Abstract
Objective
To determine the efficacy and safety of dalteparin postoperative bridging treatment versus placebo for patients with atrial fibrillation or mechanical heart valves when warfarin is temporarily interrupted for a planned procedure.
Design
Prospective, double blind, randomised controlled trial.
Setting
10 thrombosis research sites in Canada and India between February 2007 and March 2016.
Participants
1471 patients aged 18 years or older with atrial fibrillation or mechanical heart valves who required temporary interruption of warfarin for a procedure.
Intervention
Random assignment to dalteparin (n=821; one patient withdrew consent immediately after randomisation) or placebo (n=650) after the procedure.
Main outcome measures
Major thromboembolism (stroke, transient ischaemic attack, proximal deep vein thrombosis, pulmonary embolism, myocardial infarction, peripheral embolism, or vascular death) and major bleeding according to the International Society on Thrombosis and Haemostasis criteria within 90 days of the procedure.
Results
The rate of major thromboembolism within 90 days was 1.2% (eight events in 650 patients) for placebo and 1.0% (eight events in 820 patients) for dalteparin (P=0.64, risk difference −0.3%, 95% confidence interval −1.3 to 0.8). The rate of major bleeding was 2.0% (13 events in 650 patients) for placebo and 1.3% (11 events in 820 patients) for dalteparin (P=0.32, risk difference −0.7, 95% confidence interval −2.0 to 0.7). The results were consistent for the atrial fibrillation and mechanical heart valves groups.
Conclusions
In patients with atrial fibrillation or mechanical heart valves who had warfarin interrupted for a procedure, no significant benefit was found for postoperative dalteparin bridging to prevent major thromboembolism.
Trial registration
Clinicaltrials.gov NCT00432796.
Introduction
Uncertainty remains as to whether patients with atrial fibrillation or mechanical heart valves who require interruption of vitamin K antagonists for invasive procedures benefit from bridging with low molecular weight heparin (LMWH) after the procedure.1 2 Many published and diverse protocols and guidelines have sought to address this common clinical problem, and the concern has always been that these patients are at high risk of thrombotic complications caused by a procoagulable state while off vitamin K antagonists.3 4
In the typical bridging strategy, warfarin is held for four or five days before the procedure and is resumed after the procedure, on the same day or one or two days later.4 5 6 7 LMWH is given once or twice a day at a therapeutic dose for three to five days before the procedure and for a minimum of four days after the procedure until the international normalised ratio (INR) is therapeutic.4 5 6 7 The goal of bridging with LMWH is to reduce the risk of perioperative thromboembolism by minimising the time without anticoagulation while reducing bleeding risks with a short acting anticoagulant that allows for minimal or no anticoagulant effect at the time of interruption.8
Bridging has been most studied in patients with atrial fibrillation in several observational or single armed studies.9 A recently completed double blind placebo controlled trial suggested that LMWH bridging is not necessary before or after the procedure for patients on warfarin.10 However, patients with mechanical heart valves were usually excluded from such studies and patients with atrial fibrillation at high risk of stroke were not well represented.4 5 6 In a previous single arm, multicentre pilot study of dalteparin bridging given once daily to 224 patients with atrial fibrillation or mechanical heart valves, we found that the rate of thrombosis after the procedure was 3.1%; 75% of the events occurred in patients who had anticoagulation held due to a bleeding event after the procedure.6 These findings suggest that bridging after the procedure caused harm potentially because of a longer period without anticoagulation due to bleeding, which then led to thrombotic events.
Based on this information we performed a randomised, double blind, placebo controlled trial to assess the necessity of postoperative bridging with the LMWH dalteparin in patients with atrial fibrillation or prosthetic mechanical valves who required warfarin to be held for a procedure. We hypothesised that patients who would receive placebo after the procedure would have fewer bleeding events and a lower rate of thromboembolism because of the association of thromboembolism with bleeding in our pilot study.6 We chose to only study the LMWH dose after the procedure because the dose before the procedure was unlikely to be causing harm and might be of benefit.
Methods
Study design and oversight
PERIOP2 was a randomised, double blind, placebo controlled trial. The protocol was designed by the steering committee and approved by the institutional review board at each participating centre. The study was funded by the Canadian Institute of Health Research (CIHR-MCT79607) and had support from Pfizer in the form of the study drug. The trial was registered at ClinicalTrials.gov (NCT00432796). Pfizer did not have any role in the design or conduct of the study, analysis of the data, or preparation of the manuscript. The study drugs (dalteparin or placebo) were prepared in each local centre by pharmacists not involved in the patient’s care.
Patients
We used the same patient population as our pilot study.6 Patients were eligible to participate in the trial if they were aged 18 years or older and had mechanical heart valves (with or without atrial fibrillation), atrial fibrillation, or atrial flutter and a CHADS2 risk factor (previous transient ischaemic attack or stroke, high blood pressure, diabetes, age greater than 75, moderate or severe left ventricular dysfunction). Additionally, eligible patients were receiving long term anticoagulation with warfarin and required elective non-cardiac surgery or an invasive procedure in which the physician requested an absence of warfarin effect. Patients were excluded for any of the following reasons: active bleeding within 30 days before stopping warfarin; platelet count <100×109/L; spinal or neurosurgery; life expectancy less than three months; calculated creatinine clearance <30 mL/min; multiple mechanical valves or a Starr-Edwards valve, mechanical valve with history of stroke or transient ischaemic attack, or a history of heparin induced thrombocytopenia. All patients provided written informed consent.
