Abstract
Foods frequently eaten supply both micro and macro nutrients to humans which are important in the total assessment of public health status of an individual. The analysis of these foods will provide evidence on their nutritional values, guide to appropriate choice of meal and encourage intake of varieties of food with better qualities during illness while preventing diet-associated disorders. In this study, the proximate and mineral composition of unripe, naturally ripe and the effects of ripening agents on plantain (Musa paradisiaca) commonly consumed in Nigeria were examined. The plantain fruits were analysed for proximate and mineral composition. Proximate composition analysis revealed an increase in moisture content and fat content for all the plantain ripened with ripening agents when compared with the naturally ripened plantain. Furthermore, the mineral composition of the plantain fruits was determined by means of Atomic Absorption Spectrophotometry (AAS). The result showed that plantain is a good source of minerals such as calcium (Ca), potassium (K) and iron (Fe). A relatively high level of K of 1690.55 ± 0.02; 1672.35 ± 0.03 mg kg−1 were found for both unripe and natural ripe plantain while the ripening agents had K values of 1677.45 ± 0.01; 1656.10 ± 0.02; 1589.45 ± 0.01 mg kg−1 for Ethylene glycol, Potassium Dihydrogen Phosphate, Calcium carbide respectively. Also, low level of Fe was obtained in plantain ripened with the different ripening agents.
Keywords: Micro and macro nutrients, Proximate and mineral composition, Nutritional quality
Micro and macro nutrients; Proximate and mineral composition; Nutritional quality.
1. Introduction
Plantain (Musa paradisiaca) is one of the fruit crops that are of high importance to people of Nigeria. It serves as food for human beings; while the peels serve as feed for animals (Adeyemi et al., 2018). The benefits of plantain to man and animals has increased the demand for plantain in various parts of Nigeria. Ripe and unripe plantain contains substantial amount of essential nutrients in a high proportion, antioxidants and other biologically active substances that makes them effective in treatment of a number of diseases (Habauzit and Horcajada, 2008; Tucker, 2009; Park et al., 2011; Maduwanthi and Marapana, 2019) as well as prevention of some diseases (e.g. goiter) and keeps the consumer energetic throughout his life (Zahir et al., 2009). Plantain contains high quantities of chemically active compounds that have positive effects on human health status in particular such as phenolic compounds, polysaccharides, sugars, vitamins, minerals and organic acids which provide their good taste and excellent medicinal properties (Hui et al., 2006). Plantain as contained in Food and Agricultural Organization (FAO) report (2003) can be eaten in many forms either ripe or unripe (Food and Agricultural Organization, 2003). Plantain is an economic crop of which parts of the crop plant can be used as food, fodder or as raw materials by some industries, majorly for manufacturing acids (Ekunwe and Ajayi, 2010; Nura et al., 2018). The leaves are also used for wrapping food items. Also, it is free from cholesterol, high in fibre and low in sodium (Adewole and Duruji, 2010). High potassium content found in plantain made it useful in the prevention of High blood pressure and muscle cramp (Ng and Fong, 2000).
Fruits account for a significant fraction of the world's agricultural output (Per et al., 2007). Research revealed that the health enriching factors of some fruits are as a result of the additive and synergistic combinations in a mixture of phytochemicals with a lot of them possessing potent antioxidant capabilities including ascorbic acid, flavonoids, carotenoids etc (Liu, 2003; Nationat Agency For Food and Drug Administration (NAFDAC), 2018; Ojetayo et al., 2018; Singh et al., 2018). In the process of hastening fruit ripening, ripening agents are used by fruit sellers as well as farmers in some emerging economies (Nigeria inclusive) (Dudley, 2004). A number of natural/chemical ripening agents are available and have been applied recurrently on fruits to hasten ripening process, but pure taste and nutritional values have become casualties of the process. According to Singh and Janes (2001) the fast ripening of fruits could lead to production of harmful substances such as ethylene. Consequently, this study was aimed at investigating the effects of selected ripening agents on the proximate, nutritional value and mineral composition of plantain fruit.
