Abstract
The novel coronavirus SARS-CoV-2, since its initial outbreak in Wuhan, China has led to a worldwide pandemic and has shut down nations. As with any outbreak, there is a general strategy of detection, containment, treatment and/or cure. The authors would argue that rapid and efficient detection is critical and required to successful management of a disease. The current study explores and successfully demonstrates the use of canines to detect COVID-19 disease in exhaled breath. The intended use was to detect the odor of COVID-19 on contaminated surfaces inferring recent deposition of infectious material from a COVID-19 positive individual. Using masks obtained from hospitalized patients that tested positive for COVID-19 disease, four canines were trained and evaluated for their ability to detect the disease. All four canines obtained an accuracy >90% and positive predictive values ranging from ~73 to 93% after just one month of training.
Keywords: Scent discriminating canines, COVID-19, Coronavirus, Volatile organic compounds
1. Introduction
Originally epi-centered in Wuhan, the capital of the Hubei Province of the People's Republic of China, COVID-19 disease (caused by the novel coronavirus SARS-CoV-2) was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020 [1,2]. The disease has devastated world populations with the latest report, at the time of writing, from the Centers for Disease Control and Prevention (CDC) indicating over 93 million reported cases of the disease and over 2 million deaths worldwide [3]. In addition to the loss of human life, COVID-19 has ruined livelihoods, businesses and economies around the world, with a long road to recovery ahead. The World Bank projects a contraction of the global gross domestic product (GDP) of 5.2% as a result of COVID-19, the deepest global recession in decades [4]. A report by Statista (2021) [5] confirms this figure and projects the dollar loss as 3.5 trillion U.S. dollars for every 0.4% drop in economic growth.
Many governments worldwide were largely ill equipped to manage the spread or contain the damage by such a sudden and large-scale threat. Never before has it been clearer that multiple solutions and preferably rapidly deployable solutions are necessary to enable a better prognosis when the inevitable next threat arises. One of the major factors that allows an epidemic to become a pandemic is the large-scale trade and travel that exists today in an open global economy. The authors believe that rapid screening methods for disease at key entry points such as airports and shipping ports, as well as internally at large scale events, schools, etc. are currently lacking but essential to successful management of an infectious disease such as COVID-19. Naturally, one of the difficulties with a new disease or threat is that little is often known, and molecular techniques can take time to develop through research. A variety of techniques have been developed for the detection of COVID-19, these include immunoassays, molecular tests for viral detection using reverse transcription polymerase chain reactions (rtPCR) and hospital imaging techniques through chest computed tomography (CT) scans. These techniques have a range of accuracies from 30% to over 99.7% [6,7] the most accurate being rtPCR, commonly referred to as the Gold Standard in COVID-19 testing [8]. However, rtPCR is arguable the most expensive and least adaptable of these detection techniques, requiring specific sampling protocols, expensive reagents (that were quickly in short supply), highly skilled personnel and sophisticated equipment and facilities that are often far removed from the persons or areas being infected. This has resulted in a high demand for an accurate, rapid and field deployable detection mechanism for COVID-19.
One rapid screening method that has been under investigation in recent times is the detection of unique volatile organic compound (VOC) markers from exhaled breath of diseased patients [9]. VOCs are metabolic products that enter the bloodstream and are released into the air via blood, urine, feces, skin and breath (Amann et al., 2014). The use of volatile organic compounds (VOCs) has been widely reported throughout literature as a means by which certain diseased states may be identified [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19]]. Analytical instruments have been tested and show promise for their use as effective screening tools [20]. However, for the detection of breath VOCs, sensitivity limitations still exist with these instruments reporting sensitivity levels in the parts per billion (ppb) range [20]. It has been reported that VOCs in human breath are released in concentrations of parts per billion to parts per trillion (ppt), in comparison to human blood and urine where VOCs are released in the parts per million (ppm) to ppb range [20]. One method to combat this limitation has been the use of trained scent detection dogs as research has shown odor detection capabilities in the ppt range [21,22].
Dogs (Canis familiaris) are one of the earliest domesticated animals, and their use as working animals date back to early foragers and hunters between 14,000–36,000 years ago [23]. Today, working dogs are used in a number of applications but most relevant to this discussion is odor detection and discrimination. Owing to their highly developed olfactory system, dogs have been extensively used for the real-time detection of target odors of interest by law enforcement, military, and private agencies [21]. Additionally, a dog's mobility, independent thinking, ability to quickly learn new odors through operant conditioning and high selectivity present significant advantages over analytical instrumental methods. Traditional law enforcement and military targets for dogs range from drugs, money, explosives and missing persons [24]. The United States Department of Agriculture (USDA) and border control agencies globally use canines for the detection of illegal food product, plants [25] and pests [[26], [27], [28], [29], [30], [31]], and more recently, canines have been used to combat wildlife trafficking. Less traditional uses of canines have been demonstrated with the detection of chronic diseases in humans such as cancer and diabetes [[32], [33], [34], [35], [36], [37]] and pre-seizure detection of epileptic individuals [38]. Other studies have also demonstrated high rates of success in detection of plant pathogens causing laurel wilt disease in avocado trees [39,40]. Other researchers have demonstrated success with a citrus pathogen [41,42]. The current hypothesis assumes that, as with other diseased individuals, persons suffering from COVID-19 will exhibit a physiological change that manifests as a disease state related VOC profile, thereby allowing detection by a well-trained scent detection canine. Indeed, other research groups have also begun to consider canines as ideal candidates for COVID-19 screening with work on saliva [43] and sweat as targets [44]. This current study demonstrates the capabilities of canines for the detection and rapid screening of the odor of COVID-19 through the use of breath volatiles.
