Abstract
Background
Minorities and women are underrepresented in cardiovascular research. Whether their higher enrollment can be predicted or influences research site performance is unclear.
Methods
We evaluated 104 sites that enrolled 4,184 patients in the U.S. Platinum Diversity (PD) and Promus Element Plus (PE Plus) studies (2012 to 2016). Research sites were ranked from lowest to highest minority and female enrollment, respectively. United States Census Bureau division and core-based statistical area (CBSA) populations were determined for each site and the following study performance metrics compared across quartiles of minority and female enrollment, respectively: (1) study subject enrollment rate (SER), (2) time to first patient enrolled, (3) rate of follow-up visits not done, (4) rate of follow-up visits out of window, and (5) protocol deviation rate (PDR). Multivariable regression was used to predict SER and PDR.
Results
Minority enrollment varied by region (P = .025) and population (P = .024) with highest recruitment noted in the Pacific, West South Central, South Atlantic, Mid-Atlantic and East North Central divisions. Female enrollment bore no relationship to region (P = .67) or population (P = .40). Median SER was similar in sites withi the highest vs lowest quartile of minority enrollment (SER of 4 vs 5 patients per month, respectively, P = 0.78) and highest vs. lowest female enrollment (SER of 4 vs 4, respectively, P = .21). Median PDR was lower in sites within the highest vs lowest minority enrollment (0.23 vs 0.50 PDs per patient per month, respectively, P = .01) and highest vs. lowest female enrollment (0.28 vs. 0.37 PDs per patient per month, respectively, P = .04). However, this relationship did not persist after multivariable adjustment. All other site performance metrics were comparable across quartiles of minority and female enrollment.
Conclusions
Minority, but not female enrollment, correlated with research site geographic region and surrounding population. High enrollment of minorities and women did not influence study performance metrics. These findings help inform future strategies aimed at increasing clinical trial diversity.
Trial registration
The PD and PE Plus studies are registered at www.clinicaltrials.gov under identifiers NCT02240810 and NCT01589978, respectively.
Cardiovascular research trials have failed to enroll adequate numbers of underrepresented minorities, women and older adults.1,2 In most studies, women comprise only 25% to 40% of study subjects, while Black patients often represent <5% and Hispanic patients only 1%2%.3,7 These disparities have persisted 4,7,8 despite efforts from the U.S. Food and Drug Administration (FDA) and the National Institutes of Health (NIH) to increase enrollment of underrepresented minorities in clinical trials.9,10 Race/ethnicity, gender and social determinants of health influence may each influence cardiovascular outcomes, including ischemic events following coronary stent procedures (CAD).11,12 Therefore, with cardiovascular disease being the leading cause of death and major morbidity among all demographic groups in the United States,13 robust inclusion of minorities and women in cardiovascular clinical research studies is pivotal to providing an adequate evidence base from which pharmaceutical and device therapies may be properly evaluated. This issue will assume greater relevance as the US population becomes increasingly diverse.14
Clinical research sites play a central role in not only diverse patient recruitment, but also the overall integrity of a research study. Optimal site performance is critical to the timely completion of a research study and its data quality.15,16 Sites with poor trial enrollment, incomplete or inaccurate patient follow-up data and/or an excessive number of protocol deviations may unduly delay study completion, affect safety and efficacy end points and compromise overall study integrity.15,16 Given that the nation’s Black and Hispanic populations vary widely across the United States,17 site characteristics such as geographic location and surrounding population might influence minority recruitment in clinical trials. Although there is no generally accepted method for evaluating the quality and performance of individual research sites, metrics that reflect a site’s rate of study subject enrollment, patient retention and adherence to the study protocol have typically been adopted.15,18 Whether higher enrollment of historically difficult to enroll and underrepresented groups, such as minorities and women, influences site performance metrics or can be predicted is unknown. The purpose of this study was to compare the research site characteristics and performance metrics of sites that enrolled a high vs low proportion of minorities and women within the context styof 2 large US coronary stent studies.
Methods
Study sample
The study sample consisted of the 104 clinical research sites and 4,184 patients who were enrolled in the PROMUS Element Plus US Post-Approval Study (PE-Plus)19 and the Platinum Diversity (PD) everolimus-eluting coronary stent study.12 The PE-Plus study was a prospective, open-label, multicenter, “all-comers” observational study designed to examine clinical outcomes in stable CAD patients treated with everolimus-eluting, platinum-chromium PROMUS Element Plus coronary stents.19 The PE Plus study enrolled 2,683 patients at 52 US sites and was completed in August 2014. The PD study was a similar prospective, multicenter, open-label “all-comers” study (1,501 patients, 52 sites) that also enrolled stable CAD patients treated with everolimus-eluting, platinum-chromium PROMUS Element stents, but self-identified as having at least 1 of the following characteristics: female sex, black race (of African heritage), Hispanic/Latino ethnicity, or American Indian/Alaskan Native or Asian/Pacific Islanders. For the purposes of this study, the minority group consisted of only Blacks and Hispanics. Other than the aforementioned diversity requirement for the PD study, the inclusion and exclusion criteria, coronary stent designs, study protocol and end point definitions were similar between the 2 studies. In the first year following stent implantation, the follow-up schedules (office or telephone follow-up) were also identical: 30-days (± 7 days), 6-months (± 14 days) and 1-year (± 30 days). Since the PE-Plus study followed patients for a longer duration (ie, annually for 5 years in PE Plus vs 1 year in PD), our analysis was restricted to the first year of follow-up. Both studies used an independent clinical events committee for all end point event adjudication. Other details of these coronary stent registries have been previously published.12,19 With only 9 research sites participating in both studies, there was minimal site overlap between these 2 studies. The PD and PE Plus study databases were combined into a large pooled coronary stent database (n = 4,184),12 which served as the database from which our data was garnered. The purpose of pooling these 2 studies into one large sample was to increase statistical power and ensure a wide range of diversity that resulted from both unrestricted enrollment (PE Plus) and through mandated inclusion (PLATINUM Diversity).
