Abstract
Tick-borne pathogens (TBPs) have complex life cycles involving tick vectors and vertebrate hosts. However, there is limited empirical evidence on the zoonotic circulation of TBPs. In this study, we used a One Health approach to study the possible circulation of TBPs in ticks, animals and humans within a rural household in the foothills of the Fruška Gora mountain, northern Serbia. The presence of TBP DNA was assessed using microfluidic PCR (25 bacterial species, 7 parasite species, 5 bacterial genera, 3 parasite genera) in animal, human and tick samples and the presence of tick-borne encephalitis virus (TBEV) RNA was screened for using RT-qPCR on tick samples. In addition, Lyme borreliosis serology was assessed in patients sera. Rhipicephalus sanguineus and Ixodes ricinus ticks were identified on dogs and Haemaphysalis punctata was identified on house walls. Rickettsia helvetica was the most common pathogen detected in pooled R. sanguineus and I. ricinus tick samples, followed by Hepatozoon canis. None of the H. punctata tick samples tested positive for the presence of TBPs. Anaplasma phagocytophilum and Rickettsia monacensis were the most frequent pathogens detected in dogs, followed by Rickettsia felis, whereas Anaplasma bovis was the only pathogen found in one of the goats tested. None of the human blood samples collected from family members tested positive for the presence of TBPs. Although microfluidic PCR did not detect Borrelia sp. in any of the tested tick or blood samples, a family member with a history of Lyme disease was seropositive for Borrelia burgdorferi sensu lato (s.l.). We conclude that, despite the presence of TBPs in tick and vertebrate reservoirs, there is no evidence of infection with TBPs across various components of the epidemiological chain in a rural Fruška Gora household.
Keywords: One Health, Ticks, Tick-borne-pathogens, Anaplasma bovis, Rickettsia helvetica
1. Introduction
Ticks (order Ixodida) are obligate blood-feeding ectoparasites of mammals, birds, and reptiles, which are globally important vectors of disease-causing agents that impact both human and animal health [1]. Ticks are second only to mosquitoes in importance as vectors species, but, among all blood-sucking arthropods, they harbor and transmit the widest variety of pathogens, including bacteria, rickettsiae, protozoa and viruses [2]. The incidence of tick-borne diseases (TBDs) is increasing and becoming a serious problem worldwide due to the public health impact and economic losses related to reduced livestock production, particularly for developing countries such as Serbia [3]. In recent years, the growing number of emerging zoonotic TBD cases has led to increased public awareness about the interrelatedness of human and animal health. The World Health Organization (WHO) developed and promotes the “One Health” concept, an interdisciplinary approach to the study of the spread of diseases between animals and humans to better protect public health [4]. One Health studies aim to capture the inherent interdependence of human and animal health and the environment [4]. The One Health approach is particularly relevant for the development of strategies to control tick infestations and TBDs.
The number of known tick-borne pathogens (TBPs) has increased dramatically since the 1980s and is now a serious problem worldwide due to its impact on public health, livestock-related economic losses and morbidity for wildlife [5]. The occurrence of TBDs is on the rise due to climate change, globalization, population movements and growth, and modifications of landscapes and natural habitats, as well as to improved surveillance and diagnostic techniques [[6], [7], [8]]. Continuous human exploitation of environmental resources and an increase in outdoor activities have led to more contacts between humans and arthropod vectors, promoting the emergence and resurgence of TBPs [[6], [7], [8]]. Humans are frequently exposed to ticks and TBP infections, some with zoonotic potential, not only during occupational and outdoor recreational activities, but also within the premises of their own households [9].
Serbia has diverse landscapes, with plains in the north, limestone ranges and basins in the east and mountains and hills in the south and southeast. As in the rest of Europe, the most common and widespread human TBD in Serbia is Lyme borreliosis, along with other less frequently diagnosed diseases such as tick-borne encephalitis, human monocytic ehrlichiosis, tick-borne lymphadenopathy, and Crimean-Congo hemorrhagic fever (CCHF) [[10], [11], [12], [13]]. Apart from the endemicity of CCHF [14], little is known about the impact of TBDs on human health in Serbia. We hypothesize that other, less frequent TBDs are underdiagnosed due to restricted access to specialized laboratories that mainly operate within tertiary health care facilities.
Studies investigating the diversity of ticks and TBDs in humans and domestic animals inhabiting the same area are scarce in Serbia. Here we used a One Health approach to assess the circulation of TBPs within one rural household in northern Serbia, an area where tick infestations in animals and humans are frequently reported. We integrated medical observation and examination of family members with molecular diagnosis to screen for major TBPs in humans and animals, as well as in the ticks collected in the household located on the foothills of Fruška Gora, the largest mountain in the Vojvodina region in northern Serbia.
2. Materials and methods
2.1. Ethics statement
This study was approved by the ethics committee of the Faculty of Medicine in Novi Sad (Ethical approval no. 01-39/206/1) and conducted according to the Helsinki Declaration and the Patient Rights Law of the Republic of Serbia. Written informed consent for publication of this clinical case report was obtained from the patients. Handling of household animals and their blood samples was carried out in accordance with the EU Directive 2010/63/EU for animal experimentation.
2.2. Study precedents and design
2.2.1. Precedents of tick infestation and TBDs in the household
On 1 August 2019, a family with a previous history of tick infestation reported the presence of ticks on the outer walls of their house. This family of four members (father, mother and two daughters under the age of 18) lives in a one-story house of old construction. The family had five dogs and keeps alpine goats near the house for goat milk production (for family consumption). The house is located in the foothills of the Fruška Gora mountain (45.1857°N, 19.8042°E). Following this report, the family was advised to collect tick specimens and contact the Pasteur Institute in Novi Sad, the nearest large city. On 8 August 2019, a member of the family delivered a plastic vial containing 15 engorged ticks to the Novi Sad Pasteur Institute. All family members were examined for the presence of clinical signs of TBDs, but none developed any signs or symptoms within the 6 months following notification of the tick infestation.
