Abstract
BACKGROUND:
This study aims to explore the characteristics of the epithelial-to-mesenchymal transition (EMT) process and its underlying molecular mechanisms in the period of paraquat (PQ)-induced pulmonary fibrosis (PF).
METHODS:
Picrosirius red staining and collagen volume fraction were utilized to evaluate the pathological changes of PQ-induced PF in rats. Immunohistochemistry, Western blot, and real-time reverse transcriptase-polymerase chain reaction (RT-PCR) were used to measure the protein and gene expression of EMT markers, EMT-associated transcription factors, and regulators of EMT-related pathways, respectively.
RESULTS:
The collagen deposition in the alveolar septum and increased PF markers were characteristics of pathological changes in PQ-induced PF, reached a peak on day 14 after PQ poisoning, and then decreased on day 21. The protein and gene expression of the fibrosis marker, EMT markers, transcription factors, and regulators of EMT-related signaling pathways significantly increased at different time points after PQ poisoning compared with corresponding controls (P<0.05), and most of them reached a peak on day 14, followed by a decrease on day 21. The gene expression of EMT markers was significantly correlated with PF markers, transcription factors, and regulators of EMT-related signaling pathways (P<0.05). The mRNA expression of transcription factors was significantly correlated with that of TGF-β1 and Smad2 (P<0.05 or P<0.01), instead of Wnt2 and β-catenin (P>0.05).
CONCLUSIONS:
EMT process plays a role in the PQ-induced PF, in which most PF and EMT markers have a peak phenomenon, and its underlying molecular mechanisms might be determined by further studies.
Keywords: Pulmonary fibrosis, Paraquat, Epithelial-to-mesenchymal transition, Rats
INTRODUCTION
Paraquat (PQ, 1,1′-dimethyl-4,4′-bipyridinium) accounts for one-third of herbicides used in the world, especially in developing countries.[1] PQ poisoning leads to the highest fatality rate in pesticide poisoning,[2] and the latter is the most common reason for suicide in the world.[3] PQ poisoning is often caused by ingestion without intention or with attempted suicide.[4] Because of its low lethal dose (PQ 20–40 mg/kg in adults), lack of specific antidotes, and high mortality (50%–70%), PQ poisoning has become a focus in emergency medicine.[5,6]
The death from PQ poisoning was attributed to rapidly progressive respiratory failure induced by extensive and irreversible pulmonary fibrosis (PF).[7,8] So far, there have been no specific therapy methods for PQ-induced PF. Unclear poisoning mechanism may be one of reasons. Thus clarifying the mechanism for PF process might be helpful to find an effective treatment strategy for PQ poisoning.
Epithelial-to-mesenchymal transition (EMT) plays a key role in developing fibrosis in various tissues, such as renal interstitium, myocardium, and liver.[9-11] Its role in idiopathic PF and bleomycin-induced PF was gradually recognized.[12,13] EMT was found to play a role in PQ-induced PF in the lung by the generation of myofibroblasts transited from epithelial cells.[14-16] During the EMT process, the gene and protein expression of E-cadherin (E-cad), as a marker of the epithelial cell, decreased. The epithelial cells gradually obtained the features of mesenchymal phenotype, e.g., significantly enhancing expression of α-smooth muscle actin (α-SMA) protein and fibroblast specific protein-1 (FSP-1) as the markers of mesenchymal cells.[17] In vitro studies[18-22] showed that the EMT process in PQ-induced PF was mediated by the transforming growth factor-β1 (TGF-β1), Smad, mitogen-activated protein kinase (MAPK), and β-catenin. However, few studies[14,23] focused on the mechanism of EMT with PQ-induced animal models. Clinical cases who died from lung fibrosis among PQ-poisoned patients were often reported,[4] indicating that the period of PQ-induced PF might be a long phase for clinical treatment. Several studies[17,20,23] already focused on EMT process in PQ-induced PF within an early stage (e.g., three days). However, the whole EMT process in PQ-induced PF and its underlying mechanisms have been rarely reported.
