Abstract
Background
Interleukin (IL)-17 and T helper 17 (TH17) cells, a distinct subset of CD4+ T cells which promotes the expression of IL-17, mediate host defensive mechanisms to various infections and are involved in the pathogenesis of autoimmune diseases including inflammatory bowel disease (IBD), psoriasis, and rheumatic diseases. IL-17 inhibitors have shown to be effective in psoriasis, but failed to demonstrate response in IBD. Further, clinical trials of IL-17 inhibitors reported some cases of new onset IBD. We aim to discuss the roles of IL-17 and TH17 cells among autoimmune diseases and the possible immunological mechanisms of new onset IBD in patients undergoing IL-17 inhibitors.
Methods
A non-systematic literature review using PubMed/Medline.
Results
IL-17 inhibitors, which either target IL-17 A (secukinumab and ixekizumab) or the IL-17 receptor (brodalumab), have demonstrated clinical benefits in plaque psoriasis, psoriatic arthritis, or axial spondyloarthritis. However, secukinumab and brodalumab have shown no clinical benefit in Crohn's disease and led to frequent serious adverse events including worsening of Crohn's disease. Further, some cases of new onset IBD were reported in clinical trials of IL-17 inhibitors. Consistently, an animal model of colitis has demonstrated that IL-17 can directly inhibit the development of T helper 1 (TH1) cells and TH1 cells can induce aggressive colitis in the absence of IL-17 signaling.
Conclusions
IL-17 and TH17 cells might have protective rather than pro-inflammatory roles in the intestine. IL-17 inhibition may induce inflammation in the intestine by favoring TH1 pathways, which explain the lack of response to IL-17 inhibitors in IBD.
Keywords: interleukin-17, T helper 17 cells, Inflammatory bowel disease, Psoriasis, Axial spondyloarthritis
Highlights
-
•
IL-17 and TH17 cells play important roles in the pathologies of autoimmune diseases.
-
•
Monoclonal antibodies targeting IL-17 have demonstrated clinical benefits.
-
•
Clinical trials of IL-17 inhibitors reported new onset inflammatory bowel disease.
-
•
IL-17 can directly inhibit the development of TH1 cells.
-
•
TH1 cells may induce aggressive colitis in the absence of IL-17 signaling.
1. Introduction
The interleukin (IL)-17 family is composed of six molecules with IL-17A (generally called as IL-17) and IL-17F being the main family members [1]. T helper 17 (TH17) cells are identified as a distinct subset of CD4+ T cells which promote the expression of IL-17A, IL-17F, and IL-22 [[2], [3], [4]]. These effector cytokines mediate host defensive mechanisms to various infections including bacteria and fungi [5], and are involved in the pathogenesis of several autoimmune diseases [6]. In mouse models of experimental autoimmune encephalitis, IL-23 was shown to be required for the differentiation of TH17 cells in vivo [7,8]. In addition, transforming growth factor-β and IL-6 are also important cytokines to induce retinoid-related orphan receptor (ROR)-γτ (RORC in human), which is a master regulator of TH17 cells in mice [9,10]. While natural killer cells, mast cells, innate lymphoid cells, and neutrophils are other cellular sources of IL-17, the respective contribution of IL-17 produced by such different cell types to the disease pathogenesis is still unclear [2].
Several experimental animal models [[11], [12], [13]] demonstrated that IL-17 and TH17 cells play important roles in the pathologies of inflammatory bowel disease (IBD), psoriasis, and rheumatic diseases, suggesting that IL-17 can be a potential therapeutic target [2]. Indeed, monoclonal antibodies targeting IL-17A (secukinumab and ixekizumab) or an antibody against the IL-17 receptor (brodalumab) have demonstrated clinical benefits and been approved for patients with plaque psoriasis [[14], [15], [16]], psoriatic arthritis [[17], [18], [19], [20], [21], [22]], or axial spondyloarthritis [23,24]. However, randomized, double-blind, placebo-controlled trials (RCTs) of secukinumab or brodalumab in patients with Crohn's disease (CD) have been terminated due to lack of clinical benefit and frequent serious adverse events including worsening of CD in the treatment group [25,26]. Further, clinical trials of IL-17 inhibitors in patients with autoimmune diseases other than IBD reported some cases of new onset IBD [27].
In this review, we discuss the roles of IL-17 and TH17 cells in animal IBD models and patients with IBD and other autoimmune inflammatory conditions, and the possible immunological mechanisms of new onset IBD in patients undergoing IL-17 inhibitors.
