Abstract
Parasympathetic nervous system functioning as indexed by respiratory sinus arrhythmia (RSA) is widely used as a measure of physiological regulation. We examined developmental patterns of children’s resting RSA and RSA reactivity from 2 to 15 years of age, a period of time that is marked by considerable advances in children’s regulatory abilities. Physiological data were collected from a community sample of 270 children (116 males) during a resting period and during a frustration laboratory task when the children were 2, 4, 5, 7, 10, and 15 years old. We examined both stability and continuity in resting RSA and RSA reactivity across time. We found stability in resting RSA but not RSA reactivity from toddlerhood to adolescence. Separate multilevel models were used to examine changes in resting RSA and RSA reactivity from Age 2 to Age 15. The rate of change in resting RSA slowed from Age 2 to Age 15 with a plateau around Age 10. A splined growth model indicated that the rate of RSA reactivity increased from Age 2 to Age 7 and a modest slowing and leveling off from Age 7 to Age 15. Understanding the developmental characteristics of RSA across childhood and adolescence is important to understanding the larger constructs of self- and emotion regulation.
Keywords: respiratory sinus arrhythmia, resting RSA, RSA withdrawal, self-regulation
Heart rate variability (HRV), or oscillations in heart rate across respiratory cycles (Zisner & Beauchaine, 2016), is the focus of much developmental work given its link to many adjustment outcomes in childhood and adolescence, including social competence (Eisenberg et al., 2008), behavioral regulation (Hastings et al., 2008), executive function (Marcovitch et al., 2010), and attention (Perry, Calkins, & Bell, 2016). When assessing HRV, researchers typically focus on respiratory sinus arrythmia (RSA), or the component of HRV that occurs at the frequency of spontaneous respiration. RSA is thought to emanate from the parasympathetic nervous system (PNS), with central nervous system input from the brain stem (Porges, 1995, 1996). Specifically, inhibitory PNS efference from the brain stem slows heart rate while exhaling, and PNS withdrawal accelerates heart rate while inhaling (Shader et al., 2018). Thus, dynamic PNS influences on cardiac activity via the central nervous system facilitate an individual’s ability to quickly and flexibility respond to changing environmental demands.
RSA is regularly measured in the study of emotion regulation (ER; Beauchaine, Gatzke-Kopp, & Mead, 2007; Calkins, Dollar, & Wideman, 2019). ER refers to a set of processes that allow individuals to modulate their emotional arousal, experience, and expression in adaptive ways (Calkins & Hill, 2007). Although early theories of ER suggested that the PNS is self-regulatory and directly underlies emotional control (e.g., Porges, 1996), more recent models suggest that brain stem pathways implicated in past theories serve as pathways from the prefrontal cortex to the PNS but are not directly responsible for ER (Beauchaine, 2015; Thayer & Lane, 2000). Thus, although RSA is not a direct measure of ER, both are believed to be strongly influenced by top-down prefrontal control, suggesting that RSA during emotion-eliciting contexts can serve as a peripheral index of ER (Shader et al., 2018; Thayer & Lane, 2000).
Measures of RSA
Developmental scientists have focused on resting RSA and RSA reactivity in response to emotional challenge. RSA measured while an individual is at rest is often considered a stable neurophysiological mechanism that reflects an individual’s capacity to flexibly engage with the environment (Calkins, 1997; Propper & Moore, 2006). In general, low resting RSA is associated with emotion dysregulation and greater psychopathology (Beauchaine, Katkin, Strassberg, & Snarr, 2001; Hinnant & El-Sheikh, 2009).
The links between RSA reactivity and adjustment are somewhat less clear. The majority of work supports a positive association between decreases in RSA (or withdrawal) to challenge and adaptive ER in the moment and over time (Buss, Goldsmith, & Davidson, 2005; Perry et al., 2016). Greater withdrawal has been linked with fewer externalizing behaviors, which are characterized by emotion dysregulation (El-Sheikh, Harger, & Whitson, 2001; Graziano, Keane, & Calkins, 2007). In one early study, Calkins and Dedmon (2000) found that 2-year-old children at high risk for externalizing behaviors showed a smaller decrease in RSA in response to emotional challenge. These children also displayed significantly more dysregulated behavior (negative affect and venting) than did low-risk children. Perry and colleagues (2014) found that the magnitude of physiological regulation at Age 3 predicted decreases in externalizing over time, suggesting that early physiological regulation may support normative declines in emotion dysregulation. Similarly, greater decreases in RSA have been linked to fewer internalizing difficulties (Gentzler, Santucci, Kovacs, & Fox, 2009). Thus, there is good evidence that RSA withdrawal in emotionally challenging situations may index better ER. It should be noted, however, that some work has found that large decreases in RSA are associated with internalizing and externalizing problems (Beauchaine, 2015; Fortunato, Gatzke-Kopp, & Ram, 2013).
The Development of RSA
Although there has been considerable work focusing on the behavioral correlates of resting RSA and RSA reactivity (Brooker & Buss, 2010; Calkins & Dedmon, 2000), there has been less work on its development. From a normative developmental perspective, one could consider change in RSA over time as occurring as a function of (a) stability versus instability in the rank ordering of individuals (i.e., individual differences), or (b) change in the magnitude of RSA that occurs at the mean level for a group of individuals, a phenomenon sometimes referred to as continuity versus discontinuity (Bornstein & Suess, 2000).
With respect to individual differences, stability implies that children who have high resting RSA or show greater RSA change, relative to peers, are likely to continue to show these patterns over time. Studies addressing RSA stability find that individual differences in the rank order of children’s resting RSA appear to be fairly stable through childhood and early adolescence. In fact, resting RSA is moderately stable (rs = .30 to .55) from infancy through Age 5 (Alkon, Boyce, Davis, & Eskenazi, 2011; Bar-Haim, Marshall, & Fox, 2000; Bornstein & Suess, 2000; Porges, Doussard-Roosevelt, Portales, & Suess, 1994), 4.5 to 7 years of age (Marshall & Stevenson-Hinde, 1998), Ages 8 through 11 (El-Sheikh, 2005; Hinnant, Elmore-Staton, & El-Sheikh, 2011), and into early adolescence (Pang & Beauchaine, 2013). The implication of this research is that individual differences in resting RSA become entrenched early in life, which is consistent with the perspective that resting RSA is a stable neurophysiological mechanism.
In contrast, individual differences in children’s RSA reactivity do not appear to be stable across early life. There is evidence of low stability of RSA reactivity from infancy through the preschool years (Bornstein & Suess, 2000), and children’s average RSA reactivity, based on multiple types of challenging tasks (social, cognitive, physical, and emotional), was not stable from 6 to 60 months of age (Alkon et al., 2011). Doussard-Roosevelt, Montgomery, and Porges (2003) found that 5- and 6-year-old children’s RSA reactivity during a negative affect task across three 2-week intervals was unstable across assessments. Moreover, low or non-significant correlations in RSA reactivity across a 3-year period have been found for both children and adolescents (Pang & Beauchaine, 2013; Salomon, 2005).
In terms of developmental change, we might expect increases in resting RSA, and perhaps reactivity during emotional contexts, as a function of physical and neural maturation and exposure to normative experiences that affect the development of ER. Increases in resting RSA have been observed from 2 months to 5 years of age (Alkon et al., 2011; Bornstein & Suess, 2000; Porges et al., 1994) and from 4.5 to 7 years of age (Marshall & Stevenson-Hinde, 1998). Moreover, the developmental pattern characterized by increasing resting RSA may be influenced by other factors. For example, 8- to 12-year-olds with increasing resting RSA across a 2-year period were better at regulating their emotions compared with children with consistent levels of resting RSA (Vasilev, Crowell, Beauchaine, Mead, & Gatzke-Kopp, 2009). These studies provide evidence of developmental growth in RSA from infancy to early adolescence.
