Abstract
Background
Integrated drug efficacy surveillance (iDES) was formally introduced nationally across Thailand in fiscal year 2018 (FY2018), building on a history of drug efficacy monitoring and interventions. According to the National Malaria Elimination Strategy for Thailand 2017–2026, diagnosis is microscopically confirmed, treatment is prescribed, and patients are followed up four times to ensure cure.
Methods
Routine patient data were extracted from the malaria information system for FY2018–FY2020. Treatment failure of first-line therapy was defined as confirmed parasite reappearance within 42 days for Plasmodium falciparum and 28 days for Plasmodium vivax. The primary outcome was the crude drug efficacy rate, estimated using Kaplan–Meier methods, at day 42 for P. falciparum treated with dihydroartemisinin–piperaquine plus primaquine, and day 28 for P. vivax treated with chloroquine plus primaquine; day 60 and day 90 efficacy were secondary outcomes for P. vivax.
Results
The proportion of patients with outcomes recorded at day 42 for P. falciparum malaria and at day 28 for P. vivax malaria has been increasing, with FY2020 follow-up rates of 61.5% and 57.2%, respectively. For P. falciparum malaria, day 42 efficacy in FY2018 was 92.4% (n = 249), in FY2019 93.3% (n = 379), and in FY2020 98.0% (n = 167). Plasmodium falciparum recurrences occurred disproportionally in Sisaket Province, with day 42 efficacy rates of 75.9% in FY2018 (n = 59) and 49.4% in FY2019 (n = 49), leading to an update in first-line therapy to pyronaridine–artesunate at the provincial level, rolled out in FY2020. For P. vivax malaria, day 28 efficacy (chloroquine efficacy) was 98.5% in FY2018 (n = 2048), 99.1% in FY2019 (n = 2206), and 99.9% in FY2020 (n = 2448), and day 90 efficacy (primaquine efficacy) was 94.8%, 96.3%, and 97.1%, respectively.
Conclusions
In Thailand, iDES provided operationally relevant data on drug efficacy, enabling the rapid amendment of treatment guidelines to improve patient outcomes and reduce the potential for the spread of drug-resistant parasites. A strong case-based surveillance system, integration with other health system processes, supporting biomarker collection and molecular analyses, and cross-border collaboration may maximize the potential of iDES in countries moving towards elimination.
Keywords: Malaria elimination, Surveillance, Drug efficacy, Antimalarial, Drug resistance
Background
The Greater Mekong Subregion (GMS) is the global epicentre of anti-malarial drug resistance. High failure rates for artemisinin-based combination therapy (ACT) against Plasmodium falciparum have been reported throughout the region, associated with resistance to both artemisinins and their partner drugs [1]. In 2008, through the Containment Project, the World Health Organization (WHO) and its partners, with extra funding from the Bill & Melinda Gates Foundation, promoted a policy of containment and eventual elimination of artemisinin-resistant P. falciparum in the Thailand–Cambodia border area [2]. This strategy was subsequently expanded to target malaria elimination across the GMS for P. falciparum by 2025 and all malaria by 2030 [3]. The National Malaria Elimination Strategy for Thailand 2017–2026 envisions the elimination of malaria by 2024 [4, 5].
Thailand is situated between two discrete malaria transmission regions, with the north-east provinces in a zone comprising Cambodia, southern Vietnam, and southern Laos; and the western provinces in a zone with eastern Myanmar and northern Malaysia. Although P. falciparum and Plasmodium vivax are the dominant parasites, malaria cases caused by Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi also occur, as do mixed infections. Malaria transmission in Thailand is concentrated in forested areas along these international borders, with forest workers, displaced people, refugees, migrants, and police and military personnel most exposed to risk [6]. High population mobility in these areas can cause malaria to re-establish in villages where the disease has been eliminated, and civil unrest in the south of the country has hampered malaria control activities [7]. Despite these challenges, malaria incidence has declined overall since 2000, though more rapidly for P. falciparum compared with P. vivax [8].
Targeting malaria elimination has required repositioning of the health system in Thailand. At a national level, the Department of Disease Control is responsible for malaria policy and strategy, whereas the Division of Vector Borne Diseases (DVBD) undertakes capacity building and provides technical support, including managing the Malaria Information System (MIS)—a national database for malaria surveillance and monitoring [9]. The electronic MIS was initially designed to track artemisinin resistance while also modernizing Thailand’s existing paper-based reporting system [2]. In addition to malaria diagnosis and treatment, case investigation is conducted to determine the origin of the infection, thereby enabling identification, classification, and elimination of transmission foci [10]. In areas deemed to be at risk, active case detection is employed and vector control measures enhanced, with the aim of interrupting transmission [4]. This case-based malaria surveillance is captured in the MIS, providing near real-time information, which the DVBD uses to stratify malaria risk down to the village level. These detailed and timely data allow local authorities to understand the potential for malaria outbreaks, and to respond quickly should they occur [4].
Given the constant threat of drug-resistant P. falciparum, a gap in surveillance would be unacceptable. Ineffective anti-malarial therapy drives the spread of resistant parasites, so it is critical to detect changes in P. falciparum susceptibility to deployed artemisinin-based combinations and switch regimens promptly where necessary to maintain momentum towards malaria elimination [11]. Therapeutic efficacy studies (TES) have been the primary tool deployed to track anti-malarial efficacy and develop responses to P. falciparum drug resistance across the GMS. Since 2000, the DVBD monitored drug efficacy from in vivo TES conducted in Thailand with mefloquine-artesunate combination therapy for P. falciparum malaria [12]. The aims of the subsequent multipronged Containment Project included increasing surveillance to provide information for the containment of artemisinin-resistant parasites [2]. By 2011, the Containment Project had supported surveillance of patients who tested positive after 3 days of treatment, and it had developed systematic processes for cross-border investigation and follow-up [12]. However, the decline in malaria incidence in Thailand means that it is more difficult to recruit the minimum patient sample size for TES—in low transmission settings, at least 50 patients are required, ideally recruited within a single malaria season [13].
Plasmodium vivax control is complicated by the persistence of hypnozoites that remain dormant in the host’s liver before activation [14]. Radical cure consists of chloroquine to address the blood-stage infection plus primaquine to target hypnozoites. It is not operationally possible to determine whether P. vivax recurrences are recrudescence (chloroquine failure), relapse (primaquine failure), or re-infection. However, chloroquine should suppress asexual parasitaemia from relapse for approximately one month [15], so recurrence on or before day 28 can be considered chloroquine treatment failure. Failures after day 28 are conservatively regarded as primaquine failures but could be caused by re-infection. Follow-up of 3 months is both sufficient to assess primaquine efficacy and operationally feasible in Thailand. By preventing relapses, radical cure both reduces malaria incidence and interrupts transmission, so ensuring effective therapy is a key component of malaria elimination.
Integrated drug efficacy surveillance (iDES) is a novel approach that incorporates drug resistance monitoring as part of routine case-based surveillance and response. iDES expanded the initiatives undertaken in the Containment Project, requiring that all malaria cases, symptomatic or asymptomatic, have a laboratory confirmed malaria diagnosis and receive treatment according to national guidelines, with parasitological follow-up to ensure parasite clearance. iDES aims to support evidence-based strategic policy development and operational decision making to realize malaria elimination while ensuring patient outcomes. This study examines the trends and status of malaria in Thailand, the implementation of iDES, initial measures of programme performance, and the potential for further development. The contribution of iDES data to decision making in the context of malaria elimination is also discussed.
Methods
iDES aims and implementation
Thailand’s malaria elimination strategy requires that all patients have malaria diagnosed by microscopy or rapid diagnostic test, receive supervised treatment in adherence to the national treatment guidelines, and are followed up four times to ensure cure [5, 16]. iDES supports these aims by monitoring adherence to treatment guidelines, tracking follow-up rates, and recording clinical and parasitological outcomes as part of routine case management [17].
