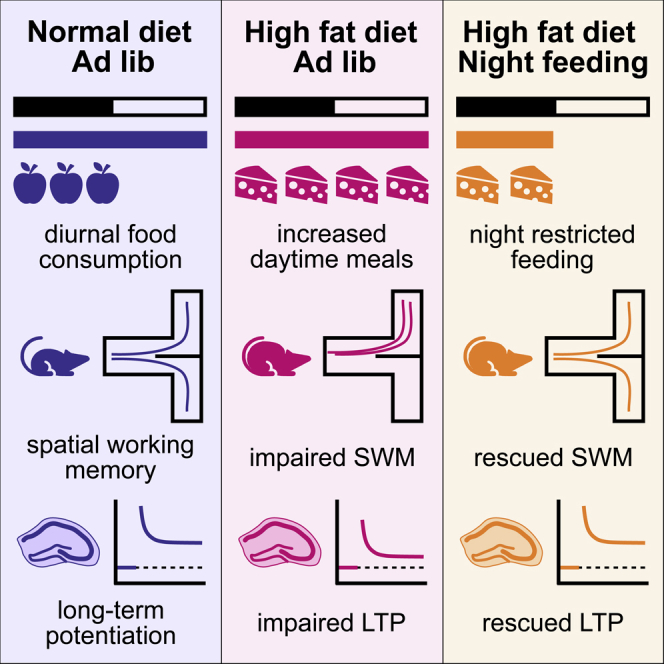
Summary
Feeding rodents a high-fat diet (HFD) disrupts normal behavioral rhythms, particularly meal timing. Within the brain, mistimed feeding shifts molecular rhythms in the hippocampus and impairs memory. We hypothesize that altered meal timing induced by an HFD leads to cognitive impairment and that restricting HFD access to the “active period” (i.e., night) rescues the normal hippocampal function. In male mice, ad-lib access to an HFD for 20 weeks increased body weight and fat mass, increased daytime meal consumption, reduced hippocampal long-term potentiation (LTP), and eliminated day/night differences in spatial working memory. Importantly, two weeks of time-restricted feeding (TRF) at the end of the chronic HFD protocol rescued spatial working memory and restored LTP magnitude, even though there was no change in body composition and total daily caloric intake. These findings suggest that short-term TRF is an effective mechanism for rescuing HFD-induced impaired cognition and hippocampal function.
Subject area: Biological Sciences, Neuroscience, Cognitive Neuroscience
Graphical abstract
Highlights
-
•
Mice fed a chronic HFD have increased daytime meal consumption and impaired LTP and memory.
-
•
HFD-impaired long-term potentiation is rescued by night-time restricted feeding.
-
•
Day/night difference in spontaneous alternation is rescued by time-restricted feeding.
Biological sciences; Neuroscience; Cognitive neuroscience
Introduction
Access to a high-fat diet (HFD) induces well-established metabolic dysfunction in many rodent models, resulting in obesity, cardiovascular disease, and insulin resistance (Hariri and Thibault, 2010; Heydemann, 2016). In addition to these physiological consequences of an HFD, cognitive dysfunction is also a common consequence of free access to an HFD in rodents. Cognitive issues range from impaired spatial memory and object recognition (Heyward et al., 2012, 2016; Pistell et al., 2010; Valladolid-Acebes et al., 2011; Davis et al., 2020) to impaired long-term potentiation (LTP) in the hippocampus, a form of synaptic plasticity (Hao et al., 2016; Hwang et al., 2010; Davis et al., 2020). Recent investigations on the impact of an HFD have found that mice provided that free access to an HFD show altered circadian behavior, including altered activity patterns and changes in meal timing (Pendergast et al., 2013; Kohsaka et al., 2007; Branecky et al., 2015; Oosterman et al., 2015). As the specific mechanism by which an HFD induces cognitive impairment remains unclear, these disrupted daily meal patterns may be a potential factor. One study reported that providing mice access to normal chow at the incorrect time of day impairs cognition and LTP, similar to the effects of HFD feeding (Loh et al., 2015). It is important to note that another study found no cognitive impact of daytime feeding in rodents (Power et al., 2018); minor methodological differences may play an impact in these disparate results. An attractive strategy to rescue these disruptions is time-restricted feeding (TRF) or restricting food access to the animal's active period – in the case of nocturnal mice, food access is allowed only during the night. TRF effectively prevents and rescues metabolic effects of an HFD on mice (Chaix et al., 2014; Chung et al., 2016; Hatori et al., 2012); however, the impact of TRF on rescuing cognitive impairment remains unknown. We aimed to test whether TRF is an effective strategy to rescue the long-term effects of an HFD on hippocampal function.
