Abstract
Rationale:
Cardiac hypertrophy, a major risk factor for heart failure, occurs when cardiomyocytes remodel in response to complex signaling induced by injury or cell stress. Although cardiomyocytes are the ultimate effectors of cardiac hypertrophy, non-myocyte populations play a large yet understudied role in determining how cardiomyocytes respond to stress.
Objective:
To identify novel paracrine regulators of cardiomyocyte hypertrophic remodeling.
Methods and Results:
We have identified a novel role for a non-myocyte-derived and TGFβ1-induced extracellular matrix protein Microfibrillar-associated protein 4 (MFAP4) in the pathophysiology of cardiac remodeling. We have determined that non-myocyte cells are the primary sources of MFAP4 in the heart in response to TGFβ1 stimulation. Furthermore, we have demonstrated a crucial role of MFAP4 in the cardiac adaptation to stress. Global knockout of MFAP4 led to increased cardiac hypertrophy and worsened cardiac function following chronic pressure overload. Also, one week of angiotensin-mediated neurohumoral stimulation was sufficient to exacerbate cardiomyocyte hypertrophy in MFAP4 null mice. In contrast, administration of exogenous MFAP4 to isolated cardiomyocytes blunted their phenylephrine-induced hypertrophic growth through an integrin-dependent mechanism. Finally, MFAP4 deficiency leads to dysregulated integration of G protein-coupled receptor and integrin signaling in the heart.
Conclusions:
Altogether, our results demonstrate a critical paracrine role of MFAP4 in the development of cardiac hypertrophy and could inform future treatment options for heart failure patients.
Keywords: TGFβ, extracellular matrix, cardiomyocyte hypertrophy, integrin signaling
Introduction
The heart undergoes adaptive remodeling in response to pressure or volume stress, loss-of-function variants in structural proteins, neurohumoral stimulation, and/or loss of myocardium through cell death. During this adaptive remodeling, coordinated signaling mechanisms between multiple cell types induce a wide range of cellular changes aimed at preserving cardiac function, including extracellular matrix remodeling, immune cell modulation, neovascularization, and cardiomyocyte hypertrophy. Although cardiomyocyte hypertrophy is a crucial response for the injured heart to maintain cardiac output short-term, it is also associated with an increased risk for development of heart failure and arrhythmia 1. Consequently, research in recent years has focused on ameliorating cardiac hypertrophy by targeting cardiomyocyte-specific pro-hypertrophic signaling 2. While significant progress has been made in understanding the complex signaling within cardiomyocytes that drives hypertrophy, the role of non-myocytes in either promoting or ameliorating this hypertrophic response is less well understood.
A primary master regulator of injury responses across organ systems, including the heart, is the secreted factor transforming growth factor beta 1 (TGFβ1). The ability of TGFβ1 to affect cardiac remodeling has been corroborated by multiple studies 3-8. Receptors for TGFβ1 (TGFβR1 and TGFβR2) are ubiquitously expressed, supporting a role for multiple cell types in the cardiac response to stress-induced activation of this pleiotropic cytokine. Although TGFβ1 is conventionally viewed as a signal for maladaptive remodeling in the injured heart, the data support a more nuanced view of the TGFβ1’s role in regulating cardiac function. For example, TGFβ1 inhibition through neutralizing antibodies does not ameliorate cardiac pathology and worsens ventricular remodeling in certain cases, highlighting the complexity of TGFβ1 signaling and discouraging a TGFβ1-specific therapeutic approach 9, 10. Recent studies adopting genetic manipulation of TGFβ1 signaling specifically in cardiomyocytes or fibroblasts highlighted the beneficial contribution of TGFβ1 for promoting adaptive cardiac remodeling 7, 8. Less is known about the contribution of endothelial cells for TGFβ1-dependent regulation of cardiac stress responses. Endothelial cells, which comprise around 60% of the non-myocyte population in the adult heart and influence cardiomyocyte signaling in a paracrine manner 11, 12.
Here we report that TGFβ1 induces cardiac expression of microfibril-associated protein 4 (MFAP4) by non-myocytes. MFAP4 is a 36-kDa secreted extracellular matrix glycoprotein that belongs to the fibrinogen-related protein superfamily and participates in elastic fiber formation 13, 14 Whereas MFAP4 appears to be redundant for cardiovascular development and homeostatic cardiovascular function, several studies have proposed a role for MFAP4 in regulating injury responses 13, 15-17 In this manuscript, we identify the previously unrecognized importance of MFAP4 as a critical downstream mediator of TGFβ1-related stress responses in the heart.
Results
MFAP4 is a TGFβ1-inducible extracellular matrix factor in the heart
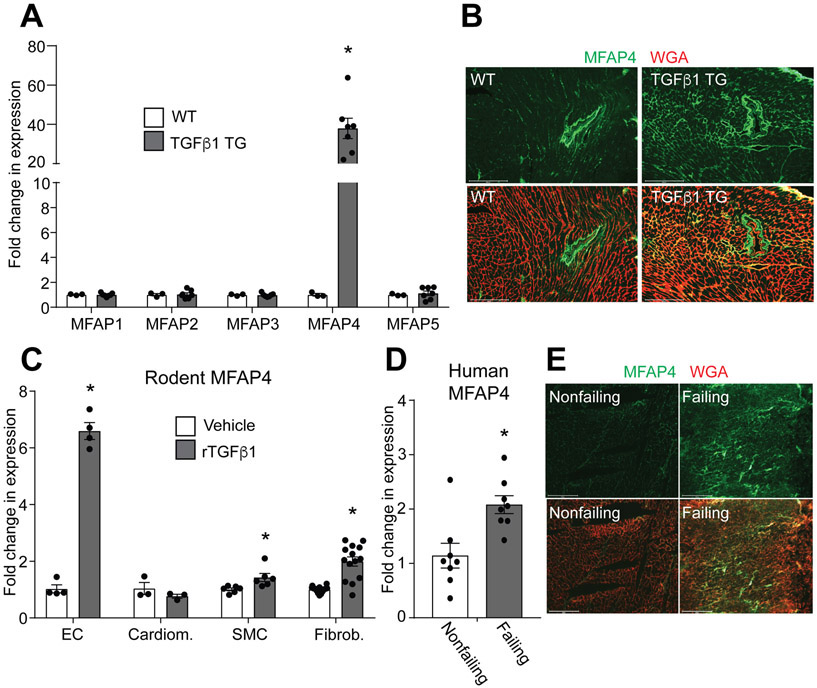
In an unbiased approach to define mediators of TGFβ1 in the heart, we performed a genome-wide transcriptome analysis on our previously characterized mouse model of inducible cardiac expression of active TGFβ1 5. This analysis revealed Mfap4 as a top upregulated gene. MFAP4 is a member of a larger family comprised of five matricellular proteins with identified roles in organization of elastins and fibrillins within the extracellular matrix, particularly important in developing tissues and large vessel remodeling 18. To determine the role of TGFβ1 in specific induction ofMfap4 expression, we performed quantitative-real time polymerase chain reaction (q-RT-PCR or qPCR) measurements on whole heart extract from wild-type (WT) and cardiomyocyte-restricted TGFβ1 overexpressing (TGFβ1 TG) mice. TGFβ1 specifically induced expression of Mfap4, and not of the other members of the microfibrillar-associated protein family (Figure 1A). To confirm MFAP4 induction by TGFβ1, we performed immunofluorescence analysis of MFAP4 protein levels in cardiac cross-sections from WT and TGFβ1-overexpressing mice. In WT animals, MFAP4 is present sparingly within the myocardial interstitium and more highly expressed around larger intra-myocardial arterioles, where previous reports have demonstrated that MFAP4 is expressed by contractile smooth muscle cells 16 (Figure 1B). TGFβ1 overexpression dramatically increased the amount of MFAP4 protein infiltrating the interstitial space between cardiomyocytes (Figure 1B). As TGFβ1 is a secreted factor that can potentially act on every cell type within a tissue, we then sought to identify the particular cell type responsible for expressing MFAP4 in response to TGFβ1. We cultured rat endothelial cells (EC), neonatal rat cardiomyocytes, mouse smooth muscle cells, and neonatal rat cardiac fibroblasts, representing the four major cardiac cell types. Ex vivo treatment with either vehicle or recombinant TGFβ1 for 48 hours revealed that endothelial cells were able to highly upregulate Mfap4 gene expression following TGFβ1 treatment (Figure 1C). In addition, smooth muscle cells and cardiac fibroblasts showed a modest increase in this gene while no significant induction was observed in cardiomyocytes, suggesting strong non-myocyte contribution for this TGFβ1-dependent cardiac event. Our group and others have previously demonstrated that TGFβ1 is activated during cardiac remodeling following various stimuli in both mice and humans 3-5, 7, 8. To test the translational potential of our finding we analyzed MFAP4 expression in human heart tissue samples from healthy donors or patients with heart failure. qPCR and immunofluorescent analyses revealed that Mfap4 is upregulated in failing human hearts compared with their nonfailing controls, suggesting evolutionary conservation of Mfap4 induction in injured hearts (Figure 1D and E). Overall, these results show that MFAP4 expression is induced in the heart by TGFβ1, that non-myocytes are a critical source for this TGFβ1-induced factor, and that MFAP4 may play a role in cardiac remodeling.
