Graphical abstract
Keywords: High-intensity ultrasound, Kiwifruit allergy, IgE binding capacity, Secondary structure, In-vitro digestibility, Circular dichroism spectroscopy
Highlights
-
•
16-min ultrasound caused a 50% reduction in the IgE binding capacity of Act d 2.
-
•
In-vitro digestibility of kiwifruit proteins increased up to 77% with ultrasound.
-
•
Ultrasonication led to a loss of alpha-helixes and an increase in beta-sheets.
-
•
Digestibility and allergenicity strongly associated its protein secondary structure.
Abstract
Kiwifruit can trigger allergic reactions that can lead to death, causing public health concerns worldwide. In the present study, we treated kiwifruit samples with high-intensity ultrasound (20 kHz, 400 W, 50% duty cycle) for 0 to 16 min to evaluate its effect on the IgE binding capacity of kiwifruit allergen Act d 2, secondary structure and in-vitro digestibility of kiwifruit proteins. The changes in the protein solubility and microstructures of kiwifruit were also analyzed. The results showed that treatment with powerful ultrasound caused a significant disruption in the microstructure of kiwifruit tissues, leading to the changes in the secondary structures of proteins, including a loss of alpha-helixes and an increase in beta-sheet structures. These structural changes were due to the ultrasound treatment, especially 16 min of treatment, resulted in a 50% reduction in Act d 2 allergen content and significantly improved in-vitro digestibility up to 62% from the initial level of 35%. Furthermore, the solubility of the total proteins present in kiwifruit samples was significantly decreased by 20% after 16-min ultrasound processing. The results of this work showed that high-intensity ultrasound treatment has a potential application in reducing the allergenicity of kiwifruit or related products.
1. Introduction
Food allergy causing public health-related issues has become a global challenge, which affects >10% of the total population, with a higher prevalence among infants and children [1], [2]. In 2004, the Food and Drug Administration (FDA) of the United States required labeling of products containing the “Big Eight” allergenic food items, including milk, eggs, fish, shellfish, tree nuts, peanuts, wheat, and soybeans [3]. Currently, kiwifruit is considered to cause the most common plant-based food allergies among fruits worldwide after the “Big Eight” [1], [4]. In Turkey, allergic reactions triggered by kiwifruit is ranked fourth among children aged 6 to 9 years old [5]. In Sweden and Denmark, studies found that 50% of individuals with food allergy history showed cross-reactivity to kiwifruit since proteins in kiwifruits share amino sequences present in other allergens [6]. In North America, up to 2% of individuals in the total population are allergic to kiwifruit or related products [7]. Since 2012, kiwifruit has been confirmed in Japan as the eighth-most prevalent food that triggers allergic reactions after shrimp, and Japan also has enacted a law for it to be listed in the ingredients of related food products [8].
In addition, many studies have found that pollen such as birch and ragweed pollen, and other fruits and vegetables showed cross-reactivity with kiwifruit since they contain similar allergenic epitopes in their proteins [9], [10], [11], [12], [13]. To date, 20–40% of the population around the world are allergic to pollen [4]. Thus, the number of people susceptible to kiwifruit allergens is increasing. Furthermore, allergic reactions to kiwifruit have symptoms ranging from mild to severe. The mild symptoms are in the form of oral allergy syndrome (OAS), which includes itching, redness, and rashes on the skin [1], [9]. Severe reactions such as a sudden decrease in the blood pressure and an increase of heart rate can result in anaphylaxis and in death [14]. Thus, kiwifruit allergy is becoming one of the primary health-related public issues worldwide.
In medical studies, there are no practical approaches to cure food allergies. A few studies on oral immunotherapy (OIT) have obtained some positive results in managing peanut and milk allergy [15], [16]. In comparison, many studies have reported that the application of thermal food processing methods showed potential in reducing the allergenicity of kiwifruit by denaturing and aggregating allergen structures [17]. Fiocchi et al. (2004) boiled raw kiwifruit samples at 100 °C for 5 min, and using the samples of the boiled kiwifruit in skin prick test found that only 5 of 20 volunteers with histories of kiwifruit allergy developed allergic reactions [18]. Recently, Uberti et al. (2015) reported that the allergenicity of kiwifruit was significantly decreased after heating at 65 to 110 °C for 15 s to 21 min [17]. However, thermal processing negatively affected the color attributes, flavor, and bioactive compounds (e.g., ascorbic acid and phenolics) present in fruit samples. Thus, novel non-thermal processing techniques are needed [4].
High-intensity ultrasound (>1 W cm−2, 20–100 kHz), a non-thermal processing technique, has attracted much attention among researchers who are working on reducing food allergenicity. Li et al. (2006) found that ultrasound treatment (30 Hz, 800 W) for 1.5 h caused structural changes of shrimp proteins, which led to a reduction in their overall allergenicity [19]. Also, the inhibition value of IgE binding to immobilized Pen a 1 (major allergen) reached 20% when compared to the untreated samples. Similarly, a study has reported that ultrasonic treatment (0–1.5 h at 50 Hz) alone significantly reduced Ara h 2 (a major peanut allergen) content, compared to the raw peanut samples [20]. However, no studies about the influence of ultrasonication on kiwifruit allergenicity have been reported. Therefore, in this study, we aimed to evaluate the effects of high-intensity ultrasound processing on the allergenicity and digestibility of kiwifruit proteins. The changes in microstructure and the secondary structures of proteins in kiwifruit were also analyzed. This study provided data that can be used to reveal the mechanisms by which ultrasound can cause a reduction in allergenicity of kiwifruits.
2. Material and methods
2.1. Sample preparation and ultrasound processing
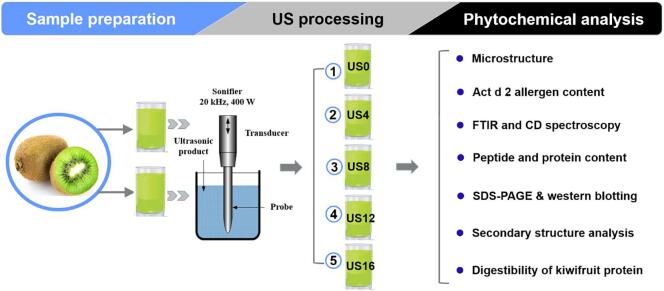
Green kiwifruits (A. deliciosa) were purchased and stored at room temperature until reaching full ripeness (15–18 °Brix, total soluble solids; firmness, 6–8 N) [21]. As described in Fig. 1, the peeled ripe kiwifruits were extracted with water at the ratio of 1:1 using a juicer. A 200 mL juice sample was treated with an ultrasonicator (20 kHz, 400 W, Model 450 Sonifier, CT, USA), and the output power was set at 50% duty cycle. Ice packs were applied to keep the low temperature of the samples during processing. Different samples were processed for 0 min (US0), 4 min (US4), 8 min (US8), 12 min (US12), and 16 min (US16) in triplicate. After each treatment, a 100 mL sample was collected and stored at 4 °C for analysis. The remainder of the juice was freeze-dried and sorted at −20 °C.