Results and discussion
An HFD alters meal timing and dampens metabolic rhythms
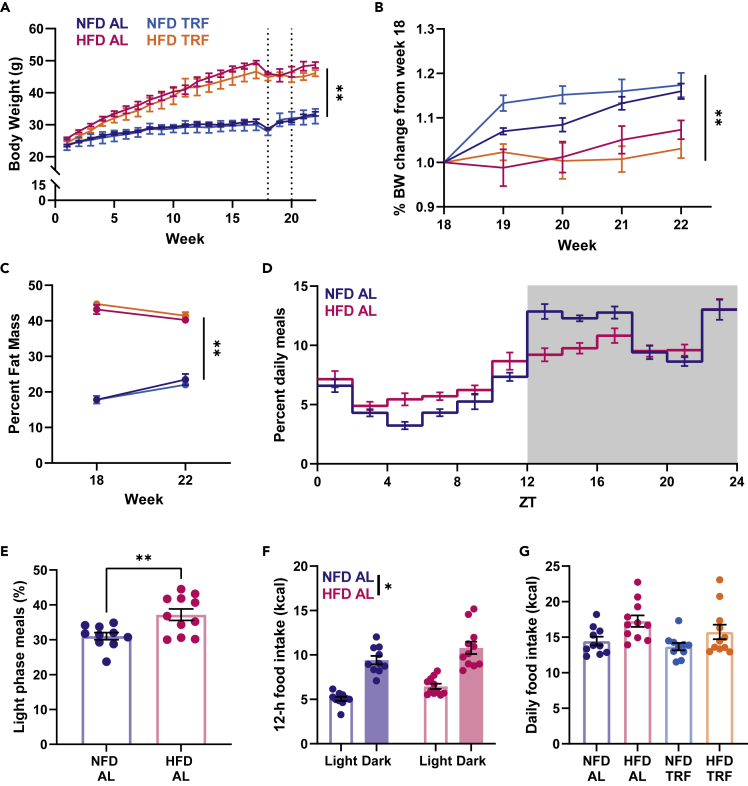
As previously mentioned, an HFD changes feeding behavior within 3 days (Pendergast et al., 2013), and these changes persist for several weeks and reverse upon return to normal chow (Branecky et al., 2015). Restricting access to food to a scheduled window (e.g., TRF) has emerged as a potential therapy for the metabolic consequences of HFD-induced obesity. Mice provided an HFD on a TRF schedule do not gain as much weight or develop HFD-induced diabetes or liver damage (Hatori et al., 2012; Chung et al., 2016; Chaix et al., 2014). Before testing the impacts of an HFD and TRF on hippocampal function, we first sought to confirm that an HFD disrupts meal timing and locomotor activity. Mice were provided ad-lib (AL) access to a normal-fat diet (NFD) (10% fat by kcal) or HFD (45% fat by kcal) for 18–20 weeks. After quantitative magnetic resonance (QMR) body scans to assess body composition and a 1- to 2-week recovery period, mice were transferred to a Comprehensive Lab Animal Monitoring System (CLAMS) and assigned to either remain on AL feeding or to begin TRF with food only accessible at night. Mice were weighed weekly over the course of the experiment, and as expected, the HFD increased body weight over the course of the feeding (Figure 1A). Owing to the drop in body weight at week 18, likely caused by the transfer stress and body scan, we examined percent body weight change from 18 to 22 weeks. In that time, there was no change in weight within the HFD-fed groups, whereas the NFD-fed animals returned to their pretransfer weight (Figure 1B); however, the TRF schedule had no impact on percent body weight change. While HFD groups have the expected higher percentage of body fat, TRF had no effect on body composition (Figure 1C). When examining the dispersion of meals across a 24-h period in the AL groups, animals on both diets consumed more meals during lights off than the lights on; however, HFD-fed animals consumed a higher percentage of meals over the 24-h day during lights on than did NFD-fed animals (Figures 1D and 1E). Overall caloric consumption was higher at night than during the day in both groups, and HFD-fed animals consumed more total calories and more calories per meal than NFD-fed animals across both times (Figures 1F, S1A, and S1B). When accounting for TRF groups, daily caloric intake was higher for both HFD-fed groups than the NFD-fed groups, but TRF did not affect caloric intake of animals fed either diet (Figure 1G).
Figure 1.
Alterations to meal timing and food intake on a high-fat diet
(A) Body weight of animals maintained on either NFD or HFD for the course of the experiment. For 1–17 weeks, there is a significant effect of diet with HFD animals weighing significantly more than the NFD animals (three-way ANOVA, F1,17 = 96.930, ∗∗p < 0.001, n = 5–6 mice per group). At week 18 (vertical dotted line), body composition was assessed via QMR and then animals were transferred to CLAMS for metabolic analysis. Second dotted vertical line at week 20 indicates the start of the TRF feeding paradigm.
