Abstract
Liver resection still represent the treatment of choice for liver malignancies, but in some cases inadequate future remnant liver (FRL) can lead to post hepatectomy liver failure (PHLF) that still represents the most common cause of death after hepatectomy. Several strategies in recent era have been developed in order to generate a compensatory hypertrophy of the FRL, reducing the risk of post hepatectomy liver failure. Portal vein embolization, portal vein ligation, and ALLPS are the most popular techniques historically adopted up to now. The liver venous deprivation and the radio-embolization are the most recent promising techniques. Despite even more precise tools to calculate the relationship among volume and function, such as scintigraphy with 99mTc-mebrofenin (HBS), no consensus is still available to define which of the above mentioned augmentation strategy is more adequate in terms of kind of surgery, complexity of the pathology and quality of liver parenchyma. The aim of this article is to analyse these different strategies to achieve sufficient FRL.
Keywords: Liver resection, future remnant liver (FRL), liver failure, liver volume, liver function, hypertrophy
Introduction
Thanks to the advances in preoperative and postoperative management of surgical patients, the rates of morbidity and mortality have been significantly reduced in the recent years (1,2), allowing the introduction and development of minimally invasive surgery also for complex cases (3). Actually the rate of perioperative mortality after major hepatectomy (≥3 segments) is less than 5% (4). One of the main goals of major liver surgery is represented by avoiding post-hepatectomy liver failure (PHLF), which still remains the main cause of mortality after major liver resection (1). PHLF is unequivocally related to quality of liver and to quantity of future remnant liver (4). In recent era, the development of even more precise tools (morphologic and functional) allow to calculate the correct value of FRL (without non-functional tumour volume), the rates of PHLF is quite low (4,5). Generally, in relation with the quality of liver parenchyma, we consider safe a FRL of ≥20% of volume in case of normal liver, ≥30–40% for severe steatosis and cholestasis, and ≥50% for cirrhotic patients. Another important correlation has been determined among FRL and Body weight, called Remnant Liver Volume to Body Weight Ratio (RLV-BWR) determining a ratio that could indicate if the future remnant liver is enough. Actually, in case of normal liver, the minimal value of RLV-BWR of liver is 0.5%, in case of steatosis and cholestasis is 0.75%, and in case of cirrhosis can be considered 1% (5).
In order to reduce the risk of PHLF, different strategies have been described to induce a compensatory hypertrophy of marginal FRLV. Most of them base their functioning on augmentation of portal pressure, who directly induce liver hypertrophy. This augmentation of pressure, overstressing the portal vein wall, induce the hyper-expression of the genes who are involved in liver regeneration (6). This capacity to induce regeneration in case of liver damage and portal flow variations (7), induce tumour necrosis factor alpha (TNFa) and interleukin 6 (IL-6) to stimulate duplication of hepatocyte under control of growth factors. In a second period, a hypertrophy of the hepatocytes induce the volume growth. Furthermore, the portal vein blood per se contains important regenerating hepatocytes factors like IGF and VGF (5,7). However, knowledge of the quality of the underlying liver parenchyma is not always obvious.
In this contest, it is important to stress the fact that today it is not only a matter of volumetric evaluation of the FRL but also of functional regeneration of FRL which can be measured through these new emerging methods: hepatobiliary scintigraphy and MRI.
Hepatobiliary scintigraphy with 99mTc-mebrofenin (HBS) is the most commonly studied non-invasive nuclear medicine imaging technique to assess liver function at global and regional (segmental) level. Mebrofenin is an amino diacetic acid (IDA) agent taken up by hepatocytes at the basal membrane by the organic anion transporter polypeptides B1 and B3. It transits through the hepatocytes to the bile canaliculi without any biotransformation. The first 6 minutes of the examination allows the calculation of the 99mTc-mebrofenin extraction rate (expressed in %/min/m2) for total liver (8) which is correlated to underlying parenchymal status (9,10) and to ICG clearance (sharing the same OATPB1/B3 transporters) (11). Then a short acquisition of less than ten minutes with SPECT (single photon emission computed tomography) is performed to assess the repartition of the radiotracer at regional and segmental levels by calculating the 99mTc-mebrofenin extraction rate in the volume of interest, especially in the FRL (12). One of the drawback of this technique is that the uptake of mebrofenin is in competition with bilirubin leading to false underestimation of liver function in patients with hyperbilirubinemia (13).
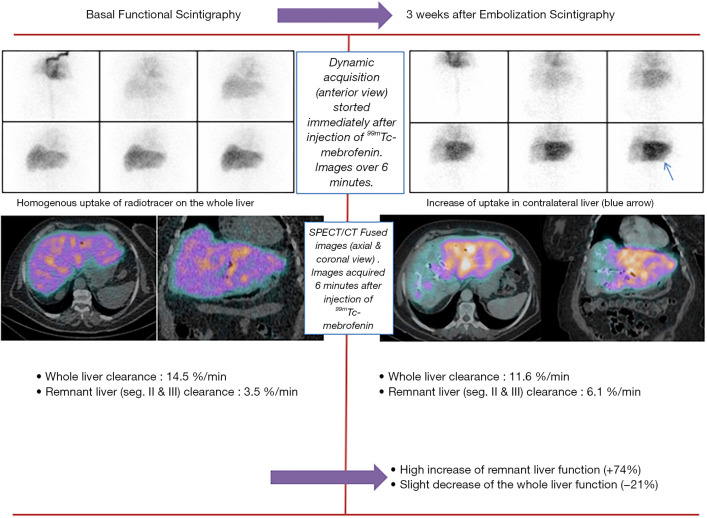
At segmental level, the FRL function (FRL-F, also expressed in %/min/m2) appears to better predict the risk of PHLF than volumetric-based parameters (10,11,14). Indeed, HBS directly reflects the quality of the underlying liver parenchyma contrary to volumetric-based methods. Moreover HBS takes into account the possible heterogeneity of the distribution of function within liver. HBS is also better than platelet-based liver scores in predicting PHLF (15-17). de Graaf et al. showed that a cut-off of 2.7%/min/m2 is of high negative predictive value (98%) to exclude the risk of PHLF in patients planned for large hepatectomy (10) (Figure 1).
Figure 1.
Functional scintigraphy basal and 3 weeks after embolization.
99Tc-GSA (Galactosyl-human serum albumin) is another scintigraphic imaging technique reflecting the functional hepatocyte mass. GSA links to the asialoglycoprotein receptor only present at the surface of hepatocytes in the sinusoidal surface. It is taken up by receptor-mediated endocytosis and degraded into the cell (no biliary excretion). Contrary to 99mTc-mebrofenin, 99mTc-GSA is not altered by hyperbilirubinemia. 99mTc-GSA scintigraphy (static and with SPECT) has proven to be predictive of postoperative liver failures (18,19). However, this radiopharmaceutical is only approved in Asia, with no availability in USA or Europe.
