Abstract
Importance:
The U.S. is in the midst of a deadly and protracted opioid crisis. While many individuals with opioid use disorder (OUD) seek treatment at residential facilities to initiate long-term recovery, the availability and utilization of medications for OUD (MOUD) in these facilities is unclear.
Objectives:
Examine differences in MOUD availability and utilization in residential facilities as a function of Medicaid policy, as well as facility-level and treatment admissions-level factors associated with MOUD availability and utilization, respectively.
Design:
Observational, cross-sectional study of deidentified facility-level and admissions-level data from residential treatment facilities across the U.S. in 2017. Statistical analyses occurred in 2019.
Setting:
Residential facilities reporting to the Substance Abuse and Mental Health Services Administration.
Participants:
Facility-level data were extracted from the 2017 National Survey of Substance Abuse Treatment Services (NSSATS), and admissions-level data from residential facilities were extracted from the 2017 Treatment Episode Data Set-Admissions (TEDS-A).
Exposures:
OUD admissions that identified opioids as their primary drug of choice at residential treatment facilities in the U.S.
Main Outcomes:
MOUD availability was defined as offering extended-release naltrexone (XR-NTX), buprenorphine, and/or methadone at a given residential facility. MOUD utilization was defined as the planned use of any MOUD during residential treatment.
Results:
A relatively low percentage of residential treatment facilities (n=2,863) offered extended-release naltrexone (XR-NTX) (29.8%), buprenorphine (33.3%), or methadone (2.1%). Regarding residential treatment admissions (n=232,414), MOUD was utilized in only 17.3% and 1.9% of admissions in states that did or did not expand Medicaid, respectively (p<0.001). Residential facilities that offered no MOUD had lower odds than facilities that offered ≥1 MOUD of also offering psychiatric medications (odds ratio [OR]=0.06, 95% confidence intervals [CI]=0.05–0.08, p<0.001), being licensed by a state/hospital authority (OR=0.39, 95% CI=0.27–0.57, p<0.001), or accredited by a health organization (OR=0.28, 95% CI=0.23–0.33, p<0.001), and had higher odds of accepting cash-only payments (OR=4.80, 95% CI=3.47–6.64, p<0.001).
Conclusions and Relevance:
In this cross-sectional study of residential addiction treatment facilities in the U.S., MOUD availability and utilization was sparse. Public health and policy efforts to improve access to and utilization of MOUD in residential treatment facilities could improve treatment outcomes for persons initiating recovery.
The opioid crisis has devastated Americans from all walks of life. To address this public health emergency, it is crucial to transition individuals with active opioid use disorder (OUD) into long-term, meaningful recovery1,2. Residential treatment facilities are frequently viewed as the highest level of care across substance use disorders (SUDs), providing an expensive3,4 yet effective means of addressing the challenges that occur in early recovery5,6, often through comprehensive behavioral interventions that provide a foundation for long-term recovery7. However, the U.S. addiction treatment infrastructure was largely developed apart from mainstream medicine8,9, and there is a pressing need to better integrate addiction specialty behavioral treatment with medical care to address the complex challenges faced by persons with OUD.
Several medications for OUD (MOUD) are now considered by the medical community to be the gold standard in initiating and sustaining long-term OUD recovery2. Despite current public health efforts to bridge paraprofessional and medical care10,11, the majority of persons with OUD still do not have access to or receive any form of MOUD12,13. Broadly speaking, FDA-approved MOUD act on the μ opioid receptor and include the full agonist methadone14, the partial agonist buprenorphine (sublingual15, subdermal implants16, extended-release depot injections17), and the antagonist naltrexone (oral, extended-release depot injections; [XR-NTX]18). Even though these medications are a front-line treatment for moderate to severe OUD, potential patients continue to face challenges with insurance coverage and treatment accessibility19, and clinicians continue to face legal and practical barriers in prescribing MOUD20–24.
While trends in access to MOUD across the U.S. are generally improving25,26, state-level disparities, which might reflect regional differences in stigma, especially towards buprenorphine and methadone, are still evident27,28. For instance, several studies have reported that MOUD availability/utilization is lower in that states that were resistant to Medicaid expansion under the Affordable Care Act (ACA) relative to states that expanded Medicaid coverage29–31. The majority of this research has focused on primary care32 or outpatient addiction specialty treatment facilities33 and there is little research on MOUD availability and/or utilization among residential facilities (which often embrace a 12-step treatment philosophy). However, differences in treatment philosophy among clinical staff who deliver 12-step care (and might themselves be in recovery)34, patients seeking MOUD versus medication-free treatment35, and medical professionals who deliver MOUD might affect the adoption of MOUD in residential settings. Regional differences in reimbursement for and access to MOUD might further compound these issues and decrease the likelihood that residential facilities will provide MOUD.
Currently, there is no medical and/or behavioral standard of care for OUD across residential facilities, and the level and quality of care can vary greatly from one facility to the next7,36. Although most OUD patients do not utilize residential treatment and instead enter outpatient treatment directly, persons with OUD often report a preference for including residential treatment in their recovery trajectory19,37. Several states have recently seen an increase in new residential addiction treatment facilities, and many have questionable standards of care38. The goals of this study were to examine national databases of treatment facility-level and treatment admission-level data regarding the availability and utilization of MOUD in residential addiction treatment programs. Specific goals included: (1) determine state-level Medicaid policy that might impact the proportion of residential treatment facilities that offered MOUD, and the proportion of residential treatment admissions that utilized any MOUD as part of their treatment plan, (2) examine facility-level characteristics that were associated with MOUD availability, including the availability of pharmacotherapies for psychiatric conditions, insurance coverage and payment options, and licensing/accreditation, (3) examine treatment admission-level characteristics that were associated with planned utilization of MOUD, including sex, race, age, veteran status, and criminal justice referral, and (4) determine whether availability and utilization of MOUD was associated with state-level opioid overdose mortality rates.
Methods
This cross-sectional study followed Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines; data sources, variable definitions, and detailed statistical methodology are reported in the Methods section and samples sizes for primary and sub-analyses are reported in the Results and Figure 1. This study was exempt from Institutional Review Board review as all data was de-identified and publicly available and thus did not qualify as human subjects research.
Figure 1.