Figure 1. TGFβ1 induces MFAP4 expression.
A. qPCR analysis for Mfap family member gene expression relative to the Rpl7 housekeeping gene in WT (n=3) and TGFβ1 overexpressing (TGFβ1 TG) (n=7) hearts. (Mann-Whitney test results: Mfap4 *p=0.0167). B. Immunofluorescence staining of MFAP4 (green) and wheat germ agglutinin (WGA; red) in hearts from the indicated genotypes. Scale bar, 200 μm. C. qPCR analysis of Mfap4 expression relative to Rpl7 in rat endothelial cells (EC) (n=4), rat cardiomyocytes (Cardiom.) (n=3), rat smooth muscle cells (SMC) (n=6) and rat cardiac fibroblasts (Fibrob.) (n=14) treated with either vehicle or recombinant TGFβ1 (rTGFβ1). (Mann-Whitney test results: EC *p=0.0286; Student’s t test results: SMC *p=0.0202; Fibrob. *p=0.0000027). D. qPCR analysis ofMFAP4 expression relative to RPL7 from nonfailing or failing human hearts (n=8). (Student’s t test results: Mfap4 *p=0.0049). E. Immunofluorescence staining of MFAP4 (green) and wheat germ agglutinin (WGA; red) in human failing and nonfailing hearts. Scale bar, 200 μm.
Loss of MFAP4 accelerates cardiac dysfunction and exacerbates cardiomyocyte hypertrophy following chronic pressure overload
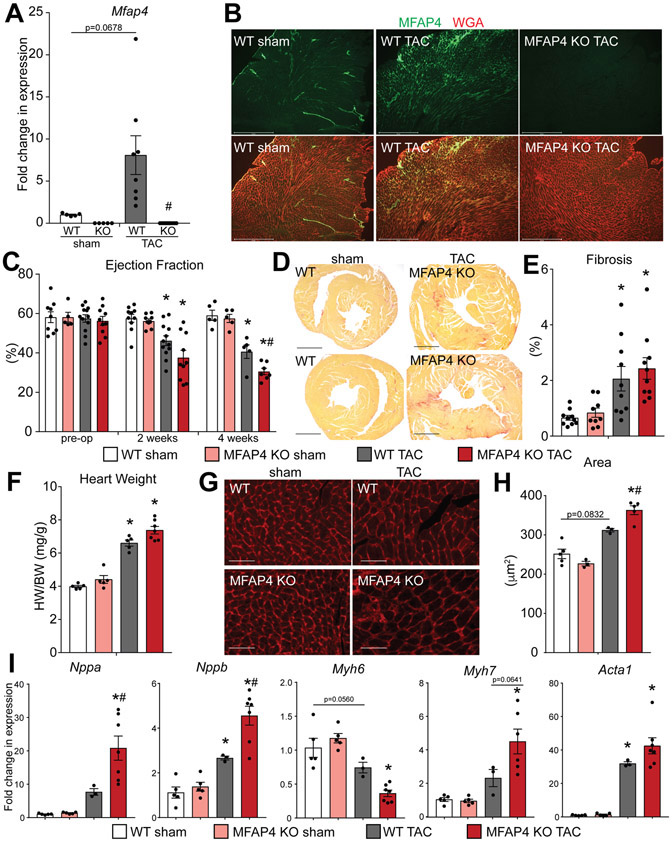
MFAP4 knockout (KO) mice 13, 14, 17 have been extensively characterized previously, and show no cardiac pathophysiology at baseline 16, 17 We first tested if MFAP4 is induced following pressure-overload-induced cardiac remodeling by transverse aortic constriction (TAC) surgery. This analysis confirmed robust induction of Mfap4 following 4 weeks of pressure overload in WT mice (Figure 2A). This test also ensured that Mfap4 is completely absent from KO mouse hearts with either sham or TAC surgery (Figure 2A). Induction and localization of MFAP4 in injured hearts was further characterized by immunostaining. Similar to the increase in MFAP4 protein amounts and distribution shown in our TGFβ1 overexpression model, we found elevated MFAP4 in the myocardial interstitial space of WT hearts injured by 4 weeks of pressure overload (Figure 2B). In an injury time course we then found that Mfap4 expression increases already at 2 weeks post-TAC and it is still upregulated at 8 weeks although to a significantly lower extent, behavior not shared by all other extracellular matrix proteins tested, suggesting an independent role for secreted MFAP4 (Online Figure IA). To test if MFAP4 exerts a primary role in regulating cardiac remodeling and function post injury, we assessed how the heart responds to stress in the absence of MFAP4 by independently assessing cardiac function at 2 and 4 weeks post-TAC. Strikingly, MFAP4 KO animals showed accelerated heart failure progression post-TAC, which was characterized by echocardiographic measurement of percent left ventricular ejection fraction which was significantly reduced in MFAP4 KO mice at the 4 weeks time point (Figure 2C). The cardiac dysfunction observed with MFAP4 loss-of-of function was accompanied by structural abnormalities such as worsened left ventricular chamber dilation post-TAC (Online Table I). Importantly, no significant defects were observed in the absence of pressure overload (sham controls), confirming a predominant role for MFAP4 as an injury response factor (Figure 2C and Online Table I). Since we have shown that MFAP4 is induced by TGFβ1 in the heart and TGFβ1 has established pro-fibrotic functions, we reasoned that MFAP4 may act in the development of cardiac fibrosis and subsequent development of cardiac dysfunction. However, when we performed picrosirius red staining and quantified the resulting fibrosis in our mouse cohorts, we found that the percentage of either total or perivascular myocardial fibrosis was not significantly different between WT and MFAP4 KO animals following pressure overload injury (Figure 2D and E, Online Figure VA and B). However, we noticed an increase in the total heart weight to body weight ratio, as well as increased cardiomyocyte cross-sectional area, in MFAP4 KO mice with TAC, two parameters indicative of increased cardiac hypertrophy (Figure 2F-H). These results were confirmed by validating the differential induction of genes canonically altered during pathological cardiac hypertrophy (Figure 2I). Specifically, pressure-overloaded MFAP4 KO mice demonstrated changes consistent with exacerbated maladaptive hypertrophy, including decreased expression of alpha myosin heavy chain (Myh6) with concomitant increase in fetal beta myosin heavy chain (Myh7), as well as significant increases over WT animals in the alpha and beta natriuretic peptides (Nppa and Nppb) and a similar trend for skeletal alpha-actin (Acta1) (Figure 2I). Furthermore, gene expression analysis of TGFβ receptors (Tgfbr) 1 and 2 revealed no significant genotype-dependent differences in WT and MFAP4 KO hearts either at baseline or after injury, as well as no significant effect on modulating the expression of these receptors on cardiomyocytes by MFAP4 supplementation (Online Figure IB and C). Importantly, inhibition of MFAP4 in WT mice post induction of pressure overload through administration of neutralizing antibodies was sufficient to recapitulate the key results observed using MFAP4 KO mice (Online Figure IIA and B). Together, the loss of MFAP4 promotes aggravated cardiac hypertrophy and resulting cardiac dysfunction following chronic pressure overload in mice.
Figure 2. MFAP4 KO mice demonstrate cardiac dysfunction and altered cardiac remodeling following chronic pressure overload.
