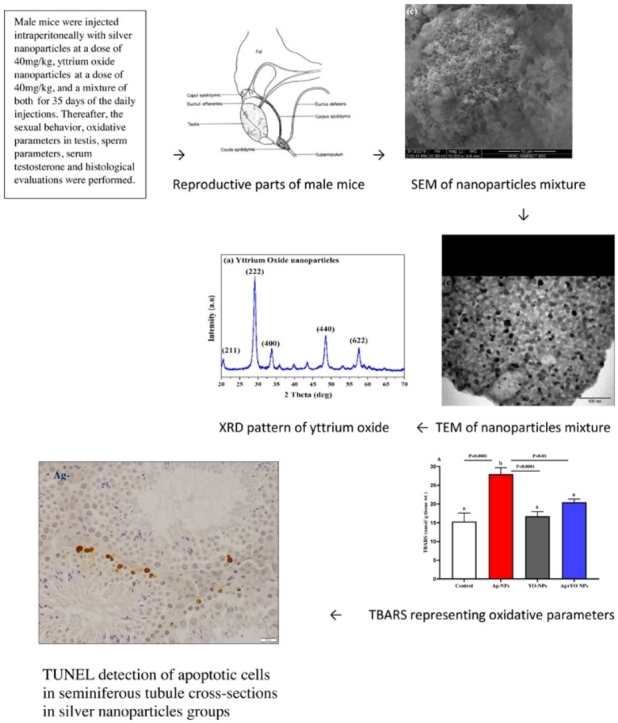
Graphical abstract
Alleviation of silver nanoparticle-induced sexual behavior and testicular parameters dysfunction in male mice by yttrium oxide nanoparticles.
Gasem Mohammad Abu-Taweel, Hani Manssor Albetran, Mohsen Ghaleb Al-Mutary, Mohammad Ahmad, It Meng Low.
Keywords: Nanoparticles, Sexual behavior, Spermatotoxicity, Testosterone, Apoptosis
Highlights
-
•
Exposure to silver nanoparticles decreased the weight of the reproductive organs, sexual behavior, oxidative defense parameters, sperm count and their motility in male mice.
-
•
In addition, serum testosterone, apoptotic germ cells and testicular histology were also disrupted due to silver nanoparticles.
-
•
Yttrium oxide nanoparticles have protective effects on sexual behavior and spermatotoxicity induced by silver nanoparticles in male mice.
-
•
The toxicity of silver nanoparticles altered testicular functions that were effectively ameliorated by yttrium oxide nanoparticles.
Abstract
Silver nanoparticles (Ag-NPs) can easily cross through the blood-testis barrier and encourage reproductive dysfunction. This study investigated the protective effects of yttrium oxide nanoparticles (YO-NPs) on sexual behavior and spermatotoxicity induced by Ag-NPs in male mice. Twenty-four male mice were separated into four groups and injected intraperitoneally once a week as the following: group I (Ag-NPs at the dose of 40 mg/kg), group II (YO-NPs at the dose of 40 mg/kg), group III (Ag + YO NPs at the doses of 40 mg/kg, each) and group IV (control; distilled water). After 35 days of the injections, the sexual behavior, oxidative parameters in testis, sperm parameters, serum testosterone, apoptotic germ cells and testicular histology were evaluated. Our findings showed that Ag-NPs decreased the weight of the reproductive organs, sexual behavior, oxidative defense parameters, sperm count and motility of male mice. In addition, the apoptotic cells in testicular cross-sections and TBARS level increased after Ag-NPs exposure when compared to other groups. However, the YO-NPs had protective effects in the studied parameters of testicles and minimized the Ag-NPs toxicity in male mice. In conclusion, the results revealed that the toxicity of Ag-NPS altered testicular functions in male mice that were effectively ameliorated by YO-NPs.
1. Introduction
Silver-nanoparticles (Ag-NPs) are extensively utilized in many applications such as food packaging, anticancer drugs, antimicrobial agents, dye effluent treatment and industrial purposes due to their physical, chemical, and biological properties [[1], [2], [3], [4], [5], [6]]. However, these usages increase the discharge of Ag-NPs in the environment and interact with the health of living organisms [[7], [8], [9]]. The major biological deteriorations of Ag-NPs are membranes injuries, protein dysfunction, mitochondrial destruction, DNA damage and ROS production [10,11]. Fetotoxicity and neurobehavioral disorders in mice offspring were found after prenatal exposure of Ag-NPs [12,13]. Furthermore, they encourage apoptosis and histological alteration in hepatocytes and associate with genotoxicity in lymphocytes of mice [14]. Meanwhile, Ag-NPs have the capability to accumulate in different organs, penetrate the biological barriers and cause toxic stress in many tissues [15].
Some studies have shown the negative effects of Ag-NPs on reproductive indicators such as increasing abnormal sperm, altering testes structure, decreasing the expression of spermatogenesis genes, lowering serum testosterone, enhancing apoptosis in germ cells and disrupting hormonal regulation of reproductive processes in the male rats [[16], [17], [18], [19], [20], [21]]. Moreover, the small size of Ag-NPs has a toxic effect on germ cells by decreasing the sperm concentration, increasing DNA damage and changing oxidative stress levels [22,23].
Recently, many studies investigated some products to relieve the toxic effects of nanoparticles (NPs). With high surface reactivity and biocompatibility, NPs of yttrium oxide (Y2O3) could be a promising agent in the field of nano-medicine [24]. Furthermore, although the toxicity effects of YO-NPs were observed at high concentrations [25], however, their protective functions were reported at low doses as a therapeutic, neuroprotective and detoxicant substance [[26], [27], [28]]. The injected rat once a day by YO-NPs at dose 45 mg/kg for two weeks recovered the pancreas from oxidative stress, apoptosis, necrosis and toxicity resulted by diazinon [29,30]. The role of YO-NPs is clearly via anti-pancreatitis activity and via decreasing the oxidative stress in mitochondria and endoplasmic reticulum induced by cerulein hyperstimulation [31]. Another study has shown that the combination of cerium oxide and YO-NPs provides potential protection against apoptosis and lipid peroxidation in the brain of rat treated with lead [32]. In addition, YO-NPs are excellent antioxidants for scavenging free radicals and treating retinal degeneration after acute light stress [33]. To our best knowledge, no studies are available for the effects of Ag-NPs and YO-NPs in combination in order to exploring the therapeutic effects of YO-NPs to reduce the toxicity of Ag-NPs on the reproductive parameters in male mice. However, the objective of this study was to investigate the ameliorating effects of YO-NPs against Ag-NPs - induced reproductive function of male mice such as organs weight, sexual behavior, oxidative stress, sperm parameters, testosterone concentration, cellular apoptosis and histological evaluations.
2. Materials and methods
2.1. Chemicals
The nanoparticles and chemicals were purchased from Sigma-Aldrich (Inc., NSW, Australia and St. Louis MO, USA) unless otherwise stated.
2.2. Animals
Twenty-four male mice (SWR/J, 25−35 g, age ∼75 days) were housed for one week before the experiment in the experimental room to adapt with stable temperature (∼25 °C), 12-h dark-light period and free access to standard food and water.
2.3. NPs preparation and examination
NPs were dissolved in distilled water and scattered by ultrasonic vibration (VGT-800, 40 kHz, China) for 40 min, followed by stirring on a vortex machine before every injection of each animal. No agglomeration of the NPS suspensions was observed visually during the short time (approximately 2−5 min).
A Rigaku Benchtop Miniflex X-ray diffractometer (XRD, analyser with Cu–Kα radiation), transmission electron microscope (TEM, FEI Morgagni 268), and scanning electron microscope (SEM, IRMC-INSPECT S50) were used to characterize the silver nanoparticles (Ag-NPs, Mw =107.87 g/mol) and yttrium-oxide nanoparticles (YO-NPs, Mw =225.81 g/mol).