Site monitoring and site performance metrics
For both the PD and PE PLUS studies, study site monitoring was outsourced to third parties (IQVIA, Durham, NC and PRA, Raleigh, NC). Site performance was monitored using online tools, on-site visits, source verification and regular study site follow-up calls. Throughout both studies, there was continued monitoring of patient enrollment, informed consent processes, data entry, protocol deviations, query resolution, data quality and safety reporting. For each site, the following site performance metrics were prospectively recorded: (1) study subject enrollment rate (defined as the number of patients enrolled per 30 days), (2) protocol deviation rate (defined as the number of all major and minor deviations from the institutional review board approved study protocol divided by the number of patients enrolled), (3) time to first patient enrolled, (defined as the time in days from site activation to the date of enrollment of the first patient), (4) rate of follow-up visits not done, (defined as the number of any follow-up visits not done divided by the number of total patients enrolled xlOO (expressed as a percent), and (5) rate of follow-up visits out of window (defined as the number of follow-up visits one outside the protocol specified time window divided by the number of patients enrolled x 100 (expressed as a percent). For the purposes of this study, site and clinical investigator identities remained anonymous to all parties except for the study sponsor (Boston Scientific, Inc.). Therefore, although 1-year clinical outcomes were available, there was limited access to other site, investigator and/or paient specific data.
Study methods and end points
Research site location was classified according to the 9 established US Census Bureau divisions.20 Site population was derived from the core-based statistical areas (CBSAs) defined by the US Office of Management and Budget.21 To measure site diversity, we calculated the percentage (%) of patients enrolled at each site who self-identified as a US minority (Black, Hispanic/Latino, American Indian/Alaskan Native or Asian/Pacific Islanders) and the % of patients who self-identified as being female. Sites were then rank ordered according to % minority enrollment and % female enrollment, respectively, and divided in quartiles from lowest to highest minority and female enrollment. We then compared site location, surrounding population and the aforementioned 5 site performance metrics across quartiles of both minority and female enrollment. Statistical comparisons were performed across the 4 quartiles of minority and female enrollment and also between the lowest and highest quartiles. The primary outcome measures were study subject enrollment rate and protocol deviation rate. Multivariable regression was used to examine for independent predictors of enrollment rate and protocol deviation rate, respectively.
Statistical analysis
Frequencies and percentages were used to summarize categorical variables and medians and interquartile ranges to describe continuous data. Wilcoxon Rank Sum tests were used to compare continuous variables and χ2 or Fisher’s Exact tests for discrete variables. The geographic distribution of study sites was displayed on a map of the US according to minority and female enrollment. Box and whisker plots were used to display the ranges of minority and female enrollment and to compare enrollment and protocol deviation rates across quartiles of minority and female enrollment. Site characteristics, including US census bureau defined geographic location (division) and CBSA population were depicted on a map and compared across quartiles of minority and female enrollment. Site performance metrics were also compared across quartiles of minority and female enrollment, both within the entire pooled PD and PE Plus cohort and separately within the PE Plus PAS and PD studies. Multivariable predictive models were constructed for enrollment and protocol deviation rates. All statistical analyses were performed using SAS System software, version 9.2 or later (SAS Institute Inc., Cary, NC).
Results
Geographic distribution of study sites according to minority and female enrollment
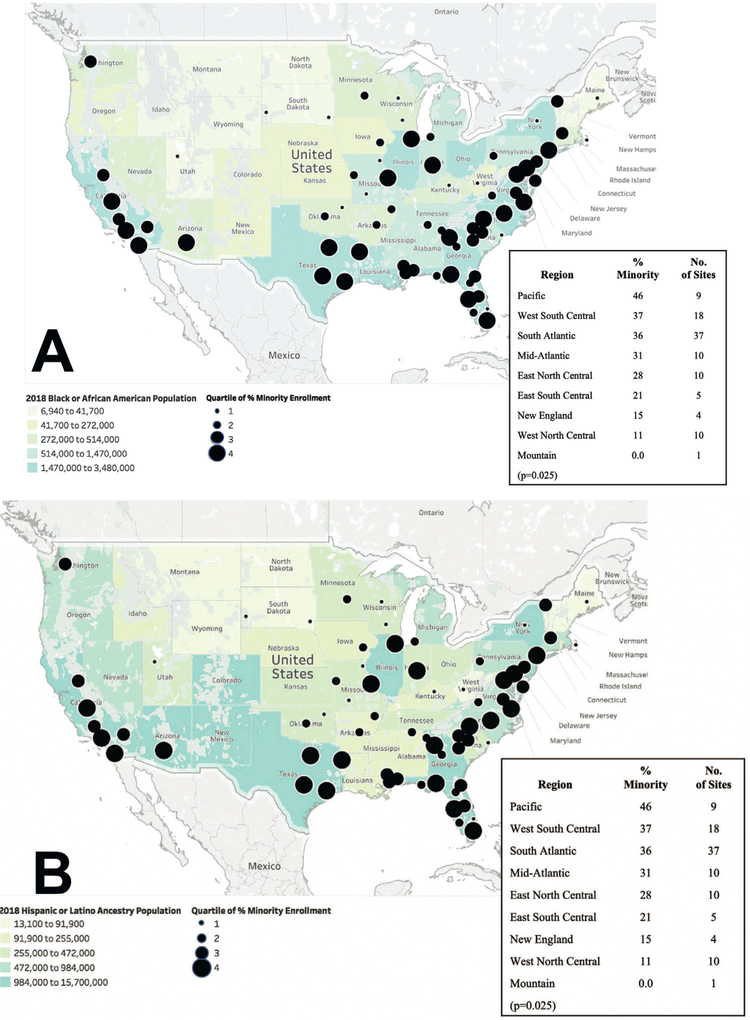
Figure 1 depicts a map of the geographic locations of all 104 PE Plus and PD study sites. Although the geographic distribution of sites was similar between the 2 studies, the PD study had fewer sites located in the Mountain and West North Central regions (Figure 1). Figures 2 and 3 depict maps of the geographic distribution of study sites according to quartiles of minority (Figure 2) and female enrollment (Figure 3), respectively. Minority enrollment varied significantly across geographic regions of the U.S. (P = .025 for trend; Figure 2) and corresponded with regional Black (Figure 2a) and Hispanic (Figure 2b) population densities. Sites with the highest minority enroll ment were more often located in the Pacific, West South Central, South Atlantic, Mid-Atlantic and East North Central US divisions, whereas sites located in the Mountain, West North Central, New England and East South-Central divisions of the US had significantly lower proportions of minority enrollment. Figure 3 depicts the geographic distribution of sites according to quartiles of female enrollment and relative to US state male: female population ratios. In contrast to minority enrollment, female enrollment was similar across all regions of the United States (P = .67 for trend).