MRI with gadolinium ethoxybenzyl diethyl enetriamine penta-acetic acid (Gd-EOB-DTPA) is a non-isotopic emerging imaging method of evaluation of liver function (20). Gd-EOB-DTPA shares pharmacokinetic properties with mebrofenin and ICG, taken up by hepatocytes and are excreted in the bile canaliculi without undergoing biotransformation. Pharmacokinetic models have been developed to estimate the uptake rate of the contrast agent based on DHCE-MRI with Gd-EOB-DTPA on a per voxel basis. Some studies have demonstrated that the assessment of liver function with DHCE-MRI is comparable with that of 99mTc-mebrofenin HBS (21,22). More studies are still needed to validate this new technique.
The aim of this paper is to analyse all different techniques available in 2020 to induce hypertrophy and function of FRL, examining all the advantages and drawback of each technique (Table 1).
Table 1. Different strategies to achieve liver hypertrophy.
| Procedures | Mechanism of action | Technique | Indications/contraindications | Pros/cons | Rate of success |
|---|---|---|---|---|---|
| Portal vein ligation (PVL) | Increasing portal pressure, peri-portal inflammation and local hypoxemia stimulate the up regulation of genes promotors of hepatocytes regeneration | Open or laparoscopic/robotic ligation of right/left portal branch | I: Patients with not adequate FLR for a major resection; first step of two-staged hepatectomy; first step of ALPPS | P: Could be performed during first step of stage hepatectomy, avoiding percutaneous embolization | Rate of cancelled surgery for inadequate FRL seems lower than PVE (23) |
| C: Ipsilateral tumor thrombus and portal hypertension; relative contraindications include non-correctable coagulopathy and renal insufficiency | C: An incomplete interruption between collateral vessels of two hemiliver; general anaesthesia; complicated dissection of hilum in surgery for dissection in the first stage; not yet clear correlation between volume and function; 4–6 weeks interval for second stage | ||||
| Portal vein embolization (PVE) | Increasing portal pressure, peri-portal inflammation and local hypoxemia stimulate the up regulation of genes promotors of hepatocytes regeneration | Percutaneous trans-hepatic ipsilateral or contralateral approach, CT-guided for embolize right/left branch (and right + seg4 when an extended right hepatectomy was planned) with various substances (lipiodol, histoacryl, gelfoam, polyvinyl alcohol and n-butyl cyanoacrylate, etc.…) | I: Patients with not adequate FLR for a major resection; first step of two staged hepatectomy; first step of ALPPS | P: Local or general anaesthesia; percutaneous approach; overall morbidity of 21.7% and overall mortality of 3.3% following major liver resection (24) | 75–80% go to major hepatectomies (25-27) |
| Transileocolic approach: laparotomy and cannulation of ileocolic trunk under fluoroscopic guidance into the portal vein for subsequent embolization | C: Ipsilateral tumor thrombus and evident portal hypertension; relative contraindications include non-correctable coagulopathy and renal insufficiency | C: It is not yet clear the correlation with function; second surgery stage can be performed after 4–6 weeks | |||
| Liver venous deprivation (LVD) | The double occlusion of the HV and PV causes an increasing pressure in the non embolized liver which induces the formation of intrahepatic collaterals, with an augmented “damage” of embolized liver and the contralateral regeneration rate. Hepatocyte atrophy is associated with a more evident sinusoid dilatation | Transhepatic RPV embolization and RHV (+/− accessory right) occlusion with an amplatzer plug positioned at about 10 mm from the origin and iodized oil and n-buty-cyanoacrylate injected behind the plug to occlude the distal part of the vein (28,29) | C: Ipsilateral tumor thrombus and evident portal hypertension; relative contraindications include uncorrectable coagulopathy and renal insufficiency | P: High rate of FLR hypertrophy | Limited series. Only 1 patient does not receive surgery for tumor progression (28). Two RCTs ongoing |
| C: Correlation with function (liver scintigraphy), second surgery stage can be performed after 4–6 weeks | |||||
| C: Augmented risk of enlarging of veno-venous collaterals compared to PVE alone and vascular congestion and increase in the risk of intraoperative bleeding and intra-operative hemorrhage (29) | |||||
| ALPPS | Portal ligation induces a portal flow redistribution which promotes releasing of proliferative factors; the preserving arterial flow represents an auxilium to FLR to tolerate portal hyper-afflux; parenchymal transection interrupts intrahepatic collaterals and actives an inflammatory response which induces augmented release of growth factors | First stage: transection of liver parenchyma associated with PVL of the diseased hemi-liver; it could be associated the removal of all lesions in the FLR Second stage: after CT volumetric assessment of the FLR, complete removal of the right liver by dividing the ipsilateral hepatic artery, bile duct and hepatic vein already taped | I: A tumor margin close to the FLR or its vascular pedicle; failure of PVE; the need for exponential hypertrophy on a very limited starting FLR | P: Second surgery stage can be performed after 1–2 weeks, often in the same hospitalisation; second step is performed with low rate of adherences | 99% of patients reach surgical resection (30) |
| C: Postoperative complication (Dindo ≥ 3b) of 27% associated with a postoperative mortality of 9% (31) | |||||
| Radio-embolization (Radiation therapy) | High concentrations of drug associated to tumoricidal dose of radiation to induce tumor cells necrosis | First stage: occlusion of all extrahepatic vessels in order to avoid 90Y diffusion in the gut an 99mTcMAA is injected to evaluated lung shunting | I: Single or multiple HCC with A-B7 Child-Pugh score; locally advanced tumor, not suitable of other treatments; bridging treatment to transplantation | P: Safe and feasible procedure; microspheres occlude only distal small vessels, reducing toxic effect on remaining normal liver | TARE reduces drop out to liver transplantation (LT) compared to TACE, with an effective bridging rate of 66% (32) |
| Second stage: 90Y microspheres are injected through hepatic artery | C: Dose to the lung in the first stage >30 Gy; portal hypertension | C: 1–4 weeks needed between two stages; more time needed to achieve hypertrophy of FLR compared to PVE |
PVE, portal vein embolization; HVE, hepatic vein embolization; TARE, trans arterial radio embolization; FLR, future liver remnant; PVE, portal vein embolization; CT, computed tomography; Gy, gray; HCC, hepatocellular carcinoma, ALPPS, Associating Liver Partition Portal and Portal Vein Ligation for Staged hepatectomy.
Search sources and study design
A systematic review of the published literature focused on the clinical impact of portal vein embolization (PVE), portal vein ligation (PVL), liver venous deprivation (LVD), associating liver partition portal vein ligation for staged hepatectomy (ALPPS), radio-embolization for the management of liver pathologies was undertaken. The search strategy was performed by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guide Lines, as well as PRISMA for abstracts.
