Abstract
Diabetes increases the prevalence of heart failure by 6–8-fold, independent of other comorbidities such as hypertension and coronary artery disease, a phenomenon termed diabetic cardiomyopathy. Several key signalling pathways have been identified that drive the pathological changes associated with diabetes-induced heart failure. This has led to the development of multiple pharmacological agents that are currently available for clinical use. While fairly effective at delaying disease progression, these treatments do not reverse the cardiac damage associated with diabetes. One potential alternative avenue for targeting diabetes-induced heart failure is the use of adeno-associated viral vector (AAV) gene therapy, which has shown great versatility in a multitude of disease settings. AAV gene therapy has the potential to target specific cells or tissues, has a low host immune response and has the possibility to represent a lifelong cure, not possible with current conventional pharmacotherapies. In this review, we will assess the therapeutic potential of AAV gene therapy as a treatment for diabetic cardiomyopathy.
Keywords: AAV, diabetes, Diabetic Cadiomyopathy, Gene Therapy
Diabetic cardiomyopathy
Diabetes is a major health problem worldwide, responsible for approximately 5 million deaths in 2017, with an increasing incidence expected to reach 693 million by the year 2045 [1]. One of the leading causes of death (approximately 50%) in people with diabetes is cardiovascular disease. In particular, the prevalence of heart failure in diabetic patients is reportedly increased by 6–8-fold in the 45- to 65-year-old age-group compared with non-diabetic individuals [2]. Moreover, diabetes alone can accelerate the development of heart failure in individuals with pre-existing cardiac pathologies (such as myocardial infarction), resulting in poorer prognosis compared with non-diabetic individuals [3]. The accompanying abnormalities in cardiac structure and function are collectively termed ‘diabetic cardiomyopathy’ or diabetes-associated heart failure, originally defined as cardiomyopathy not directly attributable to hypertension or coronary disease. This phenomenon has been known for at least 5-decades [4–7]; however, diabetic cardiomyopathy still remains the subject of intense research to understand the key causal signalling pathways to target for future therapeutic development.
Key signalling pathways implicated in diabetes-induced cardiac dysfunction and remodelling
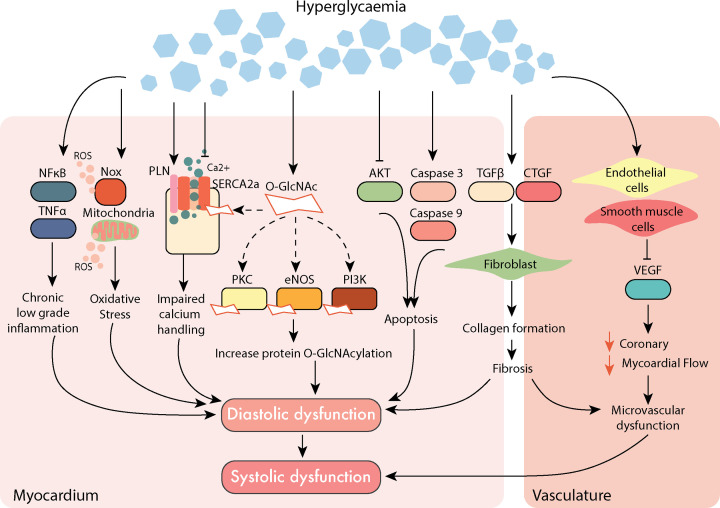
To date, several contributing mechanisms have been implicated in the development of diabetic cardiomyopathy, each of which has an impact on cardiac function and pathological cardiac remodelling (summarised in Figure 1). For instance, long-term hyperglycaemia is associated with chronic systemic low-grade inflammation. Impaired glucose handling leads to the activation of pro-inflammatory factors such as nuclear-factor-κB (NF-κB) and tumour necrosis factor-α (TNF-α) that have been linked to insulin resistance and an array of pathological consequences of diabetes [8,9]. This is paired with excessive reactive oxygen species (ROS) production in the myocardium, via the dedicated ROS-producing enzyme, NADPH oxidase (Nox), and as a result of mitochondrial electron transport chain uncoupling [7]. Excessive ROS, their dysregulation and/or impaired antioxidant defences result in oxidative stress, a key hallmark observed in the diabetic heart [10]. Diabetes-induced impairments in Ca2+ handling can impact cardiomyocyte function, prolonging relaxation and impairing contractile function [11]. In preclinical animal models of both type-1 and type-2 diabetes, a reduction in the expression and activity of the Ca2+ handling protein, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA2a), and/or an increase in its regulator, phospholamban (Pln), impairs cardiac function [12]. Likewise, these Ca2+ handling proteins have been shown to undergo post-translational modification in the setting of hyperglycaemia, altering their cellular function. O-GlcNAcylation is one such example, where a rapid and dynamic attachment of the sugar moiety, O-GlcNAc, to nuclear and cytoplasmic proteins at serine (Ser) or threonine (Thr) residues, results in altered function of a broad range of proteins [13]. In the cardiovascular system, SERCA2a, protein-kinase C, endothelial nitric oxide synthase (eNOS) and phosphoinositide-3 kinase (PI3K) are all O-GlcNAc modified [14]. For example, in mice with diabetic cardiomyopathy, left ventricular (LV) diastolic dysfunction develops in conjunction with elevated levels of global cardiac protein O-GlcNAcylation [15]. This occurs in conjunction with the inhibition of pro-survival kinase Akt, leading to an increase in apoptosis in the diabetic heart [16]. Although programmed cell death rarely occurs in the healthy myocardium (as cardiomyocytes rarely proliferate in adult cardiac muscle), it is a feature of the diabetic heart and end-stage heart failure [17]. This is driven by diabetes-induced activation of cell death factors, including caspase-3 and caspase-9 [18]. Over time, excessive cell death can cause tissue damage and scar formation. The deposition of extracellular matrix consists predominantly of collagen produced by activated fibroblasts cells (known as myofibroblasts) that stimulate transforming growth factor-β (TGF-β) and connective tissue growth factor (CTGF) to activate cell surface receptors and their response to cytokines [19,20]. Maladaptive remodelling eventually leads to a clinically observable phenotype, characterised by cardiac fibrosis and LV diastolic dysfunction that eventually leads to systolic dysfunction [21]. In addition to the direct cardiac effects, diabetes can cause microvascular dysfunction via impairment in endothelial and smooth muscles cells, impacting coronary and myocardial blood flow [7]. This vascular dysfunction may often not be evident until later in the disease progression, with initial inflammatory infiltration of the microvascular that lead to vascular remodelling and perivascular fibrosis [22]. This further contributes to increased thickness of the arterioles with a reduction in the number of capillaries and arterioles [23]. Similarly, pre-clinical models of type-1 diabetes have been demonstrated to have a similar phenotype, in addition to the reduction of important angiogenic growth factors such as the vascular endothelial growth factor (VEGF) [24]. These signalling responses contribute to the overall pathophysiology of diabetic cardiomyopathy and have been comprehensively reviewed recently [7].
Figure 1. Overview of dysregulated major signalling pathways dysregulated in diabetic cardiomyopathy.
Diabetes-induced molecular impairments lead to cardiac remodelling, inflammation, oxidative stress and impaired calcium handling, contributing to cardiac dysfunction that initially develops as diastolic dysfunction. Over time, impairment of endothelial and smooth muscle cells in the vasculature can lead to reduced coronary microvascular blood flow, as a result of this microvascular dysfunction. The dysfunction in the vasculature and the myocardium can eventually lead to LV systolic dysfunction; Akt, protein kinase B; CTGF, connective tissue growth factor; eNOS, endothelial nitric oxide synthase; HBP, hexosamine biosynthesis pathway; LV, left ventricular; NFκB, nuclear factor-κB; Nox, NADPH oxidase; PI3K, phosphoinositide-3 kinase; PKC, protein kinase-C; PLN, phospholamban; ROS, reactive oxygen species; SERCA2a, sarcoplasmic/endoplasmic reticulum ATPase- Ca2+; TGF-β, transforming growth factor-β; TNFα, tumour necrosis factor-α (see text for references).
Current treatments for the diabetic heart
Although the pathophysiology of diabetic cardiomyopathy is well-established, effective treatment options approved for clinical use are limited. Early studies indicated that lower glycated haemoglobin (a surrogate measure of long-term blood glucose) is associated with a lower incidence of cardiovascular disease in diabetic patients [25]. However, when this is applied to high-risk diabetic patients, the opposite occurs; it worsens the outcome of cardiovascular events [26]. Thus, lowering blood glucose alone is often not sufficient to prevent the development of diabetic cardiomyopathy.
Currently in the clinic, several different pharmacological agents are routinely used to manage diabetic cardiomyopathy. First-line treatments include those targeting glucose control as well as those targeting cardiovascular pathology. This includes for example renin–angiotensin system inhibitors (angiotensin converting-enzyme inhibitors, ACEi, and angiotensin receptor blockers) and metformin, which have been shown to be safe and to delay cardiac disease progression in diabetic patients, at least to some extent [27,28]. Thiazolidinediones (TZDs), a class of drug effective in lowering blood glucose levels, have been shown clinically to increase the incidence of hospitalisation due to heart failure, thus rendering them unsuitable as a treatment for diabetic cardiomyopathy [29]. More recently, treatments that modulate blood glucose levels via the incretin system have shown some efficacy in terms of cardiovascular outcomes. Although the incretin system potentiators, dipeptidyl peptidase-4 (DPP4) inhibitors, are associated with a neutral or increased risk of developing heart failure in the clinic, new-generation incretin system mimetics (glucagon-like peptide-1 (GLP-1) receptor agonists) have demonstrated favourable cardiovascular outcomes with a good safety profile [30,31]. Sodium-glucose co-transporter-2 (SGLT-2) inhibitors, initially indicated specifically for the management of type-2 diabetes, have demonstrated a marked beneficial effect in terms of cardiovascular complications in the setting of diabetes [32,33]. The beneficial effects of SGLT2 inhibitors were observed as a result of the now mandated requirement for clinical investigation of cardiovascular outcomes in diabetic drugs [34]. Whilst SGLT2 inhibitors reduce cardiovascular risk, mechanistic insights into how they impact heart failure in T2D is lacking [7,35]. Further, one recent clinical trial on SGLT-2 inhibitors suggests these beneficial effects may extend to patients with heart failure irrespective of diabetes, suggesting a mechanism independent of blood glucose-lowering [36]. For a small number of patients, risk of adverse outcomes (e.g. increased risk of amputation, genital infections or worsening kidney function) may preclude their suitability [37,38]. For the majority of diabetic patients, these newer glucose-lowering agents have proven to be favourable for limiting the risk of heart failure hospitalisation. There remain some patients however who will still require alternative therapeutic options to address the underlying triggers of, or reverse the cardiac damage associated with, diabetes. As the body of evidence continues to grow with respect to the treatment of diabetic cardiomyopathy (whether via SGLT2 inhibition or other approaches), this will also influence the need for more targeted approach for future interventions. One such potential avenue of treatment, which may also provide a longer-term treatment solution, is the use of adeno-associated viral vector-mediated (AAV) gene therapy.
