Abstract
Recent studies have reported that methylmercury (MeHg) induces neuronal apoptosis, which is accompanied by abnormal neurological development. Despite the important role of docosahexaenoic acid (DHA) in maintaining the structure and function of the brain, as well as improving neuronal apoptosis induced by MeHg, the exact mechanism remains unknown. The present study hypothesized that the reactive oxygen species (ROS)-mediated JNK signaling pathway may be associated with the protective effect of DHA against MeHg-induced PC12 cell apoptosis. Cell Counting Kit-8, TUNEL staining, flow cytometry, ROS detection, PCR and western blot analysis were performed. The results demonstrated that MeHg inhibited the activity of PC12 cells, causing oxidative damage and promoting apoptosis; however, DHA significantly attenuated this effect. Mechanistic studies revealed that MeHg increased intracellular ROS levels and JNK protein phosphorylation, and decreased the expression levels of the anti-apoptotic protein Bcl-2, whereas DHA reduced ROS levels and JNK phosphorylation, and increased Bcl-2 expression. In addition, the ROS inhibitor N-acetyl-l-cysteine (NAC) was used to verify the experimental results. After pretreatment with NAC, expression levels of Bcl-2, Bax, phosphorylated-JNK and JNK were assessed. Bcl-2 protein expression was increased and the Bcl-2/Bax ratio was increased. Moreover, the high expression levels of phosphorylated-JNK induced by MeHg were significantly decreased. Based on the aforementioned results, the present study indicated that the effects of DHA against MeHg-induced PC12 cell apoptosis may be mediated via the ROS/JNK signaling pathway.
Keywords: docosahexaenoic acid, methylmercury, apoptosis, reactive oxygen species, JNK pathway
Introduction
Epidemiological studies have reported that global methylmercury (MeHg) pollution has become increasingly serious in recent years and humans are suffering from the effects of MeHg, which has become a concern for several countries (1). The development of industry and agriculture; the discharge of wastewater, residue and exhaust gas in the process of smelting mercury ores; the combustion of fossil fuel; and the irresponsible use of medical devices, such as use of amalgam as filling material have led to increasingly serious environmental mercury pollution (2,3). Moreover, mercury released into the environment can be converted into MeHg under certain conditions, such as transformation by aquatic microorganisms, thereby becoming enriched in the aquatic food chain. Once food containing MeHg is consumed, MeHg derivatives are formed and accumulate in the body, particularly in the brain, severely affecting human health (4).
The toxic effects of MeHg have been characterized by a long incubation period before symptoms appear in humans. The main symptoms include blurred vision, weight loss, ataxia and neurodevelopmental abnormalities (5). In addition, MeHg has been reported to serve an important role in early fetal neurodevelopment. It has been reported that the brain MeHg content of exposed individuals may be 3–6 times higher compared with that in the blood (6). Furthermore, maternal exposure to MeHg from the consumption of fish and seafood may have irreversible effects on the neurobehavioral development of children, including cognitive impairment, memory impairment and motor developmental abnormalities (7,8).
It was recently demonstrated that the neurotoxicity produced by MeHg is closely associated with cell apoptosis, and oxidative stress induced by reactive oxygen species (ROS) is the most likely predisposing factor (9). As a secondary messenger, ROS serve a dual role in the body. Stable physiological ROS levels can suppress harmful cellular processes; however, high concentrations of ROS can cause cell apoptosis and death (10). A previous study reported that excessive ROS may directly damage DNA and activate the MAPK signaling pathway, initiate mitochondria-related apoptosis and depolarize the mitochondrial membrane, thus damaging the integrity of the mitochondrial membrane (11). Eventually, cytochrome c may be released into the cytoplasm from the mitochondrial intermembrane space, thereby activating caspases to induce cell apoptosis (12).
JNK, also known as stress-activated protein kinase, is a member of the MAPK superfamily, and is involved in various stress responses, particularly oxidative damage (13,14). It has previously been reported that MeHg can increase intracellular ROS levels, causing changes in glutathione content and lipid peroxidation, resulting in oxidative damage (9). Therefore, MeHg-induced apoptosis may be associated with activation of the ROS-regulated JNK signaling pathway.
Docosahexaenoic acid (DHA) is an essential n-3 long-chain polyunsaturated fatty acid that is mainly present in the brain, and is involved in neurotransmitter pathways, synaptic transmission and signal transduction (15). DHA is essential for the development of the nervous system in children. Epidemiological studies have identified that the presence of more n-3 unsaturated fatty acids may promote in utero fetal neurodevelopment in women who are exposed to low levels of MeHg (16,17). In addition, consumption of certain fish, such as salmon, with a higher DHA content may effectively decrease the neurotoxicity of MeHg in children (18).
Previous studies have revealed that DHA can effectively reduce MeHg-induced oxidative damage and inhibit apoptosis in different types of cells (19–21). Kaur et al (20) reported that DHA pretreatment effectively reduced cell-associated MeHg and the prooxidant response from MeHg in cerebellar astrocytes and neurons, thus supporting the hypothesis that fish-derived nutrients offer possible neuroprotection from MeHg. However, there is limited research on the mechanism underlying the effects of DHA against MeHg-induced apoptosis. Therefore, as PC12 cells are widely used in neurological research, a MeHg-induced PC12 cell apoptosis model was established in the present study to further examine the anti-apoptotic mechanism of DHA against MeHg based on regulation of the JNK signaling pathway.
Materials and methods
Materials
MeHg chloride and cis-4,7,10,13,16,19-DHA were obtained from Sigma-Aldrich (Merck KGaA). Cell Counting Kit-8 (CCK-8), TUNEL Apoptosis Assay kit, Annexin V-FITC Apoptosis Assay kit, Reactive Oxygen Assay kit, cell lysis buffer for western blotting and N-acetyl-l-cysteine (NAC) were purchased from Beyotime Institute of Biotechnology. Antibodies against Bax (cat. no. 2772S), JNK (cat. no. 9252S) and phosphorylated (p)JNK (cat. no. 4668S) were purchased from Cell Signaling Technology, Inc. Anti-Bcl-2 (cat. no. AB112) was purchased from Beyotime Institute of Biotechnology and anti-GAPDH (cat. no. 30202ES40) was purchased from Shanghai Yeasen Biotechnology Co., Ltd. The gene-specific primers were designed by Sangon Biotech Co., Ltd.
