Abstract
Disentangling the mechanisms that mediate the relationships between species diversity and disease risk has both theoretical and applied implications. We employed a model system of rodents and their Mycoplasma pathogens, in which an extreme negative diversity–disease relationship was demonstrated, to test the assumptions underlying three mechanisms that may explain this field pattern. Through quantifying the long-term dynamics and effects of the pathogen in its three host species, we estimated the between-host differences in pathogen spreading and transmission potentials, and host recovery potential and vulnerability to infection. The results suggest that one of the hosts is a pathogen amplifier and the other two hosts function as diluters. Considering the similarity in infection success and intensity among hosts, and the failure to detect any pathogen-induced damage, we could not validate the assumption underlying the hypotheses that diluters reduce the overall transmission or increase the mortality of infected hosts in the system. Instead, the results demonstrate that diluters clear the infection faster than amplifiers, supporting the possibility that the addition of diluters to the community may reduce the overall number of infected hosts through this mechanism. This study highlights the contribution of experimental studies that simultaneously explore different aspects of host–pathogen interactions in multiple hosts, in diversity–disease research.
Keywords: dilution effect, diversity–disease relationship, infected host mortality, multi-host communities, recovery augmentation, transmission reduction
1. Background
In this era, characterized by the profound impacts of emerging diseases and biodiversity loss [1,2], determining the relationships between species diversity and disease risk is an especially timely endeavour [3–5].
Accumulated correlative evidence in diverse natural systems shows both negative (e.g. [6–8]) and positive (e.g. [9–11]) relationships, broadly termed as dilution and amplification effects, respectively. To this end, the core of current diversity–disease research is directed towards revealing the underlying mechanisms promoting either dilution or amplification [4,5,12]. Disentangling the mechanisms that mediate diversity–disease relationships has both theoretical and applied implications. For instance, tightening the connectivity between fragmented areas will achieve the goal of disease reduction when it increases the mortality rate of infected individuals (‘infected host mortality’ in [13]). Otherwise, it may amplify disease risk, by, for example, increasing the susceptible host density (the inverse of ‘susceptible host regulation’ in [13]).
Keesing et al.'s [13] conceptual work constitutes a landmark in the mechanistic understanding of diversity–disease relationships (table 1). They offered five mechanisms through which the addition of a species to a single-host–single-pathogen system may decrease or amplify disease prevalence, and thus disease risk. Specifically, their framework suggests that disease risk will be diluted when the addition of a new species reduces (i) the encounters (encounter reduction) or (ii) the probability of transmission (transmission reduction) between susceptible and infected individuals, or reduces (iii) the number of susceptible hosts (susceptible host regulation). Disease risk is also expected to be diluted when the addition of a new host species increases (iv) the recovery (recovery augmentation) or (v) the mortality rates (infected host mortality) of infected individuals. By contrast, disease amplification is expected when the opposite trends occur. This framework has been adjusted to various community situations, including pathogens' different transmission modes and differences in host transmission capacities, scenarios in which hosts are involved in inter- and intraspecific competition, and under changing environmental conditions [14–19]. Importantly, many of these extensions emphasize the role that the specific traits of the added species play in predicting dilution or amplification effects [14,16].
Table 1.
A summary of the hypotheses, underlying assumptions and results used to disentangle three of the five mechanisms offered by Keesing et al. [13]. The hypotheses and assumptions were formulated as potential explanations for the Mycoplasma dilution in rodent communities in the sand dunes of Israel's northwestern Negev Desert. They are based on the assumed role that the host species plays in pathogen spread, where the amplifier host (Gerbillus andersoni, GA), behaviourally or physiologically, enriches and the diluter hosts (Gerbillus gerbillus, GG; Gerbillus pyramidum, GP), behaviorally or physiologically, impede the pathogen population.
| mechanism | hypothesis | underlying assumptions | result |
|---|---|---|---|
| general | the community includes both pathogen amplifiers and diluters; GA is an amplifier, and GG and GP are diluters | susceptible host perspective:
|
|
| transmission reduction | diluters are less competent for the pathogen, reducing the overall transmission in a system | susceptible host perspective:
|
|
| recovery augmentation | diluters clear the infection faster, decreasing the overall number of infected hosts | the probability and efficiency of diluters’ clearance are higher than of amplifiers | |
| infected host mortality | diluters are more vulnerable to pathogen-induced mortality than amplifiers, decreasing the overall number of infected hosts | diluters are more vulnerable to infection than amplifiers, as measured by mortality-correlative traits |
|
✓ = full support; × = no support
Nevertheless, this theoretical work has outpaced its experimental testing. At present, there have been several major theoretical–experiment gaps that limit our mechanistic understanding of diversity–disease relationships in nature. The two relevant for this study are the limited response metrics and the single hypothesis bias.
Considering the limited response metrics, some experimental studies tend to focus primarily on a single static measure of the pathogen's interactions with its most competent host (i.e. infection success, intensity of infection, host mortality or immune activation), which may lead to erroneous conclusions and incomplete understanding of the underlying mechanisms [5,20]. Others have quantified one of these measurements or a broader response metrics in hosts differing in competence levels [21–25]. However, since they focus mainly on aspects related to the transmission potential (but see [26]), the relative importance of these aspects in determining diversity–disease relationships, relative to interspecific differences in other aspects of host–pathogen interactions, such as pathogen spread and recovery potentials, and vulnerability to infection, remains obscure [20]. Considering the theoretical role that the less competent hosts play in determining diversity–disease relationships [14,27–29], this bias restricts the mechanistic understanding of natural systems.
The common single hypothesis approach in experimental studies further impedes the feedback between theory and data, as failure in empirical support for a tested hypothesis is frequently uninformative in developing future theory. The potential for disentangling multiple hypotheses is illustrated by Luis et al. [12]; through integrating field data and modelling, they showed that both amplification and dilution components exist in their system, but because the dilution effect is stronger, it determines the observed dynamics.