2. Materials and methods
2.1. Sample collection and sterilization
Florida International University (FIU) researchers collaborated with a Baptist Health South Florida team to obtain discarded masks previously worn by patients admitted to the hospital and diagnosed with COVID-19 and confirmed with PCR tests. These patients, while being monitored by medical staff, wore personal protective equipment (PPE) during treatment and afterwards, their PPE were discarded. Discarded masks were set aside and collected by the Baptist Health South Florida team for both analytical tests via GCMS and canine trials by FIU researchers. Masks were typically worn by patients for 30–45 min during doctor visits and then discarded. Masks were subsequently collected and sealed in a biohazard bag and then an aluminum barrier bag before being transported to FIU for further processing. The larger aluminum bag served to restrict VOC sample loss prior to use. In addition, masks from sick patients with similar symptoms but who tested negative for COVID-19 were also submitted as controls as well as clean, unworn masks to serve as blank training aids. The masks were carefully removed from their biohazard bags under a laboratory chamber/hood and made safe to handle by subjecting them to UV-C light (254 nm, germicidal light) for 10 min per side to sterilize and inactivate any viral particles [45], using a UV benchtop decontamination chamber (Dosage >500 mj/cm2) (Air Science USA LLC. Fort Myers, FL). Additionally, samples were allowed to sit for 24–72 h before being presented to a canine, thus adding an additional layer of safety as it has been shown the virus viability rapidly declines within hours to days [46].
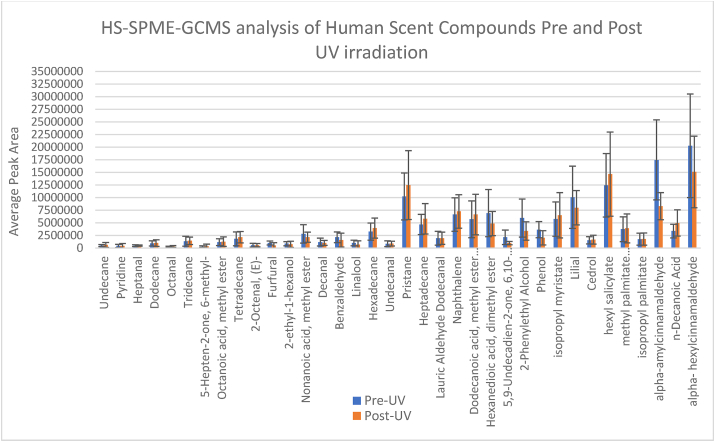
2.2. Chemical analyses of UV radiated samples
To ensure that UV radiation did not affect the VOCs, a prior analysis of pre- and post-UV treated samples, using headspace solid phase microextraction gas chromatography mass spectrometry (HS-SPME-GC-MS), was conducted with a mixture of common human scent VOCs. A 50 μl aliquots of a compound mix was pipetted onto separate 1-inch x 1-inch pieces of masks (n=12), half of which were subjected to UV radiation. The HS-SPME-GC-MS method parameters are described later in the chemical analysis section. The results (Fig. 3) showed that there was no significant effect of UV irradiation on the VOCs based on performing a Student's T-test at 95% confidence to compare the pre and post UV results. The GCMS used was an Agilent 6890 GC coupled to an Agilent 5973 MSD (Agilent Technologies). The column used was a Solgel 30 M × 0.25 mm ID with a 0.25 μm phase thickness (SGE Analytical Science). Carrier gas was ultra-high purity helium from Airgas. Solid phase microextraction fibers were Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS), needle size 24 ga, for use with a manual holder (Sigma Aldrich). Headspace vials were 40 ml clear screw top vials (Sigma Aldrich). Samples were irradiated using a UV-Box benchtop decontamination chamber from Air Science USA LLC.
Fig. 3.
Impact of 10-min UV-C irradiation on a mixture of volatiles. A Student's T-test indicated no significant difference between peak areas of the compounds before and after UV-C treatment (p > 0.05).
2.3. Chemical analysis of patient masks using HS-SPME-GCMS
An analysis of the VOCs from the PPE samples (masks previously worn by patients) was conducted utilizing headspace sampling with solid phase microextraction followed by analysis by gas chromatography mass spectrometry (HS-SPME-GCMS). After irradiating masks for 10 min, 1″ x 1” portions were cut and fitted into 40 ml headspace vials. Samples were heated at 40 °C and extracted for 15 h utilizing a DVB/CAR/PDMS SPME fiber. After extraction, the fibers were then injected in to the GCMS for analysis.
2.4. Data analysis
The identification of individual compounds present in the headspace of each sample was confirmed utilizing a mass spectral library from the National Institute of Standards and Technology (NIST) version 2017. These identified compounds were used for analysis to classify the samples as positive or negative for COVID-19, performed with the PLS-DA algorithm in the MetaboAnalyst software version 5.0 (https://www.metaboanalyst.ca). PLS is a supervised method that uses multivariate regression techniques to extract via linear combination of original variables (X), the information that can predict the class membership (Y). The raw peak area tables were utilized, and all compounds were assigned numbers rather than names for the purposes of this analysis. The resulting graph demonstrated sufficient differences in peak intensities and compound presence to separate COVID-19 positive and negative patients (Fig. 4).