Cell culture
The PC12 rat pheochromocytoma cell line (serial no. TCR8) was purchased from The Cell Bank of Type Culture Collection of The Chinese Academy of Sciences. The purchased cells were commercialized poorly differentiated PC12 cells. The cells were incubated in a 60-mm culture dish in DMEM (HyClone; Cytiva) supplemented with 5% horse serum (Sigma-Aldrich; Merck KGaA), 105% FBS (Sigma-Aldrich; Merck KGaA) and 100 U/ml penicillin-streptomycin in a humidified atmosphere containing 55% CO2/955% air at 37°C.
Cell viability assay
The CCK-8 reagent was applied to evaluate the viability ability of cells. The cells were treated with MeHg (1.25, 2.5, 5, 10 and 20 µmol/l) for 24, 48 and 72 h in a 55% CO2 incubator at 37°C, and with different concentrations of DHA (5, 10, 20, 40 and 80 µmol/l) for 24 h in a 55% CO2 incubator at 37°C to screen for the suitable drug treatment concentration. In addition, to evaluate the protective effect of DHA against MeHg-induced damage in PC12 cells, the cells pretreated with DHA (5, 10, 20, 40 and 80 µmol/l) for 24 h were treated with MeHg (2.5 µmol/l) for 48 h. After treatment, PC12 cells were cultured in a 55% CO2 incubator at 37°C, then incubated with 105% CCK-8 reagent for 1 h at 37°C. The optical density of PC12 cells was measured at a wavelength of 450 nm using a microplate reader. A cell growth curve was drawn with GraphPad Prism 5 (GraphPad Software, Inc.).
TUNEL staining assay
A TUNEL apoptosis detection kit was used to observe cell apoptosis under a fluorescence microscope. Firstly, cells were grown on culture plates until they reached 805% confluence and were then treated with DHA followed by MeHg. Cells were washed with PBS and incubated with the 10% TUNEL solution at 37°C in the dark for 60 min. The samples were immediately observed under a fluorescence microscope, where the excitation wavelength was 450–500 nm and the emission wavelength range was 515–565 nm. Green fluorescence represented apoptotic cells. In addition, nuclei were stained by 100 ng/ml DAPI (Beyotime Institute of Biotechnology) for 5 min at 25°C.
Flow cytometric analysis of apoptosis
A total of 1×105 PC12 cells were added to 100 µl binding buffer for 20 min at 25°C following centrifugation at 1,000 × g for 5 min at 25°C and were then added to the Annexin V-FITC and PI mixture, with separate Annexin V-FITC and PI control tubes and an unstained tube. Finally, each sample tube was placed on ice in the dark for 20 min, and 500 µl 1X binding buffer was added. The samples were analyzed on a LSR Fortessa flow cytometer (BD Biosciences) to detect the apoptotic rate within 30 min, and the upper- and lower-right quadrants show apoptotic cells with FlowJo V10.0.7 (BD Biosciences). The position of the gates of other groups was relative to the control plot in the flow cytometric analysis.
Assessment of ROS
Accumulation of intracellular ROS was detected using the peroxide-sensitive fluorescent probe DCFH-DA. PC12 cells (1×105) were cultured for 48 h, after which MeHg and DHA were added to the culture media. At the end of the treatment, the cells were incubated with 10 µmol/l DCFH-DA for 40 min at 37°C. Fluorescence detection was performed using a fluorescence microplate reader at an excitation wavelength of 488 nm and an emission wavelength of 525 nm, in order to determine the generation of ROS.
Western blot analysis
After treatment for the indicated duration, the cells were washed three times with cold PBS and lysed. Total cytoplasmic and nuclear protein was collected using cell lysis buffer for western blotting, according to the manufacturer's instructions. The protein concentration was measured using a BCA assay. Equal amounts of protein (25 µg) were separated by 10% SDS-PAGE, followed by transfer to PVDF membranes. The membranes were blocked in TBS containing 55% skimmed milk and 0.15% Tween-20 at 25°C for 1 h, and then incubated with primary antibodies against Bcl-2 (1:1,000), Bax (1:1,000), JNK (1:1,000), pJNK (1:1,000) and GAPDH (1:10,000) at 4°C overnight. Anti-GAPDH antibody was selected as an internal reference. Subsequently, the membranes were washed with TBS and incubated with HRP-labeled secondary antibodies (1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology) at room temperature for 1 h. Finally, the blots were visualized using ECL (BeyoECL Moon; cat. no. P0018FS; Beyotime Institute of Biotechnology) and the results were analyzed using Image Lab (5.2.1; Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative (RT-q)PCR
Total RNA was isolated from PC12 cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The extracted RNA was then reverse transcribed to obtain stable cDNA as follows: 37°C for 15 min, 85°C for 5 sec, 4°C. RT primers, including Oligo dT primer and random hexamer primers, were used to generate cDNA with the PrimeScript™ RT reagent kit with gDNA Eraser (Takara Bio, Inc.). The ABI 7500 real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to amplify the specific PCR products and analyze the threshold cycle number (Cq value) with TB Green Premix Ex Taq kit from Takara Bio, Inc. The thermocycling conditions as follows: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 20 sec. The primers for the amplification of Bax, Bcl-2 and GAPDH mRNA were designed and synthesized by Sangon Biotech Co., Ltd., as follows: Bax forward, 5′-AGGACGCATCCACCAAGAAG-3′ and reverse, 5′-CAGTTGAAGTTGCCGTCTGC-3′; Bcl-2 forward, 5′-TGGCCTTCTTTGAGTTCGGT-3′ and reverse, 5′-GATGCCGGTTCAGGTACTCA-3′; and GAPDH forward, 5′-GGGTCCCAGCTTAGGTTCATC-3′ and reverse, 5′-TGAGGTCAATGAAGGGGTCG-3′. GAPDH was used as an internal standard. The qPCR results were normalized to the Cq value of GAPDH from the same sample, and the 2−∆∆Cq (22) method was used to calculate the fold change of each gene expression. The amplification reaction for each sample was repeated three times (23). A non-template control was included in each experiment.