On 18 June 2020, all family members (n = 4) came to the Novi Sad Pasteur Institute for Lyme borreliosis serology testing upon their request, because one of the daughters developed itching at sites of previous tick bites (upper arms and back). The itching started after a recent tick bite on the lower left leg: the location of itching corresponded to earlier tick bites, not to the recent tick bite. Because the lesions associated with the itching were similar to urticarial lesions, and there were no signs of any TBD at the site of the most recent tick bite, the patient was advised to apply chloropyramine cream (Synopen, Pliva, Croatia) locally. None of the family members reported any history of TBDs, except the father who developed Lyme arthritis in the shoulders and elbows more than 10 years ago. He did not recall any tick bite or lesion similar to erythema migrans before the manifestation of Lyme arthritis. He was hospitalized and treated with ceftriaxone intravenously (Longaceph, Galenika AD, Belgrade, Serbia). After the treatment, he was discharged from the hospital. Despite negative history of TBDs, all other family members reported numerous tick bites, as well as heavy tick infestation in the house and on animals in earlier months.
2.2.2. Study design using a One Health approach
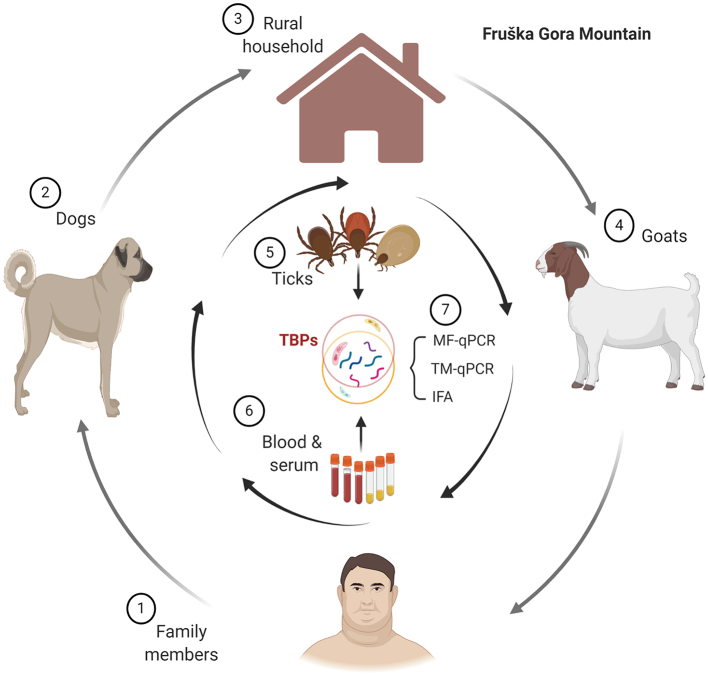
Based on these antecedents, a study was carried out to assess the circulation of TBPs in this rural household located in the Fruška Gora foothills using a One Health approach (Fig. 1). To do so, blood samples were collected from family members and the family's dogs and alpine goats. Ticks from dogs and the walls of the house were also collected. No ticks were found on the goats or the family members during the sampling period. The presence of TBP DNAs was assessed using microfluidic PCR (25 bacterial species, 7 parasite species, 5 bacterial genera, 3 parasite genera) on blood and tick samples and tick-borne encephalitis virus (TBEV) RNA was screened for using quantitative reverse-transcription PCR (RT-qPCR) on tick samples. In addition, Lyme borreliosis serology was carried out on patient sera.
Fig. 1.
Schematic diagram of the One Health approach used in this study. An epidemiological study was conducted in a rural household located in the foothills of the Fruška Gora mountain. The analysis included the molecular and serological diagnosis of several TBPs using microfluidic qPCR (MF-qPCR), TaqMan RT-qPCR (TQ-qPCR) and an indirect fluorescent antibody test (IFA). The analyses were performed on blood samples from humans (family members), dogs and alpine goats. In addition, tick samples were directly collected on the dogs and the walls of the house. Created with BioRender.com.
2.2.3. Sample collection and nucleic acid extraction
Blood samples were collected from family members (n = 4), the family's dogs (n = 5), and the female (n = 5) and male (n = 2) alpine goats. For each patient, 3 to 3.5 mL of blood was extracted in BD Vacutainer® SST™ or BD Vacutainer® spray-coated K2EDTA tubes (BD, Oakville, USA), respectively. For dogs and goats, 3 mL of blood was collected in BD Vacutainer® spray-coated K2EDTA tubes). Blood DNA was isolated using the Nucleospin Tissue kit (Macherey Nagel, Düren, Germany), according to the manufacturer's instructions.
Ticks were found and removed from four of the five dogs (n = 7) and collected on the house walls (n = 15). All collected ticks were identified with regard to species, sex and life stage, based on morphological features and standard taxonomic keys described in Estrada-Peña et al. [15], and conserved in 70% ethanol at 8 °C until further use. The ticks were pooled resulting in three and four pools for the ticks collected on the walls (five ticks per pool) and the dogs (one pool per dog), respectively.
Pooled ticks were homogenized using a Precellys 24 lyser/homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France) at 3724 ×g for 20 s with 2.8 mm stainless steel beads in 180 μL of Lysis buffer (T1 buffer) and 25 μL of proteinase K from the Nucleospin Tissue kit (Macherey Nagel). Homogenates were incubated for 3 h at 56 °C and DNA was extracted according to the manufacturer's instructions. Purified DNA was eluted in 50 μL elution buffer. Haemaphysalis punctata homogenates were also used for RNA extraction using the RNeasy Mini Kit (Qiagen, Germany).
2.3. DNA pre-amplification for microfluidic real-time PCR
To allow for better detection of pathogen DNA, total DNA was pre-amplified using the PreAmp Master Mix (Fluidigm, San Francisco, CA, USA), employed according to the manufacturer's instructions. Primers (targeting all pathogens) were pooled combining an equal volume of each primer for a final concentration of 200 nM for each primer. The reaction was performed in a final volume of 5 μL containing 1 μL PreAmp Master Mix, 1.25 μL pooled primers mix, 1.5 μL distilled water and 1.25 μL DNA. The thermocycling program consisted of one cycle at 95 °C for 2 min, 14 cycles at 95 °C for 15 s and 4 min at 60 °C. At the end of the cycling program the reactions were diluted 1:10 in Milli-Q ultrapure water. Pre-amplified DNAs were stored at −20 °C until needed.