2. The role of IL-17/TH17 cells in IBD
In the pathogenesis of IBD, CD is primarily mediated by TH1 cells and ulcerative colitis (UC) by TH2 cells [27]. TH17 cells and their cytokines are crucial mediators in both conditions [27,28]. Although increased levels of IL-23, TH17 cells, and IL-17 were found in intestinal mucosa, plasma, and serum of patients with IBD, both CD and UC [29], either protective or pro-inflammatory roles of IL-17 or TH17 cells have been shown in animal models of IBD.
2.1. Animal colitis models showing protective functions of IL-17/TH17 cells in IBD
In a dextran sulfate sodium colitis model, an experimental mouse model of UC [30], blocking of IL-17 activity using anti-IL-17 antibody enhanced the expression of tumor necrosis factor (TNF)-α, interferon (IFN)-γ and IL-6, and the infiltration of T cells (CD3+ cells, particularly CD4+ TH cells) and granulocytes-monocytes in the intestinal mucosa, resulting in the progression of severe colitis [31]. They also found that recombinant IL-17 attenuated the effect of anti-IL-17 antibody [31].
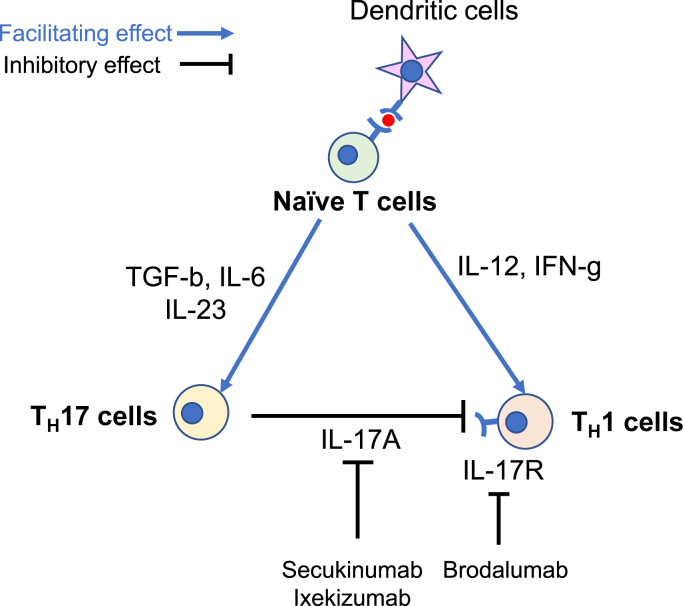
In another study assessing the function of IL-17A in a T-cell transfer model of colitis, they transferred CD45RBhighCD25−CD4+ T cells from IL-17A-knockout (KO) mice or wild type mice into Rag-KO recipients and demonstrated that IL-17A-deficient CD45RBhigh T cells induced an aggressive wasting colitis with a higher expression of genes encoding TH1-type cytokines in the colon. They also found that IL-17 receptor-KO CD45RBhigh donor T cells elicited an accelerated wasting disease in Rag-KO recipients [32], suggesting a protective role for IL-17A in the T-cell transfer colitis model. It has been also postulated that IL-17A can directly inhibit developing TH1 cells by suppressing the expression of key TH1-effector genes and TH1 cells can induce aggressive colitis in the absence of IL-17 signaling (Fig. 1) [33].
Fig. 1.
The possible immunological mechanism to explain how interleukin-17 inhibitors can induce new onset inflammatory bowel disease. IFN, interferon; IL, interleukin; IL-17R, interleukin-17 receptor; TGF, transforming growth factor; TH17 cells, T helper 17 cells; TH1 cells, T helper 1 cells.
2.2. Animal colitis models showing pro-inflammatory functions of IL-17/TH17 cells in IBD
In a colitis model in which CD25−CD4+ T cells were transferred to immunodeficient SCID mice, the development of colitis was associated with an increase in IL-17A-producing TH17 cells in the spleen, mesenteric lymph nodes, and lamina propria [34]. On the other hand, the expression of IL-17F declined in the TH17 cells in the spleen and mesenteric lymph nodes, suggesting that IL-17F was inversely corelated with the activity of colitis in this model [34]. This study also found that simultaneous neutralization of IL-17A and IL-17F ameliorated colitis, whereas neutralization of IL-17A or IL-17F alone was inefficient in vivo, suggesting overlapping and interdependent proinflammatory roles of these cytokines in colitis pathology [34].