Statistical analysis
SPSS 20.0 (version 20.0; IBM Corp.) was used for data analysis. Measurement data are presented as the mean ± SD (n=3), and one-way ANOVA was performed among multiple groups. When meeting the homogeneity of variance, the data were subsequently analyzed with a Dunnett's T3 test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of DHA on MeHg-induced PC12 cell viability
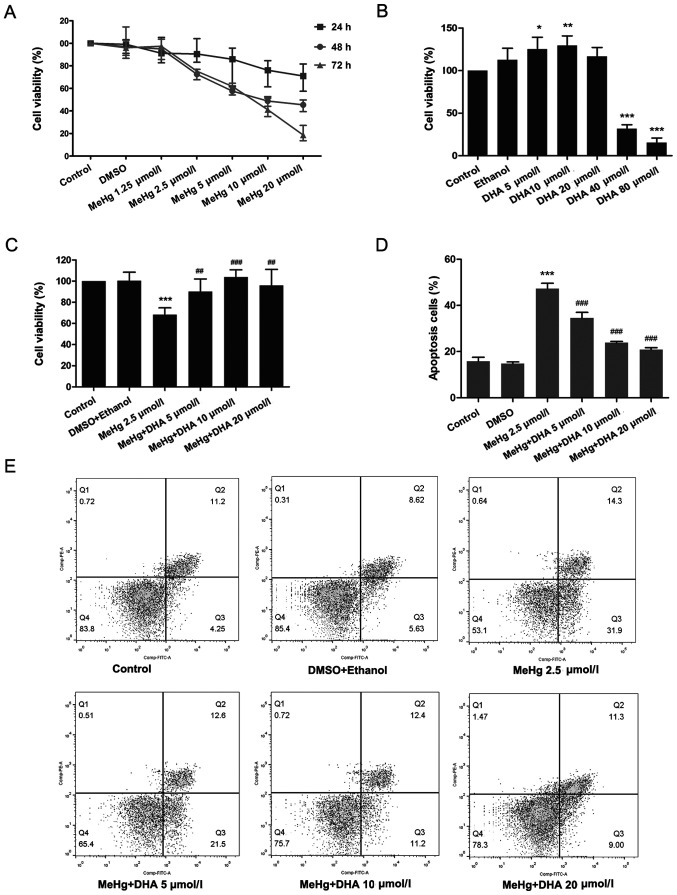
PC12 cells were treated with DHA (5, 10, 20, 40 or 80 µmol/l) for 24 h to select the appropriate concentration. In addition, the appropriate MeHg concentration was screened; PC12 cells were treated with MeHg (1.25, 2.5, 5, 10 and 20 µmol/l) for 24, 48 and 72 h. As presented in Fig. 1A, compared with the untreated control group, cell viability was significantly decreased in the 2.5 µmol/l MeHg group treated for 48 h. Moreover, it was revealed that treatment with 5 and 10 DHA increased cell viability (Fig. 1B). According to the principle of minimizing the dose and maximizing the curative effect, treatments with 2.5 µmol/l MeHg for 48 h, and 10 and 20 µmol/l DHA for 24 h were selected as the conditions for subsequent experiments. The results demonstrated that MeHg significantly inhibited the viability of PC12 cells, whereas DHA could effectively alleviate this effect, which suggested a significant dose-response relationship (Fig. 1C).
Figure 1.
Effects of DHA on MeHg-induced proliferation inhibition and apoptosis of PC12 cells. (A) Changes in cell viability following treatment with MeHg (1.25, 2.5, 5, 10 and 20 µmol/l) for 24, 48 or 72 h. (B) Changes in cell viability following treatment with DHA (5, 10, 20, 40 and 80 µmol/l) for 24 h. (C) PC12 cells were treated with 2.5 µmol/l MeHg for 48 h and were pretreated with DHA (5, 10 and 20 µmol/l) for 24 h; cell viability was detected using a Cell Counting Kit-8 assay. (D and E) Annexin V-FITC apoptosis detection of PC12 cells pretreated with DHA followed by exposure to MeHg. The apoptosis rate was subsequently analyzed (n=3). Data are presented as the mean ± SD. *P<0.05, **P<0.01 and ***P<0.001 vs. control group; ##P<0.01 and ###P<0.001 vs. MeHg group. MeHg, methylmercury; DHA, docosahexaenoic acid.
Effect of DHA on MeHg-induced PC12 cell apoptosis
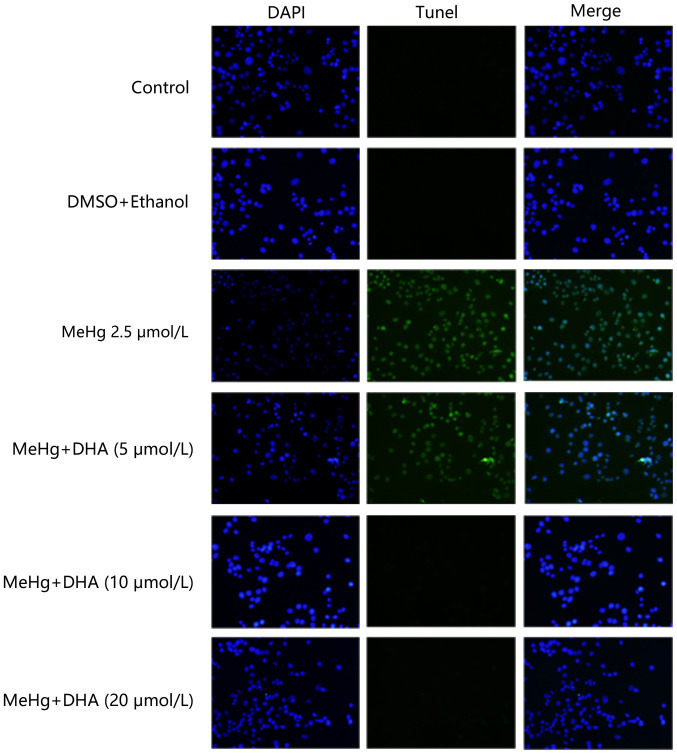
TUNEL staining was conducted to examine the protective effect of DHA on MeHg-induced PC12 cell apoptosis. PC12 cells were treated with 2.5 µmol/l MeHg and marked apoptosis was observed; however, the fluorescence intensity of DHA was markedly decreased (Fig. 2). In addition, the results of flow cytometry revealed similar findings, and there was a significant dose-response relationship (Fig. 1D and E).