From May 2017, the iDES protocol, updated from previous in vivo studies, was piloted in three provinces in northern Thailand (Chiang Mai, Chiang Rai, and Mae Hong Son), with expansion to eight provinces in fiscal year 2017 (FY2017; i.e., 1 October 2017 to 31 September 2018) [17]. National rollout of the iDES protocol commenced in FY2018. The MIS was upgraded to allow data capture from iDES activities, and analytics and visualizations were developed to allow routine data interrogation [9]. The iDES system aims to systematically capture epidemiological and laboratory data from all patients diagnosed with malaria in Thailand as part of routine malaria care. The iDES protocol outlines aims, treatment, follow-up, data recording, and sample collection and submission [17]. The methods described here are consistent with ongoing iDES management and operational procedures.
Treatment
All treatment was provided free of charge in all health facilities responsible for malaria case management—malaria posts, border malaria posts, malaria clinics, health-promoting hospitals, and public and private hospitals [18]. Treatment was prescribed as per the Practice Guidelines for the Treatment of Patients with Malaria, Thailand, 2019 (Table 1) [16]. Artesunate–mefloquine was replaced in 2015 with dihydroartemisinin–piperaquine as first-line therapy for P. falciparum malaria, with rollout in 2016. In FY2019, dihydroartemisinin–piperaquine was withdrawn from Sisaket and Ubon Ratchathani Provinces in north-east Thailand owing to the high treatment failure rates observed from iDES and confirmed by another study [19]; it was replaced with pyronaridine–artesunate. Single-dose primaquine (30 mg) was required for P. falciparum cases to clear gametocytes [20]. Radical cure of P. vivax or P. ovale malaria was recommended to clear hypnozoites with chloroquine plus 14-day primaquine (0.25 mg/kg/day). Note that primaquine was not given if the patient was pregnant or under 2 years of age. In the case of confirmed glucose-6-phosphate dehydrogenase (G6PD) deficiency, an 8-week treatment of weekly primaquine (0.75 mg/kg/week) was recommended for P. vivax/ovale radical cure. Any patient with a blood smear suspected to be P. knowlesi was referred to the district hospital for polymerase chain reaction (PCR)-based diagnosis and treatment.
Table 1.
National treatment guidelines for malaria in Thailand
| P. falciparum |
|
First-line: DHA–PIP for 3 days + PQ single dose (30 mg or 0.5 mg base/kg, started on day 1 pending patient’s condition) (except for Sisaket and Ubon Ratchathani, where PY–AS for 3 days + PQ single dose was adopted in FY2019) Second-line (ACT): 1. PY–AS 3 days + PQ single dose; 2. AL for 3 days + PQ single dose; 3. AS–MQ for 3 days + PQ single dose Second-line (non-ACT): 1. Quinine + clindamycin–doxycycline–tetracycline for 7 days + PQ single dose; 2. Atovaquone–proguanil for 3 days + PQ single dose |
| P. vivax or P. ovale |
|
First-line: CQ for 3 days + PQ (0.25 mg base/kg per day, started on day 3) for 14 days Second-line: DHA–PIP for 3 days + PQ for 14 days |
| P. malariae or P. knowlesi |
|
First-line: CQ for 3 days Second-line: DHA–PIP for 3 days |
| P. falciparum plus P. vivax or P. ovale |
| DHA–PIP for 3 days + PQ for 14 days |
| P. falciparum plus P. malariae or P. knowlesi |
| DHA–PIP for 3 days + PQ single dose |
| Severe malaria |
|
First-line: Artesunate injection within 24 h, followed by first-line/second-line regimen when tolerated + supportive care Second-line: Quinine injection within first 24 h followed by first-line/second-line regimen when tolerated + supportive care |
| Uncomplicated malaria in pregnancy 1st trimestera |
|
Quinine + clindamycin for 7 days (P. falciparum or P. knowlesi) CQ for 3 days (P. vivax, P. ovale, or P. malariae) |
| Uncomplicated malaria in pregnancy 2nd or 3rd trimestera |
|
DHA–PIP for 3 days (P. falciparum or P. knowlesi) CQ for 3 days (P. vivax, P. ovale, or P. malariae) |
aPrimaquine and doxycycline–tetracycline are contraindicated during pregnancy
ACT artemisinin-based combination therapy, DHA–PIP dihydroartemisinin–piperaquine, PQ primaquine, PY–AS pyronaridine–artesunate, AS–MQ artesunate–mefloquine, AL Artemether–lumefantrine, CQ chloroquine
Follow-up
Patients with falciparum malaria infection were followed up at days 3, 7, 28, and 42 (from the day of diagnosis and prescribed treatment) and those with vivax malaria at days 14, 28, 60, and 90. For P. falciparum cases, the PCR genotyping results could be added later to the patient record (see below). For each follow-up visit, the clinical team noted the date, patient temperature, microscopy findings, and confirmation of outcome as recrudescence or re-infection. Patients were asked if they had been consuming the anti-malarial drugs as prescribed, and the drug bag was requested for observation as a proxy of treatment compliance.
Data recording
The officer from the laboratory reference centre was responsible for data recording, either at a reference laboratory (DVBD) or a regional laboratory (Department of Disease Control). The reporting requirements were consistent with those used in TES to enable comparison with historical data and between countries [13]. The patient’s name, age, gender, weight, height, temperature, address, and residency background [i.e., resident Thai, long-term migrant (≥ 6 months residency), or short-term migrant (< 6 months residency)], were recorded, as well as the date that blood samples were taken and the date on which the person visited the clinic. Malaria species, parasite density, and the presence of gametocytes were assessed using standard methods by trained microscopists and reported [13, 21]. The Thailand Ministry of Public Health organizes regular quality control microscopy testing and training, including competency assessments and training of trainers, with assistance from partner agencies to develop standard operating procedures [22]. The prescribed anti-malarial drug and number of tablets/capsules were noted. All of these data were captured on the Malaria Case Follow-Up form and stored in the MIS (Fig. 1).
Fig. 1.
Malaria case follow-up form
Sample collection and submission
Blood spots were collected onto filter paper from all patients at first consultation and at follow-up visits. Samples were sent to regional and national laboratories as part of the microscopy quality assurance system. In the case of P. falciparum, PCR genotyping was used to compare samples from the initial and recurrent infection to differentiate recrudescence from re-infection, according to published methods [23]. For P. falciparum cases, blood samples were also collected for analysis of kelch 13 gene mutations associated with artemisinin resistance and Pfmdr1 gene copy number [24, 25]. Data on molecular markers will be reported separately.
Outcomes and statistical analysis
The primary outcome of the analysis was the crude drug efficacy rate among patients who had microscopically confirmed malaria with an identified Plasmodium species at baseline, received first-line treatment (dihydroartemisinin–piperaquine + primaquine for P. falciparum or chloroquine + primaquine for P. vivax), and had at least one post-baseline follow-up visit. Plasmodium falciparum treatment failure was defined as microscopically confirmed parasite reappearance within 42 days of first-line therapy. Crude cure rates were reported, as PCR data were sparse. For P. vivax, confirmed parasite reappearance within 28 days was considered chloroquine treatment failure, whereas reappearance at day 60 and day 90 was conservatively considered primaquine treatment failure (though could be caused by re-infection). Drug efficacy was estimated using Kaplan–Meier methods. Patients were censored if they had a re-infection with a different species from their initial infection or at their final treatment outcome assessment. In order to assess programmatic efficacy, all re-treatments with first-line therapy following failure were considered to be new malaria episodes, and the patients were requested to report for another four follow-up visits to ensure complete cure. All other outcomes were reported using descriptive statistics. Data were downloaded from the MIS on 16 November 2020. Statistical analysis used Stata (version 16) and GraphPad Prism (version 9.0.0).
