Abstract
Purpose
The role of tumor deposits (TDs) in the staging of gastric cancer is currently debatable. TDs are defined as tumoral nodules in perigastric adipose tissue with no evidence of lymphatic, vascular, or neural structures. Clinicopathological factors related to the presence of TDs are not well defined. This study aimed to identify the clinicopathological factors associated with the presence of TDs in resected gastric cancer patients.
Materials and methods
This prospective study included patients diagnosed with gastric cancer and treated with D2 radical gastrectomy from January 2019 to January 2020. Univariate and multivariate analyses were performed to determine the factors related to the presence of TDs.
Results
A total of 111 patients were eligible and TDs were present in 31 of them (28%). In the univariate analysis, male gender (p = 0.027), tumor size ≥ 5cm (p = ≤0.001), serosa and adjacent organs invasion (pT4a and pT4b) (p = ≤0.001), ≥16 metastatic lymph nodes (pN3b) (p = ≤0.001), and TNM stage III tumors (p = ≤0.001) were significantly associated with the presence of TDs. The multivariate analysis showed that a tumors size ≥5 cm (OR = 3.69, 95% CI: 1.17–11.6), serosa and adjacent organs invasion (pT4a and pT4b) (OR = 3.78, 95% CI: 1.31–10.86) and ≥16 metastatic lymph nodes (pN3b) (OR = 3.21, 95%CI:1.06–9.7) were independent risk factors for the presence of TDs.
Conclusions
Larger tumors (tumor size ≥ 5cm), serosa and adjacent organs invasion (pT4 and pT4b), and ≥16 metastatic lymph nodes (pN3b) were independent risk factors for the presence of TDs.
Keywords: Gastric cancer, Surgery, Pathology, Tumor deposits (TDs)
Gastric cancer; Surgery; Pathology; Tumor deposits (TDs)
1. Introduction
Gastric cancer is an aggressive disease with a high recurrence rate even with appropriate management. It is the sixth most common cancer worldwide, with a considerable incidence in Latin America and eastern countries [1]. In Peru, gastric cancer is the first cause of cancer mortality and, in most patients, is diagnosed in an advanced stages [2]. An accurate disease staging is essential to ensure a proper diagnosis and treatment. Currently, gastric cancer stage is determined by the TNM (Tumor, Lymph nodes, and Metastasis) staging system which considers the length of invasion of the primary tumor (pT), the number of metastatic lymph nodes (pN), and the presence of distant metastasis (pM) [3]. Several prognosis factors, including histological type, HER 2 status, lymphovascular and perineural invasion, have been identified as independent survival predictors in previous studies [4, 5, 6].
Tumor deposits (TDs) were not included as a prognostic factor in the classification of carcinomas for a relevant period. In 1935, Gabriel first described TDs in a study of patients with rectal cancer and reported an association between TDs and lower survival [7]. Evidence from several reports in the last decades has further consolidated the association of poor prognosis with TDs in patients with rectal cancer [8, 9, 10, 11]. In 2009, in the 7th edition of the TNM classification for colorectal cancer, the presence of TDs without lymph node metastasis was classified as pathological node stage 1c (pN1c) [12].
Gastric cancer is the pathology with the second largest number of TDs studies only after colorectal cancer [13, 14, 15, 16]. By consensus, TDs are defined as tumoral nodules in perigastric adipose tissue within the primary tumor's drainage area without evidence of lymphatic, vascular, or neural tissue [3]. In 1991, Di Giorgio reported for the first time, extracapsular nodal metastasis in gastric cancer patients [17]. Since then, a higher incidence of TDs has been reported in patients with poor histopathological factors as advanced stages, larger tumors, lymphovascular invasion, perineural invasion, and poorly differentiated tumors. However, results for the most part have been inconclusive. Furthermore, studies have been mostly focused on the role of TDs as an independent prognostic factor in gastric cancer but not on the pathological factors related to their presence. Thus, the objective of this study is to identify the clinicopathological factors associated with the presence of TDs in resected gastric cancer patients.
2. Materials and methods
2.1. Study design and patient's selection
This prospective single-center study conducted at the Department of Abdominal Surgery, included patients diagnosed with gastric cancer and treated with D2 radical gastrectomy with curative intent between January 2019 and January 2020. Patient inclusion criteria were an age of ≥18 years, histological diagnosis of primary gastric carcinoma, no other malignancies, undergoing D2 radical gastrectomy, elective surgery and R0 resection. Exclusion criteria included patients with R1 o R2 resection, emergency surgery, palliative surgery, stage IV according to the AJCC Cancer Staging System (8th edition), and incomplete data required for analysis [3]. Patients were divided into two groups depending on the presence or absence of TDs.