Procedures
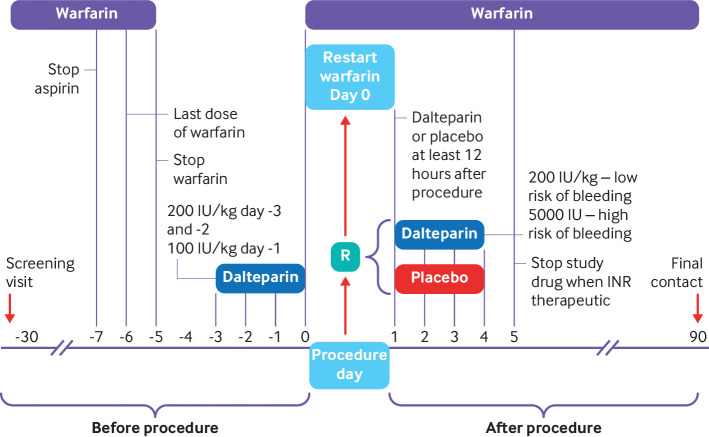
We chose this protocol because it was used in our pilot study.6 Warfarin was discontinued five days before the procedure (day 0); that is, five doses were held (fig 1). All patients were given dalteparin 200 IU/kg subcutaneously (a therapeutic dose) 72 and 48 hours before the procedure and 100 IU/kg 24 hours before the procedure. The maximum dose of dalteparin was 18 000 IU. Warfarin was resumed the evening of the procedure at twice the usual daily dose for the first day and then according to the INR result. After the procedure, eligibility was reconfirmed for all patients before randomisation, which occurred on day 0 or day +1. Patients were randomised to receive dalteparin or placebo, which started the day after the procedure, in the morning, at least 12 hours after the procedure. Patients considered at high risk of bleeding (cancer surgery (genitourinary, head and neck, intra-abdominal, breast), orthopaedic joint replacement, kidney or liver biopsy, prostate resection or biopsy, pacemaker insertion, abdominal hysterectomy, hernia repair, or vascular surgery; supplementary appendix S1) were given dalteparin at 5000 IU (a prophylactic dose) or placebo subcutaneously every day for at least four doses and until the INR was greater than 1.9. Patients who underwent procedures considered at low risk of bleeding (non-cancer abdominal surgery, cholecystectomy, haemorrhoidectomy, node dissection, vaginal hysterectomy, hand surgery, skin procedures, non-joint replacement orthopaedic surgery (shoulder, foot surgery, arthroscopic surgery), line insertion, endoscopic biopsies; supplementary appendix S1) received dalteparin at 200 IU/kg (maximum dose of 18 000 IU) subcutaneously or placebo for at least four days and until the INR was greater than 1.9. The final determination of high or low bleeding risk was left to the local investigator and attending physician. Patients on aspirin discontinued treatment seven days before the procedure and resumed treatment after the procedure at the discretion of the site investigator. Patients had daily contact with research staff while on study drugs and were assessed one week, four weeks, and 90 days after the procedure by telephone.
Fig 1.
PERIOP2 bridging study design. Screening visits occurred between 30 days and five days before planned procedure, and randomisation (R) occurred on the day of procedure (day 0) or within 24 hours of procedure (day+1) when haemostasis was achieved (no earlier than 30 minutes after procedure). INR=international normalised ratio
Study outcomes
We assessed study outcomes from the time of randomisation to 90 days, which was the same period as in our pilot study6 and was common practice at the time this study started in 2007. The primary efficacy outcome was major thromboembolism, which included any one of ischaemic stroke, transient ischaemic attack, myocardial infarction, peripheral arterial embolism, valve thrombosis, vascular death, or symptomatic acute venous thromboembolism (supplementary appendix). Secondary efficacy endpoints included the individual major thromboembolism outcomes and a composite outcome of major thromboembolism, or major bleeding (net clinical benefit). The primary safety outcome was major bleeding according to International Society on Thrombosis and Haemostasis (ISTH) criteria (supplementary appendix). The secondary safety outcomes were clinically relevant non-major bleeding and trivial bleeding (supplementary appendix). All study outcomes were independently and blindly adjudicated by an adjudicating committee that included a thrombosis expert, neurologist, and cardiologist. At the request of the reviewers, a 30 day post hoc secondary analysis of major thromboembolism, major thromboembolism or major bleeding, and major thromboembolism, major bleeding, or death was added.
Statistical analysis
The primary efficacy outcome was major thromboembolism within 90 days of the procedure. From our pilot study (that used the same inclusion and exclusion criteria) we expected 3.1% of patients in the control arm to have major thromboembolism (postoperative bridging).6 The minimum clinically important difference was a 2% absolute difference; that is, if two or more thromboembolic events occurred per 100 patients in one arm compared with the other, we would consider this outcome clinically meaningful. The 2% difference in event rates would translate into a mortality difference of 0.3% at 90 days, assuming that 15% of thromboembolic events were fatal.1 11 The null hypothesis was that the experimental intervention (no postoperative bridging) would have the same primary outcome event rate. We accepted a 5% chance of falsely rejecting the null hypothesis (two tailed α=0.05) and a 20% chance of falsely not rejecting the hypothesis (power=80%). To achieve these standards we required a sample size of 1612. In our previous study, 8% of patients did not receive the intended treatment (non-compliance, withdrawal, or lost to follow-up)6 and so to be conservative we assumed that an additional 2% would be lost to follow-up. Consequently, the sample size was increased by 10% to 1773. The study was stopped early because of funding limitations and declining enrolment due to the widespread use of direct oral anticoagulants rather than warfarin for atrial fibrillation.
Data were analysed by intention to treat. Patients who were lost to follow-up were censored from the time of their last known follow-up. Proportions of patients experiencing a primary outcome event were compared in the placebo and dalteparin groups by an unadjusted Fisher’s exact test of proportions. A priori we planned to analyse primary and secondary outcomes in subgroups of patients with prosthetic valves with or without atrial fibrillation and atrial fibrillation only. We planned two interim analyses (at 33% and 66% enrolment).
Patient and public involvement
There was no formal patient involvement in the design of this study because that was not common practice when this study was designed in 2005. Patients were informally involved in the design of this study due to their questioning of the need for LMWH injections as bridging treatment as part of their routine care. Quality of life assessments were not performed. We asked a member of the public to review the manuscript after the submission.
