Highlights
-
•
Learning Point #1: Small ulcers on esophagography are usually attributable to herpes esophagitis and drug-induced esophagitis. Although rare, Crohn’s disease may produce small apthoid ulcers. Large ulcers are usually attributable to CMV or HIV esophagitis.
-
•
Learning Point #2: The early findings of candida are plaques that mimic glycogenic acanthosis. When plaques are seen, consider early candida or glycogenic acanthosis. When shaggy esophagus is identified, consider candidiasis.
-
•
Learning Point #3: Varices and varicoid esophageal carcinoma may appear similar on imaging. The presence of obstruction and lack of change with time and position should sway the Radiologist to diagnosing varicoid esophageal carcinoma.
-
•
Learning Point #4: Transverse esophageal lines should suggest the entities of feline esophagus and idiopathic eosinophilic esophagitis.
-
•
Learning Point #5: Esophageal contour abnormalities may suggest extrinsic or intrinsic lesions. Extrinsic lesions include aberrant vessels. Intrinsic lesions include intramural pseudodiverticulosis, gastroesophageal reflux, Barrett’s esophagus, and esophageal cancer.
Keywords: Oesophaghus, Oesophagram, Fluoroscopy, Oesophageal patterns
Abstract
Esophageal pathologies encountered on fluoroscopic examination may pose a diagnostic challenge to the interpreting Radiologist. Understanding the varied imaging appearances of esophageal pathology requires a thorough understanding of barium esophagography. This article reviews the various fluoroscopic imaging findings of different esophageal pathologies by describing an approach to image interpretation centered on dots, lines, contours, and ends. By utilizing this approach, the Radiologist will be better positioned to reconcile seemingly disparate pathologies into a cogent and succinct differential diagnosis.
1. Introduction
The spectrum of esophageal pathology encountered on esophagography is vast; and reconciling disparate imaging findings towards a cogent differential diagnosis may be a daunting endeavor for the interpreting Radiologist. Furthermore, recognizing esophageal pathology on imaging requires a thorough understanding of barium esophagography, a modality that is less commonly utilized compared to years past, and which has become something of a lost art. The purpose of this article is to describe common and uncommon entities of esophageal pathology by utilizing an innovative approach of esophageal dots, lines, contours, and ends. Esophageal dots refer to distinct intraluminal foci on double contrast exams, which can be appear either as bright spots or focal dark lucencies or cavities. Esophageal lines refer to prominent horizontal or longitudinal folds, as may be seen with feline esophagus or eosinophilic esophagitis. Esophageal contour refers to aberrations of normal esophageal contour, as may be seen with diverticula, vascular rings, Barrett’s esophagus, and malignancies. Finally, esophageal ends refer to caliber irregularities, as may be seen with achalasia and scleroderma. By using this approach, the Radiologist is well positioned to accurately recognize pathology, offer appropriate management recommendations to the referring provider, and ultimately render better patient care.
2. Technical considerations
Before delving into a discussion of esophageal pathology, a brief discussion of fluoroscopy protocols is warranted. Barium esophagography serves as the workhorse of esophageal imaging and is integral in providing both anatomic and functional information. The techniques utilized may include upright double-contrast views with a high-density barium suspension (105 % w/v), prone single-contrast views with a low-density barium suspension (60 % w/v), and mucosal-relief views [1]. For the double-contrast technique, the patient ingests an effervescent agent and quickly consumes high-density barium solution, allowing for evaluation of esophageal profile. To avoid overlap with the spine, this is performed with the patient in the left posterior oblique (LPO) position. At our institutions, we use 3 frames per second to capture excellent distention in the lower esophagus. The single-contrast phase includes views of the esophagus distended with barium and is useful for identifying esophageal cancers, hiatal hernias, and upper and lower esophageal rings and webs. This is performed in the right anterior oblique (RAO) position. These images may be supplemented with mucosal-relief views, which may be useful in demonstrating irregular folds, varices, small esophageal neoplasms, and esophagitis. The reader is directed to more comprehensive and thorough resources for further discussion on fluoroscopic technique; these resources also offer discussion on technique pitfalls [2,3].
3. Normal esophagus on fluoroscopy
The esophagus is a cylindrical organ that connects the pharynx to the stomach. It may be subdivided into three portions based on anatomical location: the cervical, thoracic, and abdominal portions. From a histologic standpoint, the esophagus is composed of the outer and inner muscularis propria, submucosa, muscularis mucosae, and mucosa. The structure of the esophagus dictates its function, with skeletal muscle fibers more pronounced within the proximal esophagus and smooth muscle fibers more pronounced within the distal esophagus; which allows for smooth transport of ingested food products towards the stomach [4]. On double contrast esophagram, the normal esophagus has a featureless intraluminal appearance, with smooth, thin walls (Fig. 1).
Fig. 1.
Normal double contrast esophagram. Note the featureless mucosa (inset).
Most esophageal pathology manifests as intra- or extraluminal changes, with interruption of the normal featureless appearance of the esophagus. These changes may be simplified into intraluminal dots, intraluminal lines, contour deformities, and proximal/distal end changes. Common esophageal pathologies may have one or more of these findings with a single pre-dominant feature. Several diseases may also develop a spectrum of findings as the disease progresses to more advanced stages, such as reflux esophagitis and candida esophagitis.
3.1. Intraluminal esophageal dots
Intraluminal esophageal dots refer to distinct intraluminal foci on double contrast exam which can be appear either as bright spots or focal dark lucencies or cavities. Focal bright dots are the radiological manifestation of esophageal ulcers secondary to contrast pooling. In contrast, focal lucencies/cavities are usually secondary to mucosal plaques with resultant contrast filling around the plaques, leading to apparent filling defects/cavity-like observations on double contrast esophagography (Fig. 2).
Fig. 2.
Intraluminal esophageal dots refer to distinct intraluminal foci on double contrast exam, which may appear either as bright spots or focal lucencies/cavities.
Ulcers may be further differentiated based on their number, size (small versus large), and distribution (mid vs distal esophagus) (Table 1). Reflux esophagitis usually involves the distal esophagus and may extend cranially. Pill induced esophagitis usually affects the mid esophagus at the level of aortic arch or left main bronchus compression. Herpes esophagitis has a diffuse distribution.
Table 1.
Esophageal Dots.
|
Small Ulcers (Bright dots) |
Herpes Esophagitis |
| Drug- Induced Esophagitis | |
| Crohn’s Disease | |
| Reflux Esophagitis | |
| Pemphigoid and epidermolysis bullosa dystrophicaa | |
| Large Ulcers | CMV Esophagitis |
| HIV Esophagitis Esophageal Carcinoma | |
| Plaques (Lucency or Cavity like) | Undissolved effervescent crystals |
| Candidiasisb | |
| Glycogenic Acanthosis | |
| Squamous Papilloma | |
| Superficial Spreading Carcinoma | |
| Esophageal Varices |
Pemphigoid and epidermolysis bullosa dystrophica develop ulcers in addition to mucosal blister and bulla.
Advanced candidiasis is associated with ulceration and wall irregularity.