(B) Percent change in body weight over 18–22 weeks. The diet × TRF interaction failed to reach significance (three-way ANVOA, F1,17 = 3.949, p = 0.063); however, there was a significant effect of diet (F1,17 = 22.420, ∗∗p < 0.0001, n = 5–6 mice per group).
(C) Percent fat mass (via QMR scan) before and after TRF. Main effect of diet (three-way ANOVA, F1,17 = 577.376, ∗∗p < 0.001) indicates that HFD animals, regardless of TRF protocol, have significantly higher percentage of body fat than NFD groups. There was no significant diet X feeding schedule interaction (F1,17 = 1.484, p = 0.240, n = 5–6 mice per group).
(D) Percentage of meals consumed within each time bin (2 h) on an average day for NFD AL and HFD AL animals. While NFD AL animals consume more meals at night (gray shaded region), HFD AL animals have a much more dispersed pattern (n = 10–11 mice per group).
(E) Percentage of meals consumed during the lights-on phase in NFD AL and HFD AL groups. The HFD AL group consumed a higher percentage of their meals during the lights-on phase than the NFD AL group (t test, t16.5 = 3.157, ∗∗p = 0.006, n = 10–11 mice per group).
(F) Food intake in calories across the light and dark phases. HFD-fed animals consume more overall calories than the NFD-fed animals (two-way ANOVA, effect of diet, F1, 19 = 7.278, ∗p = 0.014, n = 10–11 mice per group; effect of time of day, F1,19 = 108.584, p < 0.001).
(G) Overall daily caloric intake across all 4 feeding groups. A main effect of diet indicated that both HFD AL and HFD TRF fed mice consume more calories than the NFD groups (two-way ANOVA, F1,38 = 9.602, p = 0.004, n = 10–11 mice per group). However, there is no effect of TRF on caloric intake; animals on TRF consume the same number of calories as the AL animals on the same diet.
Data are plotted as mean ± SEM with individual data points visible where possible.
These food intake and meal timing results validate that the HFD disturbs normal eating patterns in mice. Importantly, forcing the animals to return to a rhythmic eating pattern via 12-h TRF did not alter total daily calorie intake. As expected, energy expenditure was higher during the dark phase than the light phase in all feeding conditions when normalized to lean body mass (Figure S1C). HFD-fed mice still exhibited diurnal activity patterns, with higher activity during the night. However, total activity levels of HFD-fed mice were reduced compared with NFD-fed animals (Figure S1D). Interestingly, the HFD-induced reduction in activity was not improved with TRF. In addition to altered meal timing, the HFD eliminated rhythmicity in respiratory exchange ratio, a measure of metabolic flexibility, which was partially rescued with TRF (Figure S1F). Overall, these results suggest that our model of HFD feeding is consistent with previous reports and that TRF is successful at reinstating metabolic rhythms without altering body composition and total daily caloric intake in HFD-fed animals. TRF protocols in other studies that aimed to prevent or rescue metabolic impairment were much longer (>12 weeks) than in the present study; thus, longer TRF protocols ultimately reduce weight and fat mass in HFD-fed animals (Chaix et al., 2014). Here, we took advantage of the shorter TRF protocol to examine whether there are neuroprotective benefits independent of the known metabolic benefits.