Figure 1. Geographic Distribution of Platinum Diversity (PD)d Promus Element Plus (PE Plus] Study Sites.
The geographic location of PD (green circles] and PE Plus (orange circles] study sites are depicted on a map of the United States. Divided circles indicate multiple sites in the same location.
Figure 2. Geographic Distribution of Research Sites According to Minority Enrollment.
The geographic locations of research sites are shown superimposed on (a)the 2018 U.S. Black population distribution and (b) the 2018 U.S. Hispanic/Latino population distribution. Smaller circles indicate quartiles of lower % minority enrollment and larger circles indicate quartiles of higher % minority enrollment.
Figure 3. Geographic Distribution of Research Sites According to Female Enrollment.
The geographic locations of research sites are shown superimposed on 2018 U.S. male to female population ratios. Smaller circles indicate quartiles of lower % female enrollment and larger circles indicate quartiles of higher % female enrollment.
Site demographics and performance metrics according to minority enrollment
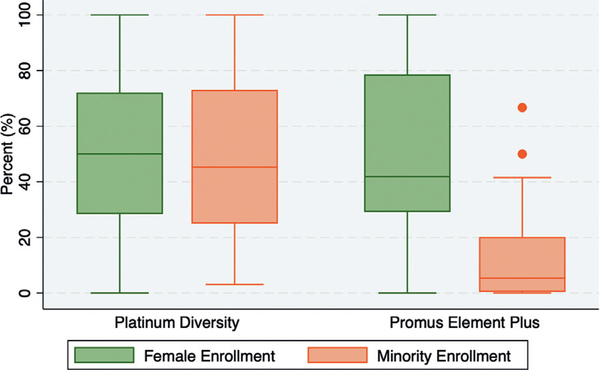
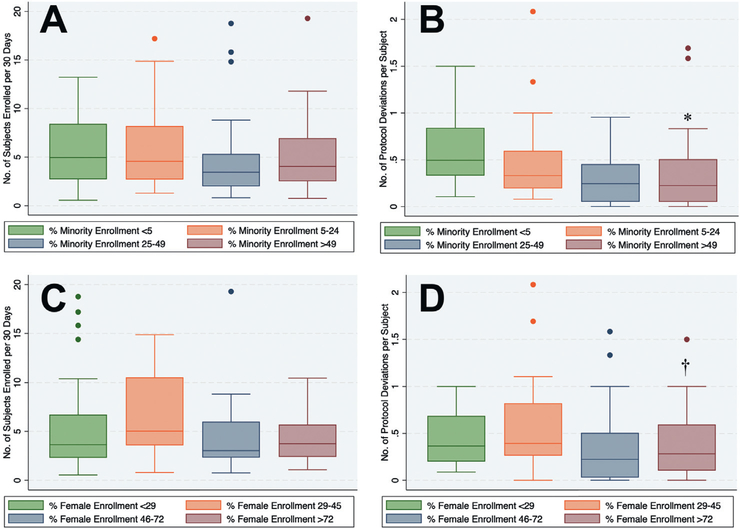
Minority enrollment varied widely (Figure 4) across PD and PE Plus study sites (range: 0–100%; median: 24% [IQR 4.5%-49%]). Median minority enrollment was higher in the PD study vs PE Plus (45 % vs 5%, P < .001). Table I compares patient demographics, site characteristics and site performance metrics across the 4 quartiles of minority and female enrollment. Median patient age was 64 years (IQR 62–67), with no differences across quartiles of minority or female enrollment. Sites that enrolled the most minorities (Q3 and Q4) tended to be located in larger metropolitan regions with greater populations (Table I, Figure 2). Hgh minority enrolling sites tended to enroll fewer patients overall, however, this was related to a shorter duration of study enrollment rather than a lower enrollment rate. The highest minority enrollment quartile enrolled more than 40 times more minorities than the lowest (75% vs 1.8%, P < .001), including more Blacks (47% vs 1.6%, P < .001), Hispanics (28% vs 0.2, P < .001) and women (54% vs 32%, P < .001). The following site performance metrics were similar (P > .05) across all quartiles of minority enrollment: (1) study subject enrollment rate, (2) time to first patient enrolled, (3) rate of follow-up visits not done and (4) rate of follow-up visits out of window (Table I, Figure 5). Median protocol deviation rates were lower in sites enrolling more minorities (P = .01 for trend, Table I, Figure 5). While the overall study subject enrollment rates were similar between PE-Plus and PD (Online Figure 1), PE-Plus had a slightly higher median protocol deviation rate than PD (Online Figure 2).
Figure 4. Ranges of Minority and Female Enrollment in the Platinum Diversity (PD] and Promus Element Plus (PE Plus)Studies.
The ranges of % minority (orange)and % female (green) rollment in the PD and PE Plus studies are shown as Box and Whisker plots. The horizontal lines within the boxes represent the medians. The lower and upper boundaries of the boxes represent the interquartile ranges (IQR). The dots located outside the whiskers represent outlier observations (i.e. defined as below lower limit [Q1 1.5 * IQR] or above upper limit [Q3 + 1.5 * IQR]].
Table I.