IL-10-KO mice have been used as a spontaneous colitis model [35]. When IL-10-KO mice were backcrossed with IL-23p19-KO mice, the colon was histologically disease free at 12 months of age, whereas IL-10-KO mice or IL-10-KO mice backcrossed with IL-12-KO mice showed active colitis by 3 months of age [36]. Further, they also transferred naïve T cells (CD45RBhighCD4+) or memory T cells (CD45RBlowCD4+) from IL-10-KO mice to Rag-KO mice to induce colitis and assessed the effect of recombinant IL-23 on colitis. They found that recombinant IL-23 accelerated colitis. Furthermore, this study revealed that IL-23 promoted productions of IL-17 and IL-6 by memory activated T cells from IL-10-KO mice with colitis and the combination of anti-IL-17 and anti-IL-6 antibodies significantly improved the severity of colitis induced by IL-23 [36]. Consistently, a T-cell transfer colitis model in which a cecal bacterial antigen-specific C3H/HeJBir CD4+ T-cell line was transferred to C3H/HeSnJ SCID mice demonstrated that bacterial-reactive CD4+ TH17 cells were potent effector cells in chronic colitis and anti-IL-23 antibody prevented colitis [37].
2.3. Animal models modulating gut microbiotas
TH17 cells are prominent in mucosal surface of the intestine in cooperation with other T cells to maintain intestinal homeostasis and protect against microorganisms [27,38]. An antibiotic treatment mice model in which conventional C57BL/6j mice were subjected to broad-spectrum antibiotics for 8 weeks showed decreased production of cytokines such as IL-17, IL-22, IFN-γ, and IL-10. These profound changes in the immune cells were restored by fecal microbiota transplantation [39], suggesting that IL-17 inhibition might interfere with its gut protective function [27].
Germ-free mice colonized with different microbiotas have been used to investigate the relationship between host and microbiotas and to understand microbiota-specific pro- or anti-inflammatory effects. TH17 cells and FoxP3+ regulatory T (Treg) cells are most highly induced upon microbiota colonization [40,41]. A study demonstrated that the colonization of germ-free mice with a single commensal microbe, segmented filamentous bacterium, was sufficient to induce TH17 cells [42]. Previous studies have also shown that human fecal microbiotas from donors with IBD can induce intestinal inflammation in susceptible mice [43,44]. Britton et al. showed that mice colonized with microbiota derived from patients with IBD exhibited abundant mucosal TH17 cells and a deficit in Treg cells, and susceptibility to disease in colitis which was induced upon transfer of naïve T cells into Rag-KO mice [44,45]. Further, they demonstrated that transplantation of healthy donor-derived microbiota suppressed mucosal TH17 cells and protected mice from intestinal inflammation in this model [44].
2.4. Clinical trials of IL-17 inhibitors in patients with IBD
RCTs (phase II) of secukinumab or brodalumab in patients with moderate to severe CD have been conducted [25,26]. In a clinical trial of secukinumab, 59 patients with CD (39 secukinumab and 20 placebo) were included [25]. This study demonstrated that patients treated with secukinumab experienced significantly worse outcomes as compared to those treated with placebo. The reduction of mean Crohn's disease activity index (CDAI) was greater in the placebo group compared to secukinumab [25]. Safety data up to week 18 showed that more patients experienced any adverse event in the secukinumab group than in the placebo group: 74% vs 50%, respectively, particularly for infections. Twenty infections were seen in 44% of patients with secukinumab, including four local fungal infections in four patients, whereas none in the placebo group. Severe adverse events (SAEs) were reported in 28% of patients in the secukinumab and 10% in the placebo groups. Of the seven SAEs suspected to be drug-related events, five events were worsening of CD, four in secukinumab and one in placebo treated patients [25]. This study suggested that inhibition of IL-17A by secukinumab was ineffective for patients with CD and IL-17A may have a protective function in CD.
In terms of brodalumab, 130 patients with CD were randomized 1:1:1:1 to receive brodalumab (210, 350, or 700 mg at baseline and week 4) or placebo. At week 6, the rates of remission (CDAI ≤150) were 3% (210 mg), 15% (350 mg), 9% (700 mg), and 3% (placebo). However, there was no significant difference in the mean change from baseline in CDAI at week 6 between brodalumab and placebo groups. Notably, a higher rate of CD worsening was detected in the brodalumab group compared with the placebo group (25.0% vs 6.3%) [26]. This study did not demonstrate a meaningful efficacy of blocking of IL-17 receptor by brodalumab in patients with CD as well, leading to early termination of this study [26].
