flesh was palmitic acid (29.78%), whereas the main unsaturated fatty acid detected was oleic acid, methyl ester (26.29%) (Table 2). Similarly, the main saturated fatty acid in seeds was stearic acid, methyl ester (1.72%) while the main unsaturated fatty acids were Elaidic acid, methyl ester (84.32%) and Ximeninic acid (12.78%). X. americana seed is one of the source for ximeninic acid that may not be found in most fruits. These results vary with the reported value of the same species by Eromosele and Eromosele (2002) and Tanko et al. (2017). This might be due to genetic variability and environmental variations.
Figure 3.
GC-MS chromatogram for the fatty acid profile of the red X. americana fruit cultivar; a. Flesh, b. Seed.
Table 2.
Fatty acid composition (%) of flesh and seed of the red X. americana fruit cultivar.
| Retention time (min) | Systematic name | Common name | Chemical formula | Flesh | Seed |
|---|---|---|---|---|---|
| 10.59 | Nonadecane | Nonadecane | C19H40 | 6.36 | ND |
| 11.44 | Hexadecanoic acid, methyl ester | Palmitic acid methyl ester | C17H34O2 | 29.78 | 1.18 |
| 11.54 | 2-Chloro-N-(hydroxymethyl)acetamide | Chloracetamide-N-methanol | C3H6ClNO2 | 1.77 | ND |
| 13.32 | 9-Octadecenoic acid (Z)-, methyl ester | Oleic acid, methyl ester | C19H36O2 | 26.29 | ND |
| 13.30 | 9-Octadecenoic acid, methyl ester (E) | Elaidic acid, methyl ester | C19H36O2 | ND | 84.32 |
| 13.38 | Octadecane, 2,6,10,14-tetramethyl | 2,6,10,14-Tetramethyloctadecane | C22H46 | 16.29 | ND |
| 13.47 | Methyl stearate | stearic acid, methyl ester | C19H38O2 | 8.18 | 1.72 |
| 14.72 | Bis(2-ethylhexyl) phthalate | Diethylhexylphthalate | C24H38O4 | 11.33 | ND |
| 15.33 | Methyl octadeca-9-yn-11-trans-enoic acid | Ximeninic acid | C18H30O2 | ND | 12.78 |
| TSFA | - | - | - | 73.71 | 2.9 |
| TUFA | - | - | - | 26.29 | 97.1 |
ND - Not detected. TSFA - Total saturated fatty acid; TUFA- Total unsaturated fatty acids.
The presence of high proportion of palmitic acid methyl ester in flesh makes it a good candidate for margarine and other fat based product manufacturing (Trabelsi et al., 2012). Oleic acid, methyl ester existence in flesh helps to maintain some situations on metabolism, cancer and cardiac diseases (Lee et al., 2012). On the other hand, the presence of high fraction of total unsaturated fatty acid makes the Ethiopian X. americana fruit seed a preferred input for the industrial application. The fatty acid profile result indicated the X. americana seed and flesh oil to be used for essential oil production.
3.5. Chemical compositions
The chemical composition result showed variation between the red and yellow X. americana fleshs and seeds (Table 3). Statistical differences between the two fruit seeds were observed in fat, and protein content. Their flesh also showed significant differences (P < 0.05) in ash content.
Table 3.
Chemical composition of red and yellow X. americana fruit cultivar expressed in dry weight basis except moisture.