2.4. Experimental design
The males were separated into four groups, six animals in each cage and they were injected intraperitoneally (0.2 mL/animal) once a week as follows: group I received Ag-NPs at a dose of 40 mg/kg of bw, group II received YO-NPs at a dose of 40 mg/kg of bw, group III received the mixture of both Ag-NPs and YO-NPs (40 mg/kg+40 mg/kg of bw), and finally group IV received distilled water (control). After 35 days of the weekly injections, the sexual behavior, oxidative parameters in testis, sperm parameters, serum testosterone and histological evaluations were performed. This study was designed as an in vivo experiment and was approved by the Institutional Review Board (IRB) of Imam Abdulrahman Bin Faisal University (IAU) (Dammam, Saudi Arabia).
2.5. Body weight assessment
The males were weighed every seven days of the experiment, while the weights of reproductive organs (testes, prostate gland, seminal vesicle and epididymidis) were recorded immediately after dissecting the animals.
2.6. Sexual behavior
According to Abu-Taweel [34], the parameters of sexual behavior of the treated male mice were estimated during three hours by placing age-matched female in the same cage. The number of times of mounting, copulation frequency, approach and following, threatening and biting, nose or vagina smelling, rears and wall rears, squat, wash and digging of male mice were recorded.
2.7. Sperm count and motility
After recording the sexual behavior, the males were sacrificed and their epididymis was separated from the right testicle of dissected mice. A small piece (two millimeters) was taken from the caudal epididymis, placed in 35-mm petri dish containing two milliliters of phosphate physiological solution (PBS) at 37 °C and placed in shaker incubator (Daiki, Seoul, Republic Korea) for eight minutes. The sperm count and their motility were evaluated according to the procedure of Li, et al. [35], Abu-Taweel [34] and Al-Mutary and Abu-Taweel [36].
2.8. Testosterone level analysis
Blood samples were collected directly from the animal heart in heparinized tube and centrifuged by refrigerated centrifuge (Hermle, Baden-Württemberg, Germany) to obtain the serum and stored at -20 °C until testosterone concentration assessment according to the commercial kits’ procedure.
2.9. Testicular histology
Some of the excised testes from experimental groups were fixed in 10 % formaldehyde saline, embedded in paraffin wax, cut into 5 μm thick sections, placed on coated glass slides (Yancheng Yongda, Jiangsu, China) and were stained by hematoxylin and eosin for morphometric examination of the testis tissue according to the method of Cordeiro, et al. [37].
2.10. Estimation of oxidative stress indices
The oxidative biomarkers in testis were measured after homogenizing a small piece of the excised testis. The testicular tissue supernatant was mixed with 1 mL TCA (20 %), 2 mL TBA (0.67 %), and then placed in a water bath at 100 °C for one hour, cooled, centrifuged, and finally obtained the supernatant for lipid peroxidation measurement. The thiobarbituric acid reactive substances (TBARS) were measured for lipid peroxidation at 535 nm in spectrophotometer (SHIMADZU, Tokyo, Japan) [38]. In addition, the supernatant was also applied for measuring some more oxidative stress indices like GSH, GST, SOD and CAT activities according to the methods described in a previous study [39].
2.11. Apoptosis detection in testicular tissue
A TUNEL Assay Kit HRP-DAB (ab206386, Abcam, UK) was used for detecting cell death in tissue sections of mice testis according to the manufacturer’s instructions. Briefly, the tissue sections were deparaffinized and rehydrated at room temperature. The sections on coated glass slides (Yancheng Yongda, Jiangsu, China) were incubated in Proteinase K solution for 20 min at room temperature and then rinsed with Tris-buffered saline (1X TBS: 20 mM Tris pH 7.6 and 140 mM sodium chloride) for five minutes. To inactivate endogenous peroxidases, the dried slides were incubated in 100 μL of 3% H2O2 at room temperature for five minutes, washed with 1X TBS and left to dry. Then, the specimen was covered with 100 μL TdT (terminal deoxynuleotidyl transferase) equilibration buffer for 30 min and with 40 μL of TdT labeling mixture solution for 90 min at room temperature. The slides were incubated in a stop buffer at room temperature for five minutes to terminate the labeling reaction, and followed by washing with 1X TBS. Then, the sections were covered with one hundred microliters of blocking buffer at room temperature for five minutes, with the conjugate solution for 30 min and with diaminobenzidine (DAB) for 15 min respectively. Finally, the sections treated with methyl green counterstain, dehydrated with ethanol and mounted with DPX. The positive germ was colored as brown. The numbers of these cells were counted in twenty seminiferous tubules (S.T.) sections of each animal, and the S.T. was selected randomly.
2.12. Statistical analysis
GraphPad Prism 8.4.3 software was used for statistical analysis. The weight of reproductive organs, oxidative biomarkers, sperm parameters, serum testosterone level, apoptotic cells and morphometric measurements of testicular seminiferous tubes in triplicates were statistically analyzed by one-way ANOVA with Tukey's multiple comparisons tests and expressed as mean ± SEM. The sexual behavior indices were statistically performed by ANOVA and Mann-Whitney U test and expressed as median with range. A p-value of 0.05 or less was considered as significant.
3. Results
3.1. Morphological investigations of NPs
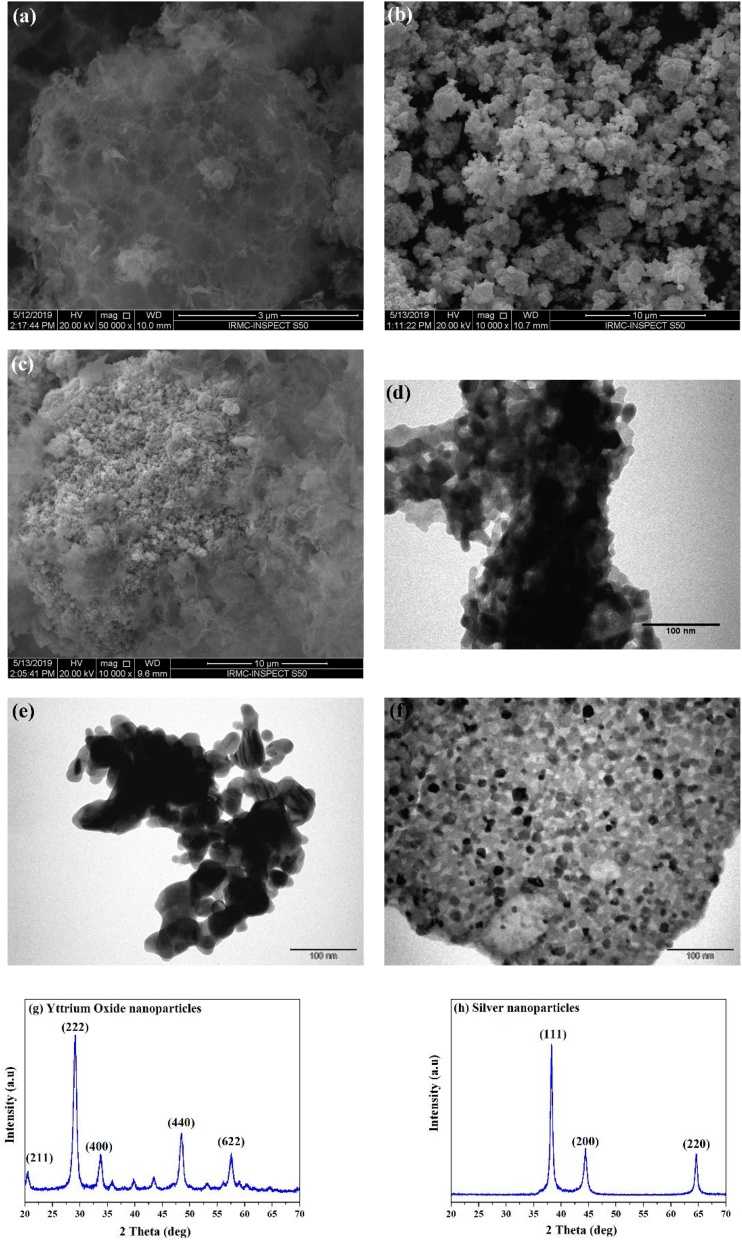
SEM and TEM were used to study the morphologies, sizes, and microstructures of nanoparticles. Images in Fig. 1(a–c) show the typical SEM micrographs of nano-sized YO-NPs, Ag-NPs, and a mixture of 50 % YO-NPs and 50 % Ag-NPs. The YO-NPs had a nebulous-structured morphology, and the Ag-NPs consisted of agglomerates of primary particles.