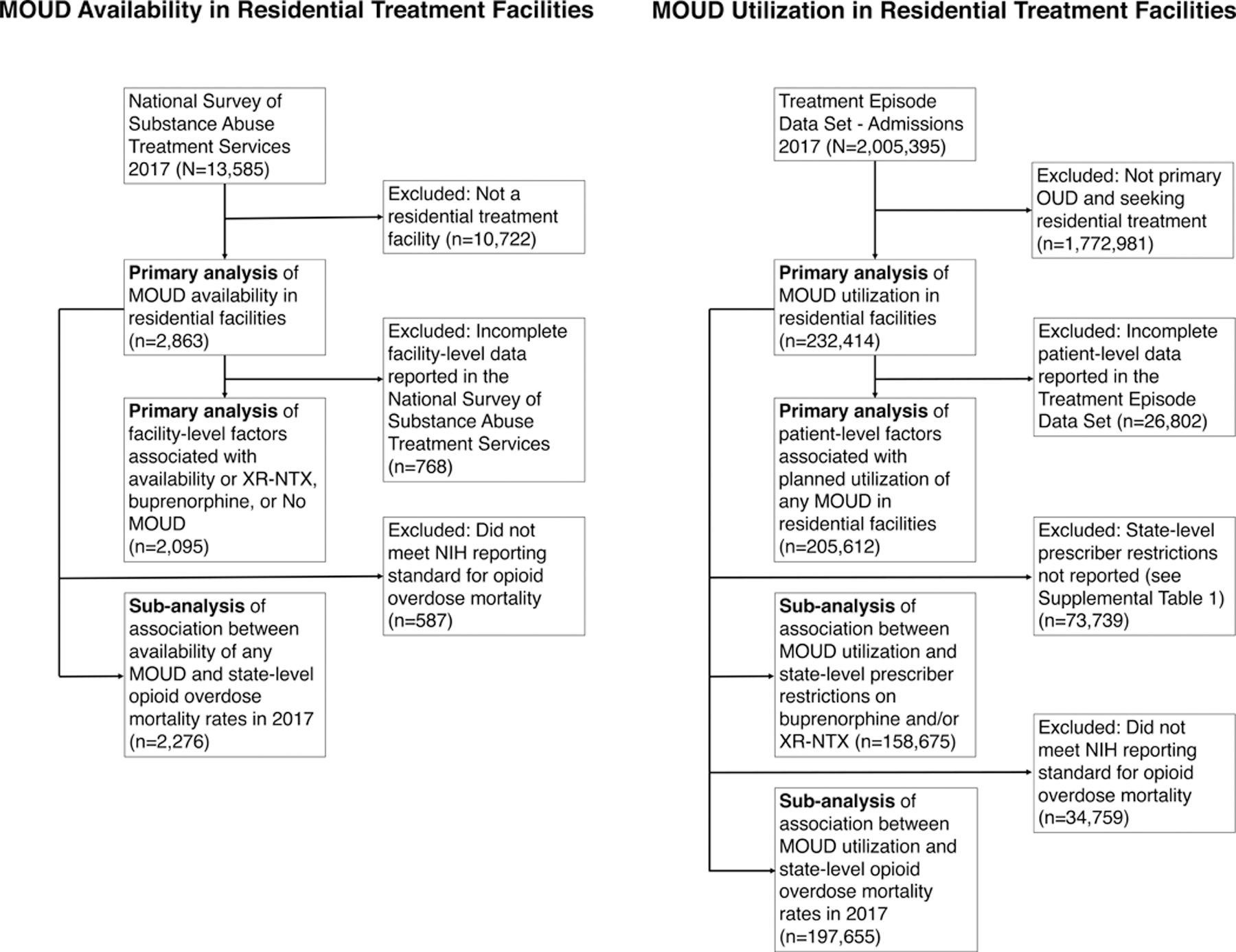
STROBE figure of data source, inclusion/exclusion factors, and numbers included in primary and sub-analyses. Left: Facility-level data reported to the National Survey of Substance Abuse Treatment Services. Right: Treatment admissions-level data reported to the Treatment Episode Data Set-Admissions. All primary and sub-analyses used all available data.
Data Sources
Data for this study were collected from four publicly available sources: the 2017 National Survey of Substance Abuse Treatment Services (N-SSATS)39 and the 2017 Treatment Episode Data Set-Admissions (TEDS-A)40, both made public through the Substance Abuse and Mental Health Services Administration (SAMHSA); state-level opioid overdose mortality rates via the Centers for Disease Control (CDC) Wide-ranging Online Data for Epidemiological Research (WONDER) website41 (only states reporting opioid overdose mortality rates that met National Institutes of Health [NIH] standards were utilized42); and state-level information on Medicaid policy and coverage, made publicly available through the Henry J. Kaiser Family Foundation43.
Database Information and Variable Definitions
Data on residential treatment facilities were collected from the 2017 (N-SSATS); a publicly-available, annual census of treatment facilities in the United States that collects facility-level data (e.g. medications available, level of care, services provided, payment options) from hospital, residential, and outpatient SUD treatment facilities39. MOUD availability was defined as offering XR-NTX; methadone; and/or sublingual or subdermal implant buprenorphine (all facilities that offered subdermal also offered sublingual buprenorphine). Independent variables were chosen based on facility characteristics that might affect quality of care or patient access to treatment, including the availability of psychiatric medications, acceptance of various forms of insurance (or cash-only, defined as not accepting insurance), whether the facility was licensed by a hospital or state authority or accredited by a health organization, and whether the facility offered long-term residential care (>28 days).
Data on treatment admissions for residential treatment facilities were collected from the 2017 TEDS-A, which collects patient-level data on persons entering state-certified substance abuse treatment facilities40. MOUD utilization was defined as planned medication-assisted therapy (a binary variable within TEDS-A representing planned use of methadone, buprenorphine, or naltrexone). Independent variables were chosen based on demographic factors known to be associated with MOUD utilization, including biological sex, race (defined for this study as white/Caucasian, black/African American, and all other), age (defined for this study as under 25, 25–54, and 55 and older), referral from the criminal justice system, and veteran status. Data on health insurance status was not included as >60% were missing/invalid.
Data on Medicaid expansion by 2017 was collected from the Henry J. Kaiser Family Foundation43. As a sub-analysis, states that did or did not have Medicaid prescribing restrictions for buprenorphine and/or XR-NTX (e.g. preauthorization requirements, time/dosage limits on buprenorphine, etc) were examined within states that did or did not implement Medicaid expansion, respectively; eleven states did not report prescribing restrictions and were thus excluded from this sub-analysis (see Supplementary Table 1).
Opioid overdose mortality rates, defined as opioid-involved deaths/100,000 persons, were collected by state and the District of Columbia from CDC WONDER41; sixteen states were excluded as they did not meet NIH reporting criteria42 (see Supplementary Table 1).
