Abstract
Objective
In an effort to reduce mortality from gastric cancer, endoscopic screening was introduced in 2016 as a nationwide screening program in Japan. Recent developments in high-definition endoscopic imaging and diagnostic strategies have enabled the simultaneous detection of other upper gastrointestinal (U-GI) malignancies. Therefore, we conducted a study to evaluate the feasibility of endoscopic screening for U-GI malignancy in a comprehensive health checkup.
Methods
We retrospectively reviewed the data of 13,120 participants who had received a comprehensive health checkup in a single institution between April 2012 and March 2018. Participants were divided into two groups [gastrointestinal endoscopy (GIE) group (n=9,142) and gastrointestinal X-ray (X-ray) group (n=3,978)] and compared with regards to the screening results, adverse events, and detection rate of U-GI malignancies (gastric cancer or other) using a propensity-score matched analysis.
Results
The gastric cancer detection rate was significantly higher in the GIE group [34/9,142 (0.48%)] than in the X-ray group [3/3,978 (0.08%)] (p=0.003). Other U-GI malignancies were found only in the GIE group and comprised two hypopharyngeal cancers, five esophageal cancers, two duodenal cancers, and one duodenal gastrointestinal stromal tumor. Adverse events occurred in 6/9,142 (0.07%) participants in the GIE group and 18/3,978 (0.45%) participants in the X-ray group (p<0.0001). A propensity-score matched analysis yielded 1,551 matched pairs, and the detection rate of gastric cancer and other U-GI malignancies remained significantly higher in the GIE group than in the X-ray group.
Conclusion
This study indicated that not only gastric cancer but also other U-GI malignancies can be detected by endoscopic screening.
Keywords: screening and diagnosis, endoscopy, upper gastrointestinal malignancies
Introduction
Upper gastrointestinal (U-GI) endoscopy is being performed increasingly frequently in clinical settings as a standardized examination procedure for U-GI diseases. In Japan, endoscopic screening was introduced in 2016 as a nationwide gastric cancer screening program by the Ministry of Health, Labour and Welfare, with reference to the guidelines published 2014 by the National Cancer Center (NCC) (Tokyo, Japan) (1). However, regular radiographic screening for U-GI malignancies introduced in 1983 has been continued. Several studies have reported a reduced mortality from gastric cancer in select cities in Japan and in nationwide programs carried out in South Korea (2-4).
In 2017, the incidence of each cancer as reported by the NCC was as follows: 129,475 gastric cancers, 22,033 oral/pharyngeal cancers, 5,247 laryngeal cancers, and 23,483 esophageal cancers (5). In addition, the numbers of mortalities were reported as follows: 44,192 gastric cancers, 7,576 oral/pharyngeal cancers, 841 laryngeal cancers, and 11,345 esophageal cancers (2018) (5). Surprisingly, the number of incidents and mortalities of cancers other than gastric cancer were not small. Therefore, the detection of these cancers by U-GI endoscopic screening might be very important.
Early detection and prompt resection are critical for reducing the mortality from U-GI malignancies. Previous reports have indicated that early mucosal gastric cancer, which can be treated by minimally invasive methods, such as endoscopic resection, are being detected at a higher rate by endoscopic screening than X-ray screening (6). In addition, the 5-year survival rate has been reported as 90% for post-resection early-stage gastric cancer patients (7), and the mortality of gastric cancer might be reduced by 40% with early detection (8,9).
An additional advantage associated with endoscopic screening is that organs other than the stomach, such as the pharynx, esophagus and duodenum, can be observed in detail during such examinations. Recent developments in high-resolution imaging with narrow-band imaging or digital-based image enhancement technologies and updated diagnostic strategies have facilitated the early detection of U-GI malignancies (10-12).
Little data exist on the extent to which U-GI malignancies other than gastric cancer are detected by endoscopic screening. Therefore, we evaluated the feasibility of endoscopic screening for detecting U-GI malignancies using the data from a comprehensive health checkup program.
