Highlights
-
•
Cyto- and receptor-based parcellation of macaque inferior parietal lobe (IPL).
-
•
Caudo-rostral gradients found in the architectonic organization of IPL areas.
-
•
Hierarchical analysis segregated IPL areas into rostral and caudal clusters.
-
•
Novel insights into homologies between human and macaque IPL areas.
Keywords: Macaque monkey, Inferior parietal, Multimodal receptor analysis, Cytoarchitecture, Homology
Abstract
The macaque monkey inferior parietal lobe (IPL) is a structurally heterogeneous brain region, although the number of areas it contains and the anatomical/functional relationship of identified subdivisions remains controversial. Neurotransmitter receptor distribution patterns not only reveal the position of the cortical borders, but also segregate areas associated to different functional systems. Thus we carried out a multimodal quantitative analysis of the cyto- and receptor architecture of the macaque IPL to determine the number and extent of distinct areas it encompasses. We identified four areas on the IPL convexity arranged in a caudo-rostral sequence, as well as two areas in the parietal operculum, which we projected onto the Yerkes19 surface. We found rostral areas to have relatively smaller receptor fingerprints than the caudal ones, which is in an agreement with the functional gradient along the caudo-rostral axis described in previous studies. The hierarchical analysis segregated IPL areas into two clusters: the caudal one, contains areas involved in multisensory integration and visual-motor functions, and rostral cluster, encompasses areas active during motor planning and action-related functions. The results of the present study provide novel insights into clarifying the homologies between human and macaque IPL areas. The ensuing 3D map of the macaque IPL, and the receptor fingerprints are made publicly available to the neuroscientific community via the Human Brain Project and BALSA repositories for future cyto- and/or receptor architectonically driven analyses of functional imaging studies in non-human primates.
1. Introduction
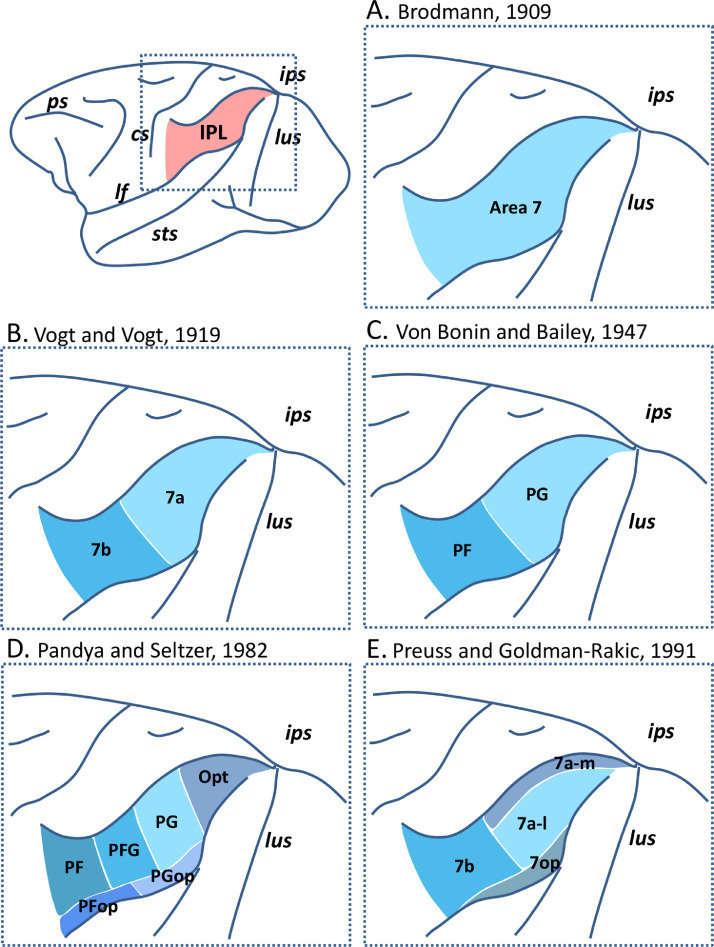
Majority of the cortex in the macaque inferior parietal lobule (IPL), which constitutes a multimodal association brain region crucial for the planning and execution of motor tasks (Rozzi et al. 2008; Binkofski et al. 2016), is occupied by Brodmann's area 7 (Brodmann 1909; Fig. 1A). Although latter studies have shown the IPL to be structurally heterogeneous (Fig. 1B-E), the exact number, precise arrangement (i.e., the size, shape, and location relative to geographic landmarks) and anatomical/functional relationship of identified subdivisions remains controversial. Furthermore, although the dorsal and ventral boundaries are well-defined by the intraparietal sulcus (ips) and the lateral fissure (lf), respectively, rostrally there is no explicit macroscopic landmark highlighting the demarcation with somatosensory area 2 of Brodmann (1909).
Fig. 1.
Architectonic parcellation schemes of the IPL from previous studies. Dashed box on the top left indicates the scope of the following schemes. A: Scheme from Brodmann (1909), B: Scheme from Vogt and Vogt (1919), C: Scheme from Von Bonin and Bailey (1947), D: Scheme from Pandya and Seltzer (1982), E: Scheme from Preuss and Goldman-Rakic (1991).
Vogt and Vogt (1919) and Von Bonin and Bailey (1947) defined two areas within BA7: area 7a of the Vogts, equivalent of area PG (Von Bonin and Bailey 1947), occupies the caudal two-thirds of BA7; area 7b, equivalent of PF, occupies the rostral portion of BA7 (Fig. 1B,C). Pandya and Seltzer (1982) and Preuss and Goldman-Rakic (1991) separated the IPL convexity from the parietal operculum. The former discerned four cytoarchitectonic divisions along the IPL convexity (caudal most area Opt, intermediate area PG, transitional area PFG, and rostral most area PF), and two subdivisions (PGop caudally and PFop rostrally) in the parietal operculum (Fig. 1D). The latter described a single area in the parietal operculum (i.e., 7op), and three areas on the IPL convexity (Fig. 1E): rostral area 7b (comparable to that of Vogt and Vogt (1919)) followed caudally by areas 7a-m (on the rim of the intraparietal sulcus) and 7a-l (on the IPL convexity). The map of Pandya and Seltzer (1982) was later confirmed by a combined cyto- and myeloarchitectonic analysis (Gregoriou et al. 2006), as well as by differences in the distribution of the serotonin 5-HT1A receptor (Geyer et al. 2005), and dominates the current view on the structural organization of the macaque IPL.
The IPL is primarily dedicated to the transformation of sensory information into a motor behavior (Rozzi et al. 2008; Borra and Luppino 2017), and each area plays different roles in the generation of motor representations integrating multi-sensory inputs (Colby 1998; Rizzolatti et al. 1997; Yokochi et al. 2003). Interestingly, these different functions are not processed at random locations throughout the IPL, but follow a gradient along the caudo-rostral axis of the IPL, where caudal areas seem to be involved in multisensory integration and visual-motor functions, whereas rostral areas are more concerned with motor planning and action-related functions (Rozzi et al. 2008). This gradient is not only apparent in the functional organization of IPL areas (Rozzi et al. 2008), but also reflected by their connectivity patterns (Andersen et al., 1990, Caspers et al., 2011, Rozzi et al., 2006).
Analyses of the regional and laminar distribution patterns of different receptors in the cerebral cortex has been shown to be a powerful tool for the detection of cortical segregation (Palomero-Gallagher and Zilles 2018; Zilles and Amunts 2009; Zilles et al. 2002a). Indeed, the organization of several cortical regions in the human (Amunts et al., 2010, Caspers et al., 2012, Caspers et al., 2015, Geyer et al., 1999, Palomero-Gallagher et al., 2009, Zilles et al., 2015)and macaque (Bozkurt et al., 2005, Geyer et al., 1998, Geyer et al., 2005, Impieri et al., 2019, Niu et al., 2020, Rapan et al., 2021, Palomero-Gallagher et al., 2013) brains was revealed with this approach. More importantly, areas with similar neurochemical organization often belong to the same functional network (Zilles and Amunts 2009; Zilles et al. 2002a; Palomero-Gallagher et al. 2009; Zilles et al. 2015). Thus, this approach provides valuable insights into both the structural and the functional segregation of the brain.
In present study, we addressed the following questions: a) does the multireceptor architectonic segregation of the macaque IPL match previous cytoarchitectonic parcellations; b) which receptors contribute most to a differentiation between areas; c) do the receptor architectonic features of IPL subdivisions reflect the functional gradient mentioned above; and d) do differences in receptor fingerprints facilitate identification of homologous areas in the macaque and human IPL?
2. Materials and methods
2.1. Subjects
We analysed 6 hemispheres from 4 adult male macaque monkeys. Both hemispheres of an adult Macaca mulatta (animal ID: DP1) obtained as a gift from Professor Deepak N. Pandya were processed for cytoarchitectonic analysis. Four hemispheres of three Macaca fascicularis monkeys (animal ID #11539: left and right hemispheres, #11543: left hemisphere, #11530: left hemisphere) obtained from Covance Laboratories (Münster, Germany) were processed for receptor autoradiography and staining of cell bodies or of myelin. The brains of Macaca mulatta and Macaca fascicularis monkeys are known to differ macroanatomically only in size, with that of the former species being slightly larger than that of the latter one Heilbroner and Holloway (1989). Furthermore, previous architectonic studies have confirmed comparability of the cyto- and chemoarchitecture of these two macaque species in diverse cortical (Peters 1994; Stanton et al. 1989; Ding et al. 2003) and subcortical (Büttner-Ennever et al. 1988; Asanuma et al. 1983) regions. All experimental protocols were carried out in accordance with the guidelines of the European Communities Council Directive for the care and use of animals for scientific purposes.
2.2. Tissue processing
2.2.1. Histological procedures
The DP1 monkey was deeply anesthetized with sodium pentobarbital, then perfused intracardially with cold saline followed by 10% buffered formalin. The brain was removed and stored in a buffered formalin solution for several months. After dehydration in graded alcohols (70% to 100% propanol), the whole brain was embedded in paraffin and sectioned in the coronal plane with a large-scale microtome, resulting in 3305.20 µm-thick whole-brain sections. Every fifth section was mounted on glass slides and stained for cell bodies with a modified silver method Merker (1983), which provides a high contrast between background (eg. neuropil) and the cell bodies, and thus enables reliable segmentation.
2.2.2. In vitro receptor autoradiography
Monkeys from Covance Laboratories (Münster, Germany) were sacrificed by a lethal intravenous injection of sodium pentobarbital, and brains were removed immediately. Each hemisphere was separated at the level of the posterior end of the central sulcus into an anterior and a posterior slab. After shock freezing at -40 °C for 10-15 min in isopentane, the slabs were stored at -80 °C until sectioning, then serially sectioned (20 μm thickness) in the coronal plane at -20 °C using a cryostat (CM 3050, Leica, Germany). Alternating sections were processed for quantitative in vitro receptor autoradiography according to previously published protocols (Zilles et al. 2002b; Palomero-Gallagher and Zilles 2018), or for cell-body (Merker 1983) or myelin (Gallyas 1979) staining.