Patient demographics and study performance metrics according to minority and female enrollment rates.
| Parameter | Q1 <4.8% Minorities |
Q2 4.8%–23% Minorities |
Q3 24%–49% Minorities |
Q4 >49% Minorities |
P value †(trend) |
P value ‡(Q1 vs Q4) |
| Number of sites | 26 | 26 | 26 | 26 | – | – |
| Population§ | 947,869 | 1,647,730 | 4,513,526 | 8,201,326 | .024 | .0001 |
| Age, years | 64 (61, 67) | 64 (63, 68) | 65 (63, 67) | 64 (62, 66) | .29 | |
| Female, n (%) | 408 (32) | 620 (48) | 558 (53) | 416 (54) | .0001 | .0001 |
| Minority, n (%) | 23 (1.8) | 151 (12) | 279 (32) | 574 (75) | .0001 | .0001 |
| Black, n (%) | 20 (1.6) | 122 (9.5) | 171 (20) | 359 (47) | .0001 | .0001 |
| Hispanic, n (%) | 3 (0.2) | 29 (2.3) | 108 (12) | 215 (28) | .0023 | .0005 |
| Study enrollment duration (days) | 169 ± 105 | 178 ± 78 | 145 ± 68 | 146 ± 95 | .55 | .42 |
| Number of patients enrolled | 1,256 | 1,291 | 867 | 770 | .14 | .08 |
| Monthly subject enrollment rate | 5 (3, 8) | 5 (3, 8) | 3 (2, 5) | 4 (3, 7) | .46 | .78 |
| Time to first patient enrolled(days) | 7 (3, 18) | 9 (2, 14) | 8 (3, 20) | 8 (3, 22) | .97 | .74 |
| Any follow-up visits not done (%) | 8 (0, 16) | 7 (3, 13) | 6 (0, 10) | 10 (0, 18) | .95 | .74 |
| Any follow-up visits out of window (%) | 10 (2, 11) | 10 (3, 17) | 0 (0, 17) | 10 (0, 18) | .36 | .61 |
| Protocol deviation rate | 0.50 (0.33, 0.84) | 0.30 (0.19, 60) | 0.20 (0.05, 0.45) | 0.23 (0.05, 0.51) | .01 | .009 |
| Parameter* | Q1 <29% Female |
Q2 29%–45% Female |
Q3 46%–72% Female |
Q4 72%–100% Female |
P value †(trend) |
P value ‡(Q1 vs Q4) |
| Number of sites | 26 | 26 | 26 | 26 | – | – |
| Population§ | 5,715,625 | 998,737 | 4,336,632 | 1,803,390 | .4 | .06 |
| Age, years | 64 (62, 67) | 64 (61,66) | 64 (62, 67) | 66 (64, 70) | .29 | |
| Female, n (%) | 281 (23) | 563 (35) | 383 (60) | 636 (88) | <.001 | <.001 |
| Minority, n (%) | 201 (17) | 244 (15) | 408 (64) | 174 (24) | <.001 | <.001 |
| Black, n (%) | 108 (8.9) | 160 (9.9) | 278 (44) | 126 (18) | <.001 | <.001 |
| Hispanic, n (%) | 93 (7.7) | 84 (5.2) | 130 (20) | 48 (6.7) | <.001 | .39 |
| Study enrollment duration (days) | 145 ± 91 | 196 ± 91 | 146 ± 87 | 151 ± 74 | .10 | .81 |
| Number of patients enrolled | 1208 | 1617 | 637 | 722 | <.001 | .07 |
| Monthly subject enrollment rate | 4 (2,7) | 5 (4, 11) | 3 (2, 6) | 4 (2, 6) | .05 | .21 |
| Time to first patient enrolled (days) | 6 (2,18) | 5 (2, 14) | 9 (4, 24) | 13 (7, 23) | .08 | .74 |
| Any follow-up visits not done (%) | 7 (0,15) | 7 (2, 15) | 6 (0, 25) | 8 (2, 16) | .95 | .74 |
| Any follow-up visits out of window (%) | 0 (0, 6) | 0 (3, 13) | 0 (0, 17) | 0 (0, 30) | .12 | .61 |
| Protocol deviation rate | 0.37 (0.20,0.69) | 0.39 (0.26,0.82) | 0.23 (0.03,0.51) | 0.28 (0.10,0.60) | .04 | .006 |
Abbreviations: MI, myocardial infarction; TVR, target vessel revascularization.
a All averages are expressed as medians with interquartile ranges.
Comparison between all 4 groups.
Comparison between Q1 and Q4.
Median US census population of metropolitan region surrounding site.
Figure 5. Relationship Between Diversity and Study Performance Metrics.
Box and whisker plots are shown comparing (a)study subject enrollment and (b) protocol deviation rates across quartiles of minority enrollment, and (c) study subject enrollment and (d) protocol deviation rates across quartiles of female enrollment. The horizontal lines within the boxes represent the medians. The lower and upper boundaries of the boxes represent the interquartile ranges (IQR). The dots located outside the whiskers represent outlier observations (i.e. defined as below lower limit [Q1 1.5 IQR] or above upper limit [Q3 + 1.5* IQR]])p = 0.01 for trend; p = 0.009 for Q1 vs Q4. +p†4 for trend; p = 0.006 for Q1 vs Q4.
Site demographics and performance metrics according to female enrollment
Female enrollment also varied widely across sites (Figure 3, Table I, from 0 to 100% (median 46% female [IQR 29%-72%]). Female enrollment rates were similar between the PD and PE Plus studies (50 % vs 42%, P = .84). Unlike minority enrollment, female enrollment showed no relationship to geographic location or surrounding population (Figure 3, Table I. Sites with the highest female enrollment tended to enroll fewer patients overall, but proportionately more minorities (Table I, including more Black and Hispanic patients, than low female enrolling sites. The following site performance metrics were comparable (P > .05) across all 4 quartiles of female enrollment: (1) study subject enrollment rate, (2) time to first patient enrolled, (3) rate of follow-up visits not done and (4) rate of follow-up visits out of window (Table I, Figure 5). Median protocol deviation rates were higher in sites that enrolled fewer females (P = .04 for trend, Table I, Figure 5d).