Results
Patients
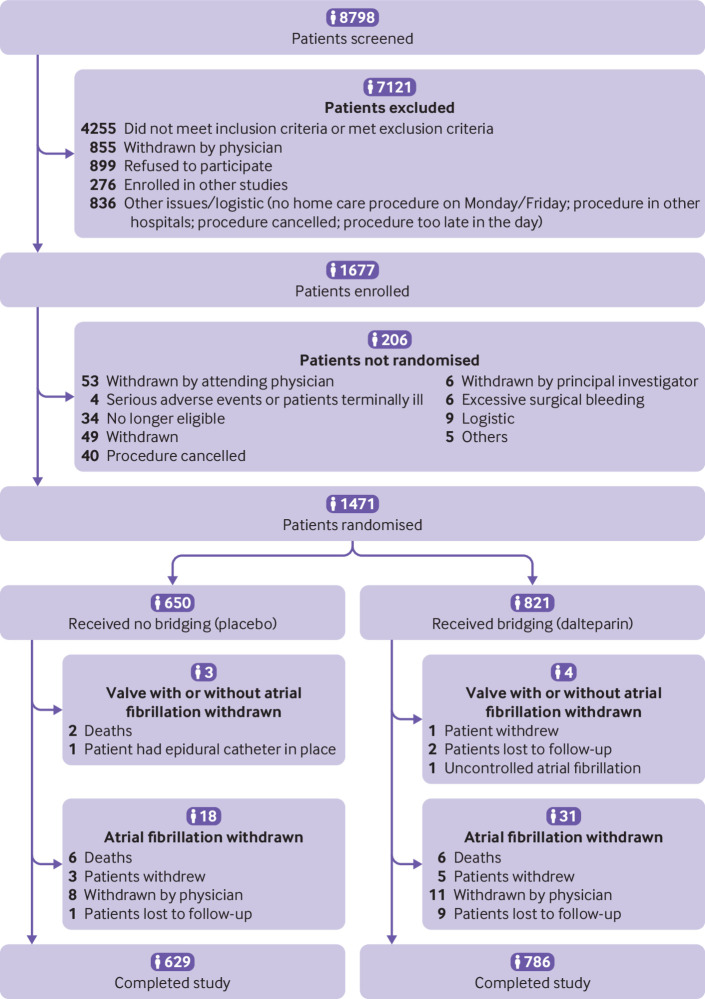
Figure 2 shows that 1677 patients provided informed consent between February 2007 and March 2016 at 10 sites in Canada and India. Subsequently 1471 patients were randomised after the procedure, 821 to dalteparin and 650 to placebo. Baseline patient characteristics were well balanced (table 1). The mean age was 69.7 years, 64.3% were men, 79.3% had atrial fibrillation, and 20.7% had mechanical valves (32.8% of patients with mechanical valves also had atrial fibrillation). One patient with a mechanical valve who was assigned to dalteparin withdrew consent immediately after randomisation before receiving the study drug. The average CHADS2 score was 2.42 and 41.2% had a score of 3.0 or higher. Aspirin was being taken by 24.5% of patients while 1.7% of patients took other antiplatelet agents. A wide range of procedures existed (supplementary appendix S2).
Fig 2.
Screening, randomisation, and follow-up of PERIOP2 bridging study
Table 1.
Baseline characteristics of entire PERIOP2 study population and subgroups. Data are numbers (%) unless indicated otherwise
| Characteristics | Total (n=1471) | Whole study population (n=1471) | Atrial fibrillation (n=1166) | Mechanical valve±atrial fibrillation (n=305) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No bridging (n=650) | Bridging (n=821) | P value | No bridging (n=496) | Bridging (n=670) | P value | No bridging (n=154) | Bridging (n=151) | P value | ||||
| Age (years), mean (SD) | 69.7 (12.3) | 69.2 (12.9) | 70.1 (11.9) | 0.19 | 73.1 (9.1) | 72.7 (9.2) | 0.45 | 56.6 (15.1) | 58.3 (15.1) | 0.32 | ||
| Male sex | 946 (64.3) | 428 (65.9) | 518 (63.1) | 0.27 | 346 (69.8) | 437 (65.2) | 0.10 | 82 (53.3) | 81 (53.6) | 0.94 | ||
| Weight (kg), mean (SD) | 85.9 (22.9) | 84.9 (22.5) | 86.6 (23.3) | 0.15 | 87.5 (21.5) | 89.1 (22.7) | 0.20 | 76.6 (23.6) | 75.5 (22.7) | 0.69 | ||
| Prosthetic mechanical valve | 305 (20.7) | 154 (23.7) | 151 (18.4) | 0.01 | — | — | — | — | — | — | ||
| Atrial fibrillation | 100 (32.8), n=305 | 47 (30.5), n=154 | 53 (35.1), n=151 | 0.39 | — | — | — | — | — | — | ||
| Mitral | 133 (43.6), n=305 | 68 (44.2), n=154 | 65 (43.1), n=151 | 0.85 | — | — | — | — | — | — | ||
| Aortic | 172 (56.4), n=305 | 86 (55.8), n=154 | 86 (57.0), n=151 | 0.85 | — | — | — | — | — | — | ||
| Tricuspid | 0, n=304 | 0, n=153 | 0, n=151 | — | — | — | — | — | — | |||
| Atrial fibrillation only | 1166 (79.3), n=1471 | 496 (76.3) | 670 (81.6), n=821 | 0.01 | — | — | — | — | — | — | ||
| Currently in atrial fibrillation* | 522 (45.4), n=1151 | 231 (47.0), n=492 | 291 (44.2), n=659 | 0.35 | — | — | — | — | — | — | ||
| Controlled with drugs (rate<100) | 995 (86.2), n=1154 | 415 (84.4), n=492 | 580 (87.6), n=662 | 0.11 | — | — | — | — | — | — | ||
| Intermittent* | 403 (35.0), n=1151 | 197 (40.0), n=492 | 206 (31.3), n=659 | 0.002 | — | — | — | — | — | — | ||
| CHADS2 (atrial fibrillation only), mean (SD) | 2.4 (1.2), n=1166 | 2.5 (1.2), n=496 | 2.4 (1.1), n=670 | 0.01 | — | — | — | — | — | — | ||
| Distribution | ||||||||||||
| 0 | 5 (0.4) | 2 (0.4) | 3 (0.5) | — | — | — | — | — | — | — | ||
| 1 | 260 (22.3) | 104 (21.0) | 156 (23.3) | — | — | — | — | — | — | — | ||
| 2 | 421 (36.1) | 166 (33.5) | 255 (38.1) | — | — | — | — | — | — | — | ||
| 3 | 276 (23.7) | 119 (24.0) | 157 (23.4) | — | — | — | — | — | — | — | ||
| 4 | 140 (12.0) | 73 (14.7) | 67 (10.0) | — | — | — | — | — | — | — | ||
| 5 | 54 (4.6) | 29 (5.9) | 25 (3.7) | — | — | — | — | — | — | — | ||
| 6 | 10 (0.9) | 3 (0.6) | 7 (1.0) | — | — | — | — | — | — | — | ||
| Congestive heart failure | 251 (17.1) | 121 (18.6) | 130 (15.8) | 0.16 | 98 (19.8) | 104 (15.5) | 0.06 | 23 (14.9) | 26 (17.2) | 0.59 | ||
