Highlights
-
•
Incisional wound closure is a critical surgical step to facilitate tissue healing.
-
•
Cyanoacrylates (Histoacryl®) have become a popular veterinary wound closure practice.
-
•
Effects of Histoacryl® over traditional suture on wounds rest undetermined.
-
•
Histoacryl® vs. Poliglecaprone (Monocryl®) have similar regenerative results in mice.
-
•
Histoacryl® or Monocryl® could be used to treat wounds in rodents.
Keywords: Histology, Microbiology, Surgical wound closure, Mouse skin, Cyanoacrylate, Histoacryl, Poliglecaprone, Monocryl, Traditional suture
Abstract
Incisional wound closure is a key surgical step to facilitate tissue healing, reduce the risk of infection and obtain esthetic and functional recovery. Cyanoacrylates such as Histoacryl® have become a popular choice in surgical veterinary practice. However, how Histoacryl® is affecting tissue regeneration and bacterial load in the wound in comparison to poliglecaprone (Monocryl®) traditional suture methods remains to be determined. This work aimed to evaluate how wounded tissue responds to traditional suture with Monocryl® (poliglecaprone 25/4-0) and Histoacryl®, as well as provide evidence of their effects on wound healing in mice. Fortyeight hours after the incisional procedure, wound tissue biopsies were prepared for histological and microbiological analysis. Biopsies were fixed and colored with Mallory's trichrome and hematoxylin-eosin stains. For microbiological assays, biopsies were suspended in tryptic soy broth (TSB) and 1/10 diluted to evaluate the number of CFU in nutrient agar plates. Our results show no differences between Histoacryl® and Monocryl® traditional suture suggesting that both methods could be used to treat wounds in small animals such as rodents.
Wound closure is a crucial step to promote tissue healing after surgical procedures. It is performed to reduce the dead space, minimize the risk of infection and dehiscence, and to achieve an esthetic and functional outcome (Suthar et al., 2020). The most common methods used to close wounds are traditional sutures, staples and surgical tapes. However, traditional closures can cause trauma as they perforate the skin and provide access for bacteria to reach underlying tissues, increasing the risk of infection. Additionally, suturing could induce complications such as loose sutures, stitch abscess, epithelial cysts, wound leaks, permanent suture tracks, foreign body reactions, tissue ischemia, fistulas and granuloma formation (Deng et al., 2019; Singh, Degala, Shetty, Rai & Das, 2019; Suthar et al., 2020). Medical tissue adhesives such as cyanoacrylates can bond hard and soft tissues rapidly without the need for sutures (Singh et al., 2019). N-butyl-2-cyanoacrylate (Histoacryl®) has hemostatic, antibacterial and bacteriostatic properties. Additionally, it has excellent tensile strength, fast polymerization and good biocompatibility when used in different types of wounds. These properties made cyanoacrylates a popular choice in surgical veterinary practice (Oladega, James & Adeyemo, 2019). However, it is still necessary to understand how cyanoacrylate works in the wounded tissue compared to poliglecaprone (Monocryl®) traditional sutures and their effects on the bacterial load. In this work, we sought to determine how tissue responds to Monocryl® sutures and Histoacryl® in order to provide evidence about their effects on wound healing and the future improvement of surgical procedures on specific veterinary patients.
The Bioethics Committee for the use of animals in research and teaching of the School of Veterinary Medicine of the Universidad San Francisco de Quito, USFQ, approved the methods used for the present work in accordance with the Animal Welfare Standards and the recommendations of Replacement, Reduction and Refinement in animal research, proposed by Russell and Burch (1959). Approval number: 2020-001.
Assays for the histological and microbiological evaluation were performed four times. Each time, five mice were used to evaluate the effects of Histoacryl® on tissue healing and traditional suturing by Monocryl®. Three mice were used for histological analysis and two for microbiological studies. A total of 20 mice were used in the assays to take into account the ethical recommendations of the committee to minimize this number. Before surgery the animals were in their respective beds in the USFQ biotherium. Their food was based on a balanced diet for rodents pre- and post-surgery and water consumption was ad libitum.
Each animal was anesthetized with xylazine 0.5 mg/kg + ketamine 80 mg/kg. Two cuts of 1 cm were performed in the dorsal skin of each mouse: one cut was bonded by simple suture Monocryl® (poliglecaprone 25 / 4–0), and the other with tissue adhesive Histoacryl®. Postoperative management was performed by placing each mouse on analgesics provided in their water to minimize pain. 48 h after the surgical procedure, the mice were euthanized and biopsies of 2 cm2 were collected for each cut. Samples were evaluated for the presence of bacteria by colony forming unit (CFU) accounts. Each tissue sample with and without cyanoacrylate suture was resuspended in 1 ml of Tryptic Soy Broth (TSB) by vortexing for 30 s. 100 µL of 1/10 dilution of the original suspension was evaluated after plating on nutrient agar plates after 48 h for CFU counting.