There is less research examining RSA reactivity across time, and the findings from these studies have been mixed. Bornstein and Suess (2000) found that at 2 months and 5 years of age, on average, children had similar levels of RSA reactivity. However, in another study, children’s average RSA reactivity increased from 6 to 60 months of age (Alkon et al., 2011). Two more recent studies suggest modest growth in RSA reactivity across early childhood (Gatzke-Kopp & Ram, 2018) and middle childhood to adolescence (Hinnant, Philbrook, Erath, & El-Sheikh, 2018). Cross-sectional studies suggest that RSA reactivity is actually greater in younger children relative to older children (Alkon et al., 2003; El-Sheikh, 2005). The diversity in the patterns of change across studies alludes to potential individual differences in mean levels of RSA reactivity over time. Importantly, these distinct patterns may be differentially associated with children’s adjustment, particularly adjustment difficulties associated with poorer ER.
In sum, limited existing research suggests that resting RSA is both stable and discontinuous, whereas the evidence for stability and continuity for RSA reactivity is less clear. However, no study has examined the same children from toddlerhood to adolescence so that both individual differences and development more broadly across developmental periods can be considered. Here, we fill this gap by examining the same children at six time points from Age 2 to Age 15.
Conceptual Challenges to Studying RSA Development
Examining developmental change in RSA and RSA reactivity across such a large span of development poses numerous conceptual and analytical challenges (see Hinnant et al., 2018, for a full discussion). Here, we address two of these, one having to do with the type of challenge used to elicit physiological responses across developmental periods and the second with the type of analytical model employed to examine change over a long period of time.
Although some researchers view RSA reactivity as an index of stress reactivity (Hinnant et al., 2018), and others view it as an indicator of more general vulnerabilities (Beauchaine & Thayer, 2015; Gatzke-Kopp & Ram, 2018), much of the literature linking RSA to behavioral processes has focused on associations with ER (Beauchaine, 2015; Buss et al., 2005; Perry et al., 2016). Thus, we adopted an approach similar to many of our colleagues (Fortunato et al., 2013; Pang & Beauchaine, 2013) and chose to focus on tasks that were emotionally challenging to the child. Given the known linkages between poor ER and externalizing behaviors, we examined children’s RSA in response to frustration. Although the nature of these tasks changed as a result of children’s age and skill level, with cognitive frustration being most effective in eliciting frustration from children at older ages, all tasks were designed to elicit frustration.
A second and related issue concerns the proper selection of the most appropriate growth model one might anticipate given that RSA in an emotion eliciting context is thought to be one index of ER. ER develops as a function of motor, language, and cognitive development, but the developmental trajectory is likely not linear across different developmental periods. Here, we assume that there is a period of growth in ER from early childhood through the transition to school, with the experience and demands of formal education supporting greater growth. Then, we expect that there will be a slowing of growth in ER and with only modest growth, on average, from middle childhood into adolescence (Calkins & Perry, 2016; Hinnant et al., 2018). Because measures of RSA that index ER may take a similar developmental trajectory, we expect an average quadratic pattern of change in RSA from toddlerhood to middle childhood and a modest linear pattern of RSA from middle childhood to adolescence.
Prior work has not addressed three developmental periods in one model (Hinnant et al., 2018) or has addressed only one developmental period (Gatzke-Kopp & Ram, 2018). Therefore, the patterns of resting RSA and RSA reactivity across developmental periods is unknown. Here, we address this gap by considering the developmental patterns of resting RSA and RSA reactivity from toddlerhood to middle adolescence in one model with a continuous growth trajectory. We use a stepwise progression of models, including splined growth modeling, which can account for a wide range of variability in some developmental processes. Notably, we also used age as a continuous variable to obtain a higher resolution into exactly how resting RSA and RSA reactivity develops. Finally, because of the role of resting levels of RSA in predicting, and confounding, RSA reactivity (Calkins, 1997; Hinnant et al., 2018), we considered resting RSA as a time-varying covariate in our models.
Method
Recruitment and Attrition
The current study utilized data from three cohorts of children who are part of an ongoing longitudinal study. The goal for recruitment was to obtain a sample of children who were at risk for developing future externalizing behavior problems and who were representative of the surrounding community in terms of race and socioeconomic status (SES). All cohorts were recruited through child day care centers, the County Health Department, and the local Women, Infants, and Children (WIC) program. Potential participants for Cohorts 1 and 2 were recruited at 2 years of age (Cohort 1, 1994–1996; Cohort 2, 2000–2001) and screened using the Child Behavior Checklist (CBCL 2–3; Achenbach, 1992), completed by the mother, in order to oversample for externalizing behavior problems. Children were identified as being at risk for future externalizing behaviors if they received an externalizing T score of 60 or above. Efforts were made to obtain approximately equal numbers of males and females. This recruitment effort resulted in a total of 307 selected children. Cohort 3 was initially recruited when infants were 6 months of age for their level of frustration, based on laboratory observation and parent report, and were followed through the toddler period (see Calkins, Dedmon, Gill, Lomax, & Johnson, 2002, for more information). Children from Cohort 3 whose mothers completed the CBCL at 2 years of age (n = 140) were then included in the larger study. Of the entire sample (N = 447), 37% of children were identified as being at risk for future externalizing problems at Age 2. There were no significant demographic differences between cohorts with regard to gender, race, or SES.
Of the 447 originally selected participants, six were dropped because they did not participate in any data collection at 2 years old. An additional 12 families participated at recruitment, did not participate at 2 years, but did participate at later years. At 4 years of age, 399 families participated. Families lost to attrition included those who could not be located, moved out of the area, declined participation, or did not respond to phone and letter requests to participate. There were no significant differences between families who did and did not participate at each age in terms of gender, race, 2-year SES, or 2-year externalizing T scores, unless otherwise noted. At Age 5, 365 families participated, including four that did not participate in the 4-year assessment. At 7 years of age, 350 families participated, including 19 that did not participate in the 5-year assessment. Families with lower 2-year SES, t(432) = −2.61, p < .01, were less likely to participate in the 7-year assessment. At Age 10, 357 families participated, including 31 families that did not participate in the 7-year assessment. At Age 15, 327 families participated, including 27 families that did not participate in the 10-year assessment. Boys were less likely to participate in the 15-year assessment, χ2(1, N = 447) = 9.31, p = .002.
Participants
This study included 270 children (57.2% girls) who had RSA data at a minimum of three time points. The sample was 66.0% White, 28% African American, 4% biracial, and 2% identified as “other.” Families were economically diverse based on Hollingshead (1975) scores at the 2-year assessment, with a range from 14 to 66 (M = 40.2, SD = 10.8). Hollingshead scores that range from 40 to 54 reflect minor professional and technical occupations considered to be representative of the middle class.
Procedure
Children and their mothers participated in an ongoing longitudinal study when children were 2, 4, 5, 7, 10, and 15 years of age. Measures of resting RSA and RSA reactivity at each time point were utilized. At each assessment they participated in a laboratory visit that included several situations designed to elicit physiological, emotional, and behavioral responding (partially derived from the Laboratory Temperament Assessment Battery; Goldsmith & Rothbart, 1993). Mothers also completed questionnaires regarding the child’s behavior and psychological functioning at each time point. The RIGHT Track study was approved by the Institutional Review Board at The University of North Carolina at Greensboro (IRB Protocol Number 07-0194). Only the tasks relevant for the current study are reported here.