Therefore, in this study, PQ-induced animal models will be used to verify the underlying mechanisms of EMT process. Both the early and late stages of PF process after PQ poisoning will be covered for the importance of PQ-induced delayed PF, which might provide treatment strategies against PF induced by PQ poisoning.
METHODS
Animals and treatment
Male Sprague-Dawley rats, 4–5 months, weighing 250±45 g (Leicester Company, China) were kept individually in a room for one week, with room temperature 23.0±0.5 °C, humidity 50.0%±0.5%, and a 12-hour light cycle. Standard rat chow and water ad libitum were provided. The control group (n=60) received saline injection, while the PQ group (n=60) received intraperitoneal injection (i.p.) of 20% PQ solution (Syngenta Nantong Crop Protection Co., Ltd., China) at a dose of 15 mg/kg per rat.[24] Rats were anesthetized with 5% chloral hydrate (300 mg/kg) at 1, 3, 7, 10, 14 and 21 days after the PQ treatment. The PF model during different periods was established through trial tests based on the pathological examination for the lung using the picrosirius staining. After euthanized, one piece of the right lung (400 mg) was frozen in liquid nitrogen and stored at –80 °C for subsequent assays. Another piece of the right lung (400 mg) was fixed in 4% paraformaldehyde solution (1:20) for histological embedding.
Picrosirius red staining
Paraffin sections of the lung tissue specimens were dewaxed and then added to a 0.1% Sirius red dye solution (Fluka Chemie Co., Ltd., Switzerland) for 20 minutes. Then, the sections were treated by the transparency and sealing process. Under the microscope, the collagen tissue was red, while the lung tissue was yellow. Collagen volume fraction (CVF) value was utilized to reflect the degree of pulmonary interstitial fibrosis. The calculation method was: eight random fields of view were selected for each section; CVF was the total area of interstitial fibrosis in each field divided by the total area of connective tissue and lung tissue.
Immunofluorescence
The EnVision two-step method (Dako, Denmark) was used to determine the protein expression of epithelial cell markers (E-cad) and fibroblast markers (α-SMA, FSP-1). The pretreated sections were incubated with primary antibodies (E-cad, α-SMA, FSP-1; 1:100; Abcam, UK) for 90 minutes and the EnVision antibody (Dako, Denmark) for 30 minutes at room temperature. Subsequently, the sections were stained with 4’,6-diamidino-2-phenylindole (DAPI) (Beyotime, China) and hematoxylin and sealed. The Leica DM2500 positive microscope with the image software analysis system LAS V3.8 (Leica, Germany) was used to estimate the positive area of each slide. The ultimate value was the average value of ten random fields of each slide.
Ribonucleic acid (RNA) isolation and real-time reverse transcriptase-polymerase chain reaction (real-time RT-PCR)
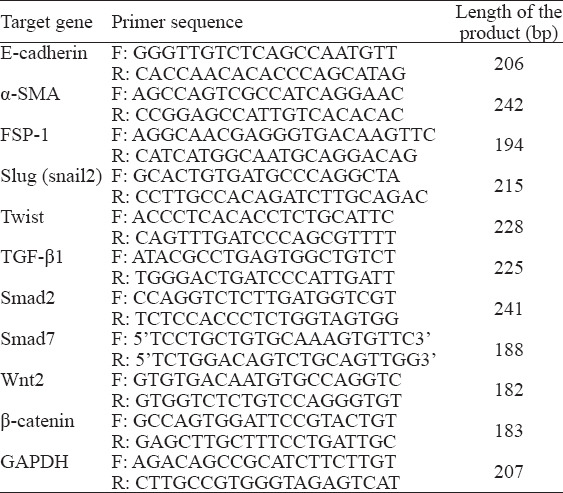
We used the Trizol kit (Invitrogen, USA) for total RNA analysis. The primer pairs (Invitrogen, China) used in real-time RT-PCR are listed in Table 1. Real-time RT-PCR was performed with reverse transcription kit (Promega, USA) and StepOne plus PCR amplification instrument with StepOne Software Version 1.0 (Applied Biosystems, USA). At the end of the reaction, the real-time RT-PCR amplification curve and fusion curve were determined. According to the mathematical method, the volume of the target gene was a 2-delta cycle threshold (Ct). In this formula, Ct was the cycle number of fluorescence that reached the fluorescence threshold. The expression of genes in the samples was compared with that of glyceraldehyde phosphate dehydrogenase (GAPDH), an internal reference, by the Ct method; three repetitions were performed.