3.2. Intraluminal esophageal dots (ulcers)
3.2.1. Herpes esophaghitis
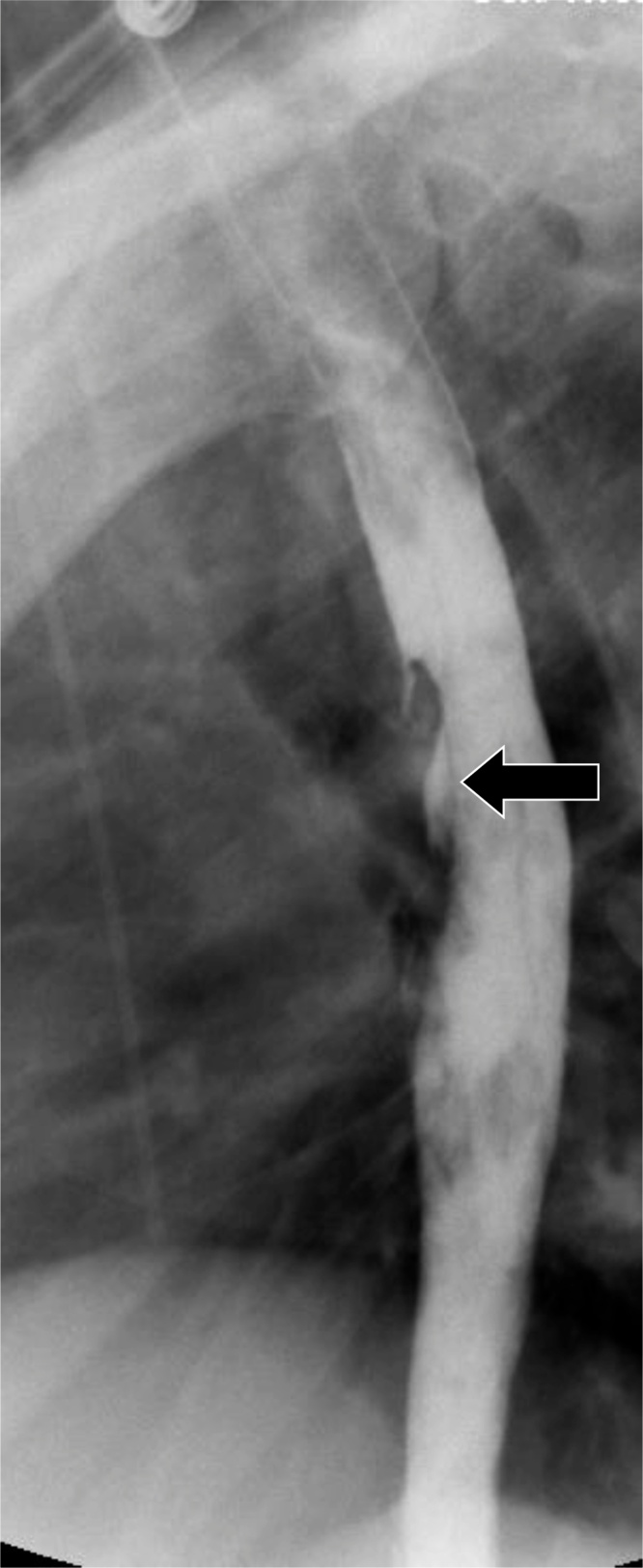
In herpes esophagitis, Herpes Simplex Virus-1 is more commonly implicated than Herpes Simplex Virus-2. Risk factors include malignancies, autoimmune disease, and HIV infection. Patients are typically immunocompromised and typically present with severe odynophagia. Discrete, punched-out ulcers are seen on endoscopy, typically located within the middle to lower third of the esophagus; and which are secondary to blistering of vesicles. The presence of Cowdry A inclusion bodies on histopathology is considered pathognomic [1,2,5]. A spectrum of appearances is seen on fluoroscopy, including small, discrete stellate-shaped ulcers within the mid esophagus (Fig. 3) and plaques without ulceration [6]. Treatment is with antiviral therapy.
Fig. 3.
Herpes esophagitis. Note the presence of small, discrete ulcers (arrows).
3.2.2. Drug-induced esophagitis
Drug-induced esophagitis may be difficult to ascertain with a patient’s provided history, as the act of taking pills is typically considered a harmless endeavor. However, certain culprit medications, including tetracycline or doxycycline capsules, when ingested without water, may lodge within the mid-aspect of the esophagus where the left mainstem bronchus or aortic arch serves as an external buttress [2,3,7]. A few example drugs implicated in drug-induced esophagitis are listed in Table 2. On double-contrast fluoroscopy, discrete ulcers are located at the characteristic site within the mid-esophagus (Fig. 4). Treatment entails discontinuation of the offending agent.
Table 2.
Common mediations associated with drug induced esophagitis.
| Tetracycline, doxycycline |
| Potassium Chloride |
| Quinidine |
| Aspirin |
| Non steroidal Antiinflammatory drugs (NSAIDs) |
| Alendronate |
Fig. 4.
Drug-induced esophagitis. Note the characteristic location within the mid esophagus, where the left mainstem bronchus or aortic arch serves as an external buttress (arrow).
3.2.3. Crohn’s esophagitis
Crohn’s disease involving the esophagus is rare, and when it does involve the esophagus, other portions of the alimentary tract are typically also involved. Commonly described endoscopic findings include apthous ulcers, superficial erosions, and strictures in cases of advanced disease [8]. These apthous ulcers may been seen on esophagography. Disease may be identified within the stomach and duodenum as well. The presence of concurrent Crohn’s disease within the terminal ileum allows for a confident diagnosis of esophageal involvement (Fig. 5). Unfortunately, no dedicated treatment guidelines exist for esophageal Crohn’s disease, although some patients may improve on medical therapy with 5-aminosalicylates and steroids.
Fig. 5.
Crohn’s esophagitis. Diffuse irregularity and small ulcers are present within the esophagus. A small-bowel follow through exam from the same patient demonstrates a terminal ileal stricture (arrow).
Epidermolysis bullosa (EB) refers to a group of skin disorders with varied inheritance patterns; and characterized by cutaneous blisters and bullae formation after minor trauma (insert sources). Although the esophagus can be involved in any EB variant, the autosomal recessive form of dystrophic EB is the most common and severe type, with esophagography recommended for this subset; as patients may be susceptible to iatrogenic esophageal from endoscopy [9]. Patients frequently complain of dysphagia. On esophagography, strictures of variable length involving the upper third of the esophagus are seen, with smooth tapered margins [10].
Pemphigoid refers to a group of heterogenous group of autoimmune blistering diseases. Cervical esophageal webs are the most common imaging finding and may be multiple; although any portion of the esophagus may be involved [11].
3.2.4. CMV/HIV esophagitis
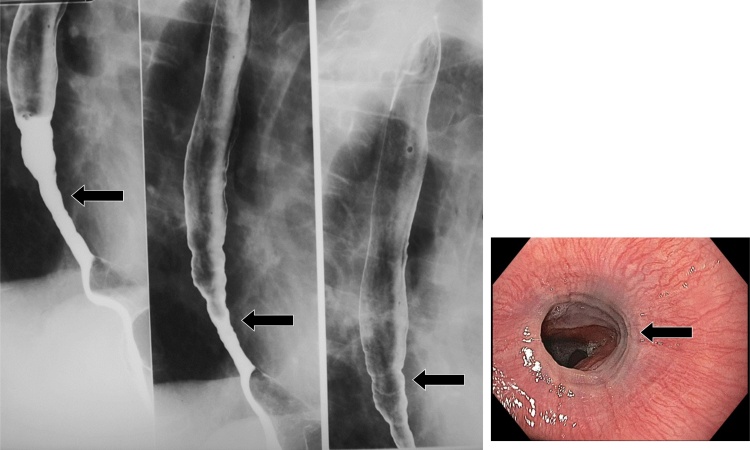
After colitis, esophagitis is the second most common manifestation of cytomegalovirus (CMV) infection involving the alimentary tract. Risk factors include immunosuppression, renal dialysis, and human immunodeficiency virus (HIV) infection. Patients typically complain of odynophagia or epigastric pain [12]. A similar clinical presentation may be seen with HIV esophagitis. On double-contrast esophagography, both CMV and HIV esophagitis may appear as large, ovoid and flat ulcers, with a surrounding lucent rim of edematous mucosa (Fig. 6). Further assessment with endoscopy and brush biopsies is required to determine the diagnosis, as the treatments for these entities are very different. CMV esophagitis requires antiviral therapy, whereas HIV esophagitis may be self-limited or resolve after oral steroid therapy [2,3,13,14].
Fig. 6.
CMV esophagitis. Large, ovoid, and flat ulcers are present (arrow). Corresponding endoscopic findings (inset) demonstrate the ulcers (arrow).
3.2.4.1. Learning point #1
Small ulcers on esophagography are usually attributable to herpes esophagitis and drug-induced esophagitis. Although rare, Crohn’s disease may produce small apthoid ulcers. Large ulcers are usually attributable to CMV or HIV esophagitis.