A. qPCR analysis for Mfap4 relative to Rpl7 in the indicated groups. (WT and KO sham n=5, WT TAC n=8, KO TAC n=12). (Two-way ANOVA results: WT sham vs TAC p=0.0678; WT vs KO TAC #p=0.00075). B. Immunofluorescence staining of MFAP4 (green) and wheat germ agglutinin (WGA; red) in hearts from the indicated groups. Scale bar, 500 μm. C. Echocardiographic quantification of percentage ejection fraction in WT and MFAP4 KO mice prior to surgery (pre-op) and every two weeks after induction of pressure overload. (WT sham pre-op n=9, KO sham pre-op n=5, WT TAC pre-op n=12, KO TAC pre-op n=10; WT sham 2 weeks n=10, KO sham 2 weeks n=9, WT TAC 2 weeks n=12, KO TAC 2 weeks n=10; WT sham 4 weeks n=5, KO sham 4 weeks n=5, WT TAC 4 weeks n=5, KO TAC 4 weeks n=7). (Two-way ANOVA results for 2 wks time point: WT sham vs TAC *p=0.0112; KO sham vs TAC *p=0.000175; 4 wks time point: WT sham vs TAC *p=0.00042; KO sham vs TAC *p=0.0000012; WT vs KO TAC #p=0.0421). D. Representative picrosirius red – stained cardiac cross-sections from WT and MFAP4 KO mice following 4 weeks of sham or pressure overload (TAC) surgery. Scale bar, 1 mm. E. Quantification of percent fibrosis from picrosirius red - stained cardiac cross-sections in the indicated groups after sham or pressure overload (TAC) surgery. (WT and KO sham n=5, WT TAC n=5, KO TAC n=7). (Two-way ANOVA results: WT sham vs TAC *p=0.0079; KO sham vs TAC *p=0.0060). F. Gravimetric analysis of heart weight to body weight ratio (HW/BW) in the indicated groups 4 weeks after sham or pressure overload (TAC) surgery. (Two-way ANOVA results: WT sham vs TAC *p=0.000017; KO sham vs TAC *p=0.0000086). G. Representative images of WGA-stained cardiac cross-sections of WT or MFAP4 KO mice 4 weeks after sham or pressure overload (TAC) surgery. Scale bar, 50 μm. H. Quantification of cardiomyocyte cross-sectional area based on WGA-stained cardiac cross-sections in the indicated groups. (WT sham n=5, KO sham n=3, WT TAC n=3, KO TAC n=5). (Two-way ANOVA results: WT sham vs TAC p=0.0832; KO sham vs TAC *p=0.00019; WT vs KO TAC #p=0.0420). I. qPCR analysis for hypertrophic gene expression relative to Rpl7 in the indicated groups. (WT and KO sham n=5, WT TAC n=3, KO TAC n=7). (Two-way ANOVA results - Nppa: KO sham vs TAC *p=0.000030; WT vs KO TAC #p=0.0037. - Nppb: WT sham vs TAC *p=0.0201; KO sham vs TAC *p=0.0000098; WT vs KO TAC #p=0.0022. - Myh6: WT sham vs TAC p=0.0560; KO sham vs TAC *p=0.00011. - Myh7: KO sham vs TAC *p=0.000087; WT vs KO TAC p=0.0641. - Acta1: WT sham vs TAC *p=0.0037; KO sham vs TAC *p=0.000055).
Further characterization of the phenotype underlying the defective stress response of MFAP4 KO mice revealed no significant alteration in gene markers associated with autophagy or cell death (Online Figure IIIA and B). However, TUNEL analysis of apoptotic cells on histological sections demonstrated a significant increase in the number of apoptotic nuclei in the MFAP4 KO animals post-TAC (Online Figure IIIC and D). This analysis also highlighted the presence of infiltrating cells in the MFAP4 KO hearts post-TAC. We therefore decided to assess if inflammation was affected by the lack of MFAP4. Indeed, we noticed a greater induction of inflammatory gene markers within the MFAP4 KO hearts after TAC (Online Figure IVA), which is supported by increased numbers of total leukocytes in the myocardium of MFAP4 KO as assessed by staining for CD45-positive cells (Online Figure IVB and C). Overall, MFAP4 KO mice exhibited increased signs of cell death and inflammation to accompany the exacerbated cardiac hypertrophy and decreased cardiac function following pressure overload.
MFAP4 loss worsens cardiac hypertrophy after neurohumoral stress
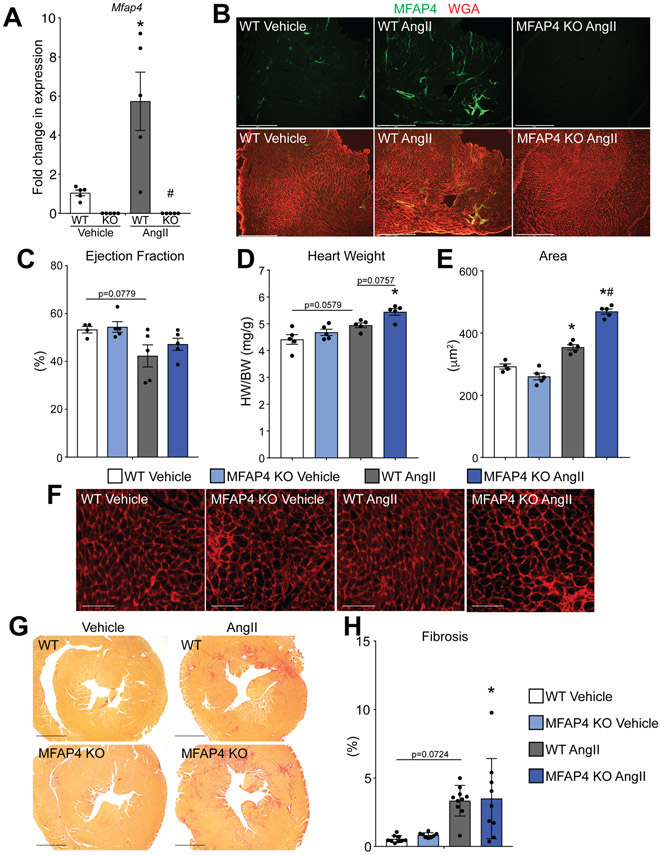
The observation that MFAP4 KO mice have exacerbated cardiac hypertrophy and rapid functional decompensation following chronic pressure overload stress prompted us to test if the hypertrophic changes in MFAP4 KO mice would precede functional decline and could be validated with alternative cardiac stressors. To address these questions, we tested the effect of MFAP4 loss in a model of neurohumoral-induced cardiac stress by infusing angiotensin II (Ang II) to mice for 1 week via implantable minipump. We first assessed if MFAP4 expression is responsive to this injury model that is also known to involve TGFβ1 signaling. Similar to our pressure overload experiments, angiotensin II infusion caused a significant increase in Mfap4 expression only in WT animals (Figure 3A), whereas Mfap4 was absent in KO animals, as expected (Figure 3A). In addition, immunofluorescence imaging demonstrated an increase in the amount of MFAP4 protein in WT animals with angiotensin II (Figure 3B). One week of angiotensin II administration did not cause significant functional deficits in either WT or KO animals via echocardiographic measurements of percent left ventricular ejection fraction (Figure 3C and Online Table 1). However, in agreement with our pressure overload studies, MFAP4 KO animals stimulated with angiotensin II exhibited exacerbated cardiac hypertrophy (Figure 3D-F). Specifically, MFAP4 KO animals had an increased heart weight to body weight ratio (Figure 3D), as well as larger cardiomyocyte cross-sectional area, compared to WT animals with angiotensin II (Figure 3E and F). Angiotensin II is a potent activator of fibrosis in mice 19; to ensure that the effects of MFAP4 loss specifically influence cardiac hypertrophy and not fibrosis, we performed picrosirius red staining on cardiac cross-sections from WT and MFAP4 KO mice treated with either vehicle or angiotensin II and quantified the resulting fibrosis percentages. Although angiotensin II was able to induce fibrotic remodeling in both genotypes, there was no significant difference in the amount of total or perivascular fibrosis between WT and MFAP4 KO mice treated with angiotensin II (Figure 3G-H, Online Figure VC and D). These data suggest that, even with a milder cardiac stress induced by infusion of a neurohumoral agent, the loss of MFAP4 exacerbates cardiomyocyte hypertrophy independent of fibrosis, and that these changes occur prior to cardiac functional decline.
Figure 3. MFAP4 KO mice develop increased hypertrophy with angiotensin II treatment.