2.4. Microfluidic real-time PCR
To detect major TBPs (25 bacterial species, 7 parasite species, 5 bacterial genera, 3 parasite genera), the BioMark™ real-time PCR system (Fluidigm, San Francisco, CA, USA) was used for high-throughput microfluidic real-time PCR amplification using 48.48 Dynamic Array™ IFC chips (Fluidigm, San Francisco, CA, USA). These chips dispense 48 PCR mixes and 48 samples into individual wells, after which on-chip microfluidics assemble real-time PCR reactions in individual chambers before thermal cycling, resulting in 2304 individual reactions. Briefly, amplifications were performed using 6-carboxyfluorescein (FAM)- and black hole quencher (BHQ1)-labeled TaqMan probes with PerfeCTa® qPCR ToughMix®, Low ROX™ (QuantaBio, Beverly, MA, USA) following the manufacturer's instructions. PCR cycling included 2 min at 50 °C, 10 min at 95 °C, followed by 40 cycles of two-step amplification of 15 s at 95 °C, and 1 min at 60 °C. One negative water control was included per chip. To determine if factors present in the sample inhibit the PCR, Escherichia coli strain EDL933 DNA was added to each sample as an internal inhibition control, and primers and probe specific for the E. coli eae gene were used. For more details regarding the development of this new high-throughput tool based on real-time microfluidic PCRs (test of sensitivity, specificity, and controls used), please see ref. [16].
2.5. Validation of microfluidic real-time PCR results using standard PCR and DNA sequencing
To confirm the microfluidic real-time PCR results, all samples positive for infectious agents underwent conventional and nested PCR assays using primers different from those of the BioMark™ system (Table 1). Amplicons were sequenced by Eurofins MWG Operon (Ebersberg, Germany) and assembled using BioEdit software (Ibis Biosciences, Carlsbad). The final nucleotide sequences were analyzed to identify the sequenced microorganisms using the GenBank database through the National Center for Biotechnology Information (NCBI; Bethesda, MD) Basic Local Alignment Sequence Tool (BLAST) search engine (www.ncbi.nlm.nih.gov/blast). Nucleotide sequence data reported in the present study are available in GenBank, EMBL and DDBJ databases under accession numbers MZ146328, MZ151073, and MZ146329.
Table 1.
Primer sets and PCR conditions used for the validation of microfluidic real-time PCR results.
| Pathogen | Primer sequences (5′ – 3′)a | Target gene | Amplicon size | PCR conditionsb | References |
|---|---|---|---|---|---|
| Hepatozoon canis | Outer primers | 18S rRNA | 660 bp | 35 cycles: | [16] |
| ATACATGAGCAAAATCTCAAC | 10 s 98 °C; 30 s 50 °C; 30 s 72 °C | ||||
| CTTATTATTCCATGCTGCAG | |||||
| Inner primers | 309 bp | 35 cycles: | [17] | ||
| GGTATGGTATTGGCTTACC | 10 s 98 °C; 30 s 51 °C; 30 s 72 °C | ||||
| CGAGCTTTTTAACTGCAACA | |||||
| Anaplasma spp. Ehrlichia spp. | Outer primers | 16S rRNA | 693 bp | 35 cycles: | [18] |
| GAACGAACGCTGGCGGCAAGC | 10 s 98 °C; 30 s 60 °C; 30 s 72 °C | ||||
| AGTAYCGRACCAGATAGCCGC | |||||
| Inner primers | 629 bp | 35 cycles: | |||
| TGCATAGGAATCTACCTAGTAG | 10 s 98 °C; 30 s 55 °C; 30 s 72 °C | ||||
| AGTAYCGRACCAGATAGCCGC | |||||
| Rickettsia spp. | Outer primers | OmpB | 475 bp | 35 cycles: | [19] |
| GTCAGCGTTACTTCTTCGATGC | 10 s 98 °C; 30 s 57 °C; 30 s 72 °C | ||||
| CCGTACTCCATCTTAGCATCAG | |||||
| Inner primers | 267 bp | 35 cycles: | |||
| CCAATGGCAGGACTTAGCTACT | 10 s 98 °C; 30 s 58 °C; 30 s 72 °C | ||||
| AGGCTGGCTGATACACGGAGTAA |
Y: T/C; R: A/G.
All PCR reactions: 30 sec 98°C initial activation; 10 min 72°C final extension.
2.6. Molecular detection of TBEV RNA using RT-qPCR on ticks
The possible circulation of TBEV in the household was evaluated using the ticks collected in the house. The presence of TBEV RNA was assessed using a probe-specific RT-qPCR targeting a 67 bp fragment of the 3′ noncoding region of the TBEV genome with the primers, F-TBE 1 (5’ GGGCGGTTCTTGTTCTCC 3′) and R-TBE 1 (5’ ACACATCACCTCCTTGTCAGACT 3′), and a TaqMan probe (5’ TGAGCCACCATCACCCAGACACA 3′) labeled with FAM [17]. RNA from TBEV isolate Ljubljana 1 [18] and water were used as positive and negative controls, respectively. The qPCR reactions were performed using a StepOne™ Real-Time PCR System (Applied Biosystems, California, USA).
2.7. Screening of human serum samples for the presence of anti-Borrelia antibodies
A fraction of the human blood samples was placed in vials and the blood allowed to clot. After 2000 ×g centrifugation for 10 min, serum samples were collected and inactivated at 56 °C. sera were used for the detection of anti-Borrelia IgM and IgG. Antigens from Borrelia afzelli, Borrelia garinii, and Borrelia burgdorferi strains CH and USA were used in a commercial immunofluorescence assay carried out as per the manufacturer's instructions (Euroimmun, Lübeck, Germany; Cat. No. FI 2138-1010-2 G and FI 2138-1010-2 M). Visible fluorescence reactions using sera diluted to ≥1:100 for IgG and to ≥1:10 for IgM were considered as positive. Fluorescence was analyzed on a microscope (Leica DM 3000, Wetzlar, Germany) with a mercury bulb light source using an N2.1 filter (Leica, Wetzlar, Germany) with an excitation wavelength of 515–560 nm.