AAV-based therapeutics represent a new frontier in clinical medicine
Treatment options for cardiovascular diseases have advanced significantly in recent years, as a result of improved understanding of the molecular pathways involved in cardiac damage [39,40]. In particular, the use of gene therapy has proven to be one of the most promising avenues to treat various types of diseases, including heart failure [40]. Aimed at the correction of key pathologies, gene therapy involves the delivery of therapeutic genes of interest to a specific tissue target. Successful incorporation of the delivered gene allows the endogenous cell machinery to produce the specific encoded protein [41,42]. The success of gene therapy depends heavily on the efficient transfer of the genetic material to the tissue of interest, which is facilitated by different delivery strategies. Early delivery methods included the use of naked plasmid DNA, liposomal DNA complexes, polymer-carrying DNA and oligonucleotides [43]. Delivery of naked plasmids to tissues, however, does not provide sufficient transfection into the tissue of interest [44,45]. Furthermore, rapid systemic degradation of plasmids and poor cellular entry are other limitations [40,46,47]. In contrast, non-pathogenic viral approaches have been shown to be a more superior method as the vector for gene delivery [45,46]. Notably, AAVs have been demonstrated to exhibit the best risk-benefit profile and will be the focus for the remainder of this review.
Brief introduction to AAV principles
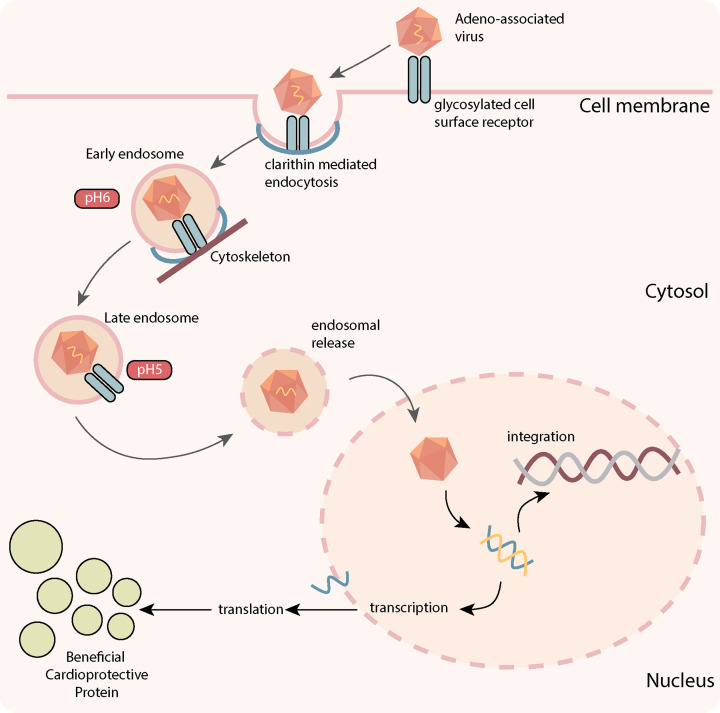
Derived from the Parvoviridae family, AAVs are non-enveloped single-stranded DNA vectors, with a favourable safety profile and the capability of achieving persistent transgene expression in a wide range of target tissues, including cardiac tissue [48]. AAVs are relatively small (20 nm) and therefore limited in their packaging capacity of only around 4.7 kb [47]. Yet one of the most attractive features of AAV vectors is the continued expression of the transgene for a prolonged period of time [40,49], despite the extrachromosomal location of the vector [47]. However, the infrequent integration of the vector means that transduction must occur in cells that either do not replicate or do so very slowly [47]. Cardiomyocytes are an excellent example of cells that are considered the most compatible for use of AAV gene therapy, as cardiomyocyte turnover is negligible in adults [50]. For in vivo gene delivery, recombinant AAVs (rAAV) are commonly used, which have the same sequence and structure as a wild-type AAV, but are devoid of all AAV-protein coding sequences [51]. AAVs enter the cell via glycosylated cell surface receptors, triggering clathrin-mediated endocytosis (Figure 2) [51]. Utilising the cytoskeletal network AAVs advance through the cytosol, undergoing conformational modification in response to a change in pH [51]. AAVs are then released by the endosome, where they enter the nucleus and release their content. The viral inverted terminal repeats (ITRs) present in the rAAV genome can drive inter- or intra-molecular recombination to form circularised episomal genomes that can persist in the nucleus. Vector genomes can also undergo integration into the host genome at very low frequencies; however, this is a very rare occurrence unlike both lentiviral and retroviral that can randomly integrate into the host genome to disrupt normal gene function [52]. Moreover, compared with adenoviruses which were a popular vector choice in the early 2000s, AAVs has been shown to be excellent to evade the innate immune system [53] and considered highly safe and potent compared with other vectors.
Figure 2. Principles of AAV-mediated gene therapy.
Adeno-associated virus (AAV) binds to the host glycosylated cell surface receptor to trigger clathrin-mediated endocytosis internalization. The AAV then moves through the cytosol via the cytoskeletal network. Conformational changes are then triggered by pH changes in the cellular environment, leading to endosomal release. The AAV undergoes transport to the nucleus, releasing its cargo, where it is then transcribed into double-stranded DNA for transcription, or undergoes integration to the host genome (which rarely occurs). Messenger RNA produced from transcription of the cargo leads to its translation to the protein-of-interest outside of the nucleus. Production of this protein-of-interest then enables the cardioprotective effects that are observed in response to AAV-mediated gene therapy.
AAV design for cardiac-targeted transduction
To achieve efficient cardiac transduction, multiple factors must be considered, including the identification of naturally cardiotropic AAVs. Currently, more than 100 serotypes of wild-type AAVs have been reported, each with distinct tissue tropism, as determined by their capsid protein structures [41,54]. Among these serotypes, AAV1, AAV6, AAV8 and AAV9 have been identified as the most cardiotropic serotypes for systemic delivery [55]. It is also important to note that these serotypes target other organs, including the liver for AAV9 and both the lung and skeletal muscle for AAV6 [55]. These have led to various efforts that include the generation of chimeric AAVs by shuffling from various subtypes to improve transduction efficacy and transgene expression [56].
In addition to identifying the most effective cardiotropic/organ-specific serotype, promoter selection is another major element to consider. The combination of both allow the mediation of AAV expression and enable the control of gene transcription that is delivered [57]. Promoters are usually located upstream of the gene of interest and are 100–1000 base pairs in length. This can either stimulate or repress transcription initiation at the transcription start site, mediated by the RNA polymerase II in the core promoter. One of the most commonly used promoters, cytomegalovirus (CMV), can achieve both strong and robust transgene expression [58]. Inducible promoters such as the tetracycline and doxycycline systems have also been used to manipulate transgene expression in preclinical studies [59,60]. For cardiac transduction, cardiac-specific promoters such as α-myosin heavy chain (αMHC), troponin and myosin light chain (MLC) have been commonly used to drive expression throughout the heart, including in the ventricles and atria [61–64]. Interestingly, the combination of AAV6 (which has strong skeletal muscle tropism) and a CMV promoter favours cardiac muscle transduction, through a mechanism that is not well understood [65]. More recently, atrial natriuretic factor (ANF) has been suggested to confer atrial-specific transduction, without observable transduction in the ventricles [66]. Therefore, promotor selection is an important consideration in AAV design, where further research will likely improve our ability to limit expression to individual organs, compartments or even cell types.
AAV-based therapies for diabetic cardiomyopathy
The increased use of AAV gene therapy in the biomedical field has led to an intensified effort to identify novel potential treatments for diabetic cardiomyopathy. This can be done by targeting the underlying mechanism of the cardiac pathology, not possible using conventional pharmacotherapies. Below we discuss recent findings that aim to treat diabetic cardiomyopathy and other cardiovascular diseases (Table 1).
Table 1. List of gene therapies investigated in preclinical and clinical studies.