2. Materials and methods
The chemicals and reagents used in this study were of analytical grade. The reagents include; ethanol, ammonia, diethyl ether, petroleum ether, 0.255 N H2SO4, 0.313 N NaOH, conc. H2SO4 (98 %), conc. HCl (35.5 %), potassium sulphate, cupric sulphate, selenium, boric acid solution, phenolphthalein indicator.
2.1. Sample collection and preparation
Freshly harvested bunch of unripe plantain was purchased from Osiele market, Abeokuta, Ogun state, Nigeria. The plantain samples were washed with clean water, air dried, and kept in clean polyethylene bags before treatment with calcium carbide, ethylene glycol and potassium dihydrogen phosphate (PDP) to induce the ripening process. 5 g of each of calcium carbide and PDP was weighed and dissolved separately in 20 mL of deionized water and 10 mL of ethylene glycol were sprinkled separately on each set of 4 plantains for each group while the control (naturally ripened) was sprinkled with deionized water and allowed to ripe naturally. The CaC2 sample group was treated for a week to fully ripe while PDP and ethylene glycol groups were treated for two weeks to ripe. After ripening, the samples were cut opened to obtain the edible part and separated from the peel. The edible part was sliced into pieces and was oven dried for 24 h at 60 °C. After oven drying, the samples were then pulverized to fine powder using mortar and pestle. The pulverized samples were stored in air tight bags for further analysis.
2.2. Proximate analysis
2.2.1. Determination of moisture content
The moisture content of the plantain samples was determined using oven dry method (Abano and Amoah, 2011). Briefly, 1 g of the sample was taken into a pre-weighed crucible, oven dried at 105 °C for 4 h then cooled and reweighed. The weight difference (wet weight – dry weight) is the moisture content in the sample.
2.2.2. Determination of fat content
The percentage fat content was determined by Rose-Gottlieb method. Briefly, 5 g of the sample was weighed into 5 mL of distilled water and transferred into a separating funnel, then 2 mL of ammonia solution and 10 mL of ethanol was added to aid breakdown of the protein content. 25 mL of diethyl ether-petroleum ether solution (1:1) was then added as the extracting solvent and shook vigorously; after separation, the upper layer was decanted into a flask (repeated thrice and combined) and boiled in a water bath for few minutes. This was oven dried at 60 °C until the solvent has completely dried.
2.2.3. Determination of ash content
About 1.0 g of the sample was taken into a pre-weighed crucible and placed in the muffle furnace at 300 °C for 30 min to pre-ash. Thereafter, the temperature was raised to 500 °C for 4 h. The crucible were cooled and reweighed.
2.2.4. Determination of crude fibre
2.0 g of the sample was weighed into 100 mL of 0.255 N H2SO4 and heated under reflux for 1 h. The hot mixture was filtered through a sieve and the residue was transferred into 100 mL of 0.313 N NaOH. The mixture was further heated under reflux for additional 1 h, again the mixture was filtered followed by addition of 10 mL of acetone to dissolve any undissolved organic compound. The residue was washed with about 50 mL hot water and transferred into a crucible. The crucible and its contents were oven dried at 105 °C overnight, cooled and then weighed. The crucible was then ignited in the muffle furnace at 550 °C for 4 h, cooled and reweighed. The loss in weight was then noted and taken as crude fibre content (AOAC, 1990).
2.2.5. Determination of protein content
The protein content was assessed by Kjeldahl method (AOAC, 1990). Briefly, 2.0 g of oven dried plantain sample was taken into a digestion flask with subsequent addition of 25 mL concentrated sulphuric acid, 0.1 g of CuSO4 and 1.0 g of selenium (tablet). This was heated until the appearance of greenish clear solution. After cooling for about 30 min, the digested sample was poured into a 100 mL volumetric flask and made up to the mark with distilled water. 10 mL of boric acid-indicator solution was put under a condenser such that the tip of the condenser was totally dipped in the solution. The digested sample was transferred through the funnel stopcock on the steam jacket into the chamber and 20 mL of NaOH was added. The funnel stopcock and the stopcock on the steam trap outlet was closed which compelled steam through the decomposition chamber and in the process pushed the liberated ammonia into the collection flask. After few minutes, the receiving flask was lowered such that the condenser tip was just above the liquid and later rinsed with distilled water. About 10 mL of the distillate (ammonia borate solution) was titrated with 0.1 M HCl solution. The titre values of the duplicate samples were noted and the percentage protein calculated equation Eq. (1):
| (1) |
2.2.6. Determination of carbohydrate content
The carbohydrate content was empirically determined using Eq. (2) (AOAC, 2002):
| (2) |
2.2.7. Determination of total titratable acidity (TTA)
The TTA was determined according to the AOAC Official Method. 942.15 (AOAC, 2000). Briefly, 100 mL of aliquot sample containing 3 drops of phenolphthalein indicator was titrated against 0.1 M NaOH. Titration was done in triplicates and titre values was recorded. The result was expressed in gram acetic acid/100 g of fruit stem (Ozgur and Koyuncu, 2002) and references therein.