Results
Malaria trends
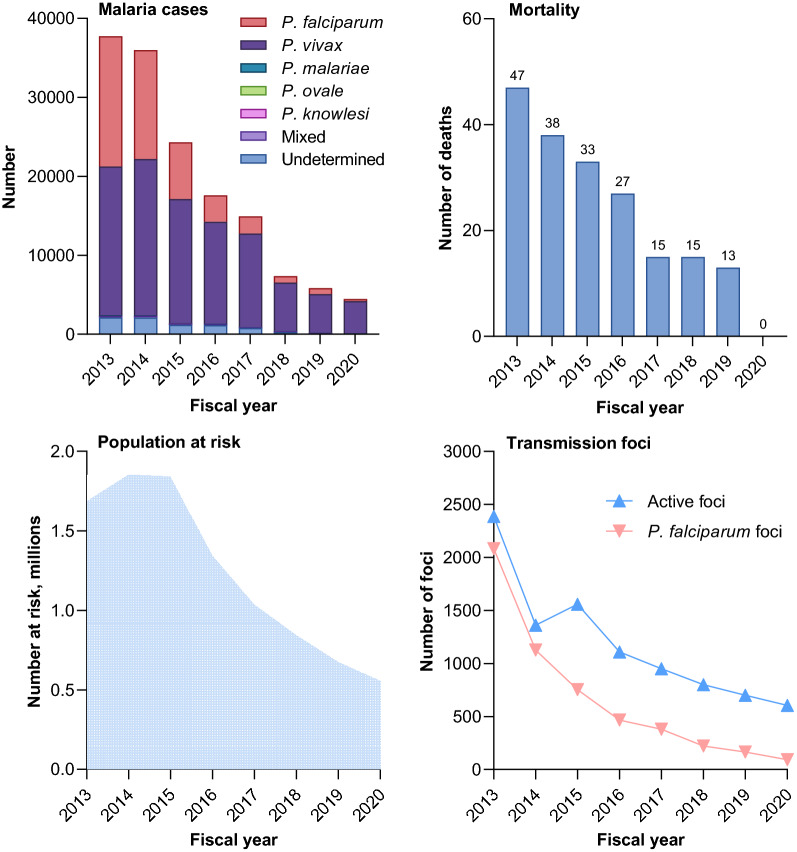
Between FY2013 and FY2020, there was an overall 88.1% decrease in malaria incidence from 37,741 to 4474 cases (Fig. 2). There was a sustained decline in the incidence of both P. falciparum and P. vivax malaria, but with a marked change in the relative proportions of infections caused by these two parasites; in FY2013, 43.7% (16,494/37,741) of infections were caused by P. falciparum, whereas in FY2020, this figure had declined to 5.7% (257/4474) (Fig. 2). Over the same period, malaria mortality declined from 47 to 0 deaths (Fig. 2). The number of active malaria foci (defined as subvillages with ongoing malaria transmission) also contracted, from 2,387 in FY2013 to 605 in FY2020, representing a 74.7% decrease (Fig. 2).
Fig. 2.
Malaria trends in Thailand FY2013–FY2020. Malaria mortality figures for FY2020 are provisional until officially approved by the Division of Planning and Strategy, Ministry of Public Health, Thailand
Current malaria situation
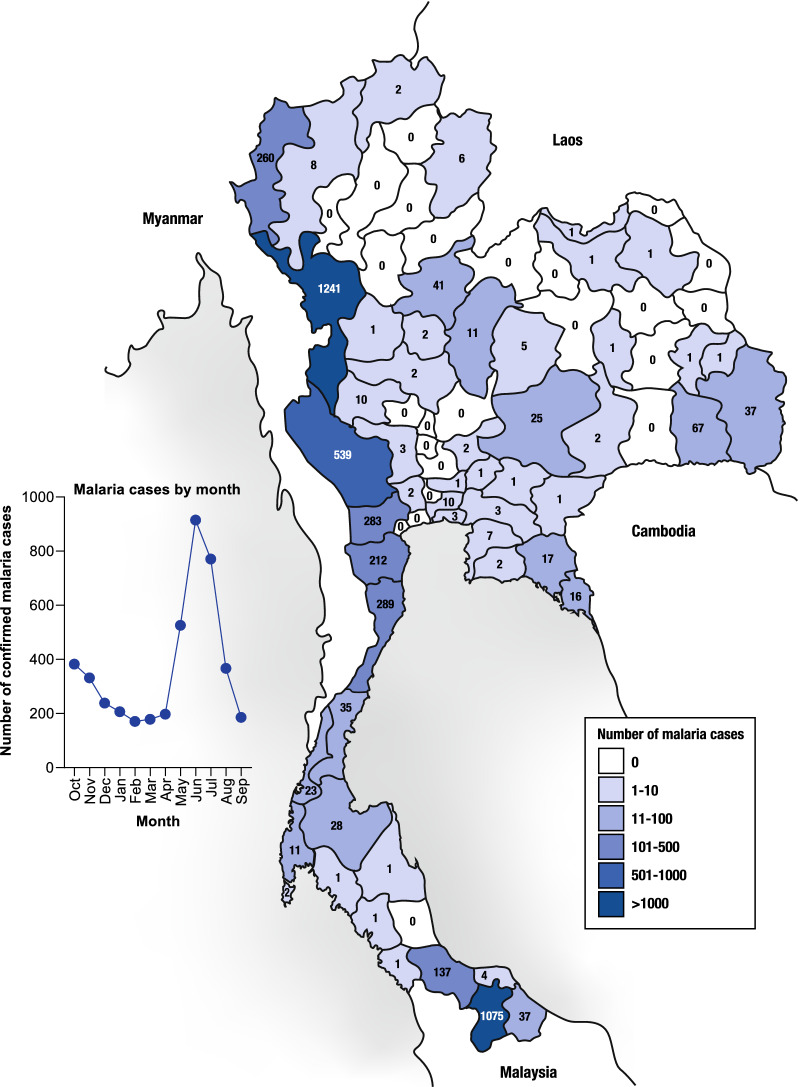
From data recorded in the MIS, in FY2020 there were 4474 malaria cases, representing a case incidence rate of 0.067/1000 population, against the FY2020 target for malaria elimination of 0.22/1000 population [5]. Plasmodium falciparum accounted for 5.7% (257/4474) of cases and P. vivax for 91.6% (4099/4474) (Fig. 2). The remaining cases were classified as P. malariae (n = 53), P. knowlesi (n = 11), or mixed (n = 18) (Fig. 2). For 36 cases, the Plasmodium species was undetermined, and no P. ovale cases were recorded. Malaria incidence was concentrated along the western border that Thailand shares with Myanmar, particularly Tak and Kanchanaburi Provinces; in the south of the country bordering Malaysia in Yala Province; and in the northeast bordering Cambodia in Sisaket and Ubon Ratchathani Provinces (Fig. 3). The peak malaria transmission season occurred between April and September, with a lower peak in October (Fig. 3).
Fig. 3.
Temporal and geographical distribution of malaria cases in Thailand FY2020
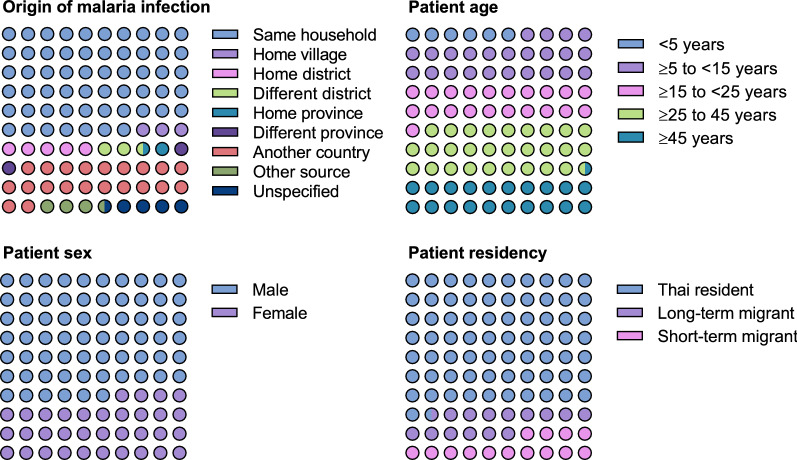
Most malaria infections occurred in individuals who were at least 15 years old (70.2% [3142/4474]), and around two-thirds occurred in males (65.9% [2950/4474]) (Fig. 4). Most infections were acquired in the same household (56.8% [2543/4474]), but around a fifth of cases were imported (21.3% [954/4474]) (Fig. 4). Thai residents accounted for 71.3% (3190/4474) of cases, with the remaining cases split between long-term migrants (14.9% [666/4474]) and short-term migrants (13.8% [618/4474]) (Fig. 4).
