Abstract
A thorough study was undertaken of the synthesis of natural rubber-silica treated with bis-(3-triethoxysilylpropyl) tetrasulfane (NR/TSi) vulcanized using electron beam irradiation (EB) and sulfur by varying the EB dose. The surface treatment of silica was confirmed using Fourier-transform infrared spectroscopy and scanning electron microscopy images. Composites were cast and vulcanized in film and latex forms compared with sulfur vulcanization. Investigation covered the mechanical properties, thermal stability, swelling resistance, and degradation under heat and humidity testing of the NR/TSi composites. It was found that the TSi had great dispersal in the NR matrix. TSi in NR matrix had a positive effect on mechanical properties, swelling in water and toluene, and thermal stability. Increasing the radiation intensity up to 250 kGy led to superior mechanical properties but for further increase in the radiation intensity, the tensile strength dropped. Degradation under thermal and humidity testing showed that the un-vulcanized composite had higher physical degradation than the vulcanized samples. Therefore, NR/TSi vulcanized using 200 kGy EB vulcanized in latex form had the greatest mechanical properties for various applications without producing any residual vulcanizing agent.
Keywords: Treated silica, Natural rubber composites, Electron beam vulcanization, Sulfur vulcanization
Highlights
-
•
Silane-69 treated silica/natural rubber composite was irradiated by electron beam.
-
•
SEM images indicated good dispersion of treated silica in the NR matrix.
-
•
10 phr NR/TSi at 200 kGy in latex form had the best mechanical properties.
-
•
EB vulcanized NR/TSi composites had less chemical residue than those by sulfur.
-
•
Degradation under thermal and humidity was performed and compared with TGA.
Treated silica; Natural rubber composites; Electron beam vulcanization; Sulfur vulcanization
1. Introduction
Natural rubber is natural polymer which is mainly isoprene. It contains water and some impurities such as organic compounds. The good properties of natural rubber are its high tensile strength, elongation, tear resistance, and abrasion resistance. It is excellent in resilience and compression. However, it is not a suitable product when subjected to aging by sunlight, ultraviolet light, heat, oxygen, and ozone explosion conditions. It also has poor petroleum and solvent resistances [1]. To overcome these disadvantages, various methods have been applied, such as rubber vulcanization with vulcanizing agent and radiation [2] reinforcement with fillers (such as silica, nano clay, carbon black, and etc) [3, 4] and treatment in the latex form. Natural rubber reinforced with silica as a filler is an option for industrial use [5, 6]. Silica is a renewable material that can improve mechanical properties, including tensile strength, elongation, and swelling parameters (swelling ratio) [7]. It is also used to improve thermal stability [8]. However, a problem with silica is that the compatibility when using as filler is poor due to its agglomeration in the latex matrix [7]. The surface of silica contains large amounts of the silanol functional group (Si–OH) that results in strong particle-particle interaction on the surface. Researchers have tried to improve or modify the filler surface to solve this problem [9, 10].
Due to the poor compatibility of silica and the rubber matrix, researchers have used chemicals such as silane coupling agents. The most widely used one are (bis-(3-triethoxysilylpropyl)) tetrasulfane (Si-69) [11,12] and 3-thiocyana-topropyl triethoxy silane (Si-264) [13]. The coupling agent provide physical improvement [14] and can enhance the chemical linkage of the NR-Si interaction by reacting with both filler and matrix [15]. One side of the hydrolysable alkoxy group react with silanol group attached on the silica surface in mixing process to form siloxane linkages and releasing alcohol, leading to reduced particle-particle interaction. At the other end is an organo-functional group that contains sulfur. Sulfur can participate in the vulcanization process and contribute to increase sulfur linkages with the matrix rubber [16]. This coupling agent could also enhance filler-natural rubber interaction and reduce particle aggregation [17].
Process ability and physical properties of NR-Si compound can be improved by other methods such as vulcanization by adding a vulcanizing agent (such as sulfur or peroxide) [18, 19] and radiation using gamma rays [20], electron beam irradiation [21], or a natural rubber latex treatment method [22].
Nowadays, there is a global focus on green technology and green products, so vulcanization of rubber using electron beam irradiation is one of the green techniques being widely used with jewelry to enhance value and used with rubber and polymers to increase the crosslink densities [23]. However, there has been a limited numbers of studies on natural rubber composites using electron beam irradiation. An electron beam was used to increase the grafting of rubbers and a polystyrene copolymer [24]. Electron beam was used to improve many mechanical properties such as that in styrene butadiene/natural rubber to increase resistance to abrasion [25]. A low dose of electron beam was used to enhance mechanical and thermal properties of vulcanized natural rubber/polymers [26]. The different aging times and electron beam radiation doses of silica/rubber were studied focusing on Mooney relaxation [27]. However, the degradation of natural rubber with silica was vulcanized between sulfur and electron beam has not been studied.
Electron beam irradiation of montmorillonite particles loaded in ethylene vinyl acetate waste rubber was studied at irradiation doses of up to 150 kGy. It was found that the crosslinking networks from the electron beam irradiation resulted in gradually increase in tensile strength of the waste rubber blends [28]. Furthermore, it was found that too much irradiation decreased the tensile properties. Combining the effects of microwave heating and electron beam irradiation to reduce the intensity of the electron beam dose and the irradiation time was studied. It was found that vulcanization based on electron beam irradiation could be obtained at a lower irradiation dose by using microwave energy [29].
This current work studied the mechanical and physical properties of silica reinforced natural rubber that had been vulcanized using sulfur and electron beam irradiation in latex and film forms. Silica contents of 10 phr was used and electron beam doses of 100, 150, 200, and 250 kGy were studied to determine the optimum values for a silica/natural rubber system.