TRF rescues hippocampal function
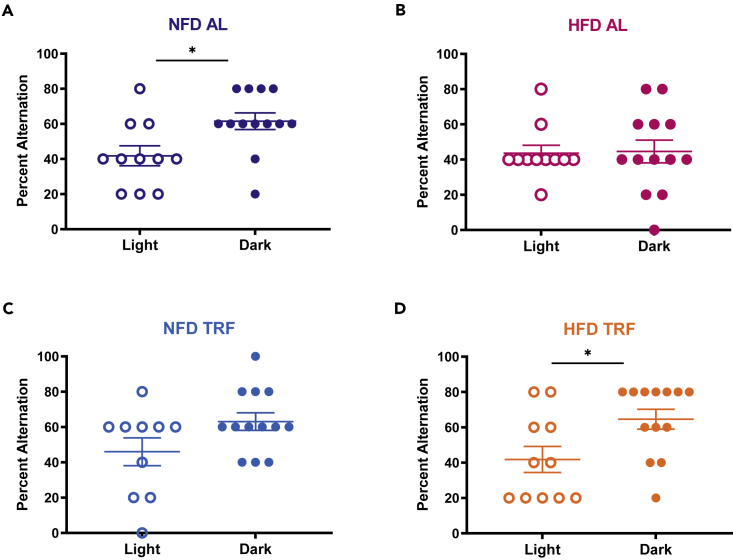
HFD-induced cognitive impairment is well documented in various rodent models (Heyward et al., 2012, 2016; Valladolid-Acebes et al., 2011; Hao et al., 2016). The specific mechanism leading to impairment remains unclear. Mistimed feeding (feeding during the daytime for a nocturnal rodent) may impair memory, similar to an HFD (Loh et al., 2015, but also refer to the study by Power et al., 2018). After confirming that mice shift meal timing while consuming an HFD (Figure 1), we investigated whether TRF rescues HFD-induced impairment of spatial working memory. After 18 weeks of AL access to a NFD or HFD, mice within each diet were divided into groups that were fed AL (NFD AL or HFD AL) or as per the TRF protocol (NFD TRF or HFD TRF). After 1 week of these new feeding schedules, spatial working memory was tested via spontaneous alternation in a T-maze, at either ZT 2 or 14. In general, nocturnal rodents show higher performance on cognitive tasks at night than during the day (Ruby et al., 2013, 2015; Shimizu et al., 2016; Snider et al., 2016). Indeed, mice in the NFD AL group alternated in the maze at a higher rate at night than during the day (Figure 2A). This day/night difference was lost in the HFD AL group with low alternation at both times of the day (Figure 2B). Excitingly, just one week of TRF rescued the night-time spatial working memory performance, restoring a significant day/night difference, even though the mice were still consuming an HFD (Figure 2D). The NFD TRF group did not have a statistical day/night difference owing to high alternation during the day in addition to high alternation at night (Figure 2C).
Figure 2.
Loss of day/night difference in spontaneous alternation rescued by TRF
(A) NFD AL animals are more likely to alternate arms of a T-maze in night trials versus day trials (ordinal regression: χ2(7) = 21.49, p = 0.003, post hoc day/night comparison, ∗p = 0.014).
(B) HFD AL animals had no day/night difference in alternation (p = 0.782).
(C) Day/night differences in NFD TRF animals failed to reach significance owing to increased daytime alteration rather than a decrease in night performance (p = 0.140).
(D) HFD TRF animals had a restored day/night difference in alternation, with higher alternation at night than during the daytime like the NFD AL group (∗p = 0.001).
Data are plotted as mean ± SEM with individual data points visible. N = 10–13 mice per group per time of the day.
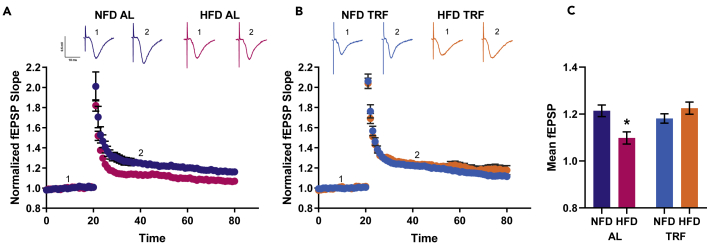
LTP, particularly between the CA3 and CA1 regions of the hippocampus, is considered a physiological correlate of learning and memory and, similar to cognitive function, is higher in magnitude at night than during the day (Besing et al., 2017; Chaudhury et al., 2005). An HFD can reduce LTP magnitude in mice compared with control-diet-fed animals (Hao et al., 2016; Hwang et al., 2010; Heyward et al., 2016; Davis et al., 2020). Because TRF improved night performance of spatial working memory, we assessed LTP at night across the 4 groups. The same mice used for behavioral testing were continued on their respective feeding protocols for an additional week and then euthanized at ~ ZT 11, and brains were prepared for LTP recordings (conducted between projected ZT 13 and ZT 20). The magnitude of LTP in hippocampal slices from HFD AL mice was significantly lower than that from NFD AL mice (Figure 3A). However, two weeks of TRF reinstated LTP in slices from HFD-fed mice, increasing their magnitude of LTP up to the level of LTP in NFD AL slices (Figure 3B). There was no difference among NFD AL, NFD TRF, or HFD TRF mice (Figure 3C).
Figure 3.
A high-fat diet impaired LTP is rescued by TRF
(A) Graph showing mean normalized fEPSP slope at baseline (1) and 20 min after high-frequency stimulus (2). In the AL feeding paradigm, NFD animals have higher magnitude of LTP (poststimulation fEPSP slopes) from 30–80 minutes than the HFD animals (p = 0.002). Data are plotted as mean ± SEM.
(B) In the TRF feeding paradigm, there is no longer a difference between the LTP magnitude of NFD and HFD animals (p = 0.188). Data are plotted as mean ± SEM.