3. Results
3.1. Identification of ticks from dogs and house walls
A total of 22 ticks were collected from the outer house walls (n = 15) and four dogs (n = 7). Following morphological characterization of the ticks, H. punctata (15 females, 68%) collected on the house walls was the most abundant tick species in this rural household (Supplementary Fig. S1), followed by Rhipicephalus sanguineus (4 males, 18%) and Ixodes ricinus (3 nymphs, 14%), which were found on the dogs. In this study, the dogs were identified as dog I-V. Mixed infestations with I. ricinus (nymphs) and R. sanguineus (adult males) were observed in dogs I, II, and III, and only R. sanguineus (adult male) was found on dog V. No tick infestation was observed on dog IV. Detailed information about tick species, developmental stage, tick size and location is available in Supplementary Table S1. No tick infestation was observed on the goats or the family members.
3.2. Evaluation of the presence of TBEV RNA in H. punctata ticks
Because the H. punctata ticks sampled from the house were engorged and this tick species was not found on dogs, we suspected that they fed on the goats nearby. Accordingly, once the goats were fenced off away from the house, ticks no longer appeared on the house. Considering that goats are known for their ability to act as a reservoir for TBEV circulation in nature, we tested for the presence of TBEV in the H. punctata ticks. However, none of the H. punctata samples tested positive for the presence of TBEV by PCR, whereas a positive signal was detected in the positive control sample (TBEV Ljubljana 1 isolate).
3.3. Tick-borne pathogens detected in tick and blood samples
The molecular diagnosis was performed on collected blood and tick samples using high-throughput microfluidic real-time PCR. For detection of pathogen DNA, the ticks were pooled, resulting in three pooled samples of H. punctata, and three of R. sanguineus and I. ricinus and one with R. sanguineus (Supplementary Table S1). Overall, five (31%, 5/16) blood and three (43%, 3/7) pooled tick samples were positive for at least one of the pathogens included in our detection system. A total of six different pathogens were identified in blood and tick samples. Positive signals were detected for the Rickettsia helvetica 23S—5S internal transcribed spacer (23S—5S ITS) in the pooled samples of R. sanguineus and I. ricinus ticks removed from dogs II and III and in the sample of R. sanguineus removed from dog V. The detection of R. helvetica 23S—5S ITS in the tick samples from dogs II, III and V concurred with positive signals for the Rickettsia spp. gltA gene in the microfluidic system. The other pathogen detected was Hepatozoon canis, present only in the R. sanguineus sample from dog V. The presence of H. canis was confirmed by positive detection of H. canis 18S rRNA, Apicomplexa 18S rRNA in the microfluidic system and by sequencing (accession number MZ146329) a fragment of H. canis 18S rRNA (Table 1). The tick infesting dog V tested positive for a mixed infection with R. helvetica and H. canis. None of the pooled H. punctata samples collected from the house walls tested positive for TBPs included in our assay.
Regarding the blood samples, Anaplasma phagocytophilum (confirmed by detection of A. phagocytophilum msp2, and Anaplasma spp. 16S rRNA in the microfluidic system) and Rickettsia monacensis (confirmed by detection of Rickettsia spp. gltA in the microfluidic system and sequencing of R. monacensis OmpB fragment (accession number MZ151073)) were detected in two of the dogs, whereas Rickettsia felis (confirmed by detection of Rickettsia spp. gltA and R. felis orfB in the microfluidic system) was detected in only one of the dogs. Anaplasma bovis was the only pathogen found in the goats tested. The presence of A. bovis was confirmed by detection of Anaplasma spp. 16S rRNA in the microfluidic system and by sequencing an A. bovis 16S rRNA fragment (accession number MZ146328). Dog II was diagnosed with the concomitant infection of A. phagocytophilum and R. felis, and dogs III and V were PCR-positive for R. monacensis and A. phagocytophilum infection, respectively. The occurrence of single and mixed infections of pathogens found in PCR-positive samples is summarized in Table 2. None of the blood samples collected from family members tested positive for the presence of TBPs.
Table 2.
Tick-borne pathogens detected using microfluidic real-time PCR.
| Tick-borne pathogens | Total |
|---|---|
| Pooled tick samples (n = 7) | |
| Total infected pooled tick samples (n = 3) | |
| Rickettsia helvetica | 3 |
| Hepatozoon canisa | 1 |
| Single infections (n = 2) | |
| R. helvetica | 2 |
| Mixed infections with two pathogens (n = 1) | |
| R. helvetica + H. canisa | 1 |
| Animal blood samples (n = 16) | |
| Number of infected blood samples (n = 5) | |
| Anaplasma phagocytophilum | 2 |
| Anaplasma bovisa | 1 |
| Rickettsia monacensisa | 2 |
| Rickettsia felis | 1 |
| Single infections (n = 4) | |
| A. phagocytophilum | 1 |
| A. bovisa | 1 |
| R. monacensisa | 2 |
| Mixed infections with two pathogens (n = 1) | |
| A. phagocytophilum + R. felis | 1 |
Species identified according to sequencing results.
3.4. Presence of anti-Borrelia antibodies in human samples
Using an immunofluorescence test, we found IgG seroreactivity against B. burgdorferi sensu stricto (s.s.) (i.e. strains USA and CH) antigens in the serum sample of one of the family members (i.e. father) at a titer of 1:400. The same sample was seronegative for IgM (cut-off titer value, 1:10) against B. burgdorferi s.s., B. afzelii, and B. garinii antigens. Other family member samples were seronegative for IgM (cut-off titer value, 1:10) and IgG (cut-off titer value, 1:100) against B. burgdorferi s.s., B. afzelii, and B. garinii antigens.