A. qPCR analysis for Mfap4 relative to Rpl7 in the indicated groups (all n=5) after 1 week of angiotensin (AngII) or vehicle (Veh) treatment. (Two-way ANOVA results: WT Veh vs AngII *p=0.0045; WT vs KO AngII #p=0.00090). B. Immunofluorescence staining of MFAP4 (green) and wheat germ agglutinin (WGA; red) in the indicated groups. Scale bar, 500 μm. C. Echocardiographic quantification of percentage ejection fraction in WT and MFAP4 KO mice following 1 week of vehicle or AngII treatment (all groups n=5). (Two-way ANOVA results: WT Veh vs AngII p=0.0779). D. Gravimetric analysis of heart weight to body weight ratio (HW/BW) in the indicated groups 1 week after vehicle or AngII administration (all groups n=5). (Two-way ANOVA results: WT Veh vs AngII p=0.0579; KO Veh vs AngII *p=0.0062; WT vs KO AngII p=0.0757). E. Quantification of cardiomyocyte cross-sectional area from WGA – stained cardiac cross-sections in the indicated groups (WT Veh n=4, KO Veh n=5, WT and KO Ang n=5). (Two-way ANOVA results: WT Veh vs AngII *p=0.0041; KO Veh vs AngII *p=0.000000030; WT vs KO AngII #p=0.000012). F. Representative images of WGA – stained cardiac cross-sections in the indicated groups. Scale bar, 50 μm. G. Representative images of picrosirius red – stained cardiac cross-sections in the indicated groups. Scale bar, 1 mm. H. Quantification of percent fibrosis from picrosirius red - stained cardiac cross-sections in the indicated groups (WT Veh n=9, KO Veh n=8, WT Ang n=10, KO Ang n=9). (Two-way ANOVA results: WT Veh vs AngII *p=0.0050; KO Veh vs AngII *p=0.0078).
Exogenous MFAP4 is sufficient to ameliorate cardiomyocyte hypertrophy ex vivo
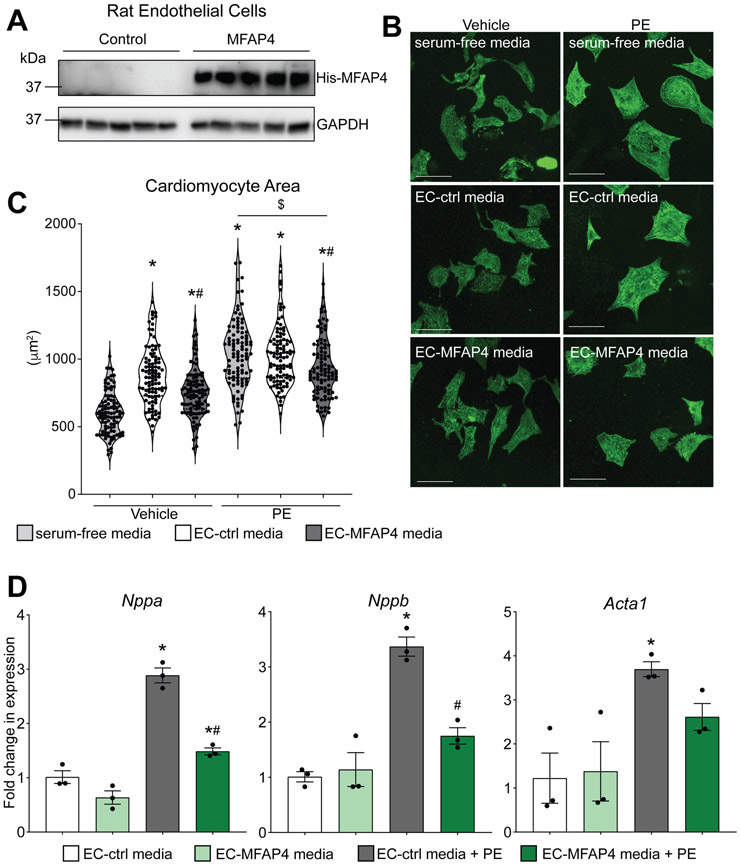
Since our in vivo models demonstrated that loss of MFAP4 leads to exacerbated cardiac hypertrophy with either acute or chronic stressors, we hypothesized that providing exogenous MFAP4 to cardiomyocytes would ameliorate hypertrophic growth in the presence of a hypertrophic stimulus. We adopted a simplified model system using primary cultured neonatal cardiomyocytes, which provides the advantage of studying the direct effects of MFAP4 independent from other confounding factors. As we found that endothelial cells are major sources for MFAP4 in the heart, we cultured rat endothelial cells (ECs) and transfected them with a control plasmid (GFP) or a plasmid encoding for his-tagged MFAP4 (Figure 4A). The cells were allowed to grow under basal conditions (i.e. without serum addition) for 48 hours, at which point we collected the media and transferred it to neonatal rat cardiomyocytes. At the same time as media transfer, we treated the cardiomyocytes with either vehicle (PBS) or the hypertrophic stimulator phenylephrine (PE). Cardiomyocytes with no endothelial cell media, but treated with either vehicle or phenylephrine in serum-free media, were used as controls. After 48 hours, we fixed and stained the cardiomyocytes with antibodies against sarcomeric alpha-actinin to visualize cardiomyocyte cell area. As expected, addition of phenylephrine to cardiomyocytes without endothelial cell media was sufficient to significantly increase cell area (Figure 4B and C). Cardiomyocytes treated with either endothelial cell-control media or endothelial cell-MFAP4 media exhibited an increase in cell area over baseline, indicating there are certain growth factors present in endothelial cell media that stimulate cardiomyocyte hypertrophy independent of phenylephrine (Figure 4B and C). However, we observed that even under vehicle-treated (non-hypertrophic) conditions, endothelial cell media containing exogenous MFAP4 decreased cardiomyocyte hypertrophy compared to endothelial cell-control media (Figure 4B and C). In addition, endothelial cell-control media and serum-free media induced a similar degree of phenylephrine-induced hypertrophy in cardiomyocytes, while cardiomyocytes exposed to endothelial cell-MFAP4 media showed reduced cell size (Figure 4B and C). Finally, in support of the negative regulation of hypertrophy by MFAP4, myocytes treated with endothelial cell-MFAP4 media had reduced expression of the phenylephrine-induced hypertrophic markers Nppa, Nppb, and Acta1 (Figure 4D). These results, supporting our area measurements, suggest that endothelial-derived MFAP4 mitigates the hypertrophic growth of cardiomyocytes.
Figure 4. Exogenous endothelial-derived MFAP4 blunts cardiomyocyte hypertrophy.
A. Western blots from rat endothelial cell transfected with GFP control plasmid (Ctrl) or His-tagged MFAP4 (MFAP4) showing MFAP4 expression through His antibodies and GAPDH loading control. B. Representative images of neonatal rat cardiomyocytes treated with either vehicle or phenylephrine (PE), in either serum-free (SF) media, endothelial cell (EC) conditioned media control (EC ctrl media), or conditioned media from EC overexpressing MFAP4 (EC MFAP4 media). Staining shows a-actinin (green). Scale bar, 50 μm. C. Quantification of cardiomyocyte area in the indicated groups (n=100). (Two-way ANOVA results: SF vs EC-Ctrl *p=0.000000000098; SF vs EC-MFAP4 *p=0.0000053; EC-Ctrl vs EC-MFAP4 #p=0.00019; SF vs SF PE *p=0.00000000011; EC-Ctrl vs EC-Ctrl PE *p=0.0000077; EC-MFAP4 vs EC-MFAP4 PE *p=0.0000000055; SF PE vs EC-MFAP4 PE $p=0.0055; EC-Ctrl PE vs EC-MFAP4 PE #p=0.0268). D. qPCR analysis of hypertrophic gene expression relative to Rpl7 in the indicated groups (n=3). (Two-way ANOVA results for - Nppa: EC-Ctrl vs EC-Ctrl PE *p=0.00019; EC-MFAP4 vs EC-MFAP4 PE *p=0.0125; EC-Ctrl PE vs EC-MFAP4 PE #p=0.00096. - Nppb: EC-Ctrl vs EC-Ctrl PE *p=0.00058; EC-Ctrl PE vs EC-MFAP4 PE #p=0.0044. - Acta1: EC-Ctrl vs EC-Ctrl PE *p=0.0435).