Fig. 1.
Experimental scheme of high-intensity ultrasound processing in kiwifruit samples.
2.2. Total soluble protein measurement
The Pierce BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA) was used to quantify the total soluble protein content in the kiwifruit samples. The ultrasound treated samples and untreated samples were determined as described by the kit protocol.
2.3. In-vitro protein digestibility (IVPD) and peptide content
According to the method described by Yao et al. (2018), two enzymes (pepsin and pancreatin) with three-stage digestion were applied to simulate in-vivo digestion of proteins in the gastrointestinal tract [22]. During the first-stage digestion, 500 mg of dried kiwifruit samples were mixed with 20 mL of phosphate buffer (0.01 M, pH 7.0) and incubated at 23 °C for 30 min, after which the mixture was centrifuged at 5000×g for 10 min. The supernatant was considered to be the initial protein extract solution. In the second-stage digestion, the pH of the initial protein extract (10 mL) was adjusted to 1.5 using 1 M HCl. Then, 100 µL of pepsin solution (10 mg pepsin/mL in 0.01 M HCl) was added. After 30-min incubation at 37 °C, 1.0 M NaOH solution (100 µL) was mixed with each sample to stop the second-stage digestion. A 1.0 M NaOH solution was used to adjust the pH of the mixture to 7.8, and 300 µL of pancreatin solution (10 mg/mL in sodium phosphate buffer, pH 7.0) was added to start the third-stage digestion, and the mixture was incubated at 40 °C for one hour. Then, 100 µL of Na2CO3 solution (150 mM) was added to stop the overall digestion stages. The total protein content of samples during these three-stages digestions was measured using the Pierce BCA Protein Assay kit. The in-vitro digestibility of kiwifruit proteins was calculated using the following equation:
where P0 is initial protein content, P1 is the final undigested protein content.
During in-vitro protein digestion, 1 mL of initial protein extract solution, pepsin digested protein solution, and pancreatin digested protein solution was collected. These samples were centrifuged at 5000×g for 10 min, and the supernatant was collected for the peptide measurement. As previously described by Iskandar et al. (2015), an o-phthalaldehyde (OPA) working solution composed of sodium tetraborate (100 mM), sodium dodecyl sulfate (20%, w/v), 40 mg/mL of OPA (methanol as solvent), 100 μL of β-mercaptoethanol was prepared [23]. A 10 μL sample from each of the three digestion stages was mixed with 150 μL OPA solution in a microplate and incubated for 2 min, after which the absorbances at 340 nm were measured and recorded using a plate reader (Spectra Max M2, Molecular Devices, QC, Canada). A standard curve was obtained using Leu-Gly (0–10000 μM) as a standard.
2.4. SDS-PAGE and western blotting test
Protein was extracted from freeze-dried kiwifruit with 0.01 M phosphate buffer (pH 7.0) for two hours at 23 °C. The mixture was centrifuged at 5000×g for 10 min at 4 °C [24]. The proteins in the supernatants were analyzed using SDS-PAGE and western blotting. Electrophoresis was performed using the Fisher brand™ FB-VE10-1 Vertical Electrophoresis System at 80 V for one hour. Western blotting was performed following the protocol from Bio-Rad through a Trans-Blot TurboTM Transfer System (Bio-Rad, QC, Canada). The related figures were obtained using the image LabTM software (Bio-Rad, QC, Canada).
2.5. Measurement of kiwifruit allergen Act d 2 using Sandwich ELISA
Act d 2 is a soluble protein that has been recognized as one of the major allergens presenting in kiwifruit [25]. A Sandwich ELISA kit based on Act d 2 was purchased from Elabscience (USA) to measure the concentration of this allergen. The kit includes biotin-conjugated rabbit polyclonal antibodies, goat anti-rabbit secondary antibody, and recombinant Act d 2. According to the protocol provided by the kit, the optical density (OD) is measured at 450 nm [26]. The recombinant Act d 2 was used as a standard to obtain the standard curve.
2.6. Fourier transform infrared (FTIR)spectroscopy
FTIR spectroscopy (Thermo Nicolet Analytical Instruments, Madison, WI, USA) was used to determine the secondary structure of kiwifruit proteins. Firstly, a spectrum of the background was recorded prior to measurement [27]. Freeze-dried kiwifruit samples (1 mg) used to perform the measurement, and the parameters were set with a 32-time scan ranging from 1000 to 2000 cm−1 [27].
2.7. Circular dichroism (CD) spectroscopy
Freeze-dried kiwifruit (0.5 g) was extracted with 5 mL of 0.01 M phosphate buffer (pH 7.0) for two hours, and the mixture was centrifuged at 5000×g for 20 min at 4 °C [24]. The supernatants obtained from each sample were collected for further analysis. The secondary structures (e.g., α-helixes and β-sheets) of kiwifruit protein extract was measured with CD spectroscopy (J-815, JASCO, Tokyo, Japan) [28]. The scanning was set in a continuous model ranging from 260 nm to 190 nm with a speed of 50 nm/min. The data obtained from 5-time scanning was analyzed by a CDPro Software [29].
2.8. Microstructure observation.
Freeze-dried kiwifruit powder was used to evaluate microstructural changes due to ultrasound. This was done by using a Scanning Electron Microscope (SEM) (TM3000, Hitachi High-Technologies Corporation., Tokyo, Japan). Images of dried kiwifruit samples that were either treated or not treated (controls) by ultrasound were taken at 100× magnification.
2.9. Statistical analysis
The data obtained from the study presented as mean ± standard deviation value was analyzed with a one-way analysis of variance (ANOVA). The SPSS software (22.0, SPSS Inc., Chicago, USA) was used to detect the significance between different ultrasound treatments including US0, US4, US8, US12, and US16 using Duncan's multiple range test (p < 0.05). All the measurements were performed in triplicate.