2.2. Surgical procedure
Distal or total gastrectomy was selected based on tumor location, and resection margins were established according to the Japanese Gastric Cancer Guidelines [18]. D2 lymphadenectomy and omentectomy were conducted in all cases. Lymph nodes 1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, 11p, and 12a were resected for distal gastrectomy, and lymph nodes 1, 2, 3,4sa, 4sb, 4d, 5, 6, 7, 8a, 9, 11p, 11d and 12a were resected for total gastrectomy. Bursectomy was performed in cases where tumors compromised the serosa of the posterior gastric wall.
2.3. Pathological examination and definition of TD
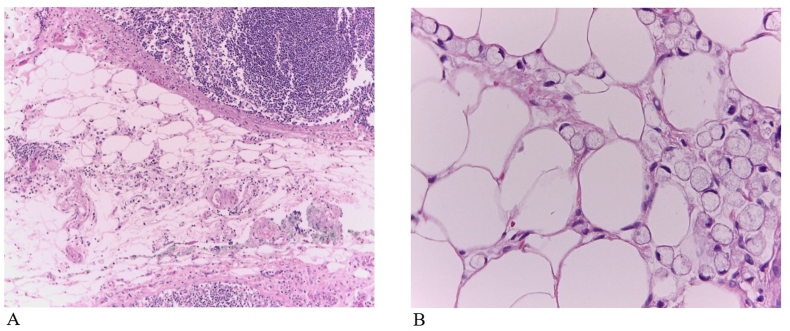
All surgical specimens were embedded in paraffin, stained in hematoxylin and eosin, and analyzed by six independent blind scorers. In this study, the stomach and all adipose connective tissue were retrieved for pathological analysis. An average of 50 slides (range, 20–65) per patient were examined. The mean distance from the TDs to the primary tumor was 3mm. TDs are defined as tumor nodules within the primary cancer drainage area without evidence of lymphatic tissue, vascular or neural structure (Figure 1). Gastric tumors were classified histologically according to the World Health Organization (WHO) classification system [19], and the pathological stage was determined according to AJCC Cancer Staging System (8th edition) [3]. Lymphovascular and perineural invasion were scored by a pathologist under direct visualization of the slides. Six (5.5%) patients were treated with neoadjuvant chemotherapy consisting of fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT protocol) [20].
Figure 1.
Hematoxylin-eosin staining (H&E) shows tumor deposits in gastric carcinoma. Tumor cells are scattered through the perinodal fat tissue. A Magnification × 100, B Magnification × 400.
2.4. Variables studied
Demographic characteristics (age and gender), pathological characteristics (tumor location, histological subtype, tumor grade, depth of invasion (T), lymph node metastasis (N), lymphovascular and perineural invasion, tumor size, clinical stage, HER2 status, neoadjuvant chemotherapy), and surgical characteristic (surgical procedure) were evaluated.
2.5. Statistical analysis
The statistical analysis was performed with IBM SPSS version 22.0. A descriptive analysis of variables was performed through frequencies, percentages, and summary measures (mean and standard deviation). The association of qualitative variables with the presence of TDs was evaluated with the Chi-square test, applying the Yates correction when appropriate. Differences between the groups of patients with and without TDs, regarding quantitative variables, were evaluated with the t-test for independent samples or its corresponding non-parametric test, after evaluation of normality. An analysis of the receiver operating characteristic curve (ROC curve) was used to obtain the optimal cut-off values for tumor size and lymph node metastasis needed to identify the presence of TDs. Optimal cut-off values were used for the dichotomization of the variables in the univariate analysis. The optimal cut-off point for tumor size was ≥5 cm and for lymph node metastasis was ≥16 metastatic lymph nodes or pN3b (AJCC classification). Each pT category and its relationship with the presence of TDs were evaluated separately; pT4 tumors showed a statistically significant relationship with the presence of TDs and were used for the variable dichotomization. A chi-square test was performed to evaluate the correlation between the variable lymphovascular invasion and the presence of TDs; however, the risk (OR) could not be calculated because all patients in the TD (+) group presented LVI (+). The variables gender (female vs male), depth of invasion (pT4 vs pT1-pT2- pT3), tumor size (≥5cm vs < 5cm), lymph node metastasis (pN3b vs pN1- pN2- pN3a), and p TNM Stage (Stage III vs Stage I-II) were used in the univariate and multivariate analysis (binary logistic regression model). In the multivariate analysis, variables with significant influence were chosen with the forward selection method. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. A p-value <0.05 was considered signific0ant for an association, difference, or influence.