Fig. 4.
Characteristics of malaria cases in Thailand FY2020. Each square represents 100% and each dot 1% of the population. Residency background was categorized as resident Thai, long-term migrant (≥ 6 months residency), or short-term migrant (< 6 months residency)
Follow-up rates and adherence to national treatment guidelines
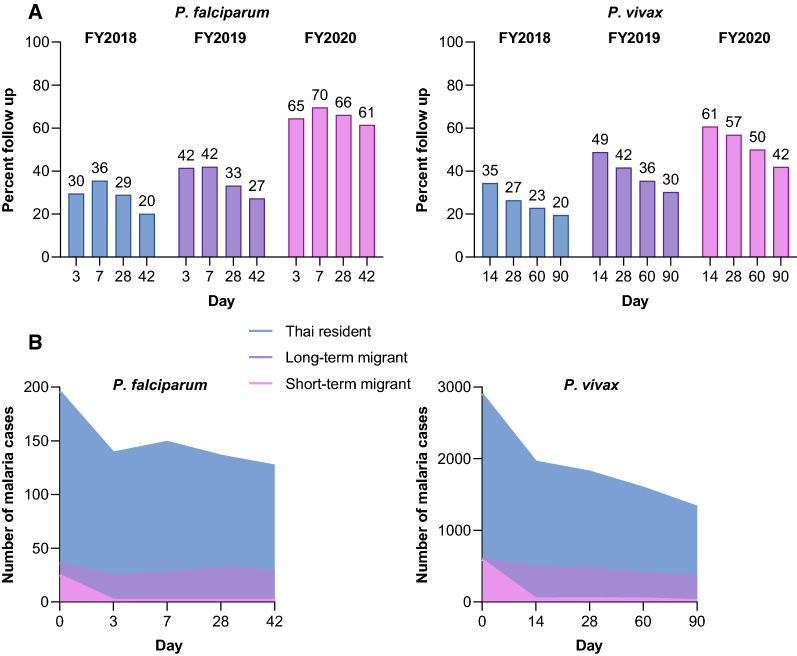
The proportion of patients with outcomes recorded at day 42 for P. falciparum malaria and for 28 days for P. vivax malaria has been increasing each year since the national launch of iDES (Fig. 5A). In FY2020, 61.5% (158/257) of P. falciparum cases had a treatment outcome recorded on day 42, and 57.2% (2344/4099) of P. vivax cases had a day 28 outcome recorded (Fig. 5A). For P. falciparum malaria, day 42 follow-up rates were highest for long-term migrants (80.6% [29/36]) and Thai residents (64.8% [127/196]), but lower for short-term migrants (8.0% [2/25]) (Fig. 5B). A similar pattern was observed for P. vivax malaria, with 77.6% (471/607) of long-term migrants, 62.7% (1821/2905) of Thai residents, and 8.9% (52/587) of short-term migrants followed up at day 28 (Fig. 5B).
Fig. 5.
Malaria case follow-up rates in Thailand. A All patients FY2018–FY2020, and B By patient origin in FY2020
The proportion of evaluable patients who received anti-malarial therapy aligned with national treatment guidelines improved over the iDES period, from 70.7% (4212/5956) in FY2018, to 77.2% (4103/5315) in FY2019, and 84.2% (3549/4215) in FY2020.
Anti-malarial drug efficacy
Anti-malarial drug efficacy was assessed for those patients who received at least one dose of first-line treatment for P. falciparum (dihydroartemisinin–piperaquine + primaquine) or P. vivax (chloroquine + primaquine) malaria, and who had at least one follow-up visit. Recurrences occurred in different provinces in different years, though Sisaket, Tak, and Yala had P. vivax recurrences in all three iDES years (Table 2). Plasmodium vivax recurrences also varied by follow-up day, with the majority of positive tests occurring on days 60 and 90 (Table 3), suggesting either primaquine treatment failure or re-infection. In FY2020, only 3.8% (2/52) of P. vivax recurrences occurred on or before day 28, suggesting high efficacy for chloroquine.
Table 2.
Summary of malaria recurrences by year, species, and province
| Species and province | Recurrences, n/N (%) | ||
|---|---|---|---|
| FY2018 | FY2019 | FY2020 | |
| P. falciparum overall | 13/249 (5.2) | 14/379 (3.7) | 3/167 (1.8) |
| Chanthaburi | – | – | 2/6 (33.3) |
| Kamphaeng Phet | – | 1/1 (100) | – |
| Phangnga | 1/2 (50.0) | – | – |
| Sakaeo | – | 1/3 (33.3) | – |
| Sisaket | 8/59 (13.6) | 10/49 (20.4) | – |
| Surat Thani | 2/33 (6.1) | – | 1/15 (6.7) |
| Surin | 1/4 (25.0) | 1/7 (14.3) | – |
| Tak | – | 1/52 (1.9) | – |
| Ubon Ratchathani | 1/10 (10.0) | – | – |
| P. vivax overall | 66/2048 (3.2) | 58/2206 (2.6) | 52/2448 (2.1) |
| Chachoengsao | 2/42 (4.8) | – | – |
| Chon Buri | 1/6 (16.7) | – | – |
| Kanchanaburi | 1/143 (0.7) | 3/235 (1.3) | – |
| Mae Hong Son | 3/184 (1.6) | 6/199 (3.0) | – |
| Nakhon Ratchasima | – | 2/2 (100) | 1/19 (5.3) |
| Phetchaburi | – | 2/79 (2.5) | 4/124 (3.2) |
| Phitsanulok | – | – | 2/26 (7.7) |
| Prachin Buri | 1/22 (4.5) | – | – |
| Prachuap Khiri Khan | – | 2/65 (3.1) | 6/110 (5.5) |
| Ratchaburi | – | 7/117 (6.0) | 11/182 (6.0) |
| Sisaket | 36/200 (18.0) | 24/198 (12.1) | 5/41 (12.2) |
| Tak | 20/385 (5.2) | 8/398 (2.0) | 21/703 (3.0) |
| Yala | 2/506 (0.4) | 4/523 (0.8) | 2/559 (0.4) |
Only provinces with malaria recurrences are shown. Recurrences are shown for patients who had at least one follow-up visit for P. falciparum malaria up to and including day 42 and received dihydroartemisinin–piperaquine plus primaquine; and for patients who had at least one follow-up visit for P. vivax malaria up to and including day 90 and received chloroquine plus primaquine
Table 3.