3. The role of IL-17/TH17 cells in autoimmune inflammatory conditions other than IBD
3.1. Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease, which can cause cartilage and bone damage [46]. IL-17 stimulates the production of IL-1 and TNF-α from human macrophages in vitro [47], resulting in IL-1-mediated IL-6 production by synoviocytes [48] and TNF-α-induced synthesis of IL-1, IL-6, and IL-8 in synovial fibroblasts [49]. Chronic inflammation via IL-17 is often associated with matrix destruction. IL-17 suppresses matrix synthesis by articular chondrocytes through enhancement of nitric oxide production [50], and also induces the production of matrix metalloproteinases from synoviocytes and chondrocytes [51,52], leading to cartilage destruction. Further, IL-17 is a potent stimulator of osteoclastogenesis as well [53]. IL-17 increases the expression of receptor activator of NF-κB ligand (RANKL) on osteoblasts and synoviocytes [54], which leads to increased RANK signaling in osteoclasts. IL-17 in combination with TNF-α also increased osteoclastic resorption in vitro [55]. These effects of IL-17 can cause bone damage in patients with RA. However, inhibition of IL-17 with secukinumab [56,57], ixekizumab [58], and brodalumab [59] have demonstrated only modest clinical benefits in patients with RA. As a result, these medications have not been approved for RA.
3.2. Psoriasis and psoriatic arthritis
Psoriasis is an inflammatory skin disease involving the skin and joints [60]. In the pathogenesis of psoriasis, complexes of host DNA and the epidermis-produced antimicrobial peptide LL-37 are thought to stimulate dermal plasmacytoid dendritic cells to produce IFN-α [61]. On the onset or exacerbation of psoriasis, activated dendritic cells produce TNF-α and IL-23 and amplifies inflammation through several inflammatory pathways including IL-23/TH17 axis [60]. Skin biopsies taken from patients with psoriasis showed a high expression of IL-17 as well as IL-23, IL-22, IL-6 [62] and increased number of TH1 and TH17 cells were found in skin lesions [63]. Activated TH17 cells also produce IL-17A, IL-17F, and IL-22 and induce keratinocyte proliferation and other clinical features of psoriasis [60]. Other cellular sources including mast cells and neutrophils also contribute to the production of IL-17 in affected skin lesions in patients with psoriasis [64]. Secukinumab [14], ixekizumab [15], and brodalumab [16] have demonstrated clinical benefits and been approved for moderate-to-severe plaque psoriasis.
Psoriatic arthritis is a form of inflammatory arthritis with destructive-joint features which may resemble the pathology of RA and occurs in up to 30% of patients with psoriasis [65]. Secukinumab [[18], [19], [20]] and ixekizumab [21,22] have also been approved for patients with psoriatic arthritis.
3.3. Axial spondyloarthritis
Axial spondyloarthritis is a chronic inflammatory disease that mainly affect the axial skeleton. It is a type of spondyloarthritis, which also includes psoriatic arthritis, arthritis associated with IBD, and reactive arthritis [66]. The term axial spondyloarthritis covers both patients who developed structural damage in the sacroiliac joints or spine visible on radiographs (radiographic axial spondyloarthritis, also termed ankylosing spondylitis) and patients without such structural damage, labeled as non-radiographic axial spondyloarthritis [66].
HLA-B27 is strongly associated with the pathogenesis of axial spondyloarthritis [67]. Further, it is clinically associated with IBD, psoriasis, or reactive arthritis in about 15–20% of cases [68], suggesting that barrier damages of intestinal mucosal or dermal surfaces and subsequent exposure of the immune system to microorganisms might be a relevant pathogenesis [66]. TNF-α and IL-17 appear to have relevant roles in pathogenesis as well. TH17 cells and IL-17-producing innate immune cells, ILC3 cells, are involved in the inflammatory process of ankylosing spondylitis and IBD [27]. While ILC3 cells are mainly located in the intestinal mucosa of healthy individuals [27], a study demonstrated that gut-derived ILC3 cells were expanded in the peripheral blood, synovial fluid, and inflamed bone marrow of patients with ankylosing spondylitis, suggesting the presence of an active homing axis between the gut and the inflamed sacroiliac joints [69]. Furthermore, systemic overexpression of IL-23 in an animal model induced an enthesitis, resembling spondyloarthritis, suggesting that IL-23 might be a therapeutic target as well [70]. Secukinumab [23] and ixekizumab [24] have been approved in patients with axial spondyloarthrtiis.