4. Discussion
Tick species identified on dogs in this study had previously been identified on domestic dogs in the Vojvodina region, in which I. ricinus and R. sanguineus were the most prevalent tick species [19]. I. ricinus and R. sanguineus are among the most common ectoparasites found infesting dogs and goats in Serbia, as well as in other countries in the Balkans such as North Macedonia, Montenegro, Romania and, Bosnia and Hercegovina [[20], [21], [22], [23]]. Infestations with R. sanguineus in houses is frequently reported in the literature [[24], [25], [26], [27]]. In contrast to urban environments, a rural household frequently includes several animal species and potential tick hosts, thereby increasing the risk of human exposure to diverse tick species that use domestic animals as hosts. H. punctata has previously been reported to have peaks of abundance in spring and autumn in Serbia and Bosnia, with adult female ticks being more prevalent than males (64.22% female ticks vs 35.78% male ticks prevalence) in one field study [28]. Although it is known that H. punctata infests goats [29], it is not common for them to be found on house walls. Anecdotally, the members of the family of the present study, had used a non-labeled substance to treat the tick infestation on the goats. It is possible that the substance was a repellent instead of a tick-killing formulation. Consequently, engorged ticks may have escaped the goat paddock to hide on the outer house walls for oviposition. Of note, the outer house walls on which the ticks were found faced the goat paddock. After the goats were fenced off in different area, no additional ticks were found on the walls of the house.
Although H. punctata has much lower importance for TBEV circulation in Central and Western Europe compared with I. ricinus [30], it is considered as a competent TBEV vector [31,32]. Knowing that TBEV foci are present in Fruška Gora [19], and that infected goats can act as reservoirs [33], engorged H. punctata may participate in the further dissemination of the virus. Here, TBEV RNA was not detected in engorged H. punctata ticks, suggesting that TBEV does not currently circulate in this rural household. However, prior to RNA extraction, H. punctata ticks were stored in ethanol, which may affect RNA quality and therefore hinder the detection of TBEV RNA. Further epidemiological screening for the presence of H. punctata in rural households in Serbia are required to address the risk posed by this tick species to human health.
In this study, A. phagocytophilum infection was confirmed in the family’'s dogs. Domestic dogs are considered as potential reservoirs for this pathogen in Serbia [34]. A. phagocytophilum is the causative agent of granulocytic anaplasmosis in humans and animals. The occurrence of this pathogen has been reported worldwide, mainly in areas of the northern hemisphere where is naturally transmitted by Ixodes ticks [35]. In Serbia, the presence of A. phagocytophilum was reported for the first time in a population of I. ricinus ticks, and the pathogen was subsequently identified in Haemaphysalis concinna and Dermacentor reticulatus ticks [36,37]. Furthermore, Rickettsia of the spotted fever group (SFG), R. monacensis and R. helvetica have previously been reported in ticks in Serbia [37]. R. helvetica and R. monacensis have also been described in I. ricinus ticks in Serbia [38], whereas R. felis was recently reported in a human blood sample [39].
Dogs cohabit with humans, and thereby confer a high risk of tick encounters and TBP infections in humans. Accordingly, dogs serve as sentinels or reservoirs and play an important role in the epidemiology of some zoonotic TBPs, including several Rickettsia spp. [40]. The three members of the SFG rickettsiae identified in this study have been associated with infections in dogs and ticks in many countries throughout Europe [41,42]. Interestingly, only R. felis and R. monacensis were detected in dogs, and R. helvetica was detected in pooled tick samples containing both R. sanguineus and I. ricinus ticks (pools from dogs II and III) or only R. sanguineus ticks (tick from dog V). These findings suggest that the ticks did not acquire R. helvetica from the tested dogs.
In Serbia, the occurrence of H. canis has been previously described in epidemiological surveys conducted on dogs [43], red foxes [44] and I. ricinus [19]. This study reports the presence of H. canis in R. sanguineus for the first time in the country. Considering that R. sanguineus is the main vector of H. canis [45], the results suggest that H. canis is transmitted by R. sanguineus in the studied area. Similarly, Gianelli et al. [46] also indicated that I. ricinus is not a biological vector of H. canis, because H. canis sporogony does not occur in I. ricinus ticks, but in R. sanguineus.
Here, we also provided the first evidence of the presence of A. bovis is an obligate intracellular bacterium of the genus Anaplasma (family Anaplasmataceae) and infects circulating monocytes [47,48]. This pathogen is commonly reported worldwide and has been associated with subclinical symptoms in small ruminants such as sheep and goats [[49], [50]]. In addition, Anaplasma ovis, also asymtomatic in goats [[50], [51], [52]], and A. bovis can coinfect small ruminants [50]. However, A. bovis was not detected in the goats sampled in this study. However, A. bovis infection, similarly to Anaplasma ovis infection [47], is asymptomatic in goats [[45], [46], [47]]. Recently, Jurković et al. [53] reported the first molecular confirmation of lethal cases of bovine anaplasmosis caused by Anaplasma marginale with concurrent infection of A. bovis and Theileria orientalis in Croatian cattle. Given that Croatia is a neighboring country of Serbia, and that goats have been described as a reservoir host of A. bovis, further studies should evaluate the impact of A. bovis in small ruminants and cattle in Serbia.
One of the family members showed IgG reactivity to B. burgdorferi s.l. complex antigens. Namely, the father was positive for B. burgdorferi s.s., a bacterial species known for its tropism for joints [54]. This bacterial species probably caused the previous disease (i.e. Lyme arthritis) reported by the father. Accordingly, the serum sample was reactive only for IgG, which is the dominant antibody isotope in late-stage Borrelia infection [55]. The bacterial members of the B. burgdorferi s.l. complex are the causative agents of Lyme borreliosis, described as a multisystemic TBD with high morbidity rates in humans. In Serbia, B. burgdorferi s.l. have been identified in competent vectors of the genus Ixodes, mainly I. ricinus, as well as in human serum samples [23,39]. The risk factors associated with human infection by Borrelia includes the presence and abundance of competent tick vectors in different types of habitats, a high prevalence of B. burgdorferi s.l. in ticks, and extended periods of possible exposure to tick bites [56]. The absence of Borrelia in the data collected from the molecular analysis of tick and animal samples echoes the low anti-Borrelia antibody seroprevalence within this family, despite a history of tick bites in this family. However, more extensive sampling of questing I. ricinus is required to obtain a better assessment of Borrelia infection prevalence in the region.
