Abstract
Emergence and spread of resistance in Plasmodium falciparum to the frontline treatment artemisinin-based combination therapies (ACTs) in the epicenter of multidrug resistance of Southeast Asia threaten global malaria control and elimination. Artemisinin (ART) resistance (or tolerance) is defined clinically as delayed parasite clearance after treatment with an ART drug. The resistance phenotype is restricted to the early ring stage and can be measured in vitro using a ring-stage survival assay. ART resistance is associated with mutations in the propeller domain of the Kelch family protein K13. As a pro-drug, ART is activated primarily by heme, which is mainly derived from hemoglobin digestion in the food vacuole. Activated ARTs can react promiscuously with a wide range of cellular targets, disrupting cellular protein homeostasis. Consistent with this mode of action for ARTs, the molecular mechanisms of K13-mediated ART resistance involve reduced hemoglobin uptake/digestion and increased cellular stress response. Mutations in other genes such as AP-2μ (adaptor protein-2 μ subunit), UBP-1 (ubiquitin-binding protein-1), and Falcipain 2a that interfere with hemoglobin uptake and digestion also increase resistance to ARTs. ART resistance has facilitated the development of resistance to the partner drugs, resulting in rapidly declining ACT efficacies. The molecular markers for resistance to the partner drugs are mostly associated with point mutations in the two food vacuole membrane transporters PfCRT and PfMDR1, and amplification of pfmdr1 and the two aspartic protease genes plasmepsin 2 and 3. It has been observed that mutations in these genes can have opposing effects on sensitivities to different partner drugs, which serve as the principle for designing triple ACTs and drug rotation. Although clinical ACT resistance is restricted to Southeast Asia, surveillance for drug resistance using in vivo clinical efficacy, in vitro assays, and molecular approaches is required to prevent or slow down the spread of resistant parasites.
Keywords: Plasmodium falciparum, Drug resistance, Artemisinin, Piperaquine, K13, pfcrt, pfmdr1, Hemoglobin digestion, Stress response, Molecular surveillance
Graphical abstract
1. Introduction
The remarkable reduction in global malaria incidence over the past two decades is truly encouraging, but a worrisome slowing-down trend has been observed since 2015. In 2019, malaria accounted for an estimated 229 million cases and 409,000 deaths globally (WHO, 2020b). Plasmodium falciparum is responsible for 97% of the global malaria incidence. Since an effective vaccine is not yet available, malaria control relies heavily on vector-based malaria interventions such as long-lasting insecticide-treated bed nets and indoor residual spraying of insecticides, and antimalarial drugs (Bhatt et al., 2015). Chemotherapy is essential for the management of clinical cases, and in some areas, for malaria elimination. However, drug resistance is a recurrent problem, and the introduction of new antimalarial treatment is soon followed by resistance development. Perhaps the most rapid development of clinical resistance in P. falciparum was to atovaquone, with resistant parasites being detected immediately in recrudescent cases following atovaquone monotherapy (Looareesuwan et al., 1996). Currently, P. falciparum has developed clinical resistance to all classes of the commonly used antimalarial drugs, including the frontline treatment – artemisinin-based combination therapies (ACTs). Antimalarial drug resistance can seriously weaken the effectiveness of chemotherapy, cause malaria resurgence, and derail the malaria elimination campaign. Therefore, resistance surveillance has received enormous attention to safeguarding the recent gains achieved.
Antimalarial drug resistance is defined as the ability of a parasite strain to survive or multiply despite the administration and absorption of a drug given in doses equal to or higher than those usually recommended, but within the tolerance of the subject (WHO, 2020a). Drug resistance arises as a result of spontaneously occurring genetic mutations with a probability of 1 in 1012 parasites, an estimate that can occur readily in a hyperparasitemic patient (White, 2004). Thus, simultaneous use of two or more antimalarials with different modes of action and different resistance mechanisms will reduce the chance of selection (White, 1999). Based on this principle, ACTs have been formulated to include an artemisinin (ART) derivative and a partner drug. The World Health Organization (WHO) currently recommends six ACTs: artemether-lumefantrine (AL), artesunate-amodiaquine (AS-AQ), artesunate-mefloquine (AS-MQ), artesunate-pyronaridine (AS-PND), artesunate-sulfadoxine/pyrimethamine (AS-SP), and dihydroartemisinin-piperaquine (DHA-PPQ). The fast-acting ART compound reduces the parasite mass by ~10,000 times per 48 h asexual life cycle, while the residual parasites (normally fewer than 105 parasites in an adult infection) are cleared by the slowly-eliminating partner drug (White, 2008). The deployment of ACTs as the frontline treatment for falciparum malaria has played an indispensable role in reducing global malaria incidence. However, the emergence of parasites resistant to both ART and the partner drugs (Table 1), resulting in marked reductions in the clinical efficacy of ACTs, is a major concern. Here we review the origin of resistance to ACTs in Southeast Asia, the resistance mechanisms, and countermeasures to slow down and deter resistance spread.
Table 1.
Modes of action and resistance mechanisms of commonly used drugs in ACTs.
| Drug | Class | Site of action | Mode of action | Molecular markers of resistance |
|---|---|---|---|---|
| PPQ | 4-Aminoquinoline | FV | Interfere with Hb digestion & heme detoxification | Plasmepsin 2 & 3 amplification, NS SNPs in PfCRT |
| AQ | 4-Aminoquinoline | FV | Interfere with heme detoxification | NS SNPs in PfMDR1 & PfCRT |
| MQ | Arylamino alcohol | FV & cytoplasm | Interfere with Hb uptake & heme detoxification; target the pf80S ribosome, purine nucleoside phosphorylase, and PfMDR1 |
Pfmdr1 amplification, NS SNPs in PfMDR1 & PfCRT |
| LMF | Arylamino alcohol | FV & cytoplasm | Interfere with Hb uptake & heme detoxification; target PfMDR1 |
Pfmdr1 amplification, NS SNPs in PfMDR1 & PfCRT |
| ARTs | Endoperoxide | Cytoplasm | Induce oxidative stress, proteasomal stress, DNA damage; alkylate and oxidate proteins, lipids, and heme | NS SNPs in K13, UBP-1, AP2-μ, Coronin, Eps15, Falcipain 2a |
Abbreviations: Piperaquine (PPQ), amodiaquine (AQ), mefloquine (MQ), lumefantrine (LMF), artemisinins (ARTs), food vacuole (FV), hemoglobin (Hb), Nonsynonymous single nucleotide polymorphisms (NS SNPs), P. falciparum chloroquine resistance transporter (PfCRT), P. falciparum multidrug resistance gene-1 (Pfmdr1), Kelch 13 (K13), ubiquitin-binding protein-1 (UBP1), adaptor protein-2μ (AP-2μ), epidermal growth factor receptor substrate-15 (Eps15) homolog.
2. Emergence of drug resistance in Southeast Asia
The Greater Mekong Subregion (GMS) in Southeast Asia has been an epicenter of antimalarial drug resistance, although it carries only a small fraction of the global malaria burden. In the 1950s, chloroquine (CQ) resistance arose here (Eyles et al., 1963; Powell et al., 1964), which was followed by resistance to the replacement drug combination SP (Hurwitz et al., 1981), MQ (Boudreau et al., 1982; Mockenhaupt, 1995; Wongsrichanalai et al., 2002), and more recently, ARTs (Dondorp et al., 2009; Noedl et al., 2008) and PPQ (Amaratunga et al., 2016; Leang et al., 2015; Saunders et al., 2014; Spring et al., 2015). Clinical ART resistance, manifested as delayed parasite clearance, was first detected in western Cambodia in 2006–2007 (Amaratunga et al., 2012; Dondorp et al., 2009; Noedl et al., 2008), and then in all GMS countries due to spread and independent emergence (Ashley et al., 2014; Bustos et al., 2013; Hien et al., 2012; Huang et al., 2015; Kyaw et al., 2013; Phyo et al., 2012). Resistance to ARTs and the partners resulted in increased failure rates of two ACTs. AS-MQ was introduced in Cambodia and Thailand amidst pre-existing MQ resistance, but it enjoyed more than a decade of satisfactory clinical efficacy and even resulted in decreased MQ resistance (Nosten et al., 1994, 2000). At the turn of the century, the clinical efficacy of AS-MQ declined steadily (Carrara et al., 2013; Rogers et al., 2009; Wongsrichanalai and Meshnick, 2008) and had to be replaced with DHA-PPQ in 2008 in Cambodia and 2015 in Thailand. With the emerging ART resistance, the partner drug PPQ quickly succumbed to resistance development, leading to high failure rates of DHA-PPQ in western Cambodia (Amaratunga et al., 2016; Leang et al., 2015; Saunders et al., 2014; Spring et al., 2015). This has led to the reconsideration of AS-MQ as the first-line treatment of P. falciparum in parts of Cambodia. Detailed events in the evolution of ACT resistance in the GMS countries have been reviewed (Hassett and Roepe, 2019).
The repeated ‘strikes’ of drug resistance in the same place deserve a better understanding of the evolutionary process, which may involve factors from the host, the parasite, and the epidemiological settings (Beez et al., 2011; Rogerson et al., 2010; White et al., 2009; Woodrow and White, 2017). In the Southeast Asian human populations, hemoglobinopathies and glucose-6-phosphate dehydrogenase deficiency are highly prevalent, and some are associated with innate resistance to malaria infection (Kariuki and Williams, 2020; Taylor et al., 2012). The infecting parasites, pre-selected to withstand the higher oxidative stress within these red blood cells (RBCs), would have a stronger antioxidative defense. This would favor resistance selection, as many antimalarials cause increased cellular oxidative stress (Kavishe et al., 2017). In addition, it has been shown that parasites infecting thalassemic RBCs are less susceptible to ART, and the suboptimal drug exposure would impose selective pressure for resistance development (Kamchonwongpaisan et al., 1994; Yuthavong et al., 1989). Also, human populations in the low-endemicity regions are less likely to develop high levels of immunity to P. falciparum, which are needed for enhanced parasite clearance and reduced chances of resistance development. Without disease-controlling immunity, patients are likely to develop symptoms after infection and seek treatment, leading to drug exposure and resistance selection for the parasites (O'Flaherty et al., 2017; Rogerson et al., 2010; White, 2004). The circulation of falsified and substandard antimalarial drugs as well as the use of artemisinin monotherapies may have also contributed to the selection of resistance (Guo et al., 2017; Newton et al., 2008).
The genetic background of the parasites from the GMS may be a predisposing factor, accelerating the development of resistance to new drugs (Beez et al., 2011; Xiong et al., 2020). In vitro studies have linked parasites from this region with the accelerated resistance to multiple drugs (ARMD) phenotype (Rathod et al., 1997) to altered DNA repair mechanisms (Castellini et al., 2011; Gupta et al., 2016; Trotta et al., 2004). Mutations in six DNA repair genes were identified in ART-resistant parasites from the GMS, which may enable the parasites to deal with DNA damage more effectively (Miotto et al., 2013; Xiong et al., 2020). Another study from the GMS found a mild mutator phenotype that allows these parasites to more quickly acquire resistance-conferring mutations while maintaining overall fitness (Lee and Fidock, 2016). During in vitro selection, a parasite strain from this region (Dd2) develops resistance to DHA much more rapidly than parasite strains from other continents (Cui et al., 2012). Furthermore, since resistant parasites often show reduced fitness (Rosenthal, 2013; Siddiqui et al., 2020; Straimer et al., 2017), the scenario of fewer polyclonal infections and less intrahost competition under low endemicity would benefit the survival of the mutant parasites. Low endemicity also means higher inbreeding of the parasites and favors the fixation of the resistant alleles and spread of the resistant parasites (Su et al., 2007). The recent trans-national hard genetic sweep of parasites with genes conferring resistance to both ARTs and PPQ in the GMS provides direct visualization of this evolutionary process (Hamilton et al., 2019; Imwong et al., 2017, Imwong et al., 2020). History has witnessed the spread of CQ- and pyrimethamine-resistant parasites from the GMS to Africa, responsible for the deaths of millions of children (Roper et al., 2004; Trape et al., 1998; Wellems and Plowe, 2001; Wootton et al., 2002). With the central position of the ACTs in the current antimalarial armamentarium, an analogous spread of ART resistance would bear a disastrous consequence on global malaria control. Out of such fear, the WHO deployed an ART resistance containment plan in the GMS (WHO, 2011). Later, the detection of ART-resistant parasites in all GMS countries (Takala-Harrison et al., 2015) and the prediction that the remaining parasites would be most drug-resistant (Maude et al., 2009) have urged the launch of a regional malaria elimination campaign intending to eliminate falciparum malaria by 2025 and all malaria by 2030 (WHO, 2016).
3. ART resistance: the unusual phenotype and underlying mechanisms
3.1. Mode of action and targets of ARTs
ART is a sesquiterpene lactone isolated from the Chinese medicinal herb Artemisia annua. It was discovered by Youyou Tu in the 1970s, who was later awarded a Nobel Prize in Medicine in 2015 for her discovery (Tu, 2011, 2016). The exact mode of action of ART is not fully understood, but a popular theory proposes that ART activation involves iron-catalyzed reductive scission of the endoperoxide bridge, producing carbon-centered radical species that alkylate cellular proteins and other biomolecules, leading to parasite death (Haynes et al., 2013; Heller and Roepe, 2019; Klonis et al., 2013a). The primary source of ferrous iron is thought to be heme, an abundant by-product of hemoglobin degradation in the food vacuole (FV) of the parasite (Haynes et al., 2013; Klonis et al., 2013a; Meunier and Robert, 2010). This explains the high-level toxicity of ARTs against the trophozoites when peak hemoglobin digestion occurs, as compared to much less activity against the 6–20 h ring stage, stage V gametocytes, and the liver stage when hemoglobin digestion is low or absent (Abu Bakar et al., 2010; Adjalley et al., 2011; Klonis et al., 2013b; Meister et al., 2011; Tilley et al., 2016). Intriguingly, very early ring-stage parasites (2–4 h post-invasion) exhibit ART hypersensitivity (Klonis et al., 2013b; Xie et al., 2016), which cannot be explained by hemoglobin digestion but may be attributed to the de novo heme biosynthesis by the parasite (Klonis et al., 2013a; Wang et al., 2015a). Additional sources of iron might include the free heme that undergoes peroxidative decomposition in the FV (Loria et al., 1999), non-polymerized heme that is supposed to be degraded by glutathione (Ginsburg et al., 1998), and/or reduced iron from a labile iron pool in the parasite cytoplasm (Clark et al., 2013).
Once ART is activated, the free radicals alkylate proteins, lipids, and also heme (O'Neill et al., 2010; Yang et al., 1994). Early studies detected proteins that are labeled by radioactive endoperoxides (Asawamahasakda et al., 1994) and characterized one of these proteins – the translationally controlled tumor protein PfTCTP (Bhisutthibhan and Meshnick, 2001; Li et al., 2016). However, recent proteomic studies indicate that the ART alkylation activity is promiscuous, involving hundreds of targets across many essential biological processes (Ismail et al., 2016a, Ismail et al., 2016b; Jourdan et al., 2019; Wang et al., 2015a). Given that this experimental procedure cannot identify non-covalent targets, the ART target spectrum may be much broader. Besides protein targets, heme also appears to be a major ART target (Asawamahasakda et al., 1994; Meunier and Robert, 2010; Robert et al., 2001). The formation of the non-polymerizable redox-active ART-heme adducts (Heller et al., 2018; Hong et al., 1994) suggests that ART may also exert a similar mechanism of action as 4-aminoquinoline drugs by interfering with the heme detoxification process (Meunier and Robert, 2010; Pandey et al., 1999). Of relevance, ART-resistant parasites showed lower ART-heme adduct abundance than ART-sensitive parasites, suggesting the formation of lower levels of reduced heme in ART-resistant parasites (Heller et al., 2018; Heller and Roepe, 2018). In addition, activated ART can lead to the generation of reactive oxygen species (ROS), which reduces the cellular antioxidant capacity and damage mitochondria and macromolecules such as parasite DNA (Gopalakrishnan and Kumar, 2015; Krungkrai and Yuthavong, 1987; Meshnick, 2002; Wang et al., 2010).
3.2. Unconventional ART resistance: in vivo and in vitro phenotypes focusing on the ring-stage parasites
Clinical ART resistance is manifested as delayed parasite clearance (DPC), with a higher proportion of patients remaining parasitemic by microscopy at day 3 after ART treatment (Dondorp et al., 2009; Noedl et al., 2008; Stepniewska et al., 2010). This definition was later more accurately updated as parasite clearance half-life (PC1/2) of >5 h for ART-resistant parasites as compared to ~2 h for sensitive parasites (Amaratunga et al., 2012; Ashley et al., 2014; Phyo et al., 2012). These ART-resistant and -sensitive parasites, however, did not show marked differences in ex vivo drug sensitivity measured by the 72-h standard drug assay (Amaratunga et al., 2012; Dondorp et al., 2009). In practice, day-3 parasitemia is more commonly used as a crude measure of ART resistance with 10% and 5% day-3 parasite positivity as the cut-off for suspected ART resistance in Southeast Asia and Africa, respectively (White et al., 2015; WWARN Artemisinin-based Combination Therapy Africa Baseline Study Group, 2015). This definition of ART resistance, which deviates from classical drug resistance, has been continuously debated (Dondorp and Ringwald, 2013; Ferreira et al., 2013; Krishna and Kremsner, 2013; Wellems et al., 2020). In fact, even before the widespread implementation of ACTs, ART monotherapies effectively cleared parasites from the blood within 7 days, but many cases recrudesced within 28 days after treatment (Li et al., 1994; Looareesuwan et al., 1992b; Nguyen et al., 1993), which is consistent with the RI resistance phenotype defined by the WHO (WHO, 1965). Despite extensive ACT usage over 30 years, RII or RIII resistance to ARTs has not emerged, which may be due to the action of this family of drugs on multiple cellular targets.
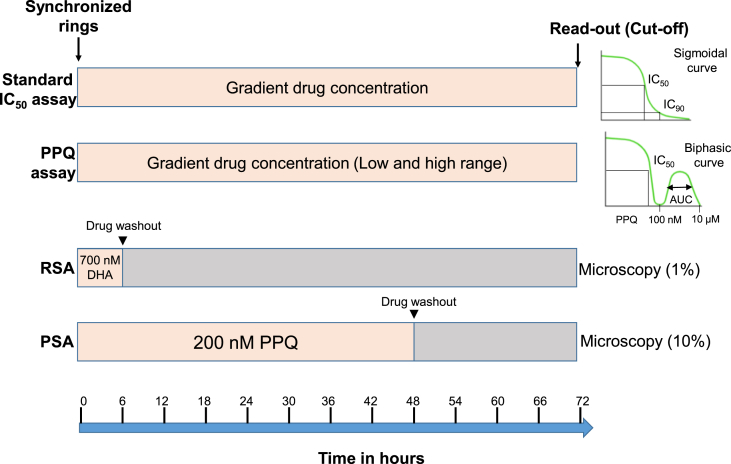
Modeling the DPC phenotype identified that ART resistance predominantly affects the ring stage (Dondorp et al., 2009; Saralamba et al., 2011). During ring-stage development, parasites can enter dormancy with reduced cellular metabolism and increased resistance to external stress (Teuscher et al., 2010; Witkowski et al., 2010). Thus, ART resistance may reflect an enhanced propensity of the ring-stage parasites to enter dormancy after ART exposure, but resuming growth days or weeks later (Cheng et al., 2012; Witkowski et al., 2013b). Besides, an altered development pattern of the resistant parasites, exhibited by decelerated progress through the ring stage when susceptibility to ARTs is low, may also contribute to the clinical phenotype of ART resistance (Hott et al., 2015; Klonis et al., 2013b; Mok et al., 2011). It is hypothesized that this change in the life cycle may be specifically selected by the ART drugs with a short half-life (~1 h), and split-dosing of an ACT would increase the chance the parasite population is treated in the more vulnerable phase of the life cycle (Khoury et al., 2020). However, increasing or splitting the ART daily dose does not seem to enhance the cure rates (Bethell et al., 2011; Das et al., 2013; White et al., 2017). To reflect the major effect of ART resistance on the ring stage, a novel in vitro/ex-vivo ring-stage survival assay (RSA) was designed (Fig. 1), where 0–3 h early ring-stage parasites are treated with a pharmacologically relevant pulse of DHA for 6 h and their survival rates are measured at 72 h (Witkowski et al., 2013a). A threshold of 1% RSA value is used to distinguish ART-resistant and ART-sensitive parasites, showing a strong correlation with slow- and fast-clearing parasites after ART therapy. Nevertheless, the current ring-stage centered ART resistance may be the preamble before the emergence of full resistance since ART-resistant parasites with elevated IC50 values have been selected from in vitro studies (Chen et al., 2010; Cui et al., 2012; Teuscher et al., 2012). Repeated drug pressure and dormancy over time may select for fast-recovering rings that can withstand the drug exposure without undergoing dormancy (Cheng et al., 2012). ART appears to temporally slow down the parasite growth and alter the periodicity of the cell cycle, and the resistant parasites are able to withstand and overcome this growth retardation with accelerated post-drug recovery (Dogovski et al., 2015; Klonis et al., 2011; Mok et al., 2021).