Fig. 4.
PLS-DA showing class separation of HS-SPME-GCMS VOCs from COVID-19 positive PPE (masks) vs COVID-19 negative PPE (masks).
2.5. Canine training aid preparation
This study was conducted with IACUC approval, number: IACUC-20-046. All canines used for this study were imprinted on COVID-19 odor utilizing COVID-19 training aids, leveraging on an existing patented technology called Controlled Odor Mimic Permeation System (COMPS) [47]. COMPS utilize a permeable polymer in which a biological sample or chemical odor is housed and delivered at a measurable and reproducible rate. From the center of treated masks approximately 1″ x 1″ squares were cut and placed into 2″ x 3” low-density polyethylene (LDPE) bags and heat sealed [48]. Each bag was then placed into a larger aluminum barrier bag and sealed until used by the canine team. Sub-samples from each mask were also placed into 40 ml glass vials for HS-SPME-GCMS analysis.
2.6. Canines
Four canines were used in this study (Fig. 1). Cobra (Belgian Malinois) and One Betta (Dutch Shepherd) are both proficient canines in scent work and were previously a part of the laurel wilt disease study mentioned above. Mac (Terrier mix) had limited detection experience and Hubble (Border Collie mix) was a green dog (no prior odor detection experience) at the beginning of this study. The canines were all first trained on the FIU patented Universal Detector Calibrant [49] (Innovative Detection Concepts Inc. Homestead, FL), which consists of a pure target VOC that is not found in the environment. Initial UDC training ensured that the canines had minimum olfaction capabilities and provided basis from which odor detection and recognition training could be performed.
Fig. 1.
The four canines used in this study Cobra (Left), Mac (Center left), Hubble (Center right) and One Betta (Right).
2.7. Canine training
The study took place on the Modesto Maidique campus (MMC) at Florida International University (Miami, FL) in both classroom and office settings. Initially using the UDC, canines were acclimatized to searching containers, as well as moving through classrooms and office spaces. Utilizing a stainless-steel training wheel (Fig. 2), positive COVID-19 masks were placed in the same container as UDC to serve as the positive target, while in other containers negative patient masks were placed as negative targets.
Fig. 2.
Canine Cobra using the training wheel.
This began the process of odor imprinting to allow the canines to detect the familiar scent of the UDC calibrant while also introducing the scent of COVID-19. This training took place for approximately 1 month with training three times per week. The next phase of training involved the removal of the UDC from the scent picture and allowing the canines to search the scent wheel. Positive alerts, indicated by the canine sitting or lying down, were recorded as well as false positives and false negative alerts. In all 156 training runs were completed, and the accuracy and positive predictive value of the canines was calculated. In addition, 40 double blind trials were conducted to evaluate canine performance training (Table 4). A summary of the training parameters and double-blind trials can be seen in Table 1. Canines were exposed to 24 different positive patient masks during the study and 10 COVID-19 negative, but sick patient masks. A total of four training aids were made from each mask and only one positive training aid was used for each training session and then discarded. A new training aid was utilized for each new session. Each dog and position on the wheel were given a number and utilizing a random number generator, the deployment order of the canines and placement of training aids on the wheel were randomized.
Table 4.
Results of 40 double blind trials utilizing healthy individual masks and unused masks as distractors.
| Canine name | Canine breed | Failure to alert (no.) | False alerts (#)z | ACC/PPV (%)y |
|---|---|---|---|---|
| Hubble | Border Collie Mix | 15 | 6 | 96.3/87.0 |
| One Betta | Dutch Shepherd | 15 | 3 | 98.1/93.0 |
| Cobra | Belgian Malinois | 20 | 1 | 99.4/97.6 |
| Mac | Terrier mix | 17 | 5 | 96.2/88.6 |
False alerts indicate when a canine sits on a negative target that does not hold a training aid.
Accuracy (ACC) is calculated as the True Positive alerts + True Negative alerts divided by the Total Positives + Total Negatives and Positive Predictive Value (PPV) as the True Positive alerts divided by the sum of the True Positive alerts and False Positive alerts.
Table 1.
The parameters utilized in the four stages of the project. Imprinting, post imprint training and double-blind trials on the training wheel.
| Training stage | Positive aid | Distractors |
|---|---|---|
| Imprinting | UDC + COVID-19 positive patient mask | COVID-19 negative patient masks |
| Post imprint | COVID-19 positive patient mask | COVID-19 negative patient masks |
| Post imprint | COVID-19 positive patient mask | Unused, clean masks |
| Double Blinds | COVID-19 positive patient mask | Healthy persons & unused, clean masks |
Once imprinting and training had been completed, the canines were deployed to search office spaces and areas to ascertain any COVID-19 contamination on surfaces. Canines Cobra and Hubble worked office spaces to demonstrate their ability to detect COVID-19 positive odors on surfaces. These searches were conducted where no prior knowledge to the handler/canine teams, or anyone present during the deployment, about any COVID-19 positive employees occupying the office building.