2.6. Ethical considerations
This article was evaluated and accepted by the Ethics Committee of the National Institute of Neoplastic Diseases INEN, Lima, Peru. It complies with current regulations on bioethical research and was performed following the Ethical Principles for Medical Research Involving Human Subjects, as outlined in The Declarations of Helsinki. The authors declare that this article does not contain personal information that allows identifying patients enrolled.
3. Results
3.1. Study population
Between January 2019 and January 2020, 145 patients were treated with D2 radical gastrectomy, of whom 111 patients were eligible to participate in this study. The mean age was 60.9 years. Sixty-five patients (58.6%) were female and 46 (41.4%) were male. Distal gastrectomy was performed in 59 patients (53.2%) and total gastrectomy in 52 patients (46.8%). Tubular adenocarcinoma was the most frequent histological subtype (n = 51; 46%), followed by signet cell carcinoma (n = 50; 45%) and mixed carcinoma (n = 10; 9%). Sixty-six and 32 patients presented poorly differentiated tumors (60%) and moderately differentiated tumors (28%), respectively. Thirty-six patients presented tumors that infiltrated the serosa (T4a) (32.4%) and 35 patients presented infiltration of the subserosa (T3) (31.5%). The mean of harvested lymph nodes was 69.5. Twenty-two patients presented ≥7 metastatic lymph nodes N3a (19.8%) and 26 patients presented ≥16 metastatic lymph nodes N3b (23.4%). The mean tumor size was 5.7 cm. Sixty-four (57.6%) patients presented negative HER2 status, 30 an equivocal score (27%), and 8 a positive HER2 status (7.2%). Six patients (5.4%) were treated with neoadjuvant chemotherapy. Clinicopathological characteristics of patients who underwent D2 radical gastrectomy are summarized in Table 1.
Table 1.
Clinicopathological characteristics of patients who underwent D2 radical gastrectomy with and without Tumor Deposits.
| All Gastrectomies |
TD∗ (-) |
TD (+) |
p-Value | |
|---|---|---|---|---|
| n† = 111 (100%) | n = 80 (72%) | n = 31 (28%) | ||
| Age‡, mean ± SD§ years |
60.9 ± 14.1 |
61.8 ± 14.2 |
58.4 ± 13.7 |
0.25 |
| Gender | ||||
| Male n (%) | 46 (41.4) | 28 (35) | 18 (58) | 0.02 |
| Female n (%) |
65 (58.6) |
52 (65) |
13 (42) |
|
| Tumor Location n (%) |
0.92 |
|||
| Distal | 57 (51.4) | 41 (51) | 16 (51.6) | |
| Middle | 45 (40.5) | 32 (40.3) | 13 (41.9) | |
| Upper |
9 (8.1) |
7 (8.7) |
2 (6.5) |
|
| Histological Subtype n (%) |
0.26 |
|||
| Tubular Adenocarcinoma | 51 (46) | 38 (47.5) | 13 (41.9) | |
| Signet Cell Carcinoma | 50 (45) | 33 (41.3) | 17 (54.8) | |
| Mixed Carcinoma |
10 (9) |
9 (11.3) |
1 (3.2) |
|
| Tumor Grade n (%) |
0.06 |
|||
| Well Differentiated | 13 (12) | 13 (16) | 0 | |
| Moderately Differentiated | 32 (28) | 22 (28) | 10 (32) | |
| Poorly Differentiated |
66 (60) |
45 (56) |
21 (68) |
|
| Neoadjuvant Chemotherapy n (%) | 0.53 |
|||
| No | 105 (94.5) | 76 (95) | 29 (93.5) | |
| Yes |
6 (5.5) |
4 (5) |
2 (6.5) |
|
| Depth of Invasion (pT) n (%) |
<0.001 |
|||
| T1a | 10 (9) | 10 (12.5) | 0 | |
| T1b | 10 (9) | 10 (12.5) | 0 | |
| T2 | 15 (13.5) | 13 (16.3) | 2 (6.5) | |
| T3 | 35 (31.5) | 28 (35) | 7 (22.5) | |
| T4a | 36 (32.4) | 18 (22.5) | 18 (58) | |
| T4b | 5 (4.5) | 1 (1.25) | 4 (13) | |
| Lymph Nodes Resected, mean ± SD |
69.5 ± 25.7 |
70.8 ± 26.8 |
66.4 ± 22.9 |
0.70 |
| Lymph Nodes Metastasis (pN) n (%) |
<0.001 |
|||
| N0 | 29 (26.1) | 27 (33.8) | 2 (6.4) | |
| N1 | 20 (18) | 16 (20) | 4 (12.9) | |
| N2 | 14 (12.6) | 10 (12.5) | 4 (12.9) | |