Fig. 1.
In vitro drug assays. Standard drug assay. In the standard IC50 assay, ring-stage parasites are treated with a gradient of drug concentrations, and readout is done at 72 h (by hypoxanthine incorporation, SYBR Green I staining, or HRP2 ELISA). IC50 and IC90 values are calculated based on the plotted sigmoid curve. PPQ (piperaquine) assay. For some PPQ-resistant parasites, drug assay is done using a much wider range of drug concentrations. In addition to the IC50 and IC90 values, area under the curve (AUC) is calculated for the second peak at PPQ concentrations of 0.1–10 μM. RSA (ring survival assay). Synchronized early ring-stage (0–3 h) parasites are treated with 700 nM DHA for 6 h, the drug is washed off, parasites are incubated for another 66 h, and surviving parasites are counted microscopically or by flow cytometry. A survival rate of 1% is used as the threshold for ART resistance. PSA (piperaquine survival assay). Synchronized ring-stage parasites are treated with 200 nM PPQ for 48 h, the drug is washed off, and parasites are incubated for an additional 24 h. Surviving parasites are counted by microscopy, and a survival rate of 10% is used as the cutoff value for PPQ resistance. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. K13 mutations as the key determinant of ART resistance
Parasite clearance rate after ART treatment has been used as a phenotype to determine the genetics underlying reduced sensitivity to ART (Amaratunga et al., 2012; Takala-Harrison et al., 2013). The DPC phenotype first identified in western Cambodia was found to be heritable (Anderson et al., 2010) and associated with genomic regions on chromosome 13 by genome-wide association studies (GWAS) (Cheeseman et al., 2012; Takala-Harrison et al., 2013). A crucial discovery was made in 2014 by Ariey et al. who identified a mutation (M476I) in the k13 gene on chromosome 13 in a Tanzanian parasite strain F32 after ~5-year in vitro selection with intermittent ART drug pressure (Ariey et al., 2014; Witkowski et al., 2010). Retrospective analysis of parasite samples from Cambodia identified numerous mutations clustered in the “propeller” domain of the K13 protein showing strong correlations with both higher in vitro RSA values and the longer PC1/2 phenotype, further implying the k13 gene as a key determinant of the DPC phenotype (Ariey et al., 2014). Many K13 mutations have been associated with DPC in clinical efficacy studies (Ashley et al., 2014; Huang et al., 2015; Miotto et al., 2015). Several most common K13 mutations (C580Y, R539T, I543T, N458Y, and Y493H) were genetically validated for in vitro ART resistance (Ghorbal et al., 2014; Siddiqui et al., 2020; Straimer et al., 2015), providing a marker gene for surveillance of ART resistance. To date, over 200 non-synonymous mutations have been identified in the k13 gene from global parasite populations (Chhibber-Goel and Sharma, 2019; MalariaGEN Plasmodium falciparum Community Project, 2016; Menard et al., 2016; Taylor et al., 2015). The WHO has also issued a list of validated K13 mutations associated with ART resistance – F446I, N458Y, M476I, Y493H, R539T, I543T, P553L, R561H, P574L, and C580Y (WHO, 2020a). Multiple K13 mutations were found in African parasite samples (Bayih et al., 2016; Conrad et al., 2014; L'Episcopia et al., 2020; Menard et al., 2016; Raman et al., 2019; Taylor et al., 2015), but those associated with DPC found in the GMS were absent or rare. For example, the most frequent A578S mutation in Africa was not associated with in vitro ART resistance when engineered in the Dd2 parasite (Menard et al., 2016). However, several recent studies made worrisome discoveries of resistance-mediating K13 mutations outside of the GMS, highlighting the emergence of ART resistance elsewhere as an imminent threat. In Rwanda, though the cure rates of ACTs remained high (>95%), the R561H mutation was identified in 7.4% of the patients, representing de novo emergence and expansion of a local lineage (Uwimana et al., 2020). A case report of AL treatment failure of a parasite with the R561H mutation imported from this region highlights the ability of K13 R561H to mediate ART resistance in non-immune traveler (Fernando et al., 2020), consistent with the fact that it has been previously associated with delayed parasite clearance in the GMS (WWARN Artemisinin-based Combination Therapy Africa Baseline Study Group, 2015). It also conferred in vitro ART resistance when engineered in the Dd2 strain (Uwimana et al., 2020). C580Y, the most prevalent K13 mutation in the eastern GMS, had independently emerged in Guyana and Papua New Guinea (PNG) (Chenet et al., 2016; Mathieu et al., 2020; Miotto et al., 2020). In Guyana, it was first detected in 2010 and has since persisted at a low frequency, most likely due to a high fitness cost as confirmed by gene editing in two parasite strains from the French Guiana (Chenet et al., 2016; Mathieu et al., 2020). In PNG, C580Y was also detected at low prevalence (2.3%) from samples collected in 2017, but the genetic background with mutations in genes associated with drug resistance including P. falciparum multidrug resistance 1 (pfmdr1), ferredoxin, autophagy-related protein 18 (atg 18) and purine nucleoside phosphorylase (pnp) suggests the potential emergence of multidrug-resistant parasites (Miotto et al., 2020). As discussed, fitness costs associated with the mutations in the parasite and high levels of immunity in the human population may have accounted for the low prevalence of the K13 mutations and persistently high ACT efficacy.
In the GMS where ART resistance first emerged, there are distinct patterns of the geographical distribution of the K13 mutations: C580Y is most prevalent in the eastern GMS (Cambodia, Laos, Vietnam, and eastern Thailand) with near fixation in some areas, whereas F446I is predominant in the western GMS (western Thailand, Yunnan province of China, and Myanmar) (Kobasa et al., 2018; Menard et al., 2016; Tun et al., 2015; Wang et al., 2015b; Ye et al., 2016). This divergence may result from different demographic histories, drug uses, genetic backgrounds, and vectors in these regions. ART resistance in Cambodia arose from multiple founder populations with mutations in ferredoxin, apicoplast ribosomal protein S10 (arps10), multidrug resistance protein 2 (mrp2), and P. falciparum chloroquine resistance transporter (pfcrt) as the genetic background (Miotto et al., 2013, 2015). Since mutant k13 alleles present a wide spectrum of growth disadvantages to the parasite, these background mutations may be compensatory. The degree of resistance conferred by a K13 mutation also appears to be contingent on the parasite's genetic background, determining its prevalence in the parasite populations (Siddiqui et al., 2020; Straimer et al., 2015). For instance, the C580Y mutation in different genetic backgrounds was found to be either less fit (Li et al., 2019; Nair et al., 2018; Tirrell et al., 2019) or fitness neutral (Siddiqui et al., 2020; Straimer et al., 2017) in in vitro competition studies. While the different prevalences of the K13 mutations are governed by their asexual proliferation rates in the human host, the ability of the mutant parasites to transmit to the mosquito vectors may also play a role. In clinical efficacy studies, the K13 mutant parasites with DPC had higher gametocytemias both before and after treatment, suggesting greater potential for transmission (Ashley et al., 2014). A subsequent in vitro study showed that clinical isolates with K13 mutations had a higher ability to initiate gametocytogenesis and infect mosquitoes under ART drug pressure (Witmer et al., 2020). Actually, exposure of trophozoites to low DHA concentrations promoted sexual conversion (Portugaliza et al., 2020), providing further support for the transmission of resistant parasites after ART treatment. Moreover, ART-resistant parasites can readily infect highly diverse Anopheles mosquitoes including the primary African vector An. gambiae, indicating the lack of a biological barrier for the spread of resistance in Africa (St Laurent et al., 2015). This is drastically different from atovaquone resistance conferred by mutations in cytochrome b, which cannot be transmitted through mosquitoes (Goodman et al., 2016).
3.4. Mechanisms of ART resistance: reduced drug activation and increased stress responses
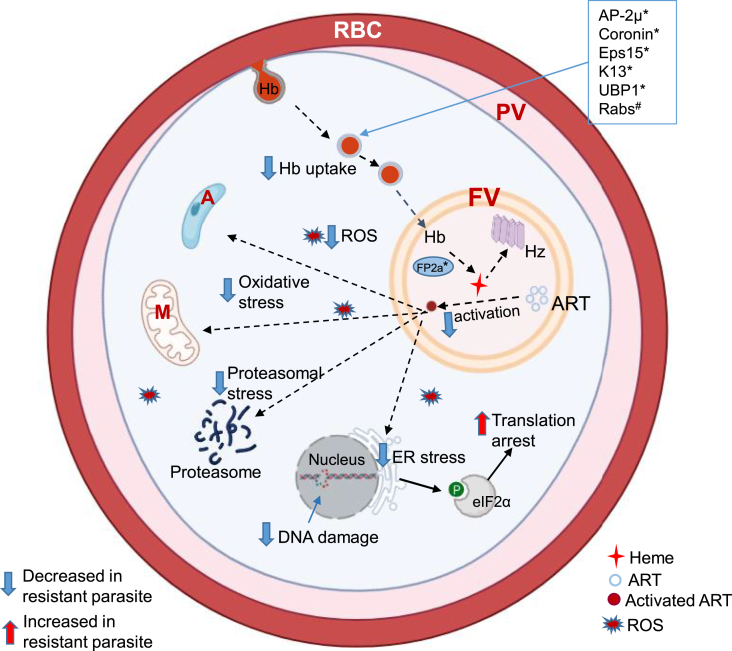
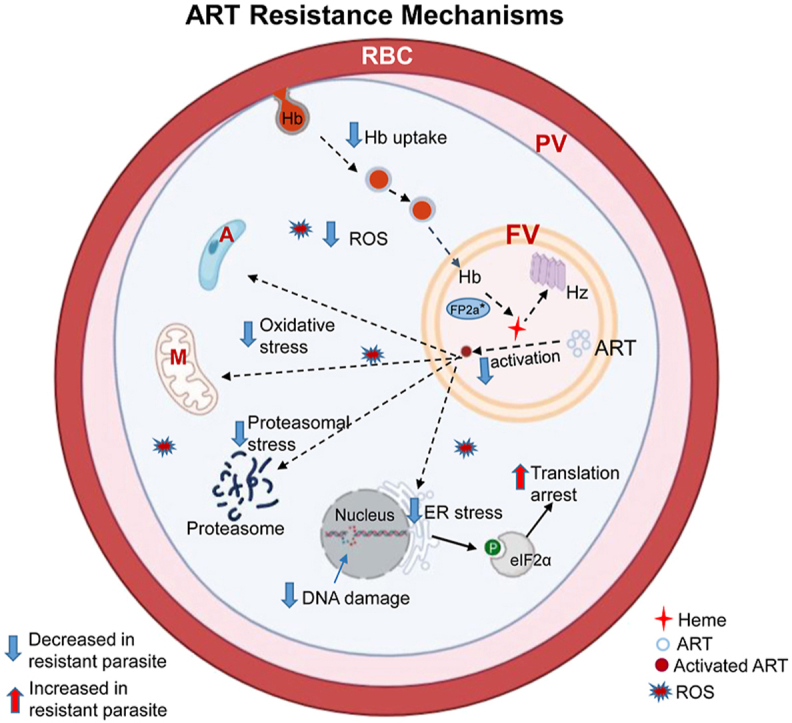
ART resistance is an active field of research, and here we present the progress on the mechanisms of ART resistance, trying to integrate both K13- and non-K13-mediated resistance into two pathways involving reduced ART activation (less production of the activator heme) and increased tolerance to oxidative stress (Fig. 2). Several excellent reviews provided more detailed accounts of the findings, mechanisms, and theories (Rosenthal and Ng, 2020; Sutherland et al., 2020; Tilley et al., 2016; Wicht et al., 2020; Xie et al., 2020).
Fig. 2.
ART resistance mechanisms. In malaria parasites, hemoglobin (Hb) is endocytosed from host cell via cytostomes and transported as Hb-containing vesicles to the food vacuole (FV). Several proteins (boxed) including AP-2μ (adaptor protein-2μ), Coronin, Eps15 (epidermal growth factor receptor substrate-15 homolog), K13, UBP1 (Ubiquitin-binding protein-1), and Rab GTPases (Rabs) may participate in the endocytosis process. The symbols * and # indicate protein mutations and post-translational modification (prenylation), respectively. Hb is digested inside the FV by multiple hemoglobinases, including Falcipain 2a (FP2a), to release heme. Heme is toxic to the parasite and is converted to the inactive crystals of hemozoin (Hz). Heme is also required for ART activation. Activated ART alkylates multiple cellular targets, leading to oxidative stress response and global translation arrest eukaryotic initiation factor 2α (eIF2α) phosphorylation. Activated ART also increase reactive oxygen species (ROS) production in the parasite cytoplasm and mitochondria and cause DNA damage. In the ART-resistant parasite, mutated forms of the proteins (marked with an asterisk *) are associated with lesser Hb uptake and digestion, resulting in less heme production, lower levels of ART activation, and lower cellular stress. The resistant parasite has increased stress responses. The dashed and solid arrows indicate down-regulated and up-regulated processes, respectively, in the ART-resistant parasite. Abbreviations: M, mitochondrion; A, apicoplast; RBC, red blood cell; PV, parasitophorous vacuole. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Partial loss of function of mutant K13. With a BTB dimerization domain and a six-blade propeller domain, the K13 protein has a structural resemblance to the human Kelch-like ECH-associated protein 1 (Keap 1) (Ariey et al., 2014), which functions to regulate the oxidative stress response pathway by binding and ubiquitylating the nuclear factor erythroid 2-related factor 2 (Nrf2) (Hayes and McMahon, 2006). Although Plasmodium does not have an Nrf2 homolog, K13 may act as a ubiquitin ligase adaptor to control the degradation of other proteins (Haldar et al., 2018; Xie et al., 2020). K13 is localized mainly to the endoplasmic reticulum (ER), cytostomes, intracellular vesicles (endocytic and secretory), and to a lesser extent, cytoplasm, FV, and apicoplast (Bhattacharjee et al., 2018; Birnbaum et al., 2017; Gnadig et al., 2020; Siddiqui et al., 2020; Yang et al., 2019), suggesting potential roles of K13 in multiple cellular processes. Interestingly, the K13 mutation was also localized in vesicular structures marked by phosphatidylinositol-3-phosphate (PI3P), which was greatly expanded in the C580Y mutant and is probably responsible for sustaining PfEMP1 export under ART treatment (Bhattacharjee et al., 2018). It is speculated that this would offer additional in vivo survival advantage to the ART-resistant parasites since the RBCs infected with the resistant parasites would better adhere to host receptors and evade splenic clearance under drug treatment. The convergent phenotype of the many K13 mutations suggests that K13-mediated ART resistance may result from a partial loss of function of the K13 protein (Xie et al., 2020). Indeed, K13 mutations do not affect its localization or interactions with other proteins but are associated with decreased K13 protein levels (Birnbaum et al., 2020; Gnadig et al., 2020; Siddiqui et al., 2017; Yang et al., 2019). K13 is essential for the asexual erythrocytic cycle but can tolerate more than 50% of reduced levels (Birnbaum et al., 2017). In vitro manipulation of the expression level and localization of K13 proteins result in similar, altered ART resistance regardless of whether the wild-type or mutant K13 is used (Birnbaum et al., 2020; Gnadig et al., 2020; Yang et al., 2019). However, a large-scale transcriptomic analysis of clinical parasites with ART resistance from the GMS did not find altered k13 expression at the transcript level (Mok et al., 2015), while a time-course analysis of wild-type k13 expression after AL treatment identified a negative correlation between k13 expression and PC1/2 (Silva et al., 2019). A comparative transcriptomic and proteomic analysis of isogenic parasites demonstrated that the impact of the K13 mutations on the K13 protein levels depended on the mutations and developmental stages, although they all showed elevated transcripts at the ring stage (Mok et al., 2021). Specifically, compared to the wild-type K13 protein level in the Cam3.II genetic background, the R539T-K13 protein levels were lower at both ring and trophozoite stages, whereas the C580Y–K13 level was reduced only at the trophozoite stage. It remains to be determined whether the reduced mutant K13 protein levels could be a result of reduced stability of the protein (Birnbaum et al., 2020; Xie et al., 2020), since molecular simulations identified some local structural destabilization in the propeller domain of the K13 C580Y and R539T mutations (Coppee et al., 2019).
K13 interactomes suggest the involvement of multiple cellular processes. To understand the functions of K13, several studies have tried to identify the K13 interactomes (Birnbaum et al., 2020; Gnadig et al., 2020; Siddiqui et al., 2020). Although a large number of proteins have been identified to be associated with K13, the data are only partially concordant, which may have resulted from the different tags (BioID-GFP/RFP or GFP) and purification methods used (immunoprecipitation with antibodies against K13/GFP or affinity purification by biotin-streptavidin) or the transient natures of the K13 interactions with other proteins. Birnbaum et al. using the BioID approach identified over 10 proteins close to K13 and named them as Kelch 13 interaction candidates (KIC1-KIC10), including the canonical endocytic factor Eps15 (epidermal growth factor receptor substrate 15), and a putative ubiquitin hydrolase – ubiquitin-binding protein −1 (UBP-1) (Birnbaum et al., 2020). This K13 compartment colocalizes with the adaptor protein complex 2 μ subunit (AP-2μ) and defines a new clathrin-independent endocytosis pathway in P. falciparum (Birnbaum et al., 2020; Henrici et al., 2020), although AP-2μ is not directly associated with the K13 complex. KIC10 may be the bridging subunit for the K13 and AP-2μ complexes since it was found in both complexes. Siddiqui et al. used GFP-Trap beads with the GFP-tagged K13 transgenic parasites and identified proteins involved in the unfolded protein response (UPR) pathway and components of the reactive oxidative stress complex of the parasite [e.g., BiP (binding immunoglobulin protein), a protein disulfide isomerase (ERp72), and the putative endoplasmin (GRP94)] (Siddiqui et al., 2020). Gnädig et al. used a set of anti-K13 monoclonal antibodies along with reporter tagging and identified significantly enriched Rab family GTPase proteins, as well as other proteins associated with the transport vesicles, chaperones, FV, and even mitochondria (Gnadig et al., 2020). These Rab proteins are known to regulate and associate with vesicles involved in secretion and endocytosis (Langsley et al., 2008). Interestingly, K13 was also co-purified with the PfEMP1 immunoproteome, which is enriched in proteins involved in proteostasis (e.g., proteins in the UPR pathway) (Bhattacharjee et al., 2018). While future studies are needed to refine the K13 interactomes, these data nonetheless point to the involvement of K13 in endocytosis, vesicular transport, and stress response.
Convergence of resistance mechanisms on the FV. As the major activator of ARTs, the main source of heme is released from the digestion of imported host hemoglobin in the FV. Alteration in cellular processes involving hemoglobin endocytosis and digestion, FV integrity, and heme detoxification all can lead to changes in ART activation. A peptidomics study revealed that the K13 mutants had lower levels of hemoglobin-derived peptides than the sensitive isolate at the trophozoite stage (Siddiqui et al., 2017), but a more recent study did not find such a difference at the ring stage (Mok et al., 2021). Several proteins that are associated with ART resistance including K13, AP-2μ, UBP-1, PfPI3K (phosphatidylinositol-3-kinase), Coronin, and Falcipain 2 may play roles in hemoglobin uptake and digestion (Sutherland et al., 2020; Xie et al., 2020). The newly identified K13 compartment is a pathway important for ART resistance, since reduced expression or mislocalization using the knock-sideways system of eight proteins associated with this compartment [UBP-1, KIC4, KIC5, KIC7, KIC9, MCA2 (metacaspase 2), AP-2μ and Eps15] rendered parasites resistant to ART, and this is most likely due to reduced endocytosis of hemoglobin (Birnbaum et al., 2020). Of these proteins associated with the K13 compartment, mutations in UBP-1 have been previously identified to be linked to selected ART resistance in the rodent malaria parasite Plasmodium chabaudi (Hunt et al., 2007). PfUBP-1 harbors multiple mutations in parasites from eastern and western Africa and was associated with in vitro resistance to DHA by GWAS, while the E1528D mutation upstream of the catalytic domain was associated with ACT treatment failure in Kenya (Adams et al., 2018; Borrmann et al., 2013; Henriques et al., 2014). Analysis of longitudinal samples from Thailand identified the R3138H mutation in PfUBP-1, showing a strong temporal change parallel to the prevalent K13 mutation C580Y (Cerqueira et al., 2017). The R3138H mutation was recently validated in vitro to confer ART resistance with an RSA value of ~1.8% (Birnbaum et al., 2020). The two PcUBP-1 mutations (orthologous to V3275F and V3306F in PfUBP-1) originally identified in P. chabaudi, when engineered in P. berghei, caused earlier recrudescence after artesunate treatment (Simwela et al., 2020), while gene manipulation confirmed the PfUBP-1 V3275F but not the V3306F mutation in conferring in vitro ART resistance in P. falciparum (Henrici et al., 2019).