Summary of P. vivax malaria recurrences by year and follow-up day
| Species and follow-up day | Recurrences, n/N (%) | ||
|---|---|---|---|
| FY2018 | FY2019 | FY2020 | |
| P. vivax overall | 66/2048 (3.2) | 58/2206 (2.6) | 52/2448 (2.1) |
| Day 14 | 1 (1.5) | 7 (12.1) | 2 (3.8) |
| Day 21 | 2 (3.0) | 1 (1.7) | 0 |
| Day 28 | 20 (30.3) | 10 (17.2) | 0 |
| Day 35 | 0 | 1 (1.7) | 0 |
| Day 42 | 0 | 0 | 1 (1.9) |
| Day 60 | 36 (54.5) | 31 (53.4) | 21 (40.4) |
| Day 90 | 7 (10.6) | 8 (13.8) | 28 (53.8) |
Recurrences are shown for patients with at least one follow-up visit for P. vivax up to and including day 90 who received chloroquine plus primaquine
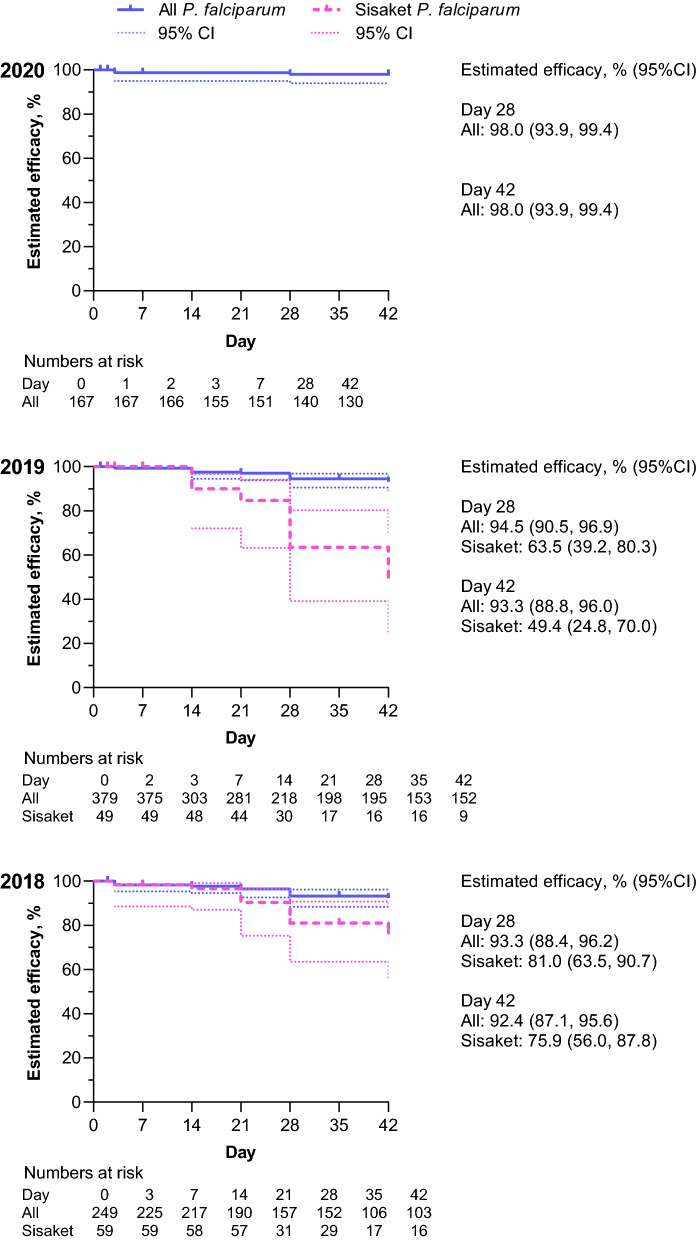
For P. falciparum malaria, day 42 efficacy (Kaplan–Meier) was 92.4% (95%CI 87.1, 95.6) in FY2018, 93.3% (95%CI 88.8, 96.0) in FY2019, and 98.0% (95%CI 93.9, 99.4) in FY2020 (Fig. 6). Most falciparum recurrences occurred in Sisaket Province, with day 42 efficacy rates of 75.9% (95%CI 56.0, 87.8) in FY2018 and 49.4% (95%CI 24.8, 70.0) in FY2019 (Fig. 6). In FY2018, all eight treatment failures in Sisaket occurred in different individuals. In FY2019, ten treatment failures occurred in six patients, three of whom received repeated treatment with dihydroartemisinin–piperaquine rather than second-line therapy. However, even when only the first treatment episode was considered for these patients, the day 42 efficacy rate in Sisaket in FY2019 was 61.5% (95%CI 31.2, 81.7). Based on the unacceptably high clinical failure rate in Sisaket, in February 2019 pyronaridine–artesunate replaced dihydroartemisinin–piperaquine as first-line therapy against uncomplicated falciparum malaria in Sisaket and neighboring Ubon Ratchathani, with rollout in FY2020. In FY2020, there were only three P. falciparum cases in Sisaket and Ubon Ratchathani. Two cases received pyronaridine–artesunate, with no recurrence. One received dihydroartemisinin–piperaquine; after parasite reappearance at day 60, the case was successfully treated with pyronaridine–artesunate.
Fig. 6.
Efficacy of dihydroartemisinin–piperaquine plus primaquine against P. falciparum malaria in Thailand FY2018–FY2020. Data are Kaplan–Meier estimates for patients who had P. falciparum monoinfection, received at least one dose of both dihydroartemisinin–piperaquine and primaquine, and attended at least one follow-up visit. In Sisaket Province, pyronaridine–artesunate was rolled out as a new first-line treatment in FY2020, so data for this province are not shown for that year (see text)
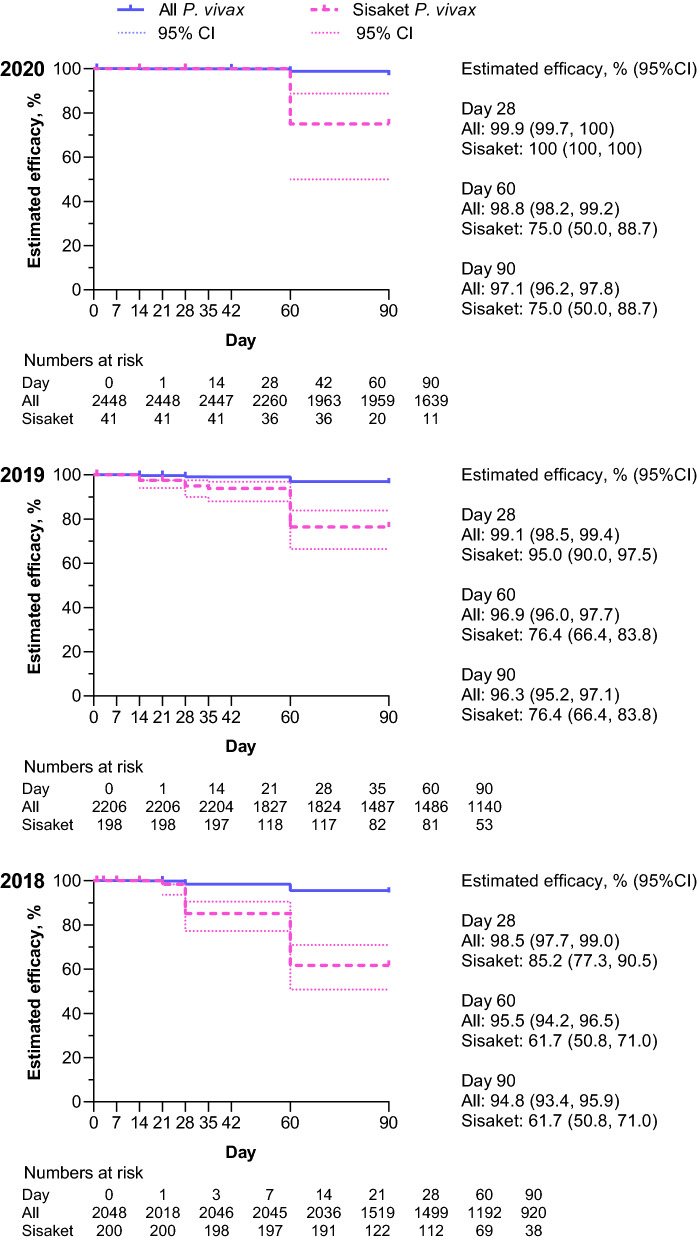
For P. vivax malaria, chloroquine/primaquine day 28 efficacy was at least 98% across all iDES years, suggesting good clinical efficacy for chloroquine (Fig. 7). Day 60 efficacy was > 95%, and Day 90 efficacy was 94.8% (95%CI 93.4, 95.9) in FY2018, but improved to 97.1% (96.2, 88.7) in 2020 which suggests good overall primaquine efficacy (Fig. 7). Sisaket Province proportionally had the greatest effect on the overall failure rate. Day 28 efficacy in Sisaket was 85.2% (95%CI 77.3, 90.5) in FY2018, but improved to 95.0% (95%CI 90.0, 97.5) in FY2019, and 100% in FY2020 (Fig. 7). However, day 60 and day 90 efficacy in Sisaket remained sub-optimal, with both at 61.7% (95%CI 50.8, 71.0) in FY2018, 76.4% (95%CI 66.4, 83.8) in FY2019, and 75.0% (95%CI 50.0, 88.7) in FY2020 (Fig. 7).