Addressing these gaps, among others (e.g. the spatial scale bias [7]), is important for achieving a more comprehensive view of diversity–disease relationships in natural systems [5,30], and the model system of rodent–Mycoplasma communities in Israel's northwestern Negev Desert's sand dunes provides an opportunity to establish the first required steps. There are conceptual and practical reasons why this system presents an ideal situation. Conceptually, this system is intriguing since it represents an extreme case of the dilution effect with potentially one amplifier host that supports most of the pathogen population (Gerbillus andersoni) and two diluter hosts that possess only a few infected individuals (G. pyramidum) or none (G. gerbillus) [31]. There are experimental indications that the main transmission route of the pathogen between G. andersoni hosts is direct, through aggressive interactions [32], while the between-species transmission route is still unknown. However, there are indications of aggressive interactions between the species [33], which are likely to allow between-host transmission. In addition, in this study system, the three rodent species are infested by the same flea species, Synosternus cleopatrae, which may occasionally transmit the pathogen from one host species to another via blood feeding [34]. Thus, we assume that the pathogen has the opportunity to reach any rodent species in the area. The dilution effect is so pronounced that in three-species communities, the pathogen is completely excluded, despite the presence of G. andersoni hosts [31]. Yet, the underlying mechanisms are unknown. We exploited these assumed host species differences to simultaneously disentangle the likelihood that three of the five alternative mechanisms offered by Keesing et al. [13] operate in our model system (table 1), avoiding the single hypothesis bias.
From a practical view, this system allows the assessment of multiple aspects of host–pathogen interactions, reducing the response metric limitations that often obscure experimental studies. This is doable through a combination of established protocols for rodent breeding and infection by Mycoplasma, as well as through protocols for pathogen quantification and rodent response evaluations [32,35].
Through quantifying the long-term dynamics and effects of the pathogen in multiple host species, we first tested whether G. andersoni individuals: (i) have higher chances to serve as infective hosts; (ii) as infective hosts, have higher chances to encounter susceptible hosts and spread the pathogen; and (iii) as recipients, have higher chances to maintain infection, compared to the other species. Our results confirmed these working assumptions, suggesting that the former species can serve as an amplifier, and the two other species as pathogen diluters (row 1 in table 1). We then quantified the pathogen's infection success and intensity within the three host species, which are assumed to be lower in the diluter species, under the ‘transmission reduction’ hypothesis (row 2 in table 1). We also assessed the pathogen's clearance and re-infection probabilities in the three host species, which are assumed to be higher in the diluter hosts under the ‘recovery augmentation’ hypothesis (row 3 in table 1). Finally, we quantified the differences in the mortality-correlative traits of the three-host species and tested whether the diluters' vulnerability to infection is higher than that of the amplifiers, as assumed by the ‘infected host mortality’ hypothesis (row 4 in table 1). Unfortunately, the assumptions underlying the ‘encounter reduction’ and the ‘susceptible host regulation’ mechanisms can only be tested in the field and thus were not tested in this study.
We collected evidence supporting the likelihood that ‘recovery augmentation’ is a dilution mechanism in our study system.
2. Methods
(a) . Study organisms
(i) . Hosts
All rodents that were used in the study were born and raised in the laboratory and were PCR negative for Mycoplasma. They were non-reproductive adult (at least three months old) males, with average body masses of 42 ± 1, 34 ± 1 and 60 ± 2 g, for G. andersoni, G. gerbillus and G. pyramidum, respectively. Non-reproductive adult males represent well the entire population of each species in the summer, when the dilution effect was observed, as in this period, only non-reproductive adults occur, and throughout the year, males and female hosts show similar pathogen prevalences and intensities (H.H. et al. 2011, unpublished data). Hosts were maintained individually in plastic cages on a 1 cm layer of autoclaved sand (34 × 24 × 13 cm3). The cages were placed in an animal facility, with an ambient temperature of 24.5 ± 1°C and a photoperiod of 12 D : 12 L. Hosts were daily provided with millet seeds ad libitum and alfalfa as a water source.
(ii) . Pathogens
The Mycoplasma haemomuris-like bacterium (hereafter, Mycoplasma or pathogen) is one of the two most dominant bacteria and the only Mycoplasma species that infects rodents in Israel's northwestern Negev Desert's sand dunes [31,36]. The prevalence of the pathogen in wild rodents can reach 95% [36]. Thus, by infecting rodents with Mycoplasma, we emulated a common infection scenario in nature.
(b) . Experimental design
Seventy individuals of G. andersoni, G. gerbillus and G. pyramidum (n = 24, 24 and 22, respectively) were used in this experiment. Within each species, pairs of siblings were randomly assigned to two treatment groups. Mycoplasma-infected hosts were inoculated with Mycoplasma-infected blood in a concentration within the natural infection range (see pathogen inoculation and quantification). Simultaneously, to control for the effects of the inoculation procedure and the blood inoculation, control hosts were inoculated with either PBS (n = 16) or Mycoplasma-negative blood (n = 19), respectively.
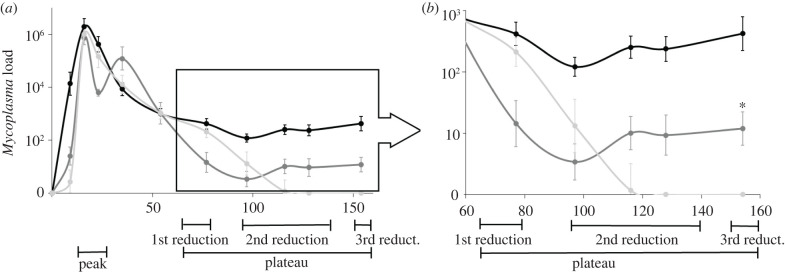
To assess the pathogen infection dynamics in Mycoplasma-infected hosts, we bled them every 10–20 days, from the inoculation day until day 154 of the experiment. Simultaneously, control hosts were bled to confirm that they are Mycoplasma-negative. In parallel, we quantified the host behavioural and physiological parameters during 1–4 days at each of the five infection stages. The infection period was divided into pre-inoculation (stage 1, days −15–0) and peak bacteremia (stage 2, days 16–23, including peak and days on either side) stages, and the plateau stage of Mycoplasma in G. andersoni (days 65–158) was further divided according to the infection dynamics in the two other host species (figure 1). Specifically, during the period of the first reduction (stage 3, days 65–77), the bacteremia levels declined in both host species; during the second reduction (stage 4, days 97–138), the pathogen levels in G. pyramidum tended to zero, and they began to lose the infection; and during the third reduction (stage 4, days 150–158), the pathogen cells were undetectable in all G. pyramidum and were steadily low or absent in G. gerbillus hosts (figure 1).
Figure 1.