Figure 2.
Effects of DHA on MeHg-induced morphological changes of apoptosis of PC12 cells. TUNEL staining was performed using a TUNEL kit to examine the apoptotic rate of PC12 cells that were pretreated with DHA followed by exposure to MeHg. Blue fluorescence represents the cell nucleus, green fluorescence represents the apoptotic cells (magnification, ×200). MeHg, methylmercury; DHA, docosahexaenoic acid.
Effects of DHA and MeHg on the expression levels of apoptosis-associated genes and proteins
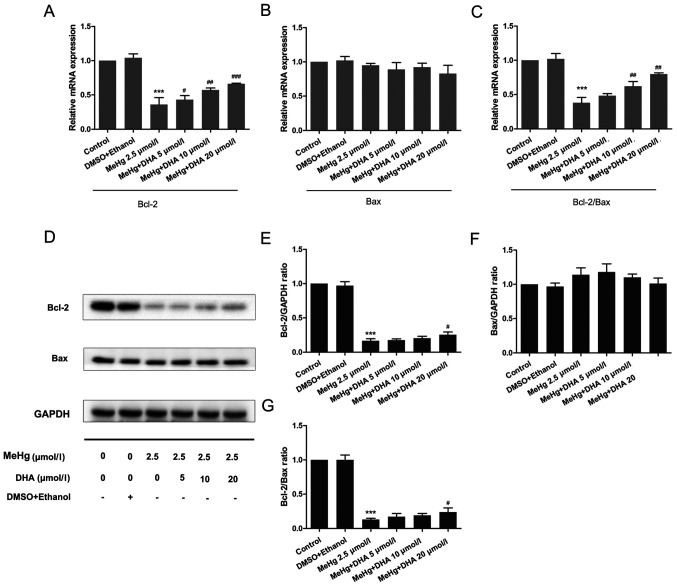
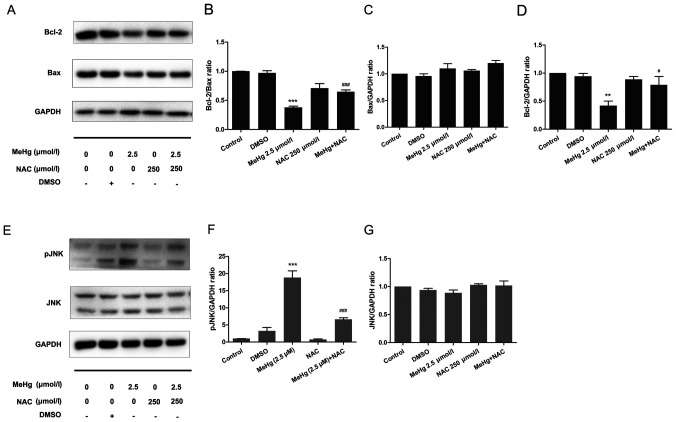
To further confirm the mechanism underlying MeHg-induced PC12 cell apoptosis, RT-qPCR and western blotting were performed to observe the changes in the mRNA and protein expression levels of apoptosis-related genes. As presented in Fig. 3, the mRNA and protein expression levels of the anti-apoptosis factor Bcl-2 were significantly inhibited after PC12 cells were treated with MeHg compared with those in the control group (Fig. 3A, D and E). However, pretreatment with DHA could markedly rescue the MeHg-induced decrease in Bcl-2. Notably, it was revealed that the relative mRNA and protein expression levels of Bax were unchanged in all groups (Fig. 3B, D and F).
Figure 3.
Effects of DHA on MeHg-induced changes in the mRNA and protein expression levels of Bcl-2 and Bax. Reverse transcription-quantitative PCR was used to detect changes in the mRNA expression levels of (A) Bcl-2 and (B) Bax, and (C) Bcl-2/Bax ratio in PC12 cells pretreated with DHA (5, 10 and 20 µmol/l) followed by exposure to 2.5 µmol/l MeHg for 48 h. Relative expression levels were normalized to the corresponding GAPDH levels. (D) Western blotting demonstrated the dynamic changes in the protein expression levels of (E) Bcl-2 and (F) Bax, and (G) Bcl-2/Bax ratio in PC12 cells pretreated with DHA (5, 10 and 20 µmol/l) followed by exposure to 2.5 µmol/l MeHg for 48 h. Protein band intensity was analyzed after normalization to the corresponding GAPDH levels, and the relative ratios were analyzed after normalization to the corresponding control group. Data are presented as the mean ± SD, n=3. ***P<0.001 vs. control group; #P<0.05, ##P<0.01 and ###P<0.001 vs. MeHg group. MeHg, methylmercury; DHA, docosahexaenoic acid.
The Bcl-2/Bax ratio has an important role in cell survival and apoptosis, with a larger ratio associated with more extensive apoptosis (24). The present study demonstrated that the Bcl-2/Bax ratio was significantly decreased in the MeHg group compared with that in the control group, and was increased in the DHA (5, 10 and 20 µmol/l) + MeHg (2.5 µmol/l) group compared with that in the MeHg group (Fig. 3C, D and G).
Effect of DHA on ROS production in PC12 cells
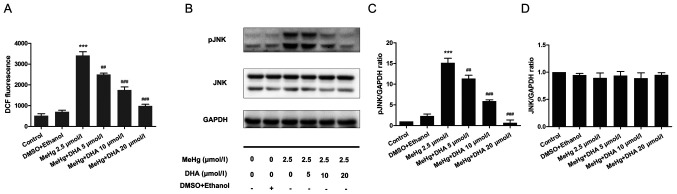
PC12 cells were stained with the DCFH-DA fluorescent probe to examine the DCF fluorescence intensity using a fluorescence microplate reader. Compared with that in the control group, the ROS level was significantly increased in the MeHg group. After DHA pretreatment, the ROS level was decreased in a dose-dependent manner compared with that in the MeHg group (Fig. 4A).
Figure 4.