3. Results
3.1. Impact of UV-C radiation on volatiles
Headspace SPME-GCMS of a mix of volatiles analyzed pre and post UV-C radiation was a critical step to evaluate whether the method of sample preparation and making training aids safe for both handlers and canines was viable. A Student's T-Test showed there was no significant difference in odor concentration pre and post UV-C (Fig. 3.)
3.2. Chemical analysis of COVID-19 positive patient masks and COVID-19 negative patient masks
Analysis to date have revealed the presence of several compounds in the headspace profile of these mask samples, many of which have been previously reported as human scent compounds [50]. Currently, further analysis is ongoing to identify the compound(s) that might be representative of a COVID-19 positive patient and in turn the compound(s) that allows the canine to detect COVID-19 as well as discriminate between COVID-19 positive and COVID-19 negative samples.
One of the ongoing tasks of the research is the identification of the chemical composition of COVID-19. Researchers attempted to determine, even with a small sample size (n=10) whether or not HS-SPME-GCMS could elucidate differences to classify positive and negative patient masks Subsequently, compound peak area data were extracted and utilizing all peak areas detected, the supervised classification method PLS-DA was employed. The clustering indicated that indeed sufficient data exist, even without compound identification, to tell positive and negative patients apart (Fig. 4).
3.3. Canine training
Of the 221 training runs using COVID-19 samples, the first 96 training sessions (Table 2.) utilized negative controls or distractors from sick patients in the hospital that had a negative rtPCR test for COVID-19 but had other illnesses. Overall, the accuracy obtained by the four canines using those negative controls was 85.2% while the positive predictive value was 64.0%, respectively. Specific accuracy and positive predictive responses for each canine from the first three weeks of the COVID-19 training were as follows: Mac 80% and 59%, Cobra 81% and 65%, One Betta 96% and 73% and Hubble 84% and 59% (Table 2).
Table 2.
Results of 96 training sessions using COVID-19 positive patient masks and distractor masks from other ill patients who tested negative as controls (negative targets).
| Canine name | Canine breed | Failure to alert (no.) | False alerts (no.)z | ACC/PPV (%)y |
|---|---|---|---|---|
| Hubble | Border Collie Mix | 10 | 13 | 84.0/59.0 |
| One Betta | Dutch Shepherd | 4 | 9 | 95.8/72.8 |
| Cobra | Belgian Malinois | 18 | 13 | 81.3/64.9 |
| Mac | Terrier mix | 18 | 16 | 80.2/58.9 |
False alerts indicate when a canine sits on a negative target that does not hold a training aid.
Accuracy (ACC) is calculated as the True Positive alerts + True Negative alerts divided by the Total Positives + Total Negatives and Positive Predictive Value (PPV) as the True Positive alerts divided by the sum of the True Positive alerts and False Positive alerts.
In subsequent test scenarios with 121 runs, the COVID-19 positive targets were again presented to the canines, but unused clean blank masks were used as negative targets. The average accuracy and positive predictive value for these sessions increased to 98.8% and 86.8%, respectively, with Mac 93.9% and 73.7%, Cobra 96.2% and 93.9%, One Betta 96.6% and 91.3% and Hubble 97.0% and 88.9% (Table 3).
Table 3.
Results of 121 training sessions using COVID-19 positive patient masks with blank unused masks as controls (negative targets).
| Canine name | Canine breed | Failure to alert (no.) | False alerts (3)z | ACC/PPV (%)y |
|---|---|---|---|---|
| Hubble | Border Collie Mix | 3 | 2 | 97.0/88.9 |
| One Betta | Dutch Shepherd | 2 | 2 | 96.6/91.3 |
| Cobra | Belgian Malinois | 2 | 4 | 96.2/93.9 |
| Mac | Terrier mix | 0 + 3 assistsx | 10 | 93.9/73.7 |
While Mac had no failures to alert he showed interest in the positive in 3 runs but required handler assistance to sit; therefore, these were counted as assisted alerts but were treated as failures in calculations.
False alerts indicate when a canine sits on a negative target that does not hold a training aid.
Accuracy (ACC) is calculated as the True Positive alerts + True Negative alerts divided by the Total Positives + Total Negatives and Positive Predictive Value (PPV) as the True Positive alerts divided by the sum of the True Positive alerts and False Positive alerts.
3.4. Double blind trials
A total of 40 blind trials was completed to evaluate the canine's performance post imprint training. Double blind trials were conducted with a mix of distractors ranging from empty containers, to blank unused, clean masks to healthy persons masks. The average accuracy and positive predictive value for these sessions were 97.5% and 91.5%, respectively, with Mac 96.2% and 88.6%, Cobra 99.4% and 97.6%, One Betta 98.1% and 93.0% and Hubble 96.3% and 87.0% (Table 4).
3.5. Deployment case study
Case #1 - On Saturday, January 30, 2021 Canine Team Cobra and Hubble were screening a building with several previous COVID-19 outbreaks. When inspecting an office shared by 10–12 individuals, the detector dogs were individually sent in and both dogs alerted to the same location, (a midline between two desks that faced each other). It was later verified that two employees with positive COVID-19 test results occupied those two desks 3–4 week prior to the canine inspection. Both canines demonstrated individual responses for a similar search in another office recently occupied by a COVID-19 positive employee. The detector dogs identified the exact location of the employee's desk among seven others without any foreknowledge of its location.