Fig. 1.
SEM micrographs of (a) yttrium-oxide nanoparticles, (b) silver nanoparticles, (c) nanoparticles mixture; TEM micrographs of (d) yttrium-oxide nanoparticles, (e) silver nanoparticles, (f) nanoparticle mixture; XRD patterns of (g) yttrium-oxide, and (h) silver nanoparticles.
The irregular-shaped, agglomerated nanoparticle formation of the YO-NPs, Ag-NPs, and the mixture were confirmed by TEM as shown in Fig. 1(d–f). Nanoparticles formed irregular-shaped agglomerates with a variation in size. The ImageJ® software (Version 1.48e, National Institutes of Health, USA) was used to measure the nanoparticle size. The mean particle sizes and standard deviations for the YO-NPs, Ag-NPs, and the mixture were 18 ± 5 nm, 19.5 ± 5 nm, and 14 ± 5, respectively. Fig. 1(f) shows the TEM image-contrast variations in the nanoparticle mixture which reflect a variation in atomic number, with the higher-atomic-number silver scattering X-rays more than the yttrium oxide nanoparticles.
3.2. 4.2. XRD analysis
The International Centre for Diffraction Data (ICDD) powder diffraction file database (Card No. 89-5591) and the Joint Committee on Powder Diffraction Standards (Card No. 04-0783) were used to obtain XRD patterns of YO-NPs (Fig. 1g) and Ag-NPs (Fig. 1h), respectively. The crystalline nanoparticles contained no impurities or second-phase peaks. The average NP crystallite size (L) was determined by using Scherer’s equation [40], where k is the shape factor (0.94), λ is the X-ray wavelength (0.15419 nm), β is the line broadening at half the maximum intensity (the full width at half maximum, FWHM, in radians), and θ is the Bragg angle.
| (1) |
Peaks at 2θ of 29.08° and 38.25° corresponded to (222) and (111) planes for the YO-NPs and Ag-NPs, respectively. The crystallite sizes YO-NPs and Ag-NPs were computed to be 11.14 and 19.97 nm, respectively. Comparable crystallite sizes were yielded by averaging particle diameters from TEM imaging.
3.3. Body and reproductive organs weight
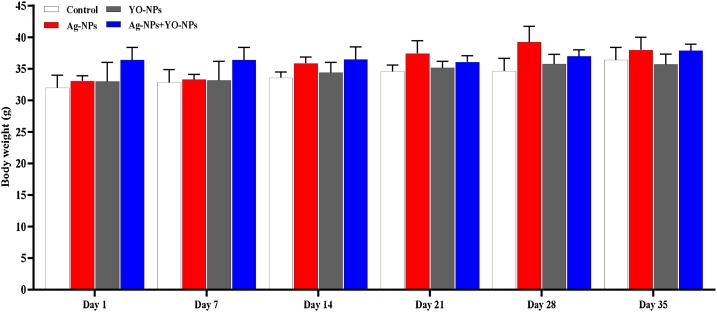
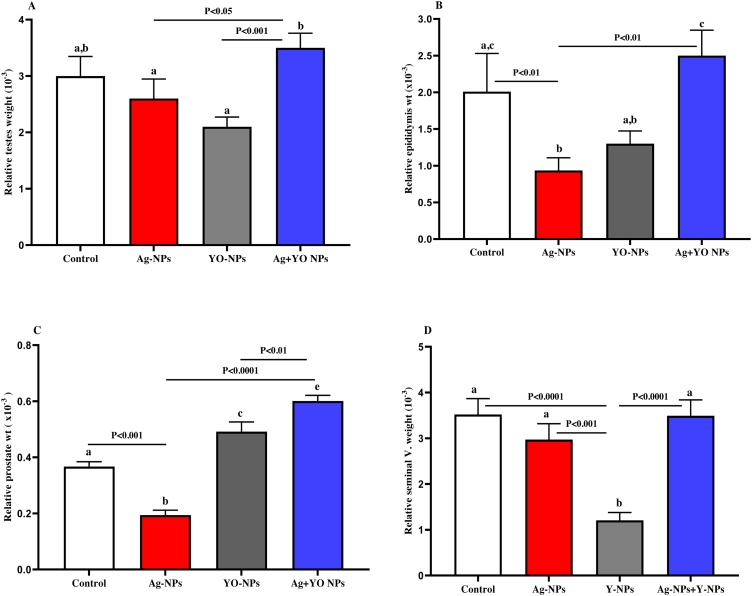
Although there was an increase in the body weight of male mice during the experiment, no significance between the groups was noted (Fig. 2). The male mice exposed to Ag-NPs did not significantly differ in the relative weight (RW) of testes as compared to the control and YO-NPs groups, while the RW of the testes was increased significantly in the Ag + YO NPs group compared to Ag-NPs group (Fig. 2A). The RW of epididymis was significantly lower in Ag-NPs compared to control group and higher in Ag + YO NPs group in comparison with Ag-NPs group (Fig. 2B). The injection of male mice with Ag-NPs caused a significant depletion in the RW of the prostate compared to control group, and an increase in Ag + YO NPs and YO-NPs groups compared with control group (Fig. 2C). The RW of seminal vesicle was decreased significantly in YO-NPs group in comparison with Ag + YO NPs, Ag-NPs and control groups (Fig. 2D).
Fig. 2.
The body weight of male mice during the experiment for 35 days, Data represented as mean ± SEM.
3.4. Sexual behavior of male mice
The male mice exposed to Ag-NPs showed a significant decrease in all sexual behaviour parameters in comparison to control group. Moreover, YO-NPs demonstrated protective activity against Ag-NPs toxicity (Table 1A, Table 1B) (Fig. 3).
Table 1A.
Parameters of sexual behavior of the treated male mice estimated during three hours by placing age-matched female in the same cage.
| Group | Median number (with ranges) of acts and postures | |||||||
| Approach | Following | Naso-Nasal contact | Naso-Genital contact | Mount | Pelvic thrust | Threat | Bite | |
| Control | 30.00 (27.00–33.00) | 21.00 (17.00–42.00) | 5200 (49.00–72.00) | 43.00 (30.00–60.00) | 10.00 (9.00–12.00) | 6.00 (6.00–9.00) | 9.00 (6.00–18.00) | 6.00 (3.00–9.00) |
| Ag-NPs | 6.00 *** (0.00–9.00) | 10.00 *** (5.00–13.00) | 22.00 *** (18.00–26.00) | 18.00 *** (8.00–2200) | 3.00 *** (0.00–3.00) | 0.00 *** (0.00–3.00) | 63.00 *** (33.00–78.00) | 12.00 ** (3.00–15.00) |
| YO-NPs | 27.00 (18.00–30.00) | 20.00 (14.00–25.00) | 55.00 (42.00−66.00) | 42.00 (28.00−45.00) | 8.00 (6.00−9.00) | 5.00 (2.00−7.00) | 15.00 (11..00 – 20.00) | 8..00 (2.00–12.00) |
| Ag + YO NPs | 16.00 ### (10.00−21.00) | 15.00 # (11.00–17 .00) | 45.00 ## (25.00–48.00) | 30.00 # (6.00–24.00) | 6.00 # (0.00–7.00) | 4..00 # (0.00–5.00) | 21.00 ## (18.00–30.00) | 9.00 (5.00–13.00) |
**and significantly different (p < 0.05, p < 0.01 and p < 0.001 ) respectively from the control.#,##and### significantly different from Ag-NPs by ANOVA and Mann-Whitney U test.