2.2.8. Determination of pH
The pH meter was calibrated using buffer solutions of 4, 7 and 10. The pH of the sample was determined by immersing the standardized electrode tip of the pH meter into the aliquot sample. The observed pH was read and recorded.
2.2.9. Determination of total soluble solid (TSS)
A portion of the aliquot sample was poured into 100 mL of distilled water and filtered. The residue was weighed and dried in the oven at 105 °C for 1 h, then cooled and reweighed. The change in weight was expressed in ppm.
2.3. Elemental determination
2.0 g of the powdered sample was weighed and 20 mL of Aqua Regia (3:1) was added and heated for 1 h. The digested sample was filtered into a 50 mL volumetric flask and made up to the mark with distilled water. The samples were run in an Atomic Absorption Spectrophotometer for the determination of the elemental composition (potassium (K), calcium (Ca), Iron (Fe)) in mg Kg−1 using 7 Point Calibration curve and the limit of quantification was taken as the least concentration of the calibration curve at 0.1 ppm. Relevant references of previous workers who used this method and references therein have been cited where necessary.
2.4. Sensory evaluation
Sensory evaluation of the samples was achieved by a highly cited method described by Ihekeronye and Ngoddy (1985). A 10-member panel was educated on sensory characteristics for the evaluation. The evaluations were based on the richness of organoleptic quality attributes of taste, colour, flavour, texture and overall acceptability using a 9-point hedonic scale, 9 for liked extremely and 1 for disliked extremely. Samples were coded for the participating judges’ ratings (Onwuka and Onwuka, 2005; Braghieri et al., 2015; Wang et al., 2019).
Ethical approval was sought and approval given by the Department of Chemistry, Federal University of Agriculture, Abeokuta, Nigeria, Ethical Committee and the consent of all evaluators was obtained.
2.5. Statistical analyses
The generated data were subjected to analysis of variance (ANOVA) at 5% level of significance using OriginPro 2019b.
3. Results and discussions
3.1. The Physico-chemical analysis of the samples
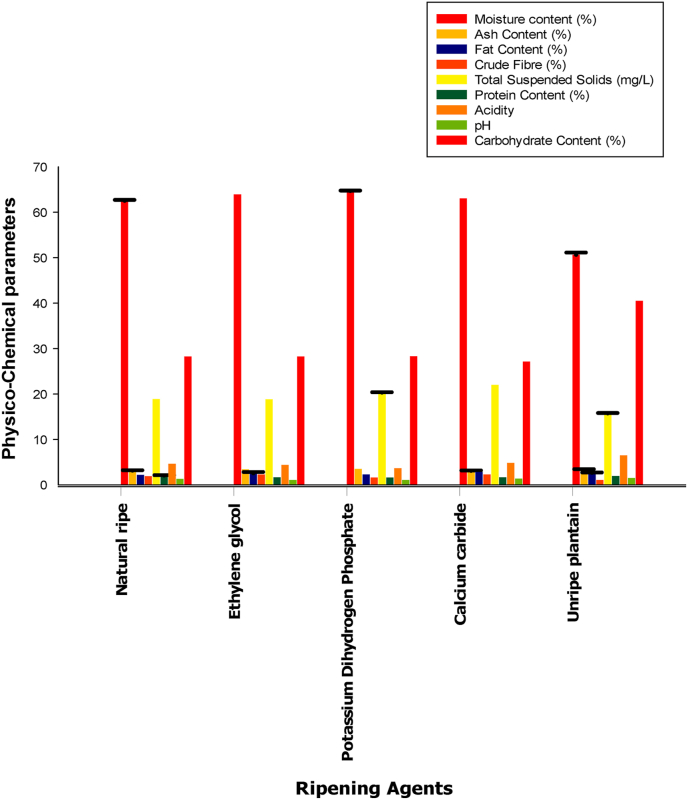
The moisture content of foods or its processed products gives an indication of its freshness and potential shelf life. The results presented in Table 1 and Figure 1 showed that the unripe plantain has the lowest moisture content (50.66 ± 0.50 %). Reduction in moisture content may be majorly due to water loss through transpiration process, while some weight loss could be due to loss of carbon in respiration process (Liu, 2003; Onwuka and Onwuka, 2005; Maduwanthi and Marapana, 2019). The plantain that was ripened using Potassium Dihydrogen Phosphate (PDP) has the highest moisture of 64.68 ± 0.10 %, while the naturally ripened plantain had a moisture content of 62.53 ± 0.20%. The results obtained showed that the use of artificial ripening agent increased the moisture content in fruits thereby subjecting such food items to faster rate of microbial degradation and shorter shelf life, which can lead to quicker deterioration (Adepoju and Adeniji, 2008; Maduwanthi and Marapana, 2019). Figure 1 showed the ash content ranging from 3.07 ± 0.10 % to 3.50 ± 0.00 % for CaC2 and PDP respectively. These findings revealed that fruits do not only lose their weight through respiration where significant carbon is lost but could also occur during ripening. However, the high ash content of the samples treated with PDP clearly suggests an increase in the metabolism of the fruits (Kulkarni et al., 2004). The results presented in Table 1 also showed that the 1.04 ± 0.00 and 2.29 ± 0.00 lowest and highest crude fibre content for the plantain samples were % for unripe and samples ripened by ethylene glycol respectively. The crude fibre contents of the samples were lower