5. Conclusions
The study provides evidence of the effectiveness of a One Health approach (i.e., testing all the possible components of a localized epidemiological chain) for the assessment of zoonotic TBDs. To the best of our knowledge, this is the first report of SFG rickettsiae R. felis and R. monacensis infections in dogs in Serbia, as well as R. helvetica and H. canis in R. sanguineus ticks These results extend our knowledge of the potential vector spectrum and diversity of TBPs affecting human and animal health in Serbia. Further larger-scale epidemiological studies including new geographic regions are necessary to determine the spatial and temporal distributions of tick populations to improve local and regional tick control programs. We conclude that, despite the presence of TBPs in tick and vertebrate reservoirs, there is no chain of TBP infection across the epidemiological chain in the rural Fruška Gora household studied here. The integration of the One Health approach in surveillance programs will improve our understanding of the circulation of zoonotic TBPs in different epidemiological settings.
Detailed information on tick samples, sample pooling, tick species, developmental stage, tick size and location.
Supplementary Fig. 1.
The dorsal (to the left) and ventral (to the right) views of an engorged female specimen.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Acknowledgments
The authors would like to thank Dr. N. Knap Gašper, Dr. M. Korva and Prof. Dr. T. Avšić-Županc (University of Ljubljana, Faculty of Medicine, Institute of Microbiology and Immunology) for providing RNA from TBEV isolate Ljubljana 1.
Conflict of interest
The authors declare no competing interests.
Contributor Information
Sara Moutailler, Email: alejandro.cabezas@vet-alfort.fr.
Alejandro Cabezas-Cruz, Email: cabezasalejandrocruz@gmail.com.
References
- 1.de la Fuente J., Estrada-Pena A., Venzal J.M., Kocan K.M., Sonenshine D.E. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. J. Virtual Libr. 2008;13:6938–6946. doi: 10.2741/3200. [DOI] [PubMed] [Google Scholar]
- 2.Boulanger N., Boyer P., Talagrand-Reboul E., Hansmann Y. Ticks and tick-borne diseases. Médecine Mal. Infect. 2019;49:87–97. doi: 10.1016/j.medmal.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Pavlović I., Milanović V., Radović B., Ivanović S., Petrović M.P., Caro-Petrović V., Bojkovski J. Tick fauna of small ruminants in south part of Serbia, with emphasis to North Kosovo. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Vet. Med. 2020;77:38–42. doi: 15835/buasvmcn-vm:2019.0034. [Google Scholar]
- 4.Dantas-Torres F., Chomel B.B., Otranto D. Ticks and tick-borne diseases: a one health perspective. Trends Parasitol. 2012;28:437–446. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Paddock C.D., Telford S.R. Inst. Med. Crit. Needs Gaps Underst. Prev. Amelior. Resolut. Lyme Tick-Borne Dis. Short-Term Long-Term Outcomes-Workshop Rep. Wash. DC Natl. Acad. Pr. 2011. Through a glass, darkly: the global incidence of tick-borne diseases; pp. 1–41. [Google Scholar]
- 6.Plowright R.K., Parrish C.R., McCallum H., Hudson P.J., Ko A.I., Graham A.L., Lloyd-Smith J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017;15:502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waltner-Toews D. Zoonoses, One Health and complexity: wicked problems and constructive conflict. Philos. Trans. R. Soc. B Biol. Sci. 2017;372:20160171. doi: 10.1098/rstb.2016.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonenshine D.E., Macaluso K.R. Microbial invasion vs. tick immune regulation. Front. Cell. Infect. Microbiol. 2017;7 doi: 10.3389/fcimb.2017.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demma L.J., Traeger M.S., Nicholson W.L., Paddock C.D., Blau D.M., Eremeeva M.E., Dasch G.A., Levin M.L., Singleton J., Zaki S.R., Cheek J.E., Swerdlow D.L., McQuiston J.H. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N. Engl. J. Med. 2005;353:587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- 10.Arsić B., Gligić A., Ristanović E., Lako B., Potkonjak A., Perunicić M., Pavlović M. A case of human monocytic ehrlichiosis in Serbia. Srp. Arh. Celok. Lek. 2014;142:79–82. doi: 10.2298/sarh1402079a. [DOI] [PubMed] [Google Scholar]
- 11.Poluga J., Barac A., Katanic N., Rubino S., Milosevic B., Urosevic A., Mitrovic N., Kelic I., Micic J., Stevanovic G. Tick-borne encephalitis in Serbia: a case series. J. Infect. Dev. Ctries. 2019;13:510–515. doi: 10.3855/jidc.11516. [DOI] [PubMed] [Google Scholar]
- 12.Gajinov Z., Roš T., Ivkov-Simić M., Gajić B., Prćić S., Matić M. Tick-borne lymphadenopathy acquired in Serbia: report of two cases. Vojnosanit. Pregl. 2018;75:1134–1137. [Google Scholar]
- 13.Banović P., Obregón D., Mijatović D., Simin V., Stankov S., Budakov-Obradović Z., Bujandrić N., Grujić J., Sević S., Turkulov V., Díaz-Sánchez A.A., Cabezas-Cruz A. Tick-borne encephalitis virus seropositivity among tick infested individuals in Serbia. Pathogens. 2021;10:301. doi: 10.3390/pathogens10030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmeti S., Berisha L., Halili B., Ahmeti F., von Possel R., Thomé-Bolduan C., Michel A., Priesnitz S., Reisinger E.C., Günther S., Krüger A., Sherifi K., Jakupi X., Hemmer C.J., Emmerich P. Crimean-congo hemorrhagic fever, Kosovo, 2013–2016. Emerg. Infect. Dis. 2019;25:321–324. doi: 10.3201/eid2502.171999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estrada-Peña A., Mihalca A.D., Petney T., editors. Ticks of Europe and North Africa: A Guide to Species Identification. Springer International Publishing; 2017. [DOI] [Google Scholar]