Table 1B.
Parameters of median numbers of acts and postures behavior of the treated male mice during sexual activities estimated during three hours by placing age-matched female in the same cage.
| Groups | Median number (with ranges) of acts and postures | ||||
| Wall rears | Rears | Wash | Squat | Digging | |
| Control | 22.00 (17.00–30.00) | 16.00 (14.00–18.00) | 4.00 (3.00–6.00) | 1.00 (0.00–2.00) | 7.00 (2.00–10.00) |
| Ag-NPs | 4.00 ** (3.00–8.00) | 2.00 *** (1.00–4.00) | 1.00 ** (0.00–2.00) | 9.00 *** (2.00–12.00) | 1.00 *** (1.00–4.00) |
| YO-NPs | 19.00 (13.00−22.00) | 13.00 (11.00–14.00) | 5.00 (00.00−7.00) | 2.00 (00.00–3.00) | 8.00 (1.00–11.00) |
| Ag + YO NPs | 14.00 ### (10.00−18.00) | 9.00 # (4.00–9.00) | 3.00 # (00.00−4.00) | 5.00 # (00.00−6.00) | 6.00 # (3.00−9.00) |
and significantly different (p < 0.01 and p < 0.001) respectively from the control.# and ### significantly different (p < 0.05 and p < 0.001).
from Ag-NPs by ANOVA and Mann-Whitney U test.
Fig. 3.
A-D. The relative weight of testes, epididymis, prostate and seminal vesicle of male mice at the end of experiment, Data represented as mean ± SEM.
3.5. Sperm parameters and testosterone level
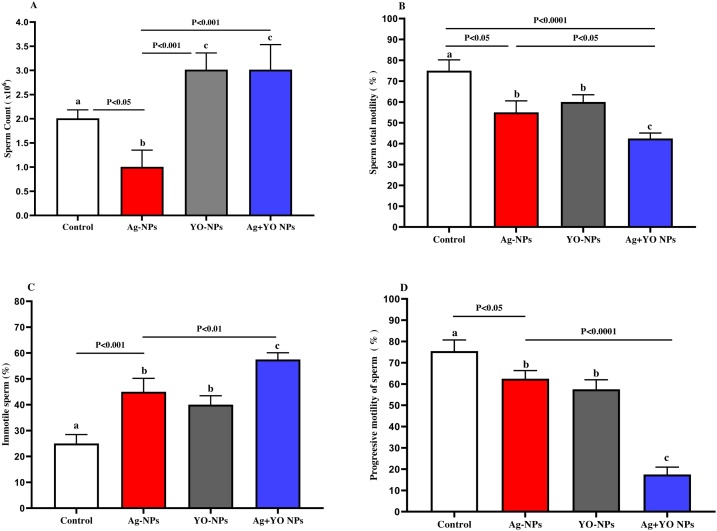
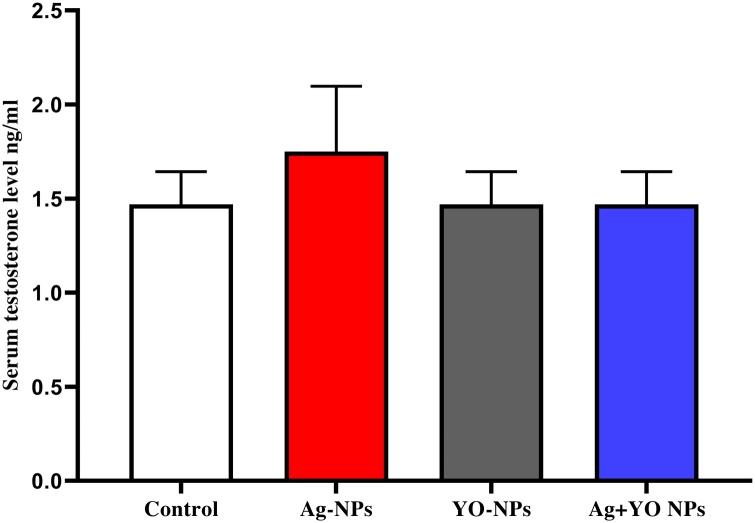
Male mice injected with Ag-NPs showed a significant decrease in epididymis sperm count compared with control, YO-NPs and Ag + YO NPs groups (Fig. 4A). The total motility of epididymal sperm was significantly greater in non-treated males compared with Ag-NPs group and lower in Ag + YO NPs group in comparison with Ag-NPs group (Fig. 4B). A lowest immotile percent of sperm was found in treated groups; Ag-NPs, YO-NPs, and Ag + YO NPs compared to untreated group and in Ag + YO NPs group compared to Ag-NPs, YO-NPs groups (Fig. 4C). Furthermore, a lower percent of progressive motile sperm was found in Ag + YO NPs group compared with other groups at a significant level of P < 0.0001, and a higher percent in the control group in comparison with Ag-NPs and YO-NPs (Fig. 4D). With regard to testosterone results, no significant differences between experimental groups were found (Fig. 5).
Fig. 4.
A-D. The sperm parameters of epididymis male mice at the end of experiment, Data represented as mean ± SEM.
Fig. 5.
The serum testosterone concentration in male mice at the end of experiment, Data represented as mean ± SEM.
3.6. Oxidative parameters in testis
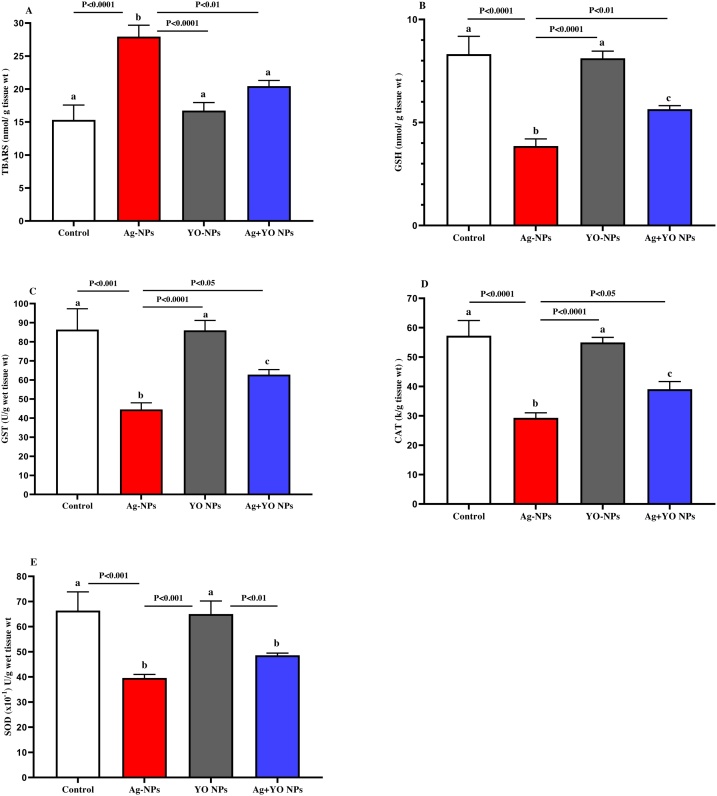
Results in Fig. 6(A–E) showed the effect of NPs on oxidative parameters in the testis. A significant increase in TBARS level was noted at the Ag-NPs group in comparison with other groups. Furthermore, the GSH, GST, SOD and CAT results showed a decrease in their activities at Ag-NPs group compared to other groups. However, YO-NPs alone or with Ag-NPs had the ability to reduce the level of TBARS and to increase GSH, GST, SOD and CAT concentrations.
Fig. 6.
A-E. The oxidative parameters in testis of male mice at the end of experiment, Data represented as mean ± SEM.