Three-dimensional structured illumination microscopy showed that GFP-labeled K13 structures are located at or near the parasite's surface, taking a doughnut shape with the dimension of a cytostome (Yang et al., 2019). This K13 localization at the cytostome has also been confirmed by immunoelectron microscopy (Gnadig et al., 2020). K13 or the K13-Eps15 compartment resides in proximity to the AP-2μ-demarcated endocytic vesicles after pinching off from the cell membrane (Birnbaum et al., 2020; Henrici et al., 2020). The canonical AP-2 complex in conjunction with clathrin constitutes the major endocytosis pathway within the cell (Yap and Winckler, 2015), but the AP-2 complex in Plasmodium defines a new endocytic pathway that is independent of clathrin. A mutation corresponding to I562T in PfAP-2μ was identified in ART-resistant P. chabaudi lines (Henriques et al., 2013; Hunt et al., 2007). Though P. falciparum parasite populations harbor many mutations in PfAP-2μ; the I562T mutation was not identified, while the S160N mutation was associated with ACT treatment failure in Kenya (Adams et al., 2018; Henriques et al., 2014). When engineered into the 3D7 parasite background using CRISPR/cas9, the I592T, but not the S160N variant, of PfAP-2μ was found to contribute to in vitro ART resistance (Henrici et al., 2019). AP-2μ appears to be essential for forming endocytic vesicles (Henrici et al., 2020) and conditional mislocalization of AP-2μ led to ART resistance at the early ring stage (Birnbaum et al., 2020). It is noteworthy that in vitro selection for ART resistance identified a mutation in PfAP-2α, further emphasizing the role of this pathway in mediating ART resistance (Rocamora et al., 2018).
The study by Mbengue et al. established a link between the K13 mutation and PI3P, a hallmark of membranes of early endosomes (Mbengue et al., 2015). In P. falciparum, PI3P is catalyzed by the only one Vps34-type PfPI3K (Hassett et al., 2017). PI3P is present in the RBC cytoplasm-filled vesicles and the FV membrane (Jonscher et al., 2019; Tawk et al., 2010), and there is pharmacological evidence showing that PfPI3K inhibition led to the accumulation of hemoglobin in endocytic vesicles, preventing its transport and digestion in the FV (Vaid et al., 2010). K13 was located close to the FV (Gnadig et al., 2020) and PI3P-labeled vesicular structures (Bhattacharjee et al., 2018), implying a link of K13 mutations with PI3K in vesicle transport and ART resistance. It was found that K13 mutations (C580Y and R539T) were associated with a 1.5–2-fold increase in PfPI3K, resulting in augmented production of PI3P, which itself induced ART resistance (Mbengue et al., 2015). However, other studies have not found altered PfPI3K levels in wild-type or K13 mutant parasites (Siddiqui et al., 2017; Yang et al., 2019), necessitating further studies.
In addition to the K13 mutations, several other resistance-associated genes may be linked to the hemoglobin uptake pathway. Selection for in vitro ART resistance in P. falciparum of an African genetic background identified mutations in an actin-binding protein, Coronin. Coronin also has a propeller-like structure with seven blades and may be involved in modulating the transport of hemoglobin-containing vesicles (Olshina et al., 2015; Xie et al., 2020). Gene manipulation by using CRISPR/cas9 validated the R100K, E107V, and G50E mutations for ring-stage ART resistance (Demas et al., 2018). Further genetic analysis indicated that the contribution of the Coronin mutations to ART resistance is masked by the presence of K13 C580Y mutation, providing additional support for Coronin's action via the same pathway as K13 (Sharma et al., 2020). Although these mutations have not been reported in field isolates, 14 other mutations were identified in the exon-3 of Coronin in African isolates with no indication of ACT resistance (Velavan et al., 2019). Analysis of P. falciparum parasites from the GMS by GWAS revealed a positive correlation of the T38I mutation in ATG18 with elevated in vivo DPC rates (Miotto et al., 2015) and in vitro reduced susceptibility to DHA (Wang et al., 2016). Given its close association with the FV in a PI3P-dependent manner (Agrawal et al., 2020; Bansal et al., 2017), ATG18 might regulate FV biogenesis and hemoglobin digestion.
The involvement of a hemoglobinase complex that directs hemoglobin to hemozoin suggests that mutations in these genes may interfere with the hemoglobin digestion and heme detoxification process, leading to ART resistance. Indeed, deletion of the cysteine protease falcipain 2a in P. falciparum results in ART resistance in vitro (Klonis et al., 2011; Xie et al., 2016). Mutations in this gene have also been identified from in vitro ART selection (Ariey et al., 2014; Rocamora et al., 2018). Falcipain 2a harbors numerous mutations and they appear to be geographically divergent, suggesting different drug selection histories. Certain haplotypes of this gene in field isolates from the China-Myanmar border area are correlated with lower falcipain activity, less hemoglobin digestion, and higher in vitro RSA values (Siddiqui et al., 2018). The presence of the Falcipain 2b paralog possibly precludes it from being one of the primary mediators of ART resistance and prevents drastic effects of the Falcipain 2a mutations on ART sensitivity.
Multiple factors leading to reduced hemoglobin uptake, vesicular transport to the FV, and digestion are consistent with reduced hemoglobin digestion and heme release, which correlates with less ART activation (Wicht et al., 2020). Reduced hemoglobin digestion also means a reduced supply of amino acids during the ring stage, which may explain the extended ring stage with certain K13 mutants (Hott et al., 2015; Siddiqui et al., 2020) as well as reduced fitness (Mathieu et al., 2020; Nair et al., 2018; Straimer et al., 2017). Furthermore, amino acid starvation can also promote dormancy development (Babbitt et al., 2012), which altogether enhances the survival of ring-stage parasites. In this regard, a Cambodian K13 mutant parasite line Cam3.IR539T was found to exhibit a much higher recovery rate after amino acid starvation (McLean and Jacobs-Lorena, 2017).
Increased tolerance to oxidative stress. Activated ARTs alkylate a wide array of cellular targets, resulting in extensive cellular damage. Likewise, an enhanced stress response is another feature of ART-resistant parasites. Transcriptomic analysis of clinical ART-resistant parasite isolates revealed upregulation of genes involved in the UPR pathway including the ER-located reactive oxidative stress complex (e.g., cyclophilin 19B, BiP, ERp72, GRP94) and the cytoplasmic T-complex protein 1 ring complex (TRiC), allowing the resistant parasites to better tolerate the proteotoxic effects of ARTs (Mok et al., 2015). Proteomic analysis of laboratory engineered isogenic parasites further confirmed that K13 mutations are associated with upregulated chaperones, including the cyclophilin 19B protein (Mok et al., 2021). Increased expression of genes involved in adaptive responses against cellular damage has also been documented in in vitro selected ART-resistant parasites (Rocamora et al., 2018). ART exposure causes protein damage, leading to the buildup of unfolded and polyubiquitinated proteins, which activates the ER stress response with hallmarks of eIF2α phosphorylation and global translational arrest. Plasmodium eIF2α phosphorylation by the PK4 protein kinase acts as a stress sensor and leads the parasite into dormancy (Zhang et al., 2017). K13 mutations were found to have pre-existing elevated eIF2α phosphorylation in the early ring stage, which may correlate with an extended ring stage and increased entry into quiescence in resistant parasites (Zhang et al., 2017). In addition, another sign of global translation arrest is the reduced tRNA expression. K13 mutant parasites may also possess a higher ability to suppress tRNA expression after ART treatment as found during amino acid starvation, thus enhancing recovery from cell-cycle arrest (McLean and Jacobs-Lorena, 2017; Mok et al., 2021). K13 mutant parasites display lesser ubiquitination of proteins, which may prevent the overwhelming of the proteasome response pathway (Bridgford et al., 2018; Dogovski et al., 2015; Siddiqui et al., 2020). Interestingly, Yang et al. found no differences in the initial proteasomal response in the K13 mutant and WT parasites against ART-induced oxidative stress, but the mutant parasite regains protein turnover more rapidly (Yang et al., 2019). In P. falciparum, K13 protein was found to be associated or colocalized with the major ER chaperone BiP, further supporting a link between K13 and the ER proteostatic response pathways (Gnadig et al., 2020; Siddiqui et al., 2020). ART-resistant parasites can also mount enhanced antioxidant responses to deal with the oxidative stress generated from activated ART (Gopalakrishnan and Kumar, 2015; Kavishe et al., 2017; Krungkrai and Yuthavong, 1987). In vitro-selected DHA-resistant parasites from the Dd2 background can upregulate the expression of over 20 genes involved in antioxidant responses, resulting in elevated levels of cellular antioxidants (glutathione and thioredoxin) and intracellular glutathione S-transferase (GST) and superoxide dismutase activity (Cui et al., 2012). A membrane-bound GST, PfEXP1 (PF3D7_1121600) was also found upregulated in in vitro-selected ART-resistant parasites (Lisewski et al., 2014; Rocamora et al., 2018). The free radicals and ROS generated from activation of ART can damage parasite DNA (Gopalakrishnan and Kumar, 2015), and the mutations in genes associated with DNA synthesis, mismatch repair, and oxidative damage response in ART-resistant parasites in the GMS bestow these parasites a greater ability to deal with DNA damage caused by ART exposure (Miotto et al., 2013; Takala-Harrison et al., 2013; Xiong et al., 2020). A recent forward genetic, phenotypic screen for heat-shock response genes using the piggyback transposon system demonstrated that the malaria parasite uses a shared crisis-response mechanism (upregulated UPR and downregulated endocytosis and ubiquitination) to relieve the build-up of damaged proteins from different stresses such as febrile temperatures and drugs (Zhang et al., 2020).
Recent studies also shed light on the significance of mitochondrion and apicoplast in stress response and ART resistance (Mok et al., 2021; Zhang et al., 2020). Mitochondria are identified as one of the cellular targets of ARTs (Wang et al., 2010) and mitochondrial activity is critical for the survival and regrowth of dormant parasites after DHA exposure (Peatey et al., 2015). Mitochondrial proteins were found associated with the K13 protein, and ART treatment induced the association of K13 with mitochondria in K13 mutant parasites (Gnadig et al., 2020; Mok et al., 2021). Combined transcriptomics, proteomics and metabolomics data revealed association of K13 mutations with up-regulated pyruvate and glutamate-linked carbon metabolism and the mitochondrial electron transport chain, and down-regulated the tricarboxylic acid cycle, indicating the rewiring of the energy requirement and metabolic activity in the mutant parasites (Mok et al., 2021). The mitochondrial cytochrome bc1 inhibitor atovaquone can delay or stop the recovery of the dormant parasites and reverse the resistance phenotype associated with the K13 mutations (Mok et al., 2021; Peatey et al., 2015; Wicht et al., 2020). Of further relevance, in vitro selection for ART resistance in Toxoplasma gondii has shown pronounced amplification of mitochondrial genome in the resistant lines (Rosenberg et al., 2019). Recent screens for altered sensitivities to ART drugs in T. gondii also identified two mitochondrial proteins, a porphyrin transporter and a protease DegP2, which regulates heme levels and ART sensitivity (Harding et al., 2020; Rosenberg et al., 2019). Interestingly, deletion of the P. falciparum homolog PfDegP2 also led to ART resistance in both the ring and schizont stages with concomitant decreases in heme levels, further linking mitochondrial heme dynamics to ART susceptibility (Harding et al., 2020). These facts highlight the involvement of mitochondria in regulating heme levels and dealing with ART-caused oxidative stress response. Following the finding that the fatty acid synthesis pathway in the apicoplast remains active in DHA-induced dormant parasites (Chen et al., 2014), a recent genetic screen further implicated the apicoplast's critical involvement in heat-shock response and K13-mediated ART resistance (Zhang et al., 2020). Especially, the isoprenoid biosynthesis pathway of the apicoplast is needed for prenylation of a restricted set of proteins (<15) that are predominated by the Rab GTPases in the blood-stage parasite (Suazo et al., 2016). The lack of prenylation disrupts Rab 5's localization, uptake and trafficking of the RBC cytoplasm to the FV, and FV integrity (Howe et al., 2013; Kennedy et al., 2019). Thus, these studies have identified a mechanistic link between elevated isoprenoid biosynthesis and ART resistance, which involves the Rab proteins and their modification by prenylation, interactions with K13, and participation in hemoglobin endocytosis (Zhang et al., 2020).
Due to the complex nature of the ART action, the resistance mechanisms encompass multiple cellular pathways. Clinically ART-resistant parasites carrying the wild-type k13 allele indicate the involvement of additional players in ART resistance (Mukherjee et al., 2017; Sutherland et al., 2017), although they also seem to converge on pathways of reduced hemoglobin uptake/digestion and increased stress response. ART-resistant founder populations contain single nucleotide polymorphisms that are in linkage disequilibrium with the k13 mutations (Amaratunga et al., 2014; Miotto et al., 2015). These genetic loci may modify the resistance phenotype through epistatic interactions with K13, underlying the display of differential levels of ART resistance of the same K13 mutation in different genetic backgrounds (Straimer et al., 2015). In addition, genes involved in multiple pathways such as ATG18, Rad5, elongation factor G and geranylgeranyltransferase have been associated with increased ART resistance in field isolates (Wang et al., 2016). Functional studies will determine whether they enhance resistance or promote fitness (Rosenthal and Ng, 2020; Wicht et al., 2020). Different in vitro ART resistance models have identified overlapping and non-overlapping resistance mechanisms (Ariey et al., 2014; Chavchich et al., 2010; Cui et al., 2012; Rocamora et al., 2018), and their potential emergence in field parasite populations might be governed by the fitness costs associated with these genetic alterations in the respective genetic backgrounds (Tirrell et al., 2019). Although it is plausible that the complex mode of action of ART involving multiple cellular targets prevents the parasite from developing full-blown resistance, the ability to induce high-grade resistance in vitro warrants continued vigilance on resistance development (Sutherland et al., 2020).
4. Resistance to ACTs: the partner drugs
Monitoring and maintaining efficacies of the ACT partner drugs is equally important for the continued success of ACTs even if resistance to ART continues. With our limited antimalarial arsenal, drug recycling may be needed and this should be done based on good knowledge of resistance to the current and previous drugs. The most commonly used partner drugs include the aminoquinoline drugs (AQ, PPQ and PND), arylamino alcohols (LMF and MQ), and antifolates (SP). MQ and PPQ are the most commonly used partner drugs in Southeast Asia, while LMF and AQ are used abundantly in Africa. Resistance mechanisms to the partner drugs have been recently reviewed (Martin et al., 2018; Ross and Fidock, 2019; Wicht et al., 2020). Here we briefly summarize resistance to the partner drugs under the scenarios of resistance to ACTs focusing on PPQ, MQ, LMF, and AQ.
4.1. Piperaquine
DHA-PPQ has been deployed in Cambodia over a decade ago when AS-MQ failure rates reached high levels. This combination was also used for malaria treatment during pregnancy (Kakuru et al., 2016) and the mass drug administration (MDA) purpose in Asia and Africa (Eisele et al., 2016; Mwesigwa et al., 2019; Parker et al., 2019; von Seidlein et al., 2019). With the emergence of ART resistance, it took only a few years before clinical resistance to DHA-PPQ emerged in Cambodia and Vietnam (Amaratunga et al., 2016; Leang et al., 2015; Saunders et al., 2014; Spring et al., 2015; Thanh et al., 2017), and the situation rapidly deteriorated in the eastern GMS (van der Pluijm et al., 2019). Surveillance for resistance used both in vivo efficacy and in vitro drug assay results as phenotypes, but the in vitro/ex vivo assay for determining the PPQ IC50 values is sometimes problematic, since PPQ dose-response curves from the traditional IC50 assays may be bi-phasic, atypical of a sigmoid pattern (Bopp et al., 2018; Duru et al., 2015). An in vitro/ex vivo PPQ survival assay (PSA) has been designed to measure parasite survival rates after exposure of the ring-stage parasites to a pharmacologically relevant dose of PPQ (200 nM) for 48 h (Fig. 1) (Duru et al., 2015). A cut-off of ≥10% is used as the threshold to determine PPQ resistance (Duru et al., 2015; Witkowski et al., 2017). In addition, the bimodal PPQ dose-response curve of PPQ-resistant parasites from in vitro assays show a second peak at high drug concentrations (0.1–10 μM), and the area under the curve for the second peak was proposed to be an alternative readout for PPQ resistance (Fig. 1) (Bopp et al., 2018).
PPQ, like other 4-aminoquinolines, is known to inhibit both hemoglobin degradation and heme detoxification in the FV (Dhingra et al., 2017; Warhurst et al., 2007). Multiple prospective and retrospective studies monitoring DHA-PPQ efficacy made two important correlations. PPQ resistance always arose in a pre-existing ART resistance background (with k13 mutations), and resulted in increased susceptibility toward MQ as indicated by the absence of pfmdr1 amplification (Amaratunga et al., 2016; Chaorattanakawee et al., 2015; Duru et al., 2015). Since pfmdr1 amplification is associated with a fitness cost (Preechapornkul et al., 2009), pfmdr1 deamplification could be either due to DHA-PPQ selection for single copy pfmdr1 or decreased use of MQ (Amato et al., 2017; Imwong et al., 2017; Parobek et al., 2017; Witkowski et al., 2017). Two independent GWAS using P. falciparum isolates from Cambodia following DHA-PPQ treatment revealed a positive correlation between PPQ resistance and amplification of two aspartic protease genes, plasmepsin 2 and 3 (Amato et al., 2017; Witkowski et al., 2017). Genetic manipulation showed that disruption of plasmepsin 2 and 3 specifically sensitized the parasites to PPQ (Mukherjee et al., 2018). However, overexpression of these two genes in the 3D7 strain did not change the parasites' susceptibility to PPQ, CQ, or artesunate (Loesbanluechai et al., 2019), suggesting that further validation of plasmepsin 2 and 3 amplification in PPQ resistance is needed.
The action of PPQ in the FV also led to focused studies on the two FV membrane resident transporters PfMDR1 and PfCRT. In vitro-selected parasites for PPQ resistance contained a new PfCRT mutation C101F (Dhingra et al., 2017; Eastman et al., 2011). A study involving French Guiana field isolates found the C350R mutation in PfCRT associated with higher PPQ IC50 values (Pelleau et al., 2015). In the same year, three novel PfCRT mutations, H97Y, M343L, or G353V, were identified in recrudescent parasites after DHA-PPQ treatment, which were correlated with high PSA values (Duru et al., 2015). Moreover, the PfCRT F145I mutation was also associated with DHA-PPQ treatment failure and decreased ex vivo PPQ sensitivity by GWAS (Agrawal et al., 2017). Many of these PfCRT mutations have been genetically validated as markers of PPQ resistance. Importantly, the introduction of some of these mutations F145I, M343L, and G353V, significantly reduced PPQ susceptibility independent of plasmepsin 2 or 3 amplification (Dhingra et al., 2017; Pelleau et al., 2015; Ross et al., 2018). Thus, these genomic amplifications might help establish a favorable genetic background on which pfcrt mutations arise or provide a low-grade PPQ resistance baseline (Ross and Fidock, 2019; Silva et al., 2020). Drug pressure and the associated fitness costs would determine the emergence and spread of these PfCRT haplotypes in combination with plasmepsin amplification. Indeed, longitudinal surveillance of parasites from Cambodia detected increased prevalence of the new PfCRT mutations after the implementation of DHA-PPQ with >98% of parasites harboring these mutations by 2017 (Shrestha et al., 2021). It is noteworthy that several different PfCRT mutations (A144Y, Q271E, and R371I) have evolved without prior K13 mutations in southern China, where PPQ monotherapy has been used extensively (Yang et al., 2007), while their roles in mediating PPQ resistance await further studies. Of note, a longitudinal follow-up of parasites at the China-Myanmar border did not find clear evidence of PPQ resistance, but the temporal fluctuation of PPQ susceptibility suggests a scenario drastically different from that in the adjacent eastern GMS (Si et al., 2021). Structural insights into PfCRT have helped elucidate its role in antimalarial drug resistance. The 7G8 isoform of PfCRT with F145I and C350R mutations can bind as well as transport PPQ out of the food vacuole in membrane potential- and pH-dependent manners resulting in PPQ resistance, whereas the wild-type protein at these two sites is only able to actively efflux CQ but not PPQ, leading to CQ resistance (Kim et al., 2019). Therefore, the selective binding of different PfCRT isoforms to expel CQ or PPQ helps regulate resistance to these drugs and causes collateral drug sensitivity (Kim et al., 2019; Pelleau et al., 2015; Ross et al., 2018).
4.2. Amodiaquine
AS-AQ is the second most widely used ACT in Africa for treating uncomplicated falciparum malaria and is also used for seasonal malaria chemoprevention (Coldiron et al., 2017). Similar to CQ, mutations in PfCRT are the primary determinant of AQ resistance, while mutations in PfMDR1 play supplemental roles (Wicht et al., 2020). A PfCRT haplotype of SVMNT is specifically associated with AQ resistance (Beshir et al., 2010; Sa et al., 2009). The PfCRT structure may also explain the different sensitivities of the parasites to CQ and AQ (Kim et al., 2019). Like CQ, AS-AQ also selects for the mutant PfMDR1 86Y, which correlates with AQ resistance (Nsobya et al., 2007).