Pathogen infection dynamics in the three rodent species. Log means and standard errors of Mycoplasma load (cells per µl of blood) in blood samples collected from individuals of G. andersoni (black), G. gerbillus (dark grey) and G. pyramidum (light grey) who established an infection. The dynamics is described from the day of inoculation (day 0) across 154 days of infection. The status and intensity of infection of the three species changed over time and, accordingly, was divided into four infection stages, through which host and pathogen parameters were evaluated. The infection stages included peak bacteremia and plateau, during which the bacteremia level in G. andersoni was steady (inside black box). The plateau stage was further divided into three periods according to the infection dynamics of the other two host species, namely the first reduction, in which bacteremia levels declined in both hosts, the second reduction, in which bacteremia levels in G. pyramidum tended to zero and they began to lose the infection and the third reduction, in which the pathogen cells were undetected in all G. pyramidum and steadily low or absent in G. gerbillus hosts. To highlight the differences between species during the plateau stage, the dynamic is zoomed in (right side). *On day 154, one G. gerbillus host showed an extreme pathogen load, following sequential undetectable levels, and thus was identified as a statistical outlier (Grubb's test: p = 7 × 10−12) and was removed from figure 1.
To assess the efficiency of each host species to resist a secondary infection, on day 158, we re-inoculated those hosts that had shown no evidence of Mycoplasma in their blood towards the end of the experiment and checked whether they became infected during an additional 127 days.
(c) . Pathogen inoculation and quantification
Mycoplasma haemomuris-like bacteria are uncultivable, and thus, hosts were subcutaneously inoculated with blood from Mycoplasma-positive G. andersoni, preserved in 20% DMSO (Sigma-Aldrich, Buchs, Switzerland) and stored at −80°C, following Cohen et al. [32]. The inoculum was diluted with PBS to reach a final concentration of 1 × 105 bacterial cells (confirmed by qPCR) and a total volume of 200 µl. This concentration was chosen since it lies within the natural range of infected rodents (102–106 cells per µl of blood [32]) and allows a significant infection success in the three host species while minimizing the amount of blood volume required for the inoculation (45 ± 8 µl of donor blood; M.G. et al. 2017, unpublished data). Mycoplasma-negative blood was added to the PBS in similar amounts for the inoculation of control hosts (36 ± 18 µl of blood in the 200 µl inoculum volume). To control for potential differences in blood donations, blood was allocated to individuals using a block design, in which each donation was used to simultaneously inoculate one or two host individuals from each species.
The pathogen infection dynamics in the three-host species was assessed following Cohen et al. [32]. Briefly, in every bleeding event, 50 µl of blood was collected under local anaesthesia (Localin drops, Fischer Pharmaceutical LTD) from the retro-orbital sinus of each individual and stored at –20°C until further molecular analyses. Then, DNA was extracted from the blood samples, using a MoBio Bacteremia DNA Isolation Kit, according to the manufacturer's instructions. In each extraction session, a negative control was included, in which all of the reagents were added to the PBS to replace the extracted blood.
Quantification of pathogen concentration was performed by a real-time PCR (Applied Biosystems 7300, Waltham, Massachusetts, USA), following Eidelman et al. [35]. To avoid overestimation of absolute numbers by plasmid standards, the standard curve was calibrated by Double Droplet PCR (ddPCR), which allows a direct count of the nucleic acid molecules.
(d) . Behavioural and physiological measurements of the three host species
Within each infection stage, the sampling days were chosen to minimize the interference between measurements. Accordingly, the behavioural measurements were always recorded at least 4 days after bleeding and before fecal collection, while excluding the behavioural records during feeding and temperature measurements (considering an extra buffer of 0.5 h). Likewise, fecal collection was conducted at least 2 days post-bleeding, and the temperature measurements were recorded at least 4 days post-bleeding.
(i) . Behavioural measurements
Movement parameters of infected and control rodents were measured throughout the infection period to test the assumption underlying the general mechanism postulating that infection increases the mobility of G. andersoni hosts, but not that of hosts from the other two species, and as such, may amplify the pathogen spread among hosts. In nature, the pathogen spreading potential may depend on either the activity (e.g. foraging) frequency, duration or both [37–40]. Accordingly, we have separated between the movement frequency and duration observed in the laboratory that may be respectively correlated with these behavioural aspects.
A motion detector (model Swan Quad; Crow Group, Airport City, Israel) was placed 40 cm above each host cage. During the activity hours (dark), it continuously recorded the host movements (every 20 s, the activity was either coded as ‘1’ or ‘0’, for movement or no movement, respectively) in data loggers. Data were then extracted, and two parameters were calculated: (i) the movement frequency, as the number of movement bouts; and (ii) the movement duration, as the mean time of a movement bout. Parameters were calculated separately for each infection stage.
(ii) . Physiological measurements
The physiological parameters of infected and control rodents were measured throughout the infection period to test the assumption underlying the ‘infected host mortality’ mechanism. Since it is not practical to measure mortality in wild mammals under laboratory conditions, we used those parameters that have been established as proxies for mortality probability and were most relevant to our model system. In particular, we quantified the rodents' packed RBC volume, body mass and body temperature, as Mycoplasma pathogens often cause severe haemolytic anaemia and body mass loss, and induce fever, which can lead, in the long run, to the death of their hosts [41,42]. Similarly, we quantified fecal corticosterone metabolites (FCM) since we have indications that Mycoplasma infections elevate the physiological stress levels in wild gerbils [35], which may reduce their longevity in nature [43]. Finally, considering the cumulative evidence relating energy expenses with survival (e.g. [44,45]), we quantified the effect of Mycoplasma on the rodents’ oxygen consumption. Methodological details on the measured packed RBC volume, body mass, surface body temperature, FCM and oxygen consumption are provided in the electronic supplementary material.
(e) . Statistical analysis
(i) . Pathogen dynamics
To quantify the differences in pathogen dynamics between the infected individuals of the three host species (independent variable), we performed generalized linear and generalized linear mixed model analyses (GLM and GLMM, respectively). The dependent variables were (i) the overall probability of a rodent to serve as an infective host (whether each individual was Mycoplasma-positive during each infection stage; GLMM with a binomial distribution and individual host as a random factor), (ii) the infection length (number of days between first and last days of infection; Gaussian distribution), (iii) the infection success (whether a Mycoplasma-inoculated individual became infected; binomial distribution), (iv) the overall intensity of infection (log-transformed mean Mycoplasma load per infected individual host throughout the experiment; Gaussian distribution), (v) clearance probability (whether each infected host was negative for Mycoplasma during at least five consecutive bleeding events; binomial distribution) and (vi) re-infection probability (whether an individual that had cleared the infection developed a new infection in response to the repeated inoculation; binomial distribution).