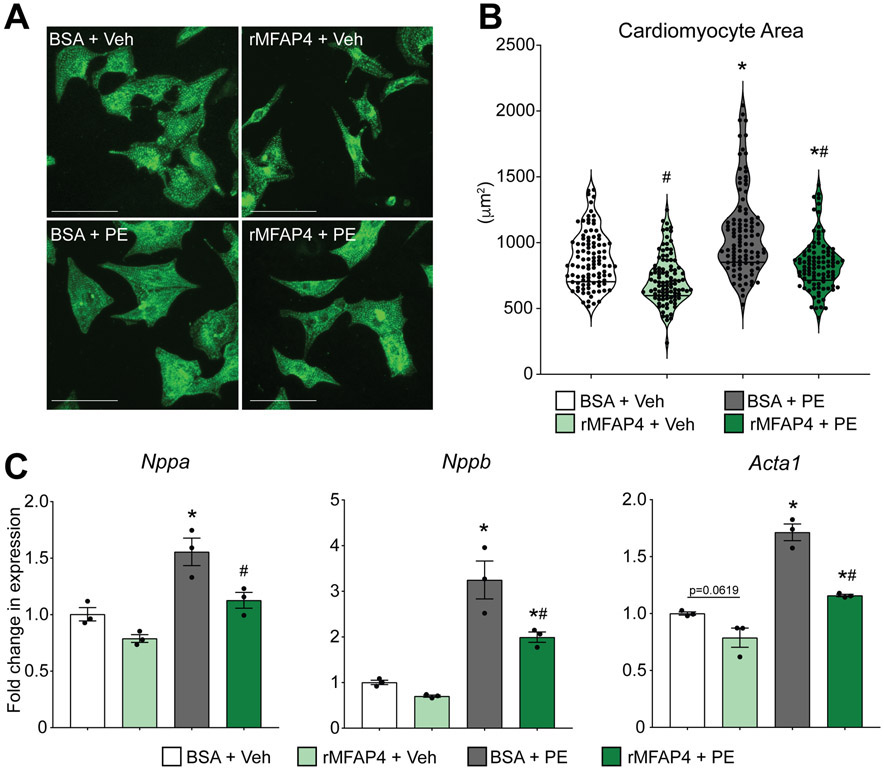
To investigate if MFAP4 alone is sufficient to recapitulate the observed effects, we then treated neonatal rat cardiomyocytes with either bovine serum albumin (BSA) control or recombinant MFAP4 (rMFAP4), along with phenylephrine, in the media for 48 hours. In support of our conditioned endothelial-cell media experiments, cardiomyocytes treated with rMFAP4 showed reduced hypertrophy following phenylephrine treatment when compared to cells treated with BSA (Figure 5A and B). Furthermore, cardiomyocytes treated with rMFAP4 also had reduced gene expression of the pro-hypertrophic gene markers Nppa, Nppb, and Acta1, reinforcing our previous results (Figure 5C). Overall, these data suggest a direct role for MFAP4 in regulating the hypertrophic growth of cardiomyocytes.
Figure 5. MFAP4 is sufficient to prevent phenylephrine-driven cardiomyocyte hypertrophy.
A. Representative images of neonatal rat cardiomyocytes treated with BSA control or recombinant MFAP4 (rMFAP4) plus vehicle (Veh) or phenylephrine (PE). Staining shows a-actinin (green). Scale bar, 50 μm. B. Quantification of cardiomyocyte area in the indicated groups (n=100). (Two-way ANOVA results: BSA vs rMFAP4 #p=0.0001; BSA vs BSA PE *p=0.000000012; rMFAP4 vs rMFAP4 PE *p=0.0058; BSA PE vs rMFAP4 PE #p=0.000000000052.) C. qPCR analysis of hypertrophic gene expression relative to Rpl7 in the indicated groups (n=3). (Two-way ANOVA results for - Nppa: BSA vs BSA PE *p=0.0150; BSA PE vs rMFAP4 PE #p=0.0454. - Nppb: BSA vs BSA PE *p=0.0014; rMFAP4 vs rMFAP4 PE *p=0.0219; BSA PE vs rMFAP4 PE #p=0.0249. - Acta1: BSA vs rMFAP4 p=0.0619; BSA vs BSA PE *p=0.00014; rMFAP4 vs rMFAP4 PE *p=0.0050; BSA PE vs rMFAP4 PE #p=0.00058).
Loss of MFAP4 impairs regulation of pro-hypertrophic signaling in the heart
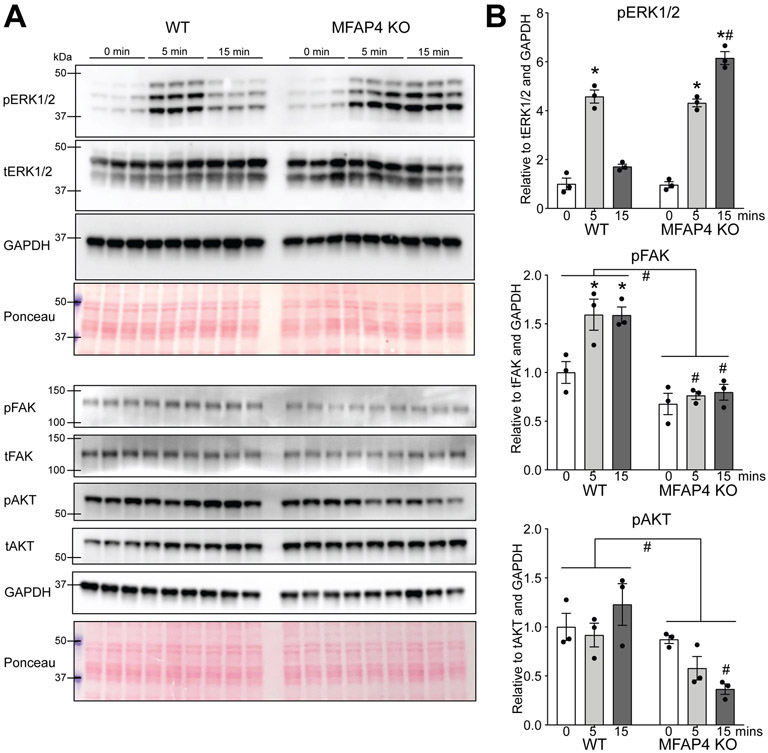
To dissect the molecular mechanisms by which expression of MFAP4 in the extracellular matrix regulates hypertrophy, we examined canonical pro-hypertrophic signaling induced downstream of cell surface receptors. To do this, we injected WT and MFAP4 KO mice with phenylephrine, a molecule known to stimulate hypertrophic signaling in cardiomyocytes through G-protein-coupled receptors. Following established protocols we analyzed the cardiac response to phenylephrine following 0, 5 and 15 minutes of stimulation 20. These time points were designed to capture the time-sensitive phosphorylation and dephosphorylation events typical of mitogen activated protein kinases such as extracellular signal-regulated kinase (ERK1/2), a critical regulator of cardiac hypertrophy. WT animals demonstrated a distinct phosphorylation of ERK1/2 at 5 minutes following phenylephrine, and this was quickly reversed such that by 15 minutes, ERK1/2 activation was not significantly different from baseline (Figure 6A and B). This tightly controlled temporal activation and deactivation of ERK1/2 is an established and essential event for adaptation to stress without maladaptive consequences 21. In MFAP4 KO animals, initial ERK1/2 phosphorylation levels were similar to WT at 5 minutes following phenylephrine; however, this phosphorylation specifically persisted in only MFAP4 KO animals at 15 minutes following stimulation (Figure 6A and B). This suggests that when MFAP4 is not present, cardiomyocytes cannot adequately regulate the temporal activation of ERK1/2, and consequently may not be able to quickly mitigate downstream hypertrophic gene expression. The MFAP4 sequence contains an Arg-Gly-Asp (RGD) attachment domain that binds integrins in vascular smooth muscle 16. To test the connection between MFAP4 and cardiac integrin signaling, we probed the same lysates for phosphorylated focal adhesion kinase (pFAK) and its downstream mediator, phosphorylated serine/threonine kinase AKT. Although phenylephrine is not a direct stimulator of this signaling cascade, our analysis revealed a mild but consistent depression of integrin signaling in MFAP4 KO hearts, demonstrated by decreased pFAK and pAKT (Figure 6A and B). This is particularly informative as integrin-mediated cardiac signaling is typically considered to be adaptive for stress responses, whereas dysregulated G-protein-coupled receptor signaling is considered to be maladaptive.
Figure 6. MFAP4 is required for regulated hypertrophic signaling.
A. Western blots for the indicated proteins from cardiac extracts from WT or MFAP4 KO mice injected with phenylephrine (PE) and sacrificed at the indicated time points. B. Quantification of expression of the indicated proteins in the indicated groups (n=3) based on the Western blots in A. p=phospho; t=total. (Two-way ANOVA results for - pERK1/2: WT baseline to 5 minutes PE *p=0.00000050; KO baseline to 5 minutes PE *p=0.0000010; KO baseline to 15 minutes PE *p=0.0000000072; WT 15 minutes PE to KO 15 minutes PE #p=0.000000043. - pFAK: WT baseline to 5 minutes PE *p=0.0252; WT baseline to 15 minutes PE *p=0.0270; WT 5 minutes PE to KO 5 minutes PE #p=0.0017; WT 15 minutes PE to KO 15 minutes PE #p=0.0026; Mann-Whitney test results for total WT to total KO #p=0.00049. - pAKT: WT 15 minutes PE to KO 15 minutes PE #p=0.0066; Mann-Whitney test results for total WT to total KO #p=0.0078).