4. New onset IBD in patients undergoing IL-17 inhibitors
Many clinical trials of IL-17 inhibitors have been undertaken for patients with rheumatological and dermatological inflammatory conditions and demonstrated its clinical benefits as previously described. However, new onset of IBD cases following the treatment with IL-17 inhibitors have been reported [27,71,72]. Hence, our group conduced a systematic review with meta-analysis to assess the risk of new onset IBD with the use of IL-17 inhibitors. We included 38 RCTs with a total 12,614 patients treated with IL-17 inhibitors and 4076 treated with placebo. The 38 RCTs included eight studies of brodalumab (4588 patients), eight of ixekizumab (4485 patients), and 22 of secukinumab (7617 patients), respectively. The study population included psoriasis, psoriatic arthritis, RA, and ankylosing spondylitis [71]. We identified a total of 12 new cases of IBD (five cases of CD, seven cases of UC). All cases were reported in patients with IL-17 inhibitors (zero on brodalumab, four on ixekizumab, and eight on secukinumab). This corresponded to an incidence of 2.4 cases of new onset IBD per 1000 patient-years. However, statistically there was no difference in the risk of developing new onset IBD with IL-17 inhibitors compared with placebo (MH RD 0.00062, 95% CI -0.00072-0.0021, P = 0.35) [71]. Other recent studies including pooled analyses from clinical trials of ixekizumab [73], secukinumab [74], and a real-world study for patients with psoriasis treated with IL-17 inhibitors [75] have been conducted and showed low incidence of new onset IBD in patients with IL-17 inhibitors. Further, an updated meta-analysis evaluating the risk of development of IBD under IL-17 inhibition was undertaken and demonstrated no differences in the pooled risk of new onset IBD in induction and maintenance studies of IL-17 inhibitors [76].
The pathogenesis of new onset IBD in patients undergoing IL-17 inhibitors is still unclear. Given that secukinumab and brodalumab frequently induced worsening of CD [25,26], IL-17 might have protective roles for patients with IBD. As previously stated, O'Connor et al. demonstrated that IL-17A-deficient CD45RBhigh T cells induced an aggressive colitis with a higher expression of genes encoding TH1-type cytokines [32], suggesting that IL-17 can directly inhibit the development of TH1 cells by suppressing expression of key TH1-effector genes and TH1 cells can induce aggressive colitis in the absence of IL-17 signaling [33]. These evidences support that inhibition of IL-17 may induce inflammation in the gastrointestinal tract by favoring TH1 pathways (Fig. 1) [72].
5. Conclusions
The roles of IL-17 and TH17 cells in the intestine, skin, and joints are different. Given that clinical trials demonstrated significant clinical benefits of IL-17 inhibitors in patients with psoriasis, psoriatic arthritis, and axial spondyloarthritis, pro-inflammatory functions of IL-17 and TH17 cells might predominate in the skin and joints. On the other hand, animal models of colitis showed either protective or pro-inflammatory roles of IL-17 or TH17 cells. In human IBD, particularly CD, IL-17 might be protective for intestinal inflammation as IL-17 inhibitors worsened CD activity in the setting of clinical trials. IL-17 inhibitors have been used in clinical practice for autoimmune diseases other than IBD, however, physicians need to know that new onset IBD can develop via induction of TH1 cell-mediated immune responses in patients undergoing treatment with IL-17 inhibitors. Hence, monitoring of gastrointestinal symptoms during the treatment of IL-17 inhibitors would be important to diagnose new onset IBD appropriately and to consider switching to alternative medications with different mechanisms.
Funding sources
No financial support for this study.
