Abstract
Background
The rate of community antibiotic use in New Zealand (NZ) is high and some may be unnecessary. Non-pharmaceutical public health interventions (Alert Levels) were implemented in 2020 to reduce the spread of COVID-19 in NZ and were likely to have affected antibiotic prescribing.
Methods
We aimed to identify the impact of these public health interventions on community antibiotic dispensing. We also examined rates of hospitalisation with infectious diseases that could be influenced by changing community antibiotic use. A retrospective review of two national databases was undertaken.
Findings
1.17 million people received 1.19 million prescriptions for antibiotics between 23/02/2020 and 18/07/2020. Antibiotic dispensing rates fell from 14 prescriptions per 1000 population per week during pre-Alert Level weeks to 9 prescriptions per 1000 population per week (a reduction of 36%) during the weeks of COVID Alert Level 3–4. Large reductions were seen with antibiotics predominantly used for respiratory- or urinary-tract infections. Hospital discharges with sentinel infections did not increase over this period; pneumonia discharges during Alert Level weeks were lower than in 2017-2019 (3 vs 6 discharges per 100,000 population).
Interpretation
A large reduction in community antibiotic dispensing was observed in NZ during the implementation of non-pharmaceutical public health interventions to eliminate COVID-19. Despite this marked reduction in antibiotic use, there was no increase in rates of hospitalisation for sentinel infections that community antibiotic use could prevent. These findings suggest that countries with high rates of antibiotic use could significantly reduce their use without an increase in morbidity.
Funding
No financial support received.
Keywords: COVID-19, Antimicrobial resistance, Antimicrobial stewardship, Antibiotic, Primary care
Research in context.
Evidence before this study
The level of community antibiotic use is high in New Zealand and in many other countries. Some of this antibiotic use may be inappropriate but there are concerns that a reduction in antibiotic consumption may lead to an increase in hospitalisation due to those infectious diseases which may be prevented by community antibiotic use. The COVID-19 pandemic, and non-pharmaceutical interventions to control COVID-19, have affected community antibiotic use but the consequences of these changes are unknown. We searched PubMed, Medline and Google Scholar on Dec 08 2020, for articles describing the impact of COVID-19, and non-pharmaceutical public health interventions to control COVID-19, on community use of antibiotics using the search terms “2019-nCOV” and “antibiotic” or “antimicrobial” with no time restrictions. Previous research describes changes in community and hospital antibiotic use but does not consider how these changes might have affected morbidity from infectious diseases.
Added value of this study
We analysed large national data sets of community antibiotic dispensing and hospital discharges with specific sentinel infectious diseases (pneumonia, peritonsillar abscess, and rheumatic fever), before and after the implementation of varying intensities of non-pharmaceutical interventions, comparing these to rates from previous years. Community antibiotic dispensing in New Zealand fell significantly during the COVID-19 pandemic. Despite this, there was no increase in the rates of hospitalisation with sentinel infectious diseases that community antibiotic use could prevent. Therefore, a large proportion of community antibiotic use might be inappropriate and large reductions in community antibiotic use are likely to be achievable without increasing morbidity.
Implication of all the available evidence
Despite concerns that reductions in community antibiotic use could result in an increase in hospitalisations with infectious diseases, our study found that community antibiotic dispensing in New Zealand was markedly lower during the early months of the COVID-19 pandemic and that this was not associated with an increase in hospitalisation due to sentinel infectious diseases. It may be possible for antimicrobial stewardship efforts to significantly reduce community antibiotic use without increasing morbidity.