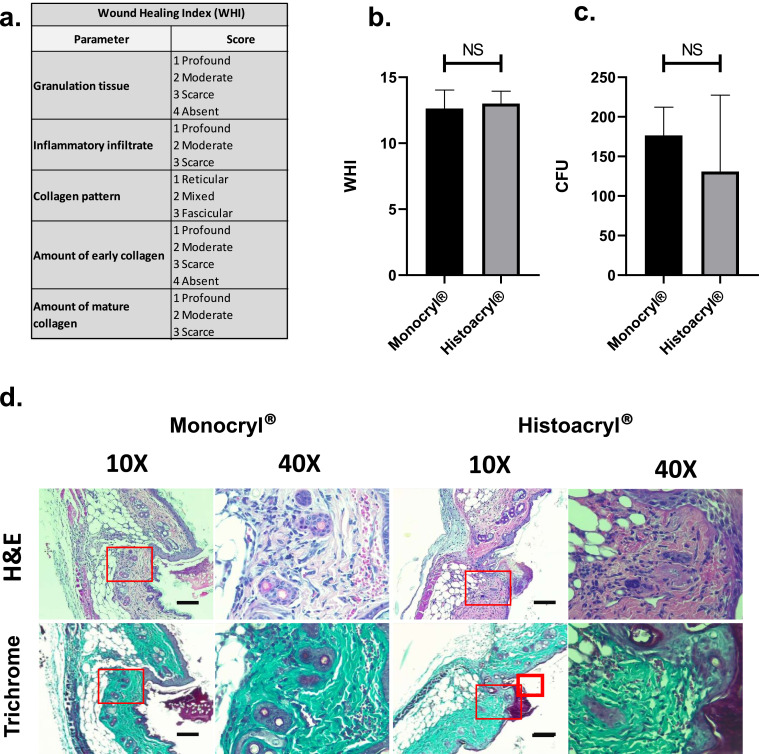
For histological analysis, biopsies were fixed with 10% buffered formalin for a minimum time of 48 h. Slides of 2 µm were made by using a microtome (Leica RM2155) and colored with Mallory's trichrome and hematoxylin-eosin (H&E) dyes. Samples were photographed with a Leica ICC50W camera and analyzed with the Airlab Leica Microsystems application. Histopathological evaluation was focused on the parameters given by Sultana et al. (2009) according to Wound Healing Index, (WHI) (Fig. 1a). The statistical analysis of the microbiological CFU and WHI were performed using the GraphPad Prism software.
Fig. 1.
48 h Histological analysis by hematoxylin and eosin (H&E), Wound Healing Index, (WHI) and microbiological evaluation (CFU in nutrient agar) of surgical wound biopsies in mice skin treated with Histoacryl® and Monocryl® a. Parameters of the WHI. b. WHI The evaluated data did not show a normal distribution (Shapiro-Wilk test ɑ = 0.05, Monocryl® p = 0.045; Histoacryl® p = 0.006) and the analysis of the non-parametric Mann-Whitney test, resulted in no differences among conditions (p = 0.74). c. CFU. CFU were counted after incubation on nutrient agar for 48 h. Data passed the normality test (Shapiro-Wilk test ɑ = 0.05, Monocryl® p = 0.07; Histoacryl® p = 0.007), and no differences or changes were observed in the comparison of normal suture (Monocryl®) compared to Histoacryl® by using an unpaired t-test analysis (p = 0.45). d. Representative images of the wounded tissue samples. We used wounded tissue samples closed with Monocryl® and Histoacryl®, fixed with 10% buffered formalin, and stained with H&E and trichrome techniques. Two image magnifications (10X and 40X, 250 µm scale) were used to analyze any differences. Red squares show the zone of the sample that was enlarged to 40X. Similar inflammation and regeneration was seen among conditions and samples, no evident changes were observed.
48 h after the surgical procedure, the WHI, based on the tissue biopsies analyzed, showed no differences among conditions by the non-parametric Mann-Whitney test (p = 0.74) (Fig. 1b, c). Additionally, histological analysis of the wounded area closed with Monocryl® and Histoacryl® samples showed similar inflammation and regeneration levels, no evident changes were seen among conditions and samples (Fig. 1d). Even if the suture condition showed the tendency to have more CFU, no differences were observed in comparison to Histoacryl® by using an unpaired t-test (p = 0.45) (Fig. 1d).