2. Materials and methods
2.1. Materials
In the preparation of the natural rubber/silica compound, the materials used were listed; Precipitated silica (purity 98%) was supplied by TTK Science Co., Ltd. High ammonia concentrated latex (60% DSC) was provided by the Rubber Research Unit, National Metal and Materials Technology Center, Thailand. Latex MST is 138 s (Test following ISO 35:2004). Deionized water (15.2 MΩ) was obtained from the Department of Chemical Engineering Kasetsart University, Bangkok, Thailand. 50% zinc oxide (ZnO), 10% potassium hydroxide (KOH), 50% zinc diethyldithiocarbamate (ZDEC), and 20% potassium lualate (K-lualate) were obtained from the Rubber Research Institute, Thailand. Bis-(3-triethoxysilylpropyl) tetrasulfide (Si69, 98% purity), ethanol (EtOH, 99.9% purity), and acetone (99.5% purity) which were supplied by Sigma-Aldrich Co., Ltd.
2.2. Preparation of surface treated silica with silane 69 (TSi)
First, 5%wt silane coupling agent (Si-69) was mixed with 800 ml ethanol, followed by stirring using a hot-plate magnetic stirrer for 1 h at 30 °C. After that, 100 g silica was put gradually into the mixture and continually stirred for 1 h. Then, it was dried in an oven at 100 °C for 12 h. It was called treated silica (TSi).
2.3. Preparation of NR/TSi composite film
Concentrated latex (60% DSC) was stirred using a magnetic stirrer before 10 phr treated silica (TSi) was added into the latex. After that, 5 ml deionization water was poured into the NR/TSi and the mixture was stirred for 1 h. Then, the composite solution was cast using a glass plate frame and put in an oven for 24 h at 55 °C. This was called Un-vulcanized (NV) and it was used as a control sample.
2.4. Preparation of NR/TSi composite vulcanized with sulfur
Concentrated latex (60% DSC) was stirred using a magnetic stirrer. Then, 5 phr treated silica was added into the concentrated latex. After that, 5 ml of deionized water was added into the NR/TSi and stirred for 1 h. Then, different vulcanizing agents (KOH, K-lualate, ZnO, ZDEC, or sulfur) were added in the ratios shown in Table 1. Each sample was stirred for 1 h and then the composite solution was cast using a glass plate frame and put in an oven for 6 h at 30 °C and continued at for 24 h 120 °C. The dry sample was named ‘sulfur’. The untreated silica with natural rubber compound was used as a control.
Table 1.
Formulation of vulcanizing agent.
| Compound in formulation | Dry weight (phr)a) |
|---|---|
| Natural rubber | 100 |
| Silica | 10 |
| 50% Sulfur | 1 |
| 10% KOH | 0.5 |
| 20% K-lualate | 1 |
| 50% ZnO | 1 |
| 50% ZDECb) | 0.8 |
phr: parts per hundred rubber.
ZDEC is Dithiocarbamates.
2.5. Preparation of NR/TSi composite vulcanized using EB in latex and film forms
First, concentrated latex (60% DSC) was stirred using a magnetic stirrer. Then, 5 phr of treated silica was added into the concentrated latex. After that, 5 ml of deionized water was added into the NR/TSi and stirred for 1 h. Then, the NR/TSi composite latex was poured into a microwave box before the irradiation process. EB accelerator used was Linear accelerator electron beam by Mevex Corporation Ltd. MB10-50 Canada. The energy range was 10 MeV and the dose rate used was 25 kGy/round. The vulcanized NR/TSi composites by EB irradiation were irradiated at doses of 150, 200, or 250 kGy using accelerated energy at 8 MeV and room temperature. This method produced the latex form. The dry sample was named ‘EB150L, EB 200L, EB250L for samples radiated in latex form irradiated at doses of 150, 200, or 250 kGy, respectively. For the film form, NR/TSi latex mixture was cast using a glass plate frame and put in an oven for 24 h at 55 °C to obtain composite film before the irradiation process. The dry sample was named ‘EB150F, EB 200F, EB250F for samples radiated in film form irradiated at doses of 150, 200, or 250 kGy, respectively.
2.6. Characterization of treated silica
The morphology of the precipitated Si-69 treated and untreated silica was investigated by a scanning electron microscope (Quanta 450 FEI) at a 15 kV accelerating voltage in vacuum. Precipitated silica and NR composites were prepared before surface coating with gold. The composition of the treated silica was analyzed using a Fourier-transform infrared (FTIR) spectrophotometer (Tensor27) in the wavelength range between 400–4000 cm−1.
2.7. Characterization of composites
2.7.1. Morphology study
The morphology of silica-reinforced natural rubber was examined using an SEM (Quanta 450 FEI) at 15 kV accelerating voltage in vacuum condition. The samples were prepared in thin sheets film and surface coated with gold. Thermogravimetric analysis (TGA) was used to observe the thermal stability of NR composites. It was conducted on an SDTA 851e thermogravimetric analyzer at a temperature range of 30–1,000 °C with flow of nitrogen at a 10 °C/min heating rate.
2.7.2. Mechanical property measurement
For elongation at break and tensile strength testing, five dumbbell-shaped samples were prepared from fabricated film sheets following ISO 37 type 2 (Standard method for vulcanized and thermoplastic rubbers in order to determine the tensile stress-strain properties). The test was performed using a universal testing machine at a constant crosshead speed of 500 mm/min. Specimens were analyzed and the average and standard deviation were calculated.
2.7.3. Swelling measurement
The vulcanized NR composites was tested to determine the crosslink density using swelling ratio. Each sample was cut into a small sheet of 1 cm × 1 cm × 0.5 cm following ASTM D471 standard (This testing procedure is for evaluation of comparative ability of rubber and rubber-like compositions to withstand the effect of liquids exposure). The samples were soaked in toluene (25 ml) at room temperature for 24 h. Then, the sample weights before and after soaking in toluene were recorded. Swelling ratio and crosslink density were then calculated according to the Flory and Rehner Eqs. (1) and (2), respectively [24, 30, 31, 32].
| Q = 1 + (ρR/ ρT) (Ws/Wo) – (ρR/ ρT) | (1) |
where Q is the swelling ratio, Wo and Ws are the sample weight before and after immersion in toluene, respectively. For toluene, ρT is 0.867 g/ml; for NR, ρR is 0.915 g/cm3.
| V = kQ−5/3 | (2) |
where Q is the swelling ratio, k is a constant (9.06 ×1020), and V is the crosslink density (cl/mol) and k can be calculated as in Eq. (3).
| k = [(0.5 - μ) NA/Vs] | (3) |
where μ is rubber-solvent interaction parameter (which is 0.34 for natural rubber with toluene solvent) [33]. Vs is the molar volume of toluene (106.29 cm3·mol−1). NA is Avogadro's number (6.02 × 1023).