The 2-year assessment included a baseline task in which the child watched a segment of the videotape Spot, a short story about a puppy that explores its neighborhood (5 min), and a frustration task that included either a toy or cookies in a locked box that the child could not retrieve (2 or 3 min, respectively). The 4-year assessment included the same baseline task and a frustration task in which the child was instructed to draw perfect circles and then told that the circles were not perfect (3.5 min). The 5-year assessment included the same baseline task and a frustration task in which the experimenter sorted candy for themselves and the child and then took the child’s candy (4 min). The 7-year assessment included a baseline task in which the child sat still without talking (2 min) and a frustration task in which the child was asked to complete a puzzle without being able to see it (5 min; Eisenberg et al., 1996). The 10-year assessment included a baseline task in which the child sat still without talking (2 min) and the same frustration task as completed at the 7-year assessment. The 15-year assessment included a baseline task in which the adolescent sat still without talking (2 min) and a frustration task in which the adolescent completed the Delis-Kaplan Executive Function System (Delis, Kaplan, & Kramer, 2001) Tower task.
Measures
At the beginning of each assessment, the experimenter placed three disposable pediatric electrodes in an inverted triangle pattern on the child’s chest. The electrodes were connected to a preamplifier, the output of which was transmitted to a portable ECG monitor (Ages 2, 4, 5, 7, and 10 [Cohort 1]: VTM-I, Delta Biometrics, Bethesda, MD; Age 10 [Cohorts 2 and 3], Age 15: Biolog 399x, UFI, Morro Bay, CA) for R-wave detection. A data file containing the interbeat intervals (IBIs) for the entire period of collection was transferred to a computer for later artifact editing and analysis. The sampling rate utilized in the current study was 250 Hz, which is adequate when analyzing data from healthy populations (Grossman, van Beek, & Wientjes, 1990; Riniolo & Porges, 1997). To edit the files, the data were scanned for outliers relative to adjacent data, and the outliers were replaced by dividing or summing them so they would be consistent with the surrounding data.
At Ages 2, 4, 5, 7, and 10 (Cohort 1), measures of children’s RSA were obtained by editing IBI files using MXEDIT software (Delta Biometrics, Bethesda, MD). Cardio Batch/Edit software (Brain-Body Center, University of Illinois at Chicago, Chicago, IL) was used to edit files for the 10-year assessment (Cohorts 2 and 3) and the 15-year assessment. Both programs used the Porges-Bohrer method of analyzing IBI data to calculate RSA (Porges & Bohrer, 1990). This method applies an algorithm to the sequential heart period (HP) data that uses a moving 21-point polynomial to detrend periodicities in HP that are slower than RSA. Next, a bandpass filter extracts variance in HP within the frequency band of spontaneous respiration: 0.24 to 1.04 Hz (Ages 2, 4, 5, and 7), 0.12 to 1.00 Hz (Age 10), and 0.12 to 0.40 Hz (Age 15). The natural log of the variance of the bandpassed time series is taken and reported in units of ln (msec)2. RSA was calculated every 30 s and the average across the epochs for each task was used. Missing RSA data were because of children not allowing the experimenter to apply the HR electrodes, equipment failure, or the child pulling on or touching the HR leads, which caused excessive movement artifact affecting greater than 10% of the data in the file, thus not allowing appropriate editing. Children missing HR data did not differ from those with HR data on any of the demographic variables.
For the 2-, 4-, and 5-year assessments, resting RSA was measured while children watched a video. Although this was not a true baseline, it was necessary to limit movement given that young children were unlikely to remain quietly seated for the duration needed. At Ages 7, 10, and 15, children sat quietly. RSA change scores (RSA reactivity) were computed for each task by subtracting the task RSA from the resting RSA (Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996). Positive change scores reflect a decrease in RSA from baseline to the challenge task indicating RSA withdrawal.
Results
Analytic Plan
Multilevel models were used to assess changes in RSA from Ages 2 to 15. Multilevel models are flexible statistical approaches that can handle missed measurement occasions and unequal measurement intervals. For instance, there is no need for all subjects to have been measured at the same ages. This creates a situation in which a greater resolution of the data is possible compared with other approaches in which time points must be grouped together based on some window of time. Full-information maximum likelihood was used to handle missing data. Model fit was assessed by comparing changes in the Akaike information criterion, the Bayesian information criterion, and the log-likelihood ratio. All statistical procedures were performed using R Statistics Version 3.5.0. Multilevel models were performed using the nlme package in R (Pinheiro, Bates, DebRoy, Sarkar, & R Core Team, 2018). Separate multilevel growth models were used to assess changes in resting RSA and RSA reactivity from toddlerhood to adolescence following the necessary progression from linear, quadratic, bilinear spline, and quadratic-linear spline models.
Preliminary Data
Descriptive statistics for study variables are presented in Table 1. There were no significant race differences for RSA reactivity, but significant race differences in resting RSA emerged at Ages 2 (t = 2.84, p < .01), 4 (t = 3.11, p < .01), 5 (t = 3.69, p < .001), and 7 (t = 2.21, p < .05). This finding indicates that African American children had higher resting RSA than White children during early childhood. Thus, race was included as a covariate in the resting RSA models. No significant associations emerged between SES or gender and resting RSA and RSA reactivity, and thus neither gender nor SES were considered further. Bivariate correlations indicated that there were four significant associations between externalizing behaviors and resting RSA and RSA reactivity (r = −0.15 to 0.16). Thus, we examined the effects of externalizing behaviors in the initial resting RSA and RSA reactivity models. Because breathing rates (which are associated with lung volume) change over time and between individuals of the same age (Cook & Hamann, 1961), we also examined the effect of height in each of the unconditional models as a means of controlling for lung size.
Table 1.
Descriptive Statistics
| Variable name | 2 years | 4 years | 5 years | 7 years | 10 years | 15 years |
|---|---|---|---|---|---|---|
| Resting RSA | 5.37 (±1.27) | 5.90 (±1.20) | 6.07 (±1.17) | 6.50 (±1.20) | 7.29 (±1.07) | 6.66 (±1.13) |
| Minimum | 1.10 | 1.05 | 3.28 | 3.01 | 3.66 | 3.53 |
| Maximum | 8.56 | 10.20 | 9.42 | 10.72 | 9.90 | 9.72 |
| White | 5.21** (±1.30) | 5.72** (±1.15) | 5.86*** (±1.16) | 6.33* (±1.16) | 7.30 (±1.10) | 6.57 (±1.16) |
| African American | 5.72 (±1.20) | 6.24 (±1.21) | 6.45 (±1.14) | 6.68 (±1.10) | 7.28 (±0.99) | 6.78 (±1.13) |
| RSA reactivity | 0.58 (±0.75) | 0.29 (±0.62) | 0.26 (±0.73) | 0.77 (±0.77) | 0.80 (±0.81) | 0.65 (±0.83) |
| Minimum | −1.92 | −1.45 | −2.66 | −1.34 | −2.11 | −3.27 |
| Maximum | 3.10 | 2.85 | 2.50 | 3.72 | 3.36 | 2.85 |
| White | 0.51 (±0.73) | 0.33 (±0.66) | 0.22 (±0.78) | 0.82 (±0.74) | 0.85 (±0.76) | 0.68 (±0.86) |
| African American | 0.71 (±0.80) | 0.24 (±0.53) | 0.30 (±0.62) | 0.63 (±0.73) | 0.61 (±0.77) | 0.52 (±0.80) |
| n | 221 | 252 | 239 | 229 | 217 | 189 |
Note. RSA = respiratory sinus arrhythmia. Differences by race.
p < .05.
p < .01.
p < .001.
