Abstract
Bacterial cellulose (BC) has excellent material properties and can be produced sustainably through simple bacterial culture, but BC‐producing bacteria lack the extensive genetic toolkits of model organisms such as Escherichia coli (E. coli). Here, a simple approach is reported for producing highly programmable BC materials through incorporation of engineered E. coli. The acetic acid bacterium Gluconacetobacter hansenii is cocultured with engineered E. coli in droplets of glucose‐rich media to produce robust cellulose capsules, which are then colonized by the E. coli upon transfer to selective lysogeny broth media. It is shown that the encapsulated E. coli can produce engineered protein nanofibers within the cellulose matrix, yielding hybrid capsules capable of sequestering specific biomolecules from the environment and enzymatic catalysis. Furthermore, capsules are produced which can alter their own bulk physical properties through enzyme‐induced biomineralization. This novel system uses a simple fabrication process, based on the autonomous activity of two bacteria, to significantly expand the functionality of BC‐based living materials.
Keywords: bacterial cellulose, biomineralization, curli nanofibers, engineered living materials, synthetic biology
A simple approach is developed to expand the capabilities of bacterial cellulose‐based living materials. Using a fabrication process based mostly on the autonomous action of bacteria, highly programmable robust cellulose capsules are produced containing high concentrations of Escherichia coli. Engineering of the encapsulated E. coli yields hybrid capsules capable of biomolecule sequestration, enzymatic catalysis, and biomineralization.
1. Introduction
Living systems have evolved the ability to produce materials—from bacterial biofilms to skeletal tissue—that can respond to environmental cues, self‐regenerate, and dynamically alter their physical properties. There is great potential for the development of engineered living materials (ELMs) with these same desirable attributes.[ 1 , 2 , 3 ]
Organisms such as bacteria and yeasts can be genetically programmed to execute sensing, computing, memory, and response functions.[ 4 ] Many approaches have been explored for producing ELMs by incorporating these living, programmable units into human‐made materials.[ 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 ] ELMs have also been fabricated using natural polymers harvested from microbes.[ 15 , 16 , 17 , 18 , 19 , 20 , 21 ] But unlike the living materials found in nature, these ELMs require specialized labor and instrumentation to assemble the biomaterial into the desired architecture. To more closely replicate the living materials found in nature, ELMs should be fabricated via self‐organization, with the cells simultaneously producing the material and imbuing it with genetically programmable functionality.
One notable example of bulk material production from living cells is bacterial cellulose (BC). When grown in sugar‐rich medium, various species of Gram‐negative acetic acid bacteria can produce extracellular cellulose with possible yields of >10 g L−1 after a few days of growth.[ 22 ] This is often in the form of a thick, floating mat—known as a pellicle—that forms atop a static liquid culture, growing at the air–water interface. This pellicle consists of a dense network of cellulose fibrils—each ≈50 nm wide and up to 9 µm in length—in which the BC‐producing bacteria are embedded.[ 23 ]
BC is both highly pure and highly crystalline, which affords its excellent mechanical properties, including a Young's modulus of 114 GPa for an individual fibril.[ 24 ] It is biocompatible and has a high capacity for water retention, which, along with its purity, has made it an attractive material for wound and surgical dressings. Furthermore, it is biodegradable and can be mass‐produced with little environmental impact. These properties have inspired a wide range of applications including wound dressings,[ 25 ] scaffolds for tissue engineering,[ 26 , 27 , 28 ] electronic paper displays,[ 29 ] substrates for enzyme immobilization,[ 30 , 31 ] and acoustic diaphragms for speakers.[ 32 ] The high cost of culture media has heretofore limited large‐scale industrialization of BC production. But, ongoing efforts to improve yields and lower costs through optimization of fermentation methods, use of industrial wastes as feedstocks, and metabolic engineering of BC‐producing organisms are promising.[ 33 , 34 ]
Several methods have been developed to better control the shape and improve the functionality of BC materials. Greca et al. exploited the tendency of BC‐producing bacteria to synthesize cellulose at the air–water interface to produce highly customizable 3D structures.[ 35 ] Song et al. utilized this same principle to develop an emulsion‐based technique for high‐throughput production of BC capsules across a wide range of sizes.[ 36 ] Caro‐Astorga et al. developed a protocol for producing millimeter‐scale BC spheroids which could be used as building blocks for patterning and repairing BC materials.[ 37 ]
Additionally, several groups have developed methods for genetically engineering BC‐producing bacteria for a range of applications. Florea et al. genetically engineered a BC‐producing strain so that cellulose production and red fluorescent protein (RFP) production could be controlled by the addition of a small molecule inducer, which allowed for spatial patterning of the cellulose pellicle.[ 38 ] Walker et al. genetically engineered “sender” and “receiver” strains of a BC‐producing bacteria to achieve boundary detection in a fused pellicle.[ 39 ] Teh et al. characterized a toolkit of synthetic biology parts in three different BC‐producing strains and demonstrated knockdown of a gene necessary for BC biosynthesis using clustered regularly interspaced short palindromic repeats interference (CRISPRi).[ 40 ] But even with encouraging early efforts in this area, the potential functionality of smart ELMs composed of BC is limited by our ability to engineer BC‐producing bacteria to secrete recombinant proteins and sense specific external cues.
By coculturing BC‐producing bacteria with other microorganisms, it is possible to significantly expand the capabilities of BC‐based ELMs. Das et al. cocultured BC‐producing bacteria with the photosynthetic microalgae Chlamydomonas reinhardtii in a symbiotic consortium wherein the oxygen produced by C. reinhardtii allowed for increased cellulose production away from the air–water interface.[ 41 ] Recently, Gilbert et al. developed a coculture system in which the model eukaryote Saccharomyces cerevisiae is stably maintained among BC‐producing bacteria during cellulose production. The yeast provides an engineerable host cell within the growing material that can be rationally programmed at the genetic level for dedicated tasks.[ 42 ] This colocalization of different cell types with specialized functions is common in naturally occurring living materials and could offer their engineered counterparts a comparable level of versatility and robustness.
In this work, we have developed a novel platform in which a BC‐producing strain of bacteria (Gluconacetobacter hansenii (G. hansenii)) is cocultured with various engineered strains of Escherichia coli (E. coli). By incorporating the engineered E. coli within the BC material, we significantly expand the functionality of BC‐based ELMs by exploiting some of the many genetic tools developed for E. coli. Our approach utilizes the BC‐producing G. hansenii as a primary “material factory,” while the engineered E. coli provide genetically encoded functionality. Through simple steps of incubation and media exchange, we produce BC capsules containing dense colonies of engineered E. coli that can respond to environmental cues. We show that the encapsulated E. coli can provide functionality by expressing engineered curli nanofibers, yielding hybrid capsules capable of biomolecule sequestration and enzymatic catalysis. Additionally, we demonstrate the ability of the engineered capsules to modulate their own bulk physical properties via urease‐induced biomineralization.
2. Results
2.1. Biological Fabrication of BC Capsules Containing Engineered E. coli
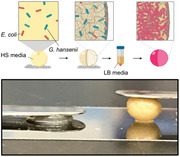
We chose to produce the BC material as a hollow spherical capsule rather than the more conventional pellicle format in the hopes of creating a material with topologically distinct regions, with G. hansenii primarily occupying the “outer” shell composed of a dense cellulose matrix, and E. coli occupying the “inner” core. To produce the BC capsules, we inoculated Hestrin–Schramm (HS) media with G. hansenii and incubated droplets of the culture on a bed of superhydrophobic polytetrafluoroethylene (PTFE) powder, as previously described.[ 35 ] After several days of incubation these droplets became robust BC capsules due to cellulose production by G. hansenii at the air–water interface. To produce capsules containing engineered E. coli, we inoculated the HS media with both G. hansenii and E. coli. Then, following the incubation in HS, the newly formed capsules were transferred to lysogeny broth (LB) media (containing antibiotic to select for engineered E. coli) and incubated under shaking conditions to proliferate E. coli inside the capsules (Figure 1A). We note that the G. hansenii are not engineered for antibiotic resistance, so are not expected to survive the incubation in LB. The HS media pH was lowered from 5.9 to 5 using citric acid because a preliminary analysis showed that E. coli survived, but did not proliferate, and that cellulose production from G. hansenii was strong at this pH level (Figure S1, Supporting Information).