2.7.4. Water absorption
For the swelling test, each sample had dimensions of about 1 cm × 1 cm. Each sample was soaked in water for 24 h at room temperature. Sample weights before and after soaking in water were recorded. Water absorption ratio was calculated based on Eq. (4).
| Water absorption ratio = wt. before soaking in toluene/wt. after soaking in toluene | (4) |
2.7.5. Degradation test
Degradation testing of the NR composites was determined by focusing on degradation under thermal and humid conditions adopted from Tomita et al (2017) [34]. Samples were cut into a 2.5 cm × 2.5 cm sheet size and put in a humidity chamber (Quanta 450) at 70 °C and 95% relative humidity condition for 720 h.
3. Results and discussion
3.1. Treated silica characterization
3.1.1. Morphology of treated silica
Morphology of untreated and Si-69 treated silica was characterized using SEM and their results are shown in Figure 1. The particle size of untreated silica was larger than that of treated silica due to hydrogen bond interactions between the silica particles themselves and the silanol group (Si–OH) on the surface of untreated silica. This caused particle accumulation [11, 17].
Figure 1.
SEM images of (a) Si-69 treated silica (TSi) and (b) untreated silica (Si).
3.1.2. Fourier transform infrared (FT-IR) analysis of treated silica (TSi) compare with untreated silica (Si)
The FTIR spectra of Si-69 treated silica and untreated silica are depicted in Figure 2. The peak of absorption bands occurred in both untreated and treated silica at 3450 cm−1 existed from the O–H stretching of the silanol group. The absorption band at 1630 cm−1 was assigned to stretching vibration of O–H bond peak, while that at 1115 cm−1 corresponded to the asymmetric stretching of Si–O–Si group. The absorption bands at around 472 and 795 cm−1 showed blending vibration of siloxane groups (Si–O–Si). For treated silica with Si-69, there were additional absorption bands at 2925 and 2970 cm−1 assigned to stretching vibration of the –CH3 and –CH2 groups, respectively, of silane coupling with Si-69 on the silica surface [35].
Figure 2.
FT-IR spectra of (a) Si-69 (b) untreated silica (c) treated silica with Si-69.
3.2. NR composites characterization
The silane-69 treated silica particle content in NR (NR/TSi) was in the range 0–20 phr. Their mechanical properties were not shown here. However, Si-69 treated silica at 10 phr in NR had the highest mechanical properties. Therefore, in this paper, Si-69 treated silica content at 10 phr. in NR (NR/TSi) was prepared for the study of composite properties following vulcanizing using EB irradiation.
3.2.1. Mechanical properties
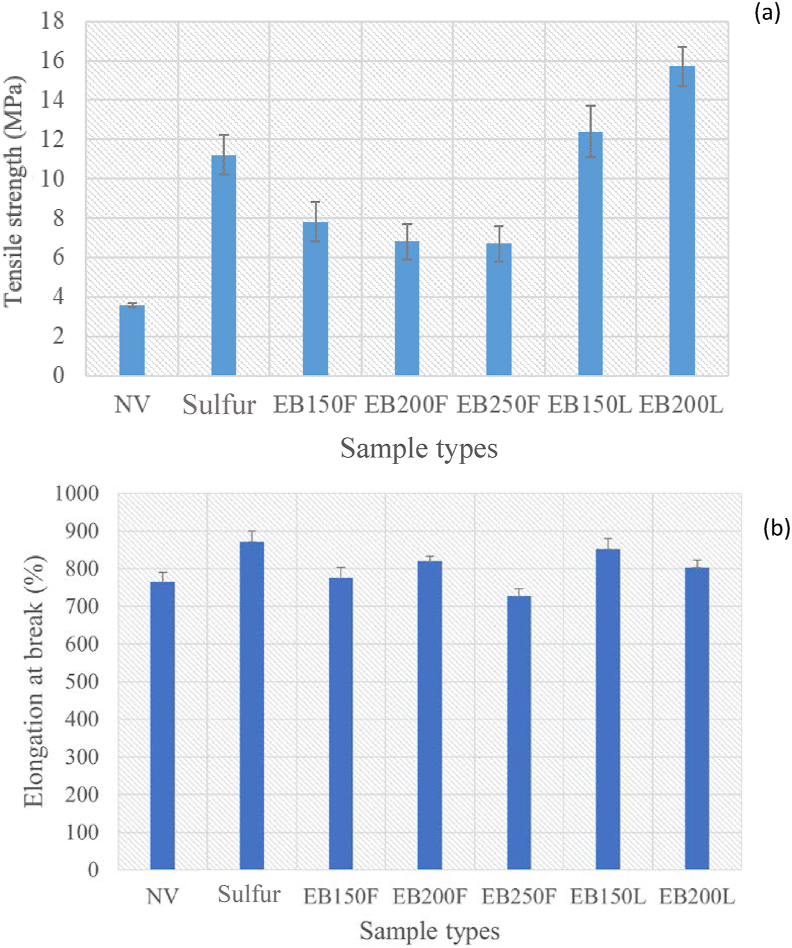
Figure 3 shows the tensile strength of NR/TSi 10 phr. The tensile strengths of NR/TSi 10 phr vulcanized with sulfur and using EB radiation was higher than that of the un-vulcanized composite due to the vulcanization process forming crosslink networks between rubber molecules. An effect of EB radiation vulcanization was observed between the film and latex forms, with the highest tensile strength of 15.7 ± 1 MPa recorded in the composite vulcanized using EB radiation at 200 kGy in the latex form. This was due to the EB radiation of NR/TSi in the latex form being associated with radiolysis which generated NR radicals from NR molecules and OH radicals from water molecules in the latex. The H radicals and hydrated electrons from the water radicals in the radiolysis process enhanced the efficiency of crosslinking of NR [36]. Elongation at break of NR composites irradiated by 150, 200, and 250 kGy, represents in Figure 3 (b). It was found that elongation at break decreased when EB irradiation dose increased. The crosslinking degree was generally increased when increased the irradiation dose [36]. It was in agreement with the previous work of Jansomboon et al. (2019) [23] who reported that the NR composites filled with the mixture of silica/graphene irradiated by EB irradiation in the range of 100–250 kGy had the elongation at break decreased when EB doses increased due to increase in crosslink density in the composites.