| Target | Mechanism of action | Vector serotype | Dose (vector genomes) | Study phase | Model | Indication | Delivery method | References |
|---|---|---|---|---|---|---|---|---|
| Preclinical studies | ||||||||
| S100A1 | Increased calcium handling protein | rAAV6 | 2.5 × 1011 vg | Small animal | Rodents | Ischaemic heart failure (myocardial infarction) | Coronary perfusion | [69] |
| AAV9 | 1.5 × 1013 vg | Large animal | Porcine | Ischaemic heart failure (myocardial infarction) | Retrograde coronary injection | [70] | ||
| AAV6 | 1.5 × 1013 vg | Large animal | Porcine | Ischaemic heart failure (myocardial infarction | Retrograde coronary injection | [71] | ||
| SUMO-1 | Increased calcium handling protein | rAAV9 | 5 × 1010 vg | Small animal | Rodents | Heart failure transverse aortic constriction | Tail vein injection | [72] |
| AAV1 | 5 × 1012 vg 1 × 1013 vg |
Large animal | Porcine | Ischaemic heart failure (myocardial infarction) | Antegrade intracoronary infusion | [73] | ||
| I-1 | Increased calcium handling protein | AAV9 | 2.8×1012 vg | Small animal | Rodents | Heart failure transverse aortic constriction | Tail vein injection | [75] |
| BNP1161 | 3 × 1012 vg 1 × 1013 vg |
Large animal | Porcine | Ischaemic heart failure (myocardial infarction) | Intracoronary infusion | [74] | ||
| BNP1161 | 1 × 1013 vg 1 × 1014 vg |
Large animal | Porcine | Non-ischaemic HF (volume overload HF) | Intracoronary injection | [76] | ||
| Urocortin | Increase calcium protein handling | AAV8 | 5 × 1011 vg | Small animal | Rodents | Ischaemic heart failure (myocardial infarction) | Jugular vein injection | [78] |
| AAV8 | 5×1011 vg | Small animal | Rodents | Cryoinjury myocardial infarction model | Jugular vein injection | [79] | ||
| VEGF-B | Increased angiogenesis | AAV9 | 1 × 1010 vg | Small animal | Rodent | Heart failure transverse aortic constriction | Direct myocardial injection | [81] |
| AAV9 | 1 × 1013 vg 2 × 1013 vg 5 × 1013 vg |
Large animal | Canine | Non-ischaemic dilated cardiomyopathy | Intracoronary infusion | [82] | ||
| NGF | Increased angiogenesis | AAV22 AAV93 |
1 × 1011 vg 1.5 × 1012 vg |
Small animal | Rodents | Diabetic cardiomyopathy | Direct myocardial injection2 and tail vein injection3 | [86] |
| YAP | Cardiac regeneration | AAV9 | N/A | Small animal | Rodents | Myocardial infarction | Direct myocardial injection | [87] |
| FGF-2 sTGFβ2 |
Growth modulators | AAV8 | 1 × 1010 vg 1 × 1011 vg |
Small animal | Rodents | Heart failure ascending aortic constriction | Retro orbital injection | [88] |
| SOD | Increase antioxidant defence | AAV | 2.5 × 1010 vg 5 × 1010 vg 2.5 × 1012 vg |
Small animal | Rodents | Ischaemia reperfusion injury | Direct myocardial injection | [90] |
| CTRP3 | Limit ROS and inflammation | AAV | 5×1011 vg | Small animal | Rodents | Diabetic Cardiomyopathy | Tail vein injection | [91] |
| HO-1 | ROS scavenger and anti-inflammatory | AAV2 | 2×1011 vg | Small animal | Rodents | Ischaemic heart failure (myocardial infarction) | Direct myocardial injection | [92] |
| AAV6 | 1 × 1013 vg | Large animal | Porcine | Ischaemic heart failure (myocardial infarction) | Retro infusion into the ventricular vein | [93] | ||
| BFIB4 | Increased longevity factors and anti-inflammatory | AAV9 | 1 × 1012 vg | Small animal | Rodents | Diabetic cardiomyopathy | Tail vein injection | [94] |
| RNR | Increase pro-survival protein (via increased energy synthesis) | rAAV6 | 2.5 × 1013 vrg | Small animal | Rodents | Ischaemic heart failure (myocardial infarction) | i.v. injection via retro-orbital sinus route | [96] |
| AAV6 | 1 × 1012 vrg 5 × 1012 vrg 1 × 1013 vrg |
Large animal | Porcine | Ischaemic heart failure (myocardial infarction) | Antegrade Intracoronary infusion | [97] | ||
| βARKct | Inhibition of β-adrenergic | AAV6 | 1 × 1013 vg | Large animal | Porcine | Ischaemic heart failure (myocardial infarction) | Retrograde injection into coronary veins | [99] |
| O-GlcNAcylation | Alteration of cardiac O-GlcNAc balance | AAV6 | 2 × 1011 vg 1 × 1012 vg |
Small animal | Rodents | Diabetic cardiomyopathy | Tail vein injection | [103,104] |
| PIM-1 | Increased pro-survival kinase | AAV9 | 1 × 1010 vg 5 × 1010 vg |
Small animal | Rodents | Diabetic cardiomyopathy | Tail vein injection | [106] |
| PI3K(p110α) | Increase pro-survival kinase and reduce ROS | AAV6 | 2 × 1011 vg | Small animal | Rodents | Diabetic cardiomyopathy | Tail vein injection | [108,109] |
| miRNA-1 | mRNA regulator | AAV9 | 5 × 1011 vg | Small animal | Rodents | Heart failure ascending aortic constriction | Tail vein injection | [111] |
| miRNA-21 | mRNA regulator through gelsolin inhibition | AAV9 | N/A | Small animal | Rodents | Diabetic cardiomyopathy | Tail vein injection | [112] |
| miRNA-30c | mRNA regulator to increase PPARα | AAV9 | 1 × 1011 vg | Small animal | Rodents | Diabetic cardiomyopathy | Tail vein injection | [113] |
| miRNA-320 | mRNA regulator to increase CD36 expression | AAV9 | 1 × 1011 vg | Small animal | Rodents | Diabetic cardiomyopathy | N/A | [114] |
| Clinical Studies | ||||||||
| SERCA2a | Increased calcium handling protein | AAV1 | 1 × 1013 vg | Phase 2b | Human | Heart failure | Intracoronary infusion | [117] |
| Adenylyl Cyclase 6 | Increased calcium handling and pro-survival kinase | Adv | 3.2 × 109 vg 3.2 × 1010 vg 1 × 1011 vg 3.2 × 1011 vg 1 × 1012 vg |
Phase 2b | Human | Heart failure | Intracoronary injection | [118] |
Summary of studies that have used AAV gene therapy to target different types of heart failure in preclinical and clinical studies. Vectors: 1Chimeric AAV between AAV2 and AAV8 2First intervention 3Second intervention. AAV, adeno-associated viral; Adv, adenoviral; βARKct; β-adrenergic receptor kinase 1 (carboxy terminus); BFIB, bactericidal/permeability-increasing fold-containing family B member 4; CTRP3, C1q/tumour necrosis factor-related protein; FGF, fibroblast growth factor-2; HO-1, heme oxygenase-1; miRNA, micro RNA; NGF, nerve growth factor; PI3K(p110α), phosphoinositide 3- kinase (p110α); PIM-1, pro-viral integration site for Moloney murine leukaemia virus; RNR, ribonucleotide reductase;S100A1, S100 calcium-binding protein A1; SERCA2a; sarcoplasmic/endoplasmic reticulum ATPase-2SOD, superoxide dismutase; sTGFβ2, soluble transforming growth factor- β2; SUMO-1, small ubiquitin-related modifier-1; I-1, constitutively active inhibitor-1; VEGF-B, vascular endothelial growth factor B; YAP, yes-associated protein 1.
Calcium cycling/handling-targeted AAVs
The S100 calcium-binding protein A1 (S100A1) is involved in all Ca2+-dependent target protein interactions and has recently been discovered to play a critical role in heart failure [67]. S100A1 acts upstream of SERCA2a to modulate a wide range of cellular effects. The protein interacts with Pln to increase the activity of SERCA2a and regulates ryanodine receptor-2 (Ryr2) function, during both systole and diastole [68]. In the setting of heart failure (where S100A1 is down-regulated), its restoration via rAAV6-S100A1 in rats restored cardiac function and improve Ca2+ handling via a SERCA2a-dependent mechanism [69]. Similar improvements were also observed in large animal models of myocardial infarction that was administered with rAAV9-S100A1 [60]. Moreover, administration of S100A1 AAV has also been proven to be safe as demonstrated in a study conducted in pigs [71]. Equally, another SERCA2a modulator, small ubiquitin-related modifier-1 (SUMO-1) stabilised cellular activity through post-translational modification of both mouse and human proteins [72]. SUMO-1 binds to a broad range of proteins at their lysine residues, resulting in SUMOylation of the protein. In the setting of heart failure (where levels of both SUMO-1 and SUMOylation are diminished), administration of AAV9-SUMO1 in mice increases survival rate and improved cardiac function [72]. This has been confirmed in a porcine model of heart failure, where administration of AAV1-SUMO1 increased specificity protein-1 (Sp-1) SUMOylation, improving cardiac function and stabilisation of LV volumes [73]. In a separate study, another upstream regulator of SERCA2a, inhibitor-1 (I-1), was demonstrated to be cardioprotective in a porcine model of heart failure [64]. I-1 reduced the activity of protein phosphatase-1, an upstream regulator of the SERCA2a-Pln complex and has been shown to decline with heart failure [74,75]. Administration of constitutively active (I-1c)-AAV to both ischaemic and non-ischaemic heart failure in pigs improved cardiac function (systolic and diastolic), in conjunction with increased Pln phosphorylation, leading to enhanced SERCA2a activity [64,65]. In addition to cardiac targeted gene therapy, administration of liver targeted AAV8-Urocortin2 or Urocortin-3 improved cardiac function in the failing murine heart [77]. The approach is unique, as the product of the liver targeted gene therapy does not directly produce the beneficial effects, but rather through the activation of corticotropin-releasing hormone receptor 2 (CRH2) by Urocortin. The activation of CRH2 exerts extensive cardioprotective activity, including increasing calcium handling proteins such as SERCA2a and troponin in the heart [78,79]. In addition to its cardioprotective effects, Urocortin-2 (but not Urocortin-3) gene transfer reduces blood glucose levels and increases glucose clearance, indicating its potential as an ideal candidate for the treatment of diabetic cardiomyopathy [77]. Thus, these studies demonstrate the central role of SERCA2a in maintaining cardiac contractility suggesting its suitability as a therapeutic target for heart failure or diabetic cardiomyopathy. However, direct targeting of other calcium handling approaches using AAV (such as Ryr2 and Pln) should also be considered as a potential therapeutic target to improve cardiac function.
Growth factor-targeted AAVs
Gene therapeutic approaches targeting growth factors, particularly to induce new blood vessel formation, has been previously explored to treat several cardiovascular diseases. The VEGF family, consisting of VEGF-A, -B, -C, -D and -E, are among the most powerful regulators of blood vessel growth [80]. In particular, VEGF-B has been associated with enhancing cardiac angiogenesis with specific effects on metabolism, cell survival and apoptosis [80]. Previous reports demonstrated that administration of cardiac-specific AAV9-VEGF-B in an aortic constriction mouse model modulated the angiogenic response, increasing proliferation and thereby attenuating systolic function [81]. These findings were corroborated in a canine model of dilated cardiomyopathy, where both LV diastolic and systolic function were preserved, in addition to increases in cardiomyocyte antioxidant defence [82]. Nerve growth factor (NGF) is another growth modulator which is implicated in the promotion of angiogenesis, similar to VEGF-B [83]. Secreted by glycoproteins, NGF elicits its biological effects mainly by binding to high-affinity tropomyosin-related receptor A, leading to inactivation of the forkhead box-O-transcription factor (Foxo) pathway [84]. Overexpression of NGF using gene therapy in mice after myocardial infarction increased both cell survival and cardiac perfusion [85]. Likewise, systemic delivery of human AAV9-NGF prevented cardiomyopathy in diabetic mice, while limiting LV diastolic dysfunction [86]. Similarly, Yes-associated protein (YAP), which has a vital role in regulating embryonic cardiomyocyte proliferation, has attracted interest as a potential mechanism to enhance heart regeneration. By introducing AAV9 carrying a human YAP sequence, contractile function and cell survival were enhanced in mice following myocardial infarction [87]. More recently, a therapeutic gene approach combining two genes (fibroblast growth factor-2 (FGF-2) and soluble transforming growth factor-β-2 (sTGFβ2)) simultaneously in an AAV8 vector limits both obesity and type-2 diabetes phenotypes, and has separately been shown to improve cardiac contraction induced by aortic constriction [88]. These reports highlight the major advantage of utilising AAV to deliver growth factors and modulators to the heart where there is a lack of cell regeneration and replication. This is especially important as off-target or systemic delivery can lead to uncontrollable cancerous growth in other unwanted tissues. Thus, the application of pin-point growth factor delivery using AAV may also favour other organ diseases that require enhance angiogenesis.