In the initial resting RSA models, race was included as a covariate, and externalizing behaviors and height were included as time-varying covariates in a stepwise manner. Race accounted for a significant amount of variance and thus was included in the final model. Neither externalizing behaviors nor height accounted for a significant amount of variance and therefore were not included in subsequent models in order to keep models as parsimonious as possible. In the initial RSA reactivity models, resting RSA, externalizing behaviors, and height were included as time-varying covariates in a stepwise manner. Resting RSA accounted for a significant amount of variance explained, but none of the other time-varying covariates added significant benefit to the model and thus were excluded.
Stability of Resting RSA and RSA Reactivity
Bivariate correlation analyses were conducted to assess stability across time in resting RSA and RSA reactivity (see Table 2). As expected, resting RSA showed low to moderate stability from Ages 2 to 15. There was much less evidence for stability in RSA reactivity across developmental periods.
Table 2.
Correlations Among Resting RSA and RSA Reactivity
| Variable name | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 2-year resting RSA | — | |||||||||||
| 2. | 4-year resting RSA | .46*** | — | ||||||||||
| 3. | 5-year resting RSA | .39*** | .56*** | — | |||||||||
| 4. | 7-year resting RSA | .43*** | .51*** | .48*** | — | ||||||||
| 5. | 10-year resting RSA | .26*** | .47*** | .32*** | .44*** | — | |||||||
| 6. | 15-year resting RSA | .19** | .30** | .21** | .41*** | .39*** | — | ||||||
| 7. | 2-year RSA reactivity | .43*** | .14* | .09 | .22* | .07 | .14 | — | |||||
| 8. | 4-year RSA reactivity | −.03 | .27*** | .18** | .01 | .15* | .00 | .12 | — | ||||
| 9. | 5-year RSA reactivity | −.02 | .09 | .39*** | .01 | .08 | −.04 | .04 | .27*** | — | |||
| 10. | 7-year RSA reactivity | .05 | .07 | .12 | .43*** | .16* | .12 | .08 | .17* | .16* | — | ||
| 11. | 10-year RSA reactivity | −.01 | .07 | .12 | .09 | .39*** | −.01 | .14 | .17* | .17* | .30*** | — | |
| 12. | 15-year RSA reactivity | −.12 | −.20* | −.10 | .00 | .00 | .46*** | .10 | .07 | .07 | .14 | .15 | — |
Note. RSA = respiratory sinus arrhythmia.
p < .05.
p < .01.
p < .001.
The intraclass correlation (ICC) of the unconditional models supported these findings. The ICC in the resting RSA model was 0.275 (intercept variance = 0.48, residual variance = 1.25), indicating that approximately 27.5% of the observed variability in resting RSA occurred across subjects (i.e., 72.5% of the variance in resting RSA was related to within-person changes over time, whereas 27.5% of was associated with between-person changes). The ICC for RSA reactivity was 0.124 (intercept variance = 0.07, residual variance = 0.53), indicating that approximately 12.4% of the observed variability in RSA reactivity occurred across subjects (i.e., 87.6% of the variance in RSA reactivity was related to within-person changes over time, whereas 12.4% was associated with between-person differences). These findings indicate that a larger percentage of the variability in resting RSA was because of differences between individuals, whereas changes in RSA reactivity were largely related to changes in RSA reactivity across time at the individual level. Moreover, the ICC denotes a more stable resting RSA compared with RSA reactivity.
Developmental Continuity of Resting RSA
Because existing work suggests a linear increase in resting RSA from early to late childhood, as a preliminary model, we estimated resting RSA from Age 2 to Age 10 before including the 15-year assessment. Each of these models were defined as
where yti is the value of the response variable, y (resting RSA), at the ith measurement for the jth individual; b1i is the random intercept for individual i; b2i is the random slope for individual i; and uti is the time-dependent residual. This model indicated a positive linear increase in resting RSA across childhood (see Table 3).
Table 3.
Model Summaries for Change in Resting RSA From Age 2 to Age 15
| Parameters | Unconditionala | Linearb | Lineara | Quadraticb | Quadratica |
|---|---|---|---|---|---|
| Fixed effects | |||||
| Intercept | 6.128 (0.064)*** | 4.688 (0.010)*** | 5.284 (0.091)*** | 4.85 (0.170)*** | 4.114 (0.132)*** |
| Slopeb | — | 0.019 (0.0009)*** | 0.010 (0.0006)*** | 0.014 (0.004)*** | 0.038 (0.002)*** |
| Accelerationb | — | — | — | 0.00003 (0.00003) | −0.0001 (0.00001)*** |
| Race | 0.370 (0.110)* | 0.363 (0.113)* | 0.329 (0.107)* | 0.363 (0.113)* | 0.327 (0.106)* |
| Random effects | |||||
| Intercept | 0.450 (0.063)*** | 1.122 (0.188)* | 1.05 (0.1557)*** | 1.188 (0.202)*** | 1.108 (0.149)*** |
| Slopeb | — | 0.00005 (0.00002)* | 0.00004 (0.00001)*** | 0.00005 (0.00002)*** | 0.00004 (0.00001)*** |
| Residual | 1.244 (0.056) | 0.704 (0.015) | 0.833 (0.045) | 0.702 (0.041) | 0.762 (0.039) |
| Model summary | |||||
| AIC | 4,130.13 | 3,190.45 | 3,876.22 | 3,191.13 | 3,739.86 |
| BIC | 4,150.69 | 3,225.43 | 3,912.20 | 3,231.10 | 3,780.98 |
| LL | −2,061.07 | −1,588.23 | −1,931.11 | −1,587.56 | −1,861.93 |
Note. The inclusion of race into the unconditional model controlled for a small amount of additional variance (~1.2%) but significantly improved model fit (p < .001) over the fully unconditional model, and thus was retained in the subsequent models for resting RSA. Model comparison: Linearb vs. Quadraticb, p = .25; Lineara vs. Quadratica, p < .001. RSA = respiratory sinus arrhythmia; AIC = Akaike information criterion; BIC = Bayesian information criterion; LL = log-likelihood.
With 15-year included in the analysis.
Without 15-year included in the analysis.
p < .05.
p < .001.
After including the Age 15 data, the residuals indicated that the model overestimated resting RSA during the early childhood and adolescent years while underestimating resting RSA during the middle childhood years. Thus, we included an acceleration term to test for nonlinear change in resting RSA from childhood through adolescence. The quadratic model is defined as
where yti is the value of the response variable, y (resting RSA), at the ith measurement for the jth individual; b1i is the random intercept for individual i; b2i is the random slope for individual i; b3i is the fixed acceleration; and uti is the time-dependent residual. Including the quadratic term to describe change in resting RSA from Age 2 to Age 15 significantly improved the fit over and above the inclusion of the linear term (p < .001), indicating that the developmental trajectory of resting RSA from Age 10 to Age 15 was significantly different when compared with the trajectory from Age 2 to Age 10. The fixed and random effects for the quadratic model fitting resting RSA from Ages 2 to 15 years old are provided in Table 3. The random intercept and slope were negatively correlated with one another (r = −0.96, p < .001), indicating that those with a higher resting RSA at Age 2 had a lower slope of change in resting RSA from Ages 2 to 15 years old. Individuals with lower resting RSA at Age 2 were more likely to show larger increases in resting RSA from 2 to 15 years old (see Figure 1).
Figure 1.