Predictors of study subject enrollment and protocol deviation rates
Multivariable logistic regression revealed no independent predictors of study subject enrollment or protocol deviation rates (Tables II and III). After adjustment for other covariates, neither minority, nor female enrollment emerged as an independent predictor of enrollment rate or protocol deviation rate. When the regression analysis was performed independently within the PD and PE Plus PAS studies, respectively, study subject enrollment rates and protocol deviation rates remained comparable across all 4 quartiles of minority and female enrollment.
Table II.
Multivariable predictors of study enrollment rate.
| Variable | DF* | Est† | SE‡ | t Value | P value | STD§ Beta |
|---|---|---|---|---|---|---|
| Intercept | 1 | −0.749 | 0.749 | −1.000 | .320 | 0.000 |
| Population | 1 | 0.000 | 0.000 | −1.170 | .245 | −0.081 |
| Age | 1 | 0.015 | 0.011 | 1.320 | .191 | 0.072 |
| Sex (Male vs female) | 1 | −0.006 | 0.005 | −1.100 | .275 | −0.218 |
| Caucasian | 1 | 0.007 | 0.017 | 0.420 | .675 | 0.324 |
| Minority | 1 | 0.012 | 0.017 | 0.750 | .457 | 0.243 |
| Rate of total protocol deviations | 1 | 0.048 | 0.104 | 0.460 | .650 | 0.025 |
| Rank of site activation date | 1 | 0.038 | 0.021 | 1.800 | .076 | 1.517 |
| Time to 1st patient enrolled | 1 | 0.004 | 0.004 | 0.850 | .400 | 0.074 |
| Total # patients enrolled | 1 | 0.014 | 0.017 | 0.850 | 0.397 | 0.736 |
| Rank of first patient enrolled | 1 | −0.032 | 0.022 | −1.490 | .139 | −1.282 |
| Rank of last patient enrolled | 1 | −0.002 | 0.003 | −0.940 | .350 | −0.096 |
| % Minority enrollment | 1 | 0.001 | 0.002 | 0.240 | .810 | 0.024 |
| Region | ||||||
| Midwest | 1 | 0.254 | 0.162 | 1.570 | .121 | 0.133 |
| Northeast | 1 | 0.273 | 0.184 | 1.490 | .141 | 0.124 |
| South | 1 | 0.188 | 0.142 | 1.320 | .190 | 0.123 |
| West (reference) | – | – | – | – | – | – |
degrees of freedom.
slope estimate.
standard error.
standard deviation.
Table III.
Multivariable predictors of protocol deviation rate.
| Variable | DF* | Est† | SE‡ | t Value | P value | STD§ Beta |
|---|---|---|---|---|---|---|
| Intercept | 1 | 0.5657 | 0.7664 | 0.74 | .462 | 0.0000 |
| Population | 1 | 0.0000 | 0.0000 | 0.99 | .327 | 0.1292 |
| Age | 1 | 0.0019 | 0.0116 | 0.17 | .868 | 0.0172 |
| Sex (Male vs female) | 1 | 0.0078 | 0.0054 | 1.45 | .150 | 0.5502 |
| Caucasian | 1 | −0.0055 | 0.0173 | −0.32 | .752 | −0.4651 |
| Minority | 1 | −0.0017 | 0.0172 | −0.10 | .923 | −0.0604 |
| Enrolment rate | 1 | 0.0021 | 0.0310 | 0.07 | .947 | 0.0210 |
| Rank of site activation date | 1 | −0.0074 | 0.0224 | −0.33 | .742 | −0.5508 |
| Time 1st patient enrolled | 1 | 0.0003 | 0.0043 | 0.07 | 0.946 | 0.0115 |
| Total # patients enrolled | 1 | −0.0026 | 0.0173 | −0.15 | 0.879 | −0.2544 |
| Rank of first patient enrolled | 1 | 0.0071 | 0.0223 | 0.32 | 0.751 | 0.5302 |
| Rank of last patient enrolled | 1 | −0.0002 | 0.0027 | −0.08 | 0.939 | −0.0157 |
| % Minority enrollment | 1 | −0.0046 | 0.0025 | −1.85 | 0.068 | −0.3504 |
| Region | ||||||
| Midwest | 1 | −0.0169 | 0.1670 | −0.10 | 0.920 | −0.0166 |
| Northeast | 1 | −0.2215 | 0.1896 | −1.17 | 0.246 | −0.1880 |
| South | 1 | 0.0158 | 0.1468 | 0.11 | 0.914 | 0.0194 |
| West (reference) | – | – | – | – | – | – |
degrees of freedom.
slope estimate.
standard error.
standard deviation.
Discussion
Race/ethnicity, gender, and social determinants of health have been shown to influence coronary stent outcomes.11,12 Therefore, study subject diversity in cardiovascular research is vital to the generation of safety and efficacy data that are reflective of an increasingly diverse US population. Due to the historical challenges associated with the recruitment and retention of minorities and women in cardiovascular trials,2–4 it is conceivable that aggressive efforts to recruit a high proportion of minorities and women might slow overall study subject enrollment or compromise other study performance metrics. From the 104 sites enrolling 4,184 patients in the PD and PE PLUS coronary stent registries, we observed the following key findings: (1) minority and female enrollment both varied widely across sites (from 0 to 100%) with a greater than 40-fold difference in highest vs. lowest minority enrollment; (2) sites that enrolled the most minorities tended to be located in more densely populated metropolitan regions in the Pacific, West South Central, South Atlantic, Mid-Atlantic and East North Central regions of the United States; (3) female enrollment showed no relationship to geographic location or population density; (4) after adjustment for covariates, site performance metrics were similar across quartiles of minority and female enrollment and (5) multivariable regression identified no specific site or patient predictors of enrollment or protocol deviation rates.
