Abstract
Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) has not been described in lymphoma patients. A 65-year-old man with refractory mantle cell lymphoma (MCL) presented typical MRI features of MERS. The patient's cerebrospinal fluid contained an increased number of reactive T-cells; a small number of MCL cells were detected by immunoglobulin heavy chain-polymerase chain reaction (IGH-PCR). His symptoms and the splenial lesion resolved in response to ibrutinib treatment, although the patient eventually died of progressive MCL with overt leptomeningeal disease. We suggest that central nervous system involvement in MCL can present clinicoradiological features of MERS and that ibrutinib could be a choice of treatment.
Keywords: mantle cell lymphoma, mild encephalitis/encephalopathy with reversible splenial lesion, polymerase chain reaction
Introduction
Approximately 5% patients with mantle cell lymphoma (MCL) have central nervous system (CNS) involvement, mostly presenting as leptomeningeal disease (1). Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) is a rare clinicoradiological entity characterized by a reversible lesion in the corpus callosum, which occasionally involves the symmetrical white matter, as revealed by magnetic resonance imaging (MRI) (2). MERS is predominantly caused by viral infections, and it is less frequently associated with antiepileptic medication withdrawal, high-altitude exposure, and metabolic disturbances (2-4). From a clinical viewpoint, patients with MERS present with mild CNS symptoms, such as consciousness disturbance, seizures, and headache and achieve spontaneous recovery within a month. We herein report a case of secondary CNS involvement in MCL presenting MRI features of MERS at the time of the onset of neurological symptoms.
Case Report
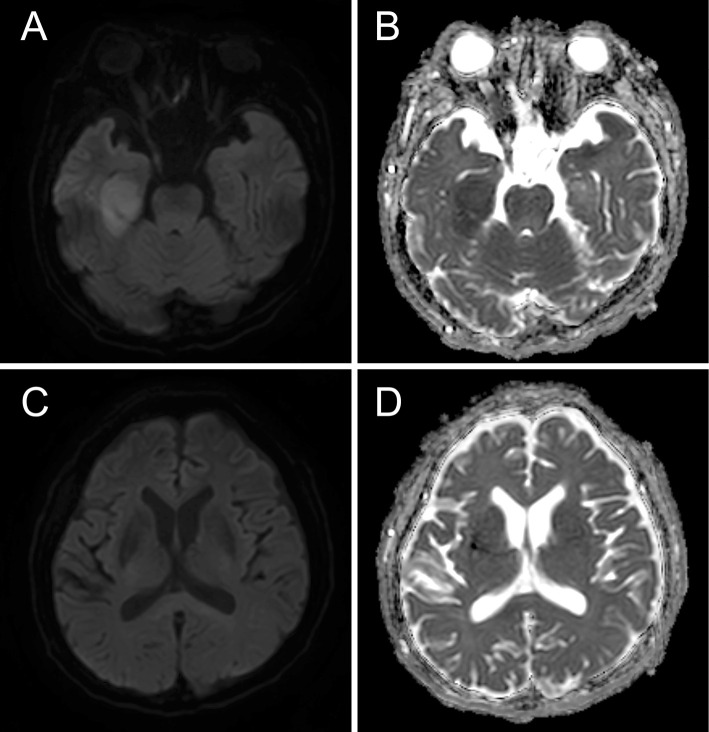
A 65-year-old man presented with a 2-week history of paraphasia and action tremor. Nine years earlier, he was diagnosed with MCL and was treated with five lines of systemic chemoimmunotherapy for multiple relapses of MCL. In the fifth remission under chemoimmunotherapy, computed tomography showed that the mesenteric lymph nodes were less than 3 cm in the long axis diameter. A physical examination revealed conduction aphasia, right oculomotor palsy-related upper eyelid ptosis, exotropia, and mydriasis. MRI revealed an isolated ovoid lesion in the central portion of the splenium of the corpus callosum, characterized by hyperintensity on diffusion-weighted images (DWI) and low apparent diffusion coefficient (ADC) values (Fig. 1A, B). An analysis of the cerebrospinal fluid (CSF) revealed a cell count of 229 cells/μL (mostly mononuclear cells) and an increased protein level (312 mg/dL; reference range, 15-50 mg/dL). Lymphoma cells were not detected by cytology or flow cytometry. Most CSF-infiltrating cells were immunophenotypically CD3+ mature T lymphocytes, expressing either CD8+ (58%) or CD4+ (21%). There were few CD20+ B cells (<1%), and immunoglobulin light chain restrictions were not detected. Polymerase chain reaction (PCR)-amplified fragments using immunoglobulin heavy chain (IGH) gene consensus primer sets (FR1-, FR2- and FR3-JH) (5) consistently revealed monoclonal IGH gene rearrangement (Fig. 1C), indicating CNS involvement in MCL. IGH-PCR did not detect rearrangement in the peripheral blood leukocytes, which excluded the possibility of sample contamination by traumatic tap. A detailed investigation that included staining, cultures and PCR assays revealed no evidence of infection in the CSF. The patient was started on ibrutinib, which has high blood-brain barrier penetration. The 2-week follow-up MRI showed resolution of the splenial lesion, with modest improvements in the neurological symptoms.