3. Results and discussion
3.1. Total soluble protein content
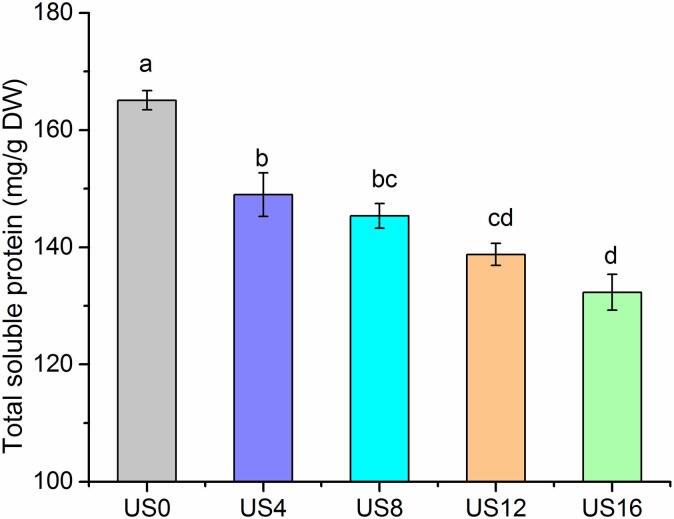
Solubility is considered as one of the most practical approaches for evaluating the denaturation and aggregation of proteins, and it is an indicator of protein functionality [30]. As shown in Fig. 2, the total soluble protein content of kiwifruit decreases as the duration of ultrasonication increases. The initial soluble protein concentration of kiwifruit (US0) was 165.09 mg/g DW, which reduced by 10% after 4-min ultrasound processing. The protein content of US8 treated samples (145.36 mg/g DW) showed a slight decrease when compared with that of US4. After 12-min ultrasound treatment, the total soluble protein of kiwifruit samples decreased to 138.78 mg/g DW. Among all the treatments, US16 treated samples contained the lowest total soluble protein content (132.33 mg/g DW), which is approximately a 20% reduction compared with the initial level.
Fig. 2.
The total soluble protein content of kiwifruit samples: untreated sample (US0) and those treated by ultrasound at 400 W, 20 kHz, for 4 min (US4), 8 min (US8), 12 min (US12), and 16 min (US16). Note: values with different letters in various columns are significantly different (p < 0.05) from each other.
These results agreed with the findings reported in previous studies, where processing black bean protein isolates with ultrasound at 150 W and 20 kHz for 12 min resulted in a reduction in solubility [31]. Similarly, Costa et al. (2013) found that 10 min of ultrasound treatment (376 W/cm) caused a 50% loss in the concentration of pineapple juice protein [32]. The decrease in protein concentration may be associated with the breakdown of hydrogen bonds and peptide chains in the proteins by the ultrasound treatment, which in turn could alter secondary and tertiary protein structures [33]. Another possibility is that the protein molecules partially unfolded and reformed, leading to the formation of macromolecular aggregates, which may have contributed to a reduction in the solubility of affected proteins [31]. This indicated that ultrasound treatment could potentially modify the structure of proteins or rupture their peptide chains, which in turn could affect the allergenicity of the proteins.
3.2. In-vitro digestibility and peptide content
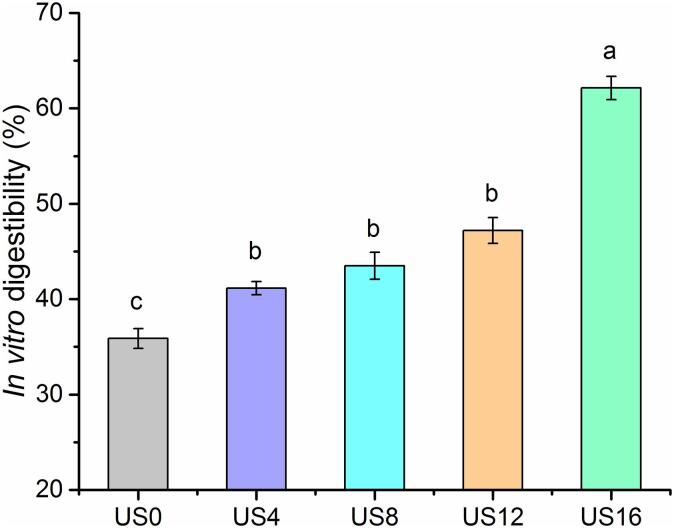
Many studies have reported that plant-based proteins have lower digestibility when compared with the proteins in meat or dairy products [34]. The in-vitro digestibilities of proteins in raw soybean and in mature pigeon pea seed are approximate 40% [35], and 58% [36], respectively. In this study, the in-vitro digestibility of kiwifruit proteins was determined after three-stage digestion using pepsin and pancreatin. The in-vitro digestibility of untreated kiwifruit proteins (US0) was 35%, and then increased to 41% after four minutes of ultrasound processing (Fig. 3). Further slight increases to 44% and 47% in the digestibility of kiwifruit proteins were observed for US8 and US12 treatments, respectively. The highest digestibility was 62% with US16 treatment, which was approximate 2-fold that of the untreated samples (US0). These results agreed with the findings reported by Zhu et al. (2018), where they treated egg white proteins with ultrasound at 400 W for 0–16 min. They found that the in-vitro digestibility of egg white proteins increased from 73% to 79% [22]. However, an opposite result was reported with faba bean protein isolates, which observed ultrasonication caused a 4% reduction in the in-vitro digestibility compared with untreated protein samples [37].
Fig. 3.
In-vitro digestibility of kiwifruit proteins treated with ultrasound.
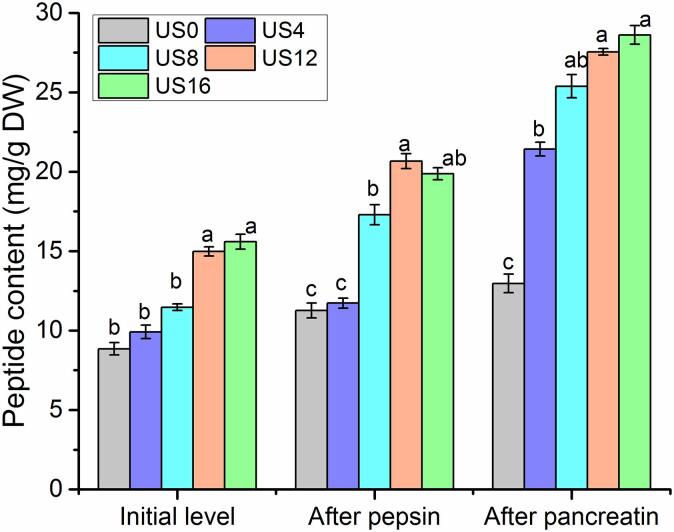
During the three-stage in-vitro digestion of kiwifruit proteins, the peptide content was measured using the OPA assay. As shown in Fig. 4, ultrasonication increased the peptide content for all stages of digestion. The initial level of peptides in raw kiwifruit protein (US0) was 8.85 mg/g DW. After 4-min and 8-min ultrasound processing, the peptide content increased slightly but not significantly compared with the control group (US0). Whereas, the peptide content significantly increased in US12 (14.98 mg/g) and US16 (15.59 mg/g) treated samples. In the second stage of in-vitro digestion (pepsin), the peptide content of pepsin digested samples was maintained at a similar level compared with the initial level. However, the peptide content was significantly enhanced in all treatments after the third-stage digestion with pancreatin. The highest concentration of peptide was observed in US16 (28.60 mg/g) samples, followed by those for US12 (27.54 mg/g) and US8 (25.38 mg/g) samples, which were approximate 3–4 time higher than in the untreated samples.