Statistical Analyses
The availability of MOUD, including methadone, buprenorphine, XR-NTX, the combination of any two MOUD, and the combination of or absence of all MOUD for residential facilities reporting to the 2017 N-SSATS, and the planned use of any MOUD for admissions to residential treatment facilities reporting to the 2017 TEDS-A were compared via logistic regression analyses and reported as unadjusted odds ratios (OR) for states that did or did not expand Medicaid as of 2017. To further examine state-level policy factors associated with MOUD utilization, states that did or did not have Medicaid prescribing restrictions for buprenorphine and/or XR-NTX (e.g. preauthorization requirements, time/dosage limits on buprenorphine prescribing, etc) were examined within states that did or did not implement Medicaid expansion, respectively.
Sensitivity analyses of patient-level TEDS-A outcomes were performed for results on Medicaid expansion and Medical prescribing restrictions by (a) restricting the MOUD utilization data to only first-time treatment admissions, (b) propensity score matching MOUD utilization data based on the core demographics age, sex, and race, and (c) in a multivariable model controlling for all demographics.
For facility-level (via the N-SSATS) and treatment admission-level (via the TEDS-A) factors that might be associated with MOUD availability and utilization, respectively, unadjusted ORs and adjusted odds ratios (AOR) were computed using unadjusted and multivariable logistic regression models. N-SSATS data used facilities offering No MOUD as the primary dependent variable (see Table 1 for independent variables), and also XR-NTX and buprenorphine availability as secondary dependent variables (see Supplemental Tables 2 and 3); methadone availability was not included in the results because the proportion of facilities offering only methadone was too low to infer meaningful results. TEDS-A data used planned use of any MOUD as the primary dependent variable (see Table 2 for independent variables). In addition to ORs and AORs, percent probabilities were calculated and reported in Supplemental Tables 4–6. In the N-SSATS, 28.8% of data were missing, and in the TEDS-A, 15.4% of data were missing in each logistic regression analysis, respectively (See Figure 1). Missing data were not imputed. In a sub-analysis, ORs were also calculated separately for availability of any MOUD within residential facilities (via the N-SSATS) and planned use of any MOUD (via the TEDS-A) as a function of state-level opioid overdose mortality rates in the 34 states (and District of Columbia) that were determined by the NIH to have provided accurate overdose mortality rates in 2017 (see Supplemental Table 1). All data were analyzed in 2019 using R Statistical Language (R Core Team).
Table 1.
Characteristics of Residential Treatment Facilities Offering No MOUD
| Descriptive Statistics | Unadjusted Analyses | Multivariable Analyses | ||||
|---|---|---|---|---|---|---|
| Residential Facility Characteristics | All Residential Facilities n (%) | Offering No MOUD n (%a) | OR | 95% CI | AOR | 95% CI |
| All Residential Facilities | 2,095 (100) | 1,300 (62.1) | ||||
| Offered Psychiatric Medications | 1,038 (49.5) | 356 (34.3) | 0.06*** | 0.05 – 0.08 | 0.08*** | 0.06 – 0.10 |
| Accepted Payment | ||||||
| Cash/self-pay | 1,786 (85.3) | 1,064 (59.6) | 0.46*** | 0.34 – 0.60 | 0.63* | 0.42 – 0.95 |
| Medicare | 313 (14.9) | 194 (62.0) | 1.00 | 0.78 – 1.28 | 1.00 | 0.72 – 1.38 |
| Medicaid | 967 (46.2) | 570 (58.9) | 0.78** | 0.66 – 0.93 | 1.41* | 1.06 – 1.86 |
| State-financed insurance | 736 (35.1) | 423 (57.5) | 0.74** | 0.62 – 0.89 | 1.44* | 1.09 – 1.91 |
| Federal military insurance | 359 (17.1) | 187 (52.1) | 0.61*** | 0.48 – 0.77 | 1.06 | 0.77 – 1.45 |
| Private insurance | 1,196 (57.1) | 582 (48.7) | 0.24*** | 0.20 – 0.29 | 0.59** | 0.42 – 0.83 |
| IHS/638 contract | 323 (15.4) | 196 (60.7) | 0.93 | 0.73 – 1.19 | 1.26 | 0.92 – 1.72 |
| Cash-only facility | 342 (16.3) | 296 (86.5) | 4.80*** | 3.47 – 6.64 | 2.20** | 1.33 – 3.62 |
| License/Accreditation | ||||||
| Hospital or State authority | 1,914 (91.4) | 1,156 (60.4) | 0.39*** | 0.27 – 0.57 | 0.51** | 0.31 – 0.82 |
| JCAHO/CARF/NCQA/COA/HFAP | 1,037 (49.5) | 491 (47.3) | 0.28*** | 0.23 – 0.33 | 0.56*** | 0.44 – 0.70 |
| Long-term residential treatment offered (>28 days) | 1,743 (83.2) | 1,152 (66.1) | 2.69*** | 2.13 – 3.39 | 1.84*** | 1.37 – 2.47 |
Table 1 displays the percent of facilities offering no MOUD that also offer other medications for psychiatric disorders, the type of payments accepted, facilities that are licensed or accredited, and facilities offering long-term residential care (n=2,095 facilities with complete data). Unadjusted odds ratios, adjusted odds ratios, and 95% confidence intervals determined via univariate and multivariable logistic regression. XR-NTX = extended-release naltrexone; OR = unadjusted odds ratios; AOR = adjusted odds ratios; CI = confidence intervals; MOUD = medications for opioid use disorder; HIS/638 = Indian Health Service federal contract; JCAHO = Joint Commission of Healthcare Organizations; CARF = Commission on Accreditation of Rehabilitation Facilities; NCQA = National Committee for Quality Assurance; COA = Council on Accreditation; HFAP = Healthcare Facilities Accreditation Program.
Percent of facilities offering No MOUD based on the number of facilities offering No MOUD divided by all facilities within each category (e.g. Offered Psychiatric Medications). Unadjusted and adjusted predicted probabilities can be found in Supplemental Tables 4 and 5.
p < .05;
p < .01;
p < .001
Table 2.