Figure 1.
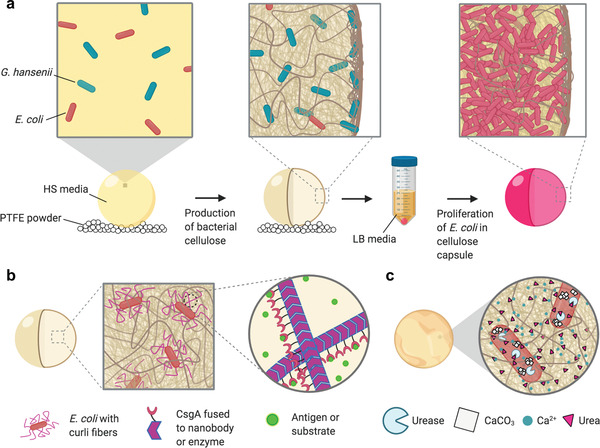
Production of programmable hybrid living materials from bacterial coculture. a) Schematic showing coculture in HS media leading to production of bacterial cellulose, followed by transfer to LB media for selective proliferation of E. coli inside the BC capsule. b) Schematic showing functionalization of the capsule via production of engineered curli fibers by the encapsulated E. coli. c) Schematic showing biomineralization of the capsule through production of urease by the encapsulated E. coli leading to CaCO3 crystal growth in the presence of urea and Ca2+.
To clearly demonstrate the programmable function of the encapsulated E. coli, we used the E. coli strain BL21 containing the plasmid pBbA8k‐RFP encoding arabinose‐inducible expression of mRFP1.[ 43 ] After the overnight incubation in LB, the capsules inoculated with E. coli had a more opaque appearance compared to the capsules without E. coli (Figure 2A) due to the growth of E. coli within the capsules. When incubated in LB with the addition of 0.1% w/w arabinose, the capsules exhibited a bright red appearance due to the production of RFP in the engineered E. coli (Figure 2A). This demonstrated that the plasmid was functional in the encapsulated E. coli, while also allowing us to clearly visualize the growth of E. coli colonies inside the capsules.
Figure 2.
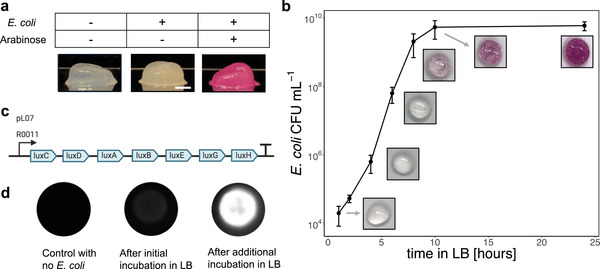
BC capsules containing a high concentration of programmable living E. coli. a) Images of BC capsules with and without engineered E. coli. The addition of arabinose during the LB incubation step induced RFP expression in the E. coli. Scale bar = 2 mm. b) Growth curve of encapsulated E. coli generated by degrading capsules after various incubation times in LB and plating on selective LB‐agar to quantify CFU. Data on E. coli cell density within the capsules is accompanied by images of the BC capsules containing RFP‐expressing E. coli. Values and error bars reflect the mean ± standard deviations (s.d.) of three biological replicates (n = 3). c) Plasmid pLO7 encodes constitutive expression of the luxCDABEGH operon. d) Capsules containing E. coli transformed with pLO7 were stored in PBS overnight following the initial incubation in LB. Some capsules were then incubated for an additional 2 h in fresh LB.
We tracked the progression of E. coli growth inside the capsules by visual inspection during the incubation in LB (Figure 2B). After 6 h, small E. coli colonies became very faintly visible. After 8 h, many more colonies were visible, with some appearing to be located near the outer wall of the capsule and others appearing to be located in the core of the capsule. The core colonies often took the form of a ring, possibly due to the rotational motion of the shaking incubator. After 10 and 24 h, the colonies became larger and more numerous, eventually covering most of the capsule surface. We also measured the growth rate of the E. coli during the incubation in LB by degrading the capsules through mechanical homogenization and cellulase treatment, and plating the degradant on selective LB‐agar to quantify E. coli colony‐forming units (CFU) inside the capsules (Figure 2B). The resulting growth curve showed that the encapsulated E. coli grew to a high density, close to 1010 CFU mL−1, after the overnight incubation in LB.
We wanted to test whether the encapsulated E. coli were capable of further activity when the media were replenished. To demonstrate that the encapsulated E. coli were metabolically active, we used the plasmid pLO7, which contained a constitutively expressed luxCDABEGH operon from the marine bacterium V. harveyii (Figure 2C). The luminescent reaction, unlike a fluorescent protein, requires constant cellular energy, so can only be maintained while the E. coli are in a metabolically active state.[ 44 ] After the initial overnight incubation in LB, capsules containing E. coli with pLO7 showed a weak luminescence signal, likely due to the cells entering stationary phase. The capsules were then stored in phosphate buffered saline (PBS) overnight to maintain their hydrated state without providing nutrients to the cells. The next day, the capsules were incubated in fresh LB for two additional hours, during which the capsules became strongly luminescent, indicating that the encapsulated E. coli were viable and capable of quickly entering a metabolically active state after a nutrient‐free storage period (Figure 2D).
Additionally, we found that the capsules usually produced a turbid culture during the incubation in LB, presumably due to E. coli cells escaping the cellulose matrix. To test the efficiency of E. coli encapsulation within the cellulose matrix following the growth period in LB, we agitated capsules containing BL21/pBbA8k‐RFP cells in PBS for 24 h and found that ≈95% of the E. coli cells were retained in the capsule, while the remaining 5% leaked into the PBS supernatant (Figure S1, Supporting Information).
2.2. Coculture Dynamics during Capsule Production
The bright red appearance of the RFP‐expressing E. coli colonies allowed us to visually observe the patterns of E. coli growth inside the capsules. We found that both the extent of E. coli growth during the incubation in LB and the permeability of the capsules varied considerably depending on at least two factors: the initial inoculation of G. hansenii and the duration of incubation in HS media before transfer to LB.
To assess the influence of these factors on E. coli growth, we synthesized capsules where the volume of week‐old G. hansenii starter culture used to inoculate the coculture was varied between 5% and 20% of the total coculture volume, and the incubation time in HS was varied between 2 and 6 d. Given a 5% inoculation of G. hansenii, the E. coli survived well during the incubation in HS, as evidenced by the growth of many E. coli colonies during the incubation in LB (Figure 3A). When the inoculation volume was increased to 10%, the survivability of the E. coli was reduced, with capsules showing no evidence of E. coli growth after 6 d of incubation in HS. When the inoculation volume was increased further to 20%, the decrease in E. coli survival was even more apparent, with no E. coli colonies visible in capsules incubated for more than 3 d in HS. For all three inoculations, when the incubation in HS was only 2 d long, the capsules were noticeably less robust, and their liquid contents quickly leaked out when placed on a glass slide.
Figure 3.