| Left ventricular dysfunction | 77 (5.2), n=1469 | 38 (5.9) | 39 (4.8), n=819 | 0.35 | 33 (6.7) | 34 (5.1) | 0.26 | 5 (3.3) | 5 (3.3) | 1 | ||
| Hypertension | 1183 (80.4) | 507 (78.0) | 676 (82.3) | 0.04 | 424 (85.5) | 582 (86.9) | 0.50 | 83 (53.90) | 94 (62.3) | 0.14 | ||
| Diabetes mellitus | 444 (30.9) | 189 (29.1) | 255 (31.1) | 0.41 | 167 (33.7) | 221 (33.0) | 0.81 | 22 (14.3) | 34 (22.5) | 0.06 | ||
| Stroke | 170 (11.6) | 84 (12.9) | 86 (10.5) | 0.14 | 79 (15.3) | 85 (12.7) | 0.12 | 5 (3.3) | 1 (0.7) | 0.21 | ||
| Stroke or transient ischaemic attack | 330 (22.4) | 164 (25.2) | 166 (20.2) | 0.02 | 153 (30.9) | 162 (24.2) | 0.01 | 11 (7.1) | 4 (2.7) | 0.07 | ||
| Transient ischaemic attack | 190 (12.9) | 94 (14.5) | 96 (11.7) | 0.12 | 85 (17.4) | 92 (13.7) | 0.11 | 9 (5.8) | 4 (2.7) | 0.17 | ||
| Ischaemic heart disease | 450 (30.6) | 206 (31.7) | 244 (29.7) | 0.41 | 166 (33.5) | 210 (31.3) | 0.44 | 40 (26.0) | 34 (22.5) | 0.48 | ||
| Angina | 265 (18.0), n=1470 | 128 (19.7) | 137 (16.7), n=820 | 0.14 | 102 (20.6) | 118 (17.6) | 0.20 | 26 (16.9), n=154, | 19 (12.7), n=150, | 0.30 | ||
| Myocardial infarction | 243 (16.5), n=1470 | 110 (16.9) | 133 (16.2), n=820 | 0.72 | 95 (19.2) | 120 (17.9) | 0.59 | 15 (9.7) n=154 | 13 (8.7) n=150 | 0.75 | ||
| Coronary artery bypass graft | 157 (10.7) | 78 (12) | 79 (9.6) | 0.14 | 56 (11.3) | 57 (8.5) | 0.11 | 22 (14.3) | 22 (14.6) | 0.94 | ||
| Previous angioplasty | 90 (6.1), n=1470 | 44 (6.8) | 46 (5.6), n=820 | 0.36 | 38 (7.7) | 41 (6.1) | 0.30 | 6 (3.9), n=154 | 5 (3.3), n=150 | 0.79 | ||
| Previous coronary stent | 76 (5.12), n=1470 | 32 (4.9) | 44 (5.4), n=820 | 0.70 | 29 (5.9) | 40 (6.0) | 0.93 | 3 (2.0), n=154 | 4 (2.7), n=150 | 0.72 | ||
| Previous thrombolytic therapy | 14 (1.0), n=1467 | 6 (0.9), n=648 | 8 (1.0), n=819 | 0.92 | 6 (1.2), n=495 | 6 (0.9), n=669 | 0.60 | 0, n=153 | 2 (1.3), n=150 | 0.24 | ||
| History of venous thromboembolism | 106 (7.2) | 47 (7.2) | 59 (7.2) | 0.97 | 44 (8.9) | 55 (8.2) | 0.69 | 3 (2.0) | 4 (2.7) | 0.72 | ||
| Deep vein thrombosis | 83 (5.6) | 35 (5.4) | 48 (5.9) | 0.70 | 32 (6.5) | 46 (6.97) | 0.78 | 3 (2.0) | 2 (1.3) | 1 | ||
| Pulmonary embolism | 39 (2.7) | 19 (2.9) | 20 (2.4) | 0.56 | 19 (3.8) | 18 (2.7) | 0.27 | 0 | 2 (1.3) | 0.24 | ||
| Laboratory values, mean (SD) | ||||||||||||
| Haemoglobin (g/L) | 134 (16.7), n=1399 | 134 (16.6), n=622 | 135 (16.8), n=777 | 0.10 | 135 (16.0), n=474 | 136 (16.4), n=641 | 0.16 | 131 (17.9), n=148 | 131 (17.8), n=136 | 0.89 | ||
| Platelet count, thrombocytes (×109/L) | 222 (78.2), n=1395 | 216 (71.1), n=620 | 227 (83.2), n=775 | 0.01 | 213 (70.1), n=473 | 224 (84.9), n=640 | 0.01 | 226 (73.7), n=147 | 239 (73.7), n=135 | 0.12 | ||
| International normalised ratio | 1.2 (0.2), n=1432 | 1.2 (0.2), n=635 | 1.2 (0.2), n=797 | 0.68 | 1.2 (0.2), n=485 | 1.2 (0.2), n=654 | 0.10 | 1.2 (0.2), n=150 | 1.2 (0.3), n=143 | 0.11 | ||
| Serum creatinine (μmol/L) | 92.7 (26.7) | 92.1 (27.0) | 93.1 (26.4) | 0.46 | 94.4 (27.9) | 94.2 (25.5) | 0.91 | 84.8 (22.5) | 88.5 (29.7) | 0.22 | ||
| Creatinine clearance (mL/min) | 79.9 (39.4) | 80.5 (40.4) | 79.3 (38.6) | 0.56 | 78.1 (41.8) | 78.6 (39.7) | 0.85 | 88.3 (34.5) | 82.8 (32.7) | 0.15 | ||
| Drug use | 377 (25.6) | 173 (26.6) | 204 (24.9) | 0.44 | 108 (21.8) | 149 (22.2) | 0.85 | 65 (42.2) | 55 (36.4) | 0.30 | ||
| Aspirin | 360 (24.5) | 167 (25.7) | 193 (23.5) | 0.33 | 102 (20.6) | 142 (21.2) | 0.79 | 65 (42.2) | 51 (33.8) | 0.13 | ||
| Clopidogrel | 25 (1.7), n=1470 | 11 (1.7) | 14 (1.7), n=820 | 0.98 | 11 (2.2), n=496 | 8 (1.2), n=669 | 0.17 | 0 | 6 (4.0) | 0.01 | ||
| Aggrenox | 2 (0.1), n=1469 | 1 (0.2), n=649 | 1 (0.1), n=820 | 1 | 1 (0.2), n=496 | 1 (0.2), n=669 | 1 | 0, n=153 | 0, n=151 | — | ||
SD=standard deviation.