Planned Use of Any MOUD Among Admissions for Opioid Use Disorder in Residential Treatment Facilities
| Descriptive Statistics | Unadjusted Analyses | Multivariable Analyses | ||||
|---|---|---|---|---|---|---|
| Patient Characteristics | All Admissions n (%) | Planned Use of Any MOUD n (%a) | OR | 95% CI | AOR | 95% CI |
| All Patients | 205,612 (100) | 33,377 (16.2) | ||||
| Sex (Male) | 136,854 (66.6) | 21,467 (15.7) | 0.89*** | 0.87 – 0.91 | 0.87*** | 0.85 – 0.89 |
| Race | ||||||
| Caucasian/White | 151,867 (73.9) | 24,102 (15.9) | Ref | Ref | Ref | Ref |
| African American/Black | 19,076 (9.3) | 2,260 (11.8) | 0.71*** | 0.68 – 0.75 | 0.67*** | 0.64 – 0.71 |
| All other | 34,669 (16.9) | 7,015 (20.2) | 1.34*** | 1.31 – 1.39 | 1.33*** | 1.29 – 1.37 |
| Age | ||||||
| Under 25 | 28,842 (14.0) | 4,376 (15.2) | Ref | Ref | Ref | Ref |
| 25–54 | 166,213 (80.8) | 26,925 (16.2) | 1.08*** | 1.04 – 1.12 | 1.09*** | 1.05 – 1.13 |
| 55 and Older | 10,557 (5.1) | 2,076 (19.7) | 1.37*** | 1.29 – 1.45 | 1.47*** | 1.39 – 1.56 |
| Veteran Status (Yes) | 4,245 (2.1) | 645 (15.2) | 0.92 | 0.85 – 1.00 | 0.92 | 0.85 – 1.01 |
| Referral from Justice System (Yes) | 23,816 (11.6) | 2,845 (11.9) | 0.67*** | 0.65 – 0.70 | 0.67*** | 0.65 – 0.70 |
Patient characteristics for opioid use disorder treatment admissions in residential settings according to the 2017 Treatment Episode Dataset-Admissions (n=205,612 with complete data). Unadjusted odds ratios, adjusted odds ratios, and 95% confidence intervals determined via univariate and multivariable logistic regression and represent increased/decreased odds of planned use of any medication for opioid use disorder. Note: Boldface indicates statistical significance (p<0.05) i.e. 95% confidence intervals that do not cross 1. MOUD = medications for opioid use disorder; OR = unadjusted odds ratio; AOR = adjusted odds ratios; CI = confidence intervals Ref = reference group.
Percent of admissions with planned use of Any MOUD based on the number of admissions with Any MOUD divided by all admissions within each category (e.g. Sex). Unadjusted and adjusted predicted probabilities can be found in Supplemental Table 6.
p < .05;
p < .01;
p < .001
Results
MOUD availability in residential treatment facilities
Of the facilities reporting to the N-SSATS in 2017 (N=13,585), only residential treatment facilities within the 50 states and District of Columbia were included in this study (n=2,863). These facilities generally accepted some form of insurance (83.7%), were licensed by a state or hospital authority (91.4%), and offered long-term residential treatment (83.2%). In 2017, 29.8% of residential treatment facilities offered XR-NTX, 33.3% offered buprenorphine, and 2.1% offered methadone. Overall, 60.0% of residential treatment facilities offered No MOUD, and only 1.3% offered all forms of MOUD (Figure 2). There was no appreciable difference in MOUD availability in residential facilities in states that did or did not expand Medicaid.
Figure 2.
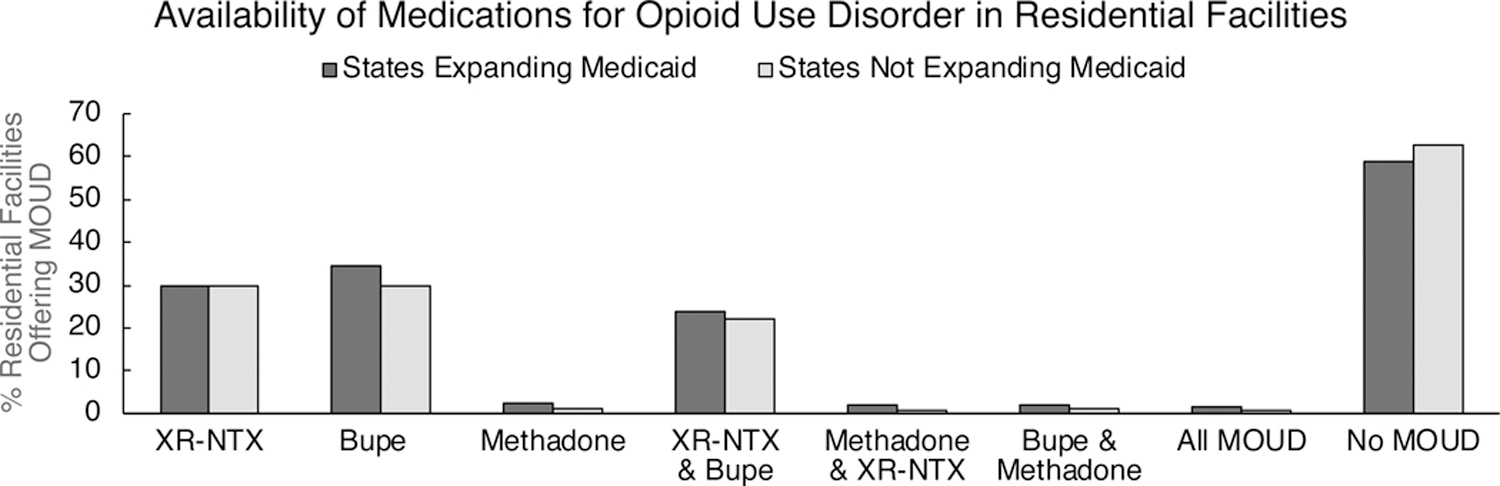
Availability of various medications for opioid use disorder (MOUD) and combinations of MOUD in residential treatment facilities in states that did or did not expand Medicaid by 2017. Facility-level data were collected from the National Survey of Substance Abuse Treatment Services. No meaningful group differences were observed. XR-NTX = extended-release naltrexone; Bupe = buprenorphine; MOUD = medication for opioid use disorder.