AAVs targeting oxidative stress and inflammation pathways
Increased production of ROS has been shown to cause cellular damage and contribute to the pathology of diabetic cardiomyopathy. One way to limit this is to target superoxide dismutase (SOD), a family of metalloenzymes that scavenge superoxide radicals and convert them to oxygen and hydrogen peroxide. Early studies indicate that the administration of the SOD gene via adenovirus protects from myocardial infarction [89]. A subsequent study revealed a similar outcome with the administration of rAAV-Ec-SOD, where it protected against damage associated with ischaemic–reperfusion injury such as cardiac infarction and dysfunction [90]. However, more recent efforts seem to target the increase in ROS further upstream, leading to the modulation of inflammatory pathways. For instance, administration of rAAV-C1q/tumour necrosis factor-related proteins (CTRPs), a key metabolic regulator of the diabetic heart, demonstrated reduced ROS-producing enzyme activity (Nox and p67 phox) and increase SOD, while also attenuating cardiac inflammation (via reduced TNF-α) [91]. Similarly, targeting the enzyme, heme oxygenase-1 (HO-1), which catalyses the degradation of heme-producing biliverdin (ROS scavenger) and carbon monoxide, has been demonstrated to limit inflammation [92]. Indeed, the administration of rAAV6-human-HO-1 to pigs prior to myocardial ischaemia reperfusion demonstrates reductions in both infarct size and extent of LV systolic dysfunction compared to the control group [93]. This improvement was attributed to reduced inflammatory activation of endothelial cells and the recruitment of leukocytes, exacerbating cardiac damage [93]. Likewise, the delivery of longevity-associated variant (LAV) of bactericidal/permeability-increasing fold-containing family B member 4 (BPIFB4) attenuates diabetes-induced cardiac dysfunction through anti-inflammatory action [94]. LAV-BFIB4 allele was demonstrated to be highly prevalent in long-living individuals with higher circulating BPIB4 levels with increased eNOS and mononuclear cells [95]. A recent study looking at liver targeted (thyroxine-binding globulin) administration AAV9-LAV-BFIB4 demonstrated improvement in cardiac function and remodelling in diabetic mice [94]. These beneficial effects have been attributed to increasing circulating BFIB4 that increases stromal-derived factor-1 (SDF)-1 release, that in turn, activate CXC chemokine receptor type 4 (CXCR-4) [94]. Despite the positive outcomes demonstrated by these studies, it is important to note that some of these studies are still in the early stages and future work looking into the use of AAV gene therapies to limit ROS production (e.g., knockdown of Nox subunits) or increase antioxidant enzymes (e.g., increase in SOD) in the diabetic heart are still required.
Myofilament/contractile function targeted AAVs
Cardiac manipulation to increase myofilament cross-bridge binding and cycling was investigated to improve cardiac function by increasing naturally occurring nucleotide, 2-deoxyadenosine triphosphate (dATP) [96]. Production of dATP is mainly maintained by ribonucleotide reductase (RNR), by converting adenosine diphosphate (ADP) to deoxy-adenosine diphosphate (dADP), leading to the final production of dATP. Targeting RNR with the administration of Rrm-1 and Rrm-2 genes (which encodes RNR) using an AAV approach improved LV systolic and diastolic function in rodent and porcine models of myocardial infarction [96,97]. This cardioprotective effect was further confirmed in Duchenne Muscular Dystrophy mice, where similar cardiac improvements were observed [98]. Similarly, inhibition of β-adrenergic receptor kinase (βARK), which is increased in heart failure, displays beneficial effects in the heart. Indeed, administration of AAV6-βARKct (the peptide responsible for βARK inhibition) showed improvements in LV haemodynamic and contractile function were evident in a pig model of myocardial infarction [99]. This was also observed in cardiomyocytes obtained from failing human hearts, where there is improved contractile function and increased adenylyl cyclase activity in response to AAV6-βARKct, indicating improved β-adrenergic receptor activity, a major regulator of cardiac contractility [100]. Further, studies investigating the toxicity and safety of AAV6-βARKct in sheep reported no toxic effects on major organ function, with robust cardiac gene expression [101], suggesting that this treatment may be close to clinical translation.
AAVs targeting post-translational modification mechanisms
Post-translational modification, a chemical process that occurs following protein biosynthesis, is an important component to cell signalling and function including the heart. Some of these modifications include phosphorylation, acetylation, nitrosylation, alkylation and glycosylation. One that is particularly attractive to target in the context of diabetes and its complications is O-GlcNAcylation, a modification that is glucose-driven and has been implicated in the development of diabetic cardiomyopathy [13]. Two enzymes regulate this post-translational modification: O-GlcNAc transferase (OGT), which facilitates the addition of the O-GlcNAc sugar moiety to Ser and Thr residues, and O-GlcNAc-ase (OGA), which facilitates its removal [102]. Our laboratory has shown that administration of rAAV6 carrying a human isoform of OGA attenuated cardiac dysfunction and remodelling in mice with established diabetic cardiomyopathy [103,104]. In contrast, administration of rAAV6 carrying a human isoform of OGT induces cardiac dysfunction and remodelling in wild-type non-diabetic mice [103,104] This suggests that regulation of this particular post-translational modification is a critical regulator of the structural and functional phenotype of the heart, and might be key in the setting of cardiomyopathy, both with and without diabetes.
AAVs targeting cardiac apoptosis
Cardiac apoptosis is a common feature of the diabetic heart and end-stage heart failure in both clinical and preclinical contexts [7]. The pro-viral integration site for Moloney murine leukaemia virus (PIM-1) has been identified as a promotor of cardiomyocyte survival in response to cell stress [105]. Increased cardiac expression of PIM-1 via AAV9 delivery increased proliferation of cardiac progenitor cells and improved cardiac contractility, with evidence of increased SERCA2a activity [106]. Likewise, the lipid kinase PI3K(p110α) responsible for membrane trafficking, cell growth and cell survival, has also been shown to be cardioprotective in multiple cardiac disorders. Administration of rAAV6-caPI3K gene therapy was demonstrated to diminish cardiac dysfunction following acute pressure-overload hypertrophy [107]. In a chronic disease such as diabetes, we have shown that administration of rAAV6-caPI3K gene therapy to mice with type-1 diabetes-induced diastolic dysfunction, attenuated cardiac dysfunction and oxidative stress [108]. Equally, in a preclinical model of type-2 diabetes we have also demonstrated that rAAV6-caPI3K administration delivers similar cardiac improvements [109].
AAVs targeting micro RNAs (miRNA)
Micro RNAs (miRNA) are small, non-coding RNAs that function via base-pairing with complementary sequences and messenger RNA molecules. These can regulate multiple different genes at the post-transcriptional level, in both healthy and disease settings [110]. Indeed, diabetes-induced oxidative stress, hypertrophy and cardiac fibrosis have been shown to be associated with changes in several miRNAs, including miRNA-1, miRNA-133a, miRNA-373, miRNA-378, miRNA-23b, miRNA-181 and miRNA-195 [110]. Thus, there is an increased interest in targeting miRNA for the treatment of diabetic cardiomyopathy. One mode of delivery that has been widely used for cardiac miRNA studies is the use of AAV as a vehicle, due to its versatility and capability to transduce cardiac tissue. For instance restoration of miR-1 using AAV9 improved fractional shortening, attenuated cardiac remodelling and improved Ca2+ cycling in rats with aortic banding [111]. In the setting of diabetic cardiomyopathy, delivery of cardiac-targeted AAV9 carrying miRNA-21 has been shown to attenuate diabetes-induced cardiomyocyte hypertrophy and diastolic dysfunction, via gelsolin inhibition, an important cardiac transcription cofactor [112]. Similarly, administration of rAAV9-miRNA-30c has also been demonstrated to limit diabetes-induced cardiac dysfunction and remodelling in T2D db/db mice [113]. This has been attributed to an increase in peroxisome proliferator-activated receptor (PPAR)-α transcriptional activity that modulates oxidative stress, lipid accumulation, ATP production and apoptosis in the diabetic heart [113]. In contrast with the cardioprotective effects demonstrated for the above miRNAs, an increase in miRNA-320 has been shown to be detrimental in the setting of diabetic cardiomyopathy [114]. Delivery of rAAV-miRNA-320 exacerbates cardiac remodelling and dysfunction in T2D mice, whereas inhibition of miRNA-320 attenuated these cardiac parameters [114]. These studies indicate that regulation of miRNA in the diabetic heart is complex and will benefit from further investigation.
Clinical translation of AAV-based therapeutics
The Calcium Upregulation by Percutaneous Administration of Gene Therapy in Patients with Cardiac Disease (CUPID) trial was the first human study that investigated the use of AAV for heart failure by targeting impaired Ca2+ handling utilising SERCA2a-AAV gene therapy [115]. This treatment aimed to treat heart failure through an increase in SERCA2a protein (responsible for regulating calcium reuptake following contraction) [40]. Results from both Phase 1 and Phase 2 clinical trials were promising, demonstrating no or few side effects in the small patient cohort [50,116]. However, the results of the Phase 2b clinical trial were neutral, as the administration of SERCA2a-AAV did not achieve improvement in primary end point in both recurring (i.e. admission to hospital due to heart failure) and terminal events (i.e. death) [117]. However, a clinical trial using adenylyl cyclase-6 gene delivery in heart failure patients demonstrated favourable outcomes in terms of improving LV ejection fraction and LV-dP/dt [118]. The improvement has been attributed to an increase in SERCA2a, Akt and phospholamban following treatment [119,120]. Currently, a Phase 3 clinical trial is being planned and reassessed for commencement to investigate the effects of intracoronary administration of Ad5 CMV hAC6 (RT-100) or placebo in 536 HFrEF (Heart Failure with reduced Ejection Fraction) patients (FLOURISH Trial) ([121]- Clinical Trials.gov NCT03360448). More recently I-1c-AAV gene therapy (a protein phosphatase-1 inhibitor), which demonstrated success in preclinical studies, has progressed to a Phase 1 clinical trial, to investigate the safety profile of NAN-101 (BNP116.sc-CMVi1c) in patients with heart failure (Clinical Trials.gov NCT04179643, start date 20 November 2019). Thus, these studies highlight that therapeutic gene approaches are in the pipeline for the treatment of heart-related diseases (Figure 3), which have proven to offer clear promise at least in the preclinical stage.
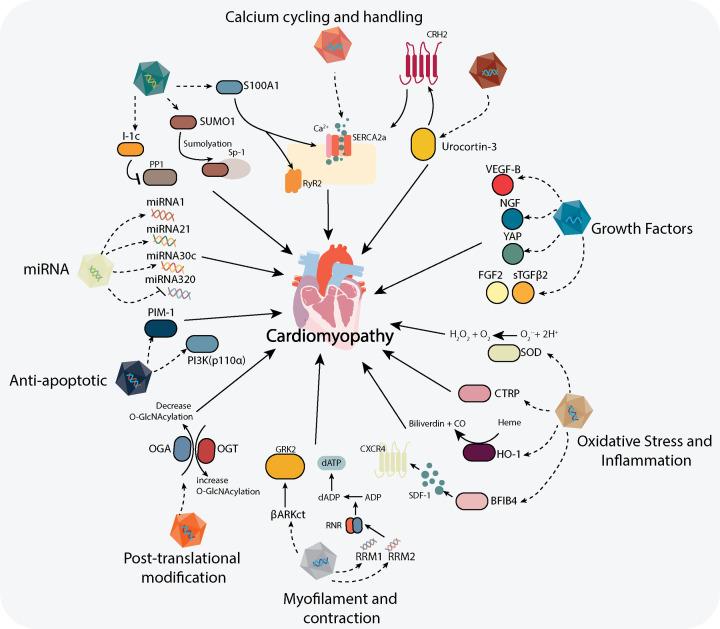
Figure 3. Outline of reported AAV-based potential therapeutics for diabetic cardiomyopathy.