4.3. Mefloquine and lumefantrine
MQ has been used extensively in Thailand, initially in combination with SP, followed by its monotherapy, which was later replaced with AS-MQ (Wongsrichanalai et al., 2004). This combination was used in the eastern GMS around 1990 to address the resistance associated with MQ monotherapy (Looareesuwan et al., 1992a). AL is the most popular ACT used in Africa, which still has no concrete evidence of clinical resistance in the field, although indications of lower efficacy were reported in some African countries (Hamed and Kuhen, 2015; Mumtaz et al., 2020; Plucinski et al., 2015, Plucinski et al., 2017). A recent report of low efficacy for AL in Angola has been debated due to the lack of convincing evidence (Dimbu et al., 2021; Rasmussen and Ringwald, 2021). The mode of action for these arylamino alcohols has not been completely delineated, but they are predicted to interfere with the detoxification of heme, albeit their effect is less pronounced than that of CQ (Combrinck et al., 2013). These drugs may also hinder the normal function of PfMDR1 (Cowman et al., 1994; Rubio and Cowman, 1996). A recent study has identified the binding of MQ to the GTPase-associated center of the large ribosomal subunit (Wong et al., 2017). Using a cellular thermal shift assay, Dzikan et al. identified PNP as a common binding target for MQ and quinine (Dziekan et al., 2019), suggesting that the major MQ target site(s) may be located in the cytoplasm.
Copy number variations or mutations in pfmdr1 are known to regulate susceptibility to several antimalarial drugs including 4-aminoquinolines and arylamino alcohols (Martin et al., 2018; Ross and Fidock, 2019). Increased copy number of pfmdr1 is linked to clinical resistance to MQ in the GMS (Pickard et al., 2003; Price et al., 2004). If the ribosomes are the principal target of MQ, it is plausible that amplified pfmdr1 would pump out more MQ from the minor site (FV) to the major site (cytoplasm) of action. Interestingly, the N86Y mutation commonly found in Africa induces resistance to CQ and AQ while restoring susceptibility to MQ, LMF, and DHA (Okell et al., 2018; Veiga et al., 2016; Venkatesan et al., 2014; Yeka et al., 2016). As expected, clinical studies have documented that AL selects for wild-type PfMDR1 at N86, while DHA-PPQ selects for the mutant allele (Taylor et al., 2017). Similarly, parasite carrying multiple copies of pfmdr1 encoding N86 and 184F are prevalent in the GMS, probably as the consequence of selection by AS-MQ (Calcada et al., 2020), whereas the deployment of DHA-PPQ selects for single-copy pfmdr1 (Amato et al., 2017; Imwong et al., 2017; Parobek et al., 2017; Witkowski et al., 2017). This selection for different forms of pfmdr1 could be due to the inhibition of drug transport between the FV and the cytoplasm, where the major sites of action for the different families of drugs are differentially located. The principle by which the selection for resistance to one drug causes hypersensitivity to another drug is currently being employed in the design of triple ACTs.
5. Conclusions and future outlook
ART resistance in P. falciparum has “struck” the GMS again, the hotspot of multidrug resistance (Dondorp et al., 2010), accelerating the resistance development to the partner drugs and leading to rapidly declining efficacies of two major ACTs in this region, AS-MQ and DHA-PPQ (Hassett and Roepe, 2019). The failure to contain ART resistance in western Cambodia motivated the GMS nations to aim for the elimination of P. falciparum by 2025 (WHO, 2016). The trans-national spread of ART-resistant parasites observed within the GMS suggests that trans-continental spread is an imminent threat (Hamilton et al., 2019; Imwong et al., 2020). Though clinical ART resistance has not been observed elsewhere, the emergence of the K13 C580Y and R561H mutations in Oceania, Africa, and South America deserves heightened vigilance to preserve the effectiveness of ACTs (Chenet et al., 2016; Mathieu et al., 2020; Miotto et al., 2020; Uwimana et al., 2020). To inform drug policies and sustain the effectiveness of ACTs, surveillance for antimalarial drug resistance should combine in vivo efficacy studies, in vitro assays, and molecular surveillance of resistance markers. Especially, with the advancement in genotyping and whole-genome sequencing technologies, molecular surveillance can be upscaled to process large field samples (Menard et al., 2016) and track the origins of resistant parasites (Hamilton et al., 2019; Miotto et al., 2020). The establishment of the worldwide antimalarial resistance network (WWARN) offers a collaborative platform for global efforts to map, track, and fight against antimalarial drug resistance (www.wwarn.org).
Elimination of the falciparum malaria from the epicenter of ART resistance is challenging. The deployment of an effective ACT to manage clinical cases may be difficult given the limited number of ACTs available. Regardless of ART resistance, the selection of the right partner drug is critical, given the relatively short window of action of ART drugs. In Cambodia, resistance to DHA-PPQ led to the recycling of a previous ACT, AS-MQ. Alternatively, re-optimizing the course of therapy for a longer duration, e.g., from 3 days to 7 days, might also help safeguard the efficacy of the current drugs (Wang et al., 2019). Triple ACTs with the inclusion of a second partner drug with different action mechanisms or opposing selection on the same targets to the currently used ACTs such as DHA-PPQ plus MQ and AM-LMF plus AQ may be another option to achieve the needed treatment effectiveness. The recent randomized clinical trials of triple ACTs have demonstrated excellent efficacy in areas with ACT resistance (van der Pluijm et al., 2020). Yet, the emergence of triple mutant parasites that contain pfmdr1 and plasmepsin 2 amplification, and the K13 C580Y mutation in Cambodia deserves close attention to resistance evolution in the GMS (Rossi et al., 2017). Moreover, in the elimination phase, it is critical to remove the infective, often asymptomatic, reservoirs, which may require an MDA approach. The recent trial of targeted MDA with three monthly rounds of DHA-PPQ showed that MDA might be a useful tool to accelerate malaria elimination in low-endemicity settings (von Seidlein et al., 2019). Further, with the infectivity of ART-resistant parasites to different mosquito species, the use of single low-dose primaquine as the gametocytocidal drug is necessary to interrupt transmission of the resistant parasites.
Drug resistance is a continuing challenge to control and eradicate malaria worldwide, and research on this topic remains a high priority. Intensified studies on ART resistance in the malaria parasite have contributed to a better understanding of the molecular mechanisms of resistance, which are linked primarily to the process of ART activation and cellular stress response. To date, we have gained a considerable understanding of the drug targets and resistance mechanisms for most of the commonly used antimalarials and those that are still in the drug development pipeline (Ross and Fidock, 2019). The identification of drug targets and resistance markers offers convenient molecular surveillance tools to detect the emergence and forestall the spread of drug resistance (Prosser et al., 2018). With the arms race between drug implementation and resistance development, there is also a pressing need to use our knowledge about drug resistance to discover antimalarials with distinct modes of action and activity against both sexual and asexual stages of the parasite. Combined efforts in resistance detection, mechanism elucidation, and strengthened surveillance will guide us to apply effective antimalarial drug policies, putting us ahead of the curve in the fight against drug resistance.
Acknowledgments
We want to acknowledge funding (U19 AI089672 and R01AI128940) from the National Institute for Allergy and Infectious Diseases.
References
- Abu Bakar N., Klonis N., Hanssen E., Chan C., Tilley L. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J. Cell Sci. 2010;123:441–450. doi: 10.1242/jcs.061499. [DOI] [PubMed] [Google Scholar]
- Adams T., Ennuson N.A.A., Quashie N.B., Futagbi G., Matrevi S., Hagan O.C.K., Abuaku B., Koram K.A., Duah N.O. Prevalence of Plasmodium falciparum delayed clearance associated polymorphisms in adaptor protein complex 2 mu subunit (pfap2mu) and ubiquitin specific protease 1 (pfubp1) genes in Ghanaian isolates. Parasites Vectors. 2018;11:175. doi: 10.1186/s13071-018-2762-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjalley S.H., Johnston G.L., Li T., Eastman R.T., Ekland E.H., Eappen A.G., Richman A., Sim B.K., Lee M.C., Hoffman S.L., Fidock D.A. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc. Natl. Acad. Sci. U.S.A. 2011;108:E1214–E1223. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal P., Manjithaya R., Surolia N. Autophagy-related protein PfATG18 participates in food vacuole dynamics and autophagy-like pathway in Plasmodium falciparum. Mol. Microbiol. 2020;113:766–782. doi: 10.1111/mmi.14441. [DOI] [PubMed] [Google Scholar]
- Agrawal S., Moser K.A., Morton L., Cummings M.P., Parihar A., Dwivedi A., Shetty A.C., Drabek E.F., Jacob C.G., Henrich P.P., Parobek C.M., Jongsakul K., Huy R., Spring M.D., Lanteri C.A., Chaorattanakawee S., Lon C., Fukuda M.M., Saunders D.L., Fidock D.A., Lin J.T., Juliano J.J., Plowe C.V., Silva J.C., Takala-Harrison S. Association of a novel mutation in the Plasmodium falciparum chloroquine resistance transporter with decreased piperaquine sensitivity. J. Infect. Dis. 2017;216:468–476. doi: 10.1093/infdis/jix334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaratunga C., Lim P., Suon S., Sreng S., Mao S., Sopha C., Sam B., Dek D., Try V., Amato R., Blessborn D., Song L., Tullo G.S., Fay M.P., Anderson J.M., Tarning J., Fairhurst R.M. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect. Dis. 2016;16:357–365. doi: 10.1016/S1473-3099(15)00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaratunga C., Sreng S., Suon S., Phelps E.S., Stepniewska K., Lim P., Zhou C., Mao S., Anderson J.M., Lindegardh N., Jiang H., Song J., Su X.Z., White N.J., Dondorp A.M., Anderson T.J., Fay M.P., Mu J., Duong S., Fairhurst R.M. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect. Dis. 2012;12:851–858. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaratunga C., Witkowski B., Dek D., Try V., Khim N., Miotto O., Menard D., Fairhurst R.M. Plasmodium falciparum founder populations in western Cambodia have reduced artemisinin sensitivity in vitro. Antimicrob. Agents Chemother. 2014;58:4935–4937. doi: 10.1128/AAC.03055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato R., Lim P., Miotto O., Amaratunga C., Dek D., Pearson R.D., Almagro-Garcia J., Neal A.T., Sreng S., Suon S., Drury E., Jyothi D., Stalker J., Kwiatkowski D.P., Fairhurst R.M. Genetic markers associated with dihydroartemisinin-piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect. Dis. 2017;17:164–173. doi: 10.1016/S1473-3099(16)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T.J., Nair S., Nkhoma S., Williams J.T., Imwong M., Yi P., Socheat D., Das D., Chotivanich K., Day N.P., White N.J., Dondorp A.M. High heritability of malaria parasite clearance rate indicates a genetic basis for artemisinin resistance in western Cambodia. J. Infect. Dis. 2010;201:1326–1330. doi: 10.1086/651562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariey F., Witkowski B., Amaratunga C., Beghain J., Langlois A.C., Khim N., Kim S., Duru V., Bouchier C., Ma L., Lim P., Leang R., Duong S., Sreng S., Suon S., Chuor C.M., Bout D.M., Menard S., Rogers W.O., Genton B., Fandeur T., Miotto O., Ringwald P., Le Bras J., Berry A., Barale J.C., Fairhurst R.M., Benoit-Vical F., Mercereau-Puijalon O., Menard D. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asawamahasakda W., Ittarat I., Pu Y.M., Ziffer H., Meshnick S.R. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob. Agents Chemother. 1994;38:1854–1858. doi: 10.1128/aac.38.8.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley E.A., Dhorda M., Fairhurst R.M., Amaratunga C., Lim P., Suon S., Sreng S., Anderson J.M., Mao S., Sam B., Sopha C., Chuor C.M., Nguon C., Sovannaroth S., Pukrittayakamee S., Jittamala P., Chotivanich K., Chutasmit K., Suchatsoonthorn C., Runcharoen R., Hien T.T., Thuy-Nhien N.T., Thanh N.V., Phu N.H., Htut Y., Han K.T., Aye K.H., Mokuolu O.A., Olaosebikan R.R., Folaranmi O.O., Mayxay M., Khanthavong M., Hongvanthong B., Newton P.N., Onyamboko M.A., Fanello C.I., Tshefu A.K., Mishra N., Valecha N., Phyo A.P., Nosten F., Yi P., Tripura R., Borrmann S., Bashraheil M., Peshu J., Faiz M.A., Ghose A., Hossain M.A., Samad R., Rahman M.R., Hasan M.M., Islam A., Miotto O., Amato R., MacInnis B., Stalker J., Kwiatkowski D.P., Bozdech Z., Jeeyapant A., Cheah P.Y., Sakulthaew T., Chalk J., Intharabut B., Silamut K., Lee S.J., Vihokhern B., Kunasol C., Imwong M., Tarning J., Taylor W.J., Yeung S., Woodrow C.J., Flegg J.A., Das D., Smith J., Venkatesan M., Plowe C.V., Stepniewska K., Guerin P.J., Dondorp A.M., Day N.P., White N.J. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbitt S.E., Altenhofen L., Cobbold S.A., Istvan E.S., Fennell C., Doerig C., Llinas M., Goldberg D.E. Plasmodium falciparum responds to amino acid starvation by entering into a hibernatory state. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E3278–E3287. doi: 10.1073/pnas.1209823109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal P., Tripathi A., Thakur V., Mohmmed A., Sharma P. Autophagy-related protein ATG18 regulates apicoplast biogenesis in apicomplexan parasites. mBio. 2017;8 doi: 10.1128/mBio.01468-17. e01468-01417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayih A.G., Getnet G., Alemu A., Getie S., Mohon A.N., Pillai D.R. A unique Plasmodium falciparum K13 gene mutation in northwest Ethiopia. Am. J. Trop. Med. Hyg. 2016;94:132–135. doi: 10.4269/ajtmh.15-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beez D., Sanchez C.P., Stein W.D., Lanzer M. Genetic predisposition favors the acquisition of stable artemisinin resistance in malaria parasites. Antimicrob. Agents Chemother. 2011;55:50–55. doi: 10.1128/AAC.00916-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beshir K., Sutherland C.J., Merinopoulos I., Durrani N., Leslie T., Rowland M., Hallett R.L. Amodiaquine resistance in Plasmodium falciparum malaria in Afghanistan is associated with the pfcrt SVMNT allele at codons 72 to 76. Antimicrob. Agents Chemother. 2010;54:3714–3716. doi: 10.1128/AAC.00358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethell D., Se Y., Lon C., Tyner S., Saunders D., Sriwichai S., Darapiseth S., Teja-Isavadharm P., Khemawoot P., Schaecher K., Ruttvisutinunt W., Lin J., Kuntawungin W., Gosi P., Timmermans A., Smith B., Socheat D., Fukuda M.M. Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial. PloS One. 2011;6 doi: 10.1371/journal.pone.0019283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., Weiss D.J., Cameron E., Bisanzio D., Mappin B., Dalrymple U., Battle K.E., Moyes C.L., Henry A., Eckhoff P.A., Wenger E.A., Briet O., Penny M.A., Smith T.A., Bennett A., Yukich J., Eisele T.P., Griffin J.T., Fergus C.A., Lynch M., Lindgren F., Cohen J.M., Murray C.L., Smith D.L., Hay S.I., Cibulskis R.E., Gething P.W. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S., Coppens I., Mbengue A., Suresh N., Ghorbal M., Slouka Z., Safeukui I., Tang H.Y., Speicher D.W., Stahelin R.V., Mohandas N., Haldar K. Remodeling of the malaria parasite and host human red cell by vesicle amplification that induces artemisinin resistance. Blood. 2018;131:1234–1247. doi: 10.1182/blood-2017-11-814665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhisutthibhan J., Meshnick S.R. Immunoprecipitation of [(3)H]dihydroartemisinin translationally controlled tumor protein (TCTP) adducts from Plasmodium falciparum-infected erythrocytes by using anti-TCTP antibodies. Antimicrob. Agents Chemother. 2001;45:2397–2399. doi: 10.1128/AAC.45.8.2397-2399.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum J., Flemming S., Reichard N., Soares A.B., Mesen-Ramirez P., Jonscher E., Bergmann B., Spielmann T. A genetic system to study Plasmodium falciparum protein function. Nat. Methods. 2017;14:450–456. doi: 10.1038/nmeth.4223. [DOI] [PubMed] [Google Scholar]
- Birnbaum J., Scharf S., Schmidt S., Jonscher E., Hoeijmakers W.A.M., Flemming S., Toenhake C.G., Schmitt M., Sabitzki R., Bergmann B., Frohlke U., Mesen-Ramirez P., Blancke Soares A., Herrmann H., Bartfai R., Spielmann T. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science. 2020;367:51–59. doi: 10.1126/science.aax4735. [DOI] [PubMed] [Google Scholar]
- Bopp S., Magistrado P., Wong W., Schaffner S.F., Mukherjee A., Lim P., Dhorda M., Amaratunga C., Woodrow C.J., Ashley E.A., White N.J., Dondorp A.M., Fairhurst R.M., Ariey F., Menard D., Wirth D.F., Volkman S.K. Plasmepsin II-III copy number accounts for bimodal piperaquine resistance among Cambodian Plasmodium falciparum. Nat. Commun. 2018;9:1769. doi: 10.1038/s41467-018-04104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrmann S., Straimer J., Mwai L., Abdi A., Rippert A., Okombo J., Muriithi S., Sasi P., Kortok M.M., Lowe B., Campino S., Assefa S., Auburn S., Manske M., Maslen G., Peshu N., Kwiatkowski D.P., Marsh K., Nzila A., Clark T.G. Genome-wide screen identifies new candidate genes associated with artemisinin susceptibility in Plasmodium falciparum in Kenya. Sci. Rep. 2013;3:3318. doi: 10.1038/srep03318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau E.F., Webster H.K., Pavanand K., Thosingha L. Type II mefloquine resistance in Thailand. Lancet. 1982;2:1335. doi: 10.1016/s0140-6736(82)91532-x. [DOI] [PubMed] [Google Scholar]
- Bridgford J.L., Xie S.C., Cobbold S.A., Pasaje C.F.A., Herrmann S., Yang T., Gillett D.L., Dick L.R., Ralph S.A., Dogovski C., Spillman N.J., Tilley L. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat. Commun. 2018;9:3801. doi: 10.1038/s41467-018-06221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos M.D., Wongsrichanalai C., Delacollette C., Burkholder B. Monitoring antimalarial drug efficacy in the Greater Mekong Subregion: an overview of in vivo results from 2008 to 2010. Southeast Asian J. Trop. Med. Publ. Health. 2013;44(Suppl. 1):201–230. discussion 306-207. [PubMed] [Google Scholar]