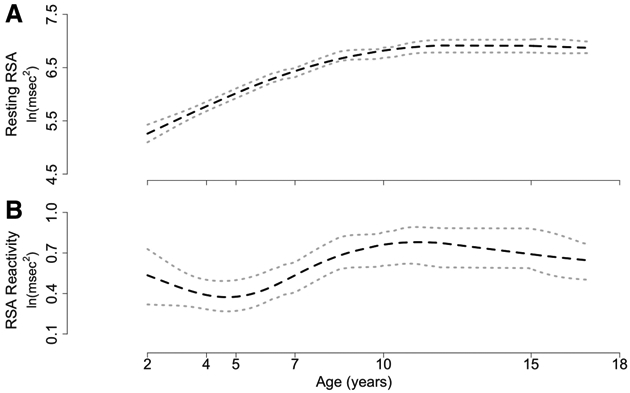
(A) Mean changes in observed resting RSA throughout childhood and adolescence (Age 2 through Age 15). (B) Mean changes in observed RSA reactivity throughout childhood and adolescence (Age 2 through Age 15). Data are presented as mean ±95% confidence interval. The frustration task at Age 15 included a cognitive component, whereas earlier tasks were solely emotional in nature. RSA = respiratory sinus arrhythmia.
To confirm these findings, we retroactively fit a quadratic model to the resting RSA data from Age 2 to Age 10. However, this model showed no improvement on the linear model (p = .25), supporting existing work that resting RSA from 2 to 10 years follows a linear trajectory.
Developmental Continuity of RSA Reactivity
To assess the developmental continuity of RSA reactivity, we first considered a multilevel model with a response variable y (RSA reactivity), measured at t time for individual i. RSA reactivity was initially modeled as a linear function of time while the intercept and slopes were permitted to vary at the individual level. The linear growth model with a time-varying covariate can be written as
where yti is the value of the response variable, y (RSA reactivity), at the ith measurement for the jth individual; b1i is the random intercept for individual i conditional on xti; b2i is the random slope for individual i conditional on xti; b4 is the effect of the time-varying covariate (resting RSA); and uti is the time-dependent residual. This model produced non-normal and highly autocorrelated residuals.
This model was expanded to include a quadratic term and permitting the intercept, slope, and acceleration term to be freely estimated at the individual level. The quadratic model with a time-varying covariate can be written as
Although the quadratic model more accurately depicted some of the rapid changes in RSA reactivity in early childhood, it failed to accurately estimate RSA reactivity in late childhood and adolescence. Therefore, we incorporated a splined growth model to account for the two apparent trends within the data. These splined models can account for a wide range of variability in trajectories, and in instances in which an acute perturbation/change results in a rapid behavioral, developmental, or physiologic response, the rapid change represented by a knot point may provide the best representation of the data (Grimm, Ram, & Estabrook, 2017). Given that ER development varies across individuals, we tested a model in which the knot point was fixed at 7 years of age as well as a model in which the knot point was fixed at 10 years of age. The model in which the knot point was fixed at 10 years of age was a significantly poorer fitting model than the model with the 7-year knot point. Thus, a fixed knot point at 7 years was used for all individuals.
A bilinear spline model with a single spline, s, at 7 years of age was first estimated. This model was estimated to provide a comparison for changes in overall model fit and can be written as
where yti is the value of the RSA reactivity at the ith measurement for the jth individual; b1i is the random intercept centered at the knot point; b2i is the preknot random slope and b3i is the postknot slope for individual i conditional on xti; b4 is the effect of the time-varying covariate (resting RSA); and uti is the time-dependent residual.
The overall fit of the bilinear spline model was not significantly better than the quadratic model (p = .09). Although the bilinear model seemed to perform better following the knot point, it failed to accurately estimate the patterns of RSA reactivity from toddlerhood to middle childhood. Thus, a quadratic-linear spline growth model was tested. A quadratic term was used to fit the growth trajectories through 7 years in order to better estimate the observed patterns of change in RSA reactivity from toddlerhood to middle childhood, whereas a linear function was used to represent the change after this point. These decisions were based on our observations of the raw data and literature suggesting that early childhood may be a period of greater developmental sensitivity compared with adolescence, which appears more stable (Hinnant et al., 2018). The intercept (centered at the knot point) and slope of the first (quadratic) spline were permitted to vary at the individual level, whereas the acceleration term of the first (quadratic) spline and the second (linear) spline were fixed. This model can be written as
where yti is the value of the RSA reactivity at the ith measurement for the jth individual; b1i is the random intercept centered at the knot point; b2i is the preknot random slope and b3i is the preknot acceleration for individual i conditional on xti; b4i is the postknot slope; b5 is the effect of the time-varying covariate (resting RSA); and uti is the time-dependent residuals.
The mean of the random intercept was −1.21, representing the predicted average RSA reactivity for an individual at the knot point after controlling for resting RSA (see Table 4). The preknot slope and acceleration were 0.02 and 0.0004, respectively, representing a gradual positive increase in the monthly change of RSA reactivity up to 7 years of age after controlling for changes in resting RSA. The postknot slope was −0.002, indicating a relatively flat to slight reduction in RSA reactivity from the 7 to 10 years after controlling for resting RSA. The random intercept was positively correlated with the random slope (r = .83, p < .001), indicating that those with higher RSA reactivity at 7 years had a higher rate of change in RSA reactivity from toddlerhood to 7 years. Predicted values from the quadratic-linear spline model are provided in Figure 1.
Table 4.
Model Summaries for Change in RSA Reactivity From Age 2 to Age 15
| Parameters | Unconditional | Linear | Quadratic | Bilinear | Quadratic-linear |
|---|---|---|---|---|---|
| Fixed effects | |||||
| Intercept | 0.544 (0.026)*** | −1.216 (0.098)* | −1.064 (0.105)*** | −1.420 (0.120)*** | −1.211 (0.119)*** |
| Slopea | — | −0.0007 (0.0004)† | −0.008 (0.002)*** | −0.003 (0.001)*** | 0.023 (0.003)*** |
| Accelerationa | — | — | 0.00003 (0.000008)*** | — | 0.0004 (0.00005)*** |
| Slopeb | — | — | — | 0.0007 (0.0006) | −0.002 (0.0007)* |
| Resting RSA | — | 0.290 (0.016)*** | 0.314 (0.017)*** | 0.300 (0.017)*** | 0.295 (0.016)*** |
| Random effects | |||||
| Intercept | 0.075 (0.016) | 0.100 (0.017) | 0.107 (0.017)*** | 0.136 (0.026)*** | 0.151 (0.026)*** |
| Slopea | — | — | — | 0.00001 (0.00002)† | 0.00002 (0.00002)* |
| Residual | 0.529 (0.023) | 0.399 (0.017) | −0.387 (0.017) | 0.387 (0.019) | 0.353 (0.018) |
| Model summary | |||||
| AIC | 3,114.34 | 2,791.96 | 2,779.52 | 2,788.45 | 2,700.49 |
| BIC | 3,129.95 | 2,820.05 | 2,810.71 | 2,830.03 | 2,747.27 |
| LL | −1,554.17 | −1,392.03 | −1,383.76 | −1,386.23 | −1,341.25 |
Note. Model comparisons: linear vs. quadratic, p < .001; linear vs. bilinear, p < .01; quadratic vs. bilinear, p < .10; quadratic vs. quad-linear, p < .001; bilinear vs. quad-linear, p < .001. RSA = respiratory sinus arrhythmia; AIC = Akaike information criterion; BIC = Bayesian information criterion; LL = log-likelihood.
Spline 1.
Spline 2.
p < .10.
p < .05.
p < .001.
Discussion
We examined longitudinal data spanning 13 years to assess developmental patterns of children’s resting RSA and RSA reactivity from toddlerhood to adolescence. There are well-documented associations between RSA and adjustment outcomes across the life span (Eisenberg et al., 2008; Hastings et al., 2008; Marcovitch et al., 2010; Perry et al., 2014). Moreover, ER, a significant predictor of adaptive functioning (Calkins & Perry, 2016), and RSA are both likely influenced by top-down prefrontal control, suggesting that RSA can serve as a peripheral index of ER (Shader et al., 2018; Thayer & Lane, 2000). Existing work has primarily considered how RSA changes over short time periods, and thus a clearer understanding of the developmental patterns of RSA from toddlerhood through adolescence is needed.