Author contribution
Literature search-SA, Figures and Tables-SA, Study design-SA, AS, Drafting of manuscript-SA, AS.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Aggarwal S., Gurney A.L. IL-17: prototype member of an emerging cytokine family. J. Leukoc. Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 2.Miossec P., Kolls J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 3.Yao Z., Painter S.L., Fanslow W.C., Ulrich D., Macduff B.M., Spriggs M.K. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 4.Fossiez F., Djossou O., Chomarat P., Flores-Romo L., Ait-Yahia S., Maat C. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAleer J.P., Kolls J.K. Mechanisms controlling Th17 cytokine expression and host defense. J. Leukoc. Biol. 2011;90:263–270. doi: 10.1189/jlb.0211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouyang W., Kolls J.K., Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 8.Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangan P.R., Harrington L.E., O'Quinn D.B., Helms W.S., Bullard D.C., Elson C.O. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 10.Yang X.O., Panopoulos A.D., Nurieva R., Chang S.H., Wang D., Watowich S.S. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 11.Bamias G., Arseneau K.O., Cominelli F. Mouse models of inflammatory bowel disease for investigating mucosal immunity in the intestine. Curr. Opin. Gastroenterol. 2017;33:411–416. doi: 10.1097/MOG.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 12.Schon M.P., Manzke V., Erpenbeck L. Animal models of psoriasis-highlights and drawbacks. J. Allergy Clin. Immunol. 2020;147(2):439–455. doi: 10.1016/j.jaci.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Bessis N., Decker P., Assier E., Semerano L., Boissier M.C. Arthritis models: usefulness and interpretation. Semin. Immunopathol. 2017;39:469–486. doi: 10.1007/s00281-017-0622-4. [DOI] [PubMed] [Google Scholar]
- 14.Langley R.G., Elewski B.E., Lebwohl M., Reich K., Griffiths C.E., Papp K. Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 15.Gordon K.B., Blauvelt A., Papp K.A., Langley R.G., Luger T., Ohtsuki M. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N. Engl. J. Med. 2016;375:345–356. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 16.Lebwohl M., Strober B., Menter A., Gordon K., Weglowska J., Puig L. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N. Engl. J. Med. 2015;373:1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 17.Mease P.J., Genovese M.C., Greenwald M.W., Ritchlin C.T., Beaulieu A.D., Deodhar A. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N. Engl. J. Med. 2014;370:2295–2306. doi: 10.1056/NEJMoa1315231. [DOI] [PubMed] [Google Scholar]
- 18.Mease P.J., McInnes I.B., Kirkham B., Kavanaugh A., Rahman P., van der Heijde D. Secukinumab inhibition of interleukin-17a in patients with psoriatic arthritis. N. Engl. J. Med. 2015;373:1329–1339. doi: 10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
- 19.McInnes I.B., Mease P.J., Kirkham B., Kavanaugh A., Ritchlin C.T., Rahman P. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 20.McInnes I.B., Sieper J., Braun J., Emery P., van der Heijde D., Isaacs J.D. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann. Rheum. Dis. 2014;73:349–356. doi: 10.1136/annrheumdis-2012-202646. [DOI] [PubMed] [Google Scholar]
- 21.Nash P., Kirkham B., Okada M., Rahman P., Combe B., Burmester G.R. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389:2317–2327. doi: 10.1016/S0140-6736(17)31429-0. [DOI] [PubMed] [Google Scholar]
- 22.Mease P.J., van der Heijde D., Ritchlin C.T., Okada M., Cuchacovich R.S., Shuler C.L. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 2017;76:79–87. doi: 10.1136/annrheumdis-2016-209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baeten D., Sieper J., Braun J., Baraliakos X., Dougados M., Emery P. Secukinumab, an interleukin-17a inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 2015;373:2534–2548. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 24.van der Heijde D., Cheng-Chung Wei J., Dougados M., Mease P., Deodhar A., Maksymowych W.P. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441–2451. doi: 10.1016/S0140-6736(18)31946-9. [DOI] [PubMed] [Google Scholar]
- 25.Hueber W., Sands B.E., Lewitzky S., Vandemeulebroecke M., Reinisch W., Higgins P.D. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Targan S.R., Feagan B., Vermeire S., Panaccione R., Melmed G.Y., Landers C. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe crohn's disease. Am. J. Gastroenterol. 2016;111:1599–1607. doi: 10.1038/ajg.2016.298. [DOI] [PubMed] [Google Scholar]