- Calcada C., Silva M., Baptista V., Thathy V., Silva-Pedrosa R., Granja D., Ferreira P.E., Gil J.P., Fidock D.A., Veiga M.I. Expansion of a specific Plasmodium falciparum PfMDR1 haplotype in Southeast Asia with increased substrate transport. mBio. 2020;11 doi: 10.1128/mBio.02093-20. e02093-02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara V.I., Lwin K.M., Phyo A.P., Ashley E., Wiladphaingern J., Sriprawat K., Rijken M., Boel M., McGready R., Proux S., Chu C., Singhasivanon P., White N., Nosten F. Malaria burden and artemisinin resistance in the mobile and migrant population on the Thai-Myanmar border, 1999-2011: an observational study. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellini M.A., Buguliskis J.S., Casta L.J., Butz C.E., Clark A.B., Kunkel T.A., Taraschi T.F. Malaria drug resistance is associated with defective DNA mismatch repair. Mol. Biochem. Parasitol. 2011;177:143–147. doi: 10.1016/j.molbiopara.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira G.C., Cheeseman I.H., Schaffner S.F., Nair S., McDew-White M., Phyo A.P., Ashley E.A., Melnikov A., Rogov P., Birren B.W., Nosten F., Anderson T.J.C., Neafsey D.E. Longitudinal genomic surveillance of Plasmodium falciparum malaria parasites reveals complex genomic architecture of emerging artemisinin resistance. Genome Biol. 2017;18:78. doi: 10.1186/s13059-017-1204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaorattanakawee S., Saunders D.L., Sea D., Chanarat N., Yingyuen K., Sundrakes S., Saingam P., Buathong N., Sriwichai S., Chann S., Se Y., Yom Y., Heng T.K., Kong N., Kuntawunginn W., Tangthongchaiwiriya K., Jacob C., Takala-Harrison S., Plowe C., Lin J.T., Chuor C.M., Prom S., Tyner S.D., Gosi P., Teja-Isavadharm P., Lon C., Lanteri C.A. Ex vivo drug susceptibility testing and molecular profiling of clinical Plasmodium falciparum isolates from Cambodia from 2008 to 2013 suggest emerging piperaquine resistance. Antimicrob. Agents Chemother. 2015;59:4631–4643. doi: 10.1128/AAC.00366-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavchich M., Gerena L., Peters J., Chen N., Cheng Q., Kyle D.E. Role of pfmdr1 amplification and expression in induction of resistance to artemisinin derivatives in Plasmodium falciparum. Antimicrob. Agents Chemother. 2010;54:2455–2464. doi: 10.1128/AAC.00947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I.H., Miller B.A., Nair S., Nkhoma S., Tan A., Tan J.C., Al Saai S., Phyo A.P., Moo C.L., Lwin K.M., McGready R., Ashley E., Imwong M., Stepniewska K., Yi P., Dondorp A.M., Mayxay M., Newton P.N., White N.J., Nosten F., Ferdig M.T., Anderson T.J. A major genome region underlying artemisinin resistance in malaria. Science. 2012;336:79–82. doi: 10.1126/science.1215966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Chavchich M., Peters J.M., Kyle D.E., Gatton M.L., Cheng Q. Deamplification of pfmdr1-containing amplicon on chromosome 5 in Plasmodium falciparum is associated with reduced resistance to artelinic acid in vitro. Antimicrob. Agents Chemother. 2010;54:3395–3401. doi: 10.1128/AAC.01421-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., LaCrue A.N., Teuscher F., Waters N.C., Gatton M.L., Kyle D.E., Cheng Q. Fatty acid synthesis and pyruvate metabolism pathways remain active in dihydroartemisinin-induced dormant ring stages of Plasmodium falciparum. Antimicrob. Agents Chemother. 2014;58:4773–4781. doi: 10.1128/AAC.02647-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenet S.M., Akinyi Okoth S., Huber C.S., Chandrabose J., Lucchi N.W., Talundzic E., Krishnalall K., Ceron N., Musset L., Macedo de Oliveira A., Venkatesan M., Rahman R., Barnwell J.W., Udhayakumar V. Independent emergence of the Plasmodium falciparum Kelch propeller domain mutant allele C580Y in Guyana. J. Infect. Dis. 2016;213:1472–1475. doi: 10.1093/infdis/jiv752. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Kyle D.E., Gatton M.L. Artemisinin resistance in Plasmodium falciparum: a process linked to dormancy? Int. J. Parasitol. Drugs Drug. Resist. 2012;2:249–255. doi: 10.1016/j.ijpddr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhibber-Goel J., Sharma A. Profiles of Kelch mutations in Plasmodium falciparum across South Asia and their implications for tracking drug resistance. Int. J. Parasitol. Drugs Drug Resist. 2019;11:49–58. doi: 10.1016/j.ijpddr.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M., Fisher N.C., Kasthuri R., Cerami Hand C. Parasite maturation and host serum iron influence the labile iron pool of erythrocyte stage Plasmodium falciparum. Br. J. Haematol. 2013;161:262–269. doi: 10.1111/bjh.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldiron M.E., Von Seidlein L., Grais R.F. Seasonal malaria chemoprevention: successes and missed opportunities. Malar. J. 2017;16:481. doi: 10.1186/s12936-017-2132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combrinck J.M., Mabotha T.E., Ncokazi K.K., Ambele M.A., Taylor D., Smith P.J., Hoppe H.C., Egan T.J. Insights into the role of heme in the mechanism of action of antimalarials. ACS Chem. Biol. 2013;8:133–137. doi: 10.1021/cb300454t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M.D., Bigira V., Kapisi J., Muhindo M., Kamya M.R., Havlir D.V., Dorsey G., Rosenthal P.J. Polymorphisms in K13 and falcipain-2 associated with artemisinin resistance are not prevalent in Plasmodium falciparum isolated from Ugandan children. PloS One. 2014;9 doi: 10.1371/journal.pone.0105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppee R., Jeffares D.C., Miteva M.A., Sabbagh A., Clain J. Comparative structural and evolutionary analyses predict functional sites in the artemisinin resistance malaria protein K13. Sci. Rep. 2019;9:10675. doi: 10.1038/s41598-019-47034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A.F., Galatis D., Thompson J.K. Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1143–1147. doi: 10.1073/pnas.91.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Wang Z., Miao J., Miao M., Chandra R., Jiang H., Su X.Z., Cui L. Mechanisms of in vitro resistance to dihydroartemisinin in Plasmodium falciparum. Mol. Microbiol. 2012;86:111–128. doi: 10.1111/j.1365-2958.2012.08180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D., Tripura R., Phyo A.P., Lwin K.M., Tarning J., Lee S.J., Hanpithakpong W., Stepniewska K., Menard D., Ringwald P., Silamut K., Imwong M., Chotivanich K., Yi P., Day N.P., Lindegardh N., Socheat D., Nguon C., White N.J., Nosten F., Dondorp A.M. Effect of high-dose or split-dose artesunate on parasite clearance in artemisinin-resistant falciparum malaria. Clin. Infect. Dis. 2013;56:e48–58. doi: 10.1093/cid/cis958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas A.R., Sharma A.I., Wong W., Early A.M., Redmond S., Bopp S., Neafsey D.E., Volkman S.K., Hartl D.L., Wirth D.F. Mutations in Plasmodium falciparum actin-binding protein coronin confer reduced artemisinin susceptibility. Proc. Natl. Acad. Sci. U.S.A. 2018;115:12799–12804. doi: 10.1073/pnas.1812317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra S.K., Redhi D., Combrinck J.M., Yeo T., Okombo J., Henrich P.P., Cowell A.N., Gupta P., Stegman M.L., Hoke J.M., Cooper R.A., Winzeler E., Mok S., Egan T.J., Fidock D.A. A variant PfCRT isoform can contribute to Plasmodium falciparum resistance to the first-line partner drug piperaquine. mBio. 2017;8 doi: 10.1128/mBio.00303-17. e00303-00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimbu P.R., Horth R., Candido A.L.M., Ferreira C.M., Caquece F., Garcia L.E.A., Andre K., Pembele G., Jandondo D., Bondo B.J., Nieto Andrade B., Labuda S., Ponce de Leon G., Kelley J., Patel D., Svigel S.S., Talundzic E., Lucchi N., Morais J.F.M., Fortes F., Martins J.F., Plucinski M.M. Continued low efficacy of artemether-lumefantrine in Angola in 2019. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.01949-20. e01949-01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogovski C., Xie S.C., Burgio G., Bridgford J., Mok S., McCaw J.M., Chotivanich K., Kenny S., Gnadig N., Straimer J., Bozdech Z., Fidock D.A., Simpson J.A., Dondorp A.M., Foote S., Klonis N., Tilley L. Targeting the cell stress response of Plasmodium falciparum to overcome artemisinin resistance. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp A.M., Nosten F., Yi P., Das D., Phyo A.P., Tarning J., Lwin K.M., Ariey F., Hanpithakpong W., Lee S.J., Ringwald P., Silamut K., Imwong M., Chotivanich K., Lim P., Herdman T., An S.S., Yeung S., Singhasivanon P., Day N.P., Lindegardh N., Socheat D., White N.J. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp A.M., Ringwald P. Artemisinin resistance is a clear and present danger. Trends Parasitol. 2013;29:359–360. doi: 10.1016/j.pt.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Dondorp A.M., Yeung S., White L., Nguon C., Day N.P., Socheat D., von Seidlein L. Artemisinin resistance: current status and scenarios for containment. Nat. Rev. Microbiol. 2010;8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- Duru V., Khim N., Leang R., Kim S., Domergue A., Kloeung N., Ke S., Chy S., Eam R., Khean C., Loch K., Ken M., Lek D., Beghain J., Ariey F., Guerin P.J., Huy R., Mercereau-Puijalon O., Witkowski B., Menard D. Plasmodium falciparum dihydroartemisinin-piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: retrospective and prospective investigations. BMC Med. 2015;13:305. doi: 10.1186/s12916-015-0539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziekan J.M., Yu H., Chen D., Dai L., Wirjanata G., Larsson A., Prabhu N., Sobota R.M., Bozdech Z., Nordlund P. Identifying purine nucleoside phosphorylase as the target of quinine using cellular thermal shift assay. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aau3174. [DOI] [PubMed] [Google Scholar]
- Eastman R.T., Dharia N.V., Winzeler E.A., Fidock D.A. Piperaquine resistance is associated with a copy number variation on chromosome 5 in drug-pressured Plasmodium falciparum parasites. Antimicrob. Agents Chemother. 2011;55:3908–3916. doi: 10.1128/AAC.01793-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele T.P., Bennett A., Silumbe K., Finn T.P., Chalwe V., Kamuliwo M., Hamainza B., Moonga H., Kooma E., Chizema Kawesha E., Yukich J., Keating J., Porter T., Conner R.O., Earle D., Steketee R.W., Miller J.M. Short-term impact of mass drug administration with dihydroartemisinin plus piperaquine on malaria in Southern Province Zambia: a cluster-randomized controlled trial. J. Infect. Dis. 2016;214:1831–1839. doi: 10.1093/infdis/jiw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles D.E., Hoo C.C., Warren M., Sandosham A.A. Plasmodium falciparum resistant to chloroquine in Cambodia. Am. J. Trop. Med. Hyg. 1963;12:840–843. doi: 10.4269/ajtmh.1963.12.840. [DOI] [PubMed] [Google Scholar]
- Fernando D., Weerasekera C.J., Gunasekera W., Hapuarachchi H.C., Koo C., Munas M., Ranaweera P. Case report: the first case of genotypically confirmed K13 propeller mutation in Sri Lanka and its implications on the elimination status of malaria. Am. J. Trop. Med. Hyg. 2020;104:964–967. doi: 10.4269/ajtmh.20-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira P.E., Culleton R., Gil J.P., Meshnick S.R. Artemisinin resistance in Plasmodium falciparum: what is it really? Trends Parasitol. 2013;29:318–320. doi: 10.1016/j.pt.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Ghorbal M., Gorman M., Macpherson C.R., Martins R.M., Scherf A., Lopez-Rubio J.J. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat. Biotechnol. 2014;32:819–821. doi: 10.1038/nbt.2925. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Famin O., Zhang J., Krugliak M. Inhibition of glutathione-dependent degradation of heme by chloroquine and amodiaquine as a possible basis for their antimalarial mode of action. Biochem. Pharmacol. 1998;56:1305–1313. doi: 10.1016/s0006-2952(98)00184-1. [DOI] [PubMed] [Google Scholar]
- Gnadig N.F., Stokes B.H., Edwards R.L., Kalantarov G.F., Heimsch K.C., Kuderjavy M., Crane A., Lee M.C.S., Straimer J., Becker K., Trakht I.N., Odom John A.R., Mok S., Fidock D.A. Insights into the intracellular localization, protein associations and artemisinin resistance properties of Plasmodium falciparum K13. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C.D., Siregar J.E., Mollard V., Vega-Rodriguez J., Syafruddin D., Matsuoka H., Matsuzaki M., Toyama T., Sturm A., Cozijnsen A., Jacobs-Lorena M., Kita K., Marzuki S., McFadden G.I. Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes. Science. 2016;352:349–353. doi: 10.1126/science.aad9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan A.M., Kumar N. Antimalarial action of artesunate involves DNA damage mediated by reactive oxygen species. Antimicrob. Agents Chemother. 2015;59:317–325. doi: 10.1128/AAC.03663-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Kyaw M.P., He L., Min M., Ning X., Zhang W., Wang B., Cui L. Quality testing of artemisinin-based antimalarial drugs in Myanmar. Am. J. Trop. Med. Hyg. 2017;97:1198–1203. doi: 10.4269/ajtmh.17-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D.K., Patra A.T., Zhu L., Gupta A.P., Bozdech Z. DNA damage regulation and its role in drug-related phenotypes in the malaria parasites. Sci. Rep. 2016;6:23603. doi: 10.1038/srep23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar K., Bhattacharjee S., Safeukui I. Drug resistance in plasmodium. Nat. Rev. Microbiol. 2018;16:156–170. doi: 10.1038/nrmicro.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed K., Kuhen K. No robust evidence of lumefantrine resistance. Antimicrob. Agents Chemother. 2015;59:5865–5866. doi: 10.1128/AAC.00329-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W.L., Amato R., van der Pluijm R.W., Jacob C.G., Quang H.H., Thuy-Nhien N.T., Hien T.T., Hongvanthong B., Chindavongsa K., Mayxay M., Huy R., Leang R., Huch C., Dysoley L., Amaratunga C., Suon S., Fairhurst R.M., Tripura R., Peto T.J., Sovann Y., Jittamala P., Hanboonkunupakarn B., Pukrittayakamee S., Chau N.H., Imwong M., Dhorda M., Vongpromek R., Chan X.H.S., Maude R.J., Pearson R.D., Nguyen T., Rockett K., Drury E., Goncalves S., White N.J., Day N.P., Kwiatkowski D.P., Dondorp A.M., Miotto O. Evolution and expansion of multidrug-resistant malaria in southeast Asia: a genomic epidemiology study. Lancet Infect. Dis. 2019;19:943–951. doi: 10.1016/S1473-3099(19)30392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C.R., Sidik S.M., Petrova B., Gnadig N.F., Okombo J., Herneisen A.L., Ward K.E., Markus B.M., Boydston E.A., Fidock D.A., Lourido S. Genetic screens reveal a central role for heme metabolism in artemisinin susceptibility. Nat. Commun. 2020;11:4813. doi: 10.1038/s41467-020-18624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett M.R., Roepe P.D. Origin and spread of evolving artemisinin-resistant Plasmodium falciparum malarial parasites in Southeast Asia. Am. J. Trop. Med. Hyg. 2019;101:1204–1211. doi: 10.4269/ajtmh.19-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett M.R., Sternberg A.R., Riegel B.E., Thomas C.J., Roepe P.D. Heterologous expression, purification, and functional analysis of Plasmodium falciparum phosphatidylinositol 3'-kinase. Biochemistry. 2017;56:4335–4345. doi: 10.1021/acs.biochem.7b00416. [DOI] [PubMed] [Google Scholar]
- Hayes J.D., McMahon M. The double-edged sword of Nrf2: subversion of redox homeostasis during the evolution of cancer. Mol. Cell. 2006;21:732–734. doi: 10.1016/j.molcel.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Haynes R.K., Cheu K.W., N'Da D., Coghi P., Monti D. Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses. Infect. Disord. - Drug Targets. 2013;13:217–277. doi: 10.2174/1871526513666131129155708. [DOI] [PubMed] [Google Scholar]
- Heller L.E., Goggins E., Roepe P.D. Dihydroartemisinin-ferriprotoporphyrin IX adduct abundance in Plasmodium falciparum malarial parasites and the relationship to emerging artemisinin resistance. Biochemistry. 2018;57:6935–6945. doi: 10.1021/acs.biochem.8b00960. [DOI] [PubMed] [Google Scholar]
- Heller L.E., Roepe P.D. Quantification of free ferriprotoporphyrin IX heme and hemozoin for artemisinin sensitive versus delayed clearance phenotype Plasmodium falciparum malarial parasites. Biochemistry. 2018;57:6927–6934. doi: 10.1021/acs.biochem.8b00959. [DOI] [PubMed] [Google Scholar]
- Heller L.E., Roepe P.D. Artemisinin-based antimalarial drug therapy: molecular pharmacology and evolving resistance. Trav. Med. Infect. Dis. 2019;4:89. doi: 10.3390/tropicalmed4020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrici R.C., Edwards R.L., Zoltner M., van Schalkwyk D.A., Hart M.N., Mohring F., Moon R.W., Nofal S.D., Patel A., Flueck C., Baker D.A., Odom John A.R., Field M.C., Sutherland C.J. The Plasmodium falciparum artemisinin susceptibility-associated AP-2 adaptin mu subunit is clathrin independent and essential for schizont maturation. mBio. 2020;11 doi: 10.1128/mBio.02918-19. e02918-02919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrici R.C., van Schalkwyk D.A., Sutherland C.J. Modification of pfap2mu and pfubp1 markedly reduces ring-stage susceptibility of Plasmodium falciparum to artemisinin in vitro. Antimicrob. Agents Chemother. 2019;64 doi: 10.1128/AAC.01542-19. e01542-01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques G., Hallett R.L., Beshir K.B., Gadalla N.B., Johnson R.E., Burrow R., van Schalkwyk D.A., Sawa P., Omar S.A., Clark T.G., Bousema T., Sutherland C.J. Directional selection at the pfmdr1, pfcrt, pfubp1, and pfap2mu loci of Plasmodium falciparum in Kenyan children treated with ACT. J. Infect. Dis. 2014;210:2001–2008. doi: 10.1093/infdis/jiu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques G., Martinelli A., Rodrigues L., Modrzynska K., Fawcett R., Houston D.R., Borges S.T., d'Alessandro U., Tinto H., Karema C., Hunt P., Cravo P. Artemisinin resistance in rodent malaria--mutation in the AP2 adaptor mu-chain suggests involvement of endocytosis and membrane protein trafficking. Malar. J. 2013;12:118. doi: 10.1186/1475-2875-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien T.T., Thuy-Nhien N.T., Phu N.H., Boni M.F., Thanh N.V., Nha-Ca N.T., Thai le H., Thai C.Q., Toi P.V., Thuan P.D., Long le T., Dong le T., Merson L., Dolecek C., Stepniewska K., Ringwald P., White N.J., Farrar J., Wolbers M. In vivo susceptibility of Plasmodium falciparum to artesunate in binh phuoc province, Vietnam. Malar. J. 2012;11:355. doi: 10.1186/1475-2875-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y.L., Yang Y.Z., Meshnick S.R. The interaction of artemisinin with malarial hemozoin. Mol. Biochem. Parasitol. 1994;63:121–128. doi: 10.1016/0166-6851(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Hott A., Casandra D., Sparks K.N., Morton L.C., Castanares G.G., Rutter A., Kyle D.E. Artemisinin-resistant Plasmodium falciparum parasites exhibit altered patterns of development in infected erythrocytes. Antimicrob. Agents Chemother. 2015;59:3156–3167. doi: 10.1128/AAC.00197-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe R., Kelly M., Jimah J., Hodge D., Odom A.R. Isoprenoid biosynthesis inhibition disrupts Rab 5 localization and food vacuolar integrity in Plasmodium falciparum. Eukaryot. Cell. 2013;12:215–223. doi: 10.1128/EC.00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Takala-Harrison S., Jacob C.G., Liu H., Sun X., Yang H., Nyunt M.M., Adams M., Zhou S., Xia Z., Ringwald P., Bustos M.D., Tang L., Plowe C.V. A single mutation in K13 predominates in southern China and is associated with delayed clearance of Plasmodium falciparum following artemisinin treatment. J. Infect. Dis. 2015;212:1629–1635. doi: 10.1093/infdis/jiv249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P., Afonso A., Creasey A., Culleton R., Sidhu A.B., Logan J., Valderramos S.G., McNae I., Cheesman S., do Rosario V., Carter R., Fidock D.A., Cravo P. Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Mol. Microbiol. 2007;65:27–40. doi: 10.1111/j.1365-2958.2007.05753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz E.S., Johnson D., Campbell C.C. Resistance of Plasmodium falciparum malaria to sulfadoxine-pyrimethamine ('Fansidar') in a refugee camp in Thailand. Lancet. 1981;1:1068–1070. doi: 10.1016/s0140-6736(81)92239-x. [DOI] [PubMed] [Google Scholar]
- Imwong M., Dhorda M., Myo Tun K., Thu A.M., Phyo A.P., Proux S., Suwannasin K., Kunasol C., Srisutham S., Duanguppama J., Vongpromek R., Promnarate C., Saejeng A., Khantikul N., Sugaram R., Thanapongpichat S., Sawangjaroen N., Sutawong K., Han K.T., Htut Y., Linn K., Win A.A., Hlaing T.M., van der Pluijm R.W., Mayxay M., Pongvongsa T., Phommasone K., Tripura R., Peto T.J., von Seidlein L., Nguon C., Lek D., Chan X.H.S., Rekol H., Leang R., Huch C., Kwiatkowski D.P., Miotto O., Ashley E.A., Kyaw M.P., Pukrittayakamee S., Day N.P.J., Dondorp A.M., Smithuis F.M., Nosten F.H., White N.J. Molecular epidemiology of resistance to antimalarial drugs in the Greater Mekong subregion: an observational study. Lancet Infect. Dis. 2020;20:1470–1480. doi: 10.1016/S1473-3099(20)30228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwong M., Suwannasin K., Kunasol C., Sutawong K., Mayxay M., Rekol H., Smithuis F.M., Hlaing T.M., Tun K.M., van der Pluijm R.W., Tripura R., Miotto O., Menard D., Dhorda M., Day N.P.J., White N.J., Dondorp A.M. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect. Dis. 2017;17:491–497. doi: 10.1016/S1473-3099(17)30048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail H.M., Barton V., Phanchana M., Charoensutthivarakul S., Wong M.H., Hemingway J., Biagini G.A., O'Neill P.M., Ward S.A. Artemisinin activity-based probes identify multiple molecular targets within the asexual stage of the malaria parasites Plasmodium falciparum 3D7. Proc. Natl. Acad. Sci. U. S. A. 2016;113:2080–2085. doi: 10.1073/pnas.1600459113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail H.M., Barton V.E., Panchana M., Charoensutthivarakul S., Biagini G.A., Ward S.A., O'Neill P.M. A click chemistry-based proteomic approach reveals that 1,2,4-trioxolane and artemisinin antimalarials share a common protein alkylation profile. Angew. Chem. 2016;128:6511–6515. doi: 10.1002/ange.201512062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonscher E., Flemming S., Schmitt M., Sabitzki R., Reichard N., Birnbaum J., Bergmann B., Hohn K., Spielmann T. PfVPS45 is required for host cell cytosol uptake by malaria blood stage parasites. Cell Host Microbe. 2019;25:166–173. doi: 10.1016/j.chom.2018.11.010. e165. [DOI] [PubMed] [Google Scholar]