Fig. 7.
Efficacy of chloroquine plus primaquine for P. vivax malaria in Thailand FY2018–FY2020. Data are Kaplan–Meier estimates for patients who had P. vivax monoinfection, received at least one dose of both chloroquine and primaquine, and attended at least one follow-up visit. Recurrences occurring on or before day 28 were considered chloroquine treatment failures. Recurrences occurring after day 28 until day 90 were assumed to be primaquine treatment failures as a conservative analysis but could be caused by re-infection
Discussion
Thailand has made significant progress towards malaria elimination, surpassing its 2020 milestone reductions for both malaria incidence and mortality [8]. However, many areas remain receptive to malaria, and re-establishment of transmission continues to be a significant risk across most of the country [26].
It was clear that as malaria incidence declined in Thailand, TES would no longer be feasible in many provinces. The reorganization of the health system to target malaria elimination offered an opportunity to integrate drug efficacy surveillance. However, retroactive adaptation of the MIS to incorporate the iDES module was complex and has required continued refinement of the platform to adapt to changing epidemiology and corresponding data needs. The DVBD can examine the data in almost real time, thereby enabling limited resources to be targeted to the provinces, villages, or health facilities most in need of support to improve iDES compliance. The DVBD and its partners are working to further improve data visualizations to facilitate the data analyses most needed by subnational officers, such as health care workers interested in patient follow-up and treatment outcomes.
In order to move from TES to iDES, it was necessary for Thailand to have a strong case-based surveillance system and the capacity for universal and supervised anti-malarial treatment. For countries that have not yet reached the elimination phase, TES remains the most appropriate drug efficacy surveillance method [18]. For iDES to have an impact on policy, other aspects of the health system need to be aligned for delivering different anti-malarial drugs to different regions. For example, purchasing and supply logistics, laboratory capacity and coordination, health financing, communication with prescribers, community outreach, and patient education. Thus, iDES cannot just be appended to an information system, but instead must be fully integrated into all health system processes.
Rapid diagnostic tests for malaria have been an invaluable tool in areas of high-to-moderate transmission to identify cases and direct appropriate therapy. However, iDES requires microscopic malaria diagnosis to confirm parasite clearance. Maintaining microscopy skills in an elimination setting is challenging as some laboratories see very few cases. With sustained support from external funding partners, Thailand has invested in a strong cadre of trained microscopists stationed throughout the country, and 33 professionals hold current expert certification from the WHO. However, other countries in the GMS and elsewhere may need to consider how to build these skills as malaria burden reduces, and as an iDES system becomes the recommended programme for case-based surveillance and follow-up.
Despite being included in the iDES protocol, the collection of dried blood spots for PCR analysis of P. falciparum has been sporadic, with difficulties in managing their storage and processing. Thus, only crude efficacy rates are reported here, but in a country aiming for elimination it is expected that all recurrences are recrudescences. The collection of samples for molecular resistance markers has also been sub-optimal while subnational officers gain new skills. The DVBD anticipates enhanced molecular surveillance and streamlined processes to triangulate clinical and laboratory data as a National Reference Laboratory database is developed and with additional training for health workers and laboratory staff.
Follow-up rates for iDES have been increasing steadily since its introduction, supported by a network of village health volunteers and through community education. High burden provinces in western Thailand (Fig. 3) have lower follow-up rates, as does the crucial Sisaket Province. Also, follow-up rates among short-term migrants have been consistently low. Maintaining contact with this population is challenging, and cross-border collaboration between countries may be required to ensure patient outcomes. There is the potential to incorporate mobile health data (mHealth) within iDES [27], boosting follow-up rates by expanding coverage of malaria follow-up services to the household and individual levels via patients’ mobile devices. iDES could also be enhanced by complementary research to identify potential bottlenecks to patient follow-up—such as patient resistance or forgetfulness, provider lack of knowledge, poor adherence, or issues with recording and reporting. A positive sign is that in FY2020, malaria case follow-up and iDES showed resilience to disruption caused by the novel coronavirus disease (COVID-19) epidemic, given that both follow-up rates and data capture improved.
Adherence to the national treatment guidelines is necessary to optimize patient outcomes and to support malaria elimination. Although adherence rates have been improving, further progress will require additional resources. For example, Thailand’s village health volunteers are key in accessing remote locations and engaging with patients on a personal level, and additional training of these health workers is planned in FY2021, alongside iDES capacity building. In Sisaket, iDES identified issues related to re-treatment with first-line therapy following failure, leading to subsequent treatment failure. These repeated treatment failures are programmatic and may result from stock-outs of second-line anti-malarial therapies, patients presenting at different clinics, or patient or prescriber choice. Being able to find and interrogate these cases allows interventions to be targeted at the causes of non-adherence to treatment guidelines in specific locations.
Although follow-up rates and treatment adherence are not perfect in terms of ensuring individual case outcome, data penetration has been sufficient to enable policy decisions on anti-malarial drug treatment at the provincial level, with dihydroartemisinin–piperaquine switched to pyronaridine–artesunate for two provinces. Although pyronaridine–artesunate shows high efficacy in regions in the GMS where multidrug-resistant P. falciparum parasites are prevalent [28–31], normally a TES study would be conducted to support a drug treatment policy change. Instead, pyronaridine–artesunate efficacy in Sisaket and Ubon Ratchathani will be monitored in FY2021 through ‘intensified iDES’ (Box 1), which aims to optimize data gathering, given the anticipated low number of P. falciparum cases.
Box 1.
Intensified iDES to support treatment policy change
| 100% adherence to the national treatment guidelines |
| No stock-outs of drugs |
| Additional training for treatment providers to ensure daily supervised drug intake |
| Target to achieve > 90% of follow-up days |
| iDES standard operating procedures are followed with increased frequency of monitoring |
| Adequate patient support for follow-up visits (providing transport, etc.) |
| Quality control on all microscopy slides |
| Collection of all day 0 dried blood spots and at recurrence for PCR and molecular markers |
| Follow-up of all treatment failures |
| Integration of laboratory data and the results of molecular markers to the online system |
iDES data also underline the importance of P. vivax malaria elimination. Although generally high efficacy rates were observed, a disparity was evident in Sisaket versus other provinces. The high day 28 failure rate in Sisaket in FY2018 suggests sub-optimal chloroquine efficacy, though efficacy was 100% in FY2020. Mutations associated with chloroquine resistance have been detected in P. vivax isolates from Thailand [32]. Notably, Sisaket borders Cambodia, where chloroquine was abandoned in 2012 because of parasite resistance, being replaced first by dihydroartemisinin–piperaquine, and then by mefloquine-artesunate in 2017. There is some evidence of increasing chloroquine susceptibility in Cambodian clinical isolates [33], which could explain the improved day 28 efficacy observed in Sisaket between FY2018 and FY2020. However, day 90 efficacy has remained unacceptably low in the province, suggesting sub-optimal primaquine efficacy. Although primaquine treatment should be fully supervised, drug consumption cannot be verified and poor primaquine adherence cannot be excluded as a cause. Cytochrome 2D6 polymorphisms can affect primaquine efficacy [34], but this was not investigated. There may also be social factors in this particular region that predispose the population to P. vivax re-infection, despite declining case numbers. Investigations and discussions on the appropriate response to P. vivax malaria in Sisaket are ongoing in FY2021, and the findings may also affect the management of P. vivax malaria elsewhere in Thailand.
iDES has provided operationally relevant data on drug efficacy; however, there are some limitations. Most importantly, it is difficult to obtain a complete dataset for data collected routinely, which may introduce a bias towards patients that are more easily reached. As follow-up improves, this bias should diminish. Similarly, although health workers attempt to verify drug adherence, not all treatment is directly observed. Thus, although malaria recurrence in this report is attributed to treatment failure, it could be a result of poor adherence. From an operational perspective, it is valuable to be able to differentiate these two sources of recurrence, so health workers are receiving continued training to observe the drug pack as a proxy for consumption. A final consideration is that MIS data can be adjusted to reflect new information received or to correct records, and so should be regarded as cross-sectional.