than the previously reported values (8.50–20.90 %) of some Nigerian fruits (US Department of Agriculture, 2005; Sajib et al., 2014). The fibre-recommended dietary allowance values for children, adults, pregnant and breast-feeding mothers are 19–25 %, 21–38 %, 28 % and 29 %, respectively. The reduced fibre content in the studied samples may be attributed to the low total dietary fibre content found in plantain samples (Haslinda et al., 2009). Protein is an important component of diet needed for survival of animals and humans, their basic role in diet is to supply adequate amount of required amino acids. Table 1 showed that the calculated protein content for the plantain samples ranged from 1.59 ± 0.00 to 2.03 ± 0.10 %. Food crops that provide more than 12 % of their calorific value of protein are a great source of protein (Elegbede, 1998; Maduwanthi and Marapana, 2019). In this context, the plantain fruits investigated are not a relatively good source of protein. Figure 1 indicated that there was a major increase in carbohydrate content for the plantain samples. Unripe plantain showed the highest carbohydrate content of 40.48 ± 0.00%. This was expected because all the starch in an unripe plantain is contained in the unripe green fruit. However, the ripened plantains showed a carbohydrate levels that ranged between 27.13 ± 0.00 to28.32 ± 0.00 and a mean value of 30.48 ± 0.00 for all the samples which is consistent with previous studies (Joon et al., 2001; Ojetayo et al., 2018). In addition, the high carbohydrate content in the plantain samples implies that the fruit is a vital source of energy for man and animals. The results obtained showed that the ripening agent had no significant effect on the carbohydrate content of the plantain samples. The results obtained in Table 1 also showed that there was no significant difference in the fat content values as the lowest and highest fat content ranged between 2.17 ± 0.00 to 2.84 ± 0.00 %. The low crude fat content of the plantain samples suggests that they may not be a good source of fat-soluble vitamins. One of the purposes of using ripening agents in fruits is to increase the level of Total Suspended Solid (TSS). The results obtained showed that there was a slight effect of ripening agents on the TSS values as it ranged between 18.81 ± 0.00 to 22.02 ± 0.00 mgL-1 for the treated samples. However, the lowest TSS level was observed in the unripe plantain at 15.71 ± 0.10 mgL-1 while TSS level recorded for the natural ripe plantain was 18.87 ± 0.00 mgL-1 (Table 1 and Figure 1). The increased TSS level in the ripened fruit may be due to the hydrolysis of starch to soluble sugars. This could be because during the process of ripening and fruit softening, starch is broken down into the simple soluble sugars-sucrose, glucose, fructose, acids and soluble pectin which increase fruit softening (Bindu et al., 2017). There was no significant difference in the pH values of the plantain samples as it ranged between 1.05 ± 0.00 and 1.50 ± 0.00 (Table 1 and Figure 1). The acidity of the samples was not significantly affected with the use of ripening agents as the interaction effect between the natural ripe, unripe and ripening agents was found to be negligible. Hence, it can be said that treatment of fruits with ripening agents resulted in a slight decrease in the pH value of 1.38 ± 0.00 for CaC2 while ethylene glycol and PDP were at pH 1.05 ± 0.00 when compared to the pH of 1.50 ± 0.00 for the unripe plantain. The low level of acidity for the plantain samples obtained ranged between 3.64 ± 0.00 and 6.48 ± 0.00. The reduction in acidity levels aligns with an increase in concentration of sugar in the plantain fruits. It has been suggested that during storage, fruits utilize organic acids for metabolic activities which results in a reduction in the acidity levels (Bindu et al., 2017).