The labelled sections were air-dried and exposed against tritium-sensitive films (Hyperfilm, Amersham, Braunschweig, Germany) together with plastic tritium standards of known radioactivity concentrations (MicroscalesⓇ, Amersham) for 4-18 weeks. The ensuing autoradiographs reveal the regional and laminar distribution of receptor binding sites.
2.3. Image acquisition
Histological sections were automatically scanned with a light microscope (Axioplan 2 imaging, Zeiss, Germany) equipped with a motor-operated stage controlled by the KS400 (Zeiss, Germany) image-analyzing system (version 3.0) and Axiovision (version 4.6), or by means of a TISSUEscope HS (Huron, Canada). And the ensuing 8-bit images were transformed into gray level index (GLI) images (Palomero-Gallagher and Zilles, 2018, Schleicher et al., 1999) by means of in-house Matlab scripts. Each pixel in the GLI image represents the local volume fraction of cell bodies in the corresponding measuring field (Schleicher and Zilles, 1990, Wree et al., 1982).
Autoradiographs were processed densitometrically using a video-based image analysing technique (Zilles et al. 2002b; Palomero-Gallagher and Zilles 2018). The plastic standards with known radioactivity were used to nonlinearly transform the gray values of the autoradiographs into binding site concentration in fmol/mg protein, thus generating linearized images, which were contrast enhanced and pseudo-colour coded for visualization purposes.
2.4. Statistical analysis
2.4.1. Identification of cortical borders
The position of all borders, including those with areas not included within the region of interest (e.g., rostrally with somatosensory area 2 or dorsally with intraparietal areas LIPd or AIP), was confirmed by means of an observer-independent and statistically testable method based on the analysis of interareal differences at the laminar level (Palomero-Gallagher and Zilles, 2018, Schleicher et al., 2005).
For detection of cytoarchitectonic borders, GLI profiles (Schleicher et al., 2000, Zilles et al., 2002b) oriented vertically to the cortical surface were extracted to quantitatively describe the laminar distribution of the volume fraction of cell bodies. The shape of each GLI profile was parametrized to create a feature vector which can be used to quantify the degree of similarity or dissimilarity in the shape of the corresponding profiles, and the Mahalanobis distance (Mahalanobis et al. 1949) was used as the distance function. Maximum differences in profile shape can be expected at the interface between the feature vectors of two groups (or blocks) of profiles which represent adjacent cortical areas. Thus, the Mahalanobis distance was calculated at each profile, and a maximum of the Mahalanobis distance function indicated position of potential architectonical border. The potential border was accepted only after confirmation of its statistical significance by means of a Hotelling's T2 test (p < 0.01) and Bonferroni correction (Palomero-Gallagher and Zilles, 2018, Schleicher et al., 2005). Moreover, the biological relevance of the potential border was confirmed by comparing spatially corresponding borders from adjoining sections.
A comparable strategy was applied for detection of receptor architectonic borders, whereby receptor profiles were extracted from the linearized autoradiographs (Schleicher et al., 2000) and also used for confirmation of identified borders. This procedure provided statistical validation of the position of a cortical border resulting from differences in receptor densities at the level of single or of multiple cortical layers. Furthermore, the receptor profiles of each area were compared with the cytoarchitecture in neighbouring sections from the same area in order to extract layer-specific receptor densities Palomero-Gallagher and Zilles (2019).
2.4.2. Quantitative comparison of mean receptor densities
Statistical testing was used to determine if there were significant differences in receptor densities between adjacent regions. Testing involved three steps and was performed using linear mixed-effects models because these can account for repeated measures within the same subject. Prior to statistical analysis, receptor density values were normalized within each receptor type. All statistical analysis was conducted using the R programming language (version: 3.6.3; Team 2013).
A first omnibus test of all regions and receptors was performed to establish if there were any significant differences in receptor density when all regions and receptor types were analysed simultaneously (Eq. 1). The model consisted of fixed effects for area. The random effects in the model consisted in a nested random intercept for each macaque brain, receptor type, and layer.
| (1) |
where D is the receptor density, A is IPL area, B is macaque brain, R is receptor type, and L is cortical layer.
Since this first test was found to be significant, a second set of tests were used to determine if pairs of adjacent regions were significantly different from one another over all receptor types. The linear mixed effect model used for the second series of tests had the same form as the omnibus test (i.e., Eq. 1), but was only applied to pairs of adjacent regions. The p-values for the main effect “area” were corrected for multiple comparisons using the Benjamini-Hochberg correction for false-discovery rate Benjamini and Hochberg (1995).
Finally, for the pairs of areas that were found to be significantly different from one another in the second level tests after correction for multiple comparisons, a third linear mixed-effect model was used to identify the receptor type driving the statistical difference (Eq. 2). The model was composed of a fixed effect for area and a random intercept for each macaque brain and for each layer. The p-values for the fixed-effect “area” from each of these tests were again corrected using the Benjamini-Hochberg correction for false-discovery rate Benjamini and Hochberg (1995).
| (2) |
where D is the receptor density, A is area, B is macaque brain, and L is cortical layer.
2.4.3. Multivariate cluster analyses
In order to display the densities of multiple receptors within and between different cortical areas more intuitively, the mean areal densities of 15 receptors were visualised for each area separately in a polar plot. The resulting graph is the receptor fingerprint of each area (Zilles et al. 2002a; Zilles et al. 2002b), which shows the multi-receptor balance in each subarea of IPL. Data contained in the fingerprints was used for descriptive statistical analyses to determine the degree of (dis)similarity between the cyto-and receptor architectonically distinct areas identified within the macaque IPL.
Multivariate cluster analyses were carried out with Matlab (The MathWorks, Inc., Natick, MA) as previously described (Palomero-Gallagher et al. 2009) to identify the grouping of IPL areas in the macaque brain based on similarities between their receptor fingerprints. Since absolute receptor concentrations differed considerably between receptor types, a Z score normalization was performed for each receptor separately to ensure an equal weighting of all receptors in the following analyses. For the hierarchical cluster analysis, Euclidean distances were computed to represent the similarities between receptor fingerprints, and a Ward linkage was applied. That means that, the smaller the Euclidean distance between two areas, the greater the similarity in shape and size of their fingerprints. The number of stabile clusters was determined by a subsequent k-means analysis and the elbow method Rousseeuw (1987). A Multi-Dimensional Scaling (MDS) analysis were carried out with performed to convert the 15-dimensional space (15 different receptor types) into two dimensions for graphical representation of the Euclidean distances between areas, whereby the first dimension explains as much of the variability in the areas as possible.
To address the question of homologies between human and macaque IPL areas, we conducted a MDS based on previously published receptor fingerprints obtained from human IPL areas (Caspers et al., 2012, Zilles and Palomero-Gallagher, 2017) and the receptor densities obtained here for macaque IPL areas. Since within a given receptor type, densities differed considerably between species, a Z score normalization encompassing both human and macaque data was performed for each receptor separately. For the MDS analysis, the Kruskal stress scaling method (Sherwood et al. 2004) was used to visualize of the pattern of Euclidean distances between putative homologous areas.
3. Results
In the present study we aimed to i) identify the number and extent of cyto- and receptor architectonically distinct areas within the macaque IPL, and ii) determine whether the degree of (dis)similarities in the neurochemical composition of each identified area can be associated with their involvement in different functional networks.
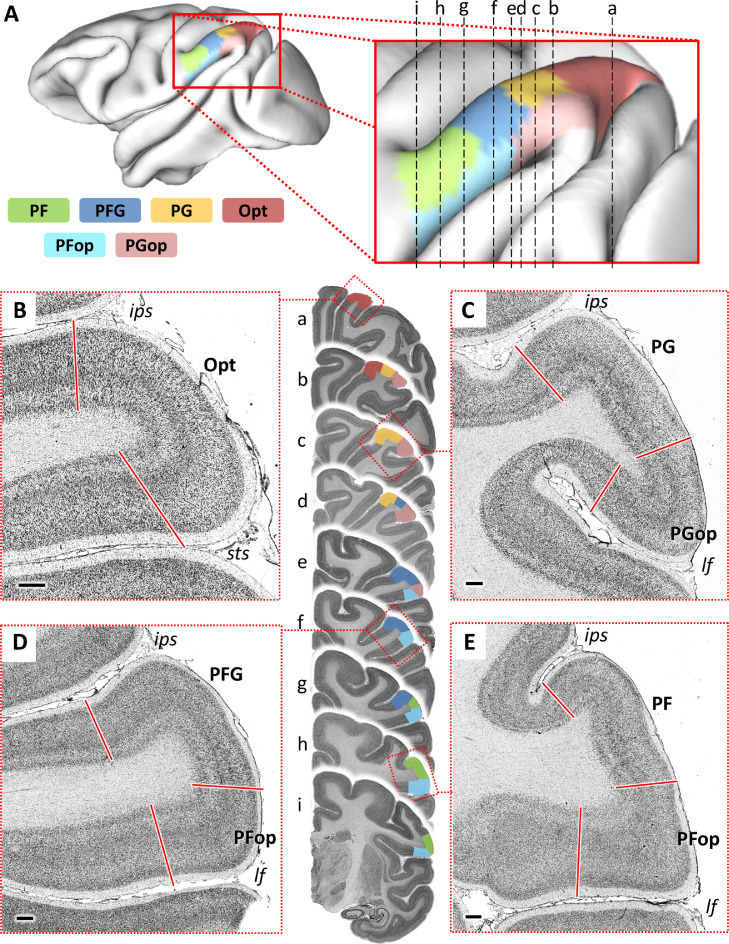
Six cyto- and receptor architectonically distinct areas were identified within the macaque IPL (Fig. 2 and S1 Figure), the location of all cortical borders, i.e. of changes in the laminar distribution patterns of cell bodies or of receptor densities was statistically identified by testing the significance of peaks in the Mahalanobis distance function with a Hotelling's T2 test. Here, we adopted the nomenclature of Pandya and Seltzer (1982): four areas are located at different caudo-rostral levels on the IPL convexity (i.e. areas Opt, PG, PFG, and PF); two additional areas in the parietal operculum (i.e. areas PGop and PFop).
Fig. 2.