3.7. Testis morphometric and apoptosis cells
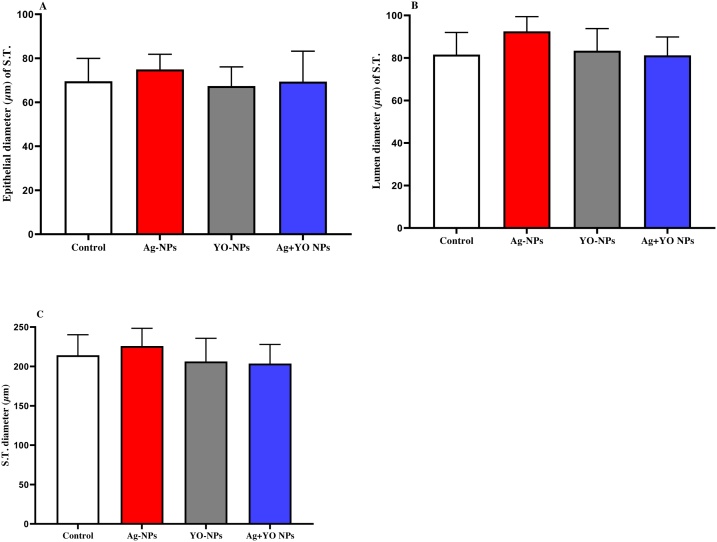
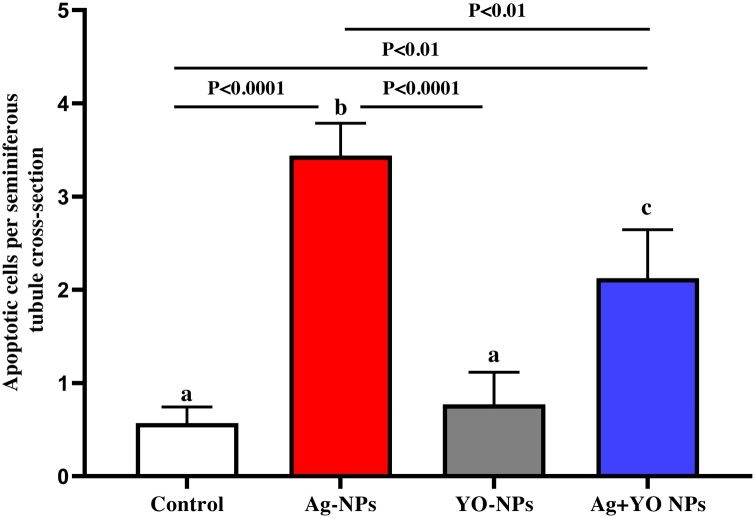
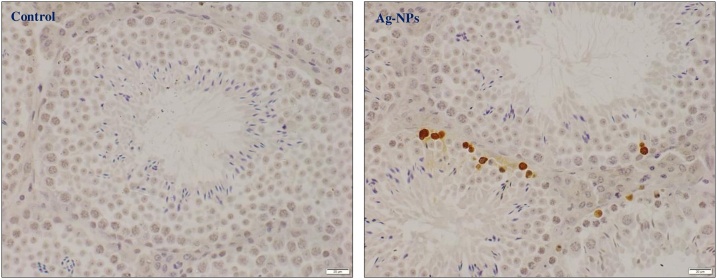
Fig. 7 shows the results of epithelial, lumen, seminiferous tubules diameters in the testis, with no significant differences between groups. In contrast, an increase was found in apoptotic cells per seminiferous tubule cross-section in Ag-NPs group compared to control and YO-NPs groups. In addition, the apoptotic cells in seminiferous tubules decreased after exposure with Ag + YO-NPs compared to Ag-NPs group (Fig. 8). The extensive apoptotic cells in testis were shown in spermatogonia and spermatocytes in Ag-NPs and Ag + YO-NPs groups (Fig. 9).
Fig. 7.
A-C. The epithelial, lumen and seminiferous tubule diameters of the testis, Data represented as mean ± SEM.
Fig. 8.
Apoptotic cells per seminiferous tubule cross-sections, Data represented as mean ± SEM.
Fig. 9.
TUNEL detection of apoptotic cells in seminiferous tubule cross-sections in control and Ag-PNs groups. Scale bar =20 μm, Data represented as mean ± SEM.
4. Discussion
Recently, with the rapid development in the uses of nanoparticles in the medical and industrial fields, many researchers have focused on devising new methods to reduce the toxicity of these materials [[41], [42], [43], [44]]. In this study, we have examined the sexual behavior, body and weight of reproductive organs, spermatotoxicity, serum testosterone level, apoptosis in germ cells, testicular histopathology and antioxidant biomarkers in the testis of mice exposed to Ag-NPs and the protective effects of YO-NPs against Ag-NPs toxicity. The results demonstrated the properties of antioxidant potency of YO-NPs against the toxic effects induced by Ag-PNs in male mice.
The animal weight is utilized as a helpful indicator to decide the toxicity of drugs on the treated animals [45]. Some studies have mentioned a decrease in body weight of animals during the experiment of Ag-NPs treatment [46,47]; however, others have not noted any significant change [16,48]. In our results, although there was no significant change observed in body weight of male mice, the Ag-NPs is clearly found to have toxic effects on testes, epididymis and prostate relative weights. These findings in the toxicity of NPs on reproductive organs of treated animals are consistent with the observations of Thakur, et al. [49], Olugbodi, et al. [50] and Greco, et al. [51], but are in conflict with other studies which revealed that the exposure to Ag-NPs has no effect on testicular weight [22,[52], [53], [54]]. Our results in the oxidative biomarkers of the testicle showed an increase in oxidative stress (TBARS molecules) and a decrease in anti-oxidative protein (GSH) and enzymes (GST, SOD and CAT) levels in males treated with Ag-NPs. The decrease in testicular weight and an increase in oxidative stress due to exposure to Ag-NPs demonstrated that there were direct interferences with the spermatogenesis process [50]. Moreover, Ag-NPs can penetrate the blood testicular barrier and reach the seminiferous tubules and affect sperm production and quality [55]. The lowering in sperm count, total and progressive motility and increasing in immotile sperm in this study are consistent with those of Lafuente, et al. [16]. The characteristics of Ag-NPs (such as a size and shape) can impair mitochondrial function before penetration their membrane [56]. In addition, the increased ROS after Ag-NPs exposure may contribute to consume the endogenous antioxidants and interfere with mitochondrial respiratory chain and lead to weak sperm motility [57,58]. A previous report mentioned that NPs accumulate in the tails and heads of spermatozoa and reduce 25 % sperm motility [59]. It is well known that testosterone contributes in the regulation of spermatogenesis. In this study, there was no change in the testosterone level between the groups despite the decrease in sperm count after Ag-NPs exposure which conflicts with the observation by Olugbodi, et al. [50]. The spermatogenesis passes through many phases before ending sperm production inside the lumen of the seminiferous tubule. The toxicity of Ag-NPs may work on these stages and induce germ cells apoptosis in mice testis which thereby reduce sperm count. Supportively, our findings in apoptosis detection in testis cross-sections confirmed extensive apoptotic cells observed after exposure to Ag-NPs. Furthermore, it is well known that germ cells do not produce androgen receptors (ARs), and Sertoli cells are the only testicle cells able to generate these ARs [[60], [61], [62]], wherefore another possible toxicity of Ag-NPs in this study may be via ARs in Sertoli cells.
The significant decreases in the sexual behavior indices of male mice after Ag-NPs treatment were noted in this study. These results do not concur with Garcia, et al. [63], who stated that no effects on sexual behavior of CD1 mice were observed after intravenous injection with Ag-NPs at a dose 1 mg/kg over 12 days. The decreasing in reproductive weights and non-significant change in testosterone level in this study may give a possible explanation of Ag-NPs effects on appetite and thereby the sexual behavior. Egecioglu, et al. [64] reported that the appetite hormone (ghrelin) is required for sexual behavior in male mice.