3.3. Intraluminal esophageal dots (plaques)
An esophageal plaque is usually secondary to mucosal/submucosal inflammation or infiltration. It appears as an intraluminal filling defect or lucency on double contrast exam. The spectrum of diseases which may cause esophageal plaques include candida esophagitis, glycogenic acanthosis, squamous papilloma, and superficial spreading carcinoma. Blistering autoimmune disease such as Mucous membrane pemphigoid may involve the esophagus with multiple blister formation at the early stage of the disease which appears as filling defect on esophagogram. Dilated submucosal venous collaterals can also look like plaques on fluoroscopy which are associated with portal hypertension or SVC obstruction.
3.3.1. Candidiasis
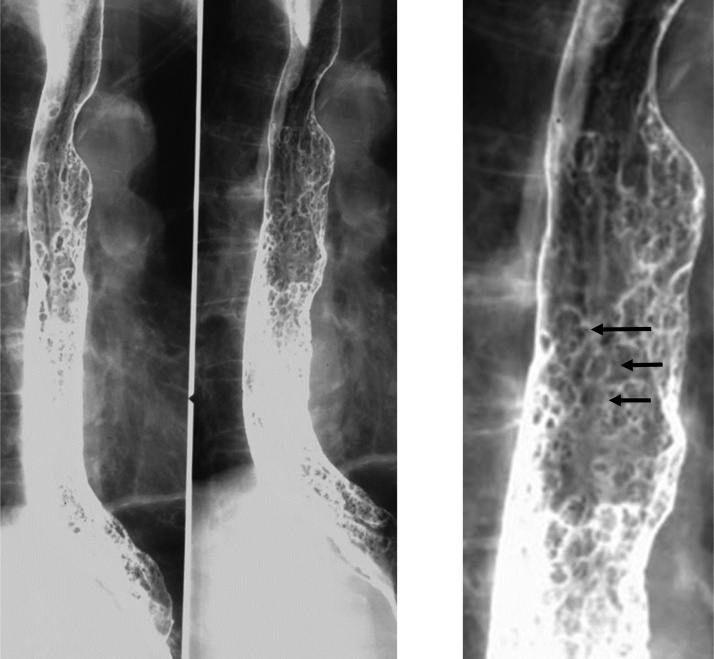
Candidiasis is the most common cause of infectious esophagitis, with Candida albicans most commonly implicated as the causative organism [5]. Esophageal candidiasis is a diagnosis with important ramifications; elderly patients with esophageal candidiasis had an increased 6-month mortality rate and decreased 1-year survival [15]. Acquired immunodeficiency syndrome (AIDS) is the most common risk factor, with other risks including immunocompromised status and esophageal stasis due to achalasia or scleroderma [16]. Patients may complain of odynophagia. On double-contrast esophagography, multiple plaques may be seen in a linear course, ranging in size from a few millimeters up to a centimeter (Fig. 7). In advanced disease, these plaques may be become fulminant and ulcerated, with the esophagus possessing a shaggy appearance and ulcers (Fig. 8). The ulcers associated with candidiasis should not be confused with herpes esophagitis ulcers, as ulcerations in candidiasis are larger than those seen in HIV and occur on a background of diffuse background plaque formation [3]. Treatment includes fluconazole, but it is important to recognize that certain Candida strains may possess a drug resistance [2,3,5].
Fig. 7.
Candida esophagus. Note the presence of multiple plaques in a linear course (arrow).
Fig. 8.
Advanced candidiasis. The plaques may be become fulminant and ulcerated, with the esophagus possessing a shaggy and irregular appearance. Note endoscopic correlate (inset).
3.3.2. Glycogenic acanthosis
Glycogenic acanthosis refers to oval mounds of hypertrophied cell layers due to increased intracellular glycogen. The incidence increases with age, with endoscopic figures ranging from 5 % to 15 % [17,18]. There is an association with Cowden syndrome, which is characterized by multiple hamartomatous polyps and autosomal dominant inheritance, and an increased risk of malignancy across several organ systems, including breast, thyroid, and endometrial cancers [19,20]. On double-contrast esophagography, glycogenic acanthosis may be characterized as multiple, tiny, raised plaques which may coalesce and form a finely nodular or cobblestone mucosal pattern (Fig. 9). These lesions are most commonly seen within the mid-esophagus. This imaging appearance may overlap with esophageal candidiasis; in this event, clinical context becomes paramount. Patients with esophageal candidiasis typically present with odynophagia and have a compromised immune status; on the other hand, glycogenic acanthosis is typically seen in older, immunocompetent patients without esophageal symptoms [3,21].
Fig. 9.
Glycogen acanthosis. There are numerous, tiny, raised plaques which may coalesce and form a finely nodular or cobblestone mucosal pattern.
3.3.3. Esophageal squamous papillomatosis
Esophageal squamous papillomatosis results in numerous 5−6 mm squamous papillomas within the esophagus. Squamous papillomas are rare, benign epithelial tumors, usually seen on endoscopy as an incidental finding. There is an association with human papilloma virus (HPV) infection. Squamous papillomatosis is an even rarer entity; with only a few cases described in the literature [22,23]. There may be an association with respiratory papillomatosis. On double contrast esophagography, a papilloma appears as a small 5−6 mm, lobulated sessile lesion. Papillomatosis appears as innumerable filling defects (Fig. 10). There is a variable clinical course, with increased risk for malignant degeneration into squamous cell carcinoma. Surgical and laser treatments may play a role in therapy [22].
Fig. 10.
Esophageal papillomatosis. Note the innumerable filling defects (arrows).
3.3.4. Varicoid esophageal carcinoma
Varicoid esophageal carcinoma refers to an uncommon pattern of dissemination within the submucosa, involving the lymphatic and vascular systems. As this causes a very gradual narrowing of the esophageal lumen, dysphagia typically does not occur until an advanced stage of disease. On fluoroscopy, localized serpentine filling defects may be identified. This appearance mimics distal esophageal “uphill” varices (downhill varices are usually present in the upper esophagus and occur due to superior vena cava obstruction) [24,25]. However, it is important to note differences on fluoroscopy: varices change shape with patient positioning, esophageal distention, and time, whereas varicoid carcinoma remains a fixed defect. Although both may cause a degree of narrowing, only varicoid carcinoma causes obstruction (Fig. 11). These changes are summarized in Table 3.
Fig. 11.
Esophageal varices. Note the serpiginous filling defects (arrow). This appearance on fluoroscopy may mimic varicoid esophageal carcinoma; however, varices change appearance with time. Endoscopic correlate of esophageal varices is also shown (inset, arrows).
Table 3.
Varices versus varicoid esophageal carcinoma.
| Varices | Varicoid CA | |
|---|---|---|
| Serpentine filling defects. | ✓ | ✓ |
| Change with time and position. | ✓ | |
| Associated with hematemesis. | ✓ | ✓ |
| Associated with obstruction. | ✓ | |
| focal luminal narrowing. | ✓ | ✓ |
3.3.4.1. Learning point #3
Varices and varicoid esophageal carcinoma may appear similar on imaging. The presence of obstruction and lack of change with time and position should sway the Radiologist towards diagnosing varicoid esophageal carcinoma.
3.4. Esophageal lines
The discussion now steers to a presentation of esophageal lines. Lines can be categorized into horizontal (perpendicular to long axis of the lumen) or longitudinal (parallel to the lumen). Horizontal lines may be seen with feline esophagus, eosinophilic esophagitis, and with the stepladder appearance of reflux esophagitis. Longitudinal lines can be seen with reflux esophagitis. The imaging spectrum of reflux esophagitis will be discussed later in this article.
3.4.1. Feline esophagus
Feline esophagus refers to transient, transverse, thinly spaced striations that are normal in cat physiology but associated with gastroesophageal reflux in humans [26]. They may also be a normal variant. On fluoroscopy, these may be identified as transverse muscular bands, secondary to intermittent contraction of longitudinally oriented muscularis mucosa (Fig. 12).
Fig. 12.
Feline esophagus. Note the transient, transverse, thinly spaced striations.
3.4.2. Eosinophilic esophagitis
Eosinophilic esophagitis is an inflammatory condition predominantly affecting young adults, with symptoms of persistent dysphagia and food impaction. It is most common in Caucasian males. There is an association with atopy and peripheral eosinophilia, with greater than 15 eosinophils per high power field [27,28]. On fluoroscopy, a spectrum of appearances may be identified. These include web-like segmental parallel areas of thin narrowing, segmental esophageal strictures producing a ringed esophagus, dysmotility, and long segment symmetric narrowing (small caliber esophagus) (Fig. 13) [2,3,27]. Although the ringed appearance of the esophagus may mimic feline esophagus, the latter entity is transient and the rings in feline esophagus are smaller. The importance of making this diagnosis by esophagram cannot be overstated; as the underlying mucosa may be friable and delicate, with a predisposition to iatrogenic injury following endoscopic procedures [29]. Treatment typically entails steroid administration.