Table 1.
The mean and standard deviation of the physico-chemical parameters of natural ripened, unripe and plantain ripened with different ripening agents.
| Samples | Moisture Cont (%) | Ash Cont (%) | Fat Cont (%) | Crude fibre Cont (%) | TSS mgL−1 | Protein Content (%) | Acidity | pH | Carbohydrate Content (%) |
|---|---|---|---|---|---|---|---|---|---|
| Natural ripe | 62.53 ± 0.20 | 3.12 ± 0.10 | 2.17 ± 0.00 | 1.90 ± 0.00 | 18.87 ± 0.00 | 2.03 ± 0.10 | 4.61 ± 0.00 | 1.29 ± 0.00 | 28.25 ± 0.00 |
| Ethylene glycol | 63.90 ± 0.00 | 3.40 ± 0.00 | 2.53 ± 0.30 | 2.29 ± 0.00 | 18.81 ± 0.00 | 1.64 ± 0.00 | 4.37 ± 0.00 | 1.05 ± 0.00 | 28.24 ± 0.00 |
| P D P | 64.68 ± 0.10 | 3.50 ± 0.00 | 2.29 ± 0.00 | 1.62 ± 0.00 | 20.28 ± 0.10 | 1.59 ± 0.00 | 3.64 ± 0.00 | 1.05 ± 0.00 | 28.32 ± 0.00 |
| Ca carbide | 63.04 ± 0.00 | 3.07 ± 0.10 | 2.84 ± 0.00 | 2.28 ± 0.00 | 22.02 ± 0.00 | 1.64 ± 0.00 | 4.81 ± 0.00 | 1.38 ± 0.00 | 27.13 ± 0.00 |
| Unripe plantain | 50.66 ± 0.50 | 3.25 ± 0.20 | 2.63 ± 0.10 | 1.04 ± 0.00 | 15.71 ± 0.10 | 1.94 ± 0.00 | 6.48 ± 0.00 | 1.50 ± 0.00 | 40.48 ± 0.00 |
| Mean | 60.96 ± 0.16 | 3.27 ± 0.08 | 2.49 ± 0.08 | 1.83 ± 0.00 | 19.14 ± 0.04 | 1.77 ± 0.02 | 4.78 ± 0.00 | 1.25 ± 0.00 | 30.48 ± 0.00 |
| Std. Dev | 5.82 | 0.18 | 0.27 | 0.52 | 2.32 | 0.20 | 1.05 | 0.20 | 5.61 |
∗TSS: Total Suspended Solid.
∗PDP: Potassium Dihydrogen Phosphate.
∗Ca: Calcium.
Figure 1.
The Physico-chemical analysis of natural ripened, unripe and plantain ripened with different ripening agents.