| N3a | 22 (19.8) | 17 (21.2) | 5 (16.2) | |
| N3b |
26 (23.4) |
10 (12.5) |
16 (51.6) |
|
| pTNM Stage n (%) |
0.001 |
|||
| Stage I | 25 (22.5) | 24 (30) | 1 (3.2) | |
| Stage II | 25 (22.5) | 20 (25) | 5 (16.1) | |
| Stage III |
61 (55) |
36 (45) |
25 (80.7) |
|
| Lymphovascular Invasion n (%) |
0.001 |
|||
| Present | 86 (77.5) | 55 (68.8) | 31 (100%) | |
| Absent |
25 (22.5) |
25 (31.2) |
0 |
|
| Perineural Invasion n (%) |
0.18 |
|||
| Present | 66 (59.5) | 45 (56.2) | 21 (67.7) | |
| Absent |
45 (40.5) |
35 (43.8) |
10 (32.3) |
|
| HER2 IHC Score System n (%) |
0.929 |
|||
| Negative Score 0 | 26 (23.4) | 18 (22.5) | 8 (25.8) | |
| Negative Score 1+ | 38 (34.2) | 28 (35) | 10 (32.2) | |
| Equivocal Score 2+ | 30 (27) | 21 (26.2) | 9 (29) | |
| Positive Score 3+ |
8 (7.2) |
5 (6.25) |
3 (9.6) |
|
| Type Of Gastrectomy |
0.33 |
|||
| Distal Gastrectomy n (%) | 59 (53.2) | 44 (55) | 15 (48) | |
| Total Gastrectomy n (%) |
52 (46.8) |
36 (45) |
16 (52) |
|
| Tumor Size, mean ± SD∥ |
5.7 ± 2.9 |
5.2 ± 2.8 |
6.96 ± 2.7 |
<0.001 |
| <5cm | 43 (53.8) | 5 (16.1) | ||
| ≥5cm | 37 (46.2) | 26 (83.9) | <0.001 | |
The values that are in bold are those that are statistically significant in the statistical analysis (p = <0.05).
TD: Tumor deposits.
n: Number of patients.
At the date of surgery.
SD: standard deviation.
mean and standard deviation in centimeters.
3.2. Clinicopathological characteristics of patients with TDs
TDs were observed in 31 patients (28%), of whom 18 (58%) were male and 13 (42%) were female (p = 0.02). The mean age was 58.4 years. Most of the patients presented distal tumors (n = 16; 51.6%), and the most frequent histological subtype was signet cell carcinoma (n = 17; 54.8%). Poorly differentiated tumors were the most frequent (n = 21; 68%) followed by moderately differentiated (n = 10; 32%). The presence of TDs was significantly associated with larger tumors (p= <0.001), with most patients in the TDs (+) group presenting a tumor size of ≥5 cm (n = 26; 83.9%). All the patients in the TDs (+) group presented lymphovascular invasion compared to 55 patients (68.8%) in the TD (-) group (p = 0.001). TDs were observed in advanced T-stage tumors, with most infiltrating the serosa or adjacent organs (T4) (n = 22; 71%). Likewise, the presence of TDs was significantly associated with advanced N stage tumors (p= <0.001), with most of the patients presenting ≥16 metastatic lymph nodes (n = 16; 51.6%). It is important to note that TDs were also observed in patients without metastatic lymph nodes (N0) (n = 2; 6.4%). According to the TNM 8th edition classification [3], clinical-stage III was the most frequent in the TDs (+) group (n = 25; 80.7%). Clinicopathological characteristics of patients who underwent D2 radical gastrectomy with TDs are summarized in Table 1.
3.3. Localization of TD
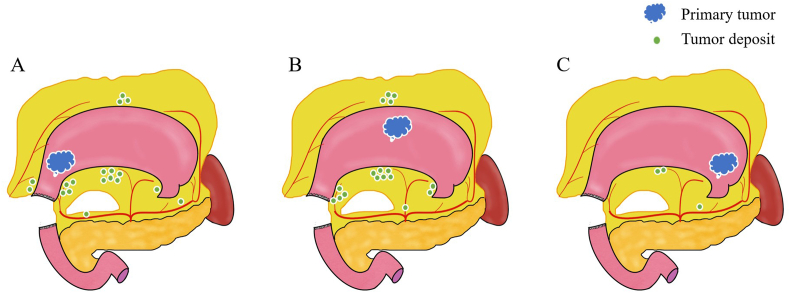
Of the 31 patients with TDs (+), 16 patients presented distal tumors. In these patients, TDs were observed in the adipose tissue of the right cardiac area (n = 1), lesser curvature (n = 6), greater curvature (n = 3), suprapyloric vessels (n = 4), infrapyloric vessels (n = 2), common hepatic artery (n = 1), and distal splenic artery (n = 1). In middle tumors, TDs were detected in the right cardiac area (n = 2), lesser curvature (n = 6), greater curvature (n = 4), suprapyloric vessels (n = 4), and coeliac axis (n = 1). In upper tumors, TDs were observed in the lesser curvature (n = 2) and proximal splenic artery (n = 1). Location of TDs is represented in Figure 2.