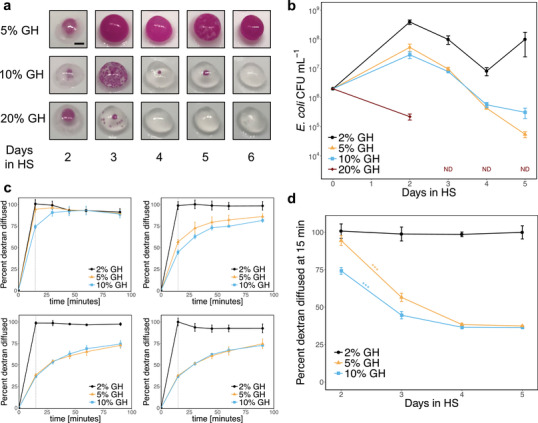
Effects of coculture parameters on E. coli survival and capsule permeability. GH refers to the relative volume of G. hansenii starter culture added to the HS coculture. a) Images of BC capsules containing RFP‐expressing E. coli. Scale bar = 2 mm. b) Survival of E. coli in capsules during the coculture in HS. After the incubation in HS, the capsules were degraded and plated on selective LB‐agar to quantify E. coli CFU. ND means that E. coli was not detected. The limit of detection for the assay was 103 CFU mL−1. Values and error bars reflect the mean ± s.d. of three biological replicates (n = 3). c) Diffusion of 500 kDa, FITC‐conjugated dextran out of the capsules. HS cocultures were supplemented with 0.5 mg mL−1 dextran and capsules were transferred to PBS after the HS incubation period. Permeability was assessed by measuring the FITC fluorescence of the surrounding solution over time. Top left: 2 d in HS. Top right: 3 d in HS. Bottom left: 4 d in HS. Bottom right: 5 d in HS. Values and error bars reflect the mean ± s.d. of three biological replicates (n = 3). Dotted lines indicate 15 min time point. d) Rate of dextran diffusion. The initial rate of dextran diffusion out of the capsules was estimated using the fluorescence of the supernatant after the capsules were incubated in PBS for 15 min. Values and error bars reflect the mean ± s.d. of three biological replicates (n = 3). For capsules inoculated with 5% or 10% G. hansenii, the amount of dextran which had diffused out after 15 min decreased significantly as HS incubation time was increased from 2 to 3 d (two‐sided Student t‐test for two means, P‐value = 0.00011 for 5% G. hansenii capsules, P‐value = 0.00010 for 10% G. hansenii capsules). *** indicates P‐value < 0.001.
To better track E. coli survival during the coculture in HS, we degraded the capsules prior to incubation in LB and plated the degradant on selective media to quantify E. coli CFU. For this experiment, the inoculation of week‐old G. hansenii starter culture was varied between 2% and 20% of the total coculture volume. Given a 5% or 10% inoculation of G. hansenii, the E. coli CFU grew by about an order of magnitude after 2 d in HS, then consistently decreased thereafter (Figure 3B). When the inoculation volume was increased to 20%, the E. coli CFU decreased by about an order of magnitude after 2 d in HS, and then decreased below detectable levels thereafter. In contrast, when the inoculation volume was lowered to 2%, E. coli CFU increased by about two orders of magnitude after 2 d in HS, then decreased for the next 2 d, before increasing again after 4 d.
Both experiments indicated that increasing the G. hansenii inoculation tends to decrease the number of surviving E. coli available to colonize the capsule upon incubation in LB. This suggests that the two microorganisms compete for resources during the coculture in HS media. It is also possible that other characteristics of the G. hansenii starter culture, such as the pH or the presence of metabolic byproducts, contribute to the inhibition of E. coli growth at the higher inoculations of G. hansenii. For most of the G. hansenii inoculations tested, the number of surviving E. coli also tended to decrease with longer incubation times in HS. However, when the G. hansenii inoculation was lowered to 2% in the second experiment, the E. coli concentration rebounded on the fifth day in HS. This could be explained by the lower inoculation volume preventing G. hansenii from dominating the culture, leading to more favorable media conditions for the E. coli to resume growth. These results reflect the importance of the initial inoculations of the two bacteria on the coculture dynamics.
Next, to evaluate the effects of the coculture dynamics on the permeability of the capsules, we produced capsules in which the HS media were inoculated with 0.5 mg mL−1 fluorescein isothiocyanate (FITC)‐conjugated, 500 kDa dextran, in addition to G. hansenii and E. coli, and then measured the rate at which the dextran diffused out of the capsules. The inoculation of G. hansenii was varied between 2% and 10% of the total coculture volume. After the incubation in HS the capsules were transferred to 5 mL PBS, and the rate at which dextran diffused out was measured by monitoring the fluorescence of the surrounding solution (Figure 3C). For capsules inoculated with 5% or 10% G. hansenii, the amount of dextran which had diffused out after 15 min decreased significantly as HS incubation time was increased from 2 to 5 d (Figure 3D), likely because of a denser cellulose matrix. In contrast, when the G. hansenii inoculation was 2%, the dextran completely diffused into the PBS supernatant within 15 min even after 5 d of incubation in HS. This could indicate that cellulose production in these capsules was hindered by the relatively low inoculation of G. hansenii. In all cases, the dextran completely diffused out of the capsules within several hours, demonstrating their permeability to a wide size range of biological molecules.
2.3. Internal Structure of BC Capsules Containing Engineered E. coli Colonies
To better understand the interactions between the cellulose matrix produced by G. hansenii and the engineered E. coli, we imaged thin sections of the capsules using fluorescence microscopy (Figure 4A–C). The cellulose matrix was stained with Calcofluor White (CFW), a blue fluorescent dye which binds cellulose, while the E. coli fluoresced due to RFP expression. To maintain the 3D structure of the mostly hollow samples, including the shape and distribution of the E. coli colonies, we equilibrated the capsules in gelatin then fixed them in paraformaldehyde (PFA) prior to cryosectioning.
Figure 4.
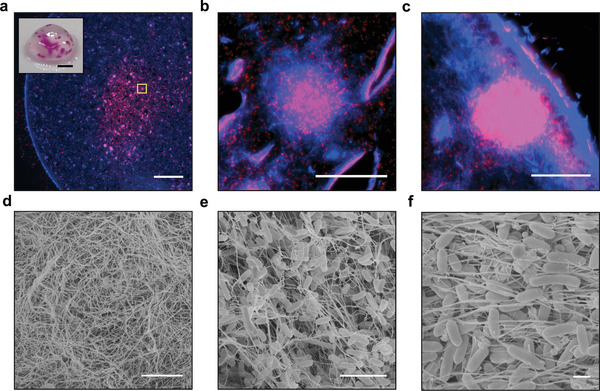
Internal structure of BC capsules containing engineered E. coli. Panels (a)–(c) show 8 µm cross‐sections of a capsule prestained with cellulose stain Calcofluor White (CFW). CFW fluorescence is shown in blue and RFP fluorescence is shown in red. a) A large region of E. coli growth is visible in the core of the capsule. The outer wall of the capsule is visible as a bright blue band. Scale bar = 500 µm. Inset: A photograph of the capsule taken before staining with CFW; scale bar = 2 mm. b) Zoomed‐in view of the region in the yellow box in panel (a). Scale bar = 50 µm. c) View of an internal E. coli colony located against the outer wall of the capsule. Scale bar = 50 µm. Panels (d)–(f) show FESEM images of capsules. d) View of the outer wall of the capsule, showing a dense network of cellulose fibers. Scale bar = 5 µm. e) View of encapsulated E. coli enmeshed within the internal cellulose matrix. Scale bar = 5 µm. f) Another view of internal, encapsulated E. coli densely packed together. Scale bar = 1 µm.
Visual inspection of the capsule prior to sectioning showed both a large internal colony and several smaller colonies along the outer wall (Figure 4A, inset). Imaging of the sections revealed that the large internal colony was not homogenous in cell density but was comprised of many smaller, highly concentrated colonies connected by more diffuse regions of E. coli (Figure 4A). At higher magnification, we found that the highly concentrated pockets of E. coli corresponded to regions of high cellulose fiber density (Figure 4B).