- 16.Michelet L., Delannoy S., Devillers E., Umhang G., Aspan A., Juremalm M., Chirico J., van der Wal F.J., Sprong H., Boye Pihl T.P., Klitgaard K., Bødker R., Fach P., Moutailler S. High-throughput screening of tick-borne pathogens in Europe. Front. Cell. Infect. Microbiol. 2014;4 doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwaiger M., Cassinotti P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2003;27:136–145. doi: 10.1016/s1386-6532(02)00168-3. [DOI] [PubMed] [Google Scholar]
- 18.Avsic-Zupanc T., Poljak M., Maticic M., Radsel-Medvescek A., LeDuc J.W., Stiasny K., Kunz C., Heinz F.X. Laboratory acquired tick-borne meningoencephalitis: characterisation of virus strains. Clin. Diagn. Virol. 1995;4:51–59. doi: 10.1016/0928-0197(94)00062-y. [DOI] [PubMed] [Google Scholar]
- 19.Potkonjak A., Gutiérrez R., Savić S., Vračar V., Nachum-Biala Y., Jurišić A., Kleinerman G., Rojas A., Petrović A., Baneth G., Harrus S. Molecular detection of emerging tick-borne pathogens in Vojvodina, Serbia. Ticks Tick-Borne Dis. 2016;7:199–203. doi: 10.1016/j.ttbdis.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Omeragic J. Ixodid ticks in Bosnia and Herzegovina. Exp. Appl. Acarol. 2011;53:301–309. doi: 10.1007/s10493-010-9402-8. [DOI] [PubMed] [Google Scholar]
- 21.Dumitrache M.O., Gherman C.M., Cozma V., Mircean V., Györke A., Sándor A.D., Mihalca A.D. Hard ticks (Ixodidae) in Romania: surveillance, host associations, and possible risks for tick-borne diseases. Parasitol. Res. 2012;110:2067–2070. doi: 10.1007/s00436-011-2703-y. [DOI] [PubMed] [Google Scholar]
- 22.Pavlović I., Jovčevski S., Rogožarski D., Csordas F., Mitrović N., Mijatovic I., Marčić D., Ćirković D., Šekler M., Ristić M. Biodiversity of ticks and fleas of dogs in the western balkans - preliminary examinations. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Vet. Med. 2016;73:220–223. doi: 15835/buasvmcn-vm:11344. [Google Scholar]
- 23.Simin V., Lalošević D., Mijatović D., Tomanović S., Miljević M., Čabrilo B., Bogdan I., Banović P. Borellia burgdorferi infection in removed ticks and anti-borrelia antibodies in infested patients admitted to the Pasteur institute, Novi Sad. Vet. Glas. 2020:8. doi: 10.2298/vetlg200527008s. [DOI] [Google Scholar]
- 24.Hansford K.M., Pietzsch M., Cull B., Medlock J.M. Brown dog tick infestation of a home in England. Vet. Rec. 2015;176:129–130. doi: 10.1136/vr.h496. [DOI] [PubMed] [Google Scholar]
- 25.Parola P., Socolovschi C., Jeanjean L., Bitam I., Fournier P.-E., Sotto A., Labauge P., Raoult D. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Negl. Trop. Dis. 2008;2 doi: 10.1371/journal.pntd.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renvoisé A., Delaunay P., Blanchouin E., Cannavo I., Cua E., Socolovschi C., Parola P., Raoult D. Urban family cluster of spotted fever rickettsiosis linked to Rhipicephalus sanguineus infected with Rickettsia conorii subsp. caspia and Rickettsia massiliae. Ticks Tick-Borne Dis. 2012;3:389–392. doi: 10.1016/j.ttbdis.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Buczek A., Buczek W. Importation of ticks on companion animals and the risk of spread of tick-borne diseases to non-endemic regions in Europe. Animals. 2021;11:6. doi: 10.3390/ani11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milutinović M.J., Radulović Ž.M. Ecological notes on ticks, Acari: Ixodidae, in Serbia, central regions. Acta Vet. (Beograd) 2002;52:49–58. [Google Scholar]
- 29.Santos-Silva M.M., Beati L., Santos A.S., De Sousa R., Núncio M.S., Melo P., Santos-Reis M., Fonseca C., Formosinho P., Vilela C., Bacellar F. The hard-tick fauna of mainland Portugal (Acari: Ixodidae): an update on geographical distribution and known associations with hosts and pathogens. Exp. Appl. Acarol. 2011;55:85–121. doi: 10.1007/s10493-011-9440-x. [DOI] [PubMed] [Google Scholar]
- 30.Liebig K., Boelke M., Grund D., Schicht S., Springer A., Strube C., Chitimia-Dobler L., Dobler G., Jung K., Becker S. Tick populations from endemic and non-endemic areas in Germany show differential susceptibility to TBEV. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-71920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de la Fuente J. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008;6938 doi: 10.2741/3200. [DOI] [PubMed] [Google Scholar]
- 32.Dobler G., Erber W., Schmitt H.-J. Global Health Press Pte Limited; 2018. The TBE Book. [Google Scholar]
- 33.Ilic M., Barbic L., Bogdanic M., Tabain I., Savic V., Kosanovic Licina M.L., Kaic B., Jungic A., Vucelja M., Angelov V., Kovacevic M., Roncevic D., Knezevic S., Stevanovic V., Slavuljica I., Lakoseljac D., Vickovic N., Bubonja-Sonje M., Hansen L., Vilibic-Cavlek T. Tick-borne encephalitis outbreak following raw goat milk consumption in a new micro-location, Croatia, June 2019. Ticks Tick-Borne Dis. 2020;11:101513. doi: 10.1016/j.ttbdis.2020.101513. [DOI] [PubMed] [Google Scholar]
- 34.Potkonjak A., Vračar V., Savić S., Lako B., Radosavljević V., Cincović M., Suvajdžić L., Jurišić A., Petrović A. The seroprevalence of Anaplasma phagocytophilum infection in dogs in the Autonomous Province of Vojvodina, Serbia. Vet. Arh. 2015;85:385–394. [Google Scholar]
- 35.Stuen S., Granquist E.G., Silaghi C. Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013;3 doi: 10.3389/fcimb.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milutinović M., Masuzawa T., Tomanović S., Radulović Ž., Fukui T., Okamoto Y. Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Francisella tularensis and their co-infections in host-seeking Ixodes ricinus ticks collected in Serbia. Exp. Appl. Acarol. 2008;45:171–183. doi: 10.1007/s10493-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 37.Tomanović S., Chochlakis D., Radulović Z., Milutinović M., Cakić S., Mihaljica D., Tselentis Y., Psaroulaki A. Analysis of pathogen co-occurrence in host-seeking adult hard ticks from Serbia. Exp. Appl. Acarol. 2013;59:367–376. doi: 10.1007/s10493-012-9597-y. [DOI] [PubMed] [Google Scholar]