Figure 2.
Schema of the relationship between the location of tumor deposits and the primary tumor in 31 patients. A distal tumors, B middle tumors, C proximal tumors.
3.4. Univariate and multivariate analysis
In the univariate analysis, male gender (p = 0.027), tumor size ≥5 cm (p = ≤ 0.001), serosa and adjacent organs invasion (pT4a and pT4b) (p = ≤ 0.001), ≥16 metastatic lymph nodes (pN3b) (p = ≤ 0.001), and TNM stage III (p = ≤ 0.001) were significantly associated with the presence of TDs (Table 2).
Table 2.
Univariate analysis of factors associated with the presence of tumor deposits.
| Univariate Analysis |
|||
|---|---|---|---|
| OR∗ | CI† 95% | p- Value | |
| Gender | |||
| Female | 1 | ||
| Male |
2.57 |
(1.10–6.01) |
0.027 |
| Tumor Size | |||
| <5cm | 1 | ||
| ≥5cm |
5.9 |
(2.05–16.93) |
≤0.001 |
| Depth of Invasion (pT) | |||
| T1-T2-T3 | 1 | ||
| T4 |
7.84 |
(3.09–19.9) |
≤0.001 |
| Lymph Nodes Metastasis (pN) | |||
| N0–N1–N2–N3a | 1 | ||
| N3b |
7.46 |
(2.83–19.63) |
≤0.001 |
| pTNM Stage n (%) | |||
| Stage I-II | 1 | ||
| Stage III | 5.35 | (1.98–14.48) | ≤0.001 |
The values that are in bold are those that are statistically significant in the statistical analysis (p = <0.05).
OR: Odds Ratio.
CI: Confidence Interval.
In the multivariate analysis, a greater incidence of TDs was found in patients with tumors ≥5 cm (OR = 3.69, 95% CI: 1.17–11.6) compared to those with tumors < 5cm.
Also, tumors with serosa and adjacent organs invasion (pT4a and p T4b) (OR = 3.78, 95% CI: 1.31–10.86) and ≥16 metastatic lymph nodes (pN3b) (OR = 3.21, 95%CI:1.06–9.7) were significantly associated with the presence of TDs (Table 3).
Table 3.
Logistic regression analysis of factors associated with the presence of tumor deposits.
| Multivariate Analysis |
|||
|---|---|---|---|
| OR∗ | CI† 95% | p- Value | |
| Tumor Size | |||
| <5cm | 1 | ||
| ≥5cm |
3.7 |
(1.17–11.6) |
0.026 |
| Depth of Invasion (pT) | |||
| T1-T2-T3 | 1 | ||
| T4 |
3.8 |
(1.31–10.86) |
0.014 |
| Lymph Nodes Metastasis (pN) | |||
| N0–N1–N2–N3a | 1 | ||
| N3b | 3.2 | (1.06–9.7) | 0.039 |
The values that are in bold are those that are statistically significant in the statistical analysis (p = <0.05).
OR: Odds Ratio.
CI: Confidence Interval.
4. Discussion
The role of TDs in gastric cancer staging is currently debatable [21]. In recent decades, of the findings of several reports have supported the concept of TDs being an independent prognostic factor in gastric cancer [22, 23, 24]. However, the clinicopathological factors associated with the presence of TDs are unclear [4, 25, 26, 27].
Our study, the first one about this topic in America, included 111 patients, and showed that a tumor size ≥5 cm, serosa and adjacent organs invasion (pT4a and pT4b), and ≥16 metastatic lymph nodes (pN3b) were significantly associated with the presence of TDs. These results support the relationship between the presence of TDs and poor prognostic factors, and clarify the relevance of TDs in gastric cancer staging. In the present study, thirty-one (28%) patients presented TDs, which is consistent with previous studies that reported a percentage between 9% to 27% [24, 26, 28, 29]. We observed a high incidence of locally advanced tumors TNM Stage II-III (75%) possibly explaining our higher incidence of TDs.
Tumor size has been reported as a clinicopathological factor related to the presence of TDs in previous studies; however, the cut-off point remains unclear [23, 24, 26, 30, 31]. Etoh et al., found that a tumor size of ≥10cm was significantly associated with the presence of TDs in a univariate analysis [23]. Nonetheless, several other studies using univariate analysis have also have reported that a tumor size of ≥5 cm is significantly related to the presence of TDs [24, 26, 30, 31, 32]. In our study, in a multivariate analysis, a tumor size of ≥5 cm was significantly associated with the presence of TDs supporting a cut-off point of 5 cm as a risk factor.