| Chemical compositions | Red X. americana |
Yellow X. americana |
||
|---|---|---|---|---|
| Seed | Flesh | Seed | Flesh | |
| Moisture (% fw) | 15.62 ± 0.60b | 68 ± 0.59a | 14.09 ± 0.49b | 71 ± 0.70a |
| Crude fat (%) | 49.58 ± 0.37b | 7.78 ± 0.31c | 52.23 ± 0.43a | 7.25 ± 0.33c |
| Total Nitrogen | 2.39 ± 0.31b | 1.65 ± 0.25c | 2.65 ± 0.41a | 1.77 ± 0.23c |
| Crude protein () | 14.96 ± 1.34b | 10.32 ± 1.35c | 16.59 ± 2.25a | 11.11 ± 1.52c |
| Crude fiber (%) | 28.09 ± 1.34a | 5.58 ± 0.51b | 25.22 ± 1.93a | 4.41 ± 0.80b |
| Ash (%) | 1.45 ± 0.23c | 7.83 ± 0.25a | 1.32 ± 0.22c | 7.02 ± 0.22b |
| pH | 4.91 ± 0.1a | 2.84 ± 0.01b | 4.79 ± 0.09a | 2.75 ± 0.01c |
| Total soluble solid | ND | 26.82 ± 3.01a | ND | 25.57 ± 1.51a |
The same letters in the same row represents no significant difference (P < 0.05). All values are expressed as mean ± standard deviation (n = 3). fw - fresh weight.
ND: Not determined.
3.5.1. Moisture content
Significant differences were not observed for the moisture content of the red (68.0 %) and yellow (71.0%) X. americana fleshs. The moisture content of the flesh of both fruits in this study were in agreement with Sarmento et al. (2015) report studied in Brazil. The study fruit fleshs had also similar moisture content as compared to berry-like fruits of Hypericum androsaemum L which ranged from 71.60% in ripe (black) fruits to 76.62% in unripe (red) fruits (Caprioli et al., 2016). The red and yellow X. americana fruit seeds were also found to be (15.62%) and (14.09%) in fresh weight basis, respectively. There were no significant variations between moisture content of both X. americana seeds. The relative low moisture content indicated that the seeds will have excellent keeping quality, decreases the probability of microbial growth, unwanted fermentation, premature seed germination and many undesirable biochemical changes normally associated with these processes (Kaleta and Górnicki, 2013).
3.5.2. Crude protein
The yellow (11.11%) and red (10.32%) X. americana flesh protein content didn't show significant differences (P < 0.05). Significant regional differences were also observed on protein content of X. americana fruit from South America to Africa. The study fruit fleshs showed higher protein content as compared to the reported value by Lockett (2000); Muhammad et al. (2019) and Sarmento et al. (2015) for the yellow X. americana. This variation in protein content could be explained by agro-ecological variations such as climate, weather and soil type.
The yellow and red X. americana seeds protein content were 16.59% and 14.69% respectively, and were significantly different (P < 0.05). Both X. americana seeds presented higher protein content as compared to the fleshs. This might be due to the seeds used as protein storage site for the germination and seedling growth (Shewry et al., 1995). Besides, the high protein content of the seeds of X. americana makes suitable for use as feed stocks for animals.
3.5.3. Crude fat
The fat content for the red (7.78%) and yellow flesh (7.25%) had no significant differences. The fat content of X. americana fruit in this study was in agreement with the reported value by Muhammad et al. (2019) and Saeed and Bashier (2010). The result obtained from the fat analysis revealed that both fruit seeds contained higher fat content than their respective fleshs. The red seed value (49.58%) was significantly lower than the yellow fruit seed (52.23%). The seeds possessed almost seven times higher fat content than the fleshs, with significant variations between the two seeds (P < 0.05). High fat content of the seed indicated as the fat is stored in the seed (Nwofia et al., 2012).
3.5.4. Crude fiber
Significant variation was not observed in crude fiber content between the red (5.58%) and yellow (4.41%) X. americana fleshs. Lower crude fiber content of X. americana flesh (3%) was reported by Muhammad et al. (2019). The study fruit flesh had low fiber content when it is compared with similar genius X. caffra (Getachew et al., 2013). The Alphonsa variety mango fruit flesh in Ethiopia had higher crude fiber content (23.07 %) as compared to the study fruit flesh (Arumugam and Manikandan, 2011). The crude fiber content of the red (28.09%) and yellow (25.22%) X. americana seeds were not statistically different. The present study indicated that fiber from X. americana seeds can be a potential source for functional food development. The crude fiber availability in the fruits promotes treating diabetics commencement (Hassan et al., 2008).