The relationship between state-level Medicaid policy and MOUD utilization among residential facility admissions
Of the total admissions reported to TEDS-A in 2017 (N=2,005,395), only admissions that identified opioids as their primary drug of choice (defined as heroin, prescription/synthetic opioids, or diverted methadone) at residential treatment facilities, and within the 50 states or District of Columbia were included in these analyses (n=232,414). Treatment admissions were predominantly male (66.6%), white (73.9%), and between the ages of 25–54 (80.8%). In 2017, only 14.3% of OUD admissions had any MOUD as part of their treatment plan. Patients admitted to residential facilities had higher odds of planned use of MOUD in states that did (17.7%) versus did not (1.9%) expand Medicaid by 2017 (OR=10.91, 95% CI, 10.15–11.73, Wald X2 = 4226.32, p<0.001). A sub-analysis was performed within states that reported whether or not they had prescribing restrictions for Medicaid reimbursement of buprenorphine or XR-NTX (n=158,675). In states that expanded Medicaid, treatment admissions had lower odds of MOUD utilization in states with prescribing restrictions (9.4%) versus those without prescribing restrictions (18.9%) (OR=0.44, 95% CI, 0.43–0.46, Wald X2 = 1456.86, p<0.001; Figure 3). The same pattern was true in states that did not expand Medicaid, where treatment admissions had lower odds of MOUD utilization in states with prescribing restrictions (1.7%) versus those without prescribing restrictions (8.1%) (OR=0.20, 95% CI, 0.17–0.23, Wald X2 = 394.52, p<0.001; Figure 3).
Figure 3.
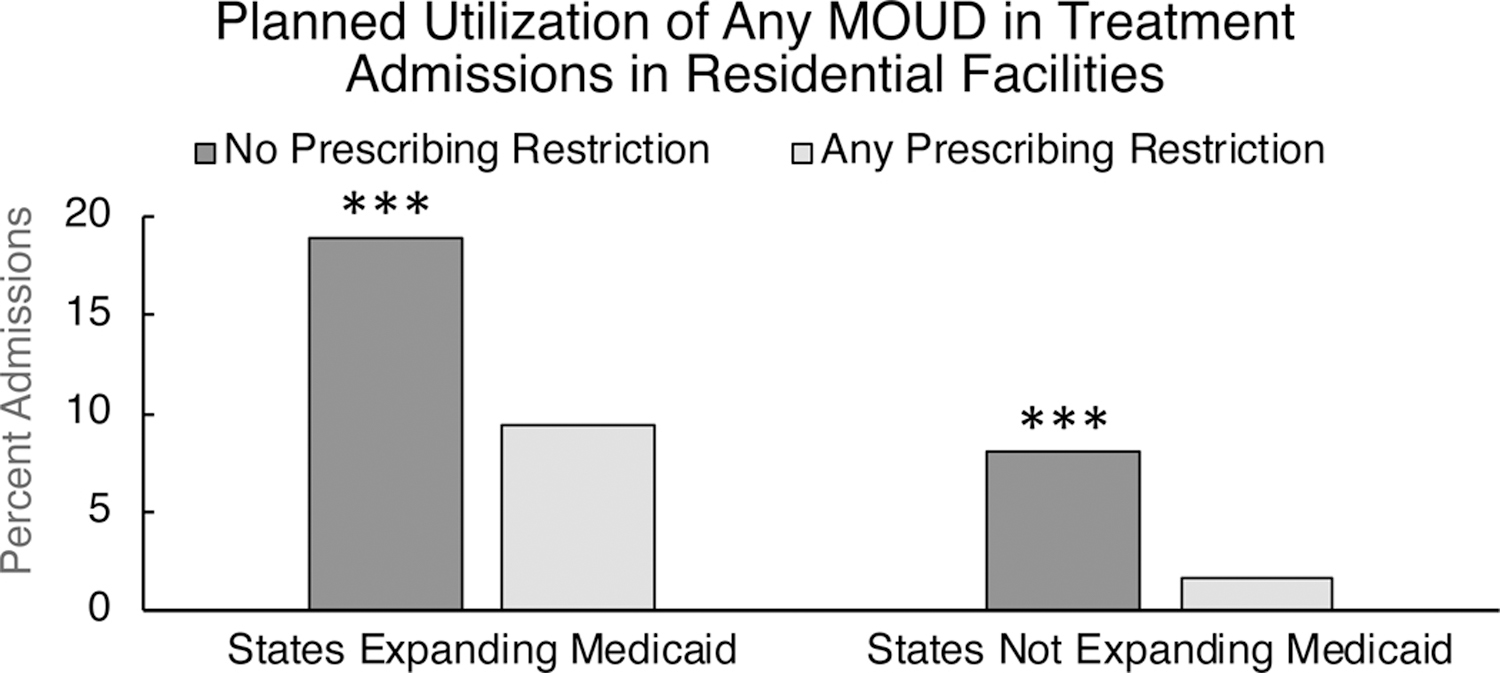
Utilization of any medication for opioid use disorder (MOUD) among opioid use disorder patients admitted to residential facilities in 2017. Results are shown for states that did or did not have any MOUD prescribing restrictions related to Medicaid reimbursement for buprenorphine or extended-release naltrexone, within states that did or did not expand Medicaid by 2017. Treatment admission-level data were collected from the Treatment Episode Data Set-Admissions. *** = p<0.001.
Sensitivity analyses
First-time OUD treatment admissions (n=48,164) followed a similar pattern as the larger population, as patients had higher odds of MOUD utilization in states that did (15.2%) versus did not (1.5%) expand Medicaid (OR=11.64, 95% CI, 10.34–13.10, Wald X2 = 1656.84, p<0.001). Propensity score matching resulted in a qualitatively and quantitatively similar results with higher odds of MOUD utilization in states that expanded Medicaid (OR=13.73, 95% CI, 12.66–14.90, Wald X2 = 3992.1, p<0.001). Prescribing restrictions also consistently resulted in reduced odds of MOUD utilization in the propensity score matched data set for both states expanding Medicaid (OR=0.43, 95% CI, 0.38–0.49, Wald X2 = 167.39, p<0.001) and not expanding Medicaid (OR=0.18, 95% CI, 0.15–0.21, Wald X2 = 389.6, p<0.001).
Facility-level factors associated with MOUD availability
Residential facilities that offered No MOUD had lower odds than those that offered ≥1 MOUD to also offer psychiatric medications, be accredited by a health organization or be licensed by a state or hospital authority, or to accept private insurance, but had higher odds to be a cash-only facility (Table 1). Residential facilities that offered XR-NTX had higher odds than those that did not to also offer buprenorphine, methadone, psychiatric medications, and be accredited by a health organization (Supplemental Table 2). Residential facilities that offered buprenorphine had higher odds than those that did not to also offer XR-NTX, methadone, psychiatric medications, to be licensed by a hospital or state authority, but lower odds of offering residential treatment >28 days (Supplemental Table 3).
Patient-level factors associated with MOUD utilization
OUD patients being admitted to residential facilities had lower odds of utilizing MOUD as part of their treatment plan if they were male, black or African American, or referred from the criminal justice system, and higher odds if they were 55 and older (Table 2).