Location and extent of IPL areas in the macaque monkey brain. A: 3D reconstruction of the left hemisphere (in lateral view) of Macaca mulatta (DP1) brain obtained using Connectome Workbench software (https://www.humanconnectome.org/software/connectome-workbench) showing the location and extent of the areas that compose the IPL (map made publicly available at https://balsa.wustl.edu/study/7qgpZ). The enlarged reconstruction scheme indicates the approximate positions of cut plane (a-i) for a series of exemplary coronal sections, which were processed for the visualization of topography of IPL areas. B-E: Low magnification views of representative fields of IPL areas taken from the four exemplary coronal sections. For each section, the dashed boxes indicate the location of the photomicrographs; arrows indicate the borders between cytoarchitectonic areas. The cytoarchitectonic subdivisions of the IPL convexity, areas Opt, PG, PFG and PF, are shown in B, C, D and E, respectively. The opercular area PGop is shown in C, PFop are shown in D and E. Dorsal is up and lateral on the right. Scale bar, 500 μm.
Subsequently, stepwise linear mixed-effects models were applied to identify significant differences in the receptor densities between adjacent areas. In the first step a single test was carried out to determine whether there are differences across all regions when all receptor types are considered simultaneously. Since this omnibus test was found to be significant, it was followed by the second level tests, with which we sought to determine, for each of the 8 possible pairs of adjacent areas (Supplementary Table 2), whether they were significantly different from one another over all receptor types. For the 7 pairs of areas found to be significantly different in the second level tests (Supplementary Table 2), the third level tests were used to see which individual receptors drive the difference between areas (Supplementary Table 3).
In addition, each IPL area has its own characteristic receptor expression pattern, and the similarities of their receptor fingerprints enabled the segregation of six IPL areas into to two functionally relevant groups.
3.1. Cytoarchitecture
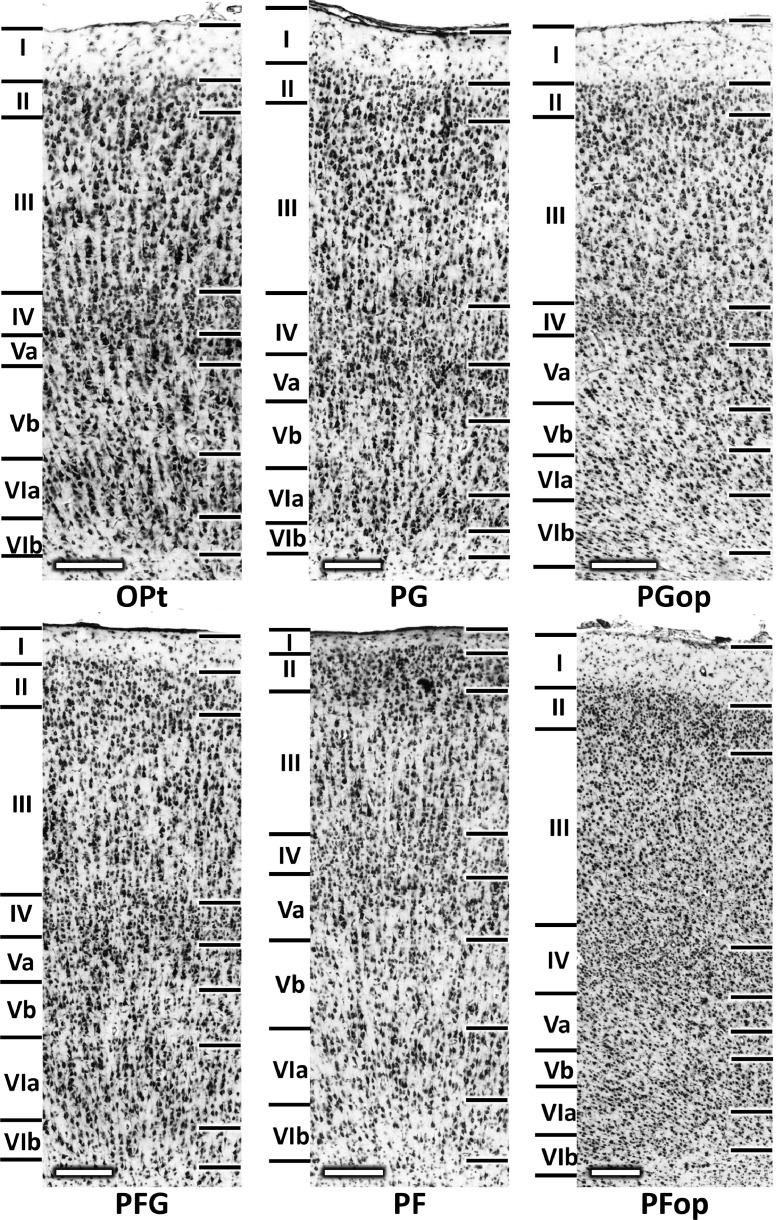
Area Opt occupies the most caudal portion of the IPL and, and encroaches onto the lateral bank of the intraparietal sulcus (Fig. 2A). It is mainly characterized by a columnar appearance, particularly in layer III, and a clear lamination (Figs. 2B and 3). Layer III contains relatively large cells. Layer IV stands out as a densely packed stripe with small-sized granule cells between layers IIIc and Va, which is extremely thin, due to the darkly stained and large pyramids in the latter layers. Layer VI has a clear border with layer Vb (Fig. 3).
Fig. 3.
High-resolution photomicrographs of the IPL areas from the left hemisphere of DP1 brain. Scale bar, 200 μm. Roman numerals indicate cytoarchitectonic layers.
Area PG lies rostral to area Opt (Fig. 2C). PG has a less columnar appearance, a more diffuse boundary between layers I and II, and a wider layer III with smaller pyramids than Opt (Fig. 3). Layer III of PG has a clear boundary with layer IV due to the densely arranged medium-sized pyramids at the interface between both layers. Layers Va and VI of PG have smaller but more densely packed pyramids than those of Opt. The border between layers V and VI of PG appears more prominent than in Opt due to the lower cell packing density in Vb of PG (Fig. 3).
Area PFG, a transition area between PG and rostral area PF, can be clearly delineated from area PG because of its fuzzier lamination and thinner layer I (Fig. 2D). A columnar organization is also visible, though less prominent than that caudal areas. The border between layers I and II is slightly blurred. Layer III of PFG is thinner than that of PG, and contains smaller pyramids, particularly at the border with layer IV. Thus, layer IV of PFG is not as prominent as in the two caudal areas. The infragranular layers of PFG are broader than those of PG and Opt. Layer Va is less cell-dense than in caudal areas and is difficult to segregate from Vb (Fig. 3).
The most rostral area, situated immediately caudal to somatosensory cortex, is area PF (Fig. 2E). A columnar pattern is recognizable in area PF, which has an overall lower cell-packing density, smaller sized pyramids and poorer lamination pattern than caudal IPL areas (Fig. 3). Layer II is exceptional due to its higher cell packing density than in PFG. This fact, together with the reduction in the size and density of layer III cells results in a prominent border between layers II and III. PF has an even thinner layer III than PFG, but considerably broader and less cell-dense layers V and VI than caudal areas (Fig. 3).
The parietal opercular region contains two cytoarchitectonically distinct areas characterized by an overall higher packing density of smaller sized cells than those of IPL areas on the convexity, and the lack of a columnar organization. The more caudal one, situated ventral to area PG and the caudal part of PFG, is termed PGop (Fig. 2A), which can be clearly delineated from the former areas due to its considerably homogeneous infragranular layers. Cells in layers II and IV are more densely packed than in layers III and V, thus enabling their demarcation. Layer III contains evenly distributed medium-sized pyramids (Fig. 3). At the apex of the parietal opercular region, area PGop gives way to area PFop (Fig. 2A, E). PFop has a higher overall cell-packing density and a poorer lamination than does PGop (Fig. 3).
3.2. Receptor architecture
The receptor-based parcellation approach led to the identification of the same 6 IPL areas as previously identified by cytoarchitecture (Fig. 4). Fig. 4, Fig. 5 and S2-S11 Figures provide examples of the receptor architectonically defined borders and the laminar distribution pattern of each IPL area. It is worth noting that not all borders are equally clearly defined by all receptor types. However, when a border was detected by differences in two or more receptor types, this happened at a comparable position to that of the corresponding cytoarchitectonically identified border.
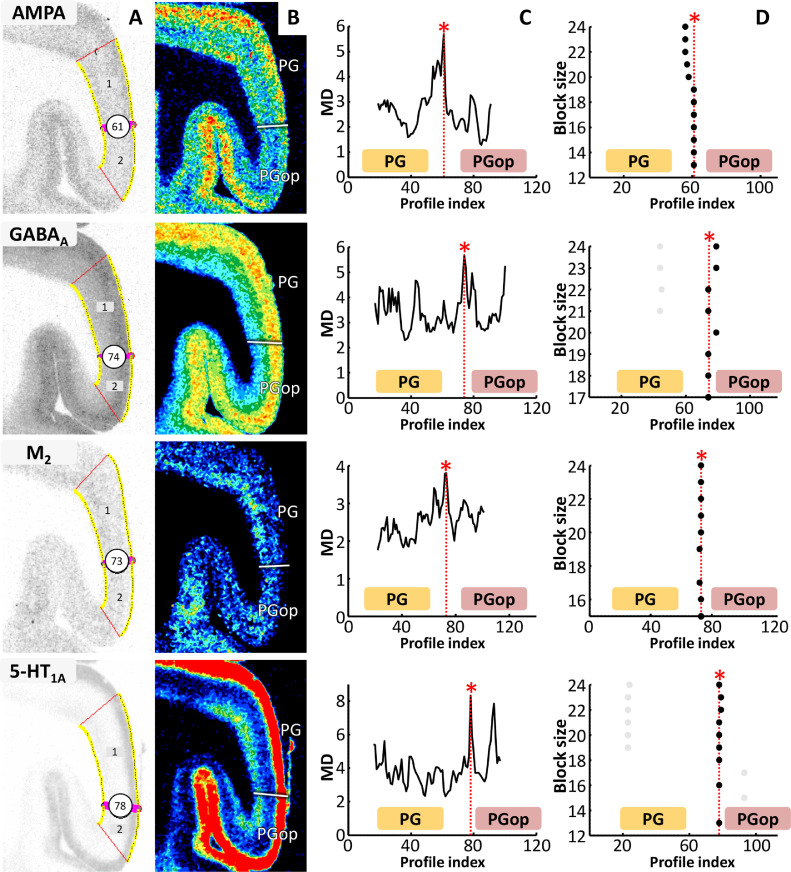
Fig. 4.
Algorithm-based detection of areal border between PG and PGop in exemplary receptor autoradiographs. Take AMPA as an example, A shows automatic labeling of the position of the statistically defined border (at position 61). B: The position of border between PG and PGop as defined by visual inspection is indicated by white bold line in the corresponding pseudocolor coded autoardiograph. C: The Mahalanobis distances between neighboring blocks of 19 profiles; significant maxima occurred at profile positions 61, which identify the border between areas PG and PGop. D: The significant maxima of varying block sizes (ranging from 12 to 24); it indicates a consistently occurring border between two cortical areas at profile positions 61. Same border on corresponding sections of GABAA, M2 and 5-HT1A receptors are shown below. Note the close resemblance of the position of the borders in different receptor sections.
