Abstract
Purpose of Review:
Evidence is growing for the positive effects of technology-delivered diabetes self-care interventions on behavioral and clinical outcomes. However, our understanding of how to effectively implement these interventions into routine clinical practice is limited. This article provides an overview of the methods and results of studies examining the implementation of technology-delivered diabetes self-care interventions into clinical care. We focus specifically on patient-facing behavioral interventions delivered with technology (e.g., text messaging, apps, websites).
Recent Findings:
Eleven articles were included in the review. Most studies (n=9) examined barriers and facilitators to implementation, while about half (n=5) integrated the intervention into clinical care and evaluated implementation and/or effectiveness. Only six studies applied a theory or framework. The most common determinants of implementation were time constraints for clinic staff, familiarity with technology, knowledge of the intervention, and perceived value. We found substantial variation in implementation outcomes, including which were reported, how they were assessed, and the results. In the four studies that evaluated effectiveness, hemoglobin A1c improved.
Summary:
Successful implementation of technology-delivered interventions has the potential to transform healthcare delivery and improve diabetes health on a population level. Promising strategies to address common determinants of implementation include appointing a clinic champion, developing staff training and educational materials, and adapting intervention processes to the clinic context. Future research should evaluate these implementation strategies to understand when and how they impact outcomes. Frameworks such as Reach Effectiveness Adoption Implementation Maintenance (RE-AIM), can help ensure outcomes are systematically reported and allow for comparison across studies.
Keywords: technology, interventions, diabetes, self-management, implementation, mobile health
Introduction
Among patients with diabetes mellitus, self-care is essential to achieve adequate glycemic control and prevent complications; however, self-care is challenging [1, 2]. Self-care behaviors include healthy eating, physical activity, blood glucose monitoring, and taking medications, which all require sufficient knowledge, motivation, and behavioral skills [1, 2]. Diabetes self-management education and support is recommended as a critical component of care for all individuals with diabetes to assist in performing and sustaining these behaviors [3]. Despite increased availability of self-management programs, numerous barriers impact access and uptake including cost, transportation, competing responsibilities, and inconvenient venues and timing [4, 5].
Technology-delivered interventions are an innovative solution to provide self-care education and support remotely and conveniently. Over 96% of U.S. adults own a cellphone that can receive text messages, 90% use the Internet, and 81% own a smartphone through which they can access the Internet and applications [6, 7]. Further, smartphone ownership is now relatively common across different economic, educational, and racial/ethnic backgrounds [8]. Personalized content can be delivered via technology to address challenges to self-care. Evidence is accumulating that technology-delivered diabetes self-care interventions are effective at improving self-management behaviors and clinical outcomes [9]; however, most of this research does not proceed past efficacy trials [9].
The research to practice gap is a significant problem in healthcare, generally. It has been widely reported that it takes 17 years to turn 14% of original research to the benefit of patient care, attributable to a loss of research along different stages of a pipeline from research to practice [10, 11]. Technology-delivered interventions may help bridge this gap given opportunities for rapid iteration, testing, and improvement [12–14]. However, despite the advantages these interventions offer – both in supporting patients’ self-care efforts and accelerating the speed of translation – integrating them into practice remains difficult [15–17]. Due to their complexity, many factors must be considered including the intervention’s cost, fit with existing systems, and impact on communication between health professionals and patients. [15]. Applying principles from implementation science allows us to systematically examine these multi-level factors and explore how to effectively integrate evidence-based programs into healthcare [18].
Understanding the implementation of technology-delivered interventions helps ensure more patients can benefit from these innovations and applications of behavioral science. The purpose of this narrative review is to provide an overview of existing evidence on the implementation of technology-delivered diabetes self-care interventions into clinical care. Specifically, we report on the current state of the science regarding the types of technology-delivered interventions being implemented, approaches to studying implementation, and relevant results. We discuss potential opportunities to grow our understanding of how to effectively integrate self-care technologies into practice.
Methods
Data Sources and Search Strategy
In December 2019, we used PubMed to search the published literature from December 2009 through December 2019 for articles reporting on the implementation of technology-delivered diabetes self-care interventions. For the purpose of this review, we focused on behavioral interventions delivered with technology (e.g., websites, apps, text messages). The online supplement reports details of the search strategy.
Study Eligibility
Eligible studies (1) focused on patients with diabetes, including type 1 (T1D) and type 2 diabetes (T2D) and prediabetes, (2) involved a behavioral intervention delivered with a patient-facing technology, and (3) assessed the proposed or actual implementation of the intervention in clinical care. We excluded studies that involved medical devices (e.g., insulin pumps), focused on telehealth, or were purely telephonic in nature. Additionally, we excluded studies if they were a systematic review, meta-analysis, or protocol paper.
Our search terms identified 234 published articles. Authors LAN and AN independently reviewed the abstract of each identified article. Studies were excluded at this stage if both reviewers agreed eligibility criteria were not met. LAN reviewed the full text of the remaining articles, excluding those not meeting eligibility criteria, resulting in 11 articles being included in our review (see Fig. 1).
Figure 1.