A search of the MEDLINE, Scopus and Cochrane Database was conducted using the following terms: “PVE” (Title/Abstract) OR “portal vein embolization” (Title/Abstract) OR “portal vein ligation” (Title/Abstract) OR “LVD” (Title/Abstract) OR “liver venous deprivation” (Title/Abstract) OR “double embolization” (Title/Abstract) OR “hepatic vein embolization” (Title/Abstract) OR “ALPPS”(Title/Abstract) OR “associating liver partition and portal vein ligation for staged hepatectomy”(Title/Abstract) OR “in situ split”(Title/Abstract) OR “in situ splitting”(Title/Abstract) OR “liver partition”(Title/Abstract) OR “radio embolization” (Title/Abstract) OR “radiation therapy” (Title/Abstract) OR “FLR” (Title/Abstract) OR “future remnant liver” (Title/Abstract) OR “PHLF” (Title/Abstract) “post hepatectomy liver failure” (Title/Abstract) up to: 2020/29/02.
The systematic qualitative review included a priori search criteria of journal articles among adult (age ≥18 years) human patients; studies were limited to the English language.
Predictors of PHLF
The definition of PHLF has been proposed by many groups (22), but actually only few definitions are used in clinical practive. The “50-50 criterion” proposed by Balzan et al. (33) consider the value of bilirubin >50 µmolL/L and PT <50% on postoperative day 5 as a predictor of liver failure. Another important definition was proposed by the International Study Group of Liver Surgery (ISGLS) (34) who proposed a standardization and grading of liver failure, based on the loss of capacity of the liver to maintain his function associated with an augmentation of bilirubin and INR after postoperative day 5. The difference in the grading of liver failure is the association with clinical impact of postoperative course, raging form a temporary disfunction (Grade A) up to multiorgan failure (Grade C) with necessity of intensive care unit assistance.
Main predictors of postoperative liver failure identified in literature (2,22,34) could be divided in patient related (diabetes, obesity, chemotherapy steatohepatitis, hepatitis, malnutrition, renal insufficiency, hyperbilirubinemia, thrombocytopenia, lung disease, cirrhosis, age >65 years old) and surgery related (high blood loss, intraoperative transfusion, vascular resection, high rate of resected volume, major resection, skeletonization of hepatic pedicle, reduced future remnant liver, postoperative haemorrhage and infections).
Portal vein embolization or ligation
The idea that liver could regenerate consequentially to a portal vein ligation was introduced in animal model in rabbit model (33) in 1920 and consequentially, few years later, the first surgical preoperative procedure was performed in a series of 21 patients who underwent percutaneous portogram used as an alternative to arterial embolization. Makuuchi et al. (34) demonstrated that this procedure could be safe and generate a contralateral hypertrophy that could allow surgical treatment of bilateral liver lesions. After this study, several variations of the percutaneous technique were described in order to obtain liver hypertrophy. The mechanism of liver regeneration remains unclear, but it’s supposed that the peri-portal inflammation associated with the diversion of portal venous flow generate a hypertrophy stimulation. Technically, this occlusion of the portal vein can be achieved either percutaneously or with surgical technique. Surgical technique varies, using either a trans-ileocolic approach or with a surgical ligation of the portal branch, called PVL that could be performed either in open than in laparoscopic/robotic surgery. Percutaneous technique requires a sterile surgical field and could be performed either in local or general anaesthesia. The procedure requires ultrasound and fluoroscopy in order to associate to ultrasound control a portogram. The portal vein could be punctured ipsilateral (preferred) or contralateral, in order to insert a catheter into the portal branch and initially occlude the smaller distal portal branches to pass after to the occlusion of the larger branches up to occlude second order branches with various embolizing agents (35,36). This kind of approach is usually used to occlude left or right portal branch, but a super selective occlusion could be achieved with isolated segment 4 or 1 in case of necessity of further volume.
Transileocolic approach and it requires a laparotomy and a cannulation of the ileocolic trunk (37) but it has been demonstrated to be an alternative to PVE or PVL in case of failure of these techniques.
The PVL has been developed to perform an occlusion of the flux during two stage hepatectomy (TSH), and it could be performed also with laparoscopic approach. The main difference from PVE, considering that the goal is to obtain the complete occlusion of the ipsilateral portal flow, is on the necessity of dissecting hepatic hilum to achieve surgical ligation that could complicate the hilum dissection of in the second step of surgery. Embolic agent could be injected, to occlude the vascular portion and to reduce the risk of recanalization of the portal branches (37).
A recent metanalysis demonstrate no significant difference in safety and rate of hypertrophy between PVE and PVL, but it focused on the lower invasiveness of percutaneous procedure, especially in patients that are not candidate to a two-stage procedure (23).
Although PVE is performed in most of cases with non resorbable products, some authors have recently described resorbable material with comparable results (38). The theoretical advantage of such procedure could be identified in the possibility to avoid accidental definitive contralateral embolization or could be used in situation in which liver could supposed to be not resected due to the possibility of tumour progression. In the study of Tranchart et al. (39), the development of an absorbable gelatine sponge which after 4–6 weeks of occlusion was temporary absorbed with a 40% hypertrophic growth of the liver that did not received embolization. Subsequently, the same group (38) expanded this concept demonstrating that repeated resorbable embolization guarantees further liver hypertrophy compared to single resorbable embolization.
Recently, it has been demonstrated that liver regeneration could impact long term disease free and overall survival. In fact, as well known, an augmented hepatocyte proliferation could stimulate remaining cancer cell. Even if it has been demonstrated a correlation among short term liver regeneration and recurrence, demonstrated also by the dropout of 23–35% patients among the TSH (40), no data were up to now available on long term results. For this reason, Early and Late Kinetic growth rate (KGR) was defined as the postoperative increase at 2/3 and 8/10 months, and has been demonstrated how a KGR ≥1% was associated with augmented risk of recurrence (41). The study by Margonis et al. (41) demonstrated that the KGR in late phase was predictive of intrahepatic recurrence, suggesting an increased attention in monitoring this subpopulation with an higher risk of recurrence.
Response to PVE can be considered an important predictor of PHLF. Generally, a rate of hypertrophy >10% in fibrosis or cirrhosis and >5% in patients with normal liver are considered safe value to proceed to surgical resection (38,42-45).
In particular, In patient with hepatocellular carcinoma (HCC) (46)where the fibrosis is often associated with the cirrhosis, it has been demonstrated that the association of PVE and trans arterial chemoembolization (TACE) guarantee a good rate of hypertrophy and better overall and disease-free survival in patients who underwent liver resection compared to PVE alone (47). The procedure started with the intraarterial injection of iodized oil and epirubicin in order to obtain a local control of the HCC nodule. After 7–10 day, when normalization of blood test has been reached, an ipsilateral portal vein embolization in performed. When the correct volume of FRL is reached, the surgical resection could be performed. However, the association of both this procedure could generate both inflammation on the hepatic pedicle and morphological variation of the liver, making the surgical procedure more complex. Despite this, some encouraging series demonstrate that liver resection is feasible and safe also with minimally invasive approach (48). Impact of PVE on FRL function has been studied. de Graaf et al. showed that there was a poor correlation between increase in FLR-F and FLR-V 3 to 4 weeks after PVE in patients with normal liver (r =0.15, P=0.068) or with compromised parenchyma (r =0.53, P=0.062) and that FLR function increased significantly more than FRL volume (P=0.003) (49). Chapelle et al. showed that HBS may be predictive of hypertrophy response after PVE (pre-PVE FRL-F of 1.72%/min/m2 was a validated cut-off value for safe resection (2.7%/min/m2) with a sensitivity of 81.3% and a specificity of 82.4% (14). In this study, the functional increase of FRL 3 weeks after PVE was not influenced by previous chemotherapy (P=0.397).