- Jourdan J., Walz A., Matile H., Schmidt A., Wu J., Wang X., Dong Y., Vennerstrom J.L., Schmidt R.S., Wittlin S., Maser P. Stochastic protein alkylation by antimalarial peroxides. ACS Infect. Dis. 2019;5:2067–2075. doi: 10.1021/acsinfecdis.9b00264. [DOI] [PubMed] [Google Scholar]
- Kakuru A., Jagannathan P., Muhindo M.K., Natureeba P., Awori P., Nakalembe M., Opira B., Olwoch P., Ategeka J., Nayebare P., Clark T.D., Feeney M.E., Charlebois E.D., Rizzuto G., Muehlenbachs A., Havlir D.V., Kamya M.R., Dorsey G. Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N. Engl. J. Med. 2016;374:928–939. doi: 10.1056/NEJMoa1509150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamchonwongpaisan S., Chandra-ngam G., Avery M.A., Yuthavong Y. Resistance to artemisinin of malaria parasites (Plasmodium falciparum) infecting alpha-thalassemic erythrocytes in vitro. Competition in drug accumulation with uninfected erythrocytes. J. Clin. Invest. 1994;93:467–473. doi: 10.1172/JCI116994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki S.N., Williams T.N. Human genetics and malaria resistance. Hum. Genet. 2020;139:801–811. doi: 10.1007/s00439-020-02142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavishe R.A., Koenderink J.B., Alifrangis M. Oxidative stress in malaria and artemisinin combination therapy: pros and Cons. FEBS J. 2017;284:2579–2591. doi: 10.1111/febs.14097. [DOI] [PubMed] [Google Scholar]
- Kennedy K., Cobbold S.A., Hanssen E., Birnbaum J., Spillman N.J., McHugh E., Brown H., Tilley L., Spielmann T., McConville M.J., Ralph S.A. Delayed death in the malaria parasite Plasmodium falciparum is caused by disruption of prenylation-dependent intracellular trafficking. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury D.S., Cao P., Zaloumis S.G., Davenport M.P., Interdisciplinary Approaches to Malaria, C Artemisinin resistance and the unique selection pressure of a short-acting antimalarial. Trends Parasitol. 2020;36:884–887. doi: 10.1016/j.pt.2020.07.004. [DOI] [PubMed] [Google Scholar]
- Kim J., Tan Y.Z., Wicht K.J., Erramilli S.K., Dhingra S.K., Okombo J., Vendome J., Hagenah L.M., Giacometti S.I., Warren A.L., Nosol K., Roepe P.D., Potter C.S., Carragher B., Kossiakoff A.A., Quick M., Fidock D.A., Mancia F. Structure and drug resistance of the Plasmodium falciparum transporter PfCRT. Nature. 2019;576:315–320. doi: 10.1038/s41586-019-1795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonis N., Creek D.J., Tilley L. Iron and heme metabolism in Plasmodium falciparum and the mechanism of action of artemisinins. Curr. Opin. Microbiol. 2013;16:722–727. doi: 10.1016/j.mib.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Klonis N., Crespo-Ortiz M.P., Bottova I., Abu-Bakar N., Kenny S., Rosenthal P.J., Tilley L. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc. Natl. Acad. Sci. U.S.A. 2011;108:11405–11410. doi: 10.1073/pnas.1104063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonis N., Xie S.C., McCaw J.M., Crespo-Ortiz M.P., Zaloumis S.G., Simpson J.A., Tilley L. Altered temporal response of malaria parasites determines differential sensitivity to artemisinin. Proc. Natl. Acad. Sci. U.S.A. 2013;110:5157–5162. doi: 10.1073/pnas.1217452110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa T., Talundzic E., Sug-Aram R., Boondat P., Goldman I.F., Lucchi N.W., Dharmarak P., Sintasath D., Fukuda M., Whistler T., MacArthur J., Udhayakumar V., Prempree P., Chinanonwait N. Emergence and spread of kelch13 mutations associated with artemisinin resistance in Plasmodium falciparum parasites in 12 Thai provinces from 2007 to 2016. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.02141-17. e02141-02117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S., Kremsner P.G. Antidogmatic approaches to artemisinin resistance: reappraisal as treatment failure with artemisinin combination therapy. Trends Parasitol. 2013;29:313–317. doi: 10.1016/j.pt.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Krungkrai S.R., Yuthavong Y. The antimalarial action on Plasmodium falciparum of qinghaosu and artesunate in combination with agents which modulate oxidant stress. Trans. R. Soc. Trop. Med. Hyg. 1987;81:710–714. doi: 10.1016/0035-9203(87)90003-4. [DOI] [PubMed] [Google Scholar]
- Kyaw M.P., Nyunt M.H., Chit K., Aye M.M., Aye K.H., Lindegardh N., Tarning J., Imwong M., Jacob C.G., Rasmussen C., Perin J., Ringwald P., Nyunt M.M. Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PloS One. 2013;8 doi: 10.1371/journal.pone.0057689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Episcopia M., Kelley J., Patel D., Schmedes S., Ravishankar S., Menegon M., Perrotti E., Nurahmed A.M., Talha A.A., Nour B.Y., Lucchi N., Severini C., Talundzic E. Targeted deep amplicon sequencing of kelch 13 and cytochrome b in Plasmodium falciparum isolates from an endemic African country using the Malaria Resistance Surveillance (MaRS) protocol. Parasites Vectors. 2020;13:137. doi: 10.1186/s13071-020-4005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsley G., van Noort V., Carret C., Meissner M., de Villiers E.P., Bishop R., Pain A. Comparative genomics of the Rab protein family in Apicomplexan parasites. Microb. Infect. 2008;10:462–470. doi: 10.1016/j.micinf.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leang R., Taylor W.R., Bouth D.M., Song L., Tarning J., Char M.C., Kim S., Witkowski B., Duru V., Domergue A., Khim N., Ringwald P., Menard D. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in western Cambodia: dihydroartemisinin-piperaquine open-label multicenter. Clinical Assessment. Antimicrob. Agents Chemother. 2015;59:4719–4726. doi: 10.1128/AAC.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.H., Fidock D.A. Evidence of a mild mutator phenotype in Cambodian Plasmodium falciparum malaria parasites. PloS One. 2016;11 doi: 10.1371/journal.pone.0154166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.Q., Guo X.B., Fu L.C., Jian H.X., Wang X.H. Clinical trials of artemisinin and its derivatives in the treatment of malaria in China. Trans. R. Soc. Trop. Med. Hyg. 1994;88(Suppl. 1):S5–S6. doi: 10.1016/0035-9203(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Li W., Zhou Y., Tang G., Xiao Y. Characterization of the artemisinin binding site for translationally controlled tumor protein (TCTP) by bioorthogonal click chemistry. Bioconjugate Chem. 2016;27:2828–2833. doi: 10.1021/acs.bioconjchem.6b00556. [DOI] [PubMed] [Google Scholar]
- Li X., Kumar S., McDew-White M., Haile M., Cheeseman I.H., Emrich S., Button-Simons K., Nosten F., Kappe S.H.I., Ferdig M.T., Anderson T.J.C., Vaughan A.M. Genetic mapping of fitness determinants across the malaria parasite Plasmodium falciparum life cycle. PLoS Genet. 2019;15 doi: 10.1371/journal.pgen.1008453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisewski A.M., Quiros J.P., Ng C.L., Adikesavan A.K., Miura K., Putluri N., Eastman R.T., Scanfeld D., Regenbogen S.J., Altenhofen L., Llinas M., Sreekumar A., Long C., Fidock D.A., Lichtarge O. Supergenomic network compression and the discovery of EXP1 as a glutathione transferase inhibited by artesunate. Cell. 2014;158:916–928. doi: 10.1016/j.cell.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesbanluechai D., Kotanan N., de Cozar C., Kochakarn T., Ansbro M.R., Chotivanich K., White N.J., Wilairat P., Lee M.C.S., Gamo F.J., Sanz L.M., Chookajorn T., Kumpornsin K. Overexpression of plasmepsin II and plasmepsin III does not directly cause reduction in Plasmodium falciparum sensitivity to artesunate, chloroquine and piperaquine. Int. J. Parasitol. Drugs Drug Resist. 2019;9:16–22. doi: 10.1016/j.ijpddr.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looareesuwan S., Kyle D.E., Viravan C., Vanijanonta S., Wilairatana P., Charoenlarp P., Canfield C.J., Webster H.K. Treatment of patients with recrudescent falciparum malaria with a sequential combination of artesunate and mefloquine. Am. J. Trop. Med. Hyg. 1992;47:794–799. doi: 10.4269/ajtmh.1992.47.794. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S., Viravan C., Vanijanonta S., Wilairatana P., Suntharasamai P., Charoenlarp P., Arnold K., Kyle D., Canfield C., Webster K. Randomised trial of artesunate and mefloquine alone and in sequence for acute uncomplicated falciparum malaria. Lancet. 1992;339:821–824. doi: 10.1016/0140-6736(92)90276-9. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S., Viravan C., Webster H.K., Kyle D.E., Hutchinson D.B., Canfield C.J. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am. J. Trop. Med. Hyg. 1996;54:62–66. doi: 10.4269/ajtmh.1996.54.62. [DOI] [PubMed] [Google Scholar]
- Loria P., Miller S., Foley M., Tilley L. Inhibition of the peroxidative degradation of haem as the basis of action of chloroquine and other quinoline antimalarials. Biochem. J. 1999;339:363–370. [PMC free article] [PubMed] [Google Scholar]
- MalariaGEN Plasmodium falciparum Community Project Genomic epidemiology of artemisinin resistant malaria. Elife. 2016;5 doi: 10.7554/eLife.08714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.E., Shafik S.H., Richards S.N. Mechanisms of resistance to the partner drugs of artemisinin in the malaria parasite. Curr. Opin. Pharmacol. 2018;42:71–80. doi: 10.1016/j.coph.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Mathieu L.C., Cox H., Early A.M., Mok S., Lazrek Y., Paquet J.C., Ade M.P., Lucchi N.W., Grant Q., Udhayakumar V., Alexandre J.S., Demar M., Ringwald P., Neafsey D.E., Fidock D.A., Musset L. Local emergence in Amazonia of Plasmodium falciparum k13 C580Y mutants associated with in vitro artemisinin resistance. Elife. 2020;9 doi: 10.7554/eLife.51015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude R.J., Pontavornpinyo W., Saralamba S., Aguas R., Yeung S., Dondorp A.M., Day N.P., White N.J., White L.J. The last man standing is the most resistant: eliminating artemisinin-resistant malaria in Cambodia. Malar. J. 2009;8:31. doi: 10.1186/1475-2875-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbengue A., Bhattacharjee S., Pandharkar T., Liu H., Estiu G., Stahelin R.V., Rizk S.S., Njimoh D.L., Ryan Y., Chotivanich K., Nguon C., Ghorbal M., Lopez-Rubio J.J., Pfrender M., Emrich S., Mohandas N., Dondorp A.M., Wiest O., Haldar K. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature. 2015;520:683–687. doi: 10.1038/nature14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean K.J., Jacobs-Lorena M. Plasmodium falciparum Maf 1 confers survival upon amino acid starvation. mBio. 2017;8 doi: 10.1128/mBio.02317-16. e02317-02316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister S., Plouffe D.M., Kuhen K.L., Bonamy G.M., Wu T., Barnes S.W., Bopp S.E., Borboa R., Bright A.T., Che J., Cohen S., Dharia N.V., Gagaring K., Gettayacamin M., Gordon P., Groessl T., Kato N., Lee M.C., McNamara C.W., Fidock D.A., Nagle A., Nam T.G., Richmond W., Roland J., Rottmann M., Zhou B., Froissard P., Glynne R.J., Mazier D., Sattabongkot J., Schultz P.G., Tuntland T., Walker J.R., Zhou Y., Chatterjee A., Diagana T.T., Winzeler E.A. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science. 2011;334:1372–1377. doi: 10.1126/science.1211936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard D., Khim N., Beghain J., Adegnika A.A., Shafiul-Alam M., Amodu O., Rahim-Awab G., Barnadas C., Berry A., Boum Y., Bustos M.D., Cao J., Chen J.H., Collet L., Cui L., Thakur G.D., Dieye A., Djalle D., Dorkenoo M.A., Eboumbou-Moukoko C.E., Espino F.E., Fandeur T., Ferreira-da-Cruz M.F., Fola A.A., Fuehrer H.P., Hassan A.M., Herrera S., Hongvanthong B., Houze S., Ibrahim M.L., Jahirul-Karim M., Jiang L., Kano S., Ali-Khan W., Khanthavong M., Kremsner P.G., Lacerda M., Leang R., Leelawong M., Li M., Lin K., Mazarati J.B., Menard S., Morlais I., Muhindo-Mavoko H., Musset L., Na-Bangchang K., Nambozi M., Niare K., Noedl H., Ouedraogo J.B., Pillai D.R., Pradines B., Quang-Phuc B., Ramharter M., Randrianarivelojosia M., Sattabongkot J., Sheikh-Omar A., Silue K.D., Sirima S.B., Sutherland C., Syafruddin D., Tahar R., Tang L.H., Toure O.A., Tshibangu-wa-Tshibangu P., Vigan-Womas I., Warsame M., Wini L., Zakeri S., Kim S., Eam R., Berne L., Khean C., Chy S., Ken M., Loch K., Canier L., Duru V., Legrand E., Barale J.C., Stokes B., Straimer J., Witkowski B., Fidock D.A., Rogier C., Ringwald P., Ariey F., Mercereau-Puijalon O., Consortium K. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N. Engl. J. Med. 2016;374:2453–2464. doi: 10.1056/NEJMoa1513137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshnick S.R. Artemisinin: mechanisms of action, resistance and toxicity. Int. J. Parasitol. 2002;32:1655–1660. doi: 10.1016/s0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- Meunier B., Robert A. Heme as trigger and target for trioxane-containing antimalarial drugs. Acc. Chem. Res. 2010;43:1444–1451. doi: 10.1021/ar100070k. [DOI] [PubMed] [Google Scholar]
- Miotto O., Almagro-Garcia J., Manske M., Macinnis B., Campino S., Rockett K.A., Amaratunga C., Lim P., Suon S., Sreng S., Anderson J.M., Duong S., Nguon C., Chuor C.M., Saunders D., Se Y., Lon C., Fukuda M.M., Amenga-Etego L., Hodgson A.V., Asoala V., Imwong M., Takala-Harrison S., Nosten F., Su X.Z., Ringwald P., Ariey F., Dolecek C., Hien T.T., Boni M.F., Thai C.Q., Amambua-Ngwa A., Conway D.J., Djimde A.A., Doumbo O.K., Zongo I., Ouedraogo J.B., Alcock D., Drury E., Auburn S., Koch O., Sanders M., Hubbart C., Maslen G., Ruano-Rubio V., Jyothi D., Miles A., O'Brien J., Gamble C., Oyola S.O., Rayner J.C., Newbold C.I., Berriman M., Spencer C.C., McVean G., Day N.P., White N.J., Bethell D., Dondorp A.M., Plowe C.V., Fairhurst R.M., Kwiatkowski D.P. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat. Genet. 2013;45:648–655. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto O., Amato R., Ashley E.A., MacInnis B., Almagro-Garcia J., Amaratunga C., Lim P., Mead D., Oyola S.O., Dhorda M., Imwong M., Woodrow C., Manske M., Stalker J., Drury E., Campino S., Amenga-Etego L., Thanh T.N., Tran H.T., Ringwald P., Bethell D., Nosten F., Phyo A.P., Pukrittayakamee S., Chotivanich K., Chuor C.M., Nguon C., Suon S., Sreng S., Newton P.N., Mayxay M., Khanthavong M., Hongvanthong B., Htut Y., Han K.T., Kyaw M.P., Faiz M.A., Fanello C.I., Onyamboko M., Mokuolu O.A., Jacob C.G., Takala-Harrison S., Plowe C.V., Day N.P., Dondorp A.M., Spencer C.C., McVean G., Fairhurst R.M., White N.J., Kwiatkowski D.P. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat. Genet. 2015;47:226–234. doi: 10.1038/ng.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto O., Sekihara M., Tachibana S.I., Yamauchi M., Pearson R.D., Amato R., Goncalves S., Mehra S., Noviyanti R., Marfurt J., Auburn S., Price R.N., Mueller I., Ikeda M., Mori T., Hirai M., Tavul L., Hetzel M.W., Laman M., Barry A.E., Ringwald P., Ohashi J., Hombhanje F., Kwiatkowski D.P., Mita T. Emergence of artemisinin-resistant Plasmodium falciparum with kelch13 C580Y mutations on the island of New Guinea. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1009133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockenhaupt F.P. Mefloquine resistance in Plasmodium falciparum. Parasitol. Today. 1995;11:248–253. doi: 10.1016/0169-4758(95)80201-0. [DOI] [PubMed] [Google Scholar]
- Mok S., Ashley E.A., Ferreira P.E., Zhu L., Lin Z., Yeo T., Chotivanich K., Imwong M., Pukrittayakamee S., Dhorda M., Nguon C., Lim P., Amaratunga C., Suon S., Hien T.T., Htut Y., Faiz M.A., Onyamboko M.A., Mayxay M., Newton P.N., Tripura R., Woodrow C.J., Miotto O., Kwiatkowski D.P., Nosten F., Day N.P., Preiser P.R., White N.J., Dondorp A.M., Fairhurst R.M., Bozdech Z. Drug resistance. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science. 2015;347:431–435. doi: 10.1126/science.1260403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S., Imwong M., Mackinnon M.J., Sim J., Ramadoss R., Yi P., Mayxay M., Chotivanich K., Liong K.Y., Russell B., Socheat D., Newton P.N., Day N.P., White N.J., Preiser P.R., Nosten F., Dondorp A.M., Bozdech Z. Artemisinin resistance in Plasmodium falciparum is associated with an altered temporal pattern of transcription. BMC Genom. 2011;12:391. doi: 10.1186/1471-2164-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S., Stokes B.H., Gnadig N.F., Ross L.S., Yeo T., Amaratunga C., Allman E., Solyakov L., Bottrill A.R., Tripathi J., Fairhurst R.M., Llinas M., Bozdech Z., Tobin A.B., Fidock D.A. Artemisinin-resistant K13 mutations rewire Plasmodium falciparum's intra-erythrocytic metabolic program to enhance survival. Nat. Commun. 2021;12:530. doi: 10.1038/s41467-020-20805-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Bopp S., Magistrado P., Wong W., Daniels R., Demas A., Schaffner S., Amaratunga C., Lim P., Dhorda M., Miotto O., Woodrow C., Ashley E.A., Dondorp A.M., White N.J., Wirth D., Fairhurst R., Volkman S.K. Artemisinin resistance without pfkelch13 mutations in Plasmodium falciparum isolates from Cambodia. Malar. J. 2017;16:195. doi: 10.1186/s12936-017-1845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Gagnon D., Wirth D.F., Richard D. Inactivation of plasmepsins 2 and 3 sensitizes Plasmodium falciparum to the antimalarial drug piperaquine. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.02309-17. e02309-02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz R., Okell L.C., Challenger J.D. Asymptomatic recrudescence after artemether-lumefantrine treatment for uncomplicated falciparum malaria: a systematic review and meta-analysis. Malar. J. 2020;19:453. doi: 10.1186/s12936-020-03520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwesigwa J., Achan J., Affara M., Wathuo M., Worwui A., Mohammed N.I., Kanuteh F., Prom A., Dierickx S., di Tanna G.L., Nwakanma D., Bousema T., Drakeley C., Van Geertruyden J.P., D'Alessandro U. Mass drug administration with dihydroartemisinin-piperaquine and malaria transmission dynamics in the Gambia: a prospective cohort study. Clin. Infect. Dis. 2019;69:278–286. doi: 10.1093/cid/ciy870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Li X., Arya G.A., McDew-White M., Ferrari M., Nosten F., Anderson T.J.C. Fitness costs and the rapid spread of kelch13-C580Y substitutions conferring artemisinin resistance. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.00605-18. e00605-00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton P.N., Fernandez F.M., Plancon A., Mildenhall D.C., Green M.D., Ziyong L., Christophel E.M., Phanouvong S., Howells S., McIntosh E., Laurin P., Blum N., Hampton C.Y., Faure K., Nyadong L., Soong C.W., Santoso B., Zhiguang W., Newton J., Palmer K. A collaborative epidemiological investigation into the criminal fake artesunate trade in South East Asia. PLoS Med. 2008;5:e32. doi: 10.1371/journal.pmed.0050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D.S., Dao B.H., Nguyen P.D., Nguyen V.H., Le N.B., Mai V.S., Meshnick S.R. Treatment of malaria in Vietnam with oral artemisinin. Am. J. Trop. Med. Hyg. 1993;48:398–402. [PubMed] [Google Scholar]