3.5.5. Total ash
The ash content of the fruits could be as an index of mineral contents. There were statistical differences (P < 0.05) in ash content between both fruits which resulted 7.83% for the red and 7.02% for the yellow X. americana fleshs. These variations might be described by environmental factors such as climate, weather and soil type (Maathuis and Diatloff, 2013). It was observed that the present study had higher ash content as compared to previous study of X. americana fruit flesh by Sarmento et al. (2015). However, the study fruit had lower values of ash content with the reported value of Muhammad et al. (2019). The ash content of the red and yellow X. americana seeds were (1.45 %) and (1.32%) respectively. Both fruit seeds didn't show significant differences statistically. Sarmento et al. (2015) reported similar values for the ash content of X. americana seed.
3.5.6. pH and total soluble solids
The pH of the fruit appears to indicate its desirability in food processing industry in relation with imparting different flavor and odor (Silva et al., 2013). There was significant difference (P < 0.05) between the pH of the red (2.84) and the yellow (2.75) X. americana fruit fleshs. In contrast, no statistical variation was observed between the red (4.91) and yellow (4.79) X. americana seeds. The acidity of the fleshs were twice higher than their respective seeds and revealed the acidic nature of the fruit (Table 3). This acidic nature of the fruit was probably due to the oxidation of pyruvic acid in kreb's cycle which results high amount of organic acids in fruits (Silva et al., 2013). Previous studies reported similar pH value of yellow X. americana fruit Almeida et al. (2016); da Silva et al. (2008) and Sarmento et al. (2015).
The total soluble solid (TSS) showed no significant variation in which 26.82 0Bx was for the red and 25.57 0Bx was for the yellow X. americana flesh. We observed that the natural flavor and taste of the fruit was correlated with total soluble solid content. TSS taken as quality indicator in sweetness of fresh and processed horticultural products (Magwaza and Opara, 2015). Besides, de Oliveira et al. (2014) reported that the higher TSS plays a great role in minimizing the energy cost for food processing required for removal of retained water. The present study showed higher TSS as compared to similar fruit (Almeida et al., 2016); (da Silva et al., 2008); (Mora et al., 2009) and (Sarmento et al., 2015).
3.6. Mineral content
Minerals are vital for the maintenance of human health. The mineral content in this study showed variations between the yellow and red X. americana fruit cultivars (Supplementary material, Table 4). The seeds contained significantly higher amount of calcium and manganese as compared to the respective fleshs.
Table 4.
Mineral composition of seed and flesh of X. americana fruit (expressed on mg/100 g) of Sample.
| Fruit parts | Calcium | Magnesium | Iron | Manganese | Copper |
|---|---|---|---|---|---|
| Red flesh | 16.56 ± 2.08d | 98.27 ± 11.02a | 2.16 ± 13a | 0.74 ± 09c | 1.35 ± 04ab |
| Red seed | 49.55 ± 6.9a | 32.64 ± 4.0d | 1.15 ± 0.05b | 33.92 ± 0.58a | 0.083 ± 0.09c |
| Yellow flesh | 23.14 ± 1.8c | 76.3 ± 5.19b | 1.74 ± 0.8a | 1.07 ± 0.35c | 1.52 ± 0.02a |
| Yellow seed | 42.45 ± 14b | 41 ± 288c | 1.29 ± 0.01b | 26.73 ± 7.08b | 0.10 ± 0.5bc |
Means followed by the same letters in the same column for each part of the fruit do not differ by Tukey's test at 5% probability. All values are expressed as mean ± standard deviation (n = 3).
The calcium content of the red X. americana flesh (16.56 mg/100g) was significantly different (P < 0.05) from the yellow one (23.14 mg/100g). The red X. americana seed (49.55 mg/100g) was significantly different (P < 0.05) with the yellow X. americana seed (42.45 mg/100g) in calcium content. Lockett (2000) and Sarmento et al. (2015) reported lower calcium content of the yellow X. americana fruit as compared to this study. The Ethiopian X. americana fruit promotes the development of bones and teeth as well as for a number of metabolic functions in the body due to its considerable content of calcium.