Confirmed on current electrocardiogram.
At the time of the first scheduled interim analysis, the Data Safety Monitoring Board (DSMB) discovered an imbalance in randomisation. This imbalance was because of a programming error that led to all patients in the atrial fibrillation and low bleeding risk group being assigned to dalteparin after the procedure at the two sites that were also leading in recruitment rates. After a review with the DSMB, our study methodologists, and independent international experts, the randomisation programme for these two sites was amended to correct for this imbalance; for the affected group, 80% of patients were allocated to placebo and 20% to dalteparin. The investigators were aware that an imbalance existed but remained blinded to its magnitude and direction.
Postoperative anticoagulant management
Table 2 shows that nine of 650 patients assigned to placebo and 14 of 821 patients assigned to dalteparin did not receive dosing after the procedure. The mean number of doses for placebo and dalteparin after the procedure was 5.6 and 5.4, respectively. The 287 patients with low bleeding risk assigned to dalteparin had a mean dose of 15 256 IU/day; the 520 patients with high bleeding risk assigned to dalteparin had a mean dose of 5024 IU/day.
Table 2.
Perioperative anticoagulant management. Data are numbers (%) unless indicated otherwise
| Variable | No bridging (n=650) | Bridging (n=821) | P value |
|---|---|---|---|
| Warfarin treatment | |||
| Time not taking warfarin before procedure | |||
| No of patients with data | 650 | 821 | — |
| No of days, mean (SD) | 5.13 (0.66) | 5.11 (0.55) | 0.61 |
| INR before procedure | |||
| INR>1.7 (day −1) | 11 (1.7), n=648 | 9 (1.1), n=817 | 0.33 |
| Vitamin K, 2 mg given | 7 (1.1), n=650 | 7 (0.9), n=819 | 0.66 |
| INR 1.5-1.7 (day −1) | 34 (5.3), n=648 | 43 (5.3), n=817 | 0.99 |
| Vitamin K, 1 mg given | 32 (4.9), n=650 | 42 (5.1), n=819 | 0.86 |
| Different dose of vitamin K given (day –1) | 5 (0.8), n=650 | 2 (0.2), n=818 | 0.25 |
| INR repeated on day of procedure (day 0) | 131 (20.2) | 128 (15.6) | 0.02 |
| Dose after procedure | |||
| Patients for whom warfarin resumed | 645 (99.2) | 814 (99.2) | 0.86 |
| Patients for whom warfarin resumed day 0 | 564/645 (87.4) | 752/814 (92.4) | 0.001 |
| Patients for whom warfarin resumed day +1 or later | 81/645 (12.6) | 62/814 (7.6) | |
| No of doses for patients who resumed on day 0, mean (SD) | 8.7 (3.5), n=564 | 8.6 (3.8), n=752 | 0.77 |
| No of doses for patients who resumed on day +1 or later, mean (SD) | 7.5 (2.9), n=81 | 7.8 (2.8), n=62 | 0.49 |
| Low molecular-weight heparin or placebo | |||
| Dose before procedure | |||
| No of patients with data | 648 | 819 | — |
| Average dose received over 3 days, mean (SD) | 13 509 (4896) | 13 484 (4137) | 0.92 |
| No of patients who received LMWH dose day −3 | 640 | 814 | — |
| Dose for day −3, mean (SD) | 16 289 (10026) | 15 980 (6361) | 0.50 |
| No of patients who received LMWH dose day −2 | 646 | 815 | — |
| Dose for day −2, mean (SD) | 15 920 (7471) | 16 029 (8076) | 0.79 |
| No of patients who received LMWH dose day −1 | 643 | 817 | — |
| Dose for day −1, mean (SD) | 8343 (2211) | 8459 (4089) | 0.49 |
| Postoperative LMWH | |||
| Patients who started LMWH day +1 | 630 (96.9) | 789 (96.1) | 0.70 |
| Patients who started LMWH day +2 or later | 11 (1.7) | 18 (2.2) | — |
| Patients who did not receive LMWH | 9 (1.4) | 14 (1.7) | — |
| Dose after procedure | |||
| No of patients who received dose | 641 | 807 | — |
| No of doses, mean (SD) | 5.6 (2.1) | 5.4 (2.0) | 0.08 |
| Average dose per day, mean (SD) | 9524 (5480) | 8663 (5271) | 0.002 |
| No of patients who started LMWH day +1 | 630 | 789 | — |
| No of doses when LMWH started day +1, mean (SD) | 5.6 (2.1) | 5.4 (1.9) | 0.08 |
| Average dose per day when LMWH started day +1, mean (SD) | 9433 (5463) | 8688 (5277) | 0.009 |
| No of patients who started LMWH day +2 or later | 11 | 18 | — |
| No of doses when LMWH started day +2 or later, mean (SD) | 4.9 (1.7) | 5.1 (2.9) | 0.83 |
| Average dose per day when LMWH started day +2 or later, mean (SD) | 14 700 (3755) | 7553 (5023) | 0.001 |
| Patients at low risk who received postoperative LMWH dose | 284 | 287 | — |
| No of doses, mean (SD) | 5.5 (2.0) | 5.5 (2.2) | 0.84 |
| Average dose per day, mean (SD) | 15 142 (3224) | 15 256 (3167) | 0.60 |
| Patients who had >5000 IU/day (Fisher’s exact test) | 281 (98.6) | 283 (98.6) | 1 |
| Patients at high risk who received postoperative LMWH dose | 356 | 520 | — |
| No of doses, mean (SD) | 5.5 (2.2) | 5.3 (1.9) | 0.05 |
| Average dose per day, mean (SD) | 5047 (685) | 5024 (568) | 0.61 |
| Patients who had >5000 IU/ day (Fisher’s exact test) | 2 (0.6) | 3 (0.6) | 1 |
| Aspirin | 167 (25.7) | 193 (23.5) | 0.33 |
| Antiplatelet drug | 173 (26.6) | 204 (24.9) | 0.44 |
| Patients who have stop day reported for antiplatelet drug | 149 | 190 | — |
| Interruption ≥7 days before procedure | 122 (81.9) | 163 (85.8) | 0.33 |
| Interruption <7 days before procedure | 27 (18.1) | 27 (14.2) | — |
| Aspirin restarted after procedure | 123 (86.0), n=143 | 150 (85.2), n=176 | 0.84 |
| Total days until aspirin resumed, mean (SD) | 9.1 (2.9), n=123 | 10.1 (5.7), n=150 | 0.05 |
LMWH dose given in IU.