Only a third of cardiovascular research studies report adequate data on race and/or ethnicity.22 When reported, the enrollment of women, minorities and older adults have been consistently poor for several decades.2 Recently, Ceron et al performed a systematic review of 964,532 patients enrolled in 130 randomized cardiovascular clinical trials from 1986 to 2018.7 Of these, 98% reported gender, but only 56% reported race. Minority representation was uniformly poor in all trials studied and did not improve over 3 decades. Women were also found to be underrepresented with no signfint improvement over time. Given that coronary artery disease and its risk factors are more prevalent in Blacks and Hispanics,23–25 these observations are concerning and run counter to directives put forth in the 2012 FDA Safety and Innovations Act (FDASLA Sec. 907) aimed at increasing patient diversity in biomedical research.26 Our study provides insight into how sites may be better selected to enrich clinical trial diversity. We found that minority enrollment was influenced by geographic location and population density. Not surprisingly, the enrollment of minorities was highest in the regions where most Blacks and Hispanics reside (ie, densely populated regions of the Pacific, West South Central, South Atlantic, Mid-Atlantic and East North Central US).27,28 In contrast, women enrollment was not associated with either geographic location or population density. This suggests that disparate strategies may be necessary when selecting sites to increase minority vs female enrollment. For example, site selection based on geographic region and population is more likely to influence minority enrollment, while other strategies may be necessary to increase female enrollment.
The selection of effective research sites is a crucial step toward the successful completion of multicenter cardiovascular clinical research studies. Poor performing sites may compromise a study in several ways. The time and cost of training, opening and maintaining underperforming sites waste resources. Sites unable to effectively recruit patients experience worse outcomes and delay study completion, thereby imposing further economic burdens.16 Research sites that provide poor quality data also tend to incur a higher than average number of protocol deviations, require more queries for data clarification, and/or have high rates of patients lost to follow-up or follow-up visits completed outside the protocol mandated window, all of which may compromise data integrity and quality.15 Prior to our study, it has not been clear how enrolling high proportions of women and minorities might affect key study performance metrics. By demonstrating that study subject diversity had no bearing on site performance metrics, our research shows that there was no “price to pay” for achieving diversity within the context of these 2 coronary stent registries. Interestingly, although sites that enrolled more minorities and women actually had lower protocol deviation rates, this relationship did not persist in the multivariable regression model. If validated by other studies, this implies that future efforts to increase the diversity of participants enrolled in cardiovascular clinical trials are unlikely to compromise a study’s time to completion and/or overall performance.
Multiple factors may influence the enrollment of minorities and women in clinical trials, including those related to patient characteristics, research sites, regulatory agencies, academic organizations, government funding bodies, industry partners, informed consent, digital technology, public awareness and patient advocacy.29–32) A better understanding of these factors, combined with our study findings that diversity (1) may be predicted by research site characteristics (location and surrounding population) and (2) does not appear to compromise study performance, should help inform the multiple stakeholders interested in enhancing diverse enrollment in clinical trials.
Limitations
There are several limitations to our study. Although we extracted data from 4,184 patients enrolled across 104 US sites, our analysis was limited to 2 national coronary stent registries. Therefore, these findings require validation from other research databases, including multicenter randomized clinical trials evaluating both pharmaceutical and medical device therapies across a broader range of cardiovascular diseases. Since all sites in the PD and PE Plus PAS studies were located in larger metropolitan regions, rural regions of the United States were poorly represented, a limitation consistent with most other research protocols. Census Bureau divisions were not normalized for minority population and divisions do not provide as much geographic location specificity as 7-digit zip code, for example, which was not available. Older adults, who are also underrepresented in clinical trials were not specifically evaluated in this study. The number of patients screened vs. enrolled at each site was not available for analysis. The characteristics (race, ethnicity, languages spoken, and gender) of the principal investigators and research coordinators of sites were also not available. This may be relevant, since patient-physician gender concordance has been shown to potentially influence the care and outcome of cardiac patients 33. The intensity and/or effectiveness of site monitoring could not be controlled for within this study. However, we believe that it is unlikely that this would have biased the results in any particular direction. The retrospective design of our study raises the potential for unknown biases which may have influenced our results. We also did not report on issues related to informed consent (missing or incorrectly dated or signed consents), since these issues were extremely rare (<1%). The fact that sites enrolling in PD vs PE Plus were distinct, with only 9 being common to both, raises the potential for bias. However, study origin (PD vs PE Plus) did not emerge as a predictor of either enrollment or protocol deviation rate using multivariable regression. Finally, beta error may have contributed to the lack of difference observed between groups.
Conclusion
In this pooled analysis of 2 large contemporary US coronary stent registries, minority and female enrollment varied markedly across research sites. The geographic location and surrounding population of the site influenced minority, but not female enrollment. Higher enrollment of minorities and women did not impact site performance metrics. If validated in randomized trials, these findings have implications on future strategies aimed at increasing clinical trial diversity.
Supplementary Material
Clinical perspective.
Competency in Medical Knowledge:
Cardiovascular clinical research studies have lacked study subject diversity for over 3 decades, despite imperatives from the FDA and NIH. Research subject diversity is pivotal to providing an adequate evidence base from which pharmaceuticals, devices and other treatment modalities may be evaluated.
Competency in Patient Care:
The geographic location and population density of a cardiovascular research site influences minority, but not female enrollment in clinical trials. Higher enrollment of minorities and women should not compromise overall study subject enrollment rates and other key study performance metrics.
Translational Outlook:
This study has implications for future strategies to improve clinical trial diversity. Future research should be directed at (1) validating our findings in other cardiovascular clinical trials, (2) refining and further delineating predictors of diversity within multicenter clinical trials and (3) determining statistical methods to predict the final demographic composition of clinical trials prior to initiation of the study based on research site characteristics and (4) determining strategies to engage the multiple stakeholders involved in clinical research in future efforts to improve representation of women, minorities and older adults in clinical trials (see central illustration).
Central Illustration.
Factors influencing clinical trial diversity and the effects of diversity on study performance metrics. Factors that may influence diverse enrollment in clinical trials are shown. The illustration highlights that in our study increased research subject diversity did not influence key study performance metrics (study subject enrollment rate, time to first patient enrolled, rate of follow-up visits not done, rate of follow-up visits out of window, and protocol deviation rate). Asterisks (*) indicate research site factors (ie, site location and surrounding population) that predicted minority enrollment. Abbreviations: EHR: electronic health record; apps: device applications (ie, for mobile devices); ICF,informed consent form; RCT,randomized clinical trial.