Alt-text: Unlabelled box
Introduction
It is widely accepted that, in many countries, a large proportion of community antimicrobial dispensing is for self-limiting illnesses and provides little or no clinical benefit. However, estimating the magnitude of this antibiotic use is difficult. Comparisons of the overall rate of community antimicrobial dispensing between countries suggest that a large proportion of antibiotic dispensing in many countries is unnecessary. For example, during 2018 the total community antimicrobial consumption in Belgium was 20.8 defined daily doses (DDDs) per 1,000 inhabitants, more than twice that seen in the Netherlands (8.9 DDDs per 1,000 inhabitants), [1]; however, rates of mortality attributable to pneumonia in the two countries were identical. [2] Comparisons of antibiotic prescribing across different populations within a country also suggest a large amount of community antibiotic use is unnecessary. Gulliford et al studied 610 general practices in the United Kingdom (UK) between 2005 and 2014 and found that between 29% and 79% of patients with respiratory tract infections (RTIs) were prescribed an antibiotic.[3] This marked variation is unlikely to be explained by differences in patient populations. It was estimated that a 10% reduction in antibiotic prescribing for RTIs at an average UK general practice with 7000 patients would result in approximately 1 more case of pneumonia per year and 1 more case of peritonsillar abscess (quinsy) each decade. [3]
Extraordinary events that result in large changes in the rates of general practice consultation across a country provide an opportunity to study the health impact of large changes in antibiotic dispensing within a country. In March 2020, the New Zealand (NZ) government implemented a tiered system of non-pharmaceutical public health interventions (COVID-19 Alert Levels 2 to 4) to manage and minimise the risk of COVID-19 transmission in the community.[4] The interventions were applied uniformly across the country and resulted in major reductions in social interactions.
During Alert Level 2, people were requested to physically distance and public gatherings were restricted to no more than 100 people. During Alert Level 3, people were additionally requested to restrict their social contacts to members of their immediate household and were encouraged to work from home, most businesses were prohibited from physically interacting with the public, public venues were closed, and gatherings were limited to no more than 10 people. During Alert Level 4, people were requested to stay within their usual residence, all but essential businesses were closed, all educational facilities and public venues were closed, and all gatherings were prohibited. The stringency of government response in NZ was significantly higher than seen in other countries such as the United States of America or the UK. [5] While these interventions imposed major restrictions on many routine activities, hospital services and General Practitioners (GPs) remained available to provide healthcare, although virtual appointments via telephone or internet services were used where physical review was not required. Rates of GP attendances are not centrally reported, but reports suggested a large reduction in visits to general practices during this period. [6]
Although antibiotic use has declined by 4.6% over the last 5 years in NZ, rates remain higher than in countries such as the UK or the Netherlands. [7] Furthermore, there is substantial seasonal variation in New Zealand community antibiotic dispensing, which increases in winter months by 25–35%, corresponding with a rise in viral upper respiratory tract infections. [8] Reducing unnecessary antibiotic use for viral upper respiratory tract infections is an important antimicrobial stewardship goal. However, there are concerns that reducing antibiotic use could lead to a rise in preventable infectious complications. In particular there is considerable concern in New Zealand that reduced access to medical care might result in a rise in the incidence of many infectious diseases, including rheumatic fever, in disadvantaged Māori and Pacific communities.[9]
We examined community antibiotic dispensing and simultaneous changes in the incidence of pneumonia, peritonsillar abscess and rheumatic fever during the different phases of the non-pharmaceutical public health interventions introduced in NZ to reduce the spread of COVID-19.
Methods
Data were obtained to correspond with the weeks preceding the introduction of the COVID-19 Alert Level system on 21/3/2020, for the weeks of public health interventions, and for 5 weeks following the step down to the lowest alert level, Level 1, on 9/6/2020. Data are displayed with alert levels for 2020 shown (Level 2 (L2): 21/3/2020; Level 3 (L3) 23/3/2020; Level 4 (L4): 25/3/2020; Return to Level 3 (L3): 27/4/2020; Return to Level 2(L2): 13/5/2020; and Level 1(L1): 9/6/2020). When the alert level changed during a week, the level in place for the majority of that week is displayed.
Data were obtained from two national healthcare databases managed by the New Zealand Ministry of Health. Data on all subsidised antibiotic medicines dispensed in the community, from 23/02/2020 to 18/07/2020, and for the same periods during 2017, 2018 and 2019, were obtained from the National Pharmaceutical Collection. [10] Data includes all dispensings generated either from in-hours or out-of-hours practices. Pharmaceuticals in this data set were classified under the Anatomical Therapeutic Chemical (ATC) system. Antibiotic dispensing was grouped, by dispensing date, into weeks of each calendar year (Week 9 to Week 29). Antibiotic dispensing was measured as number of prescriptions dispensed per 1000 population and, for ethnicity analyses, as number of people dispensed one or more antibiotic prescriptions per 1000 population. Rates were calculated using population estimates for 30 June 2017, 2018, 2019 and 2020 obtained from StatsNZ Tatauranga Aotearoa. [11] The database also included information on each patient's prioritised ethnicity, which was reported as Māori, Pacific, Asian or Other.