This study has many strengths and significantly adds to our understanding of resting RSA and RSA reactivity development from toddlerhood to adolescence. We used a stepwise progression of models to best capture the complex developmental patterns of resting RSA and RSA reactivity across multiple developmental periods. Age was used as a continuous variable to obtain higher resolution into exactly how resting RSA and RSA reactivity develop. We also included resting RSA as a time-varying covariate to control for baseline changes in physiological arousal over time when assessing changes in children’s RSA reactivity. Moreover, given that race was significantly associated with resting RSA at multiple time points, we included race as a covariate in models assessing changes in resting RSA over time. Our data indicated that African American children showed higher baseline RSA than White children at multiple time points, similar to the recent findings by Hinnant and colleagues (2011). However, because examination of associations between RSA and demographic or child characteristics, such as race, rarely are considered directly, additional work is needed to replicate these findings.
Consistent with prior work (Alkon et al., 2011; Bornstein & Suess, 2000; El-Sheikh, 2005; Pang & Beauchaine, 2013), we found evidence of stability in resting RSA from toddlerhood through adolescence. Children with high resting RSA in toddlerhood were more likely to continue to show high RSA over time, relative to their peers, although the strength of the association was weaker across longer periods of time, as would be expected. For instance, greater stability was found in resting RSA from Age 2 to Age 7 (rs = .39 to .46) than Age 2 to Age 15 (r = .19). The implication of this finding is that individual differences in resting RSA become entrenched early in life, which is consistent with the notion that resting RSA is a central biomarker of children’s characteristic level of arousal or physiological reactivity.
We found no evidence of stability in RSA reactivity from toddlerhood to adolescence (i.e., Age 2 to Age 15). However, we found some indication of rank-order stability from Age 4 to Age 5, and again from Age 7 to Age 10, but no significant associations in toddlerhood or adolescence. Although there are various ways one could interpret these findings (i.e., times of more substantial brain growth, self-regulation changes), one explanation could be that there are periods of more substantial physical growth that influence changes in RSA reactivity when compared with other developmental periods. For instance, there is considerable physical growth that takes place in the first few years of life, but by Age 4, the lungs are almost fully developed, and height changes have slowed substantially, generally following a fixed rate of change until puberty. Indeed, the lowest childhood growth rates occur during the fourth year of life and in the prepubertal nadir (the time immediately prior to pubertal linear growth; Rogol, Clark, & Roemmich, 2000). However, in middle childhood (around the onset of puberty), for most children, there is a substantial increase in growth rate (3–5 in. per year for girls, 4–6 in. for boys; Nwosu & Lee, 2008). Thus, it may be important for future work to consider multiple physical developmental processes and how these changes affect stability in RSA across development.
We also examined developmental patterns of change in resting RSA and RSA reactivity. Existing work on resting RSA across shorter periods of time suggests discontinuity, with increases in resting RSA up to middle childhood and much less change in late childhood and adolescence (El-Sheikh, 2005; Salomon, 2005). Our data indicated a linear increase in resting RSA from toddlerhood to middle childhood and a flattening out of resting RSA from middle childhood to adolescence, suggesting that there is more interindividual variability in how RSA changes by late childhood. Indeed, these findings support the hypothesis that this system is reaching close to adult level functioning by middle childhood (Bornstein & Suess, 2000).
In addition, we found that children who had higher levels of resting RSA in toddlerhood showed less-steep increases in resting RSA over time, whereas those with lower RSA in toddlerhood were more likely to show larger increases in resting RSA across development. This suggests that there may be a ceiling effect in resting RSA, such that children who have optimal levels of resting RSA early in life do not need, or are not able, to “grow” as much in their capacity to react to the environment appropriately. On the other hand, those children who have lower resting RSA—who are often thought to be at risk for developing various poor outcomes through their lowered capacity to appropriately respond to challenge—are, in fact, capable of developing a greater capacity to respond to stressors across development.
This finding raises questions about the importance of early resting RSA for child functioning. Some studies fail to find associations between resting RSA and psychological functioning (e.g., externalizing behaviors) in young children, which some researchers suggest reflects that RSA is not an implicit marker of vulnerability but perhaps a consequence of risk (Beauchaine et al., 2007; Fortunato et al., 2013). Indeed, in the current sample significant associations between resting RSA and externalizing behavior problems only occurred at two early time points (Ages 2 and 4), which is notable given that one cohort was initially oversampled for externalizing behaviors (although by early childhood this sample was no longer high in clinically relevant levels of externalizing behavior problems). Moreover, externalizing behaviors did not change the pattern of resting RSA or RSA reactivity over time. Thus, one interpretation of our findings is that it may be inappropriate to consider children with relatively lower resting RSA in toddlerhood at greater psychological risk early in life because they may show greater growth in resting RSA later in development. However, it is also reasonable to suggest that even if children with lower resting RSA “catch up” to their peers in subsequent years, there may be consequences of having lower resting RSA in early development, which could cascade into later challenges (Perry et al., 2014). For example, children with low resting RSA may have more difficulties self-regulating their behavior, which, in turn, is associated with a host of later challenges. More research is needed on the consequences of low resting RSA in development to address these questions.
We also examined developmental patterns of RSA reactivity from Age 2 to Age 15. We found that the initial rate of change in RSA reactivity from toddlerhood to middle adolescence varied across individuals but that the quadratic growth in RSA reactivity across this period was positive across all individuals. This suggests that the initial developmental trajectories varied significantly, perhaps because they are associated with processes occurring internally (i.e., temperament) and externally (i.e., caregiver behaviors) to the child, but that, overall, all children showed a similar acceleration of RSA reactivity across early development.
The patterns of RSA reactivity considered in the current study were examined across a period of time that is marked by significant maturation of the frontal brain regions that influence self-regulation. The same brain regions that influence the development of self-regulation skills across this time frame likely also influence RSA (Beauchaine & Thayer, 2015; Thayer & Lane, 2000). Our data support the idea that RSA reactivity changes in a pattern that we might also expect for ER development. Developmentalists propose that ER trajectories are likely not linear across developmental periods, such that there is a period of significant growth from toddlerhood through the transition to school, with the experience and demands of formal education supporting greater growth. Then, there is a slowing of growth in ER, with only modest growth on average from middle childhood into adolescence (Calkins & Perry, 2016).
Although our results indicated the expected leveling off of RSA reactivity in middle to late childhood, interestingly, we found that RSA reactivity declined across the preschool and early childhood years before increasing again around Age 5. Although unexpected, this pattern may reflect more general development changes and the context in which this development is occurring. For example, RSA reactivity is only one correlate of self-regulatory functioning, which is changing significantly during the preschool and early childhood years. There are increasing demands placed on children across different environments during the transition to preschool/formal schooling. These demands include managing the different expectations between home and school, navigating new social experiences, and regulating behavior in response to changing tasks in the school environment itself. As such, it is possible that this developing physiological system is especially taxed through this period and limited maturation occurs, but then as experience and demands support increased growth, it may continue to mature.
Although this study adds to our understanding of RSA development, there are issues that should be considered when interpreting these findings. First, this study was faced with the challenge of most longitudinal research: how to best measure constructs across time given the developmental level of the child. In this study, different baseline tasks were used across time. At earlier ages, children participated in a “vanilla” baseline (watching a neutral video), whereas at later ages, children engaged in baseline tasks by sitting silently. It is often necessary to use vanilla baseline tasks to ensure that young children sit still for a set period of time, and the film clip used in the study was selected to be neutral and unengaging. However, an RSA response can be evoked when attending to a video clip even if it is not an emotional response.