Table 1.
Sequences of the primers used in real-time RT-PCR
Statistical analysis
All collected data were coded and entered into a dataset in SPSS version 22.0 (IBM corporation, USA). Analysis of variance (ANOVA) with repeated measures was used to compare the difference of variables at different time points. The correlation between the expression of EMT markers and fibrosis markers and regulators of the signaling pathway was analyzed by Pearson’s coefficient. A priori level of significance was set at 0.05 (two-tailed analysis).
RESULTS
PF induced by PQ
Picrosirius red staining showed that the collagen deposition was observed in the alveolar septum in the first week, and reached a peak on day 14, then decreased on day 21 (Figure 1A). The CVF value, which represented the extent of collagen deposition, began to increase on day 3 and reached a peak in the second week, then decreased on day 21 after treatment. There were significant differences in CVF between the PQ group and the control group from day 3 to day 21 (P<0.05, Figure 1B).
Figure 1.
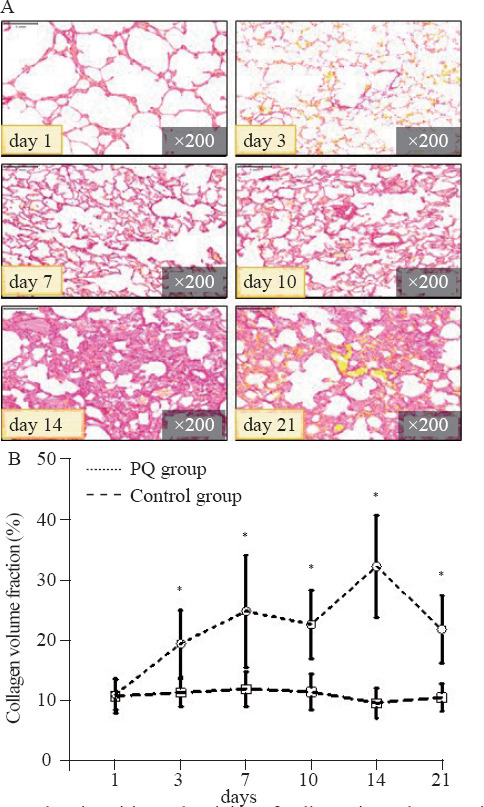
The picrosirius red staining of collagen in rat lung sections of the PQ group (A) and collagen volume fraction (B) at different time points after PQ injection (15 mg/kg) or saline. Compared with the control group, *P<0.05.
PQ-induced EMT changes in epithelial cells and mesenchymal cells in lung tissues
Positive staining of α-SMA and FSP-1 (as markers of the myofibroblast phenotype derived from epithelial cells via EMT) and weak staining of E-cad (an epithelial cell marker) were found in lung tissues by the immunofluorescent staining (Figures 2A–C). Figures 2D and 2E illustrated that the immunofluorescent semi-quantification levels of FSP-1 and α-SMA in the PQ group gradually rose on day 3, and then decreased on day 21, which were significantly different from those in the control group from day 3 to day 21 (P<0.05).
Figure 2.