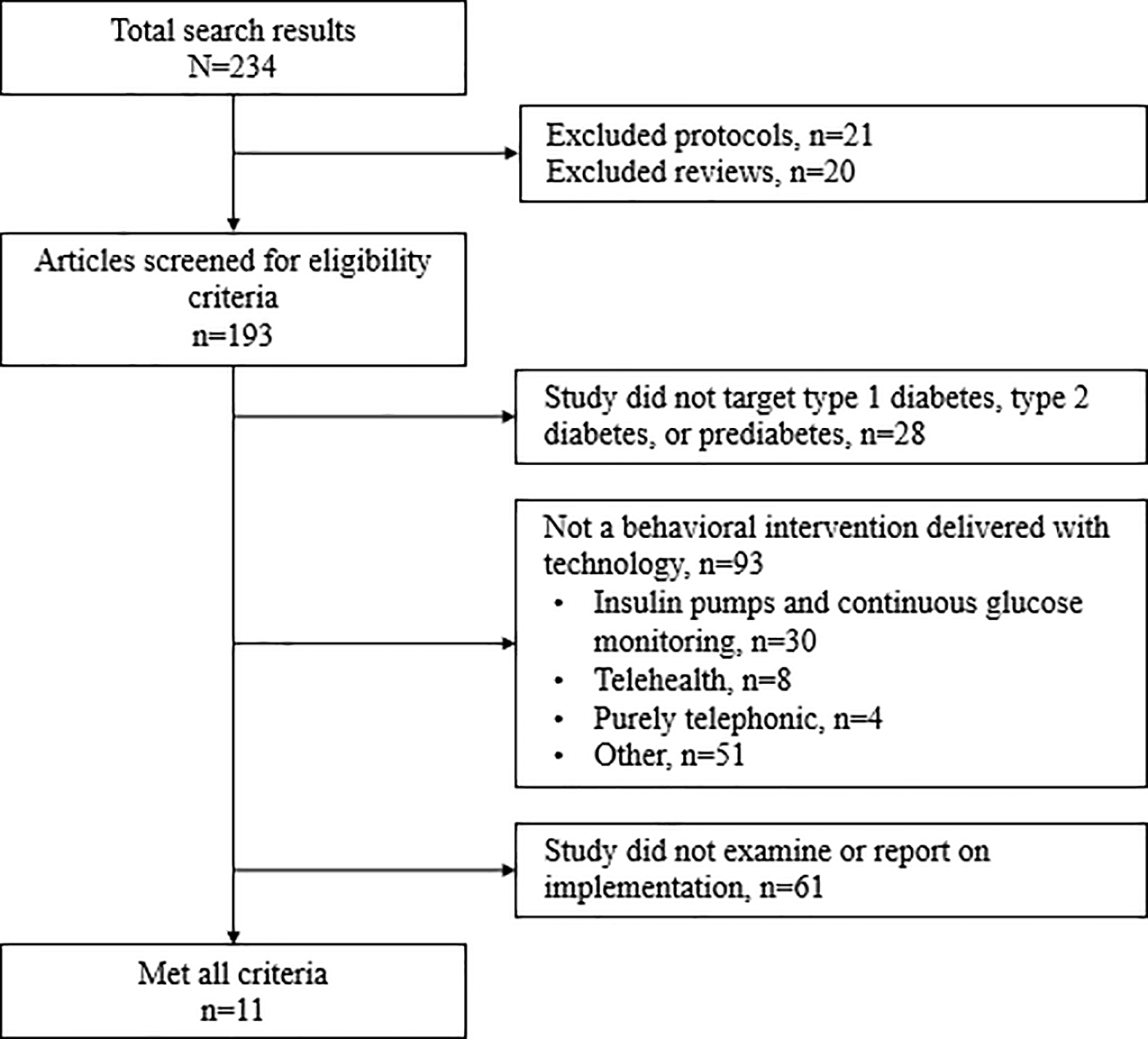
Flowchart for excluding articles from the review
Data Abstraction and Narrative Synthesis
Given variation in the types of technologies, methodologies employed, and outcomes assessed between the 11 included studies, we used a narrative synthesis approach [19] for mapping and understanding the breadth of research available. Based on the content of the articles, we organized our results by commonly reported elements: types of interventions implemented, theories and frameworks, barriers and facilitators, implementation strategies, and implementation and clinical outcomes. Table 1 reports on the study characteristics. Although all the studies involved a patient-facing technology, some also included a technology with a clinician-facing component (n=4). In addition, all the studies included a technology-delivered intervention, however several also included a human component for patients (either in-person or via phone calls) (n=3).
Table 1.
Characteristics of studies included in this review
| Authors [reference] | Country | Study Design; Assessed proposed or actual implementation | Sample size; Population/Setting | Intervention characteristics/technology | Purpose/Area of Implementation Studied | Results |
|---|---|---|---|---|---|---|
| Abidi et al. [20] | Canada | Qualitative study; Proposed implementation | Usability study: N=11 patients with T2D; Focus group study: N=3 PCPs & 4 patients with T2D | Diabetes Web-Centric Information and Support Environment (DWISE) is a computerized decision support platform with PCP (accessible via web) and patient (accessible via web and mobile app) tools for behavior change support. | Explore PCP and patient perspectives on DWISE usefulness, and implementation barriers and facilitators | Usability study: most issues related to screen layout, design features, clarity of content, and navigation Focus group study: barriers included PCP time constraints and PCPs’ and patients’ lack of technology adeptness; facilitators included personalized self-management |
| Ayre et al. [21] | Australia | Qualitative study; Proposed implementation | N=25 PCPs recruited from 50 clinics | Self-management mobile app for patients featuring individualized care plan, self-monitoring data, prompts to visit PCP, and education. | Explore PCP perspectives on proposed features for a self-management mobile app, including implementation barriers and facilitators | Barriers included PCPs’ concerns about increased workload and liability, and preference for communicating information face-to-face. Facilitators included PCPs’ perception the app might facilitate communication between visits and complement rather than replace in-person visits. |
| Bolin et al. [22] | United States | Mixed-methods pilot study; 11-month study period; Actual implementation | Tracked usage across 5 sites serving low-income populations in South Texas; Collected exit interviews with N=179 users; 45% T2D, 21% at-risk for T2D, 66% Latino | Diosk is a touch-screen kiosk providing diabetes self-management education “on-demand” to patients in English and Spanish language; 12 modules on self-management topics (e.g., exercise, meal planning) | Examine the implementation, use, and sustainability of Diosk | Diosk was accessed 5377 times; 1328 users were return users. Challenges maintaining Diosk included organizational capacity to host wireless Internet and establishing “office champions” responsible for overseeing Diosk. Users unfamiliar with technology experienced challenges with the touch screen. 3 of 5 study sites anticipated sustaining Diosk past the study period. |
| Cooper et al. [23] | England | Mixed methods feasibility study, pre-post design; 6-month study period; Actual implementation | Evaluation: N=89 adolescents with T1D; Survey: N=11 clinic staff; Focus group: N=12 clinic and research staff; Recruited from three pediatric diabetes centers in the North West of England | Adolescent Diabetes Needs Assessment Tool (ADNAT) app supports diabetes self-care decision-making. Accessed via Internet on mobile devices. Results of a needs assessment are communicated to providers to guide patients’ care plan. | Examine the feasibility of implementing ADNAT in pediatric diabetes care | Adoption: 89 patients recruited of which 44 patients (49%) completed ADNAT and 45 (51%) were non-completers. After adjusting for baseline HbA1c and site, completers had a post-intervention mean HbA1c 0.5% (5.42 mmol/mol) lower than non-completers. Survey and focus group data from diabetes care teams demonstrated positive perceptions of ADNAT’s effectiveness. |
| Dickenson et al. [24] | United States | 3-arm cluster randomized trial; 18-month study period; Actual implementation | N=36 primary care practices (18 in Colorado, 18 in California); 27 were community health centers; practices randomized to one of 3 arms | Connection to Health (CTH) uses interactive behavior change technology to help clinics deliver diabetes self-management support; Components include an online patient assessment, decision support tools for clinicians, and online self-management resources for patients. | Compare implementation and effectiveness of the 3 study arms: (1) self-management support, (2) self-management support plus CTH, and (3) self-management support plus CTH with brief practice facilitation | In intent-to-treat analysis, HbA1c trajectories did not differ significantly between study arms. However, patients who used CTH at study sites assigned to self-management education plus CTH with practice facilitation showed an improvement in HbA1c trajectory compared to patients at practices assigned to non-technology self-management support education (p=.0422). |