Figure 1 Geographic distribution of platinum diversity (PD) and promus element plus (PE Plus) study sites. The geographic location of PD (blue circles) and PE Plus (red circles) study sites are depicted on a map of the United States.
Figure 2.Geographic distribution of research sites according to quartiles of minority enrollment. The geographic locations of research sites are shown superimposed on (a) the 2018 US Black population distribution and (b) the 2018 US Hispanic/Latino population distribution. Smaller circles indicate lower quartiles of % minority enrollment and larger circles indicate higher quartiles of % minority enrollment.
Figure 3. Geographic distribution of research sites according to quartiles of female enrollment. The geographic locations of research sites are shown superimposed on 2018 US male to female population ratios. Smaller circles indicate lower quartiles of % female enrollment and larger circles indicate higher quartiles of % female enrollment.
Figure 4. Ranges of minority and female enrollment in the platinum diversity (PD) and promus element plus (PE Plus) Studies. The ranges of % minority (orange) and % female (green) enrollment in the PD and PE Plus studies are shown as Box and Whisker plots. The horizontal lines within the boxes represent the medians. The lower and upper boundaries of the boxes represent the interquartile ranges (IQR). The dots located outside the whiskers represent outlier observations (ie, defined as below lower limit [Q1 – 1.5 * IQR] or above upper limit [Q3 + 1.5 * IQR]).
Figure 5. Relationship between diversity and study performance metrics. Box and whisker plots are shown comparing (a) study subject enrollment and (b) protocol deviation rates across quartiles of minority enrollment, and (c) study subject enrollment and (d) protocol deviation rates across quartiles of female enrollment. The horizontal lines within the boxes represent the medians. The lower and upper boundaries of the boxes represent the interquartile ranges (IQR). The dots located outside the whiskers represent outlier observations (ie, defined as below lower limit [Q1 – 1.5 * IQR] or above upper limit [Q3 + 1.5 * *IQR]). *p=0.01 for trend; p=0.009 for Q1 vs Q4. †p = 0.04 for trend; p = 0.006 for Q1 vsQ4.
Key Points.
Question:
Does the enrollment of more Blacks, Hispanics and women in US cardiovascular research studies influence the overall rate of study subject enrollment and/or other key study site performance metrics and can diverse enrollment be predicted?
Findings:
In this pooled analysis of 104 sites that enrolled 4,184 patients in the Platinum Diversity and Promus Element Plus Post-Approval Studies, we found that the enrollment of higher proportions of underrepresented minorities and women was univariately associated with lower protocol deviation rates while having no effect on other site performance metrics. A site's geographic location and surrounding population predicted minority, but not female enrollment.
Meaning:
These findings suggest that cardiovascular research subject diversity may be predicted from site characteristics and enhanced without compromising key study performance metrics. These insights help inform future strategies aimed at improving clinical trial diversity. (Am Heart J 2021;236:37–48.)
Acknowledgements
The authors thank the Dudley Family for their continued contributions and support of the Inova Dudley Family Center for Cardiovascular Innovation.
Funding and financial disclosures
Dr. Damluji receives research funding from the Pepper Scholars Program of the Johns Hopkins University (NIA) P30-AG021334. Dr Yong receives research support from a VA HSR&D Career Development Award. Other authors report the following conflicts of interest/disclosures: WBB - Institutional grant/research support: Abbott, Boston Scientific; Consultant: Abbott, Medtronic, V-Wave Medical, Boston Scientific (minor); AAD - None; CY - None; MF - None; SDB - None; DEK - Research/grant support: Medtronic, Boston Scientific, Biotronik, St. Jude Medical/Abbott; Consulting: Medtronic, Boston Scientific, Micell Technologies (all minor); MSW - Speaker’s Honararia: Medtronic; KCE - None; BNT - None; DJA - Full-time Employee and Stockholder: Boston Scientific; ITM - Full-time Employee and Stockholder: Boston Scientific; JL - Consultant: Abbott, Astra-Zeneca, Boehringer-Ingelheim, CVRx, Edwards Lifesciences, Impulse Dynamics, V-Wave; Research Grant: Astra-Zeneca; CMC - Consulting: Merck, Bayer, Bristol Myers Squibb, Windtree, and Arena. RM - Institutional grant/research support: Eli Lilly/Daiichi-Sankyo, Bristol- Myers Squibb, AstraZeneca, The Medicines Company, OrbusNeich, Bayer, CSL Behring, Abbott Laboratories, Watermark Research Partners, Novartis Pharmaceuticals Medtronic, AUM Cardiovascular, Beth Israel Deaconess Medical Center, Consultant/Executive Committee: Janssen Pharmaceuticals, Osprey Medical, Watermark Research Partners, Medscape, The Medicines Company, Boston Scientific, Merck, Cardiovascular Systems, Sanoh USA, Shanghai BraccoSine Pharmaceutical, AstraZeneca (all minor) and Equity: Claret Medical, Elixir Medical Corporation.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ahj.2021.02.003.
Disclaimer
The submitted manuscript is currently not under consideration elsewhere and none of the paper’s contents have been previously published. All authors involved in this project have read and approved the manuscript. There is no portion of the text that has been copied from other material in the literature (unless in quotation marks, with citation).There is no direct financial relation with the commercial identities mentioned in the paper that might lead to a conflict of interests.