Effects of DHA on MeHg-induced changes in ROS levels, and JNK and pJNK expression. (A) A fluorescence microplate reader was used to analyze the changes in ROS levels in PC12 cells pretreated with DHA (5, 10 and 20 µmol/l) followed by exposure to 2.5 µmol/l MeHg. DCF fluorescence intensity was recorded. (B) Western blotting demonstrated the dynamic changes in (C) JNK and (D) pJNK expression levels in PC12 cells pretreated with DHA (5, 10 and 20 µmol/l) followed by exposure to 2.5 µmol/l MeHg for 48 h. Protein band intensity was analyzed after normalization to the corresponding GAPDH levels, and the relative ratios were analyzed after normalization to the corresponding control group. Double bands indicate different isoforms. Data are presented as the mean ± SD, n=3. ***P<0.001 vs. control group; ##P<0.01 and ###P<0.001 vs. MeHg group. MeHg, methylmercury; DHA, docosahexaenoic acid; p, phosphorylated; ROS, reactive oxygen species.
Effect of DHA on the JNK signaling pathway in MeHg-induced PC12 cells
Western blotting was performed to examine phosphorylation of the JNK pathway. The results demonstrated that MeHg significantly activated pJNK compared with in the control group; however, DHA significantly inhibited the effect of MeHg (Fig. 4B-D).
Inhibitory effect of NAC on MeHg-induced PC12 cell apoptosis
To further support the results of the mechanistic research, NAC was used as an ROS inhibitor to pretreat PC12 cells to further confirm the relationship between ROS and the JNK pathway. The results demonstrated that, after pretreatment with 500 µmol/l NAC at 37°C for 30 min, the expression levels of the anti-apoptotic protein Bcl-2 were significantly increased compared with those in the MeHg group (Fig. 5A and B). Moreover, the expression levels of Bax were not affected (Fig. 5A and C), whereas the Bcl-2/Bax ratio was significantly increased (Fig. 5A and B). In addition, in PC12 cells exposed to MeHg, the expression levels of pJNK were significantly increased (Fig. 5E-G). By contrast, NAC significantly downregulated the expression levels of pJNK. These findings indicated that ROS may mediate the expression levels of the downstream protein pJNK, and NAC may exert a significant protective effect against MeHg-induced apoptosis. These findings also suggested that DHA may protect PC12 cells from apoptosis induced by MeHg via antioxidant activity.
Figure 5.
Effects of NAC on MeHg-induced changes in the expression levels of apoptosis-related proteins in PC12 cells. (A) Western blotting demonstrated the dynamic changes in (B) Bcl-2/Bax ratio, and the protein expression levels of (C) Bax and (D) Bcl-2 in PC12 cells pretreated with 250 µmol/l NAC for 60 min followed by exposure to 2.5 µmol/l MeHg for 48 h. (E) Western blotting demonstrated the dynamic changes in the protein expression levels of (F) pJNK and (G) JNK in PC12 cells pretreated with 250 µmol/l NAC for 60 min followed by exposure to 2.5 µmol/l MeHg for 48 h. Protein band intensity was analyzed after normalization to the corresponding GAPDH levels, and the relative ratios were analyzed after normalization to the corresponding control group. Data are presented as the mean ± SD, n=3. **P<0.01 and ***P<0.001 vs. control group; #P<0.05 and ###P<0.001 vs. MeHg group. MeHg, methylmercury; DHA, docosahexaenoic acid; p, phosphorylated; NAC, N-acetyl-l-cysteine.
Discussion
Oxidative stress has been widely recognized as an initiator of apoptosis, in which it plays a key role (25,26). Kuo et al (27) reported that oxidative stress caused by ROS may activate the JNK pathway and regulate the transcriptional expression of related downstream genes, leading to apoptosis. Moreover, studies have shown that ROS-induced apoptosis is closely associated with activation of the JNK signaling pathway, which can regulate the expression of apoptosis-related genes and ultimately lead to cell apoptosis (28–30). Therefore, the JNK/ROS signaling pathway was selected as the focus of the mechanistic research in the present study.
MeHg has been recognized as a highly toxic pollutant in the environment, which is mainly absorbed by humans in the gastrointestinal tract, and easily crosses the blood-brain barrier and the placental barrier, exerting adverse effects on neurodevelopment (31), including IQ reduction and language impairment (32). It has been reported that a low dose of MeHg may exert a toxic effect on nerve precursor cells (33). In addition, Petroni et al (34) observed that low concentrations of MeHg significantly decreased the viability of SY-SY5Y neuroblastoma cells, resulting in permanent cell damage. As an essential unsaturated fatty acid in humans, DHA has been reported to possess antioxidant and antiapoptotic effects (35). DHA has been demonstrated to be crucial for the development of the nervous system, particularly vision and cognition of infants and young children (36). Therefore, the present study investigated the potential protective effect of DHA against MeHg poisoning and the possible molecular mechanisms. In the current study, PC12 cells, which are widely applied in neuronal cell models in vitro, were used to identify the mechanism of MeHg-induced PC12 cell apoptosis via the ROS/JNK signaling pathway and to examine the protective role of DHA in this process.
First, the MeHg poisoning model was established in vitro. DHA pretreatment efficiently rescued MeHg-induced cell viability inhibition and apoptosis. Notably, MeHg at 1.25 µmol/l had no toxic effect on PC12 cells, and it was suggested that low-dose MeHg may initiate the toxic excitatory effect of cells and trigger a series of repair activation mechanisms, which was verified in the research of Tan et al (37). In addition, it is well known that DHA has significant antioxidant effects; however, the present study revealed that PC12 cell survival was reduced following treatment with high concentrations of DHA, which was consistent with the finding of Iuchi et al (38). In addition, Srikanth et al (39) revealed that higher concentrations of DHA induced profound cell swelling and a reduction in viability, which was accompanied by increased expression levels of inflammatory cytokine and lipoxygenase genes, activation of caspase-1 activity and release of IL1β, indicating that cells were undergoing a proinflammatory cell death program known as pyroptosis; this may be one of the reasons that high concentrations of DHA become toxic.
A number of studies have demonstrated that oxidative stress may serve an important role in MeHg-induced apoptosis (40,41). Therefore, the present study examined the effect of MeHg and DHA on intracellular ROS levels to evaluate the potential mechanism underlying the effects of DHA on MeHg-induced apoptosis of PC12 cells. The results demonstrated that MeHg significantly increased the levels of ROS, whereas DHA effectively inhibited MeHg-induced oxidative injury to PC12 cells. As members of the MAPK superfamily, JNKs serve a crucial role in cell survival and apoptosis (42,43). Therefore, the protective effects of DHA on MeHg-induced apoptosis at the level of the JNK signaling pathway were further investigated. The results demonstrated that the JNK signaling pathway was markedly activated and that the expression of pJNK was significantly increased after PC12 cells were exposed to MeHg.