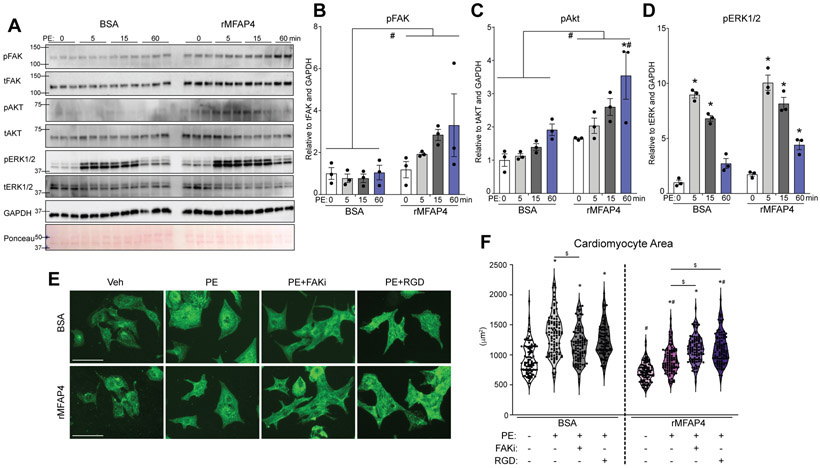
To further confirm the direct role of MFAP4 upstream of cardiac integrins, we then studied its ability to modulate this intracellular signaling in isolated neonatal primary cardiomyocytes treated with BSA control or rMFAP4 as well as phenylephrine for a short time course of 0, 5, 15, and 60 minutes. In agreement with a role for MFAP4 upstream of integrins, cardiomyocytes treated with rMFAP4 showed increased pFAK expression already at 5 and 15 minutes following phenylephrine, as well as globally increased pFAK when compared to total FAK and GAPDH loading control (Figure 7A and B). Similarly, pAKT was globally increased in rMFAP4-treated cardiomyocytes (Figure 7A and C). In contrast, pERK1/2 did not show significant differences in expression except at the terminal time point (Figure 7A and D), likely a result derived by integrin converging onto this pathway. Furthermore, we have inhibited MFAP4 binding to integrin (using competitive RGD peptides) and downstream FAK effector in cultured cardiomyocytes treated with phenylephrine and either BSA control or rMFAP4. Strikingly, although rMFAP4 was able (as previously seen) to ameliorate phenylephrine-induced hypertrophy in isolated cardiomyocytes, this effect is lost upon either FAK inhibition or RGD-peptide competition, indicating that MFAP4’s beneficial effect on cardiomyocyte hypertrophy is a direct result of integrin binding and downstream FAK signaling (Figure 7E and F).
Figure 7. MFAP4 directly regulates integrin signaling in cardiomyocytes.
A. Western blots for the indicated proteins from neonatal rat cardiomyocyte extracts treated with BSA control or recombinant MFAP4 (rMFAP4) plus PE for the indicated times (min=minutes). B-D. Quantification of expression of the indicated proteins in the indicated groups (n=3) based on western blots in A. p=phospho. t=total. (Two-way ANOVA results for - pFAK: total BSA vs total rMFAP4 #p=0.0004. - pAKT: rMFAP4 0 vs 60 minutes PE *p=0.0065; BSA vs rMFAP4 at 60 minutes PE #p=0.0223; total BSA vs total rMFAP4 #p=0.0002. - pERK1/2: BSA 0 vs 5 minutes PE *p=0.000000010; BSA 0 vs 15 minutes PE *p=0.00000091; rMFAP4 0 vs 5 minutes PE *p=0.0000000055; rMFAP4 0 vs 15 minutes PE *p=0.00000023; rMFAP4 0 vs 60 minutes PE *p=0.0070). E. Representative images for neonatal rat cardiomyocytes treated with BSA control or recombinant MFAP4 (rMFAP4) plus vehicle (Veh), phenylephrine (PE), FAK inhibitor (FAKi), or cyclic RGD peptide (RGD). Staining shows a-actinin (green). Scale bar, 50 μm. F. Quantification of cardiomyocyte area in the indicated groups (n=100). (Two-way ANOVA results: BSA vs BSA PE *p=9.91x10−18; BSA vs BSA PE FAKi *p=0.0000000072; BSA vs BSA PE RGD *p=6.01x10−16; BSA PE vs BSA PE FAKi $p=0.000080; rMFAP4 vs rMFAP4 PE *p=0.00000089; rMFAP4 vs rMFAP4 PE FAKi *p=1.92x10−30; rMFAP4 vs rMFAP4 PE RGD *p=1.75x10−27; rMFAP4 PE vs rMFAP4 PE FAKi $p=0.00012; rMFAP4 PE vs rMFAP4 PE RGD $p=0.0000055; BSA vs rMFAP4 #p=0.00000024; BSA PE vs rMFAP4 PE #p=7.35x10−20; BSA PE RGD vs rMFAP4 PE RGD #p=0.0099).
In summary, our data demonstrate a crucial role for non-myocyte-derived TGFβ1-induced MFAP4 as a regulator of cardiac remodeling and controlled hypertrophic signaling following acute and chronic stress.
Methods
All data, material and methods are available on request. Detailed methods can be found as online supplementary material.
Discussion
The cardiovascular extracellular matrix, comprised of hundreds of proteins that support the myocardium and vasculature of the heart, also serves a crucial purpose in transducing molecular signals during homeostatic and injury-response states. Nearly all pathological conditions in the heart are associated with an expansion and change in the composition of the cells within the cardiac interstitial matrix. This composition change leads to altered signaling between cells and complex communication networks between myocytes and non-myocytes, ultimately modifying cardiac systolic and diastolic function 22, 23. Whereas the major structural component of the cardiac extracellular matrix is type 1 collagen, other proteins such as fibronectin, glycosaminoglycans, and proteoglycans contribute to matrix structure and act as storage sites for growth factors and proteases that may be activated and released following injury 24. Microfibrillar associated protein 4 (MFAP4) is one such extracellular matrix protein belonging to the fibrinogen-related domain family, which is intimately involved in maintaining tissue homeostasis 14 MFAP4 can bind elastin, fibrillin, and collagen within the extracellular matrix, particularly within large vessel walls, and thereby regulate the proliferation and migration of smooth muscle cells following injury 16. Its expression from vascular smooth muscle cells within large arteries and arterioles and importance in vascular remodeling therein has been extensively studied previously, but the role of MFAP4 in cardiomyocyte remodeling is not known 16, 25. Our work has revealed MFAP4 as a novel factor implicated in TGFβ1 downstream signaling and tissue remodeling during stress responses in the heart.
TGFβ1 is activated in the stressed myocardium and regulates the function of all cardiac cell populations involved in remodeling through mechanisms that can be dependent upon the targeted cell type. This study defined MFAP4 as a central factor for cell-specific TGFβ1 signaling in the heart. We showed that TGFβ1 induced MFAP4 expression in non-myocyte cell populations (predominantly in endothelial cells, only mildly in cardiac fibroblasts or smooth muscle cells), and not at all in cardiomyocytes. Although prior studies have demonstrated a correlation between MFAP4 expression and hepatic and pulmonary fibrotic disease 26-28, our models of cardiac injury did not highlight a strong contribution of MFAP4 to fibrosis. However, we observed greater remodeling of the myocardial interstitium in injured MFAP4 knockout mice, which was characterized by increased infiltrating inflammatory cells and evidence of cell death.
Using chronic pressure overload and neurohumoral stimulation, as well as ex vivo isolated cardiomyocyte systems, we elucidated a protective role for non-myocyte-derived MFAP4 on maladaptive hypertrophy. Importantly, two recent reports have also identified MFAP4 as consistently upregulated with pressure overload in mice through multiple gene expression datasets 15, 29. Also, MFAP4 was recently identified as a factor uniquely induced by non-myocytes following cardiac stress using single cell sequencing, and was also bioinformatically-identified as a critical non-myocyte-derived secreted factor potentially affecting cardiomyocyte behaviors 30.