INR=international normalised ratio; LMWH=low molecular weight heparin; SD=standard deviation.
Study outcomes
Table 3 shows that the rate of major thromboembolism at 90 days was 1.2% (eight events in 650 patients) for placebo and 1.0% (eight events in 820 patients) for dalteparin (P=0.64, risk difference −0.3%, 95% confidence interval −1.3 to 0.8). Major bleeding occurred in 13 (2.0%) patients assigned to placebo compared with 11 (1.3%) assigned to dalteparin (P=0.32, −0.7, −2.0 to 0.7).
Table 3.
Study outcomes for whole population and subgroups of patients with atrial fibrillation and mechanical valves at 90 days. Data are numbers (%) unless indicated otherwise
| Outcomes | Whole study population | Atrial fibrillation | Mechanical valve† | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No bridging (n=650) | Bridging (n=820) | P value | Risk difference (95% CI) | No bridging (n=496) | Bridging (n=670) | P value | Risk difference (95% CI) | No bridging (n=154) | Bridging (n=150) | P value | Risk difference (95% CI) | |||
| Primary | ||||||||||||||
| Major thromboembolism* | 8 (1.2) | 8 (1.0) | 0.64 | −0.3 (−1.3 to 0.8) | 8 (1.6) | 7 (1.0) | 0.39 | −0.6 (−1.9 to 0.8) | 0 | 1 (0.7) | 0.49 | 0.7 (−0.6 to 2.0) | ||
| Secondary | ||||||||||||||
| Ischaemic stroke | 3 (0.5) | 1 (0.1) | 0.33 | — | 3 (0.6) | 1 (0.2) | 0.32 | — | — | — | — | — | ||
| Transient ischaemic attack | 0 | 1 (0.1) | 1 | — | — | — | — | — | 0 | 1 (0.7) | 0.49 | — | ||
| Symptomatic myocardial infarction | 3 (0.4) | 3 (0.5) | 1 | — | 3 (0.6) | 3 (0.5) | 0.70 | — | — | — | — | — | ||
| Peripheral embolism | — | — | — | — | — | — | — | — | — | — | — | — | ||
| Valve thrombosis | — | — | — | — | — | — | — | — | — | — | — | — | ||
| Venous thromboembolism | 2 (0.3) | 3 (0.4) | 1 | — | 3 (0.6) | 3 (0.5) | 1 | — | — | — | — | — | ||
| Vascular death | 3 (0.5) | 0 | 0.09 | — | 3 (0.6) | 0 | 0.08 | — | — | — | — | — | ||
| All deaths | 8 (1.2) | 6 (0.7) | 0.33 | −0.5 (−1.5 to 0.5) | 6 (1.2) | 5 (0.8) | 0.54 | −0.5 (−1.6 to 0.7) | 2 (1.3) | 1 (0.7) | 1 | −0.6 (−2.8 to 1.6) | ||
| Major bleeding | 13 (2.0) | 11 (1.3) | 0.32 | −0.7 (−2.0 to 0.7) | 10 (2.0) | 10 (1.5) | 0.49 | −0.5 (−2.1 to 1.0) | 3 (2.0) | 1 (0.7) | 0.62 | −1.3 (−3.8 to 1.3) | ||
| Clinically relevant non-major bleeding | 25 (3.9) | 50 (6.1) | 0.05 | 2.3 (0.1 to 4.5) | 20 (4.0) | 42 (6.3) | 0.09 | 2.2 (−0.3 to 4.8) | 5 (3.3) | 8 (5.3) | 0.37 | 2.1 (−2.5 to 6.6) | ||
| Trivial bleeding | 16 (2.5) | 22 (2.7) | 0.79 | — | 14 (2.8) | 18 (2.7) | 0.89 | — | 2 (1.3) | 4 (2.7) | 0.44 | — | ||
| Major thromboembolism or major bleeding | 21 (3.2) | 19 (2.3) | 0.28 | −0.9 (−2.6 to 0.8) | 18 (3.6) | 17 (2.5) | 0.28 | −1.1 (−3.1 to 0.9) | 3 (2.0) | 2 (1.3) | 1 | −0.6 (−3.5 to 2.2) | ||
| Major thromboembolism or major bleeding, or death | 25 (3.9) | 24 (2.9) | 0.33 | −0.9 (−2.8 to 1) | 20 (4.0) | 21 (3.1) | 0.41 | −0.9 (−3.1 to 1.3) | 5 (3.3) | 3 (2.0) | 0.72 | −1.3 (−4.8 to 2.3) | ||
Major thromboembolism—any one of first seven secondary outcomes: ischaemic stroke, transient ischaemic attack, symptomatic myocardial infarction, peripheral embolism, valve thrombosis, venous thromboembolism (pulmonary embolism or deep vein thrombosis), or vascular death.
With or without atrial fibrillation.
For the prespecified secondary endpoint of major bleeding or major thromboembolism, 21 (3.2%) events occurred in patients assigned to placebo compared with 19 (2.3%) in patients assigned to dalteparin (P=0.28, −0.9, −2.6 to 0.8). No differences were found for any of the additional secondary outcomes. Table 4 shows the secondary post hoc analyses at day 30.
Table 4.
Study outcomes for whole study population at 30 days. Data are numbers (%)
| Outcome | No bridging (n=650) | Bridging (n=820) | P value | Risk difference (95% CI) |
| Major thromboembolism | 8 (1.2) | 3 (0.4) | 0.06 | −0.9 (−1.8 to 0.1) |
| Major thromboembolism or major bleeding | 16 (2.5) | 12 (1.5) | 0.16 | −1.0 (−2.5 to 0.5) |
| Major thromboembolism or major bleeding, or death | 16 (2.5) | 13 (1.6) | 0.23 | −0.9 (−2.3 to 0.6) |
In the prespecified subgroup analyses according to indication for anticoagulation, no significant differences were found for any of the outcomes among the subgroups of atrial fibrillation and mechanical valves. Only one patient in the mechanical valve group had a major thromboembolism, and that patient was in the dalteparin arm.