(ii) . Host behavioural and physiological changes
To quantify the effects of pathogen infection, the host species and the species × treatment interaction (independent variable) on the host behavioural and physiological responses, we performed Gaussian GLM analyses. The dependent variables were the proportional change in the measured variables between each post-inoculation and pre-inoculation stage. When significant, we ran specific planned comparisons of the least square means between the infected and control hosts of each species.
To exclude the possibility that the blood injection itself generated the differences between the Mycoplasma-inoculated and PBS-inoculated groups, for each significant difference found between these groups, we ran parallel statistical comparisons between the two types of control groups.
All statistical analyses were conducted in R software, v. 4.0.0 [46] using the car, lme4 and lsmeans packages.
3. Results
(a) . General results
From the 70 individuals that were used for the experiment, 35 were inoculated with Mycoplasma-infected blood, 16 with PBS and 19 with Mycoplasma-negative blood.
All of the individuals that were inoculated with Mycoplasma (12 G. andersoni, 12 G. gerbillus and 11 G. pyramidum) developed an infection, except one G. andersoni and one G. gerbillus. Both of these uninfected individuals were inoculated with a blood donation that had successfully infected other individuals. Thus, we included these two individuals and their paired siblings only in the analysis of infection success. Accordingly, all 35 Mycoplasma-inoculated individuals were used for the infection success analysis, and 11 infected individuals from each species were used for the analyses of the probability to serve as an infective host and of intensity of infection.
To evaluate whether the hosts had cleared the infection by the end of the experiment, clearance was determined when an individual received five sequential qPCR negative results. This was a conservative assumption that was based on observations in three individuals, in which the Mycoplasma load increased after three to four sequential sampling events with values below the qPCR sensitivity. Due to practical problems, we missed the last sampling point (on day 154) of two G. pyramidum individuals, and therefore could only determine the infection length and clearance status for 11 G. andersoni, 11 G. gerbillus and 9 G. pyramidum hosts. Following the above clearance definition, no G. andersoni cleared the infection, whereas seven G. gerbillus and all nine G. pyramidum cleared the infection. These 16 individuals that cleared the infection were used to determine the re-infection probability of G. gerbillus and G. pyramidum.
For the analyses of the host behavioural and physiological responses, two additional G. andersoni that became infected and their paired control siblings were excluded due to their injury during the experiment. Accordingly, we compared the 9 G. andersoni, 11 G. gerbillus and 11 G. pyramidum that were successfully inoculated with their sibling controls (PBS-inoculated: five G. andersoni, four G. gerbillus and five G. pyramidum; blood-inoculated: four G. andersoni, seven G. gerbillus and six G. pyramidum).
The remaining results are provided with respect to the tested assumptions (table 1; electronic supplementary material, table S1; figure 2).
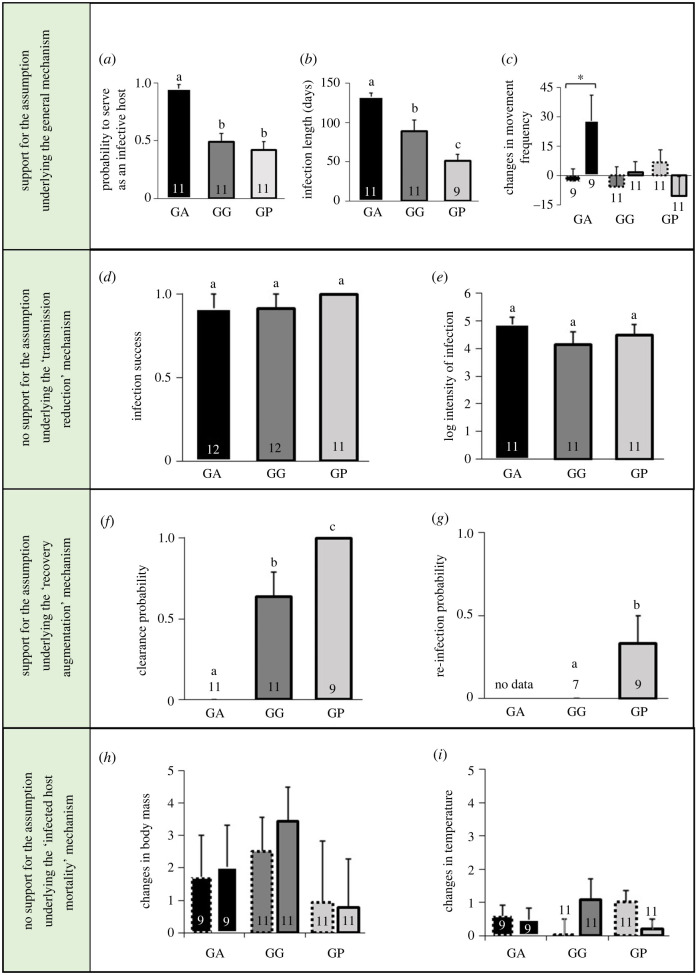
Figure 2.
Results supporting or not supporting the assumptions underlying potential diversity–disease mechanisms. Means and standard errors of the various variables measured in the three host species. These variables were used to test the assumptions underlying the general mechanism of one amplifier and two diluter species (a–c), the ‘transmission reduction’ (d,e), ‘recovery augmentation’ (f,g) and ‘infected host mortality’ (h,i) mechanisms. (a) The overall probability of each host species to serve as an infective host, which was calculated throughout the experiment following the first infection. (b) The infection length of each host species, which was calculated from the first to the last days of confirmed infection. (c) Changes in the movement frequency (number of movement bouts in 12 h) of infected (columns with a continuous frame) and control (columns with a dashed frame) individuals of the three species, between the stages of the first reduction and pre-inoculation. The changes were standardized for each individual by dividing the differences by the pre-inoculation values. (d) The probability of a host from each species to become infected by the first inoculation. (e) The log-transformed intensity of infection per infected individual of each host species (cells per µl of blood) throughout the experiment. (f) The probability of a host from each species, who became infected, to clear the infection. (g) The probability of a host from each species, who had cleared the infection, to become infected by a repeated inoculation. (h,i) Changes in the body mass (grams; h) and surface body temperature (°C; i) of infected (columns with a continuous frame) and control (columns with a dashed frame) individuals of the three species, between the stages of the first reduction and pre-inoculation. The changes were standardized for each individual by dividing the differences by the pre-inoculation values. Numbers in columns represent the sample sizes. Different letters above columns and asterisk indicate significant planned comparisons.