| Levy et al. [25] | United States | Single group, pre-post design; 12-week study period; Actual implementation | N=113 patients with T2D from 2 safety net health care systems in New York City; 79% Hispanic | Mobile Insulin Titration Intervention (MITI) uses daily weekday text messaging and weekly phone calls from registered nurses to help patients achieve their optimal (basal) insulin dose without coming into clinic for care. Texts available in English and Spanish. | Examine MITI’s effectiveness and implementation outside of a randomized controlled trial | Clinicians referred 170 patients to MITI, 129 (76%) were eligible and 113 (88%) enrolled. 95/113 (84%) reached their optimal (basal) insulin dose in an average of 24 days. HbA1c decreased from 11.4% to 10.0%, p<.001. Patients responded to 90% of text message prompts, and 85% of attending physicians made ≥1 referral to MITI. Patients reported comfort sharing information over text and texts reminded them to take insulin, check glucose, and eat healthy. |
| Oberg et al. [26] | Sweden | Qualitative study; Proposed implementation | N=20 primary care nurses from 5 health centers in northern Sweden; 100% female | Did not study a specific intervention, but rather focused generally on eHealth services to support self-management | Describe nurses’ perceptions of using eHealth systems and services to support patient self-management | Nurses perceived eHealth interventions as inevitable and with some advantages. However, nurses expressed concerns about loss of visibility and control in their daily routines as care transitions from in-person visits to eHealth. Nurses were concerned about losing their expert role in providing advice to patients and noted the growth of eHealth required them to not only have clinical knowledge but also be tech savvy. |
| Okazaki et al. [27] | Japan | Cross-sectional survey; Proposed implementation | N=471 physicians across Japan; diverse medical specialties; 87% male | Mobile diabetes monitoring (MDM) is accessible via mobile device and enables self-monitoring of blood glucose and other behaviors (e.g., exercise and medication taking) data export, physician-patient communication, and synchronization with patients’ personal health data at the hospital’s information hub. | Validate the explanatory model of factors affecting adoption of MDM among physicians | Physicians’ intention to use MDM was primarily influenced by perceived net benefits and value, and subjective norms. Privacy and security concerns had no significant influence on the intention to use MDM. |
| Rogers et al. [28] | United States | Qualitative study; Actual implementation | N= 39 patients with T2D; N=19 clinic staff members | MITI, see above (Levy, 2018) | Identify barriers and facilitators to MITI implementation | Facilitators to implementation included: convenience, no cost to patients, ease of use, patients’ confidence in their ability to use it, compatibility with patients’ daily routines and clinic workflow, patients’ and staff’s perceptions of value, and strong implementation climate and program champion. Barriers included: language limitations, nursing concerns about scope of practice, clinicians’ initial lack of knowledge about the program and perceptions that MITI might not be appropriate for some patients (e.g., older patients). |
| Ross et al. [29] | England | Mixed methods, descriptive study; Actual implementation | N=21 clinic staff (including physicians, nurses, administrators, and support staff); recruited from 34 general practices in London | Healthy Living for People with Type 2 Diabetes (HeLP-Diabetes) is an online self-management program accessible via computer, tablet, or mobile device; Content includes 8 topics in self-managements including emotional management, lifestyle change, etc. | Describe the development of a theoretically-based implementation plan for HeLP-Diabetes and identification of barriers and facilitator to implementation | Normalization Process Theory informed the development and selection of individual implementation strategies including engagement of local leaders, provision of educational materials, visits and meetings, and audits, feedback and reminders. Facilitators to implementation included strong understanding about HeLP-Diabetes and its value among clinic staff. Barriers included time constraints and limited resources. |
| Watterson et al. [30] | United States | Mixed-methods, quasi experimental pilot study; 12-week study period; Actual implementation | Evaluation: N=50 patients with T2D from 2 FQHCs in Los Angeles, CA; 66% Spanish-speaking Interviews: N=11 patient users and N=8 clinic staff | CareMessage is a text message-based program sending 3–4 educational messages per week; Most messages are bidirectional; Available in English and Spanish language. Includes 10 self-management themes including understanding diabetes, medication adherence, and nutrition. | Examine clinical effectiveness and implementation of CareMessage including barriers and facilitators to implementation | Mean HbA1c decreased by 0.4% among patients receiving CareMessage relative to the comparison group (p=0.06). Highly engaged patients (response rate ≥median of 64.5%) had a 2.2% reduction in HbA1c compared to patients who were less engaged (p<.001). Implementation facilitators included the need for relatively few resources. Barriers included reliance on volunteers to enroll patients in program limiting integration into routine care. |
PCP primary care practitioner, T2D type 2 diabetes T1D type 1 diabetes; FQHCs federally qualified health centers, HbA1c hemoglobin A1c
Results
Most of the studies in the review (n=10) were focused on T2D; only one was focused on T1D. Nearly three fourths (73%) of the articles were published in the past two years (i.e., 2018–2019) and 55% were studies conducted outside of the U.S. Nine of the studies collected qualitative data from patients and/or healthcare professionals on an intervention’s implementation potential, including barriers and facilitators to implementation [20–23, 26–30]. Five studies integrated an intervention into clinical care and evaluated implementation and/or effectiveness outcomes (three of which also assessed barriers and facilitators [22–25, 30]), and one study focused primarily on the development of an implementation strategy [29].