Figure 3.
(a) Tensile strength and (b) Elongation at break of un-vulcanized NR/10 phr TSi composites (NV) and vulcanized NR/TSi composite.
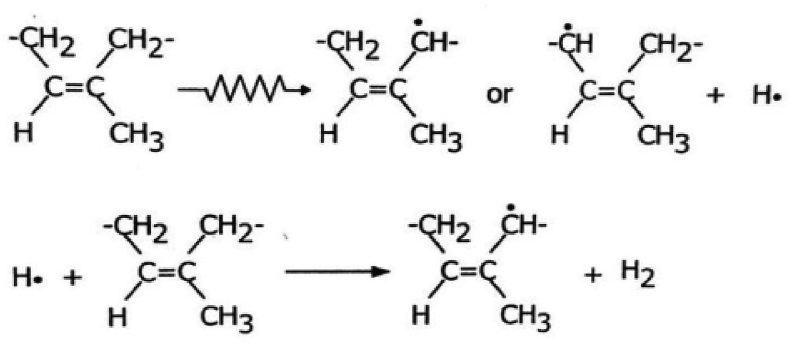
The reaction is shown in Figure 4. On the other hand, there was an absence of water in the film form sample, resulting in a low crosslink density compared to the latex form sample. The preparation method for vulcanization and the vulcanization method had strong effects on the tensile strength of NR/TSi composites [37]. Composite vulcanized with sulfur had a lower tensile strength than that vulcanized using EB radiation in both the latex and film forms due to the crosslink network of C–C bonding formed in the NR/TSi latex with EB radiation [38]. On the other hand, a crosslink network of C–S bondingwas formed by sulfur vulcanization [39]. Due to the C–C bond having a higher energy bond than the C–S bond [40], NR/TSi composites vulcanized using EB radiation in the latex form showed higher tensile strength than those vulcanized with sulfur.
Figure 4.
The radiolysis of cis, 1,4 polyisoprene to form free radical sites.
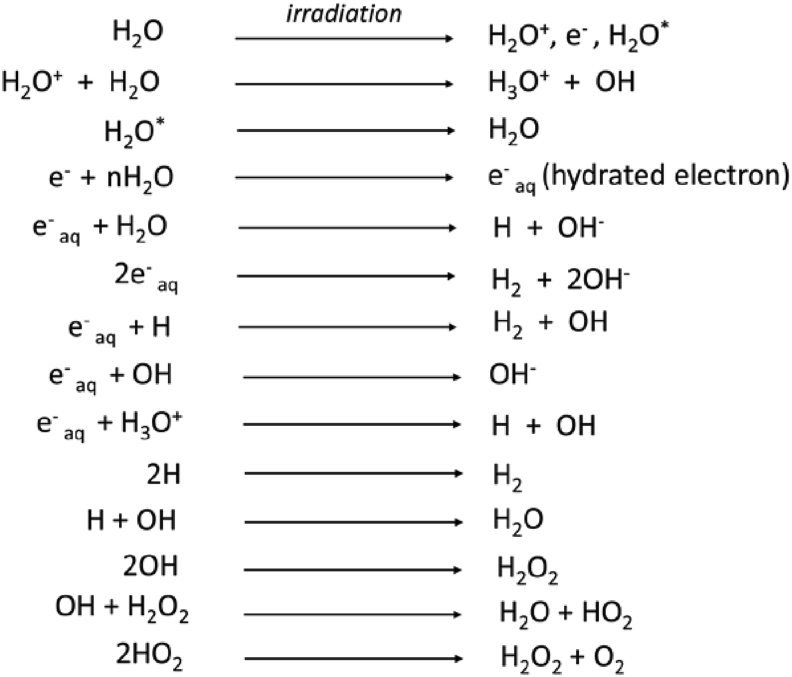
The mechanism of the effect of EB irradiation on NR matrix might be that EB radiation of NR/TSi in the latex form was associated with radiolysis which generated NR radicals from NR molecules and OH radicals from water molecules in the latex liquid. The concise chemical reaction of crosslinking is shown in Figure 4. When the irradiation was applied for NR molecule, an appearance of allyl radicals could be observed. These NR radicals can be final products via various reaction such as recombination, hydrogen formation, addition of C =C bonds. The short-lived free radicals added into C=C bonds was the results in crosslink formation in molecular chain. Additionally, the radiolysis of water occurred by irradiation in NR latex, represented in Figure 5, could affect degree of crosslinking. The hydrated electrons and H radicals from water radicals occurred by radicalization reaction in the radiolysis process can penetrate into NR molecular chains and then react with molecule of NR. This results in enhancement of crosslinking efficiency of NR [36]. It is commonly recognized that the high irradiation energy can be applied for crosslink and curing in elastomer materials such as natural rubber [41].
Figure 5.
Radiolysis reaction of EB radiation vulcanization.
3.2.2. Swelling property
A sample of NR/TSi vulcanized using an electron beam at 200 kGy was selected for the swelling test. The NR/TSi sample vulcanized with sulfur in this work used a low sulfur formula to be environmentally friendly. Hosseini et al. showed that a high sulfur content resulted in high crosslinking [42]. Where the formula of the sulfur system is changed, the crosslink density may be higher or lower than the standard [43].