- 27.Fauny M., Moulin D., D'Amico F., Netter P., Petitpain N., Arnone D. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann. Rheum. Dis. 2020;79:1132–1138. doi: 10.1136/annrheumdis-2020-217927. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi T., Okamoto S., Hisamatsu T., Kamada N., Chinen H., Saito R. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008;57:1682–1689. doi: 10.1136/gut.2007.135053. [DOI] [PubMed] [Google Scholar]
- 29.Allocca M., Furfaro F., Fiorino G., Gilardi D., D'Alessio S., Danese S. Can IL-23 be a good target for ulcerative colitis? Best Pract. Res. Clin. Gastroenterol. 2018;32–33:95–102. doi: 10.1016/j.bpg.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa A., Andoh A., Araki Y., Bamba T., Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor W., Jr., Kamanaka M., Booth C.J., Town T., Nakae S., Iwakura Y. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Awasthi A., Kuchroo V.K. IL-17A directly inhibits TH1 cells and thereby suppresses development of intestinal inflammation. Nat. Immunol. 2009;10:568–570. doi: 10.1038/ni0609-568. [DOI] [PubMed] [Google Scholar]
- 34.Wedebye Schmidt E.G., Larsen H.L., Kristensen N.N., Poulsen S.S., Lynge Pedersen A.M., Claesson M.H. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis, Inflamm. Bowel Dis. 2013;19:1567–1576. doi: 10.1097/MIB.0b013e318286fa1c. [DOI] [PubMed] [Google Scholar]
- 35.Berg D.J., Davidson N., Kuhn R., Muller W., Menon S., Holland G. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J. Clin. Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elson C.O., Cong Y., Weaver C.T., Schoeb T.R., McClanahan T.K., Fick R.B. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 38.Ohnmacht C., Marques R., Presley L., Sawa S., Lochner M., Eberl G. Intestinal microbiota, evolution of the immune system and the bad reputation of pro-inflammatory immunity. Cell Microbiol. 2011;13:653–659. doi: 10.1111/j.1462-5822.2011.01577.x. [DOI] [PubMed] [Google Scholar]
- 39.Ekmekciu I., von Klitzing E., Fiebiger U., Escher U., Neumann C., Bacher P. Immune responses to broad-spectrum antibiotic treatment and fecal microbiota transplantation in mice. Front. Immunol. 2017;8:397. doi: 10.3389/fimmu.2017.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivanov, Frutos Rde L., Manel N., Yoshinaga K., Rifkin D.B., Sartor R.B. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivanov, Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eun C.S., Mishima Y., Wohlgemuth S., Liu B., Bower M., Carroll I.M. Induction of bacterial antigen-specific colitis by a simplified human microbiota consortium in gnotobiotic interleukin-10-/- mice. Infect. Immun. 2014;82:2239–2246. doi: 10.1128/IAI.01513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Britton G.J., Contijoch E.J., Spindler M.P., Aggarwala V., Dogan B., Bongers G. Defined microbiota transplant restores Th17/RORgammat(+) regulatory T cell balance in mice colonized with inflammatory bowel disease microbiotas. Proc. Natl. Acad. Sci. U.S.A. 2020;117:21536–21545. doi: 10.1073/pnas.1922189117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Britton G.J., Contijoch E.J., Mogno I., Vennaro O.H., Llewellyn S.R., Ng R. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORgammat(+) regulatory T cells and exacerbate colitis in mice. Immunity. 2019;50:212–224 e214. doi: 10.1016/j.immuni.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 47.Jovanovic D.V., Di Battista J.A., Martel-Pelletier J., Jolicoeur F.C., He Y., Zhang M. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 48.Chabaud M., Fossiez F., Taupin J.L., Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J. Immunol. 1998;161:409–414. [PubMed] [Google Scholar]
- 49.Katz Y., Nadiv O., Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a "fine-tuning cytokine" in inflammation processes. Arthritis Rheum. 2001;44:2176–2184. doi: 10.1002/1529-0131(200109)44:9<2176::aid-art371>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 50.LeGrand A., Fermor B., Fink C., Pisetsky D.S., Weinberg J.B., Vail T.P. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001;44:2078–2083. doi: 10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 51.Koshy P.J., Henderson N., Logan C., Life P.F., Cawston T.E., Rowan A.D. Interleukin 17 induces cartilage collagen breakdown: novel synergistic effects in combination with proinflammatory cytokines. Ann. Rheum. Dis. 2002;61:704–713. doi: 10.1136/ard.61.8.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Hamburg J.P., Asmawidjaja P.S., Davelaar N., Mus A.M., Colin E.M., Hazes J.M. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- 53.Lubberts E., Koenders M.I., van den Berg W.B. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res. Ther. 2005;7:29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kotake S., Udagawa N., Takahashi N., Matsuzaki K., Itoh K., Ishiyama S. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van bezooijen R.L., Farih-Sips H.C., Papapoulos S.E., Lowik C.W. Interleukin-17: a new bone acting cytokine in vitro. J. Bone Miner. Res. 1999;14:1513–1521. doi: 10.1359/jbmr.1999.14.9.1513. [DOI] [PubMed] [Google Scholar]