Overview of Types of Technology-Delivered Interventions
Among the 11 articles included in the review, the types of technologies varied. Two articles reported on different facets (i.e., implementation potential and evaluation) of the same intervention [25, 28], and one reported more generally on eHealth services supporting diabetes self-management [26], resulting in 9 unique interventions. Most interventions were delivered with mobile phones including five app-based systems [20, 21, 23, 27, 29] (two of which could also be accessed via websites [20, 29]), one text message-based system [30], and one that relied on both text messages and phone calls [25, 28]. One intervention was a web-based program which could be accessed on a computer, tablet, or smartphone [24]. Lastly, one intervention was delivered via a kiosk [22]. Nearly all the interventions were focused on providing diabetes self-management support broadly and covered a variety of topics, although some were designed with more specific goals (e.g., helping patients find their optimal basal insulin dose [25, 28]. Four interventions shared patients’ health data collected via the technology with clinic staff [21, 23–25]. Intervention details are listed in Table 1.
Theories/Frameworks
About half (n=5) of the studies noted the use of a theory or framework to inform the planning/understanding of implementation or to guide implementation evaluation. Descriptions of these theories/frameworks are reported in Table 2. Normalization Process Theory (NPT) [31] was used in two studies, both to inform the selection of strategies for implementing an online self-management program [29], and for organizing example strategies for implementing a diabetes app [21]. The Consolidated Framework for Implementation Research [32] was used in one study [28] to inform the interview and analytic approach for assessing barriers and facilitators to implementation. To guide which outcomes to assess in an implementation evaluation, two studies [23, 24] employed the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework [33, 34], while one study [25] used Proctor et al.’s implementation outcomes framework [35].
Table 2.
Implementation Theories and Frameworks Included in Narrative Review
| Theory/Framework | Description | Relevant studies in review |
|---|---|---|
| Normalization Process Theory (NPT) | An explanatory model for understanding the social processes through which new technologies or complex interventions are implemented. NPT is based on 4 constructs: coherence, cognitive participation, collection action, and reflexive monitoring. |
Ayre et al.
(2019); a diabetes self-management app Ross et al. (2018); HeLP-Diabetes |
| Consolidated Framework for Implementation Research (CFIR) | A planning framework that provides a taxonomy of constructs associated with effective implementation. Constructs are arranged across 5 domains: inner setting, outer setting, intervention, individuals involved, and process. The CFIR is often used to assess potential barriers and facilitation to implementation. | Rogers et al. (2019); Mobile Insulin Titration Intervention (MITI) |
| Reach Effectiveness Adoption Implementation Maintenance (RE-AIM) Framework | A planning and evaluation framework that provides specific and standard ways of measuring key factors for improving the implementation and sustainability of evidence-based interventions (reach, effectiveness, adoption, implementation, and maintenance). | Cooper et al. (2019); Adolescent Diabetes
Needs Assessment Tool (ADNAT) app Dickinson et al. (2019); Connection to Health (CTH) |
| Proctor’s Framework for Implementation Research | A planning and evaluation framework that provides a taxonomy for conceptualizing and measuring 8 implementation outcomes (acceptability, adoption, appropriateness, feasibility, fidelity, implementation cost, penetration, and sustainability). | Levy et al. (2018); MITI |
Barriers and Facilitators to Implementation
Methods of Study
Most studies (n=9) examined barriers and facilitators to implementation, either as part of a proposed (n=4) or actual (n=5) implementation. Information regarding barriers and facilitators was most commonly elicited via qualitative interviews with patients and/or clinic staff (including clinicians, nurses, and administrators) involved in the implementation process, although two studies used focus groups.
Barriers
Clinic staff commonly reported time constraints, and concern the intervention would increase workload/burden, as barriers to implementation [20–23, 29, 30]. Most programs required patient identification and registration which clinic staff mentioned would be difficult due to their demanding schedules and understaffing [29, 30]. In the Diabetes Web-Centric Information and Support Environment (DWISE) study, clinicians noted their already limited time with patients to deliver all necessary care and felt inclusion of DWISE in the patient encounter might not be feasible [20]. Similarly, clinicians were concerned that adopting an intervention would change the scope of their practice/work including added responsibilities and liability [20, 21, 28]. For example, clinicians in two studies had concerns the interventions’ real-time alerts about patients’ blood glucose results would create pressure to respond in a timely matter [21, 28].
Patients’ and clinicians’ lack of familiarity or comfort with technology was also a common barrier to implementation [20–22, 28]. In the DWISE study, patients and clinicians noted not being ‘tech-savvy’ could dissuade them from using the intervention [20]. Similarly, clinicians viewed interventions as potentially inappropriate for patients they perceived as having difficulty with technology (e.g., older patients and those with visual impairments) [21, 28]. In the Diosk study, some users unfamiliar with technology experienced challenges using the kiosk touch screen [22].
Limited awareness and knowledge of the intervention among clinic staff was also identified as a barrier to implementation [28–30]. In the early implementation of MITI, staff lacked knowledge of the intervention components, available languages, and protocols for intervention administration [28]. In the CareMessage study, clinic volunteers helped enroll patients in their text message-based intervention; although this helped minimize workflow disruption, it limited integration into routine practice and clinicians lacked knowledge of the program [30]. Ross et al. [29] attributed staff’s unawareness of the HeLP-Diabetes app to a lack of communication about the program among staff members. Other barriers, though less commonly mentioned, included a preference for communicating information face-to-face [20, 21], the need for improved Internet access in clinics [22, 23], and a need for program updates [22].
Facilitators
The most common facilitator of implementation was perceived value of the intervention by both patients and clinic staff [20–23, 28, 30]. Patients appreciated receiving diabetes education via their mobile devices and felt the interventions could help improve awareness of their diabetes and diabetes management [20, 22, 28]. Similarly, clinicians felt interventions helped keep patients engaged in their self-care between clinic visits, and valued the personalized education and teaching tools that interventions provided [20, 21, 30]. In addition, clinicians valued interventions could gather and provide access to information on patients outside of the clinic [12, 13] which could help inform clinic visits [20]. Clinicians also appreciated interventions’ potential to facilitate and improve communication with their patients [20, 21, 28].
Another facilitator of implementation was low cost for use, delivery, and upkeep of the intervention [28, 30]. Rogers et al. [28] reported the free cost of MITI facilitated use with many patients noting uncertainty about whether they would enroll if there were an associated cost. Likewise, in the CareMessage study, staff appreciated the texting program allowed them to provide education to patients using relatively few resources, making the implementation more feasible in a resource-limited setting [30].