3.2. Mineral composition of the samples
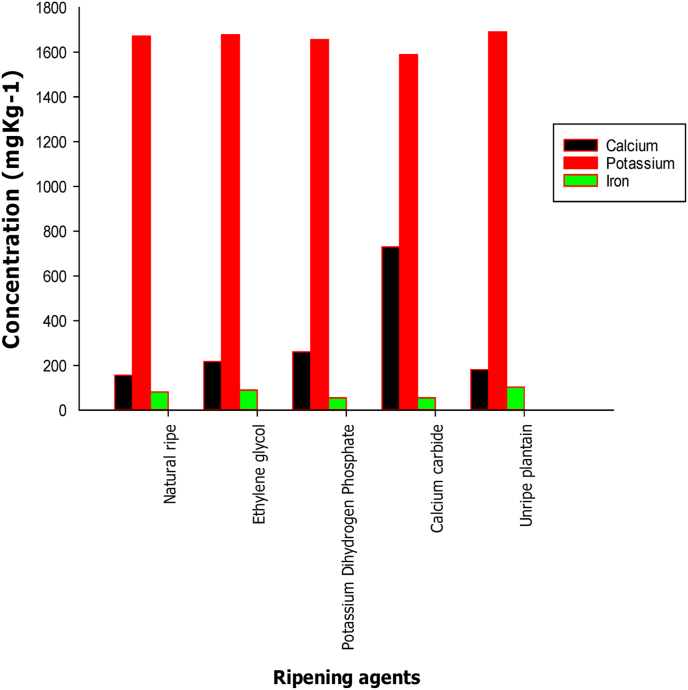
The mineral composition of plantain shows that plantain is a good source of minerals such as calcium (Ca), potassium (K) and iron (Fe). In Figure 2, a relatively high amount of K (1589.45 ± 0.01–1690.55 ± 0.02 mg kg−1) which is essential to help balance body sodium level to keep blood pressure from getting too high as well as playing an important role in heart, muscle and digestive function was reported in all the samples investigated (Ng and Fong, 2000). The source of the excess calcium found in the samples treated with CaC2 could be traced to the calcium in the ripening agent (730.02 ± 0.01 mg kg−1). Excessive level of calcium can cause constipation. This high calcium concentration might also impede the body's ability to absorb other minerals like iron and zinc. In addition, the unripe plantain was found to have the highest concentration of iron (103.03 ± 0.03 mg kg−1) which is necessary for the cure of diabetes; while the naturally ripened plantain gave a concentration of 89.60 ± 0.03 mg kg−1, this implies that ripening definitely has decreasing effect on the concentration of iron. In the study of Wills and Tirmazi (1979) on the effect of calcium and other minerals on ripening of Tomatoes they concluded that calcium, manganese, cobalt and magnesium inhibited ripening while sodium and potassium were less effective as the divalent metals. These metals also caused reduction in respiration and ethylene generation (Wills and Tirmazi, 1979). The lowest concentrations of iron were reported in plantain ripened with the different ripening agents. This low content of Fe might be due to the chemical interaction between the ripening agent and iron as previously reported (Bergman et al., 2009). However, further studies might be needed to establish the reduction of iron and other essential nutrients during ripening of fruits.
Figure 2.
Mineral composition of natural ripened, unripe and plantain ripened with different ripening agents.
The results obtained in the sensory evaluation analysis showed that there is no significant difference in the colour, taste, texture and aroma among the various forms of ripening agents used.
4. Conclusions
Food safety and security are frontline issues among food chemists, as such there is the need to continue to monitor the safety of food. With this mind set, this study was conceptualized and executed. From the findings in this study, the proximate composition of the fruit samples showed that the naturally ripened fruits will have a longer shelf-life than the artificially ripened fruits due to their higher moisture content. Also, the administration of ripening agent for fruit may result in the reduction of some food nutrients such as ash content, protein and carbohydrate contents.
In addition, there was a very higher concentration of Ca in the plantain ripened with CaC2 as compared with other samples, which could cause constipation. The result obtained from the study also showed a low level of Fe when compared to the naturally ripened and the unripe plantain fruits. This could be due to the chemical interaction between the ripening agent and iron. It can be concluded that the use of ripening agent is not encouraged due to the reduction in the quality of the fruit as seen in this study. However further study will be required to understand the reasons for the reduction of some the mineral contents in the studied samples.