Relationship between MOUD availability, utilization, and opioid-overdose mortality
In a sub-analysis of residential facilities (n=2,276), the odds offering any MOUD was higher in states with higher opioid overdose fatalities per 1000 residents in 2017 (OR=1.010, 95% CI, 1.002–1.019, Wald X2 = 5.55, p=0.010). Conversely, in a sub-analysis of treatment admissions to residential facilities (n=197,655), the odds of planned MOUD use were lower in states with higher opioid overdose fatalities per 1000 residents in 2017 (OR=0.983, 95% CI, 0.981–0.984, Wald X2 = 550.32, p<0.001).
Discussion
The majority (60.0%) of residential facilities in the U.S. did not offer any FDA-approved MOUD in 2017 (Figure 2). While states that did and did not expand Medicaid under the ACA did not differ significantly in the availability of MOUD in residential facilities, OUD patients being admitted to residential facilities were far more likely to have MOUD as part of their treatment plan in states that expanded Medicaid (17.7%) versus those that did not (1.9%) (Figure 3). Within states that did or did not expand Medicaid, MOUD prescriber restrictions for Medicaid reimbursement were further associated with MOUD utilization, such that MOUD utilization was highest in states that expanded Medicaid and had no prescriber restrictions, and lowest in states that did not expand Medicaid and had any prescriber restrictions. Importantly, none of the sub-groupings of states reported in these analyses had MOUD utilization in greater than 20% of treatment admissions, suggesting that the vast majority of OUD patients admitted to residential facilities, which are expected to offer a high level of care, did not receive the gold-standard for OUD treatment.
These findings have clear implications for public health officials regarding the effects of state-level Medicaid policy on the use of MOUD in residential facilities. By the end of 2017, Medicaid covered buprenorphine and XR-NTX - but not methadone - in all 50 states, yet there continue to be many nuances in MOUD coverage within states that might further complicate access to treatment44. Prescriber restrictions include prior authorization to prescribe buprenorphine or XR-NTX, the requirement that buprenorphine to be distributed by an opioid treatment program, or lifetime limits on doses of buprenorphine >8mg43. In addition, American Society for Addiction Medicine (ASAM) criteria plays a large role in assigning levels of care to OUD patients and determining reimbursement for OUD treatment providers45. Indeed, the assigned level of care, which is often determined by insurance coverage, is not always in concert with what the patient and/or clinician believes is appropriate for treatment. The ACA and Mental Health Parity and Addiction Equity Act have aimed to increase access to care, especially in vulnerable populations46, yet stave-level restrictions on Medicaid, which also affect private insurance coverage47, continue to restrict the treatment community’s response to the opioid crisis.
The results of the facility-level analyses indicate that there is a relationship between licensing and accreditation of residential treatment facilities and MOUD availability. For instance, facilities that did not offer any MOUD compared with those that offered ≥1 MOUD had lower odds of being licensed by a hospital or state authority and/or accredited by a health organization, and facilities that offered extended-release naltrexone or buprenorphine had higher odds of being licensed by a hospital or state authority and/or accredited by a health organization. Collectively, these data suggest that policy efforts focused on increasing MOUD availability in residential facilities through the licensing/accreditation process could quickly improve care for persons with OUD. Persons who are misusing opioids and are not yet in treatment have reported positive views of both residential facilities and MOUD19, and public health efforts to improve adoption of MOUD in residential facilities might increase the number of persons with OUD who are initiated into long-term, meaningful recovery.
Both facility-level and treatment admission-level data suggest that MOUD availability and utilization may be lacking for vulnerable populations, as residential facilities that did not offer any MOUD compared the those that offered ≥1 MOUD option had higher odds of accepting cash-only payments (Table 1), and treatment admissions who were black/African American and referred from the criminal justice system had lower odds of receiving MOUD as part of their treatment plan. This is concerning given recent evidence suggesting these groups derive substantial benefits from MOUD48,49. Policy efforts that remove barriers to MOUD prescribing and require availability of MOUD for Medicaid recipients could be used to increase the utilization of these efficacious treatments. In addition, sex/gender and age are important factors to consider in MOUD induction and maintenance50–52. Harnessing the expertise of medical (physicians, nurses), behavioral (psychologists, counselors, social workers), and peer recovery specialists (e.g. persons in recovery) to work in concert across the continuum of residential and outpatient care could better address the complex issues that arise in early recovery and improve the long-term trajectory of persons with OUD.
A previous study on availability of MOUD in outpatient facilities reported a relationship between increased MOUD availability and increased opioid overdose mortality33; the current study extends this finding, as residential facilities had higher odds of offering any MOUD in states reporting higher rates of opioid overdose mortality. At the same time, the utilization of MOUD in treatment admissions at residential facilities was lower in states with higher opioid overdose mortality. The opioid overdose epidemic continues to devastate Americans from all walks of life, especially in geographic areas where poverty is rampant and heroin/fentanyl is widely available53–55, and macro-level initiatives will be necessary to deliver care to these highly impacted communities. The current study provides evidence that MOUD availability/utilization is uncommon within most residential SUD facilities, similar to reports from outpatient SUD facilities33. Residential facilities are expected to offer a transient yet very high level of care, and their model of direct patient supervision could provide an ideal opportunity for persons with OUD to be inducted onto MOUD before transitioning to outpatient care33,34,56.
Limitations
This study has several limitations. The N-SSATS only reported data on residential facilities reporting to SAMHSA, although it is the largest database of U.S. residential SUD facilities. It is unknown whether facilities reporting to the N-SSATS refer patients to off-site medical professionals for MOUD prescribing, but the planned use of MOUD for treatment (via the TEDS-A) suggests that the vast majority of patients in residential treatment did not receive MOUD. The TEDS-A reports on planned use of any MOUD (as a single binary variable) for treatment admissions, and persons with OUD could account for more than one admission per year. This study used all OUD admissions to gain insight on total planned MOUD utilization in residential facilities. In addition, there are nuances within state-level Medicaid coverage of MOUD that are beyond the scope of this manuscript; future studies could examine state-level barriers to specific MOUD options to further elucidate the effect of policy on medical treatment of OUD. There are also philosophical differences in MOUD prescribing that are not captured by the data sources used in this study, but should be examined more thoroughly via clinician surveys and focus groups.