Several studies reported that the presence of TDs is related to a deeper length of invasion (pT) but only a few report the relationship of TDs with a specific pT category [23, 26, 29, 30, 31].
Graham-Martinez et al., in a systematic review, reported that TDs occurred more frequently in tumors with a higher T-category (T4 vs T1-T2-T3) (RR = 2.24, 95% CI = 1.62–3.09) [4]. Anup et al. [27] reported that patients with TDs had a similar survival rate compared to pT4 stage patients without TDs. Similarly, Sun et al. reported that pT1-pT4a patients with TDs had a similar prognosis with pT4a patients without TDs, and proposed that the presence of TDs might be reclassified and treated as tumors with serosa invasion (pT4a) [28]. Our finding that the presence of TDs significantly associates to tumors with serosa and adjacent organs invasion (pT4a and pT4b), further supports the classification proposed by Sun et al. It is also important to note that in our study there were no patients with early gastric cancer (pT1a and pT1b) and TDs. Xie et al. [33] reported one patient with early gastric cancer (T1b) with positive TD but with lymph node metastasis. Likewise, in a study including 1250 patients, it was reported that pT1 patients with TDs had worse survival compared to pT1 patients without TDs [27].
The relationship in terms of survival between the presence of TDs and N stage has been evaluated in multiple studies worldwide [21, 23, 24, 25, 27, 30, 32]. In a Chinese study consisting of 1518 patients [30], a worse prognosis was found in pN0, pN1, pN2, and pN3a patients with TDs compared to the same subgroups without TDs; however, there was no significant difference between patients with and without TDs in the pN3b subgroup. Similarly, Etoh et al. reported that the presence of extranodal metastasis had no significant impact on survival in pN3 patients [23]. In a propensity score matching study from China, it was evidenced that the survival curves were similar between patients with TDs and patients with N3a and N3b disease [32]. Based on our results and those of previous studies we suggest that there is a close relationship between an N3b stage and the presence of TDs. Likewise, our results support the new classification proposed by Chen [30], in which for patients with TDs, the initial N stage is increased by one stage except for patients with N3b disease.
In our study TDs were observed in 2 (6.4%) patients without lymph node metastasis (pN0). In these two cases, tumors penetrated the muscularis propria (pT2) and the serosa (pT4a), respectively. Similar results have also been found in multiple studies worldwide, with a percentage as high as 12.9% [25]. Likewise, it has been reported that the overall survival of N0 patients with TDs is significantly worse compared to N0 patients without TDs (p < 0.001) [29].
Male gender (p = 0.027) and TNM Stage III (p = ≤ 0.001), in the univariate analysis, were significantly associated with the presence of TDs; nonetheless, no association was found in the logistic regression analysis. In a retrospective study consisting of 3098 patients, after propensity score matching, no significant difference was evidenced in survival curves between Stage IIIC patients with and without TDs [31].
In our study, all the patients in the TD (+) group presented lymphovascular invasion (p = ≤ 0.001) and 21 of them (67.7%) presented perineural invasion (p = 0.18). Chen et al. [30], reported, in a univariate analysis, that lymphovascular and perineural invasion were significantly associated with the presence of TDs. Other clinicopathological factors have been reported to be related to the presence of TDs, such as Bormann type, histology, and histological grade; however, they have not been evaluated in a multivariate analysis [22, 26, 28, 34].
There were some limitations in this study. First, given the short follow-up at the time of the analysis, overall survival and disease-free survival were not evaluated. This survival analysis should be performed in a future study. Also, the lymphovascular invasion could not be evaluated in the univariate and multivariate analyses because we did not have negative patients compared in the TD (+) group and therefore the OR could not be calculated.
In conclusion, the presence of TDs was independently associated to larger tumors (tumor size ≥ 5cm), tumors with infiltration of serosa and adjacent organs (pT4a and pT4b), and the presence of ≥16 metastatic lymph nodes.
Declarations
Author contribution statement
Francisco Berrospi Espinoza: Conceived and designed the experiments; Wrote the paper.
Sofia Prado Cucho and Luis Taxa Rojas: Conceived and designed the experiments; Performed the experiments.
Carlos Luque-Vasquez: Performed the experiments; Wrote the paper.
Ivan Chavez, Eloy Ruiz Figueroa: Analyzed and interpreted the data; Wrote the paper.
Eduardo Payet Meza: Contributed reagents, materials, analysis tools or data.