Fig. 4.
Peptide content of kiwifruit samples during different digestion stages.
The increase of peptide in the processed samples could be due to the improvement of the in-vitro digestibility in kiwifruit proteins when treated with ultrasound processing (Fig. 3). In addition, high-intensity ultrasound can form “cavitation effects” causing intense shear stress and pressure in a brief time, which can lead to either change in secondary structures in the proteins or disruptions in peptide chains, thereby improving in-vitro digestibility and peptide content [22], [38], [39].
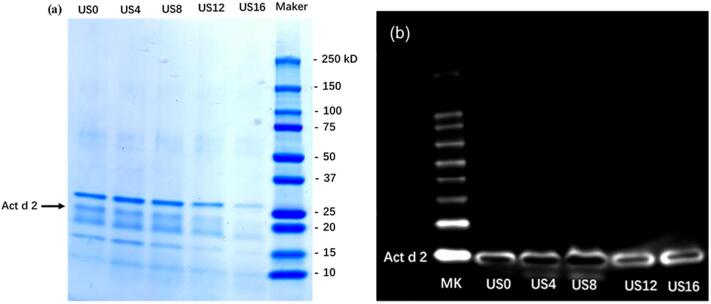
3.3. SDS-PAGE and western blotting
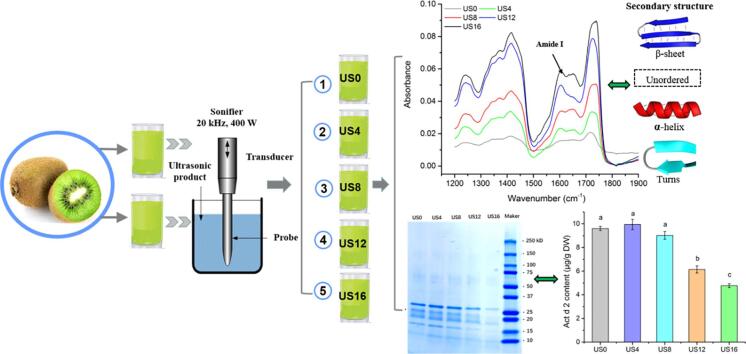
As shown in Fig. 5a, the intensity of each protein band is decreasing as processing duration is increasing, especially after 16 min, which agrees with the changes in total soluble protein content during ultrasound processing (Fig. 2). The intensities of the Act d 2 band for US4 and US8 samples were slightly but visibly lower compared to the untreated sample. After 16-min ultrasonication, the Act d 2 protein band could hardly be seen. In order to confirm these results, we performed western blotting to evaluate the IgE binding capacity of kiwifruit protein that have undergone ultrasound processing. As shown in Fig. 5b, no obvious differences in the IgE binding capacity of US4 and US8 treated samples were seen when compared with the untreated samples (US0). Whereas the IgE binding capacity of Act d 2 was significantly inhibited after 12 and 16 min of ultrasound processing. Similar results have been reported with peach juice, soybean milk, cow milk, and shrimp when treated with ultrasound at frequencies of 26–30 kHz, 150–800 W, for 0–30 min [40], [41], [42], [43]. This indicated that high-intensity ultrasound processing has a potential application in reducing the immunoactivity of allergens in different food products.
Fig. 5.
SDS-PAGE protein band (a) western blotting (b) of kiwifruit protein extract.
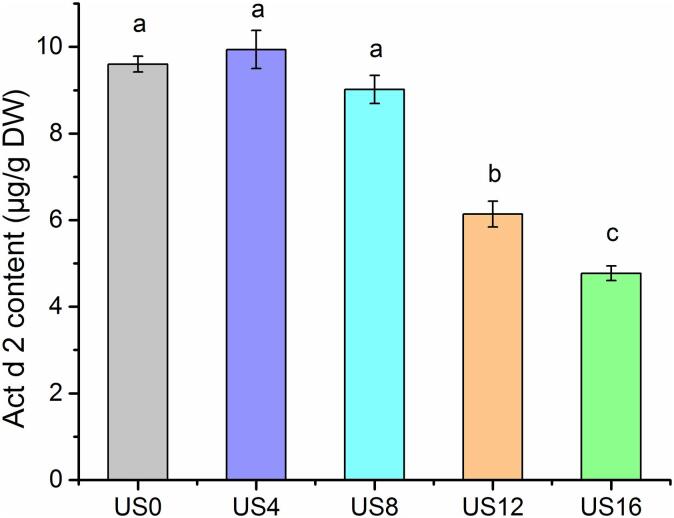
3.4. Act d 2 measurement using Sandwich ELISA test
The Sandwich ELISA test was used to quantify Act d 2 content in the kiwifruit samples. The initial concertation of Act d 2 in the raw kiwifruit samples was 9.6 μg/g DW, and no significant changes in the allergen concentration in the samples were detected during the first 8 min of ultrasound processing (Fig. 6). With 12 min of processing, Act d 2 content was significantly decreased by 36%, compared with the initial level (US0). The highest reduction in Act d 2 content was observed in US16 treated samples (4.77 μg/g DW), which is 50% lower than the untreated sample. Thus, ultrasound processing has a potential application for reducing the allergenicity of kiwifruit.
Fig. 6.
Kiwifruit allergen Act d 2 content using ELISA test.
Li et al. (2006) treated shrimp samples with high-intensity ultrasound at 50 °C for 1.5 h, and resulted in a reduction in the overall allergenicity of shrimp samples because of the disruption in the integrity and structure of shrimp proteins by ultrasound processing [19]. Also, the inhibition of IgE binding to immobilized Pen a 1, a major shrimp allergen, reached 20% when compared to untreated samples. Similarly, studies have reported that ultrasonic pre-treatment (0–1.5 h at 50 Hz) significantly reduced the Ara h 2 content, a major peanut allergen, compared to the raw peanut samples [20]. These reductions in these allergens were strongly associated with changes in their secondary structures resulting from “cavitation effects” occurring during the ultrasound processing [4], [7], [38], [44]. Further studies regarding the mechanisms of ultrasonication, causing a reduction in the allergenicity of proteins are needed.