Current AAV gene therapies reported to date to target multiple signalling pathways and molecules in cardiac tissue proposed for the treatment of diabetic cardiomyopathy. ADP, adenosine diphosphate; βARKct; β-adrenergic receptor kinase 1 (carboxy terminus); BPIFB4, bactericidal/permeability-increasing fold-containing family B member 4; CRH2, corticotrophin-releasing hormone receptor-2; CTRP, C1q/tumour necrosis factor-related protein; dADP, deoxyadenosine diphosphate; dATP, 2-deoxyadenosine triphosphate; FGF, fibroblast growth factor-2; GRK2; G protein-coupled receptor kinase-2; HO-1, heme oxygenase-1; I-1c, constitutively active inhibitor-1; miRNA, micro RNA; NGF, nerve growth factor; OGA, O-GlcNAc-ase; OGT, O-GlcNAc-transferase; PI3K(p110α), phosphoinositide 3- kinase (p110α); PIM-1, pro-viral integration site for Moloney murine leukaemia virus; PP1, protein phosphatase-1; RNR, ribonucleotide reductase; Ryr2, ryanodine receptor-2; S100A1, S100 calcium-binding protein A1; SDF-1, stromal-derived factor-1; SOD, superoxide dismutase; Sp-1; specificity protein-1; sTGFβ2, soluble transforming growth factor-β2; SUMO-1, small ubiquitin-related modifier-1; SERCA2a, sarcoplasmic/endoplasmic reticulum ATPase-2; VEGF-B, vascular endothelial growth factor-B; YAP, yes-associated protein-1 (see text for references).
Challenges and hurdles posed by AAV-mediated gene delivery approaches
Over the last few decades, the field of gene therapy has advanced significantly in terms of vector design and biology. Although the findings of the first gene therapy for heart failure was negative [117], leading gene technology experts have pointed out that further optimisation is still required to translate AAV use from bench to bedside [122]. Findings from the CUPID trial highlighted poor uptake of the AAV vector into the hearts of patients [117]. Other factors, including the presence of neutralising antibodies, have been considered a hurdle, where even at low concentration can inactivate AAV activity and block transduction to target tissue [123]. However, new strategies have been developed to tackle this limitation. Formation of synthetic AAVs through structural guided evolution and generation of chimeric AAV via fusion of wild-type AAV have been some of the few technologies that have in the developmental pipeline [124,125]. Recent findings have also highlighted the potential for toxic effects of AAVs in non-human primates [126]. Administration of a high dose AAV (2 × 1014 vg/kg) demonstrated severe hepatotoxicity, with side effects evident in the dorsal root ganglia [126]. These findings have been debated in the field of gene therapy and argued to be a variant-specific effect; in this case AAVhu68 (an AAV9 variant) was used, a variant that has not been used in the clinic [127]. Moreover, the dose that was administered was very high compared with that which is currently being used in the clinic (e.g. 1.5 × 1011 vg based on the current, clinically approved therapy for retinal dystrophy, voretigene neparvovec-rzyl). Despite these findings, a gene therapy product, Gendicine (recombinant human p53 adenoviral), to treat squamous cell carcinoma, was approved for clinical use in China over a decade ago [128]. There have been no adverse events reported and this therapy has continued to demonstrate an exemplary safety profile in the clinic [128].
The advantage of gene therapy over conventional pharmacological therapies is their potential to last for a prolonged period, if not a lifetime [51]. As these therapies only need to be administered once, or up to a handful of times, the cost per administration is likely to be eye-watering, as pharmaceutical companies look to recoup the funds invested in research and development (ranging from US$500,000 to $2 million per course of treatment) [129]. The high price-tag has been associated with low commercialisation of this therapy in the marketplace, as most of the currently approved therapies target rare monogenic genetic defects that only occur in a small number of people. For example, Zolgensma, a recently approved gene therapy treatment for spinal muscular atrophy (a disorder that affects 1 in 10,000 live births www.rarediseases.org) costs US$2 million per treatment course [130]. Although not unique to AAV gene therapy, new therapies had led pharmaceutical companies to devise innovative schemes to cover the costs of gene therapy, including pay for performance; where payment is only made when maintenance or improvement of health is maintained over a period of time. Additionally, subscription-based or money-back guarantees/rebate model is also another option that had been recently adopted by health care providers throughout the world [129]. Nonetheless, like all technologies, the cost of gene therapy will eventually reduce as research and development costs decrease over time, increasing the feasibility of AAV gene delivery as a therapy from an economic perspective. Finally, the increasing evidence and research in the treatment of diabetic cardiomyopathy may also influence the need for such targeted interventions.
AAV-mediated gene delivery therapeutic approaches – are we there yet?
The use of gene therapy has emerged to be one of the most versatile approaches in the biomedical field. It can be used to manipulate targets of interest by either up-regulating or down-regulating the activity of the target gene or protein in a tissue-specific manner, which is desirable to dissect specific pathways in different disease pathologies. Likewise, the use of AAVs in the clinic is a viable therapeutic strategy to deliver beneficial genetic materials, in place or in conjunction with pharmacological interventions. In addition, the advancement of gene therapeutics is highlighted by the increase in clinical trials (364 clinical trials based on literature search for ‘cardiovascular diseases’ and ‘gene therapy’ in www.clinicaltrials.gov as of June 2021). At time of writing, there were 3 AAV gene therapies (2 available in the clinic and 1 (Glybera) withdrawn due to expensive regulatory costs and low revenues) that have been approved for clinical use (Table 2). Indeed AAV gene technology has also been investigated as a COVID-19 vaccine and has been shown to be stable at room temperature with a favourable safety profile compared to first-generation vaccine [131]. With this resurgence and our ever-increasing knowledge in the field, it will lead to reduced research and development costs, coupled with innovative ways to recoup costs and increased level of industry-sponsored funding in the academic sector can further expand the gene therapy developmental pipeline. Moreover, significant investment is being made to optimise the protocols required for manufacturing vector stocks for clinical use, such that production can be scalable and yield a vector at the highest possible titre [132].
Table 2. Current list of approved AAV gene therapy products for clinical use.
| Therapeutic name | Year of approval | Approving agency | Indication | Type of therapy | Vector | Dose | Route of administration | Manufacturer |
|---|---|---|---|---|---|---|---|---|
| Glybera* (alipogene tiparvovec) | 2012 | EMA | Lipoprotein lipase deficiency | AAV gene therapy | AAV1-LPL | 1 × 1012 vg/kg body weight | Intramuscular injection | UniQure (Amsterdam, Netherlands) |
| Luxturna (voretigene neparvovec-rzyl) | 2017/2018 | FDA/EMA | Retinal dystrophy (biallelic RPE65 mutation) | AAV gene therapy | AAV2-RPE65 | 1.5 × 1011 vg/eye | Subretinal injection | Spark Therapeutics, Inc (Philadelphia, Pennsylvania, U.S.A.) |
| Zolgensma (onasemnogene aberparvovec xioi) | 2019 | FDA | Spinal muscular atrophy | AAV gene therapy | AAV9- SMN1 | 1.1 × 1014 vg/kg body weight | Intravenous infusion | AveXis Inc (Chicago, Illinois, U.S.A.) |
EMA, European Marketing Authorization; FDA, Food and Drug Administration.
prohibitive cost of regulatory body precluding commercial viability.
As discussed here, AAV gene therapy for diabetic cardiomyopathy in the clinic is now getting closer to becoming a reality. This can be attributed to a better understanding of the delivery vectors and promoters, their modes of delivery and improved knowledge of the molecular targets. However, challenges to achieve efficient gene transduction remain as the limiting factor for the translation of preclinical models to the clinic. In addition, the decision regarding which molecular pathways are targeted is also critical to achieving successful gene therapy transduction and outcome. Nevertheless, the capacity for gene therapy to cure human disease is now an established reality in multiple diseases. It is now only a matter of time before one will be available for the treatment of diabetic cardiomyopathy, for which there is currently no cure.
Abbreviations
- αMHC
α-myosin heavy chain
- βARK
β-adrenergic receptor kinase
- AAV
Adeno-associated viral vector
- ACEi
Angiotensin converting-enzyme inhibitors
- BPIFB4
Bactericidal/permeability-increasing fold-containing family B member 4
- CMV
cytomegalovirus
- CRH2
corticotropin-releasing hormone receptor 2
- CTGF
connective tissue growth factor
- CTRP
C1q/tumour necrosis factor-related protein
- CXCR4
CXC chemokine receptor type 4 (CXCR4)
- dATP
2-deoxyadenosine triphosphate
- DPP4
dipeptidyl peptidase-4
- eNOS
endothelial nitric oxide synthase
- FGF-2
fibroblast growth factor-2
- Foxo
Forkhead box-O-transcription factor
- GLP-1
glucagon-like peptide-1
- HO-1
heme oxygenase-1
- LAV
longevity-associated variant
- LV
left ventricle
- MLC
myosin light chain
- NF-κB
nuclear-factor-κB
- NGF
nerve growth factor
- Nox
NADPH Oxidase
- O-GlcNAc
O-linked N-acetylglucosamine
- OGA
O-GlcNAc-ase
- OGT
O-GlcNAc transferase
- PI3K
phosphoinositide-3 kinase
- PIM-1
Pro-viral integration site for Moloney murine leukaemia virus
- Pln
phospholamban
- rAAV
recombinant AAV
- RNR
ribonucleotide reductase
- ROS
reactive oxygen species
- Ryr2
ryanodine receptor-2
- S100A1
S100 calcium-binding protein A1
- SDF-1
stromal derived factor-1
- Ser
serine
- SERCA2a
sarcoplasmic/endoplasmic reticulum Ca2+-ATPase
- SGLT-2
sodium-glucose co-transporter-2
- SOD
superoxide dismutase
- Sp-1
specificity protein-1
- SUMO-1
small ubiquitin-related modifier-1
- TGF-β
transforming growth factor-β
- Thr
threonine
- TNF-α
tumour necrosis factor-α
- TZD
thiazolidinedione
- VEGF
vascular endothelial growth factor
- YAP
Yes-associated protein
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia, in the form of a Senior Research Fellowship to RHR [grant number ID1059960] and a Project Grant [grant number ID 1158013 (to R.H.R. and M.J.D.)].
Open Access
Open access for this article was enabled by the participation of University of Monash in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with CAUL.
Author Contribution
D.P. conducted the literature review and drafted the manuscript. M.T. provided comments and suggestions for the manuscript content. R.H.R. and M.J.D. provided critique on the manuscript, contributed expert advice on the topic and edited the manuscript. All authors read and approved the final manuscript.