- Noedl H., Se Y., Schaecher K., Smith B.L., Socheat D., Fukuda M.M., Artemisinin Resistance in Cambodia 1 Study, C Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- Nosten F., Luxemburger C., ter Kuile F.O., Woodrow C., Eh J.P., Chongsuphajaisiddhi T., White N.J. Treatment of multidrug-resistant Plasmodium falciparum malaria with 3-day artesunate-mefloquine combination. J. Infect. Dis. 1994;170:971–977. doi: 10.1093/infdis/170.4.971. [DOI] [PubMed] [Google Scholar]
- Nosten F., van Vugt M., Price R., Luxemburger C., Thway K.L., Brockman A., McGready R., ter Kuile F., Looareesuwan S., White N.J. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–302. doi: 10.1016/s0140-6736(00)02505-8. [DOI] [PubMed] [Google Scholar]
- Nsobya S.L., Dokomajilar C., Joloba M., Dorsey G., Rosenthal P.J. Resistance-mediating Plasmodium falciparum pfcrt and pfmdr1 alleles after treatment with artesunate-amodiaquine in Uganda. Antimicrob. Agents Chemother. 2007;51:3023–3025. doi: 10.1128/AAC.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty K., Maguire J., Simpson J.A., Fowkes F.J.I. Immunity as a predictor of anti-malarial treatment failure: a systematic review. Malar. J. 2017;16:158. doi: 10.1186/s12936-017-1815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill P.M., Barton V.E., Ward S.A. The molecular mechanism of action of artemisinin--the debate continues. Molecules. 2010;15:1705–1721. doi: 10.3390/molecules15031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okell L.C., Reiter L.M., Ebbe L.S., Baraka V., Bisanzio D., Watson O.J., Bennett A., Verity R., Gething P., Roper C., Alifrangis M. Emerging implications of policies on malaria treatment: genetic changes in the Pfmdr-1 gene affecting susceptibility to artemether-lumefantrine and artesunate-amodiaquine in Africa. BMJ Glob Health. 2018;3 doi: 10.1136/bmjgh-2018-000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshina M.A., Angrisano F., Marapana D.S., Riglar D.T., Bane K., Wong W., Catimel B., Yin M.X., Holmes A.B., Frischknecht F., Kovar D.R., Baum J. Plasmodium falciparum coronin organizes arrays of parallel actin filaments potentially guiding directional motility in invasive malaria parasites. Malar. J. 2015;14:280. doi: 10.1186/s12936-015-0801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A.V., Tekwani B.L., Singh R.L., Chauhan V.S. Artemisinin, an endoperoxide antimalarial, disrupts the hemoglobin catabolism and heme detoxification systems in malarial parasite. J. Biol. Chem. 1999;274:19383–19388. doi: 10.1074/jbc.274.27.19383. [DOI] [PubMed] [Google Scholar]
- Parker D.M., Tun S.T.T., White L.J., Kajeechiwa L., Thwin M.M., Landier J., Chaumeau V., Corbel V., Dondorp A.M., von Seidlein L., White N.J., Maude R.J., Nosten F. Potential herd protection against Plasmodium falciparum infections conferred by mass antimalarial drug administrations. Elife. 2019;8 doi: 10.7554/eLife.41023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parobek C.M., Parr J.B., Brazeau N.F., Lon C., Chaorattanakawee S., Gosi P., Barnett E.J., Norris L.D., Meshnick S.R., Spring M.D., Lanteri C.A., Bailey J.A., Saunders D.L., Lin J.T., Juliano J.J. Partner-drug resistance and population substructuring of artemisinin-resistant Plasmodium falciparum in Cambodia. Genome Biol. Evol. 2017;9:1673–1686. doi: 10.1093/gbe/evx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peatey C.L., Chavchich M., Chen N., Gresty K.J., Gray K.A., Gatton M.L., Waters N.C., Cheng Q. Mitochondrial membrane potential in a small subset of artemisinin-induced dormant Plasmodium falciparum parasites in vitro. J. Infect. Dis. 2015;212:426–434. doi: 10.1093/infdis/jiv048. [DOI] [PubMed] [Google Scholar]
- Pelleau S., Moss E.L., Dhingra S.K., Volney B., Casteras J., Gabryszewski S.J., Volkman S.K., Wirth D.F., Legrand E., Fidock D.A., Neafsey D.E., Musset L. Adaptive evolution of malaria parasites in French Guiana: reversal of chloroquine resistance by acquisition of a mutation in pfcrt. Proc. Natl. Acad. Sci. U.S.A. 2015;112:11672–11677. doi: 10.1073/pnas.1507142112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phyo A.P., Nkhoma S., Stepniewska K., Ashley E.A., Nair S., McGready R., ler Moo C., Al-Saai S., Dondorp A.M., Lwin K.M., Singhasivanon P., Day N.P., White N.J., Anderson T.J., Nosten F. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard A.L., Wongsrichanalai C., Purfield A., Kamwendo D., Emery K., Zalewski C., Kawamoto F., Miller R.S., Meshnick S.R. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob. Agents Chemother. 2003;47:2418–2423. doi: 10.1128/AAC.47.8.2418-2423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plucinski M.M., Dimbu P.R., Macaia A.P., Ferreira C.M., Samutondo C., Quivinja J., Afonso M., Kiniffo R., Mbounga E., Kelley J.S., Patel D.S., He Y., Talundzic E., Garrett D.O., Halsey E.S., Udhayakumar V., Ringwald P., Fortes F. Efficacy of artemether-lumefantrine, artesunate-amodiaquine, and dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in Angola, 2015. Malar. J. 2017;16:62. doi: 10.1186/s12936-017-1712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plucinski M.M., Talundzic E., Morton L., Dimbu P.R., Macaia A.P., Fortes F., Goldman I., Lucchi N., Stennies G., MacArthur J.R., Udhayakumar V. Efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for treatment of uncomplicated malaria in children in Zaire and Uige Provinces, Angola. Antimicrob. Agents Chemother. 2015;59:437–443. doi: 10.1128/AAC.04181-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugaliza H.P., Miyazaki S., Geurten F.J., Pell C., Rosanas-Urgell A., Janse C.J., Cortes A. Artemisinin exposure at the ring or trophozoite stage impacts Plasmodium falciparum sexual conversion differently. Elife. 2020;9 doi: 10.7554/eLife.60058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell R.D., Brewer G.J., Alving A.S., Millar J.W. Studies on a strain of chloroquine-resistant Plasmodium falciparum from Thailand. Bull. World Health Organ. 1964;30:29–44. [PMC free article] [PubMed] [Google Scholar]
- Preechapornkul P., Imwong M., Chotivanich K., Pongtavornpinyo W., Dondorp A.M., Day N.P., White N.J., Pukrittayakamee S. Plasmodium falciparum pfmdr1 amplification, mefloquine resistance, and parasite fitness. Antimicrob. Agents Chemother. 2009;53:1509–1515. doi: 10.1128/AAC.00241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.N., Uhlemann A.C., Brockman A., McGready R., Ashley E., Phaipun L., Patel R., Laing K., Looareesuwan S., White N.J., Nosten F., Krishna S. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser C., Meyer W., Ellis J., Lee R. Evolutionary ARMS race: antimalarial resistance molecular surveillance. Trends Parasitol. 2018;34:322–334. doi: 10.1016/j.pt.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Raman J., Kagoro F.M., Mabuza A., Malatje G., Reid A., Frean J., Barnes K.I. Absence of kelch13 artemisinin resistance markers but strong selection for lumefantrine-tolerance molecular markers following 18 years of artemisinin-based combination therapy use in Mpumalanga Province, South Africa (2001-2018) Malar. J. 2019;18:280. doi: 10.1186/s12936-019-2911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C., Ringwald P. Continued low efficacy of artemether-lumefantrine in Angola? Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.00220-21. AAC.00220-00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod P.K., McErlean T., Lee P.C. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A., Cazelles J., Meunier B. Characterization of the Alkylation product of heme by the antimalarial drug artemisinin. Angew Chem. Int. Ed. Engl. 2001;40:1954–1957. doi: 10.1002/1521-3773(20010518)40:10<1954::aid-anie1954>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Rocamora F., Zhu L., Liong K.Y., Dondorp A., Miotto O., Mok S., Bozdech Z. Oxidative stress and protein damage responses mediate artemisinin resistance in malaria parasites. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers W.O., Sem R., Tero T., Chim P., Lim P., Muth S., Socheat D., Ariey F., Wongsrichanalai C. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar. J. 2009;8:10. doi: 10.1186/1475-2875-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson S.J., Wijesinghe R.S., Meshnick S.R. Host immunity as a determinant of treatment outcome in Plasmodium falciparum malaria. Lancet Infect. Dis. 2010;10:51–59. doi: 10.1016/S1473-3099(09)70322-6. [DOI] [PubMed] [Google Scholar]
- Roper C., Pearce R., Nair S., Sharp B., Nosten F., Anderson T. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- Rosenberg A., Luth M.R., Winzeler E.A., Behnke M., Sibley L.D. Evolution of resistance in vitro reveals mechanisms of artemisinin activity in Toxoplasma gondii. Proc. Natl. Acad. Sci. U.S.A. 2019;116(52):26881–26891. doi: 10.1073/pnas.1914732116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M.R., Ng C.L. Plasmodium falciparum artemisinin resistance: the effect of heme, protein damage, and parasite cell stress response. ACS Infect. Dis. 2020;6:1599–1614. doi: 10.1021/acsinfecdis.9b00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P.J. The interplay between drug resistance and fitness in malaria parasites. Mol. Microbiol. 2013;89:1025–1038. doi: 10.1111/mmi.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L.S., Dhingra S.K., Mok S., Yeo T., Wicht K.J., Kumpornsin K., Takala-Harrison S., Witkowski B., Fairhurst R.M., Ariey F., Menard D., Fidock D.A. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat. Commun. 2018;9:3314. doi: 10.1038/s41467-018-05652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L.S., Fidock D.A. Elucidating mechanisms of drug-resistant Plasmodium falciparum. Cell Host Microbe. 2019;26:35–47. doi: 10.1016/j.chom.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G., De Smet M., Khim N., Kindermans J.M., Menard D. Emergence of Plasmodium falciparum triple mutant in Cambodia. Lancet Infect. Dis. 2017;17:1233. doi: 10.1016/S1473-3099(17)30635-7. [DOI] [PubMed] [Google Scholar]
- Rubio J.P., Cowman A.F. The ATP-binding cassette (ABC) gene family of Plasmodium falciparum. Parasitol. Today. 1996;12:135–140. doi: 10.1016/0169-4758(96)10003-x. [DOI] [PubMed] [Google Scholar]
- Sa J.M., Twu O., Hayton K., Reyes S., Fay M.P., Ringwald P., Wellems T.E. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc. Natl. Acad. Sci. U.S.A. 2009;106:18883–18889. doi: 10.1073/pnas.0911317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saralamba S., Pan-Ngum W., Maude R.J., Lee S.J., Tarning J., Lindegardh N., Chotivanich K., Nosten F., Day N.P., Socheat D., White N.J., Dondorp A.M., White L.J. Intrahost modeling of artemisinin resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 2011;108:397–402. doi: 10.1073/pnas.1006113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders D.L., Vanachayangkul P., Lon C. Dihydroartemisinin-piperaquine failure in Cambodia. N. Engl. J. Med. 2014;371:484–485. doi: 10.1056/NEJMc1403007. [DOI] [PubMed] [Google Scholar]
- Sharma A.I., Shin S.H., Bopp S., Volkman S.K., Hartl D.L., Wirth D.F. Genetic background and PfKelch13 affect artemisinin susceptibility of PfCoronin mutants in Plasmodium falciparum. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1009266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B., Shah Z., Morgan A.P., Saingam P., Chaisatit C., Chaorattanakawee S., Praditpol C., Boonyalai N., Lertsethtakarn P., Wojnarski M., Deutsch-Feldman M., Adams M., Sea D., Chann S., Tyner S.D., Lanteri C.A., Spring M.D., Saunders D.L., Smith P.L., Lon C., Gosi P., Sok S., Satharath P., Rekol H., Lek D., Vesely B.A., Lin J.T., Waters N.C., Takala-Harrison S. Distribution and temporal dynamics of P. falciparum chloroquine resistance transporter mutations associated with piperaquine resistance in Northern Cambodia. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y., Zeng W., Li N., Wang C., Siddiqui F., Zhang J., Pi L., He X., Zhao L., Wang S., Zhao H., Li X., Yang Q., Miao J., Yang Z., Cui L. In vitro susceptibility of Plasmodium falciparum isolates from the China-Myanmar border area to piperaquine and association with candidate markers. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.02305-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui F.A., Boonhok R., Cabrera M., Mbenda H.G.N., Wang M., Min H., Liang X., Qin J., Zhu X., Miao J., Cao Y., Cui L. Role of Plasmodium falciparum Kelch 13 protein mutations in P. falciparum populations from northeastern Myanmar in mediating artemisinin resistance. mBio. 2020;11 doi: 10.1128/mBio.01134-19. e01134-01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui F.A., Cabrera M., Wang M., Brashear A., Kemirembe K., Wang Z., Miao J., Chookajorn T., Yang Z., Cao Y., Dong G., Rosenthal P.J., Cui L. Plasmodium falciparum falcipain-2a polymorphisms in Southeast Asia and their association with artemisinin resistance. J. Infect. Dis. 2018;218:434–442. doi: 10.1093/infdis/jiy188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui G., Srivastava A., Russell A.S., Creek D.J. Multi-omics based identification of specific biochemical changes associated with PfKelch13-mutant artemisinin-resistant Plasmodium falciparum. J. Infect. Dis. 2017;215:1435–1444. doi: 10.1093/infdis/jix156. [DOI] [PubMed] [Google Scholar]
- Silva M., Calcada C., Teixeira M., Veiga M.I., Ferreira P.E. Multigenic architecture of piperaquine resistance trait in Plasmodium falciparum. Lancet Infect. Dis. 2020;20:26–27. doi: 10.1016/S1473-3099(19)30689-9. [DOI] [PubMed] [Google Scholar]
- Silva M., Ferreira P.E., Otienoburu S.D., Calcada C., Ngasala B., Bjorkman A., Martensson A., Gil J.P., Veiga M.I. Plasmodium falciparum K13 expression associated with parasite clearance during artemisinin-based combination therapy. J. Antimicrob. Chemother. 2019;74(7):1890–1893. doi: 10.1093/jac/dkz098. [DOI] [PubMed] [Google Scholar]
- Simwela N.V., Hughes K.R., Roberts A.B., Rennie M.T., Barrett M.P., Waters A.P. Experimentally engineered mutations in a ubiquitin hydrolase, UBP-1, modulate in vivo susceptibility to artemisinin and chloroquine in Plasmodium berghei. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.02484-19. e02484-02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring M.D., Lin J.T., Manning J.E., Vanachayangkul P., Somethy S., Bun R., Se Y., Chann S., Ittiverakul M., Sia-ngam P., Kuntawunginn W., Arsanok M., Buathong N., Chaorattanakawee S., Gosi P., Ta-aksorn W., Chanarat N., Sundrakes S., Kong N., Heng T.K., Nou S., Teja-isavadharm P., Pichyangkul S., Phann S.T., Balasubramanian S., Juliano J.J., Meshnick S.R., Chour C.M., Prom S., Lanteri C.A., Lon C., Saunders D.L. Dihydroartemisinin-piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect. Dis. 2015;15:683–691. doi: 10.1016/S1473-3099(15)70049-6. [DOI] [PubMed] [Google Scholar]
- St Laurent B., Miller B., Burton T.A., Amaratunga C., Men S., Sovannaroth S., Fay M.P., Miotto O., Gwadz R.W., Anderson J.M., Fairhurst R.M. Artemisinin-resistant Plasmodium falciparum clinical isolates can infect diverse mosquito vectors of Southeast Asia and Africa. Nat. Commun. 2015;6:8614. doi: 10.1038/ncomms9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska K., Ashley E., Lee S.J., Anstey N., Barnes K.I., Binh T.Q., D'Alessandro U., Day N.P., de Vries P.J., Dorsey G., Guthmann J.P., Mayxay M., Newton P.N., Olliaro P., Osorio L., Price R.N., Rowland M., Smithuis F., Taylor W.R., Nosten F., White N.J. In vivo parasitological measures of artemisinin susceptibility. J. Infect. Dis. 2010;201:570–579. doi: 10.1086/650301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straimer J., Gnadig N.F., Stokes B.H., Ehrenberger M., Crane A.A., Fidock D.A. Plasmodium falciparum K13 mutations differentially impact ozonide susceptibility and parasite fitness in vitro. mBio. 2017;8 doi: 10.1128/mBio.00172-17. e00172-00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straimer J., Gnadig N.F., Witkowski B., Amaratunga C., Duru V., Ramadani A.P., Dacheux M., Khim N., Zhang L., Lam S., Gregory P.D., Urnov F.D., Mercereau-Puijalon O., Benoit-Vical F., Fairhurst R.M., Menard D., Fidock D.A. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2015;347:428–431. doi: 10.1126/science.1260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X., Hayton K., Wellems T.E. Genetic linkage and association analyses for trait mapping in Plasmodium falciparum. Nat. Rev. Genet. 2007;8:497–506. doi: 10.1038/nrg2126. [DOI] [PubMed] [Google Scholar]
- Suazo K.F., Schaber C., Palsuledesai C.C., Odom John A.R., Distefano M.D. Global proteomic analysis of prenylated proteins in Plasmodium falciparum using an alkyne-modified isoprenoid analogue. Sci. Rep. 2016;6:38615. doi: 10.1038/srep38615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C.J., Henrici R.C., Artavanis-Tsakonas K. Artemisinin susceptibility in the malaria parasite Plasmodium falciparum: propellers, adaptor proteins and the need for cellular healing. FEMS Microbiol. Rev. 2020 doi: 10.1093/femsre/fuaa1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C.J., Lansdell P., Sanders M., Muwanguzi J., van Schalkwyk D.A., Kaur H., Nolder D., Tucker J., Bennett H.M., Otto T.D., Berriman M., Patel T.A., Lynn R., Gkrania-Klotsas E., Chiodini P.L. Pfk13-independent treatment failure in four imported cases of Plasmodium falciparum malaria treated with artemether-lumefantrine in the United Kingdom. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.02382-16. e02382-02316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala-Harrison S., Clark T.G., Jacob C.G., Cummings M.P., Miotto O., Dondorp A.M., Fukuda M.M., Nosten F., Noedl H., Imwong M., Bethell D., Se Y., Lon C., Tyner S.D., Saunders D.L., Socheat D., Ariey F., Phyo A.P., Starzengruber P., Fuehrer H.P., Swoboda P., Stepniewska K., Flegg J., Arze C., Cerqueira G.C., Silva J.C., Ricklefs S.M., Porcella S.F., Stephens R.M., Adams M., Kenefic L.J., Campino S., Auburn S., MacInnis B., Kwiatkowski D.P., Su X.Z., White N.J., Ringwald P., Plowe C.V. Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in Southeast Asia. Proc. Natl. Acad. Sci. U.S.A. 2013;110:240–245. doi: 10.1073/pnas.1211205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala-Harrison S., Jacob C.G., Arze C., Cummings M.P., Silva J.C., Dondorp A.M., Fukuda M.M., Hien T.T., Mayxay M., Noedl H., Nosten F., Kyaw M.P., Nhien N.T., Imwong M., Bethell D., Se Y., Lon C., Tyner S.D., Saunders D.L., Ariey F., Mercereau-Puijalon O., Menard D., Newton P.N., Khanthavong M., Hongvanthong B., Starzengruber P., Fuehrer H.P., Swoboda P., Khan W.A., Phyo A.P., Nyunt M.M., Nyunt M.H., Brown T.S., Adams M., Pepin C.S., Bailey J., Tan J.C., Ferdig M.T., Clark T.G., Miotto O., MacInnis B., Kwiatkowski D.P., White N.J., Ringwald P., Plowe C.V. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J. Infect. Dis. 2015;211:670–679. doi: 10.1093/infdis/jiu491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawk L., Chicanne G., Dubremetz J.F., Richard V., Payrastre B., Vial H.J., Roy C., Wengelnik K. Phosphatidylinositol 3-phosphate, an essential lipid in Plasmodium, localizes to the food vacuole membrane and the apicoplast. Eukaryot. Cell. 2010;9:1519–1530. doi: 10.1128/EC.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A.R., Flegg J.A., Holmes C.C., Guerin P.J., Sibley C.H., Conrad M.D., Dorsey G., Rosenthal P.J. Artemether-lumefantrine and dihydroartemisinin-piperaquine exert inverse selective pressure on Plasmodium falciparum drug sensitivity-associated haplotypes in Uganda. Open Forum Infect Dis. 2017;4:ofw229. doi: 10.1093/ofid/ofw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.M., Parobek C.M., DeConti D.K., Kayentao K., Coulibaly S.O., Greenwood B.M., Tagbor H., Williams J., Bojang K., Njie F., Desai M., Kariuki S., Gutman J., Mathanga D.P., Martensson A., Ngasala B., Conrad M.D., Rosenthal P.J., Tshefu A.K., Moormann A.M., Vulule J.M., Doumbo O.K., Ter Kuile F.O., Meshnick S.R., Bailey J.A., Juliano J.J. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in Sub-Saharan Africa: a molecular epidemiologic study. J. Infect. Dis. 2015;211:680–688. doi: 10.1093/infdis/jiu467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.M., Parobek C.M., Fairhurst R.M. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:457–468. doi: 10.1016/S1473-3099(12)70055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher F., Chen N., Kyle D.E., Gatton M.L., Cheng Q. Phenotypic changes in artemisinin-resistant Plasmodium falciparum lines in vitro: evidence for decreased sensitivity to dormancy and growth inhibition. Antimicrob. Agents Chemother. 2012;56:428–431. doi: 10.1128/AAC.05456-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher F., Gatton M.L., Chen N., Peters J., Kyle D.E., Cheng Q. Artemisinin-induced dormancy in plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J. Infect. Dis. 2010;202:1362–1368. doi: 10.1086/656476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh N.V., Thuy-Nhien N., Tuyen N.T., Tong N.T., Nha-Ca N.T., Dong L.T., Quang H.H., Farrar J., Thwaites G., White N.J., Wolbers M., Hien T.T. Rapid decline in the susceptibility of Plasmodium falciparum to dihydroartemisinin-piperaquine in the south of Vietnam. Malar. J. 2017;16:27. doi: 10.1186/s12936-017-1680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley L., Straimer J., Gnadig N.F., Ralph S.A., Fidock D.A. Artemisinin action and resistance in Plasmodium falciparum. Trends Parasitol. 2016;32:682–696. doi: 10.1016/j.pt.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirrell A.R., Vendrely K.M., Checkley L.A., Davis S.Z., McDew-White M., Cheeseman I.H., Vaughan A.M., Nosten F.H., Anderson T.J.C., Ferdig M.T. Pairwise growth competitions identify relative fitness relationships among artemisinin resistant Plasmodium falciparum field isolates. Malar. J. 2019;18:295. doi: 10.1186/s12936-019-2934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trape J.F., Pison G., Preziosi M.P., Enel C., Desgrees du Lou A., Delaunay V., Samb B., Lagarde E., Molez J.F., Simondon F. Impact of chloroquine resistance on malaria mortality. C. R. Acad. Sci. III. 1998;321:689–697. doi: 10.1016/s0764-4469(98)80009-7. [DOI] [PubMed] [Google Scholar]