3.5. FTIR spectroscopy
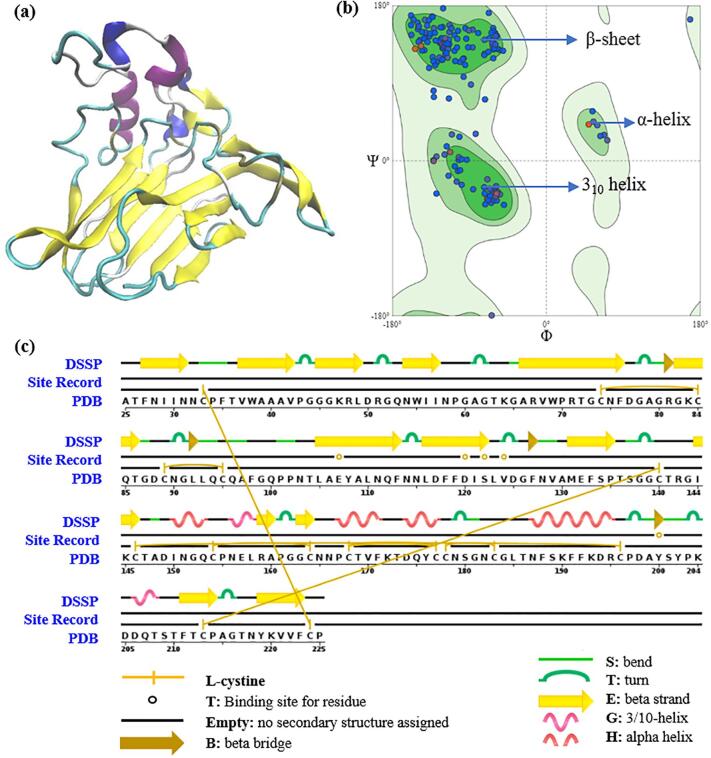
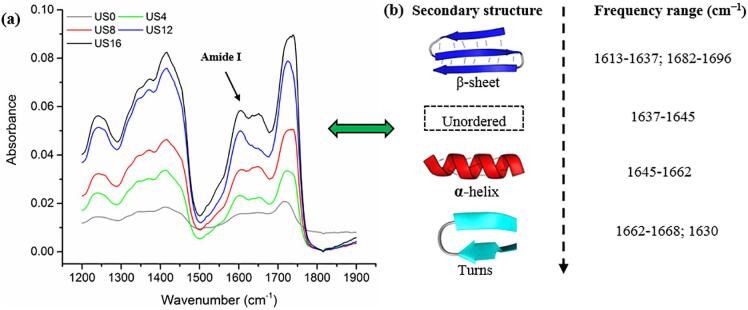
Act d 2 is major kiwifruit allergen with a molecular weight of 24 kDa. As shown in Fig. 7, Act d 2 belongs to the thaumatin-like protein family, and its secondary structure includes 13% helix (alpha-helix and 310 helices) and 38% beta-sheet [4]. In the present study, Fourier-transform infrared (FTIR) spectroscopy was used to analyze for changes in the secondary structure of kiwifruit proteins caused by ultrasonication. The results showed that the absorbance of kiwifruit samples increased as ultrasound processing time increased (Fig. 8a). The amide I region which ranges from 1600 cm−1 to 1700 cm−1 is considered as the most representative area to evaluate secondary structural changes in the proteins due to minor effects resulting from C-H groups [22], [45], [46]. As shown in Fig. 8b, within the amide I region, beta-sheet structures have peaks in the 1613–1673 cm−1 and 1682–1696 cm−1 ranges, and peaks in the 1637–1645 cm−1 and 1645–1662 cm−1 ranges are related to unordered and alpha-helix structures, respectively. The turns structures have peaks in the frequency ranges of 1662–1668 cm−1 and 1630 cm−1 [22], [45]. In this study, the results showed that the secondary structures of kiwifruit samples were positively affected by the increase in processing time, especially in the case of US16, as compared with the untreated samples (US0). This indicated that ultrasound processing has an impact on secondary structures.
Fig. 7.
(a) Secondary structure of kiwifruit allergen; (b) Ramachandran plots of kiwifruit allergen Act d 2; (c) Sequence chain of kiwifruit allergen. (Source: protein data bank, PDB accession code: 4BCT).
Fig. 8.
Secondary structural changes of kiwifruit proteins using FTIR spectroscopy analysis.
3.6. CD spectroscopy
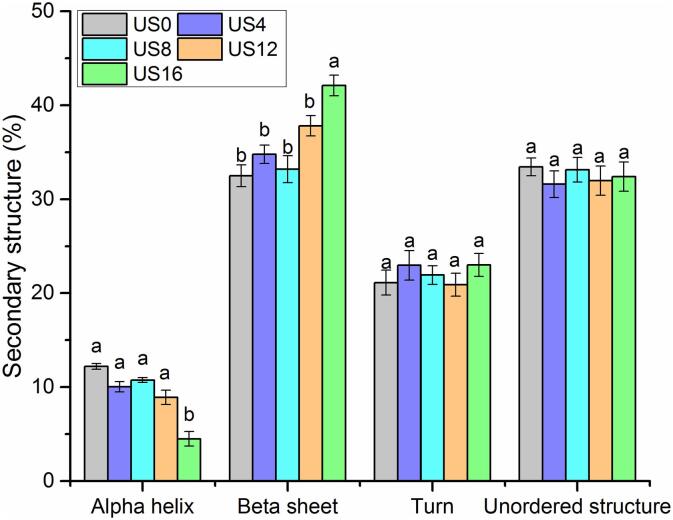
CD spectroscopy analysis was performed to quantify the secondary structural changes in protein due to ultrasound processing. As shown in Fig. 9, the beta-sheet is the major structure accounting for 30–45% of the total secondary structures, followed by turns and unordered structures, which accounted for 20–25% and 30–35% of secondary structures, respectively. Alpha-helixes only represented approximately 5–15%. Alpha helixes and beta-sheets are two major structures in the proteins that are considered to be responsible for the maintenance of the protein structure, which affect the functional properties of the protein [22]. As a result of ultrasound processing, the amount of alpha helixes in kiwifruit proteins decreased as processing time increased, especially after 16 min, while an enhancement in the beta-sheet of kiwifruit proteins was observed as compared with untreated samples (US0). No significant differences in the amount of turns and unordered structure in kiwifruit proteins were detected after ultrasound processing.
Fig. 9.
Secondary structural changes of kiwifruit proteins using CD spectroscopy analysis.
In egg white, Zhu et al. (2018) ultrasonicated samples at 400 W for 0–16 min. A 50% reduction in the amount of alpha-helixes and a 2-fold increase in the beta-sheets of egg white proteins were observed as compared with untreated samples [22]. Similarly, in blackberries, Dou et al. (2019) found that the alpha-helix content decreased after ultrasonication (300 W, 8–24 h), while no significant differences were observed in the content of beta-turns and random structures [47]. These changes in the secondary structure of the proteins might be due to shear forces, shock waves, and turbulence caused by ultrasonication. The cavitation effects can disrupt interactions between the local sequences of amino acids and between different parts of the protein molecule, resulting in the changes in the secondary structures of proteins [47]. However, in soy protein isolate, Ding et al. (2019) found that while ultrasonication decreased the contents of α-helixes and β-sheets, random and coil β-turn structures contents increased [48]. In jackfruit seed proteins, Resendiz-Vazquez et al. (2019) reported that ultrasound processing (200–600 W, 15–30 min) significantly enhanced the content of alpha-helixes and random coils, but decreased the beta-sheet content [49]. Thus, there are noticeable differences in the effect of ultrasound on the secondary structure of proteins extracted from different food matrixes.