Other facilitators were less commonly mentioned and based on specific intervention characteristics. These included: (1) low complexity of the intervention for user-friendliness [28]; (2) intervention elements that allow for personalization to support users’ preferences and self-management needs [20, 21]; and (3) automation of intervention components to reduce burden and improve integration with existing clinic workflows [21].
Implementation Strategies
Implementation strategies are the specific actions taken to enhance the adoption, integration, and sustainability of EBIs and are intended to address the determinants of implementation (e.g., barriers and facilitators) [37]. Of the 11 articles in the review, four described strategies for implementing the intervention.
The most detailed account of strategy selection was reported for the HeLP-Diabetes intervention [29]. Ross et al. first conducted a literature review to identify barriers and facilitators to digital health implementation, and then engaged key stakeholders (e.g., diabetes clinicians and educators) to understand the implementation context. Next, they used the Cochrane Effective Practice and Organisation Care (EPOC) taxonomy of implementation strategies [38], evidence gathered in the previous phases, and NPT constructs to select the most appropriate strategies to implement the intervention. The full list of strategies and operational definitions are listed in their article and include educational meetings and materials, local opinion leaders, and continuous quality improvement [29].
Three studies described strategies for implementing their intervention but did not justify strategy selection [23–25]. Each of these studies referenced using a point-person in the clinic who was responsible for overseeing the intervention and providing support. The MITI study [25] used a full-time study coordinator, while in CareMessage [23], they designated an on-site research nurse in each clinic. Similarly, in Connection to Health (CTH), they used practice facilitation [39] as their implementation strategy which includes a trained practice facilitator who assists with and supports implementation. Also mentioned in all three studies was training pre-implementation for clinic staff about the intervention and how to use it [23, 25]. The MITI study specifically trained on technical requirements (e.g., logging into the system, monitoring text messages) [25]. During implementation, the studies mentioned different strategies to offer continued support. For MITI, the study team attended routine staff meetings to provide updates and get feedback [25], whereas in CareMessage, they provided clinic staff with access to a developer who staff could contact via email to receive technical support [30]. In CTH, the practice facilitator offered “booster” sessions to address any ongoing problems [24].
Two studies detailed whom in the clinic was responsible for specific intervention processes. In the CareMessage study [30], clinic front desk staff identified eligible patients from a preprinted list when they checked in for their appointment and then referred patients to a volunteer who informed the patient about the program and helped them enroll. In MITI, clinicians referred patients to the program during a clinic visit but different enrollment models were used based on clinic site (i.e., enrollment was either completed with an on-site study coordinator or with a team nurse as part of a discharge process) [28].
Common across the studies were strategies that involved a point-person in the clinic to facilitate the implementation and training both prior to and during implementation. Specific actions to employ these strategies varied based on the intervention and clinic context.
Evaluation of Implementation and Clinical Outcomes
Five studies in the review evaluated the implementation of an intervention and measured implementation and effectiveness outcomes. Three studies were pilot studies intended to evaluate implementation feasibility [22, 23, 30], one was an implementation study using a single group pre-post design [25], and one used a cluster-randomized trial to compare the effectiveness of three implementation strategies [24]. More details about the study design, sites, and duration are reported in Table 1.
Implementation Outcomes
Reach, or the absolute number and proportion of individuals willing to participate in the intervention, was reported in three studies. In the CTH study, a random sample of charts was audited for diabetes outcomes as part of an intention to treat (ITT) sample; enrollment was slightly higher in the sites that received practice facilitation to implement the CTH program (6%; 23/385) versus sites that only received the CTH program (1.4%; 5/360), but overall was very low (~4%) [24]. Other studies reported the percentage of participants enrolled based on those approached. In MITI, of the 170 patients approached, 76% (129/170) were eligible, and of those, 88% (113/129) chose to enroll [25]. In CareMessage, 77% of those approached were both eligible and enrolled in the program [30]. Finally, in Diosk, total usage of the kiosks included 5377 uses during the 11-month study period (450–600 uses/month); of these, 1328 were return users resulting in 4044 unique users [22]; because the kiosks were made available at varied settings including clinics, a grocery store, and a community center, the study did not report a number of potentially eligible users.
Adoption, or the absolute number and proportion of settings and clinic staff involved in the intervention who are willing to initiate the program, was seldom reported. During MITI’s implementation, most clinicians did make at least one patient referral to the program supporting its adoption [25]. Patient adoption or engagement with the intervention was reported in a few studies and levels tended to vary. In MITI, patients responded to 90.1% of daily text messages over 12 weeks asking for their fasting blood glucose each morning [25]. Alternatively, in CareMessage, the average response rate to texts asking about diabetes education was 57.1% (SD:33.2%) over 12 weeks [30]. In Diosk, the average time spent per viewing session on the kiosk was 6.92 minutes (range:1–20 minutes) [22].
The only study that reported on cost was the MITI intervention [25]. Based on their analysis, the value of patients’ time and the cost of prevented clinic visits outweighed the cost of the MITI program. They calculated the per-patient per-week savings at each of their sites which ranged from $0.94 to $185.80 (based on site and number of patients who would participate per year). Lastly, sustainability or maintenance was only reported in Diosk [22]. Concerns related to sustainability included funding, Internet support, and dedicated staff members to check the Diosk daily. The researchers asked the sites to provide a plan to help enable sustainability. Three of the five partnering sites asked to sustain their Diosk delivery [22].
Effectiveness Outcomes
Four studies assessed the clinical effectiveness of the intervention. After adjusting for baseline hemoglobin A1c (HbA1c) and site, patients who completed the Adolescent Diabetes Needs Assessment Tool (ADNAT) had a post-intervention mean HbA1c 0.5% lower than non-completers [23]. The MITI study showed a statistically significant decrease in HbA1c from 11.4% (pre-intervention) to 10.0% (post-intervention) and 84% reached optimal basal insulin dose in an average of 24 days [25]. The CareMessage study showed a reduction in HbA1c of 0.4% relative to the comparison group at follow-up and higher levels of engagement with the intervention were associated with greater HbA1c reduction [30]. Finally, while the ITT analysis of patients at practices assigned to receive CTH did not show a significant reduction in HbA1c, patients who used CTH at sites receiving practice facilitation did show an improvement in HbA1c trajectory compared to patients from study sites assigned to non-technology self-management education [24]. Overall, the findings across the four studies demonstrate potential efficacy of technology-delivered diabetes self-care interventions to improve glycemic control when implemented in routine clinical care.