Because of the inadvertent randomisation error, we did an analysis to exclude bias. The model for the primary outcome was fit with an interaction between the treatment effect before and after the correction. The interaction was not statistically significant (P=0.67), meaning we found no evidence that treatment effect differed before and after the correction.
Discussion
Principal findings
Our multicentre, placebo controlled, randomised trial showed that patients with atrial fibrillation or prosthetic mechanical heart valves (with or without atrial fibrillation) who require warfarin treatment to be interrupted for a procedure do not benefit from bridging treatment after the procedure with the LMWH dalteparin. We hypothesised that the placebo arm would have a lower rate of major thromboembolism, however the rates of major thromboembolism (stroke, transient ischaemic attack, venous thromboembolism, myocardial infarction) were similar for the dalteparin and placebo groups at approximately 1.0%, which was lower than the anticipated 3.1% based on our pilot study.6 The major bleeding rate was similar for the two groups. At the request of a reviewer, a post hoc secondary analysis was performed at day 30 as shown in Table 4. It is plausible but unlikely that bridging could prevent major thromboembolism more proximate to interruptions and that our choice of a 90 day outcome diluted this effect.
Comparison with other studies
Our results in the atrial fibrillation subgroup are consistent with the findings of the BRIDGE trial, another randomised, placebo controlled trial that compared a strategy of no bridging treatment before or after the procedure with LMWH bridging before and after the procedure.10 The BRIDGE trial showed a rate of arterial thromboembolism of 0.4% in the no bridging group and 0.3% in the bridging group, while major bleeding rates were 1.3% in the no bridging group and 3.2% in the bridging group. Our bleeding rates for dalteparin were probably lower because we used a prophylactic dose of dalteparin after the procedure for patients who had a procedure that was considered a high bleeding risk. The mean CHADS2 score in our trial was 2.4 and 41.2% had a score >3.0; these results were slightly higher than those in the BRIDGE trial at 2.3 and 38.3%, respectively. The proportion of patients at high risk was low in both studies, but probably represents the general patient population and was actually higher than that of a recent perioperative direct oral anticoagulant trial.12 Our randomised controlled trial includes a mechanical heart valve patient subgroup, which is especially important because vitamin K antagonists remain the anticoagulant of choice for these patients. Our results suggest that postoperative bridging is not necessary for this group.
Strengths and limitations of the study
Strengths include the double blind design carried out in multiple centres and including a wide range of patients. Central blinded adjudication was performed by investigators not involved in the care of study patients. Our study also has limitations. Firstly, we did not achieve the full sample size because of the decreased availability of patients with atrial fibrillation on warfarin because of the introduction of direct oral anticoagulant treatment.4 Secondly, the total number of patients with mechanical heart valves was relatively low, although to our knowledge this is the largest population and the only randomised trial in this group of patients to date. Also, the rate of major thromboembolism in this subgroup was very low, with only one event, and that patient was in the bridging arm. Further randomised studies in this group would be ideal but are likely to be logistically difficult, which was our experience. Thirdly, all of our patients had bridging with dalteparin before the procedure. Given the results of the BRIDGE trial, LMWH bridging for atrial fibrillation before the procedure is probably not necessary.10 Moreover, a recent trial suggests that bridging before and after the procedure is not necessary for patients receiving direct oral anticoagulants for atrial fibrillation.12 We cannot make any conclusions about bridging before the procedure for patients with mechanical heart valves and high risk atrial fibrillation given that all patients in our study received this treatment; this aspect requires further study. Finally, because we excluded patients with mechanical heart valves and a history of stroke or transient ischaemic attack, or those with multiple mechanical valves, the results from this study should not be applied to those patients.
Although our study started in 2007, the results are valid today. Vitamin K antagonists are still the treatment of choice for mechanical heart valves, and are widely used worldwide for patients with atrial fibrillation who cannot afford newer oral agents.
Conclusions
Postoperative bridging with dalteparin is not beneficial in preventing major thromboembolism in patients with atrial fibrillation or mechanical heart valves (with or without atrial fibrillation) who are managed with warfarin. We did not find a difference in major bleeding between groups with our protocol. Although warfarin is being used less frequently in patients with atrial fibrillation, it is still the long term anticoagulant of choice for patients with mechanical heart valves and in other populations, such as patients receiving dialysis, those with limited supply of direct oral anticoagulants, and in resource constrained settings. Our results are important for such patients. Further studies are needed to determine the need for bridging before procedures in patients with mechanical heart valves.
What is already known on this topic
Uncertainty remains about whether postoperative bridging with low molecular weight heparin is necessary when patients with atrial fibrillation or mechanical valves stop taking warfarin before a procedure
What this study adds
This study of 1471 patients with atrial fibrillation or mechanical valves reported major thromboembolism rates of 1.2% for placebo and 1.0% for dalteparin, and major bleeding rates of 2.0% and 1.3%, respectively, which were not significantly different
Postoperative dalteparin bridging was not found to be beneficial in preventing major thromboembolism in patients with atrial fibrillation or mechanical heart valves who stopped taking warfarin before a procedure
Acknowledgments
The authors dedicate this work to the memory of Clive Kearon, Linda Vickars, and Susan Solymoss, accomplished researchers and dear friends. We acknowledge Guillaume Feugere and Christian Caldji from Pfizer. This study would not have been possible without the contribution of all of the investigators and research coordinators. Finally, we thank all of the patients involved for their vital contribution to this study. MJK, PSW, DRA, ALL, CK, SMB, MB, SRK, SSc, SSo, TR, EY, and MAR are investigators of the CanVECTOR Network; the Network receives grant funding from the Canadian Institutes of Health Research (CDT-142654). The results reported in this paper were presented in part at the American Society of Hematology (ASH) annual meeting in December 2018 in San Diego, CA, USA.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary appendix
Contributors: MJK (lead investigator) conceived the study; obtained peer reviewed and non-peer reviewed funding; chaired the steering committee; wrote the first draft of the protocol; recruited patients; developed the analysis plan; interpreted the study results; and wrote the first draft of the manuscript. MAR (lead investigator) conceived the study; obtained peer reviewed and non-peer reviewed funding; wrote the first draft of the protocol; recruited patients; developed the analysis plan; interpreted the study results; and wrote the first draft of the manuscript. PSW, DRA, SMB, MB, SRK, CK, and SSc conceived the study; obtained peer reviewed funding; were members of the steering committee; and recruited patients. SSo and EY obtained peer reviewed funding and recruited patients. ALL recruited patients and wrote the first draft of the manuscript. TR contributed to the study design, particularly the data analysis plan (lead statistician) and supervised the statistical analysis. ES did the statistical analysis. All authors critically revised and approved the final manuscript. CK and SSo died before publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. PERIOP2 study investigators were responsible for local implementation of the study; recruited patients; and critically revised and approved the final manuscript. PERIOP2 study adjudicators reviewed suspected study events. PERIOP2 study data safety monitoring committee reviewed study safety reports and the two interim analyses. MJK is the guarantor of the study.