(b) . Support for the assumptions underlying the general mechanism
As expected by the field pattern [31], the pathogen dynamics in G. andersoni suggests that this host species is its amplifier. In particular, G. andersoni hosts (hereafter designated as an amplifier host) were more likely to serve as infective hosts (, p = 1 × 10−4; figure 2a) and to maintain a longer and more persistent infection once infected (F2,28 = 16, p = 2 × 10−5; figures 1 and 2b), compared to the other two host species (hereafter designated as diluters). In addition, consistent with the underlying assumption, there was a significant increase in the movement frequency of infected amplifier hosts at the first reduction stage, whereas no similar behavioural responses were detected in the two diluter hosts (F2,56 = 4, p = 0.04; table 1 and figure 2c). Infected G. andersoni showed the same, though non-significant, tendency at the periods of peak infection and the second reduction stage (F2,56 = 2, p = 0.1 and F2,56 = 2, p = 0.2, for the two periods, respectively). The increase in the amplifier host's movement frequency can be attributed to Mycoplasma infection rather than as a side effect of blood inoculation, since there were no significant differences in the behaviour of the two types of control rodents (F1,7 = 0.04, p = 0.9 at the first reduction stage). There was no significant species × treatment interaction effect on the movement duration (peak: F2,56 = 2, p = 0.2; first reduction: F2,56 = 1, p = 0.3; second reduction: F2,54 = 3, p = 0.08; and third reduction: F2,22 = 1, p = 0.3).
(c) . No support for the assumption underlying the ‘transmission reduction’ mechanism
In contrast with our expectations and despite the inoculation concentration that lay within the natural infection range, there were no significant differences between the three-host species in the infection success (, p = 0.5; figure 2d) or in the overall intensity of infection of the infected hosts throughout the experiment (F2,30 = 1, p = 0.4; figure 2e). Note that on day 154, one G. gerbillus host showed an extreme pathogen load, following sequential undetectable levels, and thus was identified as a statistical outlier (Grubb's test: p = 7 × 10−12) and was removed from the intensity of infection analysis. However, even after its exclusion, there were no significant differences in the overall intensity of infection between the three host species.
(d) . Support for the assumption underlying the ‘recovery augmentation’ mechanism
As expected, the pathogen clearance probabilities of the two diluter species were significantly higher than that of the amplifier host (, p = 6 × 10−7), with no amplifier hosts clearing the infection, compared to seven G. gerbillus and all G. pyramidum hosts who lost the pathogen (figures 1 and 2f). Importantly, from the diluter hosts who cleared the infection, 13 out of 16 individuals resisted the second inoculation. Yet there was a significant difference between the diluter hosts; while no G. gerbillus were re-infected, three G. pyramidum acquired a second infection (, p = 0.05 for the comparison between the two diluter species; figure 2g).
(e) . No support for the assumption underlying the ‘infected host mortality’ mechanism
Contrary to our expectations, there were no significant effects for any of the physiological parameters correlated with the host mortality probability (0.1 < F2,56 < 2.6, 1 > p > 0.09, for all tests; figure 2h,i; electronic supplementary material, table S1).
4. Discussion
The time is now ripe to improve our mechanistic understanding of diversity–disease relationships [3,4,30]. By simulating multiple host systems in mesocosms, recent studies deciphered some of the causal effects underlying diversity–disease relationships that emerge from species interactions [14,27–29]. Here, we complemented this ‘whole system’ approach by adopting a reductionist approach. Accordingly, we partitioned a three-host–single-pathogen model system into its single-host–single-pathogen components and studied each of them in isolation. To reduce the theoretical–experimental gaps, we implemented this approach by extending traditional designs. In particular, we quantified multiple aspects of host–pathogen interactions to reduce the response metric limitations and assessed the likelihood that three alternative mechanisms operate in our model system, avoiding the single hypothesis bias. As detailed below, our results suggest that an extended view of host variation generated insights that could not have been revealed through simpler designs. They also highlight the gained understandings, projected from the dynamics of single-host–single-pathogen components into observed diversity–disease relationships.
(a) . Extending the response metrics is important for disentangling the likelihood that alternative mechanisms underlie diversity–disease relationships
The exploration of various aspects of the interaction (i.e. spreading, transmission, and recovery potentials, and host vulnerability to infection) between the pathogen and each of the potential hosts revealed four insights that could not have been attained by a narrower view. First, we confirmed that both G. gerbillus and G. pyramidum are potential hosts for Mycoplasma, despite the low rates of infected G. pyramidum individuals, and the absence of infected G. gerbillus individuals, in natural communities [31]. This was demonstrated by the significant infection success of individuals from all species who were inoculated with a total of 105 cells, a value within the natural range of infected hosts (102–106 cells per µl of blood [32]).
Second, our experimental results confirm the role of G. andersoni as a pathogen amplifier host and the role of the two other species as pathogen diluters. We found that infection increased the movement frequency of G. andersoni, which in nature may amplify the pathogen spread among hosts. The two other species were able to acquire the pathogen but maintained shorter infections, relative to the amplifier host. This distinction between amplifier and diluter hosts allowed disentangling the likelihood that three potential mechanisms underlie the observed diversity–disease relationships (table 1).
Third, based on the vulnerability assessments that were conducted for the three species, we showed that it is unlikely that the ‘infected host mortality’ mechanism, which was a plausible mechanism, based on our field observations and evidence from other disease systems [28,47] exists in our system. As the assessment of wild rodent mortality under laboratory conditions is not practical, the broad physiological response metrics, including health-related (packed blood volume, body mass and surface body temperature), hormonal (stress-related hormones), and energetic (oxygen consumption) parameters, across the different infection stages, were essential to support this conclusion.
Fourth, neither standard response metric, namely, infection success or intensity, showed any differences between host species, whereas the extended approach reflected core differences in long-term pathogen persistence. In particular, the pathogen was indefinitely able to persist in its amplifier host. By contrast, it was not able to persist in the diluter hosts, owing to their high clearance rates, predominantly in G. pyramidum, and the low re-infection success rates, which became a permanent pathogen loss, mainly in G. gerbillus hosts.
Thus, our study, as well as others [20,30,48,49], emphasizes the range of advantages that can be gained by considering multiple aspects of host–pathogen interactions in diversity–disease research.