Imaging of the sections also revealed an E. coli colony located along the outer wall of the capsule (Figure 4C). This likely corresponds to one of the colonies forming dots on the surface of the capsule. The dense outer wall of the capsule, visible as a bright blue band roughly 20 µm thick, seemed to mostly contain this concentrated pocket of E. coli. The localization of both this E. coli colony and the internal colony in regions dense with cellulose fibers suggests that the E. coli may use the cellulose matrix as a solid substrate for growth within the capsule.
Additionally, we noted that the preparation and sectioning of the capsules led to the formation of apparent “rips” in the imaged sections. These are likely artifacts of the sample preparation and cryosectioning procedures, possibly due to nonuniform embedding in the gelatin or tearing of the tissue during sectioning. We noted that the gelatin‐embedding procedure did not affect the visual appearance of the capsules, preserving the macroscopic features of the cellulose sphere. As such, we felt this technique adequately captured the spatial distribution of the dense bacterial populations within the capsules.
To further elucidate the structure of the capsules, we performed field emission scanning electron microscopy (FESEM) analysis on capsules cut open with a scalpel. Images of the exterior of the capsule showed the dense but porous nature of the outer cellulose wall (Figure 4D). Images of the capsule interior showed E. coli growing along the cellulose matrix (Figure 4E) and in tightly packed conformations (Figure 4F).
2.4. Functional Hybrid Capsules Containing Engineered Curli Nanofibers
To demonstrate the potential functionality of the capsules, we produced hybrid capsules containing engineered curli nanofibers displaying various functional protein domains. Curli are insoluble and robust functional protein fibers anchored to the surface of E. coli cells, whose versatility as a platform for ELMs has been explored in many applications, including adhesion to abiotic surfaces,[ 16 ] in vivo display of therapeutic domains,[ 45 ] and sequestration of pathogens from drinking water,[ 46 ] among others.[ 47 ] We hypothesized that we could add a range of functionality to the capsules by expressing curli‐tethered proteins, which would remain fixed within the BC matrix while interacting with the external solution.
First, we attempted to use the capsules to sequester specific proteins from surrounding solution. We used E. coli engineered to express curli fibers in which the major curli subunit CsgA was fused to a single‐domain antibody (sometimes known as a “nanobody”) specific for green fluorescent protein (NbGFP) (Figure 1B).[ 48 ] Plasmid pBbB8k‐csg‐NbGFP, which contained an arabinose‐inducible synthetic curli operon csgBACEFG with CsgA fused to NbGFP via a flexible linker, was transformed into curli knockout strain PQN4 (Figure 5A). When curli production was induced, the NbGFP‐expressing capsules sequestered over 1 µg of GFP per capsule from a purified GFP solution (Figure 5B). When the capsules contained curli fibers displaying an alternate antibody domain NbStx2, which is specific Shiga toxin 2,[ 49 ] GFP was not sequestered, demonstrating the specificity and efficacy of NbGFP.
Figure 5.
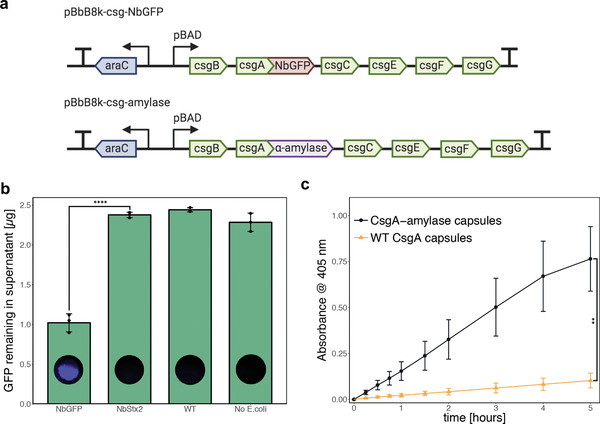
Functional hybrid capsules containing engineered curli nanofibers. a) Plasmids encoding arabinose‐inducible expression of engineered curli fibers. b) Bar graph showing green fluorescent protein (GFP) levels in the supernatant after overnight incubation of capsules in 0.5 mL of purified GFP solution (4.6 µg mL−1 in PBS). Values and error bars reflect the mean ± s.d. of three biological replicates (n = 3). When the capsules contained curli fibers displaying a nanobody domain specific for GFP, there was a significant reduction in the GFP concentration (NbGFP vs NbStx2, two‐sided Student t‐test for two means, P‐value = 3.9 × 10−5). **** indicates P‐value < 0.0001. After the incubation in GFP solution, the capsules were washed and imaged for GFP fluorescence. c) Time course of α‐amylase activity in capsules, measured by incubation of the capsules in 0.5 mL of solution containing the chromogenic substrate 4‐nitrophenyl α‐d‐maltohexaoside (3 mg mL−1 in PBS), which produces a yellow solution upon cleavage by α‐amylase. Values and error bars reflect the mean ± s.d. of three biological replicates (n = 3). Capsules containing α‐amylase‐displaying curli fibers exhibited significantly more enzyme activity than capsules containing wild‐type curli fibers (comparison of values at 5 h time point, two‐sided Student t‐test for two means, P‐value = 0.0031). ** indicates P‐value < 0.01.
As another demonstration of capsule functionality, we encapsulated E. coli cells in which CsgA was fused to a model enzyme: α‐amylase from the soil bacterium Bacillus licheniformis. α‐Amylases catalyze the hydrolysis of starch molecules into smaller oligosaccharides. They have applications in the food, fermentation, textile, paper, detergent, and pharmaceutical industries.[ 50 ] α‐Amylase activity was assessed by incubating the capsules in the presence of a model substrate, 4‐nitrophenyl α‐d‐maltohexaoside, which produces a yellow product upon cleavage by α‐amylases.[ 51 ] We observed significantly more enzyme activity in capsules containing curli fibers with α‐amylase compared to capsules containing wild‐type CsgA curli fibers (Figure 5C). After 5 h of incubation in the presence of the model substrate, a single capsule's amylase activity was equivalent to roughly 400 mU of purified amylase (Figure S7, Supporting Information).
2.5. Programmable Biomineralization of Capsules
One hallmark of natural living materials is the ability to alter their own bulk physical properties. To demonstrate this ability in our system, we sought to produce capsules capable of biomineralization. To achieve this, we used a commercially available E. coli strain, HB101, transformed with the plasmid pBR322‐Ure, which contains the urease gene cluster from the soil bacterium S. pasteurii.[ 52 ] Urease catalyzes hydrolysis of urea, which leads to the formation of carbonate ions and an increase in pH. In the presence of soluble calcium ions, this leads to the precipitation of CaCO3 (Figure 1C).
When capsules containing HB101/pBR322‐Ure cells were incubated in growth media supplemented with urea and CaCl2, the levels of soluble calcium in the culture supernatant decreased significantly faster compared to cultures containing capsules with untransformed HB101 cells, presumably due to urease‐induced precipitation of CaCO3 (Figure S9, Supporting Information). Mechanical testing showed a change in the physical properties of the HB101/pBR322‐Ure capsules following the incubation in the urea and CaCl2‐containing media, as more force was required to compress the capsules (Figure 6A). We fit regression lines to these data and found that the average slope of the lines increased significantly after 1 and 2 h of incubation (Supporting Information, Figure S10, Supporting Information), indicating a significant increase in the stiffness of the capsules.
Figure 6.