Declarations
Author contribution statement
O. S. Sojinu: Conceived and designed the experiments.
N. T. Biliaminu: Performed the experiments; Wrote the paper.
A. M. Mosaku: Analyzed and interpreted the data.
K. O. Makinde, T. H. Adeniji, B. M. Adeboye: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abano E.E., Amoah L.K.S. Effect of different parameters on drying characteristics of banana slice. ARPN J. Eng. Appl. Sci. 2011;6(3):121–129. [Google Scholar]
- Adepoju O.T., Adeniji P.O. Nutrient composition, anti-nutritional factors and contribution of native pear (Dacryoides edulis) pulp to nutrient intake of consumers. Nig. J. Nutr. Sci. 2008;29(2):15–23. [Google Scholar]
- Adewole M.B., Duruji R.W. Quality assessment of plantain (Musa para disiaca L) as affected by different ripening method. Afr. J. Biotechnol. 2010;9:38. [Google Scholar]
- Adeyemi M.M., Bawa M.H., Muktar B. Evaluation of the effect of calcium carbide on induce ripening of banana, pawpaw and mango cultivated within kaduna metropolis. Nigeria. J. Chem. Soc. Nigeria. 2018;43(2):108–118. [Google Scholar]
- AOAC . fifteenth ed. AOAC.; Arlington, Virginia, USA: 1990. Official Methods of Analysis of the Association of Official Analytical Chemists. [Google Scholar]
- AOAC, editor. Official Methods of Analysis of AOAC. Association of Analytical Communities; 2000. [Google Scholar]
- AOAC . sixteenth ed. Association of Official Analytical; Washington DC, USA: 2002. Official Method of Analysis. [Google Scholar]
- Bergman C., Gray-Scott D., Chen J.J., Meacham S. What is next for the dietary reference intake for bone metabolism related nutrients beyond calcium, phosphorus, magnesium, vitamin D, and fluoride? Critical reviews. Food Sci. Nutr. 2009;49:136–144. doi: 10.1080/10408390701764468. [DOI] [PubMed] [Google Scholar]
- Bindu M.S., Jatinder M., Amandeep K. Effect of fruit ripening agents on composition and storage quality of muskmelon. Int. J. Curr. Microbiol. App. Sci. 2017;6(9):2012–2018. [Google Scholar]
- Braghieri A., Piazzolla N., Romaniello A., Paladino F., Ricciardi A., Napolitano F. Effect of adjuncts on sensory properties and consumer liking of Scamorza cheese. J. Dairy Sci. 2015;98:1479–1491. doi: 10.3168/jds.2014-8555. [DOI] [PubMed] [Google Scholar]
- Dudley R. Ethanol, fruit ripening, and the Historical origins of human alcoholism in primate Frugivory. Integrat. Composition. Biol. 2004;4(4):315–322. doi: 10.1093/icb/44.4.315. [DOI] [PubMed] [Google Scholar]
- Ekunwe P.A., Ajayi H.I. Economics of plantain production in Edo state Nigeria. Res. J. Agric. Biol. Sci. 2010;6(6):902–905. [Google Scholar]
- Elegbede J.A. University of Benin; 1998. Legumes in: Nutritional Quantity of Plant Foods O. A.U; and O. U. Eka. Post Harvest Research Units; pp. 53–83. [Google Scholar]
- Food and Agricultural Organization . 2003. Report on Plantain nursery Practice. A Reference Manual. Rome. [Google Scholar]
- Phenolic phytochemicals and bone. Habauzit V., Horcajada M.N., editors. Phytochemistry Rev. 2008 [Google Scholar]
- Haslinda W.H., Cheng L.H., Chong L.C., Noor Aziah A.A. Chemical composition and physicochemical properties of green banana flour. Food Sci. Nutr. 2009;60(S4):232–239. doi: 10.1080/09637480902915525. [DOI] [PubMed] [Google Scholar]
- Hui Y.H., Barta J., Cano M.P., Gusek T.W., Sidhu J.S., Sinha N.K. Wiley-Blackwell; USA: 2006. Handbook of Fruits and Fruit Processing. [Google Scholar]
- Ihekeronye I.A., Ngoddy P.O. London Macmillan; 1985. Integrated Food Science and Tech. For the Tropics. [Google Scholar]
- Joon M.S., Bhutani V.P., Dahiya S.S. Effect of soil and foliar application of nitrogen on the storage quality of ‘‘Santa Rosa’’ plum. IJTA (Int. J. Trop. Agric.) 2001;1990(9):255–258. [Google Scholar]
