Abstract
Cattle are one of the most intensively bred domestic animals, providing humans with a multitude of products and uses. Using data from the fossil record, we test if their domestication, as for other taxa, has resulted in a reduction of their brain size. We not only conclude that Bos taurus (domestic cattle) have smaller brains than their wild ancestor, Bos primigenius (aurochs), but that brain size varies significantly by breed, with some having much smaller brains than others. Differences in husbandry practices between several breed categories align with a range of human engagement, which also aligns with the degree of selection for docility. Sampling 317 domestics from 71 breeds, we investigate if differences in brain size correlate with the intensity of human contact. A clear pattern emerges whereby a brain reduction gradient parallels a gradient in behavioural selection. Bullfighting cattle, which are bred for fighting and aggressive temperament, have much larger brains than dairy breeds, which are intensively selected for docility. Our results add to a fundamental aspect of animal domestication theory: the interplay between basic features of the domestic environment—selection for docility, absence of predators and human provision of resources—seems to explain differences in brain size.
Keywords: allometry, phylogeny, behaviour, aurochs, encephalization, domestication
1. Introduction
Nearly all domestic animals have been shown to have smaller brains than their wild counterparts [1–7]. Those that are most important to humans, mostly for consumption or companionship, display the greatest amount of reduction. These include pigs (approx. 34%) [8] and sheep (approx. 24%) [2], and dogs (approx. 29%) [3] and cats (approx. 24%) [3], whose brains reduce more than twice as much as those of other domestics [6,9–12]. Quantifying these changes has significant implications for assessing differences in information-processing [13,14] and the speed and mode of brain evolution [15–18], particularly since different sensory systems are variably affected in different domestic taxa [7].
Cattle are globally one of the most populous and widespread livestock [19], and yet have not been examined for brain size change, due to the extinction of their wild form (Bos primigenius, ‘aurochs’) nearly 400 years ago [20]. In many ways, however, cattle are a prime model for studying domestication. Genomic advances have highlighted the difficulty in identifying the wild ancestors of domestic populations [21], but the aurochs has been confirmed as the wild progenitor of all taurine cattle (Bos taurus) [22]. Moreover, extinction has prevented introgression between the aurochs and domestic cattle for hundreds of years, which may mean a clearer distinction between wild and domestic populations, in contrast with other wild/domestic comparisons [23]. Like most domestics today, the state of cattle is far beyond ‘initial domestication’ [24]; they have been specifically bred for a variety of purposes and phenotypes [25]. Here, we capture this range of variation with a sample of 317 specimens across 71 breeds. Using an approach that circumvents the limitations of an extinct ancestor, we test for relative brain size change between wild and domestic forms.
(a) . Brain reduction in specialized breeds
Brain reduction in domestic taxa has been consistently linked to two pillars of domestication: selection for tameness and engagement with humans [24,26–30]. Anatomical studies support this, reporting that overall brain reduction is driven by the reduction of the limbic system, a composite of brain regions responsible for the processing of fear, reactivity and aggression [7,11,29]. Experimental studies also support a correlation between human engagement, behavioural selection and a reductive influence on the brain and/or cranium [9,30–33].
However, not all domestics are tame or equally selected for tameness, nor do they experience equal amounts of human exposure [25,34]. Among domestic cattle, the bullfighting breed (Andalusian Black) is specifically selected for aggressiveness, while other breeds have no human contact throughout most of their lives [25,35]. More so than other livestock, cattle breeds are specialized for a broad range of uses [36]. These specializations correspond to different husbandry practices which invariably correlate with different degrees of human contact and selection for docility. Consequently, cattle provide a model for testing whether the amount of brain reduction in domestics (if any) depends on the degree of docility or aggressiveness of different breeds.
Most specialized beef and dairy cattle are bred under tailored human care, yet both types differ greatly in the intensity and quality of human contact [37,38]. A few breeds are bred for sport, like the bullfighting cattle, which are intensively selected for aggressive temper and a tendency to fight [25]. The free-ranging park breeds, like the British White Park and Chillingham herds—referred to as ‘wild white beasts’—were originally enclosed for private sport or to decorate the landscape and are barely managed compared to other breeds [25,35,39]. Most breeds, however, are non-specialized and are used for a combination of dairy, meat or draught [25]. We sample this broad spectrum of domestic cattle types (71 breeds) and test if breeds less intensively selected for tameness or devoid of human contact have larger brains.
(b) . Brain reduction and phylogeny
Our sample of domestic cattle (n = 317) is broad enough to be classified into breed groups based on genealogical, morphological and geographic relatedness [40]. Thus, we also test if breed relatedness has an effect on brain size in cattle.