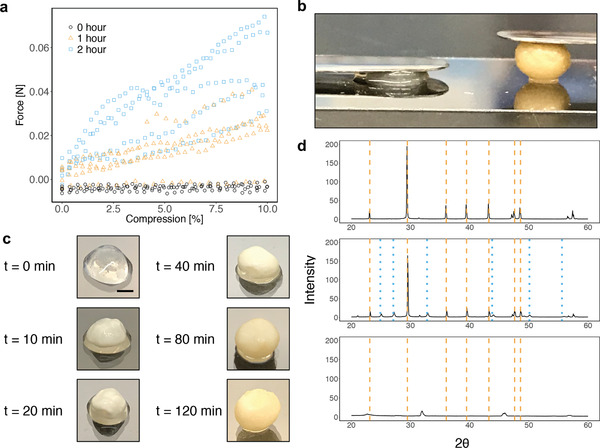
Biomineralization of capsules via urease expression in E. coli. a) Data from compression testing of capsules containing HB101 cells with plasmid pBR322‐Ure. The x‐axis shows the normalized distance between the compression plates where 0% represents the point of first contact between the capsule and top plate and 100% represents touching of the compression plates. Capsules were incubated in urea‐ and CaCl2‐containing media for 0, 1, or 2 h prior to testing. b) Image of capsules after an 8 h incubation in urea and CaCl2‐containing media. The left capsule contained HB101 cells with no plasmid and right capsule contained HB101 cells with plasmid pBR322‐Ure encoding the urease gene cluster from S. pasteurii. Each capsule has a 7 g stainless steel spatula resting on it. c) Images of HB101/pBR322‐Ure capsules incubated in urea and CaCl2‐containing media for various amounts of time. Scale bar = 2 mm. d) XRPD patterns. After the incubation in urea and CaCl2‐containing media, capsules were dried at room temperature and ground into a fine powder using a mortar and pestle prior to XRPD analysis. Top: precipitate from a culture of nonencapsulated HB101/pBR322‐Ure cells. Middle: Capsules containing HB101/pBR322‐Ure capsules. Bottom: Capsules containing HB101 with no plasmid. Dashed orange lines indicate peaks for calcite and dotted blue lines indicate peaks for vaterite.[ 53 , 54 ]
We also observed that the incubation led to a drastic change in the physical appearance of the HB101/pBR322‐Ure capsules (Figure 6B). We observed this mineralization process by imaging capsules at different time points after incubation in the urea and CaCl2‐containing media. We saw that the capsules changed in appearance quite rapidly, and took on a yellowish, pebble‐like appearance after only 2 h of incubation (Figure 6C). By comparison, the capsules containing E. coli without pBR322‐Ure did not change in appearance during such an incubation.
To confirm the presence of CaCO3 mineral forms in the yellowed capsules, we performed X‐ray powder diffraction analysis (XRPD). After the incubation in the urea and CaCl2‐containing media, the capsules were left to dry at room temperature and then ground into a fine powder using a mortar and pestle. As a control, we also analyzed precipitate from a culture of nonencapsulated HB101/pBR322‐Ure cells. The analysis showed that the precipitate from the nonencapsulated cell culture contained mostly calcite (Figure 6D). Interestingly, while the mineralized capsules indeed contained calcite, the XRPD profile also indicated the presence of vaterite, another CaCO3 mineral form. This suggests that the presence of the cellulose matrix influences the mineralization process.
3. Conclusion
We have developed an approach involving only simple microbial culture steps to produce robust, programmable materials containing a high concentration of engineered, living cells. Our coculture system leverages the strengths of two different microbes by enabling BC‐producing bacteria to produce a robust cellulose matrix colonized by programmable engineered E. coli. Since E. coli are known to have a much faster growth rate than G. hansenii, we first incubated the coculture in pH‐lowered HS media to suppress E. coli growth and encourage cellulose production from G. hansenii, then transferred the capsules to LB to proliferate the engineered E. coli. Our work significantly extends the capability of BC‐based materials by leveraging the extensive genetic engineerability of E. coli.
We were initially motivated to incubate the coculture as spherical droplets in the hopes that the dense cellulose matrix would prevent E. coli escape into the surrounding environment. Unfortunately, during the incubation in LB, the culture usually became turbid due to E. coli cells escaping from within the capsule. Despite this, the spherical shape of the capsules offers several advantages over the more conventional flat pellicle format. The dense outer wall of the capsule provides a protective barrier for the cells and other possible cargo contained within, while still allowing macromolecular diffusion. Others have also shown that relatively large cargo—such as nanoparticles larger than 10 nm—can be loaded into the capsules by inoculation into the initial coculture and be stably contained by the capsule wall thereafter.[ 35 ] Moreover, like other BC materials, the capsules are quite robust and can be easily handled and transported without breaking after several months of storage in a hydrated state at 4 °C.
The extensive genetic engineerability of E. coli facilitates the fabrication of living materials with many potential functions. In this work, we showed that robust BC capsules can be genetically programmed to respond to stimuli from the environment. We also used curli fiber‐based display to produce hybrid capsules containing engineered functional domains. Using this approach, we produced capsules containing single‐domain antibodies that could specifically sequester GFP from solution. Since BC is a robust, biocompatible material, these capsules could be used for the sequestration of specific biomolecules from a variety of complex biological samples. We also used curli fiber display to integrate α‐amylase, an industrially relevant enzyme used in ethanol production and many other applications, into the capsules. During the growth in LB, the CsgA‐enzyme fusion protein is secreted from E. coli and immobilized in the capsules through incorporation in cell‐anchored curli fibers, circumventing the need for costly protein purification and enzyme immobilization steps. Our approach is also highly adaptable. We demonstrate encapsulation of several different E. coli strains expressing recombinant proteins from a range of different organisms. Furthermore, since the capsules can respond to signals from their environment, they can be programmed for various functions and adapted to specific situations.
As highlighted above, we have demonstrated that the capsules can support immobilized enzymes for catalysis or antibody domains for sequestration. However, this platform would likely require significant development and optimization depending on the intended application. One limitation is the exposure of the genetically engineered E. coli to the environment, which precludes this technology from implementation in open environmental settings. Various biocontainment strategies have been developed which could circumvent this issue, including engineered auxotrophy,[ 55 ] adhesin‐mediated trapping,[ 56 ] and physical containment.[ 57 ] Alternatively, one could sterilize the capsules after production of the relevant functional protein. Another potential limitation of the system is the yield of secreted protein. Though the curli system offers certain advantages, such as multivalent display of the functional domain, overexpression of the curli operon causes a significant decrease in growth, limiting the amount of protein that can be secreted. However, our platform benefits from the many genetic tools for E. coli, so that other approaches such as cell surface protein display,[ 58 ] or the secretion of proteins tagged with cellulose‐binding domains,[ 59 ] could be used to functionalize the BC capsules.
The 3D BC capsules described here are produced spontaneously, under mild conditions, and through simple biological processes that largely occur autonomously. No exogenous polymers were added to the system to provide a template for growth or to reinforce the mechanical properties of the final product. The straightforward and economical fabrication of the capsules could be easily implemented in many facilities. Given the extensive set of well‐characterized genetic parts designed specifically for E. coli, we believe this work represents a significant step forward in the design and fabrication of functional BC materials and ELMs that mimic the autonomous structure building programs employed by natural living systems.
4. Experimental Section
Strains and Plasmids Used in This Study
Strains used in this study are listed in Table S1 in the Supporting Information. Plasmids used in this study are listed in Table S2 in the Supporting Information. All plasmids from this study were constructed using standard cloning techniques (polymerase chain reaction and one‐step isothermal Gibson assembly). Oligonucleotides were obtained from Integrated DNA Technologies (IDT). All plasmids were transformed into Mach1 (Invitrogen C862003) for amplification. Constructs were verified by Sanger sequencing (Genewiz).