Figure 6 presents the swelling ratios and crosslink densities of sulfur and 200 kGy electron beam irradiated natural rubber reinforced with 10 phr-treated silica (NR/TSi) in film and latex forms compared with the un-vulcanized composite. The composites were examined using immersion in toluene. The rubber molecules were able to absorb the toluene solvent leading to swelling of the NR composites [44, 45]. Vulcanization using electron beam irradiation caused the formation of carbon-carbon bond interactions between rubber molecules and vulcanization with sulfur caused the formation of monomeric polysulfides [39]. The interaction of sulfur with the composite rubbers to form polymeric polysulfides led to vulcanization of NR composites with high crosslink densities. Comparing between EB200F (film) and EB200L (latex), the NR latex became rigid or film after the heat treatment process (at 55 °C for 24 h) due to water evaporation. Water and moisture in such NR material were very low when compare to NR latex. As a consequence, during the irradiation process, crosslink network occurred in NR film compound cannot be enhanced by hydrolysis due to lack of water. On the other hands, the radical from hydrolysis in NR latex occurred by irradiation increased the crosslink reaction of NR latex compound resulting in the large crosslink density. This was the reason of lower crosslink density of NR film than that of NR latex.
Figure 6.
NR/10 phr TSi composites: (a) crosslink density; and (b) swelling ratio.
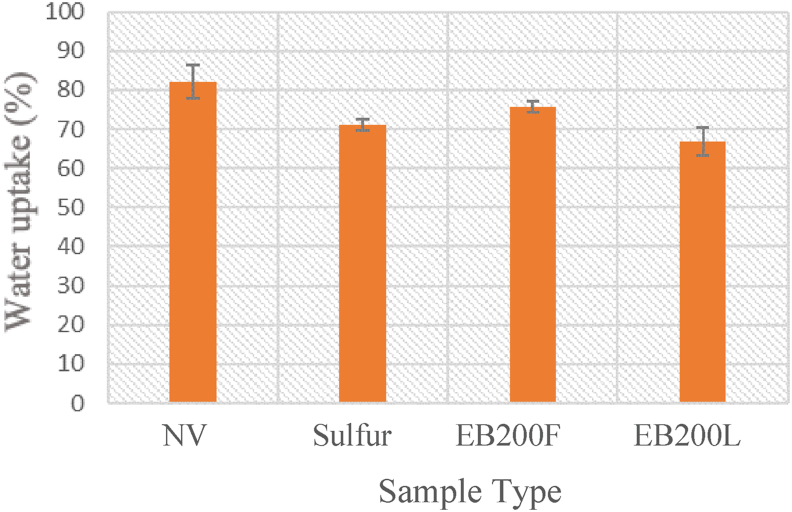
3.2.3. Water absorption property
Figure 7 shows water absorption of 10 phr NR/TSi samples vulcanized using electron beam irradiation at 200 kGy in latex and film forms, sulfur, and un-vulcanized one. The %water uptake of the vulcanized samples decreased compared to the un-vulcanized ones, as the former had greater crosslink densities than the latter [46].
Figure 7.
%Water absorption of NR/10 phr TSi vulcanized and un-vulcanized (NV) samples.
3.2.4. Thermal property
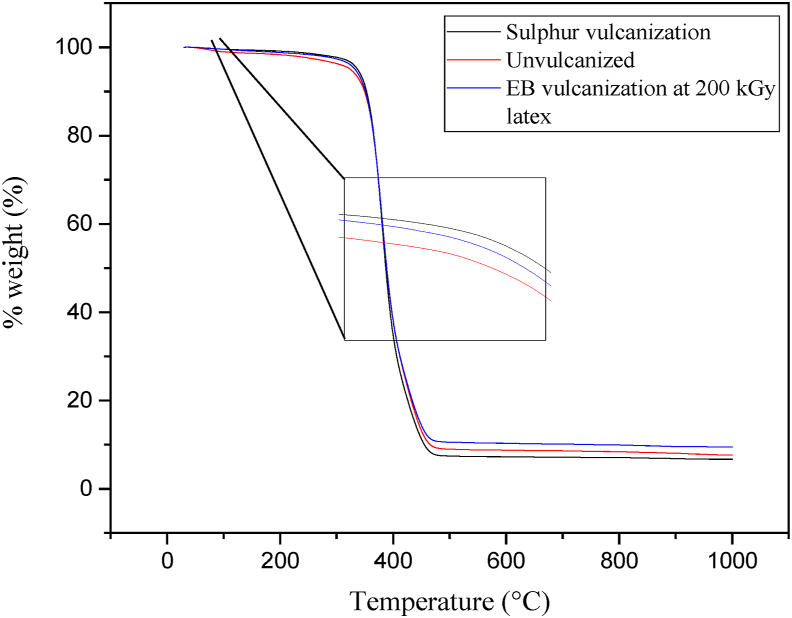
The thermal stabilities analysis of 10 phr NR/TSi vulcanized using EB radiation at 200 kGy in the latex form, sulfur, and un-vulcanized composite are shown in Figure 8. The thermogravimetric analysis showed that NR composites had one step of mass loss as shown in the weight loss curve between 330 °C and 455 °C [47]. At temperatures below 250 °C, no thermal degradation was observed due to the scission and the crosslinking of carbon chains in rubber molecules not causing a loss of volatility in the bulk rubber [48]. From Table 2, NR composites vulcanized using EB radiation and sulfur had higher initial degradation temperatures (Td) than those of the un-vulcanized NR composites. This was because the vulcanization formed a dense crosslink structure in the NR/TSi composites [49].
Figure 8.
TGA curve of natural rubber composites vulcanized using EB radiation at 200 kGy latex, sulfur vulcanization, and un-vulcanized rubber.
Table 2.