Case # 2 - On Monday, February 1, 2021 at an employee's request, an inspection was conducted of an office occupied by a person with COVID-19 positive results 4 weeks prior. Canine Cobra was sent in first, off leash to work independently. She was immediately drawn to a small trash can, where she paused and sniffed for a prolonged period (1–2 s), she then showed slight interest on a desk chair. Returning to the trash can, she demonstrated an alert. The handler inspected the trash can and noted two used soda cans along with paper. The second canine was sent in for confirmation. Canine Hubble immediately picked up odor with a change of behavior as he passed the same trash can. As he worked around the room, Hubble alerted to a stack of disarranged papers on the desk.
4. Discussion
This study is the first COVID-19 canine detection study focused on the volatiles in exhaled breath. Previous work on COVID-19 have utilized axillary sweat [44] and saliva or tracheobronchial secretions [43]. Current GC-MS data revealed some differences in the headspace VOCs of masks of patients from these two populations, i.e., those positive for COVID-19 and those negative for COVID-19. This provided evidence that the masks worn by patients could be used as a potential training aid for canines. The current study involved training with two different negative controls [1]: masks from ill, hospitalized patients who tested negative for COVID-19 and [2] blank unused masks. Utilizing the former, the study demonstrated an average accuracy (ACC) across four canines of 85.2% and a positive predictive value (PPV) of 64%. The range of accuracies were between 80.2% and 95.8% while the PPV was between 58.9% and 72.8%. There was a marked increase in ACC and PPV when utilizing blank masks as a negative control, 98.8% and 86.8% respectively, with an observed range of 93.9%–97.0% (ACC) and 73.7%–93.9% (PPV). Jendrny et al. demonstrated a PPV range of 68%–95% using eight dogs. Grandjean et al. reported success rates (number of correct identifications over number of trials) ranging from 83% to 100%. The rates observed in the current study are well within the expected ranges for detector dogs for both ACC and PPV and in line with or better than current analytical methods used to detect COVID-19 (Dong et al., 2020). In addition, post training double blind trials were also incorporated, where neither the handler nor the dog knew the location of the positive training aid. Forty [40] such trials were completed using negative distractors from healthy individual masks as well as unused masks. The recorded ranges were 96.2%–99.4% (ACC) and 87%–97.6% (PPV).
PPV is perhaps a stronger indicator of success as it takes into consideration the number of false positive alerts and is an indicator of how sure that one can be if a dog does alert when COVID-19 odor is, in fact, present, and the challenge when working with any biological samples has to be mentioned. In general, there are a number of potential confounding factors, primarily the fact that the disease system is in constant flux, or is dynamic, and at different points in the disease, metabolic changes are possible that may, in turn, alter the VOC profile being detected. With respect to human disease, there are a number of conditions, and health status questions that can be raised, one major question raised by the authors for future studies is how COVID-19 odors presents itself in asymptomatic patients versus those with symptoms. This study was unable to control for pre-existing conditions, age, gender, diet etc. as the masks were randomly acquired from positive and negative COVID-19 patients. Due to the urgency of the study and a lack of IRB approval to conduct research that would involve patient data, useful metrics such as patient symptom status, age, underlying health conditions etc., were not obtained and will be a consideration for future studies. One issue encountered with the study was the fact that initial training took place using PCR negative patient masks as non-targets. The lower ACC and PPV presented for this portion of the training could be attributed to: (a) the dogs were early in their training, (b) breath VOCs were very similar to COVID-19 as these patients presented at the hospital because they were ill, or (c) the patients had tested negative and were asymptomatic, but those data were not available for this study. These early results and those in other publications discussed are encouraging and demonstrate the usefulness of scent discriminating canines for the detection of COVID-19. It is important to note that experienced canines can be trained on new scents in a matter of weeks, and a well-established canine program can be employed to rapidly respond to novel threats and diseases. Lastly, the canine teams in this study have been successfully deployed to office spaces and classrooms, demonstrating that they can be deployed to enhance the safe repopulation of areas such as college campuses and office spaces. For example, the canines in this study detected and alerted to items such as garbage bins, office desks, folders/files, chairs and air filters in filtration units. A recent study by Harvey et al. reported 8.3% of surfaces swabbed and sampled, including garbage can lids, crosswalk buttons, and door handles, were positive for SARS CoV-2 RNA [51]. While this is an ongoing study, these four canines do support other studies that have conducted COVID-19 environmental surveillances. It is important to highlight that while mask mandates are in place to reduce the potential for viral spread through breath droplets that masks are still removed for certain activities such as eating and drinking. In high turnover areas such as classrooms, auditoriums or offices, this method of spread may become more of an issue. Nonetheless, canine behavior or search patterns can be modified, and they can be trained for different scenarios. It is feasible to transition or include training to detect these odors from individuals rather than on surfaces.