2. Material and methods
(a) . Wild versus domestic cattle
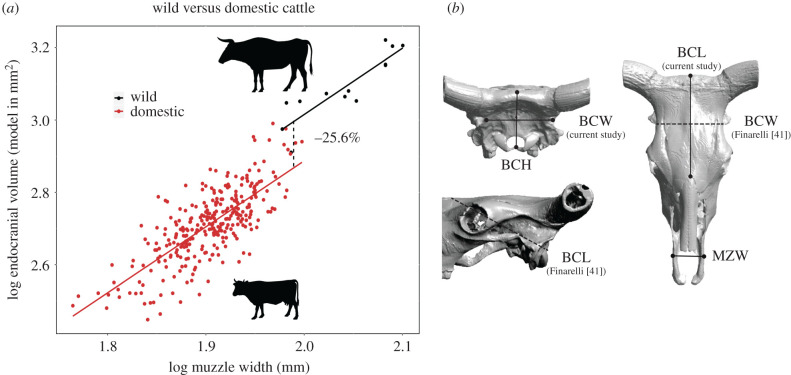
Skull measurements were taken from 13 Pleistocene adult aurochsen and 317 adult domestic taurine cattle (71, mostly European breeds) (electronic supplementary material, data D1). Measurements included braincase length, width, height and muzzle width (MZW) as in figure 1 (electronic supplementary material, data D2). Aurochsen data were taken with mechanical calipers (mm) by the first author. Domestic cattle data were taken from Veitschegger et al. [42] and converted to linear measurements. Equivalency of measurements between the two datasets was confirmed by first authors of both studies. Endocranial volume (EV) was used as a proxy for brain size, and estimated with the ‘Bovidae’ model of Finarelli [41]:
Figure 1.
(a) Relative brain size difference between wild and domestic cattle. Regression of EV estimate versus MZW; table 1 for statistics. (b) Skull measurements used for EV estimation in current study (bold) and in Finarelli [41]: BCL = braincase length and BCW = braincase width. BCH = braincase length was omitted from analyses as it is a poor estimator of EV in bovids (Finarelli [41]). MZW = muzzle width, was used for allometric size correction. Silhouettes credited to: DFoidl (modified by T. Michael Keesey) (aurochs), https://creativecommons.org/licenses/by-sa/3.0/; and Steven Traver (dairy). (Online version in colour.)
In order to maintain a uniform dataset, our cranial measurements were necessarily different from those of Finarelli [41], as depicted in figure 1b; electronic supplementary material, data D2. Greater precision would be achieved by matching Finarelli's measurements. However, when estimating EV as the geometric mean of cranial dimensions, reduction results are very similar to those when using the Finarelli model (electronic supplementary material, data D3).
For the estimation of relative brain size differences between wild and domestic cattle, EV was regressed against MZW in log-log space, thus simulating brain-to-body size allometry in both samples [43,44]. The slopes of the allometric lines were compared with an interactive ANCOVA. If allometry did not differ, a non-interactive ANCOVA was performed to test for intercept differences, which were then interpreted as differences in encephalization [7,8]. P-values were considered significant at values below 0.05. P-values corrected for multi-test comparisons using the Benjamini–Hochberg (BH) method are also provided [45]. Calculations for all analyses were made in R Studio and conducted in R [46] using the packages ‘dplyr’ (v. 0.8.4) [47], ‘MASS’ (v. 7.3-49) [48] and ‘ggplot2’ (v. 3.2.1) [49].
MZW (figure 1) has been used previously to estimate body size in ungulates [50]. It was chosen here as a proxy for body size due to its preservation in many of the aurochsen skulls sampled, and for being separate from the cranial module. We found no indication of MZW being a selected feature in any particular breed [25,51], only that soft tissue patterns on the snout can be used like fingerprints for individual identifications [52]. Rostral shortening has been reported in many domestics [53–55], but appears more correlated with morphological disparity across breeds than between wild/domestic populations [56], and it has not been reported in domestic cattle [42]). A test of the correlation between MZW and mean breed body masses is detailed in the electronic supplementary material, data D4.
Even though there is little evidence to suggest this [57–59], one could suspect that selection for a high food intake capacity in beef and dairy cattle could have led to particularly wide muzzles. To test this potentially confounding factor, we compared MZW against posterior tooth row length (PTL), which is a more typical body size proxy, in those specimens that presented both variables. If, in this comparison, those species with presumably the smallest brains in the brain-muzzle-width comparison would have the highest MZW for a given body size (=PTL), then this paper's main finding would be compromised. Therefore, domestic cattle breed MZW was regressed against PTL to test for differences in MZW between breeds.
(b) . Brain reduction in specialized breeds
Domestic specimens were classified into four breed types according to their distinct specializations: dairy production (n = 41), beef production (n = 68), bullfighting (n = 7) and park cattle (n = 4) [25,35,39]. Brain-to-body size allometry was compared for these four groups against wild cattle, using the same method as for wild versus domestic cattle [7,8]. Non-specialized, i.e. multi-purpose breeds (n = 120), were compared in a supplementary analysis (electronic supplementary material, data D5).
(c) . Brain reduction and phylogeny
Domestic specimens were categorized into six phylogenetic clusters, as per Felius et al. [40]. This classification system incorporates continental origin, geographic distribution, breed history and morphological criteria [40,51]. Geographic data appear to be most influential in determining breed relationships—a theory that is supported by molecular evidence [40]. A subset of our data (n = 293, from 60 breeds) could be categorized into the six breed clusters: (i) Northern Polled Celtic (n = 40), (ii) North-Western Lowland (n = 63), (iii) Western-Central Highland (n = 130), (iv) Highland Solid-coloured (n = 26), (v) Iberian (n = 14) and (vi) Podolian (n = 7) [40] (electronic supplementary material, data D1). The bullfighting cattle belonged to the Iberian cluster, and park cattle to the Northern Polled Celtic. Brain-to-body size allometry was compared for these six groups and wild cattle, using the same method as for wild versus domestic cattle [7,8].