Initial degradation temperature of un-vulcanized and vulcanized samples.
| Sample type | Initial degradation temperature Td (oC) |
Initial degradation temperature at 50%wt T50 (oC) |
|---|---|---|
| Un-vulcanized | 330 | 387 |
| Sulfur vulcanization | 332 | 389 |
| EB vulcanization at 200 kGy in latex form | 332 | 389 |
From Table 2, T50 of NR/TSi latex by EB radiation and NR/TSi with sulfur vulcanization were at the same values. This could occurred with some reasons as it was found in the previous work that at high-irradiation-dose sample, the initial degradation of EB irradiated styrene butadiene rubber was quicker at low temperature [50]. It can be explained that during irradiation, not only crosslinking of the network of NR was formed but also chain scission fragments were occurred. Moreover, there was more chain fragmentations produced high irradiation dose compared to that at lower irradiation dose. Therefore, irradiation dose increased can result in a decrease of Td. This was also wound in the previous study of the effect of EB irradiation on NR latex mixed with silica/graphene mixture [23]. In the case of this study, it might be possible that the Td of the NR composite decreased at EB irradiation dose of 200 kGy. The similar values of Td for both composites found in this study might accidentally happened. Therefore, an explanation above indicating the equal values of Td of NR/TSi with sulfur vulcanization and that of irradiated NR/Tsi.
3.2.5. Composite morphology
The morphology of 10 phr-treated silica particles in NR is shown in Figure 9 and was used to investigate the dispersion of filler in NR matrix interfaces [51].
Figure 9.
Micrographs of NR/10 phr TSi composites: (a) un-vulcanized; (b) sulfur vulcanization; and (c) EB radiation of at 200 kGy in latex form.
It was shown that silica particles treated with Si-69 in NR composite vulcanized by EB irradiation exhibited better homogeneity and phase-continuity between the silica filler-NR interfaces when compared with silica particle distributed in uncured NR composite and sulfur-cured NR composite. The NR/TSi vulcanized using EB radiation in the latex form had less silica filler agglomeration and good dispersion in the rubber matrix because during EB radiation, the NR composite latex produced heat from radiolysis. This heat resulted in latex viscosity decreased and led to a better silica filler-NR interaction [51, 52].
3.2.6. Degradation property
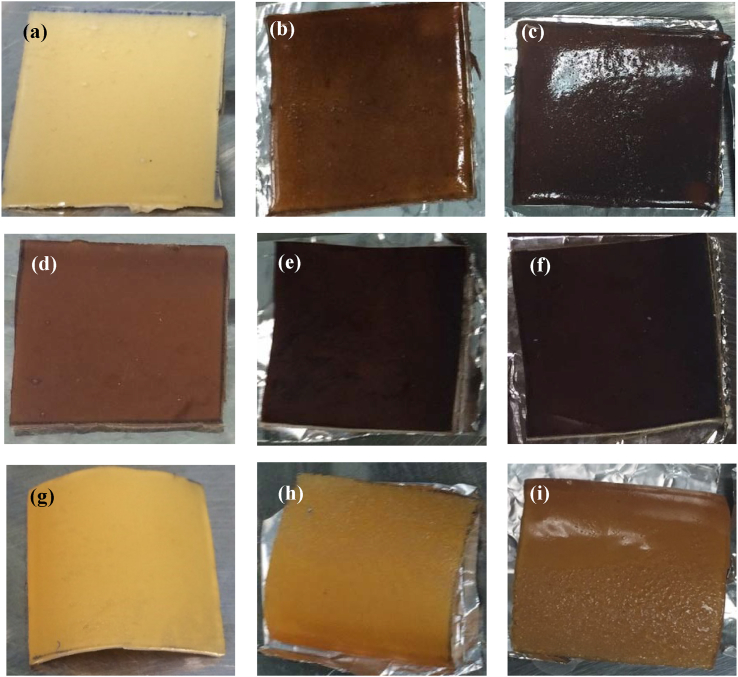
Degradation test shows physical appearance of the samples with vulcanized differently. They were used to predict the appearance when the samples are accelerated with heat and humidity or exposed to the severe condition. The results of the thermal and humidity degradation testing of 10 phr NR/TSi vulcanized at 200 kGy EB radiation in the latex form, sulfur, and un-vulcanized are shown in Figure 10. Figure 10 shows films (a-c) of NV NR/TSi10: Un-vulcanized natural rubber/silica treated 10 phr; (d-f) EB NR/TSi10L: EB radiation of natural rubber/silica treated 10 phr in latex form; (g-i) S NR/TSi10: Sulfur vulcanization of natural rubber/silica treated 10 phr. Before degradation testing (at 0 h), the composite un-vulcanized and sulfur vulcanized samples had colors similar to natural rubber while the composite sample using EB radiation vulcanization had a dark brown color due to the heat accumulation during beam radiation. After degradation testing, the longer the degradation time of a sample, the darker and stickier it became, especially for the un-vulcanized sample. This was due to low thermal stability as described in the section on thermogravimetric analysis. Stickiness of the sample indicated the degradation of NR due to heat and humidity exposition [53].
Figure 10.
Thermal and humidity degradation testing of (a–c) NV NR/TSi10, (d–f) EB NR/TSi10L, and (g–i) S NR/TSi10 at 0 h (left), 360 h (middle), 720 h (right), respectively.
4. Conclusion
Samples of natural rubber-reinforced bis(triethoxysilylpropyl) tetrasulfide (Si-69) treated silica vulcanized using EB irradiation and sulfur and cast in film and latex forms were studied for their mechanical properties, thermal stability, swelling resistance in toluene, water absorption, and degradation under thermal and humidity testing. The results showed that 200 kGy irradiation of the composite in the latex form produced the highest tensile strength of 15.7 ± 1 MPa. With EB vulcanization in the film form, tensile strength reduced after increasing the dose up to 250 kGy. This was due to the excessive crosslink density. Samples with a partial water uptake in NR/TSi 10 phr vulcanized using EB and by sulfur had lower %water absorption than that of the un-vulcanized NR composites. Degradation under thermal and humidity testing of the composites increased as the degradation time increased. The un-vulcanized NR/TSi 10 phr had the most physical changes, which was consistent with its low thermal stability. The NR/TSi composites vulcanized using EB had less chemical residue than those vulcanized using sulfur. Therefore, using EB irradiation as a green crosslinking method is suggested to vulcanize NR/TSi composites and other rubber composites.