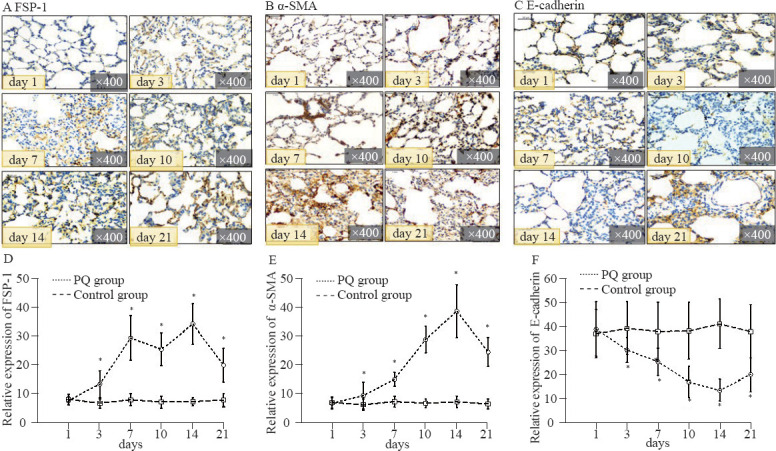
Immunofluorescent staining (A–C), protein relative expression (D–F) of FSP-1, α-SMA and E-cadherin in the rat lung tissue at different time points. Compared with the control group, *P<0.05.
The immunofluorescent semi-quantification results showed that the protein expression of E-cad in the PQ group significantly decreased from day 3 to day 21, compared with that in the control group (P<0.05, Figure 2F).
Figures 3A–C showed the Western blot photos of FSP-1, α-SMA, and E-cad in the PQ group. Figures 3D–E displayed that the protein expression levels of FSP-1 and α-SMA were significantly elevated from day 10 to day 21, compared with those in the control group (P<0.05). There was remarkable downregulation of E-cad expression from day 10 to day 21 compared with that in the control group (P<0.05, Figure 3F).
Figure 3.
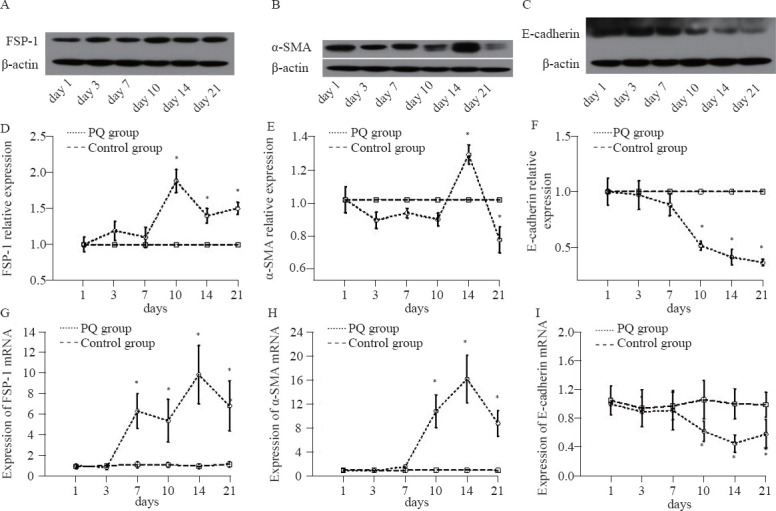
The Western blot photos (A–C), relative protein expression levels (D–F) and the mRNA expression levels (G–I) of FSP-1, α-SMA, and E-cadherin in the rat lung tissue at different time points. Compared with the control group, *P<0.05.
The mRNA expression levels of FSP-1 in the PQ group began to rise on day 7 and declined on day 21 and had significant differences compared with those in the control group from day 7 to day 21 (P<0.05, Figure 3G). Figure 3H demonstrated that the gene expression level of α-SMA started to ascend on day 7 and descend on day 21. There were significant differences in α-SMA gene expression between the PQ group and the control group from day 7 to day 21 (P<0.05). The E-cad gene expression was remarkably downregulated in the PQ group from day 10 to day 21, compared with that in the control group (P<0.05, Figure 3I).