- 56.Genovese M.C., Durez P., Richards H.B., Supronik J., Dokoupilova E., Mazurov V. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann. Rheum. Dis. 2013;72:863–869. doi: 10.1136/annrheumdis-2012-201601. [DOI] [PubMed] [Google Scholar]
- 57.Blanco F.J., Moricke R., Dokoupilova E., Codding C., Neal J., Andersson M. Secukinumab in active rheumatoid arthritis: a phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheum. 2017;69:1144–1153. doi: 10.1002/art.40070. [DOI] [PubMed] [Google Scholar]
- 58.Genovese M.C., Greenwald M., Cho C.S., Berman A., Jin L., Cameron G.S. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheum. 2014;66:1693–1704. doi: 10.1002/art.38617. [DOI] [PubMed] [Google Scholar]
- 59.Martin D.A., Churchill M., Flores-Suarez L., Cardiel M.H., Wallace D., Martin R. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res. Ther. 2013;15:R164. doi: 10.1186/ar4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boehncke W.H., Schon M.P. Psoriasis, Lancet. 2015;386:983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 61.Nestle F.O., Conrad C., Tun-Kyi A., Homey B., Gombert M., Boyman O. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 63.Pene J., Chevalier S., Preisser L., Venereau E., Guilleux M.H., Ghannam S. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J. Immunol. 2008;180:7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 64.Lin A.M., Rubin C.J., Khandpur R., Wang J.Y., Riblett M., Yalavarthi S. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ritchlin C.T., Colbert R.A., Gladman D.D. Psoriatic Arthritis, N. Engl. J. Med. 2017;376:957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 66.Sieper J., Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73–84. doi: 10.1016/S0140-6736(16)31591-4. [DOI] [PubMed] [Google Scholar]
- 67.Ellinghaus D., Jostins L., Spain S.L., Cortes A., Bethune J., Han B. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 2016;48:510–518. doi: 10.1038/ng.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vander Cruyssen B., Ribbens C., Boonen A., Mielants H., de Vlam K., Lenaerts J. The epidemiology of ankylosing spondylitis and the commencement of anti-TNF therapy in daily rheumatology practice. Ann. Rheum. Dis. 2007;66:1072–1077. doi: 10.1136/ard.2006.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciccia F., Guggino G., Rizzo A., Saieva L., Peralta S., Giardina A. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann. Rheum. Dis. 2015;74:1739–1747. doi: 10.1136/annrheumdis-2014-206323. [DOI] [PubMed] [Google Scholar]
- 70.Sherlock J.P., Joyce-Shaikh B., Turner S.P., Chao C.C., Sathe M., Grein J. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells, Nat. Med. 2012;18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 71.Yamada A., Wang J., Komaki Y., Komaki F., Micic D., Sakuraba A. Systematic review with meta-analysis: risk of new onset IBD with the use of anti-interleukin-17 agents. Aliment. Pharmacol. Ther. 2019;50:373–385. doi: 10.1111/apt.15397. [DOI] [PubMed] [Google Scholar]
- 72.Wang J., Bhatia A., Krugliak Cleveland N., Gupta N., Dalal S., Rubin D.T. Rapid onset of inflammatory bowel disease after receiving secukinumab infusion. ACG Case Rep. J. 2018;5:e56. doi: 10.14309/crj.2018.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strober B., Leonardi C., Papp K.A., Mrowietz U., Ohtsuki M., Bissonnette R. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: etanercept comparisons and integrated data. J. Am. Acad. Dermatol. 2017;76:432–440 e417. doi: 10.1016/j.jaad.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 74.Schreiber S., Colombel J.F., Feagan B.G., Reich K., Deodhar A.A., McInnes I.B. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann. Rheum. Dis. 2019;78:473–479. doi: 10.1136/annrheumdis-2018-214273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright S., Alloo A., Strunk A., Garg A. Real-world risk of new-onset inflammatory bowel disease among patients with psoriasis exposed to interleukin 17 inhibitors. J. Am. Acad. Dermatol. 2020;83:382–387. doi: 10.1016/j.jaad.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 76.Burisch J., Eigner W., Schreiber S., Aletaha D., Weninger W., Trauner M. Risk for development of inflammatory bowel disease under inhibition of interleukin 17: a systematic review and meta-analysis. PloS One. 2020;15 doi: 10.1371/journal.pone.0233781. [DOI] [PMC free article] [PubMed] [Google Scholar]