(b) . Avoiding single hypothesis bias is essential to obtain a complete understanding of diversity–disease relationships
To date, there has been a collection of evidence from diverse disease systems and host species, including invertebrates, amphibians, birds and mammals, supporting each of the five mechanisms offered by Keesing et al. [13] to explain diversity–disease relationships, namely ‘encounter reduction’ and ‘transmission reduction’ [12,27,50], ‘recovery augmentation’ [51], ‘susceptible host regulation’ [12,14], and ‘infected host mortality’ [47]. However, the tendency of experimental studies to focus on a single mechanism precludes a definitive inference about the relative importance of these mechanisms in observed diversity–disease relationships [12]. Based on the amplifier–diluter distinction, we were able to disentangle the likelihood that three out of the five offered mechanisms operate in our model system, and we collected evidence suggesting that the occurrence of each of the diluter species may lead to ‘recovery augmentation’ but probably not to ‘transmission reduction’ or ‘infected host mortality.’
(c) . From single host-single pathogen to diversity–disease relationships
The reductionist view assumes that the behaviour of a system is equal to the sum of its parts [52]. However, in the case of complex systems, it may lead to incomplete, sometimes misleading, perspectives on natural communities due to overlooking higher level interactions, indirect effects and the natural context [52]. For example, by isolating the host species from each other, we might have obscured additional effects that are caused by direct or indirect (i.e. through pathogen response) interspecific competition between the host species (e.g. [14,28]). These effects could have changed the infection success and intensity, clearance probability and recovery probability of the pathogen in the hosts, as well as the reciprocal host behavioural and physiological responses to the pathogen, potentially proposing alternative dilution mechanisms.
Nevertheless, our study shows that despite these disadvantages and limited information, a detailed exploration of single-host–single-pathogen components may complement the field patterns and may sometimes provide an initial assessment of the processes operating in nature. In particular, in the field, we described a reduction in amplifier host densities and an increase in diluter host densities in the more diverse communities [31]. This supports the occurrence of the ‘susceptible host regulation’ mechanism. However, the field patterns suggested that the observed dilution cannot be driven solely by this mechanism as we did not find remarkable differences between the numbers of susceptible amplifiers in single host species and three host species communities (H.H. et al. 2011, unpublished data). Our experimental results support a co-occurring mechanism through which the increase in diluter host densities may lead to the dilution of Mycoplasma. This increase may reduce the pathogen population by enhancing pathogen clearance and resistance rates.
If indeed Mycoplasma is diluted, at least partially, through the ‘recovery augmentation’ mechanism, but not through the ‘infected host mortality’ mechanism, it should be easier to control the pathogen. One way to do this would be by introducing grazers to the system. The grazers would be expected to damage the soil crust, lessening the sand stabilization and modifying the habitat to be more suitable to the diluter species [53,54]. Considering that the ‘recovery augmentation’ mechanism underlies the dilution effect, the resulting increase in diluter densities would be expected to dilute the pathogen without damaging any of the host species. If alternatively, the ‘infected host mortality’ mechanism operated, then the same management decision would imply extensive mortality of the diluters—both are locally endangered species—which would make this management strategy ineffective.
5. Conclusion
By integrating information on three host species' differences in spreading, transmission and recovery potentials, and in vulnerability to a shared pathogen, this experimental study provides the initial step towards a functional connection between host diversity and disease in a natural system. The application of a reductionist (i.e. considering the simplest study units), yet extended (i.e. considering multiple hosts, processes, response metrics, and hypotheses), approach highlighted the importance of the diluter species behind the diversity–disease scenes. Now that we have characterized the amplifier and diluter hosts, and rejected two unlikely mechanisms, the next steps would be: to (i) test the assumptions underlying the ‘encounter reduction’ and the ‘susceptible host regulation’ mechanisms and assess within- and between-species contact rates through long-term community observations and sampling in the field; and (ii) to make connections between the results revealed in this study and community dynamics (e.g. transmission) in the field through matrices of community competence (a density-weighted competence; [23]) and contact networks (reviewed in [38,55]) for each community type, and theoretical modelling (e.g. [12,14,56].
More broadly, our study emphasizes the value of identifying the relative importance of the various components of host–pathogen interactions for a better mechanistic understanding of how species characteristics shape diversity–disease relationships. Accordingly, we encourage future extended experiments in other disease systems and the integration between diversity–disease models, which rank each host species as competent or not, and estimate the basic reproduction number (R0) for the former (e.g. [16,56,57]), and ‘competency’ models, which estimate the within-host R0 in more detail (e.g. [20,30,48,49]. Such an extended view will bridge the theoretical–experimental gaps in diversity–disease research (see [14] for an example).
Supplementary Material
Acknowledgements
We thank N. Kronfeld-Schor, N. Shahar, M. Einav, T. Foerster, Y. Shani, O. Altstein, A. Eidelman and Y. Romach for valuable help during this study.
Data accessibility
The data and statistical codes used to conduct all analyses reported in this manuscript are available through the Figshare digital repository [58].
Authors' Contributions
M.G.: conceptualization, formal analysis, investigation, methodology, validation, visualization and writing-original draft; S.H.: conceptualization, formal analysis, investigation, methodology, writing-review and editing; R.F.: investigation, methodology and validation; C.C.: investigation and methodology; Á.N.-C.: investigation, methodology, validation, writing-review and editing; I.B.: investigation, methodology, resources, supervision, validation, writing-review and editing; H.H.: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, visualization and writing-original draft
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Israel Science Foundation (no. 1391/15 to HH) and by the United States-Israel Binational Science Foundation (no. 2012063 to H.H. (PI), Clay, Fuqua, and Dong).