(C) Bar graph showing the average fEPSP slope between 30 and 80 minutes of recording. The slope depended on diet and feeding schedule (linear mixed model, diet x feeding schedule interaction, F1,208 = 4.381, p = 0.038), and as previously mentioned, HFD AL animals had lower LTP than NFD AL animals (∗p = 0.002). TRF increases the HFD LTP such that there is no difference between NFD TRF and HFD TRF LTP (p = 0.188).
Data graphed as estimated marginal means ± 95% confidence intervals. N = 12–20 slices per diet per feeding schedule for LTP recordings. Representative traces above each graph show traces during baseline (labeled as 1) and 20 min after high-frequency stimulus (labeled as 2, minute 40). Scale bar on traces indicates 0.2 mv/5ms.
These results indicate that the timing of meals is important for improving hippocampal function and synaptic plasticity. When mice are provided free access to HFD, changes to their eating behavior may trigger downstream effects on the hippocampus. As previously mentioned, feeding normal chow to mice only during lights on may impair LTP and memory (refer to the study by Loh et al., 2015, but also refer to the study by Power et al., 2018). Here, we found similar results such that HFD AL mice have both reduced spatial working memory and LTP, specifically at night where NF animals have high performance and greater LTP magnitude. The TRF protocol forced animals to return eating behavior to the night phase, restoring both spatial working memory and LTP magnitude back to the levels observed in NFD AL mice. It is important to note that this rescue occurred rapidly. Spatial working memory was rescued with just one week of TRF, meaning the animals did not have time to lose significant amounts of weight (Figure 1). TRF has been used as a preventative treatment for the metabolic consequences of HFD and is effective even when the mice lack a functional molecular clock (Chaix et al., 2019; Hatori et al., 2012). TRF also has been used to rescue metabolic dysfunction after extended AL access to an HFD (Chaix et al., 2014); however, this study assessed the outcomes after many weeks of TRF, and no studies have examined the potential for rescuing HFD-induced cognitive impairment via TRF. Our findings that both synaptic plasticity via LTP and spatial working memory are rapidly rescued by TRF strongly suggests that meal timing is important for normal rhythmicity in hippocampal function.
In conclusion, long-term exposure to an HFD is disruptive to metabolism, cognition, and synaptic plasticity in mice. Brief (1–2 weeks) exposure to TRF is capable of restoring rhythmicity to eating, metabolic, and cognitive rhythms while also restoring hippocampal synaptic plasticity to normal levels. However, the specific mechanism by which TRF restores day/night differences in cognition remains unknown. Genetic ablation of the molecular clock impairs memory (Sakai et al., 2004; Wardlaw et al., 2014; Snider et al., 2016) like an HFD. While the impact of an HFD on the hippocampal molecular clock remains unknown, AL HFD feeding does greatly reduce rhythmicity of the molecular clock in the liver (Hatori et al., 2012; Kohsaka et al., 2007), a change likely linked to the altered behavior and meal timing. Future studies examining the effects of an HFD and TRF on the hippocampal molecular clock, as well as the impact of nutrient and metabolic rhythms on cognition may shed light on this topic.
Limitations of the study
There were several limitations to the present study. First, our study was limited to only male mice, so these experiments would need to be repeated in female mice as well before our findings could be generalized to both sexes. Second, our study only examined one type of hippocampal-dependent memory task. Spontaneous alternation was chosen for this study because of previous reports that performance shows a day/night difference and is sensitive to HFD feeding (Davis et al., 2020; Ruby et al., 2013). Finally, this study did not examine potential mechanisms, such as the molecular clock, underlying TRF-induced rescue of hippocampal dysfunction.
STAR★Methods
Key resources table
| REAGENT OR RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: organisms/strains | ||
| Mouse: C57BL/6J | The Jackson Laboratory | JAX: 000664; RRID:IMSR_JAX:000664 |
| Software and algorithms | ||
| CLAMS/Oxymax V4.30 | Columbus Instruments | N/A |
| ClockLab Analysis V2.72 | Actimetrics | RRID:SCR_014309 |
| SPSS V25 | IBM | RRID:SCR_019096 |
| Other | ||
| Rodent diet with 45% kcal fat | Research Diets Inc. | D12451 |
| Rodent diet with 10% kcal fat | Research Diets Inc. | D12450K |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Karen L. Gamble (klgamble@uab.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The data are available upon request by contacting Lead Contact, Karen Gamble (klgamble@uab.edu). No new code was generated during the course of this study.