Because of the need to include measures that were appropriately demanding for children at each age, children’s RSA reactivity was assessed through different tasks. Assessments were primarily emotional in nature, but the 15-year frustration task included a cognitive and emotional component. The 15-year frustration task was chosen because cognitively demanding tasks, as the one used in the study, often induce frustration (Bray, Martin Ginis, Hicks, & Woodgate, 2008), and thus are considered an age-appropriate frustration-eliciting task. Nevertheless, because different tasks cannot be considered equivalent when interpreting the association between psychophysiology and behavior associations, it cannot be ruled out that differences in tasks are confounded with the child’s age and unavoidably contributed to the developmental shifts in RSA. Another point to consider is that the timing of the frustration task varied across assessment and could have influenced children’s RSA reactivity scores at different time points. Finally, it should be noted that we employed a change in average RSA scores from a resting state to a task that elicits frustration as opposed to assessing real-time changes in RSA during the frustrating task. Other studies (i.e., Brooker & Buss, 2010) have considered RSA reactivity changes across a task, which may be more useful in reflecting emotional responses to the task.
An additional challenge is the analytical approach used to generate RSA measures. Typically, RSA is derived using time series analysis that isolates the variability in the frequency of spontaneous respiration as opposed to directly measuring respiration. Recent work has raised concerns regarding historically used parameters for the analysis of children’s heart rate data using respiratory rates (see Shader et al., 2018). We acknowledge that best practices for collecting and analyzing RSA change over time, and it is possible that the methodology used in the current study added nonrespiratory noise to RSA estimates, as suggested by Shader and colleagues (2018). Although we do not believe that this would alter our findings regarding the pattern of change in RSA over time, additional research is warranted to duplicate these findings using different methodological/analytical approaches (i.e., autoregressive [AR] spectral analysis with extraction of respiratory peak directly from AR output) to examine patterns of change in RSA over time. Finally, there is some discussion regarding the most appropriate sampling rate to employ when analyzing RSA data (Beauchaine et al., 2019); by some standards, this study’s sampling rate is slow and must be considered when interpreting our findings.
The current findings contribute significantly to our understanding of children’s RSA by identifying developmental patterns of both baseline RSA and RSA reactivity across a 13-year period. Clearly articulating the developmental pattern of change in children’s PNS functioning will contribute to a more nuanced understanding of the link between children’s physiological regulation and their adjustment across childhood.
Acknowledgments
This research was supported by a National Institute of Mental Health (NIMH) Behavioral Science Track Award for Rapid Transition (MH 55625), an NIMH FIRST Award (MH 55584) to Susan D. Calkins, and an NIMH Grant (MH 58144) awarded to Susan D. Calkins, Susan P. Keane, and Marion O’Brien. The authors also thank the families who generously gave their time to participate in the study.
Contributor Information
Jessica M. Dollar, University of North Carolina at Greensboro
Susan D. Calkins, University of North Carolina at Greensboro
Nathaniel T. Berry, University of North Carolina at Greensboro
Nicole B. Perry, University of Minnesota
Susan P. Keane, University of North Carolina at Greensboro
Lilly Shanahan, University of Zurich.
Laurie Wideman, University of North Carolina at Greensboro.
References
- Achenbach TM (1992). Manual for the child behavior checklist/2–3, 1992 profile. Burlington, VT: University of Vermont Department of Psychiatry. [Google Scholar]
- Alkon A, Boyce WT, Davis NV, & Eskenazi B (2011). Developmental changes in autonomic nervous system resting and reactivity measures in Latino children from 6 to 60 months of age. Journal of Developmental and Behavioral Pediatrics, 32, 668–677. 10.1097/DBP.0b013e3182331fa6 [DOI] [PubMed] [Google Scholar]
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, & Boyce WT (2003). Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology, 42, 64–78. 10.1002/dev.10082 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, & Fox NA (2000). Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology, 37, 44–56. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology, 3, 43–47. 10.1016/j.copsyc.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Bell Z, Knapton E, McDonough-Caplan H, Shader T, & Zisner A (2019). Respiratory sinus arrhythmia reactivity across empirically based structural dimensions of psychopathology: A meta-analysis. Psychophysiology, 56, e13329. 10.1111/psyp.13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, & Mead HK (2007). Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology, 74, 174–184. 10.1016/j.biopsycho.2005.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, & Snarr J (2001). Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology, 110, 610–624. 10.1037/0021-843X.110.4.610 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, & Thayer JF (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology, 98, 338–350. 10.1016/j.ijpsycho.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Bornstein MH, & Suess PE (2000). Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Developmental Psychology, 36, 54–65. 10.1037/0012-1649.36.1.54 [DOI] [PubMed] [Google Scholar]
- Bray SR, Martin Ginis KA, Hicks AL, & Woodgate J (2008). Effects of self-regulatory strength depletion on muscular performance and EMG activation. Psychophysiology, 45, 337–343. 10.1111/j.1469-8986.2007.00625.x [DOI] [PubMed] [Google Scholar]
- Brooker RJ, & Buss KA (2010). Dynamic measures of RSA predict distress and regulation in toddlers. Developmental Psychobiology, 52, 372–382. 10.1002/dev.20432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Goldsmith HH, & Davidson RJ (2005). Cardiac reactivity is associated with changes in negative emotion in 24-month-olds. Developmental Psychobiology, 46, 118–132. 10.1002/dev.20048 [DOI] [PubMed] [Google Scholar]
- Calkins SD (1997). Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology, 31, 125–135. [DOI] [PubMed] [Google Scholar]
- Calkins SD, & Dedmon SE (2000). Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology, 28, 103–118. 10.1023/A:1005112912906 [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE, Gill KL, Lomax LE, & Johnson LM (2002). Frustration in infancy: Implications for emotion regulation, physiological processes, and temperament. Infancy, 3, 175–197. 10.1207/S15327078IN0302_4 [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dollar JM, & Wideman L (2019). Temperamental vulnerability to emotion dysregulation and risk for mental and physical health challenges. Development and Psychopathology, 31, 957–970. 10.1017/S0954579419000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, & Hill A (2007). Caregiver influences on emerging emotion regulation: Biological and environmental transactions in early development. In Gross JJ (Ed.), Handbook of emotion regulation (pp. 229–248). New York, NY: Guilford Press. [Google Scholar]
- Calkins SD, & Perry NB (2016). The development of emotion regulation: Implications for child adjustment. In Cicchetti D (Ed.), Developmental psychopathology. Volume 3: Maladaptation and psychopathology (pp. 187–242). Hoboken, NJ: Wiley. 10.1002/9781119125556.devpsy306 [DOI] [Google Scholar]