Conclusions
This study reports that the majority of residential treatment facilities in the U.S. did not offer MOUD in 2017, and that the utilization of MOUD in residential settings is especially lacking in states that have been resistant to Medicaid expansion under the ACA, and in states with MOUD prescribing restrictions for Medicaid reimbursement. There are several factors that might prevent residential facilities from offering one or more MOUD, including legal and regulatory barriers associated with buprenorphine or methadone, variability in insurance reimbursement, and the lack of integration between paraprofessional and clinicians. Given that the U.S. is in the midst of a deadly and protracted opioid crisis, public health efforts should focus on bridging the gap between residential treatment facilities with deep knowledge of behavioral interventions and medical professionals who can provide MOUD to improve the trajectory of long-term recovery for individuals with OUD. The data presented here suggest that Medicaid expansion and relaxation of MOUD prescribing restrictions for Medicaid reimbursement could improve MOUD availability and utilization in residential facilities.
Supplementary Material
Key Points.
Question:
Are residential addiction treatment facilities in the U.S. utilizing medications for opioid use disorder (MOUD)?
Findings:
This cross-sectional study used national databases to demonstrate that availability and utilization of MOUD was relatively low in residential addiction treatment facilities. Residential facilities in states that resisted Medicaid expansion and/or had prescriber restrictions for Medicaid reimbursement had particularly poor utilization of MOUD.
Meaning:
While residential treatment facilities may offer a high level of behavioral treatment in a structured environment, access to MOUD in these facilities is lacking.
Acknowledgements
The authors would like to thank Kevin Psoter, PhD at the Johns Hopkins Biostatistics, Epidemiology, and Data Management core (BEAD-core) for his thoughtful advise regarding the data anayles presented in this study.
Conflict of Interest Statement: The work described in this manuscript was funded by the National Institute on Drug Abuse: NIDA UG3DA048734 (Huhn). Support for ASH, AU, and KED was provided by UG3DA048734. Support for JGH and GAO was provided by Ashley Addiction Treatment. Support for CLB and JCS was provided by NIDA T32DA07209.
Footnotes
Financial Disclosures: ASH receives research funding from Ashley Addiction Treatment through his university. In the past 3 years, KD has served as a consultant for Beckley Canopy Therapeutics and Grünenthal. All other authors report no financial disclosures.
References
- 1.Collins FS, Koroshetz WJ, Volkow ND. Helping to end addiction over the long-term: The research plan for the NIH HEAL initiative. JAMA. 2018. [DOI] [PMC free article] [PubMed]
- 2.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–2066. [DOI] [PubMed] [Google Scholar]
- 3.Wenzel SL, Burnam MA, Koegel P, et al. Access to inpatient or residential substance abuse treatment among homeless adults with alcohol or other drug use disorders. Med Care. 2001:1158–1169. [DOI] [PubMed]
- 4.Mojtabai R, Graff Zivin J. Effectiveness and cost-effectiveness of four treatment modalities for substance disorders: A propensity score analysis. Health Serv Res. 2003;38(1p1):233–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gossop M, Stewart D, Browne N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: Protective effect of coping responses. Addiction. 2002;97(10):1259–1267. [DOI] [PubMed] [Google Scholar]
- 6.Gossop M, Stewart D, Marsden J. Attendance at narcotics anonymous and alcoholics anonymous meetings, frequency of attendance and substance use outcomes after residential treatment for drug dependence: A 5-year follow-up study. Addiction. 2008;103(1):119–125. [DOI] [PubMed] [Google Scholar]
- 7.Drake RE, O’Neal EL, Wallach MA. A systematic review of psychosocial research on psychosocial interventions for people with co-occurring severe mental and substance use disorders. J Subst Abuse Treat. 2008;34(1):123–138. [DOI] [PubMed] [Google Scholar]
- 8.White WL. Slaying the dragon: The history of addiction treatment and recovery in America. Chestnut Health Systems/Lighthouse Institute; Bloomington, IL; 1998. [Google Scholar]
- 9.Aiken LS, Losciuto LA, Ann Ausetts M, Brown BS. Paraprofessional versus professional drug counselors: The progress of clients in treatment. Int J Addict. 1984;19(4):383–401. [DOI] [PubMed] [Google Scholar]
- 10.Bunn TL, Quesinberry D, Jennings T, et al. Timely linkage of individuals to substance use disorder treatment: Development, implementation, and evaluation of FindHelpNowKY. org. BMC Public Health. 2019;19(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooklyn JR, Sigmon SC. Vermont hub-and-spoke model of care for opioid use disorder: Development, implementation, and impact. J Addict Med. 2017;11(4):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu L, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the united states. Drug Alcohol Depend. 2016;169:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: A randomized controlled trial. JAMA. 2000;283(10):1303–1310. [DOI] [PubMed] [Google Scholar]
- 15.Ling W, Wesson DR. Clinical efficacy of buprenorphine: Comparisons to methadone and placebo. Drug Alcohol Depend. 2003;70(2):S57. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal RN, Lofwall MR, Kim S, Chen M, Beebe KL, Vocci FJ. Effect of buprenorphine implants on illicit opioid use among abstinent adults with opioid dependence treated with sublingual buprenorphine: A randomized clinical trial. JAMA. 2016;316(3):282–290. [DOI] [PubMed] [Google Scholar]
- 17.Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2019;393(10173):778–790. [DOI] [PubMed] [Google Scholar]
- 18.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: A double-blind, placebo-controlled, multicentre randomised trial. The Lancet. 2011;377(9776):1506–1513. [DOI] [PubMed] [Google Scholar]
- 19.Huhn AS, Tompkins DA, Dunn KE. The relationship between treatment accessibility and preference amongst out-of-treatment individuals who engage in non-medical prescription opioid use. Drug Alcohol Depend. 2017;180:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heit HA, Covington E, Good PM. Dear DEA. Pain Medicine. 2004;5(3):303–308. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham CO, Kunins HV, Roose RJ, Elam RT, Sohler NL. Barriers to obtaining waivers to prescribe buprenorphine for opioid addiction treatment among HIV physicians. J Gen Intern Med. 2007;22(9):1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: A randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017:appi. ajp. 2016.16050548. [DOI] [PMC free article] [PubMed]
- 23.Olsen Y, Sharfstein JM. Confronting the stigma of opioid use disorder—and its treatment. JAMA. 2014;311(14):1393–1394. [DOI] [PubMed] [Google Scholar]