3. Results
(a) . Wild versus domestic cattle
Domestic cattle have 25.6% smaller brains than wild cattle, according to regressions of EV versus MZW (figure 1). The slope (allometry) of their regressions is similar (ANCOVA, p = 0.982), but their intercepts differ significantly (ANCOVA, p < 0.001) (table 1). When EV is estimated with the geometric mean of cranial dimensions, the reduction estimate is similar: 22.0% (electronic supplementary material, data D3).
Table 1.
ANCOVA statistics for regression of EV versus MZW for wild versus domestic cattle. Logged data. Adj. R2 = 0.73, F-statistic = 437, DF2,314, p-value < 0.001. CI = 95% confidence interval. Interactive ANCOVA (slope difference) p-value = 0.982. BH = Benjamini–Hochberg multi-test correction.
| intercept coefficient | CI | s.e. | t-value | p-value | pairwise Wilcoxon (BH) test p-value | brain reduction | |
|---|---|---|---|---|---|---|---|
| wild intercept | −0.6317 | −0.9890 to −0.2745 | 0.181 | −3.48 | <0.001 | <0.001 | — |
| intercept difference | −0.1282 | −0.1710 to −0.0854 | 0.022 | −5.89 | <0.001 | <0.001 | 25.6% |
(b) . Brain reduction in specialized breeds
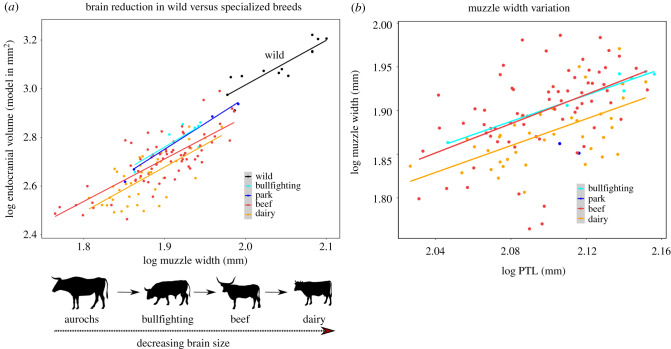
Relative brain size varies widely among domestic cattle (figure 1). This variation correlates with the primary use, or selection pressure, of specialized breeds (figure 2a; electronic supplementary material, data D6). Confidence intervals of several regressions overlap to different degrees, but mean encephalizations (y-intercepts) depict a clear pattern of differential reduction by breed type. Dairy and beef cattle exhibit the most distinct reductions in EV compared to wild cattle, at 30.6% and 24.9% reduction, respectively. Both differences are highly significant (ANCOVA, p < 0.001), even after multi-test correction (electronic supplementary material, data D6). The difference between beef and dairy breeds is also significant (ANCOVA, p = 0.010).
Figure 2.
(a) Brain size variation between breeds; table 2 in electronic supplementary material, data D6 for statistics. (b) MZW variation between breeds, measured against overall size (PTL = posterior tooth row length); table 3 in electronic supplementary material, data D6 for statistics. Silhouettes credited to: DFoidl (modified by T. Michael Keesey) (aurochs), https://creativecommons.org/licenses/by-sa/3.0/; Steven Traver (dairy); Tracy Heath (beef); and Mariana Ruiz Villarreal (bullfighting). (Online version in colour.)
MZW does not seem to increase, in relation to body mass, from bullfighting cattle to dairy cattle—on the contrary, dairy cattle have significantly smaller relative MZWs compared to bullfighting cattle (p = 0.028) (electronic supplementary material, data D5). The finding of small brains per MZW in dairy thus cannot be explained by dairy cattle having particularly wide muzzles (figure 2b). In fact, this means the dairy brain reduction estimate is likely an underestimation. The finding of larger brains in bullfighting cattle also cannot be explained by them having smaller muzzles than other breed types (figure 2b). Brain reduction in bullfighting cattle is half that observed in dairy breeds: 15.3% (electronic supplementary material, data D6). The results for park cattle (n = 4) we report with caution due to their smaller sample size, but they display similar encephalization to that of bullfighting cattle, with brain reduction of 18.2% relative to the aurochs. ANCOVA tests indicate these reduction magnitudes are borderline significant: bullfighting cattle p = 0.038, park cattle p = 0.035. These values become significant after multi-test correction (electronic supplementary material, data D6).
Non-specialized breeds lie in an intermediate space between dairy and beef breeds (electronic supplementary material, data D6). Among these, we looked closely at two unique breeds: Heck cattle (n = 3), which were initially bred to resemble the aurochs [60], and Hérens ‘Swissfighting’ cattle (n = 3), which often engage in female-to-female competitive fighting [61,62]. They show no outstanding pattern (electronic supplementary material, data D6).
(c) . Brain reduction and phylogeny
When categorized phylogenetically, all breed clusters display significant brain reduction compared to wild cattle (electronic supplementary material, data D9). The domestic sample size (n = 280) here is more than double that in the ‘wild versus specialized breeds' analysis (n = 120). Therefore, we compare the results of both analyses considering that their statistics are not directly comparable.