This current study has shown evidence that, in the areas or workspaces to which the canines alerted, the sites had been recently occupied by an employee that was later confirmed to have tested positive for COVID-19. It is recognized, however, that in the future additional biological and chemical profiles from those surfaces should be tested to verify whether or not the residual odor correlates to live virus and possible community transmission. Additional testing needs to be incorporated into the future studies in order to decipher how long a person's metabolism, the origin of the VOCs, remains “COVID-like” even after they test negative for the viral load. In other words, the body's metabolism may not recover as rapidly as the decrease in viral load, as evidenced by the ‘long haulers' that continue to have persistent symptoms, including respiratory complications. In an Italian study that followed patients for 60 days after acute symptoms were recorded, 55.2% of the 143 patients in the study, reported having >3 persistent symptoms, 32.2% reported they were experiencing 1–2 symptoms while 12.6% felt no symptoms [52]. It is of interest to analyze the breath samples from these individuals to see what the odor ‘decay’ curve may indicate. If this is the case, then the areas to which the canines alerted may indicate that even though the person has tested negative after infection, the metabolism has not returned to pre -COVID 19 levels, and the canines are still able to recognize the disease-specific volatilome. And, one must keep in mind, canine olfaction capabilities have often been shown to be more sensitive than the lower level of instrumental detection [53]. While ongoing studies will help answer some of these questions, this study has shown that, with a high degree of accuracy, canines, trained to recognize COVID-19 odor profiles, can provide one more tool, one more layer of protection, that can be utilized in the fight to dampen and perhaps even extinguish the community transmission of SARS-CoV-2 within the working environment.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The researchers would like to thank the Baptist Health Hospital Team Dr. Agueda Hernandez, Luis Collado and Miguel Diaz who partnered with FIU to be able to safely obtain the samples needed to conduct this study. This work was made possible through seed funding from the FIU Office of Research and Economic Development.
Contributor Information
Kenneth G. Furton, Email: furtonk@fiu.edu.
DeEtta Mills, Email: millsd@fiu.edu.
References
- 1.WHO COVID-19 weekly epidemiological update. World Heal. Organ. 2020;1:4. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20201012-weekly-epi-update-9.pdf [Google Scholar]
- 2.Wu Y.C., Chen C.S., Chan Y.J. The outbreak of COVID-19: an overview. J. Chin. Med. Assoc. 2020;83 doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weekly epidemiological update. 19 January 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update---19-january-2021 n.d. accessed June 3, 2021.
- 4.The global economic outlook during the COVID-19 pandemic: a changed world. https://www.worldbank.org/en/news/feature/2020/06/08/the-global-economic-outlook-during-the-COVID-19-pandemic-a-changed-world (n.d.) accessed February 8, 2021.
- 5.Impact of the coronavirus pandemic on the global economy - statistics & Facts | Statista. https://www.statista.com/topics/6139/covid-19-impact-on-the-global-economy/ (n.d.) accessed March 8, 2021.
- 6.Dong L., Zhou J., Niu C., Wang Q., Pan Y., Sheng S., Wang X., Zhang Y., Yang J., Liu M., Zhao Y., Zhang X., Zhu T., Peng T., Xie J., Gao Y., Wang D., Zhao Y., Dai X., Fang X. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. MedRxiv. 2020 doi: 10.1101/2020.03.14.20036129. 2020.03.14.20036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caruso D., Zerunian M., Polici M., Pucciarelli F., Polidori T., Rucci C., Guido G., Bracci B., De Dominicis C., Laghi A. Chest CT features of COVID-19 in rome, Italy. Radiology. 2020;296:E79–E85. doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böger B., Fachi M.M., Vilhena R.O., Cobre A.F., Tonin F.S., Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am. J. Infect. Contr. 2021;49:21–29. doi: 10.1016/j.ajic.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grassin-Delyle S., Roquencourt C., Moine P., Saffroy G., Carn S., Heming N., Fleuriet J., Salvator H., Naline E., Couderc L.J., Devillier P., Thévenot E.A., Annane D. Metabolomics of exhaled breath in critically ill COVID-19 patients: a pilot study. EBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun X., Shao K., Wang T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal. Bioanal. Chem. 2016;408(11):2759–2780. doi: 10.1007/s00216-015-9200-6. [DOI] [PubMed] [Google Scholar]
- 11.Lubes G., Goodarzi M. GC–MS based metabolomics used for the identification of cancer volatile organic compounds as biomarkers. J. Pharm. Biomed. 2018;147:313–322. doi: 10.1016/j.jpba.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Jalal A.H., Alam F., Roychoudhury S., Umasankar Y., Pala N., Bhansali S. Prospects and challenges of volatile organic compound sensors in human healthcare. ACS Sens. 2018;3(7):1246–1263. doi: 10.1021/acssensors.8b00400. [DOI] [PubMed] [Google Scholar]