Data on hospital discharges were obtained from the National Minimum Dataset (NMDS) for weeks 9 to 29 for 2017, 2018, 2019 and 2020. Discharges were described using ICD-10 codes and other variables as defined in the NMDS data dictionary. [12] Data were obtained for all hospital discharges coded for pneumonia (ICD-10 J12-18), peritonsillar abscess (ICD-10 J36) or acute rheumatic fever (ICD-10 I00-I02) together with ethnicity, length of hospital stay, and hours of intensive care unit (ICU) stay. Discharges were reported per 100,000 population using population estimates as described above.
Antibiotics were classified by spectrum of activity: broad spectrum (J01(CR+DC+DD+(F-FA01)+MA)), narrow spectrum (J01(CA+CE+CF+DB+FA01)), or other, using the European Centre for Disease Prevention and Control (ECDC) definitions [13] and also classified by the predominant indication for use in NZ.
Specific periods of interest were defined: prior to the introduction of alert levels (weeks 9–12), during the period when stringent non-pharmaceutical interventions - alert levels 3 and 4 (weeks 13–20) were in place, and during the period when most restrictions were lifted alert levels 1 and 2(weeks 20–29). Antibiotic dispensing rates and hospital discharge rates for a given period in 2020 were compared with other periods in 2020 or with the corresponding period from previous years. Formal statistical comparisons were not undertaken as any changes observed were based on whole population data and considered error free.
Antibiotic dispensing rates and hospital discharge time trend data were displayed using GraphPad Prism version 9.0.0.
Ethical approval was not required under the Health and Disability Ethics Committee criteria.
Role of the funding source
The authors received no financial support for the research, authorship, and/or publication of this article.
Results
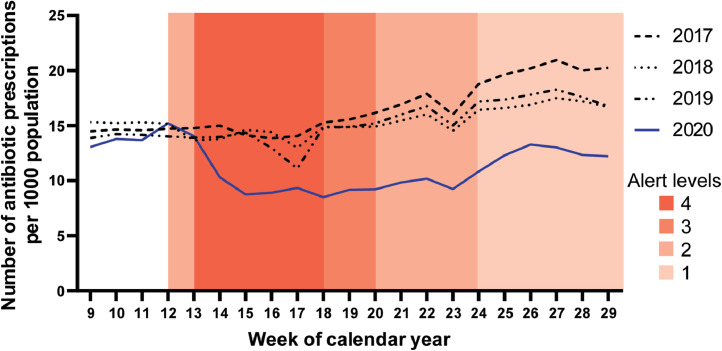
1.17 million people were dispensed 1.19 million prescriptions for antibiotics during the 20-week period between 23/02/2020 and 18/07/2020. The number of antibiotic dispensings per 1000 population for 2017 to 2020, together with the periods in which each of the COVID-19 Alert Levels were employed during 2020, are shown in Fig. 1. Compared to previous years, in 2020 antibiotic dispensing rapidly declined by 35.6%, from a mean of 13.93 prescriptions per week per 1000 population prior to COVID-19 public health interventions (weeks 9 to 12) to a mean of 8.97 prescriptions per week per 1000 population during COVID-19 Alert Levels 3 and 4 (weeks 15 to 20). Antibiotic dispensing gradually increased during early winter 2020 (weeks 21 to 29), consistent with the pattern seen in the same periods during 2017, 2018 and 2019. However, the rate of antibiotic dispensing remained lower in 2020 than during the same weeks in 2017, 2018 and 2019 (Table 1).
Fig. 1.
Number of antibiotic prescriptions dispensed per 1000 population by week of calendar year; weeks 9 to 29 of 2017 to 2020.