Furthermore, many studies have reported that the secondary structure of proteins is strongly associated with their in-vitro digestibility and allergenicity [22], [50]. In egg white proteins, a study observed a negative correlation between alpha-helix structures and in-vitro digestibility of proteins [22]. In faba bean proteins, Martínez-Velasco et al. (2018) found ultrasound processing resulted in an increase in β-sheets and turns in the secondary structures, and a slight decrease in digestibility from 68.4% to 66% [37]. In addition, Li et al. (2012) reported that high hydrostatic pressure processing resulted in a 45% reduction in the allergenicity of soybean proteins, accompanying an increase in turns and alpha-helix structures, and a loss in the beta-sheet structures [51]. In apples, Meyer-Pittroff et al. (2007) reported that high pressure (100–600 MPa) caused a reduction in the IgE binding of apple allergen Mal d 1, resulting from the secondary structure deviations in the protein allergen [52]. Similarly, in the present study, high-intensity processing, especially US16, significantly reduced by 50% allergenicity (Fig. 5, Fig. 6) and improved the in-vitro digestibility (55%) of kiwifruit proteins (Fig. 3), while contributing to changes in the secondary structure of proteins, namely a decrease in alpha-helix structures and an increase in beta-sheet structures (Fig. 9).
3.7. Microstructure
As shown in Fig. 10, the microstructures of ultrasound treated kiwifruit samples were analyzed using a scanning electron microscope (SEM). For US4 samples, a slight disruption or tears in microstructures as compared with untreated samples (US0) was observed. More disordered structures and irregular fragments were observed with increasing processing time, especially with 12 min of ultrasound treatment. These disruptions were strongly attributed to cavitational forces and microstreaming stresses generated during high-intensity ultrasound treatment [31]. The most significant disruption in the kiwifruit microstructures was detected when the longest processing duration (16 min) was applied. Specifically, numerous small particles and fragments were clearly visible in the US16 treated kiwifruit samples, which might be due to the formation of transient cavitation that resulted in high pressure and stress over the longer duration of ultrasound processing [33], [53].
Fig. 10.
The microstructure changes of kiwifruit tissues treated with ultrasonication.
Similar results have been reported for strawberries, kiwifruit tissues, and black beans when processed with high-intensity ultrasound [21], [31], [39]. High-intensity ultrasound can generate incredible high pressures (>1000 atm) within a brief time due to the rapid collapse of cavitation bubbles, which provides high energy to modify the protein structures [54], [55]. These intense shear forces and high pressures could result in the breakdown of hydrogen bonds and peptide chains in the proteins, contributing to the improvement of their in-vitro digestibility [22], [44]. Also, the secondary structures (e.g., alpha-helix and beta-sheet) of proteins were affected by these intense stresses formed during ultrasound treatment, which may lead to a reduction in the allergenicity of foods [4], [44], [54].
4. Conclusion
Kiwifruit is one of the most popular fruits worldwide because of its nutritional properties and health benefits to human beings. However, kiwifruit allergy is becoming a common fruit allergy, causing a worldwide public health concern. In the present study, kiwifruit samples were processed with ultrasound (25 kHz, 400 W) for 0–16 min. The results found that 16-min ultrasound processing reduced the solubility of total proteins by 20%, while the in-vitro digestibility and peptide content were enhanced up to 62% and 3-fold, respectively, compared with the initial level. Furthermore, intense shear stress and pressure generated from ultrasonication led to a significant disruption in the cell and protein structures, resulting in the changes in the secondary structures, including a decrease in alpha-helix content and an increase in beta-sheets. Finally, a 16 min of ultrasound treatment significantly inhibited the IgE binding capacity of Act d 2, contributing to a 50% reduction in Act d 2 allergen content. Therefore, high-intensity ultrasound processing has potential applications in improving the digestibility and reducing the allergenicity of kiwifruit and related products. However, further studies regarding the mechanisms of ultrasonication in causing a reduction in the allergenicity of proteins and relevant clinical studies are needed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the China Scholarship Council (CSC), the Natural Sciences and Engineering Research Council of Canada (NSERC), and Research Start-up Funding [2019-Z1090219188] from Northwest A&F University, China. We thank the technical support of Dr. Judith Kornblatt at the Department of Chemistry and Biochemistry, Concordia University, and the input of Dr. Darwin Lyew of the Department of Bioresource Engineering, McGill University, in the editing of this paper.
Contributor Information
Jin Wang, Email: jin.wang6@mail.mcgill.ca.
Jun Wang, Email: jun.wang@nwafu.edu.cn.
References
- 1.Wang J., Vanga S.K.K., Raghavan V.G. Structural responses of kiwifruit allergen Act d 2 to thermal and electric field stresses based on molecular dynamics simulation and experiment. Food Funct. 2020 doi: 10.1039/c9fo02427a. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer S.H., Sampson H.A. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018;141:41–58. doi: 10.1016/j.jaci.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Thompson T., Kane R.R., Hager M.H. Food allergen labeling and consumer protection act of 2004 in effect. J. Am. Diet. Assoc. 2006;106:1742–1744. doi: 10.1016/j.jada.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Vanga S.K., McCusker C., Raghavan V. A comprehensive review on kiwifruit allergy: pathogenesis, diagnosis, management, and potential modification of allergens through processing. Compr. Rev. Food Sci. Food Saf. 2019;18:500–513. doi: 10.1111/1541-4337.12426. [DOI] [PubMed] [Google Scholar]