The mitochondrial pathway is the most important pathway that mediates cell apoptosis. The change in mitochondrial outer membrane permeability (MOMP) is considered to be the main switch of the mitochondrial apoptosis pathway, and MOMP is strictly regulated by the Bcl-2 family and promotes apoptosis (44). Some members of the Bcl-2 family, such as Bax, can directly promote changes in MOMP. When apoptosis is induced, Bax migrates from the cytosol to the mitochondria and nuclear membrane, and initiates the caspase cascade via cytochrome c and second mitochondria-derived activator caspase pathways (45). The present study demonstrated that the mRNA and protein expression levels of anti-apoptotic Bcl-2 were almost completely inhibited by MeHg; however, there was no significant change in the mRNA or protein expression levels of pro-apoptotic Bax, which was similar to other research findings (46). Notably, Hou et al (47) revealed that Bax may not always be a potent inducer of apoptosis in tumor cells. It has been reported that the Bcl-2/Bax ratio is essential for determining whether cells undergo apoptosis, which is consistent with the current study (24). Additionally, the present study revealed that restoration of Bcl-2 transcription by DHA seemed to be more obvious than that of Bcl-2 protein expression, which may be related to the length of administration time. This phenomenon was also reported in a previous study (28).
To further confirm the protective mechanism of DHA and to elucidate the relationship between ROS and signaling pathways, NAC, an efficient ROS scavenger, was used to pretreat cells in the present study. The results demonstrated that all of the adverse effects of MeHg were improved; notably, NAC pretreatment inhibited JNK signaling, and increased the expression levels of the anti-apoptosis protein Bcl-2 and the ratio of Bcl-2 to Bax. Therefore, these results suggested that DHA may ameliorate MeHg-induced apoptosis via the ROS-regulated JNK signaling pathway.
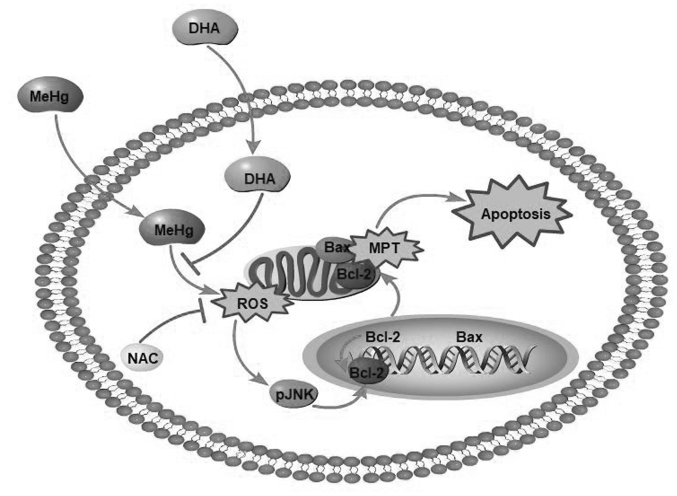
In conclusion, MeHg significantly increased the ROS content in PC12 cells and induced oxidative stress, whereas DHA effectively decreased levels of intracellular ROS to relieve oxidative stress-induced PC12 cell apoptosis (Fig. 6). Moreover, DHA may reverse the MeHg-induced adverse effects, including the elevated phosphorylation of JNK, and decreased mRNA and protein expression levels of anti-apoptotic Bcl-2. To the best of our knowledge, the present study was the first to attempt to elucidate the protective effects of DHA against MeHg-induced PC12 cell apoptosis via the ROS/JNK signaling pathway, which may provide a theoretical basis for the treatment of MeHg poisoning. Our future studies will focus on MeHg target gene prediction by full transcriptome sequencing of samples, in order to further elucidate the protective mechanism of DHA on MeHg-induced apoptosis of PC12 cells.
Figure 6.
Schematic diagram of protective effects of DHA against MeHg-induced apoptosis mediated by ROS via the JNK signaling pathway MeHg, methylmercury; DHA, docosahexaenoic acid; ROS, reactive oxygen species; p, phosphorylated; NAC, N-acetyl-l-cysteine; MPT, mitochondrial phosphate transporter.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by grants from the National Science Foundation of China (grant no. 81973062) and the National Key R&D Program of China (grant no. 2017YFC1600500).
Funding
The present study was supported by grants from the National Science Foundation of China (grant no. 81973062) and the National Key R&D Program of China (grant no. 2017YFC1600500).
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to privacy of laboratory content but are available from the corresponding author on reasonable request.