Figure 1.
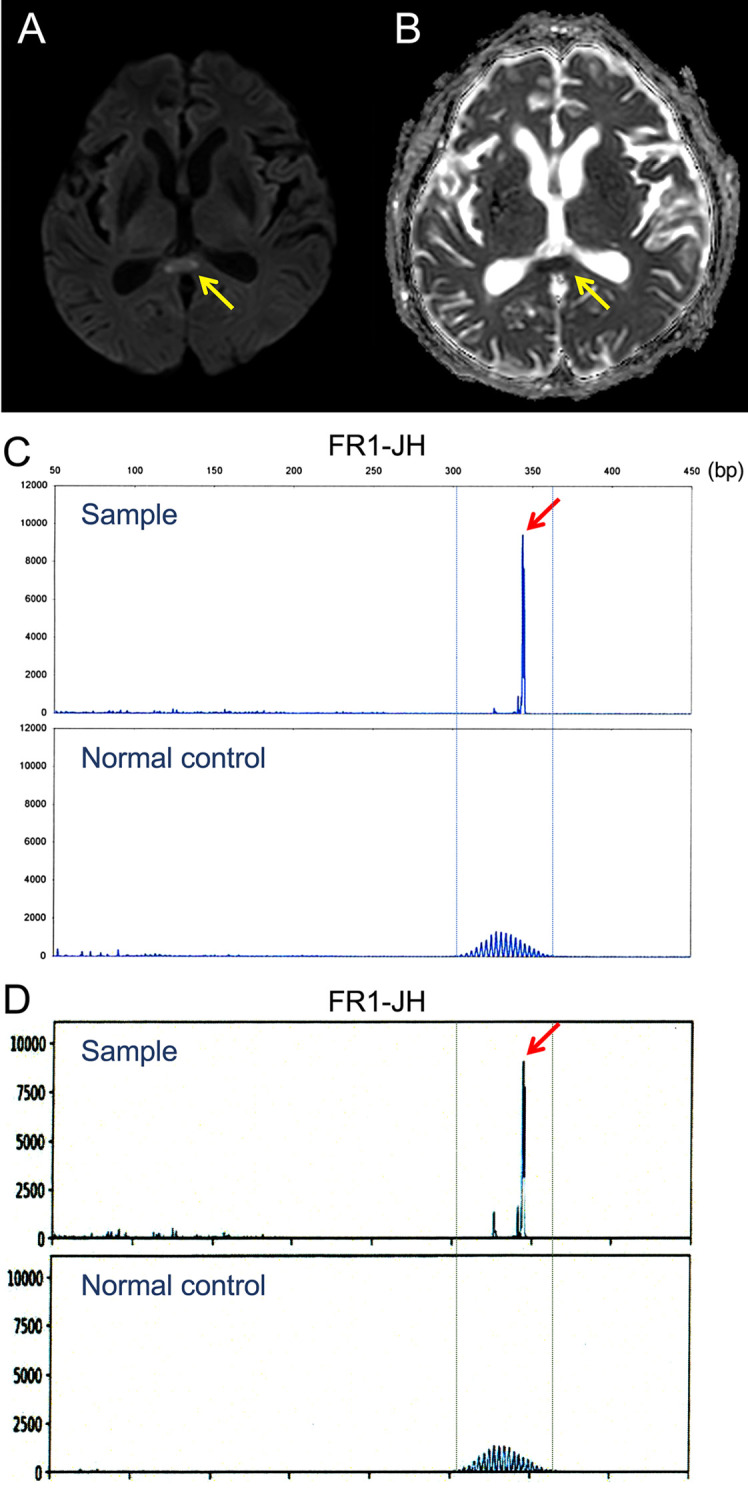
(A, B) Magnetic resonance imaging showing a hyperintense ovoid lesion in the mid-splenium of the corpus callosum on diffusion-weighted imaging (A) and a decreased apparent diffusion coefficient value (B) (arrows). (C-D) Detection of a monoclonal immunoglobulin heavy chain gene rearrangement in the cerebrospinal fluid cells using the FR1-JH consensus primers (arrow), at the time of the onset of neurological symptoms (C) and full-blown CNS lymphoma (D). The expected polymerase chain reaction product sizes for FR1-JH in normal control is also shown.