The red X. americana flesh showed the higher concentration in magnesium content (98.27mg/100g) as compared to the yellow flesh (76.3 mg/100g). Muhammad et al. (2019) and Sarmento et al. (2015) observed lower amount of magnesium content in yellow X. americana flesh. On the other hand, Lockett (2000) reported the highest value of magnesium in yellow X. americana flesh, 145 mg/100g. Significant variation in magnesium content (P < 0.05) was observed in seeds and fleshs of the yellow and red X. americana fruits. The higher magnesium content in X. americana flesh helps in making cofactor for different crucial enzyme systems, it's needed for DNA and RNA synthesis increment and energy generation for mitochondria to carry out oxidative phosphorylation (Hegazy et al., 2019).
The iron content of yellow (1.74 mg/100g) and red (2.16 mg/100g) X. americana fleshs didn't show significant differences. No significant variations were also observed for the red (1.15 mg/100g) and yellow (1.29 mg/100g) X. americana fruit seeds. These values were higher than the reported value by Sarmento et al. (2015) and lower than Lockett (2000) report for the yellow X. americana. Consuming such fruit will helps in maintaining a healthy immune system and disease prevention and aiding in energy production due to its higher iron content (Beard and Dawson, 1997).
The red and yellow X. americana fleshs didn't show significant variations in manganese content (0.74 mg/100g) and (1.07 mg/100g), respectively. However, the red X. americana seed (33.92mg/100g) was significantly higher (P < 0.05) than the yellow seed (26.73 mg/100g) in manganese content. The result revealed that the study fruits had higher manganese content in seed and lower values in its flesh. Sarmento et al. (2015) reported lower values of manganese content than the present study. Sereno et al. (2018) reported lower manganese content (0.04 mg/100 g) of Solanum sessiliflorum Dunal wild fruit which had lower values as compared to the study fruits.
The copper content of the red (1.35 mg/100g) and yellow (1.52 mg/100g) X. americana fleshs were significantly different (P < 0.05). On the other hand, the red seed (0.083 mg/100g) had lower copper content than the yellow (0.10 mg/100g) X. americana fruit seed. Lockett (2000) and Sarmento et al. (2015) reported lower copper content in flesh and seed of yellow X. americana fruit as compared to the current study.
The X. americana fruit parts investigated in the present study showed variation in their mineral content. The fruits might be considered as a good source for minerals which could be vital to improve the human body immune system and helps in fighting many diseases.
4. Conclusion
The results of the present study illustrate characterization of the red and yellow X. americana fruit grown in Ethiopia. The yellow X. americana fruit had higher flavonoid content while the red X. americana fruit contained higher total phenol content. Both the red and yellow X. americana fruit fleshs showed more than 90% of DPPH∗ free radical scavenging. The total phenol content had significant effect for the higher antioxidant activity of the red X. americana fruit. The study also revealed that the seed part of X. americana fruit had higher total unsaturated fatty acid as compared to the flesh. Palmitic acid methyl ester was the major fatty acid detected in the flesh whereas elaidic acid, methyl ester was the dominant fatty acid observed in the seed. The variations in nutritional and health imparting compounds might be explained by the genetic variability of the two colored fruits. This study confirmed that X. americana fruits grown in Ethiopia are rich in nutrients, phytochemicals contents and essential fatty acids. The fruits need to be fully studied to exploit its medicinal values to be used as functional foods.