Declarations
Author contribution statement
Manuchet Reowdecha: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Peerapan Dittanet, Pongdhorn Sae-oui: Analyzed and interpreted the data.
Surapich Loykulnant: Contributed reagents, materials, analysis tools or data.
Paweena Prapainainar: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Thailand Research Fund (Grant No. MRG5980252), the Center of Excellence on Petrochemical and Materials Technology, Department of Chemical Engineering, Faculty of Engineering, Research Development Institute (KURDI), Research Network of NANOTEC-KU on NanoCatalysts and NanoMaterials for Sustainable Energy and Environment, Kasetsart University, Bangkok, Thailand.
Data availability statement
The data that has been used is confidential.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Thanks is also to the Institute of Nuclear Technology (Public Organization), Ministry of Science and Technology, Thailand for permission to use their electron accelerating machine for irradiation.
References
- 1.Zhang B.L. Study on molecular structure and property of highly purified natural rubber. J. Anal. Appl. Pyrol. 2018;134:130–135. [Google Scholar]
- 2.Jong L. Improved mechanical properties of silica reinforced rubber with natural polymer. Polym. Test. 2019;79:106009. [Google Scholar]
- 3.Blattmann C.O., Pratsinis S.E. Nanoparticle filler content and shape in polymer nanocomposites. KONA Powder Part. J. 2019:advpub. [Google Scholar]
- 4.Du X. Preparation and properties of modified porous starch/carbon black/natural rubber composites. Compos. B Eng. 2019;156:1–7. [Google Scholar]
- 5.Harito C. Polymer nanocomposites having a high filler content: synthesis, structures, properties, and applications. Nanoscale. 2019;11(11):4653–4682. doi: 10.1039/c9nr00117d. [DOI] [PubMed] [Google Scholar]
- 6.Prasertsri S., Rattanasom N. Fumed and precipitated silica reinforced natural rubber composites prepared from latex system: mechanical and dynamic properties. Polym. Test. 2012;31(5):593–605. [Google Scholar]
- 7.Xu T. Influence of acetone extract from natural rubber on the structure and interface interaction in NR/silica composites. Appl. Surf. Sci. 2017;423:43–52. [Google Scholar]
- 8.Boonkerd K., Deeprasertkul C., Boonsomwong K. Effect of sulfur to accelerator ratio on crosslink structure, reversion, and strength in natural rubber. Rub. Chem. Technol. 2016;89 [Google Scholar]
- 9.Nuntang S. Mesostructured natural rubber/in situ formed silica nanocomposites: a simple way to prepare mesoporous silica with hydrophobic properties. Microporous Mesoporous Mater. 2018;259:79–88. [Google Scholar]
- 10.Ying P.T. Preparation and properties of spherical natural rubber/silica composite powders via spray drying. KONA Powder Part. J. 2020 [Google Scholar]
- 11.Sae-oui P. Comparison of reinforcing efficiency between Si-69 and Si-264 in a conventional vulcanization system. Polym. Test. 2004;23(8):871–879. [Google Scholar]
- 12.Weng P. Promoted dispersion of silica and interfacial strength in rubber/silica composites by grafting with oniums. J. Appl. Polym. Sci. 2019;136:48243. [Google Scholar]
- 13.Kim K.-J., VanderKooi J. Temperature effects of silane coupling on moisture treated silica surface. J. Appl. Polym. Sci. 2005;95:623–633. [Google Scholar]
- 14.Bayat H., Fasihi M. Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams. E-Polymers. 2019;19:430–436. [Google Scholar]
- 15.Yan H. Effect of silane coupling agent on the polymer-filler interaction and mechanical properties of silica-filled NR. J. Polym. Sci. B Polym. Phys. 2005;43:573–584. [Google Scholar]
- 16.Ansarifar A. The use of a silanised silica filler to reinforce and crosslink natural rubber. Int. J. Adhesion Adhes. 2005;25:77–86. [Google Scholar]
- 17.Sae-oui P. Comparison of reinforcing efficiency between Si-69 and Si-264 in an efficient vulcanization system. Polym. Test. 2005;23:871–879. [Google Scholar]
- 18.Sriring M. Viscoelastic and mechanical properties of large- and small-particle natural rubber before and after vulcanization. Polym. Test. 2018;70:127–134. [Google Scholar]
- 19.Tohsan A. Novel biphasic structured composite prepared by in situ silica filling in natural rubber latex. Polym. Adv. Technol. 2012;23 [Google Scholar]
- 20.Moustafa A.B. Effect of gamma irradiation on the properties of natural rubber/styrene butadiene rubber blends. Arab. J. Chem. 2016;9:S124–S129. [Google Scholar]
- 21.Smitthipong W. Adhesion and self-adhesion of rubbers, crosslinked by electron beam irradiation. Int. J. Adhesion Adhes. 2007;27(5):352–357. [Google Scholar]
- 22.Jayadevan J., Rosamma A., Unnikrishnan G. Deproteinised natural rubber latex grafted poly(dimethylaminoethyl methacrylate) - poly(vinyl alcohol) blend membranes: synthesis, properties and application. Int. J. Biol. Macromol. 2017;107 doi: 10.1016/j.ijbiomac.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 23.Jansomboon W., Loykulnant S., Prapainainar P. Electron beam radiation crosslinking of natural rubber prepared by latex mixing filled silica-graphene blend. Key Eng. Mater. 2019;803:361–365. [Google Scholar]
- 24.Khamplod T. Preparation of graft copolymer of natural rubber and polystyrene by electron beam irradiation. Adv. Mater. Res. 2014;931–932:73–77. [Google Scholar]
- 25.Shen J. The network and properties of the NR/SBR vulcanizate modified by electron beam irradiation. Radiat. Phys. Chem. 2013;92:99–104. [Google Scholar]
- 26.Loo K.-H. Current development of electron beam irradiation of natural rubber–polymer blends. Polym. Plast. Technol. Eng. 2017;56:1–24. [Google Scholar]