Experimental model and subject details
Adult male C57BL/6J mice (JAX stock #000664) were used for all experiments in this study (Jackson Laboratory, Bar Harbor, ME, USA). C57BL/6J mice were ordered at 6 weeks of age and then group-housed where they had ad-lib access to standard laboratory chow (Purina Mills: Rodent Laboratory Chow) and water until otherwise noted. Mice were housed under normal 12:12 lighting conditions unless otherwise noted for specific experiments. Starting at 8 weeks of age, mice were provided ad-lib access to either a high-fat diet (HFD; 45% fat, D12451, Research Diets, New Brunswick, NJ, USA) or a normal-fat control diet (NFD; 10% fat, D12450K, Research Diets, New Brunswick, NJ, USA) for 18 weeks. Mice were group-housed 4 per cage and assessed regularly for any visible signs of fighting within cages. If fighting was detected, cages were split to reduce stress for the animals. At the end of 18 weeks, mice were moved to cages of 2 mice per cage with half of the cages maintaining an ad-lib feeding schedule (20 weeks ad lib) and half the cages only receiving food during the 12-hour lights-off phase (18 weeks ad lib, 2 weeks TRF). Mice were weighed weekly throughout the duration of the study. Animals used for behavior experiments were used for slice electrophysiology approximately one week after completion of behavior tests. Sample size (N) is indicated for each experiment in the figure legends. All other experiments had unique cohorts. For CLAMS analysis and behavior/electrophysiology cohorts, restricted feeding was automated. All animals were handled in accordance with the University of Alabama at Birmingham Institutional Animal Care and Use Committee and National Institutes of Health guidelines.
Method details
Metabolic and food intake data
After 18 weeks of ad-lib feeding, animals had body composition assessed via quantitative magnetic resonance (QMR; EchoMRI™ 3-in-1 QMR machine; Echo Medical Systems, Houston, TX). After QMR assessment, mice were transferred to the Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments Inc., Columbus, OH). Mice were single housed for accurate individual metabolic and food intake measurements and were maintained on ad-lib diets with free access to water in a 12:12 light cycle while acclimating to the new environment for 2 weeks. At 20 weeks, half of the animals on each diet were transitioned to TRF, where food access was limited to the 12-h dark period. During the next week, 24-h patterns of energy expenditure (indirect calorimetry), physical activity (beam breaks), and food intake were measured. CLAMS data were imported to ClockLab software (Actimetrics, Wilmette, IL, USA) and analyzed using the batch analysis function. Cycles 2–7 in the CLAMS cages were used for analysis. Meals were measured using the CLAMS feeding bout detection which defined a “bout” as a period where food intake was greater than 0.02 g and interbout interval was greater than 10 s. During periods of restricted feeding, food access was enforced in an automated fashion by the CLAMS. After completion of TRF (week 21), mice underwent a second QMR assessment for post-TRF body composition analysis.
Spontaneous alternation task
Spatial working memory was tested during the day and night (ZT 2 and 14) with a T-maze, spontaneous alternation protocol (adapted from (Deacon and Rawlins, 2006)). Half the mice were transitioned to a reverse entrainment room (lights-on 9 pm–lights-off 9am) at approximately 15 weeks of feeding, while the other half were maintained on a normal-light cycle. After completing 18 weeks of ad-lib feeding on respective diets, mice were moved to cages with automated food access (custom large rotating wheel cage tops programmed to provide either continued ad-lib access or only allow access to food during the lights off phase) and housed 2 per cage. After 1 week in these automated feeders, mice were tested for spatial working memory via alternation in a T-maze. Cages were assigned to be tested during either the light or dark phase. Mice were handled for 4 consecutive days before testing. On the day of the test, mice were brought to the testing room 30 minutes before beginning the test to acclimate. During the test, mice were placed in the maze at the stem of the T and allowed to freely choose an arm of the T. Upon entry to an arm, a gate was closed, trapping the mouse in the arm for 30 seconds. The mouse was removed, the gate opened, and the mouse returned to the base of the maze. An alternation was recorded if the mouse chose the opposite arm as the last entry. Each mouse performed 5 trials, and an alternation percentage was calculated as (# alternations / # of trials). All of the trials at night (ZT 14) were conducted under dim red light (< 5 lux), and animals were transferred in light-tight boxes, to ensure minimal circadian disturbances, while day trials (ZT 2) were conducted under normal room lights.