- 5.Orhan F., Karakas T., Cakir M., Aksoy A., Baki A., Gedik Y. Prevalence of immunoglobulin E-mediated food allergy in 6–9-year-old urban schoolchildren in the eastern Black Sea region of Turkey. Clin. Exp. Allergy. 2009;39:1027–1035. doi: 10.1111/j.1365-2222.2009.03263.x. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson N.E., Moller C., Werner S., Magnusson J., Bengtsson U., Zolubas M. Self-reported food hypersensitivity in Sweden, Denmark, Estonia, Lithuania and Russia. J. Investig. Allergol. Clin. Immunol. 2004;14:70–79. [PubMed] [Google Scholar]
- 7.Vanga S.K., Jain M., Raghavan V. Significance of fruit and vegetable allergens: Possibilities of its reduction through processing. Food Rev. Int. 2016;1–23 [Google Scholar]
- 8.Shoji M., Adachi R., Akiyama H. Japanese food allergen labeling regulation: an update. J. AOAC Int. 2018;101:8–13. doi: 10.5740/jaoacint.17-0389. [DOI] [PubMed] [Google Scholar]
- 9.Lucas J.S., Lewis S.A., Hourihane J.O.B. Kiwi fruit allergy: a review. Pediatr. Allergy Immunol. 2003;14:420–428. doi: 10.1046/j.0905-6157.2003.00095.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Vanga S.K., Raghavan V. Effect of pre-harvest and post-harvest conditions on the fruit allergenicity: A review. Crit. Rev. Food Sci. Nutr. 2017;1–17 doi: 10.1080/10408398.2017.1389691. [DOI] [PubMed] [Google Scholar]
- 11.Sirvent S., Cantó B., Gómez F., Blanca N., Cuesta-Herranz J., Canto G., Blanca M., Rodríguez R., Villalba M., Palomares O. Detailed characterization of Act d 12 and Act d 13 from kiwi seeds: implication in IgE cross-reactivity with peanut and tree nuts. Allergy. 2014;69:1481–1488. doi: 10.1111/all.12486. [DOI] [PubMed] [Google Scholar]
- 12.Sakai Y., Ishihata K., Nakano S., Yamada T., Yano T., Uchida K., Nakao Y., Urisu A., Adachi R., Teshima R. Specific detection of banana residue in processed foods using polymerase chain reaction. J. Agric. Food Chem. 2010;58:8145–8151. doi: 10.1021/jf100675c. [DOI] [PubMed] [Google Scholar]
- 13.Bublin M., Pfister M., Radauer C., Oberhuber C., Bulley S., DeWitt Å.M., Lidholm J., Reese G., Vieths S., Breiteneder H. Component-resolved diagnosis of kiwifruit allergy with purified natural and recombinant kiwifruit allergens. J. Allergy Clin. Immunol. 2010;125 doi: 10.1016/j.jaci.2009.10.017. 687-694. e681. [DOI] [PubMed] [Google Scholar]
- 14.Ciardiello M.A., Giangrieco I., Tuppo L., Tamburrini M., Buccheri M., Palazzo P., Bernardi M.L., Ferrara R., Mari A. Influence of the natural ripening stage, cold storage, and ethylene treatment on the protein and IgE-binding profiles of green and gold kiwi fruit extracts. J. Agric. Food Chem. 2009;57:1565–1571. doi: 10.1021/jf802966n. [DOI] [PubMed] [Google Scholar]
- 15.Assa'ad Amal H., Lierl Michelle B. Oral immunotherapy to peanut in children with mild and moderate peanut allergy results in long term tolerance. J. Allergy Clin. Immunol. 2019;143(2):AB248. doi: 10.1016/j.jaci.2018.12.758. [DOI] [Google Scholar]
- 16.Kauppila T.K., Paassilta M., Kukkonen A.K., Kuitunen M., Pelkonen A.S., Makela M.J. Outcome of oral immunotherapy for persistent cow’s milk allergy from 11 years of experience in Finland. Pediatr. Allergy Immunol. 2019;30:356–362. doi: 10.1111/pai.13025. [DOI] [PubMed] [Google Scholar]
- 17.Uberti F., Peñas E., Manzoni Y., di Lorenzo C., Ballabio C., Fiocchi A., Terracciano L., Restani P. Molecular characterization of allergens in raw and processed kiwifruit. Pediatr. Allergy Immunol. 2015;26:139–144. doi: 10.1111/pai.12345. [DOI] [PubMed] [Google Scholar]
- 18.Fiocchi A., Restani P., Bernardo L., Martelli A., Ballabio C., D'auria E., Riva E. Tolerance of heat‐treated kiwi by children with kiwifruit allergy. Pediatr. Allergy Immunol. 2004;15:454–458. doi: 10.1111/j.1399-3038.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhenxing L., Caolimin L., Jamil K. Reduction of allergenic properties of shrimp (Penaeus Vannamei) allergens by high intensity ultrasound. Eur. Food Res. Technol. 2006;223:639. [Google Scholar]
- 20.Li H., Yu J., Ahmedna M., Goktepe I. Reduction of major peanut allergens Ara h 1 and Ara h 2, in roasted peanuts by ultrasound assisted enzymatic treatment. Food Chem. 2013;141:762–768. doi: 10.1016/j.foodchem.2013.03.049. [DOI] [PubMed] [Google Scholar]
- 21.Wang J., Vanga S.K., Raghavan V. High-intensity ultrasound processing of kiwifruit juice: Effects on the ascorbic acid, total phenolics, flavonoids and antioxidant capacity. LWT. 2019;107:299–307. [Google Scholar]
- 22.Zhu Y., Vanga S.K., Wang J., Raghavan V. Effects of ultrasonic and microwave processing on avidin assay and secondary structures of egg white protein. Food Bioproc. Tech. 2018;11:1974–1984. [Google Scholar]
- 23.Iskandar M., Lands L., Sabally K., Azadi B., Meehan B., Mawji N., Skinner C., Kubow S. High hydrostatic pressure pretreatment of whey protein isolates improves their digestibility and antioxidant capacity. Foods. 2015;4:184–207. doi: 10.3390/foods4020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinaciyan T., Nagl B., Faustmann S., Frommlet F., Kopp S., Wolkersdorfer M., Wöhrl S., Bastl K., Huber H., Berger U. Efficacy and safety of 4 months of sublingual immunotherapy with recombinant Mal d 1 and Bet v 1 in patients with birch pollen–related apple allergy. J. Allergy Clin. Immunol. 2018;141:1002–1008. doi: 10.1016/j.jaci.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 25.Moreno Álvarez A., Sexto L.V., Bardina L., Grishina G., Sampson H.A. Kiwifruit allergy in children: characterization of main allergens and patterns of recognition. Children. 2015;2:424–438. doi: 10.3390/children2040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L., Lucas J.S., Hourihane J.O., Lindemann J., Taylor S.L., Goodman R.E. Evaluation of IgE binding to proteins of hardy (Actinidia arguta), gold (Actinidia chinensis) and green (Actinidia deliciosa) kiwifruits and processed hardy kiwifruit concentrate, using sera of individuals with food allergies to green kiwifruit. Food Chem. Toxicol. 2006;44:1100–1107. doi: 10.1016/j.fct.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y., Pan Z.J., Liao W., Li J., Gruget P., Kitts D.D., Lu X. Determination of antioxidant capacity and phenolic content of chocolate by attenuated total reflectance-Fourier transformed-infrared spectroscopy. Food Chem. 2016;202:254–261. doi: 10.1016/j.foodchem.2016.01.130. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y., Vanga S.K., Wang J., Raghavan V. Effects of ultrasonic and microwave processing on avidin assay and secondary structures of egg white protein. Food Bioproc. Tech. 2018:1–11. [Google Scholar]