Production of G. hansenii Starter Culture
G. hansenii colonies were obtained by streaking the strain 53582 from the American Type Culture Collection (ATCC) onto an HS‐agar plate. The streaked plate was incubated at 25 °C until rough‐edged colonies formed. One of these colonies was used to inoculate 10 mL liquid HS media. HS media are glucose‐rich and standard for the production of BC with bacteria. HS media were prepared with glucose (20 g L−1), yeast extract (5 g L−1), peptone (5 g L−1), Na2HPO4 (2.7 g L−1), citric acid (1.5 g L−1), and agar where required (15 g L−1). The culture was incubated for 7 d at 25 °C, forming a thick cellulose pellicle. To produce capsules, this starter culture was vortexed for 1 min to shake cells loose from the pellicle. The liquid portion of the vortexed culture was then used to inoculate fresh HS media for capsule production. This procedure generally yielded starter cultures with concentration 2–4 million CFU mL−1, prior to dilution into the final coculture.
Evaluation of Media pH on E. coli Survival and BC Production
Before defining a standard protocol for producing BC capsules with encapsulated E. coli, it was tested how the pH of the HS media would affect E. coli survival and BC production (Figure S1, Supporting Information). E. coli BL21 colonies were inoculated in LB (2 mL) in a test tube and grown overnight with agitation (Infors Multitron, 230 rpm) to produce starter cultures. The starter cultures were diluted to OD600 = 0.2 and then diluted 1/100 into HS media (10 mL) in a Falcon tube. G. hansenii starter cultures were prepared as described above and diluted 1/10 into the coculture. This was repeated using HS media across a range of pH values, with the pH of the HS media adjusted using citric acid (0.1 m, pH 1). Unadjusted HS media had a pH of 5.9. The coculture was then incubated at 25 °C for 5 d. The tube was thoroughly vortexed for 1 min and the liquid media underlying the pellicles was plated on selective LB‐agar to quantify the E. coli concentration. The BC pellicles were removed, washed overnight in NaOH (0.1 m), and washed overnight again in deionized water, before being allowed to air‐dry at room temperature. The dried pellicles were then weighed.
Standard Protocol for Production of BC Capsules with Encapsulated E. coli
HS media were adjusted to pH 5 with citric acid before inoculation with G. hansenii and E. coli starter cultures. The E. coli starter cultures were diluted to OD600 = 0.2 (≈200 million CFU mL−1) and then diluted 1/100 into the final co‐culture. G. hansenii starter cultures were prepared as described above and diluted 1/10 into the final co‐culture (unless otherwise specified). This results in a final inoculation ratio of roughly 10:1 E. coli to G. hansenii. The co‐culture was drop casted in 50 µL volumes onto a bed of PTFE powder, forming “liquid marbles.” These droplets were incubated at 25 °C in a humid environment for 3 d (unless otherwise specified) to produce cellulose capsules. A stainless‐steel spatula was used to transfer individual capsules to 50 mL Falcon tubes containing LB (5 mL) supplemented with antibiotic to selectively grow the engineered E. coli. It is noted that the G. hansenii are not engineered for antibiotic resistance, so are not expected to survive the incubation in LB. The LB and capsule were incubated overnight at 37 °C with agitation (230 rpm) for 18–24 h. Notably, in most cases, the LB would become turbid after the overnight incubation due to E. coli escaping from within the capsule.
Degradation of Capsules
The capsules were homogenized in sterile PBS (0.5 mL) at 50 Hz for 10 min using a 5 mm stainless‐steel ball bearing. Then, 30 µL of aqueous cellulase solution (Sigma‐Aldrich #C2730) was added to the mixture to reach a final concentration of 6% v/v cellulase, corresponding to a concentration of ≥ 42 units mL−1. The mixture was then incubated at 37 °C for 2 h. At 30 min intervals during the 2 h incubation period, the suspension was homogenized at 50 Hz for 2 min to accelerate degradation, including a final round of homogenization at the end of the 2 h period. The mixture containing the degraded capsule was then plated on selective LB‐agar to quantify E. coli concentration.
Measuring Diffusion Rate of Dextran Out of Capsules
To produce dextran‐containing capsules, HS media were supplemented with 0.5 mg mL−1 FITC‐labeled 500 kDa dextran (Sigma‐Aldrich #46947) in addition to being inoculated with the G. hansenii and E. coli starter cultures (BL21/pBbB8k‐RFP). The capsules were produced as described above, until the end of the incubation in HS, at which point each capsule was transferred to PBS (5 mL) in a 50 mL Falcon tube and incubated at 37 °C with agitation (230 rpm). 150 µL samples of the supernatant were taken at various time points and FITC fluorescence was measured on a BioTek Synergy Neo plate reader (485 nm excitation, 528 nm emission) to determine the kinetics of dextran diffusion from within the capsule into the surrounding PBS. Using a calibration curve of FITC‐labeled dextran in PBS, fluorescence measurements of the supernatant were used to calculate to the percentage of the total dextran added to the capsules which had diffused out of the capsules.
Cross‐Sectional Imaging of Capsule
A capsule was produced using the standard protocol described above with the strain BL21 and plasmid pBbB8k‐RFP. LB was supplemented with kanamycin (50 µg mL−1) and 0.1% w/w arabinose to induce mRFP1 expression. Following growth in LB, the capsule was washed three times in PBS (1 mL) to remove loosely attached cells. The capsule was then immersed in Calcofluor White Stain (Sigma‐Aldrich #18909) for 2 h to stain cellulose. The capsule was then embedded in gelatin following a previously established protocol.[ 60 ] This was done to maintain the 3D structure of the capsule throughout the optimal‐cutting temperature (OCT) compound embedding procedure. First, the stained capsule was incubated in 12.5% w/w sterile gelatin solution (2 mL, Sigma‐Aldrich #G2500) at 37 °C with agitation (230 rpm) in a 15 mL Falcon tube for 24 h. The capsule was then transferred to 25% (w/w) gelatin (2 mL) and incubated for another 24 h at 37 °C with agitation (230 rpm). The capsule was then incubated at 4 °C on a glass slide for 15 min to solidify the gelatin. The solidified capsule was then transferred to 25% w/w gelatin (2 mL) and incubated for 24 h at 37 °C with agitation (230 rpm). Finally, the capsule was fixed by immersion in 4% w/v PFA for 24 h at room temperature. The fixed capsule was then stored in PBS at 4 °C. 8 µm sections were obtained via OCT embedding and cryosectioning. The sections were imaged using an EVOS fluorescent microscope with a 4′,6‐diamidino‐2‐phenylindole (DAPI) light cube for CFW (357/44 nm excitation; 447/60 nm emission), and an RFP light cube for mRFP1 (531/40 nm excitation; 593/40 nm emission).
GFP Sequestration Assay
Capsules were produced using the standard protocol described above using the strain PQN4 and plasmids pBbB8k‐csg‐NbGFP, pBbB8k‐csg‐WT, or pBbB8k‐csg‐NbStx2. LB was supplemented with kanamycin (50 µg mL−1) and 0.001% w/w arabinose to induce expression of the curli operon. After the incubation in LB, the capsules were washed three times in PBS (1 mL) to remove loosely attached cells. The capsules were then added to purified GFP solution (0.5 mL, 4.6 µg mL−1 in PBS) and incubated for 24 h at 37 °C with agitation (800 rpm in Eppendorf Thermomixer R). Following this incubation, 150 µL samples of the supernatant were taken and GFP fluorescence was measured on a BioTek Synergy Neo plate reader (485 nm excitation, 528 nm emission) to determine the amount of GFP that had been sequestered inside the capsules. Fluorescence measurements were converted to GFP concentrations using a calibration curve of purified GFP in PBS. The capsules were then thoroughly washed three times in PBS (10 mL) for a total of 3 h and imaged for GFP fluorescence using a FluorChem M imager.