- 38.Radulović Ž., Chochlakis D., Tomanović S., Milutinović M., Tselentis Y., Psaroulaki A. First detection of spotted fever group Rickettsiae in ticks in Serbia. Vector-Borne Zoonotic Dis. 2010;11:111–115. doi: 10.1089/vbz.2009.0254. [DOI] [PubMed] [Google Scholar]
- 39.Banović P., Díaz-Sánchez A.A., Galon C., Simin V., Mijatović D., Obregón D., Moutailler S., Cabezas-Cruz A. Humans infested with Ixodes ricinus are exposed to a diverse array of tick-borne pathogens in Serbia. Ticks Tick-Borne Dis. 2020:101609. doi: 10.1016/j.ttbdis.2020.101609. [DOI] [PubMed] [Google Scholar]
- 40.Chomel B. Tick-borne infections in dogs-an emerging infectious threat. Vet. Parasitol. 2011;179:294–301. doi: 10.1016/j.vetpar.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 41.Hii S.F., Kopp S.R., Abdad M.Y., Thompson M.F., O’Leary C.A., Rees R.L., Traub R.J. Molecular evidence supports the role of dogs as potential reservoirs for rickettsia felis. Vector-Borne Zoonotic Dis. 2011;11:1007–1012. doi: 10.1089/vbz.2010.0270. [DOI] [PubMed] [Google Scholar]
- 42.Lauzi S., Maia J.P., Epis S., Marcos R., Pereira C., Luzzago C., Santos M., Puente-Payo P., Giordano A., Pajoro M., Sironi G., Faustino A. Molecular detection of Anaplasma platys, Ehrlichia canis, Hepatozoon canis and Rickettsia monacensis in dogs from Maio Island of Cape Verde archipelago. Ticks Tick-Borne Dis. 2016;7:964–969. doi: 10.1016/j.ttbdis.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Gabrielli S., Otašević S., Ignjatović A., Savić S., Fraulo M., Arsić-Arsenijević V., Momčilović S., Cancrini G. Canine Babesioses in noninvestigated areas of Serbia. Vector Borne Zoonotic Dis. Larchmt. N. 2015;15:535–538. doi: 10.1089/vbz.2015.1797. [DOI] [PubMed] [Google Scholar]
- 44.Juwaid S., Sukara R., Penezić A., Mihaljica D., Veinović G., Kavallieratos N.G., Ćirović D., Tomanović S. First evidence of tick-borne protozoan pathogens, Babesia sp. and Hepatozoon canis, in red foxes (Vulpes vulpes) in Serbia. Acta Vet. Hung. 2019;67:70–80. doi: 10.1556/004.2019.008. [DOI] [PubMed] [Google Scholar]
- 45.Baneth G. Perspectives on canine and feline hepatozoonosis. Vet. Parasitol. 2011;181:3–11. doi: 10.1016/j.vetpar.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 46.Giannelli A., Ramos R.A.N., Dantas-Torres F., Mencke N., Baneth G., Otranto D. Experimental evidence against transmission of Hepatozoon canis by Ixodes ricinus. Ticks Tick-Borne Dis. 2013;4:391–394. doi: 10.1016/j.ttbdis.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Dumler J.S., Barbet A.F., Bekker C.P., Dasch G.A., Palmer G.H., Ray S.C., Rikihisa Y., Rurangirwa F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 48.Raoult D., Parola P. CRC Press; 2007. Rickettsial Diseases. [Google Scholar]
- 49.Uilenberg G. International collaborative research: significance of tick-borne hemoparasitic diseases to world animal health. Vet. Parasitol. 1995;57:19–41. doi: 10.1016/0304-4017(94)03107-8. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z., Ma M., Wang Z., Wang J., Peng Y., Li Y., Guan G., Luo J., Yin H. Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl. Environ. Microbiol. 2012;78:464–470. doi: 10.1128/AEM.06848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ceci L., Iarussi F., Greci B., Lacinio R., Fornelli S., Carreli G. Retrospective study of hemoparasites in cattle in southern Italy by reverse line blot hybridization. J. Vet. Med. Sci. 2014;76:869–875. doi: 10.1292/jvms.13-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cabezas-Cruz A., Gallois M., Fontugne M., Allain E., Denoual M., Moutailler S., Devillers E., Zientara S., Memmi M., Chauvin A., Agoulon A., Vayssier-Taussat M., Chartier C. Epidemiology and genetic diversity of Anaplasma ovis in goats in Corsica, France. Parasit. Vectors. 2019;12:3. doi: 10.1186/s13071-018-3269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jurković D., Mihaljević Ž., Duvnjak S., Silaghi C., Beck R. First reports of indigenous lethal infection with Anaplasma marginale, Anaplasma bovis and Theileria orientalis in Croatian cattle. Ticks Tick-Borne Dis. 2020;11:101469. doi: 10.1016/j.ttbdis.2020.101469. [DOI] [PubMed] [Google Scholar]
- 54.van der Heijden I.M., Wilbrink B., Rijpkema S.G., Schouls L.M., Heymans P.H., van Embden J.D., Breedveld F.C., Tak P.P. Detection of Borrelia burgdorferi sensu stricto by reverse line blot in the joints of Dutch patients with Lyme arthritis. Arthritis Rheum. 1999;42:1473–1480. doi: 10.1002/1529-0131(199907)42:7<1473::AID-ANR22>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 55.Petzke M., Schwartz I. Borrelia burgdorferi pathogenesis and the immune response. Clin. Lab. Med. 2015;35:745–764. doi: 10.1016/j.cll.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Zanzani S.A., Rimoldi S.G., Manfredi M., Grande R., Gazzonis A.L., Merli S., Olivieri E., Giacomet V., Antinori S., Cislaghi G., Bestetti G., Nan K., Sala V., Gismondo M.R., Atzori C., De Faveri E. Lyme borreliosis incidence in Lombardy, Italy (2000–2015): spatiotemporal analysis and environmental risk factors. Ticks Tick-Borne Dis. 2019;10:101257. doi: 10.1016/j.ttbdis.2019.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed information on tick samples, sample pooling, tick species, developmental stage, tick size and location.