Fig. 13.
Eosinophilic esophagitis. The esophagram demonstrates long segment symmetric narrowing. Endoscopic correlate of the ringed esophagus variant from a different patient is also shown (inset).
3.4.2.1. Learning point #4
Transverse esophageal lines should suggest the entities of feline esophagus and eosinophilic esophagitis.
3.5. Esophageal contour deformities
The normal esophagus has a smooth gently undulating contours - which tapers at gastroesophageal junction. There are three normal anatomical extrinsic indentations secondary to the aortic arch, left main bronchus, and left atrium (Fig. 14). There are two normal muscular rings within the distal esophagus: An A ring, which is typically not seen, and a B ring, located at the gastroesophageal junction. The portion of the esophagus between these rings is termed the vestibule. The contour deformities can be broadly divided into focal wall irregularities, focal out-pouching, and focal inward folding (Table 4). The focal out-pouching is usually secondary to congenital or acquired diverticula. Focal inward folding can be secondary to spectrum of pathology such as esophageal webs, submucosal mesenchymal tumors, and external vascular deformities. Assessing the surface of the contour deformity (smooth vs irregular) is an important feature to differentiate benign and malignant pathology. All wall irregularity or inward folding with irregular surface should be consider malignant until proven otherwise. Further management of contour deformities with cross sectional imaging or upper endoscopy is usually required (Fig. 15).
Fig. 14.
Normal contour deformities of the esophagus. The aortic arch, left mainstem bronchus, and left atrium form gentle indentations of the esophageal contour.
Table 4.
A Summary of Contour Deformities (Fig. 15).
| Category | Pathology | Key features |
|---|---|---|
| Focal Outpouching | Zenker’s Diverticula | Above CP, Posterior to esophagus |
| Killian-Jameson | Below CP, lateral to esophagus | |
| Traction | Transient during exam, Triangular | |
| Pulsion | Persistent | |
| Focal inward folding | Esophageal Web | Smooth margin; at proximal and distal ends of esophagus |
| Vascular ring | Smooth margin | |
| Benign mesenchymal tumor | Smooth margin | |
| Wall irregularity | Esophagitis ulcer/scarring | Associated other features of esophagitis |
| Barrett’s | Distal or mid esophagus; associated reflux esophagitis | |
| Malignant esophageal cancer | Irregular border |
Fig. 15.
A pictorial summary of esophageal diverticula. Also see Table 4.
3.5.1. Diverticula
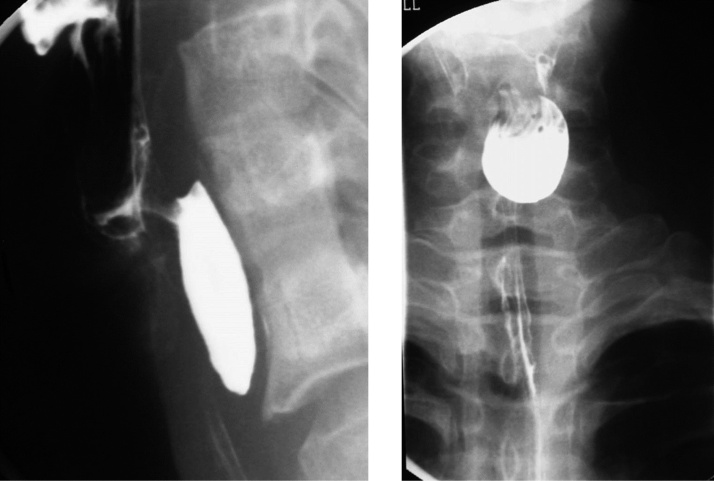
Zenker’s diverticulum is a rare acquired pseudo-diverticulum, occurring posteriorly at the pharyngoesophageal junction, through a gap between the inferior pharyngeal constrictor and cricopharyngeus muscle known as Killian’s dehiscence. Patients typically present in the 6–8th decade of life with a wide spectrum of symptoms and signs; ranging from odynophagia to halitosis and aspiration pneumonia. As the mouth of the diverticulum is aligned with the pharynx, food particles preferentially enter the diverticulum; this may result in compression of the adjacent esophageal lumen. There is a strong association between Zenker’s diverticulum and gastroesophageal reflux disease [30,31]. On esophagography, Zenker’s diverticulum has a characteristic location above the cricopharyngeus muscle (Fig. 16). Surgical diverticulectomy may be warranted for severe symptoms.
Fig. 16.
Zenker’s diverticulum. This is located posteriorly at the pharyngoesophageal junction, and inferior to the cricopharyngeus muscle.
Killian-Jameson diverticulum is another rare variable-sized outpouching from the lateral wall of the proximal cervical esophagus. These are secondary to protrusion through a muscular gap at the anterolateral wall of the proximal cervical esophagus, just below the cricopharyngeus and lateral to the longitudinal tendon of the esophagus [32]. This characteristic location dictates its appearance on esophagography, as an outpouching best seen on lateral view (Fig. 17).
Fig. 17.
Killian-Jameson diverticulum. Note the characteristic outpouching from the lateral wall of the proximal cervical esophagus.
Esophageal traction diverticula are typically found within the mid-esophagus, near the carina. These diverticula are associated with granulomatous infectious processes (such as histoplasmosis or tuberculosis) within adjacent mediastinal nodes; this leads to adjacent inflammation and adhesion formation, with resultant traction on the adjacent esophageal wall and formation of diverticula [33]. On esophagography, these traction diverticula manifest as outpouchings within the mid-esophagus. The presence of adjacent calcified lymph nodes or other evidence of granulomatous disease may suggest the diagnosis (Fig. 18).
Fig. 18.
Esophageal traction diverticulum. Note the ancillary finding of calcified granulomas within the spleen on upper GI from the same patient (arrow), which may support the diagnosis.
Esophageal epiphrenic diverticula are outpouchings of the esophageal mucosa and submucosa through the fibers of the esophageal muscular layer, occurring within the distal esophagus usually secondary to a functional or mechanical obstruction. They are associated with hiatal hernias and underlying motility disorders such as achalasia [34]. These diverticula have a characteristic appearance as focal outpouchings within the distal esophagus (Fig. 19).
Fig. 19.
Epiphrenic pulsion diverticulum. Note the characteristic outpouching within the distal esophagus. There is an associated small sliding hiatal hernia (arrow).
Esophageal hematoma refers to blood dissecting within the submucosal plane. These may be spontaneous in the setting of anticoagulation, iatrogenic in the setting of endoscopy, or post-traumatic [35]. This entity is typically managed conservatively.
3.5.2. Pseudodiverticulosis
Esophageal intramural pseudodiverticulosis is rare, mostly affecting middle aged men. They tend to occur near esophageal strictures. These are inflamed submucosal glands, and do not reflect true diverticuli. These pseudodiverticuli may be segmental or diffuse. Comorbidities include stasis from any cause, gastroesophageal reflux, eosinophilic esophagitis, alcoholism, and diabetes mellitus. Strictures may be identified on endoscopy. An association with esophageal malignancy has also been reported [36,37]. On fluoroscopy, multiple, 2−3 mm flask-shaped outpouchings are seen (Fig. 20, Fig. 21). A distal stricture may also be seen (Fig. 20).
Fig. 20.
Pseudodiverticulosis (arrow) with distal esophageal stricture.
Fig. 21.
A more pronounced case of pseudodiverticulosis.
3.5.3. Vascular ring
Most symptomatic vascular rings are diagnosed in children. Vessels crossing posterior to the esophagus include aberrant right subclavian artery, right aortic arch with aberrant origin of the left subclavian artery, and double aortic arch. If a vessel crosses anterior to the esophagus, this is reflective of a vascular sling. Vascular rings are rare congenital anomalies, which may be either asymptomatic or present in the neonate with stridor, dysphagia, or wheezing. These rings occur due to anomalies of normal aortic arch development, with resultant encircling of the esophagus and trachea. Vascular slings, on the other hand, reflect anomalous origin of the pulmonary arteries; with the left pulmonary artery arising from the right [38]. On barium swallow, the esophageal contours will be distorted in a characteristic fashion, with indentations at the site of the aberrant anatomy (Fig. 22).