Fig. 5.
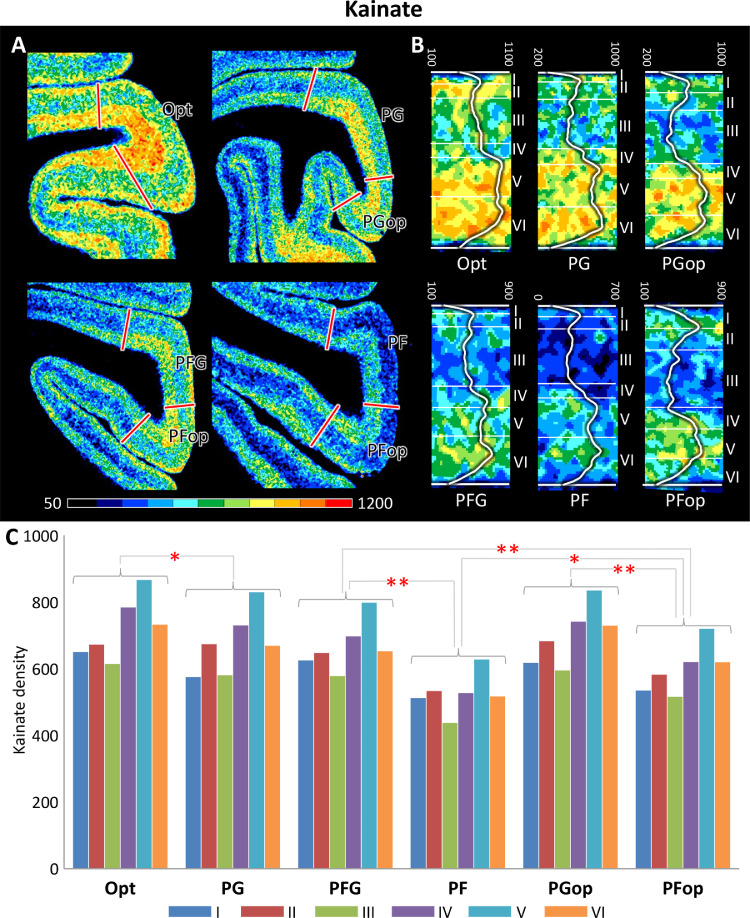
Comparison of laminar receptor densities of kainate receptor in IPL areas. A: Coronal sections through four caudo-rostral levels of a macaque hemisphere showing kainate receptor distribution patterns in the IPL. The borders between the IPL areas (red lines) are charted on the pseudocolor-coded autoradiographs. The color bar beneath each autoradiograph indicates receptor concentrations by the different colors, from dark blue for low to red for high concentrations (fmol/mg protein). B: Laminar distribution pattern of representative fields of IPL areas. Roman numerals indicate cytoarchitectonic layers. C: Bar plot shows receptor densities in each layer for the IPL areas, separately. Receptor densities (y axis) are given in fmol/mg protein. Brackets across bars denote the areas which have significant inter-area differences in laminar densities (* p < 0.05, ** p < 0.01).
3.2.1. Quantitative analysis of receptor densities
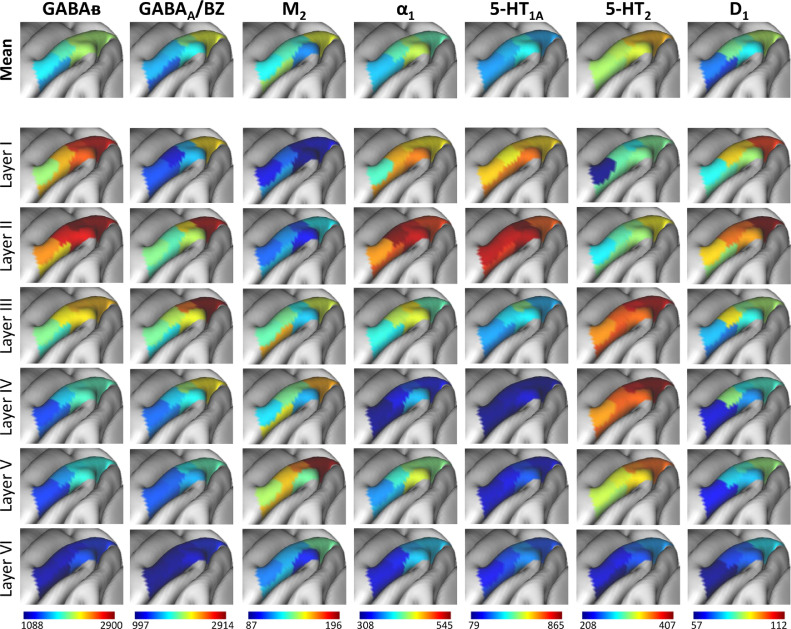
The absolute mean receptor concentration varies considerably between the different receptors in each area. Maximal values are reached in GABAB receptors, with the exception of area Opt, which reach its maximal density in GABAA/BZ binding site densities. GABAA, M1 and NMDA receptors are also present at considerably high densities, whereas lowest absolute densities are reached by the M2 and D1 receptors (Figs. 6 and S12 Figure).
Fig. 6.
The absolute mean areal densities and laminar densities of seven exemplary receptor types (i.e. GABAB, GABAA/BZ, M2, α1, 5-HT1A, 5-HT2 and D1) have been projected onto the corresponding IPL areas. Color bars code for receptor densities in fmol/mg protein. The projections of other receptor types onto the Yerkes 19 surface are shown in S12 Figure. The file coding for the densities of all 15 receptors in each area is provided as Supplementary data 1, and is made available to the neuroscientific community via the Human Brain Project (https://kg.ebrains.eu/search/instances/Project/e39a0407-a98a-480e-9c63-4a2225ddfbe4) and BALSA (https://balsa.wustl.edu/study/7qgpZ) repositories.
Differences in laminar receptor distribution patterns largely contribute to the segregation of the IPL areas. As revealed by the statistical analysis, almost all neighbouring areas (except for PG and PGop) show significant differences in their laminar receptor densities (Supplementary Table 3).
Receptor distribution patterns of the cortex occupying the inferior parietal convexity are shown in Fig. 5A and S2-S5 Figures. The border between Opt and PG is significantly indicated by most receptors (except AMPA, GABAB, α1 and α2). Specifically, Opt contains a higher NMDA, GABAA, GABAA/BZ, M3, D1 receptor density in the supragranular layers and a higher kainate, M2 receptor density in the infragranular layers than PG. In addition, Opt shows a lower 5-HT1A receptor density than PG, especially in layers II and III (S6-S7 Figures; Supplementary Table 3).
Rostrally, PFG could be distinguished from PG mainly by its significantly lower densities in AMPA, NMDA, GABAB, GABAA/BZ, 5-HT1A and 5-HT2 receptors, especially in the infragranular layers (S7-S8 Figures; Supplementary Table 3). As compared with PFG, the rostro-most area PF presented lower densities of almost all receptors (except GABAA and A1). Specifically, the most prominent differences between PFG and PG appeared in kainate, GABAB, M2, M3, α1, α2 and D1 receptors. These differences are more prominent in the supragranular layers for kainate, GABAB, M3 and α1 receptors and in the infragranular layers for α2 receptor, the densities of Μ2 and D1 receptors were lower in the all layers of PF than in those of PFG (S8-S9 Figures; Supplementary Table 3).
Regarding the two IPL opercular areas, significant inter-area differences appeared in kainate, GABAergic, M2, α1, α2, 5-HT1A and D1 receptors. For kainate, GABAB, α1, α2 and 5-HT1A receptors, almost all layers of PGop have higher densities as compared with those of PFop, whereas the opposite holds true for M2 receptors. For GABAA, GABAA/BZ and D1 receptors, inter-area differences mainly appeared in the supragranular layers, which presented higher densities in PGop (S9-S10 Figures; Supplementary Table 3).
Additionally, PGop and PFop have similar distribution patterns with their corresponding convexity areas (PG and PF), respectively. Although PGop and PG are cytoarchitectonically different, they present comparable receptor distribution patterns. Nevertheless, PFop could be distinguished from area PF most clearly by kainate, GABAB, M2, M3 and 5-HT2 receptors, which are present at higher densities in the former area (Figures S5, S9 and S11; Supplementary Table 3). Both IPL opercular areas differed considerably with PFG regarding to the laminar receptor densities. Interestingly, PFG showed significantly lower concentrations of almost all examined receptors (except for AMPA, kainate, muscarinic cholinergic, A1 and D1; Supplementary Table 3) than PGop, but significantly higher concentrations of kainate, GABAB, α1, 5-HT2 and D1 than PFop (Supplementary Table 3).
3.2.2. Multivariate analyses of receptor fingerprints
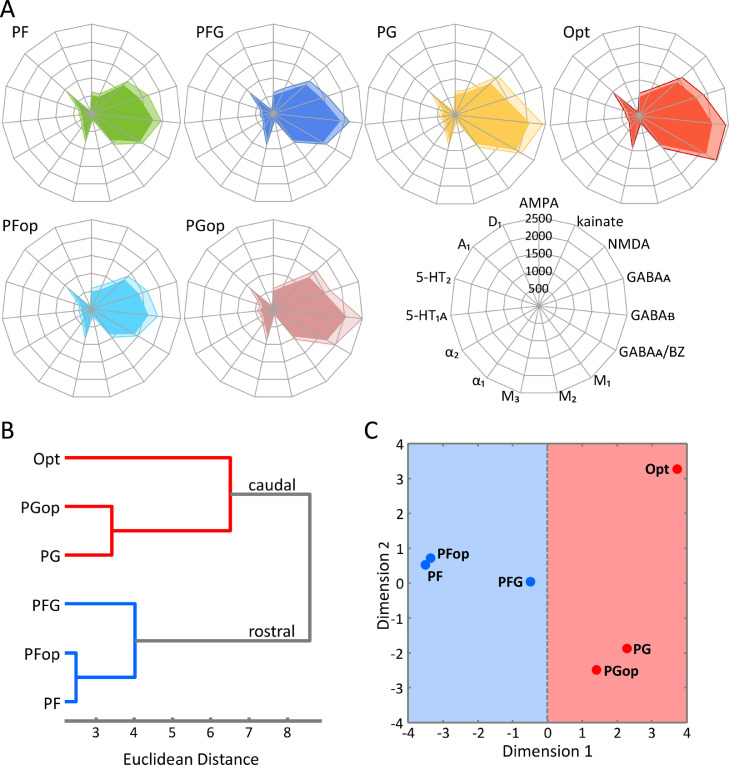
The variances in receptor expression levels between IPL areas can be determined by comparing their receptor fingerprints (Fig. 7). Differences in sizes of fingerprints reveal a rostro-caudal gradient in receptor expression levels: rostral areas (PF/PFop) have the smallest fingerprints, while caudal areas (Opt/PGop) have the largest receptor fingerprint. In particular, the progression from Opt to PG, PFG, and finally to PF was characterized by a decrease in kainite, NMDA, GABAA, GABAA/BZ, M1 and 5-HT1A receptor densities. Moreover, the absolute densities of most receptor types (except for M2, M3, A1 and D1) decreased when moving from PG through PFG to PF. Meanwhile, most receptors (except for M2 and A1) showed concurrent decreases in mean binding site concentrations from PGop to PFop reflecting the rostro-caudal gradient as well.