Declarations
Author contribution statement
Asfawosen Mamo Bazezew: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Shimelis Admassu Emire, Mulugeta Teamir Sisay: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Adama Science and Technology University and Addis Ababa University.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Ethiopian Environment and Forest research institute, especially Ato Zewdu Yilma for his sample collection guidance. Besides we would like to thank department of Food Science and Nutrition at Addis Ababa University for laboratory facility support.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Adom K.K., Liu R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002;50(21):6182–6187. doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- Al-Alawi R.A., Al-Mashiqri J.H., Al-Nadabi J.S., Al-Shihi B.I., Baqi Y. Date palm tree (Phoenix dactylifera L.): natural products and therapeutic options. Front. Plant Sci. 2017;8:845. doi: 10.3389/fpls.2017.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M.L.B., de Souza Freitas W.E., de Morais P.L.D., Sarmento J.D.A., Alves R.E. Bioactive compounds and antioxidant potential fruit of Ximenia americana L. Food Chem. 2016;192:1078–1082. doi: 10.1016/j.foodchem.2015.07.129. [DOI] [PubMed] [Google Scholar]
- AOAC . AOAC; Gaithersburg, MD: 2000. Official Methods of Analysis. [Google Scholar]
- Arumugam R., Manikandan M. Fermentation of pretreated hydrolyzates of banana and mango fruit wastes for ethanol production. Asian J. Exp. Biol. Sci. 2011;2(2):246–256. [Google Scholar]
- Beard J.L., Dawson H., Piñero D.J. Iron metabolism: a comprehensive review. Nutr. Rev. 1996;54(10):295–317. doi: 10.1111/j.1753-4887.1996.tb03794.x. [DOI] [PubMed] [Google Scholar]
- Bouhlali E. Phytochemical compositions and antioxidant capacity of three date (Phoenix dactylifera L.) seeds varieties grown in the South East Morocco. J. Saudi Soc. Agric. Sci. 2017;16:350–357. [Google Scholar]
- Brand-Williams W., Cuvelier M.-E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995;28(1):25–30. [Google Scholar]
- Caprioli G. Polar constituents and biological activity of the berry-like fruits from Hypericum androsaemum L. Front. Plant Sci. 2016;7:232. doi: 10.3389/fpls.2016.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva G.G. Wild plum fruit characterization (Ximenia americana L.) Rev. Bras. Frutic. 2008;30(2):311–314. [Google Scholar]
- Dadi D.W., Emire S.A., Hagos A.D., Assamo F.T. Influences of different drying methods and extraction solvents on total phenolic and flavonoids, and antioxidant capacity of Moringa stenopetala leaves. J. Pharmacogn. Phytochem. 2018;7(1):962–967. [Google Scholar]
- David F., Sandra P., Vickers A. Vol. 19. Agilent Technologies; Palo Alto, CA: 2005. Column Selection for the Analysis of Fatty Acid Methyl Esters. Food Analysis Application; p. 19. [Google Scholar]
- de Freitas V.A.P., Fernandes A., Oliveira J., Teixeira N., Mateus N. A review of the current knowledge of red wine colour. Oeno One. 2017;51(1) [Google Scholar]
- de Oliveira G.A., Bureau S., Renard C.M.-G.C., Pereira-Netto A.B., de Castilhos F. Comparison of NIRS approach for prediction of internal quality traits in three fruit species. Food Chem. 2014;143:223–230. doi: 10.1016/j.foodchem.2013.07.122. [DOI] [PubMed] [Google Scholar]
- Eromosele C., Eromosele I. Fatty acid compositions of seed oils of Haematostaphis barteri and Ximenia americana. Bioresour. Technol. 2002;82(3):303–304. doi: 10.1016/s0960-8524(01)00179-1. [DOI] [PubMed] [Google Scholar]
- Erşan S. Phytochemical and mineral composition of fruits and seeds of wild-growing Bactris guineensis (L.) HE Moore palms from Costa Rica. J. Food Compos. Anal. 2020;94:103611. [Google Scholar]