Fig. 22.
Vascular ring. Note the impression on the posterior esophagus (arrow). This is secondary to a right aortic arch with aberrant right subclavian artery.
3.5.4. Esophagitis scarring
Gastroesophageal reflux disease (GERD) is the most common gastrointestinal disorder in the United States, with risk factors including advanced age, abdominal obesity, alcohol, and tobacco use. Patients typically present with heartburn and postprandial acid regurgitation due to abnormal peristalsis. Prolonged GERD may lead to Barrett’s esophagus and esophageal carcinoma [3,39,40]. This spectrum of clinical appearances lends itself to myriad manifestations on fluoroscopy, including classic granular mucosal profile, ulcers, thickened longitudinal folds, inflammatory pseudopolyps, strictures, and scarring (Figs. 23). Inflammatory pseudopolyps reflect hypertrophy of the gastroesophageal junction folds and are characteristic of GERD. Distal esophageal scarring secondary to a healing ulcer may demonstrate a horizontal line stepladder appearance; it may also take a circumferential course and ultimately lead to a peptic stricture. Although these strictures may mimic a symptomatic B ring, they are typically larger in height and asymmetric in character [3].
Fig. 23.
An example of gastroesophageal reflux disease on fluoroscopy. These may appear as short segment strictures and scarring. Note corresponding focal luminal narrowing on endoscopy (inset, arrow).
3.5.5. Barrett’s esophagus
Barrett’s esophagus reflects metaplasia of normal squamous distal esophageal epithelium into columnar epithelium. Short segment Barrett’s esophagus spans <3 cm or less, whereas long segment spans >5 cm, and it is more likely associated with dysplasia and progression to cancer [41]. This condition, prevalent in up to 6% of American adults, occurs as an adaption response to chronic GERD. It is a risk factor for esophageal adenocarcinoma, with 7 % of patients with chronic reflux being diagnosed with Barrett’s esophagus, and with 0.1–0.3% of Barrett’s esophagus undergoing malignant transformation [42,43]. On fluoroscopy, a mid-esophageal stricture is the hallmark appearance, although this feature is uncommon. A distal esophageal reticular mucosal pattern may also rarely be observed (Fig. 24) [3]. Double contrast esophagography may play a vital role in stratifying patient risk. If the hallmark features of mid-esophageal stricture with distal esophageal reticular mucosal pattern are present on esophagography, the patient is likely to have Barrett’s esophagus and will benefit from endoscopy. Patients without these characteristic features on esophagography are at lower risk, and endoscopy may not be entirely warranted [3,44]. Treatment options for GERD include proton pump inhibitors, with antireflux surgery reserved for patients who may be unable to comply with long-term medical therapy [45].
Fig. 24.
Barrett’s esophagus. Note the mid-esophageal stricture (arrow). Corresponding endoscopic image demonstrates epithelial metaplasia (inset, arrow).
3.5.6. Esophageal Cancer
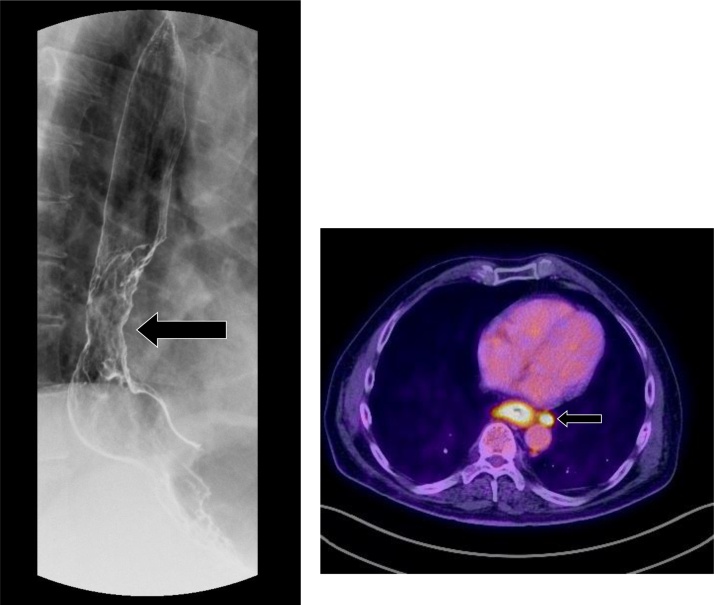
Esophageal cancer is the third most common gastrointestinal malignancy and amongst the most prevalent malignancies on a worldwide basis. Most esophageal cancers are either squamous cell carcinomas or adenocarcinomas. Adenocarcinomas have an association with Barrett’s esophagus, as previously described, and are most commonly found at the gastroesophageal junction. Patients may present with progressive dysphagia. A full review of esophageal cancer staging, multimodality imaging, and treatment is beyond the scope of this article; the reader is directed to excellent references for further discussion [4,54,55]. On fluoroscopy, esophageal malignancy may occur as a mass with irregular narrowing, shouldering, and mucosal irregularities (Fig. 25). Early cancer may appear as a plaque-like lesion with or without ulcer, whereas advanced cancer may appear as a large ulcerated mass with or without partial obstruction of the esophagus. Most GE junction malignancies are secondary to adenocarcinoma from Barrett’s esophagus. Squamous cell carcinomas do not typically occur at the ends.
Fig. 25.
Esophageal adenocarcinoma. There is an irregular mass of the distal esophagus with corresponding mucosal irregularities and luminal narrowing (arrow). Positron emission tomography-CT of the same patient (inset) demonstrates a hypermetabolic esophageal mass with para-esophageal adenopathy (arrow).
3.5.6.1. Learning point #5
Esophageal contour abnormalities may suggest extrinsic or intrinsic lesions. Extrinsic lesions include aberrant vessels. Intrinsic lesions include intramural pseudodiverticulosis, gastroesophageal reflux, Barrett’s esophagus, and esophageal cancer.
3.5.7. Esophageal ends
A spectrum of diseases which affect either the proximal or distal esophagus is described. These entities include achalasia, Chagas disease, scleroderma, esophageal webs, and finally esophageal cancer which is almost always adenocarcinoma at the gastroesophageal junction due to Barrett’s Esophagus.