Fig. 7.
A: Receptor fingerprints of the examined IPL areas. Absolute densities (fmol/mg protein) of 15 receptors are displayed in polar coordinate plots (scaling 0-2500 fmol/mg protein) of 6 brain areas. The corresponding transparent surface indicates the standard deviation. The positions of the different receptor types and the axis scaling are identical in all polar plots, and specified in the polar plot at the bottom right corner of the figure. B: Receptor-driven clustering of the monkey IPL areas. Hierarchical cluster analysis reveals 2 receptor-architectonically distinct clusters: a caudal cluster (red) consisting of areas Opt, PG and PGop; and a rostral cluster (blue) consisting of areas PFG, PF and PFop. C: Multi-Dimensional Scaling (MDS) analysis of the monkey IPL areas. The distances between areas represent the Eigenvalues of the first and second dimensions, two clusters are segregated by the first dimension, same color coding as in A.
The degree of (dis)similarity in the fingerprints of IPL areas was analysed by means of hierarchical cluster, k-means and MDS. The k-means analysis revealed two major branches in the hierarchical tree (Fig. 7B). Areas Opt, PG and PGop cluster together as a “caudal cluster”, and areas PFG, PF, and PFop build a “rostral cluster”. Interestingly, PG and PF are more similar to their corresponding opercular subdivisions (PGop and PFop, respectively) as compared with the other convexity areas in their own cluster (Fig. 7B).
The MDS analysis confirms this organization into two clusters. The segregation between rostral and caudal clusters is clearly revealed by the first dimension of the MDS (Fig. 7C). Interestingly, within the rostral cluster, the first dimension also segregates PFG from areas PF and PFop, whereas within the caudal cluster, area Opt is clearly separated from PG and PGop by the second dimension. Thus, area PFG might be considered as a transitional area between area PG and PF.
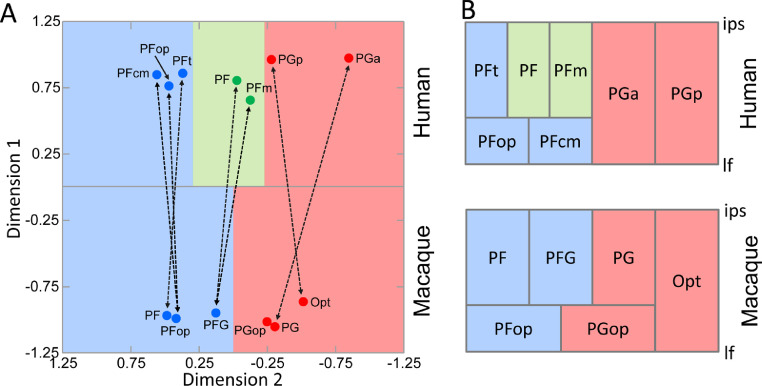
We carried out a MDS in order to address the issue of homologies between human and macaque IPL areas. The first dimension clearly segregates human and macaque areas, whereas the second dimension generally reflects differences in the fingerprints along the rostro-caudal axis of each species (Fig. 8A). Interestingly, as indicated by the smaller normalized Euclidean distance, macaque Opt was more similar to human PGp, whereas macaque PG shows shorter Euclidean distance to human PGp as compared with PGa. Smallest Euclidean distances are found between human areas PF and PFm and macaque area PFG. Macaque areas PF and PFop, close together, had more similarity with the areas belong to human rostral cluster.
Fig. 8.
Comparative analysis of human and macaque IPL areas. A: Multidimensional scaling analysis reveals the segregation of human and macaque areas by the first dimension, whereas the second dimension reflects changes in receptor fingerprints along the rostro-caudal axis of each species. Note, that to facilitate interpretation of the results, the MDS plot has been rotated counter-clockwise by 90° and flipped horizontally. Thus, human areas are found at the top of the plot and macaque areas at the bottom. Rostral areas are to the left, caudal ones to the right. Dashed lines with arrows connect homolog areas. B: Schematic representation of the topographic relationship between IPL areas in the human (top) and macaque (bottom) brains. Colour coding represents clusters as revealed by hierarchical cluster analyses, the detailed information of macaque clusters can be found in Figure 6. Three receptor-architectonically distinct clusters in human were found by Caspers S et al. (2012): a caudal cluster (red) consisting of areas PGp and PGa; an intermediate cluster (green) consisting of areas PFm and PF, and a rostral cluster (blue) consisting of areas PFt, PFcm and PFop. Abbreviations: ips intraparietal sulcus, lf lateral fissure.
4. Discussion
The present study shows that six cyto- and receptor architectonically distinct areas can be identified in the macaque IPL, and provides a detailed description of their architectonic properties. The extent and location of the borders between the identified areas were projected onto the Yerkes19 surface (Donahue et al., 2016), thus providing for the first time a 3D map integrating information on the cyto- and receptor architecture of IPL areas in the macaque monkey brain. This data is made available to the neuroscientific community via the Human Brain Project and BALSA platforms. We could identify caudo-rostral and dorso-ventral gradients in cyto- and receptor architectonic organization. Multivariate analyses revealed a segregation between rostral and caudal areas, hinted at a transitional nature of area PFG, and highlighted the unique role of Opt within the IPL.
4.1. Architectonic gradients
We identified gradual architectonic changes when moving from caudalmost area Opt to rostral areas PFop and PF, as well as principal differences between areas of the convexity and those located in the parietal operculum.
Reflecting the gradients in functional activation patterns which have been described for the IPL (Caspers et al., 2011, Rozzi et al., 2008), we here found two gradual direction trends in the cytoarchitecture of IPL areas: a caudo-rostral and a dorso-ventral gradient. On one hand, the cytoarchitectonic features of caudal areas are the rather heterogeneous size and distribution of the cells with a clear laminar distribution pattern and a fine columnar arrangement (Fig. 2, Fig. 3). From caudal to rostral, as cells become smaller and more homogeneous, the cell density increases, which makes the borders between layers become blurred. One of the most prominent cytoarchitectonic variations from caudal to rostral is the relative thickness of layers III, V and VI. Whereas layer III becomes thinner when moving from caudal to rostral, the opposite holds true for layers V and VI (Fig. 3). At the same time, the number of large pyramidal cells in deep layer III decreases gradually. On the other hand, compared with the areas located on the IPL convexity (dorsal areas), the cytoarchitectonic features of opercular areas (ventral areas) are the practical absence of the columnar appearance and a poor lamination due to the lack of large pyramidal cells in layers III and V (Fig. 3).
The receptor architecture of IPL areas also reflects the caudo-rostral cytoarchitectonic gradient. Areas Opt, PG, PFG and PF of the IPL convexity show a caudal-to-rostral trend in receptor densities, with generally higher values in the caudalmost area. Opercular areas also present a caudo-rostral gradient in receptor densities, with PGop generally containing higher concentrations than PFop (Fig. 6 and S12 Figure).
All in all, a gradual variation in cyto- and receptor architectonic organization of areas is appreciable when moving from the dorsocaudal to the ventrorostral of the IPL areas. These findings will now be discussed in the framework of functional heterogeneity within the IPL, since somatosensory, motor and visual areas have been shown to differ in their receptor architecture not only in the macaque (Impieri et al. 2019; Niu et al. 2020), but also in the human brain (Caspers et al., 2012, Caspers et al., 2014, Eickhoff et al., 2008, Scheperjans et al., 2005).
4.2. Two-cluster model of the macaque IPL
As revealed by the cluster analysis of receptor fingerprints, the organization of the identified IPL areas show a bipartite segregation in the rostro-caudal direction (Fig. 7). As previously shown, variations in the shape and size of fingerprints are associated not only with differences in the functional properties of cortical areas (Barnes and Sharp 1999; Goldman-Rakic et al. 2000; Bredt and Nicoll 2003; Hanks and González-Maeso 2013), but also in their connectivity patterns (Mengod et al., 2015, Rakic et al., 1988, Rapan et al., 2021). Thus, our two-cluster model of macaque IPL could provide an integrated organizational framework within structural, functional, and connectivity information.
The IPL is primarily dedicated to the transformation of sensory information into a motor behavior (Rozzi et al. 2008; Borra and Luppino 2017). That is, areas of the IPL are involved in associating different sensory modalities, with special emphasis on visual information, and then use this information to guide motor behavior. The caudal areas thereby seem to be involved in multisensory integration and visual-motor functions whereas rostral part is more concerned with motor planning and action-related functions (Rozzi et al. 2008).
4.2.1. Caudal cluster
The caudal cluster includes areas Opt, PG, and PGop, which occupy the same position as area 7a of Vogt and Vogt (1919). Activation of caudal IPL areas is associated with visual and oculomotor responses (Hyvarinen 1981; Leinonen et al. 1979; Leinonen and Nyman 1979; Andersen et al. 1990; Rozzi et al. 2008), combined with a poor representation of somatosensory responses (Rozzi et al. 2008). Furthermore, motor properties were widely though weakely represented in caudal IPL (Rozzi et al. 2008). Functionally, the areas in caudal cluster are important for visually guided action (Rozzi et al. 2008), spatial working memory (Leavitt et al. 2017; Froudist-Walsh et al. 2020)and navigation (Guariglia et al. 2005; Doeller et al. 2010). From a connectivity-based perspective, caudal IPL areas in macaques showed connections to superior parietal areas, extrastriate visual areas (e.g. PO, DP), and multiple temporal areas (Caspers et al., 2011). Specifically, it links the dorsal visual pathway to the medial temporal lobe, including parahippocampal cortex and hippocampus, supporting navigation, which may make it a likely node in the default mode network .