Slice electrophysiology
After completion of the spontaneous alternation task, reverse entrained mice were returned to the automated feeding cages for the remaining week of the protocol. After approximately 2 weeks from the start of the restricted feeding protocol timeline (+/- 3 days to account for limited number of animals to be used for electrophysiology at a time), mice were euthanized for electrophysiology. To record changes in LTP after long-term feeding, mice were euthanized by cervical dislocation and rapid decapitation, brains were then extracted and sliced in ice cold, oxygenated high-sucrose artificial cerebrospinal fluid (aCSF) (in mM: 85 NaCl, 2.5 KCl, 4 MgSO4, 0.5 CaCl2, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose and 75 sucrose saturated in 95% O2 and 5% CO2). Coronal slices (400 μm) were prepared via a vibratome (Campden 7000SMZ, World Precision Instruments) and transferred to a chamber (Kerr Tissue Recording System, Kerr Scientific Instruments) with standard aCSF (in mM: 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 11 glucose, saturated in 95% O2 and 5% CO2) and stored at room temperature until recording. Slices were prepared between ZT 11 and ZT 11.5 and recorded from ZT 13 to 20. Slice recordings were performed as previously described (Besing et al., 2017). Briefly, baseline recordings were established for 20 min, and then, LTP of the Schaffer collateral pathway was induced using a high-frequency stimulation (HFS) consisting of 2 trains of 100 Hz (0.5 duration, 15-s interval) and field excitatory postsynaptic potential (fEPSP) slopes were recorded for 60 min following HFS. Slices were excluded from analysis in the following cases: the after was not stable; after HFS, the fEPSP slope dropped below baseline; contamination of electrical noise in the traces; or technical issues. Data were normalized to baseline (0–20 min) before analysis. Thirty to 80 minutes (10–60 minutes after LTP induction) were used for analysis.
Quantification and statistical analysis
To assess differences between groups, data were analyzed with two- or three-way ANOVA (with repeated measures for analysis of body weight data or day/night comparisons) or t-tests as appropriate. Spontaneous alternation was analyzed using ordinal regression analysis (Logit link function) with the day-night/diet group as a fixed factor and alternation as an ordinal outcome (four categories: 0–20%, 40%, 60%, 80–100% alternations). Analysis of LTP data was carried out with a three-way linear mixed model with minute as a repeated measures covariate. Statistical analyses were performed using SPSS (version 25). Significance is ascribed as p < 0.05 unless otherwise noted.
Acknowledgments
This research was supported by NIH grants R01NS082413 (KLG), P30DK056336, T32HL105349, and the UAB School of Medicine Planning Grant for Multi-Investigator Programs. We thank Jackson Colson for his assistance in managing the animal colonies and feeding schedules. We thank additional members of the Pollock, Bailey, and Gamble laboratories for assistance in experiments involving food restriction and Martin Young for the use of the Comprehensive Lab Animal Monitoring System (CLAMS). We also thank the UAB NORC Small Animal Phenotyping Core for assistance in collecting QMR data.
Author contributions
KLG, SMB, JSP, and DMP, conceived the study. JAD, KLG, and SDY performed the experiments, JRP, JAD, KLG, and EJC analyzed data. JAD wrote the manuscript, and JRP and KLG edited the manuscript, with assistance from all other coauthors.
Declaration of interests
No relevant disclosures.
Inclusion and diversity
One or more of the authors of this article self-identifies as an underrepresented ethnic minority in science. One or more of the authors of this paper received support from a program designed to increase minority representation in science. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Published: June 25, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102532.
Supplemental information
References
- Besing R.C., Rogers C.O., Paul J.R., Hablitz L.M., Johnson R.L., McMahon L.L., Gamble K.L. GSK3 activity regulates rhythms in hippocampal clock gene expression and synaptic plasticity. Hippocampus. 2017;27:890–898. doi: 10.1002/hipo.22739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branecky K.L., Niswender K.D., Pendergast J.S. Disruption of daily rhythms by high-fat diet is reversible. PLoS One. 2015;10:e0137970. doi: 10.1371/journal.pone.0137970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A., Lin T., Le H.D., Chang M.W., Panda S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 2019;29:303–319.e4. doi: 10.1016/j.cmet.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A., Zarrinpar A., Miu P., Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D., Wang L.M., Colwell C.S. Circadian regulation of hippocampal long-term potentiation. J. Biol. Rhythms. 2005;20:225–236. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H., Chou W., Sears D.D., Patterson R.E., Webster N.J., Ellies L.G. Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism. 2016;65:1743–1754. doi: 10.1016/j.metabol.