- 13.Rahimpour E., Khoubnasabjafari M., Jouyban-Gharamaleki V., Jouyban A. Non-volatile compounds in exhaled breath condensate: review of methodological aspects. Anal. Bioanal. Chem. 2018;410(25):6411–6440. doi: 10.1007/s00216-018-1259-4. [DOI] [PubMed] [Google Scholar]
- 14.Wallace M.A.G., Pleil J.D. Evolution of clinical and environmental health applications of exhaled breath research: review of methods and instrumentation for gas-phase, condensate, and aerosols. Anal. Chim. Acta. 2018;1024:18–38. doi: 10.1016/j.aca.2018.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannoukos S., Agapiou A., Brkić B., Taylor S. Volatolomics: a broad area of experimentation. J. Chromatogr. B. 2019;1105:136–147. doi: 10.1016/j.jchromb.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez C., Santos J.P., Lozano J. Use of electronic noses for diagnosis of digestive and respiratory diseases through the breath. Biosensors. 2019;9(1):35. doi: 10.3390/bios9010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rondanelli M., Perdoni F., Infantino V., Faliva M.A., Peroni G., Iannello G., Nichetti M., Alalwan T.A., Perna S., Cocuzza C. Volatile organic compounds as biomarkers of gastrointestinal diseases and nutritional status. J Anal Methods Chem. 2019;2019 doi: 10.1155/2019/7247802. 14 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh C., Singh V., Grandy J., Pawliszyn J. Recent advances in breath analysis to track human health by new enrichment technologies. J. Separ. Sci. 2020;43(1):226–240. doi: 10.1002/jssc.201900769. [DOI] [PubMed] [Google Scholar]
- 19.Alizadeh N., Jamalabadi H., Tavoli F. Breath acetone sensors as non-invasive health monitoring systems: a review. IEEE Sensor. J. 2019;20(1):5–31. [Google Scholar]
- 20.Schmidt K., Podmore I. Current challenges in volatile organic compounds analysis as potential biomarkers of cancer. J. Biomarkers. 2015 doi: 10.1155/2015/981458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craven B.A., Paterson E.G., Settles G.S. The fluid dynamics of canine olfaction: unique nasal airflow patterns as an explanation of macrosmia. J. R. Soc. Interface. 2010 doi: 10.1098/rsif.2009.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Concha A.R., Guest C.M., Harris R., Pike T.W., Feugier A., Zulch H., Mills D.S. Canine olfactory thresholds to amyl acetate in a biomedical detection scenario. Front. Vet. Sci. 2019 doi: 10.3389/fvets.2018.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galeta P., Lázničková-Galetová M., Sablin M., Germonpré M. Morphological evidence for early dog domestication in the European Pleistocene: new evidence from a randomization approach to group differences. Anat. Rec. 2021;304 doi: 10.1002/ar.24500. [DOI] [PubMed] [Google Scholar]
- 24.Furton K.G., Myers L.J. The scientific foundation and efficacy of the use of canines as chemical detectors for explosives. Talanta. 2001 doi: 10.1016/S0039-9140(00)00546-4. [DOI] [PubMed] [Google Scholar]
- 25.Goodwin K.M., Engel R.E., Weaver D.K. Trained dogs outperform human surveyors in the detection of rare spotted knapweed (Centaurea stoebe) Invasive Plant Sci. Manag. 2010 doi: 10.1614/ipsm-d-09-00025.1. [DOI] [Google Scholar]
- 26.Brooks S.E., Oi F.M., Koehler P.G. Ability of canine termite detectors to locate live termites and discriminate them from non-termite material. J. Econ. Entomol. 2009 doi: 10.1603/0022-0493-96.4.1259. [DOI] [PubMed] [Google Scholar]
- 27.Cablk M.E., Heaton J.S. Accuracy and reliability of dogs in surveying for desert tortoise (Gopherus agassizii) Ecol. Appl. 2006 doi: 10.1890/1051-0761(2006)016[1926:AARODI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Savidge J.A., Stanford J.W., Reed R.N., Haddock G.R., Adams A.A.Y. Canine detection of free-ranging brown treesnakes on Guam. N. Z. J. Ecol. 2011;35(2):174–181. [Google Scholar]
- 29.Griffith R.T., Jayachandran K., Shetty K.G., Whitstine W., Furton K.G. Differentiation of toxic molds via headspace SPME-GC/MS and canine detection. Sensors. 2007 doi: 10.3390/s7081496. [DOI] [Google Scholar]
- 30.Nussear K.E., Esque T.C., Heaton J.S., Cablk M.E., Drake K.K., Valentin C., Yee J.L., Medica P.A. Are wildlife detector dogs or people better at finding Desert Tortoises (Gopherus agassizii)? Herpetol. Conserv. Biol. 2008;3:103–115. doi: 10.1155/2019/7247802. [DOI] [Google Scholar]
- 31.Pfiester M., Koehler P.G., Pereira R.M. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J. Econ. Entomol. 2008 doi: 10.1603/0022-0493(2008)101[1389:AOBBCT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.McCulloch M., Jezierski T., Broffman M., Hubbard A., Turner K., Janecki T. Diagnostic accuracy of canine scent detection in early- and late-stage lung and breast cancers. Integr. Canc. Ther. 2006 doi: 10.1177/1534735405285096. [DOI] [PubMed] [Google Scholar]
- 33.Moser E., McCulloch M. Canine scent detection of human cancers: a review of methods and accuracy. J. Vet. Behav. Clin. Appl. Res. 2010 doi: 10.1016/j.jveb.2010.01.002. [DOI] [Google Scholar]