- Everette J.D. Thorough study of reactivity of various compound classes toward the Folin− Ciocalteu reagent. J. Agric. Food. Chem. 2010;58:8139–8144. doi: 10.1021/jf1005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz V., Morales P., Ruiz-Rodríguez B.M., TorijaIsasa E. Nutrients and Bioactive Compounds in Wild Fruits Through Different Continents. 2017. pp. 263–314. [Google Scholar]
- Getachew G.A. Dietary values of wild and semi-wild edible plants in Southern Ethiopia. Afr. J. Food Nutr. Sci. 2013;13(2) [Google Scholar]
- Hassan L., Dangoggo S., Umar K., Saidu I., Folorunsho F. Proximate, minerals and anti-nutritional factors of Daniellia oliveri seed kernel. Chemclass J. 2008;5:31–36. [Google Scholar]
- Hegazy A.K. Chemical ingredients and antioxidant activities of underutilized wild fruits. Heliyon. 2019;5(6) doi: 10.1016/j.heliyon.2019.e01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich M., Nebel S., Leonti M., Rivera D., Obón C. ‘Local Food-Nutraceuticals’: bridging the gap between local knowledge and global needs. Forum. Nutr. 2006:1–17. doi: 10.1159/000095205. [DOI] [PubMed] [Google Scholar]
- Holcroft D.M., Kader A.A. Controlled atmosphere-induced changes in pH and organic acid metabolism may affect color of stored strawberry fruit. Postharvest Biol. Technol. 1999;17(1):19–32. [Google Scholar]
- Hunde D. 2012. Uses and Management of Ximenia Americana, Olacaceae in Semi-arid East Shewa, Ethiopia. [Google Scholar]
- Islary A., Sarmah J., Basumatary S. Proximate composition, mineral content, phytochemical analysis and in vitro antioxidantactivities of a wild edible fruit (Grewia sapidaRoxb. ex DC.) found in Assam of North-East India. Am. J. Physiol. Biochem. Pharmacol. 2016;5(1):1–11. [Google Scholar]
- Kaleta A., Górnicki K. Criteria of determination of safe grain storage time-a review. Adv. Agrophys. Res. 2013:295–318. [Google Scholar]
- Lamien-Meda A. Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules. 2008;13(3):581–594. doi: 10.3390/molecules13030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N.H.T. Bioactive polyphenols in Ximenia americana and the traditional use among Malian healers. J. Ethnopharmacol. 2012;139(3):858–862. doi: 10.1016/j.jep.2011.12.031. [DOI] [PubMed] [Google Scholar]
- Lee B.-Y., Shin D.H., Cho S., Seo K.-S., Kim H. Genome-wide analysis of copy number variations reveals that aging processes influence body fat distribution in Korea Associated Resource (KARE) cohorts. Hum. Genet. 2012;131(11):1795–1804. doi: 10.1007/s00439-012-1203-1. [DOI] [PubMed] [Google Scholar]
- Lockett C.T. 2000. Nutritional Consequences of Food-Related Behaviors during Drought and Chemical Composition of Edible Wild Plants Consumed by Groups Most Vulnerable during the Dry Season. A Study of the Rural Fulani of Northeast Nigeria. [Google Scholar]
- Maathuis F.J., Diatloff E. Springer; 2013. Roles and Functions of Plant mineral Nutrients, Plant Mineral Nutrients; pp. 1–21. [DOI] [PubMed] [Google Scholar]
- Magwaza L.S., Opara U.L. Analytical methods for determination of sugars and sweetness of horticultural products—a review. Sci. Hortic. 2015;184:179–192. [Google Scholar]
- Maikai V., Maikai B., Kobo P. Antimicrobial properties of stem bark extracts of Ximenia americana. J. Agric. Sci. 2009;1(2):30. [Google Scholar]
- Metoui M., Essid A., Bouzoumita A., Ferchichi A. Chemical composition, antioxidant and antibacterial activity of Tunisian date palm seed. Pol. J. Environ. Stud. 2019;28(1) [Google Scholar]
- Mohamed K., Feyissa T. In vitro propagation of Ximenia americana L. from shoot tip explants: a multipurpose medicinal plant. Sinet. 2020;43(1):1–10. [Google Scholar]