Authors' contributions
CY made substantial contributions to the conception and design of the study, analysis and interpretation of data, acquisition of funding and supervision of the research. HZ and SW performed the experiments. YW, AL and CH made substantial contributions to the analysis and interpretation of data. HZ and SW confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jacobs S, Sioen I, Jacxsens L, Domingo JL, Sloth JJ, Marques A, Verbeke W. Risk assessment of methylmercury in five European countries considering the national seafood consumption patterns. Food Chem Toxicol. 2017;104:26–34. doi: 10.1016/j.fct.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Hylander LD. Global Mercury Pollution and its Expected Decrease after a Mercury Trade Ban. Water Air Soil Pollut. 2001;125:331–344. doi: 10.1023/A:1005231017807. [DOI] [Google Scholar]
- 3.Borowska S, Brzóska MM. Metals in cosmetics: Implications for human health. J Appl Toxicol. 2015;35:551–572. doi: 10.1002/jat.3129. [DOI] [PubMed] [Google Scholar]
- 4.Kim W, Kim DW, Yoo DY, Jung HY, Kim JW, Kim DW, Choi JH, Moon SM, Yoon YS, Hwang IK. Antioxidant effects of Dendropanax morbifera Léveille extract in the hippocampus of mercury-exposed rats. BMC Complement Altern Med. 2015;15:247. doi: 10.1186/s12906-015-0786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarkson TW, Magos L, Myers GJ. The toxicology of mercury - current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- 6.Syversen T, Kaur P. The toxicology of mercury and its compounds. J Trace Elem Med Biol. 2012;26:215–226. doi: 10.1016/j.jtemb.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Bellinger DC, Devleesschauwer B, O'Leary K, Gibb HJ. Global burden of intellectual disability resulting from prenatal exposure to methylmercury, 2015. Environ Res. 2019;170:416–421. doi: 10.1016/j.envres.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Sheehan MC, Burke TA, Navas-Acien A, Breysse PN, McGready J, Fox MA. Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: A systematic review. Bull World Health Organ. 2014;92:F254–F269. doi: 10.2471/BLT.12.116152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ke T, Gonçalves FM, Gonçalves CL, dos Santos AA, Rocha JBT, Farina M, Skalny A, Tsatsakis A, Bowman AB, Aschner M. Post-translational modifications in MeHg-induced neurotoxicity. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2068–2081. doi: 10.1016/j.bbadis.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ying Z, Chen K, Zheng L, Wu Y, Li L, Wang R, Long Q, Yang L, Guo J, Yao D, et al. Transient Activation of Mitoflashes Modulates Nanog at the Early Phase of Somatic Cell Reprogramming. Cell Metab. 2016;23:220–226. doi: 10.1016/j.cmet.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Liu Z, Qiu L, Hao L, Guo J. Ipatasertib sensitizes colon cancer cells to TRAIL-induced apoptosis through ROS-mediated caspase activation. Biochem Biophys Res Commun. 2019;519:812–818. doi: 10.1016/j.bbrc.2019.09.063. [DOI] [PubMed] [Google Scholar]
- 12.Tan BL, Norhaizan ME, Chan LC. ROS-Mediated Mitochondrial Pathway is Required for Manilkara Zapota (L.) P. Royen Leaf Methanol Extract Inducing Apoptosis in the Modulation of Caspase Activation and EGFR/NF-κ B Activities of HeLa Human Cervical Cancer Cells. Evid Based Complement Alternat Med. 2018;2018:6578648. doi: 10.1155/2018/6578648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangali S, Bhat A, Udumula MP, Dhar I, Sriram D, Dhar A. Inhibition of protein kinase R protects against palmitic acid-induced inflammation, oxidative stress, and apoptosis through the JNK/NF-κB/NLRP3 pathway in cultured H9C2 cardiomyocytes. J Cell Biochem. 2019;120:3651–3663. doi: 10.1002/jcb.27643. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Feng X, Hu X, Sha J, Li B, Zhang H, Fan H. Dexmedetomidine Ameliorates Acute Stress-Induced Kidney Injury by Attenuating Oxidative Stress and Apoptosis through Inhibition of the ROS/JNK Signaling Pathway. Oxid Med Cell Longev. 2018;2018:4035310. doi: 10.1155/2018/6717212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niu S-L, Mitchell DC, Lim S-Y, Wen ZM, Kim HY, Salem N, Jr, Litman BJ. Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. J Biol Chem. 2004;279:31098–31104. doi: 10.1074/jbc.M404376200. [DOI] [PubMed] [Google Scholar]
- 16.Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ Health Perspect. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oken E, Østerdal ML, Gillman MW, Knudsen VK, Halldorsson TI, Strøm M, Bellinger DC, Hadders-Algra M, Michaelsen KF, Olsen SF. Associations of maternal fish intake during pregnancy and breastfeeding duration with attainment of developmental milestones in early childhood: A study from the Danish National Birth Cohort. Am J Clin Nutr. 2008;88:789–796. doi: 10.1093/ajcn/88.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardoso C, Bernardo I, Bandarra NM, Louro Martins L, Afonso C. Portuguese preschool children: Benefit (EPA+DHA and Se) and risk (MeHg) assessment through the consumption of selected fish species. Food Chem Toxicol. 2018;115:306–314. doi: 10.1016/j.fct.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Nøstbakken OJ, Bredal IL, Olsvik PA, Huang TS, Torstensen BE. Effect of marine omega 3 fatty acids on methylmercury-induced toxicity in fish and mammalian cells in vitro. J Biomed Biotechnol. 2012;2012:417652. doi: 10.1155/2012/417652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur P, Heggland I, Aschner M, Syversen T. Docosahexaenoic acid may act as a neuroprotector for methylmercury-induced neurotoxicity in primary neural cell cultures. Neurotoxicology. 2008;29:978–987. doi: 10.1016/j.neuro.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Takanezawa Y, Nakamura R, Hamaguchi M, Yamamoto K, Sone Y, Uraguchi S, Kiyono M. Docosahexaenoic acid enhances methylmercury-induced endoplasmic reticulum stress and cell death and eicosapentaenoic acid potentially attenuates these effects in mouse embryonic fibroblasts. Toxicol Lett. 2019;306:35–42. doi: 10.1016/j.toxlet.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Δ Δ C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Huang H, Wang S, Chen F, Zheng G. Dehydrocorydaline inhibits the tumorigenesis of breast cancer MDA MB 231 cells. Mol Med Rep. 2020;22:43–50. doi: 10.3892/mmr.