Today, no significant differences in safety between PVL and PVE were reported. The PVE is less invasive than the PVL. However, the two techniques are not comparable in term of rate of hypertrophy, because of PVE is often associated with an embolization of the Seg. IV portal branches determining a major FRL hypertrophy.
Liver venous deprivation (LVD)
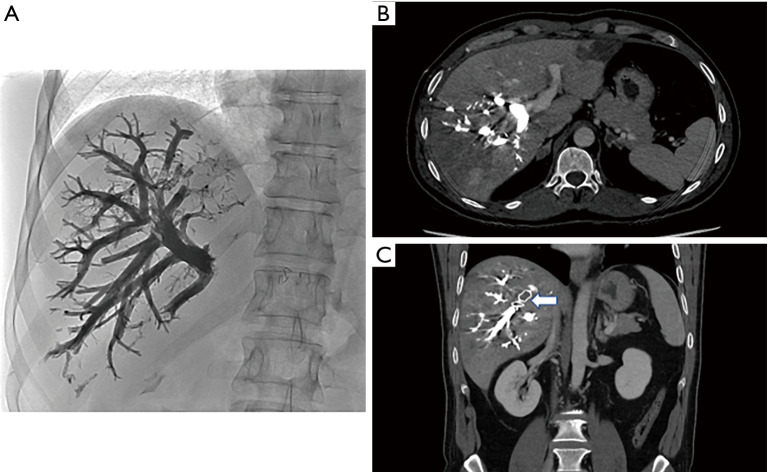
The Hepatic vein embolization (HVE), was born as an attempt to improve the results obtained by portal embolization PVE, in terms of growth of the future remnant liver FRL, before a major hepatectomy. Portal embolization therefore represents a safe and feasible technique (24), allowing a degree of hypertrophy of 10% in about 4–6 weeks, based on the phenomenon of interlobar volume shifting (50,51); but these results are not always sufficient to allow a subsequent surgical treatment. In 2009, Hwang et al. reported a first study based on sequential hepatic vein embolization at the ipsilateral portal vein (52) (Figure 2). The outflow obstruction represented by the HVE induced greater damage associated with that given by the ipsilateral PVE, promoting the increase of the contralateral regeneration mechanism. The double obstruction of the hepatic vein and the portal vein causes an increase in pressure which induces the formation of intrahepatic collaterals, resulting in an augmented damage of the territory with total deprivation [mostly in the posterior sector (53)] and on the other hand in an increase in the contralateral regeneration rate. The presence of a residual arterial flow seems to protect against the risk of bile duct ischemia and subsequent abscess formation (54). In another study, Hwang reports the data of 42 patients with low volume FLR (<40%) who underwent sequential HVE-PVE about 2 weeks apart, with a degree of hypertrophy of 13.3% after PVE and 28.9%. after HVE-PVE. These preliminary results suggested an excellent result in terms of hypertrophy and seemed not to be associated with significant percentages of complications related to procedure (55). The sequential association of the two procedures, however, does not allow time savings, a crucial aspect in the field of oncological surgery. In this perspective, the so-called “liver venous deprivation” is formulated which provides for the embolization of the hepatic vein and the ipsilateral portal vein simultaneously. In a pilot study, Guiu et al. combined during the same procedure, both right portal vein embolization and right (+/− accessory right) hepatic vein occlusion with an amplatzer plug positioned at about 10 mm from the origin and glue (n-buty-cyanoacrylate) injected behind the plug to occlude the distal part of the vein as well as potential veno-venous collaterals. The evaluation of the peri-operative outcome showed no complications related to the procedure (with the exception of a temperature >38° managed with paracetamol) and an increase in the kinetic growth factor KGB of 75% compared to the PVE alone (28). Guiu et al. also described the extended venous deprivation technique where both the right (and accessory right) and middle hepatic vein were occluded using the same technique, in addition to right portal system. Preliminary results from 99m-Tc mebrofenin hepatobiliary scintigraphy showed +66% FLR function at day 7 after extended liver venous deprivation. At day 21, the FRL-V increased by 63.3% with an increase of 64.3% of the FLR-F. These encouraging results obtained in only 3 patients, must be confirmed in a larger study.
Figure 2.
Radiological view of the hepatic vein embolization. (A) Abdomen X-ray after liver venous deprivation (portal vein embolization with glue combined with right hepatic vein embolization with plug and glue). (B) CT-scan (axial view) at day 1 after liver venous deprivation (portal vein embolization with glue combined with right hepatic vein embolization with plug and glue). (C) CT-scan (coronal view) at day 1 after liver venous deprivation [portal vein embolization with glue combined with right hepatic vein embolization with plug (arrow) and glue].
Histology showed an extensive lobular central necrosis, a marked sinusoidal dilation and a more evident atrophy of the hepatocytes compared to PVE alone. Results confirmed in a recent work by Panaro et al. (29), which found an accentuation of the damage of the liver parenchyma on the specimen, after liver venous deprivation, LVD, with mortality/morbidity rates after surgery comparable to PVE alone. These results outline liver venous deprivation as a feasible and well tolerated technique in ensuring a greater and faster increase in FRL, but further comparative studies are needed.
The LVD is a recent promising technique to hypertrophy the FLR with mortality/morbidity rates after surgery comparable to PVE alone. However, these preliminary results must be validated by a prospective RCT. A French national RCT is ongoing comparing the PVE to the LVD before major hepatectomy.
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS)
ALPPS is a TSH with a shorter delay of time among first and second surgery. To achieve this goal, this technique initially described in 2012 consist of a classical first stage hepatectomy in which all lesion of the lobe that we want to preserve are resected, associated with the anticipation of the transection line of the second stage and the PVL of the diseased liver that should be resected in “second stage” (56). Differently from classical TSH, in which a mean time among the two procedure could be estimated in 4–6 weeks, with ALPPS procedure second stage could be performed in 1–2 week after that a CT scan based volumetry has assessed the correct volume of FRL. The main advantage of this procedure is the successful rate of second procedure in almost 99% of cases (30), compared with traditional TSH, in which the rate of dropout for patient could reach 25% as described by Lam et al. (25). Indeed, the main problem of the 4–6 weeks that are required to increase the liver volume is that in addition to the growth of normal liver, tumoral liver receive the same boost and could induce dropout from two stage resection initial program. ALPPS instead, in association with the partitioning of the cancer-bearing liver, with the short period required for hypertrophy drastically reduce the time for spread of cancer (30).
The second stage consists in the division of the ipsilateral artery and bile duct and hepatic vein in order to complete the hepatectomy. The main advantage is in the possibility to perform the two surgeries during the same hospitalisation with the second step performed with low rate of adherences, respecting the quote of future remnant liver.