- Kulkarni A., Aradhya S., Divakar S. Isolation and identification of a radical scavenging antioxidant – punicalagin from pith and carpellary membrane of pomegranate fruit. J. Food Chem. 2004;87:551–557. [Google Scholar]
- Liu R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003;78(3):517–520. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- Maduwanthi S.D.T., Marapana R.A.U.J. Induced ripening agents and their effect on fruit quality of banana. Int. J. Food Sci. 2019;2019:8pp. doi: 10.1155/2019/2520179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nationat Agency For Food and Drug Administration (NAFDAC) 2018. Fruits Ripened with Calcium Carbide Dangerous to Health. [Google Scholar]
- Ng S.P., Fong C.S. Health Discovery. Petaling Jaya Malalysia; 2000. Banana enhances your anti-cancer power. [Google Scholar]
- Nura A., Dandago M.A., Wali N.R. Effects of artificial ripening of banana (Musa spp) using calcium carbide on acceptability and nutritional quality. J. Postharvest Technol. 2018;6(2):14–20. [Google Scholar]
- Ojetayo A., Bodunde J., Odeyemi O.M. Evaluation of different ripening inducers on the quality, proximate composition and mineral residue in plantain (Musa AAB) fruits. Acta Hortic. 2018;1225:199–204. [Google Scholar]
- Onwuka G.I., Onwuka N.D. The effects of ripening on the functional properties of plantain and plantain based cake. Int. J. Food Prop. 2005;8(2):347–353. [Google Scholar]
- Ozgur M.U., Koyuncu I. Determination of ternary mixtures of vitamins (B1; B6, B12) by zero-crossing derivative Spectrophotometry. Turk. J. Chem. 2002;26:385–391. [Google Scholar]
- Park B., Shin A., Park S.K., Ko K.-P., Ma S.H., Lee E.-H., Gwack J., Jung E.-J. Ecological study for refrigerator use, salt, vegetable, and fruit intakes, and gastric cancer. Canc. Causes Contr. 2011;22:1497–1502. doi: 10.1007/s10552-011-9823-7. [DOI] [PubMed] [Google Scholar]
- Per H., Kurtoglu F., Yagmur H., Gumus S., Kumandaş D., Poyrazoglu D. Calcium carbide poisoning via food in childhood. J. Emer. Med. 2007;32:179–180. doi: 10.1016/j.jemermed.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Sajib M.A.M., Jahan S., Islam M.Z., Khan T.A., Saha B.K. Nutritional evaluation and heavy metals content of selected tropical fruits in Bangladesh. Int. Food Res. J. 2014;21(2):609–615. [Google Scholar]
- Singh J., Bal J.S., Singh S., Mirza A. Assessment of chemicals and growth regulators on fruit ripening and quality : a review. Plant Arch. 2018;18(2):1215–1222. [Google Scholar]
- Singh Z., Janes J. Effect of postharvest application of ethephon on fruit ripening, quality and shelf life of mango under modified atmosphere packaging. Acta Hortic. 2001;55(3):599–601. [Google Scholar]
- Tucker K.L. Vol. 7. 2009. Osteoporosis Prevention and Nutrition Current Osteoporosis Reports; pp. 111–117. [DOI] [PubMed] [Google Scholar]
- US Department of Agriculture . Dietary Guidelines for Americans. US Department of Agriculture; Washington, DC: 2005. US department of health and human services. [Google Scholar]
- Wang Q., Liu C., Jing Y.-p., Fan S.-h., Cai J. Evaluation of fermentation conditions to improve the sensory quality of broomcorn millet sour porridge. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2019;104:165–172. [Google Scholar]
- Wills R.B.H., Tirmazi S.I.H. Effect of calcium and other minerals on ripening of Tomatoes. Aust. J. Plant Physiol. 1979;6(221-7) [Google Scholar]
- Zahir E., Naqvi I.I., Uddin S.M. Market basket survey of selected metals in fruits from Karachi city (Pakistan) J. Basic Appl. Sci. 2009;5(2):47–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.