Elevated expression of slug and twist mRNA
The mRNA expression of the EMT-associated transcription factor slug regulating the migration of epithelial cells, rose on day 10 and reached a peak on day 14, then declined on day 21. Significantly elevated expression was found in the PQ group compared with the control group on day 10 and day 14 (P<0.05, Figure 4A). Gene expression of transcription factor twist began to gradually increase from day 3 to day 14, and decreased on day 21 (Figure 4B). There were significant differences in the twist gene expression between the two groups from day 7 to day 21 (P<0.05).
Figure 4.
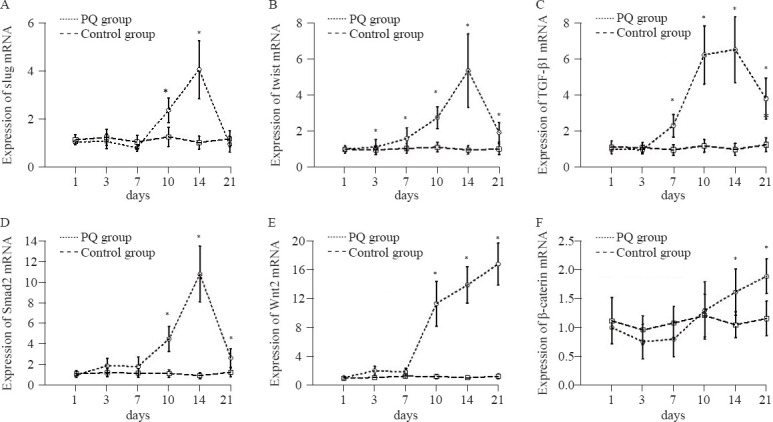
Gene expression of slug (A), twist (B), TGF-β1 (C), Smad2 (D), Wnt2 (E), and β-catenin (F) in lung slices at different time points. Compared with the control group, *P<0.05.
Gene expression of regulators in the TGF-β1-Smad signaling pathway
Increased mRNA levels of TGF-β1 and Smad2 were observed in the PQ group compared with those in the control group from day 7 to day 21 and from day 10 to day 21, respectively (P<0.05). The mRNA levels of TGF-β1 and Smad2 began to increase on day 7 and day 10, respectively, and both peaked on day 14 and decreased on day 21 after PQ poisoning (Figures 4C-D). No significant differences were found in the Smad7 mRNA levels at different time points in either the PQ group or the control group (P>0.05).
Gene expression of regulators of the Wnt2-β-catenin signaling pathway
The expression of Wnt2 mRNA and β-catenin mRNA was increased in the PQ group compared with that in the control group (P<0.05). In the PQ group, the expression of Wnt2 mRNA and β-catenin mRNA suddenly increased on day 10 and day 14, respectively, and peaked on day 21 (P<0.05, Figures 4E–F).
Correlation analysis between observation indices
The protein expression levels of FSP-1 (γ=0.947) and α-SMA (γ=0.829) were positively correlated with CVF, while the expression of E-cad (γ= –0.885) was negatively related with CVF (P<0.05, data not shown).
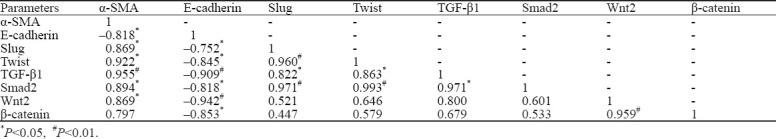
Table 2 demonstrates that gene expression levels of EMT-markers (α-SMA and E-cad) were significantly correlated with those of transcription factors (α-SMA, slug, and twis; E-cad and twist; P<0.05 or P<0.01) and regulators of signaling pathway (α-SMA, TGF-β1, Smad2, and Wnt2; E-cad, TGF-β1, Smad2, Wnt2, and β-catenin; P<0.05 or P<0.01). The mRNA expression of transcription factors was significantly correlated with that of TGF-β1 and Smad2 (P<0.05 or P<0.01), instead of Wnt2 and β-catenin (both P>0.05).
Table 2.
Correlation analysis of gene expression levels between EMT-markers and regulators of signaling pathway
DISCUSSION
In this study, we explored the characteristics of the whole EMT process in PQ-induced PF animal models. The CVF, as a PF marker, significantly elevated from day 3 to day 21 with a peak on day 14, which indicated that the process of PQ-induced PF occurred from day 3 to day 21. Among the PF process, protein and gene expression levels of myofibroblast phenotype markers showed a significant increase in the PQ group compared with those in the control group, while epithelial cell markers (e.g., E-cad) showed a significant decrease. Moreover, the correlation analysis showed that the expression levels of EMT-related markers were positively correlated with PF markers (e.g., CVF), while epithelial cell marker was negatively correlated with CVF. These findings indicated that there was an EMT and PF process during the period from day 3 to day 21, reaching a peak on day 14. Animal studies showed that PQ treatment led to lung fibrosis and increased levels of mesenchymal markers (e.g., α-SMA) at the very early stage within three days[20,23,25] or from day 7 to day 28,[14,22,26] without characteristic changes of PF and EMT markers.
The transcription factors (e.g., slug and twist) that downregulated the levels of tight junction proteins (e.g., E-cad) might be involved in the PF and EMT process in PQ animal models.[14,22,25] In this study, the EMT markers and expression levels of slug and twist mRNA increased significantly from day 3 to day 10, and reached the peak on day 14, and then decreased on day 21. The expression levels of transcription factors were significantly correlated with EMT-related markers (E-cad and α-SMA). Therefore, transcription factors (slug and twist) might play a role in processes of PQ-induced PF and EMT. TGF-β1 was recognized as a profibrotic cytokine that initiated the PF process by multiple signal pathways, and TGF-β1-Smad2 pathway was involved in the PQ-induced EMT process in animal models.[26] In our study, the mRNA expression levels of TGF-β1 and Smad2 increased with the EMT markers in the PQ-induced rat model. Moreover, the expression levels of TGF-β1 and Smad2 were correlated with those of EMT-related markers (E-cad and α-SMA) and transcription factors. Thus, TGF-β1 might participate in the EMT process of the PQ-induced PF through phosphorylating the downstream receptor Smad2 or transcription factors, and triggering EMT-related mRNA expression. These results were supported by some studies, which showed that the anti-TGF-β1 treatment might reverse the developed EMT in PQ-induced cell models with increased mesenchymal markers and decreased epithelial cell markers.[15,27] We found that the Smad7, as a negative regulatory factor of the TGF-β1-Smad2 signal transduction pathway,[28] did not participate in the EMT and PF process as no differences in the Smad7 mRNA expression between the PQ group and the control group were observed. The reason might be the slow response of the negative regulatory mechanism. Therefore, TGF-β1 and Smad2 might be involved in the EMT and PF process from day 3 to day 21 in PQ-induced rat models, and the negative regulatory mechanism might not work within 21 days.
The TGF-β1-β-catenin signaling pathway might join in the early PF process in PQ-poisoned rats.[14] Moreover, the lysyl oxidase (LOX) [21] or Wnt1[29,30] could regulate the PQ-induced EMT by activating β-catenin that participated in the development of PF process in cell models. Our study showed that the mRNA expression of Wnt2 and β-catenin in rat lung dramatically increased with a peak on day 21 after PQ injection. Correlation analysis showed a significant correlation between the gene expression of β-catenin and the Wnt2 and EMT markers. The correlation between gene expression of regulators of the Wnt2-β-catenin pathway and transcription factors or regulators of the TGF-β1-Smad2 pathway was not found. These findings showed that the Wnt2-β-catenin signaling pathway might affect the expression of genes and proteins involved in the EMT process in the PQ rat model through triggering an extracellular signaling pathway that was independent on regulating the TGF-β1, Smad2 or transcription factors. Further studies are required to elucidate the extensive mechanisms of signal pathway in PQ-induced EMT.
CONCLUSIONS
EMT process plays a role in PF induced by PQ in rats, most of PF and EMT markers have a peak phenomenon, and its underlying molecular mechanisms might be determined by further study.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China (81472961), the Natural Science Foundation of Zhejiang Province (LY13H150001), and the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents.
Ethical approval: The animal experiment was approved by the Animal Ethical Committee of the Second Affiliated Hospital of Zhejiang University, Hangzhou, China.
Conflicts of interests: The authors have no competing interests relevant to the present study.
Contributors: JHY and ZCZ contributed equally to this work. All authors read and approved the final version of the manuscript.
REFERENCES
- 1.Sabzghabaee AM, Eizadi-Mood N, Montazeri K, Yaraghi A, Golabi M. Fatality in paraquat poisoning. Singapore Med J. 2010;51(6):496–500. [PubMed] [Google Scholar]
- 2.Zhang Y, Yu B, Wang N, Li T. Acute poisoning in Shenyang, China:a retrospective and descriptive study from 2012 to 2016. BMJ Open. 2018;8(8):e021881. doi: 10.1136/bmjopen-2018-021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Suicide. Available at https://www.who.int/news-room/fact-sheets/detail/suicide .
- 4.Jiang YF, Kang J, Huang PP, Yao JX, Wang ZH, Jiang L, et al. Evaluation of gastric lavage efficiency and utility using a rapid quantitative method in a swine paraquat poisoning model. World J Emerg Med. 2020;11(3):174–81. doi: 10.5847/wjem.j.1920-8642.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Chen Y, Mao L, Zhao GJ, Hong GL, Li MF, et al. Effects of hemoperfusion and continuous renal replacement therapy on patient survival following paraquat poisoning. PLoS One. 2017;12(7):e0181207. doi: 10.1371/journal.pone.0181207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu YG, Lu YQ. Systematic review and meta-analysis of the efficacy and safety of immunosuppressive pulse therapy in the treatment of paraquat poisoning. J Zhejiang Univ Sci B. 2019;20(7):588–97. doi: 10.1631/jzus.B1800640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagiwara S, Iwasaka H, Matsumoto S, Noguchi T. An antisense oligonucleotide to HSP47 inhibits paraquatinduced pulmonary fibrosis in rats. Toxicology. 2007;236(3):199207. doi: 10.1016/j.tox.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML, Carvalho F. Paraquat poisonings:mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38(1):13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 9.Zeisberg EM, Kalluri R. Origins of cardiac fibroblasts. Circ Res. 2010;107(12):1304–12. doi: 10.1161/CIRCRESAHA.110.231910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwano M. EMT and TGF-beta in renal fibrosis. Front Biosci (Schol Ed) 2010;2:229–38. doi: 10.2741/s60. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–28. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabro AT, Minatel IO, Rangel MP, Halbwedl I, Parra ER, Capelozzi VL, et al. Usual interstitial pneumonia and smoking-related interstitial fibrosis display epithelial to mesenchymal transition in fibroblastic foci. Respir Med. 2014;108(9):1377–86. doi: 10.1016/j.rmed.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180(7):657–65. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie H, Tan JT, Wang RL, Meng XX, Tang X, Gao S. Expression and significance of HIF-1αin pulmonary fibrosis induced by paraquat. Exp Biol Med (Maywood) 2013;238(9):1062–68. doi: 10.1177/1535370213498978. [DOI] [PubMed] [Google Scholar]
- 15.Yamada A, Aki T, Unuma K, Funakoshi T, Uemura K. Paraquat induces epithelial-mesenchymal transition-like cellular response resulting in fibrogenesis and the prevention of apoptosis in human pulmonary epithelial cells. PLoS One. 2015;10(3):e0120192. doi: 10.1371/journal.pone.0120192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian R, Zhu Y, Yao J, Meng X, Wang J, Xie H, et al. NLRP3 participates in the regulation of EMT in bleomycin-induced pulmonary fibrosis. Exp Cell Res. 2017;357(2):328–34. doi: 10.1016/j.yexcr.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, Yang X, Xin S, Cao Y, Wang N. Paraquat poisoning induced pulmonary epithelial mesenchymal transition through Notch1 pathway. Sci Rep. 2017;7(1):924. doi: 10.1038/s41598-017-01069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang M, Wang YP, Zhu LQ, Cai Q, Li HH, Yang HF. MAPK pathway mediates epithelial-mesenchymal transition induced by paraquat in alveolar epithelial cells. Environ Toxicol. 2016;31(11):1407–14. doi: 10.1002/tox.22146. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Wang J, Meng X, Xie H, Tan J, Guo X, et al. A positive feedback loop promotes HIF-1αstability through miR-210-mediated suppression of RUNX3 in paraquat-induced EMT. J Cell Mol Med. 2017;21(12):3529–39. doi: 10.1111/jcmm.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J, Qian Y, Jin W, Tian R, Zhu Y, Wang J, et al. Hypoxia-inducible factor-1αregulates epithelial-to-mesenchymal transition in paraquat-induced pulmonary fibrosis by activating lysyl oxidase. Exp Ther Med. 2018;15(3):2287–94. doi: 10.3892/etm.2017.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Tai W, Qu X, Wu W, Li Z, Deng S, et al. Rapamycin protects against paraquat-induced pulmonary fibrosis:activation of Nrf2 signaling pathway. Biochem Biophys Res Commun. 2017;490(2):535–40. doi: 10.1016/j.bbrc.2017.06.074. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Tan J, Xie H, Wang J, Meng X, Wang R. HIF-1αregulates EMT via the snail and β-catenin pathways in paraquat poisoning-induced early pulmonary fibrosis. J Cell Mol Med. 2016;20(4):688–97. doi: 10.1111/jcmm.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhi QM, Sun HC, Qian XM, Nie SN, Xu BH, Tang WJ, et al. Experimental study on lung injury model induced by paraquat poisoning in rats. J Med Postgradu. 2008;21(2):134–36. [Google Scholar]
- 25.Xu L, Xu J, Wang Z. Molecular mechanisms of paraquat-induced acute lung injury:a current review. Drug Chem Toxicol. 2014;37(2):130–4. doi: 10.3109/01480545.2013.834361. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Zhu Y, Tan J, Meng X, Xie H, Wang R. Lysyl oxidase promotes epithelial-to-mesenchymal transition during paraquat-induced pulmonary fibrosis. Mol Biosyst. 2016;12(2):499–507. doi: 10.1039/c5mb00698h. [DOI] [PubMed] [Google Scholar]
- 27.Han YY, Shen P, Chang WX. Involvement of epithelial-to-mesenchymal transition and associated transforming growth factor-β/Smad signaling in paraquat-induced pulmonary fibrosis. Mol Med Rep. 2015;12(6):7979–84. doi: 10.3892/mmr.2015.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie L, Zhou D, Xiong J, You J, Zeng Y, Peng L. Paraquat induce pulmonary epithelial-mesenchymal transition through transforming growth factor-β1-dependent mechanism. Exp Toxicol Pathol. 2016;68(1):69–76. doi: 10.1016/j.etp.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Xu GP, Li QQ, Cao XX, Chen Q, Zhao ZH, Diao ZQ, et al. The effect of TGF-β1 and Smad7 gene transfer on the phenotypic changes of rat alveolar epithelial cells. Cell Mol Biol Lett. 2007;12(3):457–72. doi: 10.2478/s11658-007-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vongphouttha C, Zhu J, Deng S, Tai W, Wu W, Li Z, et al. Rapamycin protects against paraquat-induced pulmonary epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway. Exp Ther Med. 2018;15(3):3045–51. doi: 10.3892/etm.2018.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]