Either THS and ALPPS procedure spread from the necessity to treat initially unresectable patients. Up to now, despite the standardization of both techniques and the consolidation of the results, the relative benefit of THS versus ALPPS for patients with bilobar liver metastasis is still a matter of debate. The main concern is about postoperative results and oncological outcome because TSH, even if related to a reduced risk of postoperative liver failure, is penalized by the dropout risk of patient between first and second stage, due to cancer progression or appearance of new lesions in the first stage resected liver. On the other side, ALPPS procedure reduce the risk of dropout during the two procedure with an increased risk of postoperative complication and mortality. The main discussion remain opened on patient selection. In order to identify which patients could benefit of one strategy better than another, because most of data in literature are focused on outcome. In a recent comprehensive review by Moris et al. (57) on the subject, from the analysis of nine comparative studies comparing data on both techniques, concluding that preoperative data were similar among techniques, an higher major morbidity and overall mortality was present in ALPPS, especially in initial part of the experience, with a benefit in favour of rapid FRL increasing in favour of ALPPS even if some authors have expressed perplexity on the association of rapid simultaneous growth of microscopic tumour cell in the FRL (58).
Data from the International ALPPS Registry were initially analysed in 2014 on 202 patients and demonstrate a dramatic rate of postoperative complication (Dindo ≥ 3b) of 27% associated with a postoperative mortality of (31), with identification in multivariate analysis of age >60 years old and non-colorectal liver metastasis as predictor of postoperative complications. A second analysis (59) of the same Registry performed in 2015 on 320 patients, demonstrate how the rate of severe morbidity and mortality remained comparable to previous analysis, identifying MELD score >10 on postoperative day 5 as predictor of poor outcome. These analyses demonstrate that the high rate of morbidity and mortality should identify a population of patients who could really have a benefit from this surgical procedure. Actually, the ALPPS registry describe mortality among 5% in series who report only patients treated for colorectal liver metastasis with an age inferior than 60 years old (30).
In recent period, some variations (Table 2) on the original technique have been proposed (Table 2), in order to minimize the invasiveness of this kind of approach, reducing the high mortality and morbidity which characterized ALPPS at the beginning of the experience. Actually, in literature we find some variation of the original technique such as partial ALPPS (56), radiofrequency or microwave ALPPS and mini ALPPS (30). These variations are important in order to reduce the rate of postoperative complications who strongly impact postoperative course. A recent metanalysis by Eshmuminov et al. (30) compared the results of ALPPS and traditional two-stage hepatectomy, concluding that ALLPS is associated with greater future remnant liver hypertrophy and higher rate on stage 2, but at the price of greater morbidity and mortality. These high rates of morbidity and mortality may be explained by two recent studies with HBS showing that the rapid hypertrophy of the FLR after stage 1 is not correlated to a functional increase. Olthof et al. showed a median increase of FLR-V of 78% compared to an increase of only 29% of the median FRL-F throughout 7 days after stage 1 (P<0.01) in patients with peri-hilar cholangiocarcinoma (71). Sparrelid et al. showed a FRL-V increase of 56.7% versus a FRL-F increase of 28.2% (P=0.021) 6 days after stage 1 in patients with colorectal liver metastases (72). Tomassini in a recent multicenter retrospective study included 98 patients underwent to ALPPS reported the role of HBS in predicting PHLF after ALPPS. The patients presenting a daily gain in volume (KGRFLR) ≤4.1%/day and a HBSFLR ≤2.7%/min/m2 are at high risk of PHLF and their second stage should be re-discussed (73). ALPPS induced a great hypertrophy of the FRL, but unfortunately, despite the variants, is still associated with a high mortality/morbidity rates. HBS may be useful to find the best time for second stage and to identify patient at high risk of PHLF.
Table 2. Different types of ALPPS procedures.
| Type | Technique description on stage I |
|---|---|
| Classic ALPPS (60) | Complete parenchymal transection, portal vein ligation |
| Partial ALPPS (61) | Partial parenchymal transection, portal vein ligation |
| RALPPS (62) | Radiofrequency ablation of the ideal transection line, portal vein ligation |
| Mini ALPPS (63) | Partial parenchymal transection, portal vein embolization (via inferior mesenteric vein) |
| Partial TIPE ALPPS (64) | Partial parenchymal transection, portal vein embolization (via ileocecal approach) |
| Anterior approach ALPPS (65) | Complete parenchymal transection using anterior approach, down to IVC. No liver mobilization |
| Hybrid ALPPS (66) | Complete parenchymal transection with anterior approach. PVE between two stages |
| ALTPS (67) | Application of tourniquet around the parenchymal transection line, portal vein ligation |
| Modified ALPPS with preservation of portal pedicles (68) | Complete parenchymal transection, selectively preserving portal pedicles |
| Salvage ALPPS (69) | Splitting of the liver along the main portal fissure after months from a radiological portal vein embolization |
| Left ALPPS (70) | Splitting along the main portal fissure. Left portal vein ligation |
| Right ALPPS (70) | Left lateral sectionectomy, multiple resections on the left medial, right anterior section and caudate lobe. Ligation of the posterolateral branch of the right portal vein |
ALPPS, Associating Liver Partition Portal and Portal Vein Ligation for Staged hepatectomy.
Radio-embolization (radiation hepatectomy)
Radio-embolization represents a novel technique that has shown good results in increasing contralateral liver lobe. One of the most important results, apart from the good response rate of the tumour (40–70%), is the contralateral hypertrophy (74) of non treated liver. This interesting results were confirmed by other series, evidencing the feasibility and safety of this approach (74-77). Even if the strength of this initial experience is biased by the heterogeneity of the patient and of different dosage of Y90, the contralateral hypertrophy varies from 20–50% of volume, with an achievement of volume that can require more time compared to other previous described techniques. This new concept has been stressed, especially in comparison with more traditional portal vein embolization. Even if no randomized control trial are still available, one study (78) has attempted to compare equivalent population with a case match study, equilibrating baseline FRL, previous chemotherapy, platelet count and degree of embolization. Results confirm the promising results of this technique, who still remain less efficient than classical PVE, concerning volume (29% vs. 61%) and time required to achieve the correct FRL (46 vs. 33 d). Despite this slower hypertrophy (78,79), that required a more gradual augmentation of volume, TARE has the possibility to associate to the increasing of volume the possibility to treat the lesion, causing a control of the pathology or even a down staging, opening interesting possibility for transforming previous unresectable patients in treatable.
Considering the promising results, some aspects remain still hidden. For example how the quality of liver parenchyma (chemotherapy, cirrhosis, viral infection) impacts the kinetic of the hypertrophy, understanding if there is a correlation among quantity of the liver and effect of treatment effect. Dosimetry of the treated liver area can also influence hypertrophy, with a proposed threshold at 88 Gy with Theraspheres (Palard et al., EJNMMI 2018). They are only few reports looking at the impact of radio-embolization on treated or non-treated liver function by HBS. In a cohort of 13 patients, van der Velden et al. showed an increase in the volume and function of the non-treated part of the liver after radio-embolization but with a large variability between volume and function changes). Further studies are needed to assess the role of HBS in radioembolization (80).