- Mora V.H.F., Franco-Mora O., López-Sandoval J.A., de Jesús Pérez-López D., Balbuena-Melgarejo A. Characterization of wild plum (Ximenia americana L. var. americana; Olacaceae) fruit growing at Tepexi de Rodríguez, Puebla, Mexico. Genet. Resour. Crop Evol. 2009;56(5):719–727. [Google Scholar]
- Muhammad A., Haruna S.Y., Birnin-Yauri A.U., Muhammad A.H., Elinge C.M. Nutritional and anti-nutritional composition of Ximenia americana fruit. Am. J. Appl. Chem. 2019;7(4):123–129. [Google Scholar]
- Nwofia G.E., Ojimelukwe P., Eji C. Chemical composition of leaves, fruit flesh and seeds in some Carica papaya (L) morphotypes. Int. J. Med. Aromatic Plants. 2012;2(1):200–206. [Google Scholar]
- Park Y.-S., Guo S., Makarov N.S., Klimov V.I. Room temperature single-photon emission from individual perovskite quantum dots. ACS Nano. 2015;9(10):10386–10393. doi: 10.1021/acsnano.5b04584. [DOI] [PubMed] [Google Scholar]
- Saeed A., Bashier R. Physico-chemical analysis of Ximenia americana L. seed oil and structure elucidation of some chemical constituents of its seed oil and fruit flesh. J. Pharmacogn. Phytotherapy. 2010;2(4):49–55. [Google Scholar]
- Sarmento J.D.A. 7. Bioactive compounds and antioxidant activity of Ximenia americana coming from different collection sites. Archivos Latinoamericanos de Nutrición. 2015;65(4) [Google Scholar]
- Sarmento J.D.A., de Morais P.L.D., de Souza F.I., de Miranda M.R.A. Physical-chemical characteristics and antioxidant potential of seed and flesh of Ximenia americana L. from the semiarid region of Brazil. Afr. J. Biotechnol. 2015;14(20):1743–1752. [Google Scholar]
- Schubert A. Comparison of antioxidant activities and total polyphenolic and methylxanthine contents between the unripe fruit and leaves of Ilex paraguariensis A. St. Hil. Die Pharmazie-Int. J. Pharmaceuti. Sci. 2007;62(11):876–880. [PubMed] [Google Scholar]
- Sereno A.B. Mineral profile, carotenoids and composition of cocona (Solanum sessiliflorum Dunal), a wild Brazilian fruit. J. Food Compos. Anal. 2018;72:32–38. [Google Scholar]
- Shewry P.R., Napier J.A., Tatham A.S. Seed storage proteins: structures and biosynthesis. Plant Cell. 1995;7(7):945. doi: 10.1105/tpc.7.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva F., Ribeiro W., França C., Araújo F., Finger F. XI International Controlled and Modified Atmosphere Research Conference. vol. 1071. 2013. Action of potassium permanganate on the shelf-life of Cucumis anguria fruit; pp. 105–111. [Google Scholar]
- Silva K., Sirasa M. Antioxidant properties of selected fruit cultivars grown in Sri Lanka. Food Chem. 2018;238:203–208. doi: 10.1016/j.foodchem.2016.08.102. [DOI] [PubMed] [Google Scholar]
- Stuart B. Kirk-Othmer Encyclopedia of Chemical Technology; 2000. Infrared Spectroscopy. [Google Scholar]
- Tanko E. Physico-chemical, fatty acid profile and amino acid composition of the fruit flesh and seeds of Ximeni aamericana L.(Tallow plum) obtained in Niger state, Nigeria. Int. J. Food Chem. 2017;1:30–34. [Google Scholar]
- Trabelsi H. Total lipid content, fatty acids and 4-desmethylsterols accumulation in developing fruit of Pistacia lentiscus L. growing wild in Tunisia. Food Chem. 2012;131(2):434–440. [Google Scholar]
- Vongsak B., Sithisarn P., Gritsanapan W. Bioactive contents and free radical scavenging activity of Moringa oleifera leaf extract under different storage conditions. Ind. Crop. Prod. 2013;49:419–421. [Google Scholar]
- Xu D. A hierarchical N/S-codoped carbon anode fabricated facilely from cellulose/polyaniline microspheres for high-performance sodium-ion batteries. Adv. Energy Mater. 2016;6(6):1501929. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.