References
- 1.Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W.et al. (2018) IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 2.Gilbert R.E. (2006) Heart failure and nephropathy: catastrophic and interrelated complications of diabetes. Clin. J. Am. Soc. Nephrol. 1, 193–208 10.2215/CJN.00540705 [DOI] [PubMed] [Google Scholar]
- 3.MacDonald M.R., Petrie M.C., Varyani F., Ostergren J., Michelson E.L., Young J.B.et al. (2008) Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur. Heart J. 29, 1377–1385 10.1093/eurheartj/ehn153 [DOI] [PubMed] [Google Scholar]
- 4.Rubler S., Dlugash J., Yuceoglu Y.Z., Kumral T., Branwood A.W. and Grishman A. (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 30, 595–602 10.1016/0002-9149(72)90595-4 [DOI] [PubMed] [Google Scholar]
- 5.Kannel W.B., Hjortland M. and Castelli W.P. (1974) Role of diabetes in congestive heart failure: the Framingham study. Am. J. Cardiol. 34, 29–34 10.1016/0002-9149(74)90089-7 [DOI] [PubMed] [Google Scholar]
- 6.Marwick T.H., Ritchie R., Shaw J.E. and Kaye D. (2018) Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 71, 339–351 10.1016/j.jacc.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 7.Ritchie R.H. and Abel E.D. (2020) Basic mechanisms of diabetic heart disease. Circ. Res. 126, 1501–1525 10.1161/CIRCRESAHA.120.315913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reis F., Nunes S., Soares XXXX and Pereira F. (2012) The role of inflammation in diabetic cardiomyopathy. Int. J. Interferon. Cytokine. Mediat. Res. 4, 59 10.2147/IJICMR.S21679 [DOI] [Google Scholar]
- 9.Ti Y., Xie G., Wang Z., Bi X., Ding W., Wang J.et al. (2011) TRB3 gene silencing alleviates diabetic cardiomyopathy in a type 2 diabetic rat model. Diabetes 60, 2963–2974 10.2337/db11-0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhileshwar V., Patel S.P. and Katyare S.S. (2007) Diabetic cardiomyopathy and reactive oxygen species (ROS) related parameters in male and female rats: a comparative study. Indian J. Clin. Biochem. 22, 84–90 10.1007/BF02912887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penpargkul S., Fein F., Sonnenblick E.H. and Scheuer J. (1981) Depressed cardiac sarcoplasmic reticular function from diabetic rats. J. Mol. Cell Cardiol. 13, 303–309 10.1016/0022-2828(81)90318-7 [DOI] [PubMed] [Google Scholar]
- 12.Pereira L., Matthes J., Schuster I., Valdivia H.H., Herzig S., Richard S.et al. (2006) Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes 55, 608–615 10.2337/diabetes.55.03.06.db05-1284 [DOI] [PubMed] [Google Scholar]
- 13.Chatham J.C., Zhang J. and Wende A.R. (2020) Role of O-linked N-acetylglucosamine (O-GlcNAc) protein modification in cellular (patho)physiology. Physiol. Rev. physrev.00043.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson S.B. and Hart G.W. (2016) New insights: a role for O -GlcNAcylation in diabetic complications. Crit. Rev. Biochem. Mol. Biol. 9238, 1–12 10.3109/10409238.2015.1135102 [DOI] [PubMed] [Google Scholar]
- 15.Fricovsky E.S., Suarez J., Ihm S.-H., Scott B.T., Suarez-Ramirez J.a., Banerjee I.et al. (2012) Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 303, R689–R699 10.1152/ajpregu.00548.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J., Gu J., Dai C., Gu J., Jin X., Sun J.et al. (2015) O-GlcNAcylation regulates ischemia-induced neuronal apoptosis through AKT signaling. Sci. Rep. 5, 14500 10.1038/srep14500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bugger H. and Abel E.D. (2014) Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57, 660–671 10.1007/s00125-014-3171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh S., Pulinilkunnil T., Yuen G., Kewalramani G., An D., Qi D.et al. (2005) Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am. J. Physiol.-Heart Circ. Physiol. 289, H768–H776 10.1152/ajpheart.00038.2005 [DOI] [PubMed] [Google Scholar]
- 19.Brower G.L., Gardner J.D., Forman M.F., Murray D.B., Voloshenyuk T., Levick S.P.et al. (2006) The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur. J. Cardiothorac. Surg. 30, 604–610 10.1016/j.ejcts.2006.07.006 [DOI] [PubMed] [Google Scholar]
- 20.Westermann D., Rutschow S., Jäger S., Linderer A., Anker S., Riad A.et al. (2007) Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes 56, 641–646 10.2337/db06-1163 [DOI] [PubMed] [Google Scholar]
- 21.Jia G., Hill M.A. and Sowers J.R. (2018) Diabetic cardiomyopathy. Circ. Res. 122, 624–638 10.1161/CIRCRESAHA.117.311586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shome J.S., Perera D., Plein S. and Chiribiri A. (2017) Current perspectives in coronary microvascular dysfunction. Microcirculation 24, e12340 10.1111/micc.12340 [DOI] [PubMed] [Google Scholar]
- 23.Strain W.D. and Paldánius P.M. (2018) Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 17, 57 10.1186/s12933-018-0703-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon C.-H., Hur J., Park K.-W., Kim J.-H., Lee C.-S., Oh I.-Y.et al. (2005) Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells. Circulation 112, 1618–1627 10.1161/CIRCULATIONAHA.104.503433 [DOI] [PubMed] [Google Scholar]
- 25.Nathan D.M., Cleary P.A., Backlund J.-Y.C., Genuth S.M., Lachin J.M., Orchard T.J.et al. (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 353, 2643–2653 10.1056/NEJMoa052187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerstein H.C., Miller M.E., Byington R.P., Goff D.C., Bigger J.T. and Action to Control Cardiovascular Risk in Diabetes Study Group et al. (2008) Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindholm L.H., Ibsen H., Dahlöf B., Devereux R.B., Beevers G., de Faire U.et al. (2002) Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet Lond. Engl. 359, 1004–1010 10.1016/S0140-6736(02)08090-X [DOI] [PubMed] [Google Scholar]
- 28.Aguilar D., Chan W., Bozkurt B., Ramasubbu K. and Deswal A. (2011) Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ. Heart Fail. 4, 53–58 10.1161/CIRCHEARTFAILURE.110.952556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loke Y.K., Kwok C.S. and Singh S. (2011) Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ 342, d1309–d1309 10.1136/bmj.d1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowes C.D., Lien L.F. and Butler J. (2019) Clinical aspects of heart failure in individuals with diabetes. Diabetologia 62, 1529–1538 10.1007/s00125-019-4958-2 [DOI] [PubMed] [Google Scholar]
- 31.Kristensen S.L., Rørth R., Jhund P.S., Docherty K.F., Sattar N., Preiss D.et al. (2019) Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diab. Endocrinol. 7, 776–785 10.1016/S2213-8587(19)30249-9 [DOI] [PubMed] [Google Scholar]
- 32.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S.et al. (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 33.Abdul-Ghani M., Del Prato S., Chilton R. and DeFronzo R.A. (2016) SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care. 39, 717–725 10.2337/dc16-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acharya T. and Deedwania P. (2019) Cardiovascular outcome trials of the newer anti-diabetic medications. Prog. Cardiovasc. Dis. 62, 342–348 10.1016/j.pcad.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 35.Seferović P.M., Coats A.J.S., Ponikowski P., Filippatos G., Huelsmann M., Jhund P.S.et al. (2020) European society of cardiology/heart failure association position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. Eur. J. Heart Fail. 22, 196–213 10.1002/ejhf.1673 [DOI] [PubMed] [Google Scholar]
- 36.McMurray J.J.V., Solomon S.D., Inzucchi S.E., Køber L., Kosiborod M.N., Martinez F.A.et al. (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 1–13 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 37.Wiviott S.D., Raz I., Bonaca M.P., Mosenzon O., Kato E.T., Cahn A.et al. (2018) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380, 347–357 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 38.Neal B., Perkovic V., Mahaffey K.W., de Zeeuw D., Fulcher G., Erondu N.et al. (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 39.Yoshida Y. and Yamanaka S. (2012) An emerging strategy of gene therapy for cardiac disease. Circ. Res. 111, 1108–1110 10.1161/CIRCRESAHA.112.278820 [DOI] [PubMed] [Google Scholar]
- 40.Hajjar R.J. (2013) Potential of gene therapy as a treatment for heart failure. J. Clin. Invest. 123, 53–61 10.1172/JCI62837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilemann L., Ishikawa K., Weber T. and Hajjar R.J. (2012) Gene therapy for heart failure. Circ. Res. 110, 777–793 10.1161/CIRCRESAHA.111.252981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hulot J.-S., Ishikawa K. and Hajjar R.J. (2016) Gene therapy for the treatment of heart failure: promise postponed. Eur. Heart J. 37, 1651–1658 10.1093/eurheartj/ehw019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawase Y., Ladage D. and Hajjar R.J. (2011) Rescuing the failing heart by targeted gene transfer. J. Am. Coll. Cardiol. 57, 1169–1180 10.1016/j.jacc.2010.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scimia M.C., Gumpert A.M. and Koch W.J. (2014) Cardiovascular gene therapy for myocardial infarction. Expert Opin. Biol. Ther. 14, 183–195 10.1517/14712598.2014.866085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daya S. and Berns K.I. (2008) Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 21, 583–593 10.1128/CMR.00008-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pacak C.a. and Byrne B.J. (2011) AAV vectors for cardiac gene transfer: experimental tools and clinical opportunities. Mol. Ther. 19, 1582–1590 10.1038/mt.2011.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naim C., Yerevanian A. and Hajjar R.J. (2013) Gene therapy for heart failure: where do we stand? Curr. Cardiol. Rep. 15, 333 10.1007/s11886-012-0333-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mingozzi F. and High K.A. (2011) Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 12, 341–355 10.1038/nrg2988 [DOI] [PubMed] [Google Scholar]