- 24.Huhn AS, Dunn KE. Why aren’t physicians prescribing more buprenorphine? J Subst Abuse Treat. 2017;78:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the department of veterans affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dick AW, Pacula RL, Gordon AJ, et al. Growth in buprenorphine waivers for physicians increased potential access to opioid agonist treatment, 2002–11. Health Aff. 2015;34(6):1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saloner B, Karthikeyan S. Changes in substance abuse treatment use among individuals with opioid use disorders in the united states, 2004–2013. JAMA. 2015;314(14):1515–1517. [DOI] [PubMed] [Google Scholar]
- 28.Wakeman SE, Rich JD. Barriers to medications for addiction treatment: How stigma kills. Subst Use Misuse. 2018;53(2):330–333. [DOI] [PubMed] [Google Scholar]
- 29.Knudsen HK, Lofwall MR, Havens JR, Walsh SL. States’ implementation of the affordable care act and the supply of physicians waivered to prescribe buprenorphine for opioid dependence. Drug Alcohol Depend. 2015;157:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grogan CM, Andrews C, Abraham A, et al. Survey highlights differences in medicaid coverage for substance use treatment and opioid use disorder medications. Health Aff. 2016;35(12):2289–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham AJ, Andrews CM, Yingling ME, Shannon J. Geographic disparities in availability of opioid use disorder treatment for medicaid enrollees. Health Serv Res. 2018;53(1):389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knudsen HK. The supply of physicians waivered to prescribe buprenorphine for opioid use disorders in the united states: A state-level analysis. J Stud Alcohol Drugs. 2015;76(4):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mojtabai R, Mauro C, Wall MM, Barry CL, Olfson M. Medication treatment for opioid use disorders in substance use treatment facilities. Health Aff. 2019;38(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galanter M, Seppala M, Klein A. Medication-assisted treatment for opioid dependence in Twelve Step–oriented residential rehabilitation settings. Subst Abus. 2016;37(3):381–383. [DOI] [PubMed] [Google Scholar]
- 35.Majer JM, Beasley C, Stecker E, et al. Oxford house residents’ attitudes toward medication assisted treatment use in fellow residents. Community Ment Health J. 2018;54(5):571–577. [DOI] [PubMed] [Google Scholar]
- 36.Brunette MF, Mueser KT, Drake RE. A review of research on residential programs for people with severe mental illness and co-occurring substance use disorders. Drug Alcohol Rev. 2004;23(4):471–481. [DOI] [PubMed] [Google Scholar]
- 37.Hay KR, Huhn AS, Tompkins DA, Dunn KE. Recovery goals and long-term treatment preference in persons who engage in nonmedical opioid use. J Addict Med. 2019; doi: 10.1097/ADM.0000000000000498. [DOI] [PMC free article] [PubMed]
- 38.Lurie J. Mom, when they look at me, they see dollar signs. Mother Jones. 2019. Retrieved from: https://www.motherjones.com/crime-justice/2019/02/opioid-epidemic-rehab-recruiters/.
- 39.Substance Abuse and Mental Health Services Administration. National survey of substance abuse treatment services (N-SSATS): 2017 data on substance abuse treatment facilities. https://www.samhsa.gov/data/report/national-survey-substance-abuse-treatment-services-n-ssats-2017-data-substance-abuse. Updated 2018. Accessed January 10th, 2019.
- 40.Center for Behavioral Health Statistics and Quality. Treatment episode data set - admissions. https://www.datafiles.samhsa.gov/study-series/treatment-episode-data-set-admissions-teds-nid13518. Updated 2018. Accessed December, 15th, 2018.
- 41.Centers for Disease Contrl. Wide-ranging online data for epidemiological research. CDC Wonder Web site. https://wonder.cdc.gov/. Updated 2019. Accessed July 10th, 2019.
- 42.National Institute on Drug Abuse. Opioid summaries by state. NIDA Advancing Addiction Science Web site. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-summaries-by-state. Updated 2019. Accessed July 7th, 2019.
- 43.Kaiser Family Foundation. Medicaid’s role in addressing the opioid epidemic. Henry J. Kaiser Family Foundation Web site. https://www.kff.org/infographic/medicaids-role-in-addressing-opioid-epidemic/. Updated 2019. Accessed July 10th, 2019.
- 44.Clemans-Cope L, Lynch V, Epstein M, Kenney GM. Medicaid coverage of effective treatment for opioid use disorder. Urban Institute. Retrieved from: https://www.urban.org/sites/default/files/publication/90461/2001287_medicaid_coverage_of_effective_treatment_for_opioid_use_disorder.pdf. Updated 2017. Accessed January 10th, 2019. [Google Scholar]
- 45.Thomas CP, Ritter GA, Harris AH, Garnick DW, Freedman KI, Herbert B. Applying american society of addiction medicine performance measures in commercial health insurance and services data. J Addict Med. 2018;12(4):287–294. [DOI] [PubMed] [Google Scholar]
- 46.Abraham AJ, Andrews CM, Grogan CM, et al. The Affordable Care Act transformation of substance use disorder treatment. Am J Public Health. 2017;107(1):31–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molfenter T, McCarty D, Jacobson N, Kim J, Starr S, Zehner M. The payer’s role in addressing the opioid epidemic: It’s more than money. J Subst Abuse Treat. 2019;101:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark RE, Samnaliev M, Baxter JD, Leung GY. The evidence doesn’t justify steps by state medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Aff. 2011;30(8):1425–1433. [DOI] [PubMed] [Google Scholar]
- 49.Chandler RK, Fletcher BW, Volkow ND. Treating drug abuse and addiction in the criminal justice system: Improving public health and safety. JAMA. 2009;301(2):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huhn AS, Strain EC, Tompkins DA, Dunn KE. A hidden aspect of the US opioid crisis: Rise in first-time treatment admissions for older adults with opioid use disorder. Drug Alcohol Depend. 2018;193:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carew AM, Comiskey C. Treatment for opioid use and outcomes in older adults: A systematic literature review. Drug Alcohol Depend. 2018;182:48–57. [DOI] [PubMed] [Google Scholar]
- 52.Huhn AS, Berry MS, Dunn KE. Sex-Based differences in treatment outcomes for persons with opioid use disorder. Am J Addict. 2019;28(4):246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monnat SM. Factors associated with county-level differences in US drug-related mortality rates. Am J Prev Med. 2018;54(5):611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruhm CJ. Geographic variation in opioid and heroin involved drug poisoning mortality rates. Am J Prev Med. 2017;53(6):745–753. [DOI] [PubMed] [Google Scholar]
- 55.Ruhm CJ. Drug mortality and lost life years among US midlife adults, 1999–2015. Am J Prev Med. 2018;55(1):11–18. [DOI] [PubMed] [Google Scholar]
- 56.Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: The impact of buprenorphine treatment. J Urban Health. 2010;87(4):592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