Radio-embolization induce a great but a slower hypertrophy of the FRL. It is of interest for patients affected by hepatocellular carcinoma treated with this technique. However, is expensive and facility demanding. Further studies are needed to assess the role of radio-embolization in this setting.
Conclusion
The introduction of even more sophisticated techniques to achieve FRL hypertrophy has shown interesting results in treatment of non-resectable patients.
Considering the advantages and drawback of each of these techniques, one of the main unsolved problem remains the difficulty in prediction correlation among volume spread and function of FRL. In most of cases, volumetry is considered the main predictor of PHLF, but this concept is still matter of debate (81). Adding functional assessment of the FRL with HBS may be more accurate for the selection of patients to large hepatectomy.
The study of the factors influencing quality of hypertrophic liver could reduce the rate of PHLF, with a more precise prediction of postoperative complications, could permit in future to identify more defined population who could benefit of such aggressive surgical approach. Further prospective randomized control trials are necessary to achieve this goal.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/hbsn-20-394). Dr. BG and Dr. FP serve as the unpaid editorial board member of Hepatobiliary Surgery and Nutrition. The other authors have no conflicts of interest to declare.
References
- 1.Pulitano C, Crawford M, Joseph D, et al. Preoperative assessment of postoperative liver function: The importance of residual liver volume. J Surg Oncol 2014;110:445-50. 10.1002/jso.23671 [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi A, Ruzzenente A, Conci S, et al. How much remnant is enough in liver resection? Dig Surg 2012;29:6-17. 10.1159/000335713 [DOI] [PubMed] [Google Scholar]
- 3.Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [DOI] [PubMed] [Google Scholar]
- 4.van den Broek MAJ, Olde Damink SWM, Dejong CHC, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int 2008;28:767-80. 10.1111/j.1478-3231.2008.01777.x [DOI] [PubMed] [Google Scholar]
- 5.Truant S, Oberlin O, Sergent G, et al. Remnant Liver Volume to Body Weight Ratio ≥ 0.5%: A New Cut-Off to Estimate Postoperative Risks after Extended Resection in Noncirrhotic Liver. J Am Coll Surg 2007;204:22-33. 10.1016/j.jamcollsurg.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 6.Schoen JM, Wang HH, Minuk GY, et al. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide 2001;5:453-64. 10.1006/niox.2001.0373 [DOI] [PubMed] [Google Scholar]
- 7.Webber EM, Bruix J, Pierce RH, et al. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology 1998;28:1226-34. 10.1002/hep.510280509 [DOI] [PubMed] [Google Scholar]
- 8.Ekman M, Fjälling M, Friman S, et al. Liver uptake function measured by iodida clearance rate in liver transplant patients and healthy volunteers. Nucl Med Commun 1996;17:235-42. 10.1097/00006231-199603000-00011 [DOI] [PubMed] [Google Scholar]
- 9.Erdogan D, Heijnen BHM, Bennink RJ, et al. Preoperative assessment of liver function: A comparison of 99mTc-Mebrofenin scintigraphy with indocyanine green clearance test. Liver Int 2004;24:117-23. 10.1111/j.1478-3231.2004.00901.x [DOI] [PubMed] [Google Scholar]
- 10.de Graaf W, van Lienden KP, Dinant S, et al. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg 2010;14:369-78. 10.1007/s11605-009-1085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinant S, De Graaf W, Verwer BJ, et al. Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J Nucl Med 2007;48:685-92. 10.2967/jnumed.106.038430 [DOI] [PubMed] [Google Scholar]
- 12.de Graaf W, van Lienden KP, Van Gulik TM, et al. 99mTc-mebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy. J Nucl Med 2010;51:229-36. 10.2967/jnumed.109.069724 [DOI] [PubMed] [Google Scholar]
- 13.Deshayes E, Guiu B. Liver function: homogenous or not? HPB (Oxford) 2016;18:871. 10.1016/j.hpb.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapelle T, Op De Beeck B, Huyghe I, et al. Future remnant liver function estimated by combining liver volumetry on magnetic resonance imaging with total liver function on 99mTc-mebrofenin hepatobiliary scintigraphy: Can this tool predict post-hepatectomy liver failure? HPB (Oxford) 2016;18:494-503. 10.1016/j.hpb.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cieslak KP, Bennink RJ, de Graaf W, et al. Measurement of liver function using hepatobiliary scintigraphy improves risk assessment in patients undergoing major liver resection. HPB (Oxford) 2016;18:773-80. 10.1016/j.hpb.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olthof PB, Coelen RJS, Bennink RJ, et al. 99mTc-mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma. HPB (Oxford) 2017;19:850-8. 10.1016/j.hpb.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 17.Chapelle T, Op de Beeck B, Driessen A, et al. Estimation of the future remnant liver function is a better tool to predict post-hepatectomy liver failure than platelet-based liver scores. Eur J Surg Oncol 2017;43:2277-84. 10.1016/j.ejso.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 18.Bennink RJ, Tulchinsky M, De Graaf W, et al. Liver function testing with nuclear medicine techniques is coming of age. Semin Nucl Med 2012;42:124-37. 10.1053/j.semnuclmed.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 19.Mizutani Y, Hirai T, Nagamachi S, et al. Prediction of Posthepatectomy Liver Failure Proposed by the International Study Group of Liver Surgery Residual Liver Function Estimation With Tc-99m-Galactosyl Human Serum Albumin Scintigraphy. Clin Nucl Med 2018;43:77-81. 10.1097/RLU.0000000000001913 [DOI] [PubMed] [Google Scholar]
- 20.Schuhmann-Giampieri G. Liver contrast media for magnetic resonance imaging: Interrelations between pharmacokinetics and imaging. Invest Radiol 1993;28:753-61. 10.1097/00004424-199308000-00018 [DOI] [PubMed] [Google Scholar]
- 21.Rassam F, Zhang T, Cieslak KP, et al. Comparison between dynamic gadoxetate-enhanced MRI and 99mTc-mebrofenin hepatobiliary scintigraphy with SPECT for quantitative assessment of liver function. Eur Radiol 2019;29:5063-72. 10.1007/s00330-019-06029-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geisel D, Lüdemann L, Fröling V, et al. Imaging-based evaluation of liver function: comparison of 99mTc-mebrofenin hepatobiliary scintigraphy and Gd-EOB-DTPA-enhanced MRI. Eur Radiol 2015;25:1384-91. 10.1007/s00330-014-3536-8 [DOI] [PubMed] [Google Scholar]