- Cook CD, & Hamann JF (1961). Relation of lung volumes to height in healthy persons between the ages of 5 and 38 years. The Journal of Pediatrics, 59, 710–714. 10.1016/S0022-3476(61)80007-3 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan Executive Function System: Technical manual. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Doussard-Roosevelt JA, Montgomery LA, & Porges SW (2003). Short-term stability of physiological measures in kindergarten children: Respiratory sinus arrhythmia, heart period, and cortisol. Developmental Psychobiology, 43, 230–242. 10.1002/dev.10136 [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Murphy BC, Maszk P, Holmgren R, & Suh K (1996). The relations of regulation and emotionality to problem behavior in elementary school children. Development and Psychopathology, 8, 141–162. 10.1017/S095457940000701X [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Hofer C, Spinrad TL, Gershoff ET, Valiente C, Losoya SH, … Maxon E (2008). Understanding mother-adolescent conflict discussions: Concurrent and across-time prediction from youths’ dispositions and parenting. Monographs of the Society for Research in Child Development, 73, vii–viii, 1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M (2005). Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental Psychobiology, 46, 66–74. 10.1002/dev.20036 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, & Whitson SM (2001). Exposure to interparental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development, 72, 1617–1636. 10.1111/1467-8624.00369 [DOI] [PubMed] [Google Scholar]
- Fortunato CK, Gatzke-Kopp LM, & Ram N (2013). Associations between respiratory sinus arrhythmia reactivity and internalizing and externalizing symptoms are emotion specific. Cognitive, Affective & Behavioral Neuroscience, 13, 238–251. 10.3758/s13415-012-0136-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzke-Kopp L, & Ram N (2018). Developmental dynamics of autonomic function in childhood. Psychophysiology, 55, e13218. 10.1111/psyp.13218 [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, & Fox NA (2009). Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology, 82, 156–163. 10.1016/j.biopsycho.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, & Rothbart MK (1993). The Laboratory Temperament Assessment Battery (LAB-TAB). Madison, WI: University of Wisconsin. [Google Scholar]
- Graziano PA, Keane SP, & Calkins SD (2007). Cardiac vagal regulation and early peer status. Child Development, 78, 264–278. 10.1111/j.1467-8624.2007.00996.x [DOI] [PubMed] [Google Scholar]
- Grimm K, Ram N, & Estabrook R (2017). Growth modeling: Structural equation and multilevel modeling approaches. New York, NY: Guilford Press. [Google Scholar]
- Grossman P, van Beek J, & Wientjes C (1990). A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology, 27, 702–714. 10.1111/j.1469-8986.1990.tb03198.x [DOI] [PubMed] [Google Scholar]
- Hastings PD, Nuselovici JN, Utendale WT, Coutya J, McShane KE, & Sullivan C (2008). Applying the polyvagal theory to children’s emotion regulation: Social context, socialization, and adjustment. Biological Psychology, 79, 299–306. 10.1016/j.biopsycho.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Hinnant JB, Elmore-Staton L, & El-Sheikh M (2011). Developmental trajectories of respiratory sinus arrhythmia and preejection period in middle childhood. Developmental Psychobiology, 53, 59–68. 10.1002/dev.20487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnant JB, & El-Sheikh M (2009). Children’s externalizing and internalizing symptoms over time: The role of individual differences in patterns of RSA responding. Journal of Abnormal Child Psychology, 37, 1049–1061. 10.1007/s10802-009-9341-1 [DOI] [PubMed] [Google Scholar]
- Hinnant JB, Philbrook LE, Erath SA, & El-Sheikh M (2018). Approaches to modeling the development of physiological stress responsivity. Psychophysiology, 55, e13027. 10.1111/psyp.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four Factor Index of Social Status. Unpublished manuscript, Department of Sociology, Yale University, New Haven, CT. [Google Scholar]
- Marcovitch S, Leigh J, Calkins SD, Leerks EM, O’Brien M, & Blankson AN (2010). Moderate vagal withdrawal in 3.5-year-old children is associated with optimal performance on executive function tasks. Developmental Psychobiology, 52, 603–608. 10.1002/dev.20462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, & Stevenson-Hinde J (1998). Behavioral inhibition, heart period, and respiratory sinus arrhythmia in young children. Developmental Psychobiology, 33, 283–292. [DOI] [PubMed] [Google Scholar]
- Nwosu BU, & Lee MM (2008). Evaluation of short and tall stature in children. American Family Physician, 78, 597–604. [PubMed] [Google Scholar]
- Pang KC, & Beauchaine TP (2013). Longitudinal patterns of autonomic nervous system responding to emotion evocation among children with conduct problems and/or depression. Developmental Psychobiology, 55, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NB, Calkins SD, & Bell MA (2016). Indirect effects of maternal sensitivity on infant emotion regulation behaviors: The role of vagal withdrawal. Infancy, 21, 128–153. 10.1111/infa.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NB, Nelson JA, Calkins SD, Leerkes EM, O’Brien M, & Marcovitch S (2014). Early physiological regulation predicts the trajectory of externalizing behaviors across the preschool period. Developmental Psychobiology, 56, 1482–1491. 10.1002/dev.21228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DJ, DebRoy S, Sarkar D, & R Core Team. (2018). nlme: Linear and nonlinear mixed effects models. R Package Version 3.1. Retrieved from https://CRAN.R-project.org/package=nlme
- Porges SW (1995). Cardiac vagal tone: A physiological index of stress. Neuroscience and Biobehavioral Reviews, 19, 225–233. 10.1016/0149-7634(94)00066-A [DOI] [PubMed] [Google Scholar]
- Porges SW (1996). Physiological regulation in high-risk infants: A model for assessment and potential intervention. Development and Psychopathology, 8, 43–58. 10.1017/S0954579400006969 [DOI] [Google Scholar]
- Porges SW, & Bohrer RE (1990). Analyses of periodic processes in psychophysiological research. In Cacioppo JT & Tassinary LG (Eds.), Principles of psychophysiology: Physical, social, and inferential elements (pp. 708–753). New York, NY: Cambridge University Press. [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, & Greenspan SI (1996). Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology, 29, 697–712. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, & Suess PE (1994). Cardiac vagal tone: Stability and relation to difficultness in infants and 3-year-olds. Developmental Psychobiology, 27, 289–300. 10.1002/dev.420270504 [DOI] [PubMed] [Google Scholar]
- Propper C, & Moore GA (2006). The influence of parenting on infant emotionality: A multi-level psychobiological perspective. Developmental Review, 26, 427–460. 10.1016/j.dr.2006.06.003 [DOI] [Google Scholar]
- Riniolo T, & Porges SW (1997). Inferential and descriptive influences on measures of respiratory sinus arrhythmia: Sampling rate, R-wave trigger accuracy, and variance estimates. Psychophysiology, 34, 613–621. 10.1111/j.1469-8986.1997.tb01748.x [DOI] [PubMed] [Google Scholar]
- Rogol AD, Clark PA, & Roemmich JN (2000). Growth and pubertal development in children and adolescents: Effects of diet and physical activity. The American Journal of Clinical Nutrition, 72(Suppl.), 521S–528S. 10.1093/ajcn/72.2.521S [DOI] [PubMed] [Google Scholar]
- Salomon K (2005). Respiratory sinus arrhythmia during stress predicts resting respiratory sinus arrhythmia 3 years later in a pediatric sample. Health Psychology, 24, 68–76. 10.1037/0278-6133.24.1.68 [DOI] [PubMed] [Google Scholar]
- Shader TM, Gatzke-Kopp LM, Crowell SE, Jamila Reid M, Thayer JF, Vasey MW, … Beauchaine TP (2018). Quantifying respiratory sinus arrhythmia: Effects of misspecifying breathing frequencies across development. Development and Psychopathology, 30, 351–366. 10.1017/S0954579417000669 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61, 201–216. 10.1016/S0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Vasilev CA, Crowell SE, Beauchaine TP, Mead HK, & Gatzke-Kopp LM (2009). Correspondence between physiological and self-report measures of emotion dysregulation: A longitudinal investigation of youth with and without psychopathology. Journal of Child Psychology and Psychiatry, 50, 1357–1364. 10.1111/j.1469-7610.2009.02172.x [DOI] [PubMed] [Google Scholar]
- Zisner AR, & Beauchaine TP (2016). Physiological methods and developmental psychopathology. In Cicchetti D (Ed.), Developmental psychopathology: Volume 2. Developmental neuroscience (3rd ed., pp. 832–884). Hoboken, NJ: Wiley. [Google Scholar]