Healing requires a sequence of events including hemostasis, inflammation, proliferation and remodeling (Velnar, Bailey & Smrkolj, 2009). The precise approximation of incisional wound edges, by sutures or glue, relieves the tension and facilitates healing (Kwon, Yun & Park, 2018; Nitsch, Pabyk, Honig, Verheggen & Merten, 2005). The incisional wounds in this study are full-thick injuries characterized by a complete destruction of epithelial-regenerative elements still observed after 48 h (Papini, 2004). Analysis of later time points could be less resolutive as mice repair mechanisms are more efficient than in other mammals, such as humans. Wound healing in mice depends more on the contraction of panniculus carnosus (Sami, Heiba & Abdellatif, 2019) than the formation of epithelial and granulation tissue formation found in human wound healing (Wong, Sorkin, Glotzbach, Longaker & Gurtner, 2011). It has been shown that the use of Histoacryl® in comparison to sutures forms a waterproof and bactericidal barrier in humans, and is less invasive and easier to apply (Mackeen, Schuster & Berghella, 2015; Toriumi, O'Grady, Desai & Bagal, 1998). Even if the regenerative process in incisional wounds in rodents is different than in humans, the use of Histoacryl® in guinea pigs showed to be more convenient than the use of silk sutures as it promotes the healing process (Giray, Sungur, Atasever & Araz, 1995; Sami et al., 2019). Similar results have been observed when using skin flaps secured with sutures or cyanoacrylate in dogs (De Carvalho Vasconcellos, Matera & Zaidan Dagli, 2005). However, the methods for evaluating wound recovery were most of the time qualitative and not consistent enough. In our study we use the WHI as it provided advantages when comparing Monoacryl® and Histoacryl®. Our study shows no differences in the use of Histoacryl® in comparison to traditional sutures by Monocryl® at 48 h, showing that both methods could be used to treat wounds in rodents.
Data availability statement
The corresponding authors can address any comments or questions regarding this publication on request.
Funding
Corporación Ecuatoriana para el Desarrollo de la Investigación y Académica, CEDIA CEPRA XIV-2020-04, MITOCHONDRIAS
Ethical statement
The Bioethics Committee for the use of animals in research and teaching of the School of Veterinary Medicine of the Universidad San Francisco de Quito, USFQ, approved the methods used for the present work in accordance with the Animal Welfare Standards and the recommendations of Replacement, Reduction and Refinement in animal research, proposed by Russell and Burch (1959). Approval number: 2020-001.
CRediT authorship contribution statement
A. Villagomez: Conceptualization, Funding acquisition. T. Borja: Formal analysis, Data curation. P. Pontón: Data curation, Formal analysis. G. Segnini: Funding acquisition. P. Barba: Resources, Data curation. A. Chiliquinga: Conceptualization, Formal analysis, Writing - original draft. I. Yamberla: Resources, Data curation. C. Pupiales: Resources, Data curation. D. Suquillo: Formal analysis, Writing - original draft. R.F. Díaz: Formal analysis, Writing - original draft. F. Cabrera: Formal analysis, Project administration. A. Caicedo: Formal analysis, Writing - original draft, Project administration, Project administration.
Declaration of Competing Interest
The authors declare that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest on this work.
Acknowledgments
We are thankful to the "Corporación Ecuatoriana para el Desarrollo de la Investigación y Académica, CEDIA" for the financial support provided to our project, promoting the development of research and innovations regarding the use of AMT/T for preclinical to clinical applications. CEPRA XIV-2020-04, MITOCHONDRIAS. All authors thank the School of Medicine at the Universidad San Francisco de Quito, USFQ for their constant support to our work and initiatives, to the “Servicio de Patología” at the VozAndes Hospital, the University of Montpellier, School of Veterinary Medicine, “Instituto de Investigaciones en Biomedicina, USFQ”, to the Mico-Act Research Consortium, Quito, Ecuador and “Sistemas Médicos”, Universidad San Francisco de Quito, USFQ. We would like to specially thank Kevin Zambrano, Serena Sanon and Dariana Argueta Zamora for revising the manuscript and suggestions.