- 23.Isfordink CJ, Samim M, Braat MNGJA, et al. Portal vein ligation versus portal vein embolization for induction of hypertrophy of the future liver remnant: A systematic review and meta-analysis. Surg Oncol 2017;26:257-67. 10.1016/j.suronc.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 24.van Lienden KP, van den Esschert JW, De Graaf W, et al. Portal vein embolization before liver resection: A systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. 10.1007/s00270-012-0440-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam VWT, Laurence JM, Johnston E, et al. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford) 2013;15:483-91. 10.1111/j.1477-2574.2012.00607.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashita S, Sakamoto Y, Yamamoto S, et al. Efficacy of Preoperative Portal Vein Embolization Among Patients with Hepatocellular Carcinoma, Biliary Tract Cancer, and Colorectal Liver Metastases: A Comparative Study Based on Single-Center Experience of 319 Cases. Ann Surg Oncol 2017;24:1557-68. 10.1245/s10434-017-5800-z [DOI] [PubMed] [Google Scholar]
- 27.Shindoh J, Tzeng CWD, Aloia TA, et al. Safety and Efficacy of Portal Vein Embolization Before Planned Major or Extended Hepatectomy: An Institutional Experience of 358 Patients. J Gastrointest Surg 2014;18:45-51. 10.1007/s11605-013-2369-0 [DOI] [PubMed] [Google Scholar]
- 28.Guiu B, Chevallier P, Denys A, et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol 2016;26:4259-67. 10.1007/s00330-016-4291-9 [DOI] [PubMed] [Google Scholar]
- 29.Panaro F, Giannone F, Riviere B, et al. Perioperative impact of liver venous deprivation compared with portal venous embolization in patients undergoing right hepatectomy: preliminary results from the pioneer center. HepatoBiliary Surg Nutr 2019;8:329-37. 10.21037/hbsn.2019.07.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eshmuminov D, Raptis DA, Linecker M, et al. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg 2016;103:1768-82. 10.1002/bjs.10290 [DOI] [PubMed] [Google Scholar]
- 31.Schadde E, Ardiles V, Robles-Campos R, et al. Early survival and safety of ALPPS first report of the international ALPPS registry. Ann Surg 2014;260:829-36. 10.1097/SLA.0000000000000947 [DOI] [PubMed] [Google Scholar]
- 32.Ettorre GM, Levi Sandri GB, Laurenzi A, et al. Yttrium-90 Radioembolization for Hepatocellular Carcinoma Prior to Liver Transplantation. World J Surg 2017;41:2977. 10.1007/s00268-017-4044-1 [DOI] [PubMed] [Google Scholar]
- 33.Balzan S, Belghiti J, Farges O, et al. The "50-50 Criteria" on Postoperative Day 5: An Accurate Predictor of Liver Failure and Death After Hepatectomy. Ann Surg 2005;242:824-8, discussion 828-9. 10.1097/01.sla.0000189131.90876.9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makuuchi M, Takayasu K, Takuma T, et al. Preoperative transcatheter embolization of the portal venous branch for patients receiving extended lobectomy due to the bile duct carcinoma. J Jpn Pract Surg Soc 1984;45:1558-64. 10.3919/ringe1963.45.1558 [DOI] [Google Scholar]
- 35.Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg 1986;10:803-8. 10.1007/BF01655244 [DOI] [PubMed] [Google Scholar]
- 36.Capussotti L, Muratore A, Baracchi F, et al. Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch Surg 2008;143:978-82. 10.1001/archsurg.143.10.978 [DOI] [PubMed] [Google Scholar]
- 37.Shimura T, Suehiro T, Suzuki H, et al. Trans-ileocecal portal vein embolization as a preoperative treatment for right trisegmentectomy with caudate lobectomy. J Surg Oncol 2007;96:438-41. 10.1002/jso.20829 [DOI] [PubMed] [Google Scholar]
- 38.Tranchart H, Koffi GM, Gaillard M, et al. Liver regeneration following repeated reversible portal vein embolization in an experimental model. Br J Surg 2016;103:1209-19. 10.1002/bjs.10153 [DOI] [PubMed] [Google Scholar]
- 39.Tranchart H, Catherine L, Maitre S, et al. Efficient liver regeneration following temporary portal vein embolization with absorbable gelatin sponge powder in humans. J Vasc Interv Radiol 2015;26:507-15. 10.1016/j.jvir.2014.11.033 [DOI] [PubMed] [Google Scholar]
- 40.Viganò L, Torzilli G, Cimino M, et al. Drop-out between the two liver resections of two-stage hepatectomy. Patient selection or loss of chance? Eur J Surg Oncol 2016;42:1385-93. 10.1016/j.ejso.2016.03.020 [DOI] [PubMed] [Google Scholar]
- 41.Margonis GA, Sasaki K, Andreatos N, et al. Increased kinetic growth rate during late phase liver regeneration impacts the risk of tumor recurrence after colorectal liver metastases resection. HPB (Oxford) 2017;19:808-817. 10.1016/j.hpb.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 42.Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: Evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540-8. 10.1097/SLA.0b013e3181b674df [DOI] [PubMed] [Google Scholar]
- 43.Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: Expert consensus statement. In: Ann Surg Oncol 2006;13:1271-80. 10.1245/s10434-006-9045-5 [DOI] [PubMed] [Google Scholar]
- 44.Schroeder RA, Marroquin CE, Bute BP, et al. Predictive indices of morbidity and mortality after liver resection. Ann Surg 2006;243:373-9. 10.1097/01.sla.0000201483.95911.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdalla EK, Denys A, Chevalier P, et al. Total and segmental liver volume variations: Implications for liver surgery. Surgery 2004;135:404-10. 10.1016/j.surg.2003.08.024 [DOI] [PubMed] [Google Scholar]
- 46.Memeo R, de’Angelis N, de Blasi V, et al. Innovative surgical approaches for hepatocellular carcinoma. World J Hepatol 2016;8:591-6. 10.4254/wjh.v8.i13.591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esposito F, Lim C, Lahat E, et al. Combined hepatic and portal vein embolization as preparation for major hepatectomy: a systematic review. HPB (Oxford) 2019;21:1099-106. 10.1016/j.hpb.2019.02.023 [DOI] [PubMed] [Google Scholar]
- 48.Goumard C, Komatsu S, Brustia R, et al. Technical feasibility and safety of laparoscopic right hepatectomy for hepatocellular carcinoma following sequential TACE-PVE: a comparative study. Surg Endosc 2017;31:2340-9. 10.1007/s00464-016-5225-y [DOI] [PubMed] [Google Scholar]
- 49.de Graaf W, Van Lienden KP, Van Den Esschert JW, et al. Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg 2011;98:825-34. 10.1002/bjs.7456 [DOI] [PubMed] [Google Scholar]
- 50.Komori K, Nagino M, Nimura Y. Hepatocyte morphology and kinetics after portal vein embolization. Br J Surg 2006;93:745-51. 10.1002/bjs.5332 [DOI] [PubMed] [Google Scholar]
- 51.Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg 2000;231:480-6. 10.1097/00000658-200004000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang S, Lee SG, Ko GY, et al. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg 2009;249:608-16. 10.1097/SLA.0b013e31819ecc5c [DOI] [PubMed] [Google Scholar]
- 53.Hwang S, Lee SG, Park KM, et al. Hepatic venous congestion in living donor liver transplantation: Preoperative quantitative prediction and follow-up using computed tomography. Liver Transpl 2004;10:763-70. 10.1002/lt.20178 [DOI] [PubMed] [Google Scholar]
- 54.Gruttadauria S, Gridelli B. Sequential preoperative ipsilateral portal and arterial embolization in patients with liver tumors: Is it really the best approach? World J Surg 2007;31:2427-8. 10.1007/s00268-007-9239-4 [DOI] [PubMed] [Google Scholar]
- 55.Hwang S, Ha TY, Ko GY, et al. Preoperative Sequential Portal and Hepatic Vein Embolization in Patients with Hepatobiliary Malignancy. World J Surg 2015;39:2990-8. 10.1007/s00268-015-3194-2 [DOI] [PubMed] [Google Scholar]
- 56.Wu X, Rao J, Zhou X, et al. Partial ALPPS versus complete ALPPS for staged hepatectomy. BMC Gastroenterol 2019;19:170. 10.1186/s12876-019-1090-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moris D, Ronnekleiv-Kelly S, Kostakis ID, et al. Operative Results and Oncologic Outcomes of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Versus Two-Stage Hepatectomy (TSH) in Patients with Unresectable Colorectal Liver Metastases: A Systematic Review and Meta-Anal. Vol. 42, World J Surg 2018;42:806-15. 10.1007/s00268-017-4181-6 [DOI] [PubMed] [Google Scholar]
- 58.Adam R, Imai K, Castro Benitez C, et al. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Br J Surg 2016;103:1521-9. 10.1002/bjs.10256 [DOI] [PubMed] [Google Scholar]
- 59.Schadde E, Raptis DA, Schnitzbauer AA, et al. Prediction of mortality after ALPPS Stage-1. Ann Surg 2015;262:780-5. 10.1097/SLA.0000000000001450 [DOI] [PubMed] [Google Scholar]
- 60.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. 10.1097/SLA.0b013e31824856f5 [DOI] [PubMed] [Google Scholar]
- 61.Petrowsky H, Györi G, De Oliveira M, et al. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg 2015;261:e90-2. 10.1097/SLA.0000000000001087 [DOI] [PubMed] [Google Scholar]
- 62.Cillo U, Gringeri E, Feltracco P, et al. Totally Laparoscopic Microwave Ablation and Portal Vein Ligation for Staged Hepatectomy: A New Minimally Invasive Two-Stage Hepatectomy. Ann Surg Oncol 2015;22:2787-8. 10.1245/s10434-014-4353-7 [DOI] [PubMed] [Google Scholar]
- 63.de Santibañes E, Alvarez FA, Ardiles V, et al. Inverting the ALPPS paradigm by minimizing first stage impact: the Mini-ALPPS technique. Langenbecks Arch Surg 2016;401:557-63. 10.1007/s00423-016-1424-1 [DOI] [PubMed] [Google Scholar]
- 64.Sakamoto Y, Matsumura M, Yamashita S, et al. Partial TIPE ALPPS for Perihilar Cancer. Ann Surg 2018;267:e18-20. 10.1097/SLA.0000000000002484 [DOI] [PubMed] [Google Scholar]
- 65.Chan ACY, Poon RTP, Chan C, et al. Safety of ALPPS procedure by the anterior approach for hepatocellular carcinoma. Ann Surg 2016;263:e14-6. 10.1097/SLA.0000000000001272 [DOI] [PubMed] [Google Scholar]
- 66.Li J, Kantas A, Ittrich H, et al. Avoid “all-touch” by hybrid alpps to achieve oncological efficacy. Ann Surg 2016;263:e6-7. 10.1097/SLA.0000000000000845 [DOI] [PubMed] [Google Scholar]
- 67.Robles R, Parrilla P, Lõpez-Conesa A, et al. Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedure. Br J Surg 2014;101:1129-34. 10.1002/bjs.9547 [DOI] [PubMed] [Google Scholar]
- 68.Tanaka K, Kikuchi Y, Kawaguchi D, et al. Modified ALPPS procedures avoiding division of portal pedicles. Ann Surg 2017;265:e14-20. 10.1097/SLA.0000000000001967 [DOI] [PubMed] [Google Scholar]
- 69.Tschuor C, Croome KP, Sergeant G, et al. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion - An extension of the ALPPS approach. Eur J Surg Oncol 2013;39:1230-5. 10.1016/j.ejso.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 70.Gauzolino R, Castagnet M, Blanleuil ML, et al. The ALPPS technique for bilateral colorectal metastases: Three “variations on a theme.” Updates Surg 2013;65:141-8. 10.1007/s13304-013-0214-3 [DOI] [PubMed] [Google Scholar]
- 71.Olthof PB, Coelen RJS, Wiggers JK, et al. High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford) 2017;19:381-7. 10.1016/j.hpb.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sparrelid E, Jonas E, Tzortzakakis A, et al. Dynamic Evaluation of Liver Volume and Function in Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. J Gastrointest Surg 2017;21:967-74. 10.1007/s11605-017-3389-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomassini F, D’Asseler Y, Linecker M, et al. Hepatobiliary scintigraphy and kinetic growth rate predict liver failure after ALPPS: a multi-institutional study. HPB (Oxford) 2020. [Epub ahead of print]. [DOI] [PubMed]
- 74.Teo JY, Goh BKP. Contra-lateral liver lobe hypertrophy after unilobar Y90 radioembolization: An alternative to portal vein embolization? World J Gastroenterol 2015;21:3170-3. 10.3748/wjg.v21.i11.3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aoki T, Imamura H, Hasegawa K, et al. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg 2004;139:766-74. 10.1001/archsurg.139.7.766 [DOI] [PubMed] [Google Scholar]
- 76.Teo JY, Allen JC, Ng DC, et al. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB (Oxford) 2016;18:7-12. 10.1016/j.hpb.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaba RC, Lewandowski RJ, Kulik LM, et al. Radiation lobectomy: Preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol 2009;16:1587-96. 10.1245/s10434-009-0454-0 [DOI] [PubMed] [Google Scholar]
- 78.Garlipp B, De Baere T, Damm R, et al. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology 2014;59:1864-73. 10.1002/hep.26947 [DOI] [PubMed] [Google Scholar]
- 79.Fernández-Ros N, Silva N, Bilbao JI, et al. Partial liver volume radioembolization induces hypertrophy in the spared hemiliver and no major signs of portal hypertension. HPB (Oxford) 2014;16:243-9. 10.1111/hpb.12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Velden S, Braat MNGJA, Labeur TA, et al. A pilot study on hepatobiliary scintigraphy to monitor regional liver function in 90Y radioembolization. J Nucl Med 2019;60:1430-6. 10.2967/jnumed.118.224394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Truant S, Baillet C, Deshorgue AC, et al. Drop of total liver function in the interstages of the new associating liver partition and portal vein ligation for staged hepatectomy technique: Analysis of the “auxiliary liver” by HIDA scintigraphy. Ann Surg 2016;263:e33-4. 10.1097/SLA.0000000000001603 [DOI] [PubMed] [Google Scholar]