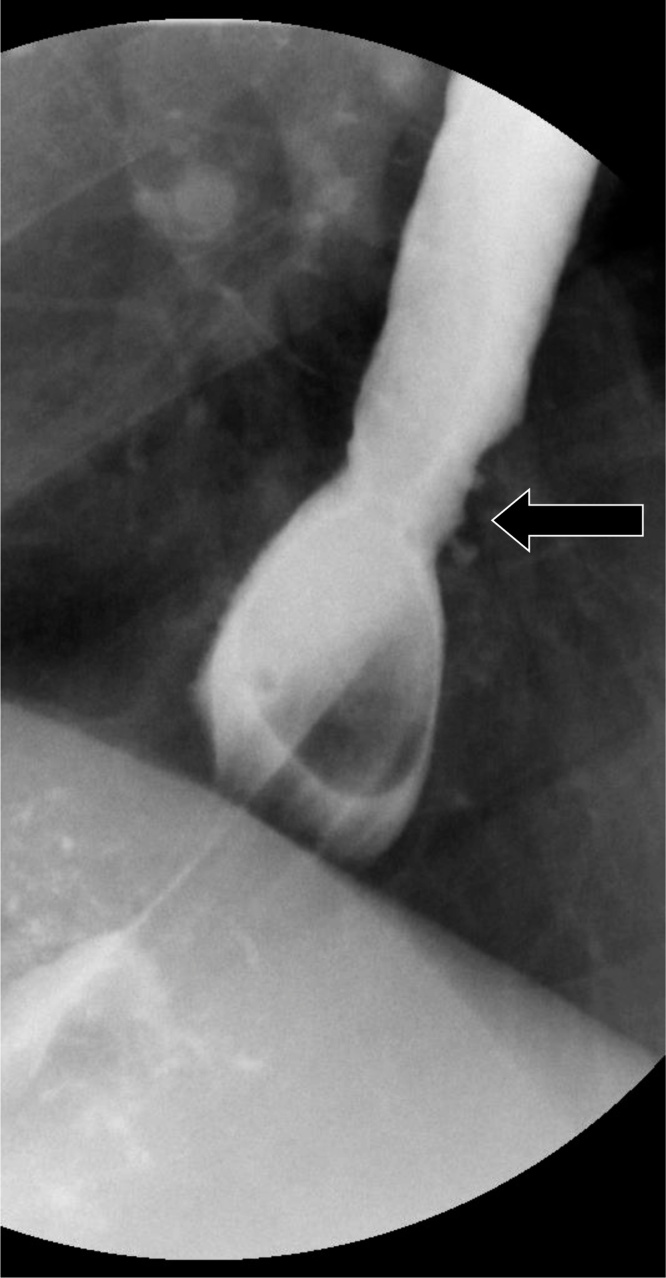
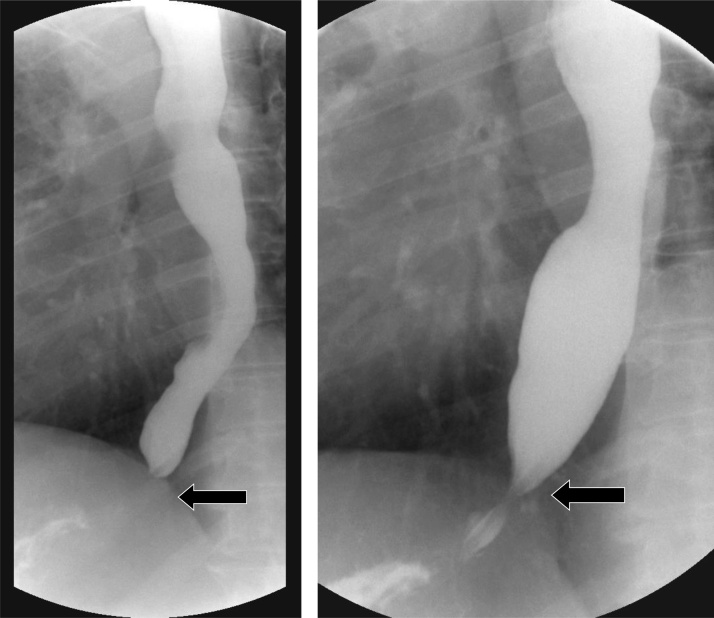
Primary achalasia is a rare esophageal motility disorder, occurring in approximately 10 in 100000 cases, and affecting patients between 30 and 60 years of age. Three subtypes of achalasia have been described under the aegis of the Chicago Classification, which utilizes high resolution manometric data; with the most common subtype, Type I, reflecting absent esophageal contractility [46,47]. The disease pathophysiology involves inflammation and degeneration of ganglion cells in the myenteric plexus of the esophageal body and the lower esophageal sphincter (LES). This results in ability of the LES to relax due to loss of primary peristalsis. Patients typically present with dysphagia [47]. The hallmark fluoroscopic finding is a distended esophagus with a tapered, beak-like configuration of the distal esophagus (Fig. 26). Secondary achalasia is due to tumor infiltration of the myenteric plexus and may appear similar to primary achalasia. However, a key difference lies in the extent of segmental narrowing, with malignant infiltration causing longer, fixed segment narrowing compared to primary achalasia. The overall similarity of imaging findings between primary and secondary achalasia must therefore prompt a thorough evaluation of the gastric cardia and fundus because cancers may involve the stomach [2,3]. Management options for primary achalasia include surgical myotomy (Heller myotomy), pneumatic dilation and peroral endoscopic myotomy (POEM) [47].
Fig. 26.
Achalasia with tapering of the distal esophagus, resembling a bird’s beak (arrow).
Chagas disease occurs due to infection from the protozoan parasite Trypanosoma Cruzi, which is most prevalent in South America. This disease affects the intramural neurons of the alimentary tract and can result in megaesophagus. Other organs that may be affected include the heart, and which may lead to cardiomyopathy in the chronic setting [48]. On fluoroscopy, this disease can mimic primary or secondary achalasia. Diagnosis therefore depends on polymerase chain reaction testing, and treatment entails administration of antitrypanosomal drugs such as nifurtimox and benznidazole [48].
Scleroderma is an immune-mediated disease with localized and systemic manifestations. The physiology is due to absent peristalsis and incomplete LES which leads to reflux esophagitis, stricture formation, and further dilatation of the esophagus. End stage scleroderma can mimic end stage achalasia. This later manifestation may result in widespread collagen deposition that results in tissue fibrosis. Gastrointestinal involvement may portend worsened morbidity and mortality due to smooth muscle atrophy secondary to collagen deposition. The esophagus is the most commonly involved organ within the alimentary tract, although interstitial lung disease is the leading cause of mortality. Fluoroscopy may demonstrate absent esophageal peristalsis below the level of the aortic arch, incompetent LES, and stigmata of reflux esophagitis (Fig. 27). It follows that most patients with scleroderma involving the esophagus develop reflux esophagitis and a substantial percentage develop Barrett’s esophagus. Immunosuppressive therapy is the most commonly used treatment regimen [3,[49], [50], [51]].
Fig. 27.
Scleroderma. The esophagus appears patulous, and there is a small reflux stricture present (arrow).
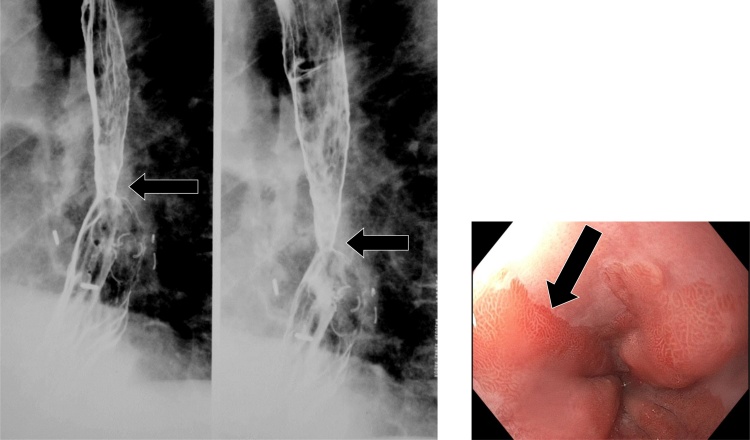
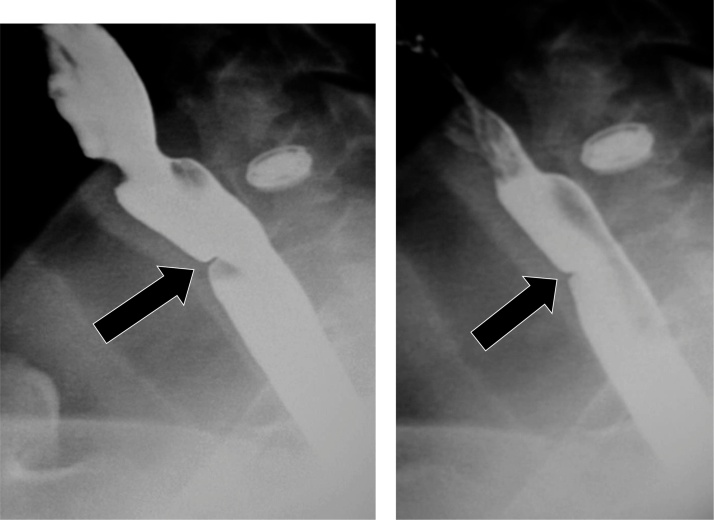
Esophageal webs are thin, 1−2 mm shelf-like linear mural projections which are most common in the cervical esophagus. A historical association with iron-deficiency anemia and dysphagia led to the characterization of Plummer-Vinson syndrome; however, more recent studies have failed to demonstrate an association between iron-deficiency anemia and esophageal webs. Other associations include epidermolysis, pemphigoid, eosinophilic esophagitis, celiac-sprue disease, graft-versus-host disease, and chronic GERD [52,53]. On fluoroscopy, these webs are typically identified within the cervical esophagus (Fig. 28). If these webs are anterolateral in location, they are frequently asymptomatic; however, if webs are circumferential, they may produce dysphagia.