- 34.Ehmann R., Boedeker E., Friedrich U., Sagert J., Dippon J., Friedel G., Walles T. Canine scent detection in the diagnosis of lung cancer: revisiting a puzzling phenomenon. Eur. Respir. J. 2012 doi: 10.1183/09031936.00051711. [DOI] [PubMed] [Google Scholar]
- 35.Godfrey A. Canine scent detection of human cancers. Vet. Nurse J. 2014 doi: 10.1111/vnj.12200. [DOI] [Google Scholar]
- 36.Brooks S.W., Moore D.R., Marzouk E.B., Glenn F.R., Hallock R.M. Canine olfaction and electronic nose detection of volatile organic compounds in the detection of cancer: a Review. Canc. Invest. 2015 doi: 10.3109/07357907.2015.1047510. [DOI] [PubMed] [Google Scholar]
- 37.Angle C., Waggoner L.P., Ferrando A., Haney P., Passler T. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front. Vet. Sci. 2016 doi: 10.3389/fvets.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis P.R.N. Florida Internationa University; 2017. The Investigation of Human Scent from Epileptic Patients for the Identification of a Biomarker for Epileptic Seizures. [DOI] [Google Scholar]
- 39.Mendel J., Burns C., Kallifatidis B., Evans E., Crane J., Furton K.G., Mills D.E. Agri-dogs: using canines for earlier detection of laurel wilt disease affecting avocado trees in South Florida. HortTechnology. 2018;28:109–116. doi: 10.21273/HORTTECH03791-17. [DOI] [Google Scholar]
- 40.Mendel J., Furton K.G., Mills D.E. An evaluation of scent-discriminating canines for rapid response to agricultural diseases. HortTechnology. 2018;28:102–108. doi: 10.21273/HORTTECH03794-17. [DOI] [Google Scholar]
- 41.Gottwald T., Poole G., McCollum T., Hall D., Hartung J., Bai J., Luo W., Posny D., Duan Y.P., Taylor E., da Graça J., Lou Polek M., Louws F., Schneider W. Canine olfactory detection of a vectored phytobacterial pathogen, Liberibacter asiaticus, and integration with disease control. Proc. Natl. Acad. Sci. U.S.A. 2020;117 doi: 10.1073/pnas.1914296117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottwald T., Poole G., Taylor E., Luo W., Posny D., Adkins S., Schneider W., McRoberts N. Canine olfactory detection of a non-systemic phytobacterial citrus pathogen of international quarantine significance. Entropy. 2020;22 doi: 10.3390/e22111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jendrny P., Schulz C., Twele F., Meller S., Von Köckritz-Blickwede M., Osterhaus A.D.M.E., Ebbers J., Pilchová V., Pink I., Welte T., Manns M.P., Fathi A., Ernst C., Addo M.M., Schalke E., Volk H.A. Scent dog identification of samples from COVID-19 patients - a pilot study. BMC Infect. Dis. 2020 doi: 10.1186/s12879-020-05281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grandjean D., Sarkis R., Lecoq-Julien C., Benard A., Roger V., Levesque E., Bernes-Luciani E., Maestracci B., Morvan P., Gully E., Berceau-Falancourt D., Haufstater P., Herin G., Cabrera J., Muzzin Q., Gallet C., Bacqué H., Broc J.M., Thomas L., Lichaa A., Moujaes G., Saliba M., Kuhn A., Galey M., Berthail B., Lapeyre L., Capelli A., Renault S., Bachir K., Kovinger A., Comas E., Stainmesse A., Etienne E., Voeltzel S., Mansouri S., Berceau-Falancourt M., Dami A., Charlet L., Ruau E., Issa M., Grenet C., Billy C., Tourtier J.P., Desquilbet L. Can the detection dog alert on COVID-19 positive persons by sniffing axillary sweat samples? A proof-of-concept study. PloS One. 2020;15 doi: 10.1371/journal.pone.0243122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heßling M., Hönes K., Vatter P., Lingenfelder C. Ultraviolet irradiation doses for coronavirus inactivation - review and analysis of coronavirus photoinactivation studies. GMS Hyg. Infect. Control. 2020;15 doi: 10.3205/dgkh000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.CDC, Epa . 2020. Guidance for Cleaning and Disinfecting Public Spaces, Workplaces, Businesses, Schools, and Homes. [Google Scholar]
- 47.Furton K.G., Harper R.J. 2008. Controlled Odor Mimic Permeation System. U.S. Patent 9706755. Filed July 19, 2017. [Google Scholar]
- 48.DeGreeff L.E., Simon A.G., Macias M.S., Holness H.K., Furton K.G. Controlled odor mimic permeation systems for olfactory training and field testing. JoVE. 2021 doi: 10.3791/60846. [DOI] [PubMed] [Google Scholar]
- 49.Furton K.G., Beltz K. 2017. Universal Detector Calibrant. U.S. Patent. 9575038. Filed Jan. 19, 2016. [Google Scholar]
- 50.Curran A.M., Rabin S.I., Prada P.A., Furton K.G. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 2005;31:1607–1619. doi: 10.1007/s10886-005-5801-4. [DOI] [PubMed] [Google Scholar]
- 51.Harvey A.P., Fuhrmeister E.R., Cantrell M.E., Pitol A.K., Swarthout J.M., Powers J.E., Nadimpalli M.L., Julian T.R., Pickering A.J. Longitudinal monitoring of SARS-CoV-2 RNA on high-touch surfaces in a community setting. Cite This Environ. Sci. Technol. Lett. 2021;8:168–175. doi: 10.1021/acs.estlett.0c00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. J. Am. Med. Assoc. 2020;324:603. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker D.B., Walker J.C., Cavnar P.J., Taylor J.L., Pickel D.H., Hall S.B., Suarez J.C. Naturalistic quantification of canine olfactory sensitivity. Appl. Anim. Behav. Sci. 2006;97:241–254. doi: 10.1016/j.applanim.2005.07.009. [DOI] [Google Scholar]