- 49.Jiang H., Pierce G.F., Ozelo M.C., de Paula E.V., Vargas J.A., Smith P.et al. (2006) Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol. Ther. 14, 452–455 10.1016/j.ymthe.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 50.Jessup M., Greenberg B., Mancini D., Cappola T., Pauly D.F., Jaski B.et al. (2011) Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 124, 304–313 10.1161/CIRCULATIONAHA.111.022889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D., Tai P.W.L. and Gao G. (2019) Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 18, 358–378 10.1038/s41573-019-0012-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baum C., von Kalle C., Staal F.J.T., Li Z., Fehse B., Schmidt M.et al. (2004) Chance or necessity? Insertional mutagenesis in gene therapy and its consequences Mol. Ther. 9, 5–13 10.1016/j.ymthe.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 53.Hulot J.-S., Ishikawa K. and Hajjar R.J. (2016) Gene therapy for the treatment of heart failure: promise postponed. Eur. Heart J. 37, 1651–1658 10.1093/eurheartj/ehw019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao G., Vandenberghe L.H., Alvira M.R., Lu Y., Calcedo R., Zhou X.et al. (2004) Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78, 6381–6388 10.1128/JVI.78.12.6381-6388.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zincarelli C., Soltys S., Rengo G., Koch W.J. and Rabinowitz J.E. (2010) Comparative cardiac gene delivery of adeno-associated virus serotypes 1-9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin. Transl. Sci. 3, 81–89 10.1111/j.1752-8062.2010.00190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziegler T., Ishikawa K., Hinkel R. and Kupatt C. (2018) Translational aspects of adeno-associated virus-mediated cardiac gene therapy. Hum. Gene Ther. 29, 1341–1351 10.1089/hum.2017.229 [DOI] [PubMed] [Google Scholar]
- 57.Domenger C. and Grimm D. (2019) Next-generation AAV vectors—do not judge a virus (only) by its cover. Hum. Mol. Genet. 28, 3–14 10.1093/hmg/ddz148 [DOI] [PubMed] [Google Scholar]
- 58.Wang B., Li J., Fu F.H., Chen C., Zhu X., Zhou L.et al. (2008) Construction and analysis of compact muscle-specific promoters for AAV vectors. Gene Ther. 15, 1489–1499 10.1038/gt.2008.104 [DOI] [PubMed] [Google Scholar]
- 59.Chtarto A., Bender H.U., Hanemann C.O., Kemp T., Lehtonen E., Levivier M.et al. (2003) Tetracycline-inducible transgene expression mediated by a single AAV vector. Gene Ther. 10, 84–94 10.1038/sj.gt.3301838 [DOI] [PubMed] [Google Scholar]
- 60.Apparailly F., Noël D., Millet V., Jacquet C., Sany J. and Jorgensen C. (2001) Development of a doxycycline inducible AAV vector for long term in vivo viral IL-10 gene transfer in rheumatoid arthritis. Arthritis Res. Ther. 3, P091 10.1186/ar260 [DOI] [Google Scholar]
- 61.Sanbe A. (2003) Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ. Res. 92, 609–616 10.1161/01.RES.0000065442.64694.9F [DOI] [PubMed] [Google Scholar]
- 62.Bostick B., Shin J.-H., Yue Y. and Duan D. (2011) AAV-microdystrophin therapy improves cardiac performance in aged female mdx mice. Mol. Ther. 19, 1826–1832 10.1038/mt.2011.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips M.I., Tang Y., Schmidt-Ott K., Qian K. and Kagiyama S. (2002) Vigilant vector: heart-specific promoter in an adeno-associated virus vector for cardioprotection. Hypertension 39, 651–655 10.1161/hy0202.103472 [DOI] [PubMed] [Google Scholar]
- 64.Konkalmatt Prasad R., Beyers Ronald J., O'Connor Daniel M., Xu Yaqin, Seaman Marc E. and French Brent A. (2013) Cardiac-selective expression of extracellular superoxide dismutase after systemic injection of adeno-associated virus 9 protects the heart against post-myocardial infarction left ventricular remodeling. Circ. Cardiovasc. Imaging 6, 478–486 10.1161/CIRCIMAGING.112.000320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weeks K.L., Gao X., Du X.-J., Boey E.J.H., Matsumoto A., Bernardo B.C.et al. (2012) Phosphoinositide 3-kinase p110α is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ. Heart Fail. 5, 523–534 10.1161/CIRCHEARTFAILURE.112.966622 [DOI] [PubMed] [Google Scholar]
- 66.Ni L., Scott L., Campbell H.M., Pan X., Alsina K.M., Reynolds J.et al. (2019) Atrial-specific gene delivery using an adeno-associated viral vector. Circ. Res. 124, 256–262 10.1161/CIRCRESAHA.118.313811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ritterhoff J. and Most P. (2012) Targeting S100A1 in heart failure. Gene Ther. 19, 613–621 10.1038/gt.2012.8 [DOI] [PubMed] [Google Scholar]
- 68.Rohde D., Busch M., Volkert A., Ritterhoff J., Katus H.A., Peppel K.et al. (2015) Cardiomyocytes, endothelial cells and cardiac fibroblasts: S100A1's triple action in cardiovascular pathophysiology. Fut. Cardiol. 11, 309–321 10.2217/fca.15.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pleger S.T., Most P., Boucher M., Soltys S., Chuprun J.K., Pleger W.et al. (2007) Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation 115, 2506–2515 10.1161/CIRCULATIONAHA.106.671701 [DOI] [PubMed] [Google Scholar]
- 70.Pleger S.T., Shan C., Ksienzyk J., Bekeredjian R., Boekstegers P., Hinkel R.et al. (2011) Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci. Transl. Med. 3, 92ra64–92ra64 10.1126/scitranslmed.3002097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weber C., Neacsu I., Krautz B., Schlegel P., Sauer S., Raake P.et al. (2014) Therapeutic safety of high myocardial expression levels of the molecular inotrope S100A1 in a preclinical heart failure model. Gene Ther. 21, 131–138 10.1038/gt.2013.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kho C., Lee A., Jeong D., Oh J.G., Chaanine A.H., Kizana E.et al. (2011) SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477, 601–605 10.1038/nature10407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tilemann L., Lee A., Ishikawa K., Aguero J., Rapti K., Santos-Gallego C.et al. (2013) SUMO-1 gene transfer improves cardiac function in a large-animal model of heart failure. Sci. Transl. Med. 5, 211ra159–211ra159 10.1126/scitranslmed.3006487 [DOI] [PubMed] [Google Scholar]
- 74.Ishikawa K., Fish K.M., Tilemann L., Rapti K., Aguero J., Santos-Gallego C.G.et al. (2014) Cardiac I-1c overexpression with reengineered AAV improves cardiac function in swine ischemic heart failure. Mol. Ther. 22, 2038–2045 10.1038/mt.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwab D.M., Tilemann L., Bauer R., Heckmann M., Jungmann A., Wagner M.et al. (2018) AAV-9 mediated phosphatase-1 inhibitor-1 overexpression improves cardiac contractility in unchallenged mice but is deleterious in pressure-overload. Gene Ther. 10.1038/gt.2017.97 [DOI] [PubMed] [Google Scholar]
- 76.Watanabe S., Ishikawa K., Fish K., Oh J.G., Motloch L.J., Kohlbrenner E.et al. (2017) Protein phosphatase inhibitor-1 gene therapy in a swine model of nonischemic heart failure. J. Am. Coll. Cardiol. 70, 1744–1756 10.1016/j.jacc.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giamouridis D., Gao M.H., Lai N.C., Tan Z., Kim Y.C., Guo T.et al. (2018) Effects of urocortin 2 versus urocortin 3 gene transfer on left ventricular function and glucose disposal. JACC Basic Transl. Sci. 3, 249–264 10.1016/j.jacbts.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai N.C., Gao M.H., Giamouridis D., Suarez J., Miyanohara A., Parikh J.et al. (2015) Intravenous AAV8 encoding urocortin-2 increases function of the failing heart in mice. Hum. Gene Ther. 26, 347–356 10.1089/hum.2014.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giamouridis D., Gao M.H., Lai N.C., Tan Z., Kim Y.C., Guo T.et al. (2019) Urocortin 3 gene transfer increases function of the failing murine heart. Hum. Gene Ther. 30, 10–20 10.1089/hum.2018.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giacca M. and Zacchigna S. (2012) VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther. 19, 622–629 10.1038/gt.2012.17 [DOI] [PubMed] [Google Scholar]
- 81.Huusko J., Lottonen L., Merentie M., Gurzeler E., Anisimov A., Miyanohara A.et al. (2012) AAV9-mediated VEGF-B gene transfer Improves systolic function in progressive left ventricular hypertrophy. Mol. Ther. 20, 2212–2221 10.1038/mt.2012.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woitek F., Zentilin L., Hoffman N.E., Powers J.C., Ottiger I., Parikh S.et al. (2015) Intracoronary cytoprotective gene therapy. J. Am. Coll. Cardiol. 66, 139–153 10.1016/j.jacc.2015.04.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emanueli C., Salis M.B., Pinna A., Graiani G., Manni L. and Madeddu P. (2002) Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 10.1161/01.CIR.0000033971.56802.C5 [DOI] [PubMed] [Google Scholar]
- 84.Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S.et al. (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 10.1016/S0092-8674(00)80595-4 [DOI] [PubMed] [Google Scholar]
- 85.Meloni M., Caporali A., Graiani G., Lagrasta C., Katare R., Van Linthout S.et al. (2010) Nerve growth factor promotes cardiac repair following myocardial infarction. Circ. Res. 106, 1275–1284 10.1161/CIRCRESAHA.109.210088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meloni M., Descamps B., Caporali A., Zentilin L., Floris I., Giacca M.et al. (2012) Nerve growth factor gene therapy using adeno-associated viral vectors prevents cardiomyopathy in type 1 diabetic mice. Diabetes 61, 229–240 10.2337/db11-0763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Z., Von Gise A., Zhou P., Gu F., Ma Q., Jiang J.et al. (2014) Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ. Res. 115, 354–363 10.1161/CIRCRESAHA.115.303632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davidsohn N., Pezzone M., Vernet A., Graveline A., Oliver D., Slomovic S.et al. (2019) A single combination gene therapy treats multiple age-related diseases. Proc. Natl. Acad. Sci. 201910073 10.1073/pnas.1910073116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Q., Bolli R., Qiu Y., Tang X.-L., Guo Y. and French Brent A. (2001) Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation 103, 1893–1898 10.1161/01.CIR.103.14.1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agrawal R.S., Muangman S., Layne M.D., Melo L., Perrella M.A., Lee R.T.et al. (2004) Pre-emptive gene therapy using recombinant adeno-associated virus delivery of extracellular superoxide dismutase protects heart against ischemic reperfusion injury, improves ventricular function and prolongs survival. Gene Ther. 11, 962–969 10.1038/sj.gt.3302250 [DOI] [PubMed] [Google Scholar]
- 91.Ma Z.-G., Yuan Y.-P., Xu S.-C., Wei W.-Y., Xu C.-R., Zhang X.et al. (2017) CTRP3 attenuates cardiac dysfunction, inflammation, oxidative stress and cell death in diabetic cardiomyopathy in rats. Diabetologia 60, 1126–1137 10.1007/s00125-017-4232-4 [DOI] [PubMed] [Google Scholar]
- 92.Liu X., Simpson J.a., Brunt K.R., Ward C.a., Hall S.R.R., Kinobe R.T.et al. (2007) Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction. Am J Physiol-Heart Circ Physiol. 293, H48–H59 10.1152/ajpheart.00741.2006 [DOI] [PubMed] [Google Scholar]
- 93.Hinkel R., Lange P., Petersen B., Gottlieb E., Ng J.K.M., Finger S.et al. (2015) Heme oxygenase-1 gene therapy provides cardioprotection via control of post-ischemic inflammation. J. Am. Coll. Cardiol. 66, 154–165 10.1016/j.jacc.2015.04.064 [DOI] [PubMed] [Google Scholar]