Cattle in the Iberian cluster (n = 14) have the highest relative brain size compared to all other clusters (electronic supplementary material, data D7). There are four different breeds within the Iberian sample, but 50% of them are bullfighting and the rest are beef breeds. Bullfighting cattle appear to express a higher encephalization plateau than other Iberian breeds including the Tudanca. A third member, the Andalusian Black (n = 4), has a nearly flat regression line so it is unclear how it compares to other members (electronic supplementary material, data D7).
The remaining phylogenetic groups express serially increasing brain reduction in the range of 21–31%, in the following order: Podolian, Northern Celtic, West-Central Highland, North-Western Lowland, and Highland Solid-coloured (ANCOVA, p-values < 0.001) (electronic supplementary material, data D7). The park cattle (Whitepark and Chillingham breeds) appear to have higher encephalization than most other members in their cluster (Northern Celtic) (electronic supplementary material, data D7). Only the Scottish Highland, a specialized beef breed, plots closer to park cattle. Further comparisons within Iberian and Northern Celtic clusters are detailed in the electronic supplementary material, data D7.
4. Discussion
The domestic cattle brain is reduced by approximately 25.6% compared to the wild aurochs, as measured by allometric regressions of EV versus MZW, adding support to the trend observed in most domestic taxa. The magnitude of reduction among domestics varies according to breed specialization. These specializations align with different husbandry practices which differ markedly in the intensity of selection for tameness or aggression. Breed genealogy also correlates with brain size, but even within these genealogical groups, bullfighting and park cattle appear to have larger brains compared to beef and dairy breeds in their cluster, suggesting a significant influence on brain size by behavioural selection.
(a) . Phylogenetic influence
Genealogy also correlated with brain size, but this also appears to support our hypothesis that breeds with less human engagement have larger brains. Iberian breeds exhibit higher encephalization than all other breed groups. This distinction may be driven by bullfighting cattle, which dominate the sample, but it may also be because the remaining sample is also comprised breeds that are infrequently handled. The Tudanca, for example, are considered ‘semi-feral’ and have only recently been classified into a formal breed [51]. Within the Northern Celtic cluster, park cattle (Whitepark and Chillingham breeds) partially overlap with the Scottish Highland (beef) (figure 3b). This may be due to their close ancient origin [51], but may also be because the typical husbandry of the Scottish Highland is also free-ranging, on large pastures [63]. This breed is referred to as a ‘less domesticated’ or ‘semi-wild’ breed [63] and is known for its ability to survive on its own in harsh environments [51].
(b) . Behavioural selection
In cattle, docility has been shown to be heritable [64,65], and among all domesticates, the brain region most affected by brain reduction is the limbic system, which is responsible for the processing of aggression and fear [7,11,66]. When examining how distinctly different husbandry practices are among specialized cattle breeds, the correlation of brain reduction and degree of human contact becomes clearer.
Bullfighting cattle are exceptional in being selected for aggressive behaviour [25]. Similar to other cattle, their environment is devoid of natural predators, and their nourishment and protection are abundantly provided [67]. However, until the day they first enter a fighting ring as adults, they experience no human contact [20] and spend most of their lives ‘roam[ing] at liberty … in unfrequented prairies' [67]. During these debut fights, docility is specifically selected against [25] when individuals that fight weakly, hesitate to attack or submit to an opponent, are eliminated from breeding [67]. These selective pressures may be maintaining the bullfighting cattle brain in a state closer to that of its wild ancestors.
Park breeds like the Chillingham and Whitepark herds have been reared in ‘baronial parks’ since 1225 AD [25,35]. They are enclosed but free-ranging, have little to no human contact, and their life cycles are not managed, as in most other breeds [35]. They are also not bred for any product or aesthetic purpose [25]. The Chillingham herd, in particular, are referred to as ‘wild’ cattle and have no human contact, even for veterinary care [68]. This lack of human engagement may be maintaining their larger brain, compared to other Northern Celtic breeds. The Scottish Highland cattle, which overlap with the park cattle, are a rare breed known for low beef productivity and are mainly used in land management [69,70]. Described as being ‘less affected by breeding’ they have thrived in open, harsh environments [71] and are able to survive in marginal landscapes [72].
(c) . Selection for commercial production
The dairy cow brain is reduced twice as much as that of bullfighting cattle, and only somewhat less than that in beef cattle. In both types, docility is a commercially important factor, so its selection is systematic [37]. Docility makes handling easier but also improves the volume and quality of milk and beef production [65,73], so animals that are aggressive or excessively fearful are culled [65]. However, dairy cattle are more intensely selected for this due to being handled much more frequently than beef cattle [37]. Since birth, dairy calves experience regular human interaction through cleaning and feeding, building bonds with humans throughout their life [38]. This can be seen in the markedly shorter flight distances for dairy versus beef breeds [37]. By contrast, beef herds tend to perceive humans as a potential danger [37].
There are, however, other factors that are likely adding to the disparity in brain size between beef and dairy cattle. This difference may be intensified by their different metabolisms and life histories [74]. Producing milk is energetically and physiologically more taxing than beef production: the ratio of calories produced to calories ingested is dramatically higher in dairy (24%) compared to beef cattle (1.9%) [19], and this conversion is not offset by richer diets—on the contrary [75,76]. Differential allocation of resources [77] could be widening their difference in brain size. In addition to this, reproductive rates, which are also associated with differences in brain size, are under stronger selection in dairy than in beef cattle [20,78].