α‐Amylase Assay
Capsules were produced using the standard protocol described above with the strain PQN4 and plasmids pBbB8k‐csg‐amylase or pBbB8k‐csg‐WT. LB was supplemented with kanamycin (50 µg mL−1) and 0.001% w/w arabinose to induce expression of the curli operon. After the incubation in LB, the capsules were washed three times in PBS (1 mL) to remove loosely attached cells. The capsules were then transferred to 0.5 mL of solution containing the substrate 4‐nitrophenyl α‐d‐maltohexaoside (Sigma‐Aldrich #73681) at a concentration of 3 mg mL−1 in PBS. The mixture was then incubated at 37 °C with agitation (800 rpm in Eppendorf Thermomixer R). 150 µL samples of the supernatant were taken with replacement at various time points and absorbance at 405 nm was measured on a BioTek Synergy Neo plate reader to determine the kinetics of α‐amylase activity. The same procedure was conducted using various concentrations of purified α‐amylase (500–1500 U mg−1, Sigma‐Aldrich #A4551) to create a calibration curve (Figure S7, Supporting Information).
Biomineralization of Capsules
Capsules were produced using the standard protocol described above with the strain E. coli HB101 and plasmid pBR322‐Ure, or with no plasmid in the control. LB media were supplemented with carbenicillin (100 µg mL−1) and NiCl2 (5 × 10−6 m). After the incubation in LB, the capsules were stored overnight in PBS (0.5 mL) at 4 °C. Urea‐containing media were prepared with Difco Nutrient Broth (3 g L−1), NaHCO3 (2.12 g L−1), NH4Cl (20 g L−1), and urea (40 g L−1). To induce mineralization, capsules were incubated in urea‐containing media (5 mL) supplemented with NiCl2 (5 × 10−6 m) and CaCl2 (50 × 10−3 m) in a 50 mL Falcon tube at 37 °C with agitation (230 rpm). Soluble calcium levels in culture supernatant were measured using Calcium Colorimetric Assay kit (Sigma‐Aldrich #MAK022).
X‐Ray Powder Diffraction of Mineralized Capsules
After the incubation in urea‐containing media, the mineralized capsules were washed thoroughly three times in MilliQ water (10 mL) for a total of 3 h, dried at room temperature, and ground into a fine powder using a mortar and pestle. X‐ray diffraction was then carried out using a D2 PHASER (Bruker) with a Cu anode operating at 30 kV and 10 mA with a 2θ range of 20°–60°, step size of 0.02°, and exposure time of 1 s per step.
Field Emission Scanning Electron Microscope (FESEM) Sample Preparation
FESEM samples were prepared by fixing the capsules with 2% w/v glutaraldehyde and 2% w/v paraformaldehyde at room temperature, overnight. The capsules were gently washed with water and the solvent was gradually exchanged to ethanol with an increasing ethanol 15 min incubation step gradient (25%, 50%, 75%, and 100% v/v ethanol). The capsules were dried in a critical point dryer, placed onto SEM sample holders using silver adhesive (Electron Microscopy Sciences) and sputtered until they were coated in a 10–20 nm layer of Pt/Pd/Au. Images were acquired using a Zeiss Ultra55/Supra55VP FESEM equipped with a field emission gun operating at 5–10 kV.
Mechanical Tests for Capsules
Capsules were produced using the standard protocol described above with the strain E. coli HB101 and plasmid pBR322‐Ure, then stored in MilliQ water at 4 °C prior to testing. Compression measurements were made using a Discovery Series Hybrid Rheometer 3 (DHR‐3) instrument (TA Instruments, New Castle, DE, USA). Testing was performed under ambient lab conditions at 25 °C. A constant linear deformation of 10 µm s−1 was applied on the capsules till they were completely compressed in a parallel plate set up. A minimum of five tests were obtained for each condition.
Statistical Analysis
All values and error bars reflect the mean ± 1 standard deviation (s.d.) of three biological replicates (n = 3, unless specified otherwise). Where appropriate, optical measurements (i.e., fluorescence or absorbance) were transformed to absolute concentrations using calibration curves constructed with purified components of known concentration. All P‐values were generated from two‐sided Student t‐tests for comparing two means (n = 3, unless specified otherwise), which were computed using R statistical software. P‐values < 0.05 were used to determine significance.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supporting Information
Acknowledgements
This work was supported by the National Science Foundation (DMR 2004875). The authors would like to thank Suzanne White (Beth Israel Deaconess Medical Center) for cryosectioning, Dr. Emily Freed (University of Colorado Boulder) for providing the pBR322‐Ure plasmid, and Dr. Shao‐Liang Zhang (Harvard University) for XRPD analysis.
Birnbaum D. P., Manjula‐Basavanna A., Kan A., Tardy B. L., Joshi N. S., Hybrid Living Capsules Autonomously Produced by Engineered Bacteria. Adv. Sci. 2021, 8, 2004699. 10.1002/advs.202004699
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Chen A. Y., Zhong C., Lu T. K., ACS Synth. Biol. 2015, 4, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nguyen P. Q., Courchesne N. D., Duraj‐Thatte A., Praveschotinunt P., Joshi N. S., Adv. Mater. 2018, 30, 1704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilbert C., Ellis T., ACS Synth. Biol. 2019, 8, 1. [DOI] [PubMed] [Google Scholar]
- 4. Way J. C., Collins J. J., Keasling J. D., Silver P. A., Cell 2014, 157, 151. [DOI] [PubMed] [Google Scholar]
- 5. Van Tittelboom K., De Belie N., De Muynck W., Verstraete W., Cem. Concr. Res. 2010, 40, 157. [Google Scholar]
- 6. Wang J., Van Tittelboom K., De Belie N., Verstraete W., Constr. Build. Mater. 2012, 26, 532. [Google Scholar]
- 7. Gerber L. C., Koehler F. M., Grass R. N., Stark W. J., Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerber L. C., Koehler F. M., Grass R. N., Stark W. J., Angew. Chem. 2012, 124, 11455. [DOI] [PubMed] [Google Scholar]
- 9. Connell J. L., Ritschdorff E. T., Whiteley M., Shear J. B., Proc. Natl. Acad. Sci.U. S. A. 2013, 110, 18380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park S. J., Gazzola M., Park K. S., Park S., Di Santo V., Blevins E. L., Lind J. U., Campbell P. H., Dauth S., Capulli A. K., Pasqualini F. S., Ahn S., Cho A., Yuan H., Maoz B. M., Vijaykumar R., Choi J. W., Deisseroth K., Lauder G. V., Mahadevan L., Parker K. K., Science 2016, 353, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu X., Tang T.‐C., Tham E., Yuk H., Lin S., Lu T. K., Zhao X., Proc. Natl. Acad. Sci.U. S. A. 2017, 114, 2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schaffner M., Rühs P. A., Coulter F., Kilcher S., Studart A. R., Sci. Adv. 2017, 3, eaao6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moser F., Tham E., González L. M., Lu T. K., Voigt C. A., Adv. Funct. Mater. 2019, 29, 30. [Google Scholar]
- 14. Smith R. S. H., Bader C., Sharma S., Kolb D., Tang T., Hosny A., Moser F., Weaver J. C., Voigt C. A., Oxman N., Adv. Funct. Mater. 2020, 30, 1907401. [Google Scholar]
- 15. Chen A. Y., Deng Z., Billings A. N., Seker U. O. S., Lu M. Y., Citorik R. J., Zakeri B., Lu T. K., Nat. Mater. 2014, 13, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nguyen P. Q., Botyanszki Z., Tay P. K. R., Joshi N. S., Nat. Commun. 2014, 5, 4945. [DOI] [PubMed] [Google Scholar]
- 17. Dorval Courchesne N. M., Duraj‐Thatte A., Tay P. K. R., Nguyen P. Q., Joshi N. S., ACS Biomater. Sci. Eng. 2017, 3, 733. [DOI] [PubMed] [Google Scholar]
- 18. Nussbaumer M. G., Nguyen P. Q., Tay P. K. R., Naydich A., Hysi E., Botyanszki Z., Joshi N. S., ChemCatChem 2017, 9, 4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seker U. O. S., Chen A. Y., Citorik R. J., Lu T. K., ACS Synth. Biol. 2017, 6, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duraj‐Thatte A. M., Courchesne N. M. D., Praveschotinunt P., Rutledge J., Lee Y., Karp J. M., Joshi N. S., Adv. Mater. 2019, 31, 1901826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang J., Liu S., Zhang C., Wang X., Pu J., Ba F., Xue S., Ye H., Zhao T., Li K., Wang Y., Nat. Chem. Biol. 2019, 15, 34. [DOI] [PubMed] [Google Scholar]
- 22. Chawla P. R., Bajaj I. B., Survase S. A., Singhal R. S., Food Technol. Biotechnol. 2009, 47, 107. [Google Scholar]
- 23. Huang Y., Zhu C., Yang J., Nie Y., Chen C., Sun D., Cellulose 2014, 21, 1. [Google Scholar]
- 24. Hsieh Y.‐C., Yano H., Nogi M., Eichhorn S. J., Cellulose 2008, 15, 507. [Google Scholar]
- 25. Portela R., Leal C. R., Almeida P. L., Sobral R. G., Microb. Biotechnol. 2019, 12, 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Svensson A., Nicklasson E., Harrah T., Panilaitis B., Kaplan D. L., Brittberg M., Gatenholm P., Biomaterials 2005, 26, 419. [DOI] [PubMed] [Google Scholar]
- 27. Bodin A., Concaro S., Brittberg M., Gatenholm P., J. Tissue Eng. Regener. Med. 2007, 1, 406. [DOI] [PubMed] [Google Scholar]
- 28. Hickey R. J., Pelling A. E., Front. Bioeng. Biotechnol. 2019, 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shah J., Brown R. M., Appl. Microbiol. Biotechnol. 2005, 66, 352. [DOI] [PubMed] [Google Scholar]
- 30. Wu S. C., Lia Y. K., J. Mol. Catal. B: Enzym. 2008, 54, 103. [Google Scholar]
- 31. Kim H. J., Jin J. N., Kan E., Kim K. J., Lee S. H., Biotechnol. Bioprocess Eng. 2017, 22, 89. [Google Scholar]
- 32. Uryu M., Kurihara N. (Sony Corp ), U.S. Patent 5274199 , 1993.