Discussion
There is a strong and growing evidence base for the efficacy and acceptability of technology-delivered diabetes self-care interventions [9, 40, 41]. However, our understanding of whether and how these interventions can be implemented into clinical care, such that their benefits can be realized by the wider population of patients with diabetes, is limited. We conducted a narrative review of studies published between 2009 and 2019 reporting on the implementation of technology-delivered diabetes self-care interventions. Most interventions in the review were focused on T2D, delivered via mobile phones, and app-based. Most studies in our review examined barriers and facilitators to implementation, while about half integrated the intervention into clinical care and evaluated implementation. The most common determinants of implementation were time constraints for clinic staff, familiarity with technology, knowledge of the intervention, and perceived value. Although rarely reported, implementation strategies included designating a core person as responsible for overseeing the implementation and training for clinic staff. In the studies that evaluated implementation, designs and measures varied, but HbA1c improved in the studies that assessed clinical effectiveness. Based on our findings, we make recommendations for studying implementation of technology-delivered diabetes self-care interventions.
As the field has evolved, the importance of establishing a theoretical basis in implementation research has been more widely recognized [36]. Implementation theories, models, and frameworks have three overarching aims: 1) describe/guide the process of implementation; 2) understand/explain determinants of implementation; and 3) evaluate implementation [36]. Among the six studies in our review that mentioned the use of an implementation theory, NPT (primarily used for understanding/planning) [31] and RE-AIM (primarily used for evaluation) [34] were most common. None of the theories/frameworks mentioned in the review were specific to technology-delivered interventions; however, both NPT and RE-AIM have been successfully applied when implementing technology-delivered interventions in healthcare settings for other conditions [17, 42, 43]. As part of a recent review, Yoshida et al. [44] recommended using RE-AIM when evaluating text message- and app-based interventions for diabetes management to improve their translation into practice.
The implementation barriers and facilitators identified in our review are consistent with those identified in other reviews. Granja et al. [45] reviewed factors contributing to the success and failures of implemented eHealth interventions and found that concerns related to clinic workflow were among the most common (e.g., increased workload, workflow disruption) ). Similarly, Alavarado et al. [46] conducted a review of barriers to the implementation of remote interventions for diabetes self-management and identified poor integration of technologies into workflow and technology illiteracy and access as common barriers. We found consistent evidence in our review that clinicians are concerned about the additional time and responsibilities necessary to adopt technology interventions in their practice. In addition, clinicians’ lack of awareness of the intervention, and both clinicians’ and patients’ lack of familiarity with technology hindered implementation. Alternatively, implementation was facilitated when patients and clinicians perceived the intervention as beneficial.
Implementation strategies are actions intended to address determinants of implementation such that they improve the adoption and integration of interventions into practice [37]. In our review, only one study detailed a very specific approach to their strategy selection; in a separate report, adoption rates for the program were high (i.e., 22 of 34 practices chose to offer the program to their patients).[47] Because technology-delivered interventions can vary considerably based on their components and delivery method, studies should assess any unique implementation determinants to guide their strategies [48]; however, based on our review and others [45, 46], there may be key strategies to consider. Designating a point-person as responsible for overseeing the intervention (i.e., a ‘clinical champion’) may help combat time constraints and related workload concerns. This person helps ensure processes are followed and provides clinic staff with ongoing support; the clinic context should guide whom in the clinic is best suited for this role. To avoid workflow disruption, clinic staff can help identify ways intervention processes can be adapted to fit the clinical context. For example, staff may enroll patients as part of another clinic process, or, when possible, enrollment could occur via the technology (e.g., text message or online portal). Staff trainings and educational materials also appear to be a critical strategy to help improve awareness and knowledge of the intervention and its perceived value, both prior to and during implementation. Especially in the context of a technology-delivered intervention, visuals and/or videos may be useful for relaying functionality aimed at improving patients’ health (e.g., tailored education). Lastly, technology support appears critical for both clinicians and patients and could take several forms (e.g., hotline number, email).
Several approaches are available for evaluating implementation of EBIs into clinical practice [49]; selection can be complex and based on many factors including the research question, existing evidence base, and organizational values [50]. In our review, most studies pursued pilot, non-randomized designs conducted in one or a small number of clinical settings. Only four studies reported clinical outcomes, but HbA1c improved in the studies that assessed it. We found considerable variation in the implementation outcomes, including which were reported, how they were assessed, and the results. At present, there is a paucity of research addressing benchmarks for these outcomes or how to decide a priori what indicates successful implementation. Reach was most commonly reported and was relatively high in both MITI and CareMessage, but low in CTH; since all three interventions used similar strategies, unique aspects of the context and/or intervention may have contributed to the difference. To advance the science of implementation surrounding technology-delivered interventions, we need more research that systematically evaluates which implementation strategies work, when, and for whom; this could technically be explored through several research designs, but involves a thorough understanding of relevant contextual factors (e.g., clinic size, policy environment, leadership engagement) [51].
Limitations include variability in the objectives, designs, and measures across the studies, preventing us from drawing conclusions about which strategies were most effective; rather we base recommendations on patterns observed. We focused on studies that included technology as the basis for their intervention, but several included a human component which may have influenced the approach and findings. In addition, few studies reported implementation outcomes, including adoption, cost, and maintenance, limiting our ability to report on these elements of implementation. Despite our attempt to identify relevant articles, it is possible some were missed, and publication bias may have led to underreporting. Finally, the studies included in our review focused predominantly on interventions for patients with T2D, limiting the generalizability of our findings to other populations.
Conclusion
Despite the significant potential of technology-delivered interventions to improve diabetes self-management, there appears to be a gap between the benefits observed in research and realizing these benefits in clinical practice. This is in part illustrated by the plethora of studies examining the efficacy of these interventions [9, 41] whereas our literature search resulted in only 11 studies examining implementation. Further, only about half of the studies in the review evaluated implementation and clinical outcomes. Successful implementation can be influenced by a variety of factors at the patient-, clinician-, and setting-level. To advance our understanding of how to effectively implement these interventions, we need more studies that develop and test implementation strategies to overcome these multi-level factors. Critical examination of context, including when and how these strategies impact process and implementation outcomes, is essential to inform best practices for implementing technology-based interventions and ultimately improving diabetes health on a population level.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K12HL137943 and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Numbers K23DK106511 and R18DK123373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank Drs. Sunil Kripalani and Kenneth Wallston for their valuable feedback on the manuscript and Camille Ivey with the Vanderbilt Eskind Biomedical Library for assisting with the literature search.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: Lyndsay A. Nelson, Sarah E. Williamson, Audriana Nigg, and William Martinez declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Ahola AJ, Groop PH. Barriers to self-management of diabetes. Diabet Med. 2013. April;30(4):413–20. doi: 10.1111/dme.12105. [DOI] [PubMed] [Google Scholar]
- 2.Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord. 2013. March 5;12(1):14. doi: 10.1186/2251-6581-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck J, Greenwood DA, Blanton L, Bollinger ST, Butcher MK, Condon JE, et al. 2017 National Standards for Diabetes Self-Management Education and Support. Diabetes Care. 2017. October;40(10):1409–19. doi: 10.2337/dci17-0025. [DOI] [PubMed] [Google Scholar]
- 4.Horigan G, Davies M, Findlay-White F, Chaney D, Coates V. Reasons why patients referred to diabetes education programmes choose not to attend: a systematic review. Diabet Med. 2017. January;34(1):14–26. doi: 10.1111/dme.13120. [DOI] [PubMed] [Google Scholar]
- 5.Morgan JM, Mensa-Wilmot Y, Bowen S-A, Murphy M, Bonner T, Rutledge S, et al. Implementing key drivers for diabetes self-management education and support programs: early outcomes, activities, facilitators, and barriers. Preventing Chronic Disease. 2018;15:170399. doi: 10.5888/pcd15.170399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pew Research Center. Mobile Fact Sheet. Washington, D.C.: Pew Research Center; 2019; Available from: https://www.pewresearch.org/internet/fact-sheet/mobile/. [Google Scholar]
- 7.Pew Research Center. Internet/Broadband Fact Sheet. Washington, D.C.: Pew Research Center; 2019; Available from: https://www.pewresearch.org/internet/fact-sheet/internet-broadband/. [Google Scholar]
- 8.Anderson M Mobile Technology and Home Broadband 2019. Pew Research Center; 2019. [09–01-2020]; Available from: https://www.pewresearch.org/internet/2019/06/13/mobile-technology-and-home-broadband-2019/. [Google Scholar]
- 9.Greenwood DA, Gee PM, Fatkin KJ, Peeples M. A Systematic Review of Reviews Evaluating Technology-Enabled Diabetes Self-Management Education and Support. J Diabetes Sci Technol. 2017. September;11(5):1015–27. doi: 10.1177/1932296817713506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balas EA, Boren SA. Managing clinical knowledge for health care improvement. In: Bemmel J, McCray A, editors. Yearbook of Medical Informatics 2000: Patient-Centered Systems. Stuttgart, Germany: Schattauer; 2000. [PubMed] [Google Scholar]
- 11.Green LW, Ottoson JM, Garcia C, Hiatt RA. Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annu Rev Public Health. 2009;30:151–74. doi: 10.1146/annurev.publhealth.031308.100049. [DOI] [PubMed] [Google Scholar]
- 12.Nelson LA, Threatt AL, Martinez W, Acuff SW, Mayberry LS. Agile science: what and how in digital diabetes research. In: Klonoff DC, Kerr D, Mulvaney SA, editors. Digital Diabetes Research. Cambridge, MA: Elsevier Inc.; 2020. [Google Scholar]
- 13.Patrick K, Hekler EB, Estrin D, Mohr DC, Riper H, Crane D, et al. The Pace of Technologic Change: Implications for Digital Health Behavior Intervention Research. Am J Prev Med. 2016. November;51(5):816–24. doi: 10.1016/j.amepre.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National Institutes of Health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012. July;102(7):1274–81. doi: 10.2105/AJPH.2012.300755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.•Ross J, Stevenson F, Lau R, Murray E. Factors that influence the implementation of e-health: a systematic review of systematic reviews (an update). Implement Sci. 2016. October 26;11(1):146. doi: 10.1186/s13012-016-0510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wienert J Understanding health information technologies as complex interventions with the need for thorough implementation and monitoring to sustain patient safety. Frontiers in ICT. 2019;6:9. [Google Scholar]
- 17.Murray E, Burns J, May C, Finch T, O’Donnell C, Wallace P, et al. Why is it difficult to implement e-health initiatives? A qualitative study. Implement Sci. 2011. January 19;6:6. doi: 10.1186/1748-5908-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM. An introduction to implementation science for the non-specialist. BMC Psychol. 2015. September 16;3:32. doi: 10.1186/s40359-015-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version. 2006;1:b92. [Google Scholar]
- 20.Abidi S, Vallis M, Piccinini-Vallis H, Imran SA, Abidi SSR. Diabetes-Related Behavior Change Knowledge Transfer to Primary Care Practitioners and Patients: Implementation and Evaluation of a Digital Health Platform. JMIR Med Inform. 2018. April 18;6(2):e25. doi: 10.2196/medinform.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayre J, Bonner C, Bramwell S, McClelland S, Jayaballa R, Maberly G, et al. Factors for Supporting Primary Care Physician Engagement With Patient Apps for Type 2 Diabetes Self-Management That Link to Primary Care: Interview Study. JMIR Mhealth Uhealth. 2019. January 16;7(1):e11885. doi: 10.2196/11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolin JN, Ory MG, Wilson AD, Salge L. Diabetes education kiosks in a latino community. Diabetes Educ. 2013. March-April;39(2):204–12. doi: 10.1177/0145721713476346. [DOI] [PubMed] [Google Scholar]
- 23.Cooper H, Lancaster GA, Gichuru P, Peak M. A mixed methods study to evaluate the feasibility of using the Adolescent Diabetes Needs Assessment Tool App in paediatric diabetes care in preparation for a longitudinal cohort study. Pilot Feasibility Stud. 2018;4:13. doi: 10.1186/s40814-017-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickinson WP, Dickinson LM, Jortberg BT, Hessler DM, Fernald DH, Cuffney M, et al. A Cluster Randomized Trial Comparing Strategies for Translating Self-Management Support into Primary Care Practices. J Am Board Fam Med. 2019. May-June;32(3):341–52. doi: 10.3122/jabfm.2019.03.180254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.•Levy NK, Orzeck-Byrnes NA, Aidasani SR, Moloney DN, Nguyen LH, Park A, et al. Transition of a Text-Based Insulin Titration Program From a Randomized Controlled Trial Into Real-World Settings: Implementation Study. J Med Internet Res. 2018. March 19;20(3):e93. doi: 10.2196/jmir.9515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberg U, Orre CJ, Isaksson U, Schimmer R, Larsson H, Hornsten A. Swedish primary healthcare nurses’ perceptions of using digital eHealth services in support of patient self-management. Scand J Caring Sci. 2018. June;32(2):961–70. doi: 10.1111/scs.12534. [DOI] [PubMed] [Google Scholar]
- 27.Okazaki S, Castañeda JA, Sanz S, Henseler J. Factors affecting mobile diabetes monitoring adoption among physicians: questionnaire study and path model. J Med Internet Res. 2012. December 21;14(6):e183. doi: 10.2196/jmir.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers E, Aidasani SR, Friedes R, Hu L, Langford AT, Moloney DN, et al. Barriers and Facilitators to the Implementation of a Mobile Insulin Titration Intervention for Patients With Uncontrolled Diabetes: A Qualitative Analysis. JMIR Mhealth Uhealth. 2019. July 31;7(7):e13906. doi: 10.2196/13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross J, Stevenson F, Dack C, Pal K, May C, Michie S, et al. Developing an implementation strategy for a digital health intervention: an example in routine healthcare. BMC Health Serv Res. 2018. October 19;18(1):794. doi: 10.1186/s12913-018-3615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watterson JL, Rodriguez HP, Shortell SM, Aguilera A. Improved Diabetes Care Management Through a Text-Message Intervention for Low-Income Patients: Mixed-Methods Pilot Study. JMIR Diabetes. 2018. October 30;3(4):e15. doi: 10.2196/diabetes.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: Normalization Process Theory. Implement Sci. 2009. May 21;4:29. doi: 10.1186/1748-5908-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009. August 7;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999. September;89(9):1322–7. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, et al. RE-AIM Planning and Evaluation Framework: Adapting to New Science and Practice With a 20-Year Review. Front Public Health. 2019;7:64. doi: 10.3389/fpubh.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011. March;38(2):65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nilsen P Making sense of implementation theories, models and frameworks. Implement Sci. 2015. April 21;10:53. doi: 10.1186/s13012-015-0242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013. December 1;8:139. doi: 10.1186/1748-5908-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Effective Practice and Organisation of Care (EPOC). EPOC Taxonomy. 2015. [08–05-2020]; Available from: https://epoc.cochrane.org/epoc-taxonomy.
- 39.Baskerville NB, Liddy C, Hogg W. Systematic review and meta-analysis of practice facilitation within primary care settings. Ann Fam Med. 2012. January-February;10(1):63–74. doi: 10.1370/afm.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson LA, Spieker A, Greevy R, LeStourgeon LM, Wallston KA, Mayberry LS. User Engagement Among Diverse Adults in a 12-Month Text Message-Delivered Diabetes Support Intervention: Results from a Randomized Controlled Trial. JMIR Mhealth Uhealth. 2020. July 21;8(7):e17534. doi: 10.2196/17534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haider R, Sudini L, Chow CK, Cheung NW. Mobile phone text messaging in improving glycaemic control for patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2019. April;150:27–37. doi: 10.1016/j.diabres.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 42.May CR, Cummings A, Girling M, Bracher M, Mair FS, May CM, et al. Using Normalization Process Theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci. 2018. June 7;13(1):80. doi: 10.1186/s13012-018-0758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palermo TM, de la Vega R, Dudeney J, Murray C, Law E. Mobile health intervention for self-management of adolescent chronic pain (WebMAP mobile): Protocol for a hybrid effectiveness-implementation cluster randomized controlled trial. Contemp Clin Trials. 2018. November;74:55–60. doi: 10.1016/j.cct.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida Y, Patil SJ, Brownson RC, Boren SA, Kim M, Dobson R, et al. Using the RE-AIM framework to evaluate internal and external validity of mobile phone-based interventions in diabetes self-management education and support. J Am Med Inform Assoc. 2020. June 1;27(6):946–56. doi: 10.1093/jamia/ocaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Granja C, Janssen W, Johansen MA. Factors Determining the Success and Failure of eHealth Interventions: Systematic Review of the Literature. J Med Internet Res. 2018. May 1;20(5):e10235. doi: 10.2196/10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarado MM, Kum HC, Gonzalez Coronado K, Foster MJ, Ortega P, Lawley MA. Barriers to Remote Health Interventions for Type 2 Diabetes: A Systematic Review and Proposed Classification Scheme. J Med Internet Res. 2017. February 13;19(2):e28. doi: 10.2196/jmir.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray E, Ross J, Pal K, Li J, Dack C, Stevenson F, et al. A web-based self-management programme for people with type 2 diabetes: the HeLP-Diabetes research programme including RCT. Programme Grants Appl Res. 2018;6(5). [PubMed] [Google Scholar]
- 48.Powell BJ, Beidas RS, Lewis CC, Aarons GA, McMillen JC, Proctor EK, et al. Methods to Improve the Selection and Tailoring of Implementation Strategies. J Behav Health Serv Res. 2017. April;44(2):177–94. doi: 10.1007/s11414-015-9475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown CH, Curran G, Palinkas LA, Aarons GA, Wells KB, Jones L, et al. An Overview of Research and Evaluation Designs for Dissemination and Implementation. Annu Rev Public Health. 2017. March 20;38:1–22. doi: 10.1146/annurev-publhealth-031816-044215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercer SL, DeVinney BJ, Fine LJ, Green LW, Dougherty D. Study designs for effectiveness and translation research :identifying trade-offs. Am J Prev Med. 2007. August;33(2):139–54. doi: 10.1016/j.amepre.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Nilsen P, Bernhardsson S. Context matters in implementation science: a scoping review of determinant frameworks that describe contextual determinants for implementation outcomes. BMC Health Serv Res. 2019. March 25;19(1):189. doi: 10.1186/s12913-019-4015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