Oscar Paredes Torres: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank all the members of the Department of Abdominal Surgery and the Department of Pathology of the National Institute of Neoplastic Diseases INEN, Lima Peru.
References
- 1.International Agency for Research on Cancer 2020 . 2018. Cancer Today [Internet]. Globacan 2018.https://gco.iarc.fr/today/home [cited 2020 Mar 31]. Available from: [Google Scholar]
- 2.Payet E., Perez P., Poquioma E., editors. fifth ed. Vol. 5. 2016. pp. 31–33.http://bvs.minsa.gob.pe/local/MINSA/3774.pdf (Registro de Cáncer De Lima Metropolitana Incidencia y Mortalidad 2010-2012). Lima [Internet] Available from: [Google Scholar]
- 3.Ajani J.A., In H., Sano T., Gaspar L.E., Erasmus J.J., Tang L.H. American Joint Committee on Cancer. AJCC Cancer Staging Manual. eighth ed. Springer; Chicago: 2018. Stomach; pp. 203–220. [Google Scholar]
- 4.Graham Martínez C., Knijn N., Verheij M., Nagtegaal I.D., Van der Post R.S. Tumour deposits are a significant prognostic factor in gastric cancer – a systematic review and meta-analysis. Histopathology. 2019;74:809–816. doi: 10.1111/his.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei D., Zhao B., Zhang J., Luo R., Lu H., Xu H. Impact of lymphovascular invasion on survival outcome in patients with gastric cancer. Am. J. Clin. Pathol. 2020;153:833–841. doi: 10.1093/ajcp/aqaa021. [DOI] [PubMed] [Google Scholar]
- 6.Aurello P., Berardi G., Tierno S.M., Rampioni Vinciguerra G.L., Socciarelli F., Laracca G.G. Influence of perineural invasion in predicting overall survival and disease-free survival in patients with locally advanced gastric cancer. Am. J. Surg. 2017;213:748–753. doi: 10.1016/j.amjsurg.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel W.B., Dukes C., Bussey H.J.R. Lymphatic spread in cancer of the rectum. Br. J. Surg. 1935;23:395–413. [Google Scholar]
- 8.Ueno H., Mochizuki H., Shirouzu K., Kusumi T., Yamada K., Ikegami M. Multicenter study for optimal categorization of extramural tumor deposits for colorectal cancer staging. Ann. Surg. 2012;255:739–746. doi: 10.1097/SLA.0b013e31824b4839. [DOI] [PubMed] [Google Scholar]
- 9.Ratto C., Ricci R., Rossi C., Morelli U., Vecchio F.M., Doglietto G.B. Mesorectal microfoci adversely affect the prognosis of patients with rectal cancer. Dis. Colon Rectum. 2002;45:733–742. doi: 10.1007/s10350-004-6288-8. [DOI] [PubMed] [Google Scholar]
- 10.Nagtegaal I.D., Quirke P. Colorectal tumour deposits in the mesorectum and pericolon; a critical review. Histopathology. 2007;51:141–149. doi: 10.1111/j.1365-2559.2007.02720.x. [DOI] [PubMed] [Google Scholar]
- 11.Prabhudesai A., Arif S., Finlayson C.J., Kumar D. Impact of microscopic extranodal tumor deposits on the outcome of patients with rectal cancer. Dis. Colon Rectum. 2003;46:1531–1537. doi: 10.1007/s10350-004-6809-5. [DOI] [PubMed] [Google Scholar]
- 12.Sobin L.H., Gospodarowicz M.W.C., Wittekind C. seventh ed. Wiley-Blackwell; Washington: 2009. TNM Classification of Malignant Tumours. [Google Scholar]
- 13.Nagatomo A., Abe N., Takeuchi H., Yanagida O., Masaki T., Mori T. Microscopic cancer cell spread in gastric cancer : whole-section analysis of mesogastrium. Langenbeck's Arch. Surg. 2009;394:655–660. doi: 10.1007/s00423-008-0427-y. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K., Ozaki N., Yamada T., Hata T., Sugimoto S., Hikino H. Evaluation of prognostic significance in extracapsular spread of lymph node metastasis in patients with gastric cancer. Surgery. 2005;137:511–517. doi: 10.1016/j.surg.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Virgilio E., D’Antonio C., Balducci G. Mesogastrium recurrence as expression of the fifth metastatic route of gastric cancer. Med. Hypotheses. 2014;82:403–404. doi: 10.1016/j.mehy.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Xie D., Osaiweran H., Liu L., Wang X., Yu C., Tong Y. Mesogastrium: a fifth route of metastasis in gastric cancer ? Med. Hypotheses. 2013;80:498–500. doi: 10.1016/j.mehy.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Di Giorgio A., Botti C., Sammartino P., Mingazzini P., Flammia M., Stipa V. Extracapsular lymphnode metastases in the staging and prognosis of gastric cancer. Int. Surg. 1991;76:218–221. [PubMed] [Google Scholar]