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.A., Paul J.R., Mcmeekin L.J., Nason S.R., Antipenko J.P., Yates S.D., Cowell R.M., Habegger K.M., Gamble K.L. High-fat and high-sucrose diets impair time-of-day differences in spatial working memory of male mice. Obesity (Silver Spring) 2020;28:2347–2356. doi: 10.1002/oby.22983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon R.M., Rawlins J.N. T-maze alternation in the rodent. Nat. Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Hao S., Dey A., Yu X., Stranahan A.M. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav. Immun. 2016;51:230–239. doi: 10.1016/j.bbi.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri N., Thibault L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- Hatori M., Vollmers C., Zarrinpar A., Ditacchio L., Bushong E.A., Gill S., Leblanc M., Chaix A., Joens M., Fitzpatrick J.A. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydemann A. An overview of murine high fat diet as a model for type 2 diabetes mellitus. J. Diabetes Res. 2016;2016:2902351. doi: 10.1155/2016/2902351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyward F.D., Gilliam D., Coleman M.A., Gavin C.F., Wang J., Kaas G., Trieu R., Lewis J., Moulden J., Sweatt J.D. Obesity weighs down memory through a mechanism involving the neuroepigenetic dysregulation of Sirt1. J. Neurosci. 2016;36:1324–1335. doi: 10.1523/JNEUROSCI.1934-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyward F.D., Walton R.G., Carle M.S., Coleman M.A., Garvey W.T., Sweatt J.D. Adult mice maintained on a high-fat diet exhibit object location memory deficits and reduced hippocampal SIRT1 gene expression. Neurobiol. Learn. Mem. 2012;98:25–32. doi: 10.1016/j.nlm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang L.L., Wang C.H., Li T.L., Chang S.D., Lin L.C., Chen C.P., Chen C.T., Liang K.C., Ho I.K., Yang W.S., Chiou L.C. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity (Silver Spring) 2010;18:463–469. doi: 10.1038/oby.2009.273. [DOI] [PubMed] [Google Scholar]
- Kohsaka A., Laposky A.D., Ramsey K.M., Estrada C., Joshu C., Kobayashi Y., Turek F.W., Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Loh D.H., Jami S.A., Flores R.E., Truong D., Ghiani C.A., O'dell T.J., Colwell C.S. Misaligned feeding impairs memories. Elife. 2015;4:e09460. doi: 10.7554/eLife.09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterman J.E., Kalsbeek A., La fleur S.E., Belsham D.D. Impact of nutrients on circadian rhythmicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R337–R350. doi: 10.1152/ajpregu.00322.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast J.S., Branecky K.L., Yang W., Ellacott K.L., Niswender K.D., Yamazaki S. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci. 2013;37:1350–1356. doi: 10.1111/ejn.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell P.J., Morrison C.D., Gupta S., Knight A.G., Keller J.N., Ingram D.K., Bruce-Keller A.J. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power S.C., Michalik M.J., Couture-Nowak S., Kent B.A., Mistlberger R.E. Midday meals do not impair mouse memory. Sci. Rep. 2018;8:17013. doi: 10.1038/s41598-018-35427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby N.F., Fernandez F., Garrett A., Klima J., Zhang P., Sapolsky R., Heller H.C. Spatial memory and long-term object recognition are impaired by circadian arrhythmia and restored by the GABAAAntagonist pentylenetetrazole. PLoS One. 2013;8:e72433. doi: 10.1371/journal.pone.0072433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby N.F., Patton D.F., Bane S., Looi D., Heller H.C. Reentrainment impairs spatial working memory until both activity onset and offset reentrain. J. Biol. Rhythms. 2015;30:408–416. doi: 10.1177/0748730415596254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Tamura T., Kitamoto T., Kidokoro Y. A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc. Natl. Acad. Sci. U S A. 2004;101:16058–16063. doi: 10.1073/pnas.0401472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Kobayashi Y., Nakatsuji E., Yamazaki M., Shimba S., Sakimura K., Fukada Y. SCOP/PHLPP1beta mediates circadian regulation of long-term recognition memory. Nat. Commun. 2016;7:12926. doi: 10.1038/ncomms12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider K.H., Dziema H., Aten S., Loeser J., Norona F.E., Hoyt K., Obrietan K. Modulation of learning and memory by the targeted deletion of the circadian clock gene Bmal1 in forebrain circuits. Behav. Brain Res. 2016;308:222–235. doi: 10.1016/j.bbr.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladolid-Acebes I., Stucchi P., Cano V., Fernandez-Alfonso M.S., Merino B., Gil-Ortega M., Fole A., Morales L., Ruiz-gayo M., Del Olmo N. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol. Learn. Mem. 2011;95:80–85. doi: 10.1016/j.nlm.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Wardlaw S.M., Phan T.X., Saraf A., Chen X., Storm D.R. Genetic disruption of the core circadian clock impairs hippocampus-dependent memory. Learn. Mem. 2014;21:417–423. doi: 10.1101/lm.035451.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available upon request by contacting Lead Contact, Karen Gamble (klgamble@uab.edu). No new code was generated during the course of this study.