- 94.Dang Z., Avolio E., Thomas A.C., Faulkner A., Beltrami A.P., Cervellin C.et al. (2020) Transfer of a human gene variant associated with exceptional longevity improves cardiac function in obese type 2 diabetic mice through induction of the SDF ‐1/CXCR4 signalling pathway. Eur. J. Heart Fail. ejhf.1840 10.1002/ejhf.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Puca A.A., Carrizzo A., Spinelli C., Damato A., Ambrosio M., Villa F.et al. (2019) Single systemic transfer of a human gene associated with exceptional longevity halts the progression of atherosclerosis and inflammation in ApoE knockout mice through a CXCR4-mediated mechanism. Eur. Heart J. ehz459 10.1093/eurheartj/ehz459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kolwicz S.C., Odom G.L., Nowakowski S.G., Moussavi-Harami F., Chen X., Reinecke H.et al. (2016) AAV6-mediated cardiac-specific overexpression of ribonucleotide reductase enhances myocardial contractility. Mol. Ther. 24, 240–250 10.1038/mt.2015.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kadota S., Carey J., Reinecke H., Leggett J., Teichman S., Laflamme M.A.et al. (2015) Ribonucleotide reductase-mediated increase in dATP improves cardiac performance via myosin activation in a large animal model of heart failure. Eur. J. Heart Fail. 17, 772–781 10.1002/ejhf.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kolwicz S.C., Hall J.K., Moussavi-Harami F., Chen X., Hauschka S.D., Chamberlain J.S.et al. (2019) Gene Therapy Rescues Cardiac Dysfunction in Duchenne Muscular Dystrophy Mice by Elevating Cardiomyocyte Deoxy-Adenosine Triphosphate. JACC Basic Transl. Sci. 10.1016/j.jacbts.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raake P.W.J., Schlegel P., Ksienzyk J., Reinkober J., Barthelmes J., Schinkel S.et al. (2013) AAV6.βARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur. Heart J. 34, 1437–1447 10.1093/eurheartj/ehr447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Williams M.L., Hata J.A., Schroder J., Rampersaud E., Petrofski J., Jakoi A.et al. (2004) Targeted beta-adrenergic receptor kinase (betaARK1) inhibition by gene transfer in failing human hearts. Circulation 109, 1590–1593 10.1161/01.CIR.0000125521.40985.28 [DOI] [PubMed] [Google Scholar]
- 101.Katz M.G., Fargnoli A.S., Swain J.D., Tomasulo C.E., Ciccarelli M., Huang Z.M.et al. (2012) AAV6-βARKct gene delivery mediated by molecular cardiac surgery with recirculating delivery (MCARD) in sheep results in robust gene expression and increased adrenergic reserve. J. Thorac. Cardiovasc. Surg. 143, 720.e3–726.e3 10.1016/j.jtcvs.2011.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hart G.W., Housley M.P. and Slawson C. (2007) Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 10.1038/nature05815 [DOI] [PubMed] [Google Scholar]
- 103.Prakoso D., Kiriazis H., Tate M., Qian H., Deo M., Parry L.et al. (2018) Manipulation of cardiac O-GlcNAc modification alters cardiac function and remodelling in the setting of diabetic cardiomyopathy. Eur. Heart J. 39, 10.1093/eurheartj/ehy566.5213 [DOI] [Google Scholar]
- 104.Prakoso D., Lim S.Y., Erickson J.R., Wallace R.S., Lees J.G., Tate M.et al. (2021) Fine-tuning the cardiac O-GlcNAcylation regulatory enzymes governs the functional and structural phenotype of the diabetic heart. Cardiovasc. Res. [cited 2021 Apr 8] 10.1093/cvr/cvab043 [DOI] [PubMed] [Google Scholar]
- 105.Muraski J.A., Rota M., Misao Y., Fransioli J., Cottage C., Gude N.et al. (2007) Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat. Med. 13, 1467–1475 10.1038/nm1671 [DOI] [PubMed] [Google Scholar]
- 106.Katare R., Caporali A., Zentilin L., Avolio E., Sala-Newby G., Oikawa A.et al. (2011) Intravenous gene therapy with PIM-1 via a cardiotropic viral vector halts the progression of diabetic cardiomyopathy through promotion of prosurvival signaling. Circ. Res. 108, 1238–1251 10.1161/CIRCRESAHA.110.239111 [DOI] [PubMed] [Google Scholar]
- 107.Weeks K.L., Gao X., Du X.-J., Boey E.J.H., Matsumoto A., Bernardo B.C.et al. (2012) Phosphoinositide 3-kinase p110α is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ Heart Fail. 5, 523–534 10.1161/CIRCHEARTFAILURE.112.966622 [DOI] [PubMed] [Google Scholar]
- 108.Prakoso D., De Blasio M.J., Qin C., Rosli S., Kiriazis H., Qian H.et al. (2017) Phosphoinositide 3-kinase (p110α) gene delivery limits diabetes-induced cardiac NADPH oxidase and cardiomyopathy in a mouse model with established diastolic dysfunction. Clin. Sci. Lond. Engl. 1979 131, 1345–1360 10.1042/CS20170063 [DOI] [PubMed] [Google Scholar]
- 109.Prakoso D., De Blasio M.J., Tate M., Kiriazis H., Donner D.G., Qian H.et al. (2020) Gene therapy targeting cardiac phosphoinositide 3-kinase (p110α) attenuates cardiac remodeling in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 318, H840–H852 10.1152/ajpheart.00632.2019 [DOI] [PubMed] [Google Scholar]
- 110.Ghosh N. and Katare R. (2018) Molecular mechanism of diabetic cardiomyopathy and modulation of microRNA function by synthetic oligonucleotides. Cardiovasc. Diabetol. 17, 1–25 10.1186/s12933-018-0684-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karakikes I., Chaanine A.H., Kang S., Mukete B.N., Jeong D., Zhang S.et al. (2013) Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J. Am. Heart Assoc. 2, 17–19 10.1161/JAHA.113.000078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dai B., Li H., Fan J., Zhao Y., Yin Z., Nie X.et al. (2018) MiR-21 protected against diabetic cardiomyopathy induced diastolic dysfunction by targeting gelsolin. Cardiovasc. Diabetol. 17, 1–17 10.1186/s12933-018-0767-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yin Z., Zhao Y., He M., Li H., Fan J., Nie X.et al. (2019) MiR-30c/PGC-1β protects against diabetic cardiomyopathy via PPARα. Cardiovasc. Diabetol. 18, 1–15 10.1186/s12933-019-0811-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li H., Fan J., Zhao Y., Zhang X., Dai B., Zhan J.et al. (2019) Nuclear miR-320 mediates diabetes-induced cardiac dysfunction by activating transcription of fatty acid metabolic genes to cause lipotoxicity in the heart. Circ. Res. 125, 1106–1120 10.1161/CIRCRESAHA.119.314898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hayward C., Banner N.R., Morley-Smith A., Lyon A.R. and Harding S.E. (2015) The current and future landscape of SERCA gene therapy for heart Failure: a clinical perspective. Hum. Gene Ther. 26, 293–304 10.1089/hum.2015.018 [DOI] [PubMed] [Google Scholar]
- 116.Jaski B.E., Jessup M.L., Mancini D.M., Cappola T.P., Pauly D.F., Greenberg B.et al. (2009) Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J. Card. Fail. 15, 171–181 10.1016/j.cardfail.2009.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Greenberg B., Butler J., Felker G.M., Ponikowski P., Voors A.A., Desai A.S.et al. (2016) Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet North Am. Ed. 387, 1178–1186 10.1016/S0140-6736(16)00082-9 [DOI] [PubMed] [Google Scholar]
- 118.Hammond H.K., Penny W.F., Traverse J.H., Henry T.D., Watkins M.W., Yancy C.W.et al. (2016) Intracoronary gene transfer of adenylyl cyclase 6 in patients with heart failure: a randomized clinical trial. JAMA Cardiol. 1, 163–171 10.1001/jamacardio.2016.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao M.H., Tang T., Guo T., Sun S.Q., Feramisco J.R. and Hammond H.K. (2004) Adenylyl cyclase type VI gene transfer reduces phospholamban expression in cardiac myocytes via activating transcription factor 3. J. Biol. Chem. 279, 38797–38802 10.1074/jbc.M405701200 [DOI] [PubMed] [Google Scholar]
- 120.Gao M.H., Miyanohara A., Feramisco J.R. and Tang T. (2009) Activation of PH-domain leucine-rich protein phosphatase 2 (PHLPP2) by agonist stimulation in cardiac myocytes expressing adenylyl cyclase type 6. Biochem. Biophys. Res. Commun. 384, 193–198 10.1016/j.bbrc.2009.04.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Penny W.F., Henry T.D., Watkins M.W., Patel A.N. and Hammond H.K. (2018) Design of a Phase 3 trial of intracoronary administration of human adenovirus 5 encoding human adenylyl cyclase type 6 (RT-100) gene transfer in patients with heart failure with reduced left ventricular ejection fraction: The FLOURISH Clinical Trial. Am. Heart J. 201, 111–116 10.1016/j.ahj.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 122.Cannatà A., Ali H., Sinagra G. and Giacca M. (2020) Gene therapy for the heart lessons learned and future perspectives. Circ. Res. 126, 1394–1414 10.1161/CIRCRESAHA.120.315855 [DOI] [PubMed] [Google Scholar]
- 123.Louis Jeune V., Joergensen J.a., Hajjar R.J. and Weber T. (2013) Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum Gene. Ther. Methods 24, 59–67 10.1089/hgtb.2012.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tse L.V., Klinc K.A., Madigan V.J., Castellanos Rivera R.M., Wells L.F., Havlik L.P.et al. (2017) Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc. Natl. Acad. Sci. 114, E4812–E4821 10.1073/pnas.1704766114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fakhiri J., Schneider M.A., Puschhof J., Stanifer M., Schildgen V., Holderbach S.et al. (2019) Novel chimeric gene therapy vectors based on adeno-associated virus and four different mammalian bocaviruses. Mol. Ther. - Methods Clin. Dev. 12, 202–222 10.1016/j.omtm.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hinderer C., Katz N., Buza E.L., Dyer C., Goode T., Bell P.et al. (2018) Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an AAV vector expressing human SMN. Hum. Gene Ther. 29, 285–298 10.1089/hum.2018.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Flotte T.R. and Büning H. (2018) Severe toxicity in nonhuman primates and piglets with systemic high-dose administration of adeno-associated virus serotype 9-like vectors: putting patients first. Hum. Gene Ther. 29, 283–284 10.1089/hum.2018.021 [DOI] [PubMed] [Google Scholar]
- 128.Zhang W.-W., Li L., Li D., Liu J., Li X., Li W.et al. (2018) The first approved gene therapy product for cancer Ad- p53 (Gendicine): 12 Years in the clinic. Hum. Gene Ther. 29, 160–179 10.1089/hum.2017.218 [DOI] [PubMed] [Google Scholar]
- 129.(2019) Gene therapy's next installment. Nat. Biotechnol. 37, 697–697 10.1038/s41587-019-0194-z [DOI] [PubMed] [Google Scholar]
- 130.(2020) First oral SMA drug. Nat. Biotechnol. 38, 1111–1111 10.1038/s41587-020-0706-x [DOI] [PubMed] [Google Scholar]
- 131.Zabaleta N., Dai W., Bhatt U., Chichester J.A., Estelien R., Sanmiguel J.et al. (2021) Immunogenicity of an AAV-based, room-temperature stable, single dose COVID-19 vaccine in mice and non-human primates. bioRxiv 2021.01.05.422952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clément N. and Grieger J.C. (2016) Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol. Ther. - Methods Clin. Dev. 3, 16002 10.1038/mtm.2016.2 [DOI] [PMC free article] [PubMed] [Google Scholar]