References
- 1.Ceballos G, Ehrlich PR, Dirzo R. 2017. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl Acad. Sci. USA 114, E6089-E6096. ( 10.1073/pnas.1704949114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990-993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halliday FW, Rohr JR. 2019. Measuring the shape of the biodiversity-disease relationship across systems reveals new findings and key gaps. Nat. Commun. 10, 5032. ( 10.1038/s41467-019-13049-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halsey S. 2019. Defuse the dilution effect debate. Nat. Ecol. Evol. 3, 145-146. ( 10.1038/s41559-018-0764-3) [DOI] [PubMed] [Google Scholar]
- 5.Rohr JR, Civitello DJ, Halliday FW, Hudson PJ, Lafferty KD, Wood CL, Mordecai EA. 2019. Towards common ground in the biodiversity–disease debate. Nat. Ecol. Evol. 4, 24-33. ( 10.1038/s41559-019-1060-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Civitello DJ, et al. 2015. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl Acad. Sci. USA 112, 8667-8671. ( 10.1073/pnas.1506279112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnusson M, Fischhoff IR, Ecke F, Hörnfeldt B, Ostfeld RS. 2020. Effect of spatial scale and latitude on diversity–disease relationships. Ecology 101, e02955. ( 10.1002/ecy.2955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson PTJ, Ostfeld RS, Keesing F. 2015. Frontiers in research on biodiversity and disease. Ecol. Lett. 18, 1119-1133. ( 10.1111/ele.12479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood CL, McInturff A, Young HS, Kim D, Lafferty KD. 2017. Human infectious disease burdens decrease with urbanization but not with biodiversity. Phil. Trans. R. Soc. B 372, 20160122. ( 10.1098/rstb.2016.0122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hechinger RF, Lafferty KD. 2005. Host diversity begets parasite diversity: bird final hosts and trematodes in snail intermediate hosts. Proc. R. Soc. B 272, 1059-1066. ( 10.1098/rspb.2005.3070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood CL, Lafferty KD, DeLeo G, Young HS, Hudson PJ, Kuris AM. 2014. Does biodiversity protect humans against infectious disease? Ecology 95, 817-832. ( 10.1890/13-1041.1) [DOI] [PubMed] [Google Scholar]
- 12.Luis AD, Kuenzi AJ, Mills JN. 2018. Species diversity concurrently dilutes and amplifies transmission in a zoonotic host–pathogen system through competing mechanisms. Proc. Natl Acad. Sci. USA 115, 7979-7984. ( 10.1073/pnas.1807106115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keesing F, Holt RD, Ostfeld RS. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9, 485-498. ( 10.1111/j.1461-0248.2006.00885.x) [DOI] [PubMed] [Google Scholar]
- 14.Strauss AT, Civitello DJ, Caceres CE, Hall SR. 2015. Success, failure and ambiguity of the dilution effect among competitors. Ecol. Lett. 18, 916-926. ( 10.1111/ele.12468) [DOI] [PubMed] [Google Scholar]
- 15.Begon M. 2008. Effects of host diversity on disease dynamics. In Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems (eds Ostfeld RS, Keesing F, Eviner VT), pp. 12-29. Princeton, NJ: Princeton University Press. [Google Scholar]
- 16.Roberts MG, Heesterbeek JAP. 2018. Quantifying the dilution effect for models in ecological epidemiology. J. R. Soc. Interface 15, 20170791. ( 10.1098/rsif.2017.0791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker CG, Rodriguez D, Toledo LF, Longo AV, Lambertini C, Corrêa DT, Leite DS, Haddad CFB, Zamudio KR. 2014. Partitioning the net effect of host diversity on an emerging amphibian pathogen. Proc. R. Soc. B 281, 20141796. ( 10.1098/rspb.2014.1796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faust CL, Dobson AP, Gottdenker N, Bloomfield LSP, McCallum HI, Gillespie TR, Diuk-Wasser M, Plowright RK. 2017. Null expectations for disease dynamics in shrinking habitat: dilution or amplification? Phil. Trans. R. Soc. B 372, 20160173. ( 10.1098/rstb.2016.0173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Searle CL, et al. 2016. Population density, not host competence, drives patterns of disease in an invaded community. Am. Nat. 188, 554-566. ( 10.1086/688402) [DOI] [PubMed] [Google Scholar]
- 20.Merrill TE S, Johnson PTJ. 2020. Towards a mechanistic understanding of competence: a missing link in diversity–disease research. Parasitology 147, 1159-1170. ( 10.1017/s0031182020000943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall SR, Becker CR, Simonis JL, Duffy MA, Tessier AJ, Cáceres CE. 2009. Friendly competition: evidence for a dilution effect among competitors in a planktonic host–parasite system. Ecology 90, 791-801. ( 10.1890/08-0838.1) [DOI] [PubMed] [Google Scholar]
- 22.Hersh MH, Tibbetts M, Strauss M, Ostfeld RS, Keesing F. 2012. Reservoir competence of wildlife host species for Babesia microti. Emerg. Infect. Dis. 18, 1951-1957. ( 10.3201/eid1812.111392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson PTJ, Preston DL, Hoverman JT, Richgels KL. 2013. Biodiversity decreases disease through predictable changes in host community competence. Nature 494, 230-233. ( 10.1038/nature11883) [DOI] [PubMed] [Google Scholar]
- 24.Keesing F, et al. 2012. Reservoir competence of vertebrate hosts for Anaplasma phagocytophilum. Emerg. Infect. Dis. 18, 2013-2016. ( 10.3201/eid1812.120919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsh ME, Cronin JP, Mitchell CE. 2020. Trait-based variation in host contribution to pathogen transmission across species and resource supplies. Ecology 101, e03164. ( 10.1002/ecy.3164) [DOI] [PubMed] [Google Scholar]
- 26.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. 2003. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 9, 311. ( 10.3201/eid0903.020628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venesky MD, Liu X, Sauer EL, Rohr JR. 2014. Linking manipulative experiments to field data to test the dilution effect. J. Anim. Ecol. 83, 557-565. ( 10.1111/1365-2656.12159) [DOI] [PubMed] [Google Scholar]
- 28.Rohr JR, Civitello DJ, Crumrine PW, Halstead NT, Miller AD, Schotthoefer AM, Stenoien C, Johnson LB, Beasley VR. 2015. Predator diversity, intraguild predation, and indirect effects drive parasite transmission. Proc. Natl Acad. Sci. USA 112, 3008-3013. ( 10.1073/pnas.1415971112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson PTJ, Calhoun DM, Riepe T, McDevitt-Galles T, Koprivnikar J. 2019. Community disassembly and disease: realistic-but not randomized-biodiversity losses enhance parasite transmission. Proc. R. Soc. B 286, 20190260. ( 10.1098/rspb.2019.0260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gervasi SS, Civitello DJ, Kilvitis HJ, Martin LB. 2015. The context of host competence: a role for plasticity in host–parasite dynamics. Trends Parasitol. 31, 419-425. ( 10.1016/j.pt.2015.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kedem H, Cohen C, Messika I, Einav M, Pilosof S, Hawlena H. 2014. Multiple effects of host-species diversity on coexisting host-specific and host-opportunistic microbes. Ecology 95, 1173-1183. ( 10.1890/13-0678.1) [DOI] [PubMed] [Google Scholar]