Multiple signaling pathways have been shown to regulate cardiomyocyte hypertrophy, the primary two being stretch-activated and hormone-activated. Integrins are crucial to stretch-activated physiological hypertrophic signaling in the heart 31-35. Importantly, MFAP4 contains an integrin binding domain that could regulate integrin function in cardiomyocytes 16. Indeed, previous studies have shown that MFAP4 interacts with vascular smooth muscle integrins ανβ3 and ανβ5 through an RGD (Arg-Gly-Asp) motif, and is therefore critical for appropriate neointimal remodeling and hyperplasia following vascular injury 16. Integrins participate in ‘outside-in signaling’, as they relay signals from the extracellular matrix through several proteins such as focal adhesion kinase (FAK), vinculin, talin, and via the downstream mediators AKT and extracellular signal-regulated kinase (ERK), among others. Integrins do not act in this way in isolation; extensive studies have shown that integrins can collaborate with growth factors, receptor tyrosine kinases, G-protein-coupled receptors, and cytokines 36, 37 In fact, the pro-hypertrophic molecule phenylephrine signals through pathways that may act in parallel, and perhaps synergistically, with integrins and extracellularly regulated kinases in particular 37 With this in mind, we observed that MFAP4 knockout animals have markedly dysregulated ERK signaling, due to prolonged temporal phosphorylation of pro-hypertrophic ERK1/2 following neurohormonal phenylephrine-dependent stimulation. In wild type conditions ERK1/2 phosphorylation is kept in check by dual specificity protein phosphatases (DUSP) 38, 39. However, this mechanism is likely insufficient to mitigate the aberrant ERK1/2 phosphorylation that occurs when cardiomyocytes are experiencing dysregulated extracellular stimuli as observed in the absence of MFAP4. Notably, since ERK1/2 is a shared signaling effector between integrins and G-protein-coupled receptors, it is experimentally challenging to distinguish which receptor is responsible for the observed phosphorylated pool, complicating our ability to differentiate downstream protective versus maladaptive consequences. However, in actuality it is plausible that signaling subdomains operate independently inside cells to differentially transduce signals from diverse extracellular messengers. Indeed, the mild increase in ERK1/2 phosphorylation that was observed with MFAP4 stimulation on isolated cardiomyocytes is likely integrin-dependent and not contributing to pathological hypertrophy. Confirming this is the fact that MFAP4 treatment protected cardiomyocytes from phenylephrine-driven hypertrophic growth, except under circumstances in which integrin binding was competitively disrupted by an RGD peptide mimic.
We also found that, while isolated cardiomyocytes supplemented with recombinant MFAP4 show globally increased activation of FAK and AKT, MFAP4 deficient mice exhibit the opposite phenomenon, namely, a globally reduced activation of focal adhesion kinase (FAK) and its downstream target AKT; this is in agreement with the proposed protective and adaptive role of integrin signaling during cardiac injury. Furthermore, inhibiting FAK signaling nullified the beneficial effect MFAP4 has in preventing hypertrophy. On the other hand, dysregulated over-activation of ERK1/2 in MFAP4 null animals is likely mediated by G-protein-coupled receptor signaling. In this way, loss of protective and adaptive integrin-mediated signaling may synergize with maladaptive neurohormonal-dependent ERK signaling to perpetuate the hypertrophic response in animals lacking MFAP4. Although not yet available for in vivo testing, development of small molecules that specifically activate RGD-binding integrins could in the future be adopted to rescue not only the MFAP4 knockout heart phenotype but general conditions where MFAP4 expression is not sufficient to provide adaptation to stress 40. Similarly, intracellular potentiation of integrin pathways in cardiomyocytes through molecules such as the cardioprotective integrin-binding protein Melusin could provide a therapeutic advantage when the levels of extracellular activators of integrins such as MFAP4 are insufficient to prevent stress-induced dysfunction 41, 42.
Our study is limited by the lack of availability of MFAP4 conditional knockout animals, for specifically investigating the importance of specific non-myocyte cell types as cardiac producers of MFAP4 in vivo. Similarly, future generation of a MFAP mouse model that would allow for temporal regulation of MFAP4 genetic inhibition will be important. Despite our current inability to temporally control MFAP4 deletion with a genetic approach, this limitation was mitigated by recapitulation of key MFAP loss-of-function phenotypes using MFAP4 neutralizing antibody treatments that started following stress-induction. Another consideration that should be highlighted is that more work will be necessary to define therapeutic dosing for in vivo modulation of cardiac hypertrophy by exogenous MFAP4 treatment.
In summary, our results support an emerging role for non-myocyte-derived, TGFβ1-dependent extracellular matrix protein MFAP4 in the regulation of cardiac remodeling following either pressure overload or neurohumoral stimulation. The loss of MFAP4 promoted accelerated hypertrophy and cardiac dysfunction in vivo, whereas exogenous administration of MFAP4 ex vivo prevented hypertrophic cell growth in isolated cardiomyocytes. Finally, the loss of MFAP4 leads to dysregulated hypertrophic signaling in the heart. Further understanding of how to modulate MFAP4 release from non-myocytes during cardiac injury may provide new insights on ameliorating hypertrophy in patients with both acute and chronic cardiac disease.
Supplementary Material
Novelty and Significance.
What is known?
Transforming growth factor-beta1 (TGFβ1) is a master regulator of cardiac stress responses
Cardiomyocyte hypertrophy is regulated by extracellular matrix components
What new information does this article contribute?
Microfibrillar-associated protein 4 (MFAP4) is secreted by non-myocyte cardiac cells following TGFβ1 activation and pro-hypertrophic stimuli
MFAP4 exerts anti-hypertrophic paracrine activities on cardiomyocytes
MFAP4 operates by modulating cardiomyocyte integrin signaling
In summary, our study identifies a pivotal role for MFAP4 in coordinating cardiac stress responses and mitigating pathologic hypertrophic remodeling of cardiomyocytes post-injuries. MFAP4 is produced by non-myocyte cells in response to TGFβ1 activation and represents a critical paracrine agent for regulation of cardiomyocyte biology by engaging physiological integrin signaling. Our work suggests MFAP4 could be exploited as a protective molecule for adaptation to stress in the heart.
Acknowledgements and Funding:
We wish to thank the Lifeline of Ohio Organ Procurement service for their collaboration in obtaining human cardiac tissue. This work was supported by NIH grants HL134616, 1F30HL145955-01A1 to L.E.D., R01-HL114893, R01-HL135096 to T.J.H., HL121284, HL136951 to F.A.