Given the low and declining malaria incidence in Thailand, without iDES it is unlikely that a pattern of treatment failure would be detected in time to avert an outbreak of drug-resistant P. falciparum. There is evidence that iDES can support appropriate management of imported malaria as part of a comprehensive prevention of reintroduction program [35]. Maintaining iDES may be crucial for Thailand as neighbouring countries in the GMS strive for elimination in the coming years.
Conclusion
The investment in malaria elimination in Thailand is considerable, but the potential benefits are even greater [36]. As countries approach elimination, it is likely that the most resistant parasites will be those that remain in circulation [11]. Ineffectual therapy promotes the spread of drug-resistant parasites, and risks malaria resurgence and the re-establishment of transmission. Thus, as it becomes more difficult to conduct drug resistance surveillance activities, it also becomes increasingly important to understand which drugs retain efficacy and whether treatment policies should be amended. iDES is designed to encompass all malaria cases, whether imported or indigenous; to encourage treatment compliance; and to follow patients until they are clinically cured and confirmed as parasite free. Thailand’s experience with iDES offers a pragmatic model for malaria-eliminating countries where TES is no longer feasible. iDES can be a useful approach to target malaria elimination by ensuring that all malaria patients receive appropriate treatment and are ultimately cured of malaria.
Acknowledgements
The authors recognize the contribution of the community volunteers and public health staff, especially from the Division of Vector Borne Diseases, Department of Disease Control, Ministry of Public Health, Thailand. Naomi Richardson developed the first draft of this paper, provided editorial and graphic services, cross-checked all data and analyses, and was funded by Inform Asia: USAID’s Health Research Program.
Disclaimer
Any opinions expressed in this supplement are those of the authors and do not necessarily reflect the views of the U.S. President’s Malaria Initiative, or the United States Agency for International Development. DG, PR, and MDB are staff members of the World Health Organization (WHO) and are responsible for the views expressed in this publication, which do not necessarily reflect the decisions or policies of the WHO.
Abbreviations
- ACT
Artemisinin-based combination therapy
- AL
Artemether–lumefantrine
- AS–MQ
Artesunate–mefloquine
- COVID-19
Coronavirus disease of 2019
- CQ
Chloroquine
- DHA–PIP
Dihydroartemisinin–piperaquine
- DVBD
Division of Vector Borne Diseases
- FY
Fiscal year
- G6PD
Glucose-6-phosphate dehydrogenase
- GMS
Greater Mekong Subregion
- iDES
Integrated drug efficacy surveillance
- MIS
Malaria Information System
- PCR
Polymerase chain reaction
- PQ
Primaquine
- PY–AS
Pyronaridine–artesunate
- TES
Therapeutic efficacy studies
- USAID
United States Agency for International Development
- WHO
World Health Organization
Authors’ contributions
PS, AS, NK, DG, MDB, and PR were instrumental in designing and establishing the iDES system for Thailand. TT and SK led routine monitoring of iDES results. PS, AS, DG, and JAS contributed to defining the scope and content of the review; JAS assembled and analyzed the data. All authors reviewed the data and critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study was made possible by the generous support of the American people through the U.S. President’s Malaria Initiative and United States Agency for International Development (USAID), under the terms of Cooperative Agreement AID-486-LA-15-00002 for Inform Asia: USAID’s Health Research Program. iDES implementation was also supported by the Thailand Division of Vector Borne Diseases (DVBD), the U.S. President’s Malaria Initiative, the World Health Organization, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria.
Availability of data and materials
All relevant data are provided in the manuscript or are available from the website of the Department of Disease Control, Ministry of Public Health Thailand: https//malaria.ddc.moph.go.th.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
PS, AS, NK, TT, SK, RS, CL, and DA are employees of the Division of Vector Borne Diseases, Ministry of Public Health, Thailand. JAS and SN are employees of RTI International, Thailand. DG, PR, and MDB are staff members of the World Health Organization; the authors alone are responsible for the views expressed in this publication that do not necessarily represent the decisions, policy, or views of the World Health Organization. NP and DS are employees of the Regional Development Mission for Asia, United States Agency for International Development, Thailand. The authors have no further conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Imwong M, Dhorda M, Myo Tun K, Thu AM, Phyo AP, Proux S, et al. Molecular epidemiology of resistance to antimalarial drugs in the Greater Mekong subregion: an observational study. Lancet Infect Dis. 2020;20:1470–1480. doi: 10.1016/S1473-3099(20)30228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khamsiriwatchara A, Sudathip P, Sawang S, Vijakadge S, Potithavoranan T, Sangvichean A, et al. Artemisinin resistance containment project in Thailand. (I): implementation of electronic-based malaria information system for early case detection and individual case management in provinces along the Thai-Cambodian border. Malar J. 2012;11:247. doi: 10.1186/1475-2875-11-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Regional Office for the Western Pacific. Strategy for malaria elimination in the Greater Mekong Subregion: 2015–2030. World Health Organization Regional Office for the Western Pacific; 2015. https://apps.who.int/iris/handle/10665/208203. Accessed 18 Feb 2021.
- 4.Bureau of Vector Borne Diseases, Department of Disease Control, Ministry of Public Health Thailand. Guide to malaria elimination for Thailand's local administrative organizations and the health network. Nonthaburi: Thailand; 2019. http://malaria.ddc.moph.go.th/downloadfiles/Guide%20to%20Malaria%20Elimination%20for%20Thailand%20LAO_EN.pdf. Accessed 9 Oct 2020.
- 5.Bureau of Vector Borne Diseases, Department of Disease Control, Ministry of Public Health Thailand. National malaria elimination strategy for Thailand 2017–2026. Nonthaburi: Thailand; 2016.
- 6.Sudathip P, Kitchakarn S, Thimasarn K, Gopinath D, Naing T, Sajjad O, et al. The evolution of the malaria clinic: the cornerstone of malaria elimination in Thailand. Trop Med Infect Dis. 2019;4:143. doi: 10.3390/tropicalmed4040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.USAID. US President's malaria initiative: Thailand, Lao PDR and regional abbreviated malaria operation plan FY 2019. United States Agency for International Development; 2019. https://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy19/fy-2019-thailand-abbreviated-malaria-operational-plan.pdf?sfvrsn=5. Accessed 9 Oct 2020.
- 8.WHO. World malaria report. Geneva, World Health Organization; 2020. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020. Accessed 13 Dec 2020.
- 9.Division of Vector Borne Diseases, Department of Disease Control, Ministry of Public Health Thailand. Malaria Online: the digital surveillance system for Thailand malaria elimination. Nonthaburi: Thailand; 2020. https://publicadministration.un.org/unpsa/Portals/0/UNPSA_Submitted_Docs/2019/3fe4c1ba-e00b-4250-8816-f513c3b209c6/2020%20UNPSA_Malaria%20online_full%20report_27112019_111848_f62725d9-bd75-4846-a44c-8524838f4e87.pdf?ver=1441-03-30-111848-927. Accessed 10 Oct 2020.
- 10.USAID. US President’s malaria initiative: Thailand, Lao PDR, and regional malaria operation plan FY 2018. United States Agency for International Development. 2018. https://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy-2018/fy-2018-thailand-regional-malaria-operational-plan.pdf?sfvrsn=8. Accessed 9 Oct 2020.