- 32.Cohen C, Shemesh M, Garrido M, Messika I, Einav M, Khokhlova I, Tasker S, Hawlena H. 2018. Haemoplasmas in wild rodents: routes of transmission and infection dynamics. Mol. Ecol. 27, 3714-3726. ( 10.1111/mec.14826) [DOI] [PubMed] [Google Scholar]
- 33.Ovadia O, Abramsky Z, Kotler B, Pinshow B. 2005. Inter-specific competitors reduce inter-gender competition in Negev Desert gerbils. Oecologia 142, 480-488. ( 10.1007/s00442-004-1726-9) [DOI] [PubMed] [Google Scholar]
- 34.Messika I, Garrido M, Kedem H, China V, Gavish Y, Dong QF, Fuqua C, Clay K, Hawlena H. 2017. From endosymbionts to host communities: factors determining the reproductive success of arthropod vectors. Oecologia 184, 859-871. ( 10.1007/s00442-017-3906-4) [DOI] [PubMed] [Google Scholar]
- 35.Eidelman A, et al. 2019. The dynamics between limited-term and lifelong coinfecting bacterial parasites in wild rodent hosts. J. Exp. Biol. 222, jeb203562. ( 10.1242/jeb.203562) [DOI] [PubMed] [Google Scholar]
- 36.Cohen C, Einav M, Hawlena H. 2015. Path analyses of cross-sectional and longitudinal data suggest that variability in natural communities of blood-associated parasites is derived from host characteristics and not interspecific interactions. Parasit. Vect. 8, 429. ( 10.1186/s13071-015-1029-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji W, White PCL, Clout MN. 2005. Contact rates between possums revealed by proximity data loggers. J. Appl. Ecol. 42, 595-604. ( 10.1111/j.1365-2664.2005.01026.x) [DOI] [Google Scholar]
- 38.White LA, Forester JD, Craft ME. 2017. Using contact networks to explore mechanisms of parasite transmission in wildlife. Biol. Rev. 92, 389-409. ( 10.1111/brv.12236) [DOI] [PubMed] [Google Scholar]
- 39.VanderWaal KL, Ezenwa VO. 2016. Heterogeneity in pathogen transmission: mechanisms and methodology. Funct. Ecol. 30, 1606-1622. ( 10.1111/1365-2435.12645) [DOI] [Google Scholar]
- 40.Clay CA, Lehmer EM, Previtali A, St. Jeor S, Dearing MD. 2009. Contact heterogeneity in deer mice: implications for Sin Nombre virus transmission. Proc. R. Soc. B 276, 1305-1312. ( 10.1098/rspb.2008.1693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barker E, Tasker S. 2013. Haemoplasmas: lessons learnt from cats. New Zeal. Vet. J. 61, 184-192. ( 10.1080/00480169.2013.771760) [DOI] [PubMed] [Google Scholar]
- 42.Messick JB. 2004. Hemotrophic mycoplasmas (hemoplasmas): a review and new insights into pathogenic potential. Vet. Clin. Pathol. 33, 2-13. ( 10.1111/j.1939-165X.2004.tb00342.x) [DOI] [PubMed] [Google Scholar]
- 43.Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R. 2011. Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166, 869-887. ( 10.1007/s00442-011-1943-y) [DOI] [PubMed] [Google Scholar]
- 44.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771-1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 45.Speakman JR. 2005. Body size, energy metabolism and lifespan. J. Exp. Biol. 208, 1717. ( 10.1242/jeb.01556) [DOI] [PubMed] [Google Scholar]
- 46.R Development Core Team. 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 47.Han BA, Kerby JL, Searle CL, Storfer A, Blaustein AR. 2015. Host species composition influences infection severity among amphibians in the absence of spillover transmission. Ecol. Evol. 5, 1432-1439. ( 10.1002/ece3.1385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin LB, Burgan SC, Adelman JS, Gervasi SS. 2016. Host competence: an organismal trait to integrate immunology and epidemiology. Integr. Comp. Biol. 56, 1225-1237. ( 10.1093/icb/icw064) [DOI] [PubMed] [Google Scholar]
- 49.Martin LB, et al. 2019. Extreme competence: keystone hosts of infections. Trends Ecol. Evol. 34, 303-314. (doi:10.1016%2Fj.tree.2018.12.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClure KM, Fleischer RC, Kilpatrick AM. 2020. The role of native and introduced birds in transmission of avian malaria in Hawaii. Ecology 101, e03038. ( 10.1002/ecy.3038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson PTJ, Hartson RB, Larson DJ, Sutherland DR. 2008. Diversity and disease: community structure drives parasite transmission and host fitness. Ecol. Lett. 11, 1017-1026. ( 10.1111/j.1461-0248.2008.01212.x) [DOI] [PubMed] [Google Scholar]
- 52.Schoener TW. 1986. Mechanistic approaches to community ecology: a new reductionism. Am. Zool. 26, 81-106. See https://www.jstor.org/stable/3883187 [Google Scholar]
- 53.Abramsky Z, Rosenzweig ML, Brand S. 1985. Habitat selection of Israel desert rodents: comparison of a traditional and a new method of analysis. Oikos 45, 79-88. ( 10.2307/3565225) [DOI] [Google Scholar]
- 54.Abramsky Z, Ovadia O, Rosenzweig ML. 1994. The shape of a Gerbillus pyramidum (Rodentia: Gerbillinae) isocline: an experimental field study. Oikos 69, 318-326. ( 10.2307/3546153) [DOI] [Google Scholar]
- 55.White LA, Forester JD, Craft ME. 2018. Dynamic, spatial models of parasite transmission in wildlife: their structure, applications and remaining challenges. J. Anim. Ecol. 87, 559-580. ( 10.1111/1365-2656.12761) [DOI] [PubMed] [Google Scholar]
- 56.Roche B, Dobson AP, Guégan JF, Rohani P. 2012. Linking community and disease ecology: the impact of biodiversity on pathogen transmission. Phil. Trans. R. Soc. B 367, 2807-2813. ( 10.1098/rstb.2011.0364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobson AP. 2004. Population dynamics of pathogens with multiple host species. Am. Nat. 164(S5), S64-S78. ( 10.1086/424681) [DOI] [PubMed] [Google Scholar]
- 58.Garrido M, Halle S, Flatau R, Cohen C, Navarro-Castilla A, Barja I, Hawlena H. 2021. Data and R codes from: Testing the underlying assumptions of dilution effect mechanisms through quantifying the long-term dynamics of a pathogen in multiple host species. Figshare. ( 10.6084/m9.figshare.13379084) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Garrido M, Halle S, Flatau R, Cohen C, Navarro-Castilla A, Barja I, Hawlena H. 2021. Data and R codes from: Testing the underlying assumptions of dilution effect mechanisms through quantifying the long-term dynamics of a pathogen in multiple host species. Figshare. ( 10.6084/m9.figshare.13379084) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data and statistical codes used to conduct all analyses reported in this manuscript are available through the Figshare digital repository [58].