5. Conclusion
We report a decrease in encephalization of 25.6% in domestic cattle compared to the aurochs, as well as great variation in brain size among domestic cattle breeds. The magnitude of reduction appears correlated with the intensity of human contact and the degree of selection for or against docility and aggression. More aggressive, ‘wilder’ breeds display larger relative brain size. Bullfighting cattle have the largest brain of all breeds, most similar to the wild aurochs. Conversely, breeds intensely selected for tameness and which have strong human contact, have smaller brains, particularly dairy cattle. We also observe a correlation between brain size and genealogy, but this may also be driven by differences in the level of human engagement.
The effect of specific selection pressures on brain size could be further explored by sampling more breeds. Hérens cattle, for example, are partly selected for competitive fighting [61] and exhibit above-average testosterone levels [62]. Their exposure to human care is otherwise similar to other dairy breeds [61]. A sampling of regionally distinct aurochs is also relevant. Ancient DNA work has reported varying mitochondrial DNA haplotypes in British and German aurochsen, which are now only recorded in some domestic Korean cattle [79]. By contrast, Italian aurochs haplotypes are more similar to European cattle ‘landraces’ [79]. The existence of breeds with distinct breeding protocols opens avenues for continued research in this area.
Supplementary Material
Acknowledgements
We thank Kristian M. Gregersen (Københavns Universitet Statens Naturhistoriske Museum, Copenhagen, Denmark), Chiara Villa (Københavns Universitet, Dept. of Forensic Medicine, Denmark), Roberto Portela Miguez and Roula Pappa (The Natural History Museum, London, UK) for access to collections under their care; M. Geiger, A. Evin, L. A. B. Wilson and A. Wilkins for discussion of ideas and advice; R. Warnock for programming guidance; A. Benitez for help with figures. We thank the editors and reviewers for their useful comments and improvements to this paper.
Data accessibility
Electronic supplementary material, data files D1–D8 provide all data used and referred to in this paper.
The data are provided in the electronic supplementary material [80].
Authors' contributions
A.M.B.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization and writing-original draft; M.R.S.-V.: conceptualization, funding acquisition, project administration, resources, supervision, validation, writing-review and editing; K.V.: methodology and validation; M.C.: formal analysis, methodology, supervision, validation, writing-review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Swiss National Science Foundation SNF grant no. 31003A-169395.
References
- 1.Kruska D. 1970. Uber die Evolution des Gehirns in der Ordnung Artiodactyla Oweb, 1848, insbesondere der Teilordnung Suina Gray, 1868. Bonn, Germany: Deutsche Forschungsgemeinschaft.
- 2.Ebinger P. 1974. A cytoarchitectonic volumetric comparison of brains in wild and domestic sheep. Z. Anat. Entwicklungsgesch 144, 267-302. ( 10.1007/BF00522811) [DOI] [PubMed] [Google Scholar]
- 3.Röhrs M, Ebinger P. 1978. Die Berteilung von Hirngrossenunterschieden. J. Zool. Syst. Evol. Res. 16, 1-14. ( 10.1111/j.1439-0469.1978.tb00916.x) [DOI] [Google Scholar]
- 4.Röhrs M, Ebinger P. 1998. Sind Zooprzewalskipferde Hauspferde. Berliner und Münchener Tieraerztliche Wochenschrift 111, 273-280. [PubMed] [Google Scholar]
- 5.Herre W, Thiede U. 1965. Studien an Gehirnen sudamerikanisther Tylopoden. Zoologische Jahrbiicher Abteilung fur Anatomie und Ontogenie Dertiere 82, 155-176. [Google Scholar]
- 6.Balcarcel AM, Sánchez-Villagra MR, Segura V, Evin A. 2021. Singular patterns of skull shape and brain size change in the domestication of South American camelids. J. Mammal. 102, 220-235. ( 10.1093/jmammal/gyaa135) [DOI] [Google Scholar]
- 7.Kruska D. 1988. Effects of domestication on brain structure and behavior in mammals. Hum. Evol. 3, 473-485. ( 10.1007/978-3-030-25865-8) [DOI] [Google Scholar]
- 8.Kruska D. 1970. Vergleichend cytoarchitektonische Untersuchungen an Gehirnen von Wild- und Hausschweinen. Anat. Embryol. 131, 291-324. ( 10.1007/bf00519973) [DOI] [PubMed] [Google Scholar]
- 9.Stuermer IW, Plotz K, Leybold A, Zinke O, Kalberlah O, Samjaa R, Scheich H. 2003. Intraspecific allometric comparison of laboratory gerbils with Mongolian gerbils trapped in the wild indicates domestication in Meriones unguiculatus (Milne-Edwards, 1867) (Rodentia: Gerbillinae). Zoologischer Anzeiger 242, 249-266. ( 10.1078/0044-5231-00102) [DOI] [Google Scholar]
- 10.Sorbe D, Kruska D. 1975. Vergleichende allometrische Untersuchungen an den Schädeln von Wander und Laborratten. Zoologischer Anzeiger 195, 124-144. [Google Scholar]
- 11.Brusini I, et al. 2018. Changes in brain architecture are consistent with altered fear processing in domestic rabbits. Proc. Natl Acad. Sci. USA 115, 7380-7385. ( 10.1073/pnas.1801024115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruska D, Steffen K. 2013. Comparative allometric investigations on the skulls of wild cavies (Cavia aperea) versus domesticated guinea pigs (C. aperea f. porcellus) with comments on the domestication of this species. Mamm. Biol. 78, 178-186. ( 10.1016/j.mambio.2012.07.002) [DOI] [Google Scholar]
- 13.MacLean EL, et al. 2014. The evolution of self-control. Proc. Natl Acad. Sci. USA 111, E2140-E2148. ( 10.1073/pnas.1323533111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerison HJ. 1975. Evolution of the brain and intelligence. Curr. Anthropol. 16, 403-426. ( 10.1086/201571) [DOI] [Google Scholar]
- 15.Jerison HJ. 2006. Fossils, brains, and behavior. See http://hjerison.bol.ucla.edu/pdf/fossilbrains.pdf.
- 16.Kruska D. 1987. How fast can total brain size change in mammals? J. für Hirnforschung 28, 59-70. [PubMed] [Google Scholar]
- 17.Weisbecker V, et al. 2021. Global elongation and high shape flexibility as an evolutionary hypothesis of accommodating mammalian brains into skulls. Evolution 75, 625-640. ( 10.1111/evo.14163) [DOI] [PubMed] [Google Scholar]
- 18.Smaers JB, et al. 2021. The evolution of mammalian brain size. Sci. Adv. 7, eabe2101. ( 10.1126/sciadv.abe2101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie H. 2017. Meat and dairy production. See https://ourworldindata.org/meat-production.
- 20.Mason IL. 1984. Evolution of domesticated animals. London, UK: Longman Group. [Google Scholar]
- 21.Frantz LAF, Bradley DG, Larson G, Orlando L. 2020. Animal domestication in the era of ancient genomics. Nat. Rev. Genet. 21, 449-460. ( 10.1038/s41576-020-0225-0) [DOI] [PubMed] [Google Scholar]
- 22.Park SD, et al. 2015. Genome sequencing of the extinct Eurasian wild aurochs, Bos primigenius, illuminates the phylogeography and evolution of cattle. Genome Biol. 16, 234. ( 10.1186/s13059-015-0790-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson G, Fuller DQ. 2014. The evolution of animal domestication. Annu. Rev. Ecol. Evol. 45, 115-136. ( 10.1146/annurev-ecolsys-110512-135813) [DOI] [Google Scholar]
- 24.Zeder MA. 2012. The domestication of animals. J. Anthropol. Res. 68, 161-190. ( 10.3998/jar.0521004.0068.201) [DOI] [Google Scholar]
- 25.Porter V. 1991. Cattle, A handbook to the breeds of the world. Singapore: The Crowood Press. [Google Scholar]
- 26.Hemmer H. 1990. Domestication: the decline of environmental appreciation, 2nd edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 27.Wilkins AS. 2017. Revisiting two hypotheses on the ‘domestication syndrome’ in light of genomic data. Vavilov J. Genet. Breeding 21, 435-442. ( 10.18699/vj17.262) [DOI] [Google Scholar]
- 28.Sanchez-Villagra MR, Geiger M, Schneider RA. 2016. The taming of the neural crest: a developmental perspective on the origins of morphological covariation in domesticated mammals. R. Soc. Open Sci. 3, 160107. ( 10.1098/rsos.160107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price EO. 2002. Animal domestication and behavior. Wallingford, UK: CABI Publishing. [Google Scholar]
- 30.Arbuckle B. 2005. First steps of animal domestication. In New archaeozoological approaches (eds Vigne JD, Peters J, Helmer D). Oxford, UK: Oxbow; Books. [Google Scholar]
- 31.Trut LN. 1999. Early canid domestication: the farm-fox experiment: foxes bred for tamability in a 40-year experiment exhibit remarkable transformations that suggest an interplay betweenbehavioral genetics and development. Am. Sci. 87, 160-169. ( 10.1511/1999.2.160) [DOI] [Google Scholar]
- 32.Kruska D, Schott U. 1977. Vergleichend-quantitative Untersuchungen an den Gehirnen von Wander- und Laborratten. III. Volumenvergleich optischer Hirnzentren. J. für Hirnforschung 18, 59-67. [PubMed] [Google Scholar]
- 33.Kruska D, Schreiber A. 1999. Comparative morphometrical and biochemical-genetic investigations in wild and ranch mink (Mustela vison: Carnivora: Mammalia). Acta Theriologica 44, 377-392. ( 10.4098/AT.arch.99-37) [DOI] [Google Scholar]
- 34.Hecht EE, Smaers JB, Dunn WD, Kent M, Preuss TM, Gutman DA. 2019. Significant neuroanatomical variation among domestic dog breeds. J. Neurosci. 39, 7748-7758. ( 10.1523/JNEUROSCI.0303-19.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall SJG. 1989. The white herd of Chillingham. J. R. Agricult. Soc. En. 150, 112-119. [Google Scholar]
- 36.Clutton-Brock J. 1999. A natural history of domesticated mammals, 2nd edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 37.Albright JL. 1986. Human/farm animal relationships. In Advances in animal welfare science 1986/87 (eds Fox MW, Mickley LD), pp. 51-66. Washington, DC: The Humane Society of the United States. [Google Scholar]
- 38.Raussi S. 2003. Human–cattle interactions in group housing. Appl. Anim. Behav. Sci. 80, 245-262. ( 10.1016/S0168-1591(02)00213-7) [DOI] [Google Scholar]
- 39.Visscher PM, Smith D, Hall SJ, Williams JL. 2001. A viable herd of genetically uniform cattle. Nature 409, 303. ( 10.1038/35053160) [DOI] [PubMed] [Google Scholar]
- 40.Felius M, Koolmees PA, Theunissen B, European Cattle Genetic Diversity, C, Lenstra JA. 2011. On the breeds of cattle—historic and current classifications. Diversity 3, 660-692. ( 10.3390/d3040660). [DOI] [Google Scholar]
- 41.Finarelli JA. 2011. Estimating endocranial volume from the outside of the skull in Artiodactyla. J. Mammal. 92, 200-212. ( 10.1644/09-MAMM-A-391.1) [DOI] [Google Scholar]
- 42.Veitschegger K, Wilson LAB, Nussberger B, Camenisch G, Keller LF, Wroe S, Sánchez-Villagra MR. 2018. Resurrecting Darwin's Niata - anatomical, biomechanical, genetic, and morphometric studies of morphological novelty in cattle. Sci. Rep. 8, 9129. ( 10.1038/s41598-018-27384-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radinsky L. 1967. Relative brain size: a new measure. Science 155, 836-838. ( 10.1126/science.155.3764.836) [DOI] [PubMed] [Google Scholar]
- 44.Kruska D. 1980. Domestikationsbedingte Hirngrößenanderungen bei Säugetieren. J. Zool. Syst. Evol. Res. 18, 161-195. ( 10.1111/j.1439-0469.1980.tb00738.x) [DOI] [Google Scholar]
- 45.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. R. Stat. Soc. 57, 289-300. ( 10.1111/j.2517-6161.1995.tb02031.x) [DOI] [Google Scholar]
- 46.R Studio Team. 2020. RStudio: integrated development for R. Boston, MA: RStudio. [Google Scholar]
- 47.Wickham H, François R, Henry L, Müller K. 2020. dplyr: a grammar of data manipulation. (v.0.8.4). See https://CRAN.R-project.org/package=dplyr. [Google Scholar]
- 48.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn. New York, NY: Springer. [Google Scholar]
- 49.Wickham H. 2016. Ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 50.Mendoza M, Janis CM, Palmqvist P. 2006. Estimating the body mass of extinct ungulates: a study on the use of multiple regression. J. Zool. 270, 90-101. ( 10.1111/j.1469-7998.2006.00094.x) [DOI] [Google Scholar]
- 51.Felius M. 1995. Cattle breeds: an encyclopedia. Doetinchem, The Netherlands: Misset. [Google Scholar]
- 52.Mahmoud HA, Hadad HMRE. 2015. Automatic cattle muzzle print classification system using multiclass support vector machine. Int. J. Image Mining 1, 126. ( 10.1504/ijim.2015.070022) [DOI] [Google Scholar]
- 53.Heck L, Wilson LAB, Evin A, Stange M, Sanchez-Villagra MR. 2018. Shape variation and modularity of skull and teeth in domesticated horses and wild equids. Front. Zool. 15, 14. ( 10.1186/s12983-018-0258-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owen J, Dobney K, Evin A, Cucchi T, Larson G, Strand Vidarsdottir U. 2014. The zooarchaeological application of quantifying cranial shape differences in wild boar and domestic pigs (Sus scrofa) using 3D geometric morphometrics. J. Archaeol. Sci. 43, 159-167. ( 10.1016/j.jas.2013.12.010) [DOI] [Google Scholar]
- 55.Stange M, Nuñez-Leon D, Sánchez-Villagra MR, Jensen P, Wilson LAB. 2018. Morphological variation under domestication: how variable are chickens? R. Soc. Open Sci. 5, 180993. ( 10.1098/rsos.180993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lord KA, Larson G, Coppinger RP, Karlsson EK. 2020. The history of farm foxes undermines the animal domestication syndrome. Trends Ecol. Evol. 35, 125-136. ( 10.1016/j.tree.2019.10.011) [DOI] [PubMed] [Google Scholar]
- 57.Gilbert RP, Bailey DR, Shannon NH. 1993a. Body dimensions and carcass measurements of cattle selected for postweaning gain fed two different diets. J. Anim. Sci. 71, 1688-1698. ( 10.2527/1993.7171688x) [DOI] [PubMed] [Google Scholar]
- 58.Gilbert RP, Bailey DRC, Shannon NH. 1993b. Linear body measurements of cattle before and after 20 years of selection for postweaning gain when fed two different diets. J. Anim. Sci. 71, 1712-1720. ( 10.2527/1993.7171712x) [DOI] [PubMed] [Google Scholar]
- 59.Williams M, Prendiville R, O'Sullivan K, McCabe S, Kennedy E, Liddane M, Buckley F. 2019. Developing and validating a model to predict the dry matter intake of grazing lactating beef cows. Animal 13, 2639-2649. ( 10.1017/S1751731119001241) [DOI] [PubMed] [Google Scholar]
- 60.Van Wijngaarden-Bakker LH. 1997. Aurochs and Heck cattle. Anthopozoologica 25–26, 193-199. [Google Scholar]
- 61.Plusquellec P, Bouissou MF, Le Pape G. 2001. Early predictors of dominance ability in heifers (Bos taurus, L.) of the Herens breed. Behaviour 138, 1009-1031. ( 10.1163/156853901753286542) [DOI] [Google Scholar]
- 62.Plusquellec P, Bouissou M-F. 2001. Behavioural characteristics of two dairy breeds of cows selected (Hérens) or not (Brune des Alpes) for fighting and dominance ability. Appl. Anim. Behav. Sci. 72, 1-21. ( 10.1016/s0168-1591(00)00198-2) [DOI] [PubMed] [Google Scholar]
- 63.Heising K, Smid J. 2013. Variation in individual behaviour of semi-wild Scottish highland cattle (Bos Taurus spp.) in relation to weather conditions, day-time and habitat. Leeuwarden, The Netherlands: University of Applied Sciences. [Google Scholar]
- 64.Boissy A, Fisher AD, Bouix J, Hinch GN, Le Neindre P. 2005. Genetics of fear in ruminant livestock. Livestock Prod. Sci. 93, 23-32. ( 10.1016/j.livprodsci.2004.11.003) [DOI] [Google Scholar]
- 65.Haskell MJ, Simm G, Turner SP. 2014. Genetic selection for temperament traits in dairy and beef cattle. Front. Genet. 5, 368. ( 10.3389/fgene.2014.00368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kruska D. 2005. On the evolutionary significance of encephalization in some eutherian mammals: effects of adaptive radiation, domestication, and feralization. Brain Behav. Evol. 65, 73-108. ( 10.1159/000082979) [DOI] [PubMed] [Google Scholar]
- 67.Chapman A, Buck WJ. 1893. Sport with rifle, rod, and gun, natural history and exploration. London, UK: Woodfall and Kinder. [Google Scholar]
- 68.CWCA. 2008. Parkland. See https://chillinghamwildcattle.com/science/parkland.
- 69.Braun U, Storni E, Hassig M, Nuss K. 2014. Eating and rumination behaviour of Scottish Highland cattle on pasture and in loose housing during the winter. Schweiz. Arch. Tierheilkd 156, 425-431. ( 10.1024/0036-7281/a000624) [DOI] [PubMed] [Google Scholar]
- 70.Pauler CM, Isselstein J, Braunbeck T, Schneider MK. 2019. Influence of highland and production-oriented cattle breeds on pasture vegetation: a pairwise assessment across broad environmental gradients. Agr. Ecosyst. Environ. 284, 106585. ( 10.1016/j.agee.2019.106585) [DOI] [Google Scholar]
- 71.Pauler CM, Isselstein J, Berard J, Braunbeck T, Schneider MK. 2020. Grazing allometry: anatomy, movement, and foraging behavior of three cattle breeds of different productivity. Front. Vet. Sci. 7, 494. ( 10.3389/fvets.2020.00494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sosa SO, et al. 2019. Impact of group management and transfer on individual sociality in Highland Cattle (Bos taurus). Front. Vet. Sci. 6, 183. ( 10.3389/fvets.2019.00183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mota-Rojas D, Broom DM, Orihuela A, Velarde A, Napolitano F, Alonso-Spilsbury M. 2020. Effects of human-animal relationship on animal productivity and welfare. J. Anim. Behav. Biometeorol. 8, 196-205. ( 10.31893/jabb.20026) [DOI] [Google Scholar]
- 74.Isler K, van Schaik CP. 2006. Metabolic costs of brain size evolution. Biol. Lett. 2, 557-560. ( 10.1098/rsbl.2006.0538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.National Academies of Sciences, Engineering, and Medicine. 2016. Nutrient requirements of beef cattle: eighth revised edition, 8th edn. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 76.National Academies of Sciences, Engineering, and Medicine. 2001. Nutrient requirements of dairy cattle: seventh revised edition, 7th edn. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 77.Beilharz RG, Luxford BG, Wilkinson JL. 1993. Quantitative genetics and evolution: is our understanding of genetics sufficient to explain evolution? J. Anim. Breed. Genet. 110, 161-170. ( 10.1111/j.1439-0388.1993.tb00728.x) [DOI] [PubMed] [Google Scholar]
- 78.Isler K, van Schaik CP. 2009. The expensive brain: a framework for explaining evolutionary changes in brain size. J. Hum. Evol. 57, 392-400. ( 10.1016/j.jhevol.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 79.MacHugh DE, Larson G, Orlando L. 2017. Taming the past: ancient DNA and the study of animal domestication. Annu. Rev. Anim. Biosci. 5, 329-351. ( 10.1146/annurev-animal-022516-022747) [DOI] [PubMed] [Google Scholar]
- 80.Balcarcel AM, Veitschegger K, Clauss M, Sánchez-Villagra MR. 2021. Intensive human contact correlates with smaller brains: differential brain size reduction in cattle types. FigShare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Balcarcel AM, Veitschegger K, Clauss M, Sánchez-Villagra MR. 2021. Intensive human contact correlates with smaller brains: differential brain size reduction in cattle types. FigShare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Electronic supplementary material, data files D1–D8 provide all data used and referred to in this paper.
The data are provided in the electronic supplementary material [80].