Fig. 28.
A small web is present in the cervical esophagus (arrow).
3.5.7.1. Learning point #6
Abnormalities of the esophageal ends may suggest achalasia, Chagas disease, scleroderma, and esophageal webs.
4. Conclusion
A broad spectrum of disease may be encountered on fluoroscopic evaluation of the esophagus. With an interpretation approach centered on esophageal dots, lines, contours, and ends, the Radiologist is well placed to discern pathology, offer an appropriate differential diagnosis, and add significant value to patient care.
Ethical statement
This is not applicable as this is a review article.
Funding statement
There has been no financial support for this work. All authors explicitly approve the content within this manuscript.
Declaration of Competing Interest
There are no known conflicts of interest associated with this publication and there has been no financial support for this work. All authors explicitly approve the content within this manuscript.
References
- 1.Levine M.S., Rubesin S.E. Diseases of the esophagus: diagnosis with esophagography. Radiology. 2005;237(November (2)):414–427. doi: 10.1148/radiol.2372050199. Radiology. 2005 Nov;237(2):414-427. [DOI] [PubMed] [Google Scholar]
- 2.Gore R.M., Levine M.S. 4th edition. Saunders; 2014. Textbook of Gastrointestinal Radiology. December 10. [Google Scholar]
- 3.Levine M.S., Ramchandani P., Rubesin S.E. Cambridge University Press; 2012. Practical Fluoroscopy of the GI and GU Tracts. [Google Scholar]
- 4.Noh H.M., Fishman E.K., Forastiere A.A., Bliss D.F., Calhoun P.S. CT of the esophagus: spectrum of disease with emphasis on esophageal carcinoma. Radiographics. 1995;15(September (5)):1113–1134. doi: 10.1148/radiographics.15.5.7501854. [DOI] [PubMed] [Google Scholar]
- 5.Hoversten P., Kamboj A.K., Katzka D.A. Infections of the esophagus: an update on risk factors, diagnosis, and management. Dis. Esophagus. 2018;31(December (12)) doi: 10.1093/dote/doy094. [DOI] [PubMed] [Google Scholar]
- 6.Levine M.S., Laufer I., Kressel H.Y., Friedman H.M. Herpes esophagitis. AJR. 1981;136(May):863–866. doi: 10.2214/ajr.136.5.863. [DOI] [PubMed] [Google Scholar]
- 7.Bova J.G., Dutton N.E., Goldstein H.M., Hoberman L.J. Medication-induced esophagitis: diagnosis by double-contrast esophagography. AJR Am. J. Roentgenol. 1987;148(April (4)):731–732. doi: 10.2214/ajr.148.4.731. [DOI] [PubMed] [Google Scholar]
- 8.Decker G.A., Loftus E.V., Jr., Pasha T.M., Tremaine W.J., Sandborn W.J. Crohn’s disease of the esophagus: clinical features and outcomes. Inflamm. Bowel Dis. 2001;7(May (2)):113–119. doi: 10.1097/00054725-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lee M.H., Lubner M.G., Peebles J.K., Hinshaw M.A., Menias C.O., Levine M.S., Pickhardt P.J. Clinical, imaging, and pathologic features of conditions with combined esophageal and cutaneous manifestations. Radiographics. 2019;39(September–October (5)):1411–1434. doi: 10.1148/rg.2019190052. [DOI] [PubMed] [Google Scholar]
- 10.Guerra-Leal J.D., Meester I., Cantu-Gonzalez J.R., Ornelas-Cortinas G., Montemayor-Martinez A., Salas-Alanis J.C. The importance of esophagography in patients with recessive dystrophic epidermolysis bullosa. AJR Am. J. Roentgenol. 2016;207(October (4)):778–781. doi: 10.2214/AJR.16.16115. [DOI] [PubMed] [Google Scholar]
- 11.Naylor M.F., MacCarty R.L., Rogers R.S., 3rd. Barium studies in esophageal cicatricial pemphigoid. Abdom. Imaging. 1995;20(March–April (2)):97–100. doi: 10.1007/BF00201511. [DOI] [PubMed] [Google Scholar]
- 12.Wang H.W., Kuo C.J., Lin W.R., Hsu C.M., Ho Y.P., Lin C.J., Su M.Y., Chiu C.T., Wang C.L., Chen K.H. The clinical characteristics and manifestations of cytomegalovirus esophagitis. Dis. Esophagus. 2016;29(May (4)):392–399. doi: 10.1111/dote.12340. [DOI] [PubMed] [Google Scholar]
- 13.Balthazar E.J., Megibow A.J., Hulnick D., Cho K.C., Beranbaum E. Cytomegalovirus esophagitis in AIDS: radiographic features in 16 patients. AJR Am. J. Roentgenol. 1987;149(November (5)):919–923. doi: 10.2214/ajr.149.5.919. [DOI] [PubMed] [Google Scholar]
- 14.Levine M.S., Loercher G., Katzka D.A., Herlinger H., Rubesin S.E., Laufer I. Giant, human immunodeficiency virus-related ulcers in the esophagus. Radiology. 1991;180(August (2)):323–326. doi: 10.1148/radiology.180.2.2068293. [DOI] [PubMed] [Google Scholar]
- 15.Weerasuriya N., Snape J. A study of candida esophagitis in elderly patients attending a district general hospital in the UK. Dis. Esophagus. 2006;19(3):189–192. doi: 10.1111/j.1442-2050.2006.00563.x. [DOI] [PubMed] [Google Scholar]
- 16.Roberts L., Gibbons R., Gibbons G., Rice R.P., Thompson W.M. Adult esophageal candidiasis: a radiographic spectrum. Radiographics. 1987;7(March (2)):289–307. doi: 10.1148/radiographics.7.2.3448636. [DOI] [PubMed] [Google Scholar]
- 17.Glick S.N., Teplick S.K., Goldstein J., Stead J.A., Zitomer N. Glycogenic acanthosis of the esophagus. AJR Am. J. Roentgenol. 1982;139(October (4)):683–688. doi: 10.2214/ajr.139.4.683. [DOI] [PubMed] [Google Scholar]
- 18.Stern Z., Sharon P., Ligumsky M., Levij I.S., Rachmilewitiz D. Glycogenic acanthosis of the esophagus: a benign but confusing endoscopic lesion. Am. J. Gastroenterol. 1980;74:261–263. [PubMed] [Google Scholar]
- 19.McGarrity T.J., Wagner Baker M.J., Ruggiero F.M., Thiboutot D.M., Hampel H., Zhou X.P., Eng C. GI polyposis and glycogenic acanthosis of the esophagus associated with PTEN mutation positive Cowden syndrome in the absence of cutaneous manifestations. Am. J. Gastroenterol. 2003;98(June (6)):1429–1434. doi: 10.1111/j.1572-0241.2003.07496.x. [DOI] [PubMed] [Google Scholar]
- 20.Pilarski R., Burt R., Kohlman W., Pho L., Shannon K.M., Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J. Natl. Cancer Inst. 2013;105(November (21)):1607–1616. doi: 10.1093/jnci/djt277. [DOI] [PubMed] [Google Scholar]
- 21.Ghahremani G.G., Rushovich A.M. Glycogenic acanthosis of the esophagus: radiographic and pathologic features. Gastrointest. Radiol. 1984;9(2):93–98. doi: 10.1007/BF01887812. [DOI] [PubMed] [Google Scholar]
- 22.Kao P.C., Vecchio J.A., Schned L.M., Blaszyk H. Esophageal squamous papillomatosis. Eur. J. Gastroenterol. Hepatol. 2005;17(November (11)):1233–1237. doi: 10.1097/00042737-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Sandvik A.K., Aase S., Kveberg K.H., Dalen A., Folvik M., Naess O. Papillomatosis of the esophagus. J. Clin. Gastroenterol. 1996;22:35–37. doi: 10.1097/00004836-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Sabedotti G., Dreweck M.O., Sabedotti V., Netto M.R. Best cases from the AFIP: carcinoma of the esophagus: varicoid pattern. Radiographics. 2006;26(January–February (1)):271–274. doi: 10.1148/rg.261045209. [DOI] [PubMed] [Google Scholar]