Recently, the uses of NPs to resist the toxic effects on reproductive system induced by some molecules have been grown [[65], [66], [67]]. Because of the redox chemistry of YO-NPs, they were completely known as an antioxidant characteristic to fight free radicals [29]. In this study, YO-NPs revealed protective activities in the testes, epididymis and prostate weights and sperm count, although we did not observe these effects on sperm motility. Interestingly, the best effect on the antioxidant defense system of treated males was evident by minimizing TBARS level and enhancing anti-oxidative parameters (GSH, GST, CAT, and SOD). Similarly, it has been found that ZnO-NPs decreased testicular-stress and enhanced the antioxidant markers [67,68]. In this study, YO-NPs effectively reduced the testicular apoptotic induced by Ag-NPs in male mice. Hosseini, et al. [69] found that YO-NPs enhanced the survival rate of rat pancreatic islets and ameliorated the oxidative stress-mediated apoptotic pathway. In addition, YO-NPs rescue cellular membranes from oxidative stress-induced cell injury and therefore form cell apoptosis [70]. Also, investigating sperm count, reproductive organs weight and testicular apoptotic showed that YO-NPs reduced the toxicity stress caused by Ag-PNs. Furthermore, Ce-NPs improved the sperm count, motility and viability after toxicity malathion-induced in rats [71]. Despite the effectiveness of YO-NPs to reduce the Ag-NPs toxicity during this study in some parameters, however, this activity is not shown in all parameters. It may be better to pre-treat the animals with YO-NPs instead of the treatment after Ag-NPs exposure [33]. However, the NPs effects differ depending on the synthesis method (chemical or biological synthesis) and coating agent used [43,44].
In conclusion, Ag-NPs were capable of suppressing the weight of reproductive organs, sexual behavior, sperm count and motility, and oxidative defense proteins in male mice. Furthermore, Ag-NPs increased MDA level and apoptotic cells in testicular tissue of male mice. Interestingly, YO-NPs improved Ag-NP-induced spermatotoxicity and testicular oxidative stress in male mice.
Author’s contribution in this work
Gasem Mohammad Abu-Taweel: Conceptualization, Methodology, Validation, Investigation, Supervision, Project Administration, Funding acquisition, Resources and Writing – Review and Editing. Hani Manssor Albetran: Methodology, Validation, Software, Formal Analysis, Investigation, Visualization, Funding acquisition and Writing – Original Draft. Mohsen Ghaleb Al-Mutary: Methodology, Validation, Software, Formal Analysis, Investigation, Visualization, Funding acquisition and Writing – Original Draft. Mohammad Ahmad: Visualization, Validation, and Writing – Original Draft. It Meng Low: Writing – Review and Editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The authors would like to thank Prof. Abdulhadi Baykal, and Dr. Sultan Akhtar at Institute for Research and Medical Consultations (IRMC), Imam Abdulrahman Bin Faisal University (IAU), Dammam, Saudi Arabia for assistance with SEM, TEM, and XRD.
Handling Editor Dr. A.M Tsatsakis
References
- 1.Iqbal S., Fakhar-e-Alam M., Akbar F., Shafiq M., Atif M., Amin N., Ismail M., Hanif A., Farooq W.A. Application of silver oxide nanoparticles for the treatment of cancer. J. Mol. Struct. 2019;1189:203–209. [Google Scholar]
- 2.Marimuthu S., Antonisamy A.J., Malayandi S., Rajendran K., Tsai P.-C., Pugazhendhi A., Ponnusamy V.K. Silver nanoparticles in dye effluent treatment: a review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B, Biol. 2020;205 doi: 10.1016/j.jphotobiol.2020.111823. [DOI] [PubMed] [Google Scholar]
- 3.Deshmukh S.P., Patil S.M., Mullani S.B., Delekar S.D. Silver nanoparticles as an effective disinfectant: a review. Mater. Sci. Eng. C. 2019;97:954–965. doi: 10.1016/j.msec.2018.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhury H., Pandey M., Lim Y.Q., Low C.Y., Lee C.T., Marilyn T.C.L., Loh H.S., Lim Y.P., Lee C.F., Bhattamishra S.K., Kesharwani P., Gorain B. Silver nanoparticles: advanced and promising technology in diabetic wound therapy. Mater. Sci. Eng. C. 2020;112 doi: 10.1016/j.msec.2020.110925. [DOI] [PubMed] [Google Scholar]
- 5.Valsalam S., Agastian P., Arasu M.V., Al-Dhabi N.A., Ghilan A.-K.M., Kaviyarasu K., Ravindran B., Chang S.W., Arokiyaraj S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. And its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B, Biol. 2019;191:65–74. doi: 10.1016/j.jphotobiol.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Carbone M., Donia D.T., Sabbatella G., Antiochia R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. - Sci. 2016;28(4):273–279. [Google Scholar]
- 7.Marambio-Jones C., Hoek E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010;12(5):1531–1551. [Google Scholar]
- 8.Tortella G.R., Rubilar O., Durán N., Diez M.C., Martínez M., Parada J., Seabra A.B. Silver nanoparticles: toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020;390 doi: 10.1016/j.jhazmat.2019.121974. [DOI] [PubMed] [Google Scholar]
- 9.Ahamed M., AlSalhi M.S., Siddiqui M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta. 2010;411(23):1841–1848. doi: 10.1016/j.cca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Liao C., Li Y., Tjong S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019;20(2):449. doi: 10.3390/ijms20020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco J., Tomás-Hernández S., García T., Mulero M., Gómez M., Domingo J.L., Sánchez D.J. Oral exposure to silver nanoparticles increases oxidative stress markers in the liver of male rats and deregulates the insulin signalling pathway and p53 and cleaved caspase 3 protein expression. Food and Chem. Toxicol.: an Int. J. Publ. Br. Indu. Biol. Res. Assoc. 2018;115:398–404. doi: 10.1016/j.fct.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Mozafari M., Khoradmehr A., Danafar A., Miresmaeili M., Kalantar S.M. Toxic effects of maternal exposure to silver nanoparticles on mice fetal development during pregnancy. Birth Defects Res. 2020;112(1):81–92. doi: 10.1002/bdr2.1605. [DOI] [PubMed] [Google Scholar]
- 13.Ghaderi S., Tabatabaei S.R.F., Varzi H.N., Rashno M. Induced adverse effects of prenatal exposure to silver nanoparticles on neurobehavioral development of offspring of mice. J. Toxicol. Sci. 2015;40(2):263–275. doi: 10.2131/jts.40.263. [DOI] [PubMed] [Google Scholar]
- 14.Al Gurabi M.A., Ali D., Alkahtani S., Alarifi S. In vivo DNA damaging and apoptotic potential of silver nanoparticles in Swiss albino mice. Onco. Ther. 2015;8:295–302. doi: 10.2147/OTT.S77572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferdous Z., Nemmar A. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020;21(7):2375. doi: 10.3390/ijms21072375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lafuente D., Garcia T., Blanco J., Sánchez D.J., Sirvent J.J., Domingo J.L., Gómez M. Effects of oral exposure to silver nanoparticles on the sperm of rats. Reprod. Toxicol. 2016;60:133–139. doi: 10.1016/j.reprotox.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Falchi L., Khalil W.A., Hassan M., Marei W.F.A. Perspectives of nanotechnology in male fertility and sperm function. Int. J. Vet. Sci. Med. 2018;6(2):265–269. doi: 10.1016/j.ijvsm.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakur M., Gupta H., Singh D., Mohanty I.R., Maheswari U., Vanage G., Joshi D.S. Histopathological and ultra structural effects of nanoparticles on rat testis following 90 days (Chronic study) of repeated oral administration. J. Nanobiotechnol. 2014;12 doi: 10.1186/s12951-014-0042-8. 42-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed S.M., Abdelrahman S.A., Shalaby S.M. Evaluating the effect of silver nanoparticles on testes of adult albino rats (histological, immunohistochemical and biochemical study) J. Mol. Histol. 2017;48(1):9–27. doi: 10.1007/s10735-016-9701-4. [DOI] [PubMed] [Google Scholar]