Materials and Methods
Participants
This study was based on the data of participants who received a comprehensive health checkup as opportunistic screening, at Hiraka General Hospital between April 2012 and March 2018. Data were collected from the hospital database and retrospectively reviewed. Participants were divided into two groups depending on their personal preference: the gastrointestinal endoscopy (GIE) group and the gastrointestinal X-ray (X-ray) group. The target population of this screening program was individuals ≥20 years old, with no upper age limit. The cost of gastric cancer screening in a comprehensive health checkup program was included in the total amount set forth by the Japan Society of Ningen Dock and did not change depending on the screening method.
Study outcomes
The outcomes of the study were as follows: the detection rate of U-GI malignancy (gastric cancer and other types of U-GI cancer), positive predictive value of the method, and adverse events related to the procedure. Outcomes were compared between the GIE group and the X-ray group using a propensity-score matched (PSM) analysis. In addition, the clinical outcomes of cases with U-GI-detected malignancies were analyzed. Follow-up of U-GI malignancy incidence was continued from the date of the first screening to the date of the gastric cancer diagnosis or up to December 2019.
Screening for U-GI malignancies
Endoscopic screening was performed by experienced endoscopists with certification from the Japanese Gastroenterological Endoscopy Society or trainees under their supervision without any sedation. In endoscopic screening, the examination targeted the pharynx, esophagus, stomach and duodenum with at least 40 images in total. The procedure was performed using high-resolution endoscopic equipment with narrow-band imaging modalities and a magnifying function (CLV-260SL; Olympus Optical, Tokyo, Japan, 2012-2014, and CLV-290SL; Olympus, 2015-2018) and scopes (GIF-H260, GIF-XP260N; Olympus; 2012-2014; and GIF-H290Z, GIF-XP290N; Olympus, 2015-2018). The recorded images were reviewed later by a second endoscopist. A biopsy was performed if necessary, and specimens were evaluated according to the criteria of the Japanese Gastric Cancer Association (13).
X-ray screening was performed by radiologists using the double-contrast upper gastrointestinal series, as per our institution's standard of care. The examination target site involved the esophagus (2 sheets), stomach (13 sheets) and duodenum (2 sheets).
The recorded images were subsequently reviewed by two medical doctors with certification from the JSGCS or the Japanese Society of Gastroenterology.
If U-GI malignancies were suspected based on the screening, participants were called directly for consultation and subsequently received a thorough evaluation, including blood tests, computed tomography and endoscopy. Helicobacter pylori (Hp) infection was evaluated in participants in the GIE group by the rapid urease test or based on endoscopic findings of chronic atrophic gastritis.
PSM analyses
The baseline characteristics of the participants reflected several possible stratification factors. Therefore, a PSM analysis was carried out to minimize potentially confounding factors and selection biases and to identify controls within the study-patient group. Since there were some duplicate participants who received a comprehensive health checkup, these duplicators were counted as one object. We used six possible confounders as matching factors. Five variables based on previous reports were used, including the age, sex, cigarette smoking, alcohol consumption and body mass index (BMI) (14-16). The examination year was added as a possible confounder because the proportion of endoscopic examinations gradually increased every year. In the multivariate logistic regression analysis, the confounders were included as independent variables, while the screening method (GIE group) was included as the dependent variable. The propensity-score for the GIE group was calculated by a logistic regression analysis. Following the estimation of the propensity-score, participants in the GIE and X-ray groups were matched. Optimal matching was achieved at a 1:1 ratio, and we used a caliper coefficient of 0.1 for the logit of the propensity-score without replacement. Covariate balance was measured using the standardized difference, whereby an absolute standardized difference above 10% represented a meaningful imbalance.
Statistical analyses
Statistical analyses of the clinical and endoscopic data were performed using the χ2 test for categorical data and Student's t-test for numerical data for the univariate analysis. We determined both the absolute differences and probability (p) values. We considered p values <0.05 as statistically significant. All statistical analyses were performed with the JMP software program, version 12.0 (SAS Institute, Cary, USA).
Results
Baseline characteristics of the study participants
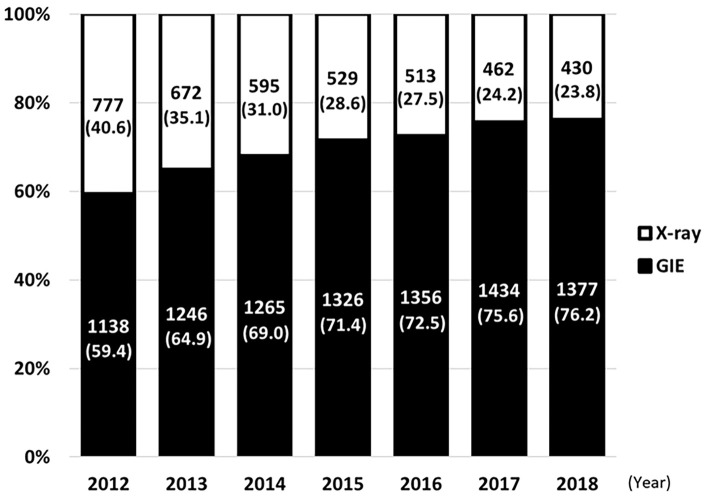
The proportion of the two screening methods in a comprehensive health checkup program are displayed in Figure. The total number of total participants was more or less fixed throughout the study years due to a full examination capacity. However, the proportion of participants who received endoscopic examinations has increased each year, moving from 59.4% to 76.2%. Endoscopic examinations were performed in 8,936/9,142 (97.8%) participants via trans-oral endoscopy, while others received trans-nasal endoscopy.
Figure.
Proportion of each screening method used in comprehensive health checkups at Hiraka General Hospital between April 2012 and March 2018. The number on the bar indicates the participant count and proportion of each screening method.
Table 1 shows the baseline characteristics for both groups. A total of 13,120 participants were evaluated over the 7 years, of which 9,142 (69.7%) individuals were assigned to the GIE group and 3,978 (30.3%) to the X-ray group. We observed a significant difference between the 2 groups with regard to the following characteristics: age (p<0.001), sex (p<0.001), alcohol consumption (p<0.001) and BMI (p<0.001). Of note, the rate of cigarette smoking did not significantly differ between the groups.
Table 1.
Baseline Characteristics of 13,120 Participants That Underwent a Comprehensive Health Checkup.
| GIE group (n=9,142) |
X-ray group (n=3,978) |
p | AS | |
|---|---|---|---|---|
| Age, median [range] (y) | 57 [24-94] | 53 [21-86] | <0.001* | 0.580 |
| Sex | ||||
| Male, n (%) | 5,737 (62.8) | 1,641 (41.3) | <0.001† | 0.441 |
| Cigarette smoking | 1,642 (18.0) | 718 (18.0) | 0.920† | 0.002 |
| positive, n (%) | ||||
| Alcohol consumption | 5,651 (61.8) | 2,184 (54.9) | <0.001† | 0.143 |
| positive, n (%) | ||||
| BMI, mean [SD] (kg/m2) | 23.7 [3.6] | 23.1 [3.6] | <0.001* | 0.163 |
BMI: body mass index, SD: standerd deviation, *: student's t-test, †: Chi-square test, ASD: Absolute standerdized difference
Table 2 shows the matched variables and outcomes in both groups after the PSM analysis. Duplicators were counted as 1 object, so 3,385 individuals were included in the GIE group and 1,578 in the X-ray group. Ultimately, 1,551 pairs of participants were matched and compared with regard to the outcomes. The propensity-score model was well-calibrated (area under the curve = 0.66) and optimally matched (caliper, 0.1; standardized difference <0.1) in terms of the baseline participant characteristics in both groups.
Table 2.
Baseline Characteristics after Propensity-score Matched Analysis.
| GIE group (n=1,551) |
X-ray group (n=1,551) |
p | ASD | |
|---|---|---|---|---|
| Age, median [range] (y) | 52 [24-83] | 53 [21-86] | 0.202* | 0.046 |
| Sex | ||||
| Male, n (%) | 742 (47.8) | 739 (47.7) | 0.914† | 0.004 |
| Cigarette smoking | 304 (19.6) | 298 (19.2) | 0.785† | 0.010 |
| positive, n (%) | ||||
| Alcohol consumption | 865 (55.8) | 881 (56.8) | 0.563† | 0.021 |
| positive, n (%) | ||||
| BMI, mean [SD] (kg/m2) | 23.5 [3.6] | 23.4 [3.7] | 0.515* | 0.003 |
BMI: body mass index, SD: standerd deviation, *: student’s t-test, †: Chi-square test, ASD: Absolute standerdized difference
Outcomes in the GIE and the X-ray groups before PSM
Table 3 shows the outcomes in both groups before PSM. The Hp infection status of the participants in the GIE group was positive in 5,056, post-eradication in 1,788 and negative in 2,298. The detection rate of U-GI malignancies was significantly higher in the GIE group [44/9,142 (0.48%)] than in the X-ray group [3/3,978 (0.08%)] (p=0.005). The positive predictive value was higher in the GIE group [3.48% (95% CI, 2.60-4.63)] than in the X-ray group [0.73% (95% CI, 0.25-2.13)] (p=0.003).
Table 3.
Outcomes before Propensity-score Matched Analysis.
| GIE group (n=9,142) |
X-ray group (n=3,978) |
p | |
|---|---|---|---|
| Hp infection, postive | 5,056 | - | |
| post eradication | 1,788 | - | |
| negative | 2,298 | - | |
| Detection rate of all U-GI malignancies, n (%) | 44 (0.48) | 3 (0.08) | 0.005* |
| Positive predictive value (95%CI) (%) | 3.48 (2.60-4.63) | 0.73 (0.25-2.13) | 0.003* |
| Detection rate of gastric cancer, n (%) | 34 (0.37) | 3 (0.08) | 0.003* |
| Stage I/II/III/IV | 32/2/0/0 | 2/1/0/0 | |
| Treatment method (endoscopic/surgical) | 31/3 | 1/2 | |
| Clinical outcome (curative/non-curative) | 33/1 | 3/0 | |
| Detection rate of other U-GI malignancies, n (%) | 10 (0.11) | 0 (0.00) | 0.036* |
| Hypopharyngeal cancer | 2 | 0 | |
| Esophageal cancer | 5 | 0 | |
| Duodenal cancer | 2 | 0 | |
| Duodenal GIST | 1 | 0 | |
| Treatment method (endoscopic/surgical/radiation) | 5/3/1 | 0 | |
| Clinical outcome (curative/non-curative) | 10/0 | 0 | |
| Adverse events | 6 (0.07) | 18 (0.45) | <0.0001* |
Hp: Helicobacter pylori, U-GI: upper-gastrointestinal, SMT: submucosal tumor, *: student's t-test
The detection rate of gastric cancer was significantly higher in the GIE group [34/9,142 (0.37%)] than in the X-ray group [3/3,978 (0.08%)] (p=0.003). Gastric cancers were observed to arise arose from only Hp-positive or Hp-eradicated chronic gastritis cases and were detected in the early stage (I or II). Thirty-three gastric cancers were curatively resected by endoscopy (n=31) or surgery (n=2), and the patients survived for the entire follow-up period. One case of gastric cancer was completely resected by surgery. However, after two years of follow-up, multiple liver metastasis had developed, and the patient is now in therapy.
In addition, the detection rate of other U-GI malignancies was significantly higher in the GIE group [10/9,142 (0.11%)] than in the X-ray group [0/3,978 (0.00%)] (p=0.036), so these 10 U-GI malignancies were detected only in the GIE group and comprised 2 hypopharyngeal cancer, 5 esophageal cancers, 2 duodenal cancers and 1 duodenal GIST. They were curatively resected by endoscopy (n=5), surgery (n=3) or radiation (n=1), and the patients survived for the entire follow-up period.
Adverse events occurred in 6 (0.07%) patients in the GIE group and in 18 (0.45%) patients in the X-ray group (p<0.0001). Specifically, adverse events comprised nasal bleeding (n=2), Mallory-Weiss syndrome (n=2) and lidocaine allergy (n=1) in the GIE group. In the X-ray group, adverse events involved problems with swallowing barium (n=8), constipation (n=5), accidental fall (n=4) and barium allergy (n=1). Serious adverse events, including anaphylactic shock and respiratory depression, were not reported, and no fatalities were noted in either of the screening programs.
Outcomes in the GIE and the X-ray groups after PSM
Table 4 shows the outcomes in both groups after PSM. The comparison demonstrated that the detection rate of U-GI malignancies was significantly higher in the GIE group [19/1,551 (1.23%)] than in the X-ray group [3/1,551 (0.19%)] (p=0.001). In addition, the detection rate of gastric cancer was significantly higher in the GIE group [16/1,551 (1.03%)] than in the X-ray group [3/1,551 (0.19%)] (p=0.003), and the detection rate of other U-GI malignancies was also significantly higher in the GIE group [3/1,551 (0.19%)] than in the X-ray group [0/1,551 (0.00%)] (p=0.005).
Table 4.
Outcomes after Propensity-score Matched Analysis.
| GIE group (n=1,551) |
X-ray group (n=1,551) |
p | |
|---|---|---|---|
| Detection rate of all U-GI malignancies, n (%) | 19 (1.23%) | 3 (0.19%) | 0.001* |
| Detection rate of gastric cancer, n (%) | 16 (1.03%) | 3 (0.19%) | 0.003* |
| Detection rate of other U-GI malignancies, n (%) | 3 (0.19%) | 0 (0.00%) | 0.005* |
U-GI: upper-gastrointestinal, *: student's t-test
Discussion
The present study evaluated the feasibility of endoscopic screening for detecting U-GI malignancies based on a review of retrospective data from comprehensive health checkup programs using PSM. Endoscopic screening has already been implemented as a nationwide gastric cancer screening program in South Korea and Japan. Our data are comparable with those of previous reports with regard to the recall rate, detection rate of gastric cancer, positive predictive value, and number of adverse events (17-21). The adverse event rate of the GIE group in this study was quite low and comparable to that (0.078%) reported by the Society of Gastroenterology Cancer Screening over a period of 3 years (22-24).
Organs other than the stomach can be observed in detail during U-GI endoscopic screening. However, little data have been published with regard to the detection rate of U-GI malignancies in screening programs, except for gastric cancer. In the present study, non-gastric malignant lesions were detected only in the GIE group. Smyth et al. claimed that routine screening was currently not recommended outside of high-risk areas or for low-risk individuals. The European Society of Gastrointestinal Endoscopy stated in 2020 that endoscopic screening may be considered only in high-risk individuals for esophageal cancer (25,26).
In addition, since it is expensive and a larger staff and greater technological expertise are needed to perform endoscopy (27), endoscopic screening for esophageal cancer, pharyngeal cancer and duodenal cancer alone for every individual might not be needed. However, our data indicate that, if gastric cancer screening is performed by endoscopy, it is important to be alert for other potential U-GI malignancies.
Hamashima et al. reported that stage shifts by endoscopic screening could lead to improved survival rates of the detected cancer by endoscopic screening (28). Furthermore, endoscopic resection of precancerous and early lesions has been associated with a reduction in the incidence and increased 5-year survival rates (29). We identified 44 cases of U-GI malignancies among 9,142 participants in the GIE group, and 97.7% (43/44) were curatively treated and survived. Ultimately, 81.8% (36/44) were endoscopically resected. These stage shifts may be explained as follows: 1) only experienced endoscopists performed the procedures, and 2) high-resolution endoscopic equipment with image-enhanced modalities and a magnifying function were used throughout the study period.
Several limitations associated with the present study warrant mention. First, the study was performed at a single institution, and only a relatively small number of patients were retrospectively enrolled. Second, our data were collected from a comprehensive health checkup program as opportunistic screening. Third, our endoscopic screening was performed using trans-oral endoscopy unless the participant refused. As a result, the 44 U-GI malignancy lesions in the endoscopic screening group were all detected by trans-oral endoscopy. However, trans-nasal endoscopy might be feasible since the image quality has recently improved. Fourth, Hp infection was evaluated only in the GIE group, as the assessment was performed by the rapid urease test or endoscopic findings of chronic atrophic gastritis. Finally, there may have been self-selection bias in the screening methods. Health-conscious people might have tended to opt for GIE, and they might have chosen to undergo endoscopic screening several times. A multicenter large-scale prospective randomized control trial with a risk stratification by age, sex, cigarette smoking, alcohol consumption and Hp infection is warranted to further clarify the feasibility of endoscopic screening for detecting U-GI malignancies.
Conclusion
This study indicated that not only gastric cancer but also other U-GI malignancies could be detected by endoscopic screening in the early stage of disease, leading to curative resection.
Written informed consent from all participants was obtained to perform the study and publish the data.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Promotion of evidence based cancer screening. National Cancer Center. Japan. The Japanese guidelines for gastric cancer screening 2015 [Internet]. [cited 2016 Feb 15]. Available from: http://canscreen.ncc.go.jp/ (in Japanese)
- 2.Jun JK, Choi KS, Lee HY, et al. Effectiveness of the Korean national cancer screening program in reducing gastric cancer mortality. Gastroenterology 152: 1319-1328, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Cho B. Evaluation of the validity of current national health screening programs and plans to improve the system. Seoul University, Seoul, 2013: 741-758. [Google Scholar]
- 4.Hamashima C, Ogoshi K, Narisawa R, et al. Impact of endoscopic screening on mortality reduction from gastric cancer. World J Gastroenterol 21: 2460-2466, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer information service of National Cancer Center Japan [Internet]. [cited 2020 Jul 6]. Available from: https://ganjoho.jp/reg_stat/statistics/stat/summary.html (in Japanese)
- 6.Hamashima C, Shabana M, Okada K, Okamoto M, Osaki Y. Mortality reduction from gastric cancer by endoscopic and radiographic screening. Cancer Sci 106: 1744-1749, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukase K, Matsuda T, Suzuki M, et al. Evaluation of the efficacy of endoscopic treatment for gastric cancer considered in terms of long-term prognosis -a comparison with surgical treatment-. Digestive endoscopy 6: 241-247, 1994. [Google Scholar]
- 8.Layke JC, Lopez PP. Gastric cancer: diagnosis and treatment options. Am Fam Physician 69: 1133-1140, 2004. [PubMed] [Google Scholar]
- 9.Zhang X, Li M, Chen S, et al. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology 155: 347-354, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process - first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res 52: 6735-6740, 1992. [PubMed] [Google Scholar]
- 11.Kaise M. Advanced endoscopic imaging for early gastric cancer. Best Pract Res Clin Gastroenterol 29: 575-587, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Li HY, Dai J, Xue HB, et al. Application of magnifying endoscopy with narrow-band imaging in diagnosing gastric lesions: a prospective study. Gastrointest Endosc 76: 1124-1132, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Chang-Qing Li, Ya Li, Xiu-Li Zuo, et al. Magnified and enhanced computed virtual chromoendoscopy in gastric neoplasia: a feasibility study. World J Gastroenterol 19: 4221-4227, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14: 101-112, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: is it needed? Gastrointest Endosc 84: 18-28, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin North Am 11: 235-256, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol 12: 17-20, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ministry of Health, Labour and Welfare. Report on regional public health services and health promotion services [Internet]. [cited 2016 Jun 20]. Available from: http://www.e-stat.go.jp/SG1/estat/GL08020103.do?_toGL08020103_&-listID=000001117956&requestSender=dsearch (in Japanese)
- 19.Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol 12: 4874-4875, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi KS, Jun JK, Park EC, et al. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS One 7: e50041, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamashima C, Ogoshi K, Okamoto M, et al. A community-based, case control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One 8: e79088, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Li M, Chen S, et al. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology 155: 347-354, 2018. [DOI] [PubMed] [Google Scholar]
- 23.Shibuya D, Ishikawa T, Ichinose M, et al. Reports on adverse effect of cancer screening, FY2010. J Gastrointestinal Cancer Screen 51: 250-255, 2013. [Google Scholar]
- 24.Shibuya D, Ishikawa T, Ichinose M, et al. Reports on adverse effect of cancer screening, FY2011. J Gastrointestinal Cancer Screen 52: 253-258, 2014. [Google Scholar]
- 25.Shibuya D, Ishikawa T, Ichinose M, et al. Reports on adverse effect of cancer screening, FY2012. J Gastrointestinal Cancer Screen 53: 233-238, 2015. [Google Scholar]
- 26.Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primer 3: 17048, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Săftoiu A, Hassan C, Areia M, et al. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 52: 293-304, 2020. [DOI] [PubMed] [Google Scholar]
- 28.Zhou HJ, Dan YY, Naidoo N, Li SC, Yeoh KG. A cost-effectiveness analysis evaluating endoscopic surveillance for gastric cancer for populations with low to intermediate risk. PLoS One 8: e83959, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamashima C, Shabana M, Okamoto M, Osaki Y, Kishimoto T. Survival analysis of patients with interval cancer undergoing gastric cancer screening by endoscopy. PLoS One 10: e0126796, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]