Within the caudal cluster, areas PG and PGop were more similar to each other than to Opt. Area Opt is considered to be a component of the dorsal visual stream (Fattori et al. 2017), and its principal functional role is the control of saccadic (Barash et al. 1991) and oculomotor (Bruce et al. 1985; Bremmer et al. 1997) movements. Indeed, Opt neurons have large visual receptive fields mainly modulated by the position of the eye (Andersen et al. 1990; Blatt et al. 1990). Interestingly, Opt is the only IPL area in which the density of GABAA/BZ binding sites is higher than that of GABAB receptors (Figure 7). In fact, of all IPL areas, the receptor fingerprint of Opt shows most pronounced similarities with those of higher extrastriate visual areas, particularly LIP (Niu et al. 2020). That means, similar to PGp in the human brain (Caspers et al., 2012), area Opt might serve as a hub, receiving input from extrastriate visual areas and sending it to areas of the parietal and temporal lobes (Kravitz et al. 2011).
Neurons of PG are characterized by visual, motor and minor somatomotor properties (Rozzi et al. 2008). Visual responses are widely represented in PG, though mainly related to fixation or movement of objects and space-related responses, in line with its connections with visual area MST and multisensory areas Tpt and STP (Andersen et al. 1990; Rozzi et al. 2006). Area PG is more strongly involved in the control of arm reaching than in oculomotor movements (Rozzi et al. 2008), which is consistent with a connectional study (Rozzi et al. 2006) showing that PG is connected with the functionally defined parietal reaching region (Andersen et al. 2014; Snyder et al. 1997). In summary, the possible functional role of PG is the organization and regulation of eye-guided reaching movement by using position and motion information (Battaglia-Mayer et al. 2006).
4.2.2. Rostral cluster
The rostral cluster encompasses areas PFG and PF of the convexity, as well as opercular area PFop. These areas were originally identified as area 7b by Vogt and Vogt (1919). Contrary to the situation described for areas of the caudal cluster, somatosensory and body-related motor responses are widely represented in the rostral cluster, whereas visual responses weaken when moving rostrally, and are absent in the most rostral part (Andersen et al. 1990; Rozzi et al. 2008). Functionally, the areas in rostral cluster are specialized for guiding action in peripersonal space (Kravitz et al. 2011). Neurons in the rostral cluster are responsive to observed and executed actions (Evangeliou et al. 2009), and are selective for particular components of observed actions (Gardner et al. 2007). The differential connectivity pattern of rostral IPL areas with (pre-) motor, inferior frontal, somatosensory, superior parietal and posterior temporal areas is in accordance with their functional profiles (Caspers et al., 2011, Petrides and Pandya, 2009, Rozzi et al., 2006). Tactile and proprioceptive input, which are important for guiding motor actions of the face, mouth, and hand/arm (Rozzi et al. 2006), particularly for the execution of precision grasping movement (Hikosaka et al. 1985), reach areas PF and PFG via the “Parietal Inferior-to-Postcentral” tract (Rozzi et al. 2006; Catani et al. 2017). Furthermore, the rostral areas receive vestibular input from cerebellum and are strongly connected with somatosensory areas.
We found PFG to be cyto- and receptor architectonically distinct from areas PG and PF. Thus, our results further support the notion that area PFG should not be considered a transitional zone between the purely visual-motor area PG and somatosensory area PF (Rozzi et al. 2008; Gregoriou et al. 2006). Most neurons of PFG were multimodal, showing sensitivity to both somatosensory, motor activity and visual stimuli. The motor act most represented in PFG is that of hand movements (eg. hand grasping), which are frequently associated with tactile responses to stimuli applied to the hand and mouth, and it is richly endowed with neurons responding to visual stimuli, particularly to peripersonal and object presentation (Rozzi et al. 2008). Thus, PFG is thought to play a crucial role in the regulation of hand or hand-to-mouth actions, aimed to manipulate the objects on the basis of their physical properties and location in peripersonal space. Furthermore, area PFG constitutes a crucial node in the mirror neurons system, since it contains mirror neurons with visuomotor properties (Rizzolatti et al. 2006; Rozzi et al. 2008) and is connected with ventral premotor area F5 (Rozzi et al. 2006).
The functional profile of anterior-most area PF is dominated by motor and somatosensory responses. The motor acts most represented in area PF are mouth related movements, such as biting and licking (Rozzi et al. 2008). Correspondingly, the most represented somatosensory responses are related to the stimuli applied to the face or the mouth (Rozzi et al. 2008). PF is connected with the mouth fields of somatosensory areas SI and SII (Petrides and Pandya 1984; Rozzi et al. 2006). In summary, PF integrates somatosensory and motor information to guide the execution of appropriate food-related mouth motor acts.
4.3. Homologies
Brodmann (1909) considered the IPL of the Old World monkey to be covered by a single cytoarchitectonic area (namely BA7) constituting an undifferentiated precursor zone for what he termed “human-specific” areas BA40 rostrally and BA39 caudally on the IPL and BA7 on the SPL. Since this assumption would not only imply an extremely fast cortical evolution of BA39 and BA40, but also a complete architectonical reorganization of the parietal lobe during brain evolution from monkeys to humans, BA7 in macaques should not be considered equivalent to human BA7 (Zilles and Palomero-Gallagher 2001). von Economo and Koskinas (1925) identified five subdivisions within BA40 which are arranged in two rostro-caudal rows: three areas (i.e. PF, PFm, and PFt) dorsally and two smaller areas (i.e. PFcm and PFop) ventrally. More recently, the existence of these subdivisions within BA40 was confirmed by means of quantitative cyto- and receptorarchitectonic analyses, which also revealed anterior (PGa) and posterior subdivisions (PGp) of BA39 (Caspers et al., 2006, Caspers et al., 2012). Thus, in human IPL, the lateral parietal surface encompasses five areas, and two areas are located within the caudal parietal operculum (Caspers et al., 2006; Fig. 8A). In monkey, a similar topological organization of IPL has been established by Pandya and Seltzer (1982) and Preuss and Goldman-Rakic (1991): four areas on the convexity of the IPL (areas Opt, PG, PFG and PF from caudal to rostral) and two areas on the parietal operculum within the Sylvian fissure (PGop caudally and PFop rostrally; Fig. 8A).
Based on the analysis of connectivity patterns, (Caspers et al., 2011) proposed macaque areas Opt/PG/PFG/PF to correspond to human areas PGp/PGa/PF/PFt, respectively. The present results not only confirm the existence of these congruencies between areas of the human and monkey IPL, but also provide new insights into for further clarifying the homologies between the IPL areas of different species. In particular, PFm is considered a human specific area that does not have an obvious homologue in macaque (Caspers et al., 2011). Given the similarity in the Euclidean distances between the fingerprints of human PFm and macaque PFG, and those of human PF and macaque PFG, we suggest that human PFm is presumably derived from a subdivision of area PFG in the macaque IPL.
Concerning the opercular areas, our results argue against an equivalence between human PFcm and macaque PGop, despite a topological correspondence, since we found macaque areas PFop and PGop to be located in different clusters. Furthermore, the cytoarchitecture of PGop closely resembled that of PG. Rather, and for the same reasons as discussed above, we propose both human PFop and PFcm to have derived from macaque PFop. Further analyses are necessary to understand the homology between macaque PGop and human areas. Since human PGa encroaches into the angular sulcus, which is the prolongation of the superior temporal sulcus, the homolog of macaque PGop could be located in the region of the temporo-parietal junction, which has not yet been analysed in detail in humans. Indeed, in the most recent map of the human brain, this region is occupied by the temporal-to-parietal GapMap (Amunts et al. 2020).
IPL areas present a functional gradient in both the human and the macaque brain, with caudal areas being strongly involved in the visual domain and rostral ones in sensorimotor processing (Rozzi et al. 2008). However, multivariate analyses of the receptor fingerprints of IPL areas reveal differences in the molecular organization between humans and macaques. Whereas for the human brain IPL areas were found to build three clusters (Caspers et al., 2012), in macaques we could only identify a rostral and a caudal cluster. Indeed, whereas human areas PF and PFm constitute a distinct intermediate cluster (Caspers et al., 2012)), their homolog in the macaque brain, area PFG, clusters together with rostral IPL areas.
To provide a possible explanation for this difference, two factors should be considered, i.e. the possible function of the corresponding areas, and evolution between species. The transformation of sensory information into a motor format appears to be the basic organizational principle of primate IPL, and areas located in the middle part, i.e. human PFm/PF or macaque PFG, play a crucial role in information integration and coordination. Additionally, some neurons in these areas are found to be involved in the organization of complex actions, and some have been further implicated in action recognition and intention understanding (Fogassi et al. 2005). It is plausible that, with the expansion of cortical surface and the fact that humans are able to perform more challenging cognitive tasks than macaques, intermediate area PFG evolved into a unique linking cluster containing areas PF and PFm. In humans, areas of the intermediate cluster would not only be responsible for integration of visual and sensorimotor information, but also be related to higher-level cognitive functions, such as attention, and the evaluation and control of visually guided motion.
5. Conclusions
The present study provides a detailed parcellation of the macaque IPL based on the quantitative analysis of its cytoarchitecture and receptor distribution patterns. We identified six cyto- and receptor architectonically distinct areas: Opt, PG, PFG, and PF on the convexity, as well as PGop and PFop within the parietal operculum. We identified caudo-rostral, and to a lesser extent dorso-ventral, gradients in the cyto- and receptor architectonic organization of IPL areas, which are in good agreement with previously described segregations in functional roles (Rozzi et al. 2006), as well as in connectivity patterns (Caspers et al., 2011, Rozzi et al., 2006), thus providing strong evidence for a general organizational principle of monkey IPL. Thus, our data provide crucial insights enabling determination of homologies between the IPL areas of human and macaque monkey. Finally, we provide for the first time a 3D map of the macaque IPL, and make it publicly available via the Human Brain Project (https://kg.ebrains.eu/search/instances/Project/e39a0407-a98a-480e-9c63-4a2225ddfbe4) and BALSA (https://balsa.wustl.edu/study/7qgpZ) repositories, thus enabling cyto- and/or receptor architectonically driven analyses of future functional imaging studies in non-human primates.
6. Author contributions
Meiqi Niu: Investigation, Data Curation, Visualization, Writing – Original Draft, Writing – Review and Editing;
Lucija Rapan: Validation, Visualization, Writing – Review and Editing;
Thomas Funck: Formal Analysis, Writing – Review and Editing;
Sean Froudist-Walsh: Formal Analysis, Visualization, Writing – Review and Editing, Funding acquisition;
Ling Zhao: Formal Analysis, Writing – Review and Editing;
Karl Zilles: Conceptualization, Resources, Supervision, Funding acquisition;
Nicola Palomero-Gallagher: Conceptualization, Resources, Supervision, Writing – Review and Editing, Project administration, Funding acquisition.
7. Data and code availability statements
Upon publication, the ensuing 3D map of the macaque IPL, and the receptor fingerprints will be made publicly available to the neuroscientific community via the Human Brain Project and BALSA repositories. The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials .
Declaration of Competing Interests
The authors declare that they have no competing financial interests.
Acknowledgements
The authors wish to thank Prof. Katrin Amunts for fruitful discussions. This project has received funding from the European Union's Horizon 2020 Research and Innovation Programme under the Specific Grant Agreements 785907 (Human Brain Project SGA2) and 945539 (Human Brain Project SGA3), from the Federal Ministry of Education and Research (BMBF) under project number 01GQ1902, from the National Institute of Health (NIH) under grant number R01MH122024-02, and from the Helmholtz Association's Initiative and Networking Fund through the Helmholtz International BigBrain Analytics and Learning Laboratory (HIBALL) under the Helmholtz International Lab grant agreement InterLabs-0015.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2021.117843.
Contributor Information
Meiqi Niu, Email: m.niu@fz-juelich.de.
Nicola Palomero-Gallagher, Email: n.palomero-gallagher@fz-juelich.de.
Appendix. Supplementary materials
References
- Amunts K., Lenzen M., Friederici A.D., Schleicher A., Morosan P., Palomero-Gallagher N. Broca's region: novel organizational principles and multiple receptor mapping. PLoS Biol. 2010;8(9) doi: 10.1371/journal.pbio.1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K., Mohlberg H., Bludau S., Zilles K. Julich-Brain: a 3D probabilistic atlas of the human brain's cytoarchitecture. Science. 2020 doi: 10.1126/science.abb4588. [DOI] [PubMed] [Google Scholar]
- Andersen R.A., Andersen K.N., Hwang E.J., Hauschild M. Optic ataxia: from Balint's syndrome to the parietal reach region. Neuron. 2014;81(5):967–983. doi: 10.1016/j.neuron.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen R.A., Asanuma C., Essick G., Siegel R. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J. Comp. Neurol. 1990;296(1):65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- Asanuma C., Thach W., Jones E. Cytoarchitectonic delineation of the ventral lateral thalamic region in the monkey. Brain Res. Rev. 1983;5(3):219–235. doi: 10.1016/0165-0173(83)90014-0. [DOI] [PubMed] [Google Scholar]
- Barash S., Bracewell R.M., Fogassi L., Gnadt J.W., Andersen R.A. Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a. J. Neurophysiol. 1991;66(3):1095–1108. doi: 10.1152/jn.1991.66.3.1095. [DOI] [PubMed] [Google Scholar]
- Barnes N.M., Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A., Mascaro M., Caminiti R. Temporal evolution and strength of neural activity in parietal cortex during eye and hand movements. Cereb. Cortex. 2006;17(6):1350–1363. doi: 10.1093/cercor/bhl046. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc. 1995;57(1):289–300. [Google Scholar]
- Binkofski F.C., Klann J., Caspers S. Neurobiology of Language. Elsevier; 2016. On the neuroanatomy and functional role of the inferior parietal lobule and intraparietal sulcus; pp. 35–47. [Google Scholar]
- Blatt G.J., Andersen R.A., Stoner G.R. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J. Comp. Neurol. 1990;299(4):421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- Borra E., Luppino G. Functional anatomy of the macaque temporo-parieto-frontal connectivity. Cortex. 2017;97:306–326. doi: 10.1016/j.cortex.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Bozkurt A., Zilles K., Schleicher A., Kamper L., Arigita E.S., Uylings H.B. Distributions of transmitter receptors in the macaque cingulate cortex. Neuroimage. 2005;25(1):219–229. doi: 10.1016/j.neuroimage.2004.10.040. [DOI] [PubMed] [Google Scholar]
- Bredt D.S., Nicoll R.A. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40(2):361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Bremmer F., Distler C., Hoffmann K.-P. Eye position effects in monkey cortex. II. Pursuit-and fixation-related activity in posterior parietal areas LIP and 7A. J. Neurophysiol. 1997;77(2):962–977. doi: 10.1152/jn.1997.77.2.962. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Barth; 1909. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. [Google Scholar]
- Bruce C.J., Goldberg M.E., Bushnell M.C., Stanton G.B. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J. Neurophysiol. 1985;54(3):714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever J., Cohen B., Pause M., Fries W. Raphe nucleus of the pons containing omnipause neurons of the oculomotor system in the monkey, and its homologue in man. J. Comp. Neurol. 1988;267(3):307–321. doi: 10.1002/cne.902670302. [DOI] [PubMed] [Google Scholar]
- Caspers J., Palomero-Gallagher N., Caspers S., Schleicher A., Amunts K., Zilles K. Receptor architecture of visual areas in the face and word-form recognition region of the posterior fusiform gyrus. Brain Struct. Function. 2015;220(1):205–219. doi: 10.1007/s00429-013-0646-z. [DOI] [PubMed] [Google Scholar]
- Caspers J., Zilles K., Amunts K., Laird A.R., Fox P.T., Eickhoff S.B. Functional characterization and differential coactivation patterns of two cytoarchitectonic visual areas on the human posterior fusiform gyrus. Hum. Brain Mapp. 2014;35(6):2754–2767. doi: 10.1002/hbm.22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S., Eickhoff S.B., Rick T., von Kapri A., Kuhlen T., Huang R. Probabilistic fibre tract analysis of cytoarchitectonically defined human inferior parietal lobule areas reveals similarities to macaques. Neuroimage. 2011;58(2):362–380. doi: 10.1016/j.neuroimage.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S., Geyer S., Schleicher A., Mohlberg H., Amunts K., Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33(2):430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Caspers S., Schleicher A., Bacha-Trams M., Palomero-Gallagher N., Amunts K., Zilles K. Organization of the human inferior parietal lobule based on receptor architectonics. Cereb. Cortex. 2012;23(3):615–628. doi: 10.1093/cercor/bhs048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Robertsson N., Beyh A., Huynh V., de Santiago Requejo F., Howells H. Short parietal lobe connections of the human and monkey brain. Cortex. 2017;97:339–357. doi: 10.1016/j.cortex.2017.10.022. [DOI] [PubMed] [Google Scholar]
- Colby C.L. Action-oriented spatial reference frames in cortex. Neuron. 1998;20(1):15–24. doi: 10.1016/s0896-6273(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Ding S.L., Morecraft R.J., Van Hoesen G.W. Topography, cytoarchitecture, and cellular phenotypes of cortical areas that form the cingulo-parahippocampal isthmus and adjoining retrocalcarine areas in the monkey. J. Comp. Neurol. 2003;456(2):184–201. doi: 10.1002/cne.10516. [DOI] [PubMed] [Google Scholar]
- Doeller C.F., Barry C., Burgess N. Evidence for grid cells in a human memory network. Nature. 2010;463(7281):657–661. doi: 10.1038/nature08704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue C.J., Sotiropoulos S.N., Jbabdi S., Hernandez-Fernandez M., Behrens T.E., Dyrby T.B., Glasser M.F. Using diffusion tractography to predict cortical connection strength and distance: A quantitative comparison with tracers in the monkey. J. Neurosci. 2016;36:6758–6770. doi: 10.1523/JNEUROSCI.0493-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Rottschy C., Kujovic M., Palomero-Gallagher N., Zilles K. Organizational principles of human visual cortex revealed by receptor mapping. Cereb. Cortex. 2008;18(11):2637–2645. doi: 10.1093/cercor/bhn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangeliou M.N., Raos V., Galletti C., Savaki H.E. Functional imaging of the parietal cortex during action execution and observation. Cereb. Cortex. 2009;19(3):624–639. doi: 10.1093/cercor/bhn116. [DOI] [PubMed] [Google Scholar]
- Fattori P., Breveglieri R., Bosco A., Gamberini M., Galletti C. Vision for prehension in the medial parietal cortex. Cereb. Cortex. 2017;27(2):1149–1163. doi: 10.1093/cercor/bhv302. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Ferrari P.F., Gesierich B., Rozzi S., Chersi F., Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308(5722):662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Froudist-Walsh, S., Bliss, D. P., Ding, X., Jankovic-Rapan, L., Niu, M., Knoblauch, K., et al. (2020). A dopamine gradient controls access to distributed working memory in monkey cortex. 2020.2009.2007.286500, doi:10.1101/2020.09.07.286500 %J bioRxiv. [DOI] [PMC free article] [PubMed]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol. Res. 1979;1(2):203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Gardner E.P., Babu K.S., Reitzen S.D., Ghosh S., Brown A.S., Chen J. Neurophysiology of prehension. I. Posterior parietal cortex and object-oriented hand behaviors. J. Neurophysiol. 2007;97(1):387–406. doi: 10.1152/jn.00558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S., Luppino G., Ekamp H., Zilles K. The macaque inferior parietal lobule: cytoarchitecture and distribution pattern of serotonin 5-HT 1A binding sites. Anatomy Embryol. 2005;210(5-6):353–362. doi: 10.1007/s00429-005-0026-4. [DOI] [PubMed] [Google Scholar]
- Geyer S., Matelli M., Luppino G., Schleicher A., Jansen Y., Palomero-Gallagher N. Receptor autoradiographic mapping of the mesial motor and premotor cortex of the macaque monkey. J. Comp. Neurol. 1998;397(2):231–250. doi: 10.1002/(sici)1096-9861(19980727)397:2<231::aid-cne6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Geyer S., Schleicher A., Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex: 1. Microstructural organization and interindividual variability. Neuroimage. 1999;10(1):63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S., Muly E.C., III, Williams G. D₁ receptors in prefrontal cells and circuits. Brain Res. Rev. 2000 doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Gregoriou G.G., Borra E., Matelli M., Luppino G. Architectonic organization of the inferior parietal convexity of the macaque monkey. J. Comp. Neurol. 2006;496(3):422–451. doi: 10.1002/cne.20933. [DOI] [PubMed] [Google Scholar]
- Guariglia C., Piccardi L., Iaria G., Nico D., Pizzamiglio L. Representational neglect and navigation in real space. Neuropsychologia. 2005;43(8):1138–1143. doi: 10.1016/j.neuropsychologia.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Hanks J.B., González-Maeso J. Animal models of serotonergic psychedelics. ACS Chem. Neurosci. 2013;4(1):33–42. doi: 10.1021/cn300138m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbroner P.L., Holloway R.L. Anatomical brain asymmetry in monkeys: frontal, temporoparietal, and limbic cortex in Macaca. Am. J. Phys. Anthropol. 1989;80(2):203–211. doi: 10.1002/ajpa.1330800208. [DOI] [PubMed] [Google Scholar]