Non-standard Abbreviations and Acronyms:
- TGFβ
transforming growth factor beta
- MFAP4
microfibrillar-associated protein 4
- TAC
transverse aortic constriction
- TG
transgenic
- PE
phenylephrine
- FAK
focal adhesion kinase
- ERK
extracellular regulated kinase
- GPCR
G-protein-coupled receptor
- TF
transcription factor
- BSA
bovine serum albumin
Footnotes
Conflicts of Interest: None
References
- 1.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: A new therapeutic target? Circulation. 2004;109:1580–1589 [DOI] [PubMed] [Google Scholar]
- 3.Rosenkranz S Tgf-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432 [DOI] [PubMed] [Google Scholar]
- 4.Hanna A, Frangogiannis NG. The role of the tgf-beta superfamily in myocardial infarction. Front Cardiovasc Med. 2019;6:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Accornero F, van Berlo JH, Correll RN, Elrod JW, Sargent MA, York A, Rabinowitz JE, Leask A, Molkentin JD. Genetic analysis of connective tissue growth factor as an effector of transforming growth factor beta signaling and cardiac remodeling. Mol Cell Biol. 2015;35:2154–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann MA, Petrosino JM, Manring HR, Wright P, Shettigar V, Kilic A, Janssen PML, Ziolo MT, Accornero F. Tgf-beta1 affects cell-cell adhesion in the heart in an ncam1-dependent mechanism. J Mol Cell Cardiol. 2017;112:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, Karch J, Molkentin JD. Fibroblast-specific tgf-beta-smad2/3 signaling underlies cardiac fibrosis. J Clin Invest. 2017;127:3770–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA. Pivotal role of cardiomyocyte tgf-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brand T, Schneider MD. The tgf beta superfamily in myocardium: Ligands, receptors, transduction, and function. J Mol Cell Cardiol. 1995;27:5–18 [DOI] [PubMed] [Google Scholar]
- 10.Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, Lonning S, Ling H, Ertl G, Bauersachs J. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol. 2008;103:485–492 [DOI] [PubMed] [Google Scholar]
- 11.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D'Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122:928–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilecki B, Schlosser A, Wulf-Johansson H, Trian T, Moeller JB, Marcussen N, Aguilar-Pimentel JA, de Angelis MH, Vestbo J, Berger P, Holmskov U, Sorensen GL. Microfibrillar-associated protein 4 modulates airway smooth muscle cell phenotype in experimental asthma. Thorax. 2015;70:862–872 [DOI] [PubMed] [Google Scholar]
- 14.Pilecki B, Holm AT, Schlosser A, Moeller JB, Wohl AP, Zuk AV, Heumuller SE, Wallis R, Moestrup SK, Sengle G, Holmskov U, Sorensen GL. Characterization of microfibrillar-associated protein 4 (mfap4) as a tropoelastin- and fibrillin-binding protein involved in elastic fiber formation. JBiol Chem. 2016;291:1103–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HB, Huang R, Yang K, Xu M, Fan D, Liu MX, Huang SH, Liu LB, Wu HM, Tang QZ. Identification of differentially expressed genes and preliminary validations in cardiac pathological remodeling induced by transverse aortic constriction. Int J Mol Med. 2019;44:1447–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlosser A, Pilecki B, Hemstra LE, Kejling K, Kristmannsdottir GB, Wulf-Johansson H, Moeller JB, Fuchtbauer EM, Nielsen O, Kirketerp-Moller K, Dubey LK, Hansen PB, Stubbe J, Wrede C, Hegermann J, Ochs M, Rathkolb B, Schrewe A, Bekeredjian R, Wolf E, Gailus-Durner V, Fuchs H, Hrabe de Angelis M, Lindholt JS, Holmskov U, Sorensen GL. Mfap4 promotes vascular smooth muscle migration, proliferation and accelerates neointima formation. Arterioscler Thromb Vasc Biol. 2016;36:122–133 [DOI] [PubMed] [Google Scholar]
- 17.Holm AT, Wulf-Johansson H, Hvidsten S, Jorgensen PT, Schlosser A, Pilecki B, Ormhoj M, Moeller JB, Johannsen C, Baun C, Andersen T, Schneider JP, Hegermann J, Ochs M, Gotz AA, Schulz H, de Angelis MH, Vestbo J, Holmskov U, Sorensen GL. Characterization of spontaneous air space enlargement in mice lacking microfibrillar-associated protein 4. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1114–1124 [DOI] [PubMed] [Google Scholar]
- 18.Mecham RP, Gibson MA. The microfibril-associated glycoproteins (magps) and the microfibrillar niche. Matrix Biol. 2015;47:13–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorn LE, Petrosino JM, Wright P, Accornero F. Ctgf/ccn2 is an autocrine regulator of cardiac fibrosis. J Mol Cell Cardiol. 2018;121:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Berlo JH, Elrod JW, Aronow BJ, Pu WT, Molkentin JD. Serine 105 phosphorylation of transcription factor gata4 is necessary for stress-induced cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2011;108:12331–12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, York AJ, Lorenz JN, Zimmermann WH, Meloche S, Molkentin JD. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berk BC, Fujiwara K, Lehoux S. Ecm remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest. 2017;127:1600–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wulf-Johansson H, Lock Johansson S, Schlosser A, Trommelholt Holm A, Rasmussen LM, Mickley H, Diederichsen AC, Munkholm H, Poulsen TS, Tornøe I, Nielsen V, Marcussen N, Vestbo J, Sækmose SG, Holmskov U, Sorensen GL. Localization of microfibrillar-associated protein 4 (mfap4) in human tissues: Clinical evaluation of serum mfap4 and its association with various cardiovascular conditions. PLoS One. 2013;8:e82243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasamatsu S, Hachiya A, Fujimura T, Sriwiriyanont P, Haketa K, Visscher MO, Kitzmiller WJ, Bello A, Kitahara T, Kobinger GP, Takema Y. Essential role of microfibrillar-associated protein 4 in human cutaneous homeostasis and in its photoprotection. Sci Rep. 2011;1:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sækmose SG, Mössner B, Christensen PB, Lindvig K, Schlosser A, Holst R, Barington T, Holmskov U, Sorensen GL. Microfibrillar-associated protein 4: A potential biomarker for screening for liver fibrosis in a mixed patient cohort. PLoS One. 2015;10:e0140418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson SL, Roberts NB, Schlosser A, Andersen CB, Carlsen J, Wulf-Johansson H, Sækmose SG, Titlestad IL, Tornoe I, Miller B, Tal-Singer R, Holmskov U, Vestbo J, Sorensen GL. Microfibrillar-associated protein 4: A potential biomarker of chronic obstructive pulmonary disease. Respir Med. 2014;108:1336–1344 [DOI] [PubMed] [Google Scholar]
- 29.Gao J, Li Y, Wang T, Shi Z, Zhang Y, Liu S, Wen P, Ma C. Analyzing gene expression profiles with preliminary validations in cardiac hypertrophy induced by pressure overload. Can J Physiol Pharmacol. 2018;96:701–709 [DOI] [PubMed] [Google Scholar]
- 30.Ren Z, Yu P, Li D, Li Z, Liao Y, Wang Y, Zhou B, Wang L. Single-cell reconstruction of progression trajectory reveals intervention principles in pathological cardiac hypertrophy. Circulation. 2020; 141:1704–1719 [DOI] [PubMed] [Google Scholar]
- 31.Babbitt CJ, Shai SY, Harpf AE, Pham CG, Ross RS. Modulation of integrins and integrin signaling molecules in the pressure-loaded murine ventricle. Histochem Cell Biol. 2002;118:431–439 [DOI] [PubMed] [Google Scholar]
- 32.Sun M, Opavsky MA, Stewart DJ, Rabinovitch M, Dawood F, Wen WH, Liu PP. Temporal response and localization of integrins beta1 and beta3 in the heart after myocardial infarction: Regulation by cytokines. Circulation. 2003;107:1046–1052 [DOI] [PubMed] [Google Scholar]
- 33.Brokat S, Thomas J, Herda LR, Knosalla C, Pregla R, Brancaccio M, Accornero F, Tarone G, Hetzer R, Regitz-Zagrosek V. Altered melusin expression in the hearts of aortic stenosis patients. Eur J Heart Fail. 2007;9:568–573 [DOI] [PubMed] [Google Scholar]
- 34.Sbroggio M, Ferretti R, Percivalle E, Gutkowska M, Zylicz A, Michowski W, Kuznicki J, Accornero F, Pacchioni B, Lanfranchi G, Hamm J, Turco E, Silengo L, Tarone G, Brancaccio M. The mammalian chord-containing protein melusin is a stress response protein interacting with hsp90 and sgt1. FEBS Lett. 2008;582:1788–1794 [DOI] [PubMed] [Google Scholar]
- 35.De Acetis M, Notte A, Accornero F, Selvetella G, Brancaccio M, Vecchione C, Sbroggio M, Collino F, Pacchioni B, Lanfranchi G, Aretini A, Ferretti R, Maffei A, Altruda F, Silengo L, Tarone G, Lembo G. Cardiac overexpression of melusin protects from dilated cardiomyopathy due to long-standing pressure overload. Circ Res. 2005;96:1087–1094 [DOI] [PubMed] [Google Scholar]
- 36.Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88:1112–1119 [DOI] [PubMed] [Google Scholar]
- 37.Ross RS. Molecular and mechanical synergy: Cross-talk between integrins and growth factor receptors. Cardiovasc Res. 2004;63:381–390 [DOI] [PubMed] [Google Scholar]
- 38.Liu R, van Berlo JH, York AJ, Vagnozzi RJ, Maillet M, Molkentin JD. Dusp8 regulates cardiac ventricular remodeling by altering erk1/2 signaling. Circ Res. 2016;119:249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu R, Molkentin JD. Regulation of cardiac hypertrophy and remodeling through the dual-specificity mapk phosphatases (dusps). J Mol Cell Cardiol. 2016;101:44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baiula M, Galletti P, Martelli G, Soldati R, Belvisi L, Civera M, Dattoli SD, Spampinato SM, Giacomini D. New β-lactam derivatives modulate cell adhesion and signaling mediated by rgd-binding and leukocyte integrins. J Med Chem. 2016;59:9721–9742 [DOI] [PubMed] [Google Scholar]
- 41.Brancaccio M, Fratta L, Notte A, Hirsch E, Poulet R, Guazzone S, De Acetis M, Vecchione C, Marino G, Altruda F, Silengo L, Tarone G, Lembo G. Melusin, a muscle-specific integrin beta1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat Med. 2003;9:68–75 [DOI] [PubMed] [Google Scholar]
- 42.Sorge M, Brancaccio M. Melusin promotes a protective signal transduction cascade in stressed hearts. Front Mol Biosci. 2016;3:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorn LE, Lasman L, Chen J, Xu X, Hund TJ, Medvedovic M, Hanna JH, van Berlo JH, Accornero F. The n(6)-methyladenosine mrna methylase mettl3 controls cardiac homeostasis and hypertrophy. Circulation. 2019;139:533–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.