- 27.Espósito L.H., Marzocca A.J. Effect of electron beam irradiation on aging time, thermal vulcanization kinetic and mechanical properties of SSBR/NR/BR compounds filled with silica. Radiat. Phys. Chem. 2020;170:108651. [Google Scholar]
- 28.Bee S.-T. Study of montmorillonite nanoparticles and electron beam irradiation interaction of ethylene vinyl acetate (EVA)/de-vulcanized waste rubber thermoplastic composites. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2018;423:97–110. [Google Scholar]
- 29.Martin D. Vulcanization of rubber mixtures by simultaneous electron beam and microwave irradiation. Radiat. Phys. Chem. 2002;65(1):63–65. [Google Scholar]
- 30.Phetarporn V. Composite properties of graphene-based materials/natural rubber vulcanized using electron beam irradiation. Mater. Today Commun. 2019;19:413–424. [Google Scholar]
- 31.Flory P.J., Rehner J. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 1943;11(11):521–526. [Google Scholar]
- 32.Minoura Y., Asao M. Studies on the γ-irradiation of natural rubber latex. J. Appl. Polym. Sci. 1961;5(14):233–239. [Google Scholar]
- 33.Orwoll R.A., Arnold P.A. Polymer–solvent interaction parameter χ. In: Mark J.E., editor. Physical Properties of Polymers Handbook. Springer New York; New York, NY: 2007. pp. 233–257. [Google Scholar]
- 34.Tomita Y. Degradation behaviors of adhesion strength of structural adhesive for weld-bonding under high temperature and humidity conditions. Procedia Eng. 2017;184:231–237. [Google Scholar]
- 35.Lopattananon N., Jitkalong D., Seadan M. Hybridized reinforcement of natural rubber with silane-modified short cellulose fibers and silica. J. Appl. Polym. Sci. 2011;120(6):3242–3254. [Google Scholar]
- 36.Chirinos H. Radiation vulcanization of natural rubber latex using 250 keV electron beam machine. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2003;208:256–259. [Google Scholar]
- 37.Prasertsri S., Rattanasom N. Mechanical and damping properties of silica/natural rubber composites prepared from latex system. Polym. Test. 2011;30(5):515–526. [Google Scholar]
- 38.Coran A.Y. Chemistry of the vulcanization and protection of elastomers: a review of the achievements. J. Appl. Polym. Sci. 2003;87:24–30. [Google Scholar]
- 39.Stephen R. Thermal stability and ageing properties of sulphur and gamma radiation vulcanized natural rubber (NR) and carboxylated styrene butadiene rubber (XSBR) latices and their blends. Polym. Degrad. Stabil. 2006;91:1717–1725. [Google Scholar]
- 40.Ahmadi-Shooli S., Tavakoli M. A comparative study of the dynamic-mechanical properties of styrene butadiene rubber/epoxidized natural rubber dual filler nanocomposites cured by sulfur or electron beam irradiation. J. Macromol. Sci., Part B. 2019;58:1–15. [Google Scholar]
- 41.Porter M. Rubber technology handbook werner hofmann, carl hanser verlag, munich, 1989. pp. xv + 611, price dm 86.00. isbn 3–446–14895–7. Br. Polym. J. 1990;23(4) 359-359. [Google Scholar]
- 42.Hosseini S.M., Razzaghi-Kashani M. Vulcanization kinetics of nano-silica filled styrene butadiene rubber. Polymer. 2014;55(24):6426–6434. [Google Scholar]
- 43.Mansilla M.A. Natural rubber/styrene-butadiene rubber blends prepared by solution mixing: influence of vulcanization temperature using a Semi-EV sulfur curing system on the microstructural properties. Polym. Test. 2017;63:150–157. [Google Scholar]
- 44.Zhang F. Reinforcement of natural rubber latex with silica modified by cerium oxide: preparation and properties. J. Rare Earths. 2016;34(2):221–226. [Google Scholar]
- 45.Norhazariah S. Effect of different preparation methods on crosslink density and mechanical properties of carrageenan filled natural rubber (NR) latex films. Procedia Chem. 2016;19:986–992. [Google Scholar]
- 46.Zhao J. Electrospun multi-scale hybrid nanofiber/net with enhanced water swelling ability in rubber composites. Mater. Des. 2015;86:14–21. [Google Scholar]
- 47.Yamamoto Y. Thermal degradation of deproteinized natural rubber. Polym. Degrad. Stabil. 2018;156:144–150. [Google Scholar]
- 48.Mathew A.P., Packirisamy S., Thomas S. Studies on the thermal stability of natural rubber/polystyrene interpenetrating polymer networks: thermogravimetric analysis. Polym. Degrad. Stabil. 2001;72(3):423–439. [Google Scholar]
- 49.Park S.-J., Cho K.-S. Filler–elastomer interactions: influence of silane coupling agent on crosslink density and thermal stability of silica/rubber composites. J. Colloid Interface Sci. 2003;267(1):86–91. doi: 10.1016/s0021-9797(03)00132-2. [DOI] [PubMed] [Google Scholar]
- 50.Bandzierz K.S. Effect of electron beam irradiation on structure and properties of styrene-butadiene rubber. Radiat. Phys. Chem. 2018;149:14–25. [Google Scholar]
- 51.Theppradit T., Prasassarakich P., Poompradub S. Surface modification of silica particles and its effects on cure and mechanical properties of the natural rubber composites. Mater. Chem. Phys. 2014;148(3):940–948. [Google Scholar]
- 52.Chakraborty S. Electron beam (EB) radiation curing-A unique technique to introduce mixed crosslinks in cured rubber matrix to improve quality and productivity. J. Appl. Polym. Sci. 2011;122 [Google Scholar]
- 53.Raji M. 5 - durability of composite materials during hydrothermal and environmental aging. In: Jawaid M., Thariq M., Saba N., editors. Durability and Life Prediction in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites. Woodhead Publishing; 2019. pp. 83–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.