- Trotta R.F., Brown M.L., Terrell J.C., Geyer J.A. Defective DNA repair as a potential mechanism for the rapid development of drug resistance in Plasmodium falciparum. Biochemistry. 2004;43:4885–4891. doi: 10.1021/bi0499258. [DOI] [PubMed] [Google Scholar]
- Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011;17:1217–1220. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- Tu Y. Artemisinin-A gift from traditional Chinese medicine to the world (Nobel Lecture) Angew. Chem. 2016;55:10210–10226. doi: 10.1002/anie.201601967. [DOI] [PubMed] [Google Scholar]
- Tun K.M., Imwong M., Lwin K.M., Win A.A., Hlaing T.M., Hlaing T., Lin K., Kyaw M.P., Plewes K., Faiz M.A., Dhorda M., Cheah P.Y., Pukrittayakamee S., Ashley E.A., Anderson T.J., Nair S., McDew-White M., Flegg J.A., Grist E.P., Guerin P., Maude R.J., Smithuis F., Dondorp A.M., Day N.P., Nosten F., White N.J., Woodrow C.J. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect. Dis. 2015;15:415–421. doi: 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwimana A., Legrand E., Stokes B.H., Ndikumana J.M., Warsame M., Umulisa N., Ngamije D., Munyaneza T., Mazarati J.B., Munguti K., Campagne P., Criscuolo A., Ariey F., Murindahabi M., Ringwald P., Fidock D.A., Mbituyumuremyi A., Menard D. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020;26:1602–1608. doi: 10.1038/s41591-020-1005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaid A., Ranjan R., Smythe W.A., Hoppe H.C., Sharma P. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum, is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood. 2010;115:2500–2507. doi: 10.1182/blood-2009-08-238972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pluijm R.W., Imwong M., Chau N.H., Hoa N.T., Thuy-Nhien N.T., Thanh N.V., Jittamala P., Hanboonkunupakarn B., Chutasmit K., Saelow C., Runjarern R., Kaewmok W., Tripura R., Peto T.J., Yok S., Suon S., Sreng S., Mao S., Oun S., Yen S., Amaratunga C., Lek D., Huy R., Dhorda M., Chotivanich K., Ashley E.A., Mukaka M., Waithira N., Cheah P.Y., Maude R.J., Amato R., Pearson R.D., Goncalves S., Jacob C.G., Hamilton W.L., Fairhurst R.M., Tarning J., Winterberg M., Kwiatkowski D.P., Pukrittayakamee S., Hien T.T., Day N.P., Miotto O., White N.J., Dondorp A.M. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect. Dis. 2019;19:952–961. doi: 10.1016/S1473-3099(19)30391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pluijm R.W., Tripura R., Hoglund R.M., Pyae Phyo A., Lek D., Ul Islam A., Anvikar A.R., Satpathi P., Satpathi S., Behera P.K., Tripura A., Baidya S., Onyamboko M., Chau N.H., Sovann Y., Suon S., Sreng S., Mao S., Oun S., Yen S., Amaratunga C., Chutasmit K., Saelow C., Runcharern R., Kaewmok W., Hoa N.T., Thanh N.V., Hanboonkunupakarn B., Callery J.J., Mohanty A.K., Heaton J., Thant M., Gantait K., Ghosh T., Amato R., Pearson R.D., Jacob C.G., Goncalves S., Mukaka M., Waithira N., Woodrow C.J., Grobusch M.P., van Vugt M., Fairhurst R.M., Cheah P.Y., Peto T.J., von Seidlein L., Dhorda M., Maude R.J., Winterberg M., Thuy-Nhien N.T., Kwiatkowski D.P., Imwong M., Jittamala P., Lin K., Hlaing T.M., Chotivanich K., Huy R., Fanello C., Ashley E., Mayxay M., Newton P.N., Hien T.T., Valecha N., Smithuis F., Pukrittayakamee S., Faiz A., Miotto O., Tarning J., Day N.P.J., White N.J., Dondorp A.M., Tracking Resistance to Artemisinin, C Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial. Lancet. 2020;395:1345–1360. doi: 10.1016/S0140-6736(20)30552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga M.I., Dhingra S.K., Henrich P.P., Straimer J., Gnadig N., Uhlemann A.C., Martin R.E., Lehane A.M., Fidock D.A. Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat. Commun. 2016;7:11553. doi: 10.1038/ncomms11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velavan T.P., Nderu D., Agbenyega T., Ntoumi F., Kremsner P.G. An alternative dogma on reduced artemisinin susceptibility: a new shadow from east to west. Proc. Natl. Acad. Sci. U. S. A. 2019;116:12611–12612. doi: 10.1073/pnas.1907142116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M., Gadalla N.B., Stepniewska K., Dahal P., Nsanzabana C., Moriera C., Price R.N., Martensson A., Rosenthal P.J., Dorsey G., Sutherland C.J., Guerin P., Davis T.M., Menard D., Adam I., Ademowo G., Arze C., Baliraine F.N., Berens-Riha N., Bjorkman A., Borrmann S., Checchi F., Desai M., Dhorda M., Djimde A.A., El-Sayed B.B., Eshetu T., Eyase F., Falade C., Faucher J.F., Froberg G., Grivoyannis A., Hamour S., Houze S., Johnson J., Kamugisha E., Kariuki S., Kiechel J.R., Kironde F., Kofoed P.E., LeBras J., Malmberg M., Mwai L., Ngasala B., Nosten F., Nsobya S.L., Nzila A., Oguike M., Otienoburu S.D., Ogutu B., Ouedraogo J.B., Piola P., Rombo L., Schramm B., Some A.F., Thwing J., Ursing J., Wong R.P., Zeynudin A., Zongo I., Plowe C.V., Sibley C.H. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: parasite risk factors that affect treatment outcomes for P. falciparum malaria after artemether-lumefantrine and artesunate-amodiaquine. Am. J. Trop. Med. Hyg. 2014;91:833–843. doi: 10.4269/ajtmh.14-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Seidlein L., Peto T.J., Landier J., Nguyen T.N., Tripura R., Phommasone K., Pongvongsa T., Lwin K.M., Keereecharoen L., Kajeechiwa L., Thwin M.M., Parker D.M., Wiladphaingern J., Nosten S., Proux S., Corbel V., Tuong-Vy N., Phuc-Nhi T.L., Son D.H., Huong-Thu P.N., Tuyen N.T.K., Tien N.T., Dong L.T., Hue D.V., Quang H.H., Nguon C., Davoeung C., Rekol H., Adhikari B., Henriques G., Phongmany P., Suangkanarat P., Jeeyapant A., Vihokhern B., van der Pluijm R.W., Lubell Y., White L.J., Aguas R., Promnarate C., Sirithiranont P., Malleret B., Renia L., Onsjo C., Chan X.H., Chalk J., Miotto O., Patumrat K., Chotivanich K., Hanboonkunupakarn B., Jittmala P., Kaehler N., Cheah P.Y., Pell C., Dhorda M., Imwong M., Snounou G., Mukaka M., Peerawaranun P., Lee S.J., Simpson J.A., Pukrittayakamee S., Singhasivanon P., Grobusch M.P., Cobelens F., Smithuis F., Newton P.N., Thwaites G.E., Day N.P.J., Mayxay M., Hien T.T., Nosten F.H., Dondorp A.M., White N.J. The impact of targeted malaria elimination with mass drug administrations on falciparum malaria in Southeast Asia: a cluster randomised trial. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Huang L., Li J., Fan Q., Long Y., Li Y., Zhou B. Artemisinin directly targets malarial mitochondria through its specific mitochondrial activation. PloS One. 2010;5 doi: 10.1371/journal.pone.0009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Xu C., Liao F.L., Jiang T., Krishna S., Tu Y. A temporizing solution to "artemisinin resistance. N. Engl. J. Med. 2019;380:2087–2089. doi: 10.1056/NEJMp1901233. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang C.J., Chia W.N., Loh C.C., Li Z., Lee Y.M., He Y., Yuan L.X., Lim T.K., Liu M., Liew C.X., Lee Y.Q., Zhang J., Lu N., Lim C.T., Hua Z.C., Liu B., Shen H.M., Tan K.S., Lin Q. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat. Commun. 2015;6:10111. doi: 10.1038/ncomms10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Cabrera M., Yang J., Yuan L., Gupta B., Liang X., Kemirembe K., Shrestha S., Brashear A., Li X., Porcella S.F., Miao J., Yang Z., Su X.Z., Cui L. Genome-wide association analysis identifies genetic loci associated with resistance to multiple antimalarials in Plasmodium falciparum from China-Myanmar border. Sci. Rep. 2016;6:33891. doi: 10.1038/srep33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Shrestha S., Li X., Miao J., Yuan L., Cabrera M., Grube C., Yang Z., Cui L. Prevalence of K13-propeller polymorphisms in Plasmodium falciparum from China-Myanmar border in 2007-2012. Malar. J. 2015;14:168. doi: 10.1186/s12936-015-0672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhurst D.C., Craig J.C., Adagu I.S., Guy R.K., Madrid P.B., Fivelman Q.L. Activity of piperaquine and other 4-aminoquinoline antiplasmodial drugs against chloroquine-sensitive and resistant blood-stages of Plasmodium falciparum. Role of beta-haematin inhibition and drug concentration in vacuolar water- and lipid-phases. Biochem. Pharmacol. 2007;73:1910–1926. doi: 10.1016/j.bcp.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Wellems T.E., Plowe C.V. Chloroquine-resistant malaria. J. Infect. Dis. 2001;184:770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- Wellems T.E., Sa J.M., Su X.Z., Connelly S.V., Ellis A.C. 'Artemisinin resistance': something new or old? Something of a misnomer? Trends Parasitol. 2020;36:735–744. doi: 10.1016/j.pt.2020.05.013. [DOI] [PubMed] [Google Scholar]
- White L.J., Flegg J.A., Phyo A.P., Wiladpai-ngern J.H., Bethell D., Plowe C., Anderson T., Nkhoma S., Nair S., Tripura R., Stepniewska K., Pan-Ngum W., Silamut K., Cooper B.S., Lubell Y., Ashley E.A., Nguon C., Nosten F., White N.J., Dondorp A.M. Defining the in vivo phenotype of artemisinin-resistant falciparum malaria: a modelling approach. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.J. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia. 1999;41:301–308. [PubMed] [Google Scholar]
- White N.J. Antimalarial drug resistance. J. Clin. Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.J. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- White N.J., Pongtavornpinyo W., Maude R.J., Saralamba S., Aguas R., Stepniewska K., Lee S.J., Dondorp A.M., White L.J., Day N.P. Hyperparasitaemia and low dosing are an important source of anti-malarial drug resistance. Malar. J. 2009;8:253. doi: 10.1186/1475-2875-8-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.J., Watson J., Ashley E.A. Split dosing of artemisinins does not improve antimalarial therapeutic efficacy. Sci. Rep. 2017;7:12132. doi: 10.1038/s41598-017-12483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Who . World Health Organization Technical Report Series; 1965. Resistance of Malaria Parasites to Drugs: Report of a WHO Scientific Group. No. 296. [PubMed] [Google Scholar]
- Who . 2011. Global Plan for Artemisinin Resistance Containment (GPARC) p. 87. [Google Scholar]
- Who Eliminating malaria in the greater Mekong subregion: united to end a deadly disease. 2016. https://www.who.int/malaria/publications/atoz/eliminating-malaria-greater-mekong/en/
- Who . 2020. Report on Antimalarial Drug Efficacy, Resistance and Response: 10 Years of Surveillance (2010–2019) [Google Scholar]
- Who . 2020. World Malaria Report 2020. [Google Scholar]
- Wicht K.J., Mok S., Fidock D.A. Molecular mechanisms of drug resistance in Plasmodium falciparum malaria. Annu. Rev. Microbiol. 2020;74:431–454. doi: 10.1146/annurev-micro-020518-115546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski B., Amaratunga C., Khim N., Sreng S., Chim P., Kim S., Lim P., Mao S., Sopha C., Sam B., Anderson J.M., Duong S., Chuor C.M., Taylor W.R., Suon S., Mercereau-Puijalon O., Fairhurst R.M., Menard D. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect. Dis. 2013;13:1043–1049. doi: 10.1016/S1473-3099(13)70252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski B., Duru V., Khim N., Ross L.S., Saintpierre B., Beghain J., Chy S., Kim S., Ke S., Kloeung N., Eam R., Khean C., Ken M., Loch K., Bouillon A., Domergue A., Ma L., Bouchier C., Leang R., Huy R., Nuel G., Barale J.C., Legrand E., Ringwald P., Fidock D.A., Mercereau-Puijalon O., Ariey F., Menard D. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study. Lancet Infect. Dis. 2017;17:174–183. doi: 10.1016/S1473-3099(16)30415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski B., Khim N., Chim P., Kim S., Ke S., Kloeung N., Chy S., Duong S., Leang R., Ringwald P., Dondorp A.M., Tripura R., Benoit-Vical F., Berry A., Gorgette O., Ariey F., Barale J.C., Mercereau-Puijalon O., Menard D. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob. Agents Chemother. 2013;57:914–923. doi: 10.1128/AAC.01868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski B., Lelievre J., Barragan M.J., Laurent V., Su X.Z., Berry A., Benoit-Vical F. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob. Agents Chemother. 2010;54:1872–1877. doi: 10.1128/AAC.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer K., Dahalan F.A., Delves M.J., Yahiya S., Watson O.J., Straschil U., Chiwcharoen D., Sornboon B., Pukrittayakamee S., Pearson R.D., Howick V.M., Lawniczak M.K.N., White N.J., Dondorp A.M., Okell L.C., Chotivanich K., Ruecker A., Baum J. Transmission of artemisinin-resistant malaria parasites to mosquitoes under antimalarial drug pressure. Antimicrob. Agents Chemother. 2020;65 doi: 10.1128/AAC.00898-20. e00898-00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Bai X.C., Sleebs B.E., Triglia T., Brown A., Thompson J.K., Jackson K.E., Hanssen E., Marapana D.S., Fernandez I.S., Ralph S.A., Cowman A.F., Scheres S.H.W., Baum J. Mefloquine targets the Plasmodium falciparum 80S ribosome to inhibit protein synthesis. Nat Microbiol. 2017;2:17031. doi: 10.1038/nmicrobiol.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongsrichanalai C., Meshnick S.R. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg. Infect. Dis. 2008;14:716–719. doi: 10.3201/eid1405.071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongsrichanalai C., Pickard A.L., Wernsdorfer W.H., Meshnick S.R. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2002;2:209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- Wongsrichanalai C., Prajakwong S., Meshnick S.R., Shanks G.D., Thimasarn K. Mefloquine--its 20 years in the Thai malaria control program. Southeast Asian J. Trop. Med. Publ. Health. 2004;35:300–308. [PubMed] [Google Scholar]
- Woodrow C.J., White N.J. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol. Rev. 2017;41:34–48. doi: 10.1093/femsre/fuw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton J.C., Feng X., Ferdig M.T., Cooper R.A., Mu J., Baruch D.I., Magill A.J., Su X.Z. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- Wwarn Artemisinin-based Combination Therapy Africa Baseline Study Group Clinical determinants of early parasitological response to ACTs in African patients with uncomplicated falciparum malaria: a literature review and meta-analysis of individual patient data. BMC Med. 2015;13:212. doi: 10.1186/s12916-015-0445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S.C., Dogovski C., Hanssen E., Chiu F., Yang T., Crespo M.P., Stafford C., Batinovic S., Teguh S., Charman S., Klonis N., Tilley L. Haemoglobin degradation underpins the sensitivity of early ring stage Plasmodium falciparum to artemisinins. J. Cell Sci. 2016;129:406–416. doi: 10.1242/jcs.178830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S.C., Ralph S.A., Tilley L. K13, the cytostome, and artemisinin resistance. Trends Parasitol. 2020;36:533–544. doi: 10.1016/j.pt.2020.03.006. [DOI] [PubMed] [Google Scholar]
- Xiong A., Prakash P., Gao X., Chew M., Tay I.J.J., Woodrow C.J., Engelward B.P., Han J., Preiser P.R. K13-Mediated reduced susceptibility to artemisinin in Plasmodium falciparum is overlaid on a trait of enhanced DNA damage repair. Cell Rep. 2020;32:107996. doi: 10.1016/j.celrep.2020.107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Yeoh L.M., Tutor M.V., Dixon M.W., McMillan P.J., Xie S.C., Bridgford J.L., Gillett D.L., Duffy M.F., Ralph S.A., McConville M.J., Tilley L., Cobbold S.A. Decreased K13 abundance reduces hemoglobin catabolism and proteotoxic stress, underpinning artemisinin resistance. Cell Rep. 2019;29:2917–2928. doi: 10.1016/j.celrep.2019.10.095. e2915. [DOI] [PubMed] [Google Scholar]
- Yang Y.Z., Little B., Meshnick S.R. Alkylation of proteins by artemisinin. Effects of heme, pH, and drug structure. Biochem. Pharmacol. 1994;48:569–573. doi: 10.1016/0006-2952(94)90287-9. [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhang Z., Sun X., Wan W., Cui L., Zhang X., Zhong D., Yan G. Molecular analysis of chloroquine resistance in Plasmodium falciparum in Yunnan Province, China. Trop. Med. Int. Health. 2007;12:1051–1060. doi: 10.1111/j.1365-3156.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- Yap C.C., Winckler B. Adapting for endocytosis: roles for endocytic sorting adaptors in directing neural development. Front. Cell. Neurosci. 2015;9:119. doi: 10.3389/fncel.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R., Hu D., Zhang Y., Huang Y., Sun X., Wang J., Chen X., Zhou H., Zhang D., Mungthin M., Pan W. Distinctive origin of artemisinin-resistant Plasmodium falciparum on the China-Myanmar border. Sci. Rep. 2016;6:20100. doi: 10.1038/srep20100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeka A., Kigozi R., Conrad M.D., Lugemwa M., Okui P., Katureebe C., Belay K., Kapella B.K., Chang M.A., Kamya M.R., Staedke S.G., Dorsey G., Rosenthal P.J. Artesunate/amodiaquine versus artemether/lumefantrine for the treatment of uncomplicated malaria in Uganda: a randomized trial. J. Infect. Dis. 2016;213:1134–1142. doi: 10.1093/infdis/jiv551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuthavong Y., Butthep P., Bunyaratvej A., Fucharoen S. Decreased sensitivity of artesunate and chloroquine of Plasmodium falciparum infecting hemoglobin H and/or hemoglobin constant spring erythrocytes. J. Clin. Invest. 1989;83:502–505. doi: 10.1172/JCI113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Gallego-Delgado J., Fernandez-Arias C., Waters N.C., Rodriguez A., Tsuji M., Wek R.C., Nussenzweig V., Sullivan W.J., Jr. Inhibiting the plasmodium eIF2alpha kinase PK4 prevents artemisinin-induced latency. Cell Host Microbe. 2017;22:766–776. doi: 10.1016/j.chom.2017.11.005. e764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Wang C., Oberstaller J., Thomas P., Otto T.D., Casandra D., Boyapalle S., Adapa S.R., Xu S., Button-Simons K., Mayho M., Rayner J.C., Ferdig M.T., Jiang R.H.Y., Adams J.H. The endosymbiotic origins of the apicoplast link fever-survival and artemisinin-resistance in the malaria parasite. bioRxiv. 2020:419788. doi: 10.1038/s41467-021-24814-1. 2020.2012.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]