Contributor Information
F. Cabrera, Email: fcabrera@usfq.edu.ec.
A. Caicedo, Email: acaicedo@usfq.edu.ec.
References
- De Carvalho Vasconcellos C.H., Matera J.M., Zaidan Dagli M.L. Clinical evaluation of random skin flaps based on the subdermal plexus secured with sutures or sutures and cyanoacrylate adhesive for reconstructive surgery in dogs. Veterinary Surgery. 2005;34:59–63. doi: 10.1111/j.1532-950X.2005.00011.x. [DOI] [PubMed] [Google Scholar]
- Deng, J., Tang, Y., Zhang, Q., Wang, C., Liao, M., Ji, P.…Tan, H. (2019). A bioinspired medical adhesive derived from skin secretion of Andrias davidianus for wound healing. Advanced Functional Materials1809110. doi:10.1002/adfm.201809110.
- Giray C.B., Sungur A., Atasever A., Araz K. Comparison of silk sutures and n-butyl-2-cyanoacrylate on the healing of skin wounds. A pilot study. Australian Dental Journal. 1995;40:43–45. doi: 10.1111/j.1834-7819.1995.tb05613.x. [DOI] [PubMed] [Google Scholar]
- Kwon J.Y., Yun H.G., Park I.Y. n-Butyl-2-cyanoacrylate tissue adhesive (Histoacryl) vs. subcuticular sutures for skin closure of Pfannenstiel incisions following cesarean delivery. PloS One. 2018;13 doi: 10.1371/journal.pone.0202074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackeen A.D., Schuster M., Berghella V. Suture versus staples for skin closure after cesarean: A metaanalysis. American Journal of Obstetrics and Gynecology. 2015;212 doi: 10.1016/j.ajog.2014.12.020. 621.e1–10. [DOI] [PubMed] [Google Scholar]
- Nitsch A., Pabyk A., Honig J.F., Verheggen R., Merten H.-.A. Cellular, histomorphologic, and clinical characteristics of a new octyl-2-cyanoacrylate skin adhesive. Aesthetic Plastic Surgery. 2005;29:53–58. doi: 10.1007/s00266-004-0096-3. [DOI] [PubMed] [Google Scholar]
- Oladega A.A., James O., Adeyemo W.L. Cyanoacrylate tissue adhesive or silk suture for closure of surgical wound following removal of an impacted mandibular third molar: A randomized controlled study. Journal of Cranio-Maxillo-Facial Surgery: Official Publication of the European Association for Cranio-Maxillo-Facial Surgery. 2019;47:93–98. doi: 10.1016/j.jcms.2018.10.018. [DOI] [PubMed] [Google Scholar]
- Papini R. Management of burn injuries of various depths. BMJ (Clinical research ed.) 2004;329:158–160. doi: 10.1136/bmj.329.7458.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami D.G., Heiba H.H., Abdellatif A. Wound healing models: A systematic review of animal and non-animal models. Wound Medicine. 2019;24:8–17. doi: 10.1016/j.wndm.2018.12.001. [DOI] [Google Scholar]
- Singh P.K., Degala S., Shetty S., Rai V.S., Das A. To evaluate the efficacy and effectiveness of N-butyl-2-cyanoacrylate glue (TRU SEAL) in closure of oral and maxillofacial laceration and surgical incisions. Journal of Maxillofacial Oral Surgery. 2019;18:131–138. doi: 10.1007/s12663-018-1111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana J., Molla M.R., Kamal M., Shahidullah M., Begum F., Bashar M.A. Histological differences in wound healing in Maxillofacial region in patients with or without risk factors. Bangladesh Journal of Pathology. 2009;24 doi: 10.3329/bjpath.v24i1.2874. [DOI] [Google Scholar]
- Suthar P., Shah S., Waknis P., Limaye G., Saha A., Sathe P. Comparing intra-oral wound healing after alveoloplasty using silk sutures and n-butyl-2-cyanoacrylate. Journal of Korean Association of Oral and Maxillofacial Surgeons. 2020;46:28–35. doi: 10.5125/jkaoms.2020.46.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriumi D.M., O'Grady K., Desai D., Bagal A. Use of octyl-2-cyanoacrylate for skin closure in facial plastic surgery. Plastic and Reconstructive Surgery. 1998;102:2209–2219. doi: 10.1097/00006534-199811000-00062. [DOI] [PubMed] [Google Scholar]
- Velnar T., Bailey T., Smrkolj V. The wound healing process: An overview of the cellular and molecular mechanisms. The Journal of International Medical Research. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- Wong V.W., Sorkin M., Glotzbach J.P., Longaker M.T., Gurtner G.C. Surgical approaches to create murine models of human wound healing. Journal of Biomedicine & Biotechnology. 2011;2011 doi: 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding authors can address any comments or questions regarding this publication on request.