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao L, Gu Q, Xiang L, Dong X, Li H, Ni J, Wan L, Cai G, Chen G. Curcumin inhibits apoptosis by modulating Bax/Bcl-2 expression and alleviates oxidative stress in testes of streptozotocin-induced diabetic rats. Ther Clin Risk Manag. 2017;13:1099–1105. doi: 10.2147/TCRM.S141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Angelis C, Galdiero M, Pivonello C, Salzano C, Gianfrilli D, Piscitelli P, Lenzi A, Colao A, Pivonello R. The environment and male reproduction: The effect of cadmium exposure on reproductive function and its implication in fertility. Reprod Toxicol. 2017;73:105–127. doi: 10.1016/j.reprotox.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Wang M, Teng S, Wang D, Li X, Wang X, Liao W, Wang D. Indolyl-chalcone derivatives induce hepatocellular carcinoma cells apoptosis through oxidative stress related mitochondrial pathway in vitro and in vivo. Chem Biol Interact. 2018;293:61–69. doi: 10.1016/j.cbi.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Kuo LM, Chen PJ, Sung PJ, Chang YC, Ho CT, Wu YH, Hwang TL. The Bioactive Extract of Pinnigorgia sp. Induces Apoptosis of Hepatic Stellate Cells via ROS-ERK/JNK-Caspase-3 Signaling. Mar Drugs. 2018;16(19) doi: 10.3390/md16010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren X, Wang S, Zhang C, Hu X, Zhou L, Li Y, Xu L. Selenium ameliorates cadmium-induced mouse leydig TM3 cell apoptosis via inhibiting the ROS/JNK /c-jun signaling pathway. Ecotoxicol Environ Saf. 2020;192:110266. doi: 10.1016/j.ecoenv.2020.110266. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Wu W, Gong B, Hou L, Dong X, Xu C, Zhao R, Yu Q, Zhou Z, Huang S, et al. Metformin attenuates cadmium-induced neuronal apoptosis in vitro via blocking ROS-dependent PP5/AMPK-JNK signaling pathway. Neuropharmacology. 2020;175:108065. doi: 10.1016/j.neuropharm.2020.108065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Yu K, Hu Y, Su F, Gao Z, Hu T, Yang Y, Cao X, Qian F. Schisantherin A induces cell apoptosis through ROS/JNK signaling pathway in human gastric cancer cells. Biochem Pharmacol. 2020;173:113673. doi: 10.1016/j.bcp.2019.113673. [DOI] [PubMed] [Google Scholar]
- 31.Zareba G, Cernichiari E, Hojo R, Nitt SM, Weiss B, Mumtaz MM, Jones DE, Clarkson TW. Thimerosal distribution and metabolism in neonatal mice: Comparison with methyl mercury. J Appl Toxicol. 2007;27:511–518. doi: 10.1002/jat.1272. [DOI] [PubMed] [Google Scholar]
- 32.Poling A, LeSage M. Evaluating psychotropic drugs in people with mental retardation: Where are the social validity data? Am J Ment Retard. 1995;100:193–200. [PubMed] [Google Scholar]
- 33.Watanabe J, Nakamachi T, Ohtaki H, Naganuma A, Shioda S, Nakajo S. Low dose of methylmercury (MeHg) exposure induces caspase mediated-apoptosis in cultured neural progenitor cells. J Toxicol Sci. 2013;38:931–935. doi: 10.2131/jts.38.931. [DOI] [PubMed] [Google Scholar]
- 34.Petroni D, Tsai J, Agrawal K, Mondal D, George W. Low-dose methylmercury-induced oxidative stress, cytotoxicity, and tau-hyperphosphorylation in human neuroblastoma (SH-SY5Y) cells. Environ Toxicol. 2012;27:549–555. doi: 10.1002/tox.20672. [DOI] [PubMed] [Google Scholar]
- 35.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuratko CN, Barrett EC, Nelson EB, Salem N., Jr The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: a review. Nutrients. 2013;5:2777–2810. doi: 10.3390/nu5072777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan Q, Zhang M, Geng L, Xia Z, Li C, Usman M, Du Y, Wei L, Bi H. Hormesis of methylmercury-human serum albumin conjugate on N9 microglia via ERK/MAPKs and STAT3 signaling pathways. Toxicol Appl Pharmacol. 2019;362:59–66. doi: 10.1016/j.taap.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Iuchi K, Ema M, Suzuki M, Yokoyama C, Hisatomi H. Oxidized unsaturated fatty acids induce apoptotic cell death in cultured cells. Mol Med Rep. 2019;19:2767–2773. doi: 10.3892/mmr.2019.9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srikanth M, Chandrasaharan K, Zhao X, Chayaburakul K, Ong W-Y, Herr DR. Metabolism of Docosahexaenoic Acid (DHA) Induces Pyroptosis in BV-2 Microglial Cells. Neuromolecular Med. 2018;20:504–514. doi: 10.1007/s12017-018-8511-0. [DOI] [PubMed] [Google Scholar]
- 40.Xue D-F, Pan S-T, Huang G, Qiu J-X. ROS enhances the cytotoxicity of cisplatin by inducing apoptosis and autophagy in tongue squamous cell carcinoma cells. Int J Biochem Cell Biol. 2020;122:105732. doi: 10.1016/j.biocel.2020.105732. [DOI] [PubMed] [Google Scholar]
- 41.Hassani S, Ghaffari P, Chahardouli B, Alimoghaddam K, Ghavamzadeh A, Alizadeh S, Ghaffari SH. Disulfiram/copper causes ROS levels alteration, cell cycle inhibition, and apoptosis in acute myeloid leukaemia cell lines with modulation in the expression of related genes. Biomed Pharmacother. 2018;99:561–569. doi: 10.1016/j.biopha.2018.01.109. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Li C, Wu G, Li W, Zhang X, Zhou J, Zhang L, Tao J, Shen M, Liu H. Involvement of JNK/FOXO1 pathway in apoptosis induced by severe hypoxia in porcine granulosa cells. Theriogenology. 2020;154:120–127. doi: 10.1016/j.theriogenology.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Li Y, Ma P, Chen J, Xie W. Ficus carica leaves extract inhibited pancreatic β-cell apoptosis by inhibiting AMPK/JNK/caspase-3 signaling pathway and antioxidation. Biomed Pharmacother. 2020;122:109689. doi: 10.1016/j.biopha.2019.109689. [DOI] [PubMed] [Google Scholar]
- 44.Kalkavan H, Green DR. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018;25:46–55. doi: 10.1038/cdd.2017.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi H, Jiang Y, Yin Z, Jiang K, Li L, Shuai J. Optimal pathways for the assembly of the Apaf-1·cytochrome c complex into apoptosome. Phys Chem Chem Phys. 2018;20:1964–1973. doi: 10.1039/C7CP06726G. [DOI] [PubMed] [Google Scholar]
- 46.Pouraminaei M, Mirzaiey MR, Khoshdel A, Hajizadeh MR, Mahmoodi M, Fahmidehkar MA. The effect of Cressa Cretica hydroalcoholic extract on apoptosis and the expression of Bcl2, Bax and P53 genes in hepatoma cell line HepG2. Gene Rep. 2020;20:100692. doi: 10.1016/j.genrep.2020.100692. [DOI] [Google Scholar]
- 47.Hou D, Che Z, Chen P, Zhang W, Chu Y, Yang D, Liu J. Suppression of AURKA alleviates p27 inhibition on Bax cleavage and induces more intensive apoptosis in gastric cancer. Cell Death Dis. 2018;9:781. doi: 10.1038/s41419-018-0823-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to privacy of laboratory content but are available from the corresponding author on reasonable request.