Two months later, MRI showed a new lesion in the temporal lobe (long-axis diameter: 3 cm), which was also characterized by hyperintensity on DWI and low ADC values (Fig. 2). The CSF contained a large number of cells with morphological features of MCL (1,440 cells/μL). Flow cytometry revealed that most infiltrating cells (approximately 90%) expressed the CD5+CD19+CD20+CD23− MCL immunophenotype with lambda light chain restriction. IGH-PCR also detected monoclonal IGH gene rearrangement. The sizes of rearrangement bands were identical to those seen in the CSF obtained at the time of the onset of neurological symptoms. The patient died of progressive disease 1 month later.
Figure 2.
(A, B) Magnetic resonance imaging showing a newly developed lesion in the temporal lobe, characterized by hyperintensity on diffusion-weighted imaging and low apparent diffusion coefficient values. (C, D) The lesion in the mid-splenium of the corpus callosum disappeared after ibrutinib treatment.
Discussion
In this report, we presented a case of MERS in a patient with evidence of secondary CNS involvement in MCL. CSF involvement in MCL was only diagnosed by IGH-PCR in the setting of negative cytology and flow cytometry results. We therefore recommend performing PCR to detect monoclonal IGH gene rearrangement in the CSF, because it has higher sensitivity than cytology and flow cytometry in samples with dense inflammatory lymphocyte infiltration.
It is unclear whether the splenial lesion was formed because of direct lymphoma involvement or an inflammatory response to the lymphoma. Although the pathophysiological mechanism of MERS is unknown, it has been hypothesized that an autoimmune response causes reversible intramyelinic edema and inflammation in the splenium (6,7). On the other hand, MERS-defining MRI features, including hyperintensity on DWI and low ADC values have also been described in CNS lymphomas (8). In addition, ibrutinib has shown clinical antineoplastic activity against secondary CNS lymphomas, providing progression-free survival of approximately 10 months (9), and anti-inflammatory properties in patients with chronic graft-versus-host disease and coronavirus disease 2019 (10,11). We therefore hypothesize that a subsided splenial lesion could form either by direct lymphoma involvement or secondarily through the influx of inflammatory T-cells. In either case, ibrutinib could be an option for MCL patients with possible CNS lesions.
Written informed consent was obtained from the patient for the publication of this case report.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Cheah CY, George A, Giné E, et al. Central nervous system involvement in mantle cell lymphoma: clinical features, prognostic factors and outcomes from the European Mantle Cell Lymphoma Network. Ann Oncol 24: 2119-2123, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 63: 1854-1858, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Ka A, Britton P, Troedson C, et al. Mild encephalopathy with reversible splenial lesion: an important differential of encephalitis. Eur J Paediatr Neurol 19: 377-382, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi M, Sahashi Y, Baba Y, Okura H, Shimohata T. COVID-19-associated mild encephalitis/encephalopathy with a reversible splenial lesion. J Neurol Sci 415: 116941, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dongen JJ, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 17: 2257-2317, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Miyata R, Tanuma N, Hayashi M, et al. Oxidative stress in patients with clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS). Brain Dev 34: 124-127, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Takanashi J, Maeda M, Hayashi M. Neonate showing reversible splenial lesion. Arch Neurol 62: 1481-1482, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Schob S, Meyer J, Gawlitza M, et al. Diffusion-weighted MRI reflects proliferative activity in primary CNS lymphoma. PLoS One 11: e0161386, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis KL, Chin CK, Manos K, et al. Ibrutinib for central nervous system lymphoma: the Australasian Lymphoma Alliance/MD Anderson Cancer Center experience. Br J Haematol. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 10.Michallet M, Dreger P, Sobh M, et al. Ibrutinib as a salvage therapy after allogeneic HCT for chronic lymphocytic leukemia. Bone Marrow Transplant 55: 884-890, 2020. [DOI] [PubMed] [Google Scholar]
- 11.Treon SP, Castillo JJ, Skarbnik AP, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood 135: 1912-1915, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]