Funding: SRK holds a Tier 1 Canada Research Chair in venous thromboembolism. SMB holds the Eli Lilly Canada/May Cohen Chair in Women’s Health at McMaster University. The study was funded by the Canadian Institute of Health Research (CIHR-MCT79607) and had support from Pfizer in the form of the study drug. Pfizer did not have any role in the design or conduct of the study, analysis of the data, or preparation of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Canadian Institute of Health Research and Pfizer for the submitted work; SSc has received honoraria from Alnylam, Boehringer Ingelheim, Bayer HealthCare, Daiichi Sankyo, Pfizer, and Sanofi, and research support from Boehringer Ingelheim and Octapharma. None of these have any connection with the study. SMB has received consultancy fees from Leo Pharma Canada. PSW declares honoraria from Bayer Healthcare, Janssen, Sanofi, Medscape, Servier Canada, Pfizer, BMS, WebMd and grant fees from Bayer Healthcare, Pfizer/BMS in the last 3 years outside the submitted work. There are no other conflicts of interest.
Ethical approval: Institutional research ethics board approval was obtained at University of Western Ontario, Western Research (REB 12559) and at all participating centres.
Data sharing: Deanonymised patient level data and the full dataset with low risk of identification are available on reasonable request from the corresponding author after approval by the trial steering committee and Lawson Health Research Institute.
The lead author (MJK) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained. No medical writer was engaged to write any part of this manuscript.
Dissemination to participants and related patient and public communities: Disseminating the results to patients will be by each site investigator if possible. This trial was designed and conducted by investigators of the CanVECTOR research network. CanVECTOR (https://www.canvector.ca) has partnered with knowledge translation and venous thromboembolism experts from across Canada to identify new, workable, effective strategies to extend the reach of knowledge translation activities. Specifically, CanVECTOR’s CLOT+ (https://plus.mcmaster.ca/clotplus) provides a continuously updated collection of high quality and highly relevant clinical research papers to over 500 subscribers, as well as evidence summaries tailored for frontline physicians. CLOT+ also partners with CanVECTOR patient partners to produce lay summaries targeted at the patient and family audience. The results of this trial will be used to produce evidence summaries directed towards frontline clinicians and patients. Further, CanVECTOR’s membership in the international INVENT network (https://www.invent-vte.com) will enable knowledge translation efforts to quickly reach researchers and clinicians in the 10 INVENT member countries. Social media will be used for promotion of the study publication; for example, CanVECTOR and INVENT have active Twitter accounts (@canvector; @INVENT_VTE) and a growing network of followers and collaborative partners.
Provenance and peer review: Not commissioned, externally peer reviewed.
References
- 1. Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997;336:1506-11. 10.1056/NEJM199705223362107 [DOI] [PubMed] [Google Scholar]
- 2. Baron TH, Kamath PS, McBane RD. Management of antithrombotic therapy in patients undergoing invasive procedures. N Engl J Med 2013;368:2113-24. 10.1056/NEJMra1206531 [DOI] [PubMed] [Google Scholar]
- 3. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 suppl):e326S-e350S. 10.1778/chest.11-2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost 2016;14:875-85. 10.1111/jth.13305. [DOI] [PubMed] [Google Scholar]
- 5. Dunn AS, Spyropoulos AC, Turpie AG. Bridging therapy in patients on long-term oral anticoagulants who require surgery: the Prospective Peri-operative Enoxaparin Cohort Trial (PROSPECT). J Thromb Haemost 2007;5:2211-8. 10.1111/j.1538-7836.2007.02729.x. [DOI] [PubMed] [Google Scholar]
- 6. Kovacs MJ, Kearon C, Rodger M, et al. Single-arm study of bridging therapy with low-molecular-weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin. Circulation 2004;110:1658-63. 10.1161/01.CIR.0000142859.77578.C9 [DOI] [PubMed] [Google Scholar]
- 7. Douketis JD, Johnson JA, Turpie AG. Low-molecular-weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen. Arch Intern Med 2004;164:1319-26. 10.1001/archinte.164.12.1319 [DOI] [PubMed] [Google Scholar]
- 8. Spyropoulos AC, Douketis JD, Gerotziafas G, Kaatz S, Ortel TL, Schulman S, Subcommittee on Control of Anticoagulation of the SSC of the ISTH . Periprocedural antithrombotic and bridging therapy: recommendations for standardized reporting in patients with arterial indications for chronic oral anticoagulant therapy. J Thromb Haemost 2012;10:692-4. 10.1111/j.1538-7836.2012.04630.x. [DOI] [PubMed] [Google Scholar]
- 9. Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012;126:1630-9. 10.1161/CIRCULATIONAHA.112.105221. [DOI] [PubMed] [Google Scholar]
- 10. Douketis JD, Spyropoulos AC, Kaatz S, et al. BRIDGE Investigators . Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015;373:823-33. 10.1056/NEJMoa1501035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodger M, Bredeson C, Wells PS, Beck J, Kearns B, Huebsch LB. Cost-effectiveness of low-molecular-weight heparin and unfractionated heparin in treatment of deep vein thrombosis. CMAJ 1998;159:931-8. [PMC free article] [PubMed] [Google Scholar]
- 12. Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative Management of Patients With Atrial Fibrillation Receiving a Direct Oral Anticoagulant. JAMA Intern Med 2019;179:1469-78. 10.1001/jamainternmed.2019.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary appendix