References
- 1.Chen MSJ, Lara PN, Dang JHT, et al. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer 2014(120 Suppl): 1091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang T, Tsang W, Wijeysundera HC, Ko DT. Reporting and representation of ethnic minorities in cardiovascular trials: a systematic review. Am Heart J 2013;166:52–7. [DOI] [PubMed] [Google Scholar]
- 3.Melloni C, Berger JS, Wang TY, et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes 2010;3:1 35–42. [DOI] [PubMed] [Google Scholar]
- 4.Dhruva $$, Bero LA, Redberg RF, et al. Gender bias in studies for Food and Drug Administration premarket approval of cardiovascular devices. Circ Cardiovasc Qual Outcomes 2011;4:165–71. [DOI] [PubMed] [Google Scholar]
- 5.Coakley M, Fadlran EO, Parrish LJ, et al. Dialogues on diversifying clinical trials: successful strategies for engaging women and minorities in clinical trials. J Women's Heal 2012;21:71 3–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22747427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheffet AJ, Howard G, Sam A, et al. Challenge and yield of enrolling racially and ethnically diverse patient populations in low event rate clinical trials. Stroke 2018;49:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceron C, Vilcant V, Verma G, et al. Minority representation in cardivascular clinical trials. J Am Coll Cardiol 2019;73(9 Supplement 1):3045. Available from: http://www.onlinejacc.org/content/73/9_Supplement_l/3045.abstract. [Google Scholar]
- 8.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N EnglJ Med 2000; 1 7:475–80. [DOI] [PubMed] [Google Scholar]
- 9.FDA. Collection of race and ethnicity data in clinical trials [Internet]. 2017. [cited 201 7 Jan 20]. Available from: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-gen/documents/document/ucm126396.pdf.
- 10.National Institutes Health. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. 2017. Available from: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-014.html
- 11.Batchelor WB, Ellis SG, Ormiston JA, et al. Racial differences in long-term outcomes after percutaneous coronary intervention with paclitaxel-eluting coronary stents. J Interv Cardiol 2013;26:49–57. [DOI] [PubMed] [Google Scholar]
- 12.Batchelor W, Kandzari DE, Davis S, et al. Outcomes in women and minorities compared with white men 1 year after everolimus-eluting stent implantation: Insights and results from the PLATINUM diversity and PROMUS element plus post-approval study pooled analysis. JAMA Cardiol 2017;2:1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC. Leading causes of death in males and females, United States. 2017. [cited 2019 Oct 6]. Available from: https://www.cdc.gov/healthequity/lcod/index.htm
- 14.The US will become 'minority white' in 2045, Census projects: Youthful minorities are the engine of future growth [Internet]. [cited 2019 Oct 15]. Available from: https://www.brookings.edu/blog/the-avenue/2018/03/14/the-us-will-become-minority-white-in-2045-census-projects/
- 15.Hurtado-Chong A, Joeris A, Hess D, Blauth M. Improving site selection in clinical studies: a standardised, objective, multistep method and first experience results. BMJ Open 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gheorghiade M, Vaduganathan M, Greene SJ, et al. Site selection in global clinical trials in patients hospitalized for heart failure: perceived problems and potential solutions. Heart Fail Rev 2014;19:135–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frey WH Six maps that reveal America's expanding racial diversity. 2019. [cited 2020 May 5]. Available from: https://www.brookings.edu/research/americas-racial-diversity-in-six-maps/
- 18.Warden D, Trivedi MH, Greer TL, et al. Rationale and methods for site selection for a trial using a novel intervention to treat stimulant abuse. Contemp Clin Trials 2012;33:29–37. 201 1 /09/1 7Available from: https://www.ncbi.nlm.nih.gov/pubmed/21946515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kandzari DE, Amjadi N, Caputo C, et al. One-year outcomes in "real-world" patients treated with a thin-strut, platinum-chromium, everolimus-eluting stent (from the PROMUS Element Plus US Post-Approval Study [PE-Plus PAS]). Am J Cardiol 2016;117:539–45. [DOI] [PubMed] [Google Scholar]
- 20.Census Regions and Divisions of the United States [Internet]. [cited 2020 Feb 3]. Available from: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
- 21.2017 Metropolitan and Micropolitan Statistical Areas (CBSAs) of the United States and Puerto Rico [Internet], [cited 2020 Jan 15]. Available from: https://www.census.gov/geographies/reference-maps/2017/geo/cbsa.html
- 22.Berger JS, Melloni C, Wang TY, et al. Reporting and representation of race/ethnicity in published randomized trials. Am Heart J 2009; 158:742–7. [DOI] [PubMed] [Google Scholar]
- 23.Graham G. Population-based approaches to understanding disparities in cardiovascular disease risk in the United States. Int J Gen Med 2014;7:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brancati FL, Kao WHL, Folsom AR, et al. Incident type 2 diabetes mellitus in African American and white adults: the atherosclerosis risk in communities study. J Am Med Assoc 2000;283:2253–9. [DOI] [PubMed] [Google Scholar]
- 25.Kramer H, Han C, Post W, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Hypertens 2004;17:963–70. [DOI] [PubMed] [Google Scholar]
- 26.FDA. FDASIA Section 907: inclusion of demographic subgroups in clinical trials. 2016. [cited 2020 Mar 15]. Available from: https://www.fda.gov/Regulatorylnformation/LawsEnforcedbyFDA/SignificantAmendmentstotheFDCAct/FDASIA/ucm389100.htm
- 27.U.S. Census Bureau: Majority of African Americans Live in 10 States; New York City and Chicago Are Cities With Largest Black Populations [Internet] Available from: https://www.census.gov/newsroom/releases/archives/census_2000/cb01cnl76.html
- 28.U.S. Census Bureau: Hispanic Heritage Month 2018. Available from: https://www.census.gov/newsroom/facts-for-features/2018/hispanic-heritage-month.html
- 29.Ortega RF, Yancy CW, Mehran R, Batchelor W. Overcoming lack of diversity in cardiovascular clinical trials: a new challenge and strategies for success. Circulation 2019;140:1690–2. [DOI] [PubMed] [Google Scholar]
- 30.Clark LT, Watkins L, Pina IL, et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr Probl Cardiol 2019;44:148–72. [DOI] [PubMed] [Google Scholar]
- 31.Braunstein JB, Sherber NS, Schulman SP, et al. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore) 2008;87:1–9. [DOI] [PubMed] [Google Scholar]
- 32.Baquet CR, Henderson K, Commiskey P, et al. Clinical trials: the art of enrollment. Semin Oncol Nurs 2008;24:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenwood BN, Carnahan S, Huang L. Patient-physician gender concordance and increased mortality among female heart attack patients. Proc Natl Acad Sci U S A 2018;115:8569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.