- Hikosaka O., Tanaka M., Sakamoto M., Iwamura Y. Deficits in manipulative behaviors induced by local injections of muscimol in the first somatosensory cortex of the conscious monkey. Brain Res. 1985;325(1-2):375–380. doi: 10.1016/0006-8993(85)90344-0. [DOI] [PubMed] [Google Scholar]
- Hyvarinen J. Regional distribution of functions in parietal association area 7 of the monkey. Brain Res. 1981;206(2):287–303. doi: 10.1016/0006-8993(81)90533-3. doi:Doi 10.1016/0006-8993(81)90533-3. [DOI] [PubMed] [Google Scholar]
- Impieri D., Zilles K., Niu M., Rapan L., Schubert N., Galletti C. Receptor density pattern confirms and enhances the anatomic-functional features of the macaque superior parietal lobule areas. Brain Struct. Function. 2019;224(8):2733–2756. doi: 10.1007/s00429-019-01930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz D.J., Saleem K.S., Baker C.I., Mishkin M. A new neural framework for visuospatial processing. Nat. Rev. Neurosci. 2011;12(4):217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt M.L., Mendoza-Halliday D., Martinez-Trujillo J.C. Sustained activity encoding working memories: not fully distributed. Trends Neurosci. 2017;40(6):328–346. doi: 10.1016/j.tins.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Leinonen L., Hyvärinen J., Nyman G., Linnankoski I. I. Functional properties of neurons in lateral part of associative area 7 in awake monkeys. Exp. Brain Res. 1979;34(2):299–320. doi: 10.1007/BF00235675. [DOI] [PubMed] [Google Scholar]
- Leinonen L., Nyman G. II. Functional properties of cells in anterolateral part of area 7 associative face area of awake monkeys. Exp. Brain Res. 1979;34(2):321–333. doi: 10.1007/BF00235676. [DOI] [PubMed] [Google Scholar]
- Mahalanobis P.C., Majumdar D., Yeatts M., Rao C.R. Anthropometric survey of the United Provinces, 1941: a statistical study. Sankhyā. 1949:89–324. [Google Scholar]
- Mengod G., Palacios J.M., Cortes R. Cartography of 5-HT1A and 5-HT2A receptor subtypes in prefrontal cortex and its projections. ACS Chem. Neurosci. 2015;6(7):1089–1098. doi: 10.1021/acschemneuro.5b00023. [DOI] [PubMed] [Google Scholar]
- Merker B. Silver staining of cell bodies by means of physical development. J. Neurosci. Methods. 1983;9(3):235–241. doi: 10.1016/0165-0270(83)90086-9. [DOI] [PubMed] [Google Scholar]
- Niu M., Impieri D., Rapan L., Funck T., Palomero-Gallagher N., Zilles K. Receptor-driven, multimodal mapping of cortical areas in the macaque monkey intraparietal sulcus. eLife. 2020;9:e55979. doi: 10.7554/eLife.55979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N., Vogt B.A., Schleicher A., Mayberg H.S., Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum. Brain Mapp. 2009;30(8):2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N., Zilles K. Cyto-and receptor architectonic mapping of the human brain. Handbook Clin. Neurol. 2018;150:355–387. doi: 10.1016/B978-0-444-63639-3.00024-4. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N., Zilles K. Cortical layers: Cyto-, myelo-, receptor-and synaptic architecture in human cortical areas. Neuroimage. 2019;197:716–741. [Google Scholar]
- Palomero-Gallagher, N., Zilles, K., Schleicher, A., & Vogt, B. A. J. J. o. C. N. (2013). Cyto-and receptor architecture of area 32 in human and macaque brains. 521(14), 3272-3286. [DOI] [PubMed]
- Pandya D.N., Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. J. Comp. Neurol. 1982;204(2):196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Peters A. Primary visual Cortex in Primates. Springer; 1994. The organization of the primary visual cortex in the macaque; pp. 1–35. [Google Scholar]
- Petrides M., Pandya D.N. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J. Comp. Neurol. 1984;228(1):105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. Distinct parietal and temporal pathways to the homologues of Broca's area in the monkey. PLoS Biol. 2009;7(8) doi: 10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss T.M., Goldman-Rakic P.S. Myelo-and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J. Comp. Neurol. 1991;310(4):429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- Rakic P., Goldman-Rakic P.S., Gallager D. Quantitative autoradiography of major neurotransmitter receptors in the monkey striate and extrastriate cortex. J. Neurosci. 1988;8(10):3670–3690. doi: 10.1523/JNEUROSCI.08-10-03670.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapan L., Froudist-Walsh S., Niu M., Xu T., Funck T., Palomero-Gallagher N. Multimodal 3D atlas of the macaque monkey motor and premotor cortex. NeuroImage. 2021;226:117574. doi: 10.1016/j.neuroimage.2020.117574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Fogassi L., Gallese V. Parietal cortex: from sight to action. Curr. Opin. Neurobiol. 1997;7(4):562–567. doi: 10.1016/s0959-4388(97)80037-2. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fogassi L., Gallese V. Mirrors in the mind. Sci. Am. 2006;295(5):54–61. doi: 10.1038/scientificamerican1106-54. [DOI] [PubMed] [Google Scholar]
- Rousseeuw P. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987;20:53–65. [Google Scholar]
- Rozzi S., Calzavara R., Belmalih A., Borra E., Gregoriou G.G., Matelli M. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb. Cortex. 2006;16(10):1389–1417. doi: 10.1093/cercor/bhj076. [DOI] [PubMed] [Google Scholar]
- Rozzi S., Ferrari P.F., Bonini L., Rizzolatti G., Fogassi L. Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur. J. Neurosci. 2008;28(8):1569–1588. doi: 10.1111/j.1460-9568.2008.06395.x. [DOI] [PubMed] [Google Scholar]
- Scheperjans F., Palomero-Gallagher N., Grefkes C., Schleicher A., Zilles K. Transmitter receptors reveal segregation of cortical areas in the human superior parietal cortex: relations to visual and somatosensory regions. Neuroimage. 2005;28(2):362–379. doi: 10.1016/j.neuroimage.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Schleicher A., Amunts K., Geyer S., Kowalski T., Schormann T., Palomero-Gallagher N. A stereological approach to human cortical architecture: identification and delineation of cortical areas. J. Chem. Neuroanat. 2000;20(1):31–47. doi: 10.1016/s0891-0618(00)00076-4. [DOI] [PubMed] [Google Scholar]
- Schleicher A., Amunts K., Geyer S., Morosan P., Zilles K. Observer-independent method for microstructural parcellation of cerebral cortex: a quantitative approach to cytoarchitectonics. Neuroimage. 1999;9(1):165–177. doi: 10.1006/nimg.1998.0385. [DOI] [PubMed] [Google Scholar]
- Schleicher A., Palomero-Gallagher N., Morosan P., Eickhoff S., Kowalski T., De Vos K. Quantitative architectural analysis: a new approach to cortical mapping. Anatomy Embryol. 2005;210(5-6):373–386. doi: 10.1007/s00429-005-0028-2. [DOI] [PubMed] [Google Scholar]
- Schleicher A., Zilles K. A quantitative approach to cytoarchitectonics: analysis of structural inhomogeneities in nervous tissue using an image analyser. J. Microsc. 1990;157(3):367–381. doi: 10.1111/j.1365-2818.1990.tb02971.x. [DOI] [PubMed] [Google Scholar]
- Sherwood C.C., Holloway R.L., Erwin J.M., Hof P.R. Cortical orofacial motor representation in old world monkeys, great apes, and humans. Brain, Behav. Evol. 2004;63(2):82–106. doi: 10.1159/000075673. [DOI] [PubMed] [Google Scholar]
- Snyder L.H., Batista A., Andersen R.A. Coding of intention in the posterior parietal cortex. Nature. 1997;386(6621):167. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Stanton G.B., Deng S.Y., Goldberg E., McMullen N. Cytoarchitectural characteristic of the frontal eye fields in macaque monkeys. J. Comp. Neurol. 1989;282(3):415–427. doi: 10.1002/cne.902820308. [DOI] [PubMed] [Google Scholar]
- Team, R. C. (2013). R: A Language and environment for statistical computing.
- Vogt C., Vogt O. Allgemeinere ergebnisse unserer hirnforschung. Journal of Neurology and Psychology. 1919;25:279–461. [Google Scholar]
- Von Bonin G., Bailey P. University of Illinois Press; Urbana, IL: 1947. The neocortex of Macaca mulatta. [Google Scholar]
- von Economo C.F., Koskinas G.N. J. Springer; 1925. Die cytoarchitektonik der hirnrinde des erwachsenen menschen. [Google Scholar]
- Wree A., Schleicher A., Zilles K. Estimation of volume fractions in nervous tissue with an image analyzer. J. Neurosci. Methods. 1982;6(1-2):29–43. doi: 10.1016/0165-0270(82)90014-0. [DOI] [PubMed] [Google Scholar]
- Yokochi H., Tanaka M., Kumashiro M., Iriki A. Inferior parietal somatosensory neurons coding face-hand coordination in Japanese macaques. Somatosens. Motor Res. 2003;20(2):115–125. doi: 10.1080/0899022031000105145. [DOI] [PubMed] [Google Scholar]
- Zilles K., Amunts K. Receptor mapping: architecture of the human cerebral cortex. Curr. Opin. Neurol. 2009;22(4):331–339. doi: 10.1097/WCO.0b013e32832d95db. [DOI] [PubMed] [Google Scholar]
- Zilles K., Bacha-Trams M., Palomero-Gallagher N., Amunts K., Friederici A.D. Common molecular basis of the sentence comprehension network revealed by neurotransmitter receptor fingerprints. Cortex. 2015;63:79–89. doi: 10.1016/j.cortex.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K., Palomero-Gallagher N. Cyto-, myelo-, and receptor architectonics of the human parietal cortex. Neuroimage. 2001;14(1):S8–S20. doi: 10.1006/nimg.2001.0823. [DOI] [PubMed] [Google Scholar]
- Zilles K., Palomero-Gallagher N. Multiple transmitter receptors in regions and layers of the human cerebral cortex. Front. Neuroanatomy. 2017;11:78. doi: 10.3389/fnana.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K., Palomero-Gallagher N., Grefkes C., Scheperjans F., Boy C., Amunts K. Architectonics of the human cerebral cortex and transmitter receptor fingerprints: reconciling functional neuroanatomy and neurochemistry. Eur. Neuropsychopharmacol. 2002;12(6):587–599. doi: 10.1016/s0924-977x(02)00108-6. [DOI] [PubMed] [Google Scholar]
- Zilles, K., Schleicher, A., Palomero-Gallagher, N., & Amunts, K. (2002b). Quantitative analysis of cyto-and receptor architecture of the human brain. In Brain Mapping: the Methods (pp. 573-602).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon publication, the ensuing 3D map of the macaque IPL, and the receptor fingerprints will be made publicly available to the neuroscientific community via the Human Brain Project and BALSA repositories. The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials .