Abstract
The Coronavirus Disease 2019 (COVID-19) is the first known pandemic caused by a coronavirus. Its causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), appears to be capable of infecting different mammalian species. Recent detections of this virus in pet, zoo, wild, and farm animals have compelled inquiry regarding the zoonotic (animal-to-human) and reverse zoonotic (human-to-animal) transmissibility of SARS-CoV-2 with the potential of COVID-19 pandemic evolving into a panzootic.
It is important to monitor the global spread of disease and to assess the significance of genomic changes to support prevention and control efforts during a pandemic. An understanding of the SARS-CoV-2 epidemiology provides opportunities to prevent the risk of repeated re-infection of humans and requires a robust One Health-based investigation. This review paper describes the known properties and the existing gaps in scientific knowledge about the zoonotic and reverse zoonotic transmissibility of the novel virus SARS-CoV-2 and the COVID-19 disease it causes.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Zoonoses, Intermediate host, Transmission
1. Zoonotic origins of human coronaviruses
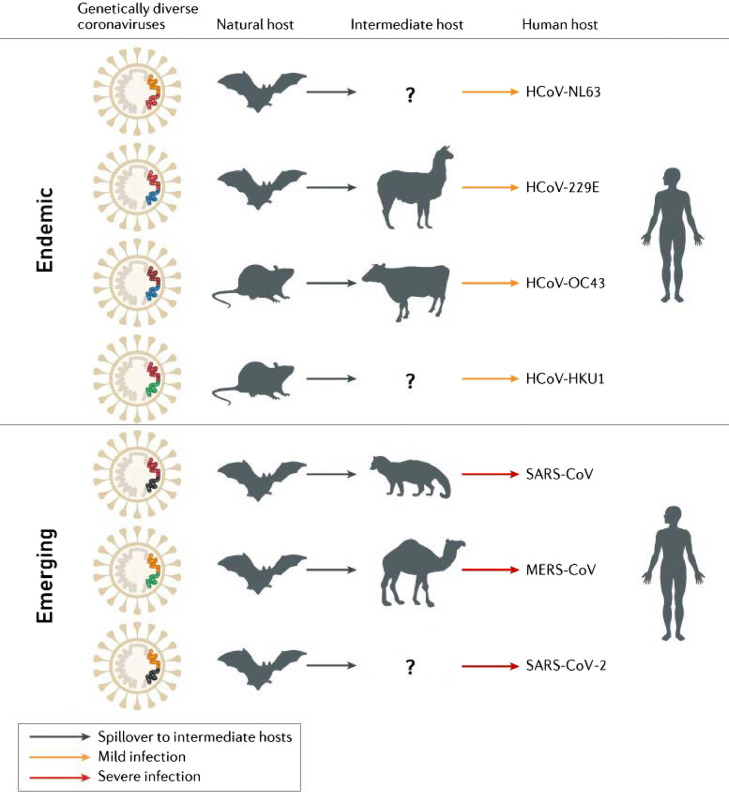
Coronaviruses are highly diverse RNA viruses that are widespread in nature and infect humans, mammals, and poultry (Su et al., 2020). Since the identification of the first human coronavirus (HCoV) in the 1960s, a total of seven coronaviruses are known to affect humans. And all seven have ancestry in other mammalian hosts (Gryseels et al., 2020). Four of these HCoVs, namely NL63, 229E, OC43, and HKU1, are endemic and generally cause asymptomatic or mild respiratory symptoms in humans, such as the common cold (Munir et al., 2020; Su et al., 2016; Ye et al., 2020). These viruses are believed to have originated in either bats or rodents (Corman et al., 2018; Gryseels et al., 2020) (Figure 1 ). The other three highly pathogenic and lethal HCoVs, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV-2, have recently emerged. These viruses have caused three global outbreaks in the last 20 years and are likely to have had an evolutionary origin from bats.
Figure 1.
Mammals as reservoirs and intermediary hosts of endemic and emerging human coronaviruses. Modified from Cui J. et al. (Cui et al., 2019).
The first HCoV known to cause severe disease in humans was SARS-CoV, which emerged in 2002-2003, with a total of 8,096 confirmed and 774 lethal cases reported to the World Health Organization from 29 countries (Louie et al., 2004; WHO, 2015). At the beginning of the SARS epidemic, most of the identified patients had animal exposure before developing the disease. The antibodies against SARS-CoV were detected in masked palm civets (Paguma larvata) and raccoon dogs (Nyctereutes procyonoides) in wet market places (Cui et al., 2019; Guan et al., 2003; Kan et al., 2005). Further research demonstrated that farmed and wild-caught civets and raccoon dogs were actually infected by other animals and more likely have acted as intermediate hosts (Gryseels et al., 2020; Tu et al., 2004). Two groups of researchers independently reported the discovery of novel coronaviruses related to human SARS-CoV, which were named SARS-CoV-related viruses. They were detected in horseshoe bats (genus Rhinolophus) demonstrating 99.8% nucleotide identity with human isolates (Cui et al., 2019; Hu et al., 2017; Li et al., 2005; Yang et al., 2015). These discoveries strengthened the evidence that bats may be the natural hosts for SARS-CoV.
The second HCoV outbreak was caused by MERS-CoV, which first emerged in humans in 2012. Currently, a total of 2,562 confirmed MERS cases with 881 related deaths were reported to the World Health Organization from 27 countries (WHO, 2020b). MERS-CoV was also detected in 14 bat species belonging to two bat families, Vespertilionidae and Nycteridae (Yang et al., 2014), which indicate that MERS-CoV probably has an evolutionary origin in bats as well (Anthony et al., 2017; Ithete et al., 2013). However, it appears to be clear that humans repeatedly acquire MERS through close contact with dromedary camels, as MERS-CoV strains allocated from camels had a strong identity with those isolated from humans (Dudas et al., 2018; Raj et al., 2014). Anti-MERS-CoV antibodies were detected in camels from the Middle Eastern, African, and Asian countries in the high titers. Also, MERS-CoV antibodies were detected in camel serum samples collected 30 years ago, which indicates that the virus has been prevalent in these animals for a long time (Müller et al., 2014) and that dromedaries were probably infected by a MERS ancestor a few decades ago, either directly from bats or via another intermediate host (Corman et al., 2014). The MERS-CoVs isolates that were derived from bats, humans, and camels have the same genomic structures, but essential differences in their genomic sequences (Anthony et al., 2017). The sequence identity between bat's MERS-CoV and human/camel's isolates is around 85% (Luo et al., 2018). Even though, dromedary camels play an important role in the continued transmission of MERS-CoV, various other animal species have been found to be susceptible to MERS-CoV: alpacas (Vicugna pacos), llamas (Lama glama), pigs (Sus scrofa), goats (Capra hircus), sheep (Ovis aries), cattle (Bos taurus), donkeys (Equus africanus), horses (Equus ferus) rabbits (Oryctolagus cuniculus), chickens (Gallus gallus), and rhesus macaques (Macaca mulatta) (Adney et al., 2016; Ali et al., 2017; Crameri et al., 2016; David et al., 2018; de Wit et al., 2013; Gautam et al., 2020; Hemida et al., 2013; Kandeil et al., 2019; Reusken et al., 2013; Reusken et al., 2016; Vergara-Alert et al., 2017a; Vergara-Alert et al., 2017b; Widagdo et al., 2019). MERS cases still occur sporadically, likely resulting from occasional spill-over from the intermediate animal hosts.
The third and still ongoing outbreak of the coronavirus disease of 2019 (COVID-19) caused by novel SARS-CoV-2 emerged in humans in December 2019. On January 30th, 2020, the World Health Organization declared the coronavirus outbreak as a Public Health Emergency of International Concern (WHO, 2020a). With its rapid spread across continents, COVID-19 was categorized as a Pandemic on March 11th, 2020, marking it as the first pandemic caused by a coronavirus (WHO, 2020c). One year later, as of June 2nd, 2021, there have been 172,045,061 confirmed cases of COVID-19 reported worldwide, with more than 3,578,246 related deaths (Worldometer 2021). The pandemic of COVID-19 caused by SARS-CoV-2 has also been designated as a zoonotic disease (Gollakner and Capua, 2020; Haider et al., 2020; Yoo and Yoo, 2020), which makes it the third zoonotic epidemic caused by a coronavirus in the 21st century.
Efforts to identify potential SARS‐CoV‐2 intermediate hosts have yet to be successful. The origin of the outbreak was associated with the Huanan seafood wholesale market in Wuhan during epidemiologic investigations of the first cases that were linked directly to this market (Huang et al., 2020a; Li et al., 2020a; Wang et al., 2020a). However, the retrospective analyses indicated that an earlier COVID‐19 human case had no exposure to that seafood market (Huang et al., 2020a). The roots of the pandemic thus remain unclear, as coronaviruses that are closely similar to SARS-CoV-2 have yet to be found in a non-human organism.
The closest relatives to human SARS‐CoV‐2 known so far are a coronavirus coming from the horseshoe bat species (Rhinolophus affinis) from China with approximately 96.2% nucleotide identity, and two sub‐lineages of SARS‐related CoVs detected in Malayan pangolins (Manis javanica) with 85.5%–92.4% nucleotide identity of the complete viral genomes (Lam et al., 2020b; Paraskevis et al., 2020; Yuan et al., 2020; Zhou et al., 2020). Moreover, phylogenetic analyses of a large subgenomic data set of bat coronaviruses from China indicate that evolutionary closely related SARS‐CoV and SARS‐CoV‐2 likely both originated in horseshoe bats (Latinne et al., 2020). However, the phylogenetic reconstruction analysis estimates that SARS-CoV and SARS-CoV-2 diverged from their respective most closely related bat CoVs approximately 40 to 70 years ago (Boni et al., 2020; Wang et al., 2021). This long divergence period suggests that the virus could have first evolved in a different animal species, yet to be sampled, before infecting humans (MacLean et al., 2021). Thus, the bat is the natural host for SARS-CoV-2, but likely involved a non-bat intermediate host animal, that introduced the virus to humans, remains to be determined (Kadam et al., 2021; Leitner and Kumar, 2020; Liu et al., 2020; Zhang and Holmes, 2020).
2. SARS-CoV-2 phylogenetic prediction of susceptible species
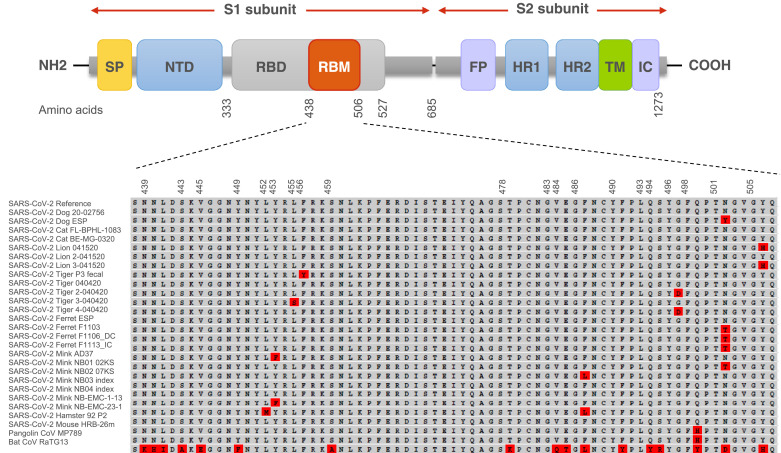
To assess the intermediate host of SARS-CoV-2 or the potential for the virus to spread to other species living with or in close proximity to humans in domestic, rural, agricultural, or zoological settings, it is vital to know which animal species are susceptible to SARS-CoV-2. As in all CoV, a surface spike (S) protein mediates viral recognition and entry into host cells via the receptor‐binding domain (RBD). The RBD binds to a particular protein on the surface of host cells. Both SARS-CoV and SARS-CoV-2 use angiotensin‐converting‐enzyme 2 (ACE2) as their receptor for entry into cells (Brooke and Prischi, 2020; Wan et al., 2020). The affinity of the viral S protein, especially the RBD, to the ACE2 receptor highly determines the corresponding host's susceptibility to infection by CoVs (Maurin et al., 2021). Unlike SARS‐CoV, SARS‐CoV‐2 also contains a polybasic cleavage site, that allows host enzymes to cleave the S protein into S1 and S2 subunits for more efficient cell entry (Figure 2 ) (Hoffmann et al., 2020). Recently, Lan et al. (Lan et al., 2020) suggested that the structure of the S protein bound to the human ACE2 receptor has 20 key amino acids for interacting with the receptor-binding motif. Prior research has already shown that certain animals are also vulnerable to SARS-CoV-2 because the same ACE2 receptor is used, and this receptor is quite conserved across mammals (Abdel-Moneim and Abdelwhab, 2020; Hoffmann et al., 2020; Li, 2013). Genetic variation in the ACE2 can affect the efficiency with which RBD binds to it, and therefore, its susceptibility to SARS‐CoV‐2 (Wan et al., 2020). Understanding the conservation of ACE2 receptors across different animal species may provide insight into the potential hosts of SARS-CoV-2 (Sun et al., 2021). It is vital to try to predict which animals could potentially be infected by SARS-CoV-2 so that the plausible extent of transmission can be estimated, and surveillance efforts can be guided appropriately.
Figure 2.
Map of SARS-CoV-2 spike protein structure from N-terminal end (NH2) to the C-terminal end (COOH). The differences in the spike amino acid sequences from pangolin coronavirus MP789, bat coronavirus RaTG13, and animal isolates compared to SARS-CoV-2 reference (Wuhan-Hu-1 isolate) are marked in red. The multiple sequence alignment is presented for the receptor-binding motif and the furin cleavage site. SP: signal peptide; NTD: N-terminal domain; RBD: receptor-binding domain; RBM: receptor-binding motif; FP: fusion peptide; HR1: heptad repeat 1; HR2: heptad repeat 2; TM: transmembrane domain; IC: intracellular domain.
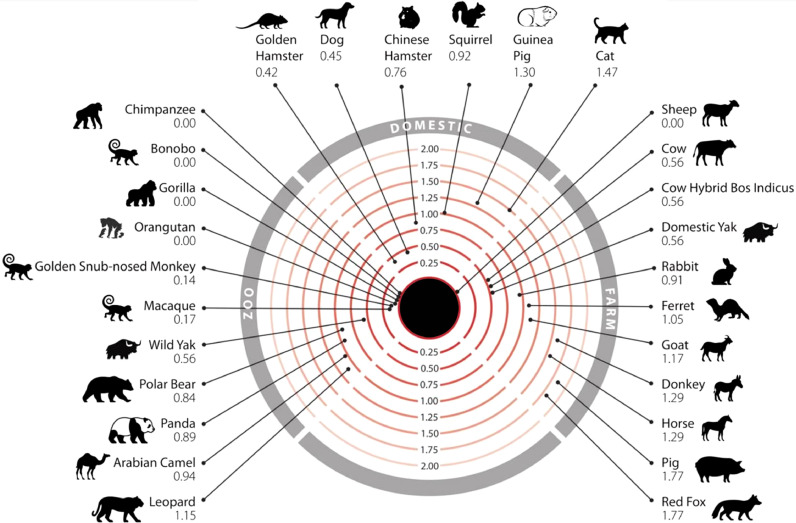
A number of recent computational studies comparing ACE2 protein sequences among different species or modeling the structure of the S protein:ACE2 complex to predict the breadth of potential viral hosts have suggested that, due to the high conservation of ACE2, many animals are vulnerable to infection by SARS-CoV-2 (Ahmed et al., 2021; Damas et al., 2020; Devaux et al., 2020; Fischhoff et al., 2021; Huang et al., 2020b; Kumar et al., 2021; Li et al., 2020b; Liu et al., 2021; Melin et al., 2020; Rodrigues et al., 2020). Humans are likely to come into contact with 26 of these species in domestic, agricultural, or zoological settings (Lam et al., 2020a) (Figure 3 ). It was predicted that SARS-CoV-2 can infect a broad range of mammals, but only a few species of fish, birds, or reptiles (Lam et al., 2020a). Of particular concern are sheep, that have no change in energy of the S protein:ACE2 complex, as these animals are farmed and come into close contact with humans. Species carrying a sequence with K31, Y41, N90, and K353 are likely to be susceptible to infection by SARS-CoV-2 (including Homo sapiens, Macaca mulatta, Felis catus, Rhinolophus sinicus, Meloidogyne javanica, and Pelodiscus sinensis) while others should be less susceptible or resistant to infection (Fischhoff et al., 2021).
Figure 3.
Mammals that humans come into contact with that are at risk of infection by SARS-CoV-2. Numbers represent the change in the binding energy of the spike protein:ACE2 complex (Lam et al., 2020a).
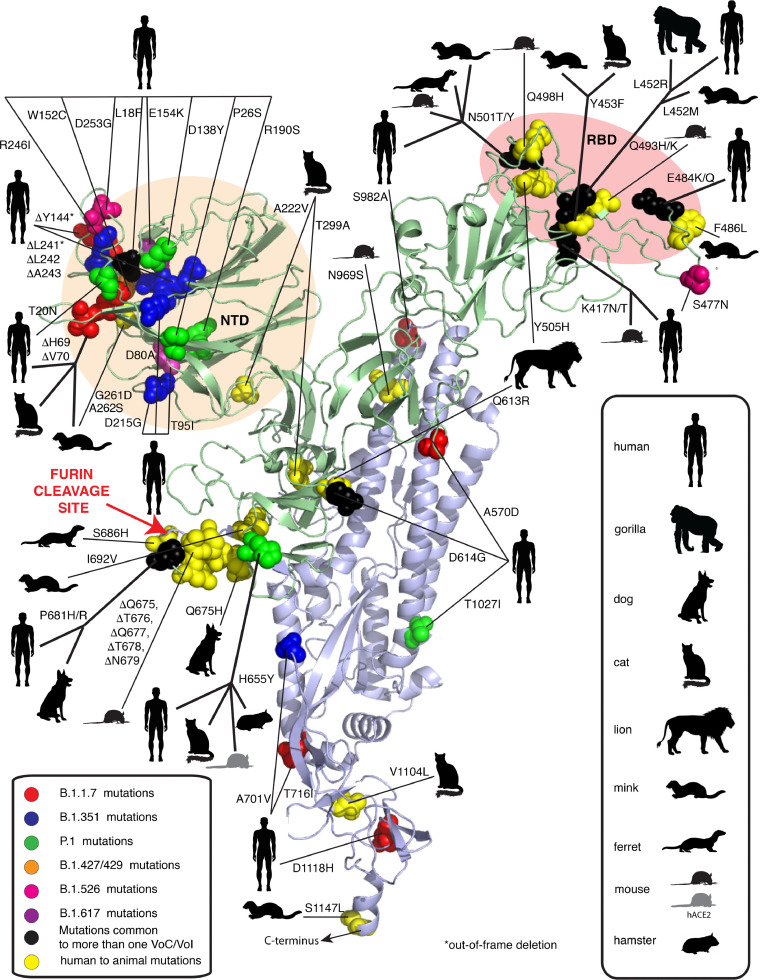
Besides currently unknown SARS-CoV-2 hosts, intermediate hosts, and susceptible animal species, new SARS-CoV-2 RBD variations could introduce compensatory mutations that render low susceptible SARS-CoV-2 species to become more susceptible to infection. Rodrigues et al. (Rodrigues et al., 2020) carried out an analysis of more than 100,000 SARS-CoV-2 RBD sequences obtained from infected patients and found that at least 17 single-point mutations suggested enhanced affinity of these RBD variants to mouse ACE2. These results are in agreement with Gu et al. recent work, which suggests that SARS-CoV-2 can readily adapt to infect house mice following serial passages. After only six passages, a single amino‐acid substitution in SARS‐CoV‐2 RBD of the S protein rendered a commonly used inbred laboratory house mouse strain to become susceptible (Gu et al., 2020). Adaptation of this viral strain in the mouse appeared to be dependent on a critical amino acid change, N501Y (Asn501 → Tyr), one of the key mutations for the emergence of several SARS-CoV-2 lineages, including B.1.1.7, B.1.351, and P.1 (Figure 2, 4 ) (Goncalves Cabecinhas et al., 2021; Gu et al., 2020). The molecular dynamics simulations research has shown that the N501Y mutation can increase the overall binding affinity of the RBD with human ACE2 through the hydrophobic interactions between them (Luan et al., 2021). Microscale thermophoresis confirmed that a tyrosine at position 501 enhances ACE2 binding affinity 1.98 times, which in turn plays an essential role in the higher transmission of this virus variant among humans (Ramanathan et al., 2021). Furthermore, a study by Wang et al. (Wang et al., 2020b) showed that the S protein of the mouse-adapted SARS-CoV-2 also had two amino acid substitutions, one in the RBM Q498H (Gln498 → His) and the other N969S (Asn969 → Ser) in the heptad repeat 1.
Figure 4.
Mutations in SARS-CoV-2 spike protein occurring in humans and animals. NTD: N-terminal domain; RBD: Receptor binding domain. Figure modified from Garry R.F. (Garry, 2021).
The rapid emergence of another N501T (Asn501 → Thr) mutation in the RBD of the S protein was observed repeatedly in farmed minks (Elaswad et al., 2020; Oude Munnink et al., 2021) and experimentally infected ferrets (Richard et al., 2020) (Figure 2). Interestingly, the N501T mutation was found in almost all SARS-CoV-2 sequences from the US minks (99%). A study by Cai, et al. (Cai and Cai, 2021) demonstrated that the N501T mutation occurred two months earlier in humans than in minks in the US, suggesting that the novel variant may have evolved in humans first and was then transmitted to the mink population in the US. Threonine at position 501 was previously shown to increase the binding affinity of the S protein with the human ACE2 receptor (Starr et al., 2020; Welkers et al., 2021). Perhaps this substitution may contribute to the virus's adaptation to efficiently bind to ferret and mink ACE2 (Bashor et al., 2021).
Another mink-associated mutation Y453F (Tyr453 → Phe) has been introduced into the human population in the Netherlands and Denmark (Figure 2, 4) (GISAID, 2020; Hoffmann et al., 2021). The rapid transmission of SARS-CoV-2 among farmed minks facilitated the accumulation of mutations that further contributed to the evolution of the virus (Oude Munnink et al., 2021). The Y453F mutation was found to be associated with a change in the S protein amino acid (Mallapaty, 2020). The biolayer interferometry analysis showed that this variant binds to the human ACE2 receptor with a 4-fold higher affinity than the original strain, suggesting the potential for enhanced transmission. The mink-derived SARS-CoV-2 isolates also contained two amino acid substitutions (G261D, A262S) in the N-terminal domain of S protein and four (L452M, Y453F, F486L, N501T) in the RBD (Figure 4) (Elaswad et al., 2020). Despite outbreaks on mink farms being a probable cause for the introduction of the Y453F SARS-CoV-2 mutation in the human population, many human carriers of this variant had no direct contact with mink farms, suggesting. indicating the possibility of sustained transmission of this variant among humans (Welkers et al., 2021). Interestingly, emerging infectious diseases previously reported in minks, such as swine pseudorabies, Newcastle disease, avian influenza, and orthoreovirus infection, all have high zoonotic potential (Fenollar et al., 2021; Jiang et al., 2017; Lian et al., 2013; Wang et al., 2018; Zhao et al., 2017).
Computational analysis predicting host susceptibility based on close phylogenetic relationships among potential host species or similarity between human ACE2 and non-human ACE2 sequences may help in the prediction of a certain species as a potential host, but need to be viewed with caution as these methods do not consistently match real-world outcomes (Fischhoff et al., 2021). For example, weak viral binding was predicted by sequence similarity for minks and ferrets, which both were later confirmed as being highly susceptible (Oude Munnink et al., 2021; Shi et al., 2020). In contrast, the highest similarity of the relative synonymous codon usage of SARS-CoV-2 and snakes led to the latter being hypothesized as a potential viral host (Ji et al., 2020). However, bioinformatics approaches that are based on the analysis of their ACE2 receptor (Luan et al., 2020; Zhang et al., 2020a) argued against this assumption (Devaux et al., 2020).
There is also always a possibility to change the range of susceptible animal species as novel SARS‐CoV‐2 strains may evolve with new mutations. Repeated interspecies transmission of a virus presents the potential for the acceleration of viral evolution. With the possibility of creating new reservoirs of SARS-CoV-2, the early identification of such events and mitigation against the risks of human-to-animal transmission (reverse zoonosis) need to be developed on national and global levels. In particular, farm animals and other animals living in close contact with humans should be monitored, protected where possible, and managed accordingly.
3. SARS-CoV-2 reverse zoonosis and secondary zoonosis
Even though recognition of the intermediate host is of much importance in preventing the transmission chain and avoiding the zoonotic event that triggered the COVID-19 pandemic in the first place, further research of SARS-CoV-2 infection in various susceptible animal species is also needed. This will help to identify and assess the extent of animals that are vulnerable to SARS-CoV-2 in order to prevent secondary zoonotic events and possible reverse zoonosis. Prior research has confirmed that reverse zoonosis events during the COVID‐19 pandemic have been documented in several countries, including Hong Kong, Belgium, United States, Netherlands, Denmark, Spain, Germany, and France (Jo et al., 2020). The transmission of SARS-CoV-2 from humans to numerous animals, as well as conducted in vitro infection experiments, make it clear that the virus is able to infect and be transmitted between a wide range of distantly related mammal species.
Case reports on cats (Felis catus) living in the same household with COVID‐19 patients in Europe, Asia, North America, and South America revealed that these animals can be infected with SARS‐CoV‐2, showing clinical manifestations ranging from asymptomatic to severe respiratory illness (de Morais et al., 2020; Garigliany et al., 2020; Michelitsch et al., 2020; Musso et al., 2020; Newman et al., 2020; OIE, 2021a; ProMED-mail, 2021e; Ruiz-Arrondo et al., 2021; Sailleau et al., 2020; Segales et al., 2020; TACC, 2021; Zhao et al., 2020). The reports show that 14% of tested cats in Hong Kong were SARS-CoV-2 positive by RT‐PCR (Barrs et al., 2020). The use of a commercial ELISA test that detects antibody reactivity against SARS‐CoV‐2 showed that the seroprevalence among sampled cats in Wuhan was 14.7% in 2020 compared with 0.0% in cats sampled in 2019 (Zhang et al., 2020b). Reports from Bosnia & Herzegovina, Canada, China, Croatia, Japan, Mexico, Thailand, and Uruguay regarding SARS‐CoV‐2‐infected dogs, whose owners were COVID‐19 patients, showed that although dogs tested positive for SARS‐CoV‐2, there were no apparent clinical signs observed (OIE, 2021a; ProMED-mail, 2021b; ProMED-mail, 2021f; Sit et al., 2020). However, other reports on SARS‐CoV‐2‐positive dogs in Argentina, Germany, the Netherlands, and the USA indicated that different symptoms can occur in infected dogs, ranging from mild to severe respiratory distress symptoms (AVMA, 2020; de Morais et al., 2020; OIE, 2021a; ProMED-mail, 2020a). The seroprevalence screening performed among pets living in SARS‐CoV‐2‐positive households in Italy demonstrated that 3.3% of dogs and 5.8% of cats were sero-positive (Patterson et al., 2020). The high seroprevalence and SARS‐CoV‐2 detection rates in cats and to some extent in dogs indicate that these animals can be infected with SARS‐CoV‐2 (Fritz et al., 2021; Hamer et al., 2020; Hosie et al., 2021; Leroy et al., 2020). Recent reports also describe sporadic cases of natural infection in household pet ferrets (Mustela putorius furo) in Slovenia and Spain (Giner et al., 2021; OIE, 2021a; ProMED-mail, 2020d). These reports suggest that the impact of SARS‐CoV‐2 infections on domestic animal health is unclear. Animals could develop COVID-19 with or without clinical signs through reverse zoonosis, which can potentially lead to the reinfection of humans.
Zoonotic SARS‐CoV‐2 transmission is not associated with domestic animals only. Several other felines’ species were found to be positive in the Bronx Zoo (New York, USA), including two Malayan tigers (Panthera tigris jacksoni), two Amur tigers (Panthera tigris altaica), and three African lions (Panthera leo) in April 2020 (McAloose et al., 2020), three Malayan tigers at Zoo Knoxville (Tennessee, USA) in October 2020, a puma (Puma concolor) in a zoo in the City of Johannesburg, Gauteng (South Africa) and at the rescue center in Santiago del Estero (Argentina) in November 2020, four lions at the Barcelona Zoo (Spain) and three snow leopards (Panthera uncia) at the Louisville Zoo (Kentucky, USA) in December 2020, one lion at the Tallin Zoo (Estonia), one Bengal tiger (Panthera tigris tigris) at the Wildcat Sanctuary in Pine County (Minnesota, USA) (Minnesota Board of Animal Health, 2021), one Amur tiger and two lions at the Boras Zoo (Sweden) in January 2021 (ProMED-mail, 2021a; Wang et al., 2020c), two Sumatran tigers (Panthera tigris sumatrae) at the Fort Wayne Children's Zoo (Indiana, USA), two lions, one tiger, and one cougar (Puma concolor) at a wild animal exhibit (Texas, USA) in February 2021 (APHIS, 2021a), two lions at the Pittsburgh Zoo & PPG Aquarium (Pennsylvania, USA), three Malayan tigers at the Virginia Zoo (Virginia, USA), and eight Asiatic lions (Panthera leo persica) at Nehru Zoological Park (India) in April 2021 (OIE, 2021a; ProMED-mail, 2021g). Three western lowland gorillas (Gorilla gorilla gorilla) at the San Diego Zoo Safari Park (California, USA) and four Asian small-clawed otters (Aonyx cinereus) at the Georgia Aquarium (Georgia, USA) were confirmed to be positive for SARS-CoV-2 (APHIS, 2021c; Daly, 2021). The source of infection in these cases were diseased zoo staff members (APHIS, 2021b; McAloose et al., 2020).
Farmed minks are highly susceptible to SARS-CoV-2 infection and, in some cases, they have transmitted the virus back to humans. SARS‐CoV‐2‐positive minks were detected in 290 fur farms in Denmark, 69 mink fur farms in the Netherlands, 13 of 40 mink farms in Sweden, 23 out of 91 mink farms in Greece, 17 fur farms in the USA, 4 farms in Lithuania, 2 farms in Canada, and one fur farm in Italy, Latvia, Poland, France, and Spain (Domańska-Blicharz et al., 2021; Fenollar et al., 2021; OIE, 2021a; Rabalski et al., 2020). The infected minks demonstrated mild respiratory distress and interstitial pneumonia on the necropsy (Oreshkova et al., 2020). The source of the outbreak in minks was linked to the farmers and their family members, who manifested with COVID‐19 infections and/or were PCR-positive for SARS‐CoV‐2. Virus transmission from infected minks to humans was proven by the phylogenetic comparison of human‐ and mink‐derived SARS‐CoV‐2 sequences, which were very similar (European Food Safety et al., 2021; Fenollar et al., 2021; Oude Munnink et al., 2021). Additionally, seven of 24 cats at the mink farms also tested positive serologically for SARS-CoV-2-specific antibodies, indicating the existence of animal-to-human (zoonotic) and animal-to-animal transmission (Enserink, 2020). The airborne transmission of SARS‐CoV‐2 between cats and between hamsters has also been reported (Halfmann et al., 2020; Shi et al., 2020). After it became known that a strain of the coronavirus can be passed between animals and humans, more than 17 million minks have been culled and mink farming was banned in Denmark until the start of 2022, devastating its fur industry (ProMED-mail, 2020b). Surveillance findings in Denmark show that SARS-CoV-2 introduced into mink populations continues to evolve through viral mutations. Viral mutations also happen in human infections, but these new mutations may also be seen while the virus adapts to a new species. Scientific investigations have confirmed that SARS-CoV-2 has been reintroduced from minks to humans. There is currently no evidence that SARS-CoV-2 is circulating or has been established in wild populations surrounding the infected mink farms. However, during the wildlife surveillance for SARS-CoV-2 in meso-carnivores and other species conducted as part of One Health investigations around infected mink farms in Utah, Michigan, and Wisconsin, USA, the US National Veterinary Services Laboratories (NVSL) has confirmed SARS-CoV-2 by real-time RT-PCR and sequencing of a nasal swab collected from a free-ranging, wild mink sampled in Utah (Shriner et al., 2021). The sequence of the viral genome obtained from the wild mink sample at NVSL was indistinguishable from those obtained from the farmed mink. The World Organisation for Animal Health acknowledges that such events could have important public health implications. There are concerns that the introduction and circulation of new virus strains in humans could result in modifications of transmissibility or virulence and decreased treatment and vaccine efficacy (OIE, 2021b).
Zoonotic infections are common hazards for humans involved in animal welfare management, including veterinarians, zoo and reserve workers, breeders, and farmers. Between March and June 2020, outbreaks of COVID-19 were reported in meat plants extending from Europe to North America (ProMED-mail, 2021d). There have been 95 confirmed COVID-19 cases linked to the workplace outbreak at the meat packing plant in Canada in February 2021 (ProMED-mail, 2021c). Other research indicated a strong relationship between US livestock-processing plants and local community transmission of COVID-19, suggesting that these plants may act as transmission vectors into the surrounding population (Taylor et al., 2020). A common occurrence among outbreaks in meat plants is a sudden sharp spike in cases, which suggests the simultaneous infection of workers from the same source rather than person-to-person spread (Donaldson, 2020). Therefore, risk assessments should be carried out to identify occupational groups that are disproportionately exposed by SARS‐CoV‐2-infected animals, similarly to how disease management was structured for the zoonotic squirrel novel bornavirus 1 that emerged as an occupation-associated disease in an animal caretaker at a zoo in northern Germany in 2013 (Tappe et al., 2018).
4. SARS-CoV-2 experimental infections
Experimental infections of SARS‐CoV‐2 were conducted in ferrets, domestic cats, raccoon dogs, Egyptian fruit bats (Rousettus aegyptiacus), Syrian hamsters (Mesocricetus auratus), Chinese hamsters (Cricetulus griseus), Roborovski's dwarf hamster (Phodopus roborovskii), deer mice (Peromyscus maniculatus), bushy-tailed woodrats (Neotoma cinerea), striped skunks (Mephitis mephitis), New Zealand white rabbits (Oryctolagus cuniculus), mice (Mus musculus), Northern tree shrews (Tupaia belangeris), white-tailed deer (Odocoileus virginianus), bank voles (Myodes glareolus), rhesus macaques (Macaca mulatta), crab‐eating macaques (Macaca fascicularis), African green monkeys (Chlorocebus aethiops), baboon (Papio hamadryas), common marmosets (Callithrix jacchus), and cattle (Bos taurus) (Bertzbach et al., 2020; Bosco-Lauth et al., 2021; Freuling et al., 2020; Imai et al., 2020; Jo et al., 2020; Lu et al., 2020b; (Montagutelli et al., 2021); Muñoz-Fontela et al., 2020; Mykytyn et al., 2021; Palmer et al., 2021; Singh et al., 2021; Temmam et al., 2020; Trimpert et al., 2020; Ulrich et al., 2021; Ulrich et al., 2020). These animals demonstrated viral replication and RNA shedding in the respiratory tract and to a lesser extent or no shedding in the gastrointestinal tract, development of SARS‐CoV‐2‐specific antibody responses, and histopathological signs of moderate inflammation in infected respiratory tissue (Chiba et al., 2021; Freuling et al., 2020; Jo et al., 2020; Muñoz-Fontela et al., 2020). Domestic cats, ferrets, Syrian hamsters, deer mice, and white-tailed deer were found to be susceptible and able to readily transmit the virus to co‐housed animals (Bosco-Lauth et al., 2020; Chan et al., 2020; Gaudreault et al., 2020; Griffin et al., 2020; Halfmann et al., 2020; Kim et al., 2020; Palmer et al., 2021; Richard et al., 2020; Schlottau et al., 2020; Sia et al., 2020). However, upon re-infection, cats do not appear to shed the virus at levels sufficient enough for the transmission to co-housed naïve cats (Gaudreault et al., 2021). Rhesus macaques, crab‐eating macaques, African green monkeys, and baboons, often used as non‐human primate model species in biomedical research, are also permissive to infection and develop similar symptoms to those of COVID-19 patients, with higher severity presenting in Rhesus macaques than in crab‐eating macaques (Deng et al., 2020; Lu et al., 2020a; Munster et al., 2020; Rockx et al., 2020; Shan et al., 2020; Woolsey et al., 2020; Yu et al., 2020; Zheng et al., 2020).
Although the first experimental infection studies demonstrated that SARS‐CoV‐2 replicated poorly in dogs, cattle, domestic pigs, and does not replicate in poultry species (Shi et al., 2020; Suarez et al., 2020), it takes only a few mutations or genetic recombination events for the virus to spill over and establish a new host (Opriessnig and Huang, 2020; Sreenivasan et al., 2020). Previous studies indicated that swine are not susceptible to SARS-CoV-2 infection (Meekins et al., 2020). However, later Canadian and US researchers measured seroconversion and viral shedding in experimentally infected domestic pigs and found them to be permissive to SARS-CoV-2 infection at low levels, as only 31.3% of infected pigs displayed low levels of viral RNA shedding and antibody production (Pickering et al., 2021; ProMED-mail, 2020c). Considering the proximity of pigs with humans as an agricultural animal, and the use of a wide range of prosthetic accessories of pig origin in the human health sector, this research highlights the need for additional livestock assessment to determine the actual role that domestic animals might play in the maintenance and spread of SARS-CoV-2 during the COVID-19 pandemic. The circumstance described indicates that SARS-CoV-2 epidemiological surveillance strategies must include susceptible animals in close contact with humans, such as pets, farm, zoo, laboratory, and biotechnology production animals, to prevent potential zoonotic and reverse zoonotic events (Mallapaty, 2021; McNamara et al., 2020; Prince et al., 2021; Valdivia-Granda and Richt, 2020). To date, in the immediacy of the COVID-19 pandemic, surveillance studies were understandably focused on human health but have already identified some of the susceptible animals as well, such as bats and minks. However, there are only a limited number of SARS-CoV-2 epidemiological surveillance studies based on the One Health approach. Therefore, reliable data are needed on the animal susceptibility to SARS-CoV-2 and the potential transmissibility within and across species.
5. Pests as vehicles for mechanical transmission of SARS-CoV-2
Rodents and arthropods are major agricultural pests worldwide, that can biologically or mechanically transmit many serious pathogens among humans and other animals. Through biological transmission, the virus undergoes development in the pests to complete its life cycle before transmission to other animals or humans. Although insects do have ACE2, these are very different from those in humans and are therefore predicted to be unable to bind efficiently with SARS-CoV-2 (Cashman et al., 2019). Recent studies demonstrated that SARS-CoV-2 replication was not supported in biting midges (Culicoides sonorensis) and mosquitoes (Aedes aegypti, Aedes albopictus, Culex pipiens, Culex quinquefasciatus, and Culex tarsalis), suggesting that these species are unable to be biological vectors of SARS-CoV-2 (Balaraman et al., 2021a; Fortuna et al., 2021; Huang et al., 2020c). However, it has been demonstrated that houseflies (Musca domestica) can acquire and harbor infectious SARS-CoV-2 for up to 24 hours post-exposure (Balaraman et al., 2021b).
Transfer of pathogens via contaminated surfaces is another transmission route for viruses from rodents and arthropods to animals and humans, called mechanical or passive transmission. Mechanical transmission involves the transfer of the virus picked up by pests’ body parts or mouths to other animals or humans with no developmental change of the virus in themselves (Franz et al., 2015; Gubler, 2010; Reuben et al., 2020). Here, the pests serve as vehicles for viral transmission which is merely incidental. Previous studies have shown that SARS-CoV-2 can survive on surfaces and objects for several hours and days depending on the nature and type of surface, humidity, and temperature (Kampf et al., 2020; Kwon et al., 2021a; Wu et al., 2020). Although the virus titer was greatly reduced over time, viable SARS-CoV-2 was measured to last on plastic up to 2-3 days, stainless steel up to 2-3 days, cardboard up to 1 day, and copper up to 4 hours (Ismail et al., 2020; van Doremalen et al., 2020). Pests such as houseflies, cockroaches, ticks, house mice, and rats exist in great abundance on farms, public places, and even in healthcare settings where they can interact with contaminated surfaces. It was reported that rodents and arthropods were involved in the mechanical transmission of the turkey coronavirus and SARS-CoV during the SARS epidemic (Calibeo-Hayes et al., 2003; Reuben et al., 2020). Experimental studies showed that houseflies were able to mechanically transmit SARS-CoV-2 genomic RNA to the surrounding environment up to 24 h post-exposure (Balaraman et al., 2021b). Some of the United States’ common rodents, such as fox squirrels (Sciurus niger), Wyoming ground squirrels (Urocitellus elegans), house mice (Mus musculus), and black-tailed prairie dogs (Cynomys ludovicianus), are not susceptible to SARS-CoV-2 infection (Bosco-Lauth et al., 2021). In contrast, several other common peridomestic rodent species are high-risk groups. Thus, deer mice (Peromyscus maniculatus) and bushy-tailed woodrats (Neotoma cinerea) are found to be susceptible to experimental inoculation of high doses of SARS-CoV-2 and can shed the virus in respiratory secretions.
Prior research has already shown that coronaviruses from contaminated surfaces can be transmitted through self-inoculation of the eyes, mouth, and nasal mucous membranes (Kampf et al., 2020; Otter et al., 2016). SARS-CoV-2 was detected in the bronchoalveolar lavage fluid specimens of 93% of COVID-19 patients, sputum – 72%, nasal swabs – 63%, fibrobronchoscope brush biopsy – 6%, pharyngeal swabs – 32%, feces – 29%, blood – 1% (Wang et al., 2020d). The virus was stable for up to 21 days in nasal mucus, sputum, saliva, tear, urine, blood, and semen (Kwon et al., 2021b). It was also shown that despite the stool samples testing positive, about 23% of patients were no longer positive for the virus in respiratory samples (Xiao et al., 2020). Another data from 98 COVID-19 patients showed viral shedding in their stool for nearly five weeks after respiratory samples were negative (Wu et al., 2020) and even asymptomatic carriers may show elevated SARS-CoV-2 in stool samples (Xie et al., 2020). With the size of the SARS-CoV-2 virus and its presence in feces, droplets, and on surfaces, there is a possibility that pests can act as mechanical vectors to transmit the virus. However, as of now, the role and importance of this mechanical transmission route of SARS-CoV-2 has not yet been fully determined and needs to be validated through further studies.
6. Discussion
Due to the process of modern world globalization, the increase in international trade, transportation of people, animals, and agricultural products, the risks of the emergence and spread of infectious diseases, as well as the spread of causative pathogens are highly increased. This explains the fact that biosafety is a key component of national security, and the control of infectious diseases is the main priority in human and veterinary medicine. The interspecies transmission among animals could make pandemic control more difficult. Up until now, the COVID-19 pandemic has continued to have a devastating impact on human life around the world, therefore epidemiological surveillance studies of SARS-CoV-2 were understandably focused mainly on the human population. This has resulted in a limited number of SARS-CoV-2 molecular and serological surveillance studies being performed on animals worldwide. Surveillance of wild, domestic, laboratory, zoo, and companion animals is the key in determining the SARS-CoV-2 intermediate host, which would help to prevent secondary zoonotic events and possible reverse zoonosis.
In combination with SARS-CoV-2 high transmissibility and its presence in a significant number of potentially asymptomatic people throughout the world, this creates a dangerous situation in which humans may unknowingly transmit the virus to a susceptible mammal population. Concerningly, these animal species might serve as reservoirs of the virus, increasing the risk of future zoonotic and reverse zoonotic events, though transmission rates across species are currently unknown. The prediction of possible hosts suggests that SARS-CoV-2 could indeed infect a broad range of mammals. Therefore, more high-quality epidemiological surveillance studies are needed. Furthermore, the need for more in-depth research has also increased due to the current lack of studies assessing the mechanical transmission of SARS-CoV-2 by rodents, insects, and arachnids, which have been already recognized as a mechanical vector of over 100 pathogens including bacteria, fungi, viruses, and parasites (El-Sherbini and Gneidy, 2012; Foil and Gorham, 2000; Gough and Jorgenson, 1983; Graczyk et al., 2005; Issa, 2019; Nayduch and Burrus, 2017; Onwugamba et al., 2018; Pitkin et al., 2009; Watson et al., 2007). Although SARS-CoV and other related coronaviruses have been reported to be mechanically transmitted by pests, together with SARS-CoV-2’s survivability on surfaces and in feces for elongated periods would undoubtedly implicate them as culprits in the role of its transmission. Thus, preventing the opportunity for the continuous spread of SARS-CoV-2, all possible transmission routes need to be ascertained and added to the current strategies for COVID-19 pandemic control.
When an outbreak happens, effective measurements include several aspects to properly address the analysis and understanding of native hosts, intermediate hosts, mutational tendency, transmission characteristics between animals-to-humans, and vice versa. The One Health approach handles the issues of emerging threats by integrating professionals from various disciplines: human medicine, veterinary medicine, environmental health, and social sciences. This correlates the interconnections between people, animals, and the environment to improve the health of both humans and animals. A shift to the One Health approach is an essential step to improve mitigation strategies of the emerging infectious disease threats, including COVID-19.
It is unlikely that SARS-CoV-2 will be the last coronavirus to jump the species barrier infecting humans and other animal species. Therefore, any human surveillance program should integrate with testing programs for animals, to capture early zoonotic pathogen circulation between human and nonhuman populations. Sources of zoonotic pathogens are frequently unclear and are often impossible to determine after the early stages of a spillover event. Active monitoring could remove much of this uncertainty, allowing epidemiologic research to inform short- and long-term responses on both local and global levels.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Iryna V. Goraichuk: Methodology, Data curtion, Writing – original draft, Writing – review & editing. Vasiliy Arefiev: Conceptualization, Writing – review & editing. Borys T. Stegniy: Conceptualization, Methodology, Supervision, Funding acquisition. Anton P. Gerilovych: Conceptualization, Methodology, Writing – review & editing, Project administration.
Declaration of Competing Interest
None
References
- Abdel-Moneim A.S., Abdelwhab E.M. Evidence for SARS-CoV-2 Infection of Animal Hosts. Pathogens. 2020;9(7) doi: 10.3390/pathogens9070529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adney D.R., Bielefeldt-Ohmann H., Hartwig A.E., Bowen R.A. Infection, Replication, and Transmission of Middle East Respiratory Syndrome Coronavirus in Alpacas. Emerg Infect Dis. 2016;22(6):1031–1037. doi: 10.3201/2206.160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Hasan R., Siddiki A.M.A.M., Islam M.S. Host range projection of SARS-CoV-2: South Asia perspective. Infect Genet Evol. 2021;87 doi: 10.1016/j.meegid.2020.104670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., El-Shesheny R., Kandeil A., Shehata M., Elsokary B., Gomaa M., Hassan N., El Sayed A., El-Taweel A., Sobhy H., Fasina F.O., Dauphin G., El Masry I., Wolde A.W., Daszak P., Miller M., VonDobschuetz S., Morzaria S., Lubroth J., Makonnen Y.J. Cross-sectional surveillance of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Euro Surveill. 2017;22(11) doi: 10.2807/1560-7917.ES.2017.22.11.30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony S.J., Gilardi K., Menachery V.D., Goldstein T., Ssebide B., Mbabazi R., Navarrete-Macias I., Liang E., Wells H., Hicks A., Petrosov A., Byarugaba D.K., Debbink K., Dinnon K.H., Scobey T., Randell S.H., Yount B.L., Cranfield M., Johnson C.K., Baric R.S., Lipkin W.I., Mazet J.A. Further Evidence for Bats as the Evolutionary Source of Middle East Respiratory Syndrome Coronavirus. mBio. 2017;8(2) doi: 10.1128/mBio.00373-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APHIS . 2021. Confirmation of COVID-19 in a Cougar at a Wild Animal Exhibitor in Texas. Stakeholder Announcement.https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-02/sars-cov-2-texas-cougar (accessed 12 May 2021) [Google Scholar]
- APHIS . 2021. Confirmation of COVID-19 in Gorillas at a California Zoo. Animal and Plant Health Inspection Service.https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-01/ca-gorillas-sars-cov-2 (accessed 11 February 2021) [Google Scholar]
- APHIS . 2021. Confirmation of COVID-19 in Otters at an Aquarium in Georgia Stakeholder Announcement.https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/SA_By_Date/SA-2021/SA-04 (accessed 12 May 2021) [Google Scholar]
- AVMA, 2020. SARS-CoV-2 in Animals. https://www.avma.org/resources-tools/animal-health-and-welfare/covid-19/sars-cov-2-animals-including-pets (accessed 13 February 2021).
- Balaraman V., Drolet B.S., Gaudreault N.N., Wilson W.C., Owens J., Bold D., Swanson D.A., Jasperson D.C., Noronha L.E., Richt J.A., Mitzel D.N. Susceptibility of Midge and Mosquito Vectors to SARS-CoV-2. J Med Entomol. 2021 doi: 10.1093/jme/tjab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaraman V., Drolet B.S., Mitzel D.N., Wilson W.C., Owens J., Gaudreault N.N., Meekins D.A., Bold D., Trujillo J.D., Noronha L.E., Richt J.A., Nayduch D. Mechanical transmission of SARS-CoV-2 by house flies. Parasit Vectors. 2021;14(1):214. doi: 10.1186/s13071-021-04703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrs V.R., Peiris M., Tam K.W.S., Law P.Y.T., Brackman C.J., To E.M.W., Yu V.Y.T., Chu D.K.W., Perera R.A.P.M., Sit T.H.C. SARS-CoV-2 in Quarantined Domestic Cats from COVID-19 Households or Close Contacts, Hong Kong, China. Emerg Infect Dis. 2020;26(12):3071–3074. doi: 10.3201/eid2612.202786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashor L., Gagne R.B., Bosco-Lauth A., Bowen R., Stenglein M., VandeWoude S. 2021. SARS-CoV-2 evolution in animals suggests mechanisms for rapid variant selection. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertzbach L.D., Vladimirova D., Dietert K., Abdelgawad A., Gruber A.D., Osterrieder N., Trimpert J. SARS-CoV-2 infection of Chinese hamsters (Cricetulus griseus) reproduces COVID-19 pneumonia in a well-established small animal model. Transbound Emerg Dis. 2020 doi: 10.1111/tbed.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni M.F., Lemey P., Jiang X., Lam T.T., Perry B.W., Castoe T.A., Rambaut A., Robertson D.L. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat Microbiol. 2020;5(11):1408–1417. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- Bosco-Lauth A.M., Hartwig A.E., Porter S.M., Gordy P.W., Nehring M., Byas A.D., VandeWoude S., Ragan I.K., Maison R.M., Bowen R.A. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc Natl Acad Sci U S A. 2020;117(42):26382–26388. doi: 10.1073/pnas.2013102117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco-Lauth A.M., Root J.J., Porter S.M., Walker A.E., Guilbert L., Hawvermale D., Pepper A., Maison R.M., Hartwig A.E., Gordy P., Bielefeldt-Ohmann H., Bowen R.A. 2021. Survey of peridomestic mammal susceptibility to SARS-CoV-2 infection. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke G.N., Prischi F. Structural and functional modelling of SARS-CoV-2 entry in animal models. Sci Rep. 2020;10(1):15917. doi: 10.1038/s41598-020-72528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.Y., Cai A. 2021. SARS-CoV-2 spike protein gene variants with N501T and G142D mutation dominated infections in minks in the US. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calibeo-Hayes D., Denning S.S., Stringham S.M., Guy J.S., Smith L.G., Watson D.W. Mechanical transmission of turkey coronavirus by domestic houseflies (Musca domestica Linnaeaus) Avian Dis. 2003;47(1):149–153. doi: 10.1637/0005-2086(2003)047[0149:MTOTCB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cashman J.S., Cozier G.E., Harrison C., Isaac R.E., Acharya K.R. Crystal structures of angiotensin-converting enzyme from Anopheles gambiae in its native form and with a bound inhibitor. Biochem J. 2019;476(22):3505–3520. doi: 10.1042/BCJ20190635. [DOI] [PubMed] [Google Scholar]
- Chan J.F., Zhang A.J., Yuan S., Poon V.K., Chan C.C., Lee A.C., Chan W.M., Fan Z., Tsoi H.W., Wen L., Liang R., Cao J., Chen Y., Tang K., Luo C., Cai J.P., Kok K.H., Chu H., Chan K.H., Sridhar S., Chen Z., Chen H., To K.K., Yuen K.Y. Simulation of the Clinical and Pathological Manifestations of Coronavirus Disease 2019 (COVID-19) in a Golden Syrian Hamster Model: Implications for Disease Pathogenesis and Transmissibility. Clin Infect Dis. 2020;71(9):2428–2446. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Halfmann P.J., Hatta M., Maemura T., Fan S., Armbrust T., Swartley O.M., Crowford L.K., Kawaoka Y. Protective Immunity and Persistent Lung Sequelae in Domestic Cats after SARS-CoV-2 Infection. Emerging Infectious Diseases. 2021;27(2):660–663. doi: 10.3201/eid2702.203884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Ithete N.L., Richards L.R., Schoeman M.C., Preiser W., Drosten C., Drexler J.F. Rooting the phylogenetic tree of middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. J Virol. 2014;88(19):11297–11303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Muth D., Niemeyer D., Drosten C. Hosts and Sources of Endemic Human Coronaviruses. Adv Virus Res. 2018;100:163–188. doi: 10.1016/bs.aivir.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri G., Durr P.A., Klein R., Foord A., Yu M., Riddell S., Haining J., Johnson D., Hemida M.G., Barr J., Peiris M., Middleton D., Wang L.F. Experimental Infection and Response to Rechallenge of Alpacas with Middle East Respiratory Syndrome Coronavirus. Emerg Infect Dis. 2016;22(6):1071–1074. doi: 10.3201/eid2206.160007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly N. Several gorillas test positive for COVID-19 at California zoo—first in the world. National Geographic. 2021 https://www.nationalgeographic.com/animals/2021/01/gorillas-san-diego-zoo-positive-coronavirus/ (accessed 11 February 2021) [Google Scholar]
- Damas J., Hughes G.M., Keough K.C., Painter C.A., Persky N.S., Corbo M., Hiller M., Koepfli K.P., Pfenning A.R., Zhao H., Genereux D.P., Swofford R., Pollard K.S., Ryder O.A., Nweeia M.T., Lindblad-Toh K., Teeling E.C., Karlsson E.K., Lewin H.A. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc Natl Acad Sci U S A. 2020;117(36):22311–22322. doi: 10.1073/pnas.2010146117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D., Rotenberg D., Khinich E., Erster O., Bardenstein S., van Straten M., Okba N.M.A., Raj S.V., Haagmans B.L., Miculitzki M., Davidson I. Middle East respiratory syndrome coronavirus specific antibodies in naturally exposed Israeli llamas, alpacas and camels. One Health. 2018;5:65–68. doi: 10.1016/j.onehlt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Morais H.A., Dos Santos A.P., do Nascimento N.C., Kmetiuk L.B., Barbosa D.S., Brandão P.E., Guimarães A.M.S., Pettan-Brewer C., Biondo A.W. Natural Infection by SARS-CoV-2 in Companion Animals: A Review of Case Reports and Current Evidence of Their Role in the Epidemiology of COVID-19. Front Vet Sci. 2020;7 doi: 10.3389/fvets.2020.591216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., Rasmussen A.L., Falzarano D., Bushmaker T., Feldmann F., Brining D.L., Fischer E.R., Martellaro C., Okumura A., Chang J., Scott D., Benecke A.G., Katze M.G., Feldmann H., Munster V.J. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A. 2013;110(41):16598–16603. doi: 10.1073/pnas.1310744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Bao L., Liu J., Xiao C., Xue J., Lv Q., Qi F., Gao H., Yu P., Xu Y., Qu Y., Li F., Xiang Z., Yu H., Gong S., Liu M., Wang G., Wang S., Song Z., Liu Y., Zhao W., Han Y., Zhao L., Liu X., Wei Q., Qin C. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020;369(6505):818–823. doi: 10.1126/science.abc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Pinault L., Osman I.O., Raoult D. Can ACE2 Receptor Polymorphism Predict Species Susceptibility to SARS-CoV-2? Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.608765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domańska-Blicharz K., Orłowska A., Smreczak M., Niemczuk K., Iwan E., Bomba A., Lisowska A., Opolska J., Trębas P., Potyrało P., Kawiak-Sadurska M., Rola J. Mink SARS-CoV-2 Infection in Poland - Short Communication. J Vet Res. 2021;65(1):1–5. doi: 10.2478/jvetres-2021-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson A.I. Aerosols in meat plants as possible cause of COVID-19 spread. Veterinary Record. 2020;187(1):34–35. doi: 10.1136/vr.m2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas G., Carvalho L.M., Rambaut A., Bedford T. MERS-CoV spillover at the camel-human interface. Elife. 2018;7 doi: 10.7554/eLife.31257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherbini G.T., Gneidy M.R. Cockroaches and flies in mechanical transmission of medical important parasites in Khaldyia Village, El-Fayoum, Governorate, Egypt. J Egypt Soc Parasitol. 2012;42(1):165–174. doi: 10.12816/0006304. [DOI] [PubMed] [Google Scholar]
- Elaswad A., Fawzy M., Basiouni S., Shehata A.A. Mutational spectra of SARS-CoV-2 isolated from animals. PeerJ. 2020;8:e10609. doi: 10.7717/peerj.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink M. Coronavirus rips through Dutch mink farms, triggering culls. Science. 2020;368(6496):1169. doi: 10.1126/science.368.6496.1169. [DOI] [PubMed] [Google Scholar]
- European Food Safety A., European Centre for Disease P., Control, Boklund A., Gortazar C., Pasquali P., Roberts H., Nielsen S.S., Stahl K., Stegeman A., Baldinelli F., Broglia A., Van Der Stede Y., Adlhoch C., Alm E., Melidou A., Mirinaviciute G. Monitoring of SARS-CoV-2 infection in mustelids. EFSA J. 2021;19(3):e06459. doi: 10.2903/j.efsa.2021.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenollar F., Mediannikov O., Maurin M., Devaux C., Colson P., Levasseur A., Fournier P.E., Raoult D. Mink, SARS-CoV-2, and the Human-Animal Interface. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.663815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff I.R., Castellanos A.A., Rodrigues J.P.G.L., Varsani A., Han B.A. 2021. Predicting the zoonotic capacity of mammal species for SARS-CoV-2. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foil L.D., Gorham J.R. In: Medical Entomology. Eldridge B.F., Edman J.D., editors. Springer; Dordrecht: 2000. Mechanical Transmission of Disease Agents by Arthropods; pp. 461–514. [Google Scholar]
- Fortuna C., Montarsi F., Severini F., Marsili G., Toma L., Amendola A., Bertola M., Michelutti A., Ravagnan S., Capelli G., Rezza G., Di Luca M., Group t.W. The common European mosquitoes Culex pipiens and Aedes albopictus are unable to transmit SARS-CoV-2 after a natural-mimicking challenge with infected blood. Parasit Vectors. 2021;14(1):76. doi: 10.1186/s13071-021-04578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz A.W., Kantor A.M., Passarelli A.L., Clem R.J. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses. 2015;7(7):3741–3767. doi: 10.3390/v7072795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freuling C.M., Breithaupt A., Müller T., Sehl J., Balkema-Buschmann A., Rissmann M., Klein A., Wylezich C., Höper D., Wernike K., Aebischer A., Hoffmann D., Friedrichs V., Dorhoi A., Groschup M.H., Beer M., Mettenleiter T.C. Susceptibility of Raccoon Dogs for Experimental SARS-CoV-2 Infection. Emerg Infect Dis. 2020;26(12):2982–2985. doi: 10.3201/eid2612.203733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M., Rosolen B., Krafft E., Becquart P., Elguero E., Vratskikh O., Denolly S., Boson B., Vanhomwegen J., Gouilh M.A., Kodjo A., Chirouze C., Rosolen S.G., Legros V., Leroy E.M. High prevalence of SARS-CoV-2 antibodies in pets from COVID-19+ households. One Health. 2021;11 doi: 10.1016/j.onehlt.2020.100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigliany M., Van Laere A.S., Clercx C., Giet D., Escriou N., Huon C., van der Werf S., Eloit M., Desmecht D. SARS-CoV-2 Natural Transmission from Human to Cat, Belgium, March 2020. Emerg Infect Dis. 2020;26(12):3069–3071. doi: 10.3201/eid2612.202223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry R.F. 2021. Mutations arising in SARS-CoV-2 spike on sustained human-to-human transmission and human-to-animal passage.https://virological.org/t/mutations-arising-in-sars-cov-2-spike-on-sustained-human-to-human-transmission-and-human-to-animal-passage/578 (accessed 26 May 2021) [Google Scholar]
- Gaudreault N.N., Carossino M., Morozov I., Trujillo J.D., Meekins D.A., Madden D.W., Cool K., Artiaga B.L., McDowell C., Bold D., Balaraman V., Kwon T., Ma W., Henningson J., Wilson D.W., Wilson W.C., Balasuriya U.B.R., García-Sastre A., Richt J.A. Experimental re-infected cats do not transmit SARS-CoV-2. Emerg Microbes Infect. 2021;10(1):638–650. doi: 10.1080/22221751.2021.1902753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault N.N., Trujillo J.D., Carossino M., Meekins D.A., Morozov I., Madden D.W., Indran S.V., Bold D., Balaraman V., Kwon T., Artiaga B.L., Cool K., García-Sastre A., Ma W., Wilson W.C., Henningson J., Balasuriya U.B.R., Richt J.A. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg Microbes Infect. 2020;9(1):2322–2332. doi: 10.1080/22221751.2020.1833687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A., Kaphle K., Shrestha B., Phuyal S. Susceptibility to SARS, MERS, and COVID-19 from animal health perspective. Open Vet J. 2020;10(2):164–177. doi: 10.4314/ovj.v10i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner J., Villanueva-Saz S., Tobajas A.P., Pérez M.D., González A., Verde M., Yzuel A., García-García A., Taleb V., Lira-Navarrete E., Hurtado-Guerrero R., Pardo J., Santiago L., Paño J.R., Ruíz H., Lacasta D., Fernández A. SARS-CoV-2 Seroprevalence in Household Domestic Ferrets. (Animals (Basel) 2021;11(3) doi: 10.3390/ani11030667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GISAID . 2020. Mutations in spike putatively linked to outbreak at Danish mink farms.https://www.gisaid.org/references/gisaid-in-the-news/mutations-in-spike-putatively-linked-to-outbreak-in-danish-mink-farms/ (accessed 25 May 2021) [Google Scholar]
- Gollakner R., Capua I. Is COVID-19 the first pandemic that evolves into a panzootic? Vet Ital. 2020;56(1):7–8. doi: 10.12834/VetIt.2246.12523.1. [DOI] [PubMed] [Google Scholar]
- Goncalves Cabecinhas A.R., Roloff T., Stange M., Bertelli C., Huber M., Ramette A., Chen C., Nadeau S., Gerth Y., Yerly S., Opota O., Pillonel T., Schuster T., Metzger C.M.J.A., Sieber J., Bel M., Wohlwend N., Baumann C., Koch M.C., Bittel P., Leuzinger K., Brunner M., Suter-Riniker F., Berlinger L., Søgaard K.K., Beckmann C., Noppen C., Redondo M., Steffen I., Seth-Smith H.M.B., Mari A., Lienhard R., Risch M., Nolte O., Eckerle I., Martinetti Lucchini G., Hodcroft E.B., Neher R.A., Stadler T., Hirsch H.H., Leib S.L., Risch L., Kaiser L., Trkola A., Greub G., Egli A. SARS-CoV-2 N501Y Introductions and Transmissions in Switzerland from Beginning of October 2020 to February 2021-Implementation of Swiss-Wide Diagnostic Screening and Whole Genome Sequencing. Microorganisms. 2021;9(4) doi: 10.3390/microorganisms9040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough P.M., Jorgenson R.D. Identification of porcine transmissible gastroenteritis virus in house flies (Musca domestica Linneaus) Am J Vet Res. 1983;44(11):2078–2082. [PubMed] [Google Scholar]
- Graczyk T.K., Knight R., Tamang L. Mechanical transmission of human protozoan parasites by insects. Clin Microbiol Rev. 2005;18(1):128–132. doi: 10.1128/CMR.18.1.128-132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B., Chan M., Tailor N., Mendoza E., Leung A., BM W. 2020. North American deer mice are susceptible to SARS-CoV-2. bioRxiv, 2020.2007.2025.221291. [DOI] [Google Scholar]
- Gryseels S., De Bruyn L., Gyselings R., Calvignac-Spencer S., Leendertz F.H., Leirs H. Risk of human-to-wildlife transmission of SARS-CoV-2. Mamm Rev. 2020 doi: 10.1111/mam.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Chen Q., Yang G., He L., Fan H., Deng Y.Q., Wang Y., Teng Y., Zhao Z., Cui Y., Li Y., Li X.F., Li J., Zhang N.N., Yang X., Chen S., Guo Y., Zhao G., Wang X., Luo D.Y., Wang H., Han G., He Y., Zhou X., Geng S., Sheng X., Jiang S., Sun S., Qin C.F., Zhou Y. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 2020;369(6511):1603–1607. doi: 10.1126/science.abc4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J., Butt K.M., Wong K.L., Chan K.W., Lim W., Shortridge K.F., Yuen K.Y., Peiris J.S., Poon L.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302(5643):276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Gubler D.J. In: Vector Biology, Ecology and Control. Atkinson P.W., editor. Springer; Dordrecht: 2010. The Global Threat of Emergent/Re-emergent Vector-Borne Diseases; pp. 39–62. [Google Scholar]
- Haider N., Rothman-Ostrow P., Osman A.Y., Arruda L.B., Macfarlane-Berry L., Elton L., Thomason M.J., Yeboah-Manu D., Ansumana R., Kapata N., Mboera L., Rushton J., McHugh T.D., Heymann D.L., Zumla A., Kock R.A. COVID-19-Zoonosis or Emerging Infectious Disease? Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.596944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann P.J., Hatta M., Chiba S., Maemura T., Fan S., Takeda M., Kinoshita N., Hattori S.I., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Kawaoka Y. Transmission of SARS-CoV-2 in Domestic Cats. N Engl J Med. 2020;383(6):592–594. doi: 10.1056/NEJMc2013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer S.A., Pauvolid-Corrêa A., Zecca I.B., Davila E., Auckland L.D., Roundy C.M., Tang W., Torchetti M., Killian M.L., Jenkins-Moore M., Mozingo K., Akpalu Y., Ghai R.R., Spengler J.R., Behravesh C.B., Fischer R.S.B., Hamer G.L. 2020. Natural SARS-CoV-2 infections, including virus isolation, among serially tested cats and dogs in households with confirmed human COVID-19 cases in Texas, USA. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida M.G., Perera R.A., Wang P., Alhammadi M.A., Siu L.Y., Li M., Poon L.L., Saif L., Alnaeem A., Peiris M. Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. 2013;18(50):20659. doi: 10.2807/1560-7917.es2013.18.50.20659. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271-280.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Zhang L., Krüger N., Graichen L., Kleine-Weber H., Hofmann-Winkler H., Kempf A., Nessler S., Riggert J., Winkler M.S., Schulz S., Jäck H.M., Pöhlmann S. SARS-CoV-2 mutations acquired in mink reduce antibody-mediated neutralization. Cell Rep. 2021;35(3) doi: 10.1016/j.celrep.2021.109017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie M.J., Hofmann-Lehmann R., Hartmann K., Egberink H., Truyen U., Addie D.D., Belak S., Boucraut-Baralon C., Frymus T., Lloret A., Lutz H., Marsilio F., Pennisi M.G., Tasker S., Thiry E., Mostl K. Anthropogenic Infection of Cats during the 2020 COVID-19 Pandemic. Viruses. 2021;13(2) doi: 10.3390/v13020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Zeng L.P., Yang X.L., Ge X.Y., Zhang W., Li B., Xie J.Z., Shen X.R., Zhang Y.Z., Wang N., Luo D.S., Zheng X.S., Wang M.N., Daszak P., Wang L.F., Cui J., Shi Z.L. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13(11) doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Zhang C., Pearce R., Omenn G.S., Zhang Y. Identifying the Zoonotic Origin of SARS-CoV-2 by Modeling the Binding Affinity between the Spike Receptor-Binding Domain and Host ACE2. J Proteome Res. 2020;19(12):4844–4856. doi: 10.1021/acs.jproteome.0c00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.S., Vanlandingham D.L., Bilyeu A.N., Sharp H.M., Hettenbach S.M., Higgs S. SARS-CoV-2 failure to infect or replicate in mosquitoes: an extreme challenge. Sci Rep. 2020;10(1):11915. doi: 10.1038/s41598-020-68882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Iwatsuki-Horimoto K., Hatta M., Loeber S., Halfmann P.J., Nakajima N., Watanabe T., Ujie M., Takahashi K., Ito M., Yamada S., Fan S., Chiba S., Kuroda M., Guan L., Takada K., Armbrust T., Balogh A., Furusawa Y., Okuda M., Ueki H., Yasuhara A., Sakai-Tagawa Y., Lopes T.J.S., Kiso M., Yamayoshi S., Kinoshita N., Ohmagari N., Hattori S.I., Takeda M., Mitsuya H., Krammer F., Suzuki T., Kawaoka Y. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci U S A. 2020;117(28):16587–16595. doi: 10.1073/pnas.2009799117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail M., Verma A.K., Abdulkadir A., Kumar A., Dhawan D.K., Bolya K.B., M Possible Mechanical Transmission of SARS-CoV-2 Causing COVID-19 by Insects: Infection, Prevention, Implications, and Control. Open Journal of Medical Microbiology. 2020;10:89–101. doi: 10.4236/ojmm.2020.102008. [DOI] [Google Scholar]
- Issa R. Musca domestica acts as transport vector hosts. Bull Natl Res Cent. 2019;43:73. doi: 10.1186/s42269-019-0111-0. [DOI] [Google Scholar]
- Ithete N.L., Stoffberg S., Corman V.M., Cottontail V.M., Richards L.R., Schoeman M.C., Drosten C., Drexler J.F., Preiser W. Close relative of human Middle East respiratory syndrome coronavirus in bat. South Africa. Emerg Infect Dis. 2013;19(10):1697–1699. doi: 10.3201/eid1910.130946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92(4):433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Wang S., Zhang C., Li J., Hou G., Peng C., Chen J., Shan H. Characterization of H5N1 highly pathogenic mink influenza viruses in eastern China. Vet Microbiol. 2017;201:225–230. doi: 10.1016/j.vetmic.2017.01.028. [DOI] [PubMed] [Google Scholar]
- Jo W.K., de Oliveira-Filho E.F., Rasche A., Greenwood A.D., Osterrieder K., Drexler J.F. Potential zoonotic sources of SARS-CoV-2 infections. Transbound Emerg Dis. 2020 doi: 10.1111/tbed.13872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S.B., Sukhramani G.S., Bishnoi P., Pable A.A., Barvkar V.T. SARS-CoV-2, the pandemic coronavirus: Molecular and structural insights. J Basic Microbiol. 2021;61(3):180–202. doi: 10.1002/jobm.202000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Corrigendum to "Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents". J Hosp Infect. 2020;104(2020):246–251. doi: 10.1016/j.jhin.2020.06.001. J Hosp Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan B., Wang M., Jing H., Xu H., Jiang X., Yan M., Liang W., Zheng H., Wan K., Liu Q., Cui B., Xu Y., Zhang E., Wang H., Ye J., Li G., Li M., Cui Z., Qi X., Chen K., Du L., Gao K., Zhao Y.T., Zou X.Z., Feng Y.J., Gao Y.F., Hai R., Yu D., Guan Y., Xu J. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J Virol. 2005;79(18):11892–11900. doi: 10.1128/JVI.79.18.11892-11900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeil A., Gomaa M., Shehata M., El-Taweel A., Kayed A.E., Abiadh A., Jrijer J., Moatasim Y., Kutkat O., Bagato O., Mahmoud S., Mostafa A., El-Shesheny R., Perera R.A., Ko R.L., Hassan N., Elsokary B., Allal L., Saad A., Sobhy H., McKenzie P.P., Webby R.J., Peiris M., Ali M.A., Kayali G. Middle East respiratory syndrome coronavirus infection in non-camelid domestic mammals. Emerg Microbes Infect. 2019;8(1):103–108. doi: 10.1080/22221751.2018.1560235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.I., Kim S.G., Kim S.M., Kim E.H., Park S.J., Yu K.M., Chang J.H., Kim E.J., Lee S., Casel M.A.B., Um J., Song M.S., Jeong H.W., Lai V.D., Kim Y., Chin B.S., Park J.S., Chung K.H., Foo S.S., Poo H., Mo I.P., Lee O.J., Webby R.J., Jung J.U., Choi Y.K. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe. 2020;27(5):704–709. doi: 10.1016/j.chom.2020.03.023. .e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Pandey S.N., Pareek V., Narayan R.K., Faiq M.A., Kumari C. Predicting susceptibility for SARS-CoV-2 infection in domestic and wildlife animals using ACE2 protein sequence homology. Zoo Biol. 2021;40(1):79–85. doi: 10.1002/zoo.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T., Gaudreault N.N., Richt J.A. Environmental Stability of SARS-CoV-2 on Different Types of Surfaces under Indoor and Seasonal Climate Conditions. Pathogens. 2021;10(2) doi: 10.3390/pathogens10020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T., Gaudreault N.N., Richt J.A. Seasonal Stability of SARS-CoV-2 in Biological Fluids. Pathogens. 2021;10(5) doi: 10.3390/pathogens10050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S.D., Bordin N., Waman V.P., Scholes H.M., Ashford P., Sen N., van Dorp L., Rauer C., Dawson N.L., Pang C.S.M., Abbasian M., Sillitoe I., Edwards S.J.L., Fraternali F., Lees J.G., Santini J.M., Orengo C.A. SARS-CoV-2 spike protein predicted to form complexes with host receptor protein orthologues from a broad range of mammals. Sci Rep. 2020;10(1):16471. doi: 10.1038/s41598-020-71936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.T., Jia N., Zhang Y.W., Shum M.H., Jiang J.F., Zhu H.C., Tong Y.G., Shi Y.X., Ni X.B., Liao Y.S., Li W.J., Jiang B.G., Wei W., Yuan T.T., Zheng K., Cui X.M., Li J., Pei G.Q., Qiang X., Cheung W.Y., Li L.F., Sun F.F., Qin S., Huang J.C., Leung G.M., Holmes E.C., Hu Y.L., Guan Y., Cao W.C. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583(7815):282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Latinne A., Hu B., Olival K.J., Zhu G., Zhang L., Li H., Chmura A.A., Field H.E., Zambrana-Torrelio C., Epstein J.H., Li B., Zhang W., Wang L.F., Shi Z.L., Daszak P. Origin and cross-species transmission of bat coronaviruses in China. Nat Commun. 2020;11(1):4235. doi: 10.1038/s41467-020-17687-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Leitner T., Kumar S. Where Did SARS-CoV-2 Come From? Mol Biol Evol. 2020;37(9):2463–2464. doi: 10.1093/molbev/msaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy E.M., Ar Gouilh M., Brugere-Picoux J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Receptor recognition and cross-species infections of SARS coronavirus. Antiviral Res. 2013;100(1):246–254. doi: 10.1016/j.antiviral.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H., Zhang J., McEachern J., Field H., Daszak P., Eaton B.T., Zhang S., Wang L.F. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang H., Tang X., Fang S., Ma D., Du C., Wang Y., Pan H., Yao W., Zhang R., Zou X., Zheng J., Xu L., Farzan M., Zhong G. SARS-CoV-2 and Three Related Coronaviruses Utilize Multiple ACE2 Orthologs and Are Potently Blocked by an Improved ACE2-Ig. J Virol. 2020;94(22) doi: 10.1128/JVI.01283-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian H., Liu Y., Zhang S., Zhang F., Hu R. Novel orthoreovirus from mink, China, 2011. Emerg Infect Dis. 2013;19(12):1985–1988. doi: 10.3201/eid1912.130043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Jiang J.Z., Wan X.F., Hua Y., Li L., Zhou J., Wang X., Hou F., Chen J., Zou J. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog. 2020;16(5) doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Hu G., Wang Y., Ren W., Zhao X., Ji F., Zhu Y., Feng F., Gong M., Ju X., Cai X., Lan J., Guo J., Xie M., Dong L., Zhu Z., Na J., Wu J., Lan X., Xie Y., Wang X., Yuan Z., Zhang R., Ding Q. Functional and genetic analysis of viral receptor ACE2 orthologs reveals a broad potential host range of SARS-CoV-2. Proc Natl Acad Sci U S A. 2021;118(12) doi: 10.1073/pnas.2025373118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie J.K., Hacker J.K., Mark J., Gavali S.S., Yagi S., Espinosa A., Schnurr D.P., Cossen C.K., Isaacson E.R., Glaser C.A., Fischer M., Reingold A.L., Vugia D.J., Group, U.D.a.C.I.W. SARS and common viral infections. Emerg Infect Dis. 2004;10(6):1143–1146. doi: 10.3201/eid1006.030863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Zhao Y., Yu W., Yang Y., Gao J., Wang J., Kuang D., Yang M., Yang J., Ma C. 2020. Comparison of SARS-CoV-2 infections among 3 species of non-human primates. bioRxiv2020.2004.2008.031807. [DOI] [Google Scholar]
- Lu S., Zhao Y., Yu W., Yang Y., Gao J., Wang J., Kuang D., Yang M., Yang J., Ma C., Xu J., Qian X., Li H., Zhao S., Li J., Wang H., Long H., Zhou J., Luo F., Ding K., Wu D., Zhang Y., Dong Y., Liu Y., Zheng Y., Lin X., Jiao L., Zheng H., Dai Q., Sun Q., Hu Y., Ke C., Liu H., Peng X. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduct Target Ther. 2020;5(1):157. doi: 10.1038/s41392-020-00269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan B., Wang H., Huynh T. Enhanced binding of the N501Y-mutated SARS-CoV-2 spike protein to the human ACE2 receptor: insights from molecular dynamics simulations. FEBS Lett. 2021;595(10):1454–1461. doi: 10.1002/1873-3468.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J., Jin X., Lu Y., Zhang L. SARS-CoV-2 spike protein favors ACE2 from Bovidae and Cricetidae. J Med Virol. 2020;92(9):1649–1656. doi: 10.1002/jmv.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C.M., Wang N., Yang X.L., Liu H.Z., Zhang W., Li B., Hu B., Peng C., Geng Q.B., Zhu G.J., Li F., Shi Z.L. Discovery of Novel Bat Coronaviruses in South China That Use the Same Receptor as Middle East Respiratory Syndrome Coronavirus. J Virol. 2018;92(13) doi: 10.1128/JVI.00116-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean O.A., Lytras S., Weaver S., Singer J.B., Boni M.F., Lemey P., Kosakovsky Pond S.L., Robertson D.L. Natural selection in the evolution of SARS-CoV-2 in bats created a generalist virus and highly capable human pathogen. PLoS Biol. 2021;19(3) doi: 10.1371/journal.pbio.3001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. COVID mink analysis shows mutations are not dangerous - yet. Nature. 2020;587(7834):340–341. doi: 10.1038/d41586-020-03218-z. [DOI] [PubMed] [Google Scholar]
- Mallapaty S. The search for animals harbouring coronavirus - and why it matters. Nature. 2021;591(7848):26–28. doi: 10.1038/d41586-021-00531-z. [DOI] [PubMed] [Google Scholar]
- Maurin M., Fenollar F., Mediannikov O., Davoust B., Devaux C., Raoult D. Current Status of Putative Animal Sources of SARS-CoV-2 Infection in Humans: Wildlife, Domestic Animals and Pets. Microorganisms. 2021;9(4) doi: 10.3390/microorganisms9040868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAloose D., Laverack M., Wang L., Killian M.L., Caserta L.C., Yuan F., Mitchell P.K., Queen K., Mauldin M.R., Cronk B.D., Bartlett S.L., Sykes J.M., Zec S., Stokol T., Ingerman K., Delaney M.A., Fredrickson R., Ivančić M., Jenkins-Moore M., Mozingo K., Franzen K., Bergeson N.H., Goodman L., Wang H., Fang Y., Olmstead C., McCann C., Thomas P., Goodrich E., Elvinger F., Smith D.C., Tong S., Slavinski S., Calle P.P., Terio K., Torchetti M.K., Diel D.G. From People to Panthera: Natural SARS-CoV-2 Infection in Tigers and Lions at the Bronx Zoo. mBio. 2020;11(5) doi: 10.1128/mBio.02220-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara T., Richt J.A., Glickman L. A Critical Needs Assessment for Research in Companion Animals and Livestock Following the Pandemic of COVID-19 in Humans. Vector Borne Zoonotic Dis. 2020;20(6):393–405. doi: 10.1089/vbz.2020.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meekins D.A., Morozov I., Trujillo J.D., Gaudreault N.N., Bold D., Carossino M., Artiaga B.L., Indran S.V., Kwon T., Balaraman V., Madden D.W., Feldmann H., Henningson J., Ma W., Balasuriya U.B.R., Richt J.A. Susceptibility of swine cells and domestic pigs to SARS-CoV-2. Emerg Microbes Infect. 2020;9(1):2278–2288. doi: 10.1080/22221751.2020.1831405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin A.D., Janiak M.C., Marrone F., Arora P.S., Higham J.P. Comparative ACE2 variation and primate COVID-19 risk. Commun Biol. 2020;3(1):641. doi: 10.1038/s42003-020-01370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelitsch A., Hoffmann D., Wernike K., Beer M. Occurrence of Antibodies against SARS-CoV-2 in the Domestic Cat Population of Germany. Vaccines (Basel) 2020;8(4) doi: 10.3390/vaccines8040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnesota Board of Animal Health Minnesota tiger tests positive for COVID-19 virus. News Release. 2021 https://www.bah.state.mn.us/news_release/minnesota-tiger-tests-positive-for-covid-19-virus/ (accessed 12 May 2021) [Google Scholar]
- Montagutelli X., Prot M., Levillayer L., Salazar E.B., Jouvion G., Conquet L., Donati F., Albert M., Gambaro F., Behillil S., Enouf V., Rousset D., Jaubert J., Rey F., van der Werf S., Simon-Loriere E. 2021. The B1.351 and P.1 variants extend SARS-CoV-2 host range to mice. bioRxiv. [DOI] [Google Scholar]
- Müller M.A., Corman V.M., Jores J., Meyer B., Younan M., Liljander A., Bosch B.J., Lattwein E., Hilali M., Musa B.E., Bornstein S., Drosten C. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983-1997. Emerg Infect Dis. 2014;20(12):2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir K., Ashraf S., Munir I., Khalid H., Muneer M.A., Mukhtar N., Amin S., Imran M.A., Chaudhry U., Zaheer M.U., Arshad M., Munir R., Ahmad A., Zhao X. Zoonotic and reverse zoonotic events of SARS-CoV-2 and their impact on global health. Emerg Microbes Infect. 2020;9(1):2222–2235. doi: 10.1080/22221751.2020.1827984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Fontela C., Dowling W.E., Funnell S.G.P., Gsell P.S., Riveros-Balta A.X., Albrecht R.A., Andersen H., Baric R.S., Carroll M.W., Cavaleri M., Qin C., Crozier I., Dallmeier K., de Waal L., de Wit E., Delang L., Dohm E., Duprex W.P., Falzarano D., Finch C.L., Frieman M.B., Graham B.S., Gralinski L.E., Guilfoyle K., Haagmans B.L., Hamilton G.A., Hartman A.L., Herfst S., Kaptein S.J.F., Klimstra W.B., Knezevic I., Krause P.R., Kuhn J.H., Le Grand R., Lewis M.G., Liu W.C., Maisonnasse P., McElroy A.K., Munster V., Oreshkova N., Rasmussen A.L., Rocha-Pereira J., Rockx B., Rodríguez E., Rogers T.F., Salguero F.J., Schotsaert M., Stittelaar K.J., Thibaut H.J., Tseng C.T., Vergara-Alert J., Beer M., Brasel T., Chan J.F.W., García-Sastre A., Neyts J., Perlman S., Reed D.S., Richt J.A., Roy C.J., Segalés J., Vasan S.S., Henao-Restrepo A.M., Barouch D.H. Animal models for COVID-19. Nature. 2020;586(7830):509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V.J., Feldmann F., Williamson B.N., van Doremalen N., Pérez-Pérez L., Schulz J., Meade-White K., Okumura A., Callison J., Brumbaugh B., Avanzato V.A., Rosenke R., Hanley P.W., Saturday G., Scott D., Fischer E.R., de Wit E. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020;585(7824):268–272. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso N., Costantino A., La Spina S., Finocchiaro A., Andronico F., Stracquadanio S., Liotta L., Visalli R., Emmanuele G. New SARS-CoV-2 Infection Detected in an Italian Pet Cat by RT-qPCR from. Deep Pharyngeal Swab. Pathogens. 2020;9(9) doi: 10.3390/pathogens9090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn A.Z., Lamers M.M., Okba N.M.A., Breugem T.I., Schipper D., van den Doel P.B., van Run P., van Amerongen G., de Waal L., Koopmans M.P.G., Stittelaar K.J., van den Brand J.M.A., Haagmans B.L. Susceptibility of rabbits to SARS-CoV-2. Emerg Microbes Infect. 2021;10(1):1–7. doi: 10.1080/22221751.2020.1868951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayduch D., Burrus R.G. Flourishing in Filth: House Fly–Microbe Interactions Across Life History. Ann Entomol Soc Am. 2017;110:6–18. doi: 10.1093/aesa/saw083. [DOI] [Google Scholar]
- Newman A., Smith D., Ghai R.R., Wallace R.M., Torchetti M.K., Loiacono C., Murrell L.S., Carpenter A., Moroff S., Rooney J.A., Barton Behravesh C. First Reported Cases of SARS-CoV-2 Infection in Companion Animals - New York, March-April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):710–713. doi: 10.15585/mmwr.mm6923e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . 2021. COVID-19 Events in Animals.https://www.oie.int/en/what-we-offer/emergency-and-resilience/covid-19/ (accessed 02 June 2021) [Google Scholar]
- OIE . 2021. Questions and Answers on COVID-19. COVID-19 Portal.https://www.oie.int/en/what-we-offer/emergency-and-resilience/covid-19/ (accessed 05 May 2021) [Google Scholar]
- Onwugamba F.C., Fitzgerald J.R., Rochon K., Guardabassi L., Alabi A., Kühne S., Grobusch M.P., Schaumburg F. The role of 'filth flies' in the spread of antimicrobial resistance. Travel Med Infect Dis. 2018;22:8–17. doi: 10.1016/j.tmaid.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Huang Y.W. Coronavirus disease 2019 (COVID-19) outbreak: Could pigs be vectors for human infections? Xenotransplantation. 2020;27(2):e12591. doi: 10.1111/xen.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova N., Molenaar R.J., Vreman S., Harders F., Oude Munnink B.B., Hakze-van der Honing R.W., Gerhards N., Tolsma P., Bouwstra R., Sikkema R.S., Tacken M.G., de Rooij M.M., Weesendorp E., Engelsma M.Y., Bruschke C.J., Smit L.A., Koopmans M., van der Poel W.H., Stegeman A. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveill. 2020;25(23) doi: 10.2807/1560-7917.ES.2020.25.23.2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink B.B., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., van der Spek A., Tolsma P., Rietveld A., Brouwer M., Bouwmeester-Vincken N., Harders F., Hakze-van der Honing R., Wegdam-Blans M.C.A., Bouwstra R.J., GeurtsvanKessel C., van der Eijk A.A., Velkers F.C., Smit L.A.M., Stegeman A., van der Poel W.H.M., Koopmans M.P.G. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021;371(6525):172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer M.V., Martins M., Falkenberg S., Buckley A., Caserta L.C., Mitchell P.K., Cassmann E.D., Rollins A., Zylich N.C., Renshaw R.W., Guarino C., Wagner B., Lager K., Diel D.G. Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2. J Virol. 2021 doi: 10.1128/JVI.00083-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevis D., Kostaki E.G., Magiorkinis G., Panayiotakopoulos G., Sourvinos G., Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol. 2020;79 doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson E.I., Elia G., Grassi A., Giordano A., Desario C., Medardo M., Smith S.L., Anderson E.R., Prince T., Patterson G.T., Lorusso E., Lucente M.S., Lanave G., Lauzi S., Bonfanti U., Stranieri A., Martella V., Basano F.S., Barrs V.R., Radford A.D., Agrimi U., Hughes G.L., Paltrinieri S., Decaro N. 2020. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering B.S., Smith G., Pinette M.M., Embury-Hyatt C., Moffat E., Marszal P., Lewis C.E. Susceptibility of Domestic Swine to Experimental Infection with Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 2021;27(1):104–112. doi: 10.3201/eid2701.203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkin A., Deen J., Otake S., Moon R., Dee S. Further assessment of houseflies (Musca domestica) as vectors for the mechanical transport and transmission of porcine reproductive and respiratory syndrome virus under field conditions. Can J Vet Res. 2009;73(2):91–96. [PMC free article] [PubMed] [Google Scholar]
- Prince T., Smith S.L., Radford A.D., Solomon T., Hughes G.L., Patterson E.I. SARS-CoV-2 Infections in Animals: Reservoirs for Reverse Zoonosis and Models for Study. Viruses. 2021;13(3) doi: 10.3390/v13030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ProMED-mail . 2020. COVID-19 update (506): Argentina (BA, SE) animal, cat, dog.https://promedmail.org/promed-post/?id=20201125.7972283 OIE. ProMED 20201125.7972283. (accessed 12 February 2021) [Google Scholar]
- ProMED-mail . 2020. COVID-19 update (549): animal, mink, Denmark, erad. Russia, vaccine, China, RFI.https://promedmail.org/promed-post/?id=8039549 ProMED 20201222.8039549. (accessed 05 May 2021) [Google Scholar]
- ProMED-mail . 2020. COVID-19 update (551): animal, pig, research, experimental infection.https://promedmail.org/promed-post/?id=8041877 ProMED 20201223.8041877. (accessed 05 May 2021) [Google Scholar]
- ProMED-mail . 2020. COVID-19 update (557): animal, Slovenia (SA) ferret, OIE.https://promedmail.org/promed-post/?id=8048809 ProMED 20201226.8048809. (accessed 12 May 2021) [Google Scholar]
- ProMED-mail . 2021. COVID-19 update (33): Sweden, zoo, tiger, lion.https://promedmail.org/promed-post/?id=20210125.8134087 ProMED 20210125.8134087. (accessed 13 February 2021) [Google Scholar]
- ProMED-mail . 2021. COVID-19 update (49): Bosnia & Herzegovina (SA) animal, dog, OIE. ProMED 20210205.8165920.https://promedmail.org/promed-post/?id=8165920 (accessed 05 May 2021) [Google Scholar]
- ProMED-mail . WHO, global; 2021. COVID-19 update (56): meat facility outbreak, vacc 2nd dose, routes.https://promedmail.org/promed-post/?id=20210210.8181892 ProMED 20210210.8181892. (accessed 05 May 2021) [Google Scholar]
- ProMED-mail . WHO, global; 2021. COVID-19 update (61): meat facility, S Korea, T lymphocytes, S Asia.https://promedmail.org/promed-post/?id=20210212.8187538 [Google Scholar]
- ProMED-mail . 2021. COVID-19 update (62): Latvia (RA) animal, cat, OIE. ProMED 20210212.8187389.https://promedmail.org/promed-post/?id=20210212.8187389 (accessed 13 February 2021) [Google Scholar]
- ProMED-mail . 8336058. 2021. https://promedmail.org/promed-post/?id=20210429.8336058 (COVID-19 update (152): animal, Croatia, dog, OIE. ProMED 20210429). (accessed 12 May 2021) [Google Scholar]
- ProMED-mail . 8344580. 2021. https://promedmail.org/promed-post/?id=20210504.8344580 (COVID-19 update (159): India, zoo, lion. ProMED 20210504). (accessed 12 May 2021) [Google Scholar]
- Rabalski L., Kosinski M., Smura T., Aaltonen K., Kant R., Szewczyk B., Grzybek M. 2020. Detection and molecular characterisation of SARS-CoV-2 in farmed mink (Neovision vision) in Poland. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Farag E.A., Reusken C.B., Lamers M.M., Pas S.D., Voermans J., Smits S.L., Osterhaus A.D., Al-Mawlawi N., Al-Romaihi H.E., AlHajri M.M., El-Sayed A.M., Mohran K.A., Ghobashy H., Alhajri F., Al-Thani M., Al-Marri S.A., El-Maghraby M.M., Koopmans M.P., Haagmans B.L. Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014. Emerg Infect Dis. 2014;20(8):1339–1342. doi: 10.3201/eid2008.140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan M., Ferguson I.D., Miao W., Khavari P.A. SARS-CoV-2 B.1.1.7 and B.1.351 spike variants bind human ACE2 with increased affinity. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R.C., Gyar S.D., Danladi M.M. COVID-19: Probable involvement of insects in the mechanical transmission of novel coronavirus (2019-nCoV) Microbes and Infectious Diseases. 2020;1(3) doi: 10.21608/MID.2020.41443.1059. [DOI] [Google Scholar]
- Reusken C.B., Haagmans B.L., Müller M.A., Gutierrez C., Godeke G.J., Meyer B., Muth D., Raj V.S., Smits-De Vries L., Corman V.M., Drexler J.F., Smits S.L., El Tahir Y.E., De Sousa R., van Beek J., Nowotny N., van Maanen K., Hidalgo-Hermoso E., Bosch B.J., Rottier P., Osterhaus A., Gortázar-Schmidt C., Drosten C., Koopmans M.P. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013;13(10):859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C.B., Schilp C., Raj V.S., De Bruin E., Kohl R.H., Farag E.A., Haagmans B.L., Al-Romaihi H., Le Grange F., Bosch B.J., Koopmans M.P. MERS-CoV Infection of Alpaca in a Region Where MERS-CoV is Endemic. Emerg Infect Dis. 2016;22(6):1129–1131. doi: 10.3201/eid2206.152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M., Kok A., de Meulder D., Bestebroer T.M., Lamers M.M., Okba N.M.A., Fentener van Vlissingen M., Rockx B., Haagmans B.L., Koopmans M.P.G., Fouchier R.A.M., Herfst S. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat Commun. 2020;11(1):3496. doi: 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M.M., Oude Munnink B.B., de Meulder D., van Amerongen G., van den Brand J., Okba N.M.A., Schipper D., van Run P., Leijten L., Sikkema R., Verschoor E., Verstrepen B., Bogers W., Langermans J., Drosten C., Fentener van Vlissingen M., Fouchier R., de Swart R., Koopmans M., Haagmans B.L. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368(6494):1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues J.P.G.L., Barrera-Vilarmau S., M C Teixeira J., Sorokina M., Seckel E., Kastritis P.L., Levitt M. Insights on cross-species transmission of SARS-CoV-2 from structural modeling. PLoS Comput Biol. 2020;16(12) doi: 10.1371/journal.pcbi.1008449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Arrondo I., Portillo A., Palomar A.M., Santibanez S., Santibanez P., Cervera C., Oteo J.A. Detection of SARS-CoV-2 in pets living with COVID-19 owners diagnosed during the COVID-19 lockdown in Spain: A case of an asymptomatic cat with SARS-CoV-2 in Europe. Transbound Emerg Dis. 2021;68(2):973–976. doi: 10.1111/tbed.13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailleau C., Dumarest M., Vanhomwegen J., Delaplace M., Caro V., Kwasiborski A., Hourdel V., Chevaillier P., Barbarino A., Comtet L., Pourquier P., Klonjkowski B., Manuguerra J.C., Zientara S., Le Poder S. First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transbound Emerg Dis. 2020;67(6):2324–2328. doi: 10.1111/tbed.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlottau K., Rissmann M., Graaf A., Schön J., Sehl J., Wylezich C., Höper D., Mettenleiter T.C., Balkema-Buschmann A., Harder T., Grund C., Hoffmann D., Breithaupt A., Beer M. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe. 2020;1(5):e218–e225. doi: 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segales J., Puig M., Rodon J., Avila-Nieto C., Carrillo J., Cantero G., Terron M.T., Cruz S., Parera M., Noguera-Julian M., Izquierdo-Useros N., Guallar V., Vidal E., Valencia A., Blanco I., Blanco J., Clotet B., Vergara-Alert J. Detection of SARS-CoV-2 in a cat owned by a COVID-19-affected patient in Spain. Proc Natl Acad Sci U S A. 2020;117(40):24790–24793. doi: 10.1073/pnas.2010817117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C., Yao Y.F., Yang X.L., Zhou Y.W., Gao G., Peng Y., Yang L., Hu X., Xiong J., Jiang R.D., Zhang H.J., Gao X.X., Peng C., Min J., Chen Y., Si H.R., Wu J., Zhou P., Wang Y.Y., Wei H.P., Pang W., Hu Z.F., Lv L.B., Zheng Y.T., Shi Z.L., Yuan Z.M. Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques. Cell Res. 2020;30(8):670–677. doi: 10.1038/s41422-020-0364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Liu R., He X., Shuai L., Sun Z., Zhao Y., Liu P., Liang L., Cui P., Wang J., Zhang X., Guan Y., Tan W., Wu G., Chen H., Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriner S.A., Ellis J.W., Root J.J., Roug A., Stopak S.R., Wiscomb G.W., Zierenberg J.R., Ip H.S., Torchetti M.K., DeLiberto T.J. SARS-CoV-2 Exposure in Escaped Mink, Utah, USA. Emerg Infect Dis. 2021;27(3):988–990. doi: 10.3201/eid2703.204444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia S.F., Yan L.M., Chin A.W.H., Fung K., Choy K.T., Wong A.Y.L., Kaewpreedee P., Perera R.A.P.M., Poon L.L.M., Nicholls J.M., Peiris M., Yen H.L. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583(7818):834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D.K., Singh B., Ganatra S.R., Gazi M., Cole J., Thippeshappa R., Alfson K.J., Clemmons E., Gonzalez O., Escobedo R., Lee T.H., Chatterjee A., Goez-Gazi Y., Sharan R., Gough M., Alvarez C., Blakley A., Ferdin J., Bartley C., Staples H., Parodi L., Callery J., Mannino A., Klaffke B., Escareno P., Platt R.N., Hodara V., Scordo J., Gautam S., Vilanova A.G., Olmo-Fontanez A., Schami A., Oyejide A., Ajithdoss D.K., Copin R., Baum A., Kyratsous C., Alvarez X., Ahmed M., Rosa B., Goodroe A., Dutton J., Hall-Ursone S., Frost P.A., Voges A.K., Ross C.N., Sayers K., Chen C., Hallam C., Khader S.A., Mitreva M., Anderson T.J.C., Martinez-Sobrido L., Patterson J.L., Turner J., Torrelles J.B., Dick E.J., Brasky K., Schlesinger L.S., Giavedoni L.D., Carrion R., Kaushal D. Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nat Microbiol. 2021;6(1):73–86. doi: 10.1038/s41564-020-00841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit T.H.C., Brackman C.J., Ip S.M., Tam K.W.S., Law P.Y.T., To E.M.W., Yu V.Y.T., Sims L.D., Tsang D.N.C., Chu D.K.W., Perera R.A.P.M., Poon L.L.M., Peiris M. Infection of dogs with SARS-CoV-2. Nature. 2020;586(7831):776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan C.C., Thomas M., Wang D., Li F. Susceptibility of livestock and companion animals to COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.26621. [DOI] [PubMed] [Google Scholar]
- Starr T.N., Greaney A.J., Hilton S.K., Ellis D., Crawford K.H.D., Dingens A.S., Navarro M.J., Bowen J.E., Tortorici M.A., Walls A.C., King N.P., Veesler D., Bloom J.D. Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding. Cell. 2020;182(5):1295–1310. doi: 10.1016/j.cell.2020.08.012. .e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M., Chen Y., Qi S., Shi D., Feng L., Sun D. A Mini-Review on Cell Cycle Regulation of Coronavirus Infection. Front Vet Sci. 2020;7 doi: 10.3389/fvets.2020.586826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez D.L., Pantin-Jackwood M.J., Swayne D.E., Lee S.A., DeBlois S.M., Spackman E. Lack of Susceptibility to SARS-CoV-2 and MERS-CoV in Poultry. Emerg Infect Dis. 2020;26(12):3074–3076. doi: 10.3201/eid2612.202989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Gu L., Ma L., Duan Y. Atlas of ACE2 gene expression reveals novel insights into transmission of SARS-CoV-2. Heliyon. 2021;7(1):e05850. doi: 10.1016/j.heliyon.2020.e05850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TACC Influenza Institute studies COVID-19 virus detected in cat first time in Russia. TACC - RUSSIAN NEWS AGENCY. 2021 https://tass.com/science/1245025 [Google Scholar]
- Tappe D., Schlottau K., Cadar D., Hoffmann B., Balke L., Bewig B., Hoffmann D., Eisermann P., Fickenscher H., Krumbholz A., Laufs H., Huhndorf M., Rosenthal M., Schulz-Schaeffer W., Ismer G., Hotop S.K., Brönstrup M., Ott A., Schmidt-Chanasit J., Beer M. Occupation-Associated Fatal Limbic Encephalitis Caused by Variegated Squirrel Bornavirus 1, Germany, 2013. Emerg Infect Dis. 2018;24(6):978–987. doi: 10.3201/eid2406.172027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.A., Boulos C., Almond D. Livestock plants and COVID-19 transmission. Proc Natl Acad Sci U S A. 2020;117(50):31706–31715. doi: 10.1073/pnas.2010115117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmam S., Barbarino A., Maso D., Behillil S., Enouf V., Huon C., Jaraud A., Chevallier L., Backovic M., Pérot P., Verwaerde P., Tiret L., van der Werf S., Eloit M. Absence of SARS-CoV-2 infection in cats and dogs in close contact with a cluster of COVID-19 patients in a veterinary campus. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimpert J., Vladimirova D., Dietert K., Abdelgawad A., Kunec D., Dökel S., Voss A., Gruber A.D., Bertzbach L.D., Osterrieder N. The Roborovski Dwarf Hamster Is A Highly Susceptible Model for a Rapid and Fatal Course of SARS-CoV-2 Infection. Cell Rep. 2020;33(10) doi: 10.1016/j.celrep.2020.108488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Crameri G., Kong X., Chen J., Sun Y., Yu M., Xiang H., Xia X., Liu S., Ren T., Yu Y., Eaton B.T., Xuan H., Wang L.F. Antibodies to SARS coronavirus in civets. Emerg Infect Dis. 2004;10(12):2244–2248. doi: 10.3201/eid1012.040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich L., Michelitsch A., Halwe N., Wernike K., Hoffmann D., Beer M. Experimental SARS-CoV-2 Infection of Bank Voles. Emerg Infect Dis. 2021;27(4):1193–1195. doi: 10.3201/eid2704.204945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich L., Wernike K., Hoffmann D., Mettenleiter T.C., Beer M. Experimental Infection of Cattle with SARS-CoV-2. Emerg Infect Dis. 2020;26(12):2979–2981. doi: 10.3201/eid2612.203799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia-Granda W.A., Richt J.A. What We Need to Consider During and After the SARS-CoV-2 Pandemic. Vector Borne Zoonotic Dis. 2020;20(7):477–483. doi: 10.1089/vbz.2020.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara-Alert J., Raj V.S., Muñoz M., Abad F.X., Cordón I., Haagmans B.L., Bensaid A., Segalés J. Middle East respiratory syndrome coronavirus experimental transmission using a pig model. Transbound Emerg Dis. 2017;64(5):1342–1345. doi: 10.1111/tbed.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara-Alert J., van den Brand J.M., Widagdo W., Muñoz M., Raj S., Schipper D., Solanes D., Cordón I., Bensaid A., Haagmans B.L., Segalés J. Livestock Susceptibility to Infection with Middle East Respiratory Syndrome Coronavirus. Emerg Infect Dis. 2017;23(2):232–240. doi: 10.3201/eid2302.161239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.S., Du Y., Wu J.Q., Tian F.L., Yu X.J., Wang J.B. Vaccine resistant pseudorabies virus causes mink infection in China. BMC Vet Res. 2018;14(1):20. doi: 10.1186/s12917-018-1334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Pipes L., Nielsen R. Synonymous mutations and the molecular evolution of SARS-CoV-2 origins. Virus Evol. 2021;7(1):veaa098. doi: 10.1093/ve/veaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Shuai L., Wang C., Liu R., He X., Zhang X., Sun Z., Shan D., Ge J., Wang X., Hua R., Zhong G., Wen Z., Bu Z. Mouse-adapted SARS-CoV-2 replicates efficiently in the upper and lower respiratory tract of BALB/c and C57BL/6J mice. Protein Cell. 2020;11(10):776–782. doi: 10.1007/s13238-020-00767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Mitchell P.K., Calle P.P., Bartlett S.L., McAloose D., Killian M.L., Yuan F., Fang Y., Goodman L.B., Fredrickson R., Elvinger F., Terio K., Franzen K., Stuber T., Diel D.G., Torchetti M.K. Complete Genome Sequence of SARS-CoV-2 in a Tiger from a U.S. Zoological Collection. Microbiol Resour Announc. 2020;9(22) doi: 10.1128/MRA.00468-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D.W., Niño E.L., Rochon K., Denning S., Smith L., Guy J.S. Experimental evaluation of Musca domestica (Diptera: Muscidae) as a vector of Newcastle disease virus. J Med Entomol. 2007;44(4):666–671. doi: 10.1093/jmedent/44.4.666. https://doi.org/10.1603/0022-2585(2007)44[666:eeomdd]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkers M.R.A., Han A.X., Reusken C.B.E.M., Eggink D. Possible host-adaptation of SARS-CoV-2 due to improved ACE2 receptor binding in mink. Virus Evol. 2021;7(1):veaa094. doi: 10.1093/ve/veaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2015. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003.https://www.who.int/publications/m/item/summary-of-probable-sars-cases-with-onset-of-illness-from-1-november-2002-to-31-july-2003 (accessed 19 December 2020) [Google Scholar]
- WHO . 2020. COVID-19 Public Health Emergency of International Concern (PHEIC) Global research and innovation forum.https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum (accessed 20 December 2020) [Google Scholar]
- WHO . 2020. Middle East respiratory syndrome (MERS) situation update, January 2020.http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html (accessed 19 December 2020) [Google Scholar]
- WHO . 2020. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 (accessed 20 December 2020) [Google Scholar]
- Widagdo W., Okba N.M.A., Richard M., de Meulder D., Bestebroer T.M., Lexmond P., Farag E.A.B.A., Al-Hajri M., Stittelaar K.J., de Waal L., van Amerongen G., van den Brand J.M.A., Haagmans B.L., Herfst S. Lack of Middle East Respiratory Syndrome Coronavirus Transmission in Rabbits. Viruses. 2019;11(4) doi: 10.3390/v11040381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey C., Borisevich V., Prasad A., Agans K., Deer D., Dobias N. 2020. Establishment of an African green monkey model for COVID-19. bioRxiv, 2020.2005.2017.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worldometer . 2021. COVID-19 Coronavirus Pandemic.https://www.worldometers.info/coronavirus accessed 02 June. [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. .e1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Jiang L., Huang G., Pu H., Gong B., Lin H., Ma S., Chen X., Long B., Si G., Yu H., Yang X., Shi Y., Yang Z. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis. 2020;93:264–267. doi: 10.1016/j.ijid.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Wu Z., Ren X., Yang F., Zhang J., He G., Dong J., Sun L., Zhu Y., Zhang S., Jin Q. MERS-related betacoronavirus in Vespertilio superans bats. China. Emerg Infect Dis. 2014;20(7):1260–1262. doi: 10.3201/eid2007.140318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.L., Hu B., Wang B., Wang M.N., Zhang Q., Zhang W., Wu L.J., Ge X.Y., Zhang Y.Z., Daszak P., Wang L.F., Shi Z.L. Isolation and Characterization of a Novel Bat Coronavirus Closely Related to the Direct Progenitor of Severe Acute Respiratory Syndrome Coronavirus. J Virol. 2015;90(6):3253–3256. doi: 10.1128/JVI.02582-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.W., Yuan S., Yuen K.S., Fung S.Y., Chan C.P., Jin D.Y. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16(10):1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H.S., Yoo D. COVID-19 and veterinarians for one health, zoonotic- and reverse-zoonotic transmissions. J Vet Sci. 2020;21(3):e51. doi: 10.4142/jvs.2020.21.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Qi F., Xu Y., Li F., Liu P., Liu J., Bao L., Deng W., Gao H., Xiang Z., Xiao C., Lv Q., Gong S., Song Z., Qu Y., Xue J., Wei Q., Liu M., Wang G., Wang S., Yu H., Liu X., Huang B., Wang W., Zhao L., Wang H., Ye F., Zhou W., Zhen W., Han J., Wu G., Jin Q., Wang J., Tan W., Qin C. Age-related rhesus macaque models of COVID-19. Animal Model Exp Med. 2020;3(1):93–97. doi: 10.1002/ame2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S., Jiang S.C., Li Z.L. Analysis of Possible Intermediate Hosts of the New Coronavirus SARS-CoV-2. Front Vet Sci. 2020;7:379. doi: 10.3389/fvets.2020.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zheng W., Huang X., Bell E.W., Zhou X., Zhang Y. Protein Structure and Sequence Reanalysis of 2019-nCoV Genome Refutes Snakes as Its Intermediate Host and the Unique Similarity between Its Spike Protein Insertions and HIV-1. J Proteome Res. 2020;19(4):1351–1360. doi: 10.1021/acs.jproteome.0c00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhang H., Gao J., Huang K., Yang Y., Hui X., He X., Li C., Gong W., Zhang Y., Zhao Y., Peng C., Gao X., Chen H., Zou Z., Shi Z.L., Jin M. A serological survey of SARS-CoV-2 in cat in Wuhan. Emerg Microbes Infect. 2020;9(1):2013–2019. doi: 10.1080/22221751.2020.1817796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.Z., Holmes E.C. A Genomic Perspective on the Origin and Emergence of SARS-CoV-2. Cell. 2020;181(2):223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Cui W., Tian B.P. The Potential Intermediate Hosts for SARS-CoV-2. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.580137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Sun L., Sun X., Li S., Zhang W., Pulscher L.A., Chai H., Xing M. Newcastle disease virus from domestic mink, China, 2014. Vet Microbiol. 2017;198:104–107. doi: 10.1016/j.vetmic.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Zheng H., Li H., Guo L., Liang Y., Li J., Wang X., Hu Y., Wang L., Liao Y., Yang F., Li Y., Fan S., Li D., Cui P., Wang Q., Shi H., Chen Y., Yang Z., Yang J., Shen D., Cun W., Zhou X., Dong X., Wang Y., Dai Q., Jin W., He Z., Li Q., Liu L. Virulence and pathogenesis of SARS-CoV-2 infection in rhesus macaques: A nonhuman primate model of COVID-19 progression. PLoS Pathog. 2020;16(11) doi: 10.1371/journal.ppat.1008949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]