- 29.Fu X., Liu Q., Tang C., Luo J., Wu X., Lu L., Cai Z. Study on structural, rheological and foaming properties of ovalbumin by ultrasound-assisted glycation with xylose. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104644. [DOI] [PubMed] [Google Scholar]
- 30.Hu H., Wu J., Li-Chan E.C., Zhu L., Zhang F., Xu X., Fan G., Wang L., Huang X., Pan S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013;30:647–655. [Google Scholar]
- 31.Jiang L., Wang J., Li Y., Wang Z., Liang J., Wang R., Chen Y., Ma W., Qi B., Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014;62:595–601. [Google Scholar]
- 32.Costa M.G.M., Fonteles T.V., de Jesus A.L.T., Almeida F.D.L., de Miranda M.R.A., Fernandes F.A.N., Rodrigues S. High-intensity ultrasound processing of pineapple juice. Food Bioprocess Technol. 2013;6:997–1006. [Google Scholar]
- 33.de São José J.F.B., de Andrade N.J., Ramos A.M., Vanetti M.C.D., Stringheta P.C., Chaves J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control. 2014;45:36–50. [Google Scholar]
- 34.van Vliet S., Burd N.A., van Loon L.J. The skeletal muscle anabolic response to plant-versus animal-based protein consumption. The J. Nutr. 2015;145:1981–1991. doi: 10.3945/jn.114.204305. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Chen S., Qi B., Sui X., Jiang L. Complexation of thermally-denatured soybean protein isolate with anthocyanins and its effect on the protein structure and in vitro digestibility. Food Res. Int. 2018;106:619–625. doi: 10.1016/j.foodres.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Tapal A., Vegarud G.E., Sreedhara A., Tiku P.K. Nutraceutical protein isolate from pigeon pea (Cajanus cajan) milling waste by-product: functional aspects and digestibility. Food Funct. 2019 doi: 10.1039/c8fo01933a. [DOI] [PubMed] [Google Scholar]
- 37.Martínez-Velasco A., Lobato-Calleros C., Hernández-Rodríguez B.E., Román-Guerrero A., Alvarez-Ramirez J., Vernon-Carter E.J. High intensity ultrasound treatment of faba bean (Vicia faba L.) protein: Effect on surface properties, foaming ability and structural changes. Ultrason. Sonochem. 2018;44:97–105. doi: 10.1016/j.ultsonch.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y., Vanga S.K., Wang J., Raghavan V. Impact of food processing on the structural and allergenic properties of egg white. Trends Food Sci. Technol. 2018;78:188–196. [Google Scholar]
- 39.Wang J., Wang J., Ye J., Vanga S.K., Raghavan V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control. 2019;96:128–136. [Google Scholar]
- 40.Garino C., Zitelli F., Travaglia F., Colsson J.D., Cravotto G., Arlorio M. Evaluation of the impact of sequential microwave/ultrasound processing on the IgE binding properties of Pru p 3 in treated peach juice. J. Agric. Food Chem. 2012;60:8755–8762. doi: 10.1021/jf302027e. [DOI] [PubMed] [Google Scholar]
- 41.Li X., Li Z., LiN H., Samee H. Effect of power ultrasound on the immunoactivity and texture changes of shrimp (Penaeus vannamei) Czech J. Food Sci. 2011;29:508–514. [Google Scholar]
- 42.Yang F., Zou L., Wu Y., Wu Z., Yang A., Chen H., Li X. Structure and allergenicity assessments of bovine β-lactoglobulin treated by sonication-assisted irradiation. J. Dairy Sci. 2020 doi: 10.3168/jds.2019-17070. [DOI] [PubMed] [Google Scholar]
- 43.Li H., Zhu K., Zhou H., Peng W., Guo X. Comparative study of four physical approaches about allergenicity of soybean protein isolate for infant formula. Food Agric. Immunol. 2016;27:604–623. [Google Scholar]
- 44.Dong X., Wang J., Raghavan V. Critical reviews and recent advances of novel non-thermal processing techniques on the modification of food allergens. Crit. Rev. Food Sci. Nutr. 2020;1–15 doi: 10.1080/10408398.2020.1722942. [DOI] [PubMed] [Google Scholar]
- 45.Vanga S.K., Singh A., Kalkan F., Gariepy Y., Orsat V., Raghavan V. Effect of thermal and high electric fields on secondary structure of peanut protein. Int. J. Food Prop. 2016;19:1259–1271. [Google Scholar]
- 46.Nazari B., Mohammadifar M.A., Shojaee-Aliabadi S., Feizollahi E., Mirmoghtadaie L. Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrason. Sonochem. 2018;41:382–388. doi: 10.1016/j.ultsonch.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Dou Z., Chen C., Fu X. The effect of ultrasound irradiation on the physicochemical properties and α-glucosidase inhibitory effect of blackberry fruit polysaccharide. Food Hydrocoll. 2019 [Google Scholar]
- 48.Ding X., Zeng N., Zhang G., Pan J., Hu X., Gong D. Influence of transglutaminase-assisted ultrasound treatment on the structure and functional properties of soy protein isolate. J. Food Process. Preserv. 2019 [Google Scholar]
- 49.Resendiz-Vazquez J., Urías-Silvas J., Ulloa J., Bautista-Rosales P., Ramírez-Ramírez J. Effect of ultrasound-assisted enzymolysis on jackfruit (artocarpus heterophyllus) seed proteins: structural characteristics. Technofunctional properties and the correlation to enzymolysis. J. Food Process Technol. 2019;10:2. [Google Scholar]
- 50.Rahaman T., Vasiljevic T., Ramchandran L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016;49:24–34. [Google Scholar]
- 51.Li H., Zhu K., Zhou H., Peng W. Effects of high hydrostatic pressure treatment on allergenicity and structural properties of soybean protein isolate for infant formula. Food Chem. 2012;132:808–814. [Google Scholar]
- 52.Meyer-Pittroff R., Behrendt H., Ring J. Specific immuno-modulation and therapy by means\of high pressure treated allergens. High Pressure Res. 2007;27:63–67. [Google Scholar]
- 53.Wang J., Wang J., Vanga S.K., Raghavan V. High-intensity ultrasound processing of kiwifruit juice: Effects on the microstructure, pectin, carbohydrates and rheological properties. Food Chem. 2020;313 doi: 10.1016/j.foodchem.2019.126121. [DOI] [PubMed] [Google Scholar]
- 54.Vanga S.K., Singh A., Raghavan V. Review of conventional and novel food processing methods on food allergens. Crit. Rev. Food Sci. Nutr. 2017;57:2077–2094. doi: 10.1080/10408398.2015.1045965. [DOI] [PubMed] [Google Scholar]
- 55.Ordóñez-Santos L.E., Pinzón-Zarate L.X., González-Salcedo L.O. Optimization of ultrasonic-assisted extraction of total carotenoids from peach palm fruit (Bactris gasipaes) by-products with sunflower oil using response surface methodology. Ultrason. Sonochem. 2015;27:560–566. doi: 10.1016/j.ultsonch.2015.04.010. [DOI] [PubMed] [Google Scholar]