- 25.Loudin M., Anderson S., Schlansky B. Bleeding’ downhill’ esophageal varices associated with benign superior vena cava obstruction: case report and literature review. BMC Gastroenterol. 2016;16(October (1)):134. doi: 10.1186/s12876-016-0548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samadi F., Levine M.S., Rubesin S.E., Katzka D.A., Laufer I. Feline esophagus and gastroesophageal reflux. AJR Am. J. Roentgenol. 2010;194(April (4)):972–976. doi: 10.2214/AJR.09.3352. [DOI] [PubMed] [Google Scholar]
- 27.White S.B., Levine M.S., Rubesin S.E., Spencer G.S., Katzka D.A., Laufer I. The small-caliber esophagus: radiographic sign of idiopathic eosinophilic esophagitis. Radiology. 2010;256(July (1)):127–134. doi: 10.1148/radiol.10091060. [DOI] [PubMed] [Google Scholar]
- 28.Dellon E. Eosinophilic esophagitis: diagnostic tests and criteria. Curr. Opin. Gastroenterol. 2012;28(July (4)):382–388. doi: 10.1097/MOG.0b013e328352b5ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan B.M., Shaffer E.A. Eosinophilic esophagitis: a newly established cause of dysphagia. World J. Gastroenterol. 2006;12(April (15)):2328–2334. doi: 10.3748/wjg.v12.i15.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nuño-Guzmán C.M., García-Carrasco D., Haro M., Arróniz-Jáuregui J., Corona J.L., Salcido M. Zenker’s diverticulum: diagnostic approach and surgical management. Case Rep. Gastroenterol. 2014;8(November (3)):346–352. doi: 10.1159/000369130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delahunty J.E., Margulies S.I., Alonso W.A., Knudson D.H. The relationship of reflux esophagitis to pharyngeal pouch (Zenker’s diverticulum) formation. Laryngoscope. 1971;81(April (4)):570–577. doi: 10.1288/00005537-197104000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Rubesin S.E., Levine M.S. Killian-jamieson diverticula radiographic findings in 16 patients. Am. J. Roentgenol. 2001;177:85–89. doi: 10.2214/ajr.177.1.1770085. [DOI] [PubMed] [Google Scholar]
- 33.Raziel A., Landau O., Fintsi Y., Fass R., Charuzi I. Sarcoidosis and giant midesophageal diverticulum. Dis. Esophagus. 2000;13(4):317–319. doi: 10.1046/j.1442-2050.2000.00139.x. [DOI] [PubMed] [Google Scholar]
- 34.Tapias L.F., Morse C.R., Mathisen D.J., Gaissert H.A., Wright C.D., Allan J.S., Lanuti M. Surgical management of esophageal epiphrenic diverticula: a transthoracic approach over four decades. Ann. Thorac. Surg. 2017;104(October (4)):1123–1130. doi: 10.1016/j.athoracsur.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Syed T.A., Salem G., Fazili J. Spontaneous intramural esophageal hematoma. Clin. Gastroenterol. Hepatol. 2018;16(February (2)):e19–e20. doi: 10.1016/j.cgh.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 36.Sinha N., Mikityanskiy Y., Mularz S.2, Trillo J. Esophageal intramural pseudodiverticulosis. Gastrointest. Endosc. 2018;88(October (4)):764–765. doi: 10.1016/j.gie.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 37.van der Putten A.B., Loffeld R.J. Esophageal intramural pseudodiverticulosis. Dis. Esophagus. 1997;10(January (1)):61–63. doi: 10.1093/dote/10.1.61. [DOI] [PubMed] [Google Scholar]
- 38.Smith B.M., Lu J.C., Dorfman A.L., Mahani M.G., Agarwal P.P. Rings and slings revisited. Magn. Reson. Imaging Clin. N. Am. 2015;23(February (1)):127–135. doi: 10.1016/j.mric.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Vela M.F., Richter J.E., Pandolfino J.E. John Wiley & Sons; 2013. Practical Manual of Gastroesophageal Reflux Disease. [Google Scholar]
- 40.Richter J.E., Rubenstein J.H. Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology. 2018;154(January (2)):267–276. doi: 10.1053/j.gastro.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudolph R.E., Vaughan T.L., Storer B. Segment length and risk for neoplastic progression in patients with Barrett esophagus. Ann. Intern. Med. 2000;133(November (9)):748. doi: 10.7326/0003-4819-133-9-200011070-00026. [DOI] [PubMed] [Google Scholar]
- 42.Westhoff B., Brotze S., Weston A., McElhinney C., Cherian R., Mayo M.S., Smith H.J., Sharma P. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest. Endosc. 2005;61(February (2)):226–231. doi: 10.1016/s0016-5107(04)02589-1. [DOI] [PubMed] [Google Scholar]
- 43.Spechler S.J., Souza R.F. Barrett’s esophagus. N. Engl. J. Med. 2014;371(August (9)):836–845. doi: 10.1056/NEJMra1314704. [DOI] [PubMed] [Google Scholar]
- 44.Gilchrist A.M., Levine M.S., Carr R.F., Saul S.H., Katzka D.A., Herlinger H., Laufer I. Barrett’s esophagus: diagnosis by double-contrast esophagography. AJR Am. J. Roentgenol. 1988;150(January (1)):97–102. doi: 10.2214/ajr.150.1.97. [DOI] [PubMed] [Google Scholar]
- 45.Sandhu D.S., Fass R. Current trends in the management of gastroesophageal reflux disease. Gut Liver. 2018;12(January (1)):7–16. doi: 10.5009/gnl16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahrilas P.J., Bredenoord A.J., Fox M., Gyawali C.P., Roman S., Smout A.J.P.M., Pandolfino J.E. Expert consensus document: advances in the management of oesophageal motility disorders in the era of high-resolution manometry: a focus on achalasia syndromes. Nat. Rev. Gastroenterol. Hepatol. 2017;14(November (11)):677–688. doi: 10.1038/nrgastro.2017.132. [DOI] [PubMed] [Google Scholar]
- 47.Stavropoulos S.N., Friedel D., Modayil R., Parkman H.P. Diagnosis and management of esophageal achalasia. BMJ. 2016;354(September):i2785. doi: 10.1136/bmj.i2785. [DOI] [PubMed] [Google Scholar]
- 48.Bern C. Chagas’ disease. N. Engl. J. Med. 2015;373(July (5)):456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 49.Rongioletti F., Ferreli C., Atzori L., Bottoni U., Soda G. Scleroderma with an update about clinico-pathological correlation. G Ital. Dermatol. Venereol. 2018;153(April (2)):208–215. doi: 10.23736/S0392-0488.18.05922-9. [DOI] [PubMed] [Google Scholar]
- 50.Pickhardt P.J. The “hide-bound” bowel sign. Radiology. 1999;213(December (3)):837–838. doi: 10.1148/radiology.213.3.r99dc21837. [DOI] [PubMed] [Google Scholar]
- 51.Schoenfeld S.R., Castelino F.V. Evaluation and management approaches for scleroderma lung disease. Ther. Adv. Respir. Dis. 2017;11(August (8)):327–340. doi: 10.1177/1753465817713680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiang H., Han J., Ridley W.E., Ridley L.J. Oesophageal web. J. Med. Imaging Radiat. Oncol. 2018;62(October (Suppl. 1)):95. doi: 10.1111/1754-9485.41_12784. [DOI] [PubMed] [Google Scholar]
- 53.Novacek G. Plummer‐Vinson syndrome. Orphanet J. Rare Dis. 2006;1:36. doi: 10.1186/1750-1172-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong S.J., Kim T.J., Nam K.B., Lee I.S., Yang H.C., Cho S., Kim K., Jheon S., Lee K.W. New TNM staging system for esophageal cancer: what chest radiologists need to know. Radiographics. 2014;34(October (6)):1722–1740. doi: 10.1148/rg.346130079. [DOI] [PubMed] [Google Scholar]
- 55.Kim T.J., Kim H.Y., Lee K.W., Kim M.S. Multimodality assessment of esophageal cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2009;29(March–April (2)):403–421. doi: 10.1148/rg.292085106. [DOI] [PubMed] [Google Scholar]