- 33. Hussain Z., Sajjad W., Khan T., Wahid F., Cellulose 2019, 26, 2895. [DOI] [PubMed] [Google Scholar]
- 34. Andriani D., Apriyana A. Y., Karina M., Cellulose 2020, 27, 6747. [Google Scholar]
- 35. Greca L. G., Lehtonen J., Tardy B. L., Guo J., Rojas O. J., Mater. Horiz. 2018, 5, 408. [Google Scholar]
- 36. Song J., Babayekhorasani F., Spicer P. T., Biomacromolecules 2019, 20, 4437. [DOI] [PubMed] [Google Scholar]
- 37. Caro‐Astorga J., Walker K. T., Ellis T., (Preprint) bioRxiv , 2020, 10.1101/2020.05.11.088138. [DOI]
- 38. Florea M., Hagemann H., Santosa G., Abbott J., Micklem C. N., Spencer‐Milnes X., de Arroyo Garcia L., Paschou D., Lazenbatt C., Kong D., Chughtai H., Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walker K. T., Goosens V. J., Das A., Graham A. E., Ellis T., Microb. Biotechnol. 2019, 12, 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Teh M. Y., Ooi K. H., Danny Teo S. X., Bin Mansoor M. E., Shaun Lim W. Z., Tan M. H., ACS Synth. Biol. 2019, 8, 708. [DOI] [PubMed] [Google Scholar]
- 41. Das A. A., Bovill J., Ayesh M., Stoyanov S. D., Paunov V. N., J. Mater. Chem. B 2016, 4, 3685. [DOI] [PubMed] [Google Scholar]
- 42. Gilbert C., Tang T.‐C., Ott W., Dorr B. A., Shaw W. M., Sun G. L., Lu T. K., Ellis T., Nat. Mater. 2021, 1. [DOI] [PubMed] [Google Scholar]
- 43. Lee T., Krupa R. A., Zhang F., Hajimorad M., Holtz W. J., Prasad N., Lee S., Keasling J. D., J. Biol. Eng. 2011, 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Byers D. M., Bognar A., Meighen E. A., J. Bacteriol. 1988, 170, 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Praveschotinunt P., Duraj‐Thatte A. M., Gelfat I., Bahl F., Chou D. B., Joshi N. S., Nat. Commun. 2019, 10, 5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pu J., Liu Y., Zhang J., An B., Li Y., Wang X., Din K., Qin C., Li K., Cui M., Liu S., Huang Y., Wang Y., Lu Y., Huang J., Cui Z., Zhao S., Zhong C., Adv. Sci. 2020, 7, 1903558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li Y., Li K., Wang X., Cui M., Ge P., Zhang J., Qiu F., Zhong C., Sci. Adv. 2020, 6, eaba1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rothbauer U., Zolghadr K., Tillib S., Nowak D., Schermelleh L., Gahl A., Backmann N., Conrath K., Muyldermans S., Cristina Cardoso M., Leonhardt H., Nat. Methods 2006, 3, 887. [DOI] [PubMed] [Google Scholar]
- 49. Tremblay J. M., Mukherjee J., Leysath C. E., Debatis M., Ofori K., Baldwin K., Boucher C., Peters R., Beamer G., Sheoran A., Bedenice D., Tzipori S., Shoemaker C. B., Infect. Immun. 2013, 81, 4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Souza P. M., de Oliveira Magalhães P., Braz. J. Microbiol. 2010, 41, 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lorentz K., J. Clin. Chem. Clin. Biochem. 1983, 21, 463. [DOI] [PubMed] [Google Scholar]
- 52. Liang L., Heveran C., Liu R., Gill R. T., Nagarajan A., Cameron J., Hubler M., Srubar W. V. III, Cook S. M., ACS Synth. Biol. 2018, 7, 2497. [DOI] [PubMed] [Google Scholar]
- 53. RRUFF , Entry R040070 in RRUFF database, https://rruff.info/calcite/display=default/R040070 (accessed: October 2020).
- 54. Luo X., Song X., Cao Y., Song L., Bu X., RSC Adv. 2020, 10, 7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee J. W., Chan C. T., Slomovic S., Collins J. J., Nat. Chem. Biol. 2018, 14, 530. [DOI] [PubMed] [Google Scholar]
- 56. Guo S., Dubuc E., Rave Y., Verhagen M., Twisk S. A. E., van der Hek T., Oerlemans G. J. M., van den Oetelaar M. C. M., van Hazendonk L. S., Brüls M., Eijkens B. V., Joostens P. L., Keij S. R., Xing W., Nijs M., Stalpers J., Sharma M., Gerth M., Boonen R. J. E. A., Verduin K., Merkx M., Voets I. K., de Greef T. F. A., ACS Synth. Biol. 2020, 9, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tang T. C., Tham E., Liu X., Yehl K., Rovner A. J., Yuk H., Isaacs F. J., Zhao X., Lu T. K., (Preprint) bioRxiv , 2020, 10.1101/2020.02.11.941120. [DOI]
- 58. Salema V., Marín E., Martínez‐Arteaga R., Ruano‐Gallego D., Fraile S., Margolles Y., Teira X., Gutierrez C., Bodelón G., Fernández L. Á., PLoS One 2013, 8, e75126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gao D., Wang S., Li H., Yu H., Qi Q., Microb. Cell Fact. 2015, 14, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hruschka V., Meinl A., Saeed A., Al Ghanami R. C., Redl H., Shakesheff K., Wolbank S., Biomed. Mater. 2013, 8, 041001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