- 18.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021;24:1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagtegaal I.D., Odze R.D., Klimstra D., Paradis V., Rugge M., Schirmacher P., editors. fifth ed. Vol. 1. International Agency for Research on Cancer; Lyon: 2019. (WHO Classification of Tumours of the Digestive System). [Google Scholar]
- 20.Al-Batran S.E., Hofheinz R.D., Pauligk C., Kopp H.G., Haag G.M., Luley K.B. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697–1708. doi: 10.1016/S1470-2045(16)30531-9. [DOI] [PubMed] [Google Scholar]
- 21.Peparini N. Beyond T, N and M: the impact of tumor deposits on the staging and treatment of colorectal and gastric carcinoma. Surg Oncol. 2018;27:129–137. doi: 10.1016/j.suronc.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Ersen A., Unlu M.S., Akman T., Sagol O., Oztop I., Atila K. Tumor deposits in gastric carcinomas. Pathol. Res. Pract. 2014;210(9):565–570. doi: 10.1016/j.prp.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Etoh T., Sasako M., Ishikawa K., Katai H., Sano T., Shimoda T. Extranodal metastasis is an indicator of poor prognosis in patients with gastric carcinoma. Br. J. Surg. 2006;93:369–373. doi: 10.1002/bjs.5240. [DOI] [PubMed] [Google Scholar]
- 24.Wang W., Li Y., Zhang Y., Yuan X., Xu D., Guan Y. Incorporation of extranodal metastasis of gastric carcinoma into the 7th edition UICC TNM staging system. PloS One. 2011;6 doi: 10.1371/journal.pone.0019557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenquan L., Yuhua L., Jianxin C., Hongqing X., Kecheng Z., Jiyang L. Tumor deposit serves as a prognostic marker in gastric cancer: a propensity score-matched analysis comparing survival outcomes. Canc. Med. 2020;9:3268–3277. doi: 10.1002/cam4.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang N., Deng J.-Y., Ding X.-W., Ke B., Liu N., Liang H. Node-extranodal soft tissue stage based on extranodal metastasis is associated with poor prognosis of patients with gastric cancer. J. Surg. Res. 2014;192:90–97. doi: 10.1016/j.jss.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 27.Anup S., Lu J., Zheng C.-H., Li P., Xie J.-W., Wang J.-B. Prognostic significance of perigastric tumor deposits in patients with primary gastric cancer. BMC Surg. 2017;17:84. doi: 10.1186/s12893-017-0280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Z., Wang Z., Xu Y., Zhu G., Huang B., Xu Y. Prognostic significance of tumor deposits in gastric cancer patients who underwent radical surgery. Surgery. 2012;151:871–881. doi: 10.1016/j.surg.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Lee H.S., Lee H.E., Yang H.K., Kim W.H. Perigastric tumor deposits in primary gastric Cancer : implications for patient prognosis and staging. Ann. Surg Oncol. 2013;20:1604–1613. doi: 10.1245/s10434-012-2692-9. [DOI] [PubMed] [Google Scholar]
- 30.Chen H., Tang Z., Chen L., Li H., Wang X., Liu F. Evaluation of the impact of tumor deposits on prognosis in gastric cancer and a proposal for their incorporation into the AJCC staging system. Eur. J. Surg. Oncol. 2018;44:1990–1996. doi: 10.1016/j.ejso.2018.10.062. [DOI] [PubMed] [Google Scholar]
- 31.Gu L., Chen P., Su H., Li X., Zhu H., Wang X. Clinical significance of tumor deposits in gastric cancer: a retrospective and propensity score - matched study at two institutions. J. Gastrointest. Surg. 2020;24:2482–2490. doi: 10.1007/s11605-019-04421-8. [DOI] [PubMed] [Google Scholar]
- 32.Tan J., Yang B., Xu Z., Zhou S., Chen Z., Huang J. Tumor deposit indicates worse prognosis than metastatic lymph node in gastric cancer: a propensity score matching study. Ann. Transl. Med. 2019;7:671. doi: 10.21037/atm.2019.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie D., Liu L., Osaiweran H., Yu C., Sheng F., Gao C. Detection and characterization of metastatic cancer cells in the mesogastrium of gastric cancer patients. PloS One. 2015;11 doi: 10.1371/journal.pone.0142970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yildiz B., Etiz D., Dal P., Junushova B., Pasaoglu O., Yilmaz E. Tumor deposits: prognostic significance in gastric cancer patients. J. BUON. 2016;21:1476–1481. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.