- 20.Dziendzikowska K., Krawczyńska A., Oczkowski M., Królikowski T., Brzóska K., Lankoff A., Dziendzikowski M., Stępkowski T., Kruszewski M., Gromadzka-Ostrowska J. Progressive effects of silver nanoparticles on hormonal regulation of reproduction in male rats. Toxicol. Appl. Pharmacol. 2016;313:35–46. doi: 10.1016/j.taap.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Elsharkawy E.E., Abd El-Nasser M., Kamaly H.F. Silver nanoparticles testicular toxicity in rat. Environ. Toxicol. Pharmacol. 2019;70 doi: 10.1016/j.etap.2019.103194. [DOI] [PubMed] [Google Scholar]
- 22.Gromadzka-Ostrowska J., Dziendzikowska K., Lankoff A., Dobrzyńska M., Instanes C., Brunborg G., Gajowik A., Radzikowska J., Wojewódzka M., Kruszewski M. Silver nanoparticles effects on epididymal sperm in rats. Toxicol. Lett. 2012;214(3):251–258. doi: 10.1016/j.toxlet.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Moradi-Sardareh H., Basir H.R.G., Hassan Z.M., Davoudi M., Amidi F., Paknejad M. Toxicity of silver nanoparticles on different tissues of Balb/C mice. Life Sci. 2018;211:81–90. doi: 10.1016/j.lfs.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Akhtar M.J., Ahamed M., Alrokayan S.A., Ramamoorthy M.M., Alaizeri Z.M. High surface reactivity and biocompatibility of Y2O3 NPs in human MCF-7 epithelial and HT-1080 fibro-blast cells. Molecules. 2020;25(5):1137. doi: 10.3390/molecules25051137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panyala A., Chinde S., Kumari S.I., Rahman M.F., Mahboob M., Kumar J.M., Grover P. Comparative study of toxicological assessment of yttrium oxide nano- and microparticles in Wistar rats after 28 days of repeated oral administration. Mutagenesis. 2019;34(2):181–201. doi: 10.1093/mutage/gey044. [DOI] [PubMed] [Google Scholar]
- 26.Song X., Shang P., Sun Z., Lu M., You G., Yan S., Chen G., Zhou H. Therapeutic effect of yttrium oxide nanoparticles for the treatment of fulminant hepatic failure. Nanomedicine. 2019;14(19):2519–2533. doi: 10.2217/nnm-2019-0154. [DOI] [PubMed] [Google Scholar]
- 27.Schubert D., Dargusch R., Raitano J., Chan S.W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 2006;342(1):86–91. doi: 10.1016/j.bbrc.2006.01.129. [DOI] [PubMed] [Google Scholar]
- 28.Sandhir R., Yadav A., Sunkaria A., Singhal N. Nano-antioxidants: An emerging strategy for intervention against neurodegenerative conditions. Neurochem. Int. 2015;89:209–226. doi: 10.1016/j.neuint.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Khaksar M.R., Rahimifard M., Baeeri M., Maqbool F., Navaei-Nigjeh M., Hassani S., Moeini-Nodeh S., Kebriaeezadeh A., Abdollahi M. Protective effects of cerium oxide and yttrium oxide nanoparticles on reduction of oxidative stress induced by sub-acute exposure to diazinon in the rat pancreas. J. Trace Elements in Med. Boil.: Organ Soc. Miner. Trace Elem. (GMS) 2017;41:79–90. doi: 10.1016/j.jtemb.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Rahimifard M., Khaksar M.R., Baeeri M., Navaei Nigjeh M., Moeini Nodeh S., Abdollahi M. Improving function of rat pancreas, by using yttrium oxide nanoparticles in sub-acute diazinon exposure. Toxicol. Lett. 2016;258:S264. [Google Scholar]
- 31.Khurana A., Anchi P., Allawadhi P., Kumar V., Sayed N., Packirisamy G., Godugu C. Yttrium oxide nanoparticles reduce the severity of acute pancreatitis caused by cerulein hyperstimulation, Nanomedicine: nanotechnology. Biol. Med. 2019;18:54–65. doi: 10.1016/j.nano.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Hosseini A., Sharifi A.M., Abdollahi M., Najafi R., Baeeri M., Rayegan S., Cheshmehnour J., Hassani S., Bayrami Z., Safa M. Cerium and yttrium oxide nanoparticles against lead-induced oxidative stress and apoptosis in rat Hippocampus. Biol. Trace Elem. Res. 2015;164(1):80–89. doi: 10.1007/s12011-014-0197-z. [DOI] [PubMed] [Google Scholar]
- 33.Mitra R.N., Merwin M.J., Han Z., Conley S.M., Al-Ubaidi M.R., Naash M.I. Yttrium oxide nanoparticles prevent photoreceptor death in a light-damage model of retinal degeneration. Free Radic. Biol. Med. 2014;75:140–148. doi: 10.1016/j.freeradbiomed.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abu-Taweel G.M. Curcumin palliative effects on sexual behavior, fertility and reproductive hormones disorders in mercuric chloride intoxicated mice offspring. J. King Saud Univ. - Sci. 2020;32(2):1293–1299. [Google Scholar]
- 35.Li X., Yi H., Wang H. Sulphur dioxide and arsenic affect male reproduction via interfering with spermatogenesis in mice. Ecotoxicol. Environ. Saf. 2018;165:164–173. doi: 10.1016/j.ecoenv.2018.08.109. [DOI] [PubMed] [Google Scholar]
- 36.Al-Mutary M.G., Abu-Taweel G.M. Effects of pomegranate juice on the sexual behavior, fertility and protective activity against aluminum exposure in male mice. J. King Saud Univ. - Sci. 2020 [Google Scholar]
- 37.Cordeiro F., Goncalves V., Jr., Moreira N., Slobodticov J.I., de Andrade Galvao N., de Souza Spinosa H., Bonamin L.V., Bondan E.F., Ciscato C.H.P., Barbosa C.M., Bernardi M.M. Ivermectin acute administration impaired the spermatogenesis and spermiogenesis of adult rats. Res. Vet. Sci. 2018;117:178–186. doi: 10.1016/j.rvsc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Esterbauer H., Cheeseman K.H. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Meth. Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 39.Mulero M., Romeu M., Giralt M., Folch J., Nogués M.R., Fortuño A., Sureda F.X., Linares V., Cabré M., Paternáin J.L., Mallol J. Oxidative stress-related markers and langerhans cells in a hairless rat model exposed to UV radiation. J. Toxicol. Environ. Res., Part A. 2006;69(14):1371–1385. doi: 10.1080/15287390500471187. [DOI] [PubMed] [Google Scholar]
- 40.Low I.M., Albetran H., Prida V.M., Vega V., Manurung P., Ionescu M. A comparative study on crystallization behavior, phase stability, and binding energy in pure and Cr-doped TiO2 nanotubes. J. Mater. Res. 2013;28(3):304–312. [Google Scholar]
- 41.Balmuri S.R., Selvaraj U., Kumar V.V., Anthony S.P., Tsatsakis A.M., Golokhvast K.S., Raman T. Effect of surfactant in mitigating cadmium oxide nanoparticle toxicity: implications for mitigating cadmium toxicity in environment. Environ. Res. 2017;152:141–149. doi: 10.1016/j.envres.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Pinzaru I., Coricovac D., Dehelean C., Moacă E.-A., Mioc M., Baderca F., Sizemore I., Brittle S., Marti D., Calina C.D., Tsatsakis A.M., Şoica C. Stable PEG-coated silver nanoparticles – a comprehensive toxicological profile. Food Chem. Toxicol. 2018;111:546–556. doi: 10.1016/j.fct.2017.11.051. [DOI] [PubMed] [Google Scholar]