- 11.Maude RJ, Pontavornpinyo W, Saralamba S, Aguas R, Yeung S, Dondorp AM, et al. The last man standing is the most resistant: eliminating artemisinin-resistant malaria in Cambodia. Malar J. 2009;8:31. doi: 10.1186/1475-2875-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satimai W, Sudathip P, Vijaykadga S, Khamsiriwatchara A, Sawang S, Potithavoranan T, et al. Artemisinin resistance containment project in Thailand. II: responses to mefloquine-artesunate combination therapy among falciparum malaria patients in provinces bordering Cambodia. Malar J. 2012;11:300. doi: 10.1186/1475-2875-11-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Methods for surveillance of antimalarial drug efficacy. Geneva, World Health Organization; 2009. https://www.who.int/malaria/publications/atoz/9789241597531/en/. Accessed 13 Dec 2020.
- 14.Olliaro PL, Barnwell JW, Barry A, Mendis K, Mueller I, Reeder JC, et al. Implications of Plasmodium vivax biology for control, elimination, and research. Am J Trop Med Hyg. 2016;95:4–14. doi: 10.4269/ajtmh.16-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu CS, Phyo AP, Lwin KM, Win HH, San T, Aung AA, et al. Comparison of the cumulative efficacy and safety of chloroquine, artesunate, and chloroquine-primaquine in Plasmodium vivax malaria. Clin Infect Dis. 2018;67:1543–1549. doi: 10.1093/cid/ciy319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministry of Public Health Thailand . Practice guidelines for the treatment of patients with malaria. Nonthaburi: Ministry of Public Health Thailand; 2019. [Google Scholar]
- 17.Division of Vector Borne Diseases . Department of Disease Control, Ministry of Public Health Thailand: Standard operating procedures (SOP) for malaria case follow up in Thailand. Nonthaburi: Division of Vector Borne Diseases; 2019. [Google Scholar]
- 18.WHO Regional Office for the Western Pacific. Fifth Meeting of the Greater Mekong Subregion (GMS) Therapeutic Efficacy Studies (TES) Network, Ho Chi Minh City, Viet Nam, 28–29 September 2017: meeting report. World Health Organization Regional Office for the Western Pacific; 2017. https://apps.who.int/iris/handle/10665/260008. Accessed 10 Oct 2020.
- 19.van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, et al. Determinants of dihydroartemisinin–piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis. 2019;19:952–961. doi: 10.1016/S1473-3099(19)30391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stepniewska K, Humphreys GS, Gonçalves BP, Craig E, Gosling R, Guerin PJ, et al. Efficacy of single-dose primaquine with artemisinin combination therapy on Plasmodium falciparum gametocytes and transmission: an individual patient meta-analysis. J Infect Dis. 2020;20:943–952. doi: 10.1093/infdis/jiaa498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO/TDR. Microscopy for the detection, identification and quantification of malaria parasites on stained thick and thin blood films in research settings. Geneva, World Health Organization Special Programme for Research and Training in Tropical Diseases; 2015. https://www.who.int/tdr/publications/microscopy_detec_ident_quantif/en/. Accessed 10 Oct 2020.
- 22.USAID. US President's malaria initiative Thailand, Lao PDR and regional malaria operational plan FY 2020. United States Agency for International Development; 2020. https://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy20/fy-2020-thailand-malaria-operational-plan.pdf?sfvrsn=6. Accessed 10 Oct 2020.
- 23.WHO. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. Geneva, World Health Organization; 2008. http://www.who.int/malaria/publications/atoz/9789241596305/en/. Accessed 13 Dec 2020.
- 24.Institut Pasteur du Cambodge. PCR and sequencing for genotyping of candidate Plasmodium falciparum artemisinin resistance SNPs in the Kelch 13 gene v1.0. Institut Pasteur du Cambodge; 2013. https://www.wwarn.org/tools-resources/procedures/pcr-and-sequencing-genotyping-candidate-plasmodium-falciparum-artemisinin. Accessed 20 Feb 2021.
- 25.WWARN. Copy number estimation of P. falciparum pfmdr1 v1.1. WorldWide Antimalarial Resistance Network; 2011. https://www.wwarn.org/sites/default/files/attachments/procedures/MOL05_CopyEstimationPfalciparum_pfmdr1.pdf. Accessed 9 Oct 2020.
- 26.Sudathip P, Kitchakarn S, Shah J, Bisanzio D, Young F, Gopinath D, et al. A foci cohort analysis to monitor successful and persistent foci under Thailand’s malaria elimination strategy. Malar J. 2021;20:118. doi: 10.1186/s12936-021-03648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. mHealth: new horizons for health through mobile technologies. Geneva: World Health Organization; 2011. https://www.who.int/goe/publications/goe_mhealth_web.pdf. Accessed 27 Oct 2020.
- 28.Quang Bui P, Hong Huynh Q, Thanh Tran D, Le Thanh D, Quang Nguyen T, Van Truong H, et al. Pyronaridine–artesunate efficacy and safety in uncomplicated Plasmodium falciparum malaria in areas of artemisinin-resistant falciparum in Viet Nam (2017–2018) Clin Infect Dis. 2020;70:2187–2195. doi: 10.1093/cid/ciz580. [DOI] [PubMed] [Google Scholar]
- 29.Leang R, Mairet-Khedim M, Chea H, Huy R, Khim N, Mey Bouth D, et al. Efficacy and safety of pyronaridine–artesunate plus single-dose primaquine for treatment of uncomplicated Plasmodium falciparum malaria in eastern Cambodia. Antimicrob Agents Chemother. 2019;63:e02242–e2318. doi: 10.1128/AAC.02242-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leang R, Khim N, Chea H, Huy R, Mairet-Khedim M, Mey Bouth D, et al. Efficacy and safety of pyronaridine–artesunate plus single-dose primaquine for the treatment of malaria in western Cambodia. Antimicrob Agents Chemother. 2019;63:e01273–e1319. doi: 10.1128/AAC.01273-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han KT, Lin K, Han ZY, Myint MK, Aye KH, Thi A, et al. Efficacy and safety of pyronaridine–artesunate for the treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax malaria in Myanmar. Am J Trop Med Hyg. 2020;103:1088–1093. doi: 10.4269/ajtmh.20-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tantiamornkul K, Pumpaibool T, Piriyapongsa J, Culleton R, Lek-Uthai U. The prevalence of molecular markers of drug resistance in Plasmodium vivax from the border regions of Thailand in 2008 and 2014. Int J Parasitol Drugs Drug Resist. 2018;8:229–237. doi: 10.1016/j.ijpddr.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roesch C, Mairet-Khedim M, Kim S, Lek D, Popovici J, Witkowski B. Impact of the first-line treatment shift from dihydroartemisinin/piperaquine to artesunate/mefloquine on Plasmodium vivax drug susceptibility in Cambodia. J Antimicrob Chemother. 2020;75:1766–1771. doi: 10.1093/jac/dkaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baird JK, Louisa M, Noviyanti R, Ekawati L, Elyazar I, Subekti D, et al. Association of impaired cytochrome P450 2D6 activity genotype and phenotype with therapeutic efficacy of primaquine treatment for latent Plasmodium vivax malaria. JAMA Netw Open. 2018;1:e181449. doi: 10.1001/jamanetworkopen.2018.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dharmawardena P, Rodrigo C, Mendis K, Gunasekera AWGW, Premaratne R, Ringwald P, et al. Response of imported malaria patients to antimalarial medicines in Sri Lanka following malaria elimination. PLoS ONE. 2017;12:e0188613. doi: 10.1371/journal.pone.0188613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sudathip P, Kongkasuriyachai D, Stelmach R, Bisanzio D, Sine J, Sawang S, et al. The investment case for malaria elimination in Thailand: a cost-benefit analysis. Am J Trop Med Hyg. 2019;100:1445–1453. doi: 10.4269/ajtmh.18-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are provided in the manuscript or are available from the website of the Department of Disease Control, Ministry of Public Health Thailand: https//malaria.ddc.moph.go.th.