Table 1.
Median weekly antibiotic dispensing, per 1000 population, during calendar weeks of Alert Level 3 – 4 (weeks 15 to 20) from 2017 to 2020.
| Antibiotic dispensings per 1000 population | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|
| All antibiotic prescriptions | 14.84 | 14.45 | 13.89 | 8.97 |
| Broad spectrum antibiotic prescriptions | 4.50 | 4.28 | 3.90 | 2.68 |
| Narrow spectrum antibiotic prescriptions | 7.85 | 7.71 | 7.62 | 4.45 |
| Antibiotics predominantly prescribed for respiratory tract infections | 8.75 | 8.41 | 8.14 | 4.55 |
| Antibiotics predominantly prescribed for urinary tract infections | 2.24 | 2.17 | 1.91 | 1.45 |
| Antibiotics predominantly prescribed for skin and soft tissue infections | 2.71 | 2.79 | 2.72 | 2.12 |
| Antibiotics predominantly prescribed for sexually transmitted infections | 0.01 | 0.02 | 0.02 | 0.02 |
| Antibiotics predominantly prescribed for acne | 1.12 | 1.06 | 1.10 | 0.84 |
| Māori people dispensed antibiotic prescriptions | 16.49 | 15.83 | 14.76 | 9.50 |
| Pacific people dispensed antibiotic prescriptions | 17.04 | 15.81 | 16.34 | 8.21 |
| Asian people dispensed antibiotic prescriptions | 8.72 | 8.34 | 8.56 | 4.05 |
| Other people dispensed antibiotic prescriptions | 15.19 | 15.04 | 14.35 | 9.99 |
Antibiotic dispensing in relation to spectrum of activity
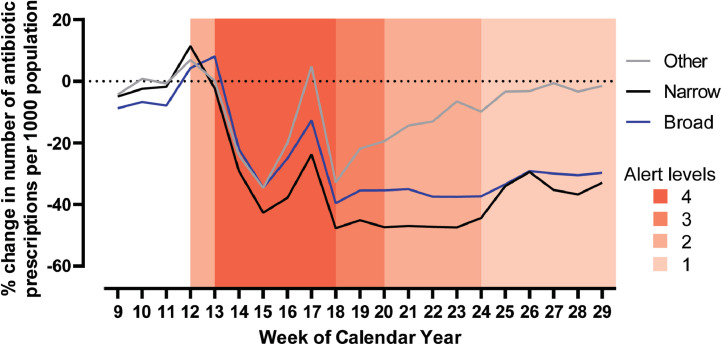
Reductions in antibiotic dispensing were seen for narrow spectrum, broad spectrum and other antibiotics (Fig. 2). During COVID-19 Alert Levels 3 and 4 (weeks 13–20 in 2020) there was a -29.4% change in dispensing of narrow spectrum antibiotics, a -21.4% change in dispensing of broad-spectrum antibiotics, and a -15.7% change in dispensing of other antibiotics in comparison with the same period in 2019. During weeks 13–20 of 2020, the mean weekly percentage of all community antibiotics that were broad spectrum was 29.8%, an increase from the same period in 2019 (28.0%). Use of narrow spectrum antibiotics, as a weekly proportion of all antibiotic use, which had previously been increasing from 2017 (53%) to 2019 (54.9%) fell in 2020 to 50.4%.
Fig. 2.
Percentage change in number of narrow, broad and other spectrum antibiotic prescriptions dispensed per 1000 population by week of calendar year; weeks 9 to 29 in 2020 compared to 2019.
Variability in change in antibiotic dispensing for different indications
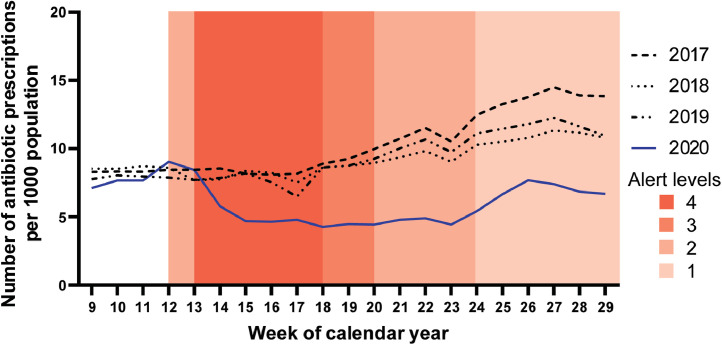
The magnitude and the duration of the reductions in antibiotic dispensing varied by antibiotic agent. When antibiotic dispensing during COVID-19 Alert Levels 3 and 4 (weeks 13–20 in 2020) was compared with the same period in 2019, there was a large and sustained reduction in the dispensing of penicillin, amoxicillin, amoxicillin-clavulanate, erythromycin and roxithromycin (agents used in NZ predominantly for treatment of RTIs). (Fig. 3) There was also a reduction in dispensing of trimethoprim, co-trimoxazole and nitrofurantoin (agents used in NZ predominantly for treatment of urinary tract infections). There was no apparent reduction in the dispensing of ceftriaxone or azithromycin (agents used in the community in NZ predominantly for treatment of sexually transmitted infections), a minor brief reduction in the dispensing of doxycycline (an agent used in NZ predominantly for treatment of acne), and a modest brief decline in the dispensing of flucloxacillin and cefalexin (agents used in NZ predominantly for treatment of skin and soft tissue infections) (Supplementary Figure 1).
Fig. 3.
Number of prescriptions for antibiotics, predominantly used to treat respiratory tract infections, dispensed per 1000 population by week of calendar year; weeks 9 to 29 of 2017 to 2020.
Antibiotic dispensing in different ethnic groups
For each ethnicity, the rate of antibiotic dispensing prior to COVID-19 public health interventions (weeks 9–11 in 2020) was consistent with that in previous years (Fig. 4). However, there were marked differences between ethnicities in their mean levels of antibiotic dispensing prior to COVID-19 public health interventions: 14.80 prescriptions per week per 1000 population in Māori people, 15.46 prescriptions per week per 1000 population in Pacific people, 7.17 prescriptions per week per 1000 population in Asian people, and 13.91 prescriptions per week per 1000 population in people from other ethnic groups.
Fig. 4.
Number of people dispensed an antibiotic, by ethnic group, per 1000 population by week of calendar year; weeks 9 to 29 of 2017 to 2020. A: Māori, B: Pacific people, C: Asian, D: People of other ethnicities.
The reductions in mean antibiotic dispensing during COVID-19 Alert Levels 3 and 4 (weeks 13–20 in 2020), when compared with the same period in 2019, were 29% for Māori, 44% for Pacific people, 47% for Asian people, and 25% for people from other ethnic groups. (Fig. 4)
Hospital discharges for patients with pneumonia, peritonsillar abscess, and rheumatic fever
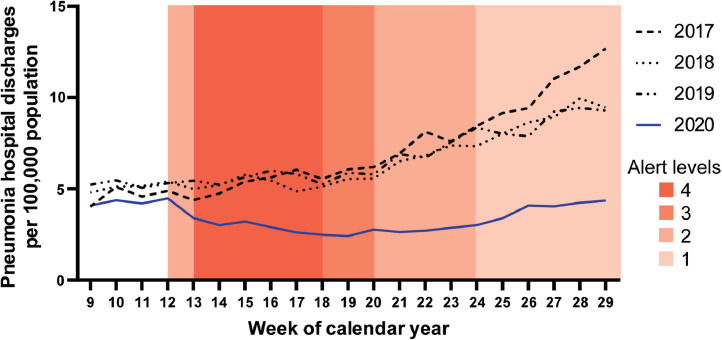
The mean weekly rates of discharge from hospital with pneumonia per 100,000 population during COVID-19 Alert Levels 2, 3, and 4 (weeks 12-24) were significantly lower during 2020 than during 2017-2019 (p < 0.0001) (Fig. 5). There was a small change in mean ICU stay per case of pneumonia admitted to ICU (5.5 days during 2020 vs 4.5 days during 2019); and in mean hospital length of stay from 2.8 days in 2019 to 3.4 days in 2020.
Fig. 5.
Number of hospital discharges with pneumonia per 100,000 population by week of calendar year; weeks 9 to 29 of 2017 to 2020.
There was a reduction in hospital discharges with peritonsillar abscess per 100,000 population (0.17 during 2020; vs 0.33 during 2017-2019) during the COVID-19 Alert Levels 2, 3 and 4. (Supplementary Figure 2A) ICU admissions were rare for peritonsillar abscess so rates were not calculated (2020 n = 1 admission; 2019 n = 2 admissions). There was a clinically unimportant difference in mean hospital length of stay during 2020 when compared to the equivalent weeks during 2019 (1.54 days during 2020 vs 1.31 days during 2019)
The mean weekly rates of hospital discharge with acute rheumatic fever per 100,000 population during COVID-19 Alert Levels 2, 3 and 4, differed trivially from the rates in 2017–2019. (0.116 during 2020 vs 0.109 during 2017–2019) (Supplementary Figure 2B) There was no change when analysed by ethnicity for Māori and Pacific People, who accounted for 95% of rheumatic fever cases but represented 24% of the population.
Discussion
Concern has been raised that the liberal use of antibiotics during the pandemic could worsen antimicrobial resistance. [14] In NZ, community antibiotic dispensing fell by more than one third during COVID-19 Alert Levels 2, 3, and 4, to rates approaching the pre-COVID-19 rates seen in Denmark, long viewed as a world leader in terms of appropriate community antibiotic use. [15] Antibiotic dispensing decreased for broad and narrow spectrum antibiotics, and for antibiotics predominantly used for skin and urinary tract infections. The most pronounced effect was seen in antibiotics predominantly used for respiratory tract infections. This marked reduction was likely to have been the result of changes in accessing healthcare, and the profound effect that COVID-19 public health measures had on reducing the incidence of community respiratory tract infections caused by other viruses, although factors such as improved public awareness of the differing treatment approaches for bacterial and viral respiratory tract infections are likely to have contributed. [4,16] Little or no effect was seen in antibiotics for acne or sexually transmitted infections suggesting a generalised reduction in antibiotic prescribing did not occur. It would be interesting to compare patterns of antibiotic use in NZ with other countries that experienced a higher burden of COVID-19, that had higher rates of other circulating respiratory viruses, and that employed less stringent non-pharmaceutical interventions.
The speed and magnitude of the reductions in community antibiotic use in NZ were much greater than have previously been observed with interventions intended to improve antimicrobial stewardship. For example, a five-year national campaign in France between 2002 and 2007, resulted in a 10% reduction in total community antibiotic dispensing during the first year and a 21% reduction after five years.[17] National campaigns in the UK, Australia and NZ have led to recent annual reductions in total community antibiotic dispensing of 2.1%, 6.5% and 4.6% respectively. [7,18,19] The 35.6% reduction in community antibiotic dispensing in NZ occurred rapidly, over a period of three weeks, and was much larger.
Antimicrobial Stewardship is not simply the drive to reduce total antibiotic use but to optimise patient safety and outcomes. This includes mitigating the risk to patients of antibiotic use (e.g. adverse effects, super-infection and antimicrobial resistance) but also ensuring antibiotics are used when required and monitoring the adverse effects of under-use. Reducing community antibiotic dispensing commonly leads to concerns about the likelihood of consequent increases in morbidity or mortality due to infectious diseases.[3] We selected three sentinel conditions to study: pneumonia, peritonsillar abscess and rheumatic fever. Previous research had suggested that a 10% reduction in GP prescribing of antibiotics for patients with respiratory tract infections at an average general practice in the UK might result in a modest increase in the incidence of pneumonia and of peritonsillar abscess. [3] Rheumatic fever is an important condition that contributes to health inequity in NZ. Despite a 36% reduction in total antibiotic use, we found no increase in the rates of admission to hospital either for pneumonia, peritonsillar abscess or rheumatic fever. Instead, the rates of admission for pneumonia and peritonsillar abscess declined while the rate of admission for rheumatic fever remained unchanged. Although differences in access to healthcare and in the medical management of patients with many conditions during the course of 2020 could have affected the rates of admission for these conditions, our findings suggest that the very large reductions in community antibiotic dispensing in NZ were not associated with increased morbidity. Further, there was no increase in all-cause mortality in NZ during this period. [20]
Antimicrobial stewardship efforts must balance the need to improve and reduce overall antibiotic use with continued access to antibiotics for populations who suffer disproportionately from infectious diseases and their complications. In NZ Māori and Pacific people are disproportionately affected by infectious disease and it has been argued that, given the high burden of infectious diseases in Māori and Pacific people, the rates of antibiotic consumption in these groups should be higher than in people from other ethnic groups to ensure equitable health outcomes.[9] We have suggested that the appropriate rate of antibiotic dispensing for Māori and Pacific people in NZ should approximate 17.5 dispensings per 1000 population per week.[8] This suggested rate of dispensing for Māori and Pacific people was similar to what occurred during COVID-19 Alert Levels 2, 3 and 4 (21.1 dispensings per 1000 population per week). Importantly, the large reduction in antibiotic dispensing did not lead to an increase in the incidence of hospital admission for pneumonia, or peritonsillar abscess or rheumatic fever for Māori or Pacific people (Supplementary Fig. 3); in fact, the rate of admissions for pneumonia and peritonsillar abscess among Māori and Pacific people declined.
The limitations of this study include using dispensing as a surrogate for antibiotic use and the lack of data on the indication for the dispensing. Dispensing data are presented as the number of prescriptions per population and do not quantify the amount of antibiotic dispensed per prescription. Non-subsidised antibiotic prescriptions are not included in the data set although these prescriptions are expected to comprise a small minority of antibiotics dispensed in NZ. [21] Hospital discharge rates with urinary tract infections were not reported as the reduction in the use of antibiotics for this indication was unexpected and the rate of hospital discharge with urinary tract infections was not a pre-specified outcome variable in the data extract.
These data suggest that NZ's high rate of pre-COVID-19 antibiotic dispensing, much of which is for people with self-limiting respiratory tract infections, [22] could be reduced significantly without risk of adverse consequences secondary to under-treatment of serious bacterial infections such as peritonsillar abscess or bacterial pneumonia. Other well-resourced countries with similarly high rates of inappropriate antibiotic use should be able to achieve similar reductions in antibiotic dispensing without increasing the incidence of admission to hospital for infectious diseases.
Declaration of Competing Interest
All authors have no conflicts of interest to declare.
Acknowledgments
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Date sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Author contribution
E.D., M.T. and S.R. conceived and designed the study. E.D and T.H. analysed the data. E.D, M.T., T.H. and S.R wrote the manuscript. The corresponding author, as guarantor, attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted
Footnotes
The lead author* (the manuscript's guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100162.
Contributor Information
Eamon Duffy, Email: eamond@adhb.govt.nz.
Mark Thomas, Email: mg.thomas@auckland.ac.nz.
Thomas Hills, Email: tom.hills@mrinz.ac.nz.
Stephen Ritchie, Email: s.ritchie@auckland.ac.nz.
Appendix. Supplementary materials
References
- 1.European Centre for Disease Prevention and Control. Trend of antimicrobial consumption by country. At: https://www.ecdc.europa.eu/en/antimicrobial-consumption/database/trend-country
- 2.Marshall DC, Goodson RJ, Xu Y. Trends in mortality from pneumonia in the Europe union: a temporal analysis of the European detailed mortality database between 2001 and 2014. Respir Res. 2018;19(1):81. doi: 10.1186/s12931-018-0781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulliford MC, Moore MV, Little P. Safety of reduced antibiotic prescribing for self limiting respiratory tract infections in primary care: cohort study using electronic health records. BMJ. 2016 Jul 4;354:i3410. doi: 10.1136/bmj.i3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jefferies S, French N, Gilkison C. COVID-19 in New Zealand and the impact of the national response: a descriptive epidemiological study. The Lancet Public Health. 2020 Nov 1;5(11):e612–e623. doi: 10.1016/S2468-2667(20)30225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roser M, Ritchie H, Ortiz-Ospina E et al (2020) - "Coronavirus Pandemic (COVID-19)". Published online at OurWorldInData.org. Retrieved from: 'https://ourworldindata.org/coronavirus' [Online Resource] on 8 October 2020
- 6.GP Pulse Newsletter; 2 April 2020. Royal New Zealand college of general practitioners. https://www.rnzcgp.org.nz/GPPulse/RNZCGP/News/College_news/2020/New-Zealands-GPs-are%20a-workforce-in-crisis.aspx. [Google Scholar]
- 7.Thomas M, Tomlin A, Duffy E. Reduced community antibiotic dispensing in New Zealand during 2015–2018: marked variation in relation to primary health organisation. NZ Med J (Online) 2020 Jul 17;133(1518):33–35. [PubMed] [Google Scholar]
- 8.Whyler N, Tomlin A, Tilyard M. Ethnic disparities in community antibacterial dispensing in New Zealand, 2015. NZ Med J. 2018;131(1480):50–60. [PubMed] [Google Scholar]
- 9.Metcalfe S, Vallabh M, Murray P. Ethnic inequities in community antibacterial prescribing. N Z Med J. 2019;132(1488):65–68. [PubMed] [Google Scholar]
- 10.New Zealand ministry of health. National Pharmaceutical Collection. At: https://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/pharmaceutical-collection
- 11.Statistics New Zealand. NZ.Stat: National ethnic population projections, by age and sex, 2013(base)-2038 update. At: http://nzdotstat.stats.govt.nz/retrieved on 8 October 2020
- 12.New Zealand Ministry of Health. National Minimum Dataset. At: https://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/national-minimum-dataset-hospital-events
- 13.ECDC EFSA Panel on Biological Hazards (BIOHAZ), EMA Committee for Medicinal Products for Veterinary Use (CVMP). ECDC, EFSA and EMA Joint Scientific Opinion on a list of outcome indicators as regards surveillance of antimicrobial resistance and antimicrobial consumption in humans and food-producing animals. Efsa J. 2017;15(10):e05017. doi: 10.2903/j.efsa.2017.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strathdee SA, Davies SC, Marcelin JR. Confronting antimicrobial resistance beyond the COVID-19 pandemic and the 2020 US election. Lancet North Am Ed. 2020 10;396(10257):1050–1053. doi: 10.1016/S0140-6736(20)32063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korsgaard HB, Ellis-Iversen J, Hendriksen RS, et al. DANMAP 2019-Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark.
- 16.Leis JA, Born KB, Theriault G. BMJ. 2020. Using antibiotics wisely for respiratory tract infection in the era of covid-19; p. 371. [DOI] [PubMed] [Google Scholar]
- 17.Sabuncu E, David J, Bernède-Bauduin C, Pépin S. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLoS Med. 2009;6(6) doi: 10.1371/journal.pmed.1000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Public Health England. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR). Report 2018–2019. At: http://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/843129/English_Surveillance_Programme_for_Antimicrobial_Utilisation_and_Resistance_2019.pdf
- 19.Australian commission on Safety and Quality in Health Care (ACSQHC) ACSQHC; Sydney: 2019. AURA 2019: third Australian report on antimicrobial use and resistance in human health.www.safetyandquality.gov.au/antimicrobial-use-and-resistance-in-australia/resources-page/ At: [Google Scholar]
- 20.Kung S, Doppen M, Black M. Reduced mortality in New Zealand during the COVID-19 pandemic. Lancet North Am Ed. 2020 doi: 10.1016/S0140-6736(20)32647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horsburgh S, Malik M, Norris P et al. Technical report No. 001/09. Prescribing and dispensing data sources in New Zealand: their usage and future directions. University of Otago. Available at: https://www.researchgate.net/publication/45637891_Prescribing_and_dispensing_data_sources_in_New_Zealand_their_usage_and_future_directions
- 22.Hobbs MR, Grant CC, Ritchie SR. Antibiotic consumption by New Zealand children: exposure is near universal by the age of 5 years. J Antimicrob Chemother. 2017;72(6):1832–1840. doi: 10.1093/jac/dkx060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.