- 43.Docea A.O., Calina D., Buga A.M., Zlatian O., Paoliello M.M.B., Mogosanu G.D., Streba C.T., Popescu E.L., Stoica A.E., Bîrcă A.C., Vasile B.Ș., Grumezescu A.M., Mogoanta L. The effect of silver nanoparticles on Antioxidant/Pro-Oxidant balance in a murine model. Int. J. Mol. Sci. 2020;21(4):1233. doi: 10.3390/ijms21041233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghosh R., Sarkhel S., Saha K., Parua P., Chatterjee U., Mana K. Synthesis, characterization & evaluation of venom neutralization potential of silver nanoparticles mediated Alstonia scholaris Linn bark extract. Toxicol. Rep. 2021;8:888–895. doi: 10.1016/j.toxrep.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen H., Larsen S., Spliid H., Christensen N.D. Multivariate statistical analysis of organ weights in toxicity studies. Toxicology. 1999;136(2):67–77. doi: 10.1016/s0300-483x(99)00056-6. [DOI] [PubMed] [Google Scholar]
- 46.Tiwari D.K., Jin T., Behari J. Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol. Mech. Methods. 2011;21(1):13–24. doi: 10.3109/15376516.2010.529184. [DOI] [PubMed] [Google Scholar]
- 47.Yin N., Yao X., Zhou Q., Faiola F., Jiang G. Vitamin E attenuates silver nanoparticle-induced effects on body weight and neurotoxicity in rats. Biochem. Biophys. Res. Commun. 2015;458(2):405–410. doi: 10.1016/j.bbrc.2015.01.130. [DOI] [PubMed] [Google Scholar]
- 48.Adeyemi O.S., Adewumi I. Biochemical evaluation of silver nanoparticles in wistar rats. Int. Sch. Res. Notices. 2014;2014 doi: 10.1155/2014/196091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thakur M., Gupta H., Singh D., Mohanty I.R., Maheswari U., Vanage G., Joshi D.S. Histopathological and ultra structural effects of nanoparticles on rat testis following 90 days (Chronic study) of repeated oral administration. J. Nanobiotechnology. 2014;12(1):42. doi: 10.1186/s12951-014-0042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olugbodi J.O., David O., Oketa E.N., Lawal B., Okoli B.J., Mtunzi F. Silver nanoparticles stimulates spermatogenesis impairments and hematological alterations in testis and epididymis of male rats. Molecules. 2020;25(5) doi: 10.3390/molecules25051063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greco F., Courbière B., Rose J., Orsière T., Sari-Minodier I., Bottero J.Y., Auffan M., Perrin J. [Toxicity of nanoparticles on reproduction] Gynecol. Obstet. Fertil. 2015;43(1):49–55. doi: 10.1016/j.gyobfe.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Park E.J., Bae E., Yi J., Kim Y., Choi K., Lee S.H., Yoon J., Lee B.C., Park K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ. Toxicol. Pharmacol. 2010;30(2):162–168. doi: 10.1016/j.etap.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Lee Y., Kim P., Yoon J., Lee B., Choi K., Kil K.H., Park K. Serum kinetics, distribution and excretion of silver in rabbits following 28 days after a single intravenous injection of silver nanoparticles. Nanotoxicology. 2013;7(6):1120–1130. doi: 10.3109/17435390.2012.710660. [DOI] [PubMed] [Google Scholar]
- 54.Sung J.H., Ji J.H., Park J.D., Yoon J.U., Kim D.S., Jeon K.S., Song M.Y., Jeong J., Han B.S., Han J.H., Chung Y.H., Chang H.K., Lee J.H., Cho M.H., Kelman B.J., Yu I.J. Subchronic inhalation toxicity of silver nanoparticles. Toxicol. Sci.: Off. J. Soc. Toxicol. 2009;108(2):452–461. doi: 10.1093/toxsci/kfn246. [DOI] [PubMed] [Google Scholar]
- 55.Castellini C., Ruggeri S., Mattioli S., Bernardini G., Macchioni L., Moretti E., Collodel G. Long-term effects of silver nanoparticles on reproductive activity of rabbit buck. Syst. Biol. Reprod. Med. 2014;60(3):143–150. doi: 10.3109/19396368.2014.891163. [DOI] [PubMed] [Google Scholar]
- 56.Akter M., Sikder M.T., Rahman M.M., Ullah A., Hossain K.F.B., Banik S., Hosokawa T., Saito T., Kurasaki M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018;9:1–16. doi: 10.1016/j.jare.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asharani P.V., Hande M.P., Valiyaveettil S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009;10:65. doi: 10.1186/1471-2121-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asare N., Instanes C., Sandberg W.J., Refsnes M., Schwarze P., Kruszewski M., Brunborg G. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells. Toxicology. 2012;291(1-3):65–72. doi: 10.1016/j.tox.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 59.Wiwanitkit V., Sereemaspun A., Rojanathanes R. Effect of gold nanoparticles on spermatozoa: the first world report. Fertil. Steril. 2009;91(1):e7–8. doi: 10.1016/j.fertnstert.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Yan H.H.N., Mruk D.D., Lee W.M., Yan Cheng C. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. Faseb J. 2008;22(6):1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lie P.P.Y., Cheng C.Y., Mruk D.D. Signalling pathways regulating the blood–testis barrier. Int. J. Biochem. Cell Biol. 2013;45(3):621–625. doi: 10.1016/j.biocel.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao Z., Huang W., Sun Y., Li Y. Deoxynivalenol induced spermatogenesis disorder by blood-testis barrier disruption associated with testosterone deficiency and inflammation in mice. Environ. Pollut. 2020;264 doi: 10.1016/j.envpol.2020.114748. [DOI] [PubMed] [Google Scholar]
- 63.Garcia T.X., Costa G.M., França L.R., Hofmann M.C. Sub-acute intravenous administration of silver nanoparticles in male mice alters Leydig cell function and testosterone levels. Reprod. Toxicol. 2014;45:59–70. doi: 10.1016/j.reprotox.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egecioglu E., Prieto-Garcia L., Studer E., Westberg L., Jerlhag E. The role of ghrelin signalling for sexual behaviour in male mice. Addict. Biol. 2016;21(2):348–359. doi: 10.1111/adb.12202. [DOI] [PubMed] [Google Scholar]
- 65.Hamza R.Z., Diab A.E.-A.A. Testicular protective and antioxidant effects of selenium nanoparticles on Monosodium glutamate-induced testicular structure alterations in male mice. Toxicol. Rep. 2020;7:254–260. doi: 10.1016/j.toxrep.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El-behery E.I., El-naseery N.I., El-Ghazali H.M., Elewa Y.H.A., Mahdy E.A.A., El-Hady E., Konsowa M.M.H. The efficacy of chronic zinc oxide nanoparticles using on testicular damage in the streptozotocin-induced diabetic rat model. Acta Histochem. 2019;121(1):84–93. doi: 10.1016/j.acthis.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 67.Anan H.H., Zidan R.A., Abd El-Baset S.A., Ali M.M. Ameliorative effect of zinc oxide nanoparticles on cyclophosphamide induced testicular injury in adult rat. Tissue Cell. 2018;54:80–93. doi: 10.1016/j.tice.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Afifi M., Almaghrabi O.A., Kadasa N.M. Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. Biomed Res. Int. 2015;2015 doi: 10.1155/2015/153573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hosseini A., Baeeri M., Rahimifard M., Navaei-Nigjeh M., Mohammadirad A., Pourkhalili N., Hassani S., Kamali M., Abdollahi M. Antiapoptotic effects of cerium oxide and yttrium oxide nanoparticles in isolated rat pancreatic islets. Hum. Exp. Toxicol. 2013;32(5):544–553. doi: 10.1177/0960327112468175. [DOI] [PubMed] [Google Scholar]
- 70.Schubert D., Dargusch R., Raitano J., Chan S.-W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 2006;342(1):86–91. doi: 10.1016/j.bbrc.2006.01.129. [DOI] [PubMed] [Google Scholar]
- 71.Moridi H., Hosseini S.A., Shateri H., Kheiripour N., Kaki A., Hatami M., Ranjbar A. Protective effect of cerium oxide nanoparticle on sperm quality and oxidative damage in malathion-induced testicular toxicity in rats: An experimental study. Int. J. Reprod. Biomed. (Yazd Iran) 2018;16